- 1Department of Child and Adolescent Psychiatry and Behavioral Sciences, Children's Hospital of Philadelphia, Philadelphia, PA, United States
- 2Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 3Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 4Department of Psychology, University of California, Berkeley, Berkeley, CA, United States
- 5Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
Background: Effective and equitable strategies to prevent youth suicidal thoughts and behaviors (STB) are an urgent public health priority. Adolescent sleep disturbances are robustly linked to STB but are rarely addressed in preventive interventions or among Black and/or Hispanic/Latinx youth for whom STB risk is increasing disproportionately. This paper describes an application of health equity-informed implementation science models and frameworks to adapt and evaluate the evidence-based Transdiagnostic Sleep and Circadian (TSC) intervention for primary care implementation with adolescents of minoritized backgrounds with depression and STB risk.
Methods: This multiphase study protocol uses the Assessment, Decision, Adaptation, Production, Topical Experts-Integration, Training, Testing (ADAPT-ITT) model to adapt and evaluate TSC for primary care implementation with adolescents who are depressed, at risk for STB, and of primarily Black and/or Hispanic/Latinx backgrounds. We integrate the Consolidated Framework for Implementation Research (CFIR) in an initial qualitative inquiry of adolescent, caregiver, and clinician perceptions of TSC. Subsequent ADAPT-ITT phases include systematically and iteratively testing adaptations based on the qualitative inquiry, with ongoing key informant input, and then evaluating the adapted TSC for feasibility, acceptability, and efficacy in a pilot randomized trial.
Anticipated results: Based on youth depression and sleep health disparities research, we expect that TSC adaptations will be needed to enhance intervention content for adolescents with depression, STB risk, and primarily Black and/or Hispanic/Latinx backgrounds. We also anticipate adaptations will be needed to align TSC delivery methods with primary care implementation.
Conclusions: Adapting evidence-based interventions with end-users and contexts in mind can help ensure that intervention strategies and delivery methods are acceptable to, and feasible with, health disparate populations. Although TSC has shown effectiveness for adolescents with sleep disturbances, we expect that additional multiphase research is necessary to optimize TSC for primary care delivery with Black and/or Hispanic/Latinx adolescents with depression and STB risk.
Introduction
Youth suicide is a significant public health concern, ranking as the second leading cause of death for young people worldwide (1). In the United States, suicide attempts and deaths have increased more rapidly among African American, Caribbean American, and other Black American (hereafter referred to as “Black”) youth compared to any other racial or ethnic group (2, 3). Disproportionate increases in suicide risk are also apparent in Hispanic/Latinx youth (hereafter, ‘Latinx’), underscoring the need for preventive efforts that are culturally tailored to address these disparities (4). However, few effective interventions exist for adolescent suicidal thoughts and behaviors (STB) (5), and those that are available have been tested with youth of primarily non-Hispanic/Latinx White (hereafter, ‘White’) backgrounds (6). This research gap raises questions about whether such treatments are similarly effective among youth of minoritized backgrounds, or whether culturally responsive adaptations would enhance effectiveness. These open questions and observed racial and ethnic disparities reflect an urgent need for effective and equitable STB prevention in adolescence.
Sleep as an optimal target of adolescent STB prevention
To effectively prevent adolescent STB, interventions must focus on risk factors that are acute, proximal, and modifiable (7). Sleep disturbances are among the few risk factors that meet these criteria, but are rarely addressed in preventive interventions for youth STB (8, 9). A range of subjective sleep and circadian problems (e.g., insomnia symptoms, poor perceived sleep quality, sleeping much of the day, daytime sleepiness) and objective indicators of poor sleep health (e.g., short sleep duration, high variability, late bedtimes) (10) are robustly associated with the continuum of STB (11, 12), from suicidal ideation (13) to death by suicide (14). In addition to these temporal linkages with STB, sleep disturbances are implicated in the onset and maintenance of depressive symptoms in adolescence (15, 16), one of the strongest risk factors for youth STB (17). Moreover, sleep disturbances are modifiable, with a growing body of research supporting the efficacy of cognitive and behavioral approaches in treating youth sleep problems as well as comorbid mood concerns (8, 18). Findings from adult research demonstrate the potential for sleep treatment to improve STB. Two randomized controlled trials (RCTs) have shown that cognitive-behavioral (19) or pharmacological treatment (20) of insomnia yields post-treatment reductions in STB among adults, supporting the value of addressing sleep disturbances to prevent STB.
Adolescent sleep health disparities
Racial and ethnic sleep health disparities are well-documented in adolescence (21). Sleep-related risk factors for increased STB, such as a short sleep duration, poor sleep quality, and variable sleep timing, are more prevalent among Black and/or Latinx youth compared to White youth (21–24). Both social and environmental factors contribute to sleep health disparities. Black youth are more likely than their White peers to live in lower socioeconomic status (SES) homes and neighborhoods (25, 26), which can contribute to poor sleep via environmental factors including high levels of light, noise, household crowding, and community violence (27–30). In addition, among Black and/or Latinx youth, exposure to racism and discrimination at multiple levels (i.e., systemic/institutional; personally mediated; internalized) (31–34) can contribute to sleep difficulties, including long sleep onset latency and poor sleep quality (35, 36). For example, in a study of Black, Latinx, and Asian American youth, experiences of discrimination were associated with same-day sleep disturbances (37). It is also possible that stressors related to racism and discrimination exacerbate the adverse impacts of sleep-disrupting environmental factors (30). For instance, in one study community violence concerns were linked to short and poor quality sleep in Black but not White adolescents, who most likely do not experience daily discrimination (38).
The need for a culturally tailored sleep intervention for youth with STB risk
Experiencing more sleep problems compared to their White counterparts may put Black and/or Latinx youth at increasingly higher risk for depression and STB (39). Figure 1 presents a proposed conceptual model in which social-environmental risks, including social determinants of health, racism, and discrimination, and well-established behavioral risks factors (e.g., prior STB, hopelessness, depression) (3, 39) collectively contribute to sleep and circadian disturbances and, ultimately, STB risk via proximal affective and behavioral dysregulation. Accordingly, treating sleep disturbances could improve affective and behavioral regulation, in turn reducing depression and risk for STB (8, 9). A sleep-focused intervention to decrease STB risk may be especially well-suited for Black and/or Latinx youth with depression and sleep disturbance, given stigmatization of mental health treatment (40, 41).
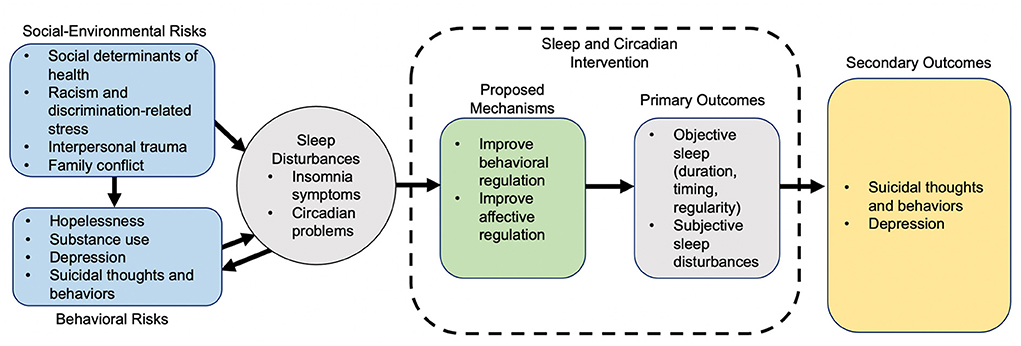
Figure 1. Conceptual model of adolescent risk factors, sleep disturbances, depression, STB risk, and sleep intervention mechanisms.
To date, however, very few sleep treatments have been tested with Black and/or Latinx youth (42). The few studies testing adolescent sleep interventions with Black and/or Latinx youth have shown lower acceptability (43) as well as diminished treatment response (44) in these groups compared to White youth. These poorer outcomes could be due to limited attention to salient socio-cultural and environmental factors (45), including the adverse impacts of racism and discrimination on sleep in minoritized youth (35–37). To ensure acceptability and effectiveness, a sleep intervention for Black and/or Latinx adolescents with depression and at risk for STB must be tailored to address these socio-cultural and environmental factors and disparities. In addition, most youth with psychiatric disorders present with comorbid conditions and a range of sleep disturbances (46, 47), such as insomnia symptoms and the (frequently co-occurring) circadian rhythm disruptions that are highly prevalent in adolescence (48). Thus, for a sleep intervention to be effective with a diverse population, it must also be transdiagnostic with regard to both sleep and psychiatric concerns.
The proposed research
The Transdiagnostic Sleep and Circadian intervention (TSC, also referred to as TranS-C) is one of the only evidence-based treatments designed to treat a range of sleep and circadian difficulties among individuals with psychiatric comorbidities (46, 47). Grounded in a dimensional model of sleep health (49), TSC builds on principles of basic sleep and circadian science, evidence-based CBT strategies, and a motivational interviewing framework (46, 47), in which the patient is viewed as the expert in behavior change to enhance personal responsibility (50). TSC is modularized to enable flexible delivery and tailoring to each patient's specific sleep and circadian difficulties (51). In a community-based RCT, TSC was effective in treating sleep disturbances among adults who had comorbid sleep and psychiatric concerns (52). In this study, Black adults in particular experienced a strong treatment benefit (53). Another RCT conducted with predominantly White adolescents with delayed circadian rhythms showed that TSC produced durable improvements in sleep and circadian disturbances, even at 12-month follow-up (54–56).
