- 1Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL, United States
- 2Charles E. Schmidt College of Science, Boca Raton, FL, United States
Introduction: This scoping review aims to highlight key social determinants of health associated with breast cancer screening behavior in United States women aged ≥40 years old, identify public and private databases with SDOH data at city, state, and national levels, and share lessons learned from United States based observational studies in addressing SDOH in underserved women influencing breast cancer screening behaviors.
Methods: The Arksey and O’Malley York methodology was used as guidance for this review: (1) identifying research questions; (2) searching for relevant studies; (3) selecting studies relevant to the research questions; (4) charting the data; and (5) collating, summarizing, and reporting results.
Results: The 72 included studies were published between 2013 and 2023. Among the various SDOH identified, those related to socioeconomic status (n = 96) exhibited the highest frequency. The Health Care Access and Quality category was reported in the highest number of studies (n = 44; 61%), showing its statistical significance in relation to access to mammography. Insurance status was the most reported sub-categorical factor of Health Care Access and Quality.
Discussion: Results may inform future evidence-based interventions aiming to address the underlying factors contributing to low screening rates for breast cancer in the United States.
Introduction
The social determinants of health (SDOH) are factors outside of the realm of medicine that impact health outcomes and quality of life on a daily basis (1). According to the World Health Organization (WHO), SDOH are defined as “the conditions in which people are born, grow, work, live, and age, and the wider set of forces and systems shaping the conditions of daily life (1).” These determinants of health can be divided into five categories: economic stability, education access and quality, health care access and quality, neighborhood and built environment, and social and community context (2). While factors within each of these categories can individually impact a different facet of a person’s health, these categories often also work collectively to create facilitators and barriers to healthy behaviors and health outcomes (1–3). Such SDOH play a significant role in creating new and worsening existing healthcare disparities and may exhibit a stronger influence on health and well-being than the care received by providers and healthcare organizations (4).
One of the most influential roles of SDOH lies within the realm of equitable access to cancer care (4–7). Specifically, when considering breast cancer, there is significant evidence that supports the influence of SDOH on screening. Despite the presence of innovative screening and treatment strategies, breast cancer remains the second most common type of cancer and is a leading cause of disability and mortality in the United States (8). Breast cancer screening, through mammography and clinical breast examination, is the method of primary prevention that is recommended by the United States Preventive Service Task Force (9). However, research studies showed that health disparities persist, as minority women within the United States are less likely to take advantage of breast cancer screening methods (10–14). Though these studies assessed primarily the role of race and ethnicity on breast cancer screening behaviors, they all found that reported associations were mediated by other SDOH such as quality of health care, education, family income, and health insurance (11–14). Hence, there is a need to explore and understand which determinants act as significant influential factors contributing to low breast cancer screening behaviors. This scoping review aims to highlight key SDOH associated with breast cancer screening behavior in United States women aged ≥40 years old, identify public and private databases with SDOH data at city, state, and national levels, and share lessons learned from United States based observational studies in addressing SDOH in underserved women influencing breast cancer screening behaviors. Findings can guide researchers, physicians, and community workers in improving accessibility, affordability, and quality of breast cancer screening opportunities through culturally competent strategies tailored to satisfy the needs of the at-risk female population group.
Methods
The review team consisted of a multidisciplinary team of health professionals with extensive knowledge on the role of SDOH in minority populations. The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) was utilized as a reference checklist for the sections of this study (15). The Arksey and O’Malley (16) York methodology was used as guidance for this review. This framework employs five steps: (1) identifying research questions; (2) searching for relevant studies; (3) selecting studies relevant to the research questions; (4) charting the data; and (5) collating, summarizing, and reporting results (16). These methods ensure transparency, permits replicability of the search strategy, and increases the reliability of study findings.
Step 1: identifying research questions
Three research questions were used for this scoping review: (1) What are the major SDOH hindering breast cancer screening in United States women aged > = 40?; (2) What were the major databases/data sources used to capture SDOH data to assess its influence on breast cancer screening behaviors in United States women?; and (3) What are the lessons learned for future recommendations to address SDOH in underserved women at-risk for the disease?
Step 2: searching for relevant articles
Keywords and MeSH terms were developed in collaboration with a research librarian (MK) who is an expert in scoping review protocols. Search terms included: breast cancer, breast cancer screening, mammography, race/ethnicity, education level, income, housing instability, insurance coverage, language preferences, health equity, health disparities, and medically underserved communities, among others. Four electronic databases (PubMed, Embase, Web of Science, and Cochrane) were selected due to their breadth and focus on psychosocial and behavioral aspects of chronic illnesses. These databases were searched to identify peer-reviewed literature from primary data sources, secondary data sources, and case reports. The review of the literature was completed over a period of 3 months, from January 2023 to March 2023. The screening of these articles was carried out by senior author (LS) and co-authors (VJ, DL, GO, YZ, SB, AM, SD, MR, and DD).
Inclusion criteria
The articles that were included were peer-reviewed observational studies, published in English between 2013 and 2023 that focused on the SDOH, including race/ethnicity, employment, education, food security, insurance status, housing, and access to quality healthcare. These observational studies specifically focused on assessing the significance of the role of SDOH in creating health inequities in breast cancer screenings, particularly for women who are 40 years or older, and are at-risk or have been diagnosed with breast cancer. The ≥40 years old age cut-off was selected based on the American Cancer Society recommended guidelines for screening, which highlight that (1) women between 40 and 44 have the option to start screening with a mammogram every year; (2) women 45–54 should get mammograms every year; and (3) women 55 and older can switch to a mammogram every other year, or they can choose to continue yearly mammograms (17).
Exclusion criteria
Excluded studies encompass narrative, scoping, and systematic reviews, as well as qualitative, descriptive, and experimental studies. Additionally, articles were excluded if they did not focus on SDOH as influential factors of breast cancer screening behavior, were assessing knowledge and attitudes rather than exploring SDOH as influencing factors of breast cancer screening, were discussing interventions addressing low breast cancer screening rates and associated disparities that might be related to SDOH, were focusing on survival and mortality rather than screening, and were looking at guideline adherence rather than breast cancer screening behavior itself. Datasets with data collected prior to 2005 were not included in the review.
Step 3: selecting studies relevant to the research questions
All co-authors (VJ, DL, GO, YZ, SB, AM, SD, MR, and DD) extracted, summarized, and tabulated the data from relevant studies. The senior author (LS) reviewed all tabulated data for accuracy and to resolve any discrepancies. Summary tables included an evidence table (Table 1) describing study characteristics, types of SDOH, and outcomes. Types of SDOH were first listed and then categorized based on Healthy People 2030 into five categories: Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context (18). The Healthy People 2030 is a set of science-based objectives with targets to monitor progress and motivate and focus action (18). The Healthy People 2030 first introduced SDOH objectives in 2010, following the World Health Organization’s (WHO) call to address SDOH to maintain health and quality of life (18). The five categories listed reflect the social conditions and environments that are shaped by a wider set of forces and influence behavioral outcomes (18).
Significance of associations between breast cancer screening as an outcome and identified SDOH were reported (Table 1). Table 2 included a list of databases from where the data was accessed, the availability status of the data (public/private), and the geographical level from where the data was extracted. Basic qualitative content analysis was carried out to identify similar themes in future directions across studies highlighted in Table 3. The three phases of qualitative content analysis for the results of primary qualitative research described by Elo and Kyngas (19) were applied: (i) preparation, (ii) organizing, and (iii) reporting.
Step 4 and 5: charting the data and collation, summarization, and reporting of results
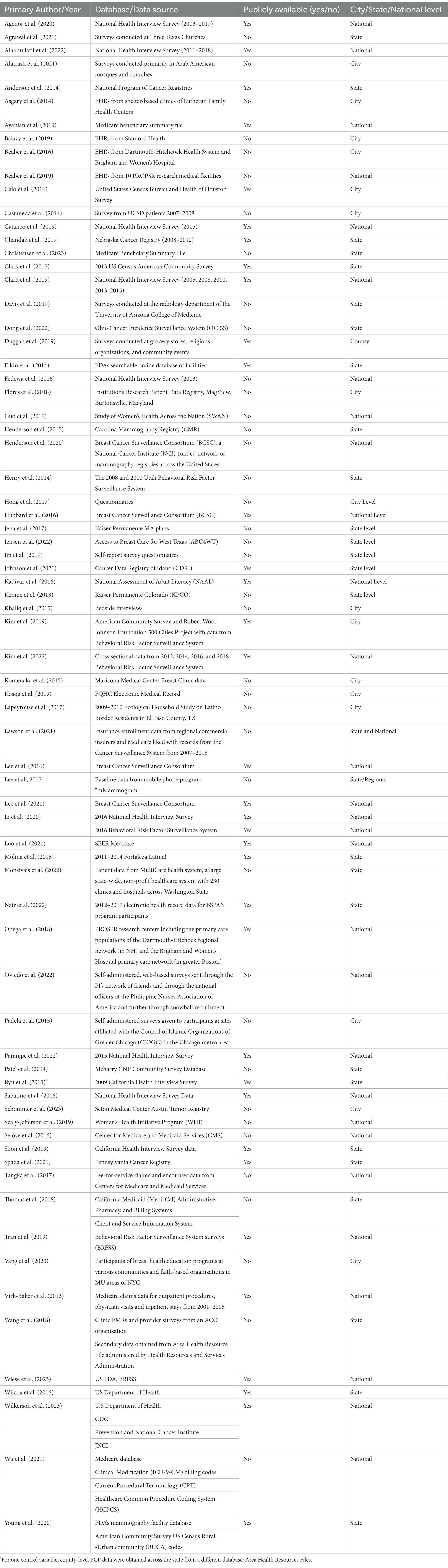
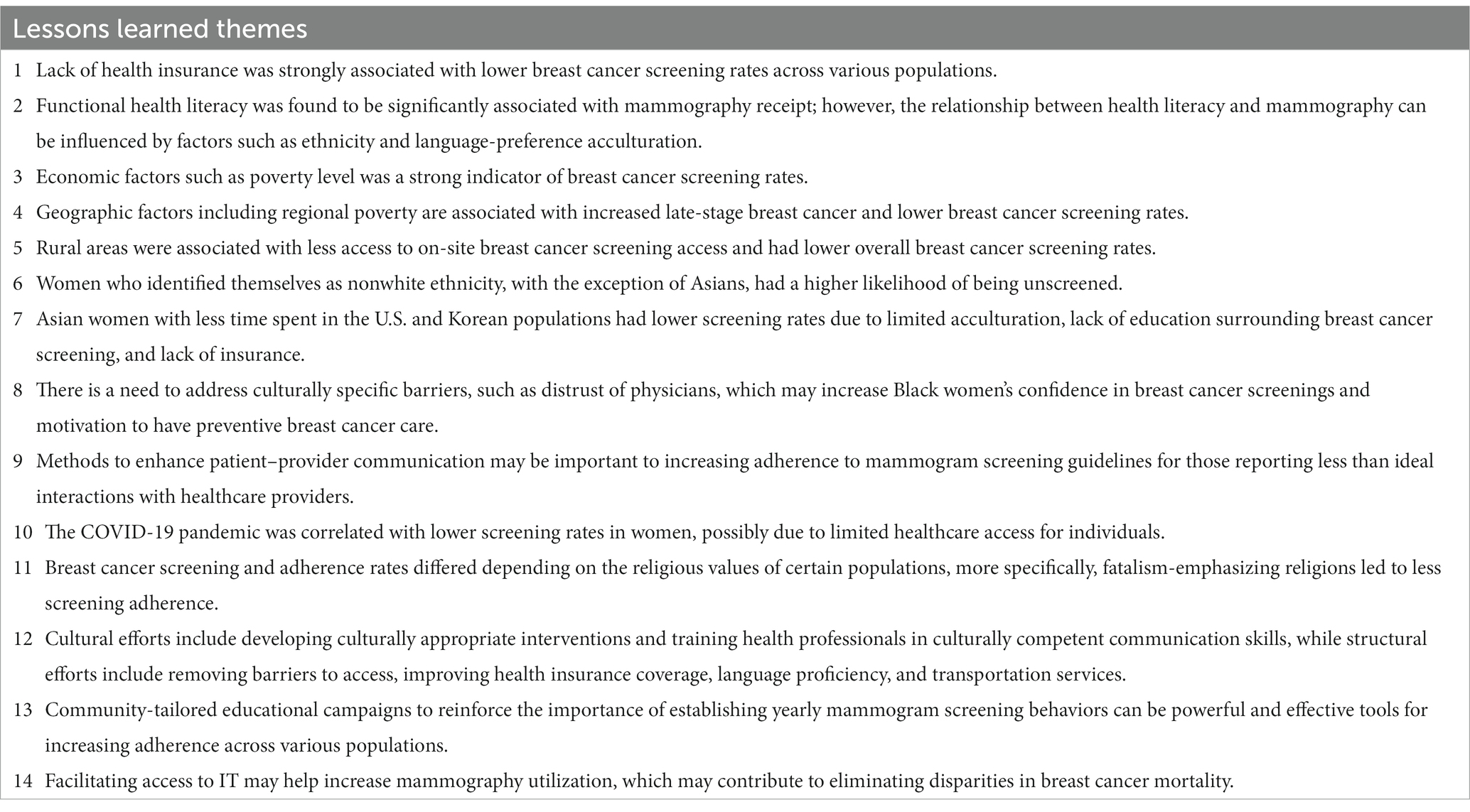
Study characteristics were tabulated for primary author/year, study design, sample size, study population, age range, study purpose, type of SDOH, SDOH category based on HP 2030, association between SDOH and outcome (significant/non-significant), and type of methodology/analysis used for data analysis (Table 1). Identified databases were tabulated by primary author/year, database/data source, public availability, and city/state/national level (Table 2). Each database was stratified based on availability (publicly available/not publicly available) and location (city/state/national level). Lessons learned from each relevant study were highlighted in Table 3.
Results
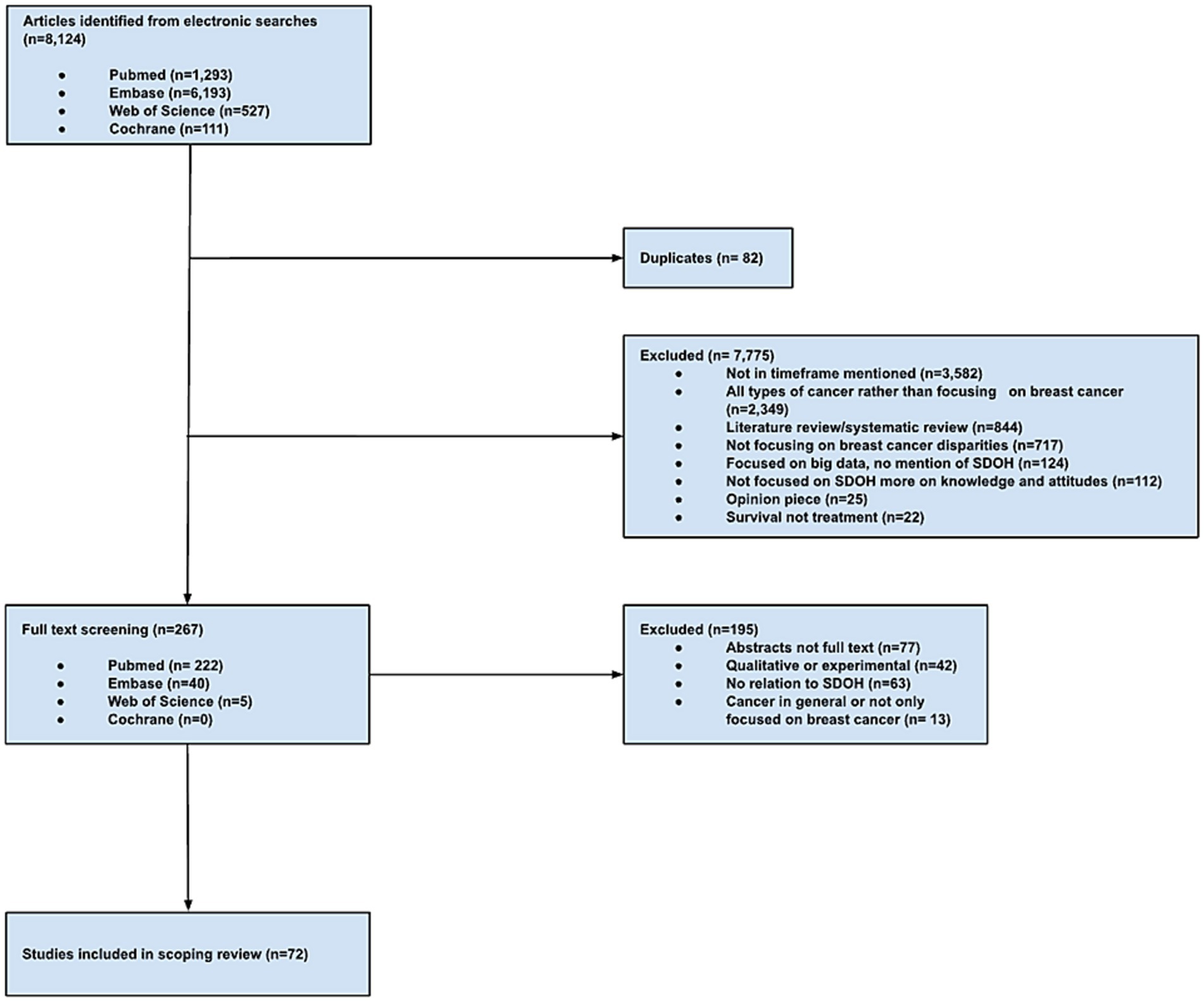
The initial study extraction resulted in 8,124 articles from PubMed (n = 1,293), EMBASE (n = 6,193), Web of Science (n = 527), and Cochrane (n = 111). Studies were excluded due to publication outside of the timeframe (n = 7,775), discussion of all types of cancer rather than focusing on breast cancer (n = 2,349), being a literature review or systematic review (n = 884), lack of focus on breast cancer disparities (n = 717), focusing on big data or no mention of SDOH (n = 124), focusing more on knowledge and attitudes rather than SDOH (n = 112), being an opinion piece or an editorial (n = 25), or emphasizing survival as an outcome rather than treatment (n = 22). Duplicate studies were also excluded (n = 82 from PubMed, n = 60 from EMBASE, n = 20 from Web of Science, and n = 2 from Cochrane). A total of 267 studies met the inclusion criteria from PubMed (n = 222), EMBASE (n = 40), and Web of Science (n = 5). An additional 195 studies were excluded after a full study review due to being an abstract and not a full text (n = 77), having a qualitative or experimental study design (n = 42), having no relation to SDOH (n = 63), and discussing cancer types in general rather than narrowing it down to breast cancer (n = 13). A total of 72 studies were retained for analysis (Figure 1).
The 72 included studies were published between 2013 and 2023. About half of the studies (58%) were published in 2018 or later (n = 42). Study designs included cross-sectional studies (n = 45); cohort studies (n = 18); and case–control studies (n = 9). Sample size ranged from n = 100 to n = 3,821,084 female adults with breast cancer while the age of this target population ranged from 40 to 89 years old (Table 1).
Priority populations
Priority populations who were actively involved (or targeted) in implementation activities were ethnically diverse female patients diagnosed with breast cancer including African American women; Muslim and Christian Arab American; Haitian women; Filipino women; and Korean American women. Another set of studies focused on women from programs, such as women from Geisel School of Medicine (n = 3,413), from the BSPAN program (n = 19,292), women who underwent mammography in Harvard Medical School (n = 9,575), female patients from a single institution undergoing breast radiotherapy (n = 1,057), presenting to radiology department (n = 758), mammogram facilities (n = 1,749), and at a quaternary care academic medical center (n = 738) (Table 1).
Additional studies focused on the characteristics of the women, such as women who have individual subscribers or employer supplemented (n = 95,661), are Medicaid-insured and Medicare fee-for service (n = 11), are insured but have not undergone mammogram in 24 months (n = 47,946), have no history of breast cancer (n = 181,755), have telephone access (n = 169,116), homeless women (n = 100), hospitalized women (n = 250), are medically underserved (n = 518), and have limited accessibility to mammogram (n = 73,718) (Table 1).
Classification of SDOH factors influencing breast cancer screening behavior based on the healthy people 2030 categories
An examination of SDOH influential factors of breast cancer screening was conducted, focusing on their classification into Healthy People 2030 categories (20). Among the various SDOH identified, those related to socioeconomic status (n = 96) exhibited the highest frequency (Table 1). Specifically, factors such as income (n = 32), education level (n = 29), employment status (n = 8), birthplace/citizenship (n = 5), acculturation/years lived in the United States (n = 5), marital status (n = 2), social support (n = 2), and number of children (n = 1) were among the key elements. Access to healthcare (n = 75) emerged as a significant theme, with subcategories like insurance status (n = 33), accessibility of healthcare services and providers (n = 18), insurance coverage (n = 8), access to mammography facilities (n = 5), insurance copayments (n = 2), time from breast cancer diagnosis to first treatment (n = 1), travel time to clinic (n = 1), and county uninsured rate (n = 1) also being identified. Race/Ethnicity (n = 79), age (n = 52), sex/gender (n = 2), and sexual orientation (n = 1) were additional factors reported. Language-related SDOH (n = 21) were observed 21 times, encompassing language proficiency/preferred language (n = 15) and health literacy (n = 6). Furthermore, location (n = 30), transportation (n = 5), housing (n = 3), county poverty rate (n = 2), internet access (n = 1), area deprivation index (n = 1), diversity index (n = 1), cultural and religious beliefs (n = 4), perceived discrimination (n = 2), health beliefs (n = 1), and trust in health care providers/systems (n = 1) were also cited. Finally, health-related factors (n = 9) that were reported include comorbidities and chronic illnesses (n = 3), BMI (n = 2), medical/family history of breast cancer (n = 1), history of mental illness (n = 1), HIV status (n = 1), and substance/alcohol abuse (n = 1) (Table 1). Among the Healthy People 2030 categories, Social and Community Context (n = 177) emerged as the most prevalent, with a striking 177 occurrences of SDOH. Following closely behind were Healthcare Access and Quality (n = 80), Economic Stability (n = 56), Neighborhood and Built Environment (n = 46), and Education Access and Quality (n = 36) (Table 1).
Database access and characteristics
Databases with the highest number of occurrences include data from the National Health Interview Survey (n = 8) [over a range of years from 2005 to 2018], the Breast Cancer Surveillance Consortium (n = 4), and the United States Department of Health (n = 2). Other databases used include the National Program of Cancer Registries, the National Assessment of Adult Literacy, and SEER Medicare. Of the 74 databases used, 47% (n = 35) are publicly available. The databases are available at the city (n = 16), county (n = 1), state (n = 28), and national (n = 30) levels (Table 2).
Significance of association between SDOH factors and access to mammography and treatment opportunities
The Health Care Access and Quality category was reported in the highest number of studies (n = 44; 61%), showing its statistical significance in relation to access to mammography. Insurance status was the most reported sub-categorical factor of Health Care Access and Quality with n = 36 (50%) articles supporting this finding. A total of n = 42 (58%) studies showed statistical significance in the social and community context category, with the highest subcategories being age and ethnicity with n = 46 (63%) and n = 40 (55%) articles denoting their significance, respectively. Language was the third highest with n = 11 (15%) studies highlighting its significance as an influential factor of screening behavior. Further, n = 28 (38%) studies exhibited statistical significance under the Economic Stability category, with income level being the most common sub-categorical indicator emphasized in n = 20 (27%) studies. Next, the Neighborhood and Built Environment category showed statistical significance in n = 18 (25%) articles, with zip code or geographic location being reported as the strongest sub-categorical indicator in n = 15 studies (20%). Moreover, n = 24 (33%) articles showed statistical significance in Education Access and Quality as strong indicators of mammography rate, with the highest level of education completed acting as the strongest sub-categorical factor in n = 24 (33%) articles (Table 1).
The methodology used across the included studies to communicate statistical data were reported as: logistic regression (n = 63), descriptive statistics (n = 23), chi-square tests (n = 20), T-tests (n = 13), linear regression (n = 9), multivariate analyses (n = 9), Wald tests (n = 8), Generalized estimating equations (n = 7), Spatial analysis (n = 7), Cox proportional hazards regression (n = 5), Kaplan–Meier cumulative incidence (n = 3), Sensitivity analysis (n = 2), Trend analysis (n = 2), and Z tests (n = 1) (Table 1).
Lessons learned
Using the three phases of qualitative content analysis delineated by Elo and Kyngas (19), qualitative themes were identified. First, data relevant to lessons learned was collected from each of the included studies in the preparation stage (Phase I) (Supplementary material 1). Second, lessons learned were organized into bullet points and tabulated by primary author to compare data across studies and explore emerging themes (Phase 2) (Supplementary material 1). Major themes were then highlighted in Table 3 (Phase III).
Many of the studies demonstrated a strong association between a lack of health insurance and a lower rate of breast cancer screening (21–25). Ethnic minority women, with the exception of those identifying as Asian, had a lower likelihood of being screened, and Black women experienced a higher risk of diagnosis upon first screening (25–29). While few studies analyze the effect of sexual orientation on breast cancer screening, initial insights reveal there are significant differences in mammography between bisexual, lesbian, and heterosexual women regardless of racial/ethnic groups (30). In considering religious values, fatalism-emphasizing religions were associated with less screening adherences and maintenance of modesty did not prove a significant limitation for women receiving mammograms (31–33). Economic factors present limitations as both high levels of poverty and impoverished rural regions were associated with lower screening rates (27, 32, 34–37). Improving patient-provider communication, addressing perceived discrimination, and improving trust in the health care system is necessary to improve screening rates across all demographics (38–42). Additionally, structural efforts to improve health insurance coverage, language proficiency, and transportation services could be beneficial (20–110). These steps will need to involve the local community to develop community-tailored educational campaigns to reinforce the importance of establishing yearly mammogram screenings (Table 3) (22, 34, 46, 49, 54, 55, 70, 76, 80, 86).
Discussion
The purpose of this scoping review was to identify the major SDOH acting as influential factors of breast cancer screening in United States women aged ≥ 40 years old. The analysis of the 72 included studies can inform which SDOH categories to focus on when designing evidence-based interventions for more effective and sustained positive behavior and health outcomes among United States women at-risk of breast cancer.
SDOH factors and healthy people 2030 categories
Of the classifications of SDOH by Healthy People 2030, the Social and Community Context Category was the most prevalent across the included studies (n = 177). However, when looking at the most frequently cited SDOH influential factors of breast cancer screening behaviors, those related to socioeconomic status exhibited the highest frequency. Such factors included income (n = 32), education level (n = 29), employment status (n = 8), birthplace/citizenship (n = 5), acculturation/years lived in the United States (n = 5), marital status (n = 2), social support (n = 2), and number of children (n = 1). Other highly reported factors include insurance status (n = 33) under the Healthcare Access and Quality category, as well as race/ethnicity (n = 79) and age (n = 52) under the Social and Community Context Category.
There is evidence to show the significance of the relationship between socioeconomic factors and breast cancer screening. Over 30 different interventions that address SDOH increased breast cancer screening rates by 12.3% (93). Social determinants such as poverty, lack of education, neighborhood disadvantage, residential segregation, racial discrimination, lack of social support, and social isolation have shown in numerous studies to play a role in the breast cancer stage at diagnosis (94, 95). Gomez et al. (94) highlighted in their review that social and built environments have been shown to factor into cancer diagnoses in 82% of 34 reviewed articles published since 2010, including breast cancer (96). Studies have found that, not only do these factors have a significant association with breast cancer screening individually, but they also work dynamically to impact screening and treatment for breast cancer (97).
Low affordability and healthcare accessibility profoundly impact breast cancer screening, leading to lower adherence in female patients. For instance, Medicaid patients who are required to pay co-payments for preventative services as well as for recommended follow-up visits are less likely to pursue such preventative services and mammograms are included in lost care (96). Co-payments of more than $10 have been associated with reduced rates of mammograms (97). Furthermore, a study investigating breast cancer screening among young military women revealed that, when removing cost and access barriers to obtaining a breast mammography, first-time screening rates were 90% (98). Similar results have been noted when patients were provided free mammograms in underserved areas. The Building Relationships and Initiatives Dedicated to Gaining Equality (BRIDGE) Healthcare Clinic, a free clinic offered by the University of South Florida, provided patients free mammograms and noted that about 84.5% of patients utilized these services (99).
Significance of associations between SDOH factors and breast cancer screening and treatment
The majority of the studies reported a significant association between the SDOH factors under each of the five Healthy People 2030 categories. Insurance status was the most reported sub-categorical factor of Health Care Access and Quality with n = 36 (50%) articles supporting this finding. Insurance status often determines whether patients seek mammography services as they often become costly without robust coverage (93). Despite stable mammography rates among women in the United States between the years 2000 and 2015, women who report being uninsured consistently have the lowest rates of mammography at 35.3% (100).
Moreover, a total of n = 42 (58%) studies showed statistical significance in the social and community context category, with the highest subcategories being age and ethnicity with n = 46 (63%) and n = 40 (55%) articles denoting their significance, respectively. Health disparities in the United States have been consistently associated with delayed screening, which then contributes to higher mortality rates among both Hispanic and Black populations (28). Inequities also exist in mammography rates between patients of different sexual orientations (111). White, bisexual women had significantly lower mammography rates than White, heterosexual women, while mammography rates were significantly higher for bisexual, Black women than for heterosexual, Black women (102).
Income (n = 20; 27%) strongly influences mammography rates since women with estimated household incomes greater than $38,100 have been found to have rates of repeat mammography higher than those of women below $25,399 (109). In addition to household income, food security acts as another influential factor of mammography rates. When patients are forced to choose between feeding their families and pursuing preventative care, mammography becomes more of a luxury than lifesaving care (110). Women facing food insecurity have shown a 54% lower likelihood of obtaining mammography (110).
Language (n = 11; 15%) and availability of translation services, health literacy, and culture also play a strong role in mammography rates since many women with limited English proficiency seek mammography care and receive abnormal results (103). Appropriate, timely follow-up in the correct language is imperative to proper care provision; however, a lack of translation services worsens the language barrier between these patients and their healthcare providers, delaying care (101). Clinics with a patient population that is majority non-English speaking also experience greater follow-up delays than those with a minority of non-English speakers due to language barriers (103). The lower a patient’s health literacy, the less likely they are to undergo up-to-date breast cancer screening according to official guidelines (104, 105). The cultural and religious beliefs in fatalism have also been continuously found to be associated with lower mammography rates, whereby women with the highest beliefs in fatalism had the lowest breast cancer screening rates (106, 107).
Finally, Education Access and Quality sub-categories were significant indicators of mammography rate, with the highest level of education completed acting as the strongest sub-categorical factor in n = 24 (33%) articles. A systematic review by Damiani et al. (109) showed that United States women with the highest level of education were more likely to screen for breast cancer, with a 36% higher rate of adherence to national screening guidelines compared to women with lower levels of education. This finding holds health professionals and community outreach efforts accountable in ensuring that the local patient population is aware of the importance of and has access to breast cancer screening measures (109, 110).
Availability of public databases
Of the 74 databases used, only 47% (n = 35) were publicly available. There is a need to establish more widely accessible databases encompassing a routine collection of data on the SDOH to allow for the examination of additional evidence on exiting associations between SDOH and health outcomes. These databases could also inform the development and implementation of longitudinal and experimental studies at the county, city, and national levels to decrease health disparities exacerbated by SDOH factors.
Strengths and limitations
Despite the importance of this study in guiding and informing the development and implementation of future SDOH-oriented evidence-based interventions for breast cancer screening, findings need to take into consideration this study’s limitations. First, despite a comprehensive search of the literature in psychosocial databases compatible with the topic at hand, this review did not include gray literature and did not encompass tracing of reference lists in included studies. Second, it also was limited to observational studies to explore SDOH factors acting as factors based on statistical tests looking at significance of reported associations. These observational studies also widely varied in reported sample sizes, ranging from 100 participants to a population of 4 million. Therefore, although statistical significance was reported across different studies, effect sizes, power, and external validity varied greatly. Future systematic reviews should assess the rigor and quality of analysis carried out, evaluate recruitment efforts and data collection methods, and critique analytical tests carried out to account for the difference in sample sizes. Third, the mesh terms included as many technical words and keywords relevant to the SDOH as possible but might have inadvertently omitted some key words due to the continuously evolving and changing definitions related to SDOH. However, the help of an expert research librarian mitigated the impact of this concern by imposing rigor in implemented scoping review protocols when developing the search strategy for this review. Fourth, formal assessment of the methodology and quality of the evidence was beyond the scope of this study and relied on the reported statistical tests to assess significance. Follow-up systematic reviews would help with addressing this limitation by focusing specifically on the analytical proportion of each study. Fifth, although various categorizations exist for SDOH such as the WHO and CDC categories, the Healthy People 2030 taxonomy was adopted for use as it is the most recently updated classification encompassing a wide range of SDOH. Future studies should compare these taxonomies by feasibility, usability, and importance for a more valid and systematic approach to SDOH categorization.
Conclusion
This scoping review describes major SDOH acting as significant influential factors of breast cancer screening behaviors among United States women aged ≥40 years old who are at-risk of the disease. Results may inform future evidence-based interventions aiming to address the underlying factors contributing to low screening rates for breast cancer in the United States. Efforts to integrate SDOH within the different components of intervention planning, implementation, and sustainability are widely gaining recognition, particularly in underserved communities, due to their substantial influence on everyday behaviors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
VJ: Conceptualization, Data curation, Methodology, Writing – original draft. DL: Conceptualization, Data curation, Methodology, Writing – original draft. GO: Conceptualization, Data curation, Methodology, Writing – original draft. YZ: Conceptualization, Data curation, Methodology, Writing – original draft. SB: Conceptualization, Data curation, Methodology, Writing – original draft. AM: Conceptualization, Data curation, Methodology, Writing – original draft. SD: Conceptualization, Data curation, Methodology, Writing – original draft. MR: Conceptualization, Data curation, Methodology, Writing – original draft. DD: Methodology, Writing – original draft. MK: Methodology, Software, Writing – review & editing. LS: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1354717/full#supplementary-material
References
1. World Health Organization (2014). World conference on social determinants of health: case studies on social determinants. Available at: http://www.who.int/sdhconference/resources/case_studies/en/
2. Office of Disease Prevention and Health Promotion (n.d.). Social determinants of health. Healthy people 2030. U.S. Department of Health and Human Services. Available at: https://health.gov/healthypeople/priority-areas/social-determinants-health
3. Krause, TM, Schaefer, C, and Highfield, L. The association of social determinants of health with health outcomes. Am J Manag Care. (2021) 27:e89–96. doi: 10.37765/ajmc.2021.88603
4. Agency for Healthcare Research and Quality (2021). 2021 National Healthcare Quality and Disparities Report: Executive Summary.
5. Liu, D, Schuchard, H, Burston, B, Yamashita, T, and Albert, S. Interventions to reduce healthcare disparities in cancer screening among minority adults: a systematic review. J Racial Ethn Health Disparities. (2021) 8:107–26. doi: 10.1007/s40615-020-00763-1
6. Zavala, VA, Bracci, PM, Carethers, JM, Carvajal-Carmona, L, Coggins, NB, Cruz-Correa, MR, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. (2021) 124:315–32. doi: 10.1038/s41416-020-01038-6
7. Ward, E, Jemal, A, Cokkinides, V, Singh, GK, Cardinez, C, Ghafoor, A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. (2004) 54:78–93. doi: 10.3322/canjclin.54.2.78
8. Mathers, C, and Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
9. U.S. Preventive Services Task Force (2016). Breast cancer screening final recommendations. Available at: http://screeningforbreastcancer.org/ (Accessed May 26, 2023).
10. Coughlin, SS, Uhler, RJ, Richards, T, and Wilson, KM. Breast and cervical cancer screening practices among Hispanic and non-Hispanic women residing near the United States-Mexico border, 1999–2000. Fam Commun Health. (2003) 26:130–9. doi: 10.1097/00003727-200304000-00006
11. Jones, AR, Caplan, LS, and Davis, MK. Racial/ethnic differences in the self-reported use of screening mammography. J Community Health. (2003) 28:301–16. doi: 10.1023/a:1025451412007
12. Rodriguez, MA, Ward, LM, and Perez-Stable, EJ. Breast and cervical cancer screening: impact of health insurance status, ethnicity, and nativity of Latinas. Ann Fam Med. (2005) 3:235–41. doi: 10.1370/afm.291
13. Lantz, PM, Mujahid, M, Schwartz, K, Janz, NK, Fagerlin, A, Salem, B, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. (2006) 96:2173–8. doi: 10.2105/AJPH.2005.072132
14. Abraido-Lanza, AF, Chao, MT, and Gammon, MD. Breast and cervical cancer screening among Latinas and non-Latina whites. Am J Public Health. (2004) 94:1393–8. doi: 10.2105/AJPH.94.8.1393
15. Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
16. Arksey, H, and O’Malley, L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
17. American Cancer Society. ACS breast cancer screening guidelines. Cancer.org. (2023). Available at: https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html (Accessed January 16, 2024).
18. Office of Disease Prevention and Health Promotion (2023). Social determinants of health. Health.gov. Available at: https://health.gov/healthypeople/objectives-and-data/social-determinants-health (Accessed August 15, 2023).
19. Elo, S, and Kyngäs, H. The qualitative content analysis process. J Adv Nurs. (2008) 62:107–15. doi: 10.1111/j.1365-2648.2007.04569.x
20. Henderson, LM, O’Meara, ES, Haas, JS, Lee, CI, Kerlikowske, K, Sprague, BL, et al. The role of social determinants of health in self-reported access to health care among women undergoing screening mammography. J Women's Health. (2020) 29:1437–46. doi: 10.1089/jwh.2019.8267
21. Beaber, EF, Tosteson, ANA, Haas, JS, Onega, T, Sprague, BL, Weaver, DL, et al. Breast cancer screening initiation after turning 40 years of age within the PROSPR consortium. Breast Cancer Res Treat. (2016) 160:323–31. doi: 10.1007/s10549-016-3990-x
22. Jin, SW, Lee, HY, and Lee, J. Analyzing factors of breast cancer screening adherence among Korean American women using Andersen’s behavioral model of healthcare services utilization. Ethn Dis. (2019) 29:427–34. doi: 10.18865/ed.29.s2.427
23. Kosog, K, Earle, M, Stellon, E, Nolan, C, Wainwright, MK, Webb, T, et al. Identifying an association between socio-demographic factors and breast cancer screening adherence in a federally qualified health Centre sample in the United States. A retrospective, cross-sectional study. Health Soc Care Commun. (2020) 28:1772–9.
