- 1National Institute of Hospital Administration, National Health Commission, Beijing, China
- 2Southern Institute of Pharmacoeconomics and Health Technology Assessment, Jinan University, Guangzhou, China
- 3School of Political Science and Public Administration, Wuhan University, Wuhan, China
- 4Health Research Institute, Fujian Medical University, Fujian, China
Objective: To evaluate the efficacy, immunogenicity and safety of HPV vaccination in Chinese population.
Methods: PubMed, Embase, Web of Science and Cochrane Library from inception to November 2022 were searched to collect information on clinical trials of HPV vaccines. Database search strategy used a combination of subject terms and free terms. Studies were first identified by two authors through reading the title, abstract and full texts and, subsequently, based on the inclusion criteria: Chinese population, with at least one of the following outcomes (efficacy, immunogenicity, and safety), and HPV vaccine RCT, those eligible were included in this paper. Efficacy, immunogenicity and safety data, pooled by random effects models, are presented as risk ratios [95% confidence intervals (CI)].
Results: Eleven RCTs and four follow-up studies were included. Meta-analysis showed that HPV vaccine had good profile of efficacy and immunogenicity. The seroconversion rates were significantly higher among the vaccinated, uninfected (initial negative serum antibody) population than the placebo population for both HPV-16 (RR 29.10; 95% CI: 8.40–100.82) and HPV-18 (RR 24.15; 95% CI: 3.82–152.84), respectively. A significant reduction of the incidence of cervical intraepithelial neoplasia grade 1 (CIN1+) (RR 0.05; 95% CI: 0.01–0.23) and CIN2+ (RR 0.09; 95% CI: 0.02–0.40) was also measured. Risk for serious adverse events after HPV vaccination indicated comparable outcomes between vaccination and placebo.
Conclusions: For Chinese populations, HPV vaccines enhance the level of HPV16- and HPV18-specific antibodies and reduce the incidence of CIN1+ and CIN2+ in uninfected population. Also, the risk of serious adverse events in both groups are almost equivalent. More data are needed to establish vaccine efficacy with cervical cancer.
1. Introduction
Cervical cancer is a preventable disease, which can be curable if detected early and treated adequately (1). Yet it is the fourth most common cancer among women and the fourth leading cause of cancer death. In 2020, there were 604,000 new cases of cervical cancer and 342,000 deaths worldwide, an increase in both new cases and deaths compared with that of 2018. Whereas in China, such number of new cases and deaths were 109,740 and 59,060 respectively. China is among the countries with the greatest disease burden of cervical cancer, which calls for prompt and effective measures to eliminate cervical cancer (2, 3).
As is well known that 99.7% of cervical cancers are caused by HPV (4), while HPV 16 and 18 are known to cause at least 70% of cervical cancers (5). Moreover, certain HPV type infection can also lead to anal cancer, vulvar cancer, penile cancer, oral cancer and head and neck cancer (6). A meta-analysis (7) estimated, amongst women with normal cytological findings, the global prevalence of infection with any HPV genotype to be 11.7%; China has a higher prevalence (15.6%) (8). Prevalence of HPV infection were generally associated with HIV infection and men who have sex with men, the former with a higher prevalence.
Evidence from clinical trials (9–12) supports HPV vaccination and clearly demonstrates that different types of HPV vaccines induce high levels of antibodies, prevent HPV vaccine type-related infection and reduce the number of people developing cervical intraepithelial neoplasia grade 1 or higher (CIN1+) and persistent infection (PI). Furthermore, most clinical trials concluded that HPV vaccine is generally safe and well tolerated, with no significant difference in adverse events (AEs) and serious adverse events (SAEs) between the vaccinated and control groups. In addition, vaccination with the 2-valent HPV vaccine and the 4-valent HPV vaccine can provide cross-protective efficacy against some 9-vaccine type HPVs (HPV types 31, 33, and 45) (13).
The 2020 WHO's Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem (1) provides a roadmap, through three key targets: vaccination, screening and treatment. This global call-to-action is aiming to reach one goal: to accelerate the elimination of cervical cancer worldwide. To achieving this vision by 2030, China and other 193 countries support this strategy and make efforts to get 90% of girls fully vaccinated with the HPV vaccine by age 15.
Previous studies with population-level evidence of HPV vaccine were generally from developed countries, and few has focused on the Chinese context. Vaccine efficacy, immunogenicity and safety may be affected by the demographical factors, such as race, individual behavior and lifestyle (14). Therefore, cautions should be taken when interpreting these studies from developed countries. In such context, a targeted review on HPV vaccine efficacy, immunogenicity and safety data in the setting of China will be of importance for future introduction of HPV vaccine into national immunization programs.
In this study, we performed a review on clinical trials and follow-up studies on HPV vaccination in China and then conduct a meta-analysis of those studies.
2. Methods
2.1. Inclusion/exclusion criteria
The inclusion criteria used in this paper focused on the following considerations: (1) Population: Chinese women and/or men of aged 9–45 years were included; (2) Intervention: 2-valent, 4-valent and 9-valent HPV vaccination were selected; (3) Control: vaccination with placebo, or 2-valent, 4-valent and 9-valent HPV vaccination were selected; (4) Outcome: efficacy indicators such as incidence of CIN1+ or CIN2+, immunogenicity indicators such as antibody seroconversion rate were measured and safety indicators such as incidence of local AEs; (5) Study type: randomized clinical trials (RCTs) and follow-up study for RCTs were assessed.
The exclusion criteria adopted were non-English studies; incomplete articles such as conference abstracts; multiple publications of the same randomized clinical trial (only the latest one included); phase I clinical trials and duplicate studies.
2.2. Search strategy
We searched PubMed, Embase, Web of Science and Cochrane Library databases for RCTs of HPV vaccines from the time of database establishment to November 2022. The search was conducted using a combination of subject terms and free terms, the detailed PubMed search strategy is (Vaccines, Papillomavirus [Title/Abstract] OR Papillomavirus Vaccine [Title/Abstract]OR Vaccine, Papillomavirus [Title/Abstract] OR Human Papillomavirus Vaccines [Title/Abstract] OR Papillomavirus Vaccines, Human [Title/Abstract] OR Vaccines, Human Papillomavirus [Title/Abstract] OR HPV Vaccine [Title/Abstract] OR Vaccine, HPV [Title/Abstract] OR Human Papilloma Virus Vaccine [Title/Abstract] OR HPV Vaccines [Title/Abstract] OR Human Papilloma Virus Vaccines [Title/Abstract] OR Human Papillomavirus Vaccine [Title/Abstract] OR Papillomavirus Vaccine, Human [Title/Abstract] OR Vaccine, Human Papillomavirus [Title/Abstract] OR Papillomavirus Vaccines [Mesh]). AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab] NOT (animals [mh] NOT humans [mh]) AND (China OR Chinese). Details of additional database search strategy see Supplementary Figure 1. In addition, ClinicalTrials.gov were searched to supplement the trial data and a manual search of references for the included articles was performed.
2.3. Data extraction and outcome assessments
All RCTs conducted in China, reporting at least one of the following indicators: efficacy, immunogenicity and safety were included in this paper. Data were extracted independently by two reviewers using a predefined data extraction form and then cross-checked by these two reviewers as well. Disagreements, if any, were resolved through discussion and consultation with a third reviewer. Based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (15), the following data were extracted: authors, year published, study types, protocol number, study outcome, participants, vaccine studied, intervention, comparator, outcomes of efficacy, outcomes of immunogenicity, outcomes of safety and funding source.
To perform the meta-analysis, efficacy (CIN1+, CIN2+, 6-month PI and incident infection), immunogenicity (seroconversion rate and Geometric Mean Titer) and safety (local, systemic and serious AEs) data from included articles were extracted. The efficacy indicators were measured by HPV DNA testing throughout the study; the immunogenicity indicators were measured 1 month after the last vaccination by enzyme-linked immunosorbent assay (2-valent) and competitive Luminex immunoassay (4-valent and 9-valent); for the safety indicators, local AEs were reported 3–7 days after each vaccination; systemic AEs were reported 30 days after each vaccination; and serious AEs were reported throughout the study.
