- 1School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 2Laboratory Bacteriology Research, Faculty of Medicine and Health Sciences, Ghent University, De Pintelaan, Belgium
Background: Staphylococcus aureus nasal carriage has been linked to higher rates of infection and morbidity. People with Methicillin-resistant Staphylococcus aureus can be a potential source of infection for others. University students living together in crowded conditions increase their risk of acquiring infections. The prevalence of S. aureus, particularly Methicillin-resistant Staphylococcus aureus nasal carriage, in Ethiopian university students is sparse.
Objective: This study aimed to determine the nasal carriage rate, associated factors, and antimicrobial susceptibility patterns of methicillin-resistant Staphylococcus aureus among pre-clinical students at the College of Health and Medical Sciences, Haramaya University, Ethiopia, from 1 July to 30 August 2022.
Methods: An institutional-based cross-sectional study was conducted among 270 randomly selected pre-clinical Health and Medical Sciences students. Data on associated factors were collected using pre-tested, structured questionnaires. A nasal swab was taken from each participant and sent to the microbiology laboratory via Amies transport media in a cold chain. There, it was cultivated using conventional techniques. The isolated colonies were found to be S. aureus, and its antimicrobial susceptibility was performed using the Kirby–Bauer disk diffusion method on Muller–Hinton agar. Methicillin-resistant Staphylococcus aureus expressing using cefoxitin based on CLSI breakpoint. Data were entered into Epi-Data version 4.4.2.1 and exported to the Statistical Package for Social Sciences (SPSS) software version 25 for analysis. Pearson’s chi-square test was performed to predict the associations between variables. A p-value less than 0.05 was regarded as statistically significant.
Result: Methicillin-resistant Staphylococcus aureus nasal carriage was 5.9% (95% CI: 3.09–8.7) of cases of S. aureus nasal colonization, which was found to be 12.96% (95% CI: 8.85–16.96). Methicillin-resistant Staphylococcus aureus nasal colonization was significantly associated with the history of cigarette smoking (p = 0.000), intake of khat (p = 0.042), nose-picking habit (p = 0.003), history of sharing personal goods (p = 0.021), and history of hospitalizations (p = 0.00). All of the Methicillin-resistant Staphylococcus aureus isolates were resistant to ampicillin and cefoxitin.
Conclusion: Based on the findings, a considerable proportion of healthy students harbored Methicillin-resistant Staphylococcus aureus strains associated with behavioral factors. Furthermore, these isolates showed high resistance to cefoxitin and ampicillin. Hence, it is crucial to regularly test pre-clinical students to prevent endogenous infections and the spread of Methicillin-resistant Staphylococcus aureus.
Introduction
Background
Staphylococcus aureus is a prevalent bacterium that frequently causes nosocomial infections and public health issues (1, 2). Methicillin-resistant Staphylococcus aureus (MRSA) is now the main source of community-acquired infections among individuals who have not been exposed to healthcare environments (3). Nasal MRSA colonizes approximately 30% of the human population (4).
Individuals with a history of hospital admissions, history of nasal polyps, HIV/AIDS, malaria, tuberculosis, malnourishment, climate change, sharing of personal items without frequent cleaning, frequent skin-to-skin contact in skin infection, crowded housing, and MRSA carriage have all been linked to an increased risk of S. aureus and MRSA infections (3–9). The MRSA strains are typically spread through direct skin-to-skin contact and can occur in shared public areas such as dorms, gyms, and barracks (10, 11), causing community-acquired infections. According to reports, the chance of contracting MRSA might increase by 7.5 times when one is close to someone who has previously been colonized or infected (2, 7, 12, 13). MRSA infections have increased in people who have not been exposed to healthcare settings since the 1990s. Consequently, strains of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) have been identified (14). Given the increasing evidence that MRSA acquired in the community is spreading among healthy individuals (15). Serious opportunistic infections in humans and systemic illnesses such as sepsis, infections related to healthcare, and infections of the skin, soft tissues, and bones are caused by S. aureus (16). It frequently results in major infections with significant rates of morbidity, mortality, and expenses related to medical care (11). Approximately 10–35% of the world’s population harbors MRSA in their anterior nares (17), and the prevalence of MRSA ranges from 5.0 to 73.0% (18). However, the burden of infection is high in developing countries (19). People with MRSA infections are 64% more likely to die than people with drug-sensitive infections (20). According to earlier research, the carriage rate of S. aureus in 2013 was 21% at Tanzanian universities (21), 22.1% at Jimma University in Ethiopia (22), and 27.1% at Arba Minch University in Ethiopia (7). These findings were significant for MRSA.
S. aureus strains have developed resistance to β-lactam antibiotics, including MRSA (23). The organism acquires resistance through the insertion of the mecA gene at a precise location on its chromosome, and the mecA encodes an alternative penicillin-binding protein with a low affinity for semisynthetic drugs, such as methicillin, nafcillin, and oxacillin (7). Antibiotic-resistant bacteria are an increasing problem worldwide, contributing to longer hospital stays, higher medical costs, and higher rates of morbidity and mortality among patients (24). MRSA strains spread quickly in vulnerable hospitalized (exposed) and healthy non-exposed individuals, profoundly changing the current therapeutic options for the prevention and treatment of staphylococcal infections (22, 25).
Carrier health students act as a reservoir for the spread of S. aureus to uncolonized susceptible patients. Besides, the chances of the transmission of MRSA from the medical student carriers to the community are a great hazard. University students living in the university dormitory are among the groups living in crowded conditions, which could make them more vulnerable to inadequate care and possibly at higher risk for MRSA colonization. However, little is known about MRSA carriage among pre-clinical undergraduate students living on the university campus or dormitory before their hospital attachment. Therefore, this study aimed to assess the MRSA nasal carriage rate, associated factors, and antimicrobial susceptibility patterns among students at Haramaya University’s College of Health and Medical Sciences (CHMS).
Materials and methods
Study area and period
The study was conducted at the CHMS, Haramaya University, Eastern Ethiopia, from 1 July to 30 August 2022. The CHMS is one of the colleges that was established in September 1996 with the objective of training health professionals who contribute to filling the gap in the health professional needs of the country, especially the rural population.
Study design and population
We carried out an institutional-based cross-sectional study. All undergraduate pre-clinical students from the selected department in the preceding 6 months at the CHMS were recruited. Pre-clinical students who had taken antibiotics within the 10 days before the data collection (26) and participants who had nasal bleeding during the time of collection were excluded. The source population for this study comprised all undergraduate students enrolled during the study period at Haramaya University’s CHMS. On the other hand, all of the selected students from each department were considered the study population during the study period.
Sample size determination and sampling technique
The sample size was determined using a single population proportion statistical formula by considering 95% confidence, p = proportion of Staphylococcus aureus in Arba Minch University, Ethiopia (27.1%) (7), and d = the level of precision (0.05). Ten percent of the sample size was added to reduce errors resulting from the likelihood of non-compliance, giving a final sample size of 270. Health and Medical Sciences students were stratified based on academic years; the final sample size was allocated proportionally to each academic year during the study period. A simple random sampling technique was used to select each pre-clinical student who fulfills the inclusion criteria from each departmental batch by using a student roster as a sampling frame that was collected from each department until it reached the allocated sample size (Figure 1).
