- 1Department of Public Health, Faculty of Medicine, University of Toyama, Toyama, Japan
- 2Toyama Regional Center for JECS Study, Faculty of Medicine, University of Toyama, Toyama, Japan
- 3Department of Public Health, Gunma University Graduate School of Medicine, Maebashi, Japan
Background: Results on the association between fish intake during pregnancy and a reduction in neurodevelopmental delays in children have been inconsistent, with some reports finding an association and others finding none. Because neurodevelopmental delays are more pronounced at the age of 3 years, their association needs to be examined at this age.
Methods: After exclusion and multiple imputation from a dataset comprising 104,057 records from the Japan Environment and Children's Study, logistic regression analysis was conducted in quintiles to evaluate the association between maternal fish intake during pregnancy and child neurodevelopment at age 3 years in 91,909 mother–child pairs. The Food Frequency Questionnaire (FFQ), validated in the Japan Public Health Center-Based Prospective Study for the Next Generation, was used to assess maternal fish intake during pregnancy. The Ages and Stages Questionnaires-3 was used to assess children's neurodevelopment in five domains: communication, gross motor, fine motor, problem-solving, and personal-social.
Results: Consistently lower odds were found for the highest vs. lowest quintile for the domains of communication, fine motor, problem-solving, and personal-social but not gross motor skills, with adjusted odd ratios (95% confidence intervals) of 0.89 (0.80–0.998), 0.90 (0.83–0.97), 0.86 (0.80–0.94), 0.87 (0.77–0.98), and 1.04 (0.94–1.16), respectively. The trend for lower odds of symptoms of neurodevelopmental delays across quintiles of higher maternal fish intake were significant for fine motor, problem-solving, and personal-social but not communication or gross motor.
Conclusions: Fish consumption during pregnancy may be associated with a reduced risk of neurodevelopmental delay in 3-year-olds, particularly in the fine motor, problem-solving, and personal-social domains. Continued investigation after the age of 3 could further clarify the association.
1 Introduction
Maternal fish intake during pregnancy has been associated with fewer adverse birth events, especially preterm birth (1), and a lower risk of wheeze, eczema, and food allergy in offspring (2). On the other hand, the relationship between maternal fish intake during pregnancy and child neurodevelopment is controversial (3–6).
Studies linking maternal seafood intake to child neurodevelopment have reported that low maternal seafood intake increases the risk of suboptimal outcomes in children's prosocial behavior, fine motor, communication, and social development scores (3) and that maternal seafood intake during pregnancy is associated with better neurodevelopment at 14 months and 5 years of age (7). A large study of 10,026 mothers also reported that maternal omega-3 docosahexaenoic acid (DHA) intake during pregnancy was positively correlated with infant DHA status at 3 months of age and with problem-solving ability at 12 months of age, that children whose mothers had higher levels of DHA had better language development, and that the positive effects of omega-3 fatty acids in seafood and n-3 polyunsaturated fatty acid (PUFA) intake during pregnancy outweighed the negative effects of mercury (8). However, some studies have shown that seafood and n-3 PUFA intake during pregnancy is not associated with neurodevelopment in children, particularly studies conducted in Japan. For example, no significant association was found between total seafood intake during pregnancy and motor cluster scores on the Neonatal Behavior Rating Scale for 3-day-old infants (5) and no significant association was identified between maternal n-3 PUFA fatty acid intake during pregnancy and development of attention-deficit/hyperactivity disorder (ADHD) at age 5 years (6).
Previously, we hypothesized that fish consumption by mothers during pregnancy might enhance psychomotor development in their children, and we examined this association in children at 6 months and 1 year of age (4). Using data from the Japan Environment and Children's Study (JECS), a nationwide survey, we assessed 81,697 and 77,751 mother–child pairs at 6 months and 1 year of age, respectively. The results showed that maternal fish intake during pregnancy was independently associated with a reduced risk of delay in problem-solving skills at 6 months of age and in fine motor and problem-solving skills at 1 year of age.
The inconsistency in results thus far may have been influenced by regional and cultural differences in fish consumption. For example, in a Japanese study, Miyake et al. (6) suggested that the reason they did not find an association between maternal eicosapentaenoic acid (EPA) or DHA intake and child behavioral problems was their study population lived in areas with high fish consumption and speculated that an association might be found in populations with low fish consumption. In our previous study, the median intake of fish by pregnant mothers was 29.9 g/day (4), which is low compared with studies reporting no association (5, 9). Given the declining trend in fish consumption in the Japanese population (10), it is important to study the long-term effects of mothers' fish consumption during pregnancy on their children's neurodevelopment in large populations.
It has been reported that developmental delays are innate (11) and gradually become more pronounced by the age of 3 years (12). Specifically, by that age, children are able to use the fingers of both hands and are able to understand the meaning of words and express their intentions (13). In addition, the ability to decide on one's own actions nears completion at the age of 3–4 years (13). For this reason, in Japan, mental developmental tests have been introduced into examinations in early childhood (14), and the importance of the early detection and follow-up of developmental delays during limited screening has been reported (15). In particular, the age between 3 and 4 years is expected to be a particularly important developmental stage because of the increase in social activities preceding compulsory education, such as kindergarten attendance.
