- 1Department of Environmental Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 2Department of Environmental Health, College of Medicine and Health Sciences, Debre Markos University, Debre Markos, Ethiopia
- 3Department of Nursing and Midwifery, Dessie Health Science College, Dessie, Ethiopia
Introduction: Air pollution is a significant global public health concern. However, there is a lack of updated and comprehensive evidence regarding the association between exposure to ambient air pollution and adverse birth outcomes (preterm birth, low birth weight, and stillbirth). Furthermore, the existing evidence is highly inconsistent. Therefore, this study aims to estimate the overall association between ambient air pollution and adverse birth outcomes.
Methods: In this study, initially a total of 79,356 articles were identified. Finally, a total of 49 articles were included. We conducted compressive literature searches using various databases, including PubMed, Scientific Direct, HINARI, and Google Scholar. Data extraction was performed using Microsoft Excel, and the data were exported to STATA 17 software for analysis. We used the Joanna Briggs Institute’s quality appraisal tool to ensure the quality of the included studies. A random effects model was employed to estimate the pooled prevalence. Publication bias was assessed using funnel plots and Egger’s regression test.
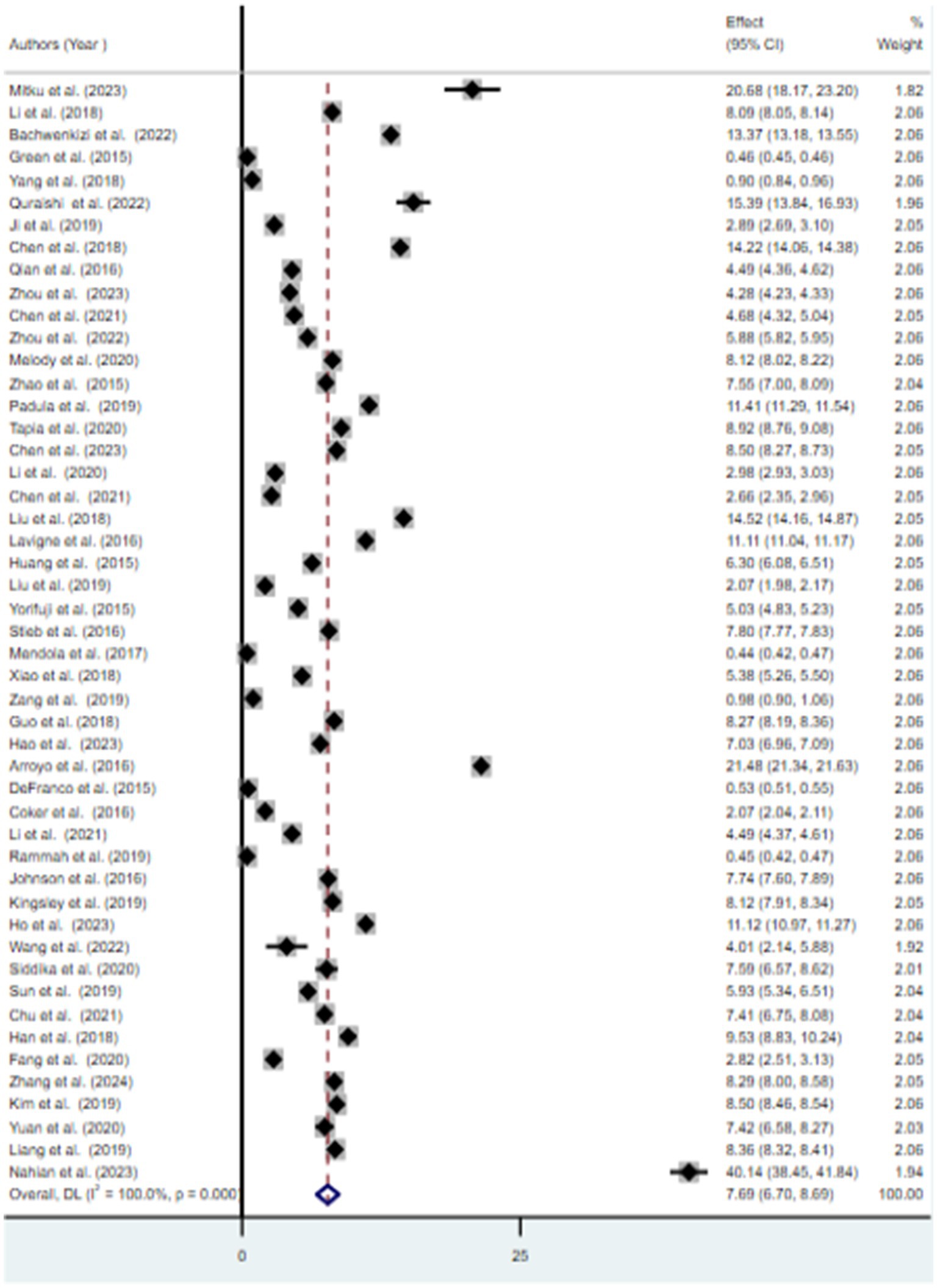
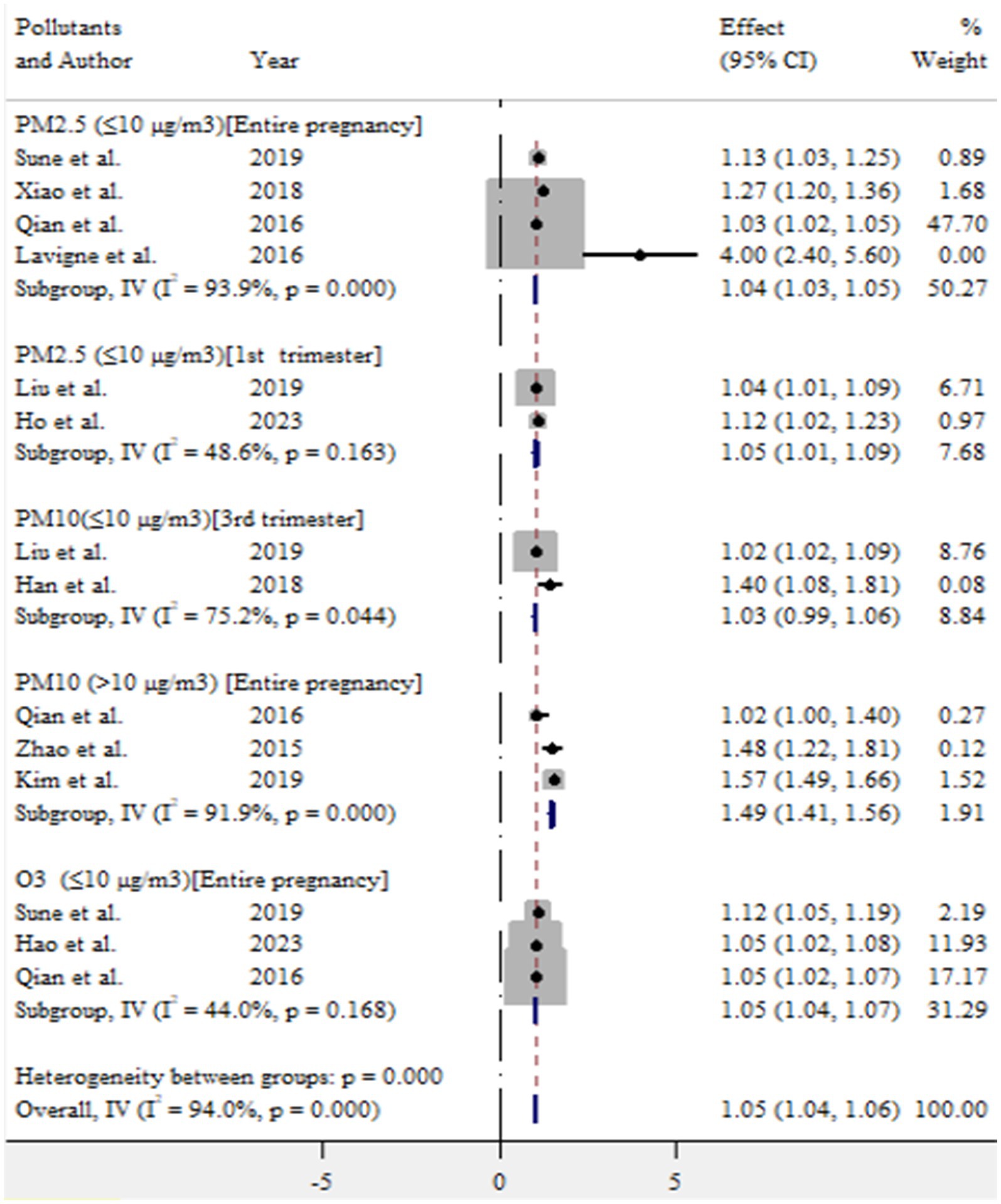
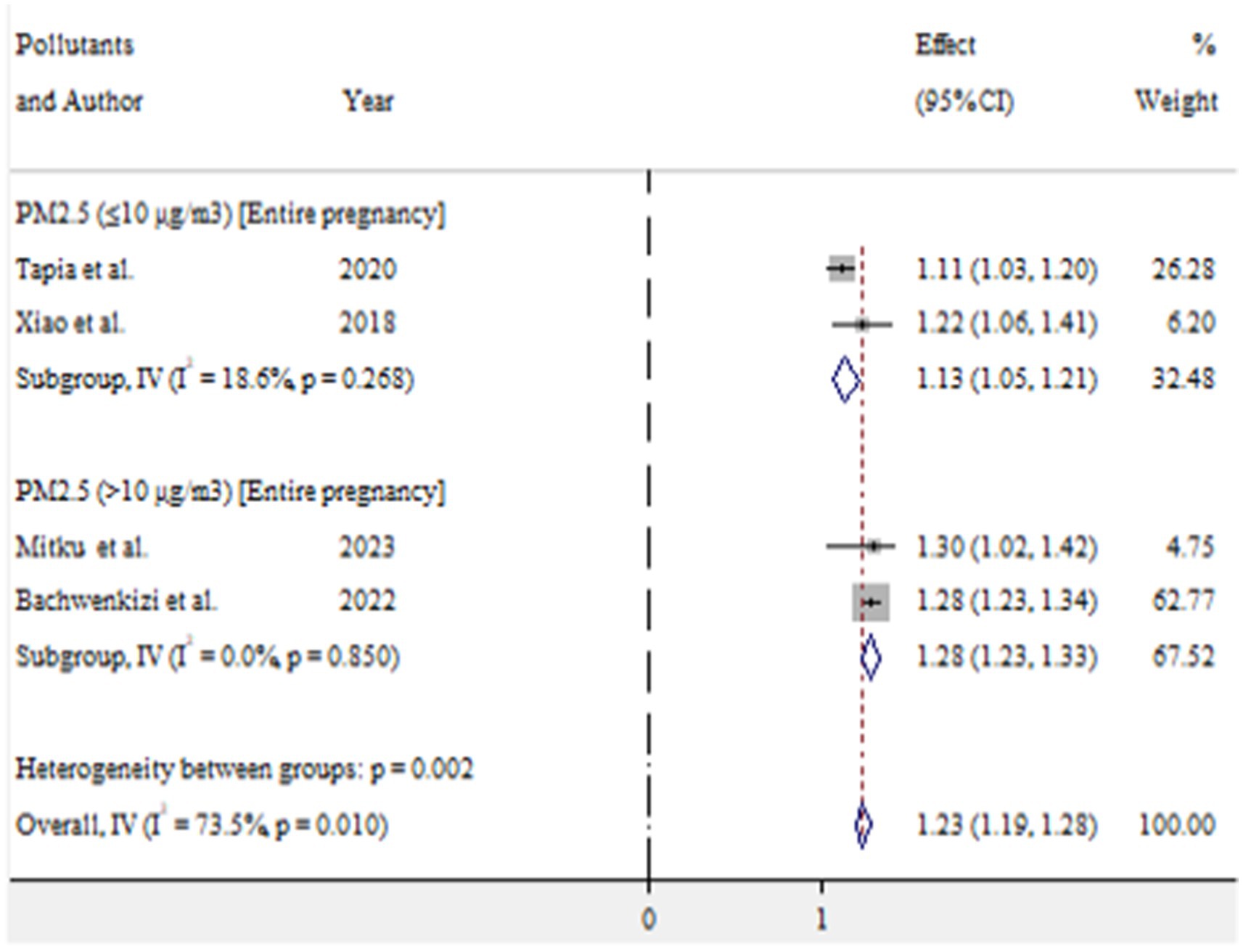
Results: In this study, the pooled prevalence of at least one adverse birth outcome was 7.69% (95% CI: 6.70–8.69), with high heterogeneity (I2 = 100%, p-value < 0.001). In this meta-analysis, high pooled prevalence was found in preterm birth (6.36%), followed by low birth weights (5.07%) and stillbirth (0.61%). Exposure to PM2.5 (≤10 μg/m3) throughout the entire pregnancy, PM2.5 (≤10 μg/m3) in the first trimester, PM10 (>10 μg/m3) during the entire pregnancy, and O3 (≤10 μg/m3) during the entire pregnancy increased the risk of preterm birth by 4% (OR = 1.04, 95% CI: 1.03–1.05), 5% (OR = 1.05, 95% CI: 1.01–1.09), 49% (OR = 1.49, 95% CI: 1.41–1.56), and 5% (OR = 1.05, 95% CI: 1.04–1.07), respectively. For low birth weight, exposure to PM2.5 (≤10 μg/m3) and PM2.5 (>10 μg/m3) throughout the entire pregnancy was associated with an increased risk of 13% (OR = 1.13, 95% CI: 1.05–1.21) and 28% (OR = 1.28, 95% CI: 1.23–1.33), respectively.
Conclusion: This study highlighted a significant association between ambient air pollution and adverse birth outcomes. Therefore, it is crucial to implement a compressive public health intervention.
Systematic review registration: The review protocol was registered with the record ID of CRD42024578630.
1 Introduction
Air pollution is a major global public health concern, with growing evidence linking exposure during pregnancy to a range of adverse birth outcomes (1, 2). Exposure to ambient air pollutants such as particulate matter (PM), ozone (O3), nitrogen dioxides (NO2), and sulfur dioxide (SO2) has been linked to a range of adverse birth outcomes, including preterm birth, stillbirth, low birth weight, and congenital anomalies (1, 3, 4). There is no evidence for the biological mechanisms underlying these associations, but they are thought to involve disruption of placental function, inflammation, and oxidative stress (5, 6). The World Health Organization (WHO) estimates that ambient air pollution caused 4.2 million premature deaths globally in 2019, highlighting significant implications for maternal and child health (7).