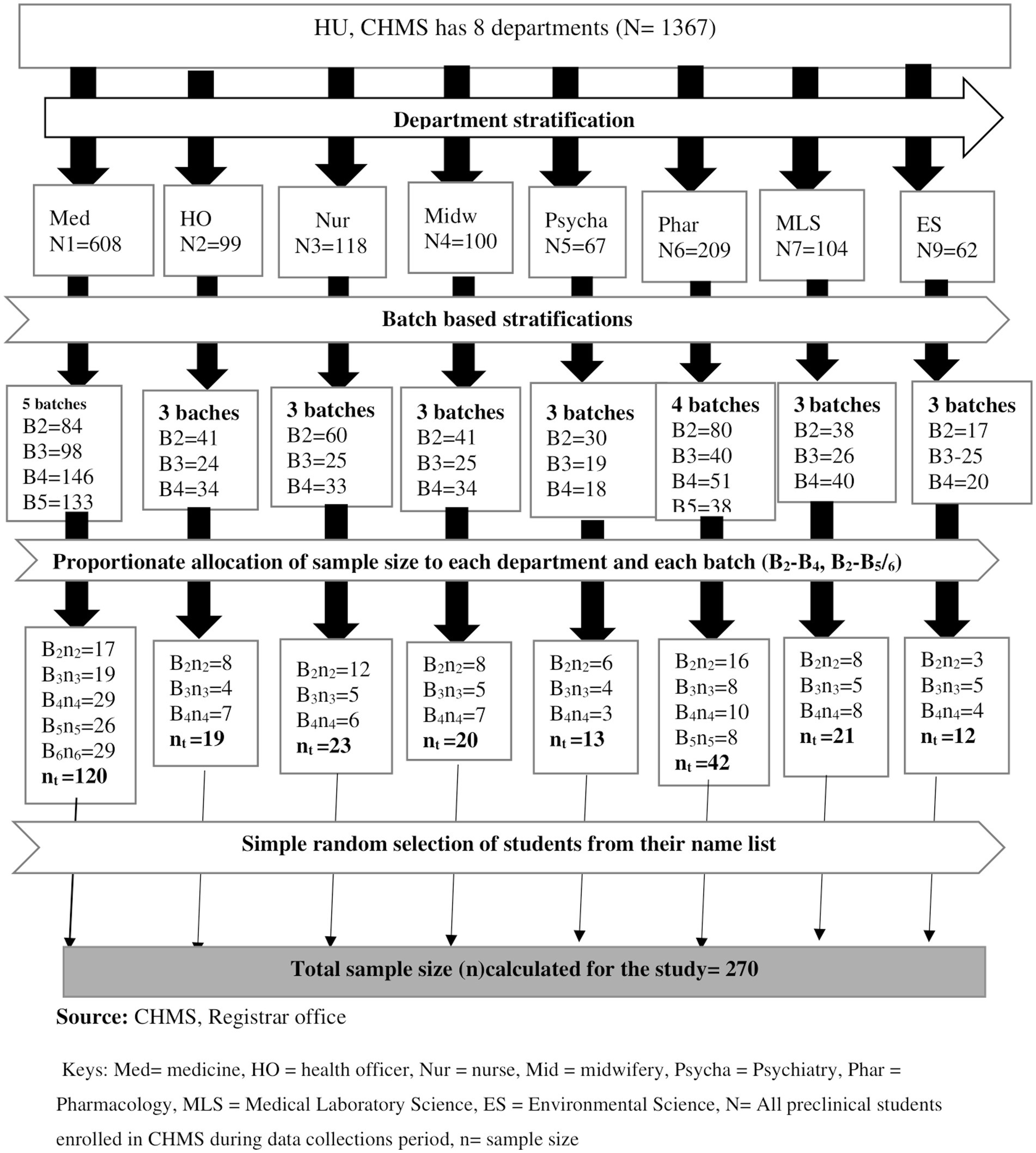
Figure 1. Flow diagram illustrating the sampling procedure of pre-clinical students in CHMS Haramaya University, Harar, Eastern Ethiopia, 2022.
Data and sample collections
Data on socio-demographic, behavioral features, clinical information, and associated factors were collected using a structured, pretested questionnaire that was developed after a review of a variety of literature sources (2, 15, 17, 27) through oral interviews of study participants, and nasal swabs were taken from the anterior nares using a sterile cotton-tipped applicator stick saturated with sterile normal saline. The specimens were transported for microbiological analysis to the microbiology laboratory at Haramaya University CHMS within 30 min of collection (28).
Isolation and identification of S. aureus
The nasal swab was inoculated on mannitol salt agar (MSA) and blood agar (Oxoid, United Kingdom) and incubated at 37°C for 24 h. Presumptive S. aureus was detected by the visual inspection of golden-yellow colonies on MSA and beta-hemolysis on sheep blood agar. Gram stain, catalase, and tube coagulase tests were used to further identify the isolates (27).
Antimicrobial susceptibility testing
Antimicrobial tests of S. aureus isolates were performed on Muller–Hinton Agar (Oxoid, United Kingdom) using the modified Kirby–Bauer disk diffusion method based on recommendations from the Clinical and Laboratory Standards Institute (CLSI) (28). The suspension of bacterial inoculum, equivalent to 0.5 McFarland standards, was uniformly spread on Mueller–Hinton agar (Oxoid, United Kingdom) plates using a sterile applicator cotton swab. The following antimicrobial disks (Oxoid, United Kingdom) were used: ciprofloxacin (5 mg), clindamycin (2 mg), gentamicin (10 mg), erythromycin (15 mg), chloramphenicol (30 mg), ampicillin (10 mg), tetracycline (30 mg), trimethoprim-sulfamethoxazole (25 mg), and cefoxitin (30 mg). These antibacterial disks were selected depending on local availability, pathogens, and 2020 CLSI recommendations (28). The antibiotic disks were applied to the surfaces of the inoculated Mueller–Hinton agar and incubated at 37°C for 24 h. After incubation, the diameter of the zone of inhibition produced by each antibiotic disk was determined based on the CLSI protocol, and the results were interpreted as sensitive (S), intermediate (I), or resistant (28).
Identification of MRSA: Cefoxitin disk (HiMedia Ltd., Mumbai) was used to identify Methicillin-resistant Staphylococcus aureus. The inoculated plate was covered with a cefoxitin disk (30 μg), which was then incubated for 16–18 h at 37°C. An inhibition diameter <22 is indicative of methicillin expression (27).
Data quality control
Standard operating procedures (SOPs) were strictly followed for each activity in the laboratory. To ensure the validity of the questionnaire, 5% of it was pre-tested at Dire Dawa University. Data collectors were trained regarding all stages of the data collection process. The completeness of each questionnaire is checked by the principal investigator and the supervisors daily. The sterility of uninoculated culture media was checked by incubating 5% of the batch at 37°C overnight, and following incubation, it must remain clear. In addition, standard positive control strains of Staphylococcus aureus (ATCC 25923) and MRSA (ATCC 29213) were used for viability and to check the quality of the reagents, culture media, and antimicrobial disks (28). The bacterial suspension’s inoculum density was calibrated using the 0.5 McFarland standard.
Methods of data analysis
Data were entered into Epi-Data version 4.4.2.1., cleaned, and then exported to the Statistical Package for Social Sciences software (SPSS) version 25 for analysis. The findings were summarized using descriptive statistical tools such as mean, standard deviation, and percentage. Pearson’s chi-square test (X2) was used to forecast the associations between variables. Variables with a p-value of less than 0.05 were considered statistically significant.
Ethical consideration
Ethical clearance was obtained from the Institutional Health Research Ethics Review Committee of the College of Health and Medical Science, Haramaya University, Haramaya University. Written, and signed consent was obtained from the respondents during the time of the interview. Anonymity and confidentiality were ensured for information obtained from study participants. Finally, the results were reported to the participants for appropriate measurement, particularly MRSA.
Results
Socio-demographic characteristics
Of a total of 270 samples analyzed, 65.2% (176/270) were males, with a mean age of 21.32 ± 1.14 and a range of 18–27 years. The majority of the study participants were within the age group of 18–22 years; 85.9, 44.4% (120/270) were medicine students, 28.9% (78/270) were second-year students, and 48.1% lived in groups of six (Table 1).