For efficacy, CIN is the term for precancerous lesion affecting the cells on the surface of the cervix. CIN1+ is defined as CIN grades 1, 2, and3, low-grade cervical glandular intraepithelial neoplasia (LCGIN), high grade cervical glandular intraepithelial neoplasia (HCGIN), adenocarcinoma in-situ (AIS) or invasive cervical cancer. CIN2+ is defined as CIN grades 2 and 3, LCGIN, HCGIN, AIS or invasive cervical cancer. 6-month PI is defined as having a persistent cervical infection for a specified HPV type if there was a sequence of positive HPV DNA samples for that HPV type, not interrupted by negative samples, such that the total range was more than 5 months (>150 days) apart. Incident infection is defined as at least one positive specified HPV type DNA PCR assay at the time point considered.
For immunogenicity, seroconversion is defined as HPV specific antibody concentration above the cut-off point, and the seroconversion rate is the proportion of participants being seropositive. Immune response to a vaccine is often measured by Geometric Mean Titer (GMT), rise in titer indicating a better vaccine immunogenicity.
For safety, local AEs have three indicators: pain, redness and swelling; systemic AEs under evaluation were fatigue, fever, headache and myalgia. SAEs assessed include medical occurrences that result in death, are life-threatening, require hospitalization or prolongation of hospitalization, result in disability/incapacity or are a congenital anomaly/birth defect in the offspring of a study subject.
2.4. Risk of bias assessment and data analysis
The Cochrane Collaboration (16) used in this paper offers a specific tool for assessing risk of bias in each included RCT and the assessing criteria is defined as “low risk,” as “high risk,” or as “unclear risk.”
We focused on efficacy, immunogenicity, and safety data from the rest of the included studies and conducted a meta-analysis of such data. Meta-analysis was performed using Review Manager Software (RevMan version 5.3) for Windows. Relative risks (RR) were calculated in the vaccinated groups and control groups using a random effects model with 95% confidence intervals (CI). Subsequently, heterogeneity between studies was addressed using Cochrane Q test results and quantifying the I2 score, where I2 value ranges from 0% to 100%, and if it is greater than or equal to 50%, a high degree of heterogeneity is considered. Lastly, Results with high heterogeneity were analyzed in order to find factors that might influence the study results.
3. Results
3.1. Article selection process
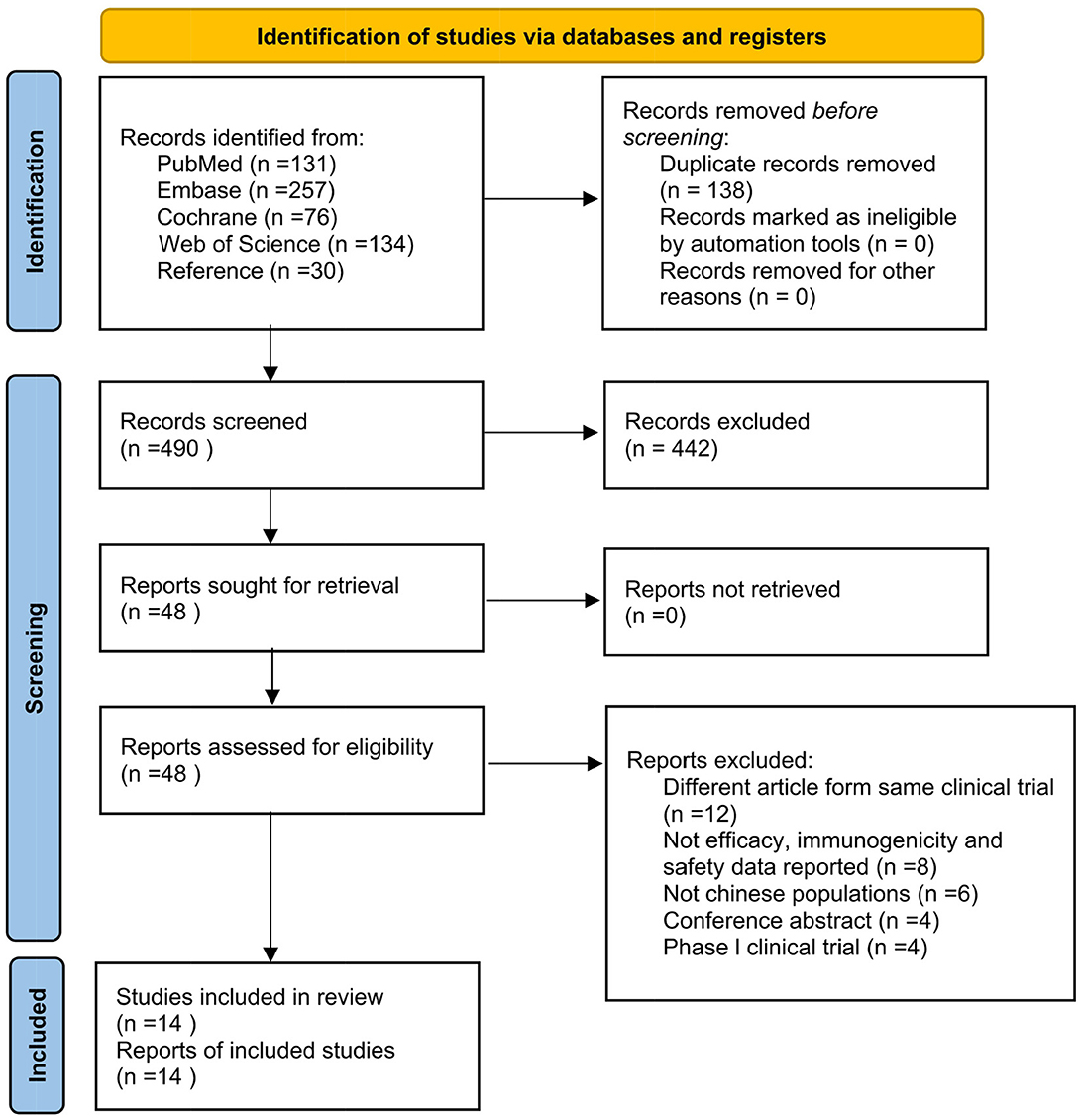
A total of 628 articles were obtained after the initial search, where two researchers independently reviewed the articles, browsed the titles and abstracts, and then read the full text. If any disagreement appeared, a third reviewer was consulted. Eventually, 14 studies were included (17–30), containing 11 RCTs (17–26) and four follow-up studies (27–30). The specific article screening process is shown in Figure 1.
