- 1Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The Second Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Emergency Department of Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou, China
- 4Guangdong Provincial Key Laboratory of Research on Emergency in TCM, Guangzhou, China
Background: Substantial research evidence supports the correlation between mental disorders and sepsis. Nevertheless, the causal connection between a particular psychological disorder and sepsis remains unclear.
Methods: For investigating the causal relationships between mental disorders and sepsis, genetic variants correlated with mental disorders, including anorexia nervosa (AN), attention-deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), bipolar disorder (BD), major depressive disorder (MDD), obsessive-compulsive disorder (OCD), panic disorder (PD), posttraumatic stress disorder (PTSD), schizophrenia (SCZ), and tourette syndrome (TS), were all extracted from the Psychiatric Genomics Consortium (PGC). The causal estimates and direction between these mental disorders and sepsis were evaluated employing a two-sample bidirectional MR strategy. The inverse variance weighted (IVW) method was the primary approach utilized. Various sensitivity analyses were performed to confirm the validity of the causal effect. Meta-analysis, multivariable MR, and mediation MR were conducted to ensure the credibility and depth of this research.
Results: The presence of AN was in relation to a greater likelihood of sepsis (OR 1.08, 95% CI 1.02–1.14; p = 0.013). A meta-analysis including validation cohorts supported this observation (OR 1.06, 95% CI 1.02–1.09). None of the investigated mental disorders appeared to be impacted when sepsis was set as the exposure factor. Even after adjusting for confounding factors, AN remained statistically significant (OR 1.08, 95% CI 1.02–1.15; p = 0.013). Mediation analysis indicated N-formylmethionine levels (with a mediated proportion of 7.47%), cystatin D levels (2.97%), ketogluconate Metabolism (17.41%) and N10-formyl-tetrahydrofolate biosynthesis (20.06%) might serve as mediators in the pathogenesis of AN-sepsis.
Conclusion: At the gene prediction level, two-sample bidirectional MR analysis revealed that mental disorder AN had a causal association with an increased likelihood of sepsis. In addition, N-formylmethionine levels, cystatin D levels, ketogluconate metabolism and N10-formyl-tetrahydrofolate biosynthesis may function as potential mediators in the pathophysiology of AN-sepsis. Our research may contribute to the investigation of novel therapeutic strategies for mental illness and sepsis.
1 Introduction
As a common syndrome in emergency departments and intensive care units (ICUs), sepsis is a potentially fatal organ dysfunction attributed to a dysregulated host reaction to infection (1). In 2017, it harmed 48.9 million individuals globally, with 11 million fatalities in relation to sepsis, representing approximately 20% of overall mortality worldwide (2), and remains one of the primary factors of patient mortality globally. In recent years, although the age standardized incidence rate and mortality of sepsis have declined worldwide, many sepsis survivors suffer from cognitive decline, mental health disorders, physical function damage, and long-term death risks (3, 4). Severe sepsis and septic shock increase the likelihood of psychiatric disorders including major depressive disorder (MDD) (5), anxiety disorder (AD) and posttraumatic stress disorder (PTSD) (6).
Mental disorders pose a serious threat to public health because they serve as a critical cause of numerous diseases. Previous studies have indicated that mental disorders are the primary risk factors related to the advancement of sepsis. For instance, retrospective multicenter research revealed that patients previously diagnosed with mental illnesses, including MDD, attention deficit hyperactivity disorder (ADHD), schizophrenia (SCZ), bipolar disorder (BD) and panic disorder (PD), possess a greater likelihood of severe sepsis and related death (7). However, a nationwide cohort study from France suggested that septic patients with BD, MDD, and SCZ, have lower 90-day case fatality rates than other individuals (8). These inconsistent research results are contradictory, and the assessment of the types of mental disorders is not comprehensive, which limits the opportunities for guiding early prevention and effective treatment of diseases.
Mendelian randomization (MR) serves as an epidemiological approach that utilizes instrumental variable single nucleotide polymorphisms (SNPs) for random allocation (9). SNPs are highly associated with exposure factors, reducing the susceptibility of MR analysis to reverse causality bias and confounding factors, independent of potential confounding factors (10). Consequently, the statistical efficacy of causal connections may be increased by analyzing summary data from GWAS applying two-sample MR (11). Multivariable MR (MVMR) is an advanced approach that extends MR by utilizing genetic variants linked to multiple possibly interconnected exposures to assess the impact of each exposure on a single outcome. This approach could avoid bias induced by confounding factors, preserving the advantages of utilizing genetic instruments for causal inference (12). The association between sepsis and different illnesses, such as insomnia (13), type I diabetes mellitus (14), atrial fibrillation, and cardioembolic stroke (15), has been extensively investigated utilizing MR analysis. Nevertheless, research has concentrated less on the relationship between sepsis and mental disorders.
Consequently, a two-sample bidirectional MR analysis was conducted utilizing the aggregated statistical data from the Large Genome Association Study (GWAS) to evaluate the causal association of 12 mental disorders, including ADHD, BD, MDD, PD, PTSD, SCZ, anorexia nervosa (AN), tourette syndrome (TS), obsessive-compulsive disorder (OCD), and autism spectrum disorder (ASD), on the risk of sepsis. Additionally, the causal involvement of sepsis in the onset of these 10 psychiatric disorders is also examined. Meta-analyses were performed to prove the credibility of the causal associations utilizing the validation cohorts. Based on previous studies, total cholesterol, triglyceride levels, and weight were recognized as possible confounding factors (16–22), and MVMR was carried out to ensure the independent causal impact of mental disorders on sepsis. Additionally, we explored the potential mediators between mental illness and sepsis. Previous studies have shown that mental illness leads to changes in inflammatory factors, blood metabolites and gut microbiome, which in turn are associated with sepsis (23–28), and several MR studies have recently demonstrated the relationship between blood metabolites and sepsis (29, 30). Therefore, mediation MR analysis was applied to explore the mediating pathway among mental illnesses and sepsis via the phenotypes of inflammatory factors, blood metabolites and gut microbiome.
2 Methods
2.1 Study design
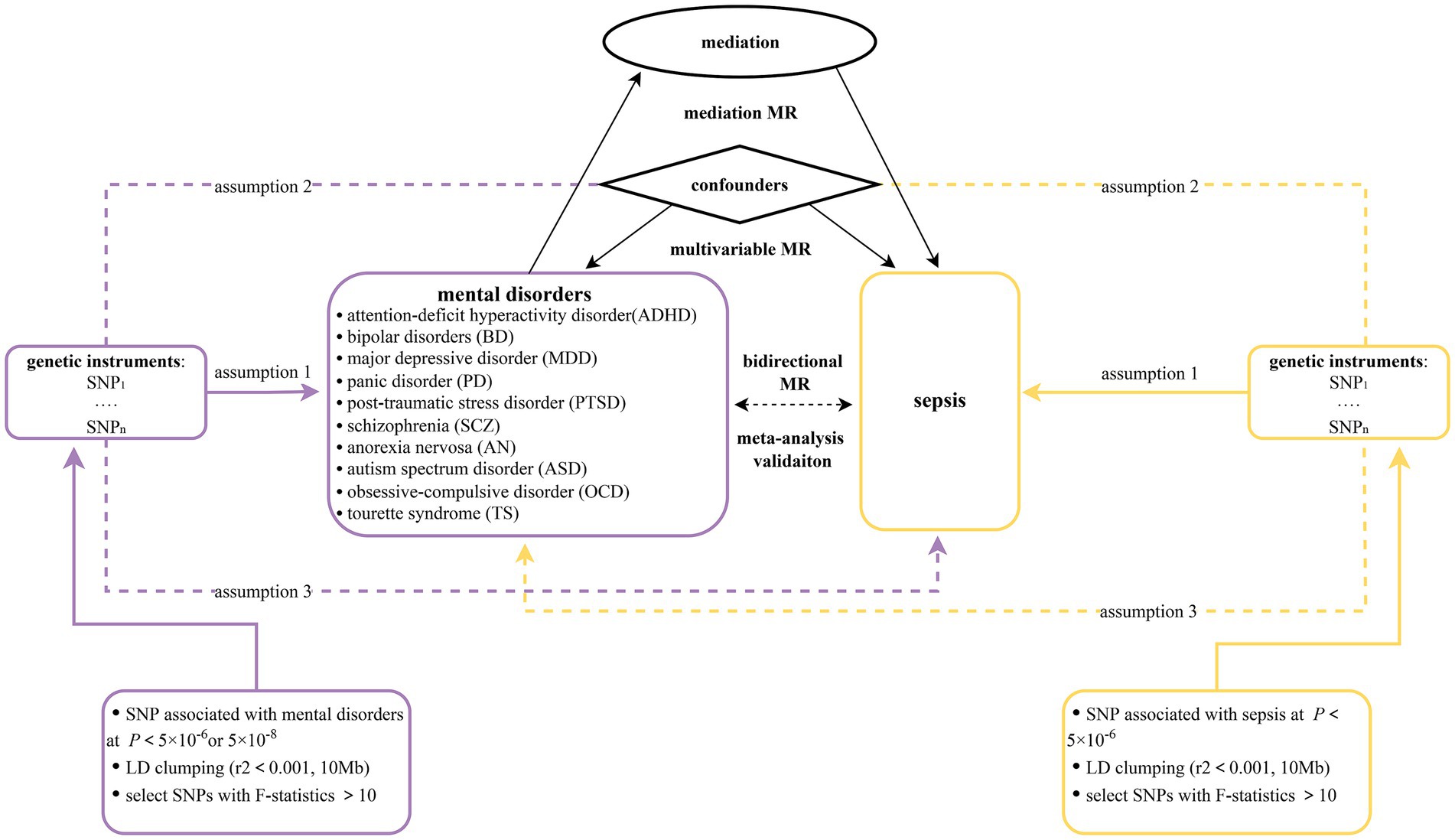
This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization guidelines (STROBE-MR, Supplementary Table S1) (31). In this research, a two-sample bidirectional MR design was applied to claim the causal effect between 10 mental disorders (ADHD, BD, MDD, PD, PTSD, SCZ, AN, TS, OCD, and ASD) and sepsis. First, we assumed 10 mental disorders as exposures and sepsis as outcome. To establish a causal connection between mental illnesses and sepsis, forward MR studies were performed. Second, the effect of sepsis on 10 mental illnesses was examined utilizing reverse MR analyses. MR analyses must meet three basic assumptions: (1) genetic variants are strongly correlated with exposures; (2) genetic variants are not linked to any confounders; and (3) genetic variants only influence outcomes only through exposure (32). The most recent GWAS datasets from the FinnGen (33) database were combined with the latest study published by Hamilton et al. (34). The reliability and stability of the causal connection between mental illnesses and sepsis were assessed using a meta-analysis methodology. The MVMR method was employed in order to reduce potential pleiotropy by adjusting for confounding variables including total cholesterol, triglyceride levels, and weight. In the mediation analysis, 91 inflammatory factors, 309 metabolite ratios, 1,091 blood metabolites, 205 gut microbiome pathways and 207 gut microbiome taxa were included. The mediation MR approach was utilized to evaluate the indirect effect of each mediator (35). Figure 1 displays the flow chart illustrating the detailed process of our MR analysis. To decrease discrimination based on population stratification, all summarized GWAS data were restricted to participants of European ancestry.
