- 1Division of Abdominal Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Radiation Oncology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Chengdu, China
Background: Atezolizumab has recently demonstrated improved prognosis in patients with advanced or metastatic non-small-cell lung cancer (NSCLC) who are not eligible for treatment with a platinum-containing regimen, as observed in a randomized phase 3 clinical trial. This study aims to evaluate the cost-effectiveness of atezolizumab for the treatment of NSCLC from the perspective of payers in both developed and developing countries.
Materials and methods: A Markov model was developed to simulate treatment scenarios involving atezolizumab or chemotherapy for patients diagnosed with NSCLC. The model estimated the transition probabilities, health care costs, and health utilities base on the risk of disease progression, survival, and toxicity using data from IPSOS clinical trials, relevant literature, and publicly available databases. A price simulation was conducted to guide the pricing strategy at the specified willingness-to-pay (WTP) threshold, and sensitivity analyses were performed to assess the model’s response to uncertainty.
Results: Among patients with NSCLC who are not suitable for treatment with a platinum-containing regimen, the use of atezolizumab led to an incremental gain of 0.35 quality adjusted life years (QALYs) compared to chemotherapy. The ICER for atezolizumab compared to chemotherapy was calculated at $220400.53 per QALY in the US and $101874.61 per QALY in China. The price simulation results indicated that atezolizumab was favored in the US when the price was less than $371.28/60 mg and $474.92/60 mg at the WTP thresholds of $100,000 and $150,000, respectively; it was cost-effective at a WTP threshold of $36023.71when the price was about 40% of the current price in China. Sensitivity analysis revealed that variables such as the price of atezolizumab and utilities influenced the r model’s outcomes, although these factors did not significantly alter the overall conclusion.
Conclusion: Atezolizumab was not considered cost-effective at the WTP thresholds of $150,000 per QALY in the US and $36,024 per QALY in China for patients with advanced NSCLC who are ineligible for platinum-based chemotherapy.
Introduction
The global cancer data from the international Agency for Research on Cancer(IARC) indicated that lung cancer was among the most common cancer, contributing to a substantial number of new cases and deaths in 2020 (1). In the United States, lung cancer was estimated to have an incidence of 234,580 new cases and caused 125,070 deaths in 2024 (2). In China, approximately 828,100 new cases were reported in 2020 (3).The rapid advancement of emerging therapies, such as targeted therapies and immunotherapies, has notably improved survival rates for patients diagnosed with non-small cell lung cancer (NSCLC), the most prevalent form of lung cancer (4). Clinical trials investigating first-line immunotherapy for patients without targeted mutations primarily focus on individuals who can tolerate standard platinum-based chemotherapy, possess good performance status, and are relatively young (5). As researchers continue their quest for treatment alternatives surpassing chemotherapy in terms of both survival outcomes and quality of life, monotherapy with immune-checkpoint inhibitors (ICIs) is emerging as a promising approach.
Atezolizumab, a programmed death-ligand 1 (PD-L1) inhibitor, has shown improved overall survival compared to single-agent chemotherapy both in previously treated metastatic NSCLC patients (6, 7) and as first-line treatment in PD-L1–high NSCLC patients (8). Critical trials of first-line immunotherapy have been primarily limited to patients with good performance status, and there is limited evidence for its efficacy in patients with poorer performance status. Recently, the IPSOS trial investigated the safety and efficacy of atezolizumab versus chemotherapy alone in patients who were ineligible for treatment with a platinum-containing regimen (9). The findings indicated that initial treatment with atezolizumab significantly prolonged the median overall survival (OS) (10.3 vs. 9.2 months), increased the 2-year survival rate (24% vs. 12%), and maintained stabilization or improvement in quality of life, compared to chemotherapy alone (9).
Therefore, atezolizumab regimens appear to provide a viable therapeutic option for patients with NSCLC who are not eligible for treatment with platinum-containing regimens. However, given the substantial prevalence of NSCLC and the considerable number of advanced cases, the selection of a therapeutic agent will substantially impact the total cost of cancer treatment. According to a report from the National Cancer Institute, expenditure specifically on lung cancer increased from $21.1 billion in 2015 to $23.8 billion in 2020 (10). Additionally, the China Health Yearbook of 2022 reported that the average cost of hospitalization for lung cancer in China reached $5538.50 (11). Therefore, the economic impact of innovative drugs or new treatments should be considered in comprehensive assessments to guide the allocation of limited healthcare resources.
Several published studies have indicated that atezolizumab monotherapy or combination regimens may not achieve cost-effectiveness in both the US and China (12–15). However, the cost-effectiveness of atezolizumab monotherapy has not been assessed in patients with NSCLC who are ineligible for platinum-based chemotherapy. This study aimed to evaluate the cost-effectiveness of atezolizumab compared to chemotherapy for managing NSCLC cases ineligible for platinum-based doublet chemotherapy, from the perspectives of payers in both the US and China.
Materials and methods
Model construction
A mathematical model was developed to evaluate both the economic and clinical outcomes of patients with NSCLC who are ineligible for platinum-based treatment. The study compares the use of atezolizumab with single-agent chemotherapy by integrating decision trees and a Markov model. To simulate NSCLC progression, a three-health-state Markov model was constructed comprising progressive disease (PD), progression-free survival (PFS), and death (see Supplementary Figure S1). The model operates in monthly cycles over a 10-year time horizon, a duration chosen based on simulations demonstrating that over 95% of patients had died within this period. Transition probabilities among health states were derived from the IPSOS clinical trial. Patients entered the model in the PFS state, marking the beginning of their treatment, and the model’s endpoint was defined as patient mortality to reflect clinical reality.
The model was employed to estimate the total costs and effectiveness associated with each treatment option, with quality-adjusted life years (QALYs) serving as the measure of effectiveness. Subsequently, the incremental cost-effectiveness ratio (ICER) of atezolizumab relative to chemotherapy was calculated. This study was conducted from the perspective of payers in both developed and developing countries—represented by US payers (including public insurance, private insurance, and out-of-pocket payments) and the Chinese healthcare system, respectively (16). The willingness to pay (WTP) thresholds were set at $150,000 and $36023.71 per QALY, respectively, (17, 18). Annual discount rates of 3% for costs and 5% for utilities were applied (19, 20).
Transition probabilities
This study employed a partitioned survival analysis to estimate transition probabilities among PFS, PD, and death states over time within the cohort. The probabilities for the states were determined by Kaplan–Meier (K-M) curves of OS and PFS from the IPSOS trial (9, 21, 22).Initially, outcomes points from the curves of OS and PFS were extracted using Plot Digitizer (version 2.6.8). Subsequently, the survival curves were reconstructed via the algorithm proposed by Guyot et al. using R statistical software (version 4.2.2; https://www.r-project.org/) (see Supplementary Figure S2) (23, 24). The reconstructed survival curves were then fitted to the Weibull, exponential, log-logistic, log-normal, gamma, Gompertz and generalized gamma distributions, respectively. The Bayesian information criterion (BIC), Akaike’s information criterion (AIC) and visual validation were employed to determine the best-fitting model. After evaluating the models’ fit, the log–normal model was selected to extrapolate the K–M curves beyond the IPSOS trial follow-up period (see Supplementary Figure S3). For details on the selected distributions and their application, refer to Supplementary Table S1. PFS and OS probabilities at time t were computed using Log-normal distributed survival functions. The proportion of patients in the PD state was calculated as the difference between the PFS and OS probabilities, while the proportion of patients in the death state was determined as 1-OS probability. Background mortality-representing the transitions from the PFS state to death, was estimated using age-specific life tables from the United States and China (25, 26).Microsoft Excel (Microsoft Corporation, Redmond, WA) was used to calculate the transition probabilities between states. The model was ultimately built and manipulated using TreeAge Pro software (Version 2020, https://www.treeage.com/).
Cost and utilities
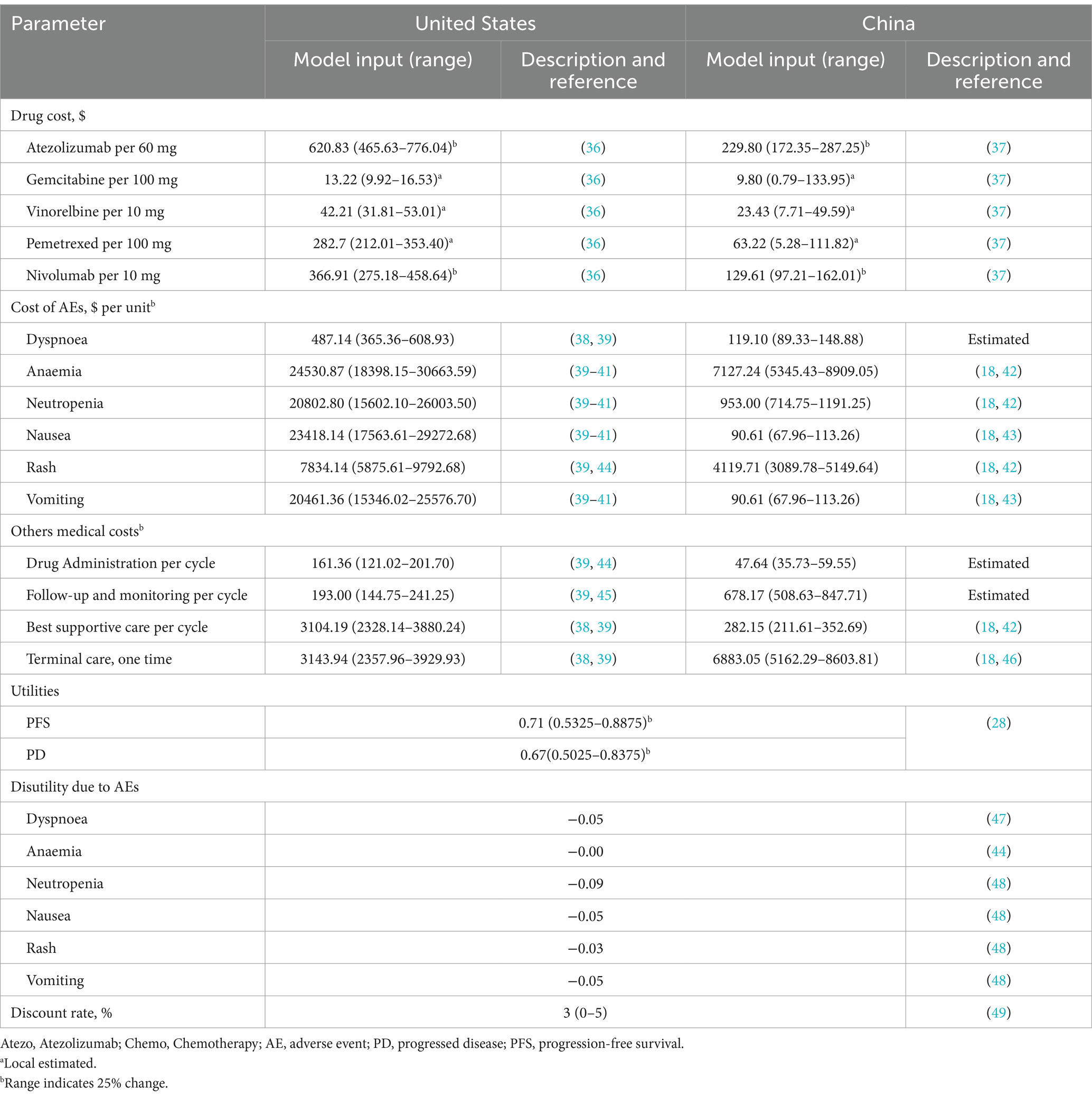
Direct healthcare costs considered in the model comprised drugs procurement, administration, best supportive care, adverse events management, and terminal care (Table 1). The cost of drug administration was based on the dosing regimen developed in the IPSOS trial: On days 1 of each 21-day cycle, atezolizumab was administered intravenously at a fixed dose of 1,200 mg, or single-agent chemotherapeutic drugs including gemcitabine (1,250 mg/m2 intravenously on days 1, 8, and 15 of a 28-day cycle) or vinorelbine (25 mg/m2 intravenously on days 1 and 8 of a 21-day cycle) were administered accordingly. Subsequent treatments and their associated costs were considered in the model. The model further incorporated a post-progression treatment regimen, including chemotherapy (pemetrexed), immunotherapy (Nivolumab), and best supportive care as subsequent treatments on a pro rata basis, based on the data provided in the IPSOS trial, and taking into account end-of-life costs (Supplementary Table S2). Additionally, the model accounted for grade 3/4 adverse events that exhibited significant differences between the study groups in the IPSOS trial. Notable events, such as neutropenia, anemia, dyspnoea, rash, nausea, and vomiting, were incorporated due to their clinical relevance (Supplementary Table S2) ($1 = ¥7.1368) (27).
