- 1Department of Sports Science and Physical Education, The Chinese University of Hong Kong (CUHK), Shatin, Hong Kong SAR, China
- 2Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Kowloon, Hong Kong SAR, China
- 3Neuroscience Research Australia, University of New South Wales, Sydney, NSW, Australia
- 4CUHK Jockey Club Institute of Aging, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
Older adults residing in long-term care often experience declines in physical and cognitive functions despite the access to in-house physical activity (PA) programs. This study aimed to evaluate the associations of PA with physical function and global cognitive function in older adults living in long-term care, while examining potential sex differences. A cross-sectional analysis of baseline data from a two-arm cluster randomized controlled trial was conducted, involving 67 participants (34 men, 33 women). PA levels were assessed using tri-axial accelerometers. Physical function, including muscular strength, postural sway, and Short Physical Performance Battery (SPPB) and cognitive function were measured. Spearman correlation analysis revealed no significant associations between PA metrics and muscular strength, postural sway, or global cognitive function across the entire samples (p ≥ 0.091). Multiple linear regression models were developed for the entire sample, males, and females to examine the associations between PA and physical function measures and global cognitive function. After adjustments for confounders, light PA was significantly associated with higher SPPB sub-scores (gait: β = 0.600, p < 0.001; sit-to-stand: β = 0.574, p < 0.001), faster usual gait speed (β = 0.659, p = 0.012), and shorter sit-to-stand times (β = −0.305, p = 0.041) across the whole sample. Similar significant associations were observed in males between light PA and SPPB scores (total: β = 0.319, p = 0.040; gait: β = 0.532, p < 0.001; sit-to-stand: β = 0.417, p = 0.009), usual gait speed (β = 0.762, p = 0.017), and sit-to-stand times (β = −0.677, p < 0.001). In females, a significant association was found between light PA and global cognitive function (β = 0.319, p = 0.012) after adjusting for confounders. This study highlights sex differences in the association of accelerometer-determined PA with physical and cognitive function in older adults living in long-term care, with LPA showing beneficial effects, especially for physical function in males and for cognitive function in females.
1 Introduction
The global population aged 60 and over is rapidly increasing (1), with projections indicating that by 2050, it will comprise 21.1% of the entire global population (2). This shift in the proportion of older adults is accompanied by increased prevalence of aging-related pathologies and disabilities, thereby affecting their daily functioning (3). Those with limited self-care abilities due to poorer health often choose long-term care to access 24-h professional care (4). However, residents in these facilities frequently experience progressive declines in both physical and cognitive functions (5).
Physical impairments often manifest early in individuals with cognitive decline, and mobility decline is predictive of disabilities in older adults (6). Therefore, it is important to assess physical function in older adults with emphasis on mobility, balance, and muscular strength, given that these are key correlates of cognitive changes (7, 8). As physical function deteriorates, older adults may limit their participation in instrumental activities of daily living (ADLs), leading to a sedentary lifestyle and an increased risk for dependency, healthcare needs, and diminished quality of life (QoL) (9–11). Cognitive impairment further exacerbates this situation by increasing the risk of dementia progression and adding burdens to families and society (12–14). It is a major risk factor for institutionalization and the need for long-term care.
Previous research highlights the positive effects of physical activity (PA) on both physical and cognitive health in older adults living in long-term care (15–18). However, the specific relationship between objectively measured PA levels and physical function and cognitive performance in this population remains underexplored. Recent studies have utilized tri-axial accelerometers to accurately evaluate PA, which is commonly categorized into sedentary behavior (SB; low-intensity activities with a Metabolic Equivalent of Task [MET] of <1.5), light PA (LPA, activities with a MET of 1.5–2.9), and moderate-to-vigorous intensity PAs (MVPA, activities with a MET of ≥ 3.0) (19, 20). While the World Health Organization recommends at least 150 min of MVPA weekly for older adults (21), achieving this can be challenging for frail individuals in long-term care (22). SB is associated with various negative health outcomes, including cardiovascular disease (23), falls (24), frailty (25), and mortality (26).
Despite PA programs are readily available in care facilities, older adults residing in long-term care exercise less (0.2 h per day, or 84 min per week of MVPA) and are more sedentary (9.2 h per day of SB) compared to their community-dwelling counterparts (27–29). The severity of physical and cognitive deficits in this population, combined with limited caregiver monitoring, reduces the accuracy of self-reported or caregiver-reported PA levels (30). Therefore, objective measurements of PA and their relationship with physical and cognitive functions in frail older adults could provide insights into the declines in PA and help develop interventions to improve QoL and survival time.
Furthermore, compared to men, women are generally less physically active and have weaker muscular strength for those dwelling in the community and long-term care (31–33). Sex differences in PA levels and physical function are well-documented in older adults, but it is unclear if these differences extend to the correlations between PA levels and physical function performance and global cognitive function in long-term care residents. Understanding these sex differences is crucial for developing tailored interventions. Men and women may respond differently to PA due to various biological, psychological, and social factors. Tailoring PA programs to address these differences could enhance their effectiveness, potentially improving physical and cognitive outcomes and QoL for both sexes. Identifying these differences could also aim in designing more effective health policies and resource allocation, ensuring that both men and women receive optimal care and support.
The study aimed to evaluate the associations between PA levels and both physical function and global cognitive function in older adults residing in long-term care. A secondary objective was to examine sex differences in these associations. We hypothesized that more time spent in SB would be associated with lower physical and cognitive performance in both men and women. By elucidating these relationships, our study may provide a better understanding of how specific PA level, such as SB and LPA, impact overall health in this vulnerable population, offering a foundation for designing tailored interventions that maximize physical and cognitive benefits for each sex group.
2 Materials and methods
2.1 Study participants
The current study is a cross-sectional, secondary analysis of baseline data from a two-arm cluster randomized controlled trial (RCT) (34). Baseline data were collected from 20 long-term care facilities in Hong Kong between September 2023 and December 2023. Inclusion criteria were as follows: (1) aged 65 years or older; (2) able to rise from a chair, with or without using armrests and stand for at least 20 s; (3) confirmed by a physician to be able to participate in the study; and (4) able to wear an accelerometer continuously for 7 days to measure PA. Exclusion criteria included: (1) inability to comprehend instructions; (2) inability to complete the exercise program due to medical conditions; and (3) legal blindness (34). The study adhered to the principles of the World Medical Association Declaration of Helsinki and Good Clinical Practice. All participants provided written informed consent, either personally or through a family member, prior to study enrolment. Ethics approval was obtained from the Research Ethics Board at the Chinese University of Hong Kong and the Joint CUHK-NTEC Clinical Research Ethics Committee (34).
2.2 Outcome measures
2.2.1 Physical activity level measurement
Participants’ PA levels were measured using a tri-axial accelerometer (ActiGraph GT9X Link IMU, Pensacola, FL, USA). The primary outcomes included the percentage of time spent in SB, LPA, and MVPA, and total step counts. Participants were instructed to wear the accelerometer on the right side of their waist for seven consecutive days, removing it only during bathing or before going to bed (35). Data were recorded in 1-min epochs at a sampling frequency of 100 Hz. Non-wearing time was defined as more than 60 consecutive minutes of inactivity (with activity intensity less than 1.0 METs), allowing up to 2 min of activity above 1.0 METs (35–38). A valid day was considered as at least 480 min (8 h) of wear time, because many participants required the assistance of a caregiver for proper wearing and charging of the accelerometer, taking into account the caregiver’s work hours. Data from at least 3 valid days analyzed using ActiLife software (39, 40). PA intensity was classified based on established cut-off points from studies on similar populations (41–43): SB, LPA, and MVPA levels were defined as 0–50 counts/min, 51–759 counts/min, and ≥760 counts/min, respectively. For this study, MVPA was categorized into bouts of activity lasting at least 3 min, since the participants were relatively frailer than their healthy counterparts (44).