2.2 GWAS data sources
The summary statistics for ADHD (36) included data from 13 cohorts, with an amount of 225,534 individuals. Among these individuals, there were 38,691 cases and 186,843 controls. Notably, 49.61% of the participants were female. The definition of ADHD was determined by using the criteria of the International Classification of Diseases, Tenth Revision (ICD-10) code or through clinical assessment, including the consumption of medication specially recommended for the treatment of ADHD. The summary statistics for BD (37) were obtained from a group of 57 cohorts containing 41,917 cases and 371,549 controls. Regarding MDD (38), a comprehensive dataset containing 135,458 cases and 344,901 controls was gathered from 35 various cohorts. The latest GWAS meta-analysis of 6 cohorts with an overall sample size of 2,248 cases and 7,992 controls, provided PD (39) summary statistics. For SCZ (40), summary statistics were collected from 76 cohorts involving a general of 55,365 cases and 21,9,884 controls. The cases diagnosed with BD, MDD, PD, or SCZ were classified based on the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV), ICD-9, or ICD-10 code, and assessed by trained interviewers, clinician-managed checklists, and records of medical review. PGC-PTSD, which comprised 9 European ancestry datasets with 2,424 cases and 7,113 controls, provided the summary statistics for PTSD (41). The diagnostic criteria involved PTSD with the presence of clinical characteristics or self-reported. AN (42) summary statistics were derived from 12 cohorts, totaling 3,495 cases and 10,982 controls. The diagnosis of AN included enduring anorexia nervosa, or long-term eating disorders. Regarding ASD (43), the summary statistics for ASD encompassed a collective count of 18,381 cases and 27,969 controls. Patients with ASD were identified based on the ICD-10 codes. Summary statistics for OCD (44) comprised a sample size of 2,688 patients and 7,037 controls. All cases satisfied the diagnostic criteria for OCD as specified in the DSM-IV. Summary statistics for TS (45) included 4,819 cases and 9,488 controls. The diagnoses of TS cases were established by highly qualified clinicians following DSM-IV-TR criteria. All data were restricted to individuals with European ancestry so as to prevent potential bias induced by population heterogeneity. We manually checked all cohorts of GWAS meta-analytic results for 10 mental disorders and discarded those results containing UK Biobank participants, which could largely minimize sample overlap and ensure independence between the exposure and outcome data.
Summarized GWAS data for sepsis were derived from the UK Biobank (46), comprising 462,918 individuals with 10,154 cases and 454,764 controls of European ancestry. The BOLT-LMM linear mixed model was applied to adjust the genetic association estimates for sepsis based on factors such as age, sex, and genotyping chip. The diagnosis of sepsis was primarily determined based on a structured assessment by a panel of physicians. An earlier published paper offered more specific information about this summarized GWAS data (47). The cohort of sepsis cases comprised 5,655 females (56%), with a median age of 60 years, a mean BMI of 28.2 kg/m2, mean low-density lipoprotein cholesterol levels of 3.4 mmol/L, and a mean systolic blood pressure of 136.1 mmHg. These baseline characteristics for sepsis cases were consistent with those observed in the comparator groups. Furthermore, we selected two datasets for validation to improve the stability of the causal association between mental illness and sepsis to a greater extent. We collected GWAS data on sepsis from a study conducted by Hamilton et al. (34). The sample comprised 486,484 individuals of European ancestry, of which 11,643 were cases and 474,841 were controls. The FinnGen R10 database was used as another validation source, the data on sepsis from which included 429,209 European-ancestry individuals with 13,531 cases and 415,678 controls (33). All the sepsis cases mentioned above had to match the criteria including the ICD-10 codes A02, A39, A40, and A41. All GWAS data were derived from individuals with European genetic ancestry. And individuals with sex mismatches, genotype missingness greater than 5 per cent and heterozygosity greater than or less than four standard deviations were excluded (33). And it is worth noting that FinnGen R10 database contains only individuals of Finnish genetic ancestry.
Summary statistics for total cholesterol levels were gathered from Nightingale Health’s metabolomic research and included a sample size of 115,078 patients. The summary data for triglyceride levels were gathered from the Within Family GWAS Consortium, which included 30,515 individuals. Both datasets were downloaded from the IEU OpenGWAS database.1 Summary statistics for weight were derived from the FinnGen database and included a sample size of 1,146 cases and 217,646 controls (33). GWAS data for 91 inflammatory factors were derived from 11 cohorts comprising a total of 14,824 individuals with European ancestry (48). Summary statistics for 309 metabolite ratios and 1,091 blood metabolites were gathered from The Canadian Longitudinal Study on Aging for 51,338 randomly selected participants aged 45–85 years (49). Summary statistics for 205 pathways and 207 taxa in relation to gut microbiome were acquired from the Dutch Microbiome Project, analyzing feces from 7,738 individuals (50). The use of data sources distinct from the outcome data was confirmed to avoid sample overlap. Further information regarding summarized GWAS data related to exposure and outcome is provided in Table 1. All original studies were ethically approved, and all original data used in this study were openly accessible.
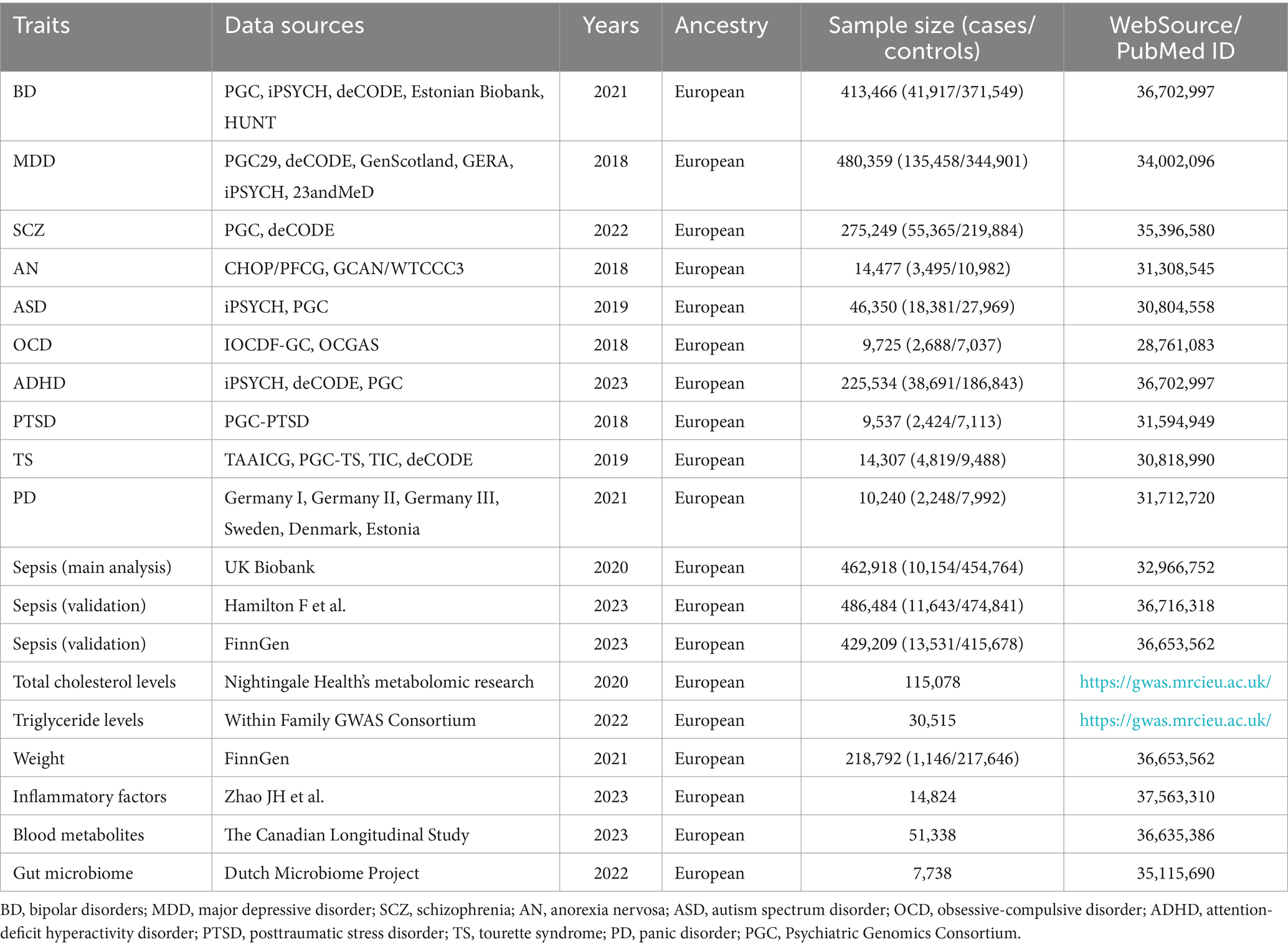
Table 1. Summary of genome-wide association studies (GWAS) datasets for 10 mental disorders and sepsis.
2.3 Selection of instrument variants
As stated previously, SNPs for exposure were selected with a stringent p-value criteria (p < 5 × 10−8). However, few independent SNPs were identified by these strict criteria. In light of this situation, we applied a less restrictive statistical threshold (p < 5 × 10−6) for the exposure variables (MDD, AN, PD, PTSD, ASD, OCD, TS, sepsis, total cholesterol, triglyceride levels, weight). Moreover, a higher cutoff (p < 1 × 10−5) was selected considering the relatively few SNPs discovered for part of mediators when they served as exposures. This strategy has been applied in several MR studies (51, 52). Then, we specified a clumping window of 10,000 kb and linkage disequilibrium R2 < 0.001 to guarantee the independence of the selected SNPs (53). Furthermore, we manually checked and removed genetic variants that were not identified in the outcome GWAS data to guarantee that the genetic variants included could directly link psychiatric disorders to sepsis. Second, SNPs that had a strong correlation with the outcome were excluded. Second, F-statistics (F = beta2/se2) were calculated for each SNP (54). In an effort to minimize the impact for weak instrumental bias, SNPs were ruled out if their corresponding F-statistics failed to reach the threshold of 10. Third, harmonization of effect estimates was performed to verify that exposure data and outcome data had corresponding effect directions and to avoid strand mismatch (55, 56).
2.4 Statistical analysis
The principal method employed in the major analysis was IVW analysis, which was chosen for its strong statistical power (57). The IVW approach combined the Wald ratios derived from each SNP, assuming SNPs with the lowest variance. For binary outcomes, the measures of causal estimates are presented as the form of odds ratio (OR) and 95% confidence interval (CI). Due to the estimation method of IVW was unbiased, it is susceptible to invalid instrument bias (13). As a result, additional complementary methods such as maximum likelihood (ML), weighted median (WM), and MR-Egger were conducted to assure the validity of the results. To facilitate the interpretation of the intercepts and directions of the different analysis methods, scatter plots were generated. Moreover, the Cochrane’s Q test for the IVW method and funnel plot were utilized to determine heterogeneity (58). The p-value of Cochran’s Q test was also applied in choosing random-effects (p < 0.05) or fixed-effects IVW MR analysis (p > 0.05). Subsequently, estimates of horizontal pleiotropy were derived using the MR-Egger intercept tests (59). Last but not least, leave-one-out analysis was applied to assess the potential presence of bias in the estimations due to the influence of a single SNP.
The GWAS datasets from distinct sources were merged via meta-analysis method to assess the stability and credibility of the previous results. By adjusting for confounding risk factors with MVMR analysis, we were able to reduce the impact of confounding variables on causal associations. Applying instrumental variables for mental diseases, we first assessed the causal influence of mental illnesses on a hypothesized mediator. In the following phase, we established the causal impact of the mediators on sepsis by utilizing instrumental variables as the mediator. We quantified the mediation proportion of each mediating component by dividing the indirect effect by the total effect. The delta approach was used to estimate confidence intervals (60). Sensitivity analyses were applied to prove the validity of the results. The entire data preparation and analyses were conducted utilizing the TwoSampleMR (version 0.5.7), MendelianRandomization (version 0.9.0), and meta (version 7.0-0) packages in the R software (version 4.3.0).
3 Results
3.1 Genetic instrumental variables
We conducted a comprehensive screening of instrumental variables for 10 psychiatric disorders, and all 294 SNPs for psychiatric disorders associated with sepsis were ultimately included in the research. There were between 8 and 122 SNPs for each psychiatric disorder. Additionally, instrumental variables for sepsis were comprised of 32 SNPs, with 12 SNPs being utilized in the validation cohorts. There were between 10 and 111 SNPs for each confounding factor. The number of SNPs utilized in mediation MR varies from 7 to 45. The F-statistics exceeding 10 were observed for each of the instrumental variables. Supplementary Tables S2–S5 provide comprehensive details on the chosen instrumental variables and the harmonized data.
3.2 Bidirectional MR analysis
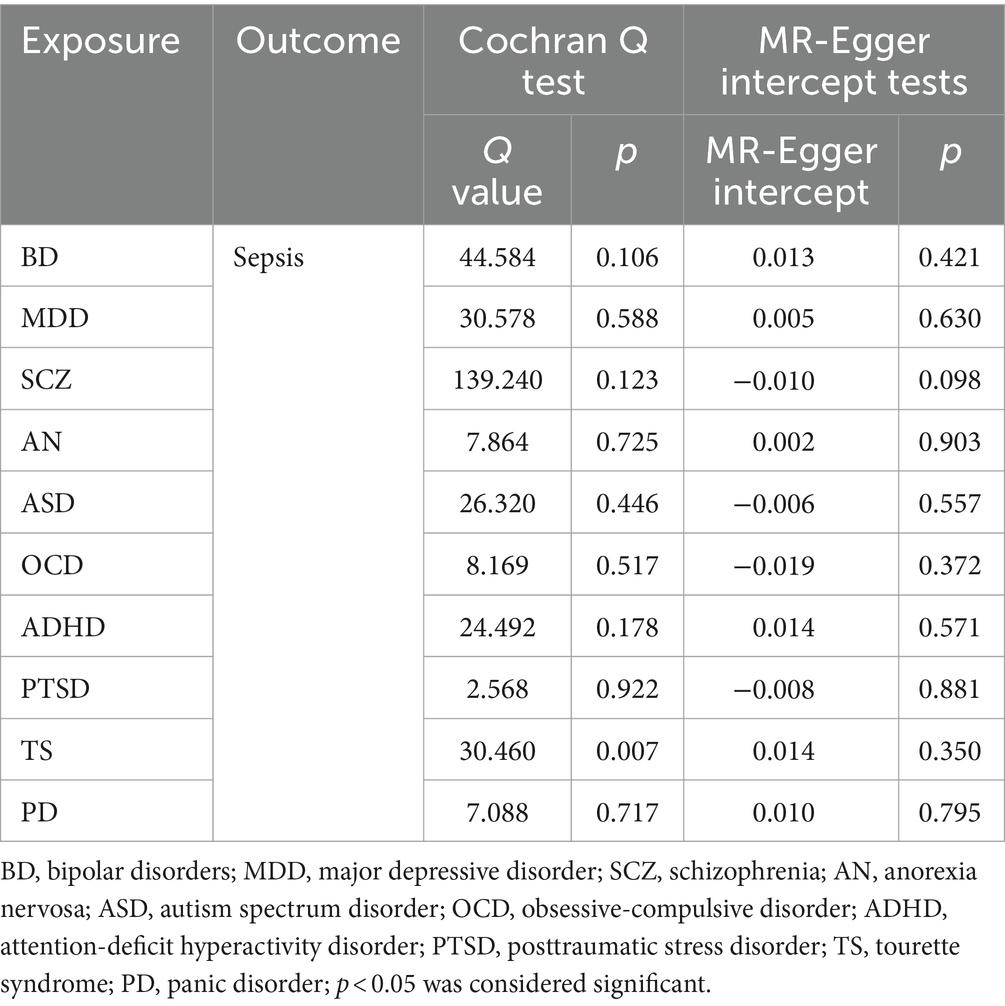
We discovered a correlation between sepsis and genetic susceptibilities to AN by using mental diseases as exposures to examine the causal effect of mental diseases on sepsis. The IVW analysis revealed a correlation between AN and an elevated risk of sepsis (OR 1.08, 95% CI 1.02–1.14; p = 0.013). ML analysis provided the same estimate (OR 1.08, 95% CI 1.02–1.14; p = 0.010), while MR-Egger and WM analyses yielded consistent yet nonsignificant results (Figure 2). The scatter plot demonstrated that the analysis results were nearly identical direction (Figure 3). Using mental disorders as the outcomes, the p-values of all 10 IVW analyses were greater than 0.05, indicating that sepsis failed to affect any of the studied mental disorders (Table 2; Supplementary Table S6). A variety of sensitivity analysis approaches, such as the Cochran’s Q test, the MR Egger intercept, the funnel plot, and the leave-one-out analysis, were used to evaluate the reliability of the positive estimates. The Cochran’s Q test performed for AN as an exposure factor showed p-values larger than 0.05, and the p-value for the MR Egger intercept was found to exceed the predetermined threshold of 0.05, suggesting no evidence of heterogeneity and horizontal pleiotropy in our study (Table 3). In addition, the funnel plot was symmetric, further demonstrating the validity of the results (Supplementary Figure S1). Finally, using leave-one-out analysis to avoid biasing effects on IVW, the correlation between AN and a higher likelihood of sepsis was meticulously confirmed (Figure 4).
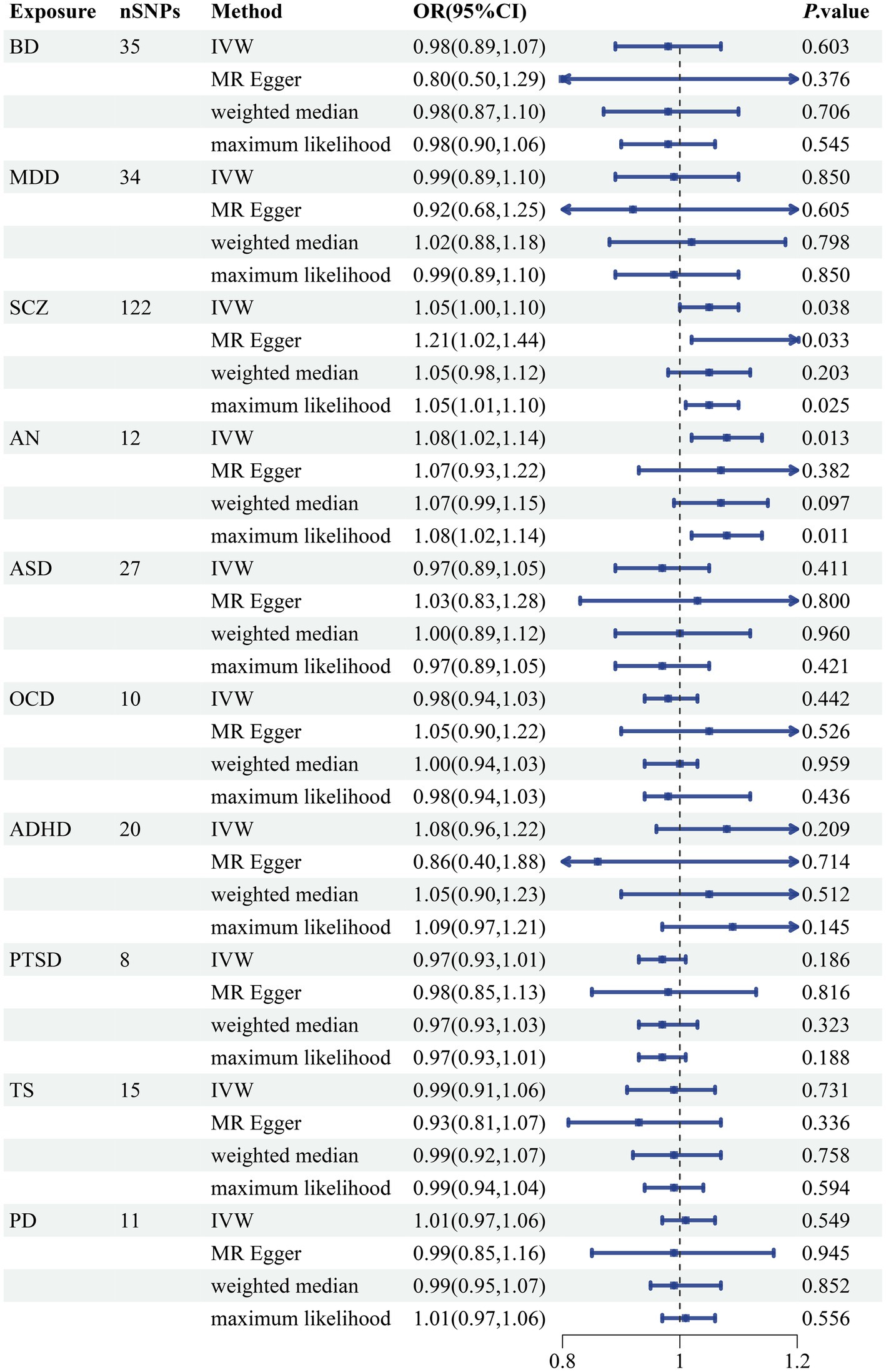
Figure 2. Forest plot of causal associations between 10 mental disorders and sepsis risk. BD, bipolar disorder; MDD, major depressive disorder; SCZ, schizophrenia; AN, anorexia nervosa; ASD, autism spectrum disorder; OCD, obsessive-compulsive disorder; ADHD, attention-deficit hyperactivity disorder; PTSD, posttraumatic stress disorder; TS, tourette syndrome; PD, panic disorder; SNP, single nucleotide polymorphism; IVW, inverse variance weighted; OR, odds ratios; CI, confidence intervals; p-value < 0.05 was considered significant.
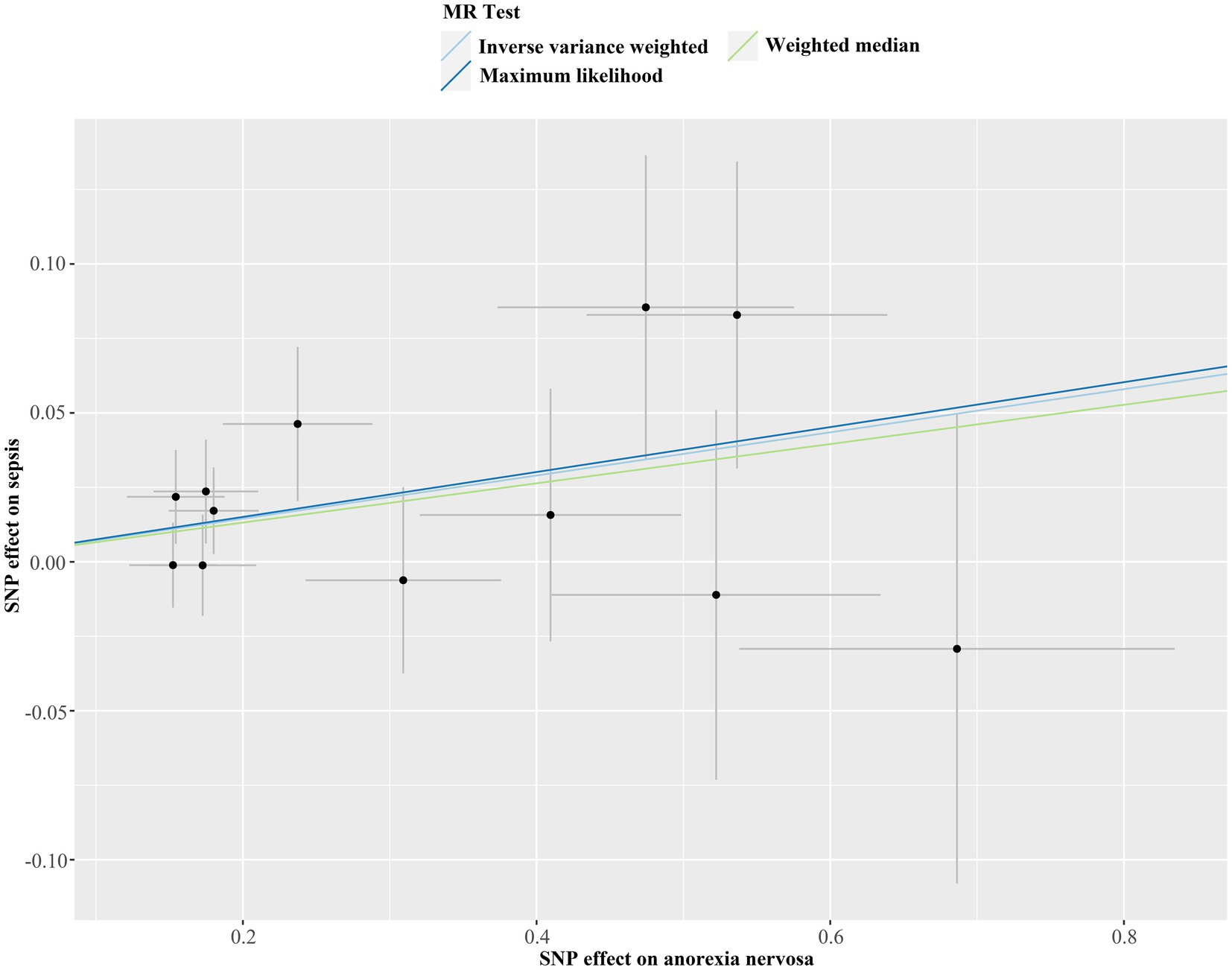
Figure 3. Scatter plot of Mendelian randomization analysis for the associations of anorexia nervosa with the risk of sepsis. SNP, single nucleotide polymorphism; MR, Mendelian randomization.
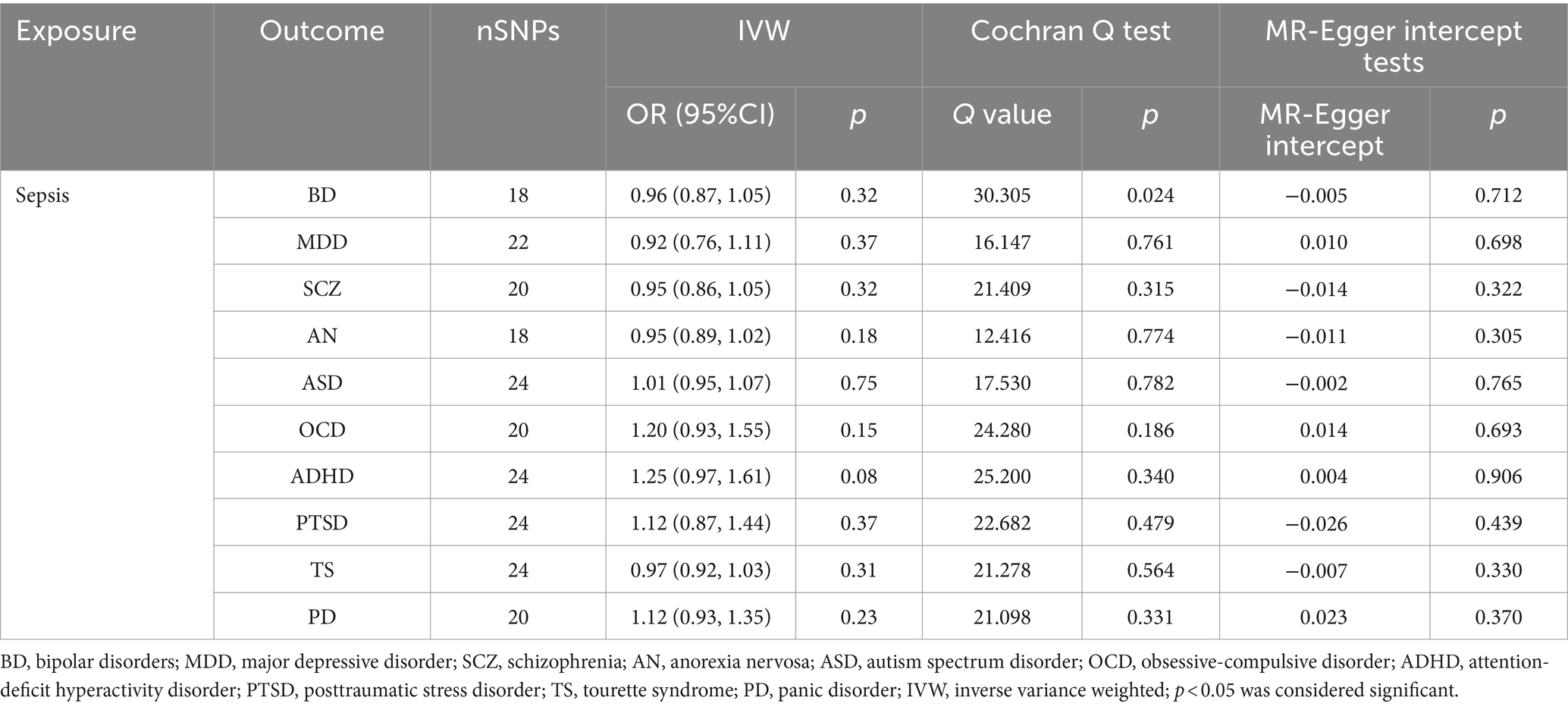
Table 2. Results of reverse MR analyses and sensitivity analysis assumed sepsis as exposure and 10 mental disorders as outcome.
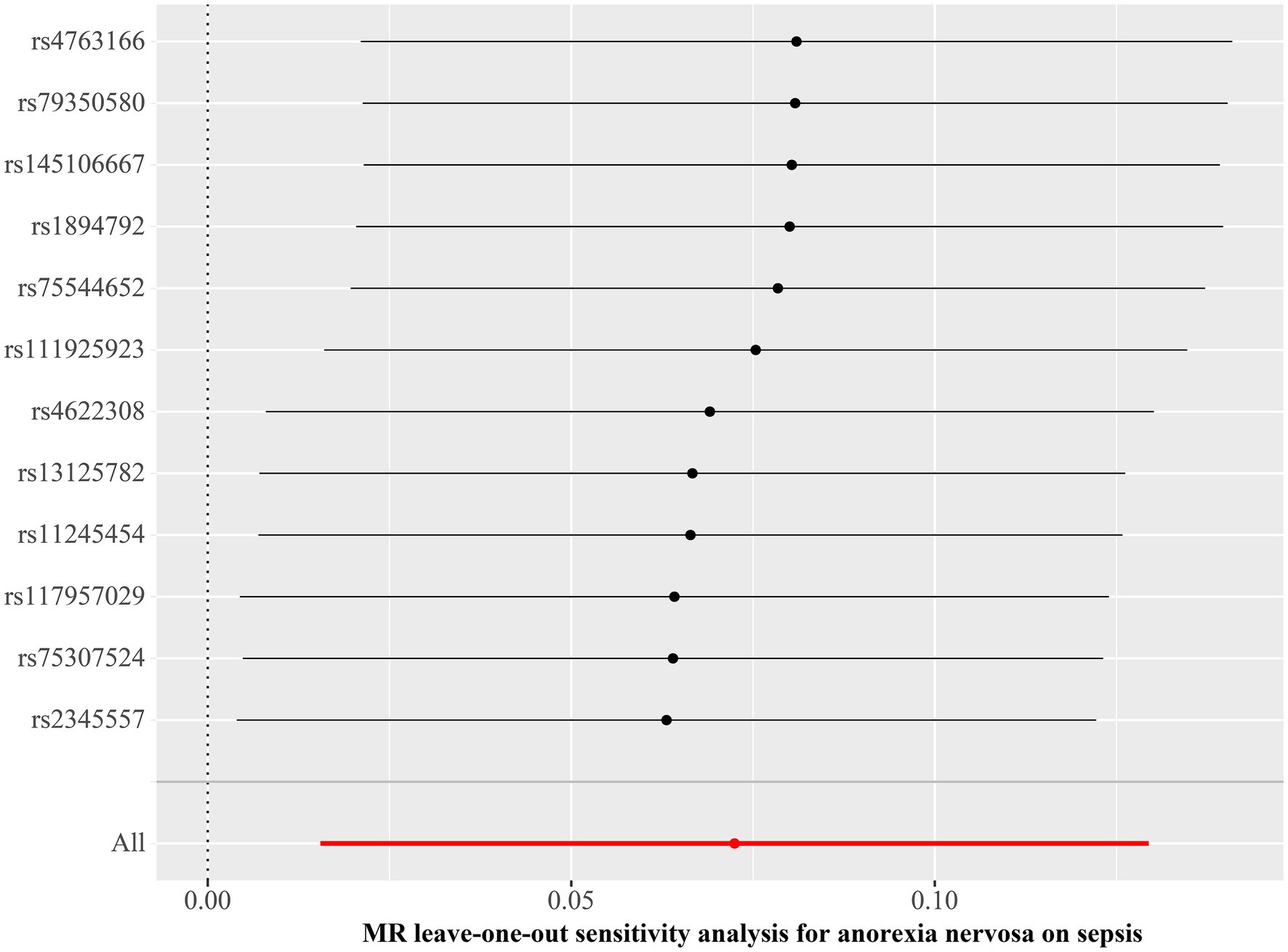
Figure 4. Leave-one-out plot for Mendelian randomization analysis of the causal effect of anorexia nervosa on the risk of sepsis. MR, Mendelian randomization.
3.3 Meta-analysis
As demonstrated in Figure 5, the ORs of the three datasets were in identical direction, validating the effect of AN on the increased risk of sepsis occurrence, and with the inclusion of the two validation datasets, the effect of AN on the increased possibility of sepsis occurrence (OR 1.08, 95% CI 1.02–1.14) remained relatively stable in the meta-analysis model (OR 1.06, 95% CI 1.02–1.09) and no heterogeneity was observed (I2 = 0%, τ2 < 0.0001, p = 0.38).

Figure 5. Meta-analysis of the causal effect of AN on sepsis. SE, standard error; OR, odds ratio; CI, confidence interval; p < 0.05 was considered significant.
3.4 MVMR
In order to more thoroughly exclude the impact of confounding variable-level pleiotropy, we employed the MVMR method. An association of statistical significance remained between AN and sepsis after adjusting for total cholesterol (OR 1.08, 95% CI 1.02–1.15; p = 0.012), triglyceride levels (OR 1.09, 95% CI 1.02–1.15; p = 0.006), and weight (OR 1.08, 95% CI 1.01–1.15; p = 0.029). Moreover, we included all the confounding factors to conduct a multivariable estimate; the investigation remained stable and confirmed previous results (OR 1.08, 95% CI 1.02–1.15; p = 0.013) (Figure 6; Supplementary Table S7). The Cochran’s Q test conducted for AN as an exposure factor indicated p-values greater than 0.05. The p-value for the MR Egger intercept in all analyses was more than the predefined threshold of 0.05 (Supplementary Table S8). Our investigation failed to identify any evidence of heterogeneity or horizontal pleiotropy.
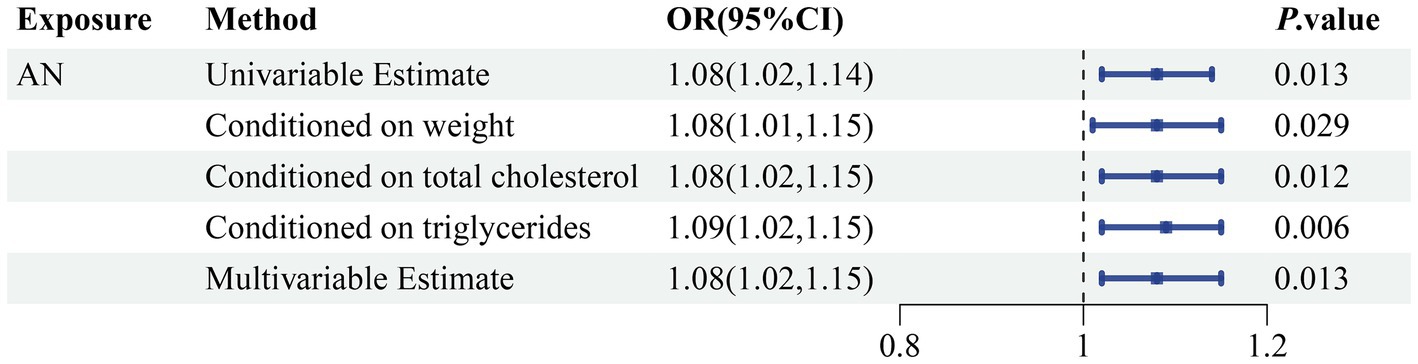
Figure 6. Genetically predicted association of AN with sepsis after adjusting for confounding factors. OR, odds ratio; CI, confidence interval; p-value < 0.05 was considered significant.
3.5 Mediation MR
Among the 91 inflammatory factors, 309 metabolite ratios, 1,091 blood metabolites, 205 gut microbiome pathways and 207 gut microbiome taxa examined, N-formylmethionine levels, cystatin D levels, ketogluconate metabolism, and N10-formyl-tetrahydrofolate biosynthesis were found to be associated with the causal association between AN and sepsis. The causative influence of AN on N-formylmethionine levels was statistically significant (OR 1.06, 95% CI 1.01–1.12; p = 0.046). Also in the causative influence of AN on cystatin D levels (OR 0.96, 95% CI 0.92–1.00; p = 0.040), ketogluconate metabolism (OR 1.16, 95% CI 1.01–1.33; p = 0.031) and N10-formyl-tetrahydrofolate biosynthesis (OR 1.12, 95% CI 1.03–1.21; p = 0.010). This research revealed a causal association between N-formylmethionine levels (OR 1.10, 95% CI 1.01–1.19; p = 0.024), cystatin D levels (OR 0.95, 95% CI 0.91–1.00; p = 0.030), ketogluconate metabolism (OR 1.09, 95% CI 1.00–1.18; p = 0.042), N10-formyl-tetrahydrofolate biosynthesis (OR 1.14, 95% CI 1.03–1.27; p = 0.015) and sepsis (Figure 7). In conjunction with previous studies establishing a causal relationship between AN and sepsis, a mediation analysis was performed. The mediation analysis revealed that N-formylmethionine levels mediated the connection between AN and sepsis and explained 7.47% of the mediation impact. Cystatin D levels (2.97%), ketogluconate metabolism (17.41%), and N10-formyl-tetrahydrofolate biosynthesis (20.06%) were additionally included. No heterogeneity or pleiotropy was detected. The detailed results and sensitivity analysis of the mediation MR can be found in Supplementary Tables S9–S11.
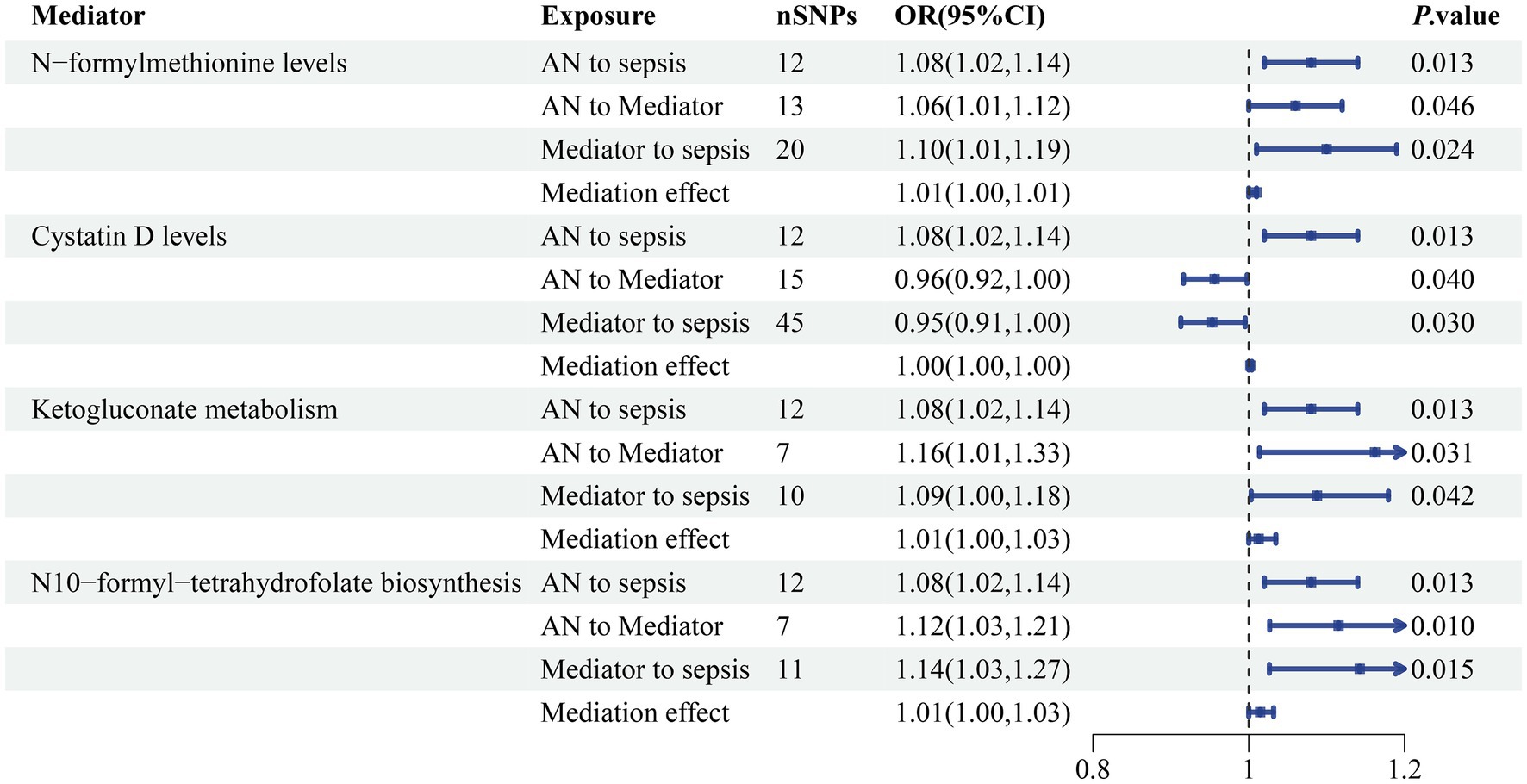
Figure 7. The mediating effect of AN on sepsis via blood N-formylmethionine levels and cystatin D levels accompanied by ketogluconate metabolism and N10-formyl-tetrahydrofolate biosynthesis in the gut microbiome. SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval; p-value < 0.05 was considered to indicate statistical significance.
4 Discussion
This study presented genetic data suggesting a causal connection between mental diseases and sepsis through bidirectional MR, meta-analysis, and MVMR analyses. Mediators between mental illness and sepsis were investigated by mediation MR analysis.
Clinical investigations have indicated a connection between mental disorders and sepsis. Large prospective cohort studies revealed that MDD and PD were linked to a higher likelihood of sepsis (6). Additionally, MDD, PD, and PTSD were observed at increased rates among individuals with sepsis (61). A clinical investigation revealed that MDD is in relation to increased 5-year all-cause mortality in individuals who survived sepsis (62). Nevertheless, a nationwide cohort study conducted in France indicated that septic patients with severe mental illnesses (BD, MDD, and SCZ) have reduced 90-day case fatality rates (8). Several research focused on the correlation between mental illness and infection by employing the Mendelian randomization method. The inflammatory factors CRP and IL-6R were found to be associated with SCZ (63). MDD and ADHD elevate the likelihood of hospitalization due to COVID-19, whereas SCZ is also strongly associated with COVID-19 (64–66). Clinical studies on the correlation between mental disorders and sepsis have not been thorough or comprehensive. There are contradictory findings in this research area. In addition, the Mendelian randomization method for establishing a causal association between mental disorders and sepsis is lacking. In order to surmount these obstacles, A MR study was implemented with the purpose of establishing a causal relationship between mental illnesses and sepsis, thereby presenting significant evidence. Our findings revealed compelling evidence that AN was correlated with a higher probability of sepsis. There is no study suggested that sepsis affects any of the studied mental disorders.
AN is a serious psychiatric disease characterized by an elevated death rate, surpassing that of all other psychiatric conditions (67). And this condition is strongly linked to several psychiatric disorders including MDD, OCD, PTSD, and substance abuse disorders (68). In contrast to other mental illnesses, its characteristics mainly include a restriction of calorie consumption relative to requirements, an intense dread of gaining weight or of turning obese, and an irrational preoccupation with shape or body weight (69). The combination of these factors will lead to a series of symptoms such as starvation, malnutrition, and weight loss (70). Research indicates that starvation and malnutrition are key factors contributing to the impairment of the human immune system, potentially resulting in a considerable decline in cellular immunity (71). Metabolic changes in AN could result in significant biochemical disorders, leading to dysfunction in multiple organs and systems, with cytokines and immune cells playing a crucial role. Severe malnutrition causes disturbances in T-cell populations, leading to a higher vulnerability to infections (72). Clinical research on individuals with AN observed decreased levels of TNF-α and IL-6, but elevated levels of IL-1 compared to the control group (73). Research conducted on neutrophils in individuals with AN revealed diminished adhesion and lower bactericidal and cellular activities, resulting in heightened vulnerability to infections (74). An 8-year cohort study investigating the causes of death among individuals with AN revealed that 29% of deaths were attributed to infections, ranking second after deaths caused by AN or malnutrition (75). Sepsis is a critical infection-related illness (1), and the possible mechanisms by which AN leads to sepsis are currently focused on inflammatory factors, blood metabolites and gut microbiome (29, 76–83). Through a mediation MR analysis involving inflammatory factors, blood metabolites and gut microbiome as potential mediators, we discovered that blood N-formylmethionine levels and cystatin D levels accompanied by ketogluconate metabolism and N10-formyl-tetrahydrofolate biosynthesis in gut microbiome may mediate the causal association between AN and sepsis (84–86).
N-Formylmethionine is an amino acid found in bacteria and related eukaryotic organelles. It is a derivative of methionine that was initially identified during translation in bacteria, chloroplasts, and mitochondria. N-formylmethionine has historically been regarded as a marker of bacteria or bacteria-derived organelles (87). However, a recent study revealed an essential connection between human cellular N-formylmethionine and the susceptibility to age-related diseases in humans. Further investigation of the blood levels of N-formylmethionine confirmed its correlation with a higher likelihood of age-related diseases including renal disease and heart failure, as well as the risk of mortality (88). The precise function of N-formylmethionine in humans remains unclear, yet it exhibits potential as a biomarker for various age-related illnesses. Metabolic changes in AN result from chronic starvation and include disturbances in amino acids, lipids, and carbohydrates (89). Prior research has shown that individuals with AN frequently exhibit hyperaminoacidemia. Researchers have conducted targeted and untargeted metabolomics analyses of methionine levels in AN patient, with varying results showing enhanced, reduced, or stable levels of methionine (90–92). Formylmethionine deformylase can convert N-formylmethionine to methionine in prokaryotes (93). However, this conversion has not been observed in humans. Human mitochondria produce danger-associated molecular patterns (DAMPs) in the form of mitochondrial DNA and N-formyl-methionyl peptides when degrading. Mitochondrial DAMPs have been connected with dramatic disruption of metabolic balance, effective activation of innate immunity, and negative events in critically ill patients (94–96). The breakdown of the DAMP N-formylmethionyl peptide results in the release of N-formylmethionine into the bloodstream, indicating a significant disturbance in physiological homeostasis (97). A comprehensive metabolomic study analyzing critically ill ICU patients, including a sepsis cohort, showed that circulating N-formylmethionine promotes metabolic shifts and increased mortality, including incomplete oxidation of mitochondrial fatty acids, increased metabolism of branched-chain amino acids, and activation of the pentose phosphate pathway (98).
Cystatins are a group of protease inhibitors capable of inhibiting cysteine cathepsins, both inside and outside cells. Cystatin D belongs to type 2 cystatin and is encoded by the CST5 gene, which serves a regulatory role in the immunological response (99). Previous cross-sectional studies have discovered the existence of AN with the level of CST5 (100), and cystatin C has been found to possess greater diagnostic efficacy than creatinine in studies related to renal disease in patients with AN (101). A large Mendelian randomized study found a negative association between CST5 and various immune-related diseases such as gout, IgA nephropathy, primary sclerosing cholangitis, and sepsis (48). Research indicates that AN is correlated with unfavorable variations in the composition of the normal gut microbiota (102). According to a previous longitudinal study, individuals with AN possess substantially great abundances of Alistipes, Clostridiales, Christensenellaceae, and Ruminococcaceae and a lower abundance of Faecalibacterium, Agathobacter, Bacteroides, Blautia, and Lachnospira (84). A recent MR study revealed that AN was associated with increased levels of gut microbiome, including Alphaproteobacteria, Christensenellaceae, and Coriobacteriia (103). Variations in the gut microbiome make a person more prone to sepsis because they promote the growth of harmful bacteria in the gastrointestinal tract and trigger a robust proinflammatory response in the immune system (85). According to preliminary studies, individuals with less variety in their microbiome and a higher relative abundance of pathogenic gram-negative bacteria and enterococci are more susceptible to sepsis (86, 104, 105). Previous related studies have not further explored the relevant metabolic pathways in the gut microbiome. This study discovered that AN may increase the possibility of sepsis by increasing ketogluconate metabolism and N10-formyl-tetrahydrofolate biosynthesis in the gut microbiome, which may serve as a foundation for subsequent explorations for the specific metabolic patterns associated with gut microbiome alterations in AN-related sepsis.
In the present study, we investigated 91 inflammatory factors, 309 metabolite ratios, 1,091 blood metabolites, 205 gut microbiome pathways and 207 gut microbiome taxa and discovered that N-formylmethionine levels, cystatin D levels, ketogluconate metabolism and N10-formyl-tetrahydrofolate biosynthesis may possess mediating functions in the process of AN-sepsis. Currently, there is a dearth of studies on N-formylmethionine levels, cystatin D levels, ketogluconate metabolism and N10-formyl-tetrahydrofolate biosynthesis in patients with AN or sepsis. Further research is required to determine the role of the identified mediators in AN-sepsis.
The major strengths of this research are the adequate inclusion of multiple psychiatric disorders and the rigorous MR design. Data associated with psychiatric disorders were acquired from the PGC database, the most authoritative GWAS database for psychiatric disorders, to ensure the richness and credibility of the data. Causality between mental illness and sepsis was determined by MR analysis, and a bidirectional analysis was performed, ruling out reverse causality because of the negative effect of sepsis on mental illness. The causal relationship between mental illness and sepsis was further confirmed using meta-analysis and the MVMR method. Mediation MR analysis was employed for an in-depth investigation of the causal relationship.
Therefore, we believe that our findings are convincing and demonstrate a dependable causal explanatory impact. These discoveries may provide valuable insights for the prospective treatment of sepsis through further study of the mechanisms associated with mental illness. However, there are some limitations to our investigation. First, the applicability of our European population-based analysis to other populations cannot be determined. Although our findings demonstrate a causal relationship and discover a possible mediator between particular psychiatric disorders and sepsis, due to the limitations of GWAS data, the underlying mechanisms need to be further investigated in order to develop effective and feasible treatments for sepsis.
Mental illnesses and sepsis are significant health concerns today, and while there appears to be a causal link between them, it remains unclear. This study pioneers the use of the MR method to examine the relationship. By using genetic variations strongly related to mental disorders as instrumental variables, we assessed the causal connection between mental disorders and sepsis. Bidirectional MR, multivariable MR and meta-analysis were utilized for validation. Additionally, mediation MR helped us understand the roles of blood metabolites, inflammatory factors and gut microbiota in this relationship. These findings lay the groundwork for advancing the understanding and treatment of both mental illnesses and sepsis, with the ultimate goal of reducing incidence rates, mortality, and the global burden on health systems.
5 Conclusion
In summary, this research revealed that AN is independently associated with an increased likelihood of sepsis. Additionally, mediation MR analysis indicated that blood N-formylmethionine levels and cystatin D levels accompanied by ketogluconate metabolism and N10-formyl-tetrahydrofolate biosynthesis in gut microbiome may mediate the causal connection between AN and sepsis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YH: Formal analysis, Writing – original draft. ZX: Methodology, Writing – original draft. PH: Investigation, Writing – original draft. WH: Investigation, Visualization, Writing – review & editing. MZ: Visualization, Writing – review & editing. DZ: Visualization, Writing – review & editing. GT: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the funding from the Guangdong Provincial Key Laboratory of Research on Emergency in TCM (2023B1212060062).
Acknowledgments
The authors express deep appreciation to the researchers for generously providing the GWAS data that were used in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1327315/full#supplementary-material
Footnotes
References
1. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Rudd, KE, Johnson, SC, Agesa, KM, Shackelford, KA, Tsoi, D, Kievlan, DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017:analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Prescott, HC, and Angus, DC. Enhancing recovery from sepsis: a review. JAMA. (2018) 319:62–75. doi: 10.1001/jama.2017.17687
4. Shankar-Hari, M, Harrison, DA, Ferrando-Vivas, P, Rubenfeld, GD, and Rowan, K. Risk factors at index hospitalization associated with longer-term mortality in adult sepsis survivors. JAMA Netw Open. (2019) 2:e194900. doi: 10.1001/jamanetworkopen.2019.4900
5. Oh, TK, Park, HY, and Song, IA. Depression and long-term survival among south Korean sepsis survivors: anationwide cohort study from 2011 to 2014. Crit Care Med. (2021) 49:1470–80. doi: 10.1097/CCM.0000000000005030
6. Askim, A, Gustad, LT, Paulsen, J, Reitan, SK, Mehl, A, Mohus, RM, et al. Anxiety and depression symptoms in a general population and future risk of bloodstream infection: the hunt study. Psychosom Med. (2018) 80:673–9. doi: 10.1097/PSY.0000000000000619
7. Schultebraucks, K, Blekic, W, Basaraba, C, Corbeil, T, Khan, Z, Henry, BF, et al. The impact of preexisting psychiatric disorders and antidepressant use on covid-19 related outcomes: a multicenter study. Mol Psychiatry. (2023) 28:2462–8. doi: 10.1038/s41380-023-02049-4
