- 1Institute for Medical Research (IMR), National Institutes of Health (NIH), Ministry of Health Malaysia, Setia Alam, Malaysia
- 2Ministry of Health Malaysia, Putrajaya, Malaysia
- 3Department of Medical Microbiology, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, Malaysia
- 4International Medical School, Management and Science University, Shah Alam, Selangor, Malaysia
Introduction: Since the COVID-19 pandemic began, it has spread rapidly across the world and has resulted in recurrent outbreaks. This study aims to describe the COVID-19 epidemiology in terms of COVID-19 cases, deaths, ICU admissions, ventilator requirements, testing, incidence rate, death rate, case fatality rate (CFR) and test positivity rate for each outbreak from the beginning of the pandemic in 2020 till endemicity of COVID-19 in 2022 in Malaysia.
Methods: Data was sourced from the GitHub repository and the Ministry of Health’s official COVID-19 website. The study period was from the beginning of the outbreak in Malaysia, which began during Epidemiological Week (Ep Wk) 4 in 2020, to the last Ep Wk 18 in 2022. Data were aggregated by Ep Wk and analyzed in terms of COVID-19 cases, deaths, ICU admissions, ventilator requirements, testing, incidence rate, death rate, case fatality rate (CFR) and test positivity rate by years (2020 and 2022) and for each outbreak of COVID-19.
Results: A total of 4,456,736 cases, 35,579 deaths and 58,906,954 COVID-19 tests were reported for the period from 2020 to 2022. The COVID-19 incidence rate, death rate, CFR and test positivity rate were reported at 1.085 and 0.009 per 1,000 populations, 0.80 and 7.57%, respectively, for the period from 2020 to 2022. Higher cases, deaths, testing, incidence/death rate, CFR and test positivity rates were reported in 2021 and during the Delta outbreak. This is evident by the highest number of COVID-19 cases, ICU admissions, ventilatory requirements and deaths observed during the Delta outbreak.
Conclusion: The Delta outbreak was the most severe compared to other outbreaks in Malaysia’s study period. In addition, this study provides evidence that outbreaks of COVID-19, which are caused by highly virulent and transmissible variants, tend to be more severe and devastating if these outbreaks are not controlled early on. Therefore, close monitoring of key epidemiological indicators, as reported in this study, is essential in the control and management of future COVID-19 outbreaks in Malaysia.
Introduction
The emergence of the COVID-19 infection caused by the SARS-CoV-2 virus was first reported in Wuhan, China, in late December 2019 (1). Within a short period since its discovery, it spread rapidly across many countries globally. The World Health Organization (WHO) subsequently declared COVID-19 a Public Health Emergency of International Concern (PHEIC) on 30 January 2020 (2). The COVID-19 public health emergency lasted for over 3 years till WHO declared it over on 5 May 2023. During this time, COVID-19 had infected almost 766 million individuals, which resulted in 6.9 million deaths globally (3).
In Malaysia, the first case of COVID-19 was reported on 25 January 2020, which marked the beginning of the pandemic, which lasted from 25 January 2020 till the disease was declared endemic in Malaysia on 1 April 2022. There was a total of 4 outbreaks caused by various COVID-19 variants over the 2-year period. These outbreaks were caused by the following 4 COVID-19 variants: Wuhan, Beta, Delta, and Omicron. Subsequently, each outbreak was then named corresponding to the circulating variants. The first outbreak by the Wuhan variants lasted for 29 weeks, from January 2020 to August 2020 [Epidemiological Week (Ep Wk)9/2020 to 37/2020] (4). This outbreak was characterized by a high case fatality rate and was when the Public Health Social Measure (PHSM), which included movement restrictions, was initiated.
The second Beta variant outbreak occurred from September 2020 to March 2021 (Ep Wk 37/2020 to 13/2021), which lasted for a duration of 30 weeks (5). This was followed by the highly virulent Delta outbreak from April 2021 to January 2022, which lasted for 43 weeks from (Ep Wk 13/2021 to 3/2022) (6). The Delta outbreak was predominantly a severe outbreak with high case burden and mortality. In addition, it was during this outbreak that the introduction of the COVID-19 vaccine commenced. The fourth outbreak, which was caused by the Omicron variant, marked the point this disease was declared endemic in Malaysia (7). The Omicron outbreak occurred from January 2022 till the disease was declared endemic in April 2022 (Ep Wk 3/2022 to 18/2022).
As the disease evolved, the demographic characteristics changed due to the various evolving variants requiring specific interventions and control measures. Hence, it is essential to examine each outbreak’s demographic, epidemiological characteristics, and trends to understand the pandemic, which would subsequently assist in improving the management and control of the disease. In addition, a detailed description of each outbreak variant during the pandemic would enable us to understand better the progression, evolution, and severity of the various COVID-19 outbreak variants (8–10). Furthermore, by systematically analyzing these epidemiological indicators, evidence of the effectiveness of pharmaceutical and non-pharmaceutical outbreak-based control measures would assist the surveillance system in monitoring the pandemic. Hence, the knowledge of the disease evolution is important in the process of initiating and adjusting PHSM and vaccination (11–13).
To date, limited published studies have described epidemiological indicators and their trends during each COVID-19 outbreak during the pandemic in Malaysia (14). Therefore, the main aim of this study is to describe and compare the epidemiological indicators during the Wuhan, Beta, Delta, and Omicron outbreak from 2020 (Ep Wk 4/2020 to 18/2022) in terms of their disease demographics (case incidences and mortality rate), hospital admissions [intensive care unit (ICU) admissions, ventilated cases] and diagnostic testing (testing capacity, test positivity rates). This paper will provide a comprehensive analysis and understanding of the various epidemiological indicators and their progression during the COVID-19 pandemic, which would provide a more comprehensive knowledge of the disease evolution and comparison of distinct characteristics during each outbreak in Malaysia (15).
Materials and methods
Data source
Data on COVID-19 cases, deaths, ICU admissions, ventilator requirements and tests was sourced from the GitHub repository (MoH-Malaysia/covid19-public) and the Ministry of Health official COVID-19 website1,2 (16, 17). The study period was from the beginning of the COVID-19 outbreak in Malaysia, which began during Ep Wk 4/2020, to when it was declared endemic, corresponding to the end of the Omicron outbreak in Ep Wk 18/2022 (18). Daily COVID-19 data were aggregated based on epidemiological week (which starts on Sunday) for each outbreak (i.e., Wuhan, Beta, Delta and Omicron) at the national level (19).
Data analysis
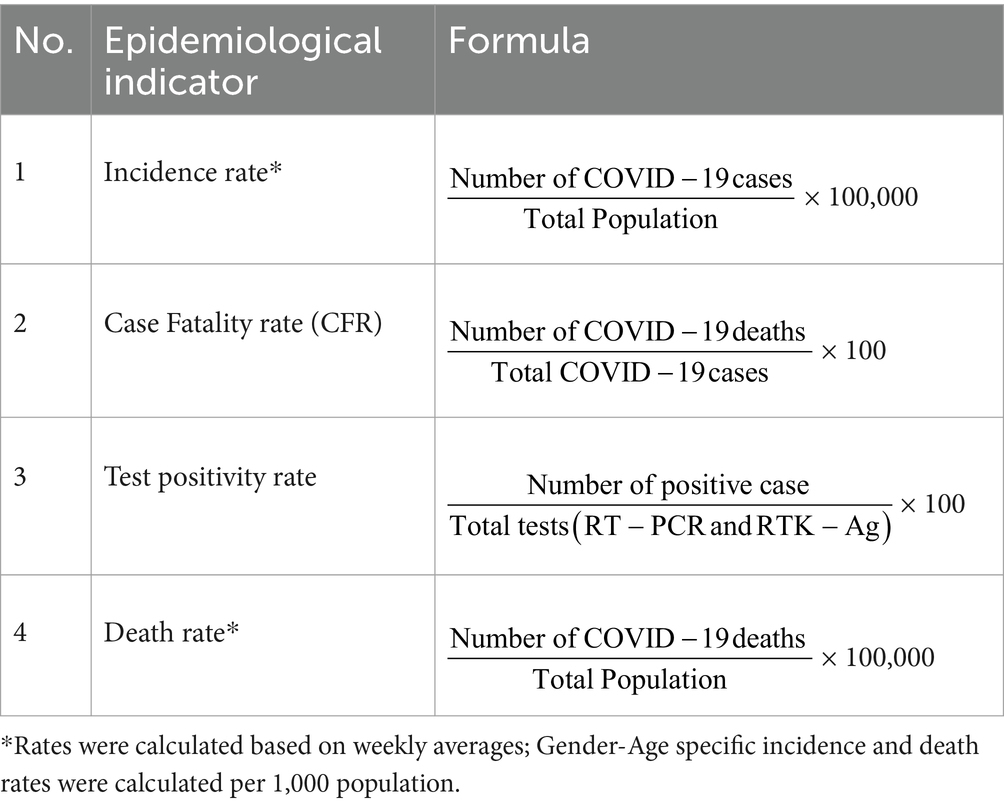
Data was analyzed using the Statistical Package for the Social Sciences (SPSS) version 26.0 (20). Table 1 shows percentages and frequencies of cases, deaths, average ICU admissions, average ventilator requirements, laboratory-confirmed test incidence rate, death rate, CFR, and test positivity rate. Average weekly incidence and death rate was estimated to allow for comparison between outbreaks. In addition, the age-gender-specific case, death, incidence rate, death rate and CFR were estimated. A 10-year interval was used to represent the age distribution as follows: 0–9 years, 10–19 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years and more than 80 years. The analysis was done for the overall duration of the COVID-19 outbreak in Malaysia from Ep Wk 4/2020 to Ep Wk 18/2022, and for each outbreak of COVID-19, namely the Wuhan (Ep Wk 9/2020 to 37/2020), Beta (Ep Wk 37/2020 to 13/2021), Delta (Ep Wk 13/2021 to 3/2022), and Omicron (Ep Wk 3/2022 to 18/2022), and presented in tabular and time series plots.
Results
Cases and incidence rate
Overall
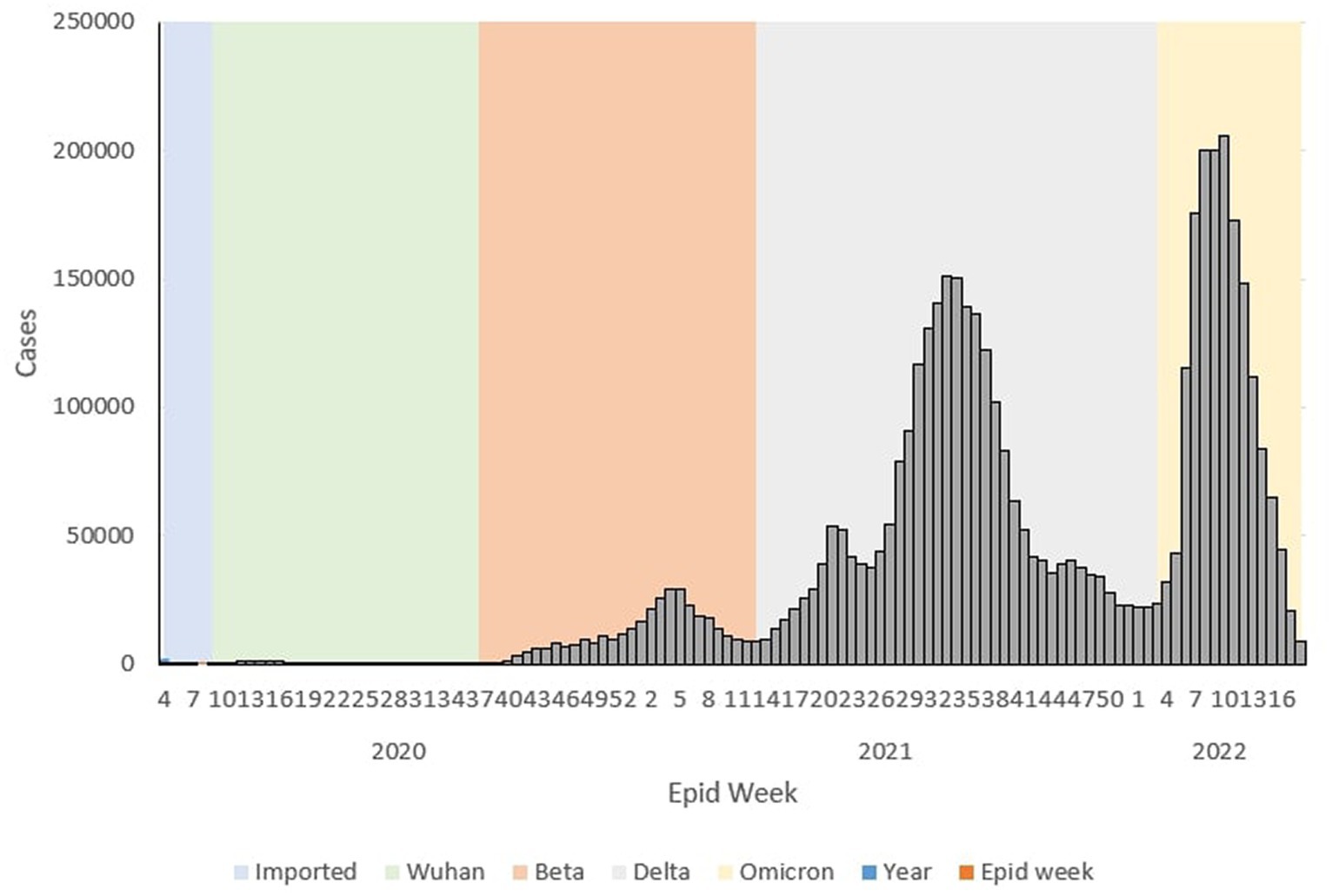
There was a total of 4,456,736 cases reported for the overall study period in which the highest and lowest weekly COVID-19 cases were reported in Ep Wk 10/2022 (n = 205,864) and Ep Wk 8/2020 (n = 0), respectively. The overall weekly average COVID-19 incidence rate was 108.51 per 100,000 population, as shown in Table 2.