TSC has not yet been tested among youth who are depressed and at risk for STB, with primarily Black and/or Latinx adolescents, or in primary care, where behavioral health services may be more accessible for minoritized youth (57, 58). Adaptations to intervention content (i.e., treatment strategies) and delivery methods (i.e., implementation strategies) are likely needed to maximize TSC acceptability, effectiveness, uptake, and scaling. Baumann and Cabassa (59, 60) recommend embedding implementation science with a health equity lens to adapt evidence-based interventions with end-users and contexts in mind, to ensure that intervention content and delivery methods are acceptable to and feasible with health disparate populations. This approach can also help to avoid perpetuating the well-documented gaps in the translation and uptake of evidence-based interventions in clinical practice settings (61, 62).
Following these recommendations, the purpose of this paper is to describe a protocol for applying health-equity informed implementation science frameworks to systematically adapt and evaluate with adolescents who are depressed, at risk for STB, and of primarily Black and/or Latinx backgrounds. Specifically, we use the Assessment, Decision, Adaptation, Production, Topical Experts-Integration, Training, Testing (ADAPT-ITT) model (63) to guide our multiphase, iterative adaptation and evaluation of TSC for this new clinical population and implementation context (Figure 2). We also integrate the Consolidated Framework for Implementation Research (CFIR) (61) to ensure assessment of, and adaptations for, contextual barriers and facilitations of implementation, such as clinician practices and organizational factors. In the following sections, we present preliminary data showing the acceptability and feasibility of TSC with a small sample of adolescents who are depressed and at risk for STB. We then describe the three sequential aims and protocol for the planned multiphase TSC adaptation and evaluation, which includes initial qualitative interviews with key informants, iterative TSC adaptation, and a pilot RCT to evaluate the adapted TSC intervention.
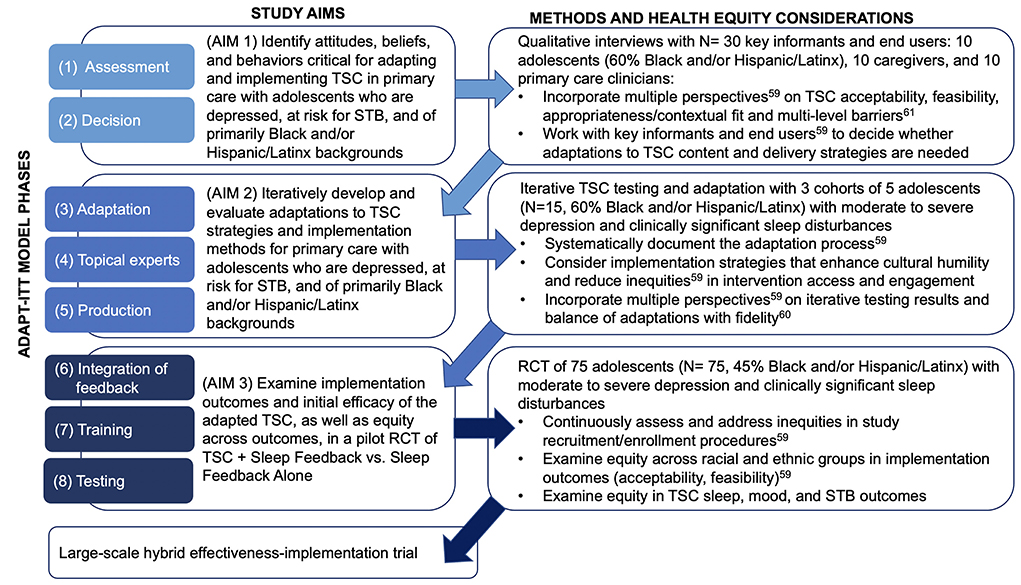
Figure 2. ADAPT-ITT phases applied to multiphase protocol aims, methods, and health equity considerations.
Initial pilot findings
Methods
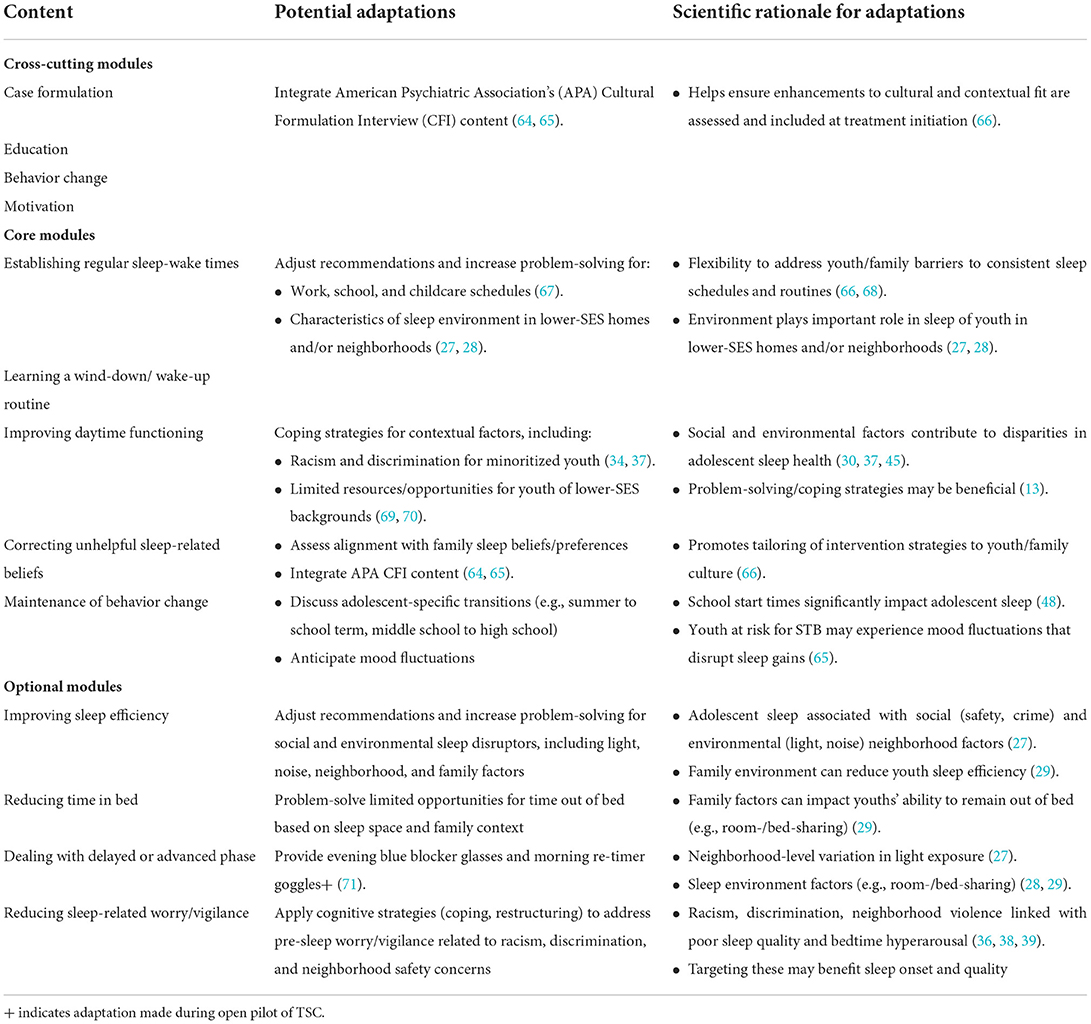
We first conducted an open trial using a convenience sample to examine preliminary feasibility and acceptability of TSC with adolescents who were experiencing depression and suicidal ideation. Standard TSC includes 8–12 sessions consisting of modules shown in Table 1. Cross-cutting, process-focused modules (case formulation, education, motivational enhancement, and goal setting) are included in each session. These cross-cutting modules are supplemented by core modules that apply to most patients (establishing regular sleep-wake times, learning a wind-down/wake-up routine, improving daytime functioning, addressing unhelpful sleep-related beliefs, and maintenance of behavior change), and optional modules for additional intervention personalization (improving sleep efficiency, reducing time in bed, dealing with delayed or advanced phase, and reducing sleep-related worry/vigilance) (47). Given prominent circadian timing changes in adolescence and late circadian preference in >75% of adolescents with depression, we enhanced TSC prior to implementation to further stabilize circadian rhythmicity (71). To this end, we integrated daily light therapy (target of 30 min in the morning delivered with Re-Timer glasses) to increase morning bright light exposure, and blue light-blocking glasses (up to 2 h before bedtime) to reduce evening light, particularly that from electronics devices. We also integrated sleep feedback through graphs constructed from sleep diaries and wrist actigraphy and used these data and participants' subjective sleep complaints to support ongoing case formulation, goal setting, and the selection of core and optional TSC modules. Adaptations to TSC for youth with depression and suicidal ideation were iteratively made, and qualitative and quantitative feedback from youth were incorporated to yield a personalized intervention.
Participant inclusion criteria were age 13–18 years, able to understand and converse in English, receiving care at a specialty clinic for youth at high risk for STB, with current moderate sleep disturbance [Pittsburgh Sleep Quality Index (72) global score >8], current suicidal ideation (per clinician report or self-report), depression, and a parent/guardian willing to consent for research. Adolescents were excluded if they had a bipolar disorder diagnosis, or were taking any photo-sensitizing medications (e.g., neuroleptics and antiarrhythmic drugs), as the bright light administered in in our adapted version of TSC is contraindicated with these medications. Participating parents/guardians provided informed consent and adolescents provided assent; youth who were age 18 or turned 18 during the study provided informed consent. Participants wore a wrist actigraph (CentrePoint Insight Watch, Actigraph Corp, Pensacola FL), completed daily sleep diaries (items described elsewhere), and attended TSC sessions every 1–3 weeks with a master's level study clinician, who delivered the program as an adjunct to youths' behavioral healthcare in the specialty clinic. The study was approved by the Institutional Review Board.
Results
Fifteen adolescents (M age = 16.1 years, SD = 1.6; 94% White and 6% Black; all non-Latinx) completed an average of 5.1 (SD = 2.6) TSC sessions (range = 1–10 sessions). Within-person average completion rates of morning and evening diaries occurred on 68 and 67% of days, respectively. Youth self-reported adherence on the daily diary to the ReTimer and blue-blocker intervention strategies was 56 and 59% of study days (among the completed diary days), respectively. For the five teens who completed acceptability ratings post-intervention, the overall mean satisfaction with the quality of TSC was 6.2 (range: 6–7) on a scale from one “very dissatisfied” to seven “very satisfied.” The mean rating of whether youth would recommend TSC to a friend who had sleep difficulties was 6.4 (range: 6–7) on a scale from one “strongly not recommended” to seven “strongly recommended,” while the mean reported likelihood of using the information and strategies learned about sleep in the future was 6.0 (range: 5–7) on a scale from 1 “not at all” to seven “very much.” Free text feedback included, “I enjoyed the bright light goggles and tracking my activity and its interactions with my sleep;” “I enjoyed the sleep therapy sessions. I found them to be helpful and beneficial to my sleep and routine;” “It helped with checking on my status of sleep and track how little and much I was sleeping;” and the morning bright light goggles “helped me get out of bed and ‘jumpstart’ my day.” All participants who completed TSC reported the length was appropriate (all rated as a four, on a scale from 1 “much too short,” four “appropriate,” to seven “much too long”).
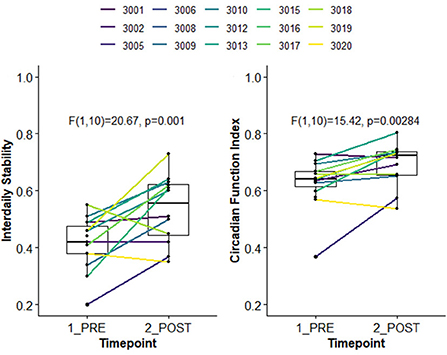
Using the first and last week of available actigraphy data, we examined change in 24-h rest activity rhythms (RARs) (73, 74), indexed by non-parametric outcomes (nparACT R package) (75). Interdaily Stability (IS) captures the degree of stability in the 24-h activity rhythm from day-to-day, varying from zero (unstable, noise) to one (stable, same activity pattern every day); here, the 24 h profile was estimated using 30-min time bins. The Circadian Function Index (CFI) (76) is a composite measure of circadian robustness, calculated as the average of three nparACT outcomes: IS, Relative Amplitude (ratio of highest 10 h of activity to lowest 5 h of activity), and inverted/normalized Intradaily Variability (within-day rhythm fragmentation). CFI ranges between zero (absence of circadian rhythmicity) and one (a robust circadian rhythm). As shown in Figure 3, compared to the baseline week, youth had significantly higher stability in 24-h activity patterns (i.e., in IS and CFI), suggesting improvement in 24-h RARs.
Implications for multiphase protocol
The open pilot establishes preliminary feasibility and acceptability of TSC with adolescents who are depressed and experiencing suicidal ideation. Results also suggest that TSC can improve 24-h RARs. However, adolescents in this sample were recruited through a specialty care clinic and were already engaged in behavioral healthcare, potentially increasing the likelihood of TSC session attendance. To tailor TSC for primary care delivery, adaptations to the number and duration of sessions may be needed, given the brief nature of primary care-based behavioral healthcare (57). Pilot results were also limited to mostly White, non-Latinx adolescents. Research on youth suicide prevention (5, 6), sleep health disparities (21, 45), and the impact of racism and/or discrimination (36, 37, 39) supports the likelihood that adaptations to TSC content will be needed to enhance its cultural relevance for Black and/or Latinx youth.
Multiphase TSC adaptation and evaluation protocol
Design
Figure 2 shows the application of the 8-phase ADAPT-ITT model (63) to the three sequential aims of the planned study, as well as considerations to enhance health equity (59). ADAPT-ITT prioritizes key informant and end-user feedback in iterative, sequential adaptations of evidence-based interventions. First developed for use in HIV prevention and intervention research, ADAPT-ITT has been applied to adaptations of many other evidence-based interventions, including a treatment for adolescent substance use (77) and a universal suicide prevention program in pediatric primary care (78). The use of ADAPT-ITT is relevant to the proposed adaptation of TSC to ensure that intervention content and delivery strategies are acceptable, feasible, and culturally responsive in a new population (i.e., adolescents who are depressed, at risk for STB, and of primarily Black and/or Latinx backgrounds) and a new context (i.e., primary care) (63).
In line with the first two ADAPT-ITT phases, Assessment and Decision (63), Aim 1 identifies attitudes, beliefs, and behaviors critical for adapting and implementing TSC in the new population and new context described above. We will qualitatively solicit the perspectives of key informants, end-users, and patients/clients, including primary care and behavioral health clinicians, adolescent patients, and their caregivers. We will use CFIR (61) to guide the development of interview questions about contextual/organizational barriers and facilitators to intervention content and delivery strategies. These methods align with prior research on adapting sleep intervention strategies for primary care implementation with minoritized young children and families (67). In our analyses and interpretation of results, we will incorporate the perspectives of the multiple key informant groups (59) to better understand the feasibility, appropriateness, contextual fit, and potential multi-level barriers (61) to TSC implementation. These results will directly inform decisions about which adaptations to TSC intervention content and delivery strategies would be necessary to maximize intervention acceptability, feasibility, and efficacy in the target population and clinical context.
Table 1 shows potential adaptations based on prior research, including our sleep intervention implementation and adaptation research (54, 55, 66, 67, 71). Potential adaptations include integrating questions from the American Psychiatric Association's Cultural Formulation Interview (64, 65) to ask about prior experiences with and preferences for enhancing cultural fit from the beginning of treatment (66). Training for TSC interventionists will likely require adaptation to more explicitly focus on enhancing interventionists' cultural humility (79) and awareness of how clinician implicit and explicit racial and ethnic biases impact the clinical encounter (80). Interventionist training may also benefit from content-related adaptations to address the impacts of racism and discrimination on sleep (35–37). More specifically, the optional module on reducing sleep-related worry and/or hypervigilance could be adapted so that cognitive coping techniques are applied to adolescents' experiences of and stress related to racism and discrimination (Table 1). Based on prior research (27–29, 66), we also anticipate that adaptations to TSC may be required for youth of lower-SES backgrounds. For instance, problem-solving can focus on potentially modifiable social and environmental sleep disruptors in the adolescent sleep environment, including light, noise, and lack of privacy for youth who room-/bed-share (27–29).
ADAPT-ITT phases three, four, and five consist of a “theater test,” in which the intervention is implemented with the target population (Administration), with additional intervention adaptation (Production), and continued input on any adaptations from key informants (Topical Experts) (63). These phases will be accomplished through Aim 2, which iteratively develops and evaluates adaptations to TSC strategies and implementation methods with primarily Black and/or Latinx adolescents recruited from primary care. The initial adaptations suggested in Aim 1 will be iteratively tested in cohorts of adolescents in Aim 2, with additional adaptations drawn from ongoing intervention participants, topical experts, and clinical advisory board feedback (Figure 2). As in Aim 1 and in line with health equity recommendations (59), we will incorporate this feedback in interpreting the Aim 2 results, as well as in balancing adaptations made with fidelity (60) to the TSC intervention. Importantly, throughout this iterative testing we will systematically document the nature and extent of any adaptations made (59).
We will then complete ADAPT-ITT phases six (Integration of feedback), seven (Training), and eight (Testing) through Aim 3, which examines implementation outcomes and initial efficacy of the adapted TSC, as well as equity across outcomes, in a pilot RCT. Activities for this aim (Figure 2) include implementing a finalized version of the adapted TSC based on the results of prior aims, ensuring adequate and adapted training as needed for TSC interventionists, and examining any observed disparities by racial and ethnic group with regard to study procedures (e.g., recruitment, enrollment) and clinical outcomes (59).
Setting and recruitment
All study aims will be conducted in pediatric primary care practices affiliated with two large academic medical centers in PA. The affiliated pediatrics practices in western PA serve over 70% of youth in the region and include 32 practices across 54 office sites in nine western PA counties. This group of practices serves over 266,000 privately and publicly insured patients, aged birth to 21 years. The second affiliated primary care network in eastern PA is the largest provider of primary care services in the region, with 31 practices across five counties. Clinicians in the network serve over 249,000 private and publicly insured patients, aged birth to 21 years. For each study aim, we will obtain caregiver/guardian consent, adolescent assent and/or consent for adolescents aged 18 years.