8. Lakbar, I, Leone, M, Pauly, V, Orleans, V, Srougbo, KJ, Diao, S, et al. Association of severe mental illness and septic shock case fatality rate inpatients admitted to the intensive care unit: a national population-based cohortstudy. PLoS Med. (2023) 20:e1004202. doi: 10.1371/journal.pmed.1004202
9. Lawlor, DA, Harbord, RM, Sterne, JA, Timpson, N, and Davey, SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
10. Emdin, CA, Khera, AV, and Kathiresan, S. Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
11. Pierce, BL, and Burgess, S. Efficient design for mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084
12. Sanderson, E. Multivariable mendelian randomization and mediation. Cold Spring Harb Perspect Med. (2021) 11:a038984. doi: 10.1101/cshperspect.a038984
13. Thorkildsen, MS, Gustad, LT, Mohus, RM, Burgess, S, Nilsen, T, Damas, JK, et al. Association of genetically predicted insomnia with risk of sepsis: a Mendelian randomization study. JAMA Psychiatry. (2023) 80:1061–5. doi: 10.1001/jamapsychiatry.2023.2717
14. Chen, XH, Liu, HQ, Nie, Q, Wang, H, and Xiang, T. Causal relationship between type 1 diabetes mellitus and six high-frequency infectious diseases: a two-sample Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1135726. doi: 10.3389/fendo.2023.1135726
15. Leng, Y, Li, Y, Wang, J, Deng, P, Wang, W, Wu, J, et al. Sepsis as an independent risk factor in atrial fibrillation and cardioembolic stroke. Front Endocrinol (Lausanne). (2023) 14:1056274. doi: 10.3389/fendo.2023.1056274
16. Beydoun, MA, Beydoun, HA, Dore, GA, Fanelli-Kuczmarski, MT, Evans, MK, and Zonderman, AB. Total serum cholesterol, atherogenic indices and their longitudinal association with depressive symptoms among us adults. Transl Psychiatry. (2015) 5:e518. doi: 10.1038/tp.2015.4
17. Lavagnino, L, Gurguis, C, and Lane, S. Risk factors for metabolic and cardiovascular disease in inpatients with severe mental illness. Psychiatry Res. (2021) 304:114148. doi: 10.1016/j.psychres.2021.114148
18. Miller, BJ, Mcevoy, JP, Mccall, WV, and Harris, RA. Insomnia and triglycerides in schizophrenia. Schizophr Res. (2022) 239:42–3. doi: 10.1016/j.schres.2021.11.021
19. Hofmaenner, DA, Kleyman, A, Press, A, Bauer, M, and Singer, M. The many roles of cholesterol in sepsis: a review. Am J Respir Crit Care Med. (2022) 205:388–96. doi: 10.1164/rccm.202105-1197TR
20. Lee, SM, Kang, JW, Jo, YH, Kim, K, Lee, JH, Lee, J, et al. Underweight is associated with mortality in patients with severe sepsis and septic shock. Intensive Care Med Exp. (2015) 3:A876. doi: 10.1186/2197-425X-3-S1-A876
21. Fricke, C, and Voderholzer, U. Endocrinology of underweight and anorexia nervosa. Nutrients. (2023):15. doi: 10.3390/nu15163509
22. Zhou, Q, Wang, M, Li, S, Zhang, J, Ma, Q, Ding, Y, et al. Impact of body mass index on survival of medical patients with sepsis: a prospective cohort study in a university hospital in China. BMJ Open. (2018) 8:e021979. doi: 10.1136/bmjopen-2018-021979
23. Yuan, N, Chen, Y, Xia, Y, Dai, J, and Liu, C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. (2019) 9:233. doi: 10.1038/s41398-019-0570-y
24. Pu, J, Liu, Y, Gui, S, Tian, L, Yu, Y, Song, X, et al. Metabolomic changes in animal models of depression: a systematic analysis. Mol Psychiatry. (2021) 26:7328–36. doi: 10.1038/s41380-021-01269-w
25. van der Poll, T, van de Veerdonk, FL, Scicluna, BP, and Netea, MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. (2017) 17:407–20. doi: 10.1038/nri.2017.36
26. Wang, J, Sun, Y, Teng, S, and Li, K. Prediction of sepsis mortality using metabolite biomarkers in the blood: a meta-analysis of death-related pathways and prospective validation. BMC Med. (2020) 18:83. doi: 10.1186/s12916-020-01546-5
27. Socala, K, Doboszewska, U, Szopa, A, Serefko, A, Wlodarczyk, M, Zielinska, A, et al. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. (2021) 172:105840. doi: 10.1016/j.phrs.2021.105840
28. Sun, S, Wang, D, Dong, D, Xu, L, Xie, M, Wang, Y, et al. Altered intestinal microbiome and metabolome correspond to the clinical outcome of sepsis. Crit Care. (2023) 27:127. doi: 10.1186/s13054-023-04412-x
29. Shang, W, Qian, H, Zhang, S, Yuan, M, Pan, X, Huang, S, et al. Human blood metabolites and risk of sepsis: a mendelian randomization investigation. Eur J Clin Investig. (2023):e14145. doi: 10.1111/eci.14145
30. Zhang, Z, Yin, Y, Chen, T, You, J, Zhang, W, Zhao, Y, et al. Investigating the impact of human blood metabolites on the sepsis development and progression: a study utilizing two-sample mendelian randomization. Front Med (Lausanne). (2023) 10:1310391. doi: 10.3389/fmed.2023.1310391
31. Skrivankova, VW, Richmond, RC, Woolf, B, Yarmolinsky, J, Davies, NM, Swanson, SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the strobe-mr statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
32. Sekula, P, Del, GMF, Pattaro, C, and Kottgen, A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
33. Kurki, MI, Karjalainen, J, Palta, P, Sipila, TP, Kristiansson, K, Donner, KM, et al. Finngen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
34. Hamilton, FW, Thomas, M, Arnold, D, Palmer, T, Moran, E, Mentzer, AJ, et al. Therapeutic potential of il6r blockade for the treatment of sepsis and sepsis-related death: a mendelian randomisation study. PLoS Med. (2023) 20:e1004174. doi: 10.1371/journal.pmed.1004174
35. Relton, CL, and Davey, SG. Two-step epigenetic mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. (2012) 41:161–76. doi: 10.1093/ije/dyr233
36. Demontis, D, Walters, GB, Athanasiadis, G, Walters, R, Therrien, K, Nielsen, TT, et al. Genome-wide analyses of adhd identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genet. (2023) 55:198–208. doi: 10.1038/s41588-022-01285-8
37. Mullins, N, Forstner, AJ, O'Connell, KS, Coombes, B, Coleman, J, Qiao, Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. (2021) 53:817–29. doi: 10.1038/s41588-021-00857-4
38. Wray, NR, Ripke, S, Mattheisen, M, Trzaskowski, M, Byrne, EM, Abdellaoui, A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. (2018) 50:668–81. doi: 10.1038/s41588-018-0090-3
39. Forstner, AJ, Awasthi, S, Wolf, C, Maron, E, Erhardt, A, Czamara, D, et al. Genome-wide association study of panic disorder reveals genetic overlap with neuroticism and depression. Mol Psychiatry. (2021) 26:4179–90. doi: 10.1038/s41380-019-0590-2
40. Trubetskoy, V, Pardinas, AF, Qi, T, Panagiotaropoulou, G, Awasthi, S, Bigdeli, TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. (2022) 604:502–8. doi: 10.1038/s41586-022-04434-5
41. Duncan, LE, Ratanatharathorn, A, Aiello, AE, Almli, LM, Amstadter, AB, Ashley-Koch, AE, et al. Largest gwas of ptsd (n=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry. (2018) 23:666–73. doi: 10.1038/mp.2017.77
42. Duncan, L, Yilmaz, Z, Gaspar, H, Walters, R, Goldstein, J, Anttila, V, et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry. (2017) 174:850–8. doi: 10.1176/appi.ajp.2017.16121402
43. Grove, J, Ripke, S, Als, TD, Mattheisen, M, Walters, RK, Won, H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. (2019) 51:431–44. doi: 10.1038/s41588-019-0344-8
44. International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. (2018) 23:1181–8. doi: 10.1038/mp.2017.154
45. Yu, D, Sul, JH, Tsetsos, F, Nawaz, MS, Huang, AY, Zelaya, I, et al. Interrogating the genetic determinants of tourette's syndrome and other tic disorders through genome-wide association studies. Am J Psychiatry. (2019) 176:217–27. doi: 10.1176/appi.ajp.2018.18070857
46. Ponsford, MJ, Gkatzionis, A, Walker, VM, Grant, AJ, Wootton, RE, Moore, L, et al. Cardiometabolic traits, sepsis, and severe covid-19: a mendelian randomization investigation. Circulation. (2020) 142:1791–3. doi: 10.1161/CIRCULATIONAHA.120.050753
47. Fatumo, S, Willer, C, Brumpton, B, and Gill, D. Cardiometabolic traits, sepsis and severe covid-19: a mendelian randomization investigation. Nat Rev Methods Prim. (2022) 2:1–22. doi: 10.1101/2020.06.18.20134676
48. Zhao, JH, Stacey, D, Eriksson, N, Macdonald-Dunlop, E, Hedman, AK, Kalnapenkis, A, et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol. (2023) 24:1540–51. doi: 10.1038/s41590-023-01588-w
49. Chen, Y, Lu, T, Pettersson-Kymmer, U, Stewart, ID, Butler-Laporte, G, Nakanishi, T, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet. (2023) 55:44–53. doi: 10.1038/s41588-022-01270-1
50. Lopera-Maya, EA, Kurilshikov, A, van der Graaf, A, Hu, S, Andreu-Sanchez, S, Chen, L, et al. Effect of host genetics on the gut microbiome in 7,738 participants of the dutch microbiome project. Nat Genet. (2022) 54:143–51. doi: 10.1038/s41588-021-00992-y
51. Choi, KW, Chen, CY, Stein, MB, Klimentidis, YC, Wang, MJ, Koenen, KC, et al. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample mendelian randomization study. JAMA Psychiatry. (2019) 76:399–408. doi: 10.1001/jamapsychiatry.2018.4175
52. Iob, E, Pingault, JB, Munafo, MR, Stubbs, B, Gilthorpe, MS, Maihofer, AX, et al. Testing the causal relationships of physical activity and sedentary behaviour with mental health and substance use disorders: a mendelian randomisation study. Mol Psychiatry. (2023) 28:3429–43. doi: 10.1038/s41380-023-02133-9
53. Davey, SG, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
54. Chen, L, Yang, H, Li, H, He, C, Yang, L, and Lv, G. Insights into modifiable risk factors of cholelithiasis: a mendelian randomization study. Hepatology. (2022) 75:785–96. doi: 10.1002/hep.32183
55. Baurecht, H, Freuer, D, Welker, C, Tsoi, LC, Elder, JT, Ehmke, B, et al. Relationship between periodontitis and psoriasis: a two-sample mendelian randomization study. J Clin Periodontol. (2022) 49:573–9. doi: 10.1111/jcpe.13620
56. Tao, H, Fan, S, Zhu, T, You, L, Zheng, D, Yan, L, et al. Psychiatric disorders and type 2 diabetes mellitus: a bidirectional mendelian randomization. Eur J Clin Investig. (2023) 53:e13893. doi: 10.1111/eci.13893
57. Lei, P, Xu, W, Wang, C, Lin, G, Yu, S, and Guo, Y. Mendelian randomization analysis reveals causal associations of polyunsaturated fatty acids with sepsis and mortality risk. Infect Dis Ther. (2023) 12:1797–808. doi: 10.1007/s40121-023-00831-z
58. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
59. Burgess, S, and Thompson, SG. Interpreting findings from mendelian randomization using the mr-egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
60. Burgess, S, Davey, SG, Davies, NM, Dudbridge, F, Gill, D, Glymour, MM, et al. Guidelines for performing mendelian randomization investigations: update for summer 2023. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.3
61. Calsavara, AJ, Costa, PA, Nobre, V, and Teixeira, AL. Prevalence and risk factors for post-traumatic stress, anxiety, and depression in sepsis survivors after icu discharge. Braz J Psychiatry. (2021) 43:269–76. doi: 10.1590/1516-4446-2020-0986
62. Oh, TK, Park, HY, and Song, IA. Prevalence and risk factors for suicide in patients with sepsis: nationwide cohort study in South Korea. BJPsych Open. (2022) 8:e61. doi: 10.1192/bjo.2022.19
63. Hartwig, FP, Borges, MC, Horta, BL, Bowden, J, and Davey, SG. Inflammatory biomarkers and risk of schizophrenia: a 2-sample mendelian randomization study. JAMA Psychiatry. (2017) 74:1226–33. doi: 10.1001/jamapsychiatry.2017.3191
64. Liu, N, Tan, JS, Liu, L, Wang, Y, Hua, L, and Qian, Q. Genetic predisposition between covid-19 and four mental illnesses: a bidirectional, two-sample mendelian randomization study. Front Psych. (2021) 12:746276. doi: 10.3389/fpsyt.2021.746276
65. Baranova, A, Cao, H, and Zhang, F. Severe covid-19 increases the risk of schizophrenia. Psychiatry Res. (2022) 317:114809. doi: 10.1016/j.psychres.2022.114809
66. Baranova, A, Zhao, Y, Cao, H, and Zhang, F. Causal associations between major depressive disorder and covid-19. Gen Psychiatr. (2023) 36:e101006. doi: 10.1136/gpsych-2022-101006
67. Sirufo, MM, Magnanimi, LM, Ginaldi, L, and De Martinis, M. Anorexia nervosa and autoimmune comorbidities: a bidirectional route? CNS Neurosci Ther. (2022) 28:1921–9. doi: 10.1111/cns.13953
68. Ulfvebrand, S, Birgegard, A, Norring, C, Hogdahl, L, and von Hausswolff-Juhlin, Y. Psychiatric comorbidity in women and men with eating disorders results from a large clinical database. Psychiatry Res. (2015) 230:294–9. doi: 10.1016/j.psychres.2015.09.008
69. American Psychiatric Association DAmerican, PA. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association (2013).