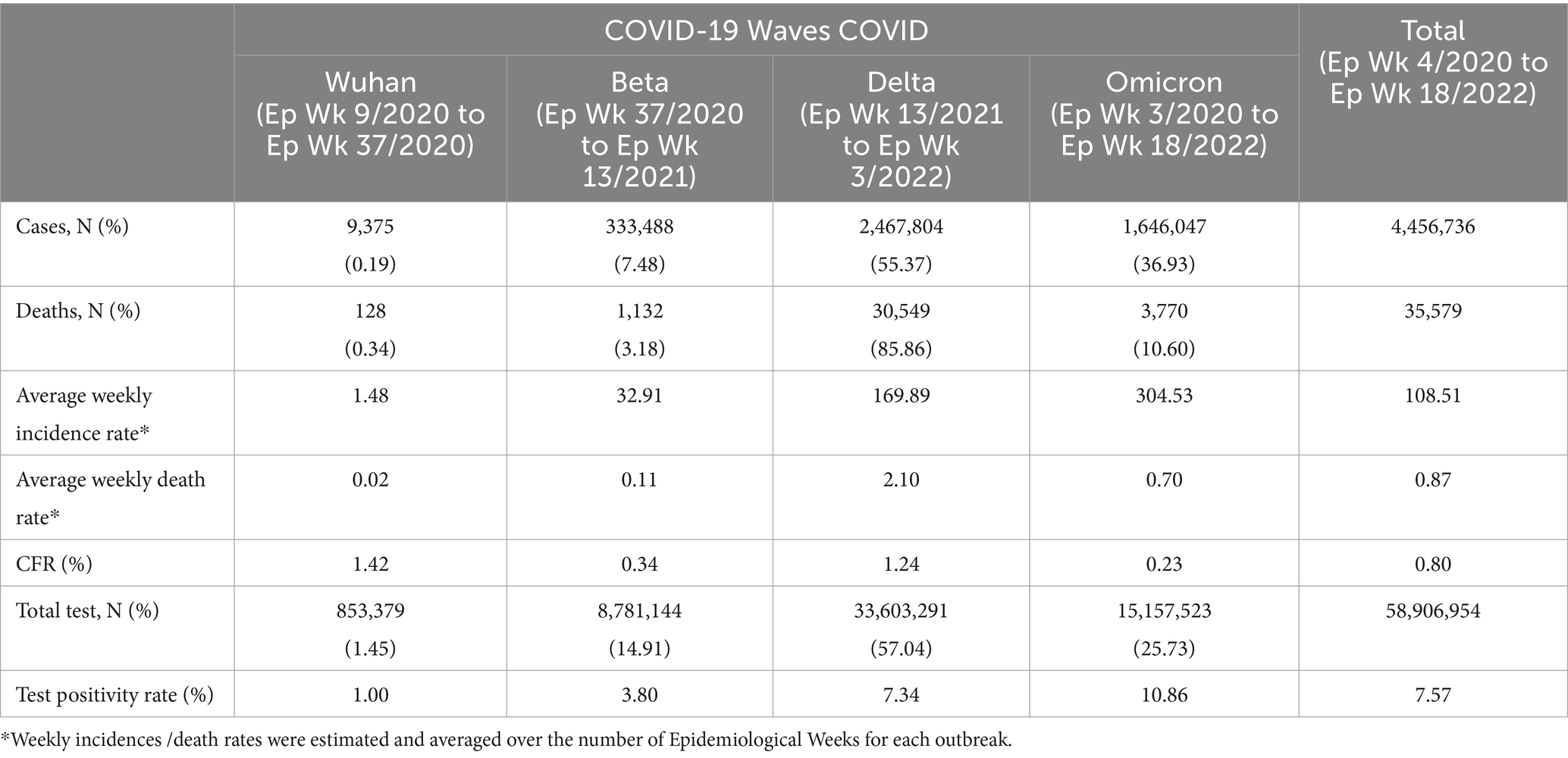
Table 2. COVID-19 cases, deaths, incidence rate, death rate, CFR, testing capacity and test positivity rate by COVID-19 variants outbreaks in Malaysia.
By outbreaks
During the Wuhan outbreak, a total of 9,375 (0.19%) cases were reported, with the highest and lowest weekly COVID-19 cases being reported in Ep Wk 14/2020 (n = 1,163) and Ep Wk 8/2020 (n = 0), respectively. On the other hand, during the Beta outbreak, a total of 333,488 (7.48%) cases were reported, with the highest and lowest weekly COVID-19 cases being reported in Ep Wk 4/2021 (n = 29,206) and Ep Wk 38/2020 (n = 299) respectively. For the Delta outbreak, a total of 2,467,804 (55.37%) cases were reported, with the highest and lowest weekly COVID-19 cases being reported in Ep Wk 33/2021 (n = 150,933) and Ep Wk 13/2021 (n = 8,968) respectively.
As for the Omicron outbreak, a total of 1,646,047 (36.93%) cases were reported, with the highest and lowest weekly COVID-19 cases being reported on Ep Wk 10/2022 (n = 205,864) and Ep Wk 18/2022 (n = 8,732) respectively. Overall, the highest number of cumulative cases was reported during the Delta (n = 2,467,804, 55.37%) outbreak, followed by the Omicron (n = 1,646,047, 36.93%), Beta (333,488, 7.48%) and Wuhan (8,493, 0.19%) outbreak, respectively. The average weekly incidence rate for the Wuhan, Beta, Delta and Omicron outbreaks was 1.48, 32.91,169.89 and 304.53 per 100,000 populations, respectively.
Trends
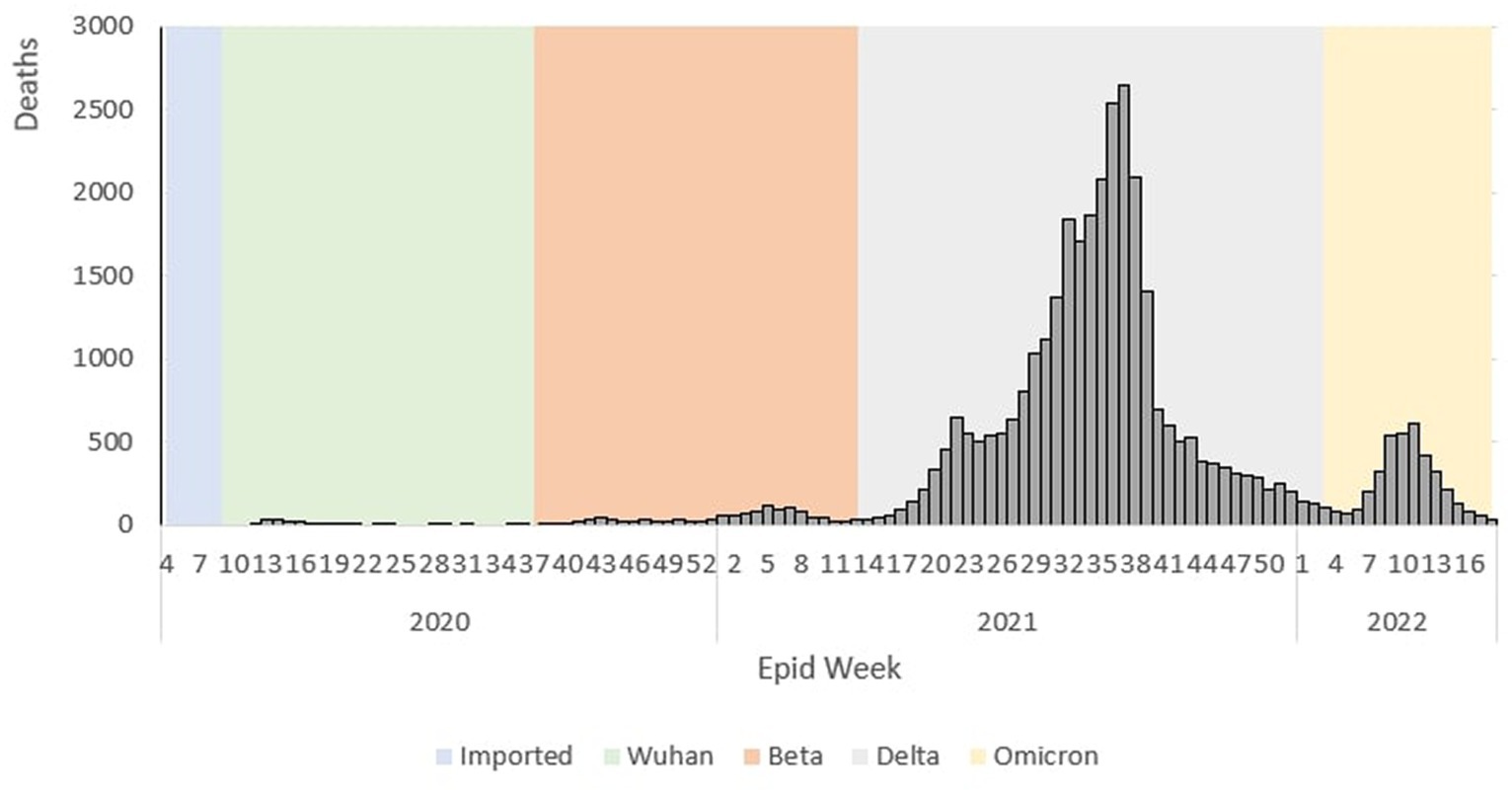
During the Wuhan outbreak, the epidemiological curve represented a slow increase in which the number of cases started to increase from Ep Wk 10/2020 (n = 68) and peaked in Ep Wk 14/2020 (n = 1,163). Following this, during the Beta outbreak, the epidemiological curve peaked on Ep Wk 4/2021 (n = 29,206). This was followed by the Delta outbreak, where the epidemiological curve peaked at Ep Wk 33/2021 (n = 150,933). Subsequently, during the Omicron outbreak, the epidemiological curve rose rapidly and peaked at Ep Wk 10/2022 (n = 205,864), corresponding to the highest number of weekly cases reported through the study duration in Malaysia (Figure 1).
ICU admission and ventilator requirements
ICU admission trends
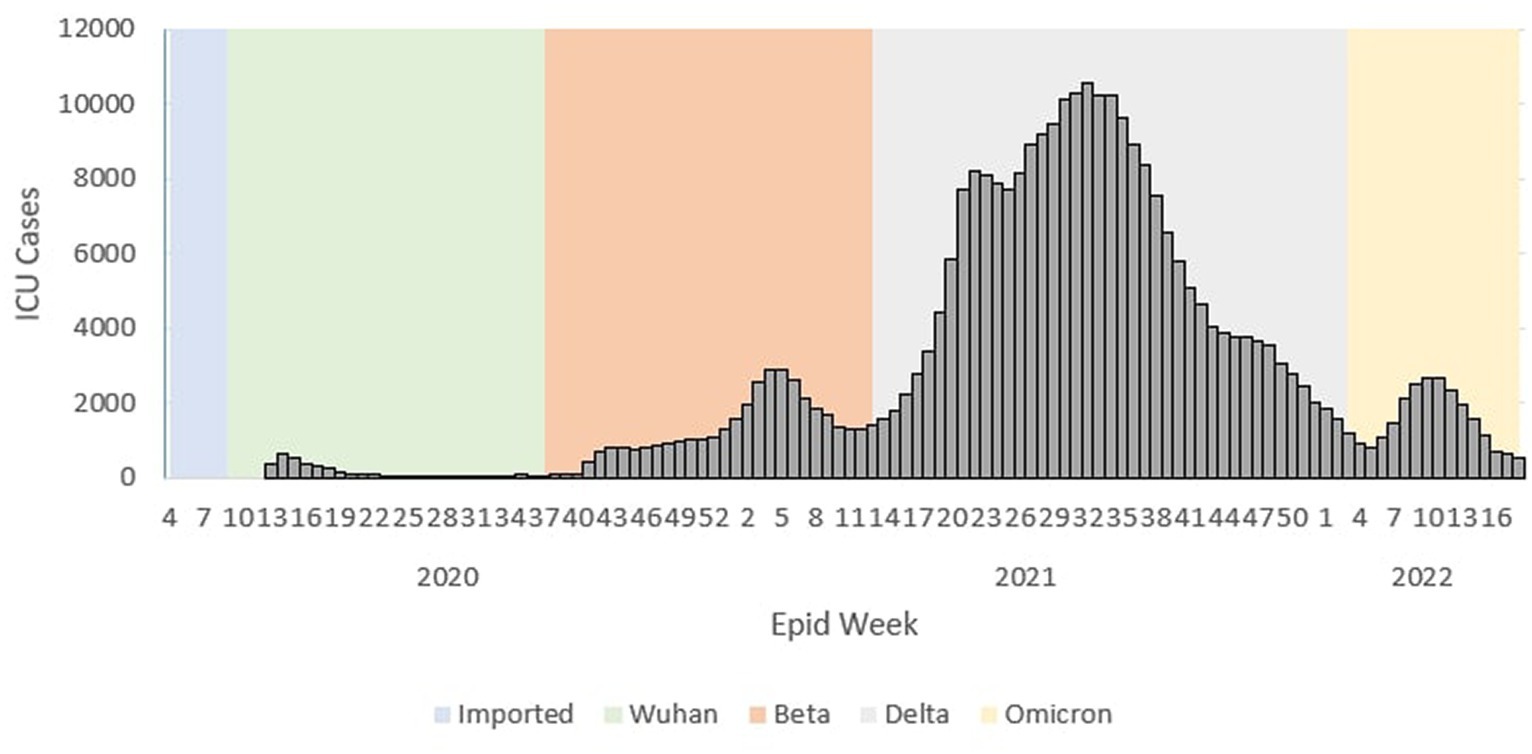
During the Wuhan outbreak, the average number of cases requiring ICU admission started to increase from Ep Wk 13/2020 (n = 369) and peaked in Ep Wk 15/2020 (n = 549). Subsequently, the average ICU case trends decreased from Ep Wk16/2020 (n = 374) to Ep Wk36/2020 (n = 35). Following this, the Beta outbreak began where the average ICU case trends started to increase from Ep Wk37/2020 (n = 53) to Ep Wk 4/2021 (n = 2,902), corresponding to the longest increasing average ICU case trends of 21 weeks. A downward trend of the average ICU cases was observed from Ep Wk 5/2021 (n = 2,873) to Ep Wk 15/2021 (n = 1,775). This was followed by the Delta outbreak, where in the ICU case trend started to increase at Ep Wk 16/2021 (n = 2,024) to Ep Wk 32/2021 (n = 10,586) and subsequently decreased from Ep Wk 35/2021 (n = 9,637) until Ep Wk 5/2022 (n = 810) which corresponded to the longest decreasing average ICU case trends of 23 weeks. Following this, the Omicron outbreak began where the average ICU case trend started to increase at Ep Wk 6/2022 (n = 1,047) to Ep Wk 15/2022 (n = 1,102) and subsequently decreased at Ep Wk 16/2022 (n = 702) till Ep Wk 18/2022 (n = 506) as shown in Figure 2.
Ventilation requirement trend
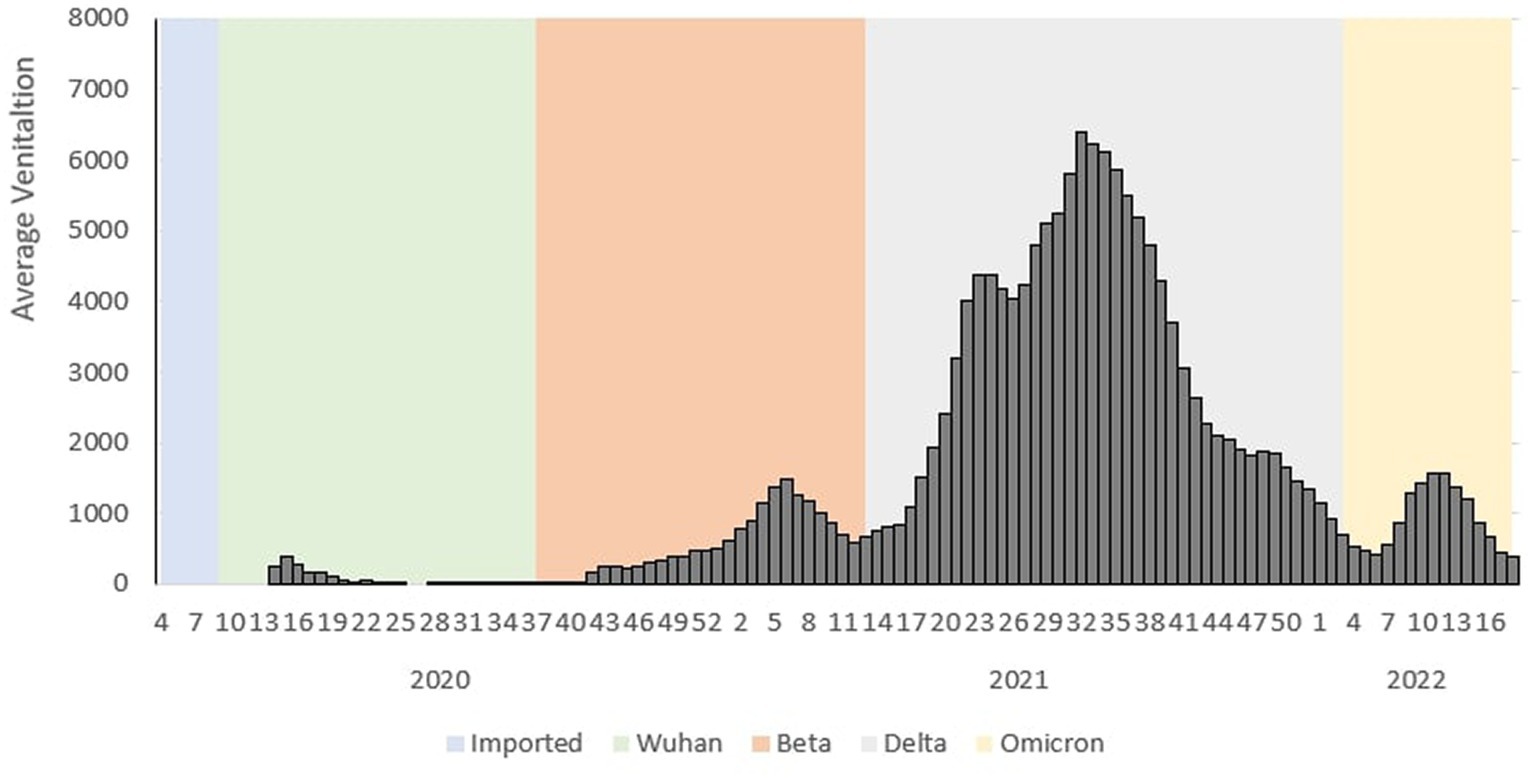
During the Wuhan outbreak, the average number of ICU cases requiring ventilation increased from Ep Wk 13/2020 (n = 252) and peaked in Ep Wk 14/2020 (n = 381). Subsequently, the average ventilated case trends decreased from Ep Wk 15/2020 (n = 279) to Ep Wk 33/2020 19 (n = 4). Following this, the Beta outbreak began where the average ventilated case trends started to once again increase from Ep Wk 34/2020 (n = 22) until Ep Wk 5/2021 (n = 1,482), and this corresponded to the longest increasing average ventilated case trends of 25 weeks. A downward trend of average ventilated cases was observed from Ep Wk 6/2021 (n = 1,270) until Ep Wk 13/2021 (n = 750).
This was followed by the Delta outbreak, wherein the average number of ICU cases requiring ventilation increased from Ep Wk 14/2021 (n = 811) and peaked in Ep Wk 31/2021 (n = 6,388). The average ventilated case trends decreased from Ep Wk 32/2021 (n = 6,217) until Ep Wk 5/2022 (n = 420), corresponding to the longest decreasing average ventilated case trend of 26 weeks. Following this, the Omicron outbreak began when the average ventilated cases started to increase Ep Wk 6/2022 (n = 558) till Ep Wk 11/2022 (n = 1,558) and subsequently decreased from Ep Wk 12/2022 (n = 1,377) till Ep Wk 18/2022 (n = 297) as shown in Figure 3.
Deaths, death rate, and CFR
Overall
There was a total of 35,579 deaths reported for the overall study period, in which the highest weekly COVID-19 death was reported in Ep Wk 37/2021 (n = 2,647), and no deaths were reported for several epidemiological weeks (17 weeks), respectively. The overall weekly average COVID-19 death rate and CFR were 0.87 per 100,000 population and 0.80%, respectively, as shown in Table 2.
By outbreak
During the Wuhan outbreak, a total of 128 (0.34%) deaths were reported, with the highest and lowest weekly COVID-19 deaths being reported in Ep Wk 13/2020 (n = 27) and several Epidemiological Weeks (6 weeks) with no deaths, respectively. While during the Beta outbreak, a total of 1,132 (3.18%) deaths were reported, with the highest and lowest weekly COVID-19 deaths being reported in week Ep Wk 5/2021 (n = 111) and Ep Wk 38 and 39/2020 (n = 3), respectively. For the Delta outbreak, a total of 30,549 (85.86%) deaths were reported, with the highest and lowest weekly COVID-19 deaths being reported in Ep Wk 37/2021 (n = 2,647) and Ep Wk 14/2021 (n = 35), respectively.
As for the Omicron outbreak, a total of 3,770 (10.6%) deaths were reported, with the highest and lowest weekly COVID-19 deaths being reported in Ep Wk 11/2022 (n = 609) and Ep Wk 18/2022 (n = 32), respectively. Overall, the highest number of cumulative deaths was reported during the Delta outbreak (n = 30,549, 85.86%), followed by the Omicron (n = 3,770, 10.60%), Beta (n = 1,132, 3.18%) and Wuhan (n = 128, 0.34%) outbreak, respectively. The CFR for the Wuhan, Beta, Delta and Omicron outbreaks was 1.42, 0.34, 1.24, and 0.23%, respectively, as shown in Table 2.
Trends
The epidemiological curve represents a sharp increase during the Wuhan outbreak, peaking at Ep Wk 14/2020 (n = 26). Following this, during the Beta outbreak, the epidemiological curve peaked at Ep Wk 5/2021 (n = 111). This was followed by the Delta outbreak, where the epidemiological curve peaked at Ep Wk 37/2021 (n = 2,647). Subsequently, during the Omicron outbreak, the epidemiological curve rose rapidly and peaked at Ep Wk 11/2022 (n = 609), as shown in Figure 4.
Gender distribution of cases
Overall
Of the total 4,456,736 cases reported during the study period, 2,379,375 (53.39%) and 2,077,361 (46.61%) were males and females, respectively. The male-to-female cases ratio was 1.2. The COVID-19 gender-specific incidence rates were 141.87 and 130.78 per 1,000 males and females, respectively (Table 3).
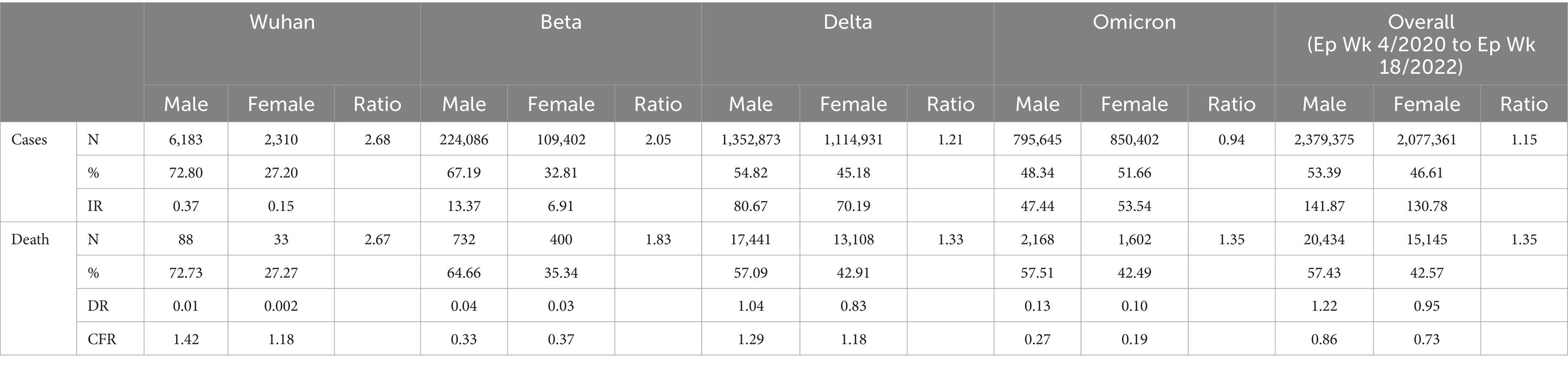
Table 3. Gender distribution of COVID-19 cases and deaths by COVID-19 variants outbreaks in Malaysia.
Outbreaks
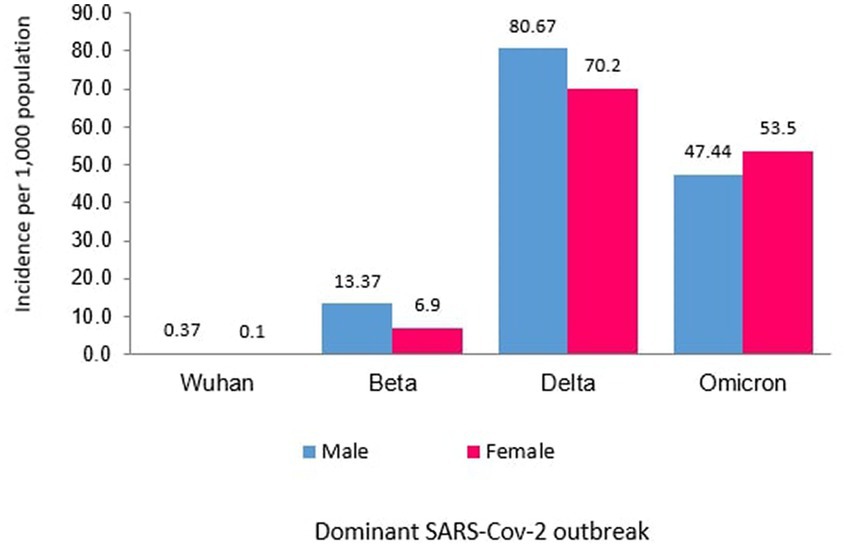
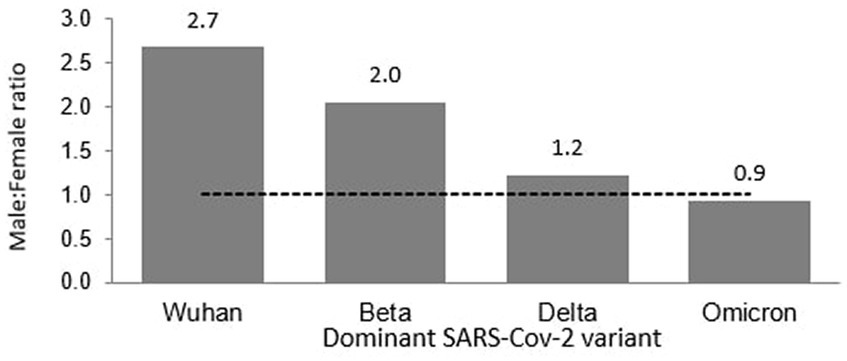
Of the 8,493 total cases reported during the Wuhan outbreak, 6,183 (72.80%) and 2,310 (27.20%) were males and females, respectively. The male-to-female cases ratio was 2.7. The COVID-19 gender-specific incidence rates per 1,000 populations for the Wuhan outbreak were 0.37 and 0.10 in males and females, respectively (Table 3; Figures 5, 6). During Beta outbreak, 333,488 cases were reported, with 224,086 (67.19%) and 109,402 (32.81%) males and females, respectively. The male-to-female cases ratio was 2.1. The COVID-19 gender-specific incidence rates per 1,000 populations for the Beta outbreak were 13.37 and 6.91 in males and females, respectively (Table 3; Figures 5, 6). Of the 2,467,804 total cases reported for the Delta outbreak, 1,352,873 (54.82%) and 1,114,931 (45.18%) were males and females, respectively. The male-to-female cases ratio was 1.2.
The COVID-19 gender-specific incidence rates per 1,000 populations for the Delta outbreak were 80.67 and 70.19 in males and females, respectively (Table 3; Figures 5, 6). As for the Omicron outbreak, 1,646,047 cases were reported, with 795,645 (48.34%) and 850,402 (51.66%) males and females, respectively. The ratio of male to female cases was 0.9. The COVID-19 gender-specific incidence rates per 1,000 populations for the Omicron outbreak were 47.44 and 53.5 in males and females, respectively (Table 3; Figures 5, 6). A higher number of COVID-19 cases and incidence rates were observed among males across all the outbreaks except for the Omicron outbreak, which reported higher numbers of COVID-19 cases and incidence rates among females (Figure 5). The male-to-female cases ratio reduced from the Wuhan outbreak to the Omicron outbreak (Figure 6).
Gender distribution of deaths
Overall
Of the 35,579 total deaths reported during the study period, 20,434 (57.43%) and 15,145 (42.57%) were males and females, respectively. The male-to-female deaths ratio was 1.4. The COVID-19 gender-specific death rates per 1,000 populations during this study period were 1.22 and 0.95 in males and females, respectively. The COVID-19 gender-specific CFR rates during this study period were 0.86 and 0.73% in males and females, respectively (Table 3).
Outbreaks
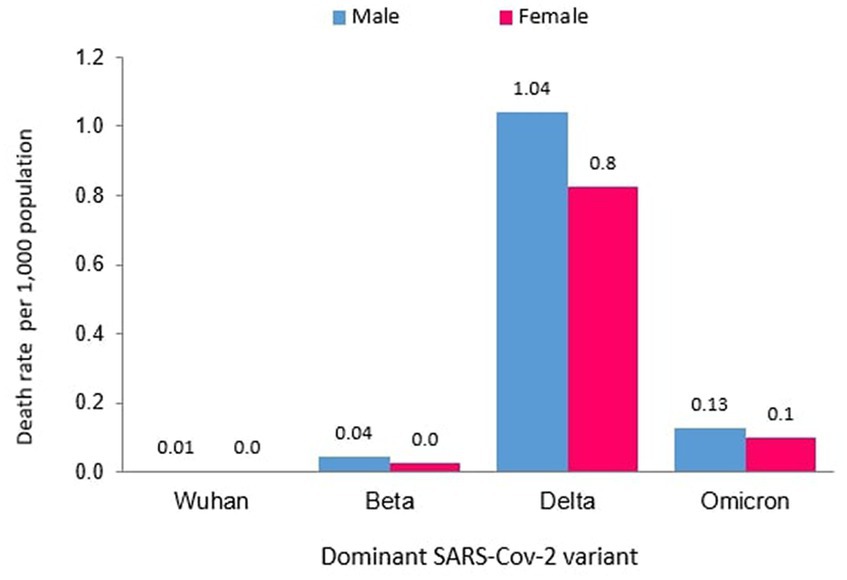
Of the 121 total deaths reported during the Wuhan outbreak, 88 (72.73%) and 33 (27.27%) were males and females, respectively. The male-to-female deaths ratio was 2.7. The COVID-19 gender-specific death rates per 1,000 populations for the Wuhan outbreak were 0.01 and 0.002 in males and females, respectively. The COVID-19 gender-specific CFR rates for the Wuhan outbreak were 1.42 and 1.18% in males and females, respectively (Table 3). During the Beta outbreak, 1,132 total deaths were reported, with 732 (64.66%) and 400 (35.34%) being males and females, respectively. The ratio of male to female deaths was 1.8. The COVID-19 gender-specific death rates per 1,000 populations for the Beta outbreak were 0.04 and 0.03 in males and females, respectively. The COVID-19 gender-specific CFR rates for the Beta outbreak were 0.33 and 0.37% in males and females, respectively (Table 3). Of the 30,549 total deaths reported in the Delta outbreak, 17,441(57.09%) and 13,108 (42.91%) were males and females, respectively. The ratio of male to female deaths was 1.3.
The COVID-19 gender-specific death rates per 1,000 populations for the Delta outbreak were 1.04 and 0.83 in males and females, respectively. The COVID-19 gender-specific CFR rates for the Delta outbreak were 1.29 and 1.18% in males and females, respectively (Table 3). As for the Omicron outbreak, 3,770 deaths were reported, with 2,168 (57.51%) and 1,602 (42.49%) males and females, respectively. The male-to-female deaths ratio was 1.4. The COVID-19 gender-specific death rates per 1,000 populations for the Omicron outbreak were 0.13 and 0.10 in males and females, respectively. The COVID-19 gender-specific CFR rates for the Omicron outbreak were 0.27 and 0.19% in males and females, respectively (Table 3; Figure 7). A higher number of COVID-19 deaths, death rate and CFR were observed among males across all the outbreaks except for the Beta outbreak, where a higher CFR was observed among females.
Age distribution of cases
Overall
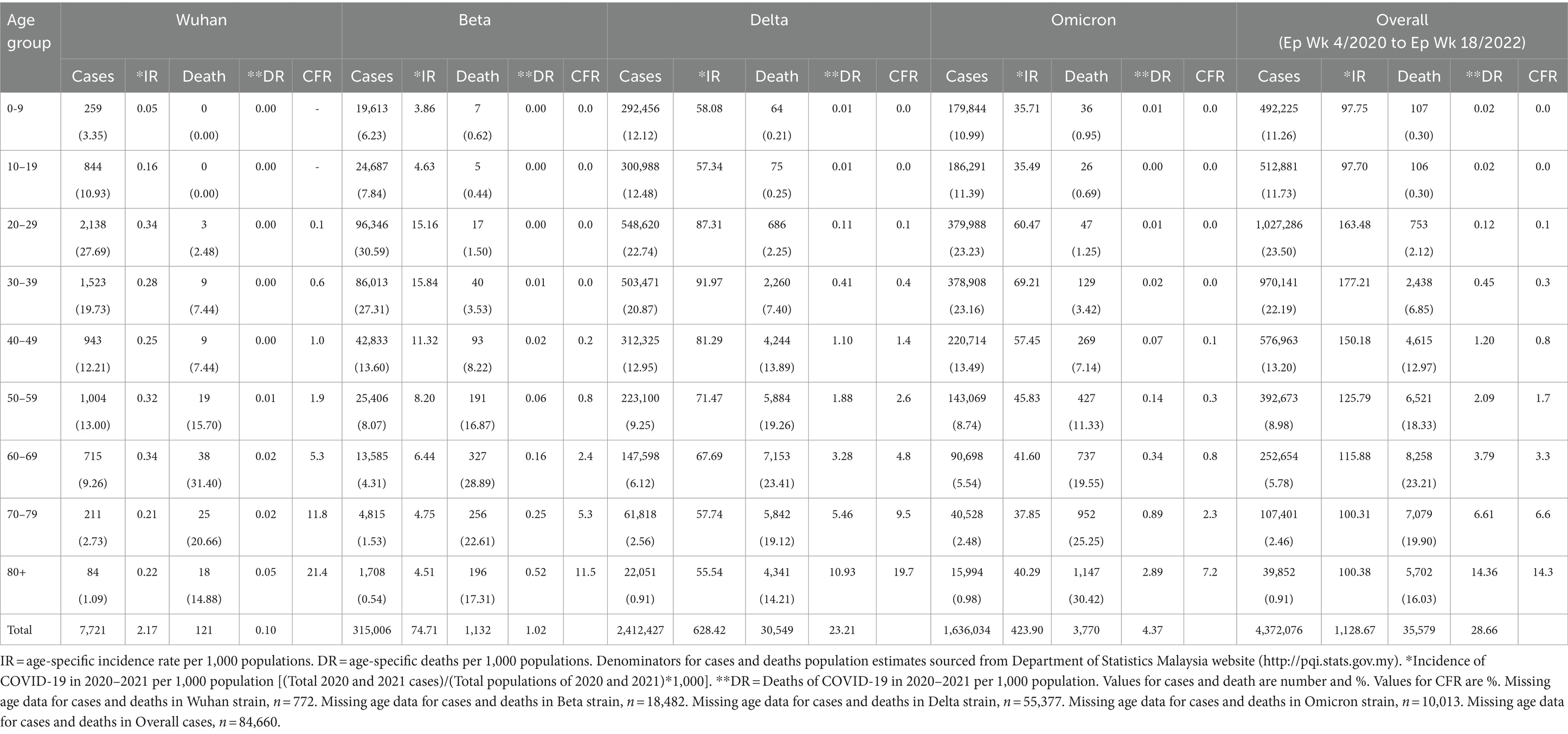
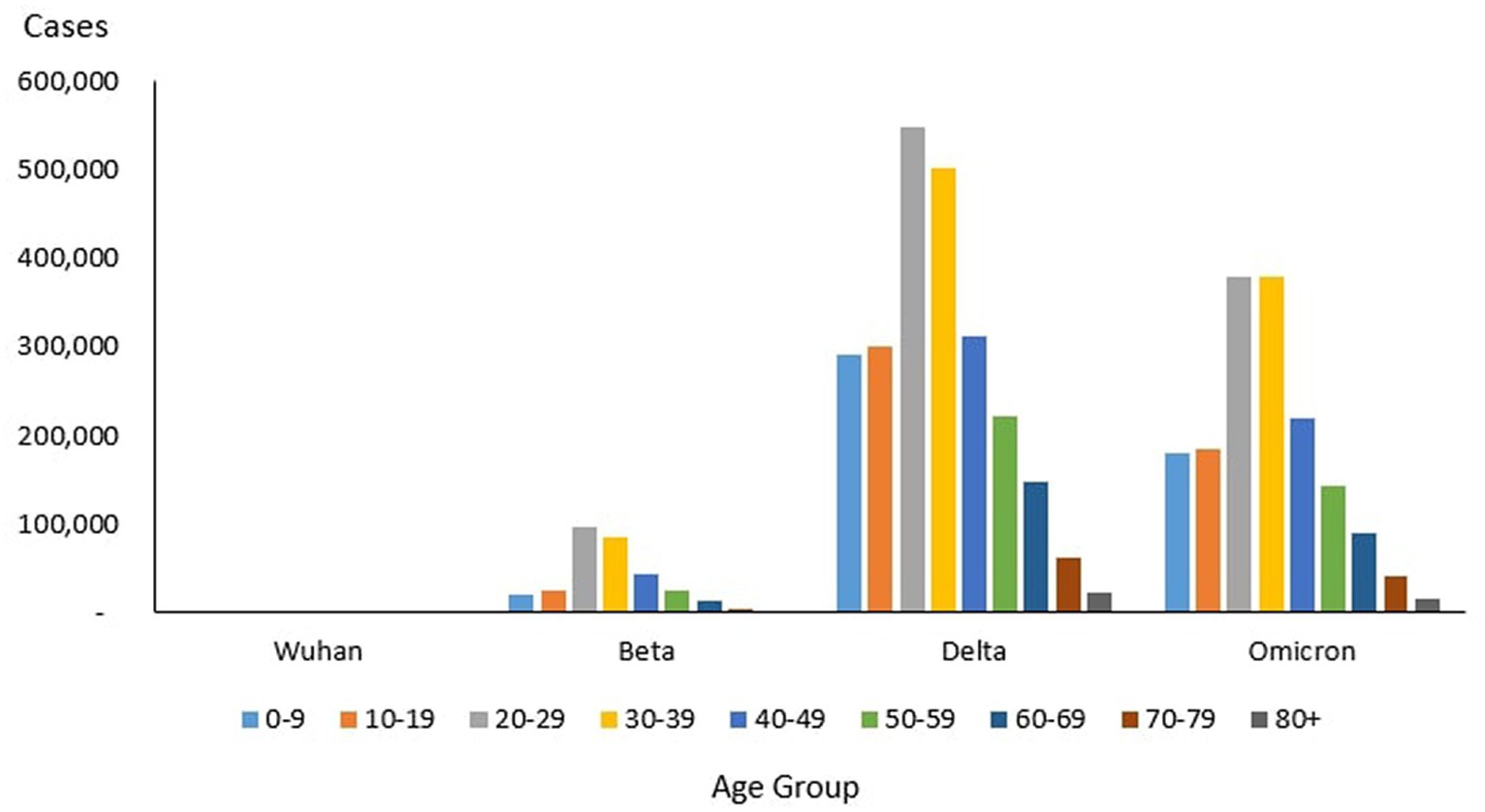
From the total of 4,456,736 cases reported during the study period, the age group with the highest and lowest cases were reported for individuals aged 20–29 years (n = 1,027,286) and 80+ years (n = 39,852), respectively. The proportion of COVID-19 cases by 10-year age during the study period are as follows: 0–9 years (11.26%), 10–19 years (11.73%), 20–29 years (23.50%), 30–39 years (22.19%), 40–49 years (13.20%), 50–59 years (8.98%), 60–69 years (5.78%), 70–79 years (2.46%) and more than 80 years (0.91%) as shown in Table 4. The COVID-19 age-specific incidence rates per 1,000 populations by the 10-year age groups for the study period were highest and lowest for individuals ages 30–39 years (incidence rates 177.21) and 10–19 years (incidence rates 97.7), respectively (Figure 8).
