- 1EcoHealth Alliance, New York, NY, United States
- 2Centre for Integrative Ecology, School of Life and Environmental Science, Deakin University, Geelong Waurn Ponds, VIC, Australia
- 3Institute of Epidemiology, Disease Control and Research (IEDCR), Dhaka, Bangladesh
- 4One Health Laboratory, International Centre for Diarrheal Diseases Research, Bangladesh (icddr,b), Dhaka, Bangladesh
- 5National Reference Laboratory for Avian Influenza, Bangladesh Livestock Research Institute (BLRI), Savar, Bangladesh
- 6Queensland Alliance for One Health Sciences, School of Veterinary Science, University of Queensland, Brisbane, QLD, Australia
Avian influenza viruses (AIV) have been frequently detected in live bird markets (LBMs) around the world, primarily in urban areas, and have the ability to spillover to other species, including humans. Despite frequent detection of AIV in urban LBMs, the contamination of AIV on environmental surfaces in rural and peri-urban LBMs in Bangladesh is poorly documented. Therefore, we conducted this study to determine the prevalence of AIV subtypes within a subset of peri-urban and rural LBMs in Bangladesh and to further identify associated risk factors. Between 2017 and 2018, we collected faecal and offal samples from 200 stalls in 63 LBMs across four sub-districts. We tested the samples for the AIV matrix gene (M-gene) followed by H5, H7, and H9 subtypes using real-time reverse transcriptase-polymerase chain reaction (rRT-PCR). We performed a descriptive analysis of market cleanliness and sanitation practices in order to further elucidate the relationship between LBM biosecurity and AIV subtypes by species, sample types, and landscape. Subsequently, we conducted a univariate analysis and a generalized linear mixed model (GLMM) to determine the risk factors associated with AIV contamination at individual stalls within LBMs. Our findings indicate that practices related to hygiene and the circulation of AIV significantly differed between rural and peri-urban live bird markets. 42.5% (95% CI: 35.56–49.67) of stalls were positive for AIV. A/H5, A/H9, and A HA/Untyped were detected in 10.5% (95% CI: 6.62–15.60), 9% (95% CI: 5.42–13.85), and 24.0% (95% CI: 18.26–30.53) of stalls respectively, with no detection of A/H7. Significantly higher levels of AIV were found in the Sonali chicken strain compared to the exotic broiler, and in offal samples compared to fecal samples. In the GLMM analysis, we identified several significant risk factors associated with AIV contamination in LBMs at the stall level. These include: landscape (AOR: 3.02; 95% CI: 1.18–7.72), the number of chicken breeds present (AOR: 2.4; 95% CI: 1.01–5.67), source of birds (AOR: 2.35; 95% CI: 1.0–5.53), separation of sick birds (AOR: 3.04; 95% CI: 1.34–6.92), disposal of waste/dead birds (AOR: 3.16; 95% CI: 1.41–7.05), cleaning agent (AOR: 5.99; 95% CI: 2.26–15.82), access of dogs (AOR: 2.52; 95% CI: 1.12–5.7), wild birds observed on site (AOR: 2.31; 95% CI: 1.01–5.3). The study further revealed a substantial prevalence of AIV with H5 and H9 subtypes in peri-urban and rural LBMs. The inadequate biosecurity measures at poultry stalls in Bangladesh increase the risk of AIV transmission from poultry to humans. To prevent the spread of AIV to humans and wild birds, we suggest implementing regular surveillance at live bird markets and enhancing biosecurity practices in peri-urban and rural areas in Bangladesh.
1. Introduction
Avian influenza viruses (AIVs) are zoonotic viruses that can infect domestic and wild bird species, along with a variety of other animals (1). Multiple subtypes of low pathogenic avian influenza (LPAI) viruses and highly pathogenic avian influenza (HPAI) have been detected from live bird markets (LBMs) and farms around Bangladesh, with H9N2 and H5N1 being the most prevalent (2–4). H5N1 and H9N2 are mostly endemic to countries in Southeast Asia, such as Bangladesh (5–7). Over 585 influenza outbreaks have been recorded in poultry in Bangladesh (4). AIVs can spillover to humans from the poultry, often presenting with severe clinical outcomes. In 1997, the H5N1 virus infected 18 people in Hong Kong, causing six fatalities. Those were the first human deaths associated with the virus (8). In Bangladesh, eight human cases of H5N1 have been detected between the years of 2008 and 2022, one of which resulted in fatality (9). Three incidences of human infection with H9N2 viruses have been reported in Bangladesh, with the most recent case involving a poultry market worker who was in contact with sick birds (6). Evidence of spillover from poultry to humans raises substantial concerns about occupational exposure to AIVs in LBMs. In addition to poultry, there have been occasional reports of spillover to house crows and evidence of AIV in captive birds at zoos and parks (10–13). These reports raise additional concerns about the potential sources of spillover to humans and implications for wildlife health.
LBMs are common sites for poultry trading, selling, and processing in Asia (14, 15). The birds are sourced from multiple locations and hoarded into densely packed cages, often with more than one breed or species in the same enclosure. It is common practice for the vendors to slaughter the birds on site and leave the offal and poultry remains in the stall (16). Moreover, LBM biosecurity is generally poor in Bangladesh, and not practiced in accordance with the guidelines recommended by the Food and Agriculture Organization (FAO) to reduce the risk of virus circulation (17). For example, one study in Bangladesh showed that LBM workers who did not follow proper biosecurity practices during daily activities, such as feeding poultry, cleaning feces from pens, and handling sick poultry were at higher risk of exposure to the virus (18). Similar findings have been reported in other countries, whereby a number of additional studies have observed risk factors associated with AIV contamination at LBMs (19, 20). As a potential hotspot for AIV infection (17, 21, 22), LBMs are in urgent need of biosecurity improvements as well as enhanced behavioral and biological surveillance.
The population of Bangladesh has increased rapidly over the past 20 years (23), resulting in a greater demand for food and intense competition for resources. This growing demand exists in urban areas as well as in peri-urban and rural areas. To meet this demand, private and governmental investment has increased to raise commercial production of protein (24). As a result, Bangladesh’s production of meat has doubled over the last 10 years (25). About one-third of the country’s protein comes from livestock and other animal products (26). In previous years, most rural households raised poultry in their backyard to support protein consumption (27). However, with the population increase, the dependency has shifted from backyard poultry to commercial poultry in both peri-urban and rural areas.
As more people turn to LBMs as a source of protein in Bangladesh (28), robust risk characterization is essential to inform targeted public health interventions at this interface. However, most studies about the risk of AIV biosecurity are conducted in urban areas and communities (7, 29–32). The risk of AIV spillover in peri-urban and rural areas remains poorly understood. To that end, we conducted a cross-sectional study on LBMs to explore the diversity and prevalence of AIV and their associated risk factors in peri-urban and rural areas in Bangladesh.
2. Methodology
2.1. Study design, site selection
Bangladesh is subdivided into an administrative hierarchy as follows: division > district > sub-district (upazilla) > union > village (33). We conducted this study in upazillas, unions, and village settings. We conducted a cross-sectional study among 63 LBMs consisting of 25 from Savar and 21 from Dhamrai in Dhaka district, 13 from Fulbaria in Mymensingh, and four from Pabna Sadar in Pabna district – covering both peri-urban and rural areas (Figure 1). We enrolled 2 to 5 vendors from each LBM based on the market size and landscape gradient, such as whether the market is in a peri-urban or rural settings. We enrolled a total of 200 vendors from 63 LBMs. We used a purposive sampling strategy, and only enlisted vendors who agreed and consented to participate in our study (34). Upon receiving consent, we conducted a behavioral risk questionnaire with each vendor, followed by biological sample collection from their stall or workspace.