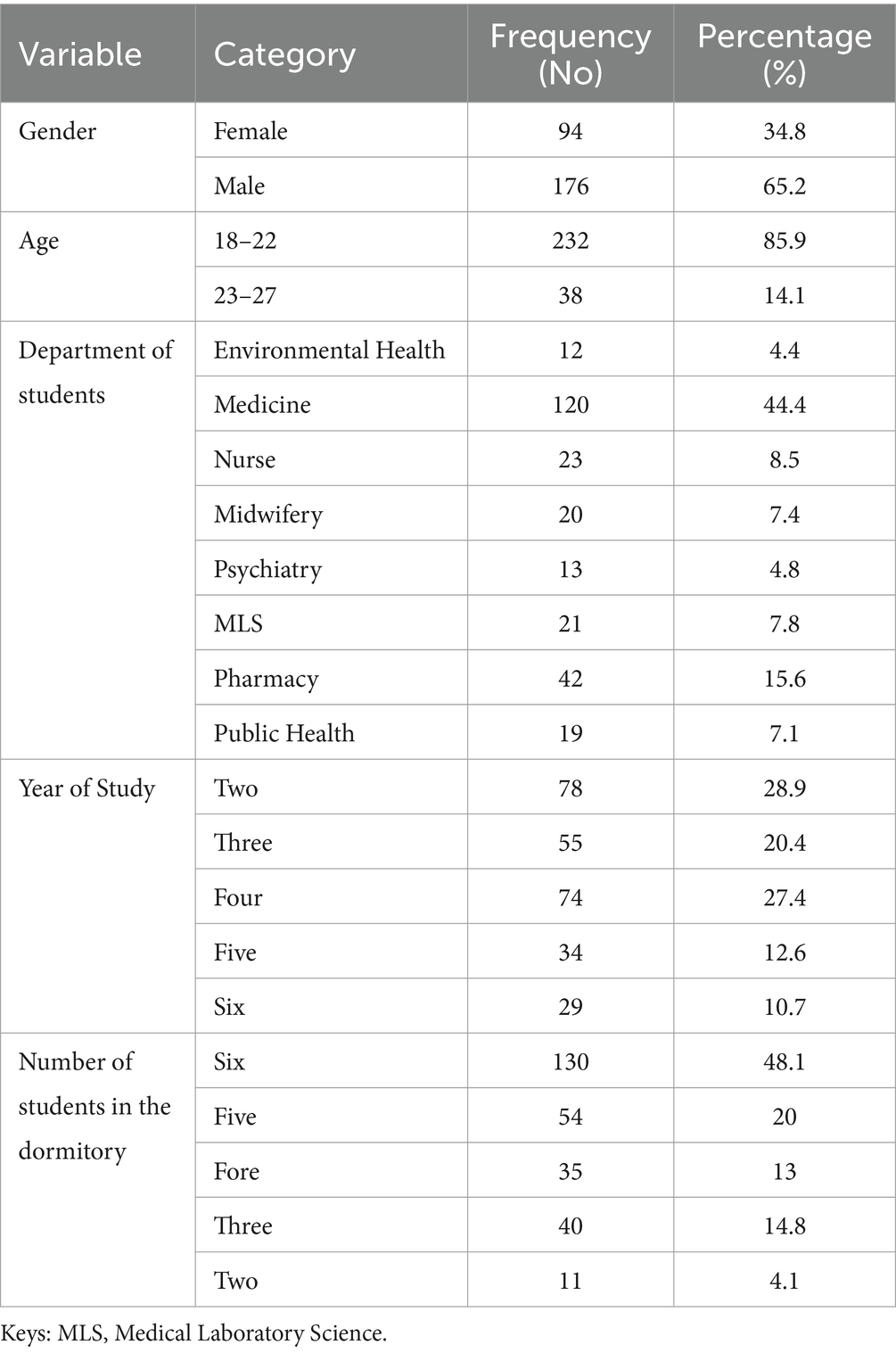
Table 1. Socio-demographic characteristics of pre-clinical students in CHMS, Haramaya University, Harar, Eastern Ethiopia, 2022.
Behavioral characteristics of the study subjects
Only 3.7% (10/270) of the students smoked cigarettes, and all of them were men, 21.1% (57/270) used khat, 38.5% (104/270) drank alcohol, and 25 females were part of this proportion. Approximately 55.6% (150/270) of students washed their hands with soap 0–3 times a day, 41.1% (111/270) had a history of sharing personal items, whereas 64.9% (72/111) shared soap with others, 23% (73/270) picked their noses, 55.6% (150/270) wiped their noses with handkerchiefs, and 38.5% (104/270) cleaned their dorm once a week (Table 2).
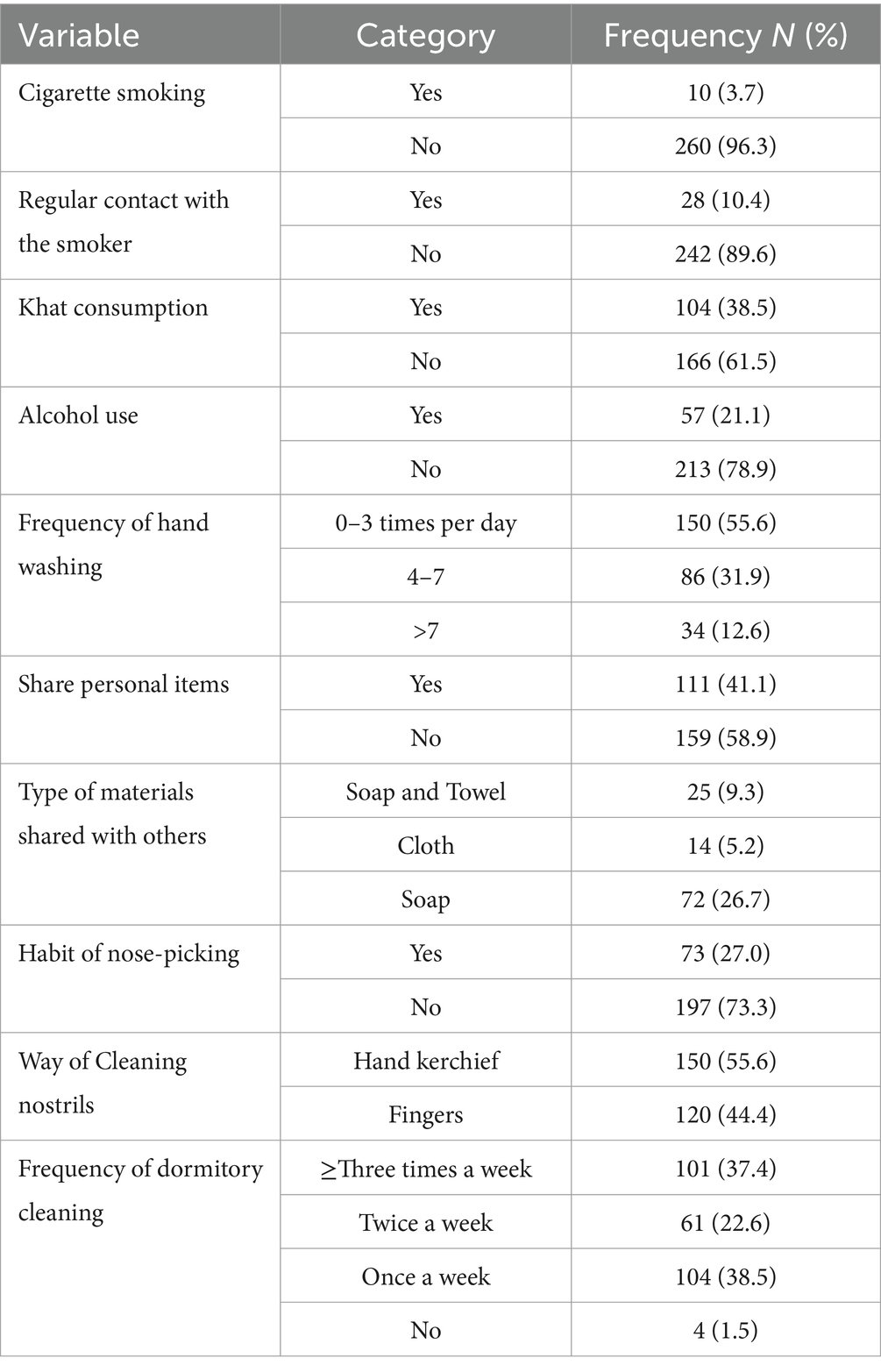
Table 2. Behavioral characteristics of study participants among pre-clinical students in CHMS, Haramaya University, Harar, Eastern Ethiopia, 2022.