Outbreaks
Of the 8,493 cases reported during the Wuhan outbreak, the highest and lowest cases were in the age group 20–29 years (n = 2,138) and 80+ years (n = 84), respectively. The proportion of COVID-19 cases by 10-year age group for the Wuhan outbreak are as follows: 0–9 years (3.35%), 10–19 years (10.93%), 20–29 years (27.69%), 30–39 years (19.73%), 40–49 years (12.21%), 50–59 years (13.0%), 60–69 years (9.26%), 70–79 years (2.73%), and 80+ years (1.09%). The COVID-19 age-specific incidence rates per 1,000 populations in 10-year age groups during the Wuhan outbreak were highest for individuals ages 20–29 years and 60–69 years (incidence rates 0.34) and lowest for individuals ages 0–9 years (incidence rates 0.05) respectively (Table 4; Figures 9, 10).
During the Beta outbreak, 333,488 cases were reported, with the highest and lowest cases in the age group 20–29 years (n = 96,346) and 80+ years (n = 1,708), respectively. The proportion of COVID-19 cases by 10-year age group for the Beta outbreak are as follows: 0–9 years (6.23%), 10–19 years (7.84%), 20–29 years (30.59%), 30–39 years (27.31%), 40–49 years (13.6%), 50–59 years (8.07%), 60–69 years (4.31%), 70–79 years (1.53%) and 80+ years (0.54%). The COVID-19 age-specific incidence rates per 1,000 populations in 10-year age groups during the Beta outbreak were highest for individuals ages 30–39 years (incidence rates 15.84) and lowest for individuals ages 0–9 years (incidence rates 3.86) respectively (Table 4; Figures 9, 10).
During the Delta outbreak, 2,467,804 cases were reported, with the highest and lowest cases in the age group 20–29 years (n = 548,620) and 80+ years (n = 2,251), respectively. The proportion of COVID-19 cases by 10-year age group for the Delta outbreak are as follows: 0–9 years (12.12%), 10–19 years (12.48%), 20–29 years (22.74%), 30–39 years (20.87%), 40–49 years (12.95%), 50–59 years (9.25%), 60–69 years (6.12%), 70–79 years (2.56%) and 80+ years (0.91%). The COVID-19 age-specific incidence rates per 1,000 populations in 10-year age groups during the Delta outbreak were highest for individuals ages 30–39 years (incidence rates 91.97) and lowest for individuals ages 80+ years (incidence rates 55.54) respectively (Table 4; Figures 9, 10).
Of the 1,646,047 cases reported during the Omicron outbreak, the age group with the highest and lowest cases were 20–29 years (n = 379,988) and 80+ years (n = 15,994), respectively. The proportion of COVID-19 cases by 10-year age group for the Omicron outbreak are as follows: 0–9 years (10.99%), 10–19 years (11.39%), 20–29 years (23.23%), 30–39 years (23.16%), 40–49 years (13.49%), 50–59 years (8.74%), 60–69 years (5.54%), 70–79 years (2.48%) and 80+ years (0.98%). The COVID-19 age-specific incidence rates per 1,000 populations in 10-year age groups during the Omicron outbreak were highest for individuals ages 30–39 years (incidence rates 69.21) and lowest for individuals ages 10–19 years (incidence rates 35.49), respectively (Table 4; Figures 11, 12).
Across all the outbreaks, the highest and lowest number of COVID-19 cases by age was reported for individuals aged 20–29 and over 80 years, respectively. The number of COVID-19 cases by age starts to reduce among individuals ages 30 and above across all the outbreaks (Figure 8). For all the outbreaks, the highest COVID-19 age-specific incidence rate was reported among individuals aged 30–39 years, except for the Wuhan outbreak, which had the highest incidence rate among individuals aged 20–29 years and 60–69 years, respectively. The lowest COVID-19 age-specific incidence rate was reported among individuals in the age groups 0–9 years and 10–19 years for all outbreaks except for the Delta outbreak, which reported the lowest COVID-19 age-specific incidence rate among individuals in the age group more than 80 years (Figure 10).
Age distribution of death
Overall
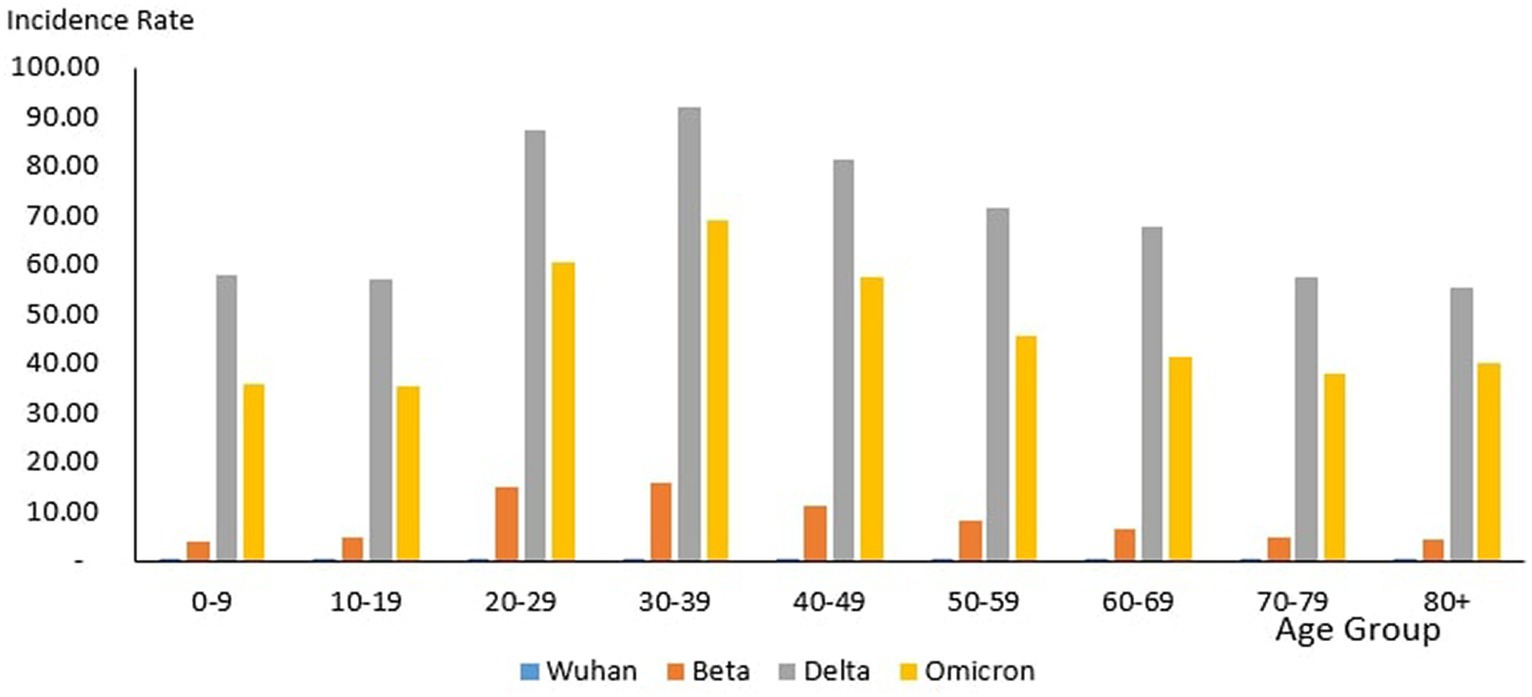
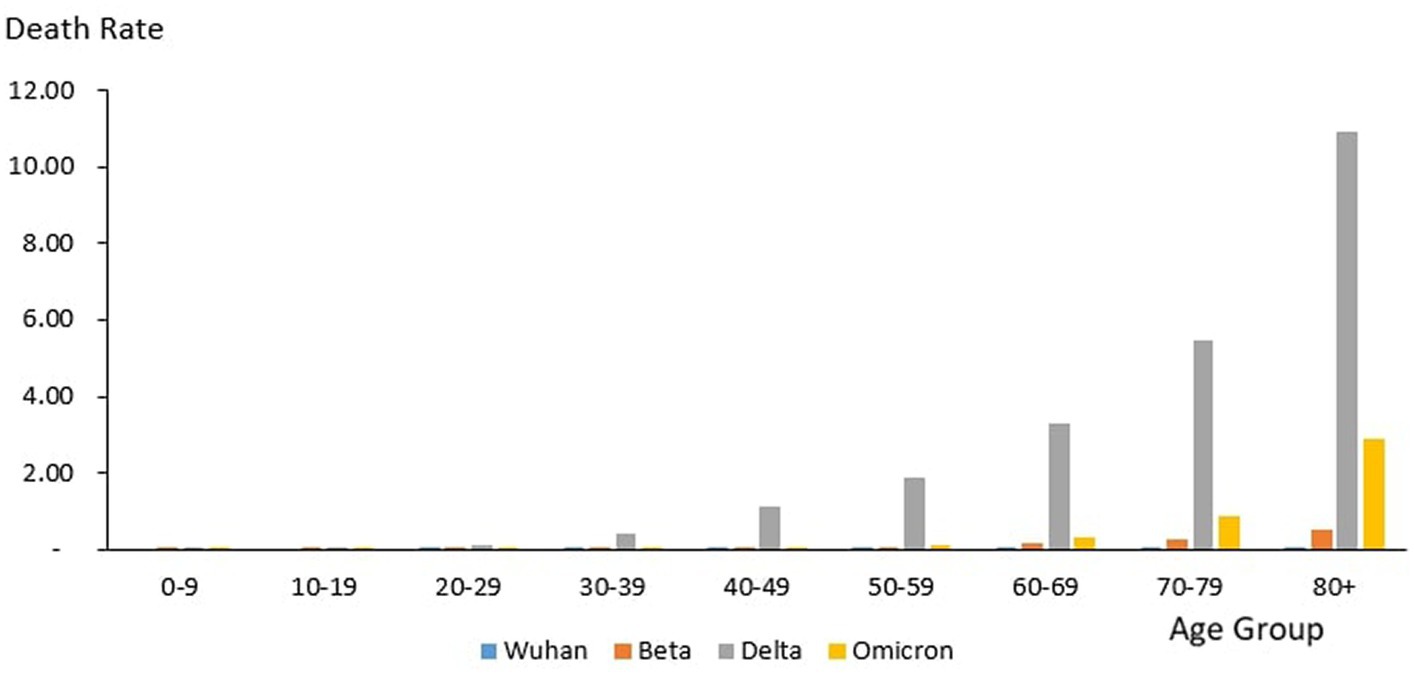
From the total of 35,579 deaths reported during the study period, the highest and lowest deaths were reported in the age group 60–69 years (n = 8,258) and 10–19 years (n = 106), respectively. The proportion of COVID-19 deaths by 10-year age group during the study period is as follows: 0–9 years (0.3%), 10–19 years (0.3%), 20–29 years (2.12%), 30–39 years (6.85%), 40–49 years (12.97%), 50–59 years (18.33%), 60–69 years (23.21%), 70–79 years (19.90%) and 80+ years (16.03%). The COVID-19 age-specific death rates per 1,000 populations in 10-year age groups during the study period were highest for individuals ages 80+ years (death rates 14.36) and lowest for individuals ages 0–9 years and 10–19 years (death rates 0.02), respectively. The COVID-19 age-specific CFR rates in 10-year age groups during the study period were highest for individuals ages 80+ years (CFR 14.3%) and lowest for individuals ages 0–9 years and 10–19 years (CFR 0%) respectively (Table 4).