The effects of ambient air pollution on pregnancy can be attributed to both direct biological mechanisms and indirect socio-environmental factors. For instance, exposure to high levels of PM during critical periods of fetal development can disrupt placental function and fetal growth (8). Socioeconomic disparities often exacerbate the risks associated with air pollution, as marginalized communities frequently reside in areas with higher pollution levels and limited access to healthcare resources (9). Furthermore, the adverse effects of ambient air pollution on fetal development may have long-term consequences, as preterm birth and low birth weight are risk factors for various health problems later in life, including neurological disorders, cardiovascular disease, and diabetes (10).
A review with meta-analysis has revealed that prenatal exposure to ambient PM2.5 is associated with an increased risk of stillbirth (11, 12) and decreased birth weights (13). According to Lamichhane et al. (3) and Sun et al. (13), a 10 μg/m3 increase in PM2.5 exposure during pregnancy was associated with a 15 and 13% higher risk of preterm birth, respectively. Zhu et al. (4) reported that a 10 μg/m3 increase in PM2.5 exposure during pregnancy was associated with a 5% increased risk of low birth weight and a 10% increased risk of preterm birth. Similarly, Stieb et al. (1) concluded that there is consistent evidence linking air pollution exposure to preterm birth and low birth weight. Maternal exposure to PM2.5 (per 10 μg/m3 increased) was associated with a 15% increased risk of stillbirth in the entire pregnancy and a 9% increased risk of stillbirth in the third trimester (12). Exposure to major air pollutants throughout pregnancy may increase the risk of low birth weight (14). Several other studies have also indicated possible associations between ambient air pollution and adverse birth outcomes (15–20).
Although several reviews and meta-analyses have explored the association between specific ambient air pollutants and adverse outcomes, their findings have been inconsistent and lack comprehensiveness. Additionally, the conflicting results from previous primary studies underscore the need for a more thorough and integrated analysis of the available evidence. The purpose of this research is to thoroughly estimate the pooled association between ambient air pollutants and adverse birth outcomes, including preterm birth, low birth weight, and stillbirth, while also identifying predictive factors. Up-to-date and comprehensive evidence is crucial for informed decision-making, the development of effective strategies, and support for policymakers and other stakeholders.
2 Methods
This study followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (21) (Figure 1). The review protocol for this study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the record ID of CRD42024578630.
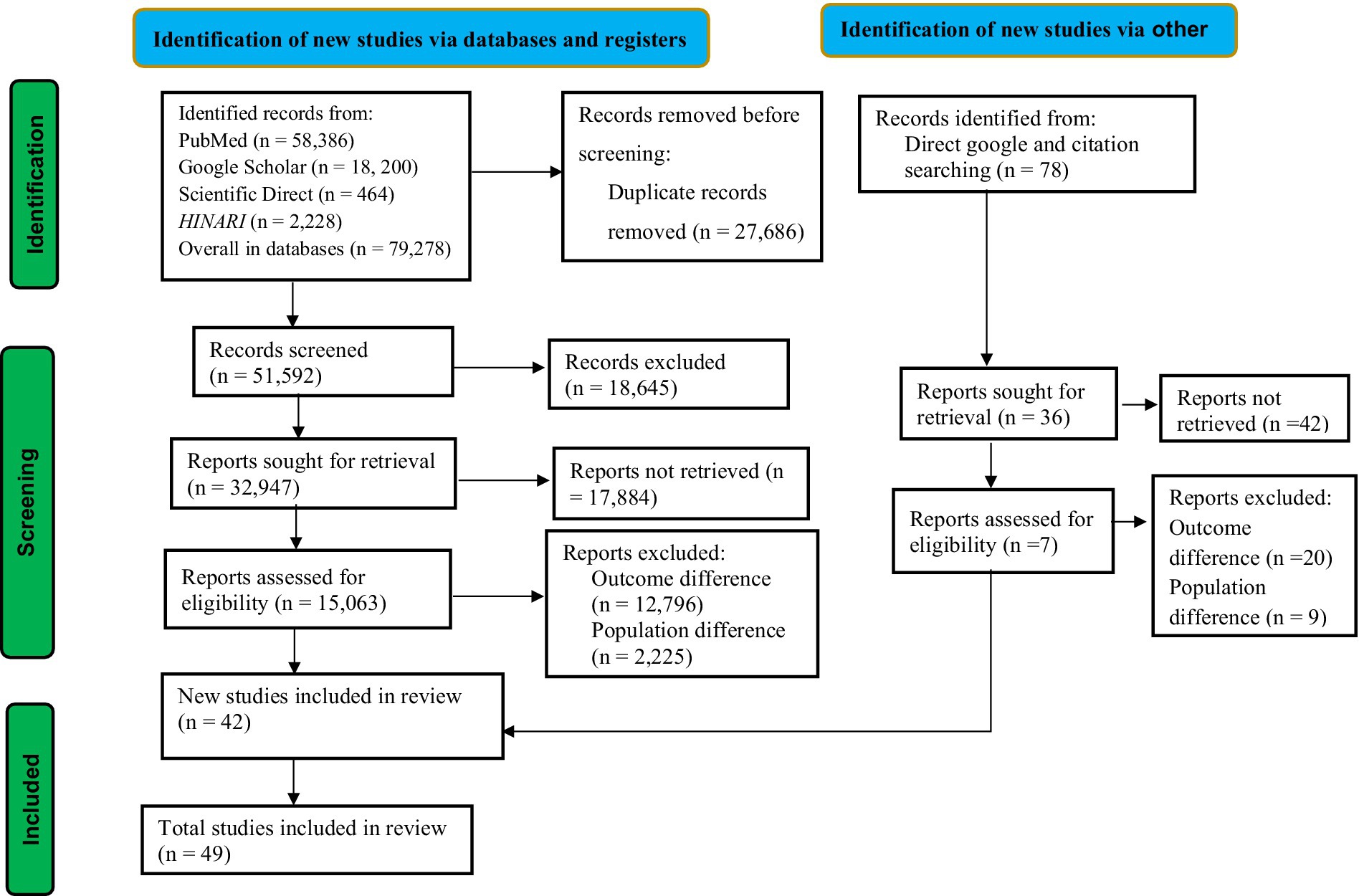
Figure 1. PRISMA flow diagram of association between ambient air pollution and adverse birth outcomes, 2024.
2.1 Eligibility criteria
2.1.1 Inclusion criteria
The eligible for this review must meet the following PECOS (Participants/Populations, Exposures, Comparators, Outcomes and Study designs) criteria.
• Participants or populations: the participants are pregnant women at any stage of pregnancy up to birth.
• Exposures: prenatal exposure to ambient air pollution.
• Comparators: pregnant women with lower exposure levels, with or without adverse birth outcomes, as compared to those exposed to higher exposure with adverse birth outcomes.
• Outcomes: the adverse birth outcomes of interest include preterm birth, low birth weight, and stillbirth (reported prevalence [%] and measure of association in adjusted odds ratio [AOR]).
• Study design: all observational studies (cross-sectional, cohort, and case control).
Moreover, articles written in English, both published and unpublished, and studies reported from January 1, 2015 to August 30, 2024 were also the inclusion criteria.
2.1.2 Exclusion criteria
Studies that investigated other pregnancy related outcomes besides the specified adverse birth outcomes (preterm birth, low birth weight, and stillbirth). Descriptive epidemiological studies (e.g., descriptive cross-sectional, case reports, and case series), studies without a full report after three personal email contacts with the primary and corresponding authors, conference abstracts, letters to the editors, qualitative studies, systematic reviews, short communications, and commentaries were not considered.
2.1.3 Operational definitions
Low birth weight: “a birth weight of <2,500 g” (22).
Stillbirth: “A baby who dies after 28 weeks of pregnancy, but before or during birth” (23).
Preterm birth: “babies born alive before 37 weeks of pregnancy” (24).
In this study, we categorized the reported concentrations of pollutants into two main groups: ≤10 μg/m3 and >10 μg/m3. This categorization was necessary due to the variability in concentration levels reported across different studies.
2.2 Information sources
Both published and grey literature were sources of information for this study. A systematic literature search was undertaken using the following databases: PubMed, Scientific Direct, Google Scholar, and HINAR. The search was conducted for studies published from January 1, 2015, to August 30, 2024. In addition to the electronic database search, further articles were obtained by searching for grey literature through direct Google searches and by reviewing the references of the eligible studies.
2.3 Search strategies
Comprehensive search terms were used to identify relevant studies. These include MeSH terms and key words such as ambient air pollution, outdoor air pollution, adverse birth outcomes, preterm birth, low birth weights, and stillbirth. These search terms were used within PubMed as a template database to finalize an advanced search strategy utilizing the Boolean operators “AND” and “OR.” The search strategy was modified as appropriate for other databases and other sources.
2.4 Study screening and selection
All stages of reviewing articles were conducted independently by the two researchers (BD and AKG), with conflict managed by evidence-based discussion with the involvement of the third researcher (GB). From the search, the titles of all identified citations with abstracts were uploaded into Zotero reference manager, and duplicates were removed. Then it was followed by screening the titles and abstracts according to the eligibility criteria. The potential full text of eligible studies was retrieved. All studies that do not meet the inclusion criteria were excluded with reasons and presented in the PRISMA flow chart (21).
2.5 Quality (risk of bias) assessment of the selected studies
The quality of selected eligible studies was evaluated using the Joana Briggs Institute (JBI) critical appraisal checklist for cohort and case–control studies (25). The quality assessment was conducted independently by two reviewers (BD and ABK). In case of any discrepancies encountered during the quality assessment, they were managed through evidence-based discussions with the involvement of a third researcher (GB). Only studies that scored more than 50% on the quality assessment were considered for inclusion in this review (25, 26), as depicted in Table 1.

Table 1. Summary of the included studies on the association between ambient air pollution and adverse birth outcomes, 2024.
2.6 Data extraction and management
Data extraction was conducted by two authors (BD and AKG) using a data extraction tool. Any disagreements between the two data extractors were resolved through consensus or with the involvement of a third author (GB). The following information was extracted from the selected studies: author information, study setting, study country, type of study, pollutants, outcomes, statistical methods, study periods, sample size, cases, prevalence, and quality scores. The extracted data was organized in a table format (Table 1).
2.7 Statistical methods and data analysis
