- 1Department of Cardiology, Lishui Hospital, College of Medicine, Zhejiang University, Lishui, China
- 2College of Medicine, Zhejiang University, Hangzhou, China
Background: Cadmium is a commonly found heavy metal with a prolonged biological half-life, which results in long-term health burden for the population. Prior studies have demonstrated an association between blood cadmium and hypertension. However, few studies examined the relationship between blood cadmium and long-term health outcomes in patients with hypertension. This study aimed to investigate the association of blood cadmium with mortality in patients with hypertension.
Methods: This study analyzed data from the National Health and Nutrition Examination Survey 1999–2012. Complex sampling-weighted multivariate Cox proportional hazards models were used to evaluate the hazard ratios (HRs) of all-cause, cardiovascular, and Alzheimer’s disease mortality in patients with hypertension classified by blood cadmium concentrations’ quantiles.
Results: The study included 12,208 patients with hypertension with a median follow-up duration of 10.8 years. During this period, there were 4,485 all-cause deaths, including 1,520 cardiovascular deaths and 180 Alzheimer’s disease deaths. Compared with the lowest quintile of blood cadmium (≤0.25 μg/L) group, the highest quintile of blood cadmium (≥0.80 μg/L) group’s adjusted HRs were 1.85 (95% CI, 1.59–2.14) for all-cause mortality, 1.76 (95% CI, 1.33–2.34) for cardiovascular mortality, and 3.41 (95% CI, 1.54–7.51) for Alzheimer’s disease mortality. Additionally, the adjusted HR for cardiovascular mortality was 2.12 (95% CI, 1.36–3.30) in never-smoking patients with hypertension.
Conclusion: Higher blood cadmium is associated with increased risks of all-cause, cardiovascular, and Alzheimer’s disease mortality in patients with hypertension. The effect of blood cadmium on cardiovascular mortality may be more pronounced in never-smoking hypertensive patients.
Introduction
Cadmium is a common toxic heavy metal that is widely present in the environment. It has an extremely long biological half-life in both the environment (approximately 10–30 year) and the human body (greater than 20 years), leading to a long-term health burden (1–3). The exposure of cadmium mainly occurs through industrial production (e.g., mining, metallurgical industries, and manufacturers of nickel-Cd batteries) (4), tobacco smoking, ambient air (5), and foods (6). Although exposure levels have decreased in recent years, cadmium still has a significant impact on various health status, including cardiovascular diseases, diabetes, cognitive dysfunction, Alzheimer’s disease, kidney injury, arthritis, and cancer (3, 7–12). Since the 1950s, multiple studies have suggested an association between cadmium and blood pressure and hypertension (13–17). A recent meta-analysis reconfirmed the positive association between blood cadmium and hypertension (18). In addition, some studies have shown the effect of cadmium exposure on mortality (19–26). Previous studies mostly focus on the general population (20, 22–24). And several studies have focused on cadmium-contaminated areas (25, 26). However, few studies have investigated the association between blood cadmium and long-term health outcomes in high-risk populations who had already suffered from related diseases (e.g., patients with hypertension). There is an urgent need to generate more scientific evidence on the relationship between cadmium exposure and mortality in high-risk populations to raise public awareness of cadmium exposure reduction. Therefore, this study aimed to evaluate the association of blood cadmium with all-cause and cause-specific mortality in patients with hypertension, which may provide reference for the long-term adverse health effects of cadmium exposure, especially in high-risk population.
Methods
Study participants
The National Health and Nutrition Examination Survey (NHANES) is a program designed to assess the health and nutritional status of the US population (27). The US National Center for Health Statistics (NCHS) is responsible for producing vital and health statistics by using a stratified, multi-stage probability sampling design that ensures participants are representative of the civilian deinstitutionalized population of the United States (27). The NHANES protocol has been approved by the NCHS Research Ethics Review Board, and written informed consent has been obtained from each participant (28).
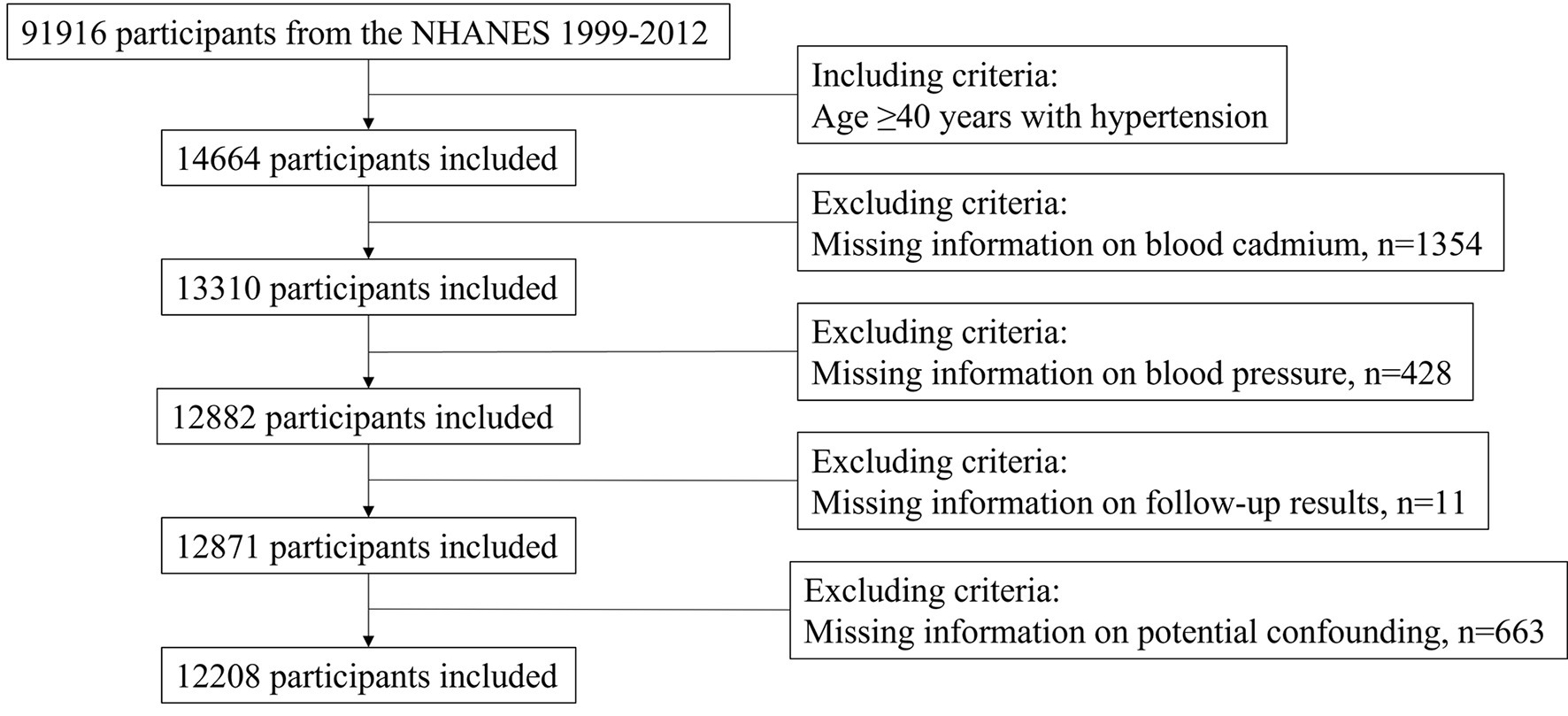
For this study, we obtained data on NHANES participants between 1999 and 2012. Since there were few mortality-related cases under the age of 40, we focused on participants aged 40 years or older with hypertension at baseline, resulting in a total of 14,664 participants. Hypertension was defined as measured systolic blood pressure (SBP) ≥ 140 mmHg or/and diastolic blood pressure (DBP) ≥ 90 mmHg, or/and previous diagnosis of hypertension, or/and taking antihypertensive prescription. We excluded patients with missing information on blood cadmium (n = 1,354), missing blood pressure data (n = 428), lacking follow-up data (n = 11), and missing information on potential confounding variables (n = 663). This left us with a final sample size of 12,208 participants for analysis (Figure 1).