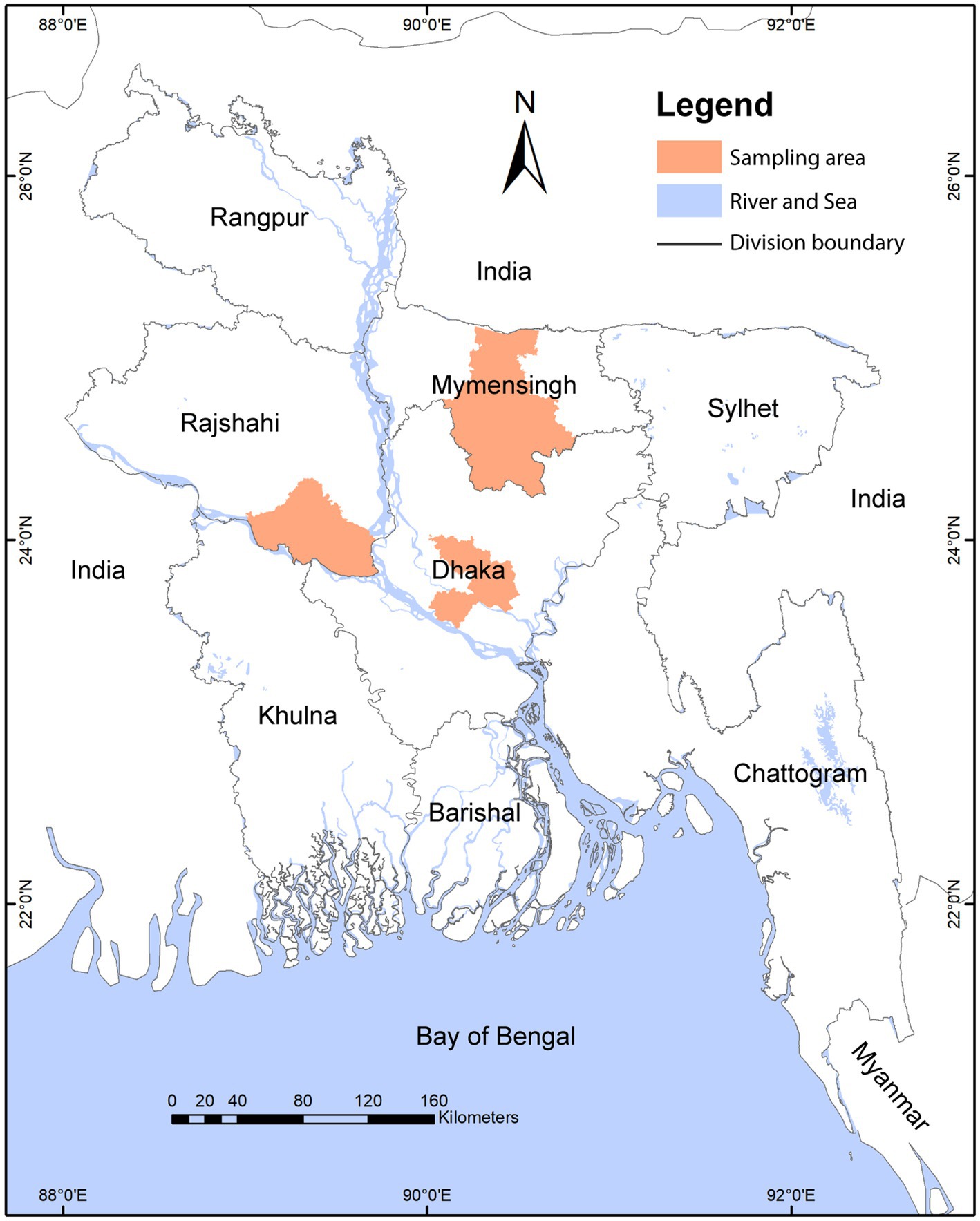
Figure 1. Map showing the study sites. Colored regions indicates the sampling districts of the study.
In our study, we defined an LBM as a facility with a physical structure where vendors sell live poultry. Birds are slaughtered and sold on-site and typically remain at the market until they are sold. A vendor is a shop owner or stall keeper who buys poultry from farms or middleman to sell to other vendors or directly to consumers. Stalls are small places within the LBM, usually owned or leased by a vendor (the shop owner) to keep, process, and sell poultry.
In Bangladesh, LBMs are regulated by different authorities, including government and private. At the peri-urban level, the LBMs are monitored and controlled by local governments (such as municipalities) or privately. In the case of rural areas, it is mainly regulated by the local government (union porishod) (35). In some cases, specific market-based associations play a vital role in the LBM operation. There are 12, 16, 13, and 10 unions under the Savar., Dhamrai, Fulbaria, and Pabna Sadar subdistrict, respectively (Figure 1).
2.2. Biological specimens and biosecurity practices data collection at the stall level
We took samples from two strains of chickens: exotic white broilers and golden colored, Sonali birds. Sonali is a crossbreed between the Rhode Island Red (RIR) cocks and Fayoumi hens (36). These extotic broilers and cross-bred Sonali chicken are sold as meat types in the LBM. We collected freshly deposited feces from the stall and offal from freshly slaughtered birds’. We obtained 2–4 fecal or offal swab samples from each stall and made them into a fecal and offal pool separately. If any dead or sick birds were available at the time of sampling, we also took pooled oropharyngeal and cloacal swab samples. We recorded all bird species present in the stall at the time of sample collection, based on observation. The swab samples were kept in a 3.6 ml cryovial, or 10 ml falcon tube containing 3 ml viral transport media (VTM) and placed in a liquid nitrogen container (−196°C). In the Laboratory, we stored the samples at −80°C in the freezer until further processing. During sample collection, the team wore appropriate personnel protective equipment like gloves and N95 masks. We prepared and pretested a questionnaire to collect data on biosafety and biosecurity practices at the stall level of LBMs. We administered the questionnaires to consenting vendors or workers through a face-to-face interview.
2.3. Ethical approval
The study protocol was approved by the Institutional Ethics Committee of the Institute of Epidemiology Disease Control and Research (IEDCR/IRB/2015/04), Chattogram Veterinary and Animal Sciences University-Animal Experimentation Ethics Committee (protocol: CVASU/Dir (R&E) AEEC/2015/751).
2.4. Laboratory testing
Following the manufacturer’s instructions, we extracted RNA using the magnetic bead-based RNA isolation technique in a KingFisher Flex 96-well robot using the MagMAXTM-96 AI/ND Viral RNA Isolation Kit (Applied BiosystemsTM, San Francisco, CA). We tested the pooled fecal and offal samples from each stall separately for the presence of the AIV viral Matrix (M) gene. We evaluated the swab samples using a real-time reverse transcription PCR detection kit and fluorescent TaqMan probes to type and subtype influenza viruses (37, 38). We used primers and probes specific to the matrix gene to detect influenza A viruses. We employed H5, H7, and H9 hemagglutinin gene-specific primers and probes to detect H5, H7, and H9 subtypes in influenza A virus-positive samples (37, 39). The samples that tested positive for AIV RNA (M-gene) but negative for H5, H7, and H9 were classified as HA Untyped.
2.5. Statistical analysis
We summarized the characteristics of biosecurity practices from the questionnaire using descriptive analyses. We then determined the prevalence of AIV subtypes at the level of stall, LBM, chicken strains, and sample category along with 95% confidence intervals (CIs) and visualized them using graphical analysis. We considered a stall AIV positive if the fecal or offal sample was positive for any of the aforementioned subtypes. In addition, we labelled an LBM as AIV positive if at least one stall sample was positive for AIV by marking each stall as positive or negative for AIV and its subtype in LBMs containing multiple stall samples (40, 41). We performed univariable and multivariable risk factors analysis at the stall level. We considered a sample positive for the binary outcome variable if it was found positive either for A/H5, A/H9, or A/HA untyped in the laboratory test. We performed Pearson’s chi-square test (42) to find the bio-security practices significantly associated with AIV. Factors associated with AIV with a value of p <=0.05 in univariate analysis were selected for multivariable analyses. We then used a generalized linear mixed model (GLMM) (43), accounting for clustering by LBM, to estimate adjusted odds ratios. We considered the value of p <=0.05 statistically significant in the final multivariable analysis. We calculated model χ2 to measure model fitness for the GLMM. We performed all statistical analyses using R (44). We used “lme4” and “tidyverse” packages for the analysis in R software. We created the maps using ArcGIS v10.4.1 (ESRI, Redlands, CA, United States). The shape file was collected from freely available DIVA-GIS.1
3. Results
3.1. Hygienic status and physiographic characteristics of the studied LBMs across landscapes
We conducted this study on two different types of landscapes: 92 peri-urban LBMs and 108 rural LBMs. We noted a number of differences in the physiographic characteristics and hygienic systems between the peri-urban and rural markets. We found that the majority of vendors kept multiple strains of chicken (56.5%; 95% CI: 49.3–63.5), but we did not detect a significant difference between peri-urban and rural LMBs. Only 17.5% of the stalls in our study had ducks, although the proportion was significantly (p = 0.04) higher at stalls in peri-urban LBMs (87.96%; 95% CI: 80.3–93.4). Concrete flooring was more common in peri-urban markets (56.52%; 95% CI: 45.8–66.8), and a mud floor was more common in rural markets (64.81%; 95% CI: 55.0–73.8). 65.5% of all vendors kept their poultry on the floor compared to the cage. We detected a signigicant difference in the (p < 0.01) use of bamboo to create a stall boundary compared to brick, in rural LBMs (65.74%; 95% CI: 56.0–74.6). 66.3% of stalls (95% CI: 55.7–75.8) of peri-urban LBMs collected their birds from middlemen rather than commercial farms, and the difference was significant (p = 0.01) compared to rural LBMs. Most stalls had no unsold birds that stayed overnight at the shop (57.5; 95% CI: 50.3–64.4), but we did not observe a difference by landscape. The number of peri-urban markets with a bird death in the 7 days prior to sampling was significant (36.96%; 95% CI: 27.1–47.7) (p = 0.05) compared to rural markets. Running water supply was more common in peri-urban areas (60.87%; 95% CI: 50.1–70.9), and the percentage of stalls with no drainage system (68.5%; 95% CI: 61.6–74.9) and no electricity (62.04%; 95% CI: 52.2–71.2) was higher in the rural LBMs. Wild birds were more commonly observed at peri-urban stalls (63.04%; 95% CI: 52.3–72.9). Most of the peri-urban vendors (69.57%; 95% CI: 59.1–78.7) kept their stalls open daily, which was notably (p < 0.01) higher than rural vendors (42.59%; 95% CI: 33.1–52.5; Table 1).
