- 1Department of Pharmacy, College of Medicine and Health Sciences, Debre Markos University, Debre Markos, Ethiopia
- 2Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Curtin Medical School, Faculty of Health Sciences, Curtin University, Bentley, WA, Australia
- 4Department of Internal Medicine, School of Medicine, College of Medicine and Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 5Department of Pharmacy, School of Health science, College of Medicine and Health sciences, Bahir Dar University, Bahir Dar, Ethiopia
Background: Dermatological problems are among the leading causes of hospital visits in Ethiopia. Extemporaneous compounded products are currently used by many patients with different disorders. The aim of the study was to evaluate the most commonly prescribed extemporaneously compounded products compounding practice and applicability of compounding guidelines at five randomly selected hospitals in Northwest Ethiopia.
Methods: A hospital-based multicenter descriptive cross-sectional study was conducted by retrospectively analyzing prescription records for dermatological compounding from January to April 2023. A total of 423 prescriptions from hospital pharmacies were systematically selected. Data related to skin condition patterns, product selection, and dosage form types were extracted using a semi-structured data extraction tool and analyzed using SPSS version 25.0 at a significance level of 5%.
Results: A total of 423 prescriptions containing dermatological products for compounding were analyzed. Most patients were female (82.1%) and aged 30–64 years (46.3%). Melasma (82.9%), acne vulgaris (68.6%), acute dermatitis (63.27%), and Rosacea (61.54%) were the four top dermatological diseases for which compounding preparations were prescribed. More than half of the prescriptions (56.26%) contained a combination of two drugs. Most compounded dosage form was semisolid preparations (95.98%), while the remaining 17 (4.02%) were liquids. Salicylic acid (35.39%) was the most frequently prescribed active ingredient, followed by Clobetasone (13.03%) and Betamethasone (10.01%). Vaseline (47.62%) and Nivea cream (44.3%) were the most commonly used excipients for compound preparations. Hydroquinone (4%) with Nivea cream (30gm) (17.0%), followed by salicylic acid (5%) + Betamethasone (75 g) + Vaseline (20 g) (10.6%) were the most commonly prescribed dermatological formulations.
Conclusion: Dermatological disease is more prevalent in the study area. and extemporaneous compounding is a common element of pharmaceutical care. Extemporaneous and topical semisolid preparations containing two or more active ingredients are the most widely compounded and prescribed products. This study suggests the application of good manufacturing practices and componding guidelines for extemporaneous compounding of dermatological formulations to ensure efficacy, quality, and safety.
Introduction
Nearly one-third of the world’s population suffers from skin disorders, which rank as the fourth most common non-fatal disease burden worldwide (1), with an annual percent increase of 46.8% between 1990 and 2017 (2, 3). Skin conditions are among the most prevalent health issues in the world and have a significant financial and health impact. The psychological, social, and financial effects of skin disease on patients, their families, and society at large are all included in the multifaceted concept of the burden of skin disease. The two most prevalent skin conditions in poor nations are scabies and psoriasis (4, 5). In Ethiopia, dermatological issues account for 25% of cases in the Outpatient Department (OPD), making it one of the main reasons patients visit hospitals. Psychosocial consequences from skin diseases can have a major negative impact on patients’ quality of life (6).
Pharmaceutical compounding involves the combination, mixing, diluting, reconstructing, or alteration of a drug/bulk drug substance to create medications tailored to individual patient needs (7). It is an art or science to combine various chemical elements into a functional medicament. Extemporaneously compounded products are prepared specifically for an individual patient for immediate use and may include modifications to commercially manufactured products, such as the preparation of a suspension from tablets or the preparation of a product from individual raw materials (8).
Extemporaneous compounding is still a vital pharmaceutical service that continues to provide crucial patient populations with access to life-saving medications despite the development of commercial pharmaceutical manufacture and the widespread availability of contemporary medications (9, 10). One advantage of extemporaneously compounded products over commercial products is their flexibility for individual needs. Moreover, they have paramount importance in cases where individuals are sensitive to the ingredients of commercially available products, and even they may become the only way someone can receive a treatment that has been deemed insufficiently profitable by pharmaceutical companies (9–11).
Errors in extemporaneous compounding can occur at any point in the process, from prescribing medication to giving the patient their ready medication. Incorrect calculations and microbiological contamination are among the most serious errors, which may result in death. There were cases reported where the active component was absent from the final compounded preparation, where the active ingredient or excipients were wrong, or where the product was packaged incorrectly. It is crucial to note that unfavorable precipitation or nonhomogeneous mixing may arise from physicochemical problems, disregard for preparation guidelines, or improper preparation technology of extemporaneous medicines (12–14).
Ethiopian pharmacy practice, which included pharmaceutical compounding and manufacturing, was discovered at a very young age and was virtually forgotten in hospital settings (15). Recently, the Ministry of Health in Ethiopia has paid some attention to this service, which is regarded as one endeavor. Dermatological preparations are one of the services that a technical working group has been formed to help public hospitals provide this service (12, 15, 16).
Currently, many hospitals and community pharmacists in Ethiopia are increasingly incorporating the practice of extemporaneous compounding into their existing services (Box 1). However, data is lacking on the practice of prescribing, compounding, and use of extemporaneously compounded products in many hospitals. In addition, gaps have been observed in the facilities with the country’s regulatory requirements and their quality, efficacy, and safety (12, 16, 17). The safety, quality, and performance of compounded preