- 1Department of Precision and Regenerative Medicine and Ionian Area, Clinic of Infectious Diseases, University of Bari, Bari, Italy
- 2Operational Research Unit, Doctors With Africa CUAMM, Padua, Italy
- 3Doctors With Africa CUAMM, Wolisso, Ethiopia
- 4Geriatric Unit, Department of Internal Medicine and Geriatrics, University of Palermo, Palermo, Italy
Introduction: Worldwide, COVID-19 pandemic lead to a large fall in the number of newly reported TB cases. In sub-Saharan Africa, microbiological diagnosis of TB is generally based on smear microscopy and Xpert MTB/RIF on sputum samples, but good quality sputum samples are often difficult to obtain, leading clinicians to rely on more invasive procedures for diagnosis. Aim of this study was to investigate pooled sensitivity and specificity of Xpert MTB/RIF on stool samples compared to respiratory microbiological reference standards in African countries.
Methods: Four investigators independently searched PubMed, SCOPUS, and Web of Science until 12th October 2022, then screened titles and abstracts of all potentially eligible articles. The authors applied the eligibility criteria, considered the full texts. All the studies reported the data regarding true positive (TP), true negative (TN), false positive (FP) and false negative (FN). Risk of bias and applicability concerns were assessed with the Quadas-2 tool.