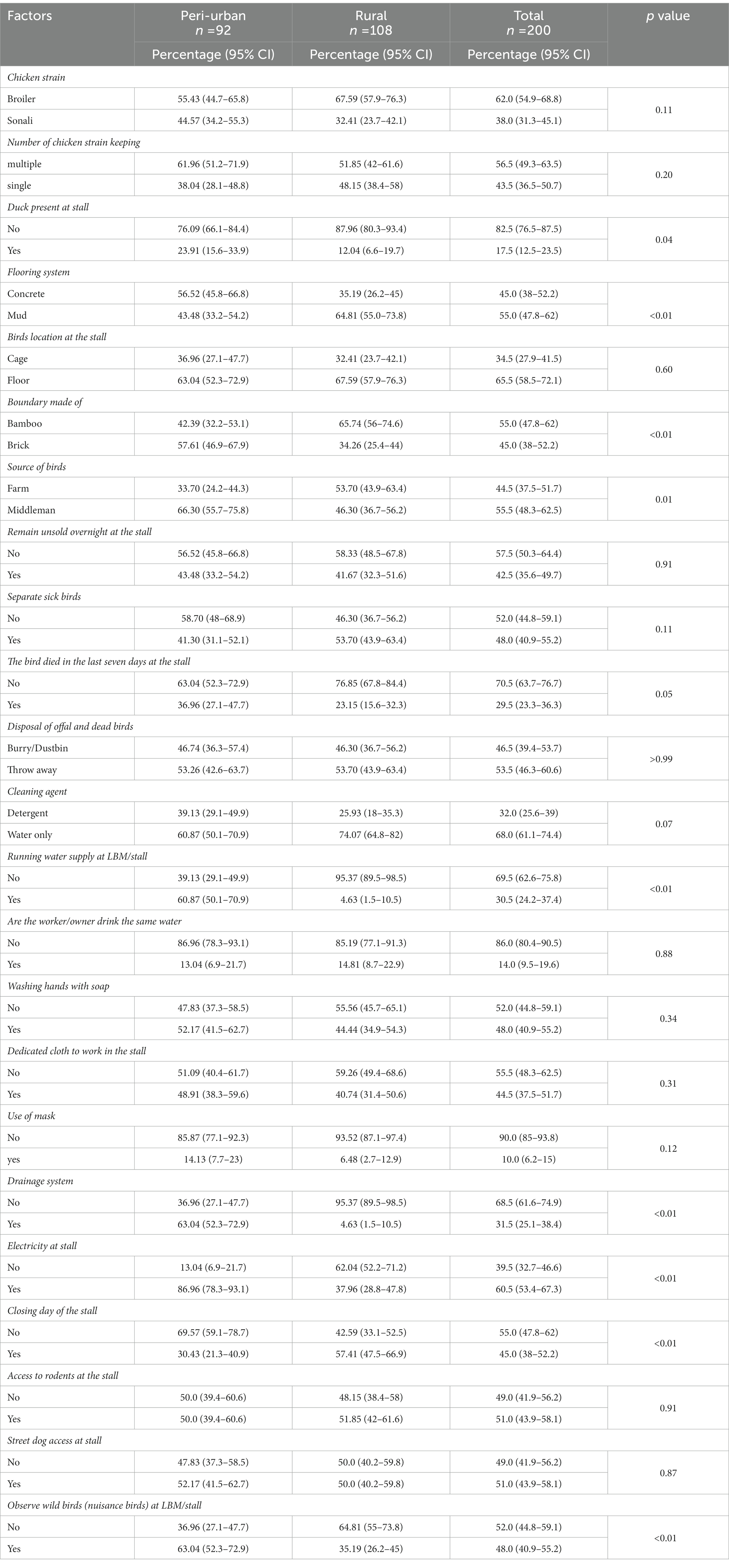
Table 1. Frequency of physiographic and hygienic status of the studied poultry markets in peri-urban and rural LBMs.
3.2. Prevalence of AIV and its subtypes by different factors
3.2.1. Prevalence of AIV subtype at the market and stall level
We collected samples from a total of 63 LBMs during our study period. Of the 63 markets, 52 (82.54%; 95% CI: 70.90–90.95) were positive for the AIV M-gene. Overall, 23.81% (95% CI: 13.98–36.21) of markets tested positive for subtype A/H5, 22.22% (95% CI: 12.72–34.46) contained A/H9, and 58.73% (95% CI: 45.62–70.99) tested positive for HA/untyped. We recorded two instances of co-contamination with subtypes A/H5 and A/H9 at two of the LBMs. The spatial distribution of AIV subtype circulation for all sub-districst is shown in Figure 2.
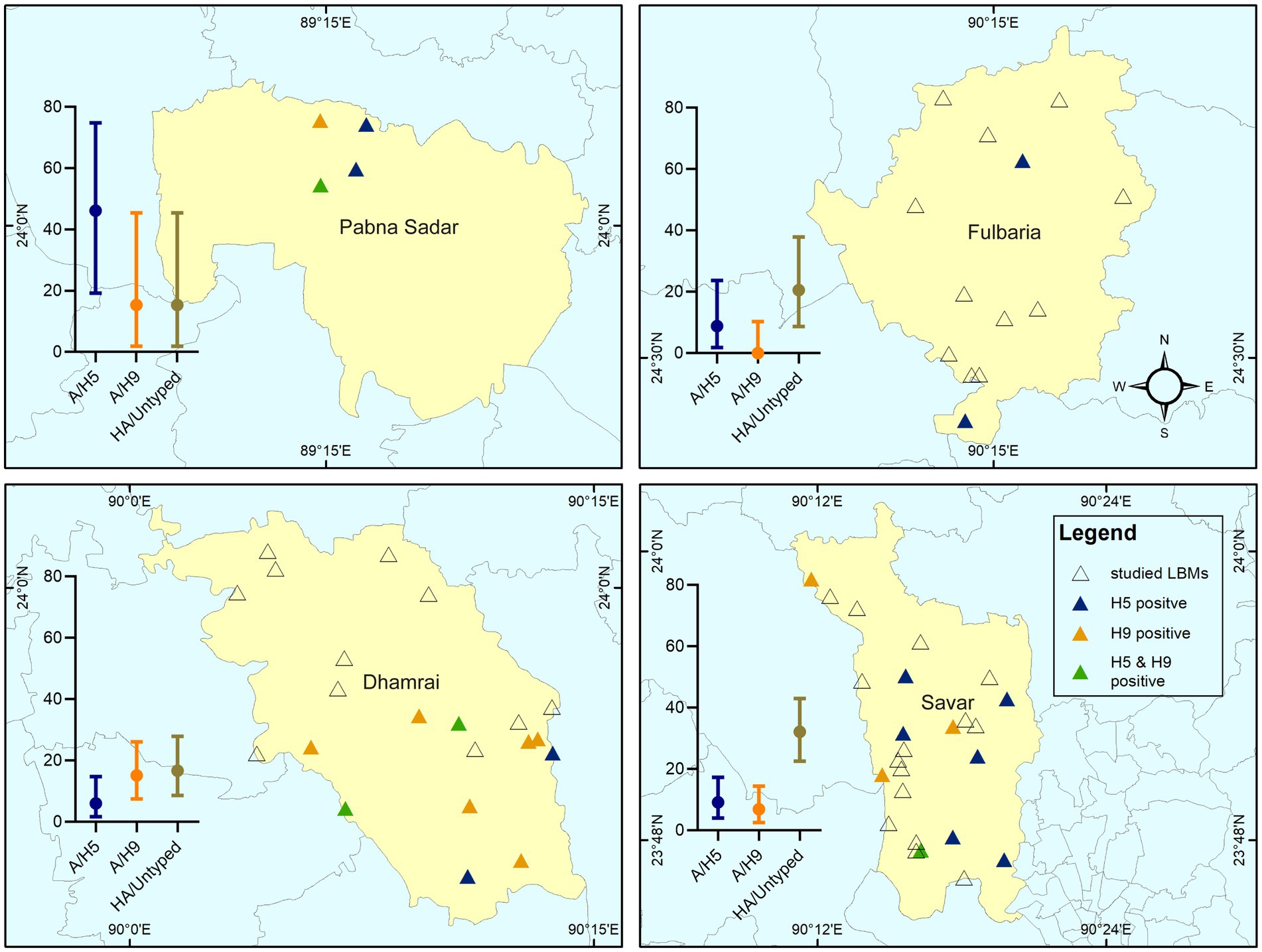
Figure 2. Spatial distribution of studied LBMs and the prevalence of H5, H9, and A/HA untyped detected in the LBMs. The error bar with mean value indicates the prevalence of subtypes of AIV at stall level in that region.
The prevalence of the AIV M-gene was 42.5% (95% CI: 35.56–49.67) at the stall level. A/H5 and A/H9 positive samples were found in 10.5% (95% CI: 6.62–15.60) and 9% (95% CI: 5.42–13.85) of stalls, respectively. We detected A/HA untyped in 24.0% (95% CI: 18.26–30.53) stalls. At the sub-district level, we found a higher prevalence for subtype H5 (46.15%; 95% CI: 19.22–74.87) in Pabna Sadar. The sub-district Dhamrai had the highest detection of subtype A/H9 (15.15%; 95% CI: 7.51–26.10), while samples from Savar had the highest detection of A/HA untyped (32.18%; 95% CI: 22.56–43.06). We did not detect subtype A/H7 in any of our samples (Figure 2).
3.2.2. Prevalence of AIV by landscape
Detection of AIV was associated with landscape (peri-urban vs. rural) when calculating prevalence at the stall level. We found that the prevalence of AIV in peri-urban regions (54.35%; 95% CI: 43.63–64.78) was significantly higher than in rural regions (p < 0.01). Additionally, we observed a significantly higher detection of subtype A/H5 (16.30%; 95% CI: 9.42–25.46) in peri-urban landscapes, at the stall level (p = 0.03; Figure 3).