Therefore, the purpose of this study was to clarify the relationship between fish intake during pregnancy and neurodevelopment at age 3 years and also to focus on the neurodevelopmental associations observed at 6 months and 1 year of age and those observed at age 3 years in a large nationwide population.
2 Methods
2.1 Study population
The JECS protocol has been described in detail elsewhere (16). Briefly, the aim of the JECS, a nationwide government-funded birth cohort study, is to evaluate the impact of various environmental factors on child health and development. Between January 2011 and March 2014, participants (expectant mothers) were recruited face to face by cooperating healthcare providers at 15 regional centers, covering rural and urban areas, throughout Japan. Eligibility criteria for the participants were as follows: (1) resident in a study area at the time of recruitment and expecting to continue living in Japan for the foreseeable future; (2) expected delivery date between 1 August 2011 and mid-2014; and (3) ability to comprehend the Japanese language and complete the self-administered questionnaires. We excluded those residing outside of a study area even if they visited a cooperating healthcare provider within a study area. The participation acceptance rate was 78.5%.
Data pertaining to the following periods were collected on nine occasions up until 3 years after delivery: during early pregnancy (mean ± SD: 16.7 ± 7.7 weeks of gestation), during mid-late pregnancy (mean ± SD: 28.2 ± 6.5 weeks of gestation), and at 1, 6, 12, 18, 24, 30, and 36 months after delivery. Mothers completed a self-administered questionnaire on demographic characteristics, socioeconomic status, diet, child development, and other topics at those times. JECS staff distributed and collected the questionnaire for the first three timepoints during the participants' health check-ups at the maternity hospital up until 1 month after delivery. When collection was not possible at these times, questionnaires were returned by mail. Subsequent questionnaires were distributed and collected by mail.
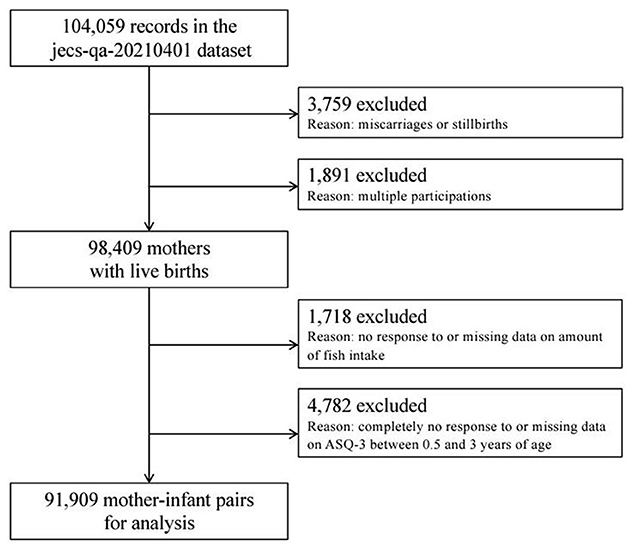
The present study is based on the JECS-QA-20210401 dataset, which was released in April 2021. The full dataset comprises 104,059 records, but we excluded 3759 records because of miscarriages/stillbirths and 1,891 records because of multiple births (Figure 1). We also excluded 1,718 mothers because of no response or missing data on fish intake and 4,782 mothers because of no response or missing data for the ASQ-3 (Ages and Stages Questionnaires®, Third Edition). This left data for 91,909 mother–child pairs for analysis.
The study protocol was reviewed and approved by the Ministry of the Environment's Institutional Review Board on Epidemiological Studies and the ethics committees of all participating institutions. All participants provided written informed consent.
2.2 Measurements of fish intake
Maternal fish intake during pregnancy was measured using the food frequency questionnaire (FFQ). The FFQ is a semiquantitative instrument that consists of three components: list of food items, average frequency of intake, and average portion size (average intake per serving). For each food item, the average frequency is multiplied by the average portion size, and the food items can be summed within a category (e.g., fish intake) in order to estimate average dietary intake over a period of several months from a single questionnaire. The FFQ has been validated for use in large-scale Japanese epidemiological studies (17). It covers more than 170 food and beverage items, including 21 related to fish or shellfish, and it has nine frequency categories ranging from “almost never” to “seven or more times per day” (or “10 or more glasses per day” for beverages) and three portion size categories of small (50% smaller than standard), medium (same as standard), or large (50% larger than standard).
Participants answered how often and how much they consumed each food type during mid-late pregnancy (average dietary intake after learning of the pregnancy to the time of questionnaire completion). For each of the 21 fish or shellfish items, the common 9 frequency categories for each item of < 1 time/month, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, every day, 2–3 times/day, 4–6 times/day, and ≥7 times/day were used. The standard portion size (with the approximate equivalent size in grams) of each of the 21 fish or shellfish items listed was as follows: slice of salted fish (70 g); one whole dried fish (50 g); quarter of a can of tuna (20 g); slice of salmon or trout (70 g); four sashimi slices of bonito or tuna (60 g);four sashimi slices of Japanese amberjack (60 g); half slice of cod or flatfish (40 g); slice of sea bream (70 g); one whole horse mackerel or sardine (80 g); one whole saury or mackerel (80 g); one tablespoons of small dried fish (10 g); one-quarter of a clutch of salted roe (20 g); half skewer of eel (50 g); three sashimi slices of squid (50 g); one-third of an octopus tentacle (50 g); two Chinese white shrimps (40 g); 10 shucked clams (20 g); 10 shucked pond snails (20 g); and fish paste products comprising one-sixth of chikuwa (20 g); two slices of kamaboko (20 g), and one-quarter of satsuma-age (20 g).