The extracted data from Microsoft Excel was transported to STATA version 17 for analysis. The Index of heterogeneity (I2 statistics) was used to assess variations among the included studies, where values of 25–50%, 50–75%, and >75% indicated low, moderate, and high heterogeneity, respectively (27). The metaprop command in STATA was used to estimate the pooled prevalence. Subgroup analysis were conducted to explore potential variations in the pooled prevalence based on study countries and the nature of the outcomes. Sensitivity analysis was performed to assess the effect of each individual study on the estimated pooled results. To evaluate publication bias, a funnel plot test and Egger’s regression test were used. A meta-regression was employed to identify potential sources of heterogeneity. Finally, the findings of this study are presented using tables, figures, forest plots, and descriptive texts.
3 Results
3.1 Overview of search process
We identified a total of 79,356 studies using a database and through direct Google and citation searching. After duplicate records were removed, 51,592 records were screened for this review. According to the records, only 32,947 studies were sought for retrieval. After being identified for retrieval, 15,063 studies were evaluated for eligibility. Following eligibility, a total of 15,021 studies were excluded due to differences in outcome interest and population differences. Ultimately, a total of 42 studies were included in this review from database sources. In addition to the database sources, seven studies were included in this review from direct Google and citation searching. Finally, a total of 49 articles were included in this study, as presented in the PRISMA flowchart (Figure 1).
3.2 Characteristics of the eligible studies
The majority of the included studies were conducted using birth cohort studies. This meta-analysis included a total of 21,019,317 study participants. The majority of the studies were conducted in China (n = 26) and the United States (n = 10). This meta-analysis examined the association between ambient air pollutants (PM2.5, PM10, SO2, NO2, O3, CO) and adverse birth outcomes (preterm birth, low birth weight, and stillbirths). In this study, the main exposure assessment methods were the daily mean concentration of air pollutants, monitoring stations, land use regression model, and real-time measurement. Among the included studies, Bangladesh had the highest at least one birth outcome (40%) (28), while the United States had the lowest rate (0.44%) (29). The quality score of the included studies was between the ranges of 62.5 and 87.5% (Table 1).
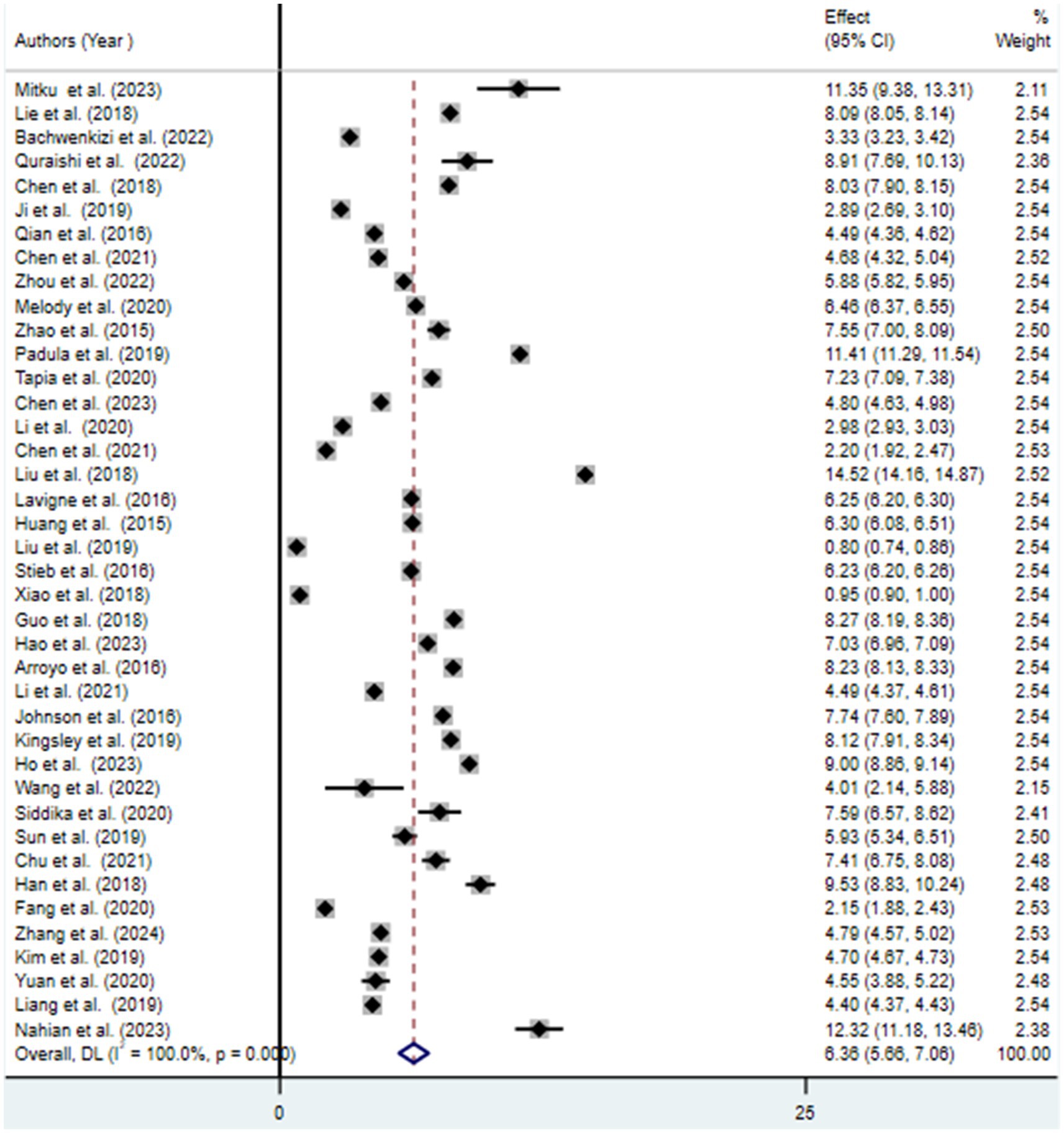
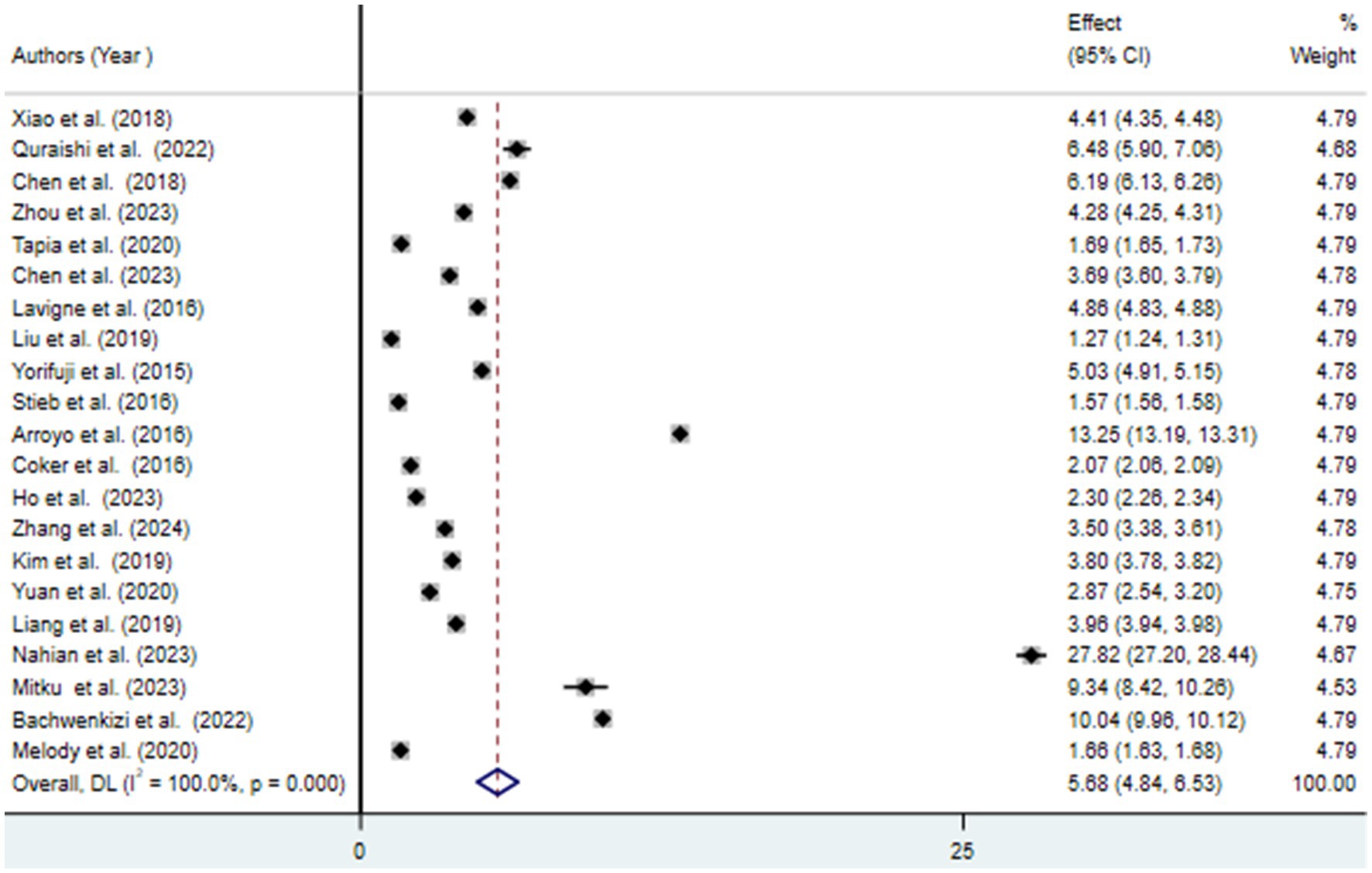
3.3 Meta-analysis
The findings from the random effects model indicated that the pooled prevalence of at least one adverse birth outcome was 7.69% (95% CI: 6.70–8.69), with high heterogeneity (I2 = 100%, p-value < 0.001) (Figure 2). In this meta-analysis, high pooled prevalence was found in preterm birth (6.36%) (Figure 3), followed by low birth weights (5.07%) (Figure 4) and stillbirth (0.61%) (Figure 5). Subgroup analysis based on study country: the highest pooled prevalence of at least one adverse birth outcome was observed in Bangladesh at 40.14% (95% CI: 38.45–41.84) and in Spain at 21.5% (95% CI: 21.34–21.63). In contrast, the lowest pooled prevalence of at least one adverse birth outcome was observed in Japan at 5.03% (95% CI: 4.83–5.24) and the United States at 5.12% (95% CI: 4.22–6.02). In addition, subgroup analysis based on the nature of outcomes found that preterm birth and low birth weight were at 11.1% (95% CI: 9.85–12.35), preterm birth at 6.96% (95% CI: 5.86–8.66), low birth weight at 3.79% (95% CI: 2.0–5.59), and stillbirth at 0.615% (95% CI: 0.53–0.699) (Table 2).
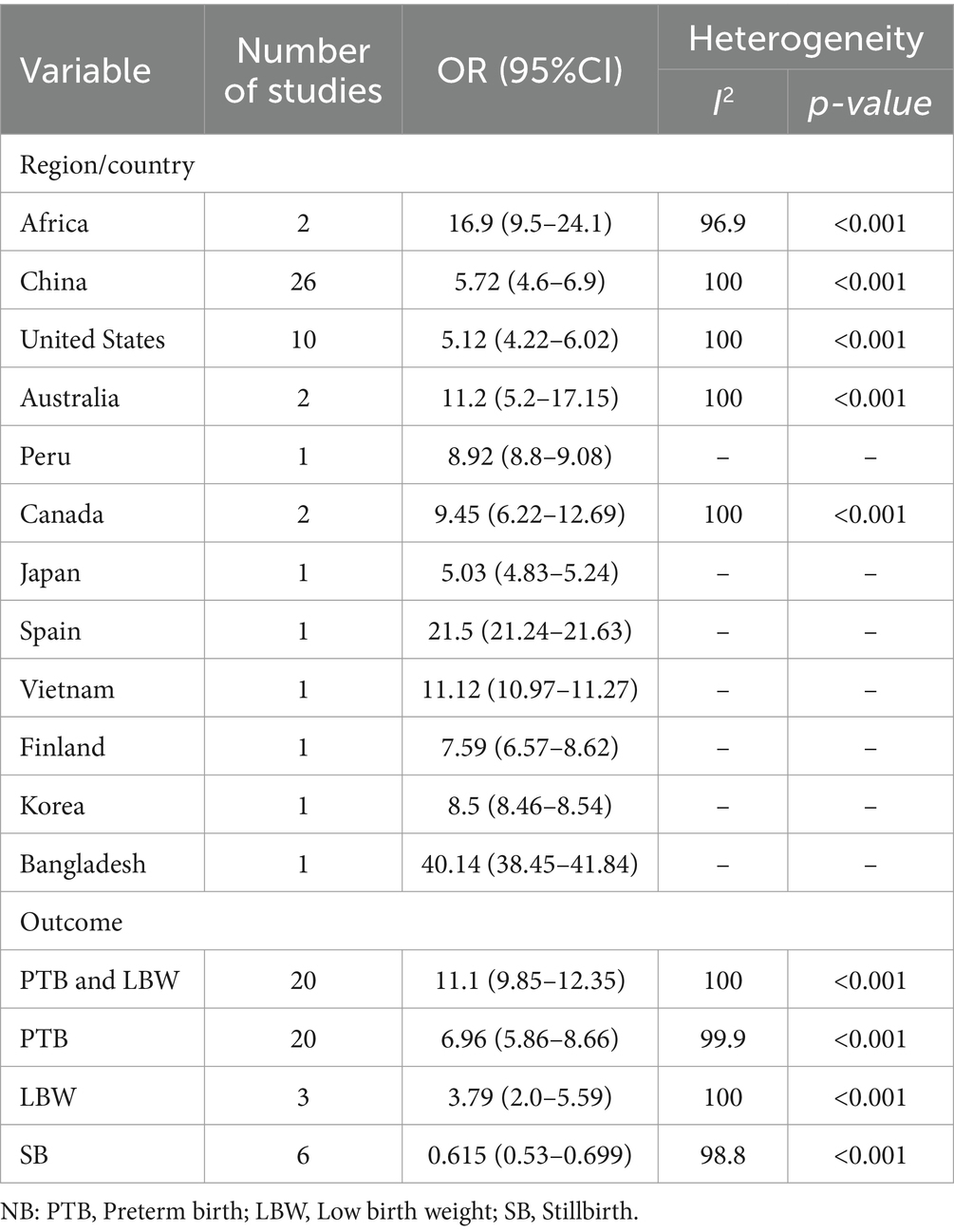
Table 2. Subgroup analysis of the association between ambient air pollution and at least one adverse birth outcome, 2024.
In this study, a meta-regression analysis was conducted using the study country and nature of outcomes as factors to identify the source of heterogeneity. The finding revealed that the study country was not a statistically significant source of heterogeneity (p = 0.196), but the outcome nature was found to be a statistically significant source of heterogeneity (p < 0.001).
A sensitivity analysis was also performed to evaluate a single study effect on the overall results. The analysis showed for the overall prevalence of at least one adverse birth outcome a slightly broader confidence interval of 7.69% (95% CI: 6.05–8.98) compared to the original pooled prevalence of 7.69% (95% CI: 6.70–8.69), but it does not suggest strong evidence for single study effects. Similarly, sensitivity analysis was also conducted for preterm birth, low birth weight, and stillbirth to examine the effect of a single study on the overall prevalence. The findings suggested that there is no evidence for a single study effect on the overall pooled prevalence (Supplementary material 1).