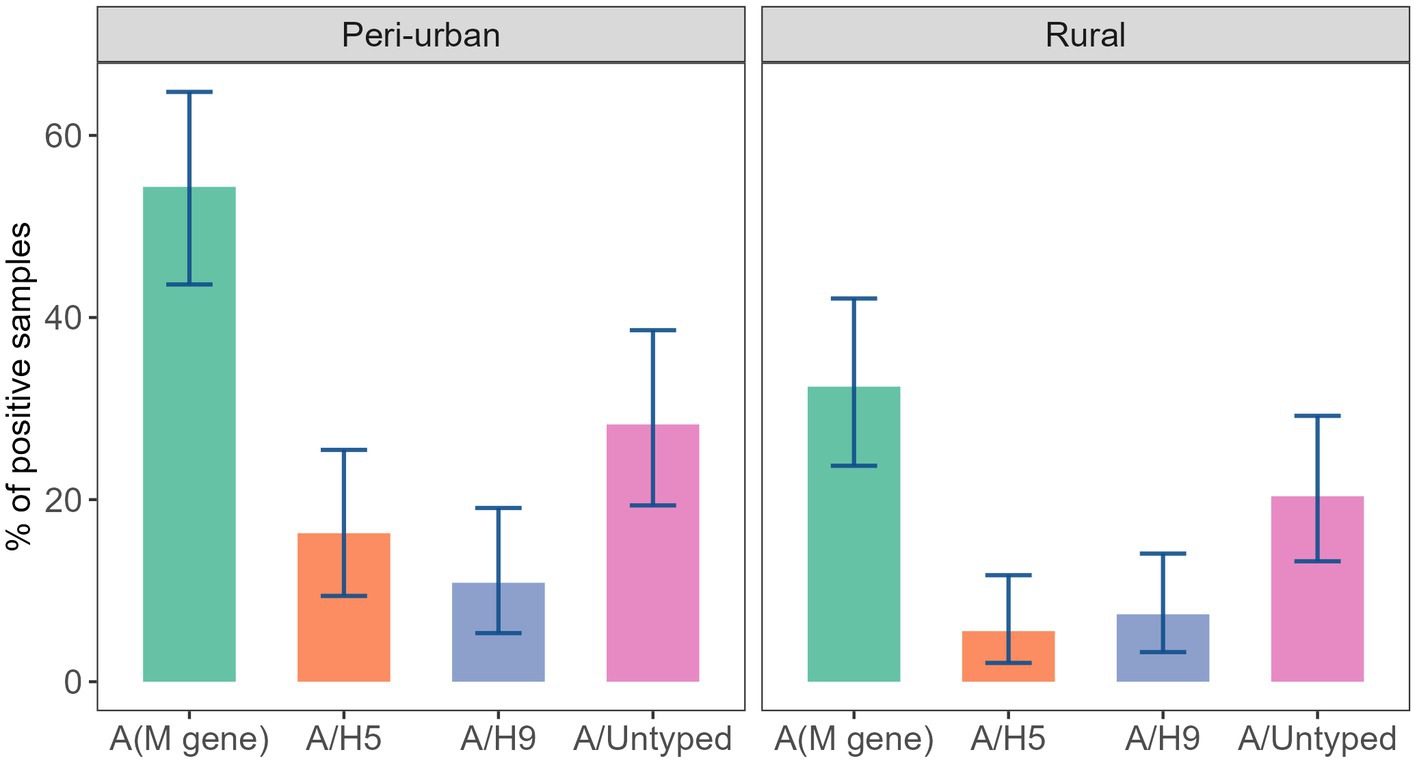
Figure 3. Prevalence of AIV subtypes in the stall across peri-urban and rural landscapes of studied LBMs.
3.2.3. Prevalence of AIV subtype by chicken strain
In our study, we sampled two types of chicken: Broiler and Sonali. On average, Sonali chickens were more positive for all subtypes compared to broiler chickens. Likewise, overall prevalence of AIV was significantly higher in the Sonali strain (55.26%; 95% CI: 43.41–66.69) with a value of p less than 0.01. HA/untyped was also more significantly associated with the Sonali strain (p = 0.03), (32.89%; 95% CI: 22.54–44.63; Figure 4).
3.2.4. Prevalence of AIV subtypes in fecal and offal samples
In total, we collected 139 pooled fecal swabs throughout our study. Of these samples, 50 tested AIV positive (35.97%; 95% CI: 28.01–44.54). We also collected a total of 61 offal swabs, 35 of which tested AIV positive (57.38%; 95% CI: 44.06–69.96) (p < 0.01). Detection of A/H5 and A/H9 was more common in offal samples (57.38%) than fecal (Figure 5). Additionally, the prevalence of A/untyped was significantly higher (36.07; 95% CI: 24.16–49.37) in the offal sample (p = 0.01; Figure 5).
3.3. Stall-level association between biosecurity practices and AIV circulation
3.3.1. Factors associated with AIV circulation using univariable analysis (results from Pearson’s chi-square test)
We considered 21 stall-level variables related to hygiene and sanitation practices that could be associated with AIV contamination, circulation, and persistence in LBMs. We considered a stall as AIV positive if any of the samples collected from the stall tested positive for AIV or any of the subtypes. We then extracted 21 variables related to biosecurity from the questionnaire to analyze for association with AIV positivity at the stall-level. We found that 13 variables were significantly associated with detection of AIV at a 5% significance level in the univariate analysis. The landscape (rural vs. peri-urban) was significantly associated with AIV prevalence (Figure 3; Table 2). Stalls with a single chicken breed had a lower prevalence (28.74%; p < 0.01) compared to stall with more than one breed. Likewise, stalls that sold ducks and chickens were more positively associated with AIV (68.60%; p = 0.03). We detected a significant difference in prevalence of AIV for the stalls that kept birds on the floor compared to a cage (48.09%; p = 0.04), as well as for stalls that used bamboo boundaries compared to brick (49.09%; p = 0.05). AIV prevalence was also significantly higher among stalls in which the vendor answered yes to purchasing their birds from a middleman (57.66%; p < 0.01), and to disposing of their waste or dead birds in an open place (57.01%; p < 0.01). Vendors who used water instead of detergent as a cleaning agent had a significantly higher prevalence (54.41%; p < 0.01). Stalls with unsold birds that remained overnight had a significantly higher prevalence (55.29%; p = 0.01) of AIV. Vendors that did not separate their sick birds from their healthy birds (59.62%; p < 0.01), did not prevent dogs from accessing the stall (55.88; p < 0.01), or wild birds from accessing the stall (58.33%; p < 0.01) had a higher prevalence (Table 2).
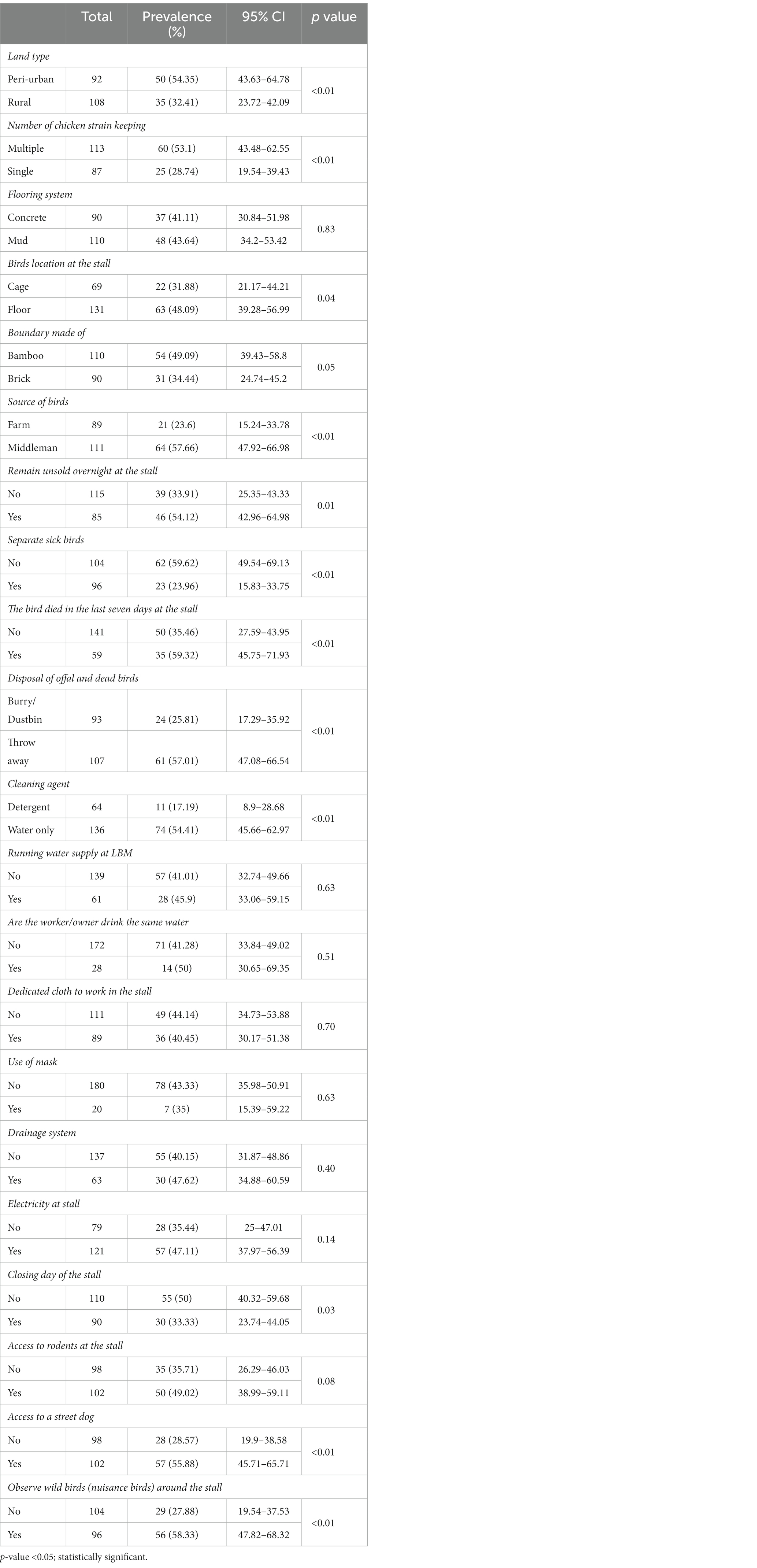
Table 2. Univariable analysis of factors to check association with AIV circulation (results from Pearson’s chi-square test).