Clinical-related characteristics
Of the 270 study participants, 4.4% (12/270) had been admitted to the hospital during the previous 3 months, and 94.8% (256/270) had no soft tissue infections. The majority of the students had no history of indwelling medical devices; 92.6, 85.2% (230/270) had no comorbidities, 24.1% (65/270) had used antibiotics in the past 3 months, and 24.24% (19/65) had used ciprofloxacin-type antibiotics (Table 3).
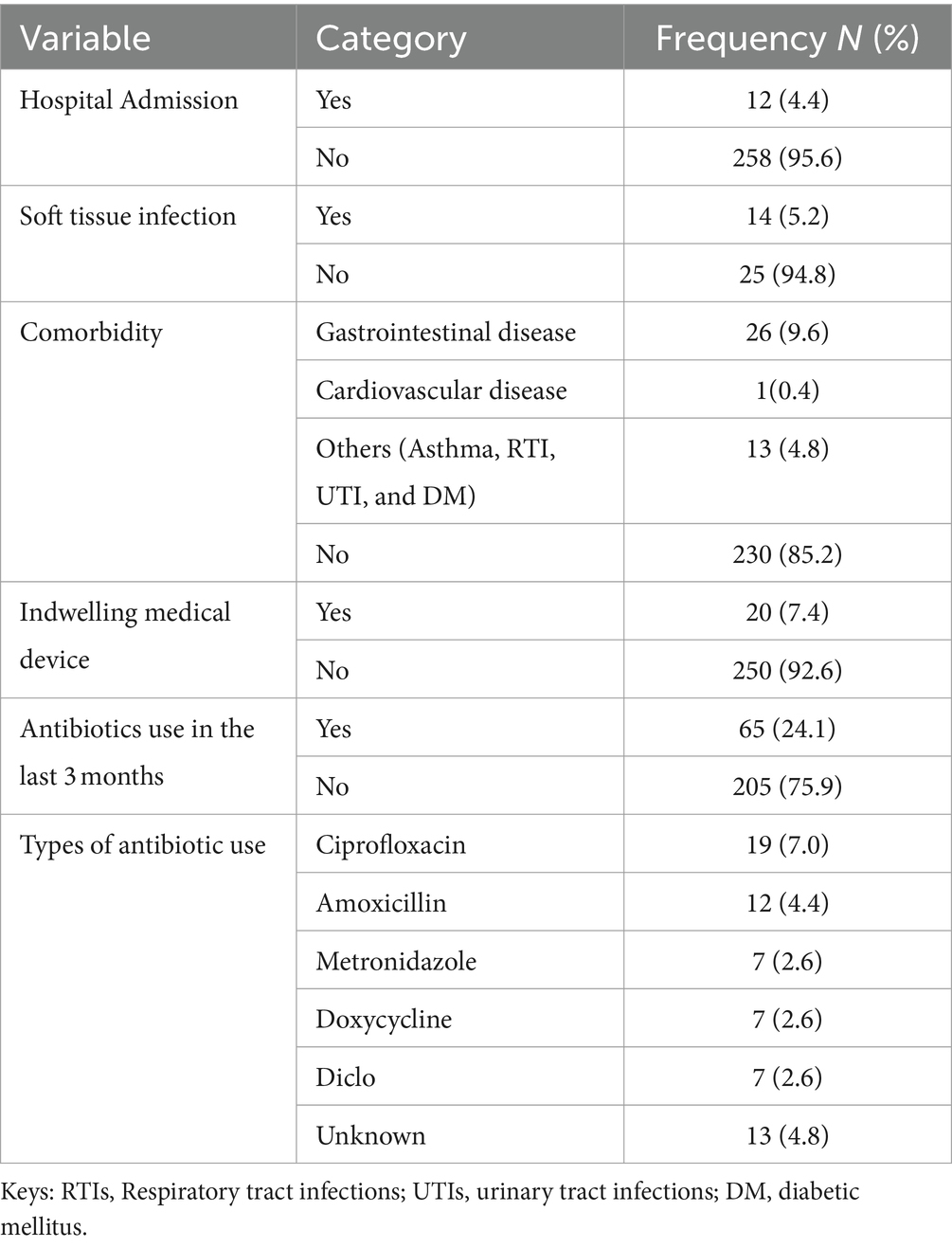
Table 3. Clinical characteristics of study participants among pre-clinical students in CHMS, Haramaya University, Harar, Eastern Ethiopia, 2022.
Prevalence of Staphylococcus aureus and MRSA
Among a total of 270 study participants, the overall prevalence of S. aureus was 35/270 (12.96%) (95% CI: 8.85–16.96); of these, 16/35 (45.7%) were MRSA strains. Therefore, 16/270 (5.9%) (95% CI, 3.09–8.7) of all students were identified as MRSA carriers. Of the total of 35 isolates, 22/270 (8.2%) were isolated from females, of which 5/270 (1.9%) were MRSA. A total of 13/270 (4.8%) isolates were from males, of which 11/270 (4.1%) were MRSA (Table 4).
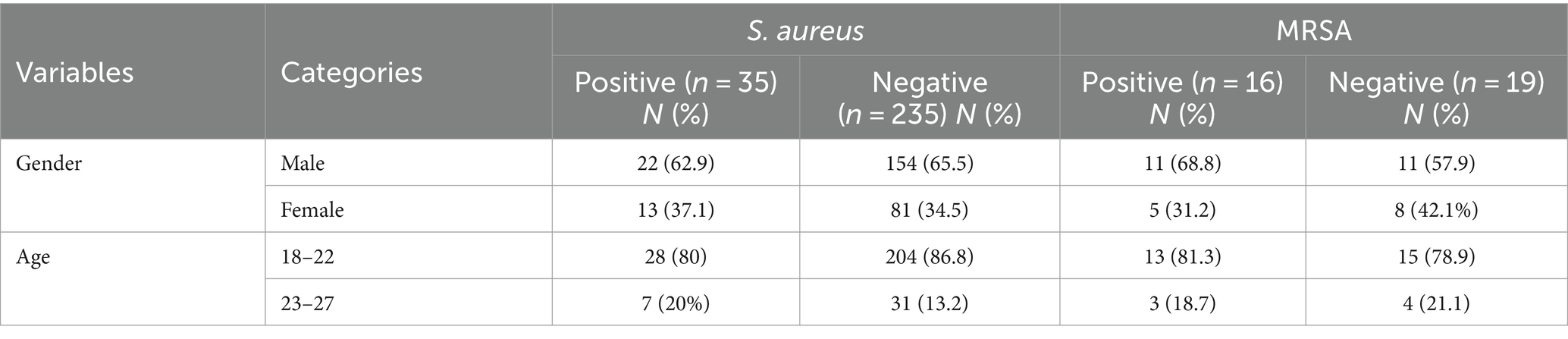
Table 4. Prevalence of S. aureus and MRSA among pre-clinical students in CHMS, Haramaya University, Harar, Eastern Ethiopia, 2022.
Factors associated with MRSA colonization
Following chi-square analysis, study participants who shared personal items such as soap, cloth, and towels (X2 = 5.367, p = 0.021), smoked cigarettes (X2 = 36.185, p = 0.000), consumed khat (X2 = 4.130, p = 0.042), picked their noses (X2 = 8.596, p = 0.003), and had a history of hospital admission before 3 months (X2 = 83.11, p = 0.00) were significantly associated with MRSA nasal colonization (Table 5).