Measurement of blood cadmium
Whole blood specimens are processed, stored, and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, and Centers for Disease Control and Prevention for analysis. Blood cadmium was determined using a PerkinElmer Model SIMAA 6000 simultaneous multi-element atomic absorption spectrometer with Zeeman background correction in NHANES 1999–2002 and using inductively coupled plasma mass spectrometry in NHANES 2003–2012 (27).
The extremely high and low values were verified by NCHS staff carefully, and numerous consistency checks were performed. The limit of detection (LOD) for blood cadmium was 0.1–0.2 μg/L. Blood cadmium below LOD was replaced with a value equal to LOD divided by the square root of two. Blood cadmium below LOD were found in 12% of participants overall (n = 1,488), which did not affect grouping according to quintile.
Outcome ascertainment
The NHANES linked mortality data used in this study were updated with follow-up data through December 31, 2019 (29). All-cause death was defined as death from any cause. According to the International Classification of Diseases, 10th revision (ICD-10), cardiovascular death was defined as death due to diseases of heart (I00-I09, I11, I13, and I20–I51) and cerebrovascular diseases (I60–I69) (30). Alzheimer’s disease death was defined as death due to Alzheimer’s disease (G30).
Covariate assessment
The following variables were obtained through the interview questionnaire: age, sex, race/ethnicity, educational level, smoking status, medical conditions (including hypertension, diabetes, asthma, heart failure, coronary heart disease, stroke, emphysema, chronic bronchitis, liver condition, and cancer), and antihypertensive medication use. The following variables were measured according to standard protocols: body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), blood pressure, total cholesterol, triglycerides, high density lipoprotein, serum creatinine, uric acid, and cotinine. The estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation (31). Obese was defined as BMI ≥ 30 kg/m2. Optimal blood pressure was defined as SBP < 140 mmHg and DBP < 90 mmHg.
Statistical analysis
Given that the NHANES data used in this study were not simple random samples, appropriate weights were used for each analysis based on the selected variables, as recommended by the US Centers for Disease Control and Prevention (32). Continuous variables were expressed as mean ± standard error (mean ± SE), and comparisons between groups were made by analysis of variance. Categorical variables were expressed as percentages, and comparisons between groups were made by the chi-square test. Complex sampling-weighted multivariate Cox models were applied to investigate the hazard ratio (HR) and 95% confidence interval (CI) between blood cadmium and mortality. Model 1 was adjusted for age, sex, race/ethnicity, and education level. Model 2 was further adjusted for BMI, eGFR, SBP, DBP, total cholesterol, triglycerides, high-density lipoprotein, and disease conditions (heart failure, coronary heart disease, stroke, diabetes, and cancer). Model 3 was further adjusted for serum cotinine and smoking status (never, former, or current smoker). Based on model 3, subgroup analyses were stratified by gender, age, BMI, blood pressure control, eGFR, and smoking status. Interactions between blood cadmium (lowest and highest quintiles) and stratification factors were assessed. Restricted cubic spline regression was used to explore the dose–response association of blood cadmium and mortality. Furthermore, we performed sensitivity analysis by using the random forest imputation method to interpolate values of blood cadmium below LOD and missing variables. All statistical analyses were performed using R version 4.1.2 (R Project for Statistical Computing). p < 0.05 was regarded as statistically significant for all tests.