2.2.2 Physical function measurement
Physical function were objectively measured by trained research assistants. Measurements included maximum handgrip strength (right and left), upper limb strength (elbow flexion and extension), lower limb strength (knee extension), postural sway in the anterior–posterior (AP) and medial-lateral (ML) directions, and the Short Physical Performance Battery (SPPB) (34). Handgrip strength was measured using a digital hand-held dynamometry (HHD) (5001 Grip-A; Takei, Niigata City, Japan), with two measurements taken for each hand, and the maximum value recorded. Upper limb strength (biceps and triceps) and lower limb strength (quadriceps) were measured on the participant’s dominant side using the Hoggan microFET2 HHD (Hoggan Scientific, LLC, Salt Lake City, UT, USA). Two trails were performed for upper limbs, and three trials for lower limbs, with the maximum value used in analysis (45–47). Postural sway was measured in accordance with the Physiological Profile Assessment, with participant standing still on a hard surface with eyes open for 30 s (48). The AP and ML sway distances were recorded twice by the sway meter, and the average values were used for analysis. The SPPB included assessments of 4-m walking speed at self-selected speed (two measurements taken, with the fastest time recorded using a stopwatch), five-repetition sit-to-stand (STS) time, and 10 s standing balance in different positions (side-by-side, semi-tandem, and tandem). Each task was scored from 0 (lowest score) to 4 (highest score), with a total SPPB score ranging from 0 to 12 (49, 50). In this study, participants were allowed to use mobility aids for walking and to hold armrests when standing up from a chair if needed, considering their physical limitations.
2.2.3 Cognitive function measurement
Global cognitive function was assessed using the Hong Kong version of the Montreal Cognitive Assessment (HK-MoCA), a validated tool widely utilized for assessing cognitive function in the Hong Kong population due to its accessibility and ease of administration (51, 52). The HK-MoCA evaluates seven cognitive domains, including visuospatial and executive function, naming, attention, language, abstraction, recall/short-term memory, and orientation. Both total and domain-specific scores were recorded, with a maximum score of 30 (Supplementary material 1).
2.2.4 Other measurements
Several variables were considered potential confounders in the relationship between PA, physical function, and cognitive function, including age, sex, body mass index (BMI), education level, mobility aid used, frailty, and facility site. Body height and weight were measured by facility staff, and BMI was calculated. The mobility aids use was categorized into four levels: independent, cane-using, walker-using, and dependent. Frailty status was assessed using the 7-item FRAIL-NH scale, which evaluates fatigue, resistance, mobility, incontinence or disease, weight loss, eating style, and assistance with dressing. Each item was scored from 0 to 2, with a total score ranging from 0 (best) to 14 (worst) (34).
2.3 Statistical analyses
Statistical analyses were conducted using IBM SPSS (version 25.0, IBM SPSS Inc., Chicago, IL, USA), with significance level set at p < 0.05. Continuous variables were presented as mean ± standard deviation (SD) for normally distributed data, or median with interquartile range for non-normally distributed data. Categorical variables were represented as frequency and percentage. Normality of continuous variables was identified by the Shapiro–Wilk test. Among the physical function measures analyzed in this study, 11 continuous variables had at least one missing value, with an overall missing rate of 4.82%. Missing data were handled using multiple imputation. Descriptive statistics were calculated, and sex differences were tested using independent sample t-tests for normally distributed continuous variables, Mann–Whitney U tests for non-normally distributed continuous variables, and the chi-square test for categorical variables.