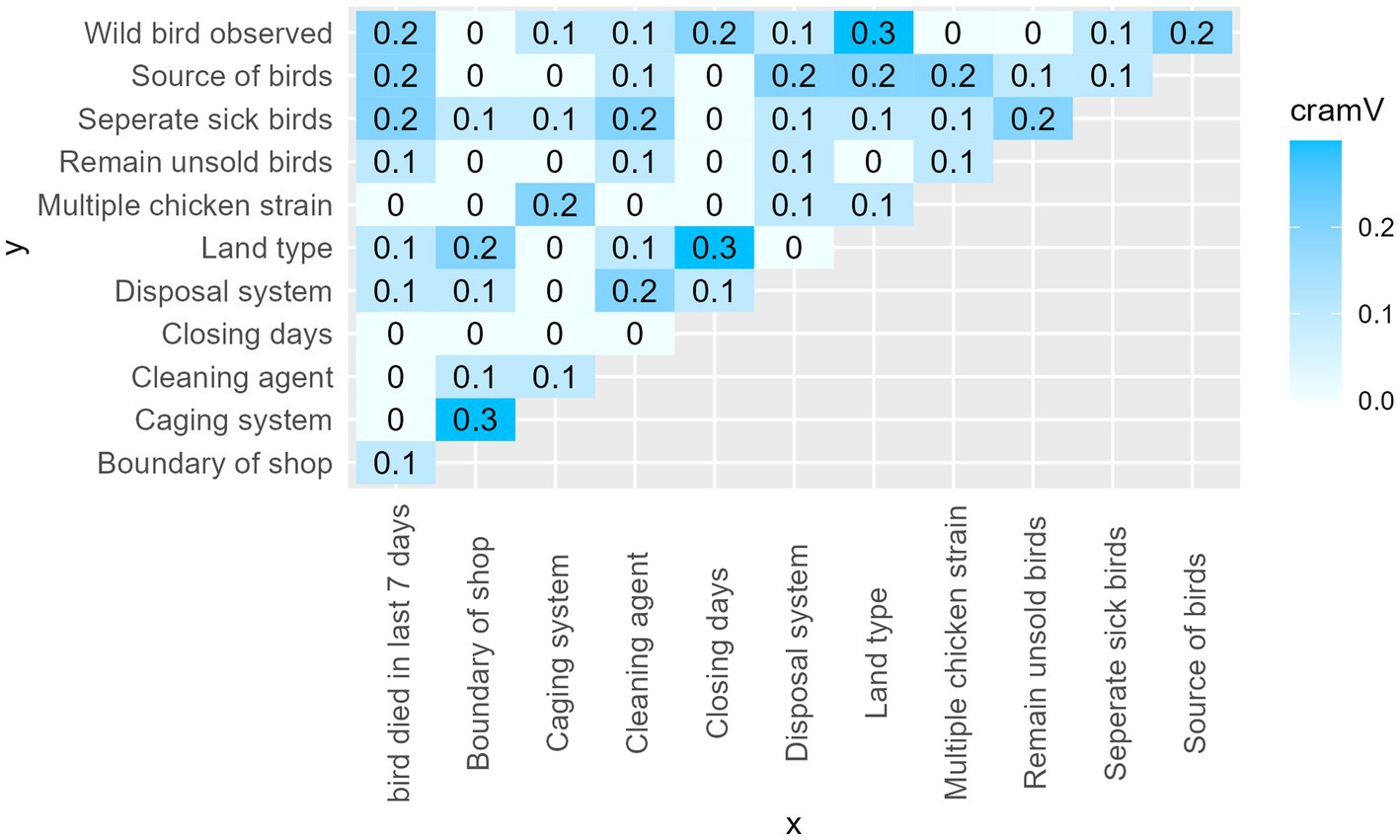
3.3.2. Matrix of Cramer’s V to check for multicollinearity
Cramer’s V measures the strength of an association between two variables. The coefficient ranges from 0 to 1. Where 0 means no association and 1 means perfect association. A value greater than 0.5 is considered a strong correlation between two variables (45). Figure 6 represents the matrix of values for Cramer’s V between the explanatory variables. There were no pairs of variables with a Cramer’s V value above our cut off point (0.5).
3.3.3. Multivariable modelling using a generalized linear mixed model
We conducted a GLMM with the variables found to be significant in the univariable analysis (Table 3). We included market as a random effect in our mixed-effect model since stalls were clustered by LBM. Poultry stalls in the peri-urban LBMs were at 3.02 times (95% CI: 1.18–7.72, p = 0.02) higher odds of AIV detection than the rural LBMs. The odds of AIV detection were 2.40 times higher for stalls with multiple chicken breeds (95% CI: 1.01–5.67, p = 0.05) compared to stalls with only one breed. The source of the birds was also found to be asscociated with AIV detection, in our model, with a middleman source at 2.35 higher odds compared to commercial farms (95% CI: 1.0–5.53, p = 0.05). Stalls where vendors did not separate their sick birds were at 3.04 times (95% CI: 1.34–6.92, p = 0.01) higher risk of infecting with AIV. Vendors who discard their waste and dead birds in open places rather than in dustbins had stall with 3.16 times (95% CI: 1.41–7.05, p < 0.01) higher risk of AIV contamination at the LBM surface. Most notably, vendors who did not use disinfectant to clean stall surfaces had 5.99 times (95% CI: 2.26–15.82, p < 0.01) higher odds of AIV detection. Lastly, we found 2.52 times (95% CI: 1.12–5.70, p = 0.03) higher risk of AIV where dogs had access to stalls and 2.31 times (95% CI: 1.01–5.30, p = 0.05) higher risk where vendors observed wild bird around their stalls (Table 3).
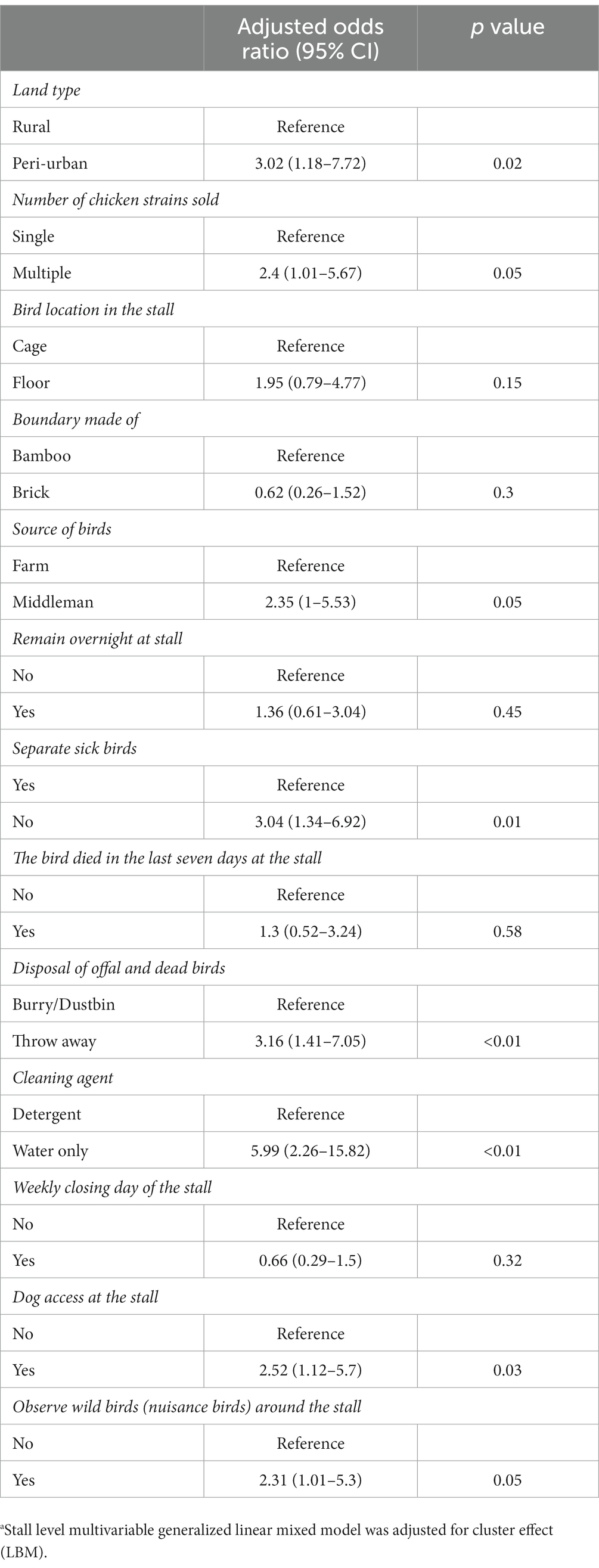
Table 3. Stall level generalized linear mixed model (GLMM) model of bio-security practices and AIV circulation in peri-urban and rural LBM.a
4. Discussion
AIV is a public health concern in the countries like Bangladesh, where people and poultry are in frequent contact without adequate biosecurity measures in place (32). LBMs are a significant source of AIV circulation, mutation, and spillover to humans or other wildlife. Over the past years, several studies have demonstrated a rising trend in AIV circulation among LBMs in Bangladesh (4, 46). However, these studies have almost exclusively targeted urban settings (7, 14, 22, 30). When we conducted our study from 2017 to 2018, only 36.63% of the total population of Bangladesh lived in urban areas, with the majority residing in peri-urban and rural areas (47). Furthermore, meat production and consumption has drastically increased in response to the growing density of the population over the years (48). As a result, there are now many more LBMs and commercial farms in rural and peri-urban areas. To address this critical gap in the research, we set out to investigate risk factors of AIV in peri-urban and rural LBMs in Bangladesh.