Results
Baseline characteristics
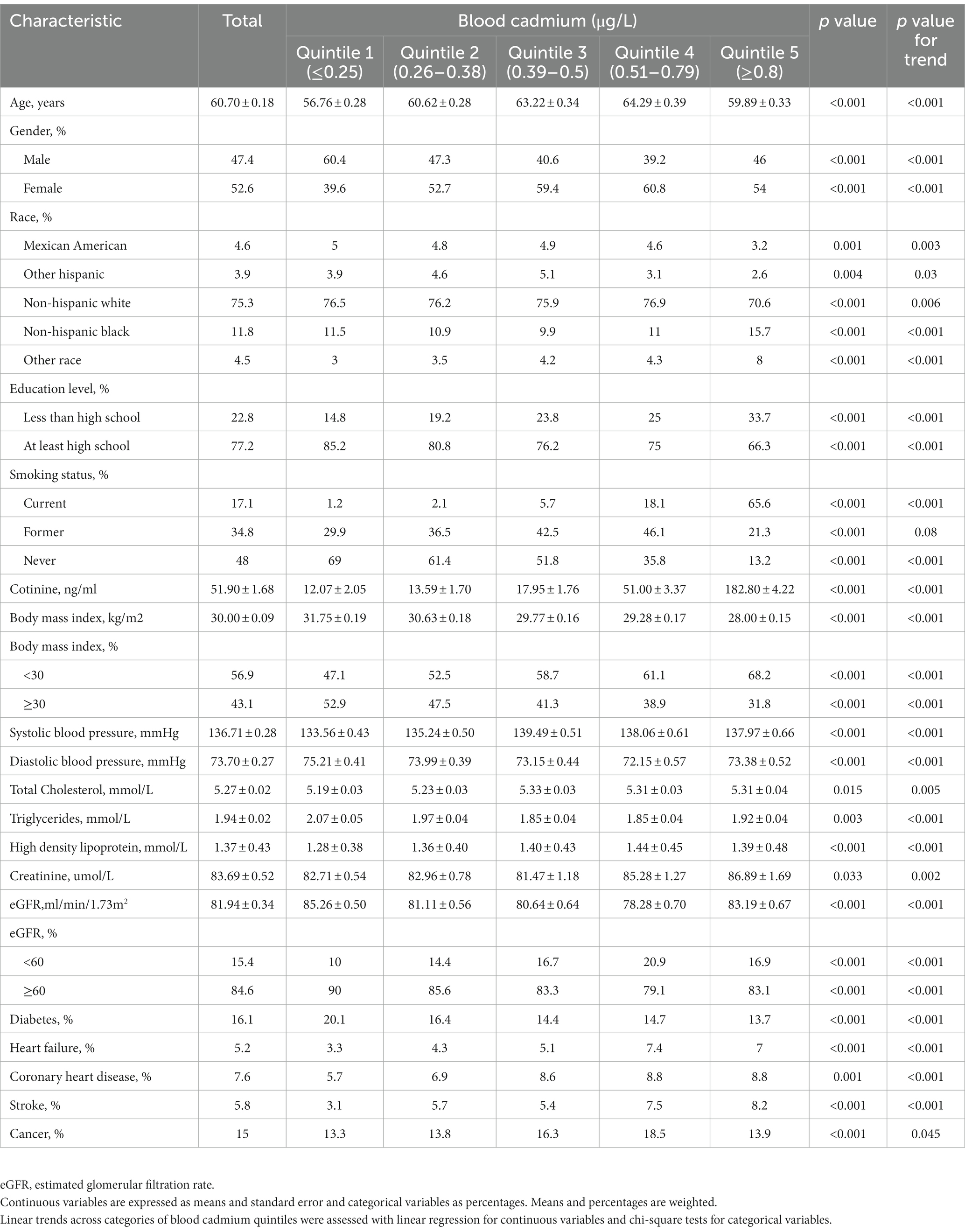
In this study, a total of 12,208 patients with hypertension aged 40 years or older were included. During a median follow-up of 10.8 years (interquartile range [IQR], 7.9–14.4), 4,485 all-cause deaths occurred, including 1,520 cardiovascular deaths and 180 Alzheimer’s disease deaths. The range and percentage of blood cadmium below the limit of detection were 0.1–0.2 μg/L and 12.2%, respectively. The baseline characteristics of the patients grouped according to blood cadmium quintiles were listed in Table 1. Participants with higher blood cadmium levels were more likely to be older, female, non-Hispanic black, non-obese, and current smokers. They also had lower education levels, lower triglycerides, lower eGFR, lower DBP, and higher SBP, higher total cholesterol, and higher high-density lipoprotein levels. In addition, participants with higher blood cadmium levels had a lower prevalence of diabetes but a higher prevalence of cardiovascular diseases (heart failure, coronary heart disease, and stroke).
Association of blood cadmium with mortality
Table 2 presented survey-weighted Cox regression results. The multivariate adjusted model 3 showed that blood cadmium was positively associated with all-cause, cardiovascular, and Alzheimer’s disease mortality in participants. Compared with participants in the lowest