The model employed health state utility (HSU) values to assign weights to survival time in each health state, thereby assessing the QALYs for different treatments. These HSU values reflect the overall well-being and functional status of patients across various health states. HSU values were assumed to be equivalent across treatment arms within the same health states, with those for PFS and PD derived from an observational cohort study (N = 263) that assessed health-related quality of life in advanced NSCLC patients using a validated EQ-5D questionnaire (28). Additionally, disutility values associated with adverse events (AEs) were incorporated into the model, derived from previously published research involving patients facing similar conditions as those in this study. Table 1 displays the HSU for the PFS and PD health states and the disutility values associated with AEs.
Price simulation
We varied the price of atezolizumab per 60 mg between $0 and $700 to analyse the possibility of cost-effectiveness when the WTP threshold for the corresponding price is $100,000 or $150,000. In China, assuming a WTP equal to three times the GDP per capita, the estimated thresholds in 2023 were $84188.15 in Beijing (highest), $20121.20 in Gansu (lowest), and $36023.71 at the national level (18). Additionally, we varied the price between $0 and $250 to evaluate cost-effectiveness under the Chinese WTP thresholds of $20121.20, $36023.71, and $84188.15.
Sensitivity analysis
Probabilistic sensitivity analysis (PSA) and one-way sensitivity analysis were conducted to assess the robustness of the model outcomes and conclusions in response to variations in key parameters. For the one-way sensitivity analysis, key parameters were varied based on their confidence intervals or by assuming a ±25% deviation from the base-case values. In the PSA, critical parameters such as cost and HSU data were incorporated. Costs were modeled using a gamma distribution, and HSU values were represented using a beta distribution. Subsequently, 1,000 simulations were performed utilizing the Monte Carlo simulation method.
Subgroup analysis
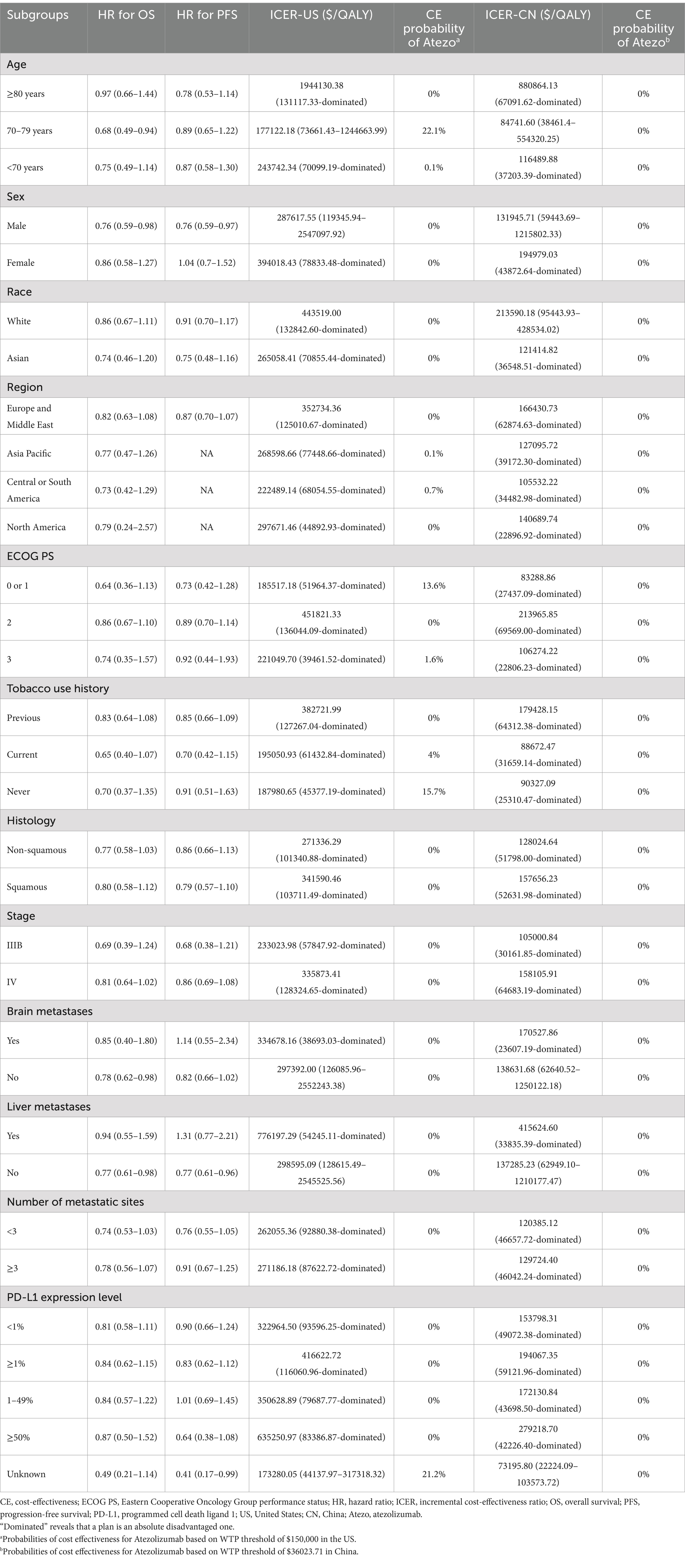
In subgroup analyses, ICER was calculated using subgroup-specific OS and PFS hazard ratios (HRs) derived from IPSOS trial (9). Because PFS data by subgroup were not available for the Region classification, we assumed that these subgroups shared the same PFS HRs as the overall population. We evaluated the cost-effectiveness of patient subgroups defined by varying age, sex, Eastern Cooperative Oncology Group (ECOG PS) performance status scores, and PD-L1 expression levels. Due to insufficient data, proportional hazards were assumed. In addition to the base case analysis status, we also used simulated prices and specific WTP for subgroup analyses.