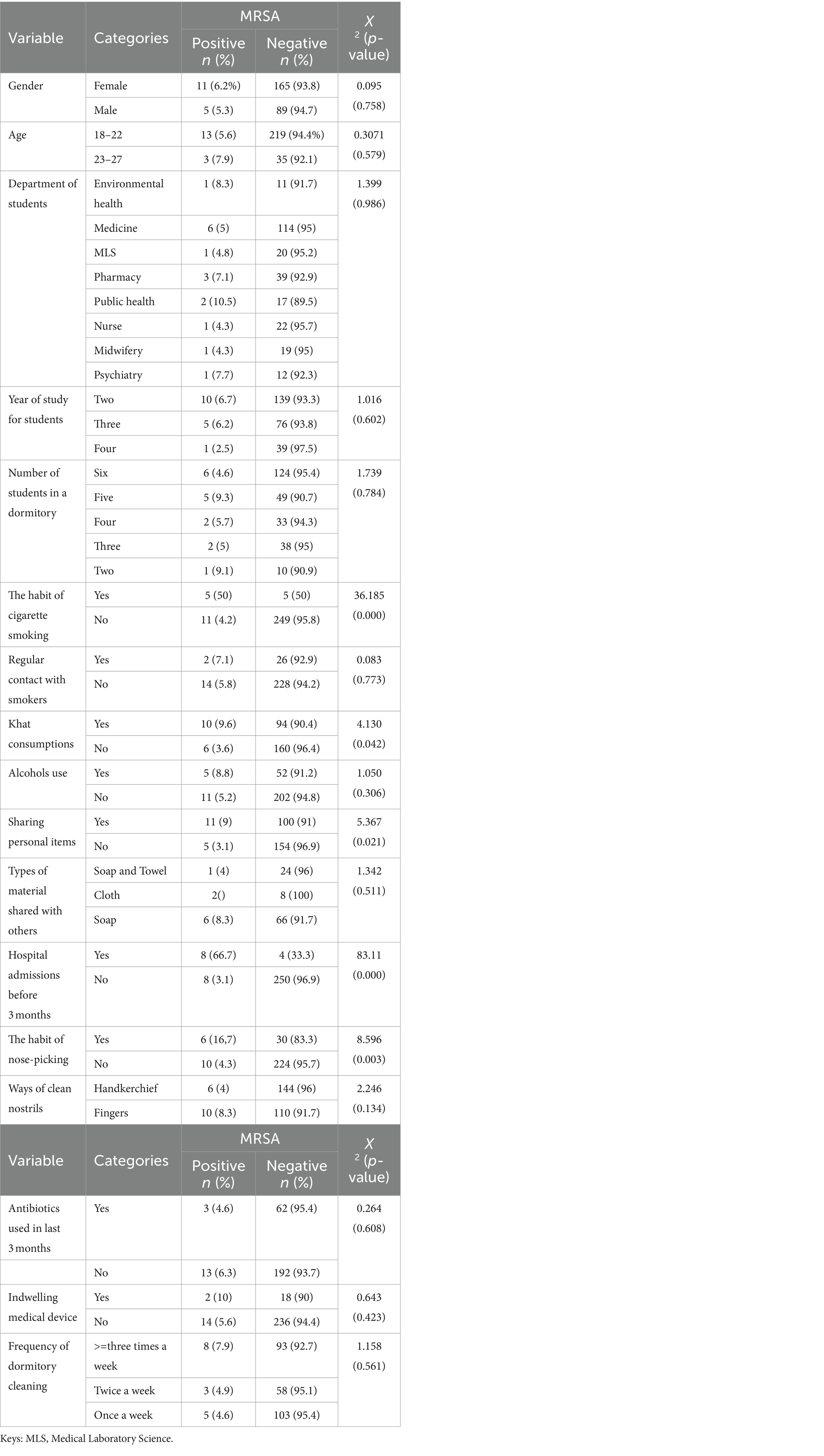
Table 5. Chi-square analysis of MRSA among pre-clinical students in CHMS, Haramaya University, Harar, Eastern Ethiopia, 2022.
Antimicrobial susceptibility patterns
The antimicrobial susceptibility test was performed on 35 S. aureus isolates. Out of 35 isolates of S. aureus, 19/35 (54.3%) were identified as MSSA, while the rest (16/35) (45.7%) were identified as MRSA. Approximately 91.4% of S. aureus were susceptible to clindamycin, 88.6% to chloramphenicol, and 85.7% to both ciprofloxacin and gentamycin. However, 94.3% of S. aureus were found to be resistant to ampicillin, 77.1% to erythromycin, and 65.7% to trimethoprim-sulfamethoxazole. Of the 16 MRSA isolates, all of them were sensitive to clindamycin, chloramphenicol, and gentamycin. However, ampicillin and cefoxitin resistance were present in all the MRSA isolates (Table 6).
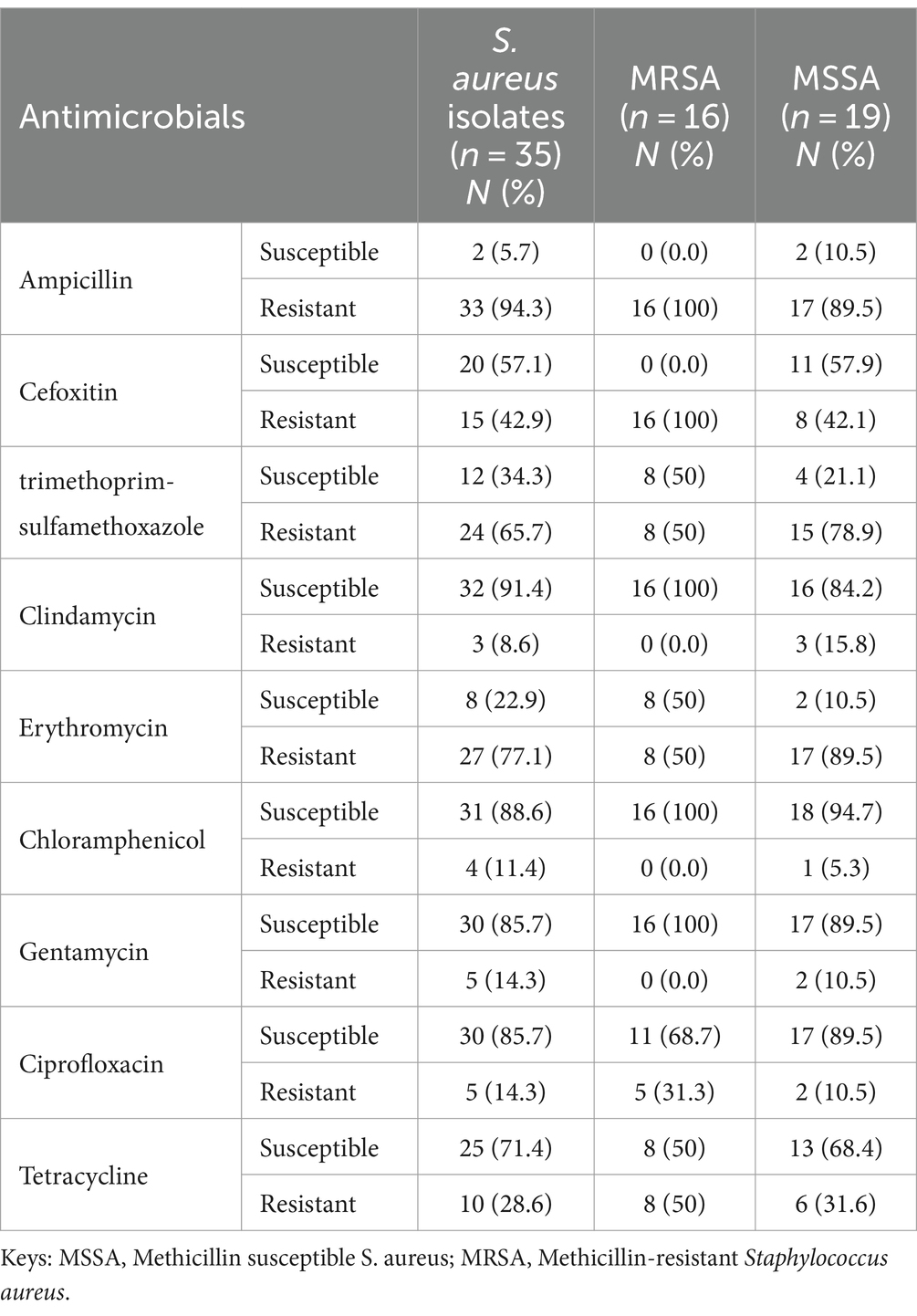
Table 6. Antimicrobial susceptibility pattern of S. aureus isolated from nasal swabs of pre-clinical students at CHMS, Haramaya University, Harar, Eastern Ethiopia, 2022.