3.2. Characteristics of the included studies
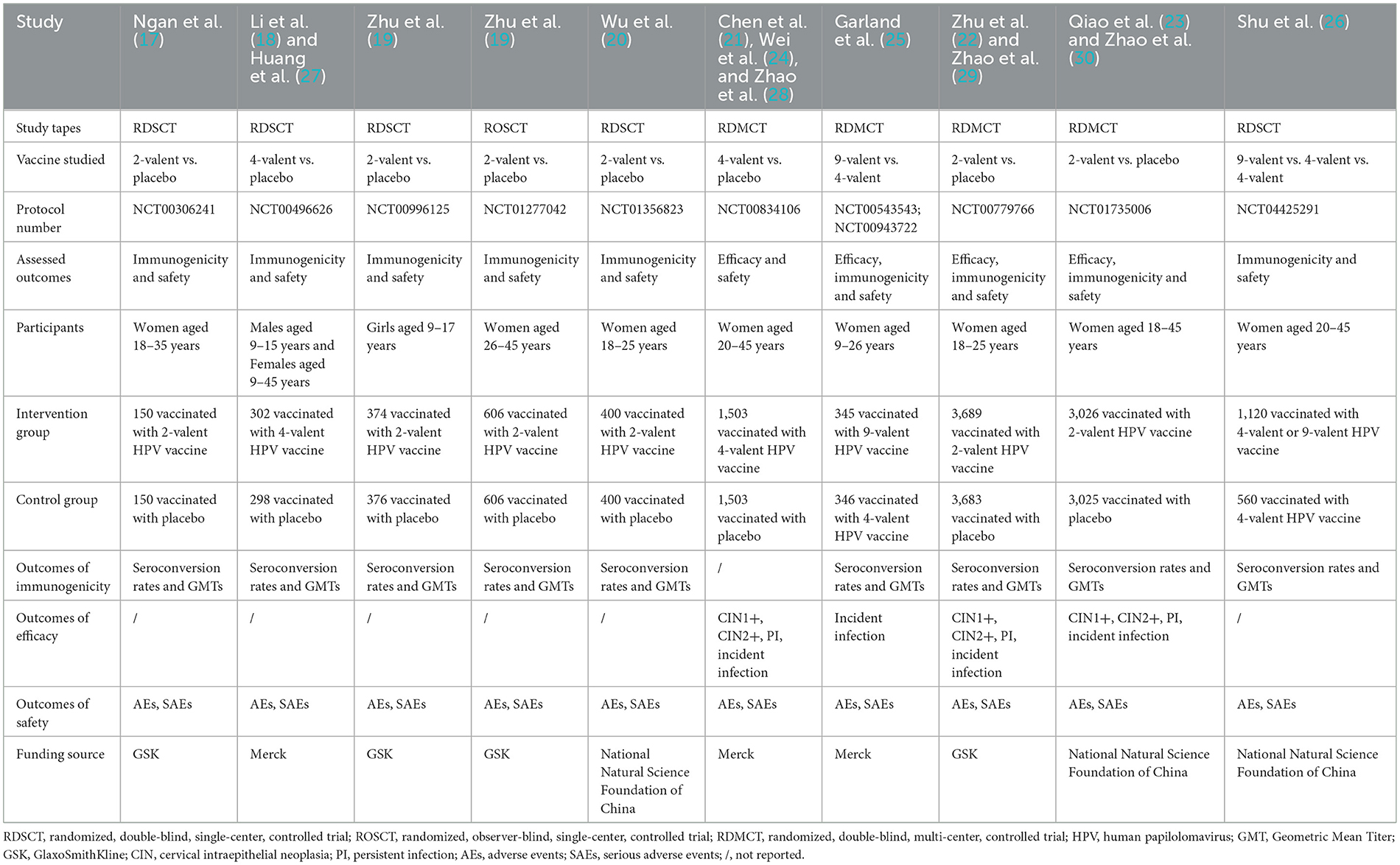
The basic characteristics of the included studies were shown in Table 1. All included RCTs were randomized double-blind controlled trials except one (19) randomized observer-blinded controlled trial; more than half (n = 6) (17–20, 26) were single-center trials, the rest being multi-center trials; All 11 (17–26) RCTs were with registration numbers, where ten (17, 19–26) out of the 11 RCTs were with women subjects only, age ranging from 9 to 45 years.
With regards to the vaccine types, subjects were vaccinated with 2-valent HPV vaccine (GSK) (14, 16, 19), domestic 2-valent HPV vaccine (the National Natural Science Foundation of China) (17, 20), domestic 4-valent HPV vaccine and domestic 9-valent HPV vaccine (the National Natural Science Foundation of China) (23), 4-valent HPV vaccine (Merck) (15, 18), and 9-valent HPV vaccine (Merck) (22). All the control groups were on a placebo except 9-valent HPV vaccine trials. For vaccination procedures, RCTs with 2-valent HPV vaccine were to vaccinate at Months 0, 1 and 6, while RCTs with 4-valent HPV vaccine and 9-valent HPV vaccine were at Months 0, 2 and 6.
Seroconversion occurs when the antibody concentration value is above the cut-off point. For 2-valent HPV vaccine (GSK), the value was 8 EU/ml for HPV-16 and 7 EU/ml for HPV-18; for domestic 2-valent HPV vaccine, one trial (20, 23) adopted an increase in antibody titers of at least fourfold and the other (23) used 3.1 IU/ml for HPV-16 antibodies and 2.0 IU/ml for HPV-18; for 4-valent HPV vaccine and 9-valent HPV vaccine, the value was 20 mMU/ml (milli-Merck unit/ml) and 24 mMU/ml as cut-off points for antibodies against HPV16 and HPV18.
For clinical outcomes assessed, five RCTs (21–23, 25) reported HPV vaccine efficacy; ten RCTs (17–20, 22, 23, 25, 26) reported immunogenicity of HPV vaccine; all 11 RCTs (17–26) reported the safety of the HPV vaccine. The efficacy, immunogenicity and safety data were collected from the total vaccinated set or per-protocol set.
Four follow-up studies (27–30) reported long-term immunogenicity and safety with follow-ups up to 3.5 years, 66 mouths, 8 years and 10 years. As follow-up study has a variable follow-up timeline and the control group of 9-valent HPV vaccine RCTs was not on a placebo, therefore a descriptive analysis on 9-valent HPV vaccine RCTs and follow-up study was conducted.
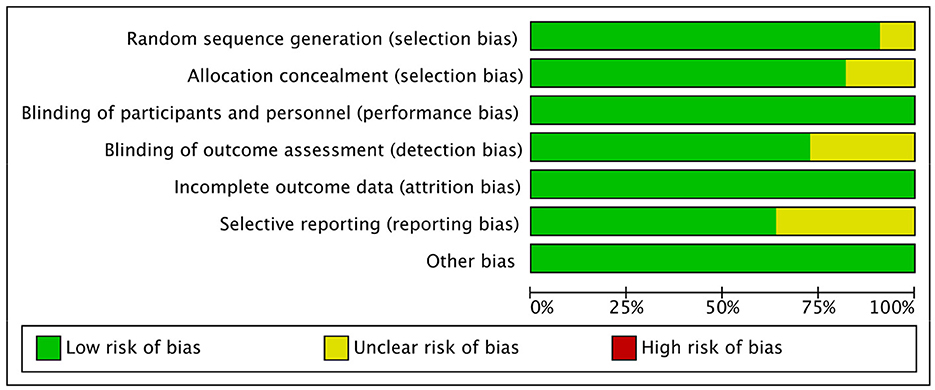
3.3. The risk of bias assessment
In terms of selection bias, all trials (n = 11) were randomized trials, with only ten trials (17, 19–26) describing the method of sequence generation, and nine (17, 19, 20, 22–26) detailing the unpredictability of random allocation of subjects. In order to maintain low risk of bias, all trials were blinded and unlikely to break; for bias in measurement of the outcomes, eight trials (19, 21–26) had explicit blindness for outcomes measurement and were unlikely to break. All 11 trials had complete results and no missing outcome data, with four (19, 22, 23) reporting bias, due to the lack of data for the control groups (Figure 2).
3.4. Immunogenicity of HPV vaccine
Based on the published clinical data, the HPV-16/18 antibody seroconversion rates at Month 7 after three doses of HPV vaccine in the initial antibody serum negative (uninfected) population were calculated. And pooled RR of seroconversion rate between vaccinated groups and control groups was analyzed with a random-effects model (Figure 3).