24. Kempe, KL, Larson, RS, Shetterley, S, and Wilkinson, A. Breast cancer screening in an insured population: whom are we missing? Perm J. (2013) 17:38–44. doi: 10.7812/TPP/12-068
25. Selove, R, Kilbourne, B, Fadden, MK, Sanderson, M, Foster, M, Offodile, R, et al. Time from screening mammography to biopsy and from biopsy to breast cancer treatment among black and white, women medicare beneficiaries not participating in a health maintenance organization. Womens Health Issues. (2016) 26:642–7. doi: 10.1016/j.whi.2016.09.003
26. Wilcox, ML, Acuña, JM, Ward-Peterson, M, Alzayed, A, Alghamdi, M, and Aldaham, S. Racial/ethnic disparities in annual mammogram compliance among households in little Haiti, Miami-Dade County, Florida: an observational study. Medicine. (2016) 95:e3826. doi: 10.1097/md.0000000000003826
27. Wilkerson, AD, Obi, M, Ortega, C, Sebikali-Potts, A, Wei, W, Pederson, HJ, et al. Young black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. (2023) 30:2856–69. doi: 10.1245/s10434-022-12995-y
28. Agénor, M, Pérez, AE, Tabaac, AR, Bond, KT, Charlton, BM, Bowen, DJ, et al. Sexual orientation identity disparities in mammography among White, black, and Latina US women. LGBT Health. (2020) 7:312–20. doi: 10.1089/lgbt.2020.0039
29. Alatrash, M. Determinants of breast cancer screening in three Arab American women subgroups. J Transcult Nurs. (2021) 32:749–56. doi: 10.1177/10436596211008215
30. Jensen, B, Khan, H, and Layeequr, RR. Sociodemographic determinants in breast cancer screening among uninsured women of West Texas. Medicina. (2022) 58:1–12. doi: 10.3390/medicina58081010
31. Anderson, RT, Yang, T-C, Matthews, SA, Camacho, F, Kern, T, Mackley, HB, et al. Breast cancer screening, area deprivation, and later-stage breast cancer in Appalachia: does geography matter? Health Serv Res. (2014) 49:546–67. doi: 10.1111/1475-6773.12108
32. Hong, HC, Ferrans, CE, Park, C, Lee, H, Quinn, L, and Collins, EG. Effects of perceived discrimination and trust on breast cancer screening among Korean American women. Womens Health Issues. (2018) 28:188–96. doi: 10.1016/j.whi.2017.11.001
33. Agrawal, P, Chen, TA, McNeill, LH, Acquati, C, Connors, SK, Nitturi, V, et al. Factors associated with breast cancer screening adherence among church-going African American women. Int J Environ Res Public Health. (2021) 18:8494. doi: 10.3390/ijerph18168494
34. Onega, T, Tosteson, TD, Weiss, J, Haas, JS, Goodrich, M, DiFlorio, R, et al. Multi-level influences on breast cancer screening in primary care. J Gen Intern Med. (2018) 33:1729–37. doi: 10.1007/s11606-018-4560-1
35. Dong, W, Rose, J, Kim, U, Cooper, GS, Tsui, J, and Koroukian, SM. Medicaid expansion associated with reduction in geospatial breast cancer stage at diagnosis disparities. J Public Health Manag Pract. (2022) 28:469–77. doi: 10.1097/PHH.0000000000001514
36. Henderson, LM, Benefield, T, Nyante, SJ, Marsh, MW, Greenwood-Hickman, MA, and Schroeder, BF. Performance of digital screening mammography in a population-based cohort of black and white women. Cancer Causes Control. (2015) 26:1495–9. doi: 10.1007/s10552-015-0631-3
37. Patel, K, Kanu, M, Liu, J, Bond, B, Brown, E, Williams, E, et al. Factors influencing breast cancer screening in low-income African Americans in Tennessee. J Community Health. (2014) 39:943–50. doi: 10.1007/s10900-014-9834-x
38. Johnson, CJ, Morawski, BM, Hobbs, L, Lewis, D, Cariou, C, and Rycroft, RK. Time from breast cancer diagnosis to treatment among Idaho’s National Breast and cervical Cancer early detection program population, 2011-2017. Cancer Causes Control. (2021) 32:667–73. doi: 10.1007/s10552-021-01407-3
39. Khaliq, W, Aamar, A, and Wright, SM. Predictors of non-adherence to breast cancer screening among hospitalized women. PLoS One. (2015) 10:e0145492. doi: 10.1371/journal.pone.0145492
40. Lee, CI, Bogart, A, Germino, JC, Goldman, LE, Hubbard, RA, Haas, JS, et al. Availability of advanced breast imaging at screening facilities serving vulnerable populations. J Med Screen. (2016) 23:24–30. doi: 10.1177/0969141315591616
41. Virk-Baker, MK, Martin, MY, Levine, RS, Wang, X, Nagy, TR, and Pisu, M. Mammography utilization among black and White Medicare beneficiaries in high breast cancer mortality US counties. Cancer Causes Control. (2013) 24:2187–96. doi: 10.1007/s10552-013-0295-9
42. Wiese, D, Islami, F, and Henry, KA. Changes in geographic accessibility to mammography by state and rural-urban status, United States, 2006-2022. J Natl Cancer Inst. (2023) 115:337–40. doi: 10.1093/jnci/djac217
43. Oviedo, AD. Mammogram adherence among Filipino American women. J Immigr Minor Health. (2022) 24:639–44. doi: 10.1007/s10903-021-01223-6
44. Nair, RG, Lee, SJC, Berry, E, Argenbright, KE, Tiro, JA, and Skinner, CS. Long-term mammography adherence among uninsured women enrolled in the breast screening and patient navigation (BSPAN) program. Cancer Epidemiol Biomarkers Prev. (2022) 31:77–84. doi: 10.1158/1055-9965.EPI-21-0191
45. Alabdullatif, N, Arrieta, A, Dlugasch, L, and Hu, N. The impact of IT-based healthcare communication on mammography screening utilization among women in the United States: National Health Interview Survey (2011-2018). Int J Environ Res Public Health. (2022) 19:12737. doi: 10.3390/ijerph191912737
46. Asgary, R, Garland, V, and Sckell, B. Breast cancer screening among homeless women of new York City shelter-based clinics. Womens Health Issues. (2014) 24:529–34. doi: 10.1016/j.whi.2014.06.002
47. Ayanian, JZ, Landon, BE, Zaslavsky, AM, and Newhouse, JP. Racial and ethnic differences in use of mammography between Medicare advantage and traditional Medicare. J Natl Cancer Inst. (2013) 105:1891–6. doi: 10.1093/jnci/djt333
48. Balazy, KE, Benitez, CM, Gutkin, PM, Jacobson, CE, von Eyben, R, and Horst, KC. Association between primary language, a lack of mammographic screening, and later stage breast cancer presentation. Cancer. (2019) 125:2057–65. doi: 10.1002/cncr.32027
49. Beaber, EF, Sprague, BL, Tosteson, ANA, Haas, JS, Onega, T, Schapira, MM, et al. Multilevel predictors of continued adherence to breast cancer screening among women ages 50-74 years in a screening population. J Women's Health. (2019) 28:1051–9. doi: 10.1089/jwh.2018.6997
50. Calo, WA, Vernon, SW, Lairson, DR, and Linder, SH. Area-level socioeconomic inequalities in the use of mammography screening: a multilevel analysis of the health of Houston survey. Womens Health Issues. (2016) 26:201–7. doi: 10.1016/j.whi.2015.11.002
51. Castañeda, SF, Malcarne, VL, Foster-Fishman, PG, Davidson, WS, Mumman, MK, Riley, N, et al. Health care access and breast cancer screening among Latinas along the California-Mexican border. J Immigr Minor Health. (2014) 16:670–81. doi: 10.1007/s10903-013-9938-x
52. Cataneo, JL, Meidl, H, Ore, AS, Raicu, A, Schwarzova, K, and Cruz, CG. The impact of limited language proficiency in screening for breast cancer. Clin Breast Cancer. (2023) 23:181–8. doi: 10.1016/j.clbc.2022.11.008
53. Chandak, A, Nayar, P, and Lin, G. Rural-urban disparities in access to breast cancer screening: a spatial clustering analysis: disparities in breast cancer screening. J Rural Health. (2019) 35:229–35. doi: 10.1111/jrh.12308
54. Christensen, EW, Pelzl, CE, Patel, BK, Carlos, RC, and Rula, EY. Urbanicity, income, and mammography-use disparities among American Indian women. Am J Prev Med. (2023) 64:611–20. doi: 10.1016/j.amepre.2023.01.013
55. Clark, CR, Tosteson, TD, Tosteson, ANA, Onega, T, Weiss, JE, Harris, KA, et al. Diffusion of digital breast tomosynthesis among women in primary care: associations with insurance type. Cancer Med. (2017) 6:1102–7. doi: 10.1002/cam4.1036
56. Clarke, TC, Endeshaw, M, Duran, D, and Saraiya, M. Breast cancer screening among women by nativity, birthplace, and length of time in the United States. Natl Health Stat Rep. (2019) 129:1–15.