quintile (≤0.25 μg/L) of blood cadmium, participants in the highest quintile (≥0.8 μg/L) had HRs of 1.85 (95% CI, 1.59–2.14) for all-cause mortality, 1.76 (95% CI, 1.33–2.34) for cardiovascular mortality, and 3.41 (95% CI, 1.54–7.51) for Alzheimer’s disease mortality. Moreover, the effect of blood cadmium on mortality may be in a dose-dependent manner.
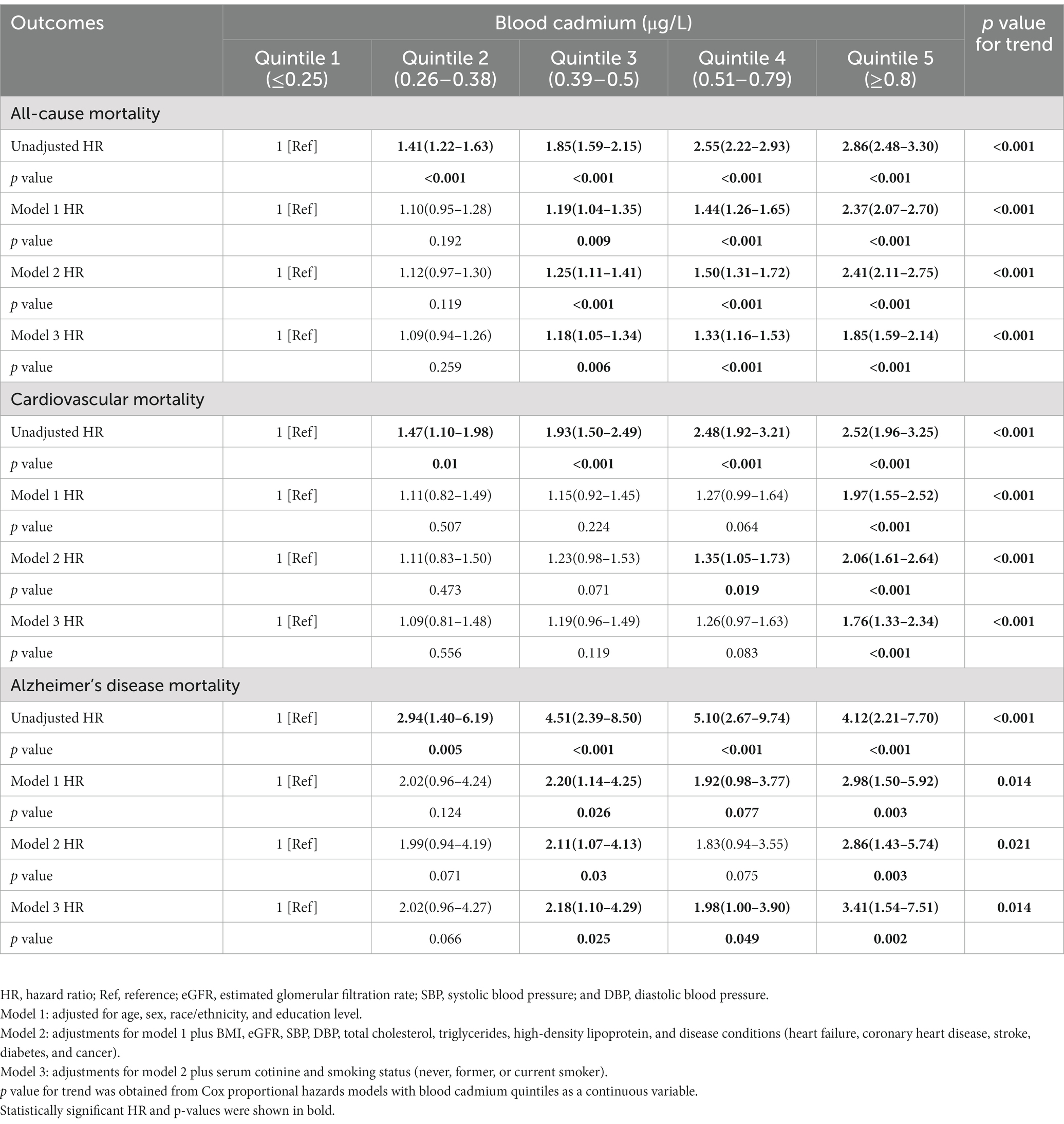
Table 2. Hazard ratios for all-cause, cardiovascular and Alzheimer’s disease mortality of all participants, stratified by blood cadmium.
Dose–response curves for blood cadmium and mortality
The restricted cubic spline analysis showed a dose–response association between blood cadmium and all-cause, cardiovascular, and Alzheimer’s disease mortality (Figure 2), which was consistent with the weighted Cox regression results. We observed a non-linear association for all-cause (p < 0.001, Nonlinear p < 0.001) and cardiovascular mortality (p < 0.001, Nonlinear p = 0.027), with adjusted HRs steeper at lower blood cadmium concentrations than at higher concentrations. For Alzheimer’s disease mortality, the association was close to a linear correlation (p = 0.055, Nonlinear p = 0.863).
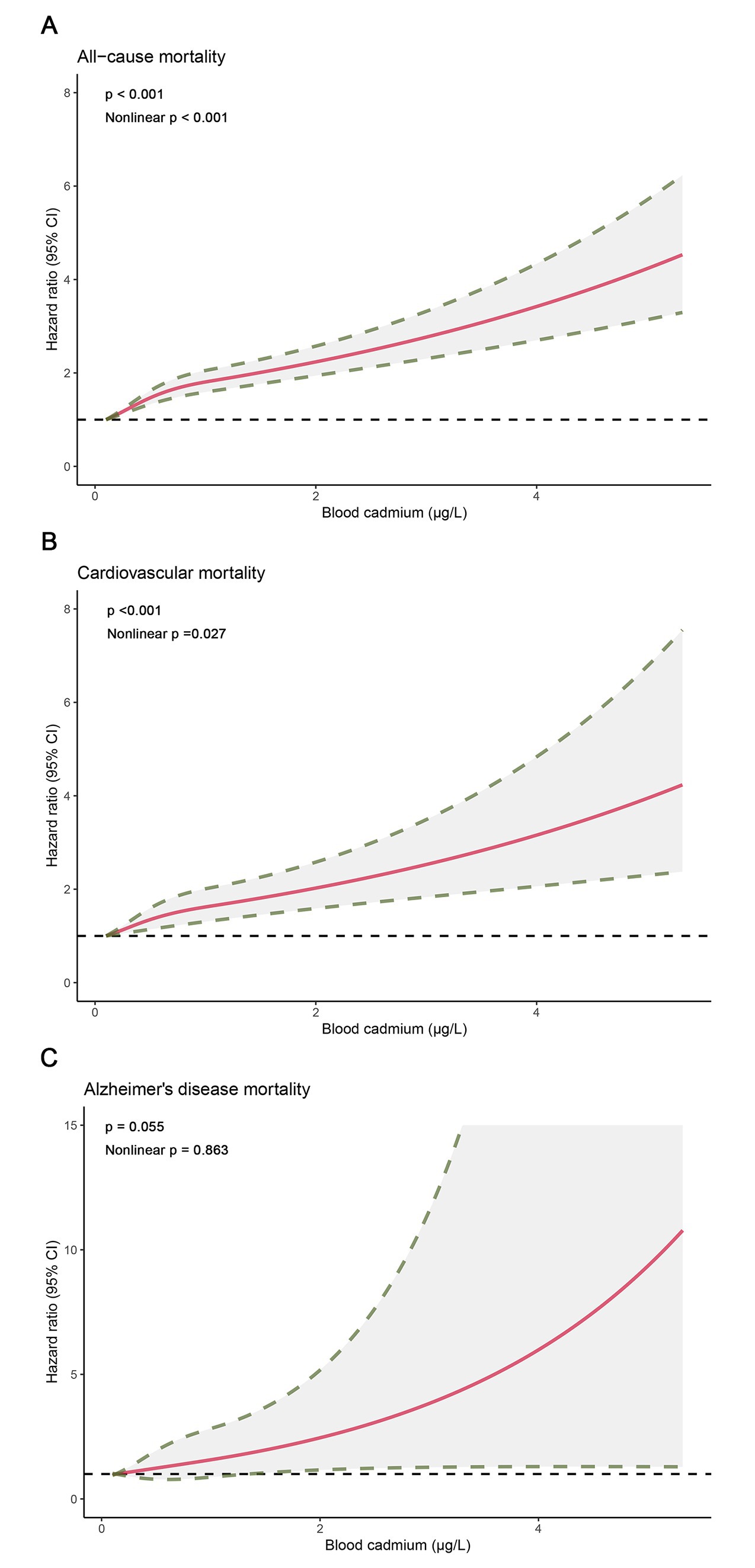
Figure 2. Dose–response curves for concentrations of blood cadmium and mortality. (A) All-cause mortality. (B) Cardiovascular mortality. (C) Alzheimer’s disease mortality.