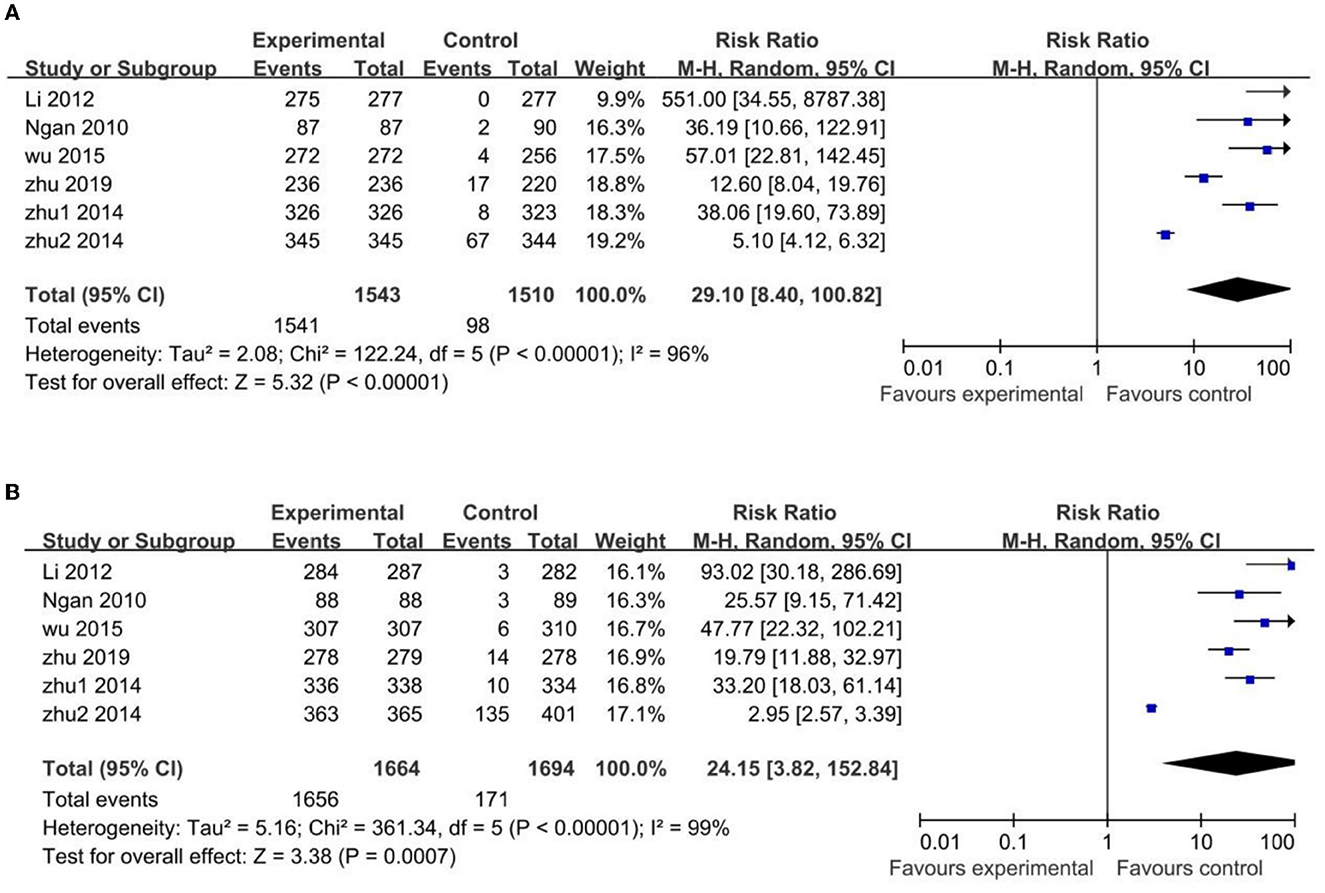
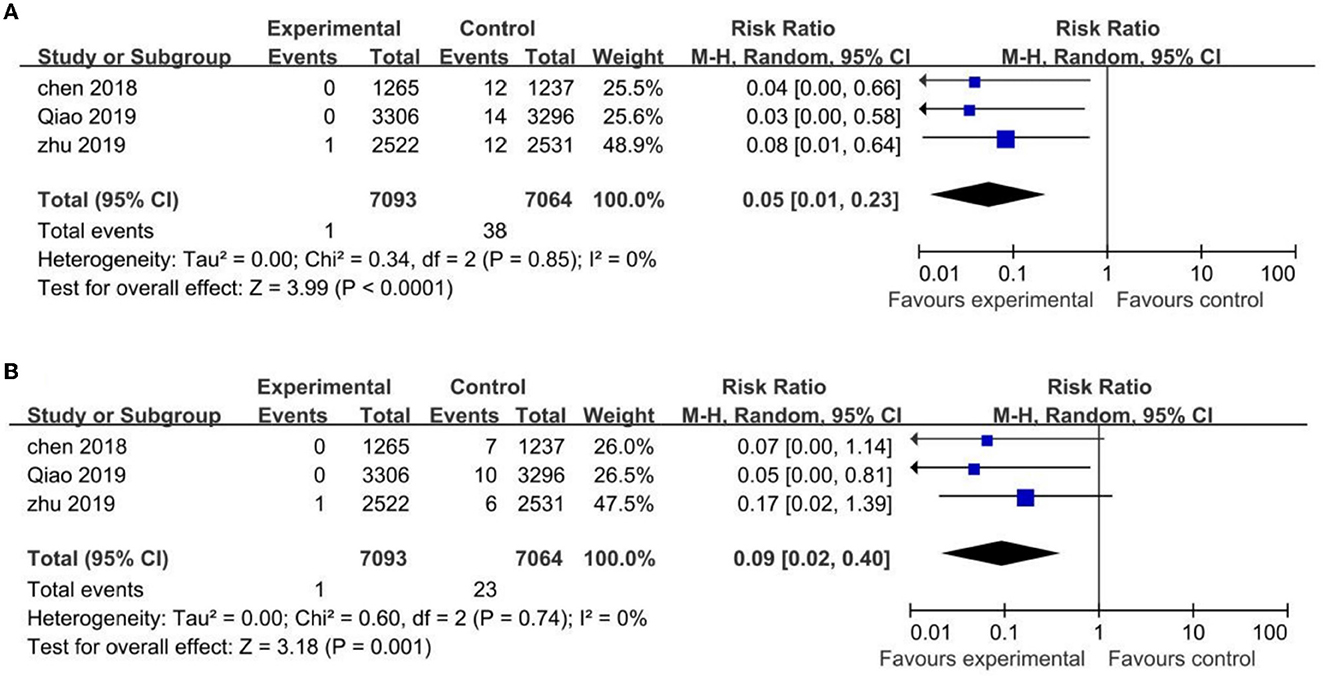
Figure 3. Comparison HPV vaccines vs. control in HPV-16 (A) and HPV-18 (B) antibody seroconversion rate in uninfected Chinese population.
The uninfected population's HPV-16 antibody seroconversion rate was higher in the vaccinated groups than those in the control groups, with a statistically significant difference (RR 29.10; 95% CI: 8.40–100.82). However, the heterogeneity among the pooled studies was high (I2 = 96%), due to two trials (19, 22) with narrow confidence intervals and disproportionate difference in their mean estimates. In particular, one (19) of these two trials with high heterogeneity showed huge significance with the other trials (RR 5.10; 95% CI: 4.12–6.32). In the sensitivity analysis excluding these two trials, the difference between the vaccinated and control groups remained statistically significant (RR 49.63; 95% CI: 24.93–98.80), but less heterogeneous (I2 = 38%) (Supplementary Figure 2).
Similar results occurred in the HPV-18 antibodies: the uninfected population's HPV-18 antibody seroconversion rate was higher in the vaccinated groups than that in the control groups, with a statistically significant difference (RR 24.15; 95% CI: 3.82–152.84). High heterogeneity was also found in these pooled studies (I2 = 99%), with the same two trials (19, 22) of heterogeneity for same reason, and the same one trial (19) of greatest heterogeneity (RR 2.95; 95% CI: 2.57–3.39). In the sensitivity analysis excluding these two trials the difference between the vaccinated and control groups remained statistically significant (RR 40.93; 95% CI: 25.96–64.53) but with reduced heterogeneity (I2 = 17%) (Supplementary Figure 3).
In addition, GMT of HPV-18 and HPV-16 in the uninfected populations were highly heterogeneous and the high heterogeneity could not be reduced. We did not perform a meta-analysis on such data, but for individual trials, GMT and seroconversion rates were significantly and statistically higher in the vaccinated groups than those in the control groups, for uninfected populations.