57. Davis, J, Liang, J, Petterson, MB, Roh, AT, Chundu, N, Kang, P, et al. Risk factors for late screening mammography. Curr Probl Diagn Radiol. (2019) 48:40–4. doi: 10.1067/j.cpradiol.2017.10.014
58. Duggan, C, Molina, Y, Carosso, E, Ibarra, G, and Thompson, B. County of residence and screening practices among Latinas and non-Latina whites in two rural communities. Ethn Dis. (2019) 29:31–8. doi: 10.18865/ed.29.1.31
59. Elkin, EB, Paige Nobles, J, Pinheiro, LC, Atoria, CL, and Schrag, D. Changes in access to screening mammography, 2008-2011. Cancer Causes Control. (2013) 24:1057–9. doi: 10.1007/s10552-013-0180-6
60. Fedewa, SA, de Moor, JS, Ward, EM, DeSantis, CE, Goding Sauer, A, Smith, RA, et al. Mammography use and physician recommendation after the 2009 U.S. preventive services task force breast cancer screening recommendations. Am J Prev Med. (2016) 50:e123–31. doi: 10.1016/j.amepre.2015.10.010
61. Flores, EJ, López, D, Miles, RC, Glover, M 4th, Lehman, CD, Harvey, HB, et al. Impact of primary care physician interaction on longitudinal adherence to screening mammography across different racial/ethnic groups. J Am Coll Radiol. (2019) 16:908–14. doi: 10.1016/j.jacr.2018.12.020
62. Guo, Y, Cheng, TC, and Yun, LH. Factors associated with adherence to preventive breast cancer screenings among middle-aged African American women. Soc Work Public Health. (2019) 34:646–56. doi: 10.1080/19371918.2019.1649226
63. Henry, KA, McDonald, K, Sherman, R, Kinney, AY, and Stroup, AM. Association between individual and geographic factors and nonadherence to mammography screening guidelines. J Women's Health. (2014) 23:664–74. doi: 10.1089/jwh.2013.4668
64. Hubbard, RA, O’Meara, ES, Henderson, LM, Hill, D, Braithwaite, D, Haas, JS, et al. Multilevel factors associated with long-term adherence to screening mammography in older women in the U.S. Prev Med. (2016) 89:169–77. doi: 10.1016/j.ypmed.2016.05.034
65. Jena, AB, Huang, J, Fireman, B, Fung, V, Gazelle, S, Landrum, MB, et al. Screening mammography for free: impact of eliminating cost sharing on cancer screening rates. Health Serv Res. (2017) 52:191–206. doi: 10.1111/1475-6773.12486
66. Kim, E, Moy, L, Gao, Y, Hartwell, CA, Babb, JS, and Heller, SL. City patterns of screening mammography uptake and disparity across the United States. Radiology. (2019) 293:151–7. doi: 10.1148/radiol.2019190647
67. Kim, SE, Bachorik, AE, Bertrand, KA, and Gunn, CM. Differences in breast cancer screening practices by diabetes status and race/ethnicity in the United States. J Women's Health. (2022) 31:848–55. doi: 10.1089/jwh.2021.0396
68. Komenaka, IK, Nodora, JN, Hsu, C-H, Martinez, ME, Gandhi, SG, Bouton, ME, et al. Association of health literacy with adherence to screening mammography guidelines. Obstet Gynecol. (2015) 125:852–9. doi: 10.1097/aog.0000000000000708
69. Lapeyrouse, LM, Miranda, PY, Morera OFHeyman, JM, and Balcazar, HG. Healthcare use and mammography among Latinas with and without health insurance near the US-Mexico border. J Racial Ethn Health Disparities. (2017) 4:282–7. doi: 10.1007/s40615-016-0227-y
70. Lee, HY, Lee, MH, Jang, YJ, and Lee, DK. Breast cancer screening disparity among Korean American immigrant women in Midwest. Asian Pac J Cancer Prev. (2017) 18:2663–7. doi: 10.22034/APJCP.2017.18.10.2663
71. Lee, CI, Zhu, W, Onega, T, Henderson, LM, Kerlikowske, K, Sprague, BL, et al. Comparative access to and use of digital breast tomosynthesis screening by women’s race/ethnicity and socioeconomic status. JAMA Netw Open. (2021) 4:e2037546. doi: 10.1001/jamanetworkopen.2020.37546
72. Li, L, Ji, J, Besculides, M, Bickell, N, Margolies, LR, Jandorf, L, et al. Factors associated with mammography use: a side-by-side comparison of results from two national surveys. Cancer Med. (2020) 9:6430–51. doi: 10.1002/cam4.3128
73. Luo, Y, Carretta, H, Lee, I, LeBlanc, G, Sinha, D, and Rust, G. Naïve Bayesian network-based contribution analysis of tumor biology and healthcare factors to racial disparity in breast cancer stage-at-diagnosis. Health Inf Sci Syst. (2021) 9:35. doi: 10.1007/s13755-021-00165-5
74. Molina, Y, Plascak, JJ, Patrick, DL, Bishop, S, Coronado, GD, and Beresford, SAA. Neighborhood predictors of mammography barriers among US-based Latinas. J Racial Ethn Health Disparities. (2017) 4:233–42. doi: 10.1007/s40615-016-0222-3
75. Monsivais, P, Amiri, S, Robison, J, Pflugeisen, C, Kordas, G, and Amram, O. Racial and socioeconomic inequities in breast cancer screening before and during the COVID-19 pandemic: analysis of two cohorts of women 50 years +. Breast Cancer. (2022) 29:740–6. doi: 10.1007/s12282-022-01352-2
76. Padela, AI, Murrar, S, Adviento, B, Liao, C, Hosseinian, Z, Peek, M, et al. Associations between religion-related factors and breast cancer screening among American Muslims. J Immigr Minor Health. (2015) 17:660–9. doi: 10.1007/s10903-014-0014-y
77. Paranjpe, A, Zheng, C, and Chagpar, AB. Disparities in breast cancer screening between Caucasian and Asian American women. J Surg Res. (2022) 277:110–5. doi: 10.1016/j.jss.2022.03.032
78. Sabatino, SA, Thompson, TD, Guy, GP, de Moor, JS, and Tangka, FK. Mammography use among medicare beneficiaries after elimination of cost sharing. Med Care. (2016) 54:394–9. doi: 10.1097/mlr.0000000000000495
79. Schommer, L, Mikulski, MF, Goodgame, B, and Brown, KM. Racial disparities in breast cancer presentation and diagnosis in COVID-era Central Texas. J Surg Res. (2023) 288:79–86. doi: 10.1016/j.jss.2023.02.021
80. Sealy-Jefferson, S, Roseland, ME, Cote, ML, Lehman, A, Whitsel, EA, Mustafaa, FN, et al. Rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: the Women’s health initiative. J Women's Health. (2019) 28:276–83. doi: 10.1089/jwh.2017.6884
81. Shon, E-J, and Townsend, AL. Predictors of never having a mammogram among Chinese, Vietnamese, and Korean immigrant women in the U.S. PLoS One. (2019) 14:e0224505. doi: 10.1371/journal.pone.0224505
82. Spada, NG, Geramita, EM, Zamanian, M, van Londen, GJ, Sun, Z, and Sabik, LM. Changes in disparities in stage of breast cancer diagnosis in Pennsylvania after the affordable care act. J Women's Health. (2021) 30:324–31. doi: 10.1089/jwh.2020.8478
83. Tangka, FK, Subramanian, S, Mobley, LR, Hoover, S, Wang, J, Hall, IJ, et al. Racial and ethnic disparities among state Medicaid programs for breast cancer screening. Prev Med. (2017) 102:59–64. doi: 10.1016/j.ypmed.2017.06.024
84. Thomas, M, James, M, Vittinghoff, E, Creasman, JM, Schillinger, D, and Mangurian, C. Mammography among women with severe mental illness: exploring disparities through a large retrospective cohort study. Psychiatr Serv. (2018) 69:48–54. doi: 10.1176/appi.ps.201600170
85. Tran, L, and Tran, P. US urban-rural disparities in breast cancer-screening practices at the national, regional, and state level, 2012-2016. Cancer Causes Control. (2019) 30:1045–55. doi: 10.1007/s10552-019-01217-8