At the market-level, the overall prevalence of AIV was 82.54% (95%CI: 70.90–90.95). This is higher than the values reported by Sayeed et al. (31) in the Chittagong Metropolitan Area, where they detected an overall prevalence of 40% (95% CI: 20–60%; N = 40). This value is also higher than the measures of AIV prevalence reported in LBM studies outside of Bangladesh, in China and Indonesia specifically (49). Findings from this study indicate a higher prevalence of AIV in LBMs in peri-urban and rural areas in Bangladesh compared to previous studies conducted in urban areas of Bangladesh as well as in other Asian countries.
We detected AIV subtypes A/H5, A/H9, and HA/Untyped in at leasat one of the LBMs included within our study. We also found evidence of co-infection with A/H5 and A/H9 at the LBM level. Similar findings have been reported by other studies conducted at urban LMBs in Bangladesh (7, 30, 50). At the stall-level, 40.5% of the stalls included in our study tested positive for AIV, which is higher than in previous research conducted in Dhaka (24%), Chattogram (20.3%), and in a country-wide estimate (26%) (6, 30, 31). Our detected prevalence is also higher than in other countries in Asia (51–53).
4.1. Physiographic and hygienic status of poultry stalls in the LBMs
Our findings provide detailed biosecurity and hygienic practices at peri-urban and rural LBMs, which have previously been unexplored for AIV. We found that most vendors mixed multiple breeds of chicken in their stalls, and nearly 1/5 of vendors used the same cage for chickens and ducks. Birds were frequently kept on the floor, and remained overnight if unsold. Many vendors did not separate their sick birds from their healthy birds or dispose of their biological waste properly, often discarding in open places. A limited number of vendors used detergent to clean their stall surfaces or utensils. They did not have dedicated clothes for their daily activities, and in some cases, there was no drainage system at all. Rodents and dogs can freely enter the stalls in most markets, and vendors noted the recurring presence of wild birds. Studies conducted by Chowdhury, Azziz-Baumgartner (30) and Sayeed, Smallwood (31) noted similar conditions among urban LBMs in Bangladesh.
4.2. Risk factors associated with the circulation of AIV in the stall of the peri-urban and rural areas
The odds of AIV infection in poultry are three times higher in peri-urban LBMs compared to rural LBMs in our study. These odds are twice as high as those found in a similar study conducted in Vietnam, which concluded that peri-urban areas had a 1.5 times higher risk of AIV than rural areas (54). With the population density in peri-urban areas, they need more nutrition, and one of the primary sources is poultry. To meet the growing demand, vendors collect their birds from inter-district or middlemen, where rural LBMs can cover the demand from nearby or backyard farms (52). Moreover, higher poultry density implies higher risk, and proximity to the highway increases the possibility of trading poultry from distant areas, which could increase the spread of AIV in peri-urban settings (54, 55).
Selling more than one breed of poultry within the stall was found to be significantly associated with AIV infection, which is aligned with the findings of Chowdhury et al. (30). Keeping multiple poultry species provides a suitable environment for effectively transmitting and amplifying AIV and allows it to spread over a large geographic area (50). In our study, birds kept on the floor rather than in cages were at greater risk of AIV. A study in Pakistan also found that keeping birds outside cages was a risk factor (56). When birds move openly on the floor, they keep in touch with the same surface that could be infected by the other sick birds (57). Additionally, birds on the floor are more likely to interact with terrestrial wild birds (58). The virus is less likely to spread in cages by keeping the birds isolated. Also, the layer of caging restricts feces and utensils from getting everywhere (59).
The source of birds is also considered a risk factor for AIV. Most vendors in our study collect their birds from a middleman, so there is no information on the conditions of transport or storage prior to purchase. As a result, birds purchased via middlemen could have been exposed to conditions which are conducive to virus transmission (51). A study in Vietnam showed that trading live birds from different sources is associated with reduced biosecurity and consequently, higher viral transmission (60). Keeping sick birds in contact with healthy ones was found to be a risk factor for AIV in our study. This is consistent with findings from a similar study in Uganda (61). The Food and Agriculture Organization (FAO) of the United Nations recommends separating sick and healthy birds as a requirement for biosecurity in LBMs (62). In peri-urban and rural areas, vendors usually throw away biological waste in the nearby pit or drain around the stall and market (63). Improper waste disposal management can facilitate opportunites for environmental exposure to AIV (64). If offal and dead birds are discarded in open areas, rodents and other birds (e.g., crows) can easily reach them, causing a significant risk for healthy birds (61).
Of all the risk factors examined in our study, the cleaning agent was found to be the most strongly associated with AIV positivity. If vendors used only water, compared to detergent/disinfectant, they had much higher odds of exposure to AIV. Detergent is essential to inactive the virus, whereas water cannot disinfect the surface properly (65). Detergent has been demonstrated to effectively inactivate AIV from wood, tiles, and hard surfaces (66). Conversely, previous studies have showed that water could not adequately eliminate AIV contamination (67). If stalls are easily accessible to dogs and wild birds, they may provide an ideal environment for virus spillover. Stray dogs and wild birds frequently eat offal or dead birds that might be infected with AIV, and they could travel to other stalls or LBMs, which threatens market biosecurity (68, 69). Several studies found unusual crow die-off events in Bangladesh due to AIV, and LBMs were considered a primary infection source (10, 12, 61). In contrast to urban areas, dogs and wild birds have easy access to stalls because most markets have no boundaries. As a result, dogs and wild birds can be carriers of AIV, and we should take steps to prevent stray dogs and wild birds from entering LBMs.
5. Conclusion
Our study demonstrates a high prevalence of AIV, with evidence of subtype H5 and H9 circulating in peri-urban and rural LBMs in Bangladesh. We identified several unhygienic practices and factors associated with detection of AIV that should be considered for future interventions or educational materials in LBMs. Most stall owners are unaware of the risks associated with mixing species, choice of caging, inadequate waste disposal, and improper disinfection. Viral transmission often goes unnoticed in LBM settings in peri-urban and rural areas, so many vendors lack a tangible motive to improve biosafety protocols. Based on the findings presented here, LBMs demonstrate inadequate safety measures to prevent viral transmission, particularly in peri-urban and rural settings. We recommend continuous awareness building and monitoring of hygienic practices at LBMs in the peri-urban and rural areas to prevent the spread of AIV to poultry, people, and wildlife in Bangladesh.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by the Institutional Ethics Committee of the Institute of Epidemiology Disease Control and Research (IEDCR/IRB/2015/04). The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Chattogram Veterinary and Animal Sciences University-Animal Experimentation Ethics Committee (protocol: CVASU/Dir (R&E) AEEC/2015/751).
Author contributions
AI and JHE: conceptualization. AI, SI, and MKR: methodology. AI and MI: software, formal analysis, visualization, and writing—original draft preparation. AI, SI, MI, and MMH: validation. AI, MKR, and MAS: investigation. MZR, AI, and MS: resources. AI, MKR, SI, and MI: data curation. AI, SI, MKR, SM, MAS, TS, and MMH: writing—review and editing. MZR, MEH, and MAS: laboratory support. TS, MSF and JHE: supervision. MSF, TS, JHE, and MZR: project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT (cooperative agreement number AID-OAA-A-14-00102).