3.5. Efficacy of HPV vaccine
Three HPV vaccine trials were included in meta-analysis of efficacy, two (22, 23) for 2-valent HPV vaccine and one (21) for 4-valent HPV vaccine. Only one (22) out of the three clinical trials reported efficacy data at Months 24, 48, 57 and 72, the rest two with only data at Months 42 and 78.
The three trials mentioned above showed statistically significant differences in the incidence rate of CIN1+ and CIN2+ associated with HPV-16/18 in the vaccinated groups compared with the control groups, with (RR 0.05; 95% CI: 0.01–0.23, I2 = 0) for CIN1+ and (RR 0.09; 95% CI: 0.02–0.40, I2 = 0) for CIN2+ (Figure 4).
Two out of the three trials (22, 23) evaluated the 6-month PI and incident infections, and for the uninfected population, such data was statistically lower in the vaccinated groups than those in the control groups. The RR values were (RR 0.04; 95% CI: 0.01–0.12, I2 = 0) for the 6-month PI and (RR 0.28; 95% CI: 0.21–0.36, I2 = 0) for incident infections (Supplementary Figures 4, 5).
3.6. Safety of HPV vaccine
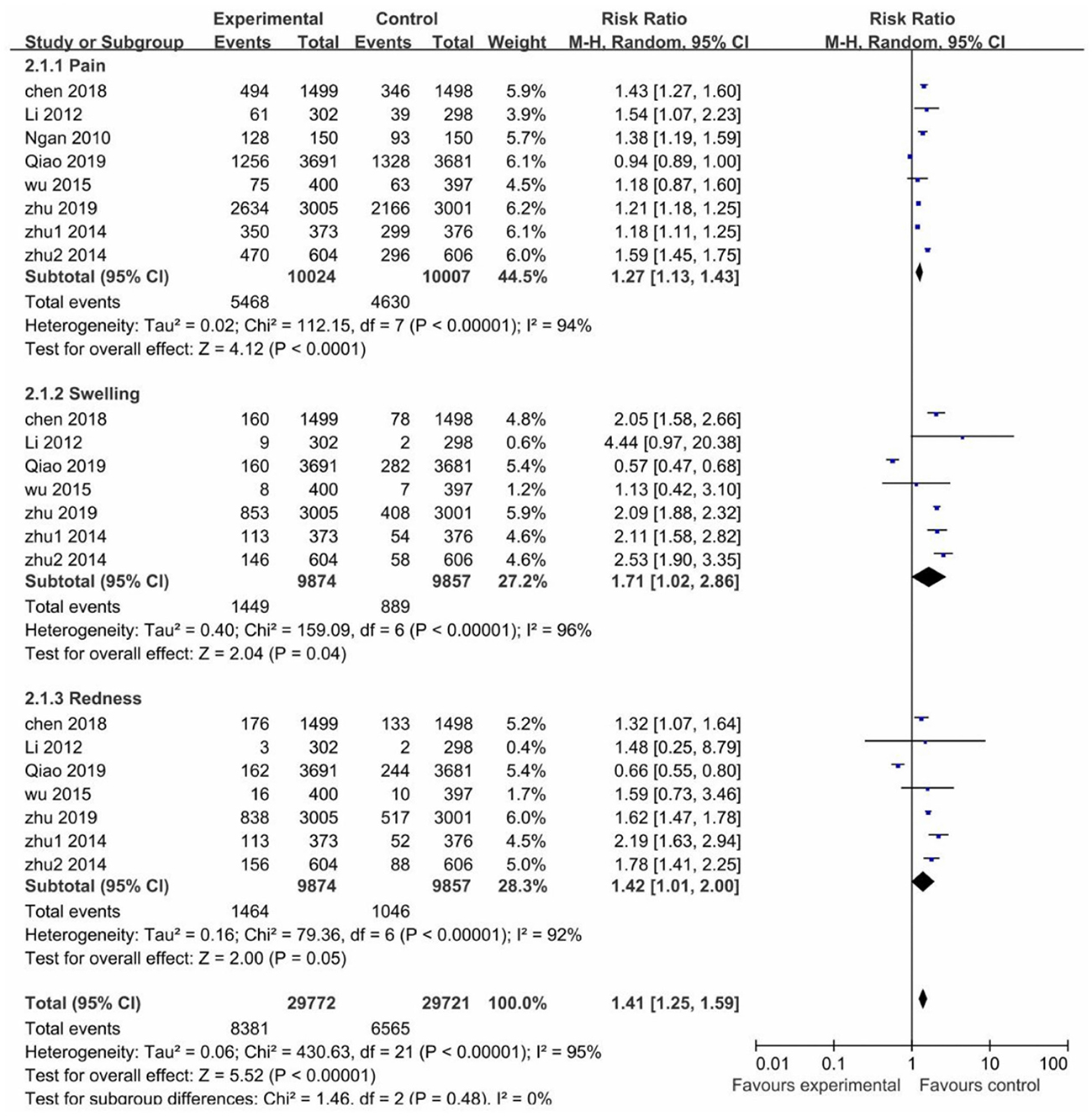
In most of the trials, local AEs were reported within 3–7 days after each vaccination in both the vaccinated and control groups. The risk of pain, swelling and redness after vaccination was higher in the vaccinated groups than the control groups, RR values were (1.27; 95% CI: 1.13–1.43) for pain, (1.71; 95% CI: 1.02–2.86) for swelling, (1.42; 95% CI: 1.01–2.00) for redness. However, all pooled studies were presented with high heterogeneity (I2 > 75%). Nevertheless, the risk of local adverse reactions was higher in the vaccinated groups than in the control groups (RR 1.41; 95% CI: 1.25–1.59) (Figure 5).