To ensure that the participant groups (adolescents, caregivers, and primary care clinicians) across study aims are reflective of the target clinical population and implementation context, we will recruit participants as part of routine well child visits. Prior to visits, potentially eligible patients based on electronic health record (EHR) screening will receive an email jointly signed by a primary care practice champion at the family's care site and research staff providing information about the research. During well child visits, the Patient Health Questionnaire (PHQ-9-M) (81) depression screener is administered as part of standard practice and integrated into the EHR. For all study phases, we will implement EHR-based clinician-directed alerts if patient scores meet criteria for potential enrollment based on their depressive symptoms. If the patient and family are interested in the research, we will conduct either in-person or remote informed consent procedures to maximize flexibility for participants. Throughout study phases, we will monitor the recruitment of Black and/or Hispanic/Latinx adolescents and caregivers and meet regularly with primary care clinical teams to solicit ongoing feedback on recruitment procedures and adjust these methods as needed to recruit a diverse sample. The protocol for this study was approved by the Institutional Review Boards of the affiliated academic medical centers. The following sections detail additional methods by aim.
Aim 1: Initial qualitative inquiry
Aim 1 participants
Aim 1 participants will include 10 adolescents, 10 caregivers of adolescents, and 10 primary care clinicians, including physicians and behavioral health providers, who are working at the affiliated primary care sites. We aim to recruit a sample with at least 50% of adolescent and caregiver participants self-identifying as Black and at least 10% self-identifying as Latinx. Adolescent inclusion criteria are as follows: age 12–18 years; able to understand and converse in English; and evidence of moderate-severe depression (PHQ-9-M >11) (81) and clinically significant sleep disturbance (PHQ-9-M sleep item 3 [“trouble falling or staying asleep”] >2, sleep trouble >50% of days in past 2 weeks). Participants with a life-threatening medical condition requiring immediate treatment or intellectual or developmental disability precluding comprehension of study procedures will be excluded, as will adolescent participants with diagnoses of obstructive sleep apnea, restless legs syndrome, bipolar disorder, a current manic or psychotic episode (per participant or caregiver report or medical record review).
Aim 1 measures
Interview guides will be developed with input from key informant groups, including adolescents, caregivers, providers, and health systems leaders to ensure the most relevant information is gathered. The dimensional CFIR framework (61) will guide interview questions on multi-level barriers and facilitators to TSC content and delivery strategies. More specifically, in the CFIR domain of intervention characteristics, we will solicit perspectives on the relative advantage of implementing TSC in primary care vs. other outpatient settings, the extent to which the content would require adaptation to meet adolescents' needs, and the ways in which the intervention is packaged and delivered to youth. Related to the CFIR outer setting domain, we will ask interviewees about the needs and resources of adolescents seen in the primary care sites. Inner setting questions will inquire about the norms and values of the primary care setting and the climate for implementation (61). This includes questions about the likelihood of future intervention implementation, dissemination, and sustainment (82) in primary care after the research concludes. In the CFIR domain of individual characteristics we will solicit perspectives about adolescents' sleep-related knowledge, beliefs, and attitudes, as well as their views of the TSC intervention content and planned delivery methods. We will embed a health equity perspective (59) and assess youth experiences of personally-mediated and systemic/institutional racism and discrimination, the impacts of these experiences on sleep, and their perceptions about addressing the sleep impacts of these experiences through TSC (31–34).
Aim 1 analytic approach
The sample size for this aim was based on guidelines for thematic saturation in qualitative research (83). We will focus our analysis on the a priori attributes of interest, specifically CFIR domains, TSC barriers, and potential adaptations (84, 85). We will initially analyze interviews using Rapid Qualitative Analysis (86), to facilitate the rapid analysis and iterative adaptation of intervention content and delivery strategies. During data collection and analysis, we will assess for thematic saturation and for a diversity of perspectives given the small number of participants proposed and the focus of this research on racially and ethnically minoritized youth. If necessary, we will increase our sample size to maximize the inclusion of a wide range of perspectives within and across key informant groups. To incorporate qualitative data from the different interview groups (adolescents; caregivers; clinicians) we will follow NIH guidelines (87) and mixed methods approaches (88) to stratify the themes that emerge according to informant groups. This will require interview transcription, the iterative development of a codebook, and the coding of qualitative data in a specialized software program.
Aim 2: Iterative TSC adaptation
Aim 2 participants
We will recruit 15 adolescents (at least 50% self-identifying as Black; 10% self-identifying as Latinx) in three cohorts of five adolescents each to participate in iteratively adapting and testing TSC. Aim 2 inclusion/exclusion criteria are identical to those in Aim 1.
Aim 2 measures
The primary Aim 2 outcomes pertain to intervention acceptability and feasibility. We will also pilot the sleep data collection methods and strategies to increase morning bright light and decrease evening light in anticipation of the Aim 3 randomized trial. Adolescents will wear actigraphs, Re-Timer glasses in the morning, blue-blocker glasses in the evening, complete a daily sleep diary, and provide ratings of their perceived sleep disturbances at pre and post intervention.
Intervention acceptability will be assessed at post-intervention using the adolescent self-reported Acceptability of Intervention Measure (AIM) (89). We will also conduct semi-structured qualitative interviews to identify participants' perspectives about intervention acceptability, barriers, and recommendations for additional adaptations to content or delivery strategies.
Intervention feasibility will be indexed by multiple outcomes (90), including the number of TSC sessions attended and rates of intervention attrition, to index intervention engagement. Intervention fidelity will be measured via the coding of a randomly selected 10% of video recorded TSC sessions. Sessions will be coded using the Cognitive Therapy Scale (CTS) (91) and a TSC session checklist (92), both used in prior TSC research.
Actigraphy is a widely used method of assessing sleep and circadian disruptions longitudinally in an individual's natural environment. Actigraphy is well-validated validated against polysomnography, the gold standard measure of overnight sleep (93). Consistent with guidelines, adolescents will continuously wear an actigraph on their non-dominant wrist, unless bathing or swimming, and complete a corresponding sleep diary for actigraphy scoring purposes. Sleep diary ratings will include time in and out of bed, sleep onset latency, night awakenings, and sleep quality (94). Actigraphy data will be scored using the Cole-Kripke algorithm in ActiLife software, which are validated against polysomnography and other actigraphs in young adults (95) and adolescents (96).
Self-reported sleep disturbances will be measured using the well-validated pediatric Patient-Reported Outcomes Measurement Information Systems (PROMIS) Sleep Disturbance and Sleep-Related Impairment Scales (97), which respectively measure perceived sleep difficulties and the impacts of sleep on daily functioning.
Aim 2 intervention procedures
TSC study clinicians will review the intervention manual and attend an initial 1-day training with the study investigators, who will conduct weekly supervision. The TSC developers (AGH and DJB) will consult on implementation as needed. Clinicians will interface with adolescents' other treatment providers and/or their caregivers in line with preferences identified in Aim 1. Therapist fidelity ratings (described above) will be monitored, with ratings < 80% prompting re-training with study investigators.
Study clinicians will implement core and optional modules weekly over 6–8 weeks via a HIPAA-compliant, secure telehealth platform. TSC sessions will supplement other mental health treatment that participants may be receiving. The selection and individualization of TSC models will be guided by the intake assessment, case conceptualization, adolescents' ongoing reports of sleep disturbances at sessions, and their actigraphy and daily diary data.
Aim 2 analytic approach
Our prior adaptation research (63, 66) and guidelines for thematic saturation (83) informed the Aim 2 sample size. As in Aim 1, we will monitor participant TSC ratings and qualitative feedback and increase our sample size if we do not reach thematic saturation. Aggregate mean AIM scores will be reviewed following each cohort of five participants. Scores for the final cohort will quantify overall acceptability, with high end-user acceptability identified as a mean AIM >80%.
Aim 3: Pilot RCT of adapted TSC
Aim 3 participants and randomization
We will recruit 75 adolescents (at least 35% self-identifying as Black; 10% self-identifying as Latinx) to participate in the RCT comparing the adapted TSC intervention plus Sleep Feedback (TSC + Sleep Feedback, described below) to a Sleep Feedback Only condition. Study inclusion/exclusion criteria are identical to those in Aim 1. Adolescents will be eligible to enroll in the study while engaged in other behavioral/mental healthcare and/or taking any sleep medications, which we will track.
Adolescents will be randomized using 2:1 allocation (2 TSC + Sleep Feedback: 1 Sleep Feedback Only) using a modification of Efron's biased coin toss procedure (98). We selected unequal allocation to maximize critical information about the intervention (e.g., adverse events). Random assignment will balance groups on age (middle vs. high school, since school start times typically shift earlier and social pressures further shorten sleep duration in high school), suicide risk (ideation/attempt history), and racial and ethnic background.
Aim 3 measures
Primary Aim 3 outcomes are related to TSC implementation, and include intervention feasibility, acceptability, appropriateness, and fidelity. Secondary Aim 3 outcomes are adolescent sleep disturbances, depressive symptoms, STB, and affective and behavioral regulation; the last of these are hypothesized intervention mechanisms (Figure 1). Sleep will be objectively assessed using actigraphy, which adolescents will wear throughout the TSC intervention period, with an accompanying daily diary to assess self-reported sleep, mood, and stressors (described below). All other secondary outcomes will be collected at baseline (pre-intervention) and at months 1, 3, 6, and 12. The type, frequency, and/or dose of behavioral, sleep, psychiatric treatment and/or medications will also be measured throughout the study using the Child and Adolescent Services Assessment (CASA) (99) throughout the study. Specific primary and secondary measures are as follows:
Intervention acceptability will be measured using the adolescent self-reported AIM (89) instrument, described in Aim 2. To further assess acceptability, adolescents will also complete the Client Satisfaction Questionnaire (CSQ) (100), adapted for the current study, as well as a semi-structured qualitative interview, with questions about TSC as described for the Aim 2 post-intervention interviews.