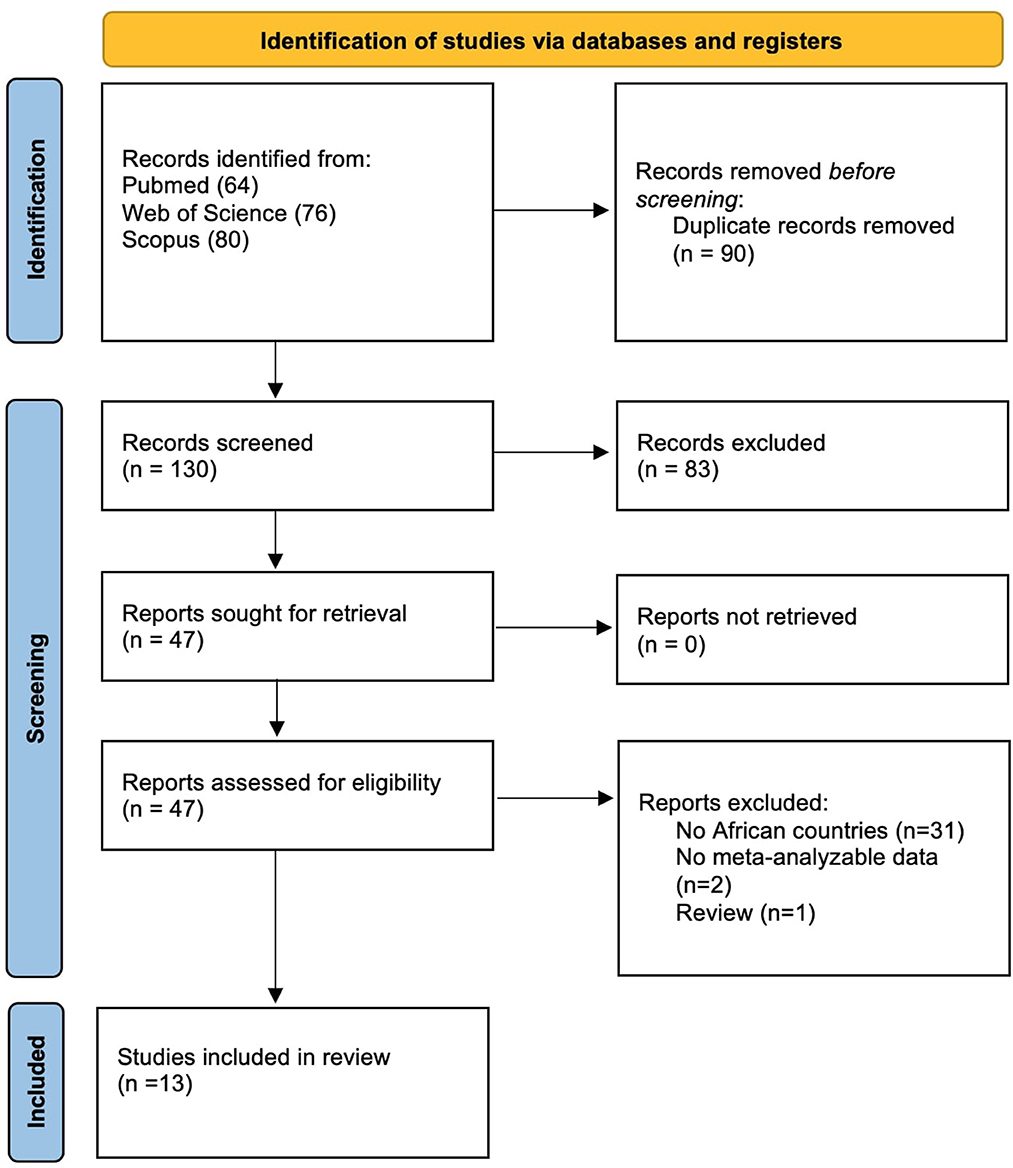
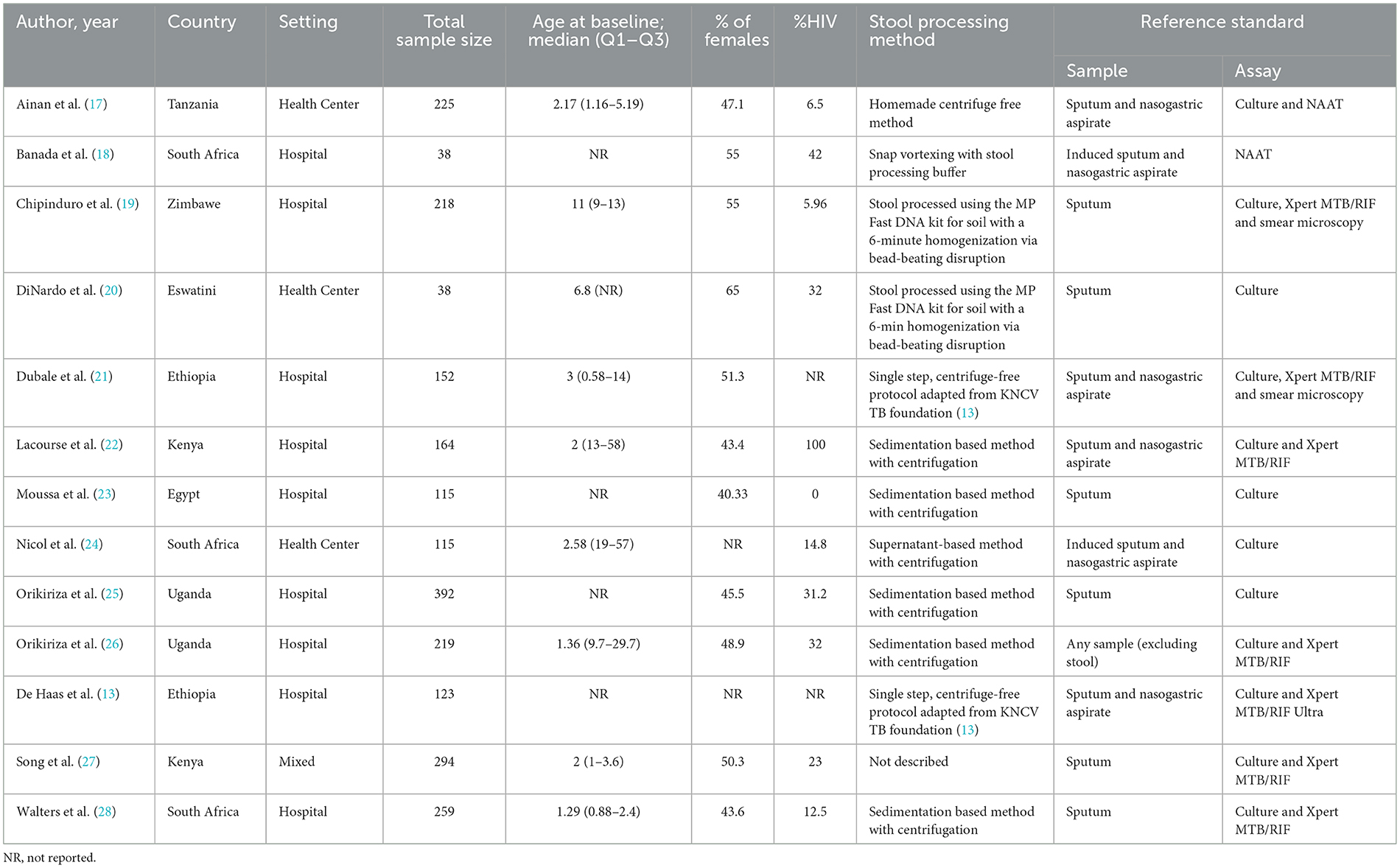
Results: overall, among 130 papers initially screened, we evaluated 47 works, finally including 13 papers for a total of 2,352 participants, mainly children. The mean percentage of females was 49.6%, whilst the mean percentage of patients reporting HIV was 27.7%. Pooled sensitivity for Xpert MTB/RIF assay for detecting pulmonary tuberculosis was 68.2% (95%CI: 61.1–74.7%) even if characterized by a high heterogeneity (I2=53.7%). Specificity was almost 100% (99%, 95%CI: 97–100%; I2 = 45.7%). When divided for reference standard, in the six studies using sputum and nasogastric aspirate the accuracy was optimal (AUC = 0.99, SE = 0.02), whilst in the studies using only sputum for tuberculosis detection the AUC was 0.85 (with a SE = 0.16). The most common source of bias was exclusion of enrolled patients in the analysis.
Conclusions: Our study confirms that, in Africa, stool Xpert MTB/RIF may be a useful rule-in test for children above and below 5 years of age under evaluation for pulmonary tuberculosis. Sensitivity increased substantially when using both sputum and nasogastric aspirate as reference samples.