Acknowledgments
The authors acknowledge the support of the Institute of Epidemiology, Disease Control, and Research (IEDCR) in Bangladesh and the EcoHealth Alliance in conducting this study. The authors also thank Bangladesh Livestock Research Institute (BLRI), and International Centre for Diarrheal Disease Research, Bangladesh (icddr,b), for their support in testing samples for avian influenza. The sample collection was partially supported by the Emerging Pandemic Threats PREDICT project (cooperative agreement number AID-OAA-A-14-00102) through EcoHealth Alliance and IEDCR. The authors thank the governments of Bangladesh, Canada, Sweden, and the United Kingdom for providing core/unrestricted support to icddr, b. The authors also acknowledge the Conservation, Food and Health Foundation(CFHF) for covering the article processing charge.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Kanaujia, R, Bora, I, Ratho, RK, Thakur, V, Mohi, GK, and Thakur, P. Avian influenza revisited: Concerns and constraints. Virus Disease. (2022) 33:456–65. doi: 10.1007/s13337-022-00800-z
2. Shanmuganatham, K, Feeroz, MM, Jones-Engel, L, Walker, D, Alam, S, Hasan, M, et al. Genesis of avian influenza H9N2 in Bangladesh. Emerg Microbes Infect. (2014) 3:e88. doi: 10.1038/emi.2014.84
3. Ansari, WK, Parvej, MS, El Zowalaty, ME, Jackson, S, Bustin, SA, Ibrahim, AK, et al. Surveillance, epidemiological, and virological detection of highly pathogenic H5N1 avian influenza viruses in duck and poultry from Bangladesh. Vet Microbiol. (2016) 193:49–59. doi: 10.1016/j.vetmic.2016.07.025
4. Islam, A, Islam, S, Amin, E, Hasan, R, Hassan, MM, Miah, M, et al. Patterns and risk factors of avian influenza a (H5) and a (H9) virus infection in pigeons and quail at live bird markets in Bangladesh, 2017–2021. Front Vet Sci. (2022) 9:1016970. doi: 10.3389/fvets.2022.1016970
5. Herfst, S, Imai, M, Kawaoka, Y, and Fouchier, R. Avian influenza virus transmission to mammals. Curr Top Microbiol Immunol. (2014) 385:137–55. doi: 10.1007/82_2014_387
6. Turner, JC, Feeroz, MM, Hasan, MK, Akhtar, S, Walker, D, Seiler, P, et al. Insight into live bird markets of Bangladesh: An overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg Microbes Infect. (2017) 6:e12. doi: 10.1038/emi.2016.142
7. Kim, Y, Biswas, PK, Giasuddin, M, Hasan, M, Mahmud, R, Chang, YM, et al. Prevalence of avian influenza a (H5) and a (H9) viruses in live bird markets, Bangladesh. Emerg Infect Dis. (2018) 24:2309–16. doi: 10.3201/eid2412.180879
8. Uyeki, TM, and Peiris, M. Novel avian influenza a virus infections of humans. Infect Dis Clin. (2019) 33:907–32. doi: 10.1016/j.idc.2019.07.003
9. WHO Cumulative number of confirmed human cases for avian influenza A (H5N1) reported to WHO, 2003-2022. (2022). Available at: https://cdn.who.int/media/docs/default-source/influenza/human-animal-interface-risk-assessments/
10. Islam, A, Islam, S, Samad, M, Hossain, M, Hassan, M, Alexandersen, S, et al. Epidemiology and molecular characterization of multiple avian influenza a/H5 subtypes circulating in house crow (Corvus splendens) and poultry in Bangladesh. Int J Infect Dis. (2022) 116:S92–3. doi: 10.1016/j.ijid.2021.12.218
11. Hassan, MM, El Zowalaty, ME, Islam, A, Rahman, MM, Chowdhury, MN, Nine, HS, et al. Serological evidence of avian influenza in captive wild birds in a zoo and two safari parks in Bangladesh. Vet Sci. (2020) 7:122. doi: 10.3390/vetsci7030122
12. Islam, A, Hossain, M, Islam, S, Samad, M, Rahman, M, Chowdhury, M, et al. Detection and genetic characterization of avian influenza a (H5N6) virus clade 2.3.4.4 in isolates from house crow and poultry in Bangladesh, 2017. Int J Infect Dis. (2020) 101:339–40. doi: 10.1016/j.ijid.2020.09.894
13. Chowdhury, M, Islam, S, Hossain, M, Rahman, M, Nine, HZ, Sadik, A, et al. Detection of influenza a and adenovirus in captive wild birds in Bangladesh. Int J Infect Dis. (2020) 101:229. doi: 10.1016/j.ijid.2020.11.034
14. Chakma, S, Osmani, MG, Akwar, H, Hasan, Z, Nasrin, T, Karim, MR, et al. Risk areas for influenza a (H5) environmental contamination in live bird markets, Dhaka, Bangladesh. Emerg Infect Dis. (2021) 27:2399–408. doi: 10.3201/eid2709.204447
15. Soares Magalhães, RJ, Ortiz-Pelaez, A, Thi, KLL, Dinh, QH, Otte, J, and Pfeiffer, DU. Associations between attributes of live poultry trade and HPAI H5N1 outbreaks: a descriptive and network analysis study in northern Vietnam. BMC Vet Res. (2010) 6:1–13. doi: 10.1186/1746-6148-6-10
16. Rahman, M, Mangtani, P, Uyeki, TM, Cardwell, JM, Torremorell, M, Islam, A, et al. Evaluation of potential risk of transmission of avian influenza a viruses at live bird markets in response to unusual crow die-offs in Bangladesh. Influenza Other Respir Viruses. (2020) 14:349–52. doi: 10.1111/irv.12716
17. Le, KT, Stevenson, MA, Isoda, N, Nguyen, LT, Chu, DH, Nguyen, TN, et al. A systematic approach to illuminate a new hot spot of avian influenza virus circulation in South Vietnam, 2016–2017. Transbound Emerg Dis. (2022) 69:e831–44. doi: 10.1111/tbed.14380
18. Nasreen, S, Khan, SU, Luby, SP, Gurley, ES, Abedin, J, Zaman, RU, et al. Highly pathogenic avian influenza a (H5N1) virus infection among workers at live bird markets, Bangladesh, 2009–2010. Emerg Infect Dis. (2015) 21:629–37. doi: 10.3201/eid2104.141281
19. Youk, S-s, Lee, D-H, Jeong, J-H, Pantin-Jackwood, MJ, Song, C-s, and Swayne, DE. Live bird markets as evolutionary epicentres of H9N2 low pathogenicity avian influenza viruses in Korea. Emerg Microbes Infect. (2020) 9:616–27. doi: 10.1080/22221751.2020.1738903
20. Jagne, JF, Bennett, J, and Collins, E. Live bird Markets of the Northeastern United States. Dela J Public Health. (2021) 7:52–6. doi: 10.32481/djph.2021.01.009
21. Dharmayanti, NLPI, Hewajuli, DA, Ratnawati, A, and Hartawan, R. Genetic diversity of the H5N1 viruses in live bird markets, Indonesia. J Vet Sci. (2020) 21:e56. doi: 10.4142/jvs.2020.21.e56
22. Hassan, MM, Hoque, MA, Ujvari, B, and Klaassen, M. Live bird markets in Bangladesh as a potentially important source for avian influenza virus transmission. Prev Vet Med. (2018) 156:22–7. doi: 10.1016/j.prevetmed.2018.05.003
23. Bank, W Total Population of Bangladesh (2022). Available at: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=BD
24. Hennessey, M, Fournié, G, Hoque, MA, Biswas, PK, Alarcon, P, Ebata, A, et al. Intensification of fragility: Poultry production and distribution in Bangladesh and its implications for disease risk. Prev Vet Med. (2021) 191:105367. doi: 10.1016/j.prevetmed.2021.105367
25. DLS (Department of Livestock Services). Livestock Economy at a glance, 2020-2021. (2021). Available at: http://www.dls.gov.bd/
26. Abu Hatab, A, Cavinato, MER, and Lagerkvist, CJ. Urbanization, livestock systems and food security in developing countries: A systematic review of the literature. Food Secur. (2019) 11:279–99. doi: 10.1007/s12571-019-00906-1
27. Sultana, R, Nahar, N, Rimi, NA, Azad, S, Islam, MS, Gurley, ES, et al. Backyard poultry raising in Bangladesh: A valued resource for the villagers and a setting for zoonotic transmission of avian influenza. A qualitative study. Rural Remote Health. (2012) 12:1–14.
28. Selvanathan, EA, Jayasinghe, M, Hossain, MM, and Selvanathan, S. Modelling the Demand for Meat in Bangladesh. Science and Technology Innovation for a Sustainable Economy. Heidelberg: Springer; (2020). p. 135–151.
29. Berry, I, Rahman, M, Flora, MS, Greer, AL, Morris, SK, Khan, IA, et al. Frequency and patterns of exposure to live poultry and the potential risk of avian influenza transmission to humans in urban Bangladesh. Sci Rep. (2021) 11:1–10. doi: 10.1038/s41598-021-01327-x
30. Chowdhury, S, Azziz-Baumgartner, E, Kile, JC, Hoque, MA, Rahman, MZ, Hossain, ME, et al. association of biosecurity and hygiene practices with environmental contamination with influenza a viruses in live bird markets, Bangladesh. Emerg Infect Dis. (2020) 26:2087–96. doi: 10.3201/eid2609.191029
31. Sayeed, MA, Smallwood, C, Imam, T, Mahmud, R, Hasan, RB, Hasan, M, et al. Assessment of hygienic conditions of live bird markets on avian influenza in Chittagong metro, Bangladesh. Prev Vet Med. (2017) 142:7–15. doi: 10.1016/j.prevetmed.2017.04.009
32. Islam, A, Islam, S, Amin, E, Shano, S, Samad, MA, Shirin, T, et al. Assessment of poultry rearing practices and risk factors of H5N1 and H9N2 virus circulating among backyard chickens and ducks in rural communities. PloS one. (2022) 17:e0275852. doi: 10.1371/journal.pone.0275852
33. Bangladesh. Bangladesh National Portal. (2023). Available at: http://www.bangladesh.gov.bd
34. Rai, N, and Thapa, B. A Study on Purposive Sampling Method in Research. Kathmandu: Kathmandu School of Law (2015).
35. Irin, N, Dilshad, SM, Al Sattar, A, Chisty, NN, Sultana, A, Hasan, M, et al. Live bird market in Bangladesh: Regulatory systems and operations. J Adv Vet Anim Res. (2021) 8:671–8. doi: 10.5455/javar.2021.h559
36. Islam, MA, Haque, A, and Nishibori, M. Growth performance, meat yield and blood lipid profile of broiler and Sonali chickens. Vet Anim Sci. (2022) 18:100272. doi: 10.1016/j.vas.2022.100272
37. Control CfD, Prevention. CDC Laboratory Support for Influenza Surveillance (CLSIS). Atlanta, GA: Centers for Disease Control and Prevention (2013).