Intervention feasibility will be assessed through multiple methods (90), including via engagement (TSC sessions attended and attrition) and intervention fidelity assessments described for Aim 2. Adolescents will also complete the Intervention Appropriateness Measure (IAM) and the Feasibility of Intervention Measure (FIM) (89) to further assess perceived fit and feasibility of the intervention for addressing their sleep disturbances, respectively.
Actigraphy will be used to evaluate behavioral sleep and circadian characteristics, with the same procedures for implementation and scoring as in Aim 2. Outcomes for actigraphy-derived sleep disturbances are duration, regularity, and timing. Consistent with Study 1, a sleep diary will be used to complement actigraphy metrics for scoring purposes. The sleep diary will include time in and out of bed, sleep onset latency, night awakenings, and sleep quality (94). The sleep-specific diary questions will be sent via SMS text messages to participants each morning. See below for additional diary items administered in the evening.
Daily mood and stressors will be measured via adolescent self-reported daily diary items implemented during the TSC intervention period. These items will be deployed using links to a web-based form sent via SMS texts or emails, as in the sleep diary implementation. These items will be assessed in the evening and will include ratings of adolescents' mood; experiences of racism, discrimination, and victimization (101); and affective and behavioral regulation (impulsivity and reactivity to the day's most positive and negative event as in our prior work) (102).
Weekly self-reported suicidal ideation and behavior will also be rated via SMS using items modeled after the Columbia—Suicide Severity Rating Scale (C-SSRS) (103, 104).
Weekly affective and behavioral regulation, which are hypothesized intervention mechanisms, will also be measured through adolescent self-report using the Childhood Affective Lability Scale (CALS) (105) to assess affective regulation and the short UPPS-P Impulsive Behavior Scale (IBS) (106) for behavioral regulation.
Self-reported sleep disturbances will be measured at baseline and follow-up assessments using the PROMIS Sleep Disturbance and Sleep-Related Impairment Scales (97), as in Aim 2.
Self-reported depressive symptoms and STB will also be measured at baseline and follow-up assessments using the adolescent PHQ-9-M (81) and the C-SSRS, (103, 104) respectively.
Aim 3 intervention procedures
Intervention training and implementation procedures for TSC will be as described for Aim 2. The Sleep Feedback Only condition consists of reports summarizing prospectively gathered actigraphy and diary data. With the mass availability of wearable devices (e.g., Fitbit) and apps, such personalized sleep tracking is now widely accessible. However, despite increasing users' awareness of sleep habits, this approach yields minimal change in sleep behavior (107, 108). Thus, the Sleep Feedback Only comparator group controls for common receipt of information related to sleep behaviors while enabling us to focus on TSC adaptations to optimize ultimate implementation. These Sleep Feedback reports will be accessible to participants via web link sent weekly by SMS. Sleep Feedback reports will also be accessible to TSC clinicians via a HIPAA-secured online portal to inform selection and personalization of TSC modules.
Aim 3 analytic approach
As Aim 3 is a pilot study, sample size considerations center on the precision of confidence interval (CI) width estimation for implementation and target outcomes. Based on best practices for pilot studies (109, 110), given our intervention sample size (TSC + Sleep Feedback) of 50 and 5% type I error rate, we will be able to estimate 95% CI widths of no more than 0.28 for primary implementation and target outcomes.
Using descriptive statistics, we will compute the proportion (and 95% confidence intervals) of participants with high ratings for TSC feasibility (session attendance >80%, attrition < 20%; FIM >80%), acceptability (AIM >80%) and appropriateness (IAM >80%). We will examine these outcomes overall and according to participant racial and ethnic groups. For implementation outcomes with both quantitative and qualitative data (feasibility, acceptability, and appropriateness), we will use established approaches for analyzing mixed methods data described as in Aim 1. We will compare participants across cells on clinical and socio-demographic baseline characteristics using standard univariate statistics.
Additionally, we will assess whether improvements in sleep (via actigraphy-derived duration, regularity, timing; and via daily diary and PROMIS measures), depressive symptoms (PHQ-9-M), and risk for STB (C-SSRS), are greater among youth randomized to TSC + Sleep Feedback vs. Sleep Feedback Only conditions using linear mixed models. Exploratory analyses will examine putative intervention mechanisms (affective/behavioral dysregulation; Figure 1) based on daily diary, CALS, and IBS ratings. Study arm, time, and their interaction will be included as primary predictors, with random effects for study subject.
Discussion
This paper describes a protocol for applying health equity-informed implementation science frameworks and models to adapt and evaluate the evidence-based, modularized TSC intervention in primary care with adolescents who are depressed, at risk for STB, and of primarily Black and/or Latinx backgrounds. This protocol expands upon our recent open pilot of TSC with predominantly White youth experiencing sleep disturbance and suicidal ideation, which demonstrated preliminary intervention feasibility. Minor adaptations to TSC during the pilot included integrating clinician feedback to youth on their sleep from both sleep diaries and actigraphy, and enhancing morning bright light exposure and evening blue light-blocking glasses, based on our prior research (71). Adolescents reported good adherence to these strategies on the daily diary, as well as high acceptability of these strategies, although the sample size for the post-intervention acceptability questionnaire was modest. Youth in the open pilot also showed evidence of improved 24-h rest activity rhythms.
Our planned multiphase protocol will rigorously develop and evaluate further adaptations of TSC for Black and/or Latinx youth who are treated in primary care settings. Developing adolescent behavioral healthcare that is both evidence-based and accessible is an urgent public health priority (111), particularly given the rising global prevalence of youth anxiety and depression over the course of the coronavirus (COVID-19) pandemic (112). Primary care may be a more accessible and less stigmatizing context for initiating behavioral healthcare (40, 41). The American Academy of Pediatrics also recommends routine adolescent depression screening as part of well child care (113), which facilitates early identification of youth at risk for depression and STB. Despite these benefits, substantial challenges remain to integrating behavioral health screening and referrals into the primary care workflow (114).
These challenges necessitate a CFIR-informed, pre-implementation inquiry to identify organizational and other contextual factors that are critical for intervention delivery methods and future sustainment (61, 82). Throughout the multiphase study, we will monitor whether planned implementation and research methods, such as the use of telehealth (115) and our initial focus on English-speaking families (116), inadvertently contribute to disparities in access to treatment for adolescents and their families presenting to primary care. In addition, we will measure participants' engagement in study evaluations (i.e., actigraphy and daily diaries) and the TSC intervention (i.e., session attendance), as these methods may require further adaptation to better align with the brief and less intensive nature of primary care-based service delivery. Our study will add to a growing body of research examining the feasibility and benefits of evidence-based adolescent behavioral health programs adapted for primary care delivery (117).
We anticipate that the results of the multiphase protocol will ensure adaptations to TSC are made to both optimize delivery methods for primary care and to maximize acceptability, feasibility, and effectiveness with adolescents of primarily Black and/or Latinx backgrounds. Although we have outlined potential intervention content adaptations based on relevant research (5, 9, 36, 37), any proposed cultural adaptations based on race and ethnicity are inherently limited. Race and ethnicity are socio-political constructs (118), and no racial or ethnic group is monolithic; considerable heterogeneity exists within racial and ethnic groups and along many other identity dimensions (e.g., gender identity and expression, language, religion, nationality, ability, etc.). Some of these dimensions, such as race and gender identity, may intersect to confer increased marginalization, and this intersectional lens (119) is needed to better personalize and enhance the cultural fit of any behavioral health treatment (120). Findings for the proposed research may have limited generalizability for these reasons, and due to the small proposed sample sizes across aims and the potential that only 10% of adolescent and caregiver participants may identify as Latinx and only 50% of participants may identify as Black for each aim.
Tailoring an intervention for every possible combination of intersectionality is not feasible and could further limit dissemination and uptake (121), particularly in under-resourced community settings where clinicians may not have time or access to needed trainings (47). At the same time, TSC is a modularized treatment that could facilitate attention to intersectionality with personalization (e.g., tailoring strategies to address adolescents' specific cultural and contextual sources of sleep disruption) across a range of sleep and circadian disturbances and comorbid psychiatric conditions (47). The modularized nature of TSC and the planned adaptations in this research could provide a foundation for the integration of suggested clinician and systems-level adaptations in the TSC training activities and treatment manuals, potentially overcoming the need for evaluating multiple adaptations in future research. Our qualitative, pre-implementation inquiry about TSC content and delivery strategies may also result in other adaptations that could enhance the cultural fit of other modules (e.g., integrating culture-specific beliefs around sleep in the module for correcting unhelpful sleep-related thoughts/beliefs). Indeed, we intend to use the results of this research to inform a fully-powered hybrid effectiveness-implementation trial (122) of TSC in primary care, to further establish the evidence base for TSC adaptations and to examine implementation outcomes with integrated behavioral health providers. Throughout this protocol, we may identify additional intervention content and delivery methods that require tailoring for optimal implementation and effectiveness.
Our research plan provides an example of how health equity-informed implementation science models (ADAPT-ITT) and frameworks (CFIR) can be applied to increase the likelihood that evidence-based interventions will be effective for health disparity populations and successfully implemented in accessible intervention contexts (59). Our goal is to ensure adaptations to TSC are systematically documented, rigorously tested, and developed with end-users in mind, so that this intervention can be scaled to equitably and effectively address adolescent STB.