Outbreaks
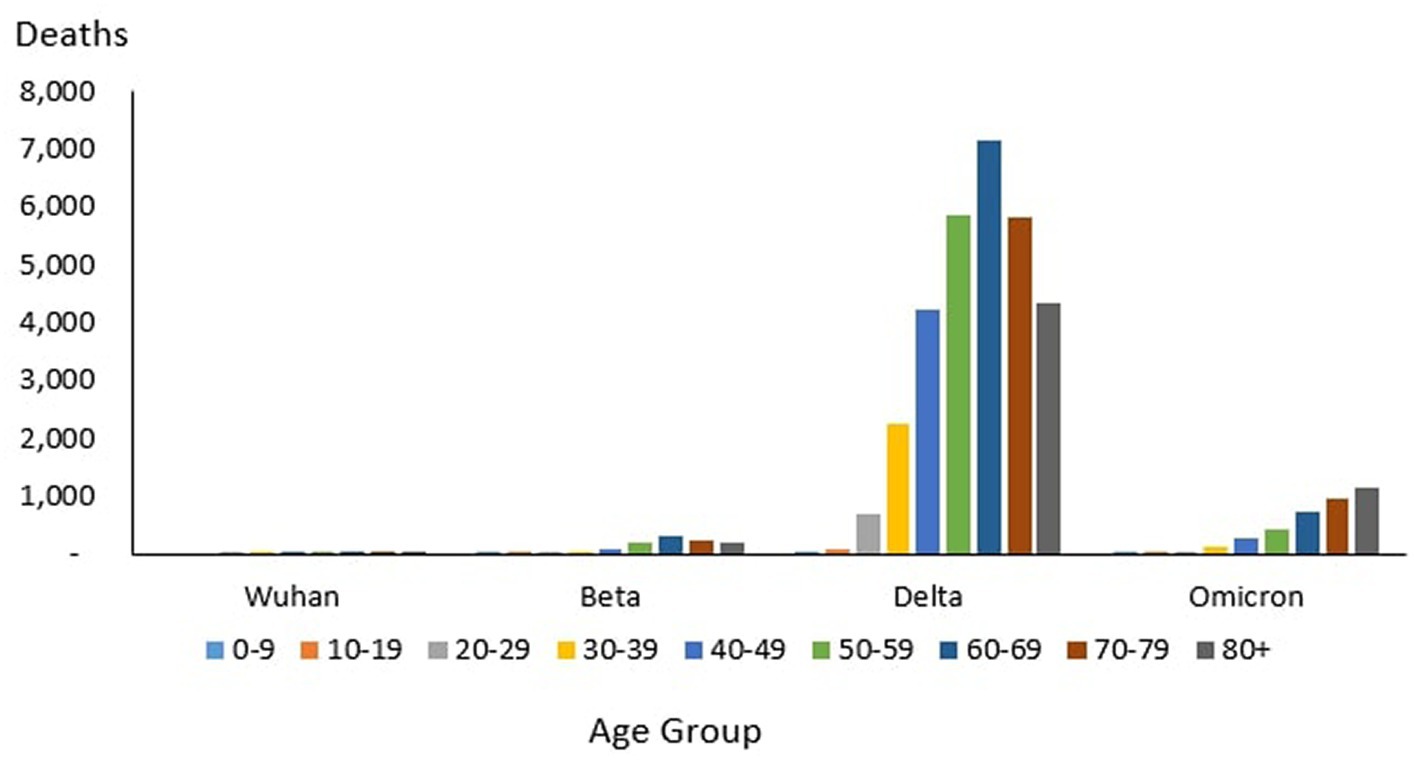
From the total of 121 deaths reported during the Wuhan outbreak, the highest and lowest deaths were reported in the age group 60–69 years (n = 38) and the group 0–9 years and 10–19 years (n = 0), respectively. The proportion of COVID-19 deaths by 10-year age group for the Wuhan outbreak is as follows: 0–9 years (0.0%), 10–19 years (0.0%), 20–29 years (2.48%), 30–39 years (7.44%), 40–49 years (7.44%), 50–59 years (15.7%), 60–69 years (31.4%), 70–79 years (20.66%) and 80+ years (14.88%). The COVID-19 age-specific death rates per 1,000 populations in 10-year age groups for the Wuhan outbreak were highest for individuals ages 80+ years (death rates 0.05) and lowest for the age group between 20 and 29 years (death rates 0.0005), respectively. The COVID-19 age-specific CFR rates in 10-year age groups for the Wuhan outbreak were highest for individuals ages 80+ years (CFR 21.4%) and lowest for individuals ages 20–29 years (CFR 0.1%), respectively (Table 4; Figure 11).
During the Beta outbreak, a total of 1,132 deaths were reported, with the highest and lowest deaths noted in the age group 60–69 years (n = 327) and 10–19 years (n = 5), respectively. The proportion of COVID-19 deaths by 10-year age group for the Beta outbreak are as follows: 0–9 years (0.62%), 10–19 years (0.44%), 20–29 years (1.50%), 30–39 years (3.53%), 40–49 years (8.22%), 50–59 years (16.87%), 60–69 years (28.89%), 70–79 years (22.61%) and 80+ years (17.31%). The COVID-19 age-specific death rates per 1,000 populations in 10-year age groups for the Beta outbreak were highest and lowest for individuals ages 80+ years (death rates 0.52) and 0–9 years and 10–19 years (death rates 0.001), respectively. The COVID-19 age-specific CFR rates in 10-year age groups for Beta outbreak were highest for individuals ages 80+ years (CFR 11.5%) and lowest for individuals ages 0–39 years (CFR 0.1%), respectively (Table 4; Figure 11).
During the Delta outbreak, 30,549 deaths were reported, with the highest and lowest deaths in the age group of 60–69 years (n = 7,153) and 0–9 years (n = 64), respectively. The proportion of COVID-19 deaths by 10-year age group for the Delta outbreak are as follows: 0–9 years (0.21%), 10–19 years (0.25%), 20–29 years (2.25%), 30–39 years (7.40%), 40–49 years (13.89%), 50–59 years (19.26%), 60–69 years (23.41%), 70–79 years (19.12%) and 80+ years (14.21%). The COVID-19 age-specific death rates per 1,000 populations in 10-year age groups for the Delta outbreak were highest for individuals ages 80 + years (death rates 10.93) and lowest for individuals aged 0–9 years and 10–19 years (death rates 0.01), respectively. The COVID-19 age-specific CFR rates in 10-year age groups for the Delta outbreak were highest for individuals ages 80+ years (CFR 19.7%) and lowest for individuals ages 0–9 and 10–19 years (CFR 0.0%) respectively (Table 4; Figure 11).
Of the 3,770 deaths reported during the Omicron outbreak, the age group with the highest number of deaths was 80+ years (n = 1,147), and the lowest was 10–19 years (n = 26), respectively. The proportion of COVID-19 deaths by 10-year age group for the Omicron outbreak are as follows: 0–9 years (0.95%), 10–19 years (0.69%), 20–29 years (1.25%), 30–39 years (3.42%), 40–49 years (7.14%), 50–59 years (11.33%), 60–69 years (19.55%), 70–79 years (25.25%) and 80+ years (30.42%). The COVID-19 age-specific death rates per 1,000 populations in 10-year age groups for the Omicron outbreak were highest for individuals aged 80+ years (death rates 2.89) and lowest for individuals aged 10–19 years (death rates 0.0), respectively. The COVID-19 age-specific CFR rates in 10-year age groups for Omicron outbreak were highest for individuals ages 80+ years (CFR 7.2%) and lowest for individuals ages 0–39 years (CFR 0.0%), respectively (Table 4; Figure 11).
Across all the outbreaks, the highest and lowest number of COVID-19 deaths by age was reported among individuals in the age groups of 60–69 years and 0–19 years, respectively, except for the Omicron outbreak, which reported the highest number of COVID-19 death by age among individual age group 80 and above. The number of COVID-19 deaths by age starts to reduce among individuals ages 70 and above across all the outbreaks (Figure 9) except during the Omicron outbreak, in which COVID-19 deaths tend to increase with advancing age. Across all the outbreaks, the highest COVID-19 age-specific death rate was reported among individuals in the age groups of 80 and above years for all the outbreaks. The lowest COVID-19 age-specific death rate was reported among individuals in the age groups 10–19 years for all outbreaks except for the Wuhan outbreak, which reported the lowest COVID-19 age-specific death rate among individuals in the age group of 20–29 years (Figure 9). Similarly, the highest age-specific CFR was reported among individuals aged 80 years and above across all the outbreaks.
Testing and test positivity rate
Overall
A total of 58,906,954 COVID-19 tests were conducted during the study period in which the highest weekly COVID-19 total test (RT-PCR and RTK-AG) and the total test positivity rate (RT PCR and RTK Ag) was reported at 1,788,410 tests (Ep Wk7/2022) and 7.57%, respectively, (Table 2).
Outbreaks
During the Wuhan outbreak, a total of 853,379 (1.5%) tests were done, wherein the highest (n = 86,018) and lowest (n = 586) weekly COVID-19 total test was reported in Ep Wk18/2020 and Ep Wk 9, respectively. The test positivity rate was reported at 1.0% during this period. As for the Beta outbreak, a total of 8,781,144 (14.91%) tests were done where the highest (n = 629,225) and lowest (n = 51,673) weekly COVID-19 total test were reported in Ep Wk 3/2021 and Ep Wk 37/2020, respectively. The test positivity rate was reported at 3.8% during this period.
In the time of the Delta outbreak, a total of 33,603,291(57.04%) tests were done where the highest (n = 1,119,105) and lowest (n = 231,648) weekly COVID-19 total tests were reported in Ep Wk 33/2021 and Ep Wk 3/2022, respectively. The test positivity rate was reported at 7.34% during this period. During the Omicron outbreak, a total of 15,157,523 (25.73%) tests were done where the highest (n = 1,788,410) and lowest (n = 221,636) weekly COVID-19 total tests were reported in Ep Wk 7/2022 and Ep Wk 18/2022, respectively. The test positivity rate was reported at 10.86%, as shown in Table 2. The Wuhan, Beta, Delta, and Omicron outbreak test positivity rates were 0.995, 3.798, 7.344, and 10.860, respectively.