Results
Base case results
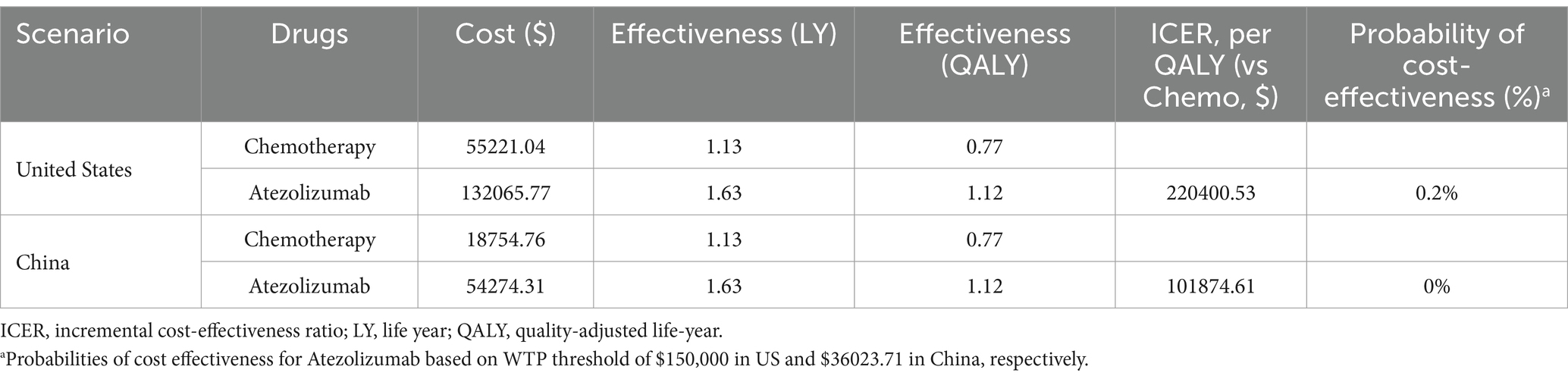
Base-case analyses were performed for both the US and China, and the detailed outcomes are presented in Table 2. In both countries, compared with chemotherapy alone, atezolizumab yielded an improvement of 0.5 life years (LYs) and 0.35 QALYs for patients with NSCLC ineligible for platinum-containing regimens. In the US, the total projected cost for the atezolizumab group was $132065.77, compared with an estimated $55221.04 for chemotherapy. This resulted in an ICER of $220400.53 per QALY gained. In China, the total anticipated costs were $54274.31 for atezolizumab and $18754.76 for chemotherapy alone, yielding an ICER of $101874.61 per QALY gained (see Table 2).
Price simulation
The results of the price simulation are presented in Figures 1A,B. In the US, when the price ranged from $0 to $700, the ICER increased as the cost of atezolizumab rose. Atezolizumab was considered cost-effective when priced below $372.28 per 60 mg and $474.92 per 60 mg, at the WTP thresholds of $100,000 and $150,000, respectively. In China, atezolizumab was considered cost-effective at prices of $193.14 per 60 mg, $60.35 per 60 mg, and $93.31 per 60 mg, corresponding to WTP thresholds of $84188.15, $20121.20, and $36023.71, respectively.

Figure 1. Results of price simulation and deterministic sensitivity analysis. (A) Dashed line perpendicular to the y-axis represents the given WTP, and the green dotted line represents the trend line of the ICER scatter point under each price in US. (B) Dashed line perpendicular to the y-axis represents the given WTP, and the green dotted line represents the trend line of the ICER scatter point under each price in China.
Sensitivity analysis
The results of the deterministic sensitivity analysis (DSA) are shown in Figures 2A,B. The base-case analysis results for both the US and China were most sensitive to the price of atezolizumab, followed by the utility values for PFS and PD states, discount rate, and chemotherapy-related AEs. According to Figure 2A, when the price of atezolizumab was below $474.92/60 mg, the ICER was less than the WTP threshold of $150,000 in the US. However, no variables were found to reduce the ICER below the WTP threshold of $36023.71 in China (Figure 2B). Through a PSA comparing the cost-effectiveness of atezolizumab to chemotherapy in both the US and China indicated that the probability of atezolizumab being cost-effective under the current WTP threshold was 0% (Figures 3A,B). The cost-effectiveness curve analyses conducted under both the base-case and simulated price scenarios are shown in Figures 3C,D. The cost-effectiveness acceptability curve provided a 0 to 57.3% probability of atezolizumab being cost-effective versus chemotherapy, at a WTP threshold of $150,000 to $225,000(1.5 × WTP) in the US. In China, the probability of atezolizumab being cost-effective ranged from 0 to 63.6% at WTP thresholds of $36023.71 to $108071.13 (3 × WTP). Moreover, as the price of atezolizumab decreased, the WTP required to achieve a 50% probability of cost-effectiveness also decreased in both the US and China (see Figures 3C,D).
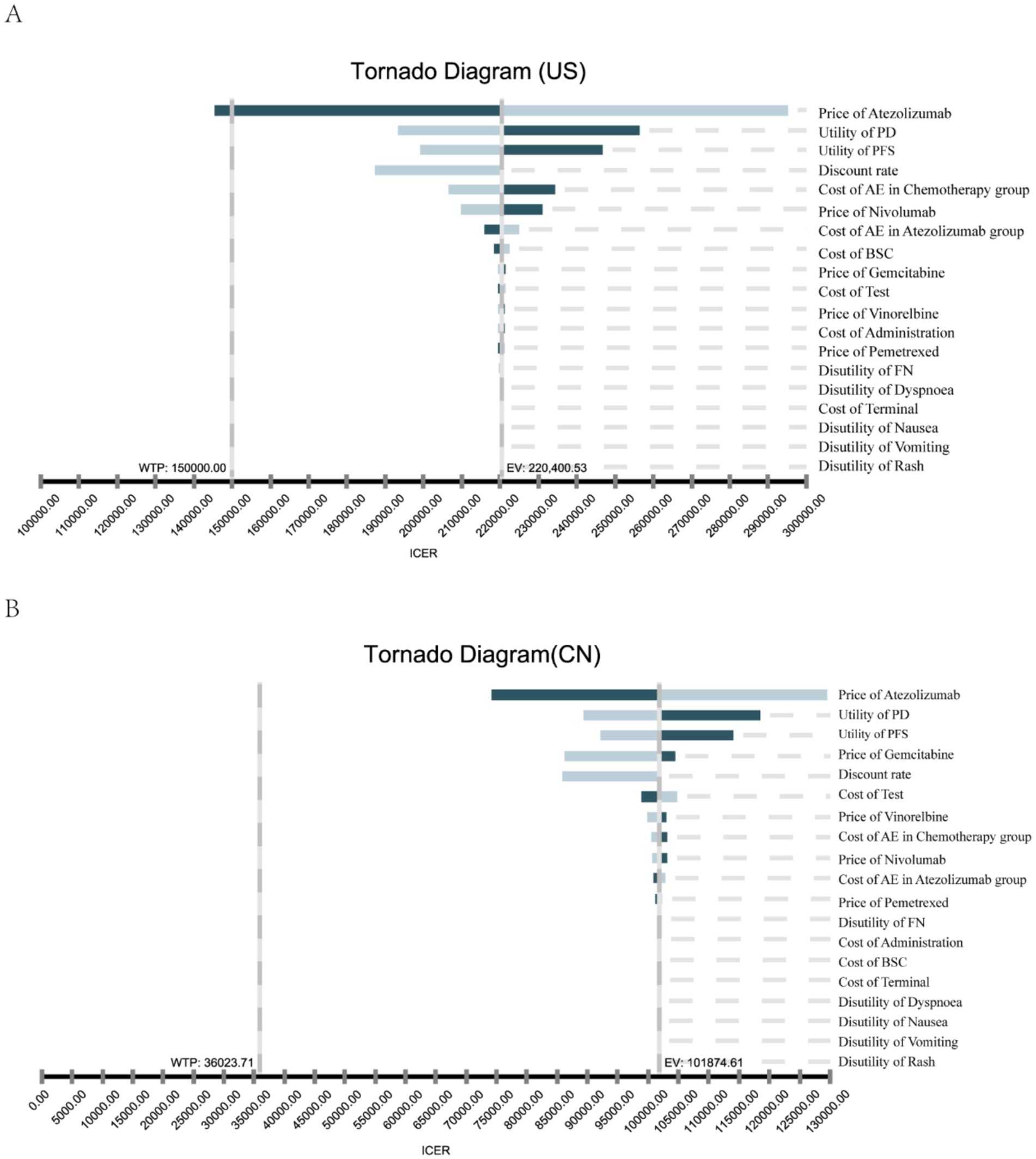
Figure 2. One-way sensitivity analysis. This diagram shows incremental cost effectiveness ratio (ICER) of atezolizumab vs. chemotherapy for different model input parameters of the United States (A) and China (B). PFS, progression-free survival; PD, progression disease; AE, adverse events febrile neutropenia; BSC, best supportive care.
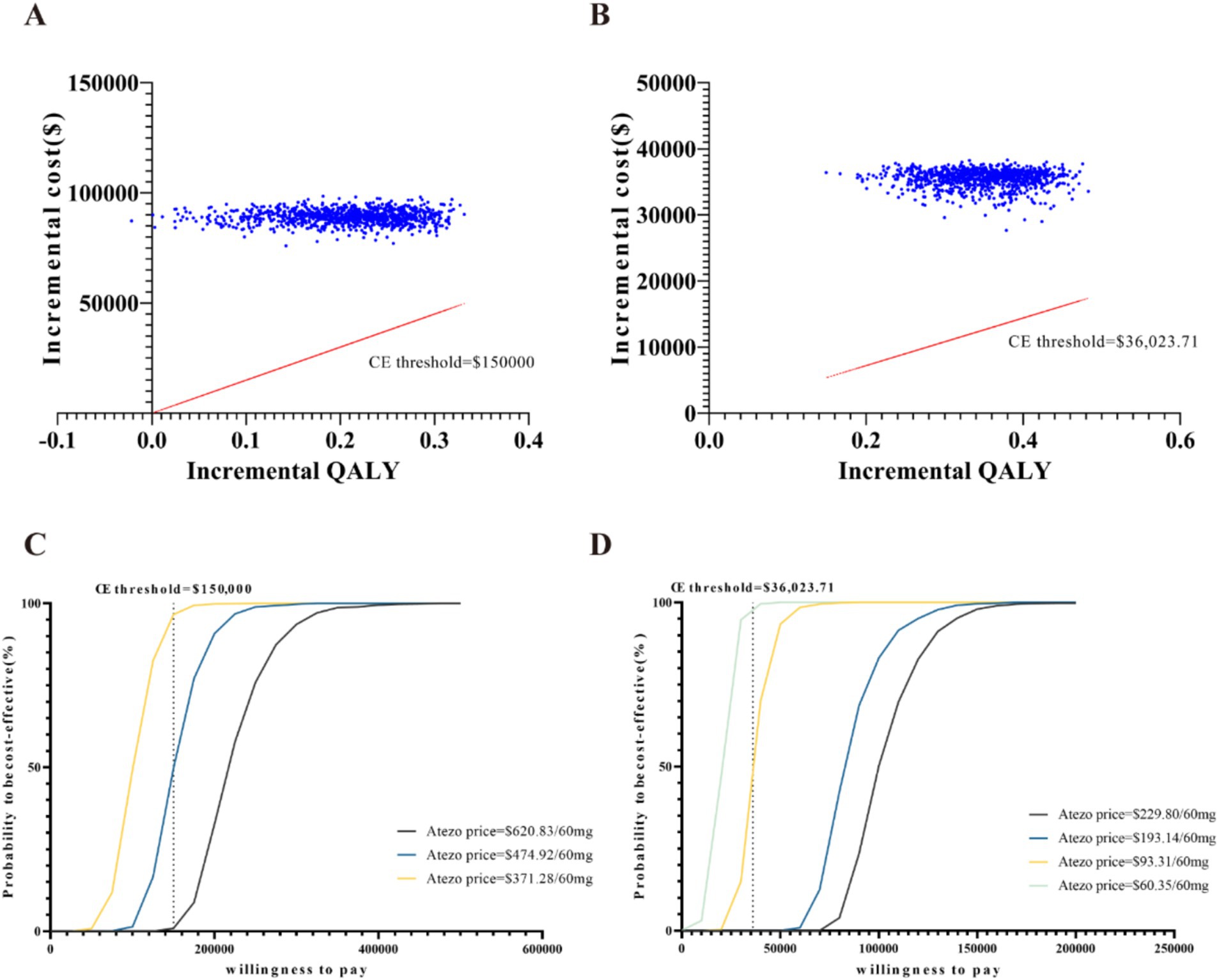
Figure 3. Probabilistic sensitivity analysis: scatter plot of the United States (A) and China (B), cost-effectiveness acceptability curves of atezolizumab versus chemotherapy (1,000 iterations) of the United States (C) and China (D). CE, cost-effectiveness; Atezo, atezolizumab; QALY, quality-adjusted life-years.
Subgroup analysis
Table 3 presents the results of the subgroup analyses. In most subgroups in the base-case analyses, the ICER was significantly influenced by the HR, with atezolizumab performing more favorably when the risk of death was lower. However, in all subgroups, the ICER for atezolizumab exceeded $150,000 per QALY in the US or $36023.71 per QALY in China compared with chemotherapy, suggesting an unfavorable cost-effectiveness profile for atezolizumab. Furthermore, simulated prices and various WTP thresholds were applied to assess the cost-effectiveness of atezolizumab across different subgroups (see Supplementary Table S3). In both the US and China, atezolizumab appeared to be favored among patients aged 70–79 years, those who never smoked or are current smokers, patients with Eastern Cooperative Oncology Group (ECOG) performance status scores of 0, 1, or 3, and patients with unknown PD-L1 status.
Discussion
The favorable outcomes associated with atezolizumab offer a viable therapeutic choice for patients with NSCLC who are ineligible for platinum-containing regimens, as it effectively delays disease progression (9). In this cost-effectiveness analysis, however, atezolizumab was not cost-effective as a first-line therapy for NSCLC patients compared with chemotherapy. The ICERs as high as $200,000/QALY in the US and nearly $100,000/QALY in China, both of which exceed the WTP thresholds.
The base-case model is most sensitive to the price of atezolizumab and the value of HSU, and adjusting the price of atezolizumab is more feasible in clinical practice than increasing the value of an HSU. To reduce the relatively considerable prices incurred by US patients, the US government has sought to align Medicare pharmaceutical prices with those paid by health systems in other developed countries (29). In China, there is an increasing trend towards the standardizing access negotiations for anticancer drugs within medical insurance frameworks, which is expected to become the primary pathway for incorporating innovative drugs into the medical insurance system. In 2019, negotiations for drug reimbursement in China covered 150 drugs, of which 97 reached agreements with the administration. Notably, within this group, 22 cancer drugs achieved average price reductions of 60.7 and 26.4% in their respective categories (30). However, our model indicates that a price reduction of 25% in the US and 60% in China would be necessary for the ICER of atezolizumab to fall below the respective WTP thresholds. Achieving such substantial price reductions presents significant challenges.
Following the publication of the IMpower110 study in 2020, numerous subsequent studies have investigated the cost-effectiveness of atezolizumab as a first-line treatment in patients with advanced or metastasis NSCLC who have favorable performance status and positive PD-L1 expression across various countries. Base-case ICER estimates range from a low of approximately $78,936 per QALY in China to a high of approximately $234,990 per QALY in the United States (13, 15, 31–33). In the US, specific studies have suggested that atezolizumab could be a cost-effective option for initial treatment in patients with high PD-L1-expressing metastatic NSCLC (31, 33). In contrast, several studies in China have indicated that atezolizumab might not represent a cost-effective solution (15, 31, 32). Moreover, due to differences in the study populations, the clinical benefit of atezolizumab compared to chemotherapy was less pronounced in the IPSOS trial than in the IMpower110 study. This suggests that further price reductions or modifications to charitable drug assistance programs may be necessary.
Another study by Jiang et al. analysed the cost-effectiveness of atezolizumab versus chemotherapy as first-line monotherapy in patients with non-small-cell lung cancer ineligible for platinum-based doublet chemotherapy (34). This analysis, also based on a Markov model but conducted in the United Kingdom, concluded that first-line atezolizumab monotherapy resulted in an additional 0.28 QALYs compared to chemotherapy monotherapy and was not considered to be cost-effective, with an ICER of £94,873 /QALY. These findings are consistent with our study conducted in the US and China. In contrast, Li et al. performed a similar analysis within the Chinese context and reported that atezolizumab was cost-effective in China (35). However, their study employed a fitted model only for the OS curve, without applying a similar fitting process to the PFS curve, and used fixed values to calculate the probability of transition to PFS, which may have further impacted the model results. Additionally, Li et al. appeared to have increased the proportion of immunotherapy in the follow-up treatment of the chemotherapy group to a greater extent, thereby directly contributing to the higher cost observed in the chemotherapy group.
Although our analysis primarily focuses on the US and China, the methodological framework and key findings offer insights that are applicable to other healthcare systems. Our examination of price simulations and cost-effectiveness acceptability curves demonstrates how fluctuations in drug prices and WTP thresholds influence cost-effectiveness outcomes. This suggests that healthcare systems in different regions can compare their local drug pricing and WTP thresholds with the scenarios we modeled. However, applying these findings to other contexts requires careful consideration of local healthcare settings, pricing structures, and demographic factors. Future research that adapts the model to specific regions will be essential for accurately assessing the cost-effectiveness of atezolizumab in diverse healthcare environments.
We acknowledge several limitations to our study. First, as the IPSOS trial individual patient data was inaccessible, the data regarding effectiveness and toxicity factors from reported studies were collected. In addition, the short-term clinical data were used to extrapolate long-term survival data from the IPSOS trial using log-normal models. Long-term survival benefits are inevitably subject to uncertainty. However, by comparing the trial curves with the simulated curves, we estimate that curve fitting and extrapolation had minimal impact on the results. Second, to simplify the analysis and enhance the generalizability of the results, we assumed that all patients in the IPSOS trial received proportional chemotherapy (Pemetrexed) and immunotherapy (Nivolumab) after progression, considering the costs of optimal supportive care and end-of-life care. Third, PFS was used as a surrogate for time on treatment in both treatment arms in the absence of available time on treatment data for chemotherapy and atezolizumab, introducing uncertainty regarding treatment duration. Finally, input data for treatment costs related to adverse events and the utility values for different states were not all from NSCLC patients. However, in the sensitivity analysis, we found that the variability of previous treatment costs, utility values, and AEs costs did not significant affect the outcomes.