1. Introduction
Before the advent of COVID-19, tuberculosis was the leading cause of death from a single infectious agent, Mycobacterium tuberculosis (MTB). Despite being a preventable and treatable disease, it infects roughly 25% of the world population and caused at least 1.6 million deaths only in 2021, reversing a long-lasting reduction trend that started in 2000 (1). Along with an increase in TB-related deaths, the immediate consequence of the pandemic was a large fall in the number of newly reported TB cases and an estimated increase of incident cases of rifampicin-resistant TB, all indicators that represent a relevant drawback in the pursue of the 2025 End TB Milestones (2).
Since mortality of untreated TB approaches 50% and cure rates are high (3), overall disease burden is strictly dependent on diagnostic capacity. In sub-Saharan Africa, microbiological diagnosis of TB is generally based on Xpert MTB/RIF (Cepheid, USA), an automated, PCR-based assay able to detect mycobacterial DNA on respiratory samples, A newer, more sensitive version of the test has been approved by WHO in 2021, Xpert MTB/RIF Ultra, with a sensitivity approaching the one reported for culture assays (4).
In sub-Saharan Africa and other high-burden, resource-limited settings, good quality sputum samples are often difficult to obtain, leading clinicians to rely on more invasive procedures for diagnosis—such as nasogastric aspirate (5) and sputum induction, that are painful, not routinely available and require additional resources and costs such as the ones related to hospitalization and the use of suction machine and nebulizers. Besides the challenges in sample collection, MTB detection on respiratory samples in high-burden TB settings is further obstacle by extra-pulmonary tuberculosis (EPTB) (6), smear-negative pulmonary tuberculosis (PTB) and pauci-bacillary TB (7), and sputum sample collection may put healthcare workers at risk of infection due to exposure to MTB infected aerosols (8). Rapid, accurate, sputum-free diagnostics for tuberculosis are of critical need (9).
In recent years, attention has been attracted by Xpert MTB/RIF on stool samples, since mycobacteria-containing sputum may be swallowed and then be available for molecular testing. The use of Xpert MTB/RIF and Xpert MTB/RIF Ultra on stool samples has been introduced in the 2020 WHO guidelines as initial diagnostic test for children with signs and symptoms of pulmonary TB (10). However, this recommendation is based on low certainty of evidence. Also, in 2022, as part of the Global Laboratory Initiative (11) the WHO endorsed two simple, centrifuge-free methods for stool processing: the optimized sucrose flotation (OSF) method developed by the TB-Speed consortium (12), and the simple one-step (SOS) method developed by the KNCV Tuberculosis Foundation (13).
Aim of this study was to investigate pooled sensitivity and specificity of Xpert MTB/RIF on stool samples compared to respiratory microbiological reference standards in African countries.
2. Materials and methods
This systematic review adhered to the MOOSE guidelines (14) and PRISMA statement (15), following a predetermined but unpublished protocol.
2.1. Inclusion and exclusion criteria
Inclusion criteria are as follows: (i) Research highlighting the comparative assessment of the Xpert MTB/RIF or Xpert MTB/RIF Ultra assay to a reference standard, which could be either the microbiological detection of MTB (MRS, with culture, molecular or smear microscopy from either respiratory or nasogastric aspirate samples) or composite reference standard (CRS) including clinical symptoms, biochemical tests reports, radiographic results, histopathological findings, and microbiology (as defined by the authors of the individual studies), (ii) Research providing sufficient information to calculate the diagnostic performance of Xpert MTB/RIF and Xper MTB/RIF Ultra and (iii) studies conducted in African countries.
Exclusion criteria are as follows: (i) Duplicate literature studies, (ii) Research with non-human samples and animal models, (iii) Conference abstracts, lectures, commentaries, letters and case reports, (iv) Research without data (e.g., only sensitivity or specificity data), (v) performed in countries other than Africa, and (vi) publications in languages other than English.
2.2. Data sources and literature search strategy
Four investigators (SC, EdV, VF, MCS) independently searched PubMed, SCOPUS, and Web of Science until 12th October 2022. The search terms used in PubMed included combinations of the following keywords: (feces OR stool) AND (tuberculosis OR Mycobacterium tuberculosis OR TB OR MTB OR EPTB OR PTB) AND (Xpert Gene OR Xpert OR Xpert MTB/RI OR GeneXpert OR GeneXpert MTB/Rif). We considered the reference lists of all included articles and of previous related reviews.
2.3. Study selection
Following the searches as outlined above, after removal of duplicates, four independent reviewers (SC, EdV, VF, MCS) screened titles and abstracts of all potentially eligible articles. The authors applied the eligibility criteria, considered the full texts, and a final list of included articles was reached through consensus with a third senior author (NV).
2.4. Data extraction
Four authors were involved in data extraction in a standardized Microsoft Excel database. For each article, we extracted information about authors, year of publication, number of patients, setting, country, study design, age, percentage of females and of patients with HIV, the use of stool GeneXpert or Xpert Ultra, number of true positive, true negative, false positive and false negative results.
2.5. Outcomes
The primary outcomes were sensitivity, specificity, positive and negative likelihood ratios, and the area under the curve (AUC) of stool Xpert MTB/RIF and stool Xpert MTB/RIF Ultra.
2.6. Assessment of study quality
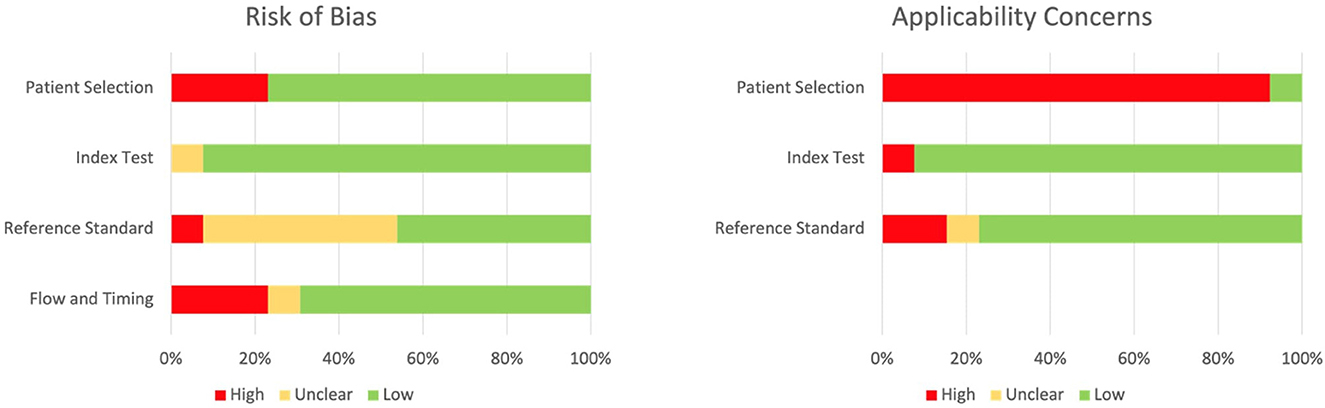
Based on the revised quality assessment of diagnosis, accuracy studies-2 (QUADAS-2) criteria, the included articles were evaluated as at high risk (–) or low risk (+) by four key domains: Patient selection, index test, reference standard, and flow and timing (16).
2.7. Data synthesis and statistical analysis
We used Meta-Disc software 5.1.4 to conduct this meta-analysis. All the studies reported the data regarding true positive (TP), true negative (TN), false positive (FP) and false negative (FN). Therefore, we were able to calculate the pooled sensitivity (TP/TP + FN), specificity (SPE) (TN/TN + FP), negative likelihood ratio (LR–), positive likelihood ratios (LR+) with their 95% confidence intervals. At the same time, we constructed the summary receiver operator characteristic (SROC) curve and calculated the area under the SROC curve based on the sensitivity and specificity of each study. Heterogeneity was estimated using the I2, with a value over 50% or a p < 0.05 as indicative of high heterogeneity. The pooled estimates were also reported by reference tool (divided in sputum vs. the association between sputum and nasogastric aspirate).
3. Results
The flow-chart of this systematic review is shown in Figure 1. Overall, among 130 papers initially screened, we evaluated 47 works, finally including 13 papers.
Table 1 reported the data of the 13 works eligible for a total of 2,352 participants, mainly children. The setting most represented was the hospital (n = 9), followed by health center (n = 3) and mixed settings (n = 1). The mean percentage of females was 49.6%, whilst the mean percentage of patients reporting HIV was 27.7%. When considering the reference standard, the use of sputum, particularly when associated with nasogastric aspirate was the most used methodology.
Considering all the 13 studies together, the pooled sensitivity for stool Xpert MTB/RIF assay for detecting tuberculosis was moderate (68.2%, 95%CI: 61.1–74.7%) even if characterized by a high heterogeneity (I2 = 53.7%) (Figure 2). In fact, the sensitivity of the studies included ranged from 44% to 100%. On the contrary the specificity of stool Xpert assay was almost 100% (99%, 95%CI: 97–100%; I2=45.7%) (Table 2, Figure 2). Almost all the studies reported a specificity higher than 95% in diagnosing tuberculosis, as shown in Figure 2. Therefore, the LR+ was optimal (38.581; 95%CI: 20.994–70.900) as well as the LR- (0.383; 95%CI: 0.295- 0.497) (Table 2). These data led to an AUC = 0.8983 with a standard error (SE) of 0.0763, even if, as shown in Figure 3, only four studies had an AUC over 0.80.

Figure 2. Forest plots of sensitivity and specificity of the Xpert MTB/RIF Ultra assay for tuberculosis detection. Power of the single studies is indicated by dot size, while horizontal lines indicate emerging from the box indicate the magnitude of the confidence interval. Dot size is proportional to the studies' sample size. and that the lozenge and box represents the pooled numbers with 95% CI error margins.
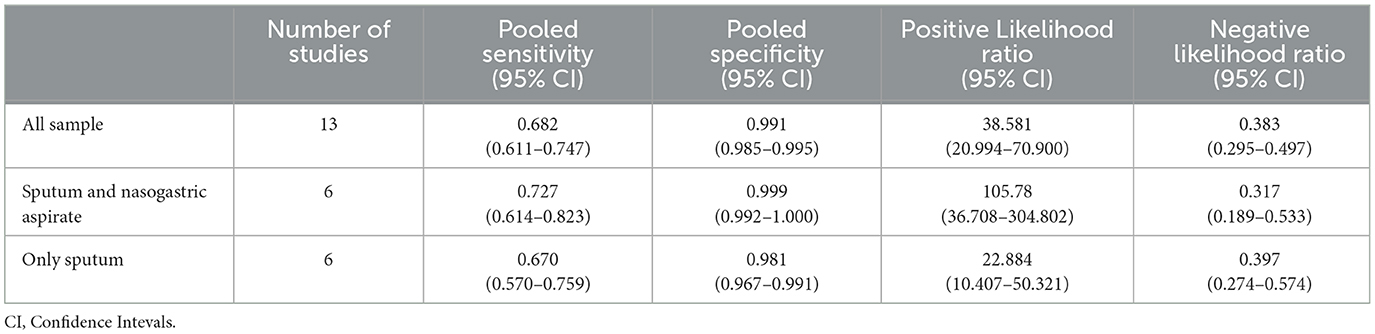
Table 2. Performance of Xpert MTB/RIF Ultra on stool sample from patients with pulmonary tuberculosis compared to reference standard type.
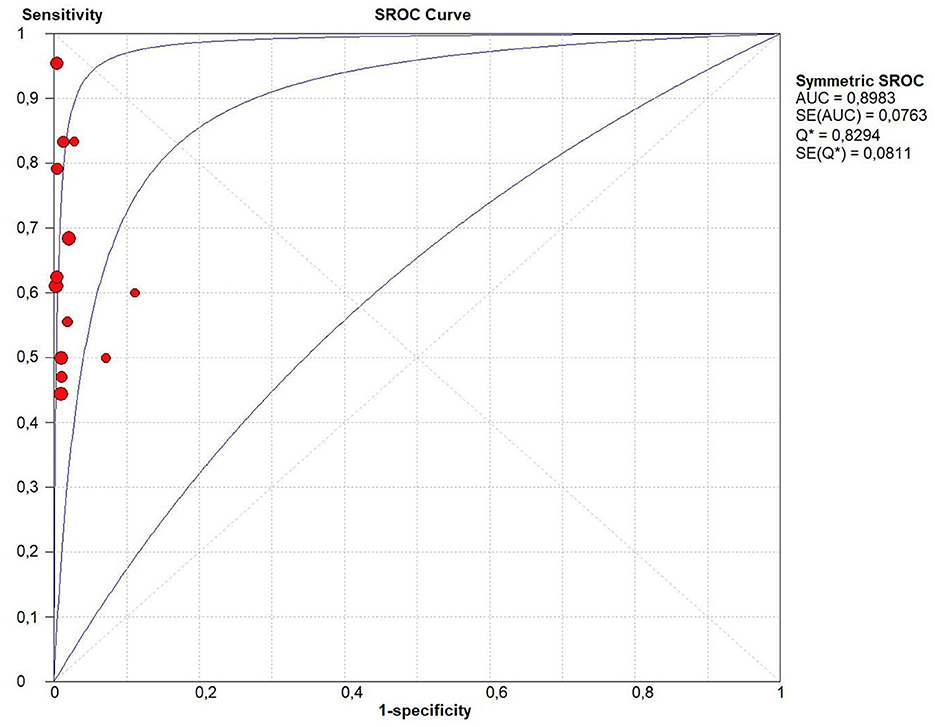
Figure 3. Summary receiver operating characteristic (SROC) curve of the diagnostic accuracy of Xpert MTB/RIF Ultra assay for tuberculosis detection. In this figure, the blue lines represent the AUC (central line) with its 95% CI (external lines) calculated with a meta-analytic approach, while red dots represent the sensitivity and specificity data for each study.
Stratifying the analysis for the reference standard, the six studies using both sputum and nasogastric aspirate showed higher sensitivity, a similar specificity, and a higher LR+ than studies using only sputum for tuberculosis detection, as shown in Table 1. In the six studies using sputum and nasogastric aspirate the accuracy was optimal (AUC = 0.99, SE = 0.02), whilst in the studies using only sputum for tuberculosis detection the AUC was 0.85 (with a SE = 0.16). In our systematic review, despite the initial protocol included both composite and microbiological reference standards, we found only one study evaluating diagnostic accuracy of stool Xpert with both CRS and MRS (26). In this case, interestingly, sensitivity dropped from 50% against MRS to 11.4% against CRS. However, for this study, we included in the meta-analysis only diagnostic accuracy data obtained against MRS.
The quality of the included studies, as assessed by the QUADAS-2, is reported on Figure 4; the most common source of bias was exclusion of enrolled patients in the analysis (Figure 4). On the other side, the most common concern in terms of applicability was due to the fact that our systematic review aimed to explore the diagnostic accuracy of stool Xpert in the general population, while most of the included studies recruited only pediatric patients. A detailed description of risk of bias and applicability concerns is provided in Supplementary Table 1.
4. Discussion
In this systematic review and meta-analysis, we investigated diagnostic accuracy of stool Xpert MTB/RIF in African settings. In our study, pooled sensitivity and specificity were, respectively, 68% (95%CI 61–75%) and 99% (95%CI 98–99%) for the diagnosis of people with presumptive pulmonary TB. Our results are consistent with the ones reported by other meta-analysis conducted on children living in both African and non-African settings (29–31). Moreover, also consistently with other studies, diagnostic accuracy reported here on stool samples is comparable to the performances of the same test on respiratory samples (32). Of note, this is the first meta-analysis including only patients living in African countries.
In low-resource settings, patients evaluated for TB often experience diagnostic delays due to several factors, such as economic constrains, lack of awareness on the importance of timely diagnosis and poor availability of diagnostic tools in primary healthcare facilities (9). This scenario was further challenged by the COVID-19 pandemic, that reversed the progresses made during the last decades and lead, worldwide, to a large drop in the reported number of newly diagnosed TB (1). This is relevant, since every loss in TB diagnostic capacity inevitably leads to an increase in the number of untreated TB and TB deaths. Despite their requirements in costs and infrastructure—which limits the availability to the settings with adequate transportation systems and funding—molecular tests such as Xpert MTB/RIF and Xpert MTB/RIF Ultra on stool sample may provide added value in TB diagnostic workflow in high burden settings. Also, another limit of rapid molecular tests is that they are sputum dependent, since population at high risk of developing TB [such as people living with HIV (33) and children (34)] is often unable to expectorate. Among children, sputum unavailability is generally replaced by nasogastric aspirate, which is invasive and poorly tolerated. In our systematic review, 7 studies out of 13 reported a median age below 5 years, accounting for 66.7% (n = 1,571) of the pooled population, that is the age category in which stool Xpert is expected to have the greatest clinical utility. The importance of implementing rapid, accurate, non-invasive, sputum-free assays for detection of MTB has been recognized by the WHO as high priority target for the development of new tuberculosis diagnostics in 2014 (35).
Consistently with other meta-analysis (29–31), we recorded a substantial between-study heterogeneity, especially in sensitivity, which ranged from 44% to 100%. This was likely a consequence of the differences in reported HIV-prevalence and in terms of used reference test. Furthermore, heterogeneity might also have been affected by the stool processing protocols used, since the majority of included articles reported non-standardized sample processing methods. In this study we found that, despite WHO endorsement of SOS and OSF methods, implementation of stool Xpert processing strategies in sub-Saharan Africa is still lacking standardization. This is relevant, since many in-house, not-standardized methods require laboratory expertise dedicated equipment which, in some settings, may discourage implementation of PCR-based diagnostics on stool samples. For future, perspective research, we emphasize the importance to adopt and report a standardized protocol for sample preparation.
A strength of this study is that diagnostic accuracy was evaluated using, in all articles, a microbiological (and non-clinical) reference standard, represented by both culture and Xpert (8/13), culture (12/13), or Xpert alone (1/13). In fact, when the reference standard used to evaluate diagnostic accuracy of stool Xpert was both sputum and nasogastric aspirate, pooled sensitivity increased to 72% and AUC was as high as 0.99.
This study has some limitations. First, data did not allow us to perform meta-regression analyses to investigate the reasons of recorded heterogeneity. Second, we could not evaluate the accuracy of stool Xpert on adults or other age groups, since we found no studies addressing this population in African countries. Third, we found only one study investigating diagnostic performances of stool Xpert Ultra (13), which contributed for 5% of the total population and reported a sensitivity of 81%. For the purposes of this study, Xpert Ultra has been included in the analysis but we recognize that it may have contributed to increase heterogeneity. Hence, future research should focus on investigating diagnostic accuracy and cost-effectiveness of Xpert Ultra on stool samples in sub-Saharan settings. Also, in the upcoming years, research should address the sensitivity advantage of this test on adults and when used in combination with other currently used assays.
5. Conclusions
Our study confirms that, in Africa, stool Xpert MTB/RIF may be a useful rule-in test for patients under evaluation for pulmonary tuberculosis. Sensitivity increased substantially when using both sputum and nasogastric aspirate as reference samples. Further studies are needed to explore stool Xpert MTB/RIF Ultra and both Xpert MTB/RIF and Xpert Ultra diagnostic performance in the adult population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FD, CM, and NV contributed to conception and design of the study. RP, SC, ED, MS, VF, OT, EF, RL, FM, DB, and GD collected the data. FV organized the database and wrote the first draft of the manuscript. NV performed the statistical analysis. GP, NV, FD, and MB wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1117709/full#supplementary-material
References
1. World Health Organization. Global tuberculosis report 2022. Geneva: World Health Organization (2022). https://apps.who.int/iris/handle/10665/363752 (accessed November 23, 2022).
2. The End TB Strategy. https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy (accessed November 25, 2022).
3. Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJD. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS ONE. (2011) 6:e17601. doi: 10.1371/journal.pone.0017601
4. Use of Xpert MTB/RIF and Xpert MTB/RIF Ultra on GeneXpert 10-colour instruments – WHO Policy Statement. Available online at: https://www.who.int/news/item/03-12-2021-use-of-xpert-mtb-rif-and-xpert-mtb-rif-ultra-on-genexpert-10-colour-instruments-who-policy-statement (accessed December 9, 2022).