Trends
During the Wuhan outbreak, the number of tests increased from Ep Wk 10 (n = 3,904) and peaked in Ep Wk 18 (n = 86,018). Subsequently, the number of tests decreased from Ep Wk 19 (n = 82,072) to Ep Wk 25 (n = 40,609). Following this, the Beta outbreak began, where testing trends started to increase from Ep Wk 38/2020 (n = 66,922) and peaked in Ep Wk 3/2021 (n = 629,225). Subsequently, a downward testing trend has been observed from Ep Wk 4/2021 (n = 544,745) till Ep Wk 13/2021 (n = 369,701). This was followed by the Delta outbreak, wherein the testing trends started to increase from Ep Wk 14/2021 (n = 382,171) and peaked in Ep Wk 33/2021 (n = 1,119,015), which corresponded to the longest increase of testing trends of 33 weeks. A downward trend of testing has been observed from Ep Wk 34/2021 (n = 1,071,056) till Ep Wk 3/2022 (n = 776,217), corresponding to the longest decrease in the testing trend of 23 weeks. Following this, the Omicron outbreak began where a testing trend started to increase from Ep Wk 4/2022 (n = 831,806) and peaked at Ep Wk 7/2022 (n = 1,788,410), which was followed by a downward trend of testing from Ep Wk 8/2022 (n = 1,596,093) to Ep Wk 18/2022 (n = 221,636) as shown in Figure 13.
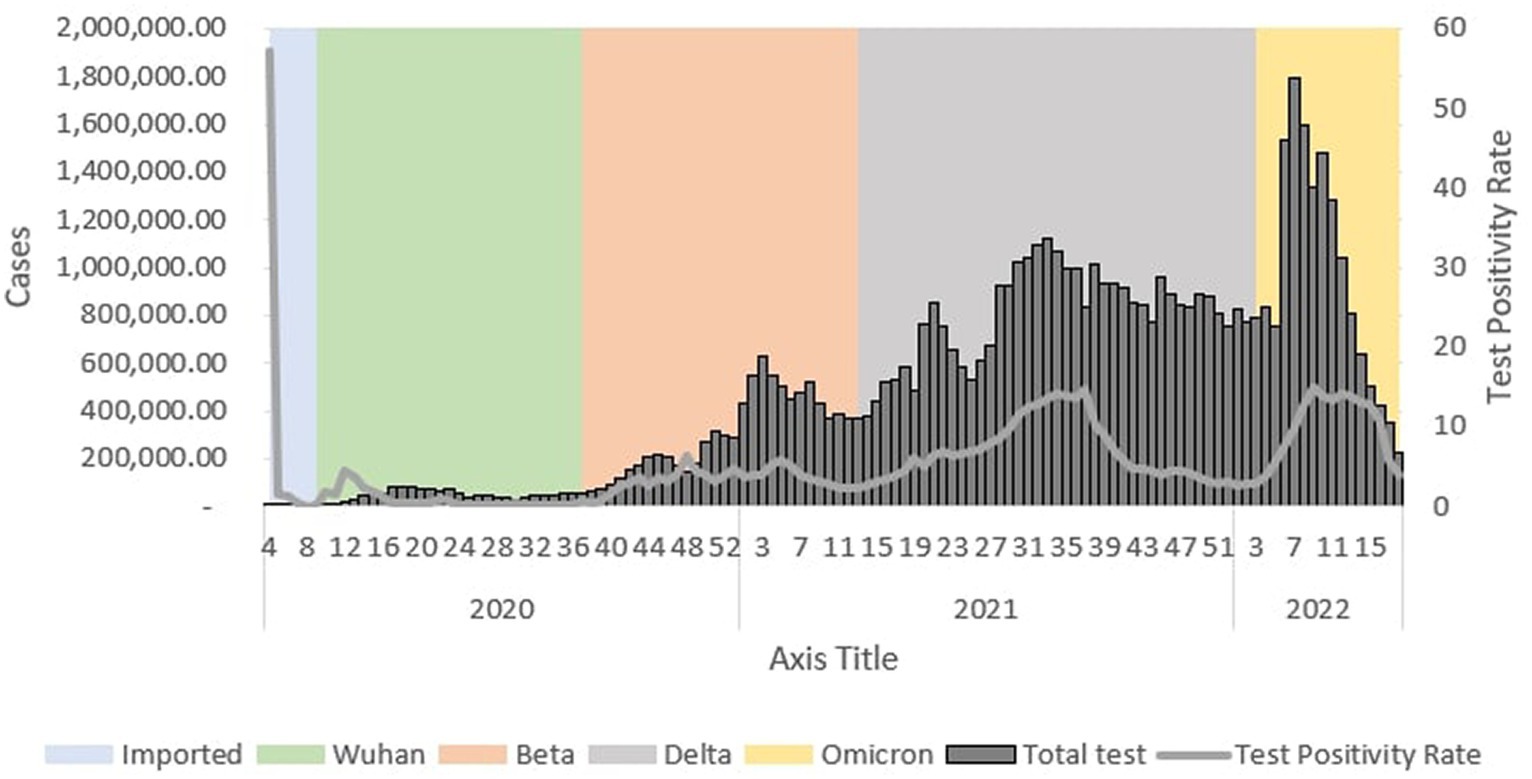
Figure 13. Weekly total COVID-19 tests and test positivity rate by outbreaks in Malaysia, 2020-2022.
Discussion
This study described the COVID-19 case demographics, hospital admissions and testing capacities for the four outbreaks, namely the Wuhan, Beta, Delta and Omicron, from the beginning of the outbreak in January 2020 till it was declared endemic in April 2022. A total of 4,456,736 cases was analyzed in this study, which showed that the Delta and Omicron outbreaks reported the highest numbers of COVID-19 cases, which was also found in studies in India and Bangladesh (21–25). These findings can be explained by the increased transmissibility in the Delta and Omicron variants of the COVID-19 virus. Moreover, studies have reported that the Delta variant is 50% more transmissible than the Alpha, Beta or Wuhan variants (26). In addition, a higher number of infections that occurred during the Delta and Omicron outbreaks can be attributed to the longer outbreak duration of these variants. Furthermore, the easing of movement control measures resulted in increased travel and population mobility during the Delta and Omicron outbreaks, which allowed for further spread of the disease. Finally, increased testing capacity during the Delta and Omicron outbreaks led to higher case detection, resulting in increased case numbers. This higher transmissibility, longer outbreak period and increased population mobility and testing contributed to conditions that caused the massive spread of the disease, causing extreme suffering of the populations as well as overwhelming the healthcare systems (27). The presence of highly transmissible variants would require an urgent need for the health systems to closely monitor the outbreak and promptly institute outbreak control measures (28).
This study also found higher COVID-19 incidence among males aged between 20 and 39. This finding can be explained by individuals in this age group being more socially active, therefore having an increased risk of being infected. In addition, differences in behavior and immunological factors may also explain the increased prevalence of the disease in this population (29). This could be attributed to high-risk behaviors such as smoking and alcohol consumption among males, resulting in more severe forms of COVID-19 disease (30). Studies conducted by the Center for Disease Control (CDC) in the United States also showed similar findings affecting males in this age group to have higher infection rates and deaths (30–32).
This study showed that the highest number of patients requiring ICU admission, ventilator support, and deaths occurred during the Delta outbreak as compared to the other outbreaks. The increased morbidity and mortality of the Delta variant can be attributed to higher virulence, resulting in more severe forms of the disease (33). In addition, the longer duration of the Delta outbreak, causing a prolonged, sustained combination of high case numbers with more severe forms of the disease, would overwhelm the healthcare system, limiting the availability of the healthcare system to provide patient care, which would ultimately increase case mortality (33–35). The increased morbidity and mortality of the Delta variant were also observed in a meta-analysis involving 16 countries worldwide, including studies in the US, UK and India, where this finding was attributed to the variant’s higher disease severity and transmissibility (36–38).
The CFR during the Delta outbreak was reported at 1.24, which corresponded to the second-highest CFR during the study period. While the highest CFR was reported during the Wuhan outbreak at 1.42, the high CFR during the Wuhan outbreak is an effect of limited testing with a low detection rate, which biased the high observed CFR during this period. Interestingly, a reduction in trends of COVID-19 patients requiring ICU admission, ventilator support and mortality was observed as the Delta outbreak progressed. This can be attributed to the vaccination programs that had resulted in 70% of the population completing two doses of COVID-19 vaccination as of mid-2022 in Malaysia (39, 40).
A cumulative of 58,906,954 COVID-19 tests were conducted during the pandemic period, and it was observed that the test positivity rate has been increasing since the beginning of the outbreak in Malaysia. The test positivity rate for the different outbreaks was reported for the Wuhan (1.0), Beta (3.8), Delta (7.34), and Omicron (10.86) outbreaks, respectively. In addition, this study found that the highest number of COVID-19 tests was reported during the Delta outbreak in Malaysia. The reason could be due to the longer duration of the Delta outbreak (n = 43 weeks) compared to other outbreaks and the increased transmissibility of the virus. The longer outbreak duration allows more testing to be conducted due to increased testing capacity during the Delta outbreak (41–44) Furthermore, there was an increased requirement for COVID-19 testing for certain socio-economic activities such as return to work, travel and pre-operative procedures during the Delta outbreak and with the introduction of simple self-testing via the rapid antigen test made accessible to the public (45–47). The weekly COVID-19 test requirement since August 2021 by the health authorities for back-to-work employees to prevent the spread of COVID-19 has also greatly contributed to the increase in testing done nationally (45–47).
Conclusion
The Delta outbreak was the most severe compared to other outbreaks during the study period in Malaysia. This is evident by the highest number of COVID-19 cases, ICU admissions, ventilatory requirements and deaths observed during the Delta outbreak. In addition, this study provides evidence that outbreaks of COVID-19, which are caused by highly virulent and transmissible variants, tend to be more severe and devastating if they are not controlled early on. This was the first attempt to describe the COVID-19 outbreak in detail. Hence, close monitoring of key epidemiological indicators, as reported in this study, is essential in the control and management of future COVID-19 outbreaks in Malaysia.
Data availability statement
The raw data that supports the findings of this study are available on request from the corresponding author.
Ethics statement
The study was registered with the National Medical Research Register (NMRR ID-22-00940-PF6).
Author contributions
MM: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. AM: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. SS: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. MS: Writing – review & editing, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. CL: Writing – review & editing, Visualization, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation. CT: Writing – review & editing, Visualization, Methodology, Investigation, Formal analysis, Data curation. TA: Writing – review & editing, Visualization, Supervision, Project administration. HM: Writing – review & editing, Validation, Supervision, Methodology, Investigation. BS: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology. N’AM: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. NM: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Methodology, Investigation. ML: Writing – review & editing, Methodology, Investigation. LA: Writing – review & editing, Methodology, Investigation. MK: Writing – review & editing, Visualization, Methodology, Investigation, Formal analysis. NA: Writing – review & editing, Methodology, Investigation, Data curation. KT: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Investigation. AZ: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Investigation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the Director General of Health Malaysia for his permission to publish this paper and the Director of the Institute for Medical Research for all the support given toward this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Jin, Y, Yang, H, Ji, W, Wu, W, Chen, S, Zhang, W, et al. Viruses virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 12:372. doi: 10.3390/v12040372
2. Khachfe, HH, Chahrour, M, Sammouri, J, Salhab, HA, Makki, BE, and Fares, MY. An epidemiological study on COVID-19: a rapidly spreading disease. Cureus. (2020) 12:e7313. doi: 10.7759/cureus.7313
3. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Available at: https://covid19.who.int/ (Accessed March 1, 2022).
4. Malaysia confirms first cases of coronavirus infection | Reuters. Available at: https://www.reuters.com/article/china-health-malaysia/malaysia-confirms-first-cases-of-coronavirus-infection-idUSL4N29U03A (Accessed December 6, 2022).
5. Tan, KK, Tan, JY, Wong, JE, Teoh, BT, Tiong, V, Abd-Jamil, J, et al. Emergence of B.1.524(G) SARS-CoV-2 in Malaysia during the third COVID-19 epidemic wave. Sci Rep. (2021) 11:1–12. doi: 10.1038/s41598-021-01223-4
6. First Indian strain Covid-19 case detected in Malaysia. Available at: https://www.nst.com.my/news/nation/2021/05/687231/first-indian-strain-covid-19-case-detected-malaysia (Accessed December 6, 2022).
7. Khairy: first Omicron case detected in Malaysia on Dec 2 | The Edge Markets. Available at: https://www.theedgemarkets.com/article/covid19-malaysia-detects-first-omicron-case-khairy (Accessed December 6, 2022).