Data availability statement
The datasets generated and/or analyzed during the current study are not available for use outside of the University of Pittsburgh at this time, due to the nature of the ethics board approvals and possible risk(s) to study participants as well as the confidentiality promised to them. Data may be made available from the corresponding author on reasonable request with permission of study investigators and ethics board approval. Requests to access the datasets should be directed to AS, c29laG5lcmFtMkB1cG1jLmVkdQ==.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board, School of Medicine, University of Pittsburgh, Pittsburgh, PA. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
Study conceptualization and design: AW, AS, RB, CJ, PF, and TG. Data collection, analysis, and interpretation of results for pilot test: AS, PF, and TG. Draft manuscript preparation: AW, AS, PF, and TG. Critical manuscript review and feedback: RB, CJ, DB, and AH. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by K23 HD094905 (AW), P50 MH115838 (Brent/Rollman), R01 MH1224907 (TG/PF), R01 MH118312 (PF), K01 MH124828 (AS).
Conflict of interest
Over the past 3 years, author DB has served as a paid consultant to National Cancer Institute, Pear Therapeutics, Sleep Number, Idorsia, Eisai, and Weight Watchers International. Author DB is an author of the Pittsburgh Sleep Quality Index, Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A), Brief Pittsburgh Sleep Quality Index (B-PSQI), Daytime Insomnia Symptoms Scale, Pittsburgh Sleep Diary, Insomnia Symptom Questionnaire, and RU_SATED (copyrights held by University of Pittsburgh). These instruments have been licensed to commercial entities for fees. He is also co-author of the Consensus Sleep Diary (copyright held by Ryerson University), which is licensed to commercial entities for a fee.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. (2016).
2. Lindsey MA, Sheftall AH, Xiao Y, Joe S. Trends of suicidal behaviors among high school students in the United States: 1991–2017. Pediatrics. (2019) 144:e20191187. doi: 10.1542/peds.2019-1187
3. Sheftall AH, Vakil F, Ruch DA, Boyd RC, Lindsey MA, Bridge JA. Black youth suicide: investigation of current trends and precipitating circumstances. J Am Acad Child Adolesc Psychiatry. (2022) 61:662–75. doi: 10.1016/j.jaac.2021.08.021
4. Xiao Y, Cerel J, Mann JJ. Temporal trends in suicidal ideation and attempts among US adolescents by sex and race/ethnicity, 1991-2019. JAMA Network. (2021) 4:e2113513–e2113513. doi: 10.1001/jamanetworkopen.2021.13513
5. Brent DA, McMakin DL, Kennard BD, Goldstein TR, Mayes TL, Douaihy AB. Protecting adolescents from self-harm: a critical review of intervention studies. J Am Acad Child Adolesc Psychiatry. (2013) 52:1260–71. doi: 10.1016/j.jaac.2013.09.009
6. Joe S, Canetto SS, Romer D. Advancing prevention research on the role of culture in suicide prevention. Suicide Life Threat Behav Jun. (2008) 38:354–62. doi: 10.1521/suli.2008.38.3.354
7. Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry. (2006) 47:372–94. doi: 10.1111/j.1469-7610.2006.01615.x
8. Blake MJ, Allen NB. Prevention of internalizing disorders and suicide via adolescent sleep interventions. Curr Opin Psychol. (2020) 34:37–42. doi: 10.1016/j.copsyc.2019.08.027
9. Goldstein TR, Franzen PL. Sleep difficulties and suicidality in youth: current reserach and future directions. Curr Opin Psychol. (2020) 34:27–31. doi: 10.1016/j.copsyc.2019.08.021
10. Meltzer LJ, Williamson AA, Mindell JA. Pediatric sleep health: It matters, and so does how we define it. Sleep Med Rev Jun. (2021) 57:101425. doi: 10.1016/j.smrv.2021.101425
11. Liu X. Sleep and adolescent suicidal behavior. Sleep. 2004;27:1351-8. doi: 10.1093/sleep/27.7.1351
12. Fitzgerald C, Messias E, Buysse D. Teen sleep and suicidality: Results from the Youth Risk Behavior Surveys of 2007 and 2009. J Clin Sleep Med. (2011) 7:351–6. doi: 10.5664/JCSM.1188
13. Bernert R, Horn M, Iwata N, Joiner T. Objectively assessed sleep variability as an acute warning sign of suicidal ideation in a longitudianl evaluation of young adults at high suicide risk. J Clin Psychiatry. (2017) 78:e678–87. doi: 10.4088/JCP.16m11193
14. Goldstein TR, Bridge JA, Brent DA. Sleep disturbance preceding completed suicide in adolescents. J Consult Clin Psychol. (2008) 76:84. doi: 10.1037/0022-006X.76.1.84
15. Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: Recommendations for future research and clinical. practice Sleep Med Rev. (2014) 18:521–9. doi: 10.1016/j.smrv.2014.03.006
16. Orchard F, Gregory AM, Gradisar M, Reynolds S. Self-reported sleep patterns and quality amongst adolescents: cross-sectional and prospective associations with anxiety and depression. J Child Psychol Psychiatry. (2020) 61:1126–37. doi: 10.1111/jcpp.13288
17. Avenevoli S, Swendsen J, He J-P, Burstein M, Merikangas KR. Major depression in the national comorbidity survey–adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. (2015) 54:37-44. e2. doi: 10.1016/j.jaac.2014.10.010
18. Blake MJ, Sheeber LB, Youssef GJ, Raniti MB, Allen NB. Systematic review and meta-analysis of adolescent cognitive–behavioral sleep interventions. Clin Child Fam Psychol Rev. (2017) 20:227–49. doi: 10.1007/s10567-017-0234-5
19. Trockel M, Karlin BE, Taylor CB, Brown GK, Manber R. Effects of cognitive behavioral therapy for insomnia on suicidal ideation in veterans. Sleep. (2015) 38:259–65. doi: 10.5665/sleep.4410
20. McCall WV, Benca RM, Rosenquist PB, Youssef NA, McCloud L, Newman JC, et al. Reducing suicidal ideation through insomnia treatment (REST-IT): a randomized clinical trial. Am J Psychiatry. (2019) 176:957–65. doi: 10.1176/appi.ajp.2019.19030267
21. Guglielmo D, Gazmararian JA, Chung J, Rogers AE, Hale L. Racial/ethnic sleep disparities in us school-aged children and adolescents: a review of the literature. Sleep Health. (2018) 4:68–80. doi: 10.1016/j.sleh.2017.09.005
22. Matthews KA, Hall M, Dahl RE. Sleep in healthy black and white adolescents. Pediatrics. (2014) 133:e1189–96. doi: 10.1542/peds.2013-2399
23. Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med. (2011) 12:239–45. doi: 10.1016/j.sleep.2010.07.020
24. James S, Chang A-M, Buxton OM, Hale L. Disparities in adolescent sleep health by sex and ethnoracial group. SSM Popul Health. (2020) 11:100581. doi: 10.1016/j.ssmph.2020.100581
25. Billings ME, Cohen RT, Baldwin CM, Johnson DA, Palen BN, Parthasarathy S, et al. Disparities in sleep health and potential intervention models: a focused review. Chest. (2021) 159:1232–40. doi: 10.1016/j.chest.2020.09.249
26. Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. (2001) 116:404–16. doi: 10.1016/S0033-3549(04)50068-7
27. Mayne SL, Mitchell JA, Virudachalam S, Fiks AG, Williamson AA. Neighborhood environments and sleep among children and adolescents: a systematic review. Sleep Med Rev. (2021) 57:101465. doi: 10.1016/j.smrv.2021.101465
28. Bagley EJ, Kelly RJ, Buckhalt JA, El-Sheik M. What keeps low-ses children from sleeping well: the role of presleep worries and sleep environment. Sleep Med. (2015) 16:496–502. doi: 10.1016/j.sleep.2014.10.008
29. Spilsbury JC, Patel SR, Morris N, Ehayaei A, Intille SS. Household chaos and sleep-disturbing behavior of family members: results of a pilot study of African American early adolescents. Sleep Health. (2017) 3:84–9. doi: 10.1016/j.sleh.2016.12.006
30. Johnson DA, Jackson CL, Williams NJ, Alcántara C. Are sleep patterns influenced by race/ethnicity–a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep. (2019) 11:79. doi: 10.2147/NSS.S169312
31. Jones CP. Levels of racism: a theoretic framework and a gardener's tale. Am J Public Health. (2000) 90:1212. doi: 10.2105/AJPH.90.8.1212
33. Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health Apr 1. (2019) 40:105–25. doi: 10.1146/annurev-publhealth-040218-043750
34. Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0138511. doi: 10.1371/journal.pone.0138511
35. Davenport MA, Landor AM, Zeiders KH, Sarsar ED, Flores M. Within-person associations between racial microaggressions and sleep among African American and Latinx young adults. J Sleep Res. (2020) 30:e13226. doi: 10.1111/jsr.13226
36. Xie M, Yip T, Cham H, El-Sheik M. The impact of daily discrimination on sleep/wake problem trajectories among diverse adolescents. Child Dev. (2021) 92:e1061–74. doi: 10.1111/cdev.13605
37. Yip T, Cheon YM, Wang Y, Cham H, Tryon W, El-Sheik M. Racial disparities in sleep: associations with discrimination among ethnic/racial minority adolescents. Child Dev. (2020) 91:914–31. doi: 10.1111/cdev.13234
38. Philbrook LE, Buckhalt JA, El-Sheik M. Community violence concerns and adolescent sleep: physiological regulation and race as moderators. J Sleep Res. (2020) 29:e12897. doi: 10.1111/jsr.12897
39. Yip T. The effects of ethnic/racial discrimination and sleep quality on depressive symptoms and self-esteem trajectories among diverse adolescents. J Youth Adolesc. (2015) 44:419–30. doi: 10.1007/s10964-014-0123-x