Multidrug resistance of Staphylococcus aureus isolates
Of all the isolates, 21 (60%) were multidrug-resistant. Fourteen isolates (66.7%) were resistant to three antimicrobial classes, whereas two (9.5%) were resistant to six antimicrobial classes (Table 7).
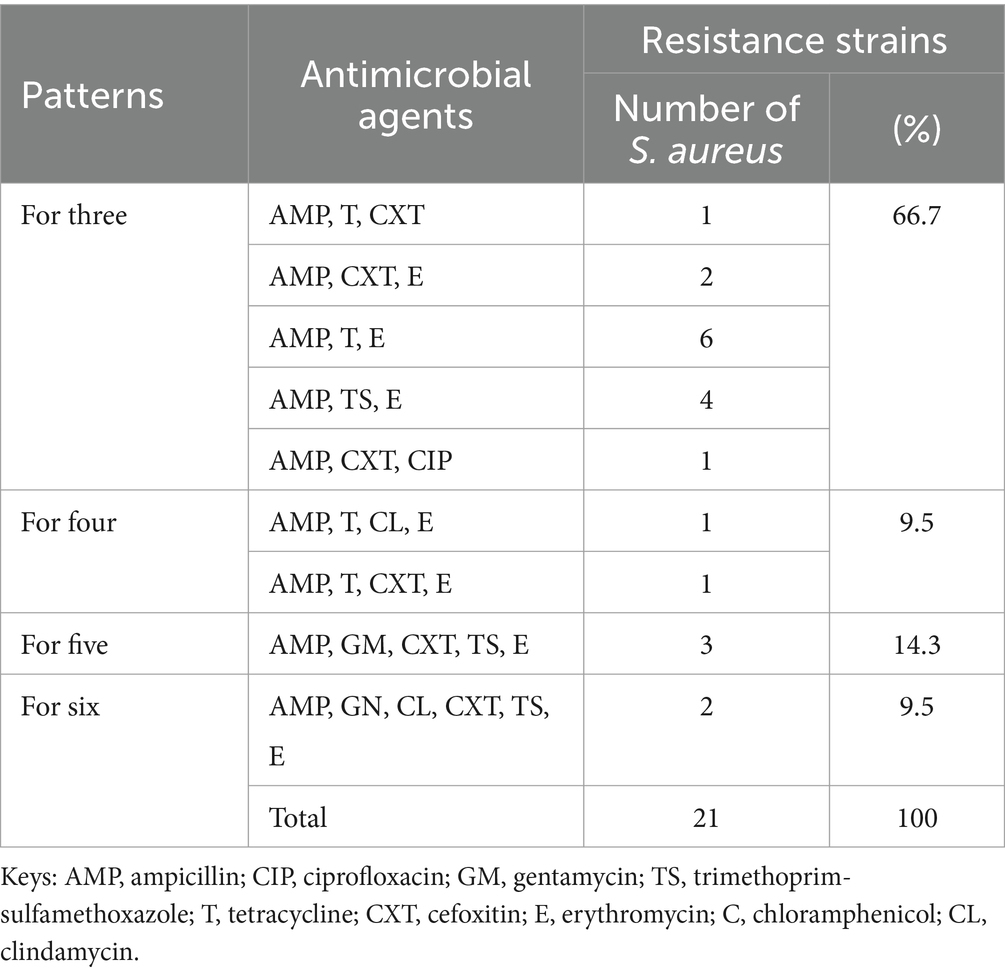
Table 7. Multidrug resistance nature of S. aureus isolates at CHMS, Haramaya University, Harar, Eastern Ethiopia 2022.
Discussion
MRSA infections are increasingly recognized worldwide as potentially fatal infections in the population, and they are no longer exclusive to healthcare settings (27). Therefore, using hospital-based infection control strategies alone will not be sufficient to reduce the community’s growing MRSA infection rate. The overall colonization rate of S. aureus was 12.96% (95% CI: 8.85–16.96). This is corroborated by a study conducted in Ethiopia (12%) (17), the Democratic Republic of Congo (16.6%) (29), and Nepal (15%) (30). But lower than a report in Ethiopia (22.1%) (22), Tanzania (21%) (21), Nigeria (51.9%) (31), India (29.7%) (6), Taiwan (24.7%) (32), Iran (19.6%) (33), Iraq (17.5%) (25), Thailand (29.7%) (34), Malaysia (31%) (35). In this study, the total prevalence of MRSA was 5.9% (95% CI: 3.09–8.7). This was in agreement with the findings from Jima, Ethiopia (22), Adigrat, Ethiopia (5.8%) (17), Iraq (4.2%) (25), North Korea (3.1%) (36), India (6.1%) (6), and Nepal (4%) (30). However, our prevalence was lower than earlier studies conducted in Nigeria (41.5%) (31) and Iran (13.14%) (33). On the other hand, our result was higher than studies reported from Tanzania (0.3%) (21), China (0.3%) (32), Thailand (0%) (34), and Malaysia (0%) (35). The variation of this prevalence across different studies could be due to differences in personal hygiene and lifestyle traits, sharing of personal items, crowded living environments, direct skin-to-skin contact, hospitalization, study durations, antimicrobial policy, and participant awareness of MRSA (17).
The incidence of MRSA nasal carriage was greater in female students than in male students in our study, but the difference was not statistically significant (p = 0.758). This is following an earlier study among clinical samples of S. aureus in Ethiopia (17) and other findings conducted by healthy students in Iraq (25) and Afghanistan (2), which shows that gender was not found to be a risk factor for MRSA infection. Additionally, nasal carriage of MRSA was studied in two age groups: 18–22 years and 23–27 years. A total of 7.9 and 5.6% of the students were 23–27 and 18–22 years old, respectively (p = 0.579). It is consistent with past findings by Naimi et al. (2) and Legese et al. (17). These indicate that gender and age were not associated factors in the acquisition of MRSA infections.
Numerous studies in different populations showed that socio-demographic traits and other risk factors have a significant role in the nasal carriage of MRSA. Similarly, in the current study, MRSA nasal colonization was significantly associated with a history of cigarette smoking (p = 0.000), the habit of consuming khat (p = 0.042), nose picking (p = 0.003), sharing personal items (p = 0.021), and a history of hospitalizations in the previous 3 months (p = 0.00). This association may result from frequent hospital visits, which are highly contagious, especially for drug-resistant pathogens; inadequate personal hygiene (the primary mechanism for the auto-transmission of contaminated hands to the nose may be the temporary hand carriage of bacteria on the hands of healthy students); and a history of person-to-person contact with tissue and skin infection (37).
In the antibiotic sensitivity testing, we found that all MRSA isolates were susceptible to clindamycin, chloramphenicol, and gentamycin, which is similar to the findings reported in Adigrat, Ethiopia (17), Nepal (30), Afghanistan (2), and India (6). However, it differs from the result documented in Iraq that indicated all MRSA isolates are sensitive to vancomycin (25). Nonetheless, all MRSA isolates were resistant to ampicillin and cefoxitin, and half of those isolates were also resistant to erythromycin and trimethoprim–sulfamethoxazole, in line with a prior study published in India (6) and Democratic Republic of the Congo, (29). Although the result contradicts previous research in Ethiopia that found a low resistance rate to ampicillin and cefoxitin (48.3%) (17). The cause of this resistance pattern in our findings may be the overuse of these antibiotics for many other infections and the replacement of sensitive strains with resistance strains.