38. Spackman, E. Avian Influenza Virus Detection and Quantitation by Real-Time RT-PCR: Animal Influenza Virus. Heidelberg: Springer (2014).
39. Ali, MZ, Hasan, M, and Giasuddin, M. Potential risk factors of avian influenza virus infection in asymptomatic commercial chicken flocks in selected areas of Bangladesh during 2019. J Adv Vet Anim Res. (2021) 8:51–7. doi: 10.5455/javar.2021.h484
40. Hernandez-Medrano, JH, Espinosa-Castillo, LF, Rodriguez, AD, Gutierrez, CG, and Wapenaar, W. Use of pooled serum samples to assess herd disease status using commercially available ELISAs. Trop Anim Health Prod. (2021) 53:1–10. doi: 10.1007/s11250-021-02939-1
41. Zhang, B, Bilder, CR, and Tebbs, JM. Group testing regression model estimation when case identification is a goal. Biom J. (2013) 55:173–89. doi: 10.1002/bimj.201200168
42. Deniz, ED. A Tool for Selecting Suitable Software Project Effort Estimation Model at Early Phases. Ankara, Türkiye: Hacettepe University (2021).
43. Hox, JJ, Moerbeek, M, and Van de Schoot, R. Multilevel Analysis: Techniques and Applications. England: Routledge (2017).
44. Team, RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2013).
46. Rimi, NA, Hassan, M, Chowdhury, S, Rahman, M, Sultana, R, Biswas, PK, et al. A decade of avian influenza in Bangladesh: Where are we now? Trop Med Infect Dis. (2019) 4:119. doi: 10.3390/tropicalmed4030119
47. Statista (2022). cited 2022. Available at: https://www.statista.com/statistics/761021/share-of-urban-population-bangladesh/
48. Rahman, MS, Jang, D-H, and Yu, C-J. Poultry industry of Bangladesh: entering a new phase. Korean J Agric Sci. (2017) 44:272–82. doi: 10.7744/kjoas.20170027
49. Bi, Y, Chen, Q, Wang, Q, Chen, J, Jin, T, Wong, G, et al. Genesis, evolution and prevalence of H5N6 avian influenza viruses in China. Cell Host Microbe. (2016) 20:810–21. doi: 10.1016/j.chom.2016.10.022
50. Khan, SU, Gurley, ES, Gerloff, N, Rahman, MZ, Simpson, N, Rahman, M, et al. Avian influenza surveillance in domestic waterfowl and environment of live bird markets in Bangladesh, 2007–2012. Sci Rep. (2018) 8:1–10. doi: 10.1038/s41598-018-27515-w
51. Sealy, JE, Fournie, G, Trang, PH, Dang, NH, Sadeyen, JR, Thanh, TL, et al. Poultry trading behaviours in Vietnamese live bird markets as risk factors for avian influenza infection in chickens. Transbound Emerg Dis. (2019) 66:2507–16. doi: 10.1111/tbed.13308
52. Lee, E-K, Kang, H-M, Song, B-M, Lee, Y-N, Heo, G-B, Lee, H-S, et al. Surveillance of avian influenza viruses in South Korea between 2012 and 2014. Virol J. (2017) 14:54. doi: 10.1186/s12985-017-0711-y
53. Zhao, G, Pan, J, Gu, X, Zhao, K, Chen, H, Chen, S, et al. Prevalence of low pathogenic avian influenza virus in one live bird market in eastern China from 2002-2011. Avian Dis. (2013) 57:155–8. doi: 10.1637/10293-062812-Case.1
54. Sumeet, S, Nong, HD, Finucane, M, Spencer, JH, Tran, CC, and Fox, J. Does Unplanned Urbanization Pose a Disease Risk in Asia? The Case of Avian Influenza in Vietnam. Honolulu, HI: East-West Center (2017).
55. Saksena, S, Fox, J, Epprecht, M, Tran, CC, Nong, DH, Spencer, JH, et al. Evidence for the convergence model: the emergence of highly pathogenic avian influenza (H5N1) in Viet Nam. PLoS One. (2015) 10:e0138138. doi: 10.1371/journal.pone.0138138
56. Chaudhry, M, Rashid, HB, Angot, A, Thrusfield, M, Bronsvoort, BMD, Capua, I, et al. Risk factors for avian influenza H9 infection of chickens in live bird retail stalls of Lahore District, Pakistan 2009-2010. Sci Rep. (2018) 8:5634. doi: 10.1038/s41598-018-23895-1
57. Collett, SR, Smith, JA, Boulianne, M, Owen, RL, Gingerich, E, Singer, RS, et al. Principles of disease prevention, diagnosis, and control. Dis. Poult. (2020):1–78. doi: 10.1002/9781119371199.ch1
58. Shriner, SA, and Root, JJ. A review of avian influenza a virus associations in synanthropic birds. Viruses. (2020) 12:1209. doi: 10.3390/v12111209
59. Hartcher, KM, and Jones, B. The welfare of layer hens in cage and cage-free housing systems. Worlds Poult Sci J. (2017) 73:767–82. doi: 10.1017/S0043933917000812
60. Fournie, G, Tripodi, A, Nguyen, TT, Nguyen, VT, Tran, TT, Bisson, A, et al. Investigating poultry trade patterns to guide avian influenza surveillance and control: a case study in Vietnam. Sci Rep. (2016) 6:29463. doi: 10.1038/srep29463
61. Islam, A, Islam, S, Hossain, M, Samad, M, Billah, M, Hassan, M, et al. One health investigation of house crow (Corvus splendens) mortality event linked to the potential circulation of H5N1 virus at live bird Markets in Northwestern Bangladesh. Int J Infect Dis. (2022) 116:S112. doi: 10.1016/j.ijid.2021.12.265
62. Fao, O. Biosecurity for Highly Pathogenic Avian Influenza: Issues and Options. Italy: FAO Rome (2008).
63. Alam, M-U, Rahman, M, Islam, MA, Asaduzzaman, M, Sarker, S, Rousham, E, et al. Human exposure to antimicrobial resistance from poultry production: Assessing hygiene and waste-disposal practices in Bangladesh. Int J Hyg Environ Health. (2019) 222:1068–76. doi: 10.1016/j.ijheh.2019.07.007
64. He, F, Chen, E-F, Li, F-D, Wang, X-Y, Wang, X-X, and Lin, J-F. Human infection and environmental contamination with avian influenza a (H7N9) virus in Zhejiang Province, China: Risk trend across the three waves of infection. BMC Public Health. (2015) 15:1–10. doi: 10.1186/s12889-015-2278-0
65. Elveborg, S, Monteil, VM, and Mirazimi, A. Methods of inactivation of highly pathogenic viruses for molecular, serology or vaccine development purposes. Pathogens. (2022) 11:271. doi: 10.3390/pathogens11020271
66. Lombardi, M, Ladman, B, Alphin, R, and Benson, E. Inactivation of avian influenza virus using common detergents and chemicals. Avian Dis. (2008) 52:118–23. doi: 10.1637/8055-070907-Reg
67. Ahrens, AK, Selinka, H-C, Mettenleiter, TC, Beer, M, and Harder, TC. Exploring surface water as a transmission medium of avian influenza viruses–systematic infection studies in mallards. Emerg Microbes Infect. (2022) 11:1250–61. doi: 10.1080/22221751.2022.2065937
68. World Health Organization. A Key Role for Veterinary Authorities and Animal Health Practitioners in Preventing and Controlling Neglected Parasitic Zoonoses: A Handbook with Focus on Taenia Solium, Trichinella, Echinococcus and Fasciola. Rome: Food & Agriculture Organization (2021).
Keywords: avian influenza virus, live bird markets, landscape, environmental contamination, biosecurity practices, risk factors, zoonoses, Bangladesh
Citation: Islam A, Islam S, Islam M, Hossain ME, Munro S, Samad MA, Rahman MK, Shirin T, Flora MS, Hassan MM, Rahman MZ and Epstein JH (2023) Prevalence and risk factors for avian influenza virus (H5 and H9) contamination in peri-urban and rural live bird markets in Bangladesh. Front. Public Health. 11:1148994. doi: 10.3389/fpubh.2023.1148994
Edited by:
Balbir Bagicha Singh, Guru Angad Dev Veterinary and Animal Sciences University, IndiaReviewed by:
Susan Catherine Cork, University of Calgary, CanadaDeepak Chandran, Amrita Vishwa Vidyapeetham University, India
Copyright © 2023 Islam, Islam, Islam, Hossain, Munro, Samad, Rahman, Shirin, Flora, Hassan, Rahman and Epstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ariful Islam, YXJpZkBlY29oZWFsdGhhbGxpYW5jZS5vcmc=
 Ariful Islam
Ariful Islam Shariful Islam
Shariful Islam Monjurul Islam
Monjurul Islam Mohammad Enayet Hossain
Mohammad Enayet Hossain Sarah Munro
Sarah Munro Mohammed Abdus Samad
Mohammed Abdus Samad Md. Kaisar Rahman
Md. Kaisar Rahman Tahmina Shirin3
Tahmina Shirin3 Meerjady Sabrina Flora
Meerjady Sabrina Flora Mohammad Mahmudul Hassan
Mohammad Mahmudul Hassan Mohammed Ziaur Rahman
Mohammed Ziaur Rahman Jonathan H. Epstein
Jonathan H. Epstein