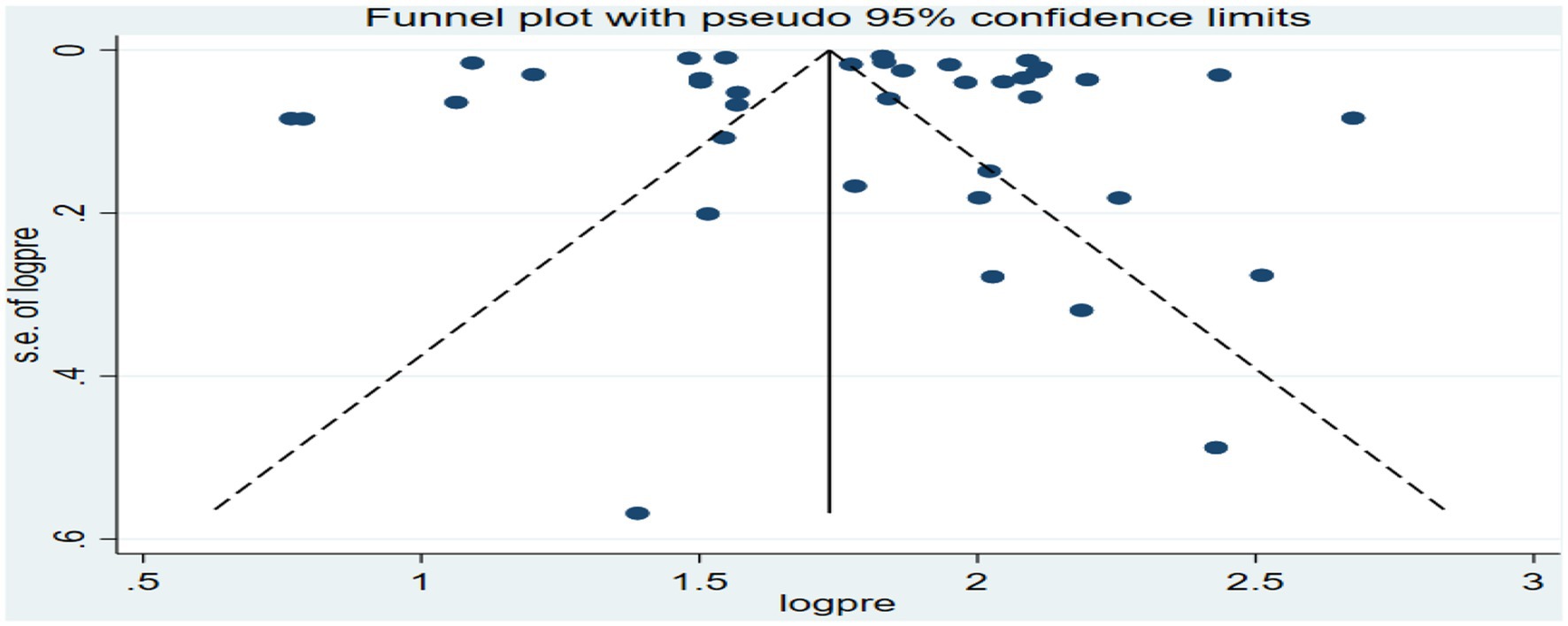
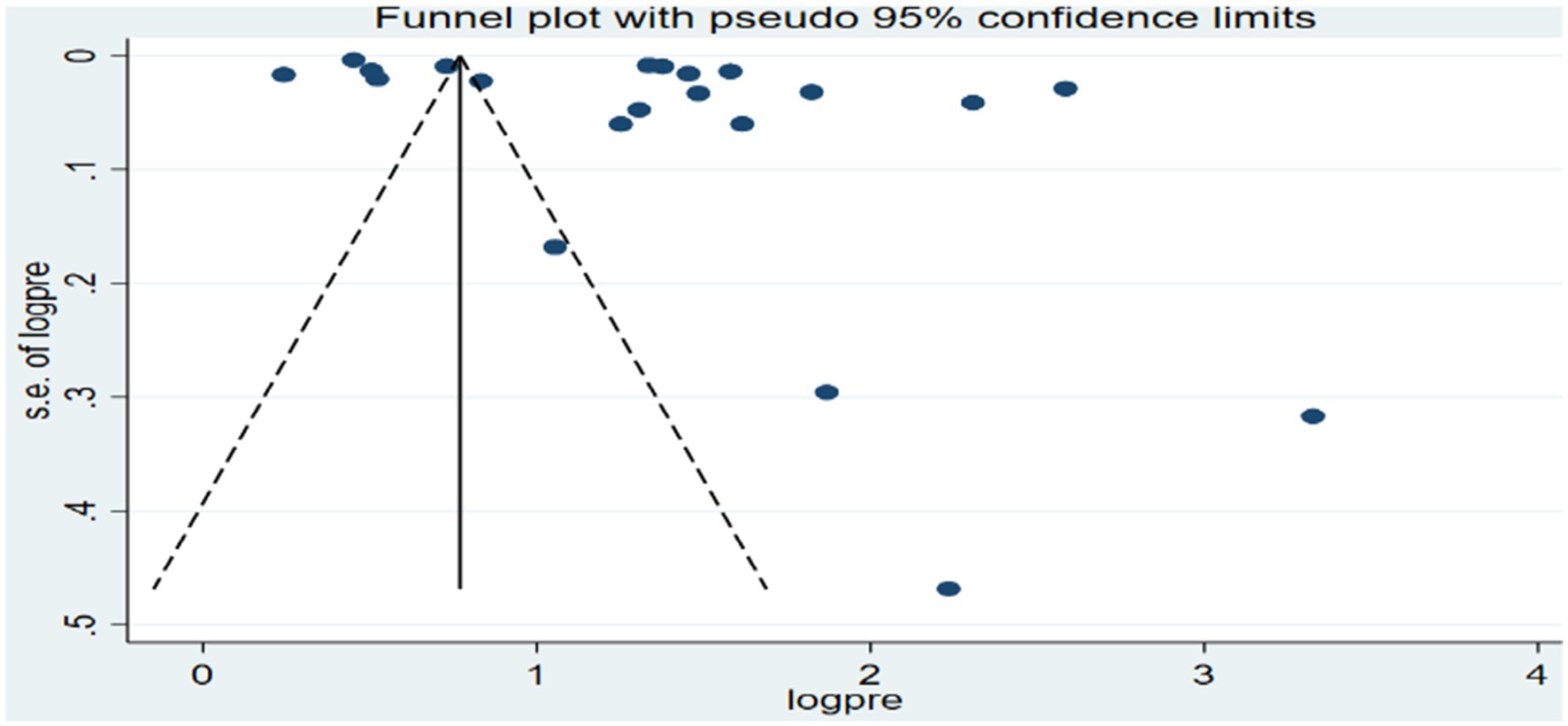
The funnel plot for the overall analysis showed an asymmetrical distribution as visualized of the included articles, revealing the potential of publication biases (Figure 6). However, the Egger-regression test confirmed that there was no statistically significant presence of publication bias (p-value = 0.1001).
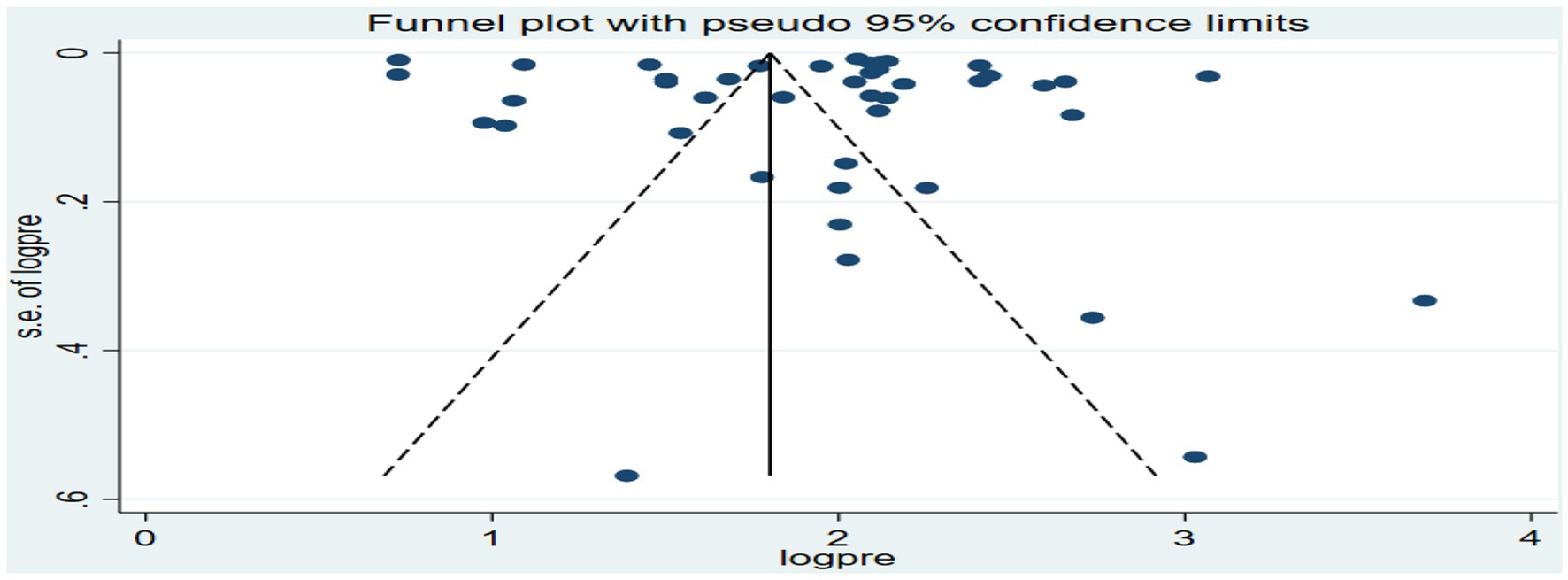
Figure 6. Funnel plot for the association between ambient air pollution and at least one adverse birth outcome, 2024.
Similarly, funnel plots and Egger-regression tests were conducted to assess publication biases for the specific birth outcomes of preterm birth and low birth weights. For preterm birth, the funnel plots showed an asymmetrical distribution as visualized of the included articles (Figure 7), but the Egger-regression test confirmed that there was no statistically significant (p-value = 0.2087) for the presence of publication bias.
In contrast, for low birth weights, the funnel plot also showed an asymmetrical distribution as visualized of the included articles (Figure 8), and the Egger-regression test confirmed the presence of publication bias with statistical significance (p-value = 0.0017). To address the publication bias identified for the low birth weight outcome, Duval and Tweedie’s “trim and fill” method was conducted (Supplementary material 2).