86. Vang, S, Margolies, LR, and Jandorf, L. Screening mammogram adherence in medically underserved women: does language preference matter? J Cancer Educ. (2022) 37:1076–82. doi: 10.1007/s13187-020-01922-y
87. Wang, H, Gregg, A, Qiu, F, Kim, J, Chen, B, Wan, N, et al. Breast cancer screening for patients of rural accountable care organization clinics: a multi-level analysis of barriers and facilitators. J Community Health. (2018) 43:248–58. doi: 10.1007/s10900-017-0412-x
88. Wu, AM, Morse, AR, Seiple, WH, Talwar, N, Hansen, SO, Lee, PP, et al. Reduced mammography screening for breast cancer among women with visual impairment. Ophthalmology. (2021) 128:317–23. doi: 10.1016/j.ophtha.2020.07.029
89. Kadivar, H, Kenzik, KM, Dewalt, DA, and Huang, I-C. The association of English functional health literacy and the receipt of mammography among Hispanic women compared to non-Hispanic U.S.-born white women. PLoS One. (2016) 11:e0164307. doi: 10.1371/journal.pone.0164307
90. Ryu, SY, Crespi, CM, and Maxwell, AE. What factors explain disparities in mammography rates among Asian-American immigrant women? A population-based study in California. Womens Health Issues. (2013) 23:e403–10. doi: 10.1016/j.whi.2013.08.005
91. Korn, AR, Walsh-Bailey, C, Correa-Mendez, M, DelNero, P, Pilar, M, Sandler, B, et al. Social determinants of health and US cancer screening interventions: A systematic review. CA Cancer J Clin. (2023) 73:461–79. doi: 10.3322/caac.21801
92. Coughlin, SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. (2019) 177:537–48. doi: 10.1007/s10549-019-05340-7
93. Alcaraz, KI, Wiedt, TL, Daniels, EC, Yabroff, KR, Guerra, CE, and Wender, RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. (2020) 70:31–46. doi: 10.3322/caac.21586
94. Gomez, SL, Shariff-Marco, S, DeRouen, M, Keegan, TH, Yen, IH, Mujahid, M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer. (2015) 121:2314–30. doi: 10.1002/cncr.29345
95. Williams, AD, and Moo, TA. The impact of socioeconomic status and social determinants of health on disparities in breast Cancer incidence, treatment, and outcomes. Curr Breast Cancer Rep. (2023) 15:30–6. doi: 10.1007/s12609-023-00473-7
96. Sabik, LM, Vichare, AM, Dahman, B, and Bradley, CJ. Co-payment policies and breast and cervical cancer screening in Medicaid. Am J Manag Care. (2020) 26:69–74. doi: 10.37765/ajmc.2020.42395
97. Trivedi, AN, Rakowski, W, and Ayanian, JZ. Effect of cost sharing on screening mammography in Medicare health plans. N Engl J Med. (2008) 358:375–83. doi: 10.1056/NEJMsa070929
98. Amin, A, Shriver, CD, Henry, LR, Lenington, S, Peoples, GE, and Stojadinovic, A. Breast cancer screening compliance among young women in a free access healthcare system. J Surg Oncol. (2008) 97:20–4. doi: 10.1002/jso.20895
99. Koç, H, O’Donnell, O, and Van Ourti, T. “Conclusion: In the absence of a universal screening program in the U.S., determinants of access—income, insurance coverage and receipt of medical advice—appear to drive the education disparities in screening mammography.” (2018) Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6163342/
100. White, A, Thompson, TD, White, MC, Sabatino, SA, de Moor, J, Doria-Rose, PV, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep. (2017) 66:201–6. doi: 10.15585/mmwr.mm6608a1
101. Tsapatsaris, A, Babagbemi, K, and Reichman, MB. Barriers to breast cancer screening are worsened amidst COVID-19 pandemic: a review. Clin Imaging. (2022) 82:224–7. doi: 10.1016/j.clinimag.2021.11.025
102. Karliner, LS, Ma, L, Hofmann, M, and Kerlikowske, K. Language barriers, location of care, and delays in follow-up of abnormal mammograms. Med Care. (2012) 50:171–8. doi: 10.1097/MLR.0b013e31822dcf2d
103. Sentell, TL, Tsoh, JY, Davis, T, Davis, J, and Braun, KL. Low health literacy and cancer screening among Chinese americans in California: a cross-sectional analysis. BMJ Open. (2015) 5:e006104. doi: 10.1136/bmjopen-2014-006104
104. Baccolini, V, Isonne, C, Salerno, C, Giffi, M, Migliara, G, Mazzalai, E, et al. The association between adherence to cancer screening programs and health literacy: a systematic review and meta-analysis. Prev Med. (2022) 155:106927. doi: 10.1016/j.ypmed.2021.106927
105. Liang, W, Wang, JH, Chen, M-Y, Feng, S, Lee, M, Schwartz, MD, et al. Developing and validating a measure of Chinese cultural views of health and cancer. Health Educ Behav. (2008) 35:361–75. doi: 10.1177/1090198106294893
106. Molaei-Zardanjani, M, Savabi-Esfahani, M, and Taleghani, F. Fatalism in breast cancer and performing mammography on women with or without a family history of breast cancer. BMC Womens Health. (2019) 19:116. doi: 10.1186/s12905-019-0810-6
107. Barton, MB, Moore, S, Shtatland, E, and Bright, R. The relation of household income to mammography utilization in a prepaid health care system. J Gen Intern Med. (2001) 16:200–3. doi: 10.1111/j.1525-1497.2001.00228.x
108. Mahmood, A, Kedia, S, Dillon, PJ, Kim, H, Arshad, H, and Ray, M. Food security status and breast cancer screening among women in the United States: evidence from the health and retirement study and health care and nutrition study. Cancer Causes Control. (2023) 34:321–35. doi: 10.1007/s10552-023-01667-1
109. Damiani, G, Basso, D, Acampora, A, Bianchi, CB, Silvestrini, G, Frisicale, EM, et al. The impact of level of education on adherence to breast and cervical cancer screening: evidence from a systematic review and meta-analysis. Prev Med. (2015) 81:281–9. doi: 10.1016/j.ypmed.2015.09.011
110. Khalil, S, Hatch, L, Price, CR, Palakurty, SH, Simoneit, E, Radisic, A, et al. Addressing breast Cancer screening disparities among uninsured and insured patients: a student-run free clinic initiative. J Community Health. (2020) 45:501–5. doi: 10.1007/s10900-019-00767-x
Keywords: social determinants of health, breast cancer screening, mammography, health inequities, underserved women, United States
Citation: Jhumkhawala V, Lobaina D, Okwaraji G, Zerrouki Y, Burgoa S, Marciniak A, Densley S, Rao M, Diaz D, Knecht M and Sacca L (2024) Social determinants of health and health inequities in breast cancer screening: a scoping review. Front. Public Health. 12:1354717. doi: 10.3389/fpubh.2024.1354717
Edited by:
Ozgur Karcioglu, Taksim Training and Research Hospital, BeyogluReviewed by:
Heidy N. Medina, University of Miami, United StatesPaula Berstad, Cancer Registry of Norway, Norway
Copyright © 2024 Jhumkhawala, Lobaina, Okwaraji, Zerrouki, Burgoa, Marciniak, Densley, Rao, Diaz, Knecht and Sacca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lea Sacca, bHNhY2NhQGhlYWx0aC5mYXUuZWR1
 Vama Jhumkhawala
Vama Jhumkhawala Diana Lobaina
Diana Lobaina Goodness Okwaraji1
Goodness Okwaraji1 Yasmine Zerrouki
Yasmine Zerrouki Sara Burgoa
Sara Burgoa Sebastian Densley
Sebastian Densley Lea Sacca
Lea Sacca