5. Bates M, O'Grady J, Maeurer M, Tembo J, Chilukutu L, Chabala C, et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infect Dis. (2013) 13:36–42. doi: 10.1016/S1473-3099(12)70245-1
6. Sharma V, Singh A, Gaur M, Rawat D, Yadav A, Rajan Kumar C, et al. Evaluating the efficacy of stool sample on Xpert MTB/RIF Ultra and its comparison with other sample types by meta-analysis for TB diagnostics. Eur J Clin Microbiol Infect Dis. (2022) 41:893–906. doi: 10.1007/s10096-022-04449-w
7. Gaur M, Singh A, Sharma V, Tandon G, Bothra A, Vasudeva A, et al. Diagnostic performance of non-invasive, stool-based molecular assays in patients with paucibacillary tuberculosis. Sci Rep. (2020) 10:7102. doi: 10.1038/s41598-020-63901-z
8. Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis. (2007) 11:593–605.
9. Nathavitharana RR, Garcia-Basteiro AL, Ruhwald M, Cobelens F, Theron G. Reimagining the status quo: how close are we to rapid sputum-free tuberculosis diagnostics for all? eBioMedicine. (2022) 78:103939. doi: 10.1016/j.ebiom.2022.103939
10. World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis: Rapid Diagnostics for Tuberculosis Detection. 2021 update. Geneva: World Health Organization (2021). Available online at: https://apps.who.int/iris/handle/10665/342331 (accessed November 24, 2022).
11. World Health Organization. Practical Manual of Processing Stool Samples for Diagnosis of Childhood TB. (2022) Available online at: https://stoptb.org/wg/gli/assets/documents/3075%20GTB%20GLI%20Stool%20processing%20manual%20ELECTRONIC%20290422.pdf (accessed April 19, 2023).
12. Lounnas M, Diack A, Nicol MP, Eyangoh S, Wobudeya E, Marcy O, et al. Laboratory development of a simple stool sample processing method diagnosis of pediatric tuberculosis using Xpert Ultra. Tuberculosis. (2020) 125:102002. doi: 10.1016/j.tube.2020.102002
13. de Haas P, Yenew B, Mengesha E, Slyzkyi A, Gashu Z, Lounnas M, et al. The simple one-step (SOS) stool processing method for use with the Xpert MTB/RIF assay for a child-friendly diagnosis of tuberculosis closer to the point of care. J Clin Microbiol. (2021) 59:e0040621. doi: 10.1128/JCM.00406-21
14. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group | EQUATOR Network. Available online at: https://www.equator-network.org/reporting-guidelines/meta-analysis-of-observational-studies-in-epidemiology-a-proposal-for-reporting-meta-analysis-of-observational-studies-in-epidemiology-moose-group/ (accessed December 6, 2022).
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
17. Ainan S, Furia FF, Mhimbira F, Mnyambwa NP, Mgina N, Zumla A, et al. Xpert® MTB/RIF assay testing on stool for the diagnosis of paediatric pulmonary TB in Tanzania. Public Health Action. (2021) 11:75–9. doi: 10.5588/pha.20.0062
18. Banada PP, Naidoo U, Deshpande S, Karim F, Flynn JL, O'Malley M, et al. Novel sample processing method for rapid detection of tuberculosis in the stool of pediatric patients using the Xpert MTB/RIF assay. PLoS ONE. (2016) 11:e0151980. doi: 10.1371/journal.pone.0151980
19. Chipinduro M, Mateveke K, Makamure B, Ferrand RA, Gomo E. Stool Xpert® MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis at primary clinics in Zimbabwe. Int J Tuberc Lung Dis. (2017) 21:161–6. doi: 10.5588/ijtld.16.0357
20. DiNardo AR, Kay AW, Maphalala G, Harris NM, Fung C, Mtetwa G, et al. Diagnostic and treatment monitoring potential of a stool-based quantitative polymerase chain reaction assay for pulmonary tuberculosis. Am J Trop Med Hyg. (2018) 99:310–6. doi: 10.4269/ajtmh.18-0004
21. Dubale M, Tadesse M, Berhane M, Mekonnen M, Abebe G. Stool-based Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children at a teaching and referral hospital in Southwest Ethiopia. PLoS ONE. (2022) 17:e0267661. doi: 10.1371/journal.pone.0267661
22. LaCourse SM, Pavlinac PB, Cranmer LM, Njuguna IN, Mugo C, Gatimu J, et al. Stool Xpert MTB/RIF and urine lipoarabinomannan for the diagnosis of tuberculosis in hospitalized HIV-infected children. AIDS. (2018) 32:69–78. doi: 10.1097/QAD.0000000000001662
23. Moussa HS, Bayoumi FS, Mohamed AMA. Gene Xpert for direct detection of mycobacterium tuberculosis in stool specimens from children with presumptive pulmonary tuberculosis. Ann Clin Lab Sci. (2016) 46:198–203.