Conclusion
Our analysis indicates that the cost of atezolizumab for NSCLC treatment is exceptionally high and would not be considered cost-effective given the current prices of atezolizumab and WTP thresholds in the US and China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
QW: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. YQ: Methodology, Software, Supervision, Writing – review & editing. QL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by China postdoctoral science foundation (NO.2023M732458).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1349645/full#supplementary-material
References
1. Sung, H, Ferlay, J, Siegel, R, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. National Cancer Institute. Cancer stat facts: Lung and bronchus cancer. Available online at: https://seer.cancer.gov/statfacts/html/lungb.html (Accessed March 28, 2025)
3. International Agency for Research on Cancer. Estimated number of new cases in 2020, worldwide both sexes all ages. Available online at: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0 (Accessed June 13, 2023)
4. Hendriks, LEL, Remon, J, Faivre-Finn, C, Garassino, MC, Heymach, JV, Kerr, KM, et al. Non-small-cell lung cancer. Nat Rev Dis Primers. (2024) 10:71. doi: 10.1038/s41572-024-00551-9
5. Rashdan, S, and Gerber, DE. Immunotherapy for non-small cell lung cancer: from clinical trials to real-world practice. Transl Lung Cancer Res. (2019) 8:202–7. doi: 10.21037/tlcr.2018.09.15
6. Rittmeyer, A, Barlesi, F, Waterkamp, D, Park, K, Ciardiello, F, von Pawel, J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. doi: 10.1016/s0140-6736(16)32517-x
7. Fehrenbacher, L, Spira, A, Ballinger, M, Kowanetz, M, Vansteenkiste, J, Mazieres, J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. (2016) 387:1837–46. doi: 10.1016/s0140-6736(16)00587-0
8. Herbst, RS, Giaccone, G, de Marinis, F, Reinmuth, N, Vergnenegre, A, Barrios, CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
9. Lee, SM, Schulz, C, Prabhash, K, Kowalski, D, Szczesna, A, Han, B, et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet. (2023) 402:451–63. doi: 10.1016/s0140-6736(23)00774-2
10. Cancer Trends Progress Report. National Cancer Institute. Available online at: https://progressreport.cancer.gov (Accessed August 20, 2023)
11. China health yearbook in 2022. Available online at: http://www.nhc.gov.cn/mohwsbwstjxxzx/tjtjnj/202305/6ef68aac6bd14c1eb9375e01a0faa1fb.shtml (Accessed July 13, 2023)
12. Wan, X, Luo, X, Tan, C, Zeng, XH, Zhang, YC, and Peng, LB. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: a United States-based cost-effectiveness analysis. Cancer. (2019) 125:3526–34. doi: 10.1002/cncr.32368
13. Peng, Y, Zeng, X, Peng, L, Liu, Q, Yi, L, Luo, X, et al. First-line atezolizumab for metastatic NSCLC with high PD-L1 expression: a United States-based cost-effectiveness analysis. Adv Ther. (2021) 38:2447–57. doi: 10.1007/s12325-021-01734-6
14. Shang, F, Zhang, B, and Kang, S. Cost-effectiveness analysis of atezolizumab plus chemotherapy as first-line treatment for patients with advanced nonsquamous non-small-cell lung cancer in China. Expert Rev Pharmacoecon Outcomes Res. (2023) 23:337–43. doi: 10.1080/14737167.2023.2170877
15. Zhang, C, Liu, Y, Tan, J, Tian, P, and Li, W. Cost-effectiveness evaluation based on two models of first-line atezolizumab monotherapy and chemotherapy for advanced non-small cell lung cancer with high-PDL1 expression. Front Oncol. (2023) 13:1093469. doi: 10.3389/fonc.2023.1093469
16. Dieleman, JL, Cao, J, Chapin, A, Chen, C, Li, Z, Liu, A, et al. US health care spending by payer and health condition, 1996-2016. JAMA. (2020) 323:863–84. doi: 10.1001/jama.2020.0734
17. Sarfaty, M, Leshno, M, Gordon, N, Moore, A, Neiman, V, Rosenbaum, E, et al. Cost effectiveness of Nivolumab in advanced renal cell carcinoma. Eur Urol. (2018) 73:628–34. doi: 10.1016/j.eururo.2017.07.041
18. National Bureau of Statistice. National data. Available online at: https://data.stats.gov.cn/english/easyquery.htm?cn=C01 (Accessed June 15 2023)
19. Sanders, GD, Neumann, PJ, Basu, A, Brock, DW, Feeny, D, Krahn, M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. (2016) 316:1093–103. doi: 10.1001/jama.2016.12195
20. Xiao, J, Sun, JF, Wang, QQ, Qi, X, and Yao, HY. Health economic evaluation reporting guideline and application status. Zhonghua Yu Fang Yi Xue Za Zhi. (2017) 51:276–80. doi: 10.3760/cma.j.issn.0253-9624.2017.03.016
21. Powles, T, Rosenberg, JE, Sonpavde, GP, Loriot, Y, Durán, I, Lee, JL, et al. Enfortumab Vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. (2021) 384:1125–35. doi: 10.1056/NEJMoa2035807
22. Lee, S, Schulz, C, Prabhash, K, Han, B, Szczesna, A, Cortinovis, D, et al. LBA11 IPSOS: results from a phase III study of first-line (1L) atezolizumab (atezo) vs single-agent chemotherapy (chemo) in patients (pts) with NSCLC not eligible for a platinum-containing regimen. Ann Oncol. (2022) 33:S1418–9. doi: 10.1016/j.annonc.2022.08.052
23. Hoyle, MW, and Henley, W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. (2011) 11:139. doi: 10.1186/1471-2288-11-139
24. Guyot, P, Ades, AE, Ouwens, MJ, and Welton, NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
26. Global Health Observatory Data Repository. Life tables China. Available online at: https://apps.who.int/gho/data/view.main.60340?lang=en (Accessed June 15, 2023)