All 35 S. aureus isolates were tested for drug susceptibility to 7 commonly used antibiotics. The strains’ resistance to ampicillin, erythromycin, and trimethoprim-sulfamethoxazole is consistent with research done in Ethiopia (17), Jima Ethiopia (22), Tanzania (21), Thailand (34), India (6), and China (32). However, a study conducted in Malaysia revealed no resistance to both cefoxitin and trimethoprim-sulfamethoxazole (35), trimethoprim-sulfamethoxazole in Thailand (34), and low resistance was revealed with trimethoprim-sulfamethoxazole (33%) and gentamycin (2%) conducted in Nigeria (5). Higher gentamycin resistance patterns have been documented in studies carried out in Nigeria (31). This could be brought on by geographical variations in the hospital settings’ approaches to infection prevention and control (2). The most often administered antibiotics in our study area are ampicillin, trimethoprim-sulfamethoxazole, and erythromycin. This might have contributed to the development of resistance to these antibiotics. This study found that S. aureus multidrug resistance was quite prevalent. Twenty-one (60%) of the isolates were resistant to three or more antimicrobial classes (38). Thirteen of them (61.9%) were MRSA, and similar susceptibility was observed in Adigrat, Ethiopia (63.6%) (17). This result is significantly higher than the study published in Afghanistan (32%) (2). The possible reasons for this increasing multidrug resistance might be the continuous genetic variation of strains caused by the mutation or cross-transmission of the resistance genetic elements from one bacterium to another, crowded wards, and administration of antibiotics without culture and sensitivity (2, 17).
Conclusion
This study found a significant rate of MRSA nasal carriage among healthy students when compared to most other similar research. Furthermore, these isolates exhibited high resistance to ampicillin and cefoxitin. Of the MRSA isolates, approximately 61.9% were deemed MDR. It was discovered that there are significant associations between the acquisition of nasal carriage of MRSA and certain student behaviors, including sharing personal items such as clothing, soap, and towels, nose picking, consuming khat, and smoking cigarettes. Hence, routine screening of pre-clinical students is crucial to prevent endogenous infections and the spread of Methicillin-resistant Staphylococcus aureus.
Limitations of the study
The authors did not perform the vancomycin minimum inhibitory concentration due to resource limitations. The species and strain type of MRSA could not be determined using more precise and sensitive molecular techniques. Moreover, phenotypic and genotypic studies are required to determine and elucidate the genetic mechanisms behind antibiotic susceptibilities for the benefit of future researchers. Consequently, further community-based research is needed to find out the prevalence of MRSA in the community.
Operational definitions
Nasal carriage rate: defined as the prevalence of S. aureus and MRSA from the participants isolated from one swab (39).
Susceptible (s): a category denoted by a breakpoint, which implies that isolates with a zone diameter above the susceptible breakpoint are inhibited by generally achievable concentrations of an antimicrobial agent when the recommended dosage is applied to treat the infection site, leading to likely clinical efficacy as determined by laboratory and clinical standards (28).
Resistant (r): a category where a breakpoint indicates that isolates with a zone diameter at or below the resistant breakpoint are not inhibited by the agent at concentrations that are typically achievable using standard dosage schedules (28). In this study, multidrug resistance was defined as resistance to three or more of the tested antimicrobial classes (38).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Health Research Ethics Review Committee of the College of Health and Medical Sciences, Haramaya University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FW: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing, Data curation, Resources, Supervision, Visualization. KU: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – review & editing. FA: Methodology, Supervision, Validation, Visualization, Writing – review & editing, Data curation, Formal analysis, Funding acquisition, Project administration, Software. KB: Data curation, Formal analysis, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing, Conceptualization. TS: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing, Investigation, Writing – original draft. MA: Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – review & editing. SD: Software, Validation, Writing – original draft, Formal analysis, Investigation, Methodology, Visualization, Conceptualization, Data curation, Funding acquisition. FT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Project administration, Resources. TT: Conceptualization, Formal analysis, Methodology, Visualization, Writing – review & editing. SM: Conceptualization, Formal analysis, Methodology, Writing – review & editing, Funding acquisition, Investigation, Project administration, Software, Validation, Writing – original draft. HA: Conceptualization, Formal analysis, Methodology, Visualization, Funding acquisition, Project administration, Software, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Human and material resource was covered by Haramaya University’s College of Health and Medical Science.
Acknowledgments
The authors were grateful for the ethical approval provided by the Institutional Health Research Ethics Review Committee of the Haramaya University Colleges of Health and Medical Sciences. The authors also extend our gratitude to the study participants and everyone else who helped us finish this research in any way.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pathak, A, Marothi, Y, Iyer, RV, Singh, B, Sharma, M, Eriksson, B, et al. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureusin healthy preschool children in Ujjain. India BMC pediatrics. (2010) 10:1–8.
2. Naimi, HM, Rahimi, MH, and Noori, AZ. Prevalence and antimicrobial susceptibility patterns of Staphylococcus aureus/methicillin-resistant Staphylococcus aureus nasal carriage among Kabul university students. International Journal of Innovative Research and Scientific Studies. (2020) 3:1–5. doi: 10.53894/ijirss.v3i1.25
3. Albusayes, NN, Binkhamis, K, Alselaimy, RM, Alsalouli, MM, Alnafisah, RA, Albawardi, LA, et al. Prevalence and factors associated with methicillin-resistant Staphylococcus aureus colonization among clinical medical students. J Nat Sci Med. (2019) 2:226–30. doi: 10.4103/JNSM.JNSM_3_19
4. Sakr, A, Brégeon, F, Mège, JL, Rolain, JM, and Blin, O. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol. (2018) 9:2419. doi: 10.3389/fmicb.2018.02419
5. Oluseun Ayepola, O, Olugbenga Taiwo, S, Anifowose, A, and Onile-ere, O. Nasal carriage of Staphylococcus aureus and associated risk factors among students in a Nigerian university. Acta scientific. Microbiology. (2018) 1:6–8. doi: 10.31080/ASMI.2018.01.0010
6. Radhakrishna, M, Taneja, A, and Rao, P. Nasal carriage of Staphylococcus aureus with special emphasis on methicillin-resistant Staphylococcus aureus among students of a south Indian medical college-prevalence and antibiogram pattern. Asian J Pharm Clin Res. (2016) 9:129–32. doi: 10.22159/ajpcr.2018.v11i11.27713
7. Mekuriya, E, Mekuriya, E, Manilal, A, Aklilu, A, Woldemariam, M, Hailu, T, et al. Nasal carriage and associated factors of methicillin-resistant Staphylococcus Aureus among medicine and health science students of Arba Minch University. Ethiopia: Arba Minch (2022).