24. Nicol MP, Spiers K, Workman L, Isaacs W, Munro J, Black F, et al. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis. (2013) 57:e18–21. doi: 10.1093/cid/cit230
25. Orikiriza P, Nansumba M, Nyehangane D, Bastard M, Mugisha IT, Nansera D, et al. Xpert MTB/RIF diagnosis of childhood tuberculosis from sputum and stool samples in a high TB-HIV-prevalent setting. Eur J Clin Microbiol Infect Dis. (2018) 37:1465–73. doi: 10.1007/s10096-018-3272-0
26. Orikiriza P, Smith J, Ssekyanzi B, Nyehangane D, Mugisha Taremwa I, Turyashemererwa E, et al. Tuberculosis diagnostic accuracy of stool Xpert MTB/RIF and urine AlereLAM in vulnerable children. Eur Respir J. (2022) 59:2101116. doi: 10.1183/13993003.01116-2021
27. Song R, Click ES, McCarthy KD, Heilig CM, Mchembere W, Smith JP, et al. Sensitive and feasible specimen collection and testing strategies for diagnosing tuberculosis in young children. JAMA Pediatr. (2021) 175:e206069. doi: 10.1001/jamapediatrics.2020.6069
28. Walters E, Scott L, Nabeta P, Demers A-M, Reubenson G, Bosch C, et al. Molecular detection of mycobacterium tuberculosis from stools in young children by use of a novel centrifugation-free processing method. J Clin Microbiol. (2018) 56:e00781–18. doi: 10.1128/JCM.00781-18
29. MacLean E, Sulis G, Denkinger CM, Johnston JC, Pai M, Ahmad Khan F. Diagnostic accuracy of stool Xpert MTB/RIF for detection of pulmonary tuberculosis in children: a systematic review and meta-analysis. J Clin Microbiol. (2019) 57:e02057–18. doi: 10.1128/JCM.02057-18
30. Mesman AW, Rodriguez C, Ager E, Coit J, Trevisi L, Franke MF. Diagnostic accuracy of molecular detection of Mycobacterium tuberculosis in pediatric stool samples: a systematic review and meta-analysis. Tuberculosis (Edinb). (2019) 119:101878. doi: 10.1016/j.tube.2019.101878
31. Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Detjen AK, Steingart KR, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst Rev. (2020) 8:CD013359. doi: 10.1002/14651858.CD013359.pub2
32. Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. (2014) 2014:CD009593. doi: 10.1002/14651858.CD009593.pub3
33. Peter JG, Theron G, Singh N, Singh A, Dheda K. Sputum induction to aid diagnosis of smear-negative or sputum-scarce tuberculosis in adults in HIV-endemic settings. European Respiratory Journal. (2014) 43:185–94. doi: 10.1183/09031936.00198012
34. Drain PK, Gardiner J, Hannah H, Broger T, Dheda K, Fielding K, et al. Guidance for studies evaluating the accuracy of biomarker-based nonsputum tests to diagnose tuberculosis. J Infect Dis. (2019) 220:S108–15. doi: 10.1093/infdis/jiz356
35. High priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. Available online at: https://www.who.int/publications-detail-redirect/WHO-HTM-TB-2014.18 (accessed March 10, 2023).
Keywords: pulmonary tuberculosis, stool Xpert MTB/RIF, meta-analysis, systematic review, diagnostic microbiology
Citation: Segala FV, Papagni R, Cotugno S, De Vita E, Susini MC, Filippi V, Tulone O, Facci E, Lattanzio R, Marotta C, Manenti F, Bavaro DF, De Iaco G, Putoto G, Veronese N, Barbagallo M, Saracino A and Di Gennaro F (2023) Stool Xpert MTB/RIF as a possible diagnostic alternative to sputum in Africa: a systematic review and meta-analysis. Front. Public Health 11:1117709. doi: 10.3389/fpubh.2023.1117709
Received: 06 December 2022; Accepted: 08 May 2023;
Published: 24 May 2023.
Edited by:
Niaina Rakotosamimanana, Institut Pasteur de Madagascar, MadagascarReviewed by:
Jonathan Hoffmann, Fondation Mérieux, FranceMaryline Bonnet, Institut de Recherche pour le Développement, France
Copyright © 2023 Segala, Papagni, Cotugno, De Vita, Susini, Filippi, Tulone, Facci, Lattanzio, Marotta, Manenti, Bavaro, De Iaco, Putoto, Veronese, Barbagallo, Saracino and Di Gennaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Vladimiro Segala, ZnZzZWdhbGFAZ21haWwuY29t
 Francesco Vladimiro Segala
Francesco Vladimiro Segala Roberta Papagni
Roberta Papagni Sergio Cotugno
Sergio Cotugno Elda De Vita1
Elda De Vita1 Valeria Filippi
Valeria Filippi Claudia Marotta
Claudia Marotta Fabio Manenti
Fabio Manenti Davide Fiore Bavaro
Davide Fiore Bavaro Giovanni Putoto
Giovanni Putoto Nicola Veronese
Nicola Veronese Mario Barbagallo
Mario Barbagallo