3.4 Factors associated with adverse birth outcomes
In this meta-analysis, exposure to ambient air pollution, such as PM2.5, PM10, and O3, was statistically significant for adverse birth outcomes (preterm birth and low birth weight). For preterm birth, exposure to PM2.5 (≤10 μg/m3) during entire pregnancy, PM2.5 (≤10 μg/m3) in first trimester, PM10 (>10 μg/m3) during entire pregnancy, and O3 (≤10 μg/m3) during entire pregnancy increased the risk by 4% (OR = 1.04, 95% CI: 1.03–1.05), 5% (OR = 1.05, 95% CI: 1.01–1.09), 49% (OR = 1.49, 95% CI: 1.41–1.56), and 5% (OR = 1.05, 95% CI: 1.04–1.07), respectively (Figure 9).
For low birth weight, exposure to PM2.5 (≤10 μg/m3) and PM2.5 (>10 μg/m3) during entire pregnancy was found to increase the risk by 13% (OR = 1.13, 95% CI 1.05–1.21) and 28% (OR = 1.28, 95% CI 1.23–1.33), respectively (Figure 10).
4 Discussion
This systematic review and meta-analysis aimed to estimate the pooled association between exposure to ambient air pollution and adverse birth outcomes (preterm birth, low birth weight, and stillbirth), as well as to identify predictive factors. The pooled prevalence of at least one adverse birth outcome was found to be 7.69% (95% CI: 6.70–8.69), with notable extreme heterogeneity among the included studies (I2 = 100, p < 0.001). Specifically, exposure to ambient air pollution was associated with a 6.36% (95% CI: 5.66–7.06) increased risk of preterm birth, a 5.07% (95% CI: 4.32–5.81) increase in low birth weight, and a 0.65% (95% CI: 0.53–0.7) increase in the risk of stillbirth. These findings suggest that exposure to ambient air pollution during pregnancy negatively affects various birth outcomes (30, 31).
Daba et al. (32) support the present findings, reporting a 15.5% (95% CI: 12.6–18.5) prevalence of adverse pregnancy outcomes linked to indoor air pollution exposure. The WHO reported low birth weight at 15.5% globally, 16.5% in developing countries, and 7% in developed countries in 2015. The current findings are also supported by numerous studies indicating that exposure to ambient air pollution increases the risk of preterm birth, low birth weight, and stillbirth (30, 31, 33). Variations in findings may be attributed to different factors such as maternal educational level, age differences, socioeconomic conditions, type of pollutants, and the duration and level of exposure during the perinatal period (34, 35). Dzekem et al. (36) highlighted the need to address disparities, such as socioeconomic issues, when examining the relationship between air pollution exposure and pregnancy outcomes. Therefore, it is crucial to ensure that the interaction between pregnant women and their environment is safe and well-maintained to prevent adverse effects.
In this study, the heterogeneity among the included studies was significantly high, a finding that is supported by previous research (14, 31–33, 37, 38). This variability may be attributed to various factors, including differences in study settings, designs, and exposure assessment methods. To identify the potential sources of this heterogeneity, we conducted a subgroup analysis based on the study country and the type of adverse birth outcomes. Subsequently, the meta-regression analysis confirmed that the primary source of heterogeneity was related to the nature of the adverse birth outcomes. This may be linked to the levels and types of pollutants, as well as the conditions of pregnant women.
In this meta-analysis, exposure to PM2.5 (≤10 μg/m3) throughout the entire pregnancy and during the first trimester was associated with an increased risk of preterm birth, with an OR of 1.04 (95% CI: 1.03–1.05) and 1.05 (95% CI: 1.01–1.04), respectively. Sapkota et al. (37) reported that exposure to PM2.5 at levels of 10 μg/m3 during pregnancy increased the risk of preterm birth with an OR of 1.15 (95% CI: 1.14–1.16). Liu et al. (39) found a similar positive association, reporting an OR of 1.15 (95% CI: 1.07–1.23) for PM2.5 exposure during pregnancy. Additionally, Lamichhane et al. (3) estimated an OR of 1.14 (95% CI = 1.06–1.22) for preterm birth per 10 μg/m3 increase in PM2.5 exposure during the entire pregnancy. Therefore, these findings highlighted the importance of protecting pregnant mothers from PM2.5 exposure to reduce the risk of adverse outcomes of preterm birth (39–41).
In this study, we found a 49% increase in the risk of preterm birth for each >10 μg/m3 increase in PM10 exposure during the entire pregnancy. Stieb et al. (1) reported a high risk of preterm birth associated with a 20 μg/m3 increase in PM10 over the same period. Similarly, Lamichhane et al. (3) noted that exposure to PM10 increased the risk of preterm birth by 23% for each 10 μg/m3 increment. The slight difference in findings may be attributed to variation in exposure levels and duration. Therefore, addressing ambient air pollution is essential for reducing the occurrence of preterm birth.
In the present study, exposure of O3 at levels of ≤10 μg/m3 during pregnancy was positively associated with a 5% increased risk of preterm birth, with (OR = 1.05, 95% CI: 1.04–1.07). This finding is consistent with previous research (31, 33), which suggests that exposure to ozone throughout pregnancy may significantly elevate the risk of preterm birth. These findings underscoring the need for protective measures to minimize pregnant women’s exposure to ozone.
In this meta-analysis, maternal exposure to PM2.5 during the entire pregnancy at levels of >10 μg/m3 was associated with a 28% increase in the risk of low birth weight (OR = 1.28, 95% CI: 1.23–1.33). Additionally, exposure to PM2.5 (≤10 μg/m3) also showed a 13% increase in risk (OR = 1.13, 95% CI: 1.05–1.21). These findings suggest that as PM2.5 exposure increases, the risk of low birth weight also increases. Supporting this, Zhu et al. (4) reported a 5% increase in low birth weight per 10 μg/m3 increment in PM2.5 exposure during the entire pregnancy (OR = 1.05, 95% CI: 1.02–1.07). The difference in findings may be attributed to variations in exposure assessment methods. Thus, exposure to PM2.5 throughout pregnancy could significantly impact the final birth weight.
4.1 Limitation of the study
This study focusses exclusively on studies conducted in the English language. Additionally, it does not explore the underlying mechanisms that link ambient air pollution to adverse birth outcomes. Furthermore, this study also focused on selected adverse birth outcomes, but other birth outcomes like congenital anomalies or birth defects and others might be important.
5 Conclusion
This meta-analysis highlighted a significant association between ambient air pollution and adverse birth outcomes. In this study, PM2.5, PM10, and O3 were found to be positively associated with these adverse birth outcomes (preterm birth and low birth weight). Given these findings, it is essential for healthcare professionals, the Ministries of Health, non-governmental organizations, and other relevant stakeholders to implement compressive public health interventions aimed at reducing the incidence of adverse birth outcomes related to ambient air pollution. Such interventions could include policies to improve air quality, strict regulations, public awareness campaigns, and targeted support for vulnerable populations of pregnant women. Furthermore, to gain a deeper understanding of the mechanisms underlying the association between ambient air pollution and adverse birth outcomes, future research is highly recommended. Investigating these mechanisms will provide valuable insights that can help inform more effective strategies for mitigating the risks associated with air pollution during pregnancy. In addition, future researchers are encouraged to investigate the impact of ambient air pollution on congenital anomalies and other significant adverse birth outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
BD: Methodology, Software, Validation, Writing – original draft, Writing – review & editing. GB: Conceptualization, Data curation, Visualization, Writing – review & editing. AG: Data curation, Methodology, Software, Writing – review & editing. LB: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing. CD: Data curation, Methodology, Software, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1488028/full#supplementary-material
References
1. Stieb, DM, Chen, L, Eshoul, M, and Judek, S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. (2012) 117:100–11. doi: 10.1016/j.envres.2012.05.007
2. Glinianaia, SV, Rankin, J, Bell, R, Pless-Mulloli, T, and Howel, D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. (2004) 15:36–45. doi: 10.1097/01.ede.0000101023.41844.ac
3. Lamichhane, DK, Leem, J-H, Lee, J-Y, and Kim, H-C. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol. (2015) 30:e2015011. doi: 10.5620/eht.e2015011
4. Zhu, X, Liu, Y, Chen, Y, Yao, C, Che, Z, and Cao, J. Maternal exposure to fine particulate matter (PM2.5) and pregnancy outcomes: a meta-analysis. Environ Sci Pollut Res Int. (2015) 22:3383–96. doi: 10.1007/s11356-014-3458-7
5. Proietti, E, Röösli, M, Frey, U, and Latzin, P. Air pollution during pregnancy and neonatal outcome: a review. J Aerosol Med Pulm Drug Deliv. (2013) 26:9–23. doi: 10.1089/jamp.2011.0932
6. Kannan, S, Misra, DP, Dvonch, JT, and Krishnakumar, A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. (2006) 114:1636–42. doi: 10.1289/ehp.9081
7. World Health Organization. (2022). Ambient (outdoor) air pollution. Available at: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (Accessed August 12, 2024).
8. Fussell, JC, Jauniaux, E, Smith, RB, and Burton, GJ. Ambient air pollution and adverse birth outcomes: a review of underlying mechanisms. BJOG. (2024) 131:538–50. doi: 10.1111/1471-0528.17727
9. Hajat, A, Hsia, C, and O’Neill, MS. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep. (2015) 2:440–50. doi: 10.1007/s40572-015-0069-5
10. Barker, DJP. The origins of the developmental origins theory. J Intern Med. (2007) 261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x
11. Zhang, H, Zhang, X, Wang, Q, Xu, Y, Feng, Y, Yu, Z, et al. Ambient air pollution and stillbirth: an updated systematic review and meta-analysis of epidemiological studies. Environ Pollut. (2021) 278:116752. doi: 10.1016/j.envpol.2021.116752
12. Xie, G, Sun, L, Yang, W, Wang, R, Shang, L, Yang, L, et al. Maternal exposure to PM2.5 was linked to elevated risk of stillbirth. Chemosphere. (2021) 283:131169. doi: 10.1016/j.chemosphere.2021.131169
13. Sun, X, Luo, X, Zhao, C, Zhang, B, Tao, J, Yang, Z, et al. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: a meta-analysis. Environ Pollut. (2016) 211:38–47. doi: 10.1016/j.envpol.2015.12.022
14. Li, C, Yang, M, Zhu, Z, Sun, S, Zhang, Q, Cao, J, et al. Maternal exposure to air pollution and the risk of low birth weight: a meta-analysis of cohort studies. Environ Res. (2020) 190:109970. doi: 10.1016/j.envres.2020.109970
15. Bekkar, B, Pacheco, S, Basu, R, and DeNicola, N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. (2020) 3:e208243. doi: 10.1001/jamanetworkopen.2020.8243
16. Ji, Y, Song, F, Xu, B, Zhu, Y, Lu, C, and Xia, Y. Association between exposure to particulate matter during pregnancy and birthweight: a systematic review and a meta-analysis of birth cohort studies. J Biomed Res. (2017) 33:56–68. doi: 10.7555/JBR.31.20170038
17. Tapia, VL, Vasquez, BV, Vu, B, Liu, Y, Steenland, K, and Gonzales, GF. Association between maternal exposure to particulate matter (PM2.5) and adverse pregnancy outcomes in Lima, Peru. J Expo Sci Environ Epidemiol. (2020) 30:689–97. doi: 10.1038/s41370-020-0223-5
18. Sapkota, A, Chelikowsky, A, Nachman, K, Cohen, A, and Ritz, B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Qual Atmos Health. (2010) 5:1–13.