Epidemiological studies have reported that total energy intake is often associated with disease risk because of the association between physical activity and body size and the likelihood of disease occurrence. In other words, to control for confounding, reduce ambulatory variation, and predict the effects of dietary interventions, epidemiological studies usually recommend adjusting for total energy intake, so fish intake was log-transformed and energy-adjusted intake was calculated using residual mode (18). Because some participants had fish intake of 0 g/day, we replaced this value with 0.03 g/day, which is one-tenth of the lowest fish intake (0.3 g/day) of all participants (excluding 0 g/day).
2.3 Neurodevelopment
The quantitative use of the Ages and Stages Questionnaires®, Third Edition (ASQ-3), a parent-completed method for screening children at risk of developmental delay in children between the ages of 1 month and 5 years, has been validated in epidemiological studies (19). The Japanese version of the ASQ-3 has also been validated (20) and has been used in several studies (21, 22). The ASQ-3 assesses the following 5 developmental domains: (a) communication: items related to language skills, such as babbling, vocalizing, listening, and understanding, including being able to answer to their own name and give simple directions using words; (b) gross motor: items related to arm, body, and leg movements during movement and play, such as being able to kick a ball and jump; (c) fine motor: items measuring hand and finger movements, such as the ability to use scissors to cut paper and to use pencils and crayons appropriately; (d) problem-solving: items related to problem-solving skills, learning, and playing with toys, such as reciting numbers and being able to arrange blocks in the same way as demonstrated; and (e) personal-social: items related to self-help skills, solitary social play, and play with toys and others, such as being able to dress oneself and keep order.
In total, the ASQ-3 comprises 30 questions (6 per domain) that can be answered with either “yes” (=10), “sometimes” (=5), or “not yet” (=0), resulting in a score of 0–60 for each domain. If 1 or 2 answers out of the 6 questions were missing for a domain, the total score for the items answered was multiplied by a correction coefficient of 1.2 or 1.5, respectively, to adjust the score to 0–60. If >2 of the 6 questions were not answered, the participant was excluded from the analysis. The timing for administering the ASQ-3 at 3 years after delivery was set to within ±1 month (from 35 months 0 days to 36 months 30 days), and participants who completed it outside of this period were excluded. Age was corrected for prematurity if a child was born ≥3 weeks before the due date. Screen-positive cases for each domain were defined as those with scores at or below the cut-offs for (a) communication: 29.95, (b) gross motor: 39.26, (c) fine motor: 27.91, (d) problem-solving: 30.03, and (e) personal-social: 29.89 (20).
2.4 Statistical analysis
To determine the association between overall fish intake and children's development at 3 years old, quintiles of fish intake during pregnancy were calculated, following previous studies (4, 23). We then calculated odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression analysis, with the 15 regional centers set as a random effect. In tests for trend, we assigned categorical numbers to the quintile distributions for fish intake and evaluated them as continuous variables. We included potential confounding factors and covariates in the statistical analysis if previous studies found them to be associated (or they are theoretically inferred to be associated) with the outcome. These were assessed by the questionnaires administered during early and mid–late pregnancy. Because birth weight, gestation length, and breastfeeding are post-exposure covariates and are considered mediators not confounders, we did not adjust for these covariates. The confounding factors and covariates used in this study were as follows: age (years, continuous variable); physical activity [METs·h/day, measured using the International Physical Activity Questionnaire (24, 25), continuous variable]; previous deliveries (nulliparous or multiparous); pre-pregnancy body mass index (BMI; kg/m2), categorized as < 18.5, 18.5–25, or ≥25; highest maternal education level (1, junior high or high school; 2, technical junior college, technical/vocational college, or associate degree; or 3, bachelor's degree or postgraduate degree); annual household income (< 4 million, 4–6 million, or ≥6 million JPY); marital status [1, married (including common-law status); 2, single (never married); or 3, divorced or widowed]; alcohol intake (1, never; 2, previously drank alcohol but quit before learning of the pregnancy; 3, previously drank alcohol but quit after learning of the pregnancy; or 4, currently drinking; because 5 participants were misclassified as belonging to category No. 5 “quit,” which was not an option, we treated them as having missing data and later imputed them using multiple imputation); smoking status (1, never; 2, previously smoked but quit before learning of the pregnancy; 3, previously smoked but quit after learning of the pregnancy; or 4, currently smoking); employment status (yes or no); child's sex (female or male); presence of a major congenital anomaly at delivery and at age 1 month (26), yes or no; and use of EPA and/or DHA supplementation (yes for ≥1–3 uses/week or no for ≤ 2–3 uses/month); and maternal psychological distress [Kessler Psychological Distress Scale (27–29) score ≥5 or not].
We performed multiple imputations for the missing values of covariates by using chained equations (30) to obtain 10 imputed datasets. We included auxiliary variables related to covariates to preserve the assumption of missing at random. Statistical significance was set at a 2-sided p < 0.05. Analyses were performed with SAS version 9.4 (SAS Institute Inc).
2.5 Additional analysis
To assess the robustness of the results, ORs were also calculated for the lowest tertile and quartile, using multivariate logistic regression analysis.
3 Results
3.1 Participant characteristics
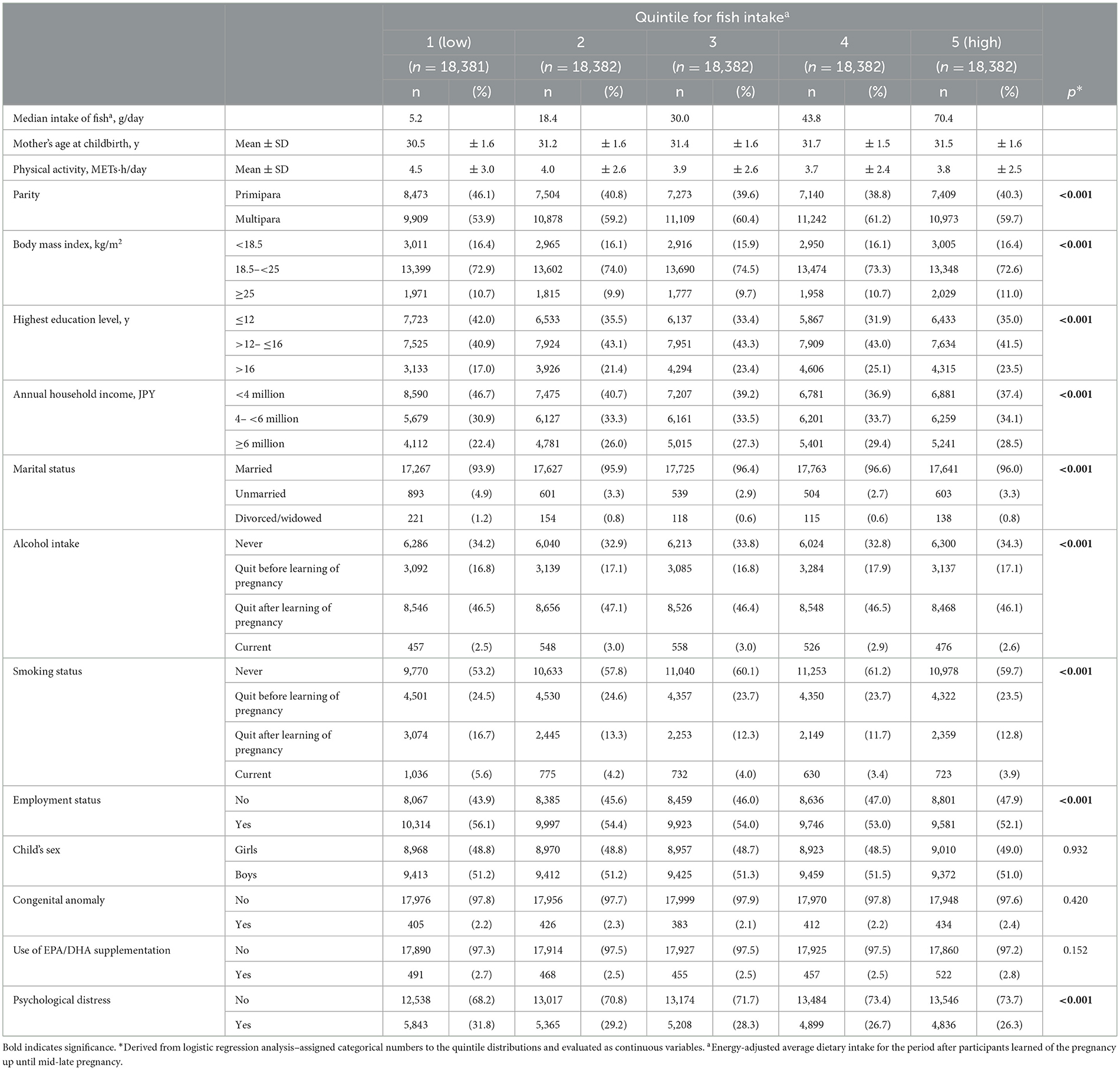
Table 1 shows the maternal characteristics of the participants according to quintile of fish intake. Women who reported eating more fish were slightly older, more likely to have multiple children, have higher educational attainment and annual household income, be unemployed, and be a non-smoker compared with women who reported eating less fish.
3.2 Multivariable analysis of psychomotor development domains at age 3 years
Table 2 shows the multivariable ORs (with 95% CIs) for the scoring of each psychomotor development domain at age 3 according to quintile of fish intake during pregnancy (n = 91,909). The results revealed reduced risks of delay at 3 years in the second and fifth quintiles for communication (0.89 [0.80–0.996] and 0.89 [0.80–0.998]), in the fourth and fifth quintiles for fine motor (0.89 [0.82–0.97] and 0.90 [0.83–0.97]), in the second, third, fourth, and fifth quintiles for problem-solving (0.90 [0.83–0.98], 0.87 [0.80–0.94], 0.89 [0.82–0.96], and 0.86 [0.80–0.94]), and in the second, fourth, and fifth quintiles for personal-social (0.88 [0.78–0.999], 0.88 [0.78–0.998], and 0.87 [0.77–0.98]). A trend test also revealed a significant linear association of fish intake with fine motor, problem-solving, and personal-social at age 3 years. However, communication and gross motor showed no significant results in the trend test, and gross motor showed no differences between groups.
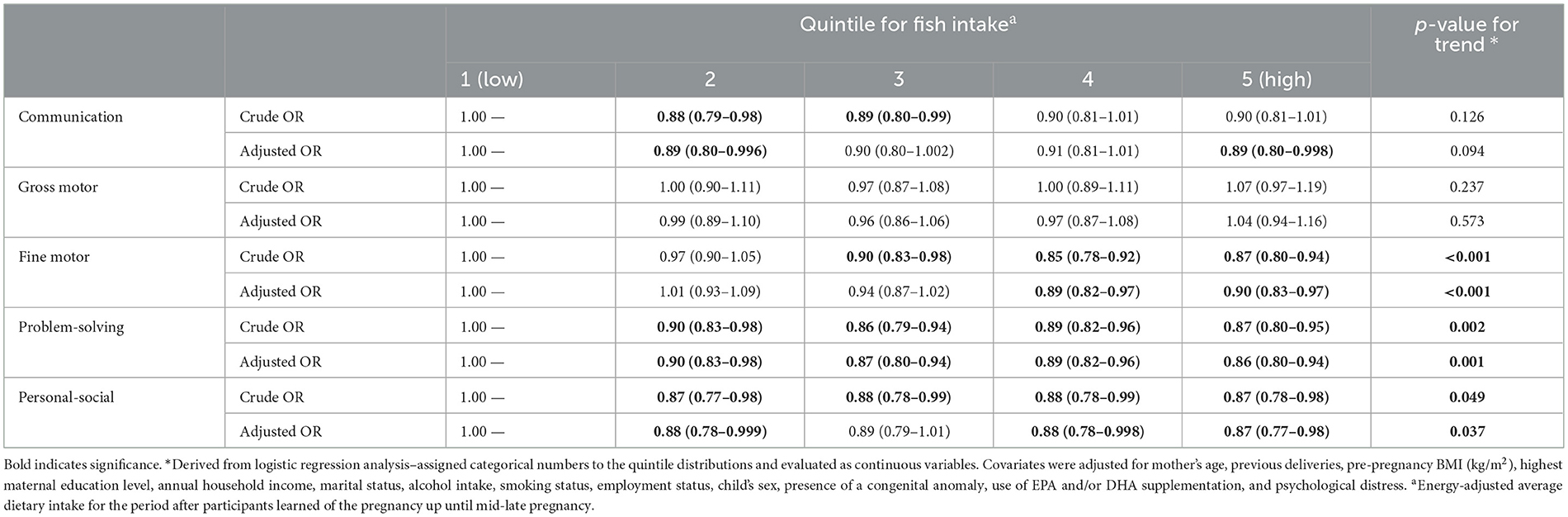
Table 2. Multivariable analysis for psychomotor development domains at age 3 years according to quintile of fish intake during pregnancy (N = 91,909).
In the additional analysis, ORs were calculated for fish intake during pregnancy and children's development at 3 years of age in both the lowest third and fourth quartiles. There were consistently lower odds in the highest vs. lowest tertile and quartile for fine motor and problem-solving but not for communication, gross motor, or personal-social skills. The trend for lower odds of symptoms of neurodevelopmental delay across tertiles or quartiles of higher maternal fish intake were significant for fine motor and problem-solving but not for communication, gross motor, or personal-social skills (Supplementary Tables 1, 2), suggesting a consistent dose-effect relationship, at least for fine motor and problem solving, regardless of the method of analysis.
4 Discussion
This study used data from 91,909 mother–child pairs from the JECS to determine the association of the dietary intake of fish during pregnancy with neurodevelopment in 3-year-old children. The results showed reduced risks of a communication delay for the second and fifth quintiles, of a fine motor delay for the fourth and fifth quintiles, of a problem-solving delay for the second, third, fourth, and fifth quintiles, and of a personal-social delay for the second, fourth, and fifth quintiles. Gross motor showed no significant results in the trend test or differences between groups.
Studies focusing on the association between maternal seafood intake and children's neurodevelopment have reported that maternal seafood intake is associated with the improved neuropsychological development of children at 14 months and, in particular, at 5 years of age (7). In addition, studies focusing on children's fish intake have reported that children with high fish intake have a higher sleep quality and intelligence quotient than those with low intake (31, 32). On the other hand, other reports failed to find an association of maternal seafood intake during pregnancy with children's neurodevelopment (33, 34). These reports were more commonly randomized controlled studies than observational studies, suggesting the presence of limitations such as an inability to actively reduce nutrient intake for ethical reasons, inability to evaluate long-term interventions, and inability to intervene in fish intake as a whole food (4). Indeed, many randomized controlled studies in animals have reported positive results.
Randomized controlled trials in animals have suggested that high levels of PUFAs, which are abundant in fish, have positive effects on development: rats with sufficient n-3 fatty acids have better memory learning ability than rats lacking n-3 fatty acids (35). In addition, it has been shown that DHA deficiency during brain maturation in rats impairs brain function in adulthood and that adequate amounts of DHA are important for long-term neuronal resilience in the brain (36).
Fish is rich in nutrients important for infant development, such as iodine and vitamins A, D, and B12 (37, 38), and has been found to function as a proxy for a healthy lifestyle (38). Given that common chronic diseases are potentially preventable when nutritional status is optimized for the fetus (39), it is expected that high fish intake during pregnancy will have a positive impact on children's neurodevelopment. However, the results of the present study showed no differences in trend tests or group ratings for gross motor activity. This was similar to the results of previous studies conducted at 6 months and 1 year of age (4), and suggests that fish consumption during pregnancy may have a positive effect on some aspects of neurodevelopment in children but may not be associated with gross motor activity. However, more research is needed because gross motor skills such as one-legged kneeling and swinging alone continue to develop after 3 years of age.
The strengths of this study are the size of the cohort, that the participants are likely to be representative of the Japanese population (40), and that the dataset is extensive, which allowed us to adjust for a large number of covariates. In addition, the ASQ-3 questionnaire used in this study as a screening tool for monitoring children who are at risk of developmental delay is well validated for quantitative use in epidemiological studies and is used worldwide (41). The prospective data collection of exposures and outcomes minimized recall bias, and almost all important confounders were included in the model. Moreover, the FFQ contains as many as 21 items related to fish or shellfish consumption (17).
However, the study also has several limitations that should be considered. First, the FFQ has been validated (17) but not specifically for pregnant women. Second, as noted above, due to the observational nature of the study, the results may have been confounded by unmeasured residual factors. This is one of the major drawbacks of observational studies. It was not possible to consider all other dietary intakes and dietary patterns. For example, fish consumption may serve as a proxy for an overall healthy lifestyle (38), and people may choose nutrient-rich options more frequently than nutritionally unbalanced and/or nutrient-deficient options, such as junk food. Furthermore, there is also geographical variation in the amount and type of fish and shellfish consumed, and shellfish in particular is reported to be affected by environmental pollution (42). In Japan, “Precautions for pregnant women regarding ingestion of fish and shellfish and mercury” that appears on the Ministry of Health, Labor and Welfare website advises caution when eating fish that may contain high levels of mercury, such as bigeye tuna, salmon, and yellowtail (43). This study did not take into account factors such as the origin of the fish consumed, the method of storage and freshness of the fish, the method of cooking, or the amount of contaminants in the fish consumed, which should be examined in future work. Third, the FFQ responses are based on the mother's memory, so recall bias cannot be ruled out, and the ASQ-3 findings are also based on parental reports and not an objective assessment. Fourth, this study dealt only with maternal fish intake during pregnancy and could not consider the children's own intake. Fifth, although we covered both rural and urbans locations across Japan in this national study, the participants may not necessarily have been a representative sample of Japanese pregnant women. Nevertheless, the basic characteristics of the mothers, fathers, and children participating in the JECS are very close to those of the Japanese government's Vital Statistics, and the JECS data are considered to reflect the birth situation in Japan. Nevertheless, even if the participants in this study were a representative sample of pregnant Japanese women, who generally consume more fish than their Western counterparts, the findings may not be generalizable to Western populations.
5 Conclusions
Fish consumption during pregnancy may be associated with a reduced risk of delay in the neurodevelopment of 3-year-olds, particularly in the fine motor, problem-solving, and personal-social domains. Continued investigation after the age of 3 could further clarify the association.
Data availability statement
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amended 9 September 2015) to publicly deposit data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of epidemiologic data. All inquiries about access to data should be sent to: amVjcy1lbkBuaWVzLmdvLmpw. The person responsible for handling enquiries sent to this e-mail address is Dr. Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Ethics statement
The studies involving humans were approved by The Ministry of the Environment's Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
MI: Conceptualization, Writing – original draft. KM: Formal analysis, Writing – review & editing. KH: Conceptualization, Investigation, Supervision, Writing – review & editing. AT: Investigation, Writing – review & editing. HI: Investigation, Project administration, Writing – review & editing.
Members of the JECS Group as of 2022
Michihiro Kamijima (Principal Investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The JECS was founded by the Ministry of the Environment, Japan. The funding source played no role in the study's design, collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit this paper for publication.
Acknowledgments
We are grateful to all participants of the JECS and to all individuals involved in data collection.
Conflict of interest
KH received speaking honoraria from the DHA&EPA Association, Why's Co., Ltd., Suntory Wellness Ltd., Maruishi Pharmaceutical Co., Ltd., JCR Pharmaceuticals Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd. and a supervision fee from Otsuka Pharmaceutical Factory.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Ministry of the Environment.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1267088/full#supplementary-material
References
1. Zhao R, Gao Q, Wang S, Yang X, Hao L. The effect of maternal seafood consumption on perinatal outcomes: a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr. (2021) 61:3504–17. doi: 10.1080/10408398.2020.1802573
2. Malmir H, Larijani B, Esmaillzadeh A. Fish consumption during pregnancy and risk of allergic diseases in the offspring: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2022) 62:7449–59. 62:7449–59. doi: 10.1080/10408398.2021.1914543
3. Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. (2007) 369:578–85. doi: 10.1016/S0140-6736(07)60277-3
4. Hamazaki K, Matsumura K, Tsuchida A, Kasamatsu H, Tanaka T, Ito M, et al. Maternal dietary intake of fish and PUFAs and child neurodevelopment at 6 months and 1 year of age: a nationwide birth cohort—the Japan Environment and Children's Study (JECS). Am J Clin Nutr. (2020) 112:1295–303. 112:1295–303. doi: 10.1093/ajcn/nqaa190
5. Suzuki K, Nakai K, Sugawara T, Nakamura T, Ohba T, Shimada M, et al. Neurobehavioral effects of prenatal exposure to methylmercury and PCBs, and seafood intake: neonatal behavioral assessment scale results of Tohoku study of child development. Environ Res. (2010) 110:699–704. 110:699–704. doi: 10.1016/j.envres.2010.07.001
6. Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal fat intake during pregnancy and behavioral problems in 5-y-old Japanese children. Nutrition. (2018) 50:91–6. 50:91–6. doi: 10.1016/j.nut.2017.12.001
7. Julvez J, Méndez M, Fernandez-Barres S, Romaguera D, Vioque J, Llop S, et al. Maternal consumption of seafood in pregnancy and child neuropsychological development: a longitudinal study based on a population with high consumption levels. Am J Epidemiol. (2016) 183:169–82. 183:169–82. doi: 10.1093/aje/kwv195
8. Strain JJ, Yeates AJ, van Wijngaarden E, Thurston SW, Mulhern MS, McSorley EM, et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr. (2015) 101:530–7. doi: 10.3945/ajcn.114.100503
9. Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. (2008) 29:767–75. doi: 10.1016/j.neuro.2008.06.001
10. Otsuka R, Yatsuya H, Tamakoshi K. Descriptive epidemiological study of food intake among Japanese adults: analyses by age, time and birth cohort model. BMC Pub Health. (2014) 14:328. 14:328. doi: 10.1186/1471-2458-14-328
11. Rutter M. Genetic studies of autism: from the 1970s into the millennium. J Abnorm Child Psychol. (2000) 28:3–14. doi: 10.1023/a:1005113900068
12. Yang CJ, Tan HP, Du YJ. The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience. (2014) 267:1–10. 267:1–10. doi: 10.1016/j.neuroscience.2014.02.021
13. Frankenburg W, Dodds J, Archer P, Bresnick B, Maschka P, Edelman N, et al. Denver II Training Manual. Denver, CO: Denver Developmental Materials Inc (1992).
14. Matsuo T, Matsuo C, Matsuoka H, Kio K. Detection of strabismus and amblyopia in 1.5- and 3-year-old children by a preschool vision-screening program in Japan. Acta Med Okayama. (2007) 61:9–16.
15. Holzinger D, Weber C, Barbaresi W, Beitel C, Fellinger J. Language screening in 3-year-olds: development and validation of a feasible and effective instrument for pediatric primary care. Front Pediatr. (2021) 9:752141. doi: 10.3389/fped.2021.752141
16. Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, et al. Rationale and study design of the Japan environment and children's study (JECS). BMC Public Health. (2014) 14:25. 14:25. doi: 10.1186/1471-2458-14-25
17. Yokoyama Y, Takachi R, Ishihara J, Ishii Y, Sasazuki S, Sawada N, et al. Validity of short and long self-administered food frequency questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan public health center-based prospective study for the next generation (JPHC-NEXT) protocol area. J Epidemiol. (2016) 26:420–32. doi: 10.2188/jea.JE20150064
18. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. (1997) 65:1220S−8S. doi: 10.1093/ajcn/65.4.1220S
19. Squires J, Bricker D, Elizabeth T, Jantina C, Robert N. Ages & Stages Questionnaires: Third Edition (ASQ-3). Baltimore, MD: Brookes Publishing (2009).
20. Mezawa H, Aoki S, Nakayama SF, Nitta H, Ikeda N, Kato K, et al. Psychometric profile of the ages and stages questionnaires, japanese translation. Pediatr Int. (2019) 61:1086–95. 61:1086–95. doi: 10.1111/ped.13990
21. Minatoya M, Araki A, Miyashita C, Itoh S, Kobayashi S, Yamazaki K, et al. Cat and dog ownership in early life and infant development: a prospective birth cohort study of japan environment and children's study. Int J Environ Res Pub Health. (2020) 17:205. doi: 10.3390/ijerph17010205
22. Matsumura K, Hamazaki K, Tsuchida A, Inadera H, Kamijima M, Yamazaki S, et al. Prospective association of air purifier use during pregnancy with the neurodevelopment of toddlers in the Japan environment and Children's study. Sci Rep. (2021) 11:19454. 11:19454. doi: 10.1038/s41598-021-98482-y
23. Hamazaki K, Matsumura K, Tsuchida A, Kasamatsu H, Tanaka T, Ito M, et al. Dietary intake of fish and n-3 polyunsaturated fatty acids and risk of postpartum depression: a nationwide longitudinal study - the Japan environment and children's study (JECS). Psychol Med. (2020) 50:2416–24. 50:2416–24. doi: 10.1017/S0033291719002587
24. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
25. Murase N, Katsumura T, Ueda C, Inoue S, Shimomitsu T. Validity and reliability of Japanese version of international physical activity questionnaire. J Health Welfare Stat. (2002) 49:1–9.
26. Mezawa H, Tomotaki A, Yamamoto-Hanada K, Ishitsuka K, Ayabe T, Konishi M, et al. Prevalence of congenital anomalies in the Japan environment and children's study. J Epidemiol. (2019) 29:247–56. 29:247–56. doi: 10.2188/jea.JE20180014
27. Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. (2002) 32:959–76. doi: 10.1017/S0033291702006074
28. Furukawa TA, Kawakami N, Saitoh M, Ono Y, Nakane Y, Nakamura Y, et al. The performance of the Japanese version of the K6 and K10 in the world mental health survey Japan. Int J Methods Psychiatr Res. (2008) 17:152–8. doi: 10.1002/mpr.257
29. Sakurai K, Nishi A, Kondo K, Yanagida K, Kawakami N. Screening performance of K6/K10 and other screening instruments for mood and anxiety disorders in Japan. Psychiatr Clin Neurosci. (2011) 65:434–41. doi: 10.1111/j.1440-1819.2011.02236.x
30. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. (2007) 16:219–42. 16:219–42. doi: 10.1177/0962280206074463
31. Aberg MA, Aberg N, Brisman J, Sundberg R, Winkvist A, Torén K. Fish intake of Swedish male adolescents is a predictor of cognitive performance. Acta Paediatr. (2009) 98:555–60. 98:555–60. doi: 10.1111/j.1651-2227.2008.01103.x
32. Liu J, Cui Y, Li L, Wu L, Hanlon A, Pinto-Martin J, et al. The mediating role of sleep in the fish consumption - cognitive functioning relationship: a cohort study. Sci Rep. (2017) 7:17961. 7:17961. doi: 10.1038/s41598-017-17520-w
33. Shulkin M, Pimpin L, Bellinger D, Kranz S, Fawzi W, Duggan C, et al. n-3 fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: a systematic review and meta-analysis. J Nutr. (2018) 148:409–18. doi: 10.1093/jn/nxx031
34. Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Datab Syst Rev. (2018) 11:Cd003402. 11:Cd003402. doi: 10.1002/14651858.CD003402.pub3
35. Fujimoto K. Effects of n-3 deficiency during pregnancy and lactation on learning ability of rats. Adv Polyunsatur Fatty Acid Res. (1993) 257–60.
36. Bhatia HS, Agrawal R, Sharma S, Huo YX, Ying Z, Gomez-Pinilla F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE. (2011) 6:e28451. doi: 10.1371/journal.pone.0028451
37. Simpson JL, Bailey LB, Pietrzik K, Shane B, Holzgreve W. Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part I-Folate Vit B12 Vit B6 J Matern Fetal Neonatal Med. (2010) 23:1323–43. 23:1323–43. doi: 10.3109/14767051003678234
38. Schiepers OJ, de Groot RH, Jolles J, van Boxtel MP. Fish consumption, not fatty acid status, is related to quality of life in a healthy population. Prostaglandins Leukot Essent Fatty Acids. (2010) 83:31–5. doi: 10.1016/j.plefa.2010.02.030
39. Fall CH. Fetal malnutrition and long-term outcomes. Nestle Nutr Inst Workshop Ser. (2013) 74:11–25. doi: 10.1159/000348384
40. Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K, et al. Baseline profile of participants in the Japan environment and children's study (JECS). J Epidemiol. (2018) 28:99–104. 28:99–104. doi: 10.2188/jea.JE20170018
41. Squires J, Bricker DD, Twombly E. Ages and Stages Questionnaires. Baltimore, MD: Paul H. Brookes (2009).
42. Giannico OV, Baldacci S, Desiante F, Basile FC, Franco E, Fragnelli GR, et al. PCDD/Fs and PCBs in Mytilus galloprovincialis from a contaminated area in Italy: the role of mussel size, temperature and meteorological factors. Food Add Contamin Part A. (2022) 39:1123–35. 39:1123–35. doi: 10.1080/19440049.2022.2059108
43. Ministry Ministry of Health Labour Welfare [For you who is going to be a mother]. (2010). Available online at: https://www.mhlw.go.jp/topics/bukyoku/iyaku/syoku-anzen/suigin/dl/051102-2a.pdf (accessed October 23, 2020).
Keywords: pregnancy, fish, neurodevelopment, infant, food life
Citation: Inoue M, Matsumura K, Hamazaki K, Tsuchida A and Inadera H (2024) Maternal dietary intake of fish and child neurodevelopment at 3 years: a nationwide birth cohort—The Japan Environment and Children's Study. Front. Public Health 11:1267088. doi: 10.3389/fpubh.2023.1267088
Received: 26 July 2023; Accepted: 11 December 2023;
Published: 24 January 2024.
Edited by:
Roberta Zupo, University of Bari Aldo Moro, ItalyReviewed by:
Tsuguhiko Kato, National Center for Child Health and Development (NCCHD), JapanJean Kerver, Michigan State University, United States
Orazio Valerio Giannico, Local Health Authority of Taranto, Italy
Copyright © 2024 Inoue, Matsumura, Hamazaki, Tsuchida and Inadera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hidekuni Inadera, aW5hZGVyYUBtZWQudS10b3lhbWEuYWMuanA=
 Mariko Inoue
Mariko Inoue Kenta Matsumura
Kenta Matsumura Kei Hamazaki
Kei Hamazaki Akiko Tsuchida
Akiko Tsuchida Hidekuni Inadera
Hidekuni Inadera