Subgroup analysis
The results of subgroup analysis according to multivariate adjusted model 3 showed that the significant association between blood cadmium and mortality still existed in most subgroups (Supplementary Table 1). The adjusted HRs of the highest quintile of blood cadmium compared with the lowest quintile of blood cadmium and interactions in subgroups were shown in Figure 3. For all-cause mortality, there was a significant interaction between age and blood cadmium (p = 0.048). The HR of the highest blood cadmium quintile compared to the lowest blood cadmium quintile in middle-aged (40–59 years) participants was 2.64 (95% CI, 1.72–4.06), while the HR of older participants (≥60 years) was only 1.66 (95% CI, 1.44–1.90). For cardiovascular mortality, there was no statistical significance in the Cox regression results of the blood cadmium quintiles of the current smokers (the highest quintile of blood cadmium, HR 1.18 [95% CI, 0.21–6.76]) or former smokers (the highest quintile of blood cadmium, HR 1.31 [95% CI, 0.83–2.08]). But blood cadmium was significantly associated with an increased risk of cardiovascular mortality in never-smoking participants (the highest quintile of blood cadmium, HR 2.12 [95% CI, 1.36–3.30]). For Alzheimer’s disease mortality, the HR of the highest blood cadmium quintile was higher in non-obese participants (HR 3.91 [95% CI, 1.71–8.97]) compared to obese participants (HR 2.31 [95% CI, 0.31–17.14]).
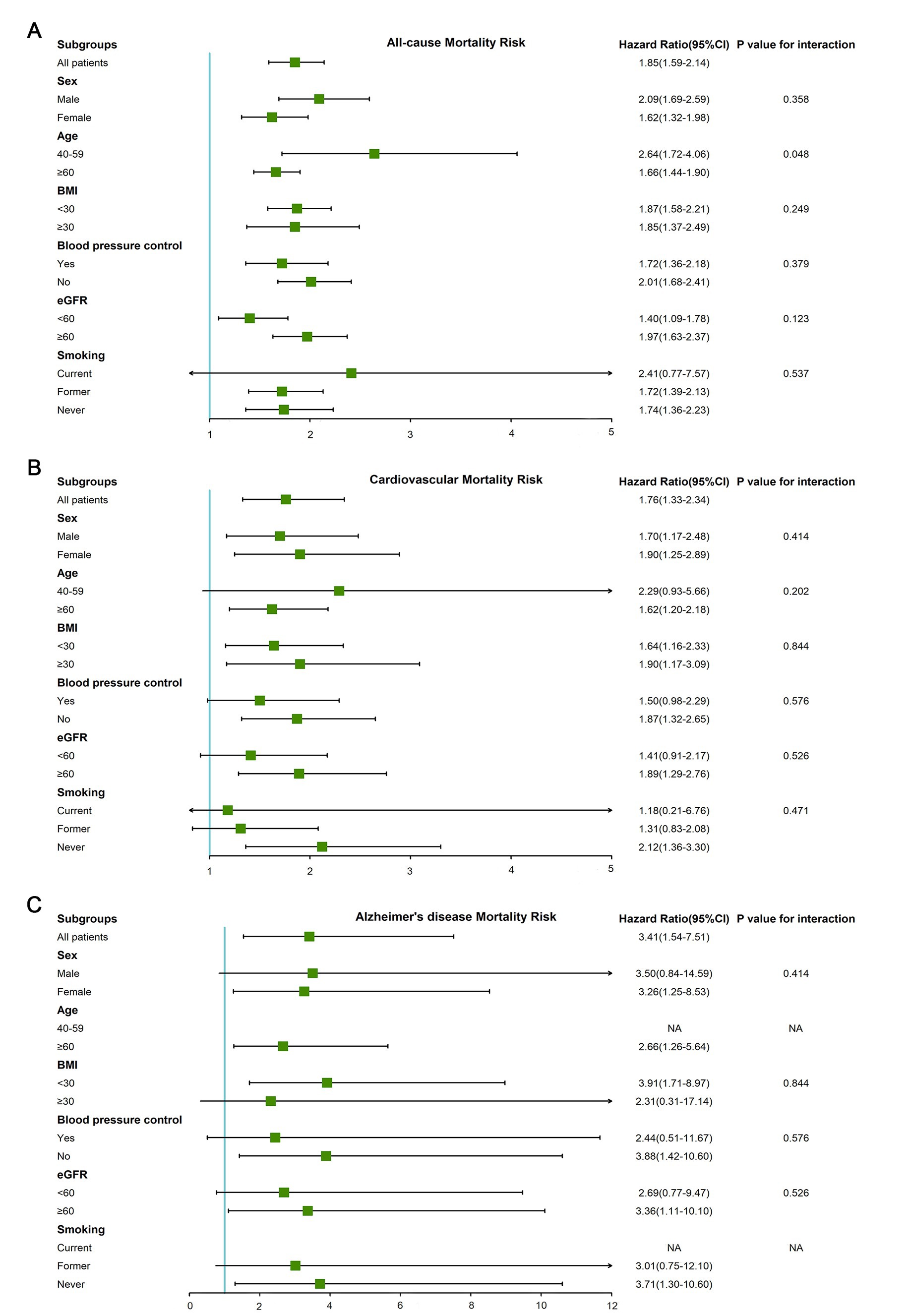
Figure 3. The adjusted HRs of the highest quintile of blood cadmium compared with the lowest quintile of blood cadmium in subgroups (gender, age, BMI, blood pressure control, eGFR, and smoking status). (A) All-cause mortality. (B) Cardiovascular mortality. (C) Alzheimer’s disease mortality.
Association of blood cadmium with mortality in sensitivity analysis
As shown in Supplementary Figure 1, triglycerides exhibited the highest proportion of missing data (11.26%, n = 1,650). Therefore, we conducted imputation for blood cadmium below LOD and missing variables. Supplementary Table 2 presented a similar positive association of blood cadmium with all-cause, cardiovascular and Alzheimer’s disease mortality. Compared to participants in the lowest quintile (≤0.25 μg/L) of blood cadmium, those in the highest quintile (≥0.8 μg/L) had the HRs of 1.76 (95%CI, 1.54–2.01) for all-cause mortality, 1.82 (95%CI, 1.44–2.31) for cardiovascular mortality and 2.57 (95%CI, 1.33–4.98) for Alzheimer’s disease mortality. Supplementary Figure 2 demonstrated the significant dose–response association of blood cadmium with all-cause and cardiovascular mortality after the interpolation.
Discussion
Our study suggests that blood cadmium levels are linked to higher risks of all-cause, cardiovascular, and Alzheimer’s mortality in patients with hypertension, even after adjusting for smoking status and cotinine concentrations. Moreover, a positive dose–response curve was observed between blood cadmium and mortality. Notably, in patients with low blood cadmium levels, the risks associated with elevated blood cadmium were even more pronounced. Furthermore, the effect of blood cadmium on cardiovascular mortality was more significant in never-smoking patients with hypertension than in smoking patients with hypertension.
Many previous studies have investigated the association of blood cadmium and mortality. Nawrot et al. (33) found that blood cadmium was associated with all-cause and non-cardiovascular mortality in a population with relatively high cadmium exposure. Several NHANES studies have suggested that blood cadmium is associated with all-cause, cardiovascular, and cancer mortality in the US general population (19, 34, 35). Similar results were also observed among patients with type 2 diabetes (36). Moreover, a few studies have suggested that higher blood cadmium is associated with higher mortality from influenza and pneumonia, as well as Alzheimer’s disease (37, 38). Our study also found a positive correlation between blood cadmium and mortality in a hypertensive population. Studies among the US general population suggest that the effect of blood cadmium in increasing the risk of cardiovascular death is more significant in smokers than in non-smokers (19, 34). However, our study among a hypertensive population found that the effect of blood cadmium in increasing the risk of cardiovascular death was more pronounced in participants who never smoked. This may be related to the different effects of blood cadmium on blood pressure in patients with different smoking status. Based on the 1999–2004 NHANES, Tellez-Plaza et al. proposed that blood cadmium is associated with increased blood pressure, and this association is mainly reflected in never-smokers.
Additionally, the dose–response curves in our study showed that the increased risk of cardiovascular mortality was more pronounced with the increase in blood cadmium at lower levels of exposure. Furthermore, the blood cadmium level of never-smokers was significantly lower than that of smokers. This finding suggests that the cardiovascular mortality risk of never-smoking patients with hypertension is more susceptible to the influence of blood cadmium than smoking patients with hypertension. However, more precisely designed prospective studies are needed to confirm this finding. It is recommended that patients with hypertension should reduce cadmium exposure as much as possible, and even non-smokers may benefit from reducing cadmium exposure from various dietary and environmental sources.
Our results also showed that higher blood cadmium had lower prevalence of diabetes, which are contrary to previous study (8). Possible explanations are as follows: Urban residents have a higher burden of cadmium pollution compared to rural residents, but they have a lower incidence of diabetes than rural residents (39, 40). The mean value±SE of blood cadmium in our study was 0.57 ± 0.01ug/L, which was much lower than the blood cadmium biological threshold limit value for non-occupational exposure recommended by the World Health Organization.1 The cadmium exposure in our study may not be high enough to induce overt diabetes (41).
Blood cadmium has been linked to increased risk of cardiovascular mortality, possibly due to its effects on blood pressure and atherosclerosis. Studies have suggested that cadmium may cause oxidative stress (42), activate calcium channels (43), inhibit nitric oxide (44), and vascular tissue damage by leading to endothelial dysfunction (45) Additionally, there is evidence to suggest that cadmium-induced oxidative stress may lead to renal injury, which in turn indirectly affects the cardiovascular system (10). Moreover, cadmium may play a role in the formation of amyloid plaques and neuronal damage, potentially contributing to Alzheimer’s disease mortality risk. Given that hypertension is a risk factor for Alzheimer’s disease, blood cadmium may indirectly affect Alzheimer’s disease through its impact on blood pressure. However, the mechanism behind the increased mortality risk associated with blood cadmium exposure requires further investigation. It is recommended that further studies be conducted to clarify the precise mechanisms involved and identify effective preventive measures.
Although this is the first study to investigate the effect of blood cadmium on the mortality of patients with hypertension, we acknowledge several limitations. Blood cadmium was measured only once, which may not accurately reflect long-term blood cadmium levels. Moreover, the low proportion of blood cadmium below the detection limit still affects the accuracy of the dose–response curve. Additionally, outcome variables identified by death certificates may not fully reflect the exact cause of death. Finally, this study is an observational study, and despite correction for multiple confounding variables, there is still a risk of confounding bias. Nonetheless, this study has several strengths, including a large sample size and a long follow-up time. The Cox model fully adjusted for many traditional risk factors, as well as smoking status and cotinine concentration.
Conclusion
Our study found that higher blood cadmium levels are associated with an increased risk of all-cause, cardiovascular, and Alzheimer’s disease mortality in patients with hypertension. The association remained significant after adjusting for demographic characteristics, serum cotinine, smoking status, BMI, eGFR, blood pressure, lipids, and various disease conditions. Notably, the effect of blood cadmium on cardiovascular mortality may be more pronounced in never-smoking patients with hypertension than in smoking patients with hypertension. Considering the risk of poor long-term health outcomes, cadmium exposure is recommended to be reduced as much as possible in patients with hypertension, even non-smokers.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The NHANES website: NHANES Questionnaires, Datasets, and Related Documentation (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
Ethics statement
The studies involving human participants were reviewed and approved by The NCHS Research Ethics Review Board approved the NHANES protocol (https://www.cdc.gov/nchs/nhanes/irba98.htm). The patients/participants provided their written informed consent to participate in this study.
Author contributions
TW, LL, and SC conceived and designed research. RS and JS processed data and performed statistical analysis. SC and RS wrote the initial paper. TW and LL reviewed and corrected the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Science and Technology Public Welfare Project of Lishui city (2019GYX28) and Key Research and Development Program of Zhejiang Province (2020ZJZC01).
Acknowledgments
We thank all participants for their supports.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1106732/full#supplementary-material
Abbreviations
HR, Hazard ratio; CI, Confidence interval; NHANES, National Health and Nutrition Examination Survey; NCHS, National Center for Health Statistics; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; ICD-10, International Classification of Diseases, 10th revision; BMI, Body mass index; eGFR, Estimated glomerular filtration rate; SE, Standard error; Ref, Reference.