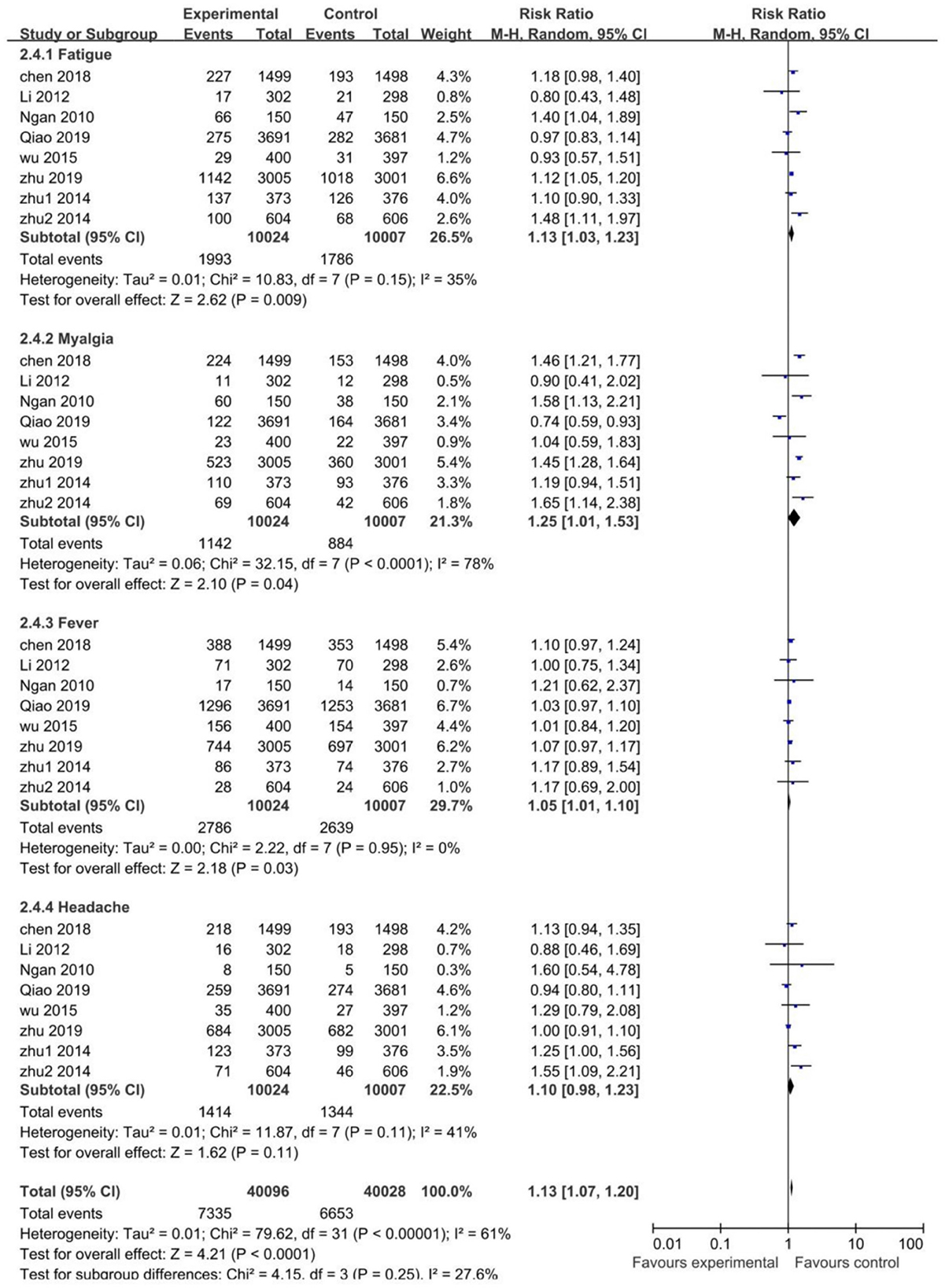
Systemic AEs occurred within 30 days after each vaccination in the studies. The risk of fatigue (RR 1.13; 95% CI: 1.03–1.23), myalgia (RR 1.25; 95% CI: 1.01–1.53), and fever (RR 1.05; 95% CI: 1.01–1.10) were higher in the vaccinated groups. The risk of headache was similar between the vaccinated and control groups (RR 1.10; 95% CI: 0.98–1.23). For the heterogeneity, it was acceptable in all pooled studies (I2 <50%), except for myalgia with high heterogeneity (I2 = 78%). Conclusively, the risk of systemic AEs was higher in the vaccinated groups than the control groups (RR 1.13; 95% CI: 1.07–1.20) (Figure 6).
Through sensitivity analysis, it is found that Qiao's study (23) contributed to the high heterogeneity. A domestic 2-valent HPV vaccine was used in Qiao's study, and we assumed that the type of vaccine may be the main reason for heterogeneity in local and systemic AEs, which led to a subgroup analysis of vaccine types. For all trials, two (20, 23) evaluated the domestic 2-valent HPV vaccine, whose subgroup analysis showed that the risk of both local and systemic AEs in the vaccinated groups was similar to that in the control groups and only pain (I2 = 51%) and redness (I2 = 78%) with heterogeneity. After removing the domestic 2-valent HPV vaccine trials, the non-domestic 2-valent HPV vaccine subgroup reported a decreased heterogeneity in most local and systemic AEs, with swelling (I2 = 96% → I2 = 0%) and myalgia (I2 = 78% → I2 = 0%) decreased to no heterogeneity and redness (I2 = 92% → I2 = 51%) to moderately high heterogeneity (Supplementary Figures 6–9).
All trials reported SAEs throughout the study. As study duration varied, a period of 7–12 months was selected for SAEs assessment. Data from six trials (17–20, 23) showed that the risk of SAEs after HPV vaccination in the vaccinated groups was similar to the control groups (RR at 1.04; 95% CI: 0.64–1.71, I2 = 0%) (Supplementary Figure 10).
3.7. Follow-up study
Four HPV vaccine RCTs with follow-ups were included in this study and the HPV vaccines used in the follow-up studies were imported 2-valent (26), domestic 2-valent (27) and imported 4-valent (24, 25). The follow-up period for these four RCTs were 10 years, 66 months, 3.5 years and 8 years, respectively.
One study (27) reported the long-term immunogenicity and the data showed that IgG antibody positivity for HPV-6,−8,−16 and−18 remained above 80% in the female population 3.5 years after the first vaccination. In the male population, all HPV-6,−8,−16 and−18 IgG antibodies remained above 90%. Three studies (28–30) reported the long-term efficacy of HPV vaccination, with a strong prevention of HPV-related precancerous diseases during the follow-up period, and over 90% efficacy on HPV16/18 CIN1+. Two studies (29, 30) showed long-term safety and HPV vaccination is safe and well tolerated, with no vaccine-related SAEs reported.
3.8. Nine-valent HPV vaccines
Two 9-valent HPV vaccine RCTs were included, including one imported 9-valent HPV vaccine trial (25) and one domestic 9-valent HPV vaccine trial (26). The imported 9-valent HPV vaccine global multi-centers RCT reported data from Hong Kong and Taiwan sub-centers, which investigated the efficacy, immunogenicity and safety of imported 9-valent HPV vaccines in two age groups (9–15 years, 16–26 years). The results showed that the seroconversion rates of each vaccinated group were >98%, no persistent infection lasting more than 6 months, and no vaccine-related SAEs reported. While the domestic 9-valent HPV vaccine trial was a non-inferiority trial compared with imported 4-valent HPV vaccines, it evaluated the immunogenicity and safety of domestic 9-valent HPV vaccines, domestic 4-valent HPV vaccines and imported 4-valent HPV vaccines in three age groups (20–26 years, 27–35 years, and 36–45 years). The results showed that the seroconversion rates of each group were >99%, and the incidence of AEs was comparable among these three groups.
4. Discussion
A search on ClinicalTrials.gov for HPV vaccines showed that most of the HPV vaccine clinical trials conducted to date have been in developed countries mostly in European countries, the United States and Japan (31–33). With good clinical practices and adequate financial support, such clinical trial results help to speed up the process of HPV vaccine into the immunization programs in developed countries. For developing countries, a more severe disease burden calls for an urgent need of domestic clinical trial data on HPV vaccines. Based on the previous experience on rotavirus vaccine (34), it is aware that the clinical trials results from developed countries cannot be simply applied to the developing countries. Demographic variables such as race would result in different levels of vaccination immunogenicity and vaccine efficacy between developed and developing countries. Following the call from the WHO's strategy for cervical cancer elimination, a growing need to conduct the state-specific clinical trials of HPV vaccines in particular among Chinese population can be foreseen.
The results of the meta-analysis showed that HPV vaccination in the uninfected population produced high vaccine efficacy and strong prevention of PI and CIN1+ in most cases, which resembles previous studies (35) in other regions. However, because the natural course of cervical cancer expands up to dozens of years, it is difficult to observe cervical cancer during the follow-up timeline set in the current studies. Further follow-up studies are suggested to fill the research gap.
This meta-analysis showed that HPV vaccination in the Chinese uninfected population produced a high degree of immunogenicity, as evidenced by high seroconversion rate in vaccinated groups, similar to previous studies and meta-analysis (36, 37) published in markets other than China. It is found that comparing only one indicator—seroconversion rates between the vaccinated and control groups is incomplete to reach a solid conclusion on vaccine immunogenicity because the GMT of seropositive subjects in the control groups was much lower than those in the vaccinated groups. Nevertheless, studies suggested that HPV vaccine has the potential to bring long-term immune efficacy.
The meta-analysis revealed vaccine immunogenicity with high heterogeneity. For HPV-16 and HPV-18 antibody seroconversion rate in the uninfected population, the sensitivity analysis found that two trials (19, 22) caused heterogeneity. This heterogeneity was remarkably induced by one trial (19). The vaccinated groups in both trials (16, 19) had comparable results with other trials, but the presence of more seropositive subjects in the control groups makes heterogeneity inevitable. Upon comparison, it is noted that subjects age ranged between 26 and 45 years in Zhu's study (16), and those in other trials ranged between 9 and 35 years, suggesting age seems to correlate with increased seropositive subjects. Although the other study (22) included Chinese women aged 18–25, due to its exclusion criteria—“women with no sexual experience due to culture and ethics were excluded,” subjects in this study may have more sexual activities than those in other studies. As a national study (38) from China stated that the median age at sexual debut was 22 years in urban China and 21 years in rural China. More sexual activity may contribute to an increase in the number of seropositive controls. Studies suggested that older age and virginity loss are the cause of heterogeneity. In fact, both factors lead to more frequent sex in the study population, which in turn increases the probability of becoming seropositive through natural infection in the control groups.
With regard to HPV vaccine safety, our meta-analysis showed that the Chinese population is tolerant to AEs from the HPV vaccines, and the risk difference of AEs for both vaccinated and control groups were low. Local AEs and some systemic AEs (fatigue, myalgia, fever), however, were more common in the vaccinated groups and with high heterogeneity. When exploring the reasons behind heterogeneity, a subgroup analysis of HPV vaccine types was performed. The analysis revealed that the risk of local and systemic AEs was similar between the vaccinated and control groups for the Chinese 2-valent HPV vaccine, and a remarkably lower heterogeneity was seen upon exclusion of both trials. This suggests that the Chinese 2-valent HPV vaccine may have a better safety profile in the Chinese population. Moreover, AEs of other HPV vaccines were also tolerable, which was consistent with previous studies (39, 40). Last but not the least, there were no significant differences between the vaccinated and control groups in terms of SAEs and vaccine-related SAEs.
There are several limitations in this study: first, a subgroup analysis of the 11 trials was impossible because the age intervals in these trials overlapped, and only one trial included Chinese women aged 26–45 years, which could not provide sufficient data to determine the age effect on the clinical outcomes of HPV vaccination; second, the clinical trials of 4-valent and 9-valent HPV vaccines in the Chinese population were lacking. Two clinical trials of 4-valent HPV vaccine were included in our study, but one missing immunogenicity data, one missing efficacy data, and the control groups in three 9-valent HPV vaccine trials included were not on placebo and lacking efficacy data, therefore it was not possible to perform a subgroup analysis of the vaccine's valence type; third, data on the outcome of HPV vaccination in Chinese men were limited with only one clinical trial of the 4-valent HPV vaccine included 100 male subjects. Fortunately, when searching ClinicalTrials.gov for ongoing clinical trials in China, several 4- and 9-valent HPV vaccine clinical trials are in progress, which could further supplement the data on HPV vaccine in the Chinese population in the future.
In the WHO position paper (41), the primary target population for HPV vaccination recommended is girls aged 9–14 years prior to their first sexual intercourse, and a vaccine catch-up program is recommended to be initiated for women aged ≥15 years. This important milestone provides China with an additional option for future HPV vaccine clinical trials: the study population of the trials could be further stratified by age subgroup. In this context, the heterogeneity among clinical trials can be reduced. Furthermore, age stratification for HPV vaccination may also help to present the real-world data once it enters the national immunization program.
Although China has promoted cervical cancer screening to control the increase in cervical cancer cases, China still accounts for 1/6 of the world's cervical cancer new cases and deaths cases in 2020 due to its large population base. What is worse, HPV vaccine, a primary strategy for preventing cervical cancer, is a self-pay vaccine in China. The high price and lack of knowledge on HPV vaccine determine low coverage of HPV vaccine in China. The inclusion of the vaccine in the national immunization program requires a comprehensive evaluation, including a cost-benefit analysis and a budget impact analysis, besides the safety and efficacy evaluation. There is still a long way to go for HPV vaccine inclusion in the national immunization program, but in the face of the current severe burden of cervical cancer, we are eager to see that happen as soon as possible.
5. Conclusion
For Chinese populations, HPV vaccines enhance the level of HPV16- and HPV18-specific antibodies and reduce the incidence of CIN1+ and CIN2+ in uninfected population. Also, the risk of serious AE in both groups are almost equivalent. More data are needed to establish vaccine efficacy with cervical cancer.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: PubMed, Embase, Web of Science, and Cochrane Library databases.
Author contributions
JG performed the material preparation, data analysis, and the first draft of the manuscript. SG contributed to data collection, the conception of the study, modification opinions, and was responsible for polishing the article. SD contributed significantly to analysis and manuscript preparation, helped perform the analysis with constructive discussions, and polish the article to make it more readable. All authors commented on previous versions of the manuscript, read, and approved the final manuscript.
Funding
This research was funded by the National Key Research and Development Plan of China (Grant No. 2020YFC2006000). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors would like to gratefully acknowledge National Institute of Hospital Administration (NIHA) and Fujian Medical University of Science and Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The findings, interpretations, and conclusions expressed in this article do not necessarily reflect the agencies' views mentioned.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1128717/full#supplementary-material
References
1. WHO. Global Strategy to Accelerate the Elimination of Cervical Cancer as a public Health Problem. Geneva: World Health Organization (2020). Licence: CC BY-NC-SA 3.0 IGO.
2. Erratum: Global Cancer Statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2020) 70:313. doi: 10.3322/caac.21609
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. (1999) 189:12–9. doi: 10.1002/(sici)1096-9896(199909)189:1<12::Aid-path431>3.0.Co;2-f
5. de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. (2010) 11:1048–56. doi: 10.1016/s1470-2045(10)70230-8
6. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to Hpv by site, country and Hpv type. Int J Cancer. (2017) 141:664–70. doi: 10.1002/ijc.30716
7. Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. (2010) 202:1789–99. doi: 10.1086/657321
8. Ma X, Wang Q, Ong JJ, Fairley CK, Su S, Peng P, et al. Prevalence of human papillomavirus by geographical regions, sexual orientation and HIV status in China: a systematic review and meta-analysis. Sex Transm Infect. (2018) 94:434–42. doi: 10.1136/sextrans-2017-053412
9. Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano-Ponce E, Salmerón J, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 As04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled Viviane study. Lancet. (2014) 384:2213–27. doi: 10.1016/s0140-6736(14)60920-x
10. Huang LM, Puthanakit T, Cheng-Hsun C, Ren-Bin T, Schwarz T, Pellegrino A, et al. Sustained immunogenicity of 2-dose human papillomavirus 16/18 As04-adjuvanted vaccine schedules in girls aged 9–14 years: a randomized trial. J Infect Dis. (2017) 215:1711–9. doi: 10.1093/infdis/jix154
11. Tay EH, Garland S, Tang G, Nolan T, Huang LM, Orloski L, et al. Clinical trial experience with prophylactic Hpv 6/11/16/18 Vlp vaccine in young women from the Asia-Pacific Region. Int J Gynaecol Obstet. (2008) 102:275–83. doi: 10.1016/j.ijgo.2008.03.021
12. Moreira ED, Jr., Block SL, Ferris D, Giuliano AR, Iversen OE, Joura EA, et al. Safety profile of the 9-valent Hpv vaccine: a combined analysis of 7 phase III clinical trials. Pediatrics. (2016) 138:4387. doi: 10.1542/peds.2015-4387
13. Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. (2012) 12:781–9. doi: 10.1016/s1473-3099(12)70187-1
14. Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K. Prevalence and risk factors of Hpv infection among women from various provinces of the world. Arch Gynecol Obstet. (2012) 285:771–7. doi: 10.1007/s00404-011-2155-8
15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
16. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
17. Ngan HY, Cheung AN, Tam KF, Chan KK, Tang HW, Bi D, et al. Human papillomavirus-16/18 As04-adjuvanted cervical cancer vaccine: immunogenicity and safety in healthy Chinese women from Hong Kong. Hong Kong Med J. (2010) 16:171–9.