19. Li, X, Huang, S, Jiao, A, Yang, X, Yun, J, Wang, Y, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ Pollut. (2017) 227:596–605. doi: 10.1016/j.envpol.2017.03.055
20. Li, Z, Tang, Y, Song, X, Lazar, L, Li, Z, and Zhao, J. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol Environ Saf. (2019) 169:248–54. doi: 10.1016/j.ecoenv.2018.10.109
21. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
22. World Health Organization. Geneva. Low birth weight. Available at: https://www.who.int/data/nutrition/nlis/info/low-birth-weight (Accessed August 27, 2024).
23. World Health Organization. Geneva. Stillbirth. Available at: https://www.who.int/health-topics/stillbirth (Accessed August 27, 2024).
24. World Health Organization. Geneva. Preterm birth. Available at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (Accessed August 27, 2024).
25. Aromataris, E, Lockwood, C, Porritt, K, Pilla, B, and Jordan, Z, editors. JBI manual for evidence synthesis - confluence. Available at: https://synthesismanual.jbi.global (Accessed October 7, 2024).
26. Munn, Z, Moola, S, Lisy, K, Riitano, D, and Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
27. Higgins, JPT, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
28. Nahian, M, Ahmad, T, Jahan, I, Chakraborty, N, Nahar, Q, and Streatfield, P. Air pollution and pregnancy outcomes in Dhaka, Bangladesh. J Climate Change Health. (2022) 9:100187. doi: 10.1016/j.joclim.2022.100187
29. Mendola, P, Ha, S, Pollack, AZ, Zhu, Y, Seeni, I, Kim, SS, et al. Chronic and acute ozone exposure in the week prior to delivery is associated with the risk of stillbirth. Int J Environ Res Public Health. (2017) 14:731. doi: 10.3390/ijerph14070731
30. Tan, Y, Yang, R, Zhao, J, Cao, Z, Chen, Y, and Zhang, B. The associations between air pollution and adverse pregnancy outcomes in China. Adv Exp Med Biol. (2017) 1017:181–214. doi: 10.1007/978-981-10-5657-4_8
31. Wang, X, Wang, X, Gao, C, Xu, X, Li, L, Liu, Y, et al. Relationship between outdoor air pollutant exposure and premature delivery in China- systematic review and meta-analysis. Int J Public Health. (2023) 68:1606226. doi: 10.3389/ijph.2023.1606226
32. Daba, C, Asmare, L, Demeke Bayou, F, Arefaynie, M, Mohammed, A, Tareke, AA, et al. Exposure to indoor air pollution and adverse pregnancy outcomes in low and middle-income countries: a systematic review and meta-analysis. Front Public Health. (2024) 12:1356830. doi: 10.3389/fpubh.2024.1356830
33. Ju, L, Li, C, Yang, M, Sun, S, Zhang, Q, Cao, J, et al. Maternal air pollution exposure increases the risk of preterm birth: evidence from the meta-analysis of cohort studies. Environ Res. (2021) 202:111654. doi: 10.1016/j.envres.2021.111654
34. Coker, E, Ghosh, J, Jerrett, M, Gomez-Rubio, V, Beckerman, B, Cockburn, M, et al. Modeling spatial effects of PM2.5 on term low birth weight in Los Angeles County. Environ Res. (2015) 142:354–64. doi: 10.1016/j.envres.2015.06.044
35. Dibben, C, and Clemens, T. Place of work and residential exposure to ambient air pollution and birth outcomes in Scotland, using geographically fine pollution climate mapping estimates. Environ Res. (2015) 140:535–41. doi: 10.1016/j.envres.2015.05.010
36. Dzekem, BS, Aschebrook-Kilfoy, B, and Olopade, CO. Air pollution and racial disparities in pregnancy outcomes in the United States: a systematic review. J Racial Ethn Health Disparities. (2024) 11:535–44. doi: 10.1007/s40615-023-01539-z
37. Sapkota, A, Chelikowsky, AP, Nachman, KE, Cohen, AJ, and Ritz, B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Qual Atmos Health. (2012) 5:369–81. doi: 10.1007/s11869-010-0106-3
38. Sun, X, Luo, X, Zhao, C, Chung Ng, RW, Lim, CED, Zhang, B, et al. The association between fine particulate matter exposure during pregnancy and preterm birth: a meta-analysis. BMC Pregnancy Childbirth. (2015) 15:300. doi: 10.1186/s12884-015-0738-2
39. Liu, C, Sun, J, Liu, Y, Liang, H, Wang, M, Wang, C, et al. Different exposure levels of fine particulate matter and preterm birth: a meta-analysis based on cohort studies. Environ Sci Pollut Res Int. (2017) 24:17976–84. doi: 10.1007/s11356-017-9363-0
40. Bosetti, C, Nieuwenhuijsen, MJ, Gallus, S, Cipriani, S, La Vecchia, C, and Parazzini, F. Ambient particulate matter and preterm birth or birth weight: a review of the literature. Arch Toxicol. (2010) 84:447–60. doi: 10.1007/s00204-010-0514-z
41. Srám, RJ, Binková, B, Dejmek, J, and Bobak, M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. (2005) 113:375–82. doi: 10.1289/ehp.6362
42. Yang, S, Tan, Y, Mei, H, Wang, F, Li, N, Zhao, J, et al. Ambient air pollution the risk of stillbirth: a prospective birth cohort study in Wuhan, China. Int J Hyg Environ Health. (2018) 221:502–9. doi: 10.1016/j.ijheh.2018.01.014
43. Quraishi, SM, Hazlehurst, MF, Loftus, CT, Nguyen, RHN, Barrett, ES, Kaufman, JD, et al. Association of prenatal exposure to ambient air pollution with adverse birth outcomes and effect modification by socioeconomic factors. Environ Res. (2022) 212:113571. doi: 10.1016/j.envres.2022.113571
44. Chen, J, Guo, L, Liu, H, Jin, L, Meng, W, Fang, J, et al. Modification effects of ambient temperature on associations of ambient ozone exposure before and during pregnancy with adverse birth outcomes: a multicity study in China. Environ Int. (2023) 172:107791. doi: 10.1016/j.envint.2023.107791
45. Chen, G, Guo, Y, Abramson, MJ, Williams, G, and Li, S. Exposure to low concentrations of air pollutants and adverse birth outcomes in Brisbane, Australia, 2003–2013. Sci Total Environ. (2018) 622-623:721–6. doi: 10.1016/j.scitotenv.2017.12.050
46. Qian, Z, Liang, S, Yang, S, Trevathan, E, Huang, Z, Yang, R, et al. Ambient air pollution and preterm birth: A prospective birth cohort study in Wuhan, China. Int J Hyg Environ Health. (2016) 219:195–203. doi: 10.1016/j.ijheh.2015.11.003
47. Zhou, W, Ming, X, Yang, Y, Hu, Y, He, Z, Chen, H, et al. Associations between maternal exposure to ambient air pollution and very low birth weight: a birth cohort study in Chongqing, China. Front Public Health. (2023) 11:1123594. doi: 10.3389/fpubh.2023.1123594
48. Chen, Q, Ren, Z, Liu, Y, Qiu, Y, Yang, H, Zhou, Y, et al. The association between preterm birth and ambient air pollution exposure in Shiyan, China, 2015-2017. Int J Environ Res Public Health. (2021) 18:4326. doi: 10.3390/ijerph18084326
49. Zhou, W, Ming, X, Yang, Y, Hu, Y, He, Z, Chen, H, et al. Association between maternal exposure to ambient air pollution and the risk of preterm birth: a birth cohort study in Chongqing, China, 2015-2020. Int J Environ Res Public Health. (2022) 19:2211. doi: 10.3390/ijerph19042211
50. Melody, S, Wills, K, Knibbs, LD, Ford, J, Venn, A, and Johnston, F. Adverse birth outcomes in Victoria, Australia in association with maternal exposure to low levels of ambient air pollution. Environ Res. (2020) 188:109784. doi: 10.1016/j.envres.2020.109784
51. Li, L, Ma, J, Cheng, Y, Feng, L, Wang, S, Yun, X, et al. Urban-rural disparity in the relationship between ambient air pollution and preterm birth. Int J Health Geogr. (2020) 19:23. doi: 10.1186/s12942-020-00218-0
52. Zhao, N, Qiu, J, Zhang, Y, He, X, Zhou, M, Li, M, et al. Ambient air pollutant PM10 and risk of preterm birth in Lanzhou, China. Environ Int. (2015) 76:71–7. doi: 10.1016/j.envint.2014.12.009
53. Padula, AM, Yang, W, Lurmann, FW, Balmes, J, Hammond, SK, and Shaw, GM. Prenatal exposure to air pollution, maternal diabetes and preterm birth. Environ Res. (2019) 170:160–7. doi: 10.1016/j.envres.2018.12.031
54. Liu, W-Y, Yu, Z-B, Qiu, H-Y, Wang, J-B, Chen, X-Y, and Chen, K. Association between ambient air pollutants and preterm birth in Ningbo, China: a time-series study. BMC Pediatr. (2018) 18:305. doi: 10.1186/s12887-018-1282-9
55. Lavigne, E, Yasseen, AS, Stieb, DM, Hystad, P, van Donkelaar, A, Martin, RV, et al. Ambient air pollution and adverse birth outcomes: differences by maternal comorbidities. Environ Res. (2016) 148:457–66. doi: 10.1016/j.envres.2016.04.026
56. Huang, C, Nichols, C, Liu, Y, Zhang, Y, Liu, X, Gao, S, et al. Ambient air pollution and adverse birth outcomes: a natural experiment study. Popul Health Metrics. (2015) 13:17. doi: 10.1186/s12963-015-0050-4