Footnotes
References
1. Franzoni, G, Ciccotelli, V, Masiello, L, De Ciucis, CG, Anfossi, AG, Vivaldi, B, et al. Cadmium and wild boar: environmental exposure and immunological impact on macrophages. Toxicol Rep. (2022) 9:171–80. doi: 10.1016/j.toxrep.2022.01.009
2. Houot, L, Floutier, M, Marteyn, B, Michaut, M, Picciocchi, A, Legrain, P, et al. Cadmium triggers an integrated reprogramming of the metabolism of Synechocystis Pcc6803, under the control of the Slr1738 regulator. BMC Genomics. (2007) 8:350. doi: 10.1186/1471-2164-8-350
3. Genchi, G, Sinicropi, MS, Lauria, G, Carocci, A, and Catalano, A. The effects of cadmium toxicity. Int J Environ Res Public Health. (2020) 17:3782. doi: 10.3390/ijerph17113782
4. Yiran, Z, Chenyang, J, Jiajing, W, Yan, Y, Jianhong, G, Jianchun, B, et al. Oxidative stress and mitogen-activated protein kinase pathways involved in cadmium-induced Brl 3a cell apoptosis. Oxidative Med Cell Longev. (2013) 2013:516051. doi: 10.1155/2013/516051
5. Cosselman, KE, Navas-Acien, A, and Kaufman, JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. (2015) 12:627–42. doi: 10.1038/nrcardio.2015.152
6. Huo, J, Huang, Z, Li, R, Song, Y, Lan, Z, Ma, S, et al. Dietary cadmium exposure assessment in rural areas of Southwest China. PLoS One. (2018) 13:e0201454. doi: 10.1371/journal.pone.0201454
7. Lin, HC, Hao, WM, and Chu, PH. Cadmium and cardiovascular disease: an overview of pathophysiology, epidemiology, therapy, and predictive value. Rev Port Cardiol. (2021) 40:611–7. doi: 10.1016/j.repce.2021.07.031
8. Filippini, T, Wise, LA, and Vinceti, M. Cadmium exposure and risk of diabetes and Prediabetes: a systematic review and dose-response Meta-analysis. Environ Int. (2022) 158:106920. doi: 10.1016/j.envint.2021.106920
9. Gade, M, Comfort, N, and Re, DB. Sex-specific neurotoxic effects of heavy metal pollutants: epidemiological, experimental evidence and candidate mechanisms. Environ Res. (2021) 201:111558. doi: 10.1016/j.envres.2021.111558
10. Yan, LJ, and Allen, DC. Cadmium-induced kidney injury: oxidative damage as a unifying mechanism. Biomol Ther. (2021) 11:1575. doi: 10.3390/biom11111575
11. Reyes-Hinojosa, D, Lozada-Pérez, CA, Zamudio Cuevas, Y, López-Reyes, A, Martínez-Nava, G, Fernández-Torres, J, et al. Toxicity of cadmium in musculoskeletal diseases. Environ Toxicol Pharmacol. (2019) 72:103219. doi: 10.1016/j.etap.2019.103219
12. Ma, S, Zhang, J, Xu, C, Da, M, Xu, Y, Chen, Y, et al. Increased serum levels of cadmium are associated with an elevated risk of cardiovascular disease in adults. Environ Sci Pollut Res Int. (2022) 29:1836–44. doi: 10.1007/s11356-021-15732-2
13. Perry, HM, and Schroeder, HA. Concentration of trace metals in urine of treated and untreated hypertensive patients compared with Normal subjects. J Lab Clin Med. (1955) 45:963–72.
14. Schroeder, HA, and Vinton, WH Jr. Hypertension induced in rats by small doses of cadmium. Am J Phys. (1962) 202:515–8. doi: 10.1152/ajplegacy.1962.202.3.515
15. Schroeder, HA. Renal cadmium and essential hypertension. JAMA. (1964) 187:358. doi: 10.1001/jama.1964.03060180044013
16. Carroll, RE. The relationship of cadmium in the air to cardiovascular disease death rates. JAMA. (1966) 198:267–9. doi: 10.1001/jama.1966.03110160095029
17. Martins, AC, Almeida Lopes, ACB, Urbano, MR, Carvalho, MFH, Silva, AMR, Tinkov, AA, et al. An updated systematic review on the association between cd exposure, blood pressure and hypertension. Ecotoxicol Environ Saf. (2021) 208:111636. doi: 10.1016/j.ecoenv.2020.111636
18. Aramjoo, H, Arab-Zozani, M, Feyzi, A, Naghizadeh, A, Aschner, M, Naimabadi, A, et al. The association between environmental cadmium exposure, blood pressure, and hypertension: a systematic review and Meta-analysis. Environ Sci Pollut Res Int. (2022) 29:35682–706. doi: 10.1007/s11356-021-17777-9
19. Li, Z, Fan, Y, Tao, C, Yan, W, Huang, Y, Qian, H, et al. Association between exposure to cadmium and risk of all-cause and cause-specific mortality in the general us adults: a prospective cohort study. Chemosphere. (2022) 307:136060. doi: 10.1016/j.chemosphere.2022.136060
20. Larsson, SC, and Wolk, A. Urinary cadmium and mortality from all causes, Cancer and cardiovascular disease in the general population: systematic review and Meta-analysis of cohort studies. Int J Epidemiol. (2016) 45:782–91. doi: 10.1093/ije/dyv086
21. Tägt, J, Helte, E, Donat-Vargas, C, Larsson, SC, Michaëlsson, K, Wolk, A, et al. Long-term cadmium exposure and fractures, cardiovascular disease, and mortality in a prospective cohort of women. Environ Int. (2022) 161:107114. doi: 10.1016/j.envint.2022.107114
22. Suwazono, Y, Nogawa, K, Sakurai, M, Watanabe, Y, Nishijo, M, Ishizaki, M, et al. Environmental cadmium exposure and noncancer mortality in a general Japanese population in cadmium nonpolluted regions. J Appl Toxicol. (2021) 41:587–94. doi: 10.1002/jat.4067
23. Jiang, A, Gong, L, Ding, H, and Wang, M. Cancer mortality and long-term environmental exposure of cadmium in contaminated community based on a third retrospective cause of death investigation of residents living in the Guangdong Province from 2004 to 2005. Biol Trace Elem Res. (2021) 199:4504–15. doi: 10.1007/s12011-021-02599-0
24. Watanabe, Y, Nogawa, K, Nishijo, M, Sakurai, M, Ishizaki, M, Morikawa, Y, et al. Relationship between Cancer mortality and environmental cadmium exposure in the general Japanese population in cadmium non-polluted areas. Int J Hyg Environ Health. (2020) 223:65–70. doi: 10.1016/j.ijheh.2019.10.005
25. Nishijo, M, Nogawa, K, Suwazono, Y, Kido, T, Sakurai, M, and Nakagawa, H. Lifetime cadmium exposure and mortality for renal diseases in residents of the cadmium-polluted Kakehashi River basin in Japan. Toxics. (2020) 8:81. doi: 10.3390/toxics8040081
26. Nogawa, K, Suwazono, Y, Nishijo, M, Sakurai, M, Ishizaki, M, Morikawa, Y, et al. Increase of lifetime cadmium intake dose-dependently increased all cause of mortality in female inhabitants of the cadmium-polluted Jinzu River basin, Toyama. Jpn Environ Res. (2018) 164:379–84. doi: 10.1016/j.envres.2018.03.019
27. Centers for Disease Control and Prevention (2022). National Health and Nutrition Examination Survey. Availbale at: https://www.cdc.gov/nchs/nhanes/index.htm (Accessed November 13, 2022).
28. Centers for Disease Control and Prevention (2022). Nchs Research Ethics Review Board Approval. Available at: https://www.cdc.gov/nchs/nhanes/irba98.htm (Accessed November 13, 2022).
29. Centers for Disease Control and Prevention (2022). 2019 Public-Use Linked Mortality Files. Available at: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm (Accessed August 25, 2022).
30. National Center for Health Statistics (2022). Underlying and Multiple Cause of Death Codes. Available at: https://www.cdc.gov/nchs/data/datalinkage/underlying-and-multiple-cause-of-death-codes-508.pdf. (Accessed August 25, 2022).
31. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YL, Castro, AF 3rd, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
32. Centers for Disease Control and Prevention (2022). Nhanes Survey Methods and Analytic Guidelines. Available at: https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx (Accessed Nov 13, 2022).
33. Nawrot, TS, Van Hecke, E, Thijs, L, Richart, T, Kuznetsova, T, Jin, Y, et al. Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environ Health Perspect. (2008) 116:1620–8. doi: 10.1289/ehp.11667
34. Tellez-Plaza, M, Navas-Acien, A, Menke, A, Crainiceanu, CM, Pastor-Barriuso, R, and Guallar, E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect. (2012) 120:1017–22. doi: 10.1289/ehp.1104352
35. Duan, W, Xu, C, Liu, Q, Xu, J, Weng, Z, Zhang, X, et al. Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and Cancer mortality: a population-based cohort study. Environ Pollut. (2020) 263:114630. doi: 10.1016/j.envpol.2020.114630
36. Zhu, K, Zhang, Y, Lu, Q, Geng, T, Li, R, Wan, Z, et al. Associations of exposure to Lead and cadmium with risk of all-cause and cardiovascular disease mortality among patients with type 2 diabetes. Environ Sci Pollut Res Int. (2022) 29:76805–15. doi: 10.1007/s11356-022-21273-z
37. Park, SK, Sack, C, Sirén, MJ, and Hu, H. Environmental cadmium and mortality from influenza and pneumonia in U.S. Adults Environ Health Perspect. (2020) 128:127004. doi: 10.1289/ehp7598
38. Min, JY, and Min, KB. Blood cadmium levels and Alzheimer's disease mortality risk in older us adults. Environ Health Glob Access Sci Source. (2016) 15:69. doi: 10.1186/s12940-016-0155-7
39. Benedetti, JL, Dewailly, E, Turcotte, F, and Lefebvre, M. Unusually high blood cadmium associated with cigarette smoking among three subgroups of the general population, Quebec, Canada. Sci Total Environ. (1994) 152:161–7. doi: 10.1016/0048-9697(94)90496-0
40. Mainous, AG 3rd, King, DE, Garr, DR, and Pearson, WS. Race, rural residence, and control of diabetes and hypertension. Ann Fam Med. (2004) 2:563–8. doi: 10.1370/afm.119
41. Nie, X, Wang, N, Chen, Y, Chen, C, Han, B, Zhu, C, et al. Blood cadmium in Chinese adults and its relationships with diabetes and obesity. Environ Sci Pollut Res Int. (2016) 23:18714–23. doi: 10.1007/s11356-016-7078-2
42. Messner, B, Knoflach, M, Seubert, A, Ritsch, A, Pfaller, K, Henderson, B, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol. (2009) 29:1392–8. doi: 10.1161/atvbaha.109.190082
43. Choong, G, Liu, Y, and Templeton, DM. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem Biol Interact. (2014) 211:54–65. doi: 10.1016/j.cbi.2014.01.007
44. Almenara, CC, Broseghini-Filho, GB, Vescovi, MV, Angeli, JK, Faria Tde, O, Stefanon, I, et al. Chronic cadmium treatment promotes oxidative stress and endothelial damage in isolated rat aorta. PLoS One. (2013) 8:e68418. doi: 10.1371/journal.pone.0068418
Keywords: blood cadmium, mortality, cardiovascular risk, hypertension, NHANES
Citation: Chen S, Shen R, Shen J, Lyu L and Wei T (2023) Association of blood cadmium with all-cause and cause-specific mortality in patients with hypertension. Front. Public Health. 11:1106732. doi: 10.3389/fpubh.2023.1106732
Edited by:
Dong-Xing Guan, Zhejiang University, ChinaReviewed by:
Natasa Krsto Rancic, University of Niš, SerbiaNayan Chandra Mohanto, Shahjalal University of Science and Technology, Bangladesh
Copyright © 2023 Chen, Shen, Shen, Lyu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiemin Wei, bHN3dG1Ac2luYS5jb20=; Lingchun Lyu, bHZsaW5nY2h1bkBtZWRtYWlsLmNvbS5jbg==
†These authors have contributed equally to this work and share first authorship
 Shuaijie Chen
Shuaijie Chen Ruming Shen
Ruming Shen Jiayi Shen1
Jiayi Shen1