40. Planey AM, Smith SM, Moore S, Walker TD. Barriers and facilitators to mental health help-seeking among African American youth and their families: a systematic review study. Child Youth Serv Rev. (2019) 101:190–200. doi: 10.1016/j.childyouth.2019.04.001
41. Lu W, Todhunter-Reid A, Mitsdarffer ML, Muñoz-Laboy M, Yoon AS, Xu L. Barriers and facilitators for mental health service use among racial/ethnic minority adolescents: a systematic review of literature. Public Health Front. (2021) 9:184. doi: 10.3389/fpubh.2021.641605
42. Schwichtenberg A, Abel E, Keys E, Honaker SM. Diversity in pediatric behavioral sleep intervention studies. Sleep Med Rev. (2019) 47:103–11. doi: 10.1016/j.smrv.2019.07.004
43. Quante M, Khandpur N, Kontos EZ, Bakker JP, Owens JA, Redline S, et al. Qualitative assessment of the acceptability of a smartphone app for improving sleep behaviors in low income and minority adolescents. Behav Sleep Med. (2019) 17:573–85. doi: 10.1080/15402002.2018.1432483
44. Tavernier R, Adam EK. Text message intervention improves objective sleep hours among adolescents: the moderating role of race-ethnicity. Sleep Health. (2017) 3:62–7. doi: 10.1016/j.sleh.2016.11.002
45. Jackson CL, Walker JR, Brown MK, Das R, Jones NL. A workshop report on the causes and consequences of sleep health disparities. Sleep. (2020) 43:zsaa037. doi: 10.1093/sleep/zsaa037
46. Harvey A. A transdiagnostic approach to treating sleep disturbance in psychiatric disorders. Cogn Behav Ther. (2009) 38:35–42. doi: 10.1080/16506070903033825
47. Harvey AG. Treating sleep and circadian problems to promote mental health: Perspectives on comorbidity, implementation science and behavior change. Sleep. (2022) 45:zsac026. doi: 10.1093/sleep/zsac026
48. Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. (2018) 67:55–65. doi: 10.1016/j.adolescence.2018.06.001
49. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. (2014) 37:9–17. doi: 10.5665/sleep.3298
50. Dilallo JJ, Weiss G. Motivational interviewing and adolescent psychopharmacology. J Am Acad Child Adolesc Psychiatry. (2009) 48:108–13. doi: 10.1097/CHI.0b013e3181930660
51. Harvey AG, Hein K, Dong L, Yu SH, Martinez AJ, Gumport NB, et al. A transdiagnostic sleep and circadian treatment to improve severe mental illness outcomes in a community setting: study protocol for a randomized controlled trial. Trials. (2016) 17:606. doi: 10.1186/s13063-016-1690-9
52. Harvey AG, Dong L, Hein K, Smith FL, Lisman M, Yu S, et al. A randomized controlled trial of the Transdiagnostic Intervention for Sleep and Circadian Dysfunction (TranS-C) to improve serious mental illness outcomes in a community setting. J Consult Clin Psychol. (2021) 89:537. doi: 10.1037/ccp0000650
53. Armstrong CC, Dong L, Harvey AG. Mediators and moderators of outcome from the Transdiagnostic Sleep and Circadian Intervention for adults with severe mental illness in a community setting. Behav Res Ther. (2022) 151:104053. doi: 10.1016/j.brat.2022.104053
54. Dolsen EA, Dong L, Harvey AG. Transdiagnostic sleep and circadian intervention for adolescents plus text messaging: randomized controlled trial 12-month follow-up. J Clin Child Adolesc Psychol. 2021:1-13. doi: 10.1080/15374416.2021.1978295
55. Dong L, Dolsen MR, Martinez AJ, Notsu H, Harvey AG, A. transdiagnostic sleep and circadian intervention for adolescents: six-month follow-up of a randomized controlled trial. J Child Psychol Psychiatry. (2020) 61:653–61. doi: 10.1111/jcpp.13154
56. Harvey AG, Hein K, Dolsen MR, Dong L, Rabe-Hesketh S, Gumport NB, et al. Modifying the impact of eveningness chronotype (“night-owls”) in youth: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. (2018) 57:742–54. doi: 10.1016/j.jaac.2018.04.020
57. Kolko DJ, Perrin E. The integration of behavioral health interventions in children's health care: Services, science, and suggestions. J Child Psychol Psychiatry. (2014) 43:216–28. doi: 10.1080/15374416.2013.862804
58. O'Loughlin K, Donovan EK, Radcliff Z, Ryan M, Rybarczyk B. Using integrated behavioral healthcare to address behavioral health disparities in underserved populations. Transl Issues Psychol Sci. (2019) 5:374. doi: 10.1037/tps0000213
59. Baumann AA, Cabassa LJ. Reframing implementation science to address inequities in healthcare delivery. BMC Health Serv Res. (2020) 20:1–9. doi: 10.1186/s12913-020-4975-3
60. Cabassa LJ, Baumann AA, A. two-way street: bridging implementation science and cultural adaptations of mental health treatments. Implement Sci. (2013) 8:1–14. doi: 10.1186/1748-5908-8-90
61. Damschroder L, Aron DC, Keith RE, Kirsh S, Alexander J, Lowery J. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. (2009) 4:50. doi: 10.1186/1748-5908-4-50
62. Parthasarathy S, Carskadon MA, Jean-Louis G, Owens J, Bramoweth A, Combs D, et al. Implementation of sleep and circadian science: recommendations from the sleep research society and national institutes of health workshop. Sleep. (2016) 39:2061–75. doi: 10.5665/sleep.6300
63. Wingood GM, DiClemente RJ. The ADAPT-ITT model: a novel method of adapting evidence-based HIV Interventions. J Acquir Immune Defic Syndr. (2008) 47:S40–6. doi: 10.1097/QAI.0b013e3181605df1
64. DeSilva R, Aggarwal NK, Lewis-Fernandez R. The DSM-5 cultural formulation interview and the evolution of cultural assessment in psychiatry. Psychiatric Times. (2015) 32:10–10. doi: 10.1176/appi.books.9781615373567.rlf01
65. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Publishing (2013). p. 668-76. doi: 10.1176/appi.books.9780890425596
66. Williamson AA, Okoroji C, Cicalese O, Evans BC, Ayala A, Harvey B, et al. Sleep Well! An adapted behavioral sleep intervention implemented in urban primary care. J Clin Sleep Med. (2022) 18:1153–66. doi: 10.5664/jcsm.9822
67. Williamson A, Milaniak I, Watson B, Cicalese O, Fiks AG, Power TJ, et al. Early childhood sleep intervention in urban primary care: caregiver and clinician perspectives. J Pediatr Psychol. (2020) 54:933–45. doi: 10.1093/jpepsy/jsaa024
68. Lee S, Hale L, Berger LM, Buxton OM. Maternal perceived work schedule flexibility predicts child sleep mediated by bedtime routines. J Child Fam Stud. (2019) 28:245–59. doi: 10.1007/s10826-018-1262-6
69. Evans GW. The environment of childhood poverty. Am Psychol. (2004) 59:77. doi: 10.1037/0003-066X.59.2.77
70. Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. (2002) 53:371–99. doi: 10.1146/annurev.psych.53.100901.135233
71. Goldstein TR, Merranko J, Krantz M, Garcia M, Franzen P, Levenson J, et al. Early intervention for adolescents at-risk for bipolar disorder: a pilot randomized trial of Interpersonal and Social Rhythm Therapy (IPSRT). J Affect Disord. (2018) 235:348–56. doi: 10.1016/j.jad.2018.04.049
72. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
73. Smagula SF. Opportunities for clinical applications of rest-activity rhythms in detecting and preventing mood disorders. Curr Opin Psychiatry. (2016) 29:389. doi: 10.1097/YCO.0000000000000283
74. Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. (1999) 16:505–18. doi: 10.3109/07420529908998724
75. Blume C, Santhi N, Schabus M. ‘nparACT'package for R: a free software tool for the non-parametric analysis of actigraphy data. MethodsX. (2016) 3:430–5. doi: 10.1016/j.mex.2016.05.006
76. Ortiz-Tudela E, Martinez-Nicolas A, Campos M, Rol MÁ, Madrid JA. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput Biol. (2010) 6:e1000996. doi: 10.1371/journal.pcbi.1000996
77. Carney T, Chimbambo V, Johnson K, Louw J, Myers B. The adaptation of an evidence-based brief intervention for substance-using adolescents and their caregivers. Psychother Res. (2020) 30:728–38. doi: 10.1080/10503307.2019.1656352
78. Davis M, Johnson C, Pettit AR, Barkin S, Hoffman BD, Jager-Hyman S, et al. Adapting safety check as a universal suicide prevention strategy in pediatric primary care. Acad Pediatr. (2021) 21:1161–70. doi: 10.1016/j.acap.2021.04.012
79. Tervalon M, Murray-Garcia J. Cultural humility versus cultural competence: a critical distinction in defining physician training outcomes in multicultural education. J Health Care Poor Underserved. (1998) 9:117–25. doi: 10.1353/hpu.2010.0233
80. Maina IW, Belton TD, Ginzberg S, Singh A, Johnson TJ, A. decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc Sci Med. (2018) 199:219–29. doi: 10.1016/j.socscimed.2017.05.009
81. Richardson LP, McCauley E, Grossman DC, McCarty CA, Richards J, Russo JE, et al. Evaluation of the Patient Health Questionnaire-9 Item for detecting major depression among adolescents. Pediatrics. (2010) 126:1117–23. doi: 10.1542/peds.2010-0852
82. Kwan BM, Brownson RC, Glasgow RE, Morrato EH, Luke DA. Designing for Dissemination and Sustainability to Promote Equitable Impacts on Health. Ann Rev Public Health. (2022) 43:331–53. doi: 10.1146/annurev-publhealth-052220-112457
83. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. (2006) 18:59–82. doi: 10.1177/1525822X05279903
84. Bradshaw C, Atkinson S, Doody O. Employing a qualitative description approach in health care research. Glob Qual Nurs Res. (2017) 4:23. doi: 10.1177/2333393617742282
85. Kim H, Sefcik JS, Bradway C. Characteristics of qualitative descriptive studies: a systematic review. Res Nurs Health. (2017) 40:23–42. doi: 10.1002/nur.21768