57. Liu, Y, Xu, J, Chen, D, Sun, P, and Ma, X. The association between air pollution and preterm birth and low birth weight in Guangdong, China. BMC Int Health Hum Rights. (2019) 19:3. doi: 10.1186/s12889-018-6307-7
58. Yorifuji, T, Kashima, S, and Doi, H. Outdoor air pollution and term low birth weight in Japan. Environ Int. (2015) 74:106–11. doi: 10.1016/j.envint.2014.09.003
59. Stieb, DM, Chen, L, Hystad, P, Beckerman, BS, Jerrett, M, Tjepkema, M, et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999-2008. Environ Res. (2016) 148:513–26. doi: 10.1016/j.envres.2016.04.025
60. Green, R, Sarovar, V, Malig, B, and Basu, R. Association of stillbirth with ambient air pollution in a California cohort study. Am J Epidemiol. (2015) 181:874–82. doi: 10.1093/aje/kwu460
61. Ji, X, Meng, X, Liu, C, Chen, R, Ge, Y, Kan, L, et al. Nitrogen dioxide air pollution and preterm birth in Shanghai, China. Environ Res. (2019) 169:79–85. doi: 10.1016/j.envres.2018.11.007
62. Guo, T, Wang, Y, Zhang, H, Zhang, Y, Zhao, J, Wang, Q, et al. The association between ambient PM2.5 exposure and the risk of preterm birth in China: a retrospective cohort study. Sci Total Environ. (2018) 633:1453–9. doi: 10.1016/j.scitotenv.2018.03.328
63. Kingsley, SL, Eliot, MN, Glazer, K, Awad, YA, Schwartz, JD, Savitz, DA, et al. Maternal ambient air pollution, preterm birth and markers of fetal growth in Rhode Island: results of a hospital-based linkage study. J Epidemiol Community Health. (2017) 71:1131–6. doi: 10.1136/jech-2017-208963
64. Ho, TH, Van Dang, C, Pham, TTB, Thi Hien, T, and Wangwongwatana, S. Ambient particulate matter (PM2.5) and adverse birth outcomes in Ho Chi Minh City, Vietnam. Hygiene Environ Health Adv. (2023) 5:100049. doi: 10.1016/j.heha.2023.100049
65. Wang, L, Fang, L, Fang, Z, Zhang, M, and Zhang, L. Assessment of the association between prenatal exposure to multiple ambient pollutants and preterm birth: a prospective cohort study in Jinan, East China. Ecotoxicol Environ Saf. (2022) 232:113297. doi: 10.1016/j.ecoenv.2022.113297
66. Xiao, Q, Chen, H, Strickland, MJ, Kan, H, Chang, HH, Klein, M, et al. Associations between birth outcomes and maternal PM2.5 exposure in Shanghai: a comparison of three exposure assessment approaches. Environ Int. (2018) 117:226–36. doi: 10.1016/j.envint.2018.04.050
67. Zang, H, Cheng, H, Song, W, Yang, M, Han, P, Chen, C, et al. Ambient air pollution and the risk of stillbirth: a population-based prospective birth cohort study in the coastal area of China. Environ Sci Pollut Res Int. (2019) 26:6717–24. doi: 10.1007/s11356-019-04157-7
68. Arroyo, V, Díaz, J, Carmona, R, Ortiz, C, and Linares, C. Impact of air pollution and temperature on adverse birth outcomes: Madrid, 2001–2009. Environ Pollut. (2016) 218:1154–61. doi: 10.1016/j.envpol.2016.08.069
69. DeFranco, E, Hall, E, Hossain, M, Chen, A, Haynes, EN, Jones, D, et al. Air pollution and stillbirth risk: exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS One. (2015) 10:e0120594. doi: 10.1371/journal.pone.0120594
70. Coker, E, Liverani, S, Ghosh, JK, Jerrett, M, Beckerman, B, Li, A, et al. Multi-pollutant exposure profiles associated with term low birth weight in Los Angeles County. Environ Int. (2016) 91:1–13. doi: 10.1016/j.envint.2016.02.011
71. Yuan, L, Zhang, Y, Wang, W, Chen, R, Liu, Y, Liu, C, et al. Critical windows for maternal fine particulate matter exposure and adverse birth outcomes: the Shanghai birth cohort study. Chemosphere. (2020) 240:124904. doi: 10.1016/j.chemosphere.2019.124904
72. Li, S, Peng, L, Wu, X, Xu, G, Cheng, P, Hao, J, et al. Long-term impact of ambient air pollution on preterm birth in Xuzhou, China: a time series study. Environ Sci Pollut Res Int. (2021) 28:41039–50. doi: 10.1007/s11356-021-13621-2
73. Rammah, A, Whitworth, KW, Han, I, Chan, W, and Symanski, E. Time-varying exposure to ozone and risk of stillbirth in a nonattainment urban region. Am J Epidemiol. (2019) 188:1288–95. doi: 10.1093/aje/kwz095
74. Mitku, AA, Zewotir, T, North, D, Jeena, P, Asharam, K, Muttoo, S, et al. Impact of ambient air pollution exposure during pregnancy on adverse birth outcomes: generalized structural equation modeling approach. BMC Public Health. (2023) 23:45. doi: 10.1186/s12889-022-14971-3
75. Li, Q, Wang, Y, Guo, Y, Zhou, H, Wang, X, Wang, Q, et al. Effect of airborne particulate matter of 2.5 μm or less on preterm birth: a national birth cohort study in China. Environ Int. (2018) 121:1128–36. doi: 10.1016/j.envint.2018.10.025
76. Bachwenkizi, J, Liu, C, Meng, X, Zhang, L, Wang, W, van Donkelaar, A, et al. Maternal exposure to fine particulate matter and preterm birth and low birth weight in Africa. Environ Int. (2022) 160:107053. doi: 10.1016/j.envint.2021.107053
77. Chu, C, Zhu, Y, Liu, C, Chen, R, Yan, Y, Ren, Y, et al. Ambient fine particulate matter air pollution and the risk of preterm birth: a multicenter birth cohort study in China. Environ Pollut. (2021) 287:117629. doi: 10.1016/j.envpol.2021.117629
78. Han, Y, Jiang, P, Dong, T, Ding, X, Chen, T, Villanger, GD, et al. Maternal air pollution exposure and preterm birth in Wuxi, China: effect modification by maternal age. Ecotoxicol Environ Saf. (2018) 157:457–62. doi: 10.1016/j.ecoenv.2018.04.002
79. Zhang, C, Yang, J, Wei, J, Liu, Y, Zhu, H, Li, X, et al. Individual ambient ozone exposure during pregnancy and adverse birth outcomes: exploration of the potentially vulnerable windows. J Hazard Mater. (2024) 464:132945. doi: 10.1016/j.jhazmat.2023.132945
80. Kim, YJ, Song, IG, Kim, K-N, Kim, MS, Chung, S-H, Choi, Y-S, et al. Maternal exposure to particulate matter during pregnancy and adverse birth outcomes in the Republic of Korea. Int J Environ Res Public Health. (2019) 16:633. doi: 10.3390/ijerph16040633
81. Liang, Z, Yang, Y, Qian, Z, Ruan, Z, Chang, J, Vaughn, MG, et al. Ambient PM2.5 and birth outcomes: estimating the association and attributable risk using a birth cohort study in nine Chinese cities. Environ Int. (2019) 126:329–35. doi: 10.1016/j.envint.2019.02.017
82. Chen, J, Fang, J, Zhang, Y, Xu, Z, Byun, H-M, Li, P, et al. Associations of adverse pregnancy outcomes with high ambient air pollution exposure: results from the project ELEFANT. Sci Total Environ. (2021) 761:143218. doi: 10.1016/j.scitotenv.2020.143218
83. Johnson, S, Bobb, JF, Ito, K, Savitz, DA, Elston, B, Shmool, JLC, et al. Ambient fine particulate matter, nitrogen dioxide, and preterm birth in New York City. Environ Health Perspect. (2016) 124:1283–90. doi: 10.1289/ehp.1510266
84. Siddika, N, Rantala, AK, Antikainen, H, Balogun, H, Amegah, AK, Ryti, NRI, et al. Short-term prenatal exposure to ambient air pollution and risk of preterm birth - a population-based cohort study in Finland. Environ Res. (2020) 184:109290. doi: 10.1016/j.envres.2020.109290
85. Sun, Z, Yang, L, Bai, X, Du, W, Shen, G, Fei, J, et al. Maternal ambient air pollution exposure with spatial-temporal variations and preterm birth risk assessment during 2013–2017 in Zhejiang Province, China. Environ Int. (2019) 133:105242. doi: 10.1016/j.envint.2019.105252
86. Hao, H, Yoo, SR, Strickland, MJ, Darrow, LA, D’Souza, RR, Warren, JL, et al. Effects of air pollution on adverse birth outcomes and pregnancy complications in the U.S. state of Kansas (2000–2015). Sci Rep. (2023) 13:21476. doi: 10.1038/s41598-023-48329-5
Keywords: ambient air pollution, outdoor air pollution, adverse birth outcomes, preterm birth, low birth weights, stillbirth
Citation: Desye B, Berihun G, Geto AK, Berhanu L and Daba C (2024) Exposure to ambient air pollutions and its association with adverse birth outcomes: a systematic review and meta-analysis of epidemiological studies. Front. Public Health. 12:1488028. doi: 10.3389/fpubh.2024.1488028
Edited by:
Paolo Lauriola, International Society Doctors for the Environment (ISDE), ItalyReviewed by:
Laura Reali, Italian National Health System, ItalyPaolo Crosignani, Fondazione IRCCS Istituto Nazionale dei Tumor, Italy
Copyright © 2024 Desye, Berihun, Geto, Berhanu and Daba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Belay Desye, YmVsYXlkZXN5ZS4yMDAxQGdtYWlsLmNvbQ==
 Belay Desye
Belay Desye Gete Berihun
Gete Berihun Abebe Kassa Geto
Abebe Kassa Geto Leykun Berhanu
Leykun Berhanu Chala Daba
Chala Daba