8. Sharma, S, Pal, S, Negi, V, Juyal, D, Sharma, M, and Prakash, R. Staphylococcus aureus including MRSA nasal carriage among hospital exposed and unexposed medical students. J Family Med Prim Care. (2020) 9:4936–41. doi: 10.4103/jfmpc.jfmpc_820_20
10. Lee, AS, de Lencastre, H, Garau, J, Kluytmans, J, Malhotra-Kumar, S, Peschel, A, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Prim. (2018) 4:1–23. doi: 10.1038/nrdp.2018.33
11. Pollitt, EJ, Szkuta, PT, Burns, N, and Foster, SJ. Staphylococcus aureus infection dynamics. PLoS Pathog. (2018) 14:e1007112. doi: 10.1371/journal.ppat.1007112
12. Algammal, AM, Hetta, HF, Elkelish, A, Alkhalifah, DHH, Hozzein, WN, Batiha, GES, et al. Methicillin-resistant Staphylococcus aureus (MRSA): one health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infection and Drug Resistance. (2020) 13:3255–65. doi: 10.2147/IDR.S272733
13. Akpinar, O, Yigit, A, Guzel, M, Akdogan, D, et al. The investigation of nasal carriage rate of methicillin-resistant Staphylococcus aureus among Turkish healthcare workers, 1990–2019: meta-analysis. Türk Hijyen ve Deneysel Biyoloji Dergisi. (2020) 77:217–26. doi: 10.5505/TurkHijyen.2020.46872
14. Boucher, HW, and Sakoulas, G. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis. (2007) 45:601–8. doi: 10.1086/520655
15. Reta, A, Mengist, A, and Tesfahun, A. Nasal colonization of methicillin resistant Staphylococcus aureus in Ethiopia: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. (2019) 18:1–12. doi: 10.1186/s12941-019-0324-y
16. Tong, SY, Davis, JS, Eichenberger, E, Holland, TL, and Fowler, VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. (2015) 28:603–61. doi: 10.1128/CMR.00134-14
17. Legese, H, Kahsay, AG, Kahsay, A, Araya, T, Adhanom, G, Muthupandian, S, et al. Nasal carriage, risk factors and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus among healthcare workers in Adigrat and Wukro hospitals, Tigray. Northern Ethiopia BMC Research Notes. (2018) 11:1–6. doi: 10.1186/s13104-018-3353-2
18. Adhikari, S, Khadka, S, Parajuli, A, Mishra, R, Kandel, P, and Tiwari, A. Nasal colonization of Staphylococcus aureus and their antibiograms among school children in Bharatpur. Nepal J Coll Med Sci. (2018) 14:172–7. doi: 10.3126/jcmsn.v14i4.19511
19. Morgenstern, M, Erichsen, C, Hackl, S, Mily, J, Militz, M, Friederichs, J, et al. Antibiotic resistance of commensal Staphylococcus aureus and coagulase-negative staphylococci in an international cohort of surgeons: a prospective point-prevalence study. PLoS One. (2016) 11:e0148437. doi: 10.1371/journal.pone.0148437
20. World Health Organization , Antimicrobial resistance, Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance [Accessed March 16, 2024]. (2020)
21. Okamo, B, Moremi, N, Seni, J, Mirambo, MM, Kidenya, BR, and Mshana, SE. Prevalence and antimicrobial susceptibility profiles of Staphylococcus aureus nasal carriage among pre-clinical and clinical medical students in a Tanzanian university. BMC Res Notes. (2016) 9:1–6. doi: 10.1186/s13104-016-1858-0
22. Efa, F, Alemu, Y, Beyene, G, Gudina, EK, and Kebede, W. Methicillin-resistant Staphylococcus aureus carriage among medical students of Jimma University, Southwest Ethiopia. Heliyon. (2019) 5:e01191. doi: 10.1016/j.heliyon.2019.e01191
23. Ali Alghamdi, B, al-Johani, I, al-Shamrani, JM, Musamed Alshamrani, H, al-Otaibi, BG, Almazmomi, K, et al. Antimicrobial resistance in methicillin-resistant staphylococcus aureus. Saudi J Biol Sci. (2023) 30:103604. doi: 10.1016/j.sjbs.2023.103604
24. Aslam, B, Wang, W, Arshad, MI, Khurshid, M, Muzammil, S, Rasool, MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. (2018) 11:1645–58. doi: 10.2147/IDR.S173867
25. Assafi, MS, Mohammed, RQ, and Hussein, NR. Nasal carriage rates of Staphylococcus aureus and CA-methicillin resistant Staphylococcus aureus among university students. Int J Microbiol Res. (2015) 5:123–7. doi: 10.5923/j.microbiology.20150504.01
26. Vandepitte, J . World Health Organization. Basic laboratory procedures in clinical bacteriology World Health Organization (2003).
27. Mulu, M, Getaneh, A, Ferede, W, and Gelaw, A. Nasal carriage rate of methicillin resistant Staphylococcus Aureus, antimicrobial susceptibility pattern and associated risk factors among pregnant women at the university of Gondar comprehensive specialized hospital northwest, Ethiopia. Ethiopian J Health Biomed Sci. (2021) 11:45–53. doi: 10.20372/ejhbs.v11i01.213
28. CLSI , Performance standards for antimicrobial susceptibility testing. M100, 30th ed. (2020); 40
29. de Boeck, H, Vandendriessche, S, Hallin, M, Batoko, B, Alworonga, JP, Mapendo, B, et al. Staphylococcus aureus nasal carriage among healthcare workers in Kisangani, the Democratic Republic of the Congo. Eur J Clin Microbiol Infect Dis. (2015) 34:1567–72. doi: 10.1007/s10096-015-2387-9
30. Ansari, S, Gautam, R, Shrestha, S, Ansari, SR, Subedi, SN, and Chhetri, MR. Risk factors assessment for nasal colonization of Staphylococcus aureus and its methicillin resistant strains among pre-clinical medical students of Nepal. BMC Res Notes. (2016) 9:1–8. doi: 10.1186/s13104-016-2021-7
31. Anueyiagu, K, Kopmut, JJ, Lagi, CA, and Okoh, KN. Nasal carriage of MRSA among healthy college students and livestock. Vet Sci Res Rev. (2020) 6:33–9. doi: 10.17582/journal.vsrr/2020/6.1.33.39
32. Chen, B, Chen, BJ, Xie, YX, Ni, LJ, Dai, XL, Lu, Y, et al. Factors associated with Staphylococcus aureus nasal carriage and molecular characteristics among the general population at a medical college campus in Guangzhou, South China. Ann Clin Microbiol Antimicrob. (2017) 16:1–10. doi: 10.1186/s12941-017-0206-0
33. Abroo, S, Jazani, NH, and Sharifi, Y. Methicillin-resistant Staphylococcus aureus nasal carriage between healthy students of medical and nonmedical universities. Am J Infect Control. (2017) 45:709–12. doi: 10.1016/j.ajic.2017.02.034
34. Treesirichod, A, Hantagool, S, and Prommalikit, O. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus among medical students at the HRH Princess Maha Chakri Sirindhorn medical Center, Thailand: a follow-up study. J Infect Public Health. (2014) 7:205–9. doi: 10.1016/j.jiph.2013.12.003
35. Aung, TS . Nasal carriage of Staphylococcus aureus and its antibiotic susceptibility pattern among medical and nursing students. Asian J Pharm. (2016) 10:e4. doi: 10.22377/ajp.v10i04.917
36. Baek, YS, Baek, S-H, and Yoo, Y-J. Higher nasal carriage rate of methicillin-resistant Staphylococcus aureus among dental students who have clinical experience. J Am Dent Assoc. (2016) 147:348–53. doi: 10.1016/j.adaj.2015.12.004
37. Gebreyesus, A, Gebre-Selassie, S, and Mihert, A. Nasal and hand carriage rate of methicillin resistant Staphylococcus aureus (MRSA) among health care workers in Mekelle hospital, North Ethiopia. Ethiop Med J. (2013) 51:41–7.
38. Magiorakos, A-P, Srinivasan, A, Carey, RB, Carmeli, Y, Falagas, ME, Giske, CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
Keywords: nasal carriage, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, students, Harar
Citation: Weldegebreal F, Urgesa K, Ayele F, Bogale K, Shume T, Ahmed M, Debebe S, Tebeje F, Asmerom H, Tesfa T and Mekonnen S (2024) Nasal carriage rate, associated factors, and antimicrobial susceptibility patterns of methicillin resistance Staphylococcus aureus among pre-clinical undergraduate students at the College of Health and Medical Sciences, Haramaya University, Ethiopia. Front. Public Health. 12:1354461. doi: 10.3389/fpubh.2024.1354461
Edited by:
Angel León-Buitimea, Monterrey Institute of Technology and Higher Education (ITESM), MexicoReviewed by:
Okon Okwong Kenneth, Federal Medical Center Makurdi, NigeriaNizami Duran, Mustafa Kemal University, Türkiye
Copyright © 2024 Weldegebreal, Urgesa, Ayele, Bogale, Shume, Ahmed, Debebe, Tebeje, Asmerom, Tesfa and Mekonnen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shambel Mekonnen, bWVrb25uZW5zaGFtYmVsOTE2QGdtYWlsLmNvbQ==
 Fitsum Weldegebreal
Fitsum Weldegebreal Kedir Urgesa
Kedir Urgesa Firayad Ayele
Firayad Ayele Kasahun Bogale
Kasahun Bogale Taddese Shume1
Taddese Shume1 Mohammed Ahmed
Mohammed Ahmed Fikru Tebeje
Fikru Tebeje Tewodros Tesfa
Tewodros Tesfa Shambel Mekonnen
Shambel Mekonnen