86. Beebe J. Rapid Qualitative Inquiry: A Field Guide to Team-Based Assessment. London: Rowman and Littlefield. (2014).
87. National Institutes of Health. Best Practices for Mixed Methods Research in the Health Sciences. (2011).
88. Palinkas LA, Aarons GA, Horwitz S, Chamberlain P, Hurlburt M, Landsverk J. Mixed method designs in implementation research. Adm Policy Ment Health Jan. (2011) 38:44–53. doi: 10.1007/s10488-010-0314-z
89. Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. (2017) 12:1–12. doi: 10.1186/s13012-017-0635-3
90. Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. (2011) 45:626–9. doi: 10.1016/j.jpsychires.2010.10.008
91. Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. University of Pennsylvania (1980). p. 36. doi: 10.1037/t00834-000
92. Gumport NB Yu SH, Mullin AC, Mirzadegan IA, Harvey AG. The validation of a provider-reported fidelity measure for the transdiagnostic sleep and circadian intervention in a community mental health setting. Behav Ther Sep. (2020) 51:800–13. doi: 10.1016/j.beth.2019.11.006
93. Marino M, Li Y, Rueschman MN, Ellenbogen JM, Solet JM, Dulin H, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. (2013) 36:1747–55. doi: 10.5665/sleep.3142
94. Monk T, Reynolds C 3rd, Kupfer D, Buysse DJ, Coble PA, Hayes AJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. (1994) 3:111–20. doi: 10.1111/j.1365-2869.1994.tb00114.x
95. Slater JA, Botsis T, Walsh J, King S, Straker LM, Eastwood PR. Assessing sleep using hip and wrist actigraphy. Sleep Biol Rhythms. (2015) 13:172–80. doi: 10.1111/sbr.12103
96. Quante M, Kaplan E, Cailler M, Rueschman M, Wang R, Weng J, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. (2018) 10:13–20. doi: 10.2147/NSS.S151085
97. Forrest CB, Meet al Pilkonis PA, Becker BD, Bevans KB. Development and validation of the PROMIS Pediatric Sleep Disturbance and Sleep-Related Impairment item banks. Sleep. (2018) 41:zsy054. doi: 10.1093/sleep/zsy054
98. Efron B. Forcing a sequential experiment to be balanced. Biometrika. (1971) 58:403–17. doi: 10.1093/biomet/58.3.403
99. Ascher BH, Farmer EMZ, Burns BJ, Angold A. The child and adolescent services assessment (CASA): description and psychometrics. J Emot Behav Disord. (1996) 4:12–20. doi: 10.1177/106342669600400102
100. Attkisson CC, Zwick R. The Client Satisfaction Questionnaire: psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. (1982) 5:233–7. doi: 10.1016/0149-7189(82)90074-X
101. Zucker RA, Gonzalez R, Ewing SWF, Paulus MP, Arroyo J, Fuligni A, et al. Assessment of culture and environment in the Adolescent Brain and Cognitive Development Study: Rationale, description of measures, and early data. Dev Cogn Neurosci. (2018) 32:107–20. doi: 10.1016/j.dcn.2018.03.004
102. Hamilton JL, Tsypes A, Zelazny J, Sewall CJR, Rode N, Merranko J, et al. Sleep influences daily suicidal ideation through affective reactivity to interpersonal events among high-risk adolescents and young adults. J Child Psychol Psychiatry. (2022). doi: 10.1111/jcpp.13651 [Epub ahead of print].
103. Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). New York, NY: Columbia University Medical Center. (2008). p. 10. doi: 10.1037/t52667-000
104. Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. (2011) 168:1266–77. doi: 10.1176/appi.ajp.2011.10111704
105. Gerson AC, Gerring JP, Freund L, Joshi PK. The children's affective lability scale: a psychometric evaluation of reliability. Psychiatry Res. (1996) 65:189–97. doi: 10.1016/S0165-1781(96)02851-X
106. Cyders MA, Littlefield AK, Coffey S, Karyadi KA. Examination of a short English version of the UPPS-P impulsive behavior scale. Addict Behav Sep. (2014) 39:1372–6. doi: 10.1016/j.addbeh.2014.02.013
107. Germain A, Moul D, Franzen PL, Miewald JM, Reynold CF, Monk TH. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. (2006) 2:403–6. doi: 10.5664/jcsm.26654
108. Vitiello MV, McCurry SM, Shortreed SM, Balderson BH, Baker LD, Keefe FJ, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc Jun. (2013) 61:947–56. doi: 10.1111/jgs.12275
109. Cook JA, Julious SA, Sones W, Hampson LV, Hewitt C, Berlin JA, et al. DELTA2 guidance on choosing the target difference and undertaking and reporting the sample size calculation for a randomized controlled trial. BMJ. (2018) 19:606. doi: 10.1136/bmj.k3750
110. Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for Planning Pilot Studies in Clinical and Translational Research. Clinical and Translational Science. (2011) 4:332–7. doi: 10.1111/j.1752-8062.2011.00347.x
111. Benton TD, Boyd RC, Njoroge WF. Addressing the global crisis of child and adolescent mental health. JAMA Pediatr. (2021) 175:1108–10. doi: 10.1001/jamapediatrics.2021.2479
112. Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, Madigan S. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. (2021) 175:1142–50. doi: 10.1001/jamapediatrics.2021.2482
113. Zuckerbrot RA, Cheung A, Jensen PS, Stein REK, Laraque D, GLAD-PC Steering Group, et al. Guidelines for adolescent depression in primary care (GLAD-PC): Part I. Practice preparation, identification, assessment, and initial management. Pediatrics. (2018) 141:e20174081. doi: 10.1542/peds.2017-4081
114. Bitar GW, Springer P, Gee R, Graff C, Schydlower M. Barriers and facilitators of adolescent behavioral health in primary care: perceptions of primary care providers. Fam Syst Health. (2009) 27:346. doi: 10.1037/a0018076
115. Crawford A, Serhal E. Digital health equity and COVID-19: the innovation curve cannot reinforce the social gradient of health. J Med Internet Res. (2020) 22:e19361. doi: 10.2196/19361
116. Cordova-Ramos EG, Tripodis Y, Garg A, Kalluri NS, Flores G, Parker MG. Linguistic disparities in child health and presence of a medical home among US Latino children. Acad Pediatr. (2021). doi: 10.1016/j.acap.2021.09.011 [Epub ahead of print].
117. Kanine RM, Bush ML, Davis M, Jones JD, Sbrilli MD, Young JF. Depression prevention in pediatric primary care: implementation and outcomes of interpersonal psychotherapy—adolescent skills training. Child Psychiatry Hum Dev. (2021) 1–13. doi: 10.1007/s10578-021-01222-6
118. Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Aff Blog. (2020) 10. doi: 10.1377/forefront.20200630.939347
119. Crenshaw KW. Demarginalizing the intersection of race and sex: a black feminist critique of antidiscrimination doctrine, feminist theory and antiracist politics. U Chi Legal F. (1989) 1989:139.
120. Roulston C, McKetta S, Price M, Fox KR, Schleider JL. Structural correlates of mental health support access among sexual minority youth of color during COVID-19. J Clin Child Adolesc Psychol. (2022) 1–10. doi: 10.1080/15374416.2022.2034633 [Epub ahead of print].
121. Weisz JR, Ng MY, Bearman SK. Odd couple? Reenvisioning the relation between science and practice in the dissemination-implementation era. Clin Psychol Sci. (2014) 2:58–74. doi: 10.1177/2167702613501307
Keywords: adolescent, adaptation, circadian, health equity, intervention, sleep, suicide, implementation science
Citation: Williamson AA, Soehner AM, Boyd RC, Buysse DJ, Harvey AG, Jonassaint CR, Franzen PL and Goldstein TR (2022) A protocol for applying health equity-informed implementation science models and frameworks to adapt a sleep intervention for adolescents at risk for suicidal thoughts and behaviors. Front. Public Health 10:971754. doi: 10.3389/fpubh.2022.971754
Received: 17 June 2022; Accepted: 16 September 2022;
Published: 12 October 2022.
Edited by:
Catherine Battaglia, United States Department of Veterans Affairs, United StatesReviewed by:
David Sommerfeld, University of California, San Diego, United StatesAlejandro Vázquez, Utah State University, United States
Copyright © 2022 Williamson, Soehner, Boyd, Buysse, Harvey, Jonassaint, Franzen and Goldstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ariel A. Williamson, d2lsbGlhbXNvYUBjaG9wLmVkdQ==
†These authors share senior authorship
 Ariel A. Williamson
Ariel A. Williamson Adriane M. Soehner
Adriane M. Soehner Rhonda C. Boyd1,2
Rhonda C. Boyd1,2 Daniel J. Buysse
Daniel J. Buysse