18. Li R, Li Y, Radley D, Liu Y, Huang T, Sings HL, et al. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, double-blind, placebo-controlled trial in Chinese males and females. Vaccine. (2012) 30:4284–91. doi: 10.1016/j.vaccine.2012.02.079
19. Zhu F, Li J, Hu Y, Zhang X, Yang X, Zhao H, et al. Immunogenicity and safety of the Hpv-16/18 As04-adjuvanted vaccine in healthy Chinese girls and women aged 9 to 45 years. Hum Vaccin Immunother. (2014) 10:1795–806. doi: 10.4161/hv.28702
20. Wu T, Hu YM Li J, Chu K, Huang SJ, Zhao H, et al. Immunogenicity and safety of an E. coli-produced bivalent human papillomavirus (type 16 and 18) vaccine: a randomized controlled phase 2 clinical trial. Vaccine. (2015) 33:3940–6. doi: 10.1016/j.vaccine.2015.06.052
21. Chen W, Zhao Y, Xie X, Liu J, Li J, Zhao C, et al. Safety of a quadrivalent human papillomavirus vaccine in a phase 3, randomized, double-blind, placebo-controlled clinical trial among Chinese women during 90 months of follow-up. Vaccine. (2019) 37:889–97. doi: 10.1016/j.vaccine.2018.12.030
22. Zhu FC, Hu SY, Hong Y, Hu YM, Zhang X, Zhang YJ, et al. Efficacy, immunogenicity and safety of the As04-Hpv-16/18 vaccine in Chinese women aged 18-25 years: end-of-study results from a phase II/III, randomised, controlled trial. Cancer Med. (2019) 8:6195–211. doi: 10.1002/cam4.2399
23. Qiao YL, Wu T, Li RC, Hu YM, Wei LH Li CG, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. (2020) 112:145–53. doi: 10.1093/jnci/djz074
24. Wei L, Xie X, Liu J, Zhao Y, Chen W, Zhao C, et al. Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: a randomized, placebo-controlled trial with 78-month follow-up. Vaccine. (2019) 37:3617–24. doi: 10.1016/j.vaccine.2018.08.009
25. Garland SM, Pitisuttithum P, Ngan HYS, Cho CH, Lee CY, Chen CA, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine: subgroup analysis of participants from Asian countries. J Infect Dis. (2018) 218:95–108. doi: 10.1093/infdis/jiy133
26. Shu Y, Yu Y, Ji Y, Zhang L, Li Y, Qin H, et al. Immunogenicity and safety of two novel human papillomavirus 4- and 9-valent vaccines in Chinese women aged 20-45 years: a randomized, blinded, controlled with gardasil (Type 6/11/16/18), Phase III non-inferiority clinical trial. Vaccine. (2022). doi: 10.1016/j.vaccine.2022.10.022
27. Huang T, Liu Y, Li Y, Liao Y, Shou Q, Zheng M. et al. Evaluation on the persistence of anti-Hpv immune responses to the quadrivalent Hpv vaccine in Chinese females and males: up to 35 years of follow-up. Vaccine. (2018) 36:1368–74. doi: 10.1016/j.vaccine.2018.02.006
28. Zhao C, Zhao Y, Li J, Li M, Su Y, Mi X, et al. The eight-year long-term follow-up on the effectiveness of the quadrivalent human papillomavirus vaccine in Chinese women 20-45 years of age. Hum Vaccin Immunother. (2022) 18:2052700. doi: 10.1080/21645515.2022.2052700
29. Zhao F, Jastorff A, Hong Y, Hu S, Chen W, Xu X, et al. Safety of As04-Hpv-16/18 vaccine in Chinese women aged 26 years and older and long-term protective effect in women vaccinated at age 18-25 years: a 10-year follow-up study. Asia Pac J Clin Oncol. (2022). doi: 10.1111/ajco.13833
30. Zhao FH, Wu T, Hu YM, Wei LH Li MQ, Huang WJ, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced human papillomavirus (16 and 18) l1 virus-like-particle vaccine: end-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infect Dis. (2022). doi: 10.1016/s1473-3099(22)00435-2
31. Konno R, Dobbelaere KO, Godeaux OO, Tamura S, Yoshikawa H. Immunogenicity, reactogenicity, and safety of human papillomavirus 16/18 As04-adjuvanted vaccine in Japanese women: interim analysis of a phase II, double-blind, randomized controlled trial at month 7. Int J Gynecol Cancer. (2009) 19:905–11. doi: 10.1111/IGC.0b013e3181a23c0e
32. Van Damme P, Leroux-Roels G, Simon P, Foidart JM, Donders G, Hoppenbrouwers K, et al. Effects of varying antigens and adjuvant systems on the immunogenicity and safety of investigational tetravalent human oncogenic papillomavirus vaccines: results from two randomized trials. Vaccine. (2014) 32:3694–705. doi: 10.1016/j.vaccine.2014.03.040
33. Esposito S, Birlutiu V, Jarcuska P, Perino A, Man SC, Vladareanu R, et al. Immunogenicity and safety of human papillomavirus-16/18 As04-adjuvanted vaccine administered according to an alternative dosing schedule compared with the standard dosing schedule in healthy women aged 15 to 25 years: results from a randomized study. Pediatr Infect Dis J. (2011) 30:e49–55. doi: 10.1097/INF.0b013e318206c26e
34. Moon SS, Tate JE, Ray P, Dennehy PH, Archary D, Coutsoudis A, et al. Differential profiles and inhibitory effect on rotavirus vaccines of nonantibody components in breast milk from mothers in developing and developed countries. Pediatr Infect Dis J. (2013) 32:863–70. doi: 10.1097/INF.0b013e318290646d
35. Porras C, Tsang SH, Herrero R, Guillén D, Darragh TM, Stoler MH, et al. Efficacy of the bivalent Hpv vaccine against Hpv 16/18-associated precancer: long-term follow-up results from the Costa Rica vaccine trial. Lancet Oncol. (2020) 21:1643–52. doi: 10.1016/s1470-2045(20)30524-6
36. Setiawan D, Luttjeboer J, Pouwels KB, Wilschut JC, Postma MJ. Immunogenicity and safety of human papillomavirus (Hpv) vaccination in Asian populations from six countries: a meta-analysis. Jpn J Clin Oncol. (2017) 47:265–76. doi: 10.1093/jjco/hyw192
37. Perez G, Lazcano-Ponce E, Hernandez-Avila M, García PJ, Muñoz N, Villa LL, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like-particle vaccine in Latin American women. Int J Cancer. (2008) 122:1311–8. doi: 10.1002/ijc.23260
38. Zhao FH, Tiggelaar SM, Hu SY, Xu LN, Hong Y, Niyazi M, et al. A multi-center survey of age of sexual debut and sexual behavior in Chinese women: suggestions for optimal age of human papillomavirus vaccination in China. Cancer Epidemiol. (2012) 36:384–90. doi: 10.1016/j.canep.2012.01.009
39. Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. (2006) 118:2135–45. doi: 10.1542/peds.2006-0461
40. Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (Hpv)-16/18 As04-adjuvanted vaccine and Hpv-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a phase III randomized trial. Hum Vaccin Immunother. (2014) 10:3435–45. doi: 10.4161/hv.36121
Keywords: HPV vaccine, meta-analysis, efficacy, immunogenicity, safety
Citation: Guo J, Guo S and Dong S (2023) Efficacy, immunogenicity and safety of HPV vaccination in Chinese population: A meta-analysis. Front. Public Health 11:1128717. doi: 10.3389/fpubh.2023.1128717
Received: 21 December 2022; Accepted: 31 January 2023;
Published: 17 February 2023.
Edited by:
Tang Shangfeng, Huazhong University of Science and Technology, ChinaReviewed by:
Gang Sun, Johns Hopkins University, United StatesZhuo Chen, University of Georgia, United States
Chengxiang Tang, Guangzhou University, China
Copyright © 2023 Guo, Guo and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siping Dong,  c2lwaW5nZEAxNjMuY29t
c2lwaW5nZEAxNjMuY29t
 Jianming Guo
Jianming Guo Shuyan Guo1
Shuyan Guo1 Siping Dong
Siping Dong