Bivariate Spearman correlation analyses (r) were conducted to explore the simple associations between objectively measured PA levels and physical function measurements and global cognitive scores for the total sample, using Matlab (version R2023b, MathWorks, Inc., Natick, MA, USA). Following r values were used to interpret the strength of correlation: 0–0.19 trivial; 0.20–0.39 weak; 0.40–0.59 moderate; 0.60–0.79 strong; and 0.80–1.00 very strong (53). Then, multiple linear regression models using the forward selection method were performed to examine the associations between PA metrics (independent variables) and physical test measures and global cognitive scores (dependent variables) for the total sample and for each sex group. Variables were retained in the final model if their p-values indicated statistical significance (p < 0.05). In the total sample models, sex was included as a covariate along with interaction terms to assess potential sex differences. Regardless of the significance of the interaction terms, we proceeded with separate models for males and females to explore potential sex-specific associations for all physical and cognitive outcomes. In sex-specific models, interaction terms were excluded, focusing on the main effects of PA metrics on the outcomes. Possible confounders, including age, BMI, education level, mobility aid used, frailty, and facility site, were included in all models. Multicollinearity of the independent variables was assessed by variance inflation factors (VIF), with values below five indicating no multicollinearity concerns. Beta coefficients were calculated to quantify the strength and direction of associations, representing the expected change in the dependent variable for each unit increase in the independent variables, holding other variables constant.
3 Results
3.1 Participant characteristics
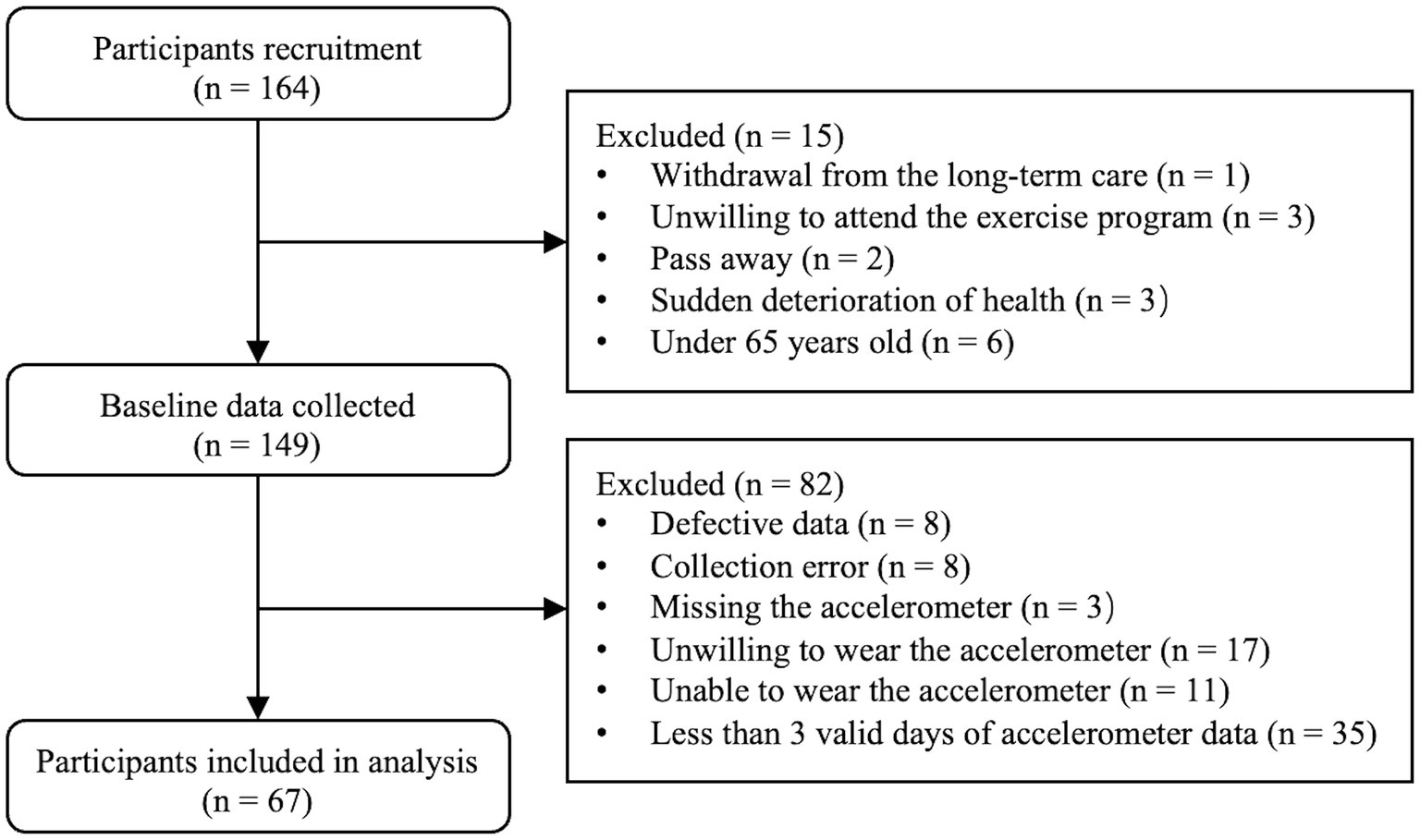
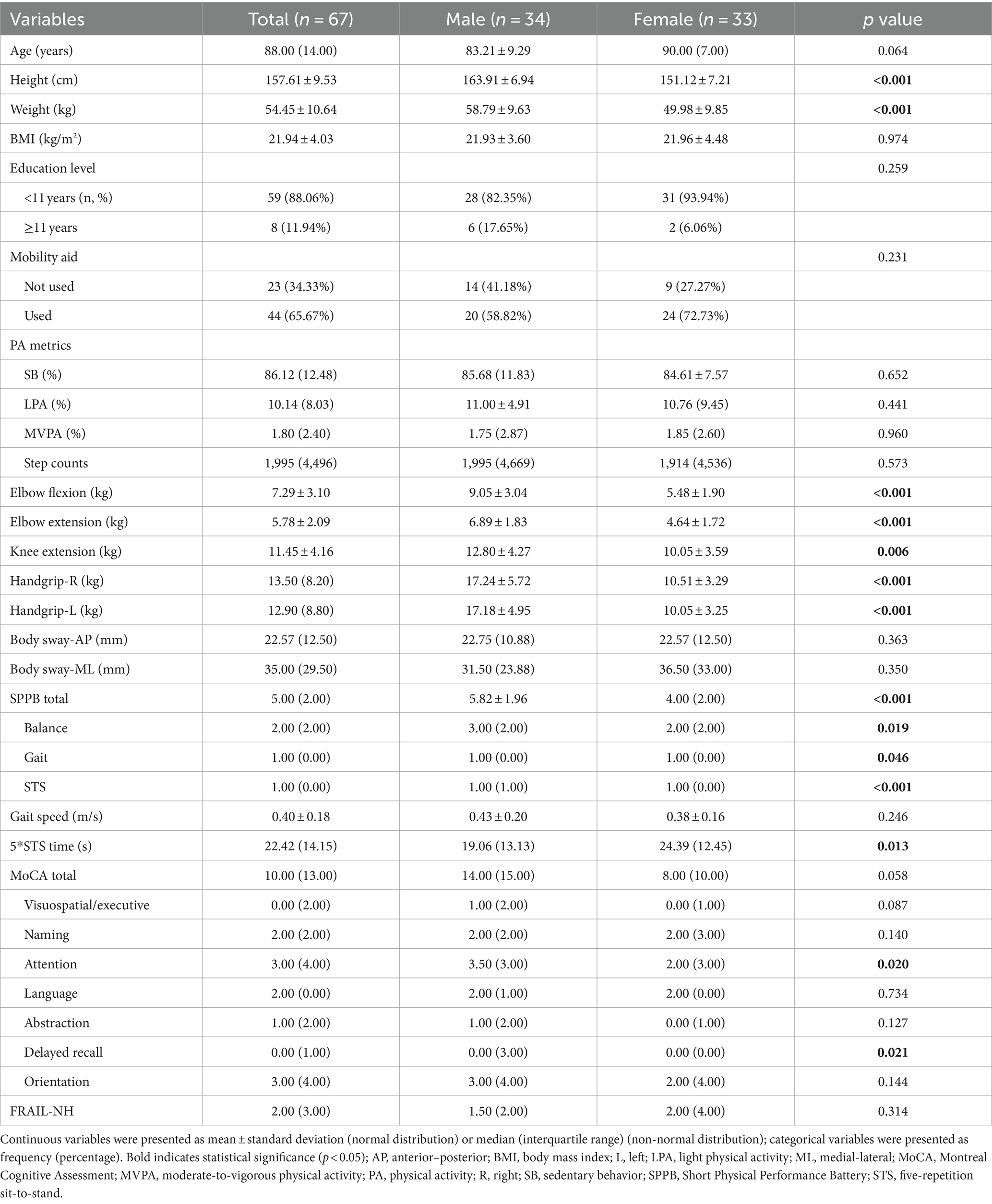
Based on the inclusion criteria, the final analyzed population comprised 67 participants (34 men and 33 women), out of an initial recruitment pool of 164 participants (Figure 1). Descriptive characteristics for the entire sample and for each sex group are depicted in Table 1. Specifically, male participants were significantly taller, heavier, and exhibited greater upper and lower limb muscle strength. They also had better SPPB (total and sub-scores), faster STS performance, and higher domain-specific cognitive scores in attention and delayed recall compared to their female counterparts (p ≤ 0.046). However, there were no significant differences between men and women in age, BMI, education level, mobility aid used, PA metrics, body sway, usual gait speed, or total MoCA score (p ≥ 0.058).
3.2 Correlations between physical activity metrics and physical function measurements and global cognitive function
Figure 2 illustrates the correlations between PA metrics and both physical and cognitive performance among older adults residing in long-term care. Significant but weak correlations were found between SB and both the SPPB gait score (r = −0.316, p = 0.009) and FRAIL-NH score (r = 0.369, p = 0.002). Weak correlations were also identified between LPA and the SPPB gait score (r = 0.390, p = 0.001) and STS score (r = 0.257, p = 0.035), usual gait speed (r = 0.303, p = 0.013), and STS time (r = −0.298, p = 0.014), while the FRAIL-NH score (r = −0.417, p = 0.009) had a significant moderate correlation with LPA. Step counts showed a significant weak correlation with both the SPPB gait score (r = 0.277, p = 0.023) and FRAIL-NH score (r = −0.338, p = 0.005). No significant correlations were identified between other PA metrics and physical function measures and total MoCA score (p ≥ 0.091).
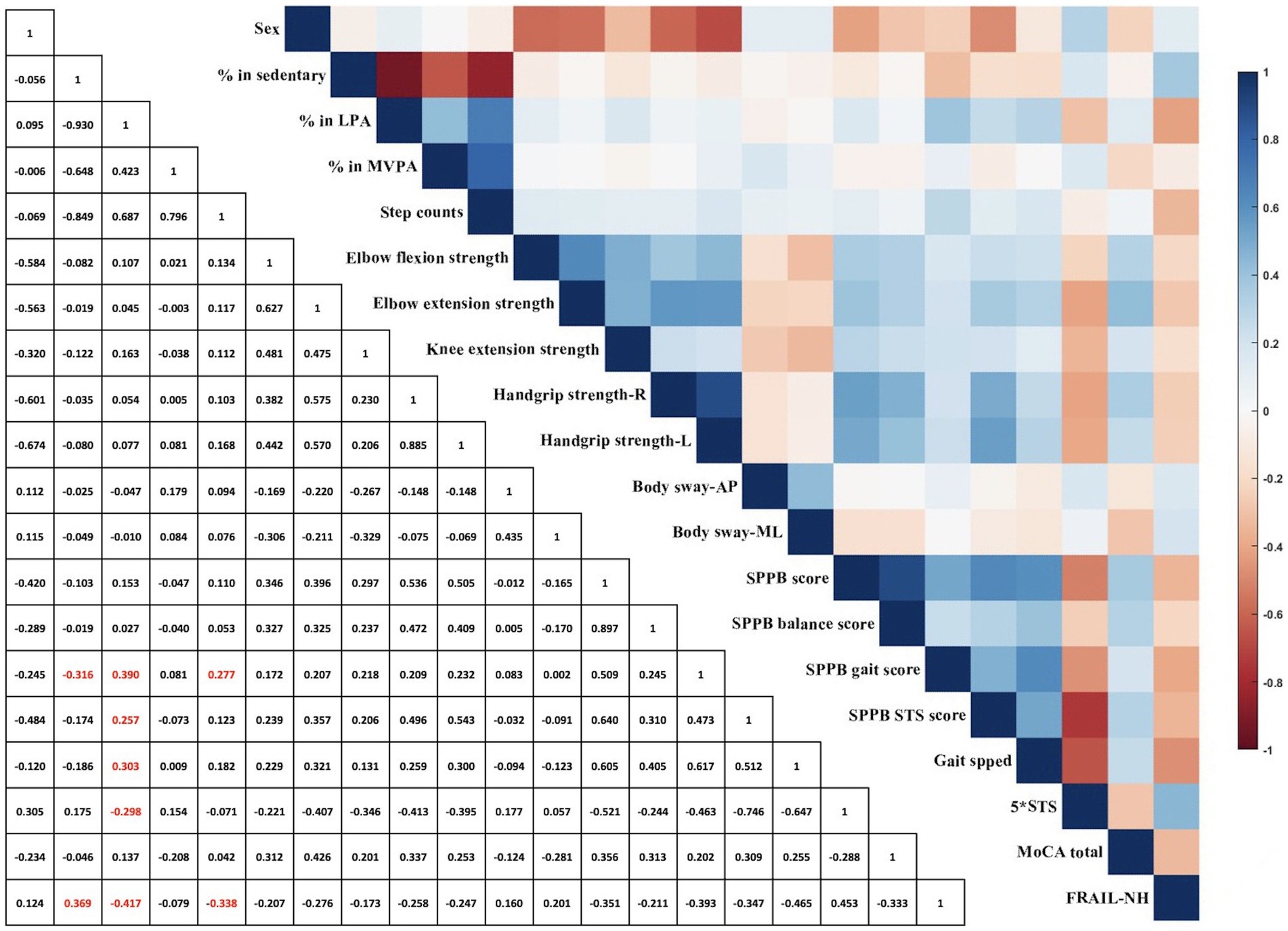
Figure 2. Spearman correlation coefficient between physical activity metrics, physical function measurements, and global cognitive function. AP, anterior–posterior; L, left; LPA, light physical activity; ML, medial-lateral; MoCA, Montreal Cognitive Assessment; MVPA, moderate-to-vigorous physical activity; R, right; SPPB, Short Physical Performance Battery; STS, five-repetition sit-to-stand; red color represents correlation is significant at the 0.05 level between physical activity metrics and physical function measurements (2-tailed).
The multiple linear regression analysis demonstrated that LPA was independently associated with physical function measurements. Specifically, in adjusted analyses, the percentage of time spent in LPA was significantly associated with the SPPB gait (β = 0.600, p < 0.001, adjusted R2 = 0.331) and STS score (β = 0.574, p < 0.001, adjusted R2 = 0.428), usual gait speed (β = 0.659, p = 0.012, adjusted R2 = 0.232), and STS time (β = −0.305, p = 0.041, adjusted R2 = 0.296). Notably, a significant interaction effect of sex was observed on the SPPB gait score (β = −0.425, p = 0.002) and STS score (β = −0.604, p < 0.001), and STS time (β = 0.319, p = 0.020). Other PA metrics were not significantly correlated with physical function measurements or global cognitive function in the total population. Full results are displayed in Table 2.
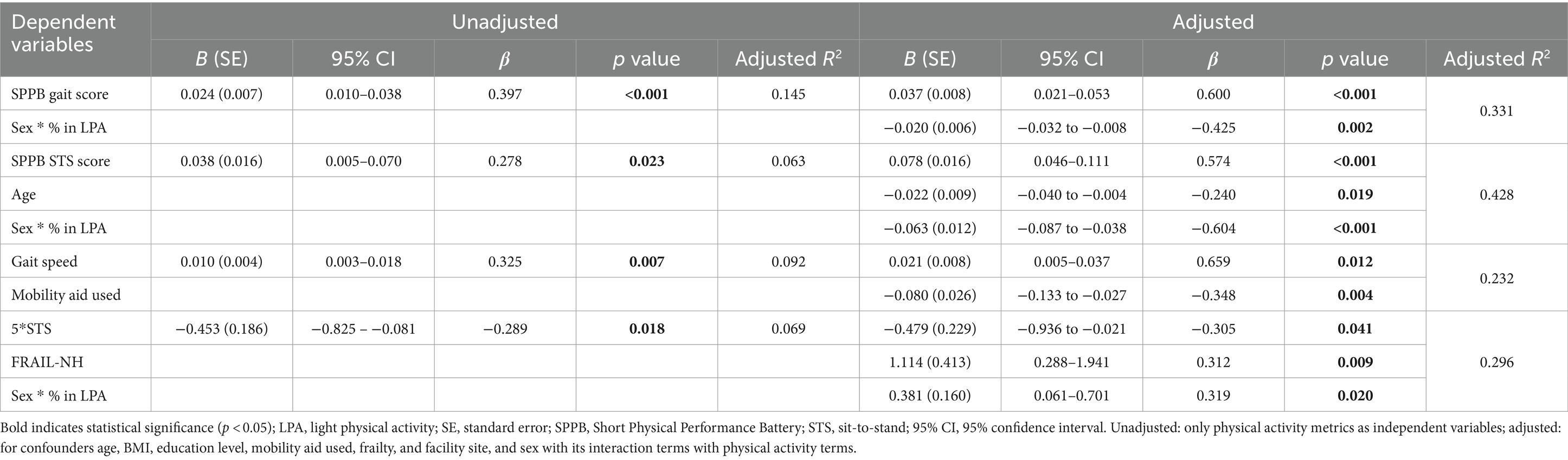
Table 2. Regression of percentage of light physical activity on physical function outcomes in total population (n = 67).
For male participants, similar associations were observed between LPA and physical function measurements. In the adjusted analysis, the percentage of LPA was independently associated with the SPPB score (total: β = 0.319, p = 0.040, adjusted R2 = 0.369; gait: β = 0.532, p < 0.001, adjusted R2 = 0.431; STS: β = 0.417, p = 0.009, adjusted R2 = 0.322), usual gait speed (β = 0.762, p = 0.017, adjusted R2 = 0.337), and STS time (β = −0.677, p < 0.001, adjusted R2 = 0.335). Full results are displayed in Table 3.
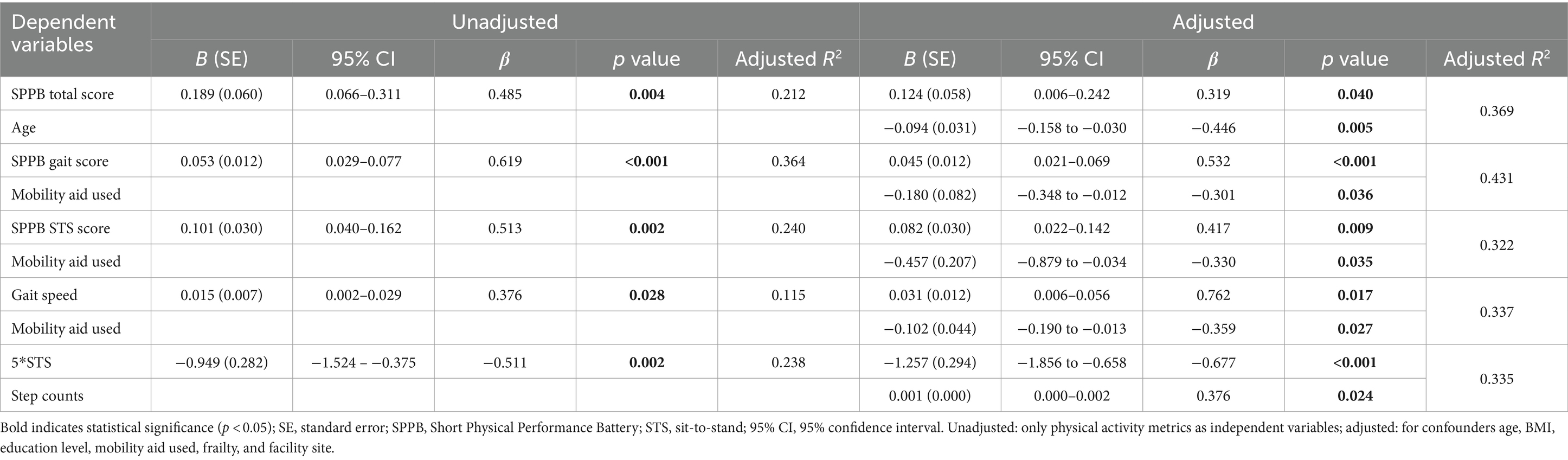
Table 3. Regression of percentage of light physical activity on physical function outcomes in male (n = 34).
For female participants, in the adjusted analysis, the percentage of time spent in LPA was only independently associated with the total MoCA score (β = 0.319, p = 0.012, adjusted R2 = 0.552). Full results are displayed in Table 4.
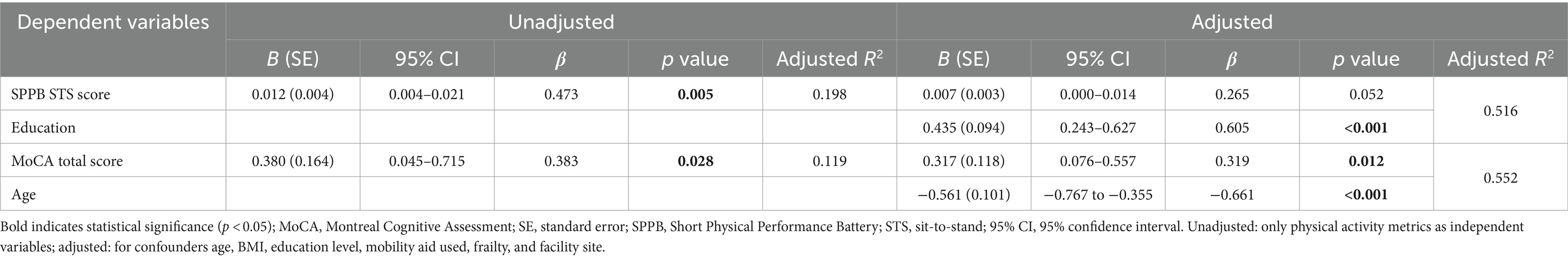
Table 4. Regression of percentage of light physical activity on physical function and cognitive function outcomes in female (n = 33).
4 Discussion
This study provides valuable insights into the associations between objectively measured PA and physical and cognitive function in older adults living in long-term care. Previous studies have highlighted the positive impact of PA on these functions in older adults, aligning with our findings (54–57). However, the nuanced relationships, particularly in the context of long-term care and sex-specific differences, have not been thoroughly explored. Our study addresses this gap, revealing significant associations between LPA and various physical function measurements, as well as global cognitive function in this population. Notably, sex differences emerged in the strength of these associations. In male participants, LPA positively correlated with several mobility functions, including SPPB performance, usual gait speed, and time spent in STS tasks, even after adjusting for confounders. In contrast, female participants showed a weaker positive correlation between LPA and physical function, with only the SPPB STS score, and total MoCA score showing positive associations that diminished after adjustment.
Surprisingly, PA metrics did not demonstrate significant associations with muscular strength, postural control, or global cognitive function in the total sample or among male participants. Previous studies have established a link between higher PA levels and improved physical functioning in older adults (58–61). However, SB is prevalent among those in long-term care, primarily due to frailty, comorbidities, and reduced independence (62, 63). Contrary to our hypothesis, SB only weakly correlated with SPPB STS scores and FRAIL-NH scores across the population. This unexpected finding may stem from a floor effect in SB measurement; individuals in this cohort are already highly sedentary, rendering variations in SB less impactful on physical function measures. Moreover, SB may not be as detrimental in this frail population, as their baseline physical function is already low, and further SB might not noticeably worsen their performance.
Interestingly, our results indicate that longer LPA durations are significantly associated with better physical performance, as evidenced by higher SPPB scores, faster gait speeds, and quicker STS times. This suggests that, within this frail population, LPA may be more beneficial and a better indicator of overall health than SB or MVPA. These findings are consistent with previous research showing that LPA is linked to improved health outcomes in older adults, and is more appealing and accessible for inactive, high-risk populations compared to MVPA (64, 65). Given that LPA often involves incidental activity (66), these results underscore the potential role of facility design in promoting LPA and enhancing physical function.
Consistent with earlier findings, male participants outperformed females in most physical performance tests and exhibited greater muscle strength (31, 49, 67). Although initial multiple regression analysis for the total population did not yield significant associations for some outcomes, such as the total MoCA scores, we still explored sex-specific models to uncover potential associations. Sex differences were also evident in the relationship between PA measures and physical function in this study, supporting prior research (68, 69). Stronger associations between LPA and physical function were demonstrated in male participants, while in females, LPA only weakly correlated with the SPPB STS score before adjusting for confounders. This discrepancy may be due to the generally poorer physical function observed in female participants compared to males in our study. Additionally, we noted a significant correlation between LPA and global cognitive function in females, suggesting that LPA might help mitigate the decline in cognitive function in this population. Previous evidence indicated that higher levels of PA are necessary to induce sufficient neuroplastic changes and cognitive benefits (70). Engaging in more intense or prolonged PA could enhance cerebral blood flow, neurogenesis, and synaptic plasticity, all critical for cognitive function (71). However, given the very low PA levels and severe mobility impairments observed in our participants (72), LPA appears to be the most feasible form of activity for this group.
Moreover, we did not observe significant correlations between PA metrics and muscular strength, which contrasts with findings in community-dwelling older adults from different countries (73, 74). Cultural differences, variations in PA levels, and disparities between living environments (e.g., living in long-term care versus independent living) may contribute to these inconsistencies (72). It is also essential to consider whether cross-sectional measurements of PA reflect current activity levels or provide insight into past behaviors. Cross-sectional data capture PA at a single time point, potentially failing to represent long-term PA habits or variations over time. Individuals who are currently active might have been sedentary in the past, or vice versa, and such variations can influence the observed relationships between PA and physical and cognitive function. While cross-sectional measurements provide a snapshot of current PA, they may not fully capture the cumulative effects over a lifetime. Longitudinal studies are necessary to elucidate how sustained PA influences physical and cognitive functions over time.
4.1 Implications of the present findings
The findings of this study underscore the importance of promoting PA to prevent declines in physical function and cognitive function among older adults living in long-term care. Our results suggest that future PA interventions should be sex-specific. For male residents, the focus could be on mobility-enhancing activities, as LPA was strongly correlated with better performance on mobility tests. Interventions could aim to increase LPA through daily walking routines, gait training, and task-based exercises. For female residents, although the correlation between LPA and physical function was weaker, the link with cognitive function was stronger. Therefore, interventions for females should emphasize cognitively stimulating activities combined with LPA, such as mind–body exercises (e.g., Tai Chi, yoga) and tasks promoting coordination and balance while engaging cognitive processes. Furthermore, longitudinal studies are needed to assess the long-term effects of PA interventions on physical and cognitive outcomes in this frail group. Understanding the role of PA in maintaining physical and cognitive function can inform tailored interventions to improve the overall well-being of long-term care residents.
4.2 Study strengths and limitations
The primary strength of this study is that it is the first of its nature in Hong Kong to examine objectively measured PA, physical function, and global cognitive function, as well as their associations in older adults residing in long-term care. Moreover, our study considered various potential confounders, including education level, mobility aid used, frailty status, and facility site, in addition to the common factors such as age and BMI. Despite the objective measurements and rigorous methodologies, including tri-axial accelerometers and comprehensive physical function assessments, we acknowledge the following limitations. First, the missing PA data due to non-cooperation, physical inadmissibility, and instrument malfunction resulted in a relatively small sample size, which may mask some potential relationships, and affect the generalizability of our findings. Our results may not be applicable to other settings or populations due to the unique characteristics of older adults in long-term care in Hong Kong. The cultural, social, and health-related aspects specific to this group could influence the outcomes. Additionally, we relied on care home staff to measure body height and weight considering comfortlessness of residents when removing shoes and personal items during assessment. This approach may have introduced biases. Lastly, the cross-sectional design restricts causal inferences regarding the relationships between PA and physical and cognitive function.
5 Conclusion
Our research addresses a gap in the literature by revealing associations between objectively measured PA and physical function and global cognitive function in older adults residing in long-term care. Our results highlight significant associations between LPA and better physical function, with sex-specific differences in these relationships. In males, LPA was strongly associated with better mobility, while in females, LPA was weakly linked to physical function but strongly correlated with global cognitive function. These findings suggest that sex-specific PA interventions are necessary to optimize the benefits of LPA in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by Joint CUHK-NTEC Clinical Research Ethics. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ZZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CH: Investigation, Methodology, Validation, Visualization, Writing – review & editing. KS: Investigation, Methodology, Validation, Visualization, Writing – review & editing. YY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Research Grants Council of Hong Kong (RGC-HK, grant number: CUHK 24618722). The funding source had no role in the design, methods, subject recruitment, data collections, analysis, and preparation of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1446286/full#supplementary-material
References
1. Liu, D. Development status, causes and countermeasures of population aging in China. Chine J Gerontol. (2022) 42:4123–6. doi: 10.3969/j.issn.1005-9202.2022.16.071
2. Sander, M, Oxlund, B, Jespersen, A, Krasnik, A, Mortensen, EL, Westendorp, RG, et al. The challenges of human population ageing. Age Ageing. (2015) 44:185–7. doi: 10.1093/ageing/afu189
3. Serrano-Urrea, R, Gomez-Rubio, V, Palacios-Cena, D, Fernandez-de-Las-Penas, C, and Garcia-Meseguer, MJ. Individual and institutional factors associated with functional disability in nursing home residents: an observational study with multilevel analysis. PLoS One. (2017) 12:e0183945. doi: 10.1371/journal.pone.0183945
4. Chu, L-W, and Chi, I. Nursing homes in China. J Am Med Dir Assoc. (2008) 9:237–43. doi: 10.1016/j.jamda.2008.01.008
5. Fried, LP, Ferrucci, L, Darer, J, Williamson, JD, and Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. (2004) 59:255–63. doi: 10.1093/gerona/59.3.m255
6. Padubidri, A, Al Snih, S, Samper-Ternent, R, Markides, KS, Ottenbacher, KJ, and Raji, MA. Falls and cognitive decline in Mexican Americans 75 years and older. Clin Interv Aging. (2014) 9:719–26. doi: 10.2147/CIA.S59448
7. Greene, BR, and Kenny, RA. Assessment of cognitive decline through quantitative analysis of the timed up and go test. IEEE Trans Biomed Eng. (2011) 59:988–95. doi: 10.1109/TBME.2011.2181844
8. Hooghiemstra, AM, Ramakers, I, Sistermans, N, Pijnenburg, YAL, Aalten, P, Hamel, REG, et al. Gait speed and grip strength reflect cognitive impairment and are modestly related to incident cognitive decline in memory clinic patients with subjective cognitive decline and mild cognitive impairment: findings from the 4C study. J Gerontol A Biol Sci Med Sci. (2017) 72:846–54. doi: 10.1093/gerona/glx0003
9. Rivera, JA, Fried, LP, Weiss, CO, and Simonsick, EM. At the tipping point: predicting severe mobility difficulty in vulnerable older women. J Am Geriatr Soc. (2008) 56:1417–23. doi: 10.1111/j.1532-5415.2008.01819.x
10. Lan, TY, Melzer, D, Tom, BD, and Guralnik, JM. Performance tests and disability: developing an objective index of mobility-related limitation in older populations. J Gerontol A Biol Sci Med Sci. (2002) 57:M294–301. doi: 10.1093/gerona/57.5.m294
11. Gill, TM, Gahbauer, EA, Han, L, and Allore, HG. Trajectories of disability in the last year of life. N Engl J Med. (2010) 362:1173–80. doi: 10.1056/NEJMoa0909087
12. Hoang, CL, Ha, GH, Pham, KTH, Tran, BX, Latkin, CA, Ho, CSH, et al. Global mapping of interventions to improve quality of life of patients with Alzheimer’s disease during 1990-2018. Dement Geriatr Cogn Disord. (2019) 48:221–33. doi: 10.1159/000505741
13. Kobayashi, LC, Wardle, J, Wolf, MS, and von Wagner, C. Cognitive function and health literacy decline in a cohort of aging English adults. J Gen Intern Med. (2015) 30:958–64. doi: 10.1007/s11606-015-3206-9
14. Roberts, R, and Knopman, DS. Classification and epidemiology of MCI. Clin Geriatr Med. (2013) 29:753–72. doi: 10.1016/j.cger.2013.07.003
15. American College of Sports Medicine Chodzko-Zajko, WJ, Proctor, DN, Fiatarone Singh, MA, Minson, CT, Nigg, CR, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. (2009) 41:1510–30. doi: 10.1249/MSS.0b013e3181a0c95c
16. Da Silva, JL, Agbangla, NF, Le Page, C, Ghernout, W, and Andrieu, B. Effects of chronic physical exercise or multicomponent exercise programs on the mental health and cognition of older adults living in a nursing home: a systematic review of studies from the past 10 years. Front Psychol. (2022) 13:888851. doi: 10.3389/fpsyg.2022.888851
17. Okamae, A, Ogawa, T, Makizako, H, Matsumoto, D, Ishigaki, T, Kamiya, M, et al. Efficacy of therapeutic exercise on activities of daily living and cognitive function among older residents in long-term care facilities: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. (2023) 104:812–23. doi: 10.1016/j.apmr.2022.11.002
18. Crocker, T, Forster, A, Young, J, Brown, L, Ozer, S, Smith, J, et al. Physical rehabilitation for older people in long-term care. Cochrane Database Syst Rev. (2013) 2:CD004294. doi: 10.1002/14651858.CD004294.pub3
19. Wild, KV, Mattek, N, Austin, D, and Kaye, JA. “Are you sure?” lapses in self-reported activities among healthy older adults reporting online. J Appl Gerontol. (2016) 35:627–41. doi: 10.1177/0733464815570667
20. Gibbs, BB, Hergenroeder, AL, Katzmarzyk, PT, Lee, IM, and Jakicic, JM. Definition, measurement, and health risks associated with sedentary behavior. Med Sci Sports Exerc. (2015) 47:1295–300. doi: 10.1249/MSS.0000000000000517
21. World Health Organization. Global recommendations on physical activity for health. Geneva, Switzerland: World Health Organization (2010).
22. Blodgett, J, Theou, O, Kirkland, S, Andreou, P, and Rockwood, K. The association between sedentary behaviour, moderate–vigorous physical activity and frailty in NHANES cohorts. Maturitas. (2015) 80:187–91. doi: 10.1016/j.maturitas.2014.11.010
23. Hajduk, AM, and Chaudhry, SI. Sedentary behavior and cardiovascular risk in older adults: a scoping review. Curr Cardiovasc Risk Rep. (2016) 10:10. doi: 10.1007/s12170-016-0485-6
24. Thibaud, M, Bloch, F, Tournoux-Facon, C, Brèque, C, Rigaud, AS, Dugué, B, et al. Impact of physical activity and sedentary behaviour on fall risks in older people: a systematic review and meta-analysis of observational studies. Eur Rev Aging Phys Act. (2012) 9:5–15. doi: 10.1007/s11556-011-0081-1
25. Song, J, Lindquist, LA, Chang, RW, Semanik, PA, Ehrlich-Jones, LS, Lee, J, et al. Sedentary behavior as a risk factor for physical frailty independent of moderate activity: results from the osteoarthritis initiative. Am J Public Health. (2015) 105:1439–45. doi: 10.2105/AJPH.2014.302540
26. Rezende, LFM, Rey-López, JP, Matsudo, VKR, and Luiz, OC. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health. (2014) 14:1–9. doi: 10.1186/1471-2458-14-333
27. Wheeler, MJ, Dempsey, PC, Grace, MS, Ellis, KA, Gardiner, PA, Green, DJ, et al. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimers Dement (N Y). (2017) 3:291–300. doi: 10.1016/j.trci.2017.04.001
28. Matthews, CE, Chen, KY, Freedson, PS, Buchowski, MS, Beech, BM, Pate, RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. (2008) 167:875–81. doi: 10.1093/aje/kwm390
29. Healy, GN, Matthews, CE, Dunstan, DW, Winkler, EA, and Owen, N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. (2011) 32:590–7. doi: 10.1093/eurheartj/ehq451
30. Leung, PM, Ejupi, A, van Schooten, KS, Aziz, O, Feldman, F, Mackey, DC, et al. Association between sedentary behaviour and physical, cognitive, and psychosocial status among older adults in assisted living. Biomed Res Int. (2017) 2017:9160504–7. doi: 10.1155/2017/9160504
31. Zeng, P, Wu, S, Han, Y, Liu, J, Zhang, Y, Zhang, E, et al. Differences in body composition and physical functions associated with sarcopenia in Chinese elderly: reference values and prevalence. Arch Gerontol Geriatr. (2015) 60:118–23. doi: 10.1016/j.archger.2014.08.010
32. Wang, CW, Yeh, JL, Li, SF, Chen, CM, Wang, HH, He, CS, et al. Functional fitness norms of community-dwelling older adults in southern rural Taiwan: a cross-sectional study. Healthcare (Basel). (2024) 12:213. doi: 10.3390/healthcare12020213
33. Chen, YM, Li, YP, and Yen, ML. Gender differences in the predictors of physical activity among assisted living residents. J Nurs Scholarsh. (2015) 47:211–8. doi: 10.1111/jnu.12132
34. Yang, Y, Zeng, Z, van Schooten, KS, Sum, RK-W, Shen, J, Ho, C-Y, et al. Effects of a multicomponent physical activity programme, mobility-fit, compared with a standard care lower limb strengthening programme, to promote safe mobility among older adults in care facilities: protocol for a cluster randomised controlled trial. BMJ Open. (2024) 14:e082403. doi: 10.1136/bmjopen-2023-082403
35. Yatsugi, H, Chen, T, Chen, S, Narazaki, K, Nagayoshi, S, Kumagai, S, et al. Normative data of objectively measured physical activity and sedentary time in community-dwelling older Japanese. Int J Environ Res Public Health. (2021) 18:18. doi: 10.3390/ijerph18073577
36. Chen, T, Narazaki, K, Haeuchi, Y, Chen, S, Honda, T, and Kumagai, S. Associations of sedentary time and breaks in sedentary time with disability in instrumental activities of daily living in community-dwelling older adults. J Phys Act Health. (2016) 13:303–9. doi: 10.1123/jpah.2015-0090
37. Honda, T, Chen, S, Kishimoto, H, Narazaki, K, and Kumagai, S. Identifying associations between sedentary time and cardio-metabolic risk factors in working adults using objective and subjective measures: a cross-sectional analysis. BMC Public Health. (2014) 14:1–9. doi: 10.1186/1471-2458-14-1307
38. Chen, T, Narazaki, K, Honda, T, Chen, S, Haeuchi, Y, Nofuji, YY, et al. Tri-axial accelerometer-determined daily physical activity and sedentary behavior of suburban community-dwelling older Japanese adults. J Sports Sci Med. (2015) 14:507–14.
39. Trost, SG, McIver, KL, and Pate, RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. (2005) 37:S531–43. doi: 10.1249/01.mss.0000185657.86065.98
40. Herrmann, SD, Barreira, TV, Kang, M, and Ainsworth, BE. How many hours are enough? Accelerometer wear time may provide bias in daily activity estimates. J Phys Act Health. (2013) 10:742–9. doi: 10.1123/jpah.10.5.742
41. Hart, TL, Swartz, AM, Cashin, SE, and Strath, SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. (2011) 8:1–7. doi: 10.1186/1479-5868-8-62
42. Strath, SJ, Greenwald, MJ, Isaacs, R, Hart, TL, Lenz, EK, Dondzila, CJ, et al. Measured and perceived environmental characteristics are related to accelerometer defined physical activity in older adults. Int J Behav Nutr Phys Act. (2012) 9:40. doi: 10.1186/1479-5868-9-40
43. Matthew, CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. (2005) 37:S512–22. doi: 10.1249/01.mss.0000185659.11982.3d
44. Haskell, WL, Lee, IM, Pate, RR, Powell, KE, Blair, SN, Franklin, BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. (2007) 39:1423–34. doi: 10.1249/mss.0b013e3180616b27
45. Vermeulen, HM, de Bock, GH, van Houwelingen, HC, van der Meer, RL, Mol, MC, Plus, BT, et al. A comparison of two portable dynamometers in the assessment of shoulder and elbow strength. Physiotherapy. (2005) 91:101–12. doi: 10.1016/j.physio.2004.08.005
46. Mentiplay, BF, Perraton, LG, Bower, KJ, Adair, B, Pua, YH, Williams, GP, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS One. (2015) 10:e0140822. doi: 10.1371/journal.pone.0140822
47. Kishimoto, H, Hata, J, Ninomiya, T, Nemeth, H, Hirakawa, Y, Yoshida, D, et al. Midlife and late-life handgrip strength and risk of cause-specific death in a general Japanese population: the Hisayama study. J Epidemiol Community Health. (2014) 68:663–8. doi: 10.1136/jech-2013-203611
48. Lord, SR, Menz, HB, and Tiedemann, A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. (2003) 83:237–52. doi: 10.1093/ptj/83.3.237
49. Guralnik, JM, Ferrucci, L, Pieper, CF, Leveille, SG, Markides, KS, Ostir, GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. (2000) 55:M221–31. doi: 10.1093/gerona/55.4.m221
50. Guralnik, JM, Simonsick, EM, Ferrucci, L, Glynn, RJ, Berkman, LF, Blazer, DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. (1994) 49:M85–94. doi: 10.1093/geronj/49.2.m85
51. Yeung, PY, Wong, LL, Chan, CC, Yung, CY, Leung, LJ, Tam, YY, et al. Montreal cognitive assessment—single cutoff achieves screening purpose. Neuropsychiatr Dis Treat. (2020) 16:2681–7. doi: 10.2147/NDT.S269243
52. Yeung, PY, Wong, LL, Chan, CC, Leung, JL, and Yung, CY. A validation study of the Hong Kong version of Montreal cognitive assessment (HK-MoCA) in Chinese older adults in Hong Kong. Hong Kong Med J. (2014) 20:504–10. doi: 10.12809/hkmj144219
53. Evans, JD. Straightforward statistics for the behavioral sciences. Pacific Grove: Brooks/Cole Publishing Co. (1996).
54. Kim, K, Hwang, G, Cho, YH, Kim, EJ, Woang, JW, Hong, CH, et al. Relationships of physical activity, depression, and sleep with cognitive function in community-dwelling older adults. Int J Environ Res Public Health. (2022) 19:19. doi: 10.3390/ijerph192315655
55. Kumar, M, Srivastava, S, and Muhammad, T. Relationship between physical activity and cognitive functioning among older Indian adults. Sci Rep. (2022) 12:2725. doi: 10.1038/s41598-022-06725-3
56. Foong, YC, Chherawala, N, Aitken, D, Scott, D, Winzenberg, T, and Jones, G. Accelerometer-determined physical activity, muscle mass, and leg strength in community-dwelling older adults. J Cachexia Sarcopenia Muscle. (2016) 7:275–83. doi: 10.1002/jcsm.12065
57. Hillsdon, MM, Brunner, EJ, Guralnik, JM, and Marmot, MG. Prospective study of physical activity and physical function in early old age. Am J Prev Med. (2005) 28:245–50. doi: 10.1016/j.amepre.2004.12.008
58. Cooper, R, Mishra, GD, and Kuh, D. Physical activity across adulthood and physical performance in midlife: findings from a British birth cohort. Am J Prev Med. (2011) 41:376–84. doi: 10.1016/j.amepre.2011.06.035
59. Balboa-Castillo, T, Leon-Munoz, LM, Graciani, A, Rodriguez-Artalejo, F, and Guallar-Castillon, P. Longitudinal association of physical activity and sedentary behavior during leisure time with health-related quality of life in community-dwelling older adults. Health Qual Life Outcomes. (2011) 9:47. doi: 10.1186/1477-7525-9-47
60. LaCroix, AZ, Guralnik, JM, Berkman, LF, Wallace, RB, and Satterfield, S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. (1993) 137:858–69. doi: 10.1093/oxfordjournals.aje.a116747
61. van Oostrom, SH, Smit, HA, Wendel-Vos, GC, Visser, M, Verschuren, WM, and Picavet, HS. Adopting an active lifestyle during adulthood and health-related quality of life: the Doetinchem cohort study. Am J Public Health. (2012) 102:e62–8. doi: 10.2105/AJPH.2012.301008
62. Hill, AD, Stukel, TA, Fu, L, Scales, DC, Laupacis, A, Rubenfeld, GD, et al. Trends in site of death and health care utilization at the end of life: a population-based cohort study. CMAJ Open. (2019) 7:E306–15. doi: 10.9778/cmajo.20180097
63. Tanuseputro, P, Chalifoux, M, Bennett, C, Gruneir, A, Bronskill, SE, Walker, P, et al. Hospitalization and mortality rates in long-term care facilities: does for-profit status matter? J Am Med Dir Assoc. (2015) 16:874–83. doi: 10.1016/j.jamda.2015.06.004
64. Fuezeki, E, Engeroff, T, and Banzer, W. Health benefits of light-intensity physical activity: a systematic review of accelerometer data of the national health and nutrition examination survey (NHANES). Sports Med. (2017) 47:1769–93. doi: 10.1007/s40279-017-0724-0
65. Perri, MG, Anton, SD, Durning, PE, Ketterson, TU, Sydeman, SJ, Berlant, NE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. (2002) 21:452–8. doi: 10.1037/0278-6133.21.5.452
66. Telford, DM, Meiring, RM, and Gusso, S. Moving beyond moderate-to-vigorous physical activity: the role of light physical activity during adolescence. Front Sports Act Living. (2023) 5:1282482. doi: 10.3389/fspor.2023.1282482
67. Goodpaster, BH, Park, SW, Harris, TB, Kritchevsky, SB, Nevitt, M, Schwartz, AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. (2006) 61:1059–64. doi: 10.1093/gerona/61.10.1059
68. Hsueh, MC, Rutherford, R, Chou, CC, Park, JH, Park, HT, and Liao, Y. Objectively assessed physical activity patterns and physical function in community-dwelling older adults: a cross-sectional study in Taiwan. BMJ Open. (2020) 10:e034645. doi: 10.1136/bmjopen-2019-034645
69. Domingos, C, Correia Santos, N, and Pêgo, JM. Association between self-reported and accelerometer-based estimates of physical activity in Portuguese older adults. Sensors. (2021) 21:2258. doi: 10.3390/s21072258
70. Hötting, K, and Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. (2013) 37:2243–57. doi: 10.1016/j.neubiorev.2013.04.005
71. Hartman, SJ, Nelson, SH, Myers, E, Natarajan, L, Sears, DD, Palmer, BW, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. (2018) 124:192–202. doi: 10.1002/cncr.30987
72. Daimiel, L, Martinez-Gonzalez, MA, Corella, D, Salas-Salvado, J, Schroder, H, Vioque, J, et al. Physical fitness and physical activity association with cognitive function and quality of life: baseline cross-sectional analysis of the PREDIMED-plus trial. Sci Rep. (2020) 10:3472. doi: 10.1038/s41598-020-59458-6
73. Park, H, Park, S, Shephard, RJ, and Aoyagi, Y. Yearlong physical activity and sarcopenia in older adults: the Nakanojo study. Eur J Appl Physiol. (2010) 109:953–61. doi: 10.1007/s00421-010-1424-8
Keywords: older adult, long-term care, sex, physical activity, cognition
Citation: Zeng Z, Hsu CL, van Schooten KS and Yang Y (2024) Sex differences in the associations of accelerometer-determined physical activity with physical and cognitive function in older adults living in long-term care. Front. Public Health. 12:1446286. doi: 10.3389/fpubh.2024.1446286
Edited by:
Sergio A. Useche, University of Valencia, SpainReviewed by:
Claudia Savia Guerrera, ItalyJuris Porozovs, University of Latvia, Latvia
Elizabeth Breeze, University of London, United Kingdom
Copyright © 2024 Zeng, Hsu, van Schooten and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yijian Yang, eXlhbmdAY3Voay5lZHUuaGs=
 Ziwei Zeng
Ziwei Zeng Chun Liang Hsu
Chun Liang Hsu Kimberley Stefanie van Schooten
Kimberley Stefanie van Schooten Yijian Yang
Yijian Yang