70. Morris, J, and Twaddle, S. Anorexia nervosa. BMJ. (2007) 334:894–8. doi: 10.1136/bmj.39171.616840.BE
71. Pomposelli, JJ, Flores, EA, and Bistrian, BR. Role of biochemical mediators in clinical nutrition and surgical metabolism. JPEN J Parenter Enteral Nutr. (1988) 12:212–8. doi: 10.1177/0148607188012002212
72. Slotwinska, SM, and Slotwinski, R. Immune disorders in anorexia. Cent Eur J Immunol. (2017) 42:294–300. doi: 10.5114/ceji.2017.70973
73. Nova, E, Gomez-Martinez, S, Morande, G, and Marcos, A. Cytokine production by blood mononuclear cells from in-patients with anorexia nervosa. Br J Nutr. (2002) 88:183–8. doi: 10.1079/BJNBJN2002608
74. Palmblad, J, Fohlin, L, and Lundstrom, M. Anorexia nervosa and polymorphonuclear (pmn) granulocyte reactions. Scand J Haematol. (1977) 19:334–42. doi: 10.1111/j.1600-0609.1977.tb01483.x
75. Westmoreland, P, Duffy, A, Rienecke, R, Le Grange, D, Joiner, T, Manwaring, J, et al. Causes of death in patients with a history of severe anorexia nervosa. J Eat Disord. (2022) 10:200. doi: 10.1186/s40337-022-00716-5
76. Dalton, B, Leppanen, J, Campbell, IC, Chung, R, Breen, G, Schmidt, U, et al. A longitudinal analysis of cytokines in anorexia nervosa. Brain Behav Immun. (2020) 85:88–95. doi: 10.1016/j.bbi.2019.05.012
77. Chousterman, BG, Swirski, FK, and Weber, GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. (2017) 39:517–28. doi: 10.1007/s00281-017-0639-8
78. Mera, S, Tatulescu, D, Cismaru, C, Bondor, C, Slavcovici, A, Zanc, V, et al. Multiplex cytokine profiling in patients with sepsis. APMIS. (2011) 119:155–63. doi: 10.1111/j.1600-0463.2010.02705.x
79. Gouel-Cheron, A, Allaouchiche, B, Guignant, C, Davin, F, Floccard, B, and Monneret, G. Early interleukin-6 and slope of monocyte human leukocyte antigen-dr: a powerful association to predict the development of sepsis after major trauma. PLoS One. (2012) 7:e33095. doi: 10.1371/journal.pone.0033095
80. Wu, HP, Chen, CK, Chung, K, Tseng, JC, Hua, CC, Liu, YC, et al. Serial cytokine levels in patients with severe sepsis. Inflamm Res. (2009) 58:385–93. doi: 10.1007/s00011-009-0003-0
81. Mira, JP, Cariou, A, Grall, F, Delclaux, C, Losser, MR, Heshmati, F, et al. Association of tnf2, a tnf-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA. (1999) 282:561–8. doi: 10.1001/jama.282.6.561
82. Jia, Y, Hui, L, Sun, L, Guo, D, Shi, M, Zhang, K, et al. Association between human blood metabolome and the risk of psychiatric disorders. Schizophr Bull. (2023) 49:428–43. doi: 10.1093/schbul/sbac130
83. Gorwood, P, Blanchet-Collet, C, Chartrel, N, Duclos, J, Dechelotte, P, Hanachi, M, et al. New insights in anorexia nervosa. Front Neurosci. (2016) 10:256. doi: 10.3389/fnins.2016.00256
84. Prochazkova, P, Roubalova, R, Dvorak, J, Kreisinger, J, Hill, M, Tlaskalova-Hogenova, H, et al. The intestinal microbiota and metabolites in patients with anorexia nervosa. Gut Microbes. (2021) 13:1–25. doi: 10.1080/19490976.2021.1902771
85. Adelman, MW, Woodworth, MH, Langelier, C, Busch, LM, Kempker, JA, Kraft, CS, et al. The gut microbiome's role in the development, maintenance, and outcomes of sepsis. Crit Care. (2020) 24:278. doi: 10.1186/s13054-020-02989-1
86. Rao, K, Patel, AR, Seekatz, AM, Bassis, CM, Sun, Y, Henig, O, et al. Gut microbiome features are associated with sepsis onset and outcomes. bioRxiv. (2021). doi: 10.1101/2021.01.08.426011
87. Lee, CS, Kim, D, and Hwang, CS. Where does n-formylmethionine come from? What for? Where is it going? What is the origin of n-formylmethionine in eukaryotic cells? Mol Cells. (2022) 45:109–11. doi: 10.14348/molcells.2021.5040
88. Cai, N, Gomez-Duran, A, Yonova-Doing, E, Kundu, K, Burgess, AI, Golder, ZJ, et al. Mitochondrial DNA variants modulate n-formylmethionine, proteostasis and risk of late-onset human diseases. Nat Med. (2021) 27:1564–75. doi: 10.1038/s41591-021-01441-3
89. Mayo-Martinez, L, Ruperez, FJ, Martos-Moreno, GA, Graell, M, Barbas, C, Argente, J, et al. Unveiling metabolic phenotype alterations in anorexia nervosa through metabolomics. Nutrients. (2021):13. doi: 10.3390/nu13124249
90. Focker, M, Cecil, A, Prehn, C, Adamski, J, Albrecht, M, Adams, F, et al. Evaluation of metabolic profiles of patients with anorexia nervosa at inpatient admission, short-and long-term weight regain-descriptive and pattern analysis. Meta. (2020):11. doi: 10.3390/metabo11010007
91. Focker, M, Timmesfeld, N, Scherag, S, Knoll, N, Singmann, P, Wang-Sattler, R, et al. Comparison of metabolic profiles of acutely ill and short-term weight recovered patients with anorexia nervosa reveals alterations of 33 out of 163 metabolites. J Psychiatr Res. (2012) 46:1600–9. doi: 10.1016/j.jpsychires.2012.08.015
92. Miyata, N, Hata, T, Takakura, S, Yoshihara, K, Morita, C, Mikami, K, et al. Metabolomics profile of japanese female patients with restricting-type anorexia nervosa. Physiol Behav. (2021) 228:113204. doi: 10.1016/j.physbeh.2020.113204
93. Wingfield, PT. N-terminal methionine processing. Curr Protoc Protein Sci. (2017) 88:6–14. doi: 10.1002/cpps.29
94. Nakahira, K, Hisata, S, and Choi, AM. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. (2015) 23:1329–50. doi: 10.1089/ars.2015.6407
95. Johansson, PI, Nakahira, K, Rogers, AJ, Mcgeachie, MJ, Baron, RM, Fredenburgh, LE, et al. Plasma mitochondrial dna and metabolomic alterations in severe critical illness. Crit Care. (2018) 22:360. doi: 10.1186/s13054-018-2275-7
96. Faust, HE, Reilly, JP, Anderson, BJ, Ittner, C, Forker, CM, Zhang, P, et al. Plasma mitochondrial DNA levels are associated with ARDS in trauma and sepsis patients. Chest. (2020) 157:67–76. doi: 10.1016/j.chest.2019.09.028
97. Raabe, CA, Groper, J, and Rescher, U. Biased perspectives on formyl peptide receptors. Biochim Biophys Acta, Mol Cell Res. (2019) 1866:305–16. doi: 10.1016/j.bbamcr.2018.11.015
98. Sigurdsson, MI, Kobayashi, H, Amrein, K, Nakahira, K, Rogers, AJ, Pinilla-Vera, M, et al. Circulating n-formylmethionine and metabolic shift in critical illness: a multicohort metabolomics study. Crit Care. (2022) 26:321. doi: 10.1186/s13054-022-04174-y
99. Zhang, Z, and Zhan, F. Type 2 cystatins and their roles in the regulation of human immune response andcancer progression. Cancers (Basel). (2023):15. doi: 10.3390/cancers15225363
100. Nilsson, I, Millischer, V, Goteson, A, Hubel, C, Thornton, LM, Bulik, CM, et al. Aberrant inflammatory profile in acute but not recovered anorexia nervosa. Brain Behav Immun. (2020) 88:718–24. doi: 10.1016/j.bbi.2020.05.024
101. Delanaye, P, Cavalier, E, Radermecker, RP, Paquot, N, Depas, G, Chapelle, JP, et al. Cystatin c or creatinine for detection of stage 3 chronic kidney disease in anorexia nervosa. Nephron Clin Pract. (2008) 110:c158–63. doi: 10.1159/000166607
102. Kleiman, SC, Watson, HJ, Bulik-Sullivan, EC, Huh, EY, Tarantino, LM, Bulik, CM, et al. The intestinal microbiota in acute anorexia nervosa and during renourishment:relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med. (2015) 77:969–81. doi: 10.1097/PSY.0000000000000247
103. Xia, X, He, SY, Zhang, XL, Wang, D, He, Q, Xiao, QA, et al. The causality between gut microbiome and anorexia nervosa: a Mendelian randomization analysis. Front Microbiol. (2023) 14:1290246. doi: 10.3389/fmicb.2023.1290246
104. Adhi, FI, Littmann, ER, Taur, Y, Maloy, MA, Markey, KA, Fontana, E, et al. Pre-transplant fecal microbial diversity independently predicts critical illness after hematopoietic cell transplantation. Blood. (2019) 134:3264. doi: 10.1182/blood-2019-124902
Keywords: anorexia nervosa, blood metabolites, gut microbiome, inflammatory factors, Mendelian randomization, mental disorders, sepsis
Citation: Hu Y, Xiong Z, Huang P, He W, Zhong M, Zhang D and Tang G (2024) Association of mental disorders with sepsis: a bidirectional Mendelian randomization study. Front. Public Health. 12:1327315. doi: 10.3389/fpubh.2024.1327315
Edited by:
Zhimin Tao, Jiangsu University, ChinaReviewed by:
Tong Wang, Chinese Academy of Sciences (CAS), ChinaYan kai Dong, Jiujiang Maternity and Child Health Care Hospital, China
Georgia Damoraki, National and Kapodistrian University of Athens, Greece
Copyright © 2024 Hu, Xiong, Huang, He, Zhong, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghua Tang, dGdoMTk3M0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yuanzhi Hu
Yuanzhi Hu Zihui Xiong
Zihui Xiong Pinge Huang1,2
Pinge Huang1,2