8. Jindahra, P, Wongboonsin, K, and Wongboonsin, P. Demographic and initial outbreak patterns of COVID-19 in Thailand. J Popul Res. (2021) 39:567–88. doi: 10.1007/s12546-021-09276-y
9. Bhuiyan, MU, Stiboy, E, Hassan, MZ, Chan, M, Islam, MS, Haider, N, et al. Epidemiology of COVID-19 infection in young children under five years: a systematic review and meta-analysis. Vaccine. (2021) 39:667–77. doi: 10.1016/j.vaccine.2020.11.078
10. Palladino, R, Bollon, J, Ragazzoni, L, and Barone-Adesi, F. Excess deaths and hospital admissions for COVID-19 due to a late implementation of the lockdown in Italy. Int J Environ Res Public Health. (2020) 17:5644. doi: 10.3390/ijerph17165644
11. Wongtanasarasin, W, Srisawang, T, Yothiya, W, Phinyo, P, Chiang Mai Hospital, N, and Mai, C. Impact of national lockdown towards emergency department visits and admission rates during the COVID-19 pandemic in Thailand: a hospital-based study. Emerg Med Australas. (2020) 33:316–23. doi: 10.1111/1742-6723.13666
12. Bernal, JL, Andrews, N, Gower, C, Robertson, C, Stowe, J, Tessier, E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. (2020) 373:n1088. doi: 10.1136/bmj.n1088
13. Raman, R, Patel, KJ, and Ranjan, K. COVID-19: unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules. (2021) 11:993. doi: 10.3390/biom11070993
14. Ng, DCE, Tan, KK, Chin, L, Ali, MM, Lee, ML, Mahmood, FM, et al. Clinical and epidemiological characteristics of children with COVID-19 in Negeri Sembilan, Malaysia. Int J Infect Dis. (2021) 108:347–52. doi: 10.1016/j.ijid.2021.05.073
15. Yarmol-Matusiak, EA, Cipriano, LE, and Stranges, S. A comparison of COVID-19 epidemiological indicators in Sweden, Norway, Denmark, and Finland. Scand J Public Health. (2021) 49:69–78. doi: 10.1177/1403494820980264
16. GitHub. MoH-Malaysia/covid19-public: official data on the COVID-19 epidemic in Malaysia. CPRC, CPRC Hospital System, MKAK, and MySejahtera. Available at: https://github.com/MoH-Malaysia/covid19-public (Accessed July 26, 2022).
17. Home | COVID-19 MALAYSIA. Available at: https://covid-19.moh.gov.my/ (Accessed July 26, 2022).
18. PM: M’sia will transition into endemic phase from April 1 | The Star. Available at: https://www.thestar.com.my/news/nation/2022/03/08/pm-msia-will-enter-endemic-phase-from-april-1 (Accessed December 23, 2022).
19. Singh, S, Herng, LC, Sulaiman, LH, Wong, SF, Jelip, J, Mokhtar, N, et al. The effects of meteorological factors on dengue cases in Malaysia. Int J Environ Res Public Health. (2022) 19:6449. doi: 10.3390/ijerph19116449
20. Downloading IBM SPSS Statistics 24. Available at: https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-24 (Accessed March 7, 2022).
21. Medley, GF. A consensus of evidence: The role of SPI-M-O in the UK COVID-19 response. Adv Biol Regul. (2022) 86:100918. doi: 10.1016/j.jbior.2022.100918
22. Delta Variant Detected in 99 percent of U.S. cases, C.D.C. Says - The New York Times. https://www.nytimes.com/2021/09/18/health/delta-covid-us-cases-cdc.html (Accessed December 6, 2022).
23. U.S. COVID-19 deaths reach 800,000 as Delta ravaged in 2021 | Reuters. Available at: https://www.reuters.com/business/healthcare-pharmaceuticals/us-covid-19-deaths-approach-800000-delta-ravaged-2021-2021-12-12/. (Accessed December 6, 2022).
24. Saha, S, Tanmoy, AM, Tanni, AA, Goswami, S, Sium, SMA, Saha, S, et al. New waves, new variants, old inequity: a continuing COVID-19 crisis. Health. (2021) 6:7031. doi: 10.1136/bmjgh-2021-007031
25. Tareq, AM, Bin, ET, Dhama, K, Dhawan, M, and Tallei, TE. Impact of SARS-CoV-2 delta variant (B16172) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum Vaccin Immunother. (2021) 17:4126–7. doi: 10.1080/2164551520211963601
26. Viral infection and transmission in a large well-traced outbreak caused by the Delta SARS-CoV-2 variant - SARS-CoV-2 coronavirus / nCoV-2019 Genomic Epidemiology - Virological. Available at: https://virological.org/t/viral-infection-and-transmission-in-a-large-well-traced-outbreak-caused-by-the-delta-sars-cov-2-variant/724 (Accessed December 6, 2022).
27. Cacciapaglia, G, Cot, C, and Sannino, F. Multiwave pandemic dynamics explained: how to tame the next wave of infectious diseases. Sci Rep. (2021) 11:6638. doi: 10.1038/s41598-021-85875-2
28. Seong, H, Hyun, HJ, Yun, JG, Noh, JY, Cheong, HJ, Kim, WJ, et al. Comparison of the second and third waves of the COVID-19 pandemic in South Korea: importance of early public health intervention. Int J Infect Dis. (2021) 104:742–5. doi: 10.1016/j.ijid.2021.02.004
29. Bwire, GM. Coronavirus: why men are more vulnerable to Covid-19 than women? SN Compr Clin Med. (2020) 2:874–6. doi: 10.1007/s42399-020-00341-w
30. CDC COVID data tracker: total cases and deaths by race/ethnicity, age, and sex. Available at: https://covid.cdc.gov/covid-data-tracker/#demographics (Accessed May 25, 2022).
31. Boehmer, TK, DeVies, J, Caruso, E, Van, SKL, Tang, S, Black, CL, et al. Changing age distribution of the COVID-19 pandemic — United States, may–august 2020. Morb Mortal Wkly Rep. (2020) 69:1404–9. doi: 10.15585/mmwr.mm6939e1
32. Nguyen, NT, Chinn, J, de Ferrante, M, Kirby, KA, Hohmann, SF, and Amin, A. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. PLoS One. (2021) 16:e0254066. doi: 10.1371/journal.pone.0254066
33. Ong, SWX, Chiew, CJ, Ang, LW, Mak, T-M, Cui, L, Toh, MPH, et al. Clinical and Virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (alpha), B.1.315 (Beta), and B.1.617.2 (Delta). SSRN Electron J. (2021). doi: 10.2139/SSRN.3861566
34. Tangcharoensathien, V, Bassett, MT, Meng, Q, and Mills, A. Are overwhelmed health systems an inevitable consequence of covid-19? Experiences from China, Thailand, and New York state. BMJ. (2021) 372:n83. doi: 10.1136/bmj.n83
35. Badalov, E, Blackler, L, Scharf, AE, Matsoukas, K, Chawla, S, Voigt, LP, et al. COVID-19 double jeopardy: the overwhelming impact of the social determinants of health. Int J Equity Health. (2022) 21:1–8. doi: 10.1186/s12939-022-01629-0
36. Yanez, ND, Weiss, NS, Romand, JA, and Treggiari, MM. COVID-19 mortality risk for older men and women. BMC Public Health. (2020) 20:1–7. doi: 10.1186/s12889-020-09826-8
37. Tandon, P, Leibner, ES, Hackett, A, Maguire, K, Mashriqi, N, and Kohli-Seth, R. The third wave: comparing seasonal trends in COVID-19 patient Data at a large Hospital system in new York City. Crit Care Explor. (2022) 4:e0653. doi: 10.1097/CCE.0000000000000653
38. Novelli, G, Colona, V, and Pandolfi, P. A focus on the spread of the delta variant of SARS-CoV-2 in India. Indian J Med Res. (2021) 153:537–41. doi: 10.4103/ijmr.ijmr_1353_21
39. Mathieu, E, Ritchie, H, Ortiz-Ospina, E, Roser, M, Hasell, J, Appel, C, et al. Coronavirus pandemic (COVID-19). Our World in Data. (2020) 5:947–53.
40. Bien-Gund, C, Dugosh, K, Acri, T, Brady, K, Thirumurthy, H, Fishman, J, et al. Factors associated with US public motivation to use and distribute COVID-19 self-tests. JAMA Netw Open. (2021) 4:e2034001–1. doi: 10.1001/jamanetworkopen.2020.34001
41. Govt to revise down COVID–19 self test kit price by end of the year – Tengku Zafrul. Available at: https://www.mof.gov.my/portal/en/news/press-citations/govt-to-revise-down-covid-19-self-test-kit-price-by-end-of-the-year-tengku-zafrul (Accessed May 25, 2022).
42. Malaysia approves two RM39.90 Covid-19 self-test kits, here’s what you need to know (VIDEO). Available at: https://malaysia.news.yahoo.com/malaysia-approves-two-rm39-90-032852008.html (Accessed May 25, 2022).
43. Self-test covid-19 test kit for conditional approval (approved) - medical device authority (MDA). Available at: https://mda.gov.my/announcement/631-kit-ujian-kendiri-covid-19-yang-telah-diberi-kelulusan-bersyarat.html. (Accessed May 25, 2022).
44. Ministry of Health Malaysia National COVID-19 testing strategy strategi pengujian COVID-19 Kebangsaan. (2021).
45. Fortnightly Covid-19 tests now mandatory for company staff | Free Malaysia Today (FMT). Available at: https://www.freemalaysiatoday.com/category/nation/2021/08/15/biweekly-covid-19-tests-now-mandatory-for-company-staff/ (Accessed May 25, 2022).
46. Miti: mandatory biweekly RTK antigen test for companies. Available at: https://www.malaysiakini.com/news/587262 (Accessed May 25, 2022).
47. Coronavirus (COVID-19) Vaccinations - Our World in Data. Available at: https://ourworldindata.org/covid-vaccinations (Accessed December 7, 2022).
Keywords: COVID-19, epidemiology, outbreak, variant, Cases
Citation: Md Nadzri MN, Md Zamri ASS, Singh S, Sumarni MG, Lai CH, Tan CV, Aris T, Mohd Ibrahim H, Gill BS, Mohd Ghazali N, Md Iderus NH, Lim MC, Ahmad LCRQ, Kamarudin MK, Ahmad NAR, Tee KK and Zulkifli AA (2024) Description of the COVID-19 epidemiology in Malaysia. Front. Public Health. 12:1289622. doi: 10.3389/fpubh.2024.1289622
Edited by:
Pacifique Ndishimye, Dalhousie University, CanadaReviewed by:
Saber Soltani, Tehran University of Medical Sciences, IranAdam Ronk, Battelle, United States
Copyright © 2024 Md Nadzri, Md Zamri, Singh, Sumarni, Lai, Tan, Aris, Mohd Ibrahim, Gill, Mohd Ghazali, Md Iderus, Lim, Ahmad, Kamarudin, Ahmad, Tee and Zulkifli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamad Nadzmi Md Nadzri, bW9oYW1hZC5uYWR6bWlAbW9oLmdvdi5teQ==
Mohamad Nadzmi Md Nadzri, https://orcid.org/0000-0001-6303-6842
†ORCID: Ahmed Syahmi Syafiq Md Zamri, orcid.org/0000-0002-3286-5493
Sarbhan Singh, https://orcid.org/0000-0002-6602-175
Mohd Ghazali Sumarni, https://orcid.org/0000-0001-5230-956
Chee Herng Lai, https://orcid.org/0000-0002-7032-0363
Tan Cia Vei, https://orcid.org/0000-0002-6060-1997
Tahir Aris, https://orcid.org/0000-0002-0205-3080
Hishamshah Mohd Ibrahim, https://orcid.org/0000-0002-0689-3095
Balvinder Singh Gill, https://orcid.org/0000-0002-0738-2991
Nur’Ain Mohd Ghazali, https://orcid.org/0009-0006-2680-9010
Lim Mei Cheng, https://orcid.org/0000-0002-7615-4669
Lonny Chen Rong Qi Ahmad, https://orcid.org/0000-0002-1478-2846
Mohd Kamarulariffin Kamarudin, https://orcid.org/0000-0002-5252-176X
Nur Ar Rabiah Ahmad, https://orcid.org/0009-0002-9176-9052
Kok Keng Tee, https://orcid.org/0000-0002-7923-5448
 Mohamad Nadzmi Md Nadzri
Mohamad Nadzmi Md Nadzri Ahmed Syahmi Syafiq Md Zamri1†
Ahmed Syahmi Syafiq Md Zamri1† Sarbhan Singh
Sarbhan Singh Mohd Ghazali Sumarni
Mohd Ghazali Sumarni Nuur Hafizah Md Iderus
Nuur Hafizah Md Iderus Mei Cheng Lim
Mei Cheng Lim Lonny Chen Rong Qi Ahmad
Lonny Chen Rong Qi Ahmad Kok Keng Tee
Kok Keng Tee Asrul Anuar Zulkifli
Asrul Anuar Zulkifli