27. State Administration of Foreign Exchange. Available online at: https://www.safe.gov.cn/en/ (Accessed June 17, 2023)
28. Chouaid, C, Agulnik, J, Goker, E, Herder, GJM, Lester, JF, Vansteenkiste, J, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. (2013) 8:997–1003. doi: 10.1097/JTO.0b013e318299243b
29. Dyer, O. US drug prices should be tied to foreign prices to tackle "global freeloading," says Trump. BMJ. (2018) 363:k4542. doi: 10.1136/bmj.k4542
30. Innovative Cancer Medicines Added to Reimbursement List. National Health Commission of the People's Republic of China. Available online at: http://en.nhc.gov.cn/2019-11/29/c_75880.html.https://clinicaltrials.gov/ct2/show/NCT04454437?cond=sacituzumab+govitecan&map_cntry=CN&draw=2&rank=1 (Accessed June 17, 2023)
31. Cheng, S, Pei, R, Li, J, Li, B, Tang, L, Yin, T, et al. Atezolizumab compared to chemotherapy for first-line treatment in non-small cell lung cancer with high PD-L1 expression: a cost-effectiveness analysis from US and Chinese perspectives. Ann Transl Med. (2021) 9:1481. doi: 10.21037/atm-21-4294
32. Liu, G, Kang, S, Wang, X, and Shang, F. Cost-effectiveness analysis of Atezolizumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung Cancer with different PD-L1 expression status. Front Oncol. (2021) 11:669195. doi: 10.3389/fonc.2021.669195
33. Lin, S, Li, Y, Gu, D, Luo, S, Huang, X, Dong, L, et al. The predictive value of PD-L1 expression level in evaluating the cost-effectiveness of Atezolizumab/Pembrolizumab. Front Oncol. (2022) 12:857452. doi: 10.3389/fonc.2022.857452
34. Jiang, Y, Zhao, M, Xi, J, Li, J, Tang, W, and Zheng, X. Cost-effectiveness analysis of atezolizumab in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen: a United Kingdom health care perspective. Front Public Health. (2023) 11:1282374. doi: 10.3389/fpubh.2023.1282374
35. Li, L-F, Qi, R, Wei, T-T, Feng, L, Zhang, X, and Liu, Q. Cost-effectiveness of first-line atezolizumab versus chemotherapy in non-small-cell lung Cancer patients ineligible for platinum-containing regimens. Risk Manag Healthc Policy. (2024) 17:927–33. doi: 10.2147/RMHP.S451846
36. IBM Micromedex Red Book. Available online at: www.micromedex.com (Accessed June 15, 2023)
37. DrugDataexpy. Available online at: https://data.yaozh.com/ (Accessed July 13, 2023)
38. Dong, L, Lin, S, Zhong, L, Nian, D, Li, Y, Wang, R, et al. Evaluation of Tucatinib in HER2-positive breast Cancer patients with brain metastases: a United States-based cost-effectiveness analysis. Clin Breast Cancer. (2022) 22:e21–9. doi: 10.1016/j.clbc.2021.06.001
39. Consumer Price Index. United States Bureau of Labor Statistics. Available online at: https://www.bls.gov/cpi/ (Accessed April 25, 2024)
40. GoodRx. Available online at: www.goodrx.com (Accessed June 15, 2023)
41. Wong, W, Yim, Y, Kim, A, Cloutier, M, Gauthier-Loiselle, M, Gagnon-Sanschagrin, P, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS One. (2018) 13:e0196007. doi: 10.1371/journal.pone.0196007
42. Gu, X, Zhang, Q, Chu, YB, Zhao, YY, Zhang, YJ, Kuo, D, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer. (2019) 127:84–9. doi: 10.1016/j.lungcan.2018.11.029
43. Xu, X, Bao, Y, Xu, K, Zhang, Z, Zhao, N, and Li, X. Economic value of Fosaprepitant-containing regimen in the prevention of chemotherapy-induced nausea and vomiting in China: cost-effectiveness and budget impact analysis. Front Public Health. (2022) 10:913129. doi: 10.3389/fpubh.2022.913129
44. Kuznik, A, Smare, C, Chen, CI, Venkatachalam, M, Keeping, S, Atsou, K, et al. Cost-effectiveness of Cemiplimab versus standard of care in the United States for first-line treatment of advanced non-small cell lung cancer with programmed death-ligand 1 expression ≥50. Value Health. (2022) 25:203–14. doi: 10.1016/j.jval.2021.08.009
45. Mistry, R, May, JR, Suri, G, Young, K, Brixner, D, Oderda, G, et al. Cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole and letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2- advanced or metastatic breast Cancer: a U.S. payer perspective. J Manag Care Spec Pharm. (2018) 24:514–23. doi: 10.18553/jmcp.2018.24.6.514
46. Zeng, X, Karnon, J, Wang, S, Wu, B, Wan, X, and Peng, L. The cost of treating advanced non-small cell lung cancer: estimates from the Chinese experience. PLoS One. (2012) 7:e48323. doi: 10.1371/journal.pone.0048323
47. Doyle, S, Lloyd, A, and Walker, M. Health state utility scores in advanced non-small cell lung cancer. Lung Cancer. (2008) 62:374–80. doi: 10.1016/j.lungcan.2008.03.019
48. Nafees, B, Stafford, M, Gavriel, S, Bhalla, S, and Watkins, J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. (2008) 6:84. doi: 10.1186/1477-7525-6-84
Keywords: atezolizumab, immunotherapy, cost-effectiveness, non-small-cell lung cancer, Markov model
Citation: Wu Q, Qin Y and Li Q (2025) Cost-effectiveness of atezolizumab versus chemotherapy in patients with non-small-cell lung cancer ineligible for platinum-based doublet chemotherapy. Front. Public Health. 13:1349645. doi: 10.3389/fpubh.2025.1349645
Edited by:
Xiaozhen Lai, Peking University, ChinaReviewed by:
Abhishesh Kumar Mehata, Indian Institute of Technology (BHU), IndiaXiaomo Xiong, University of Cincinnati, United States
Copyright © 2025 Wu, Qin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu Li, bGlxaXVAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Qiuji Wu
Qiuji Wu Yi Qin
Yi Qin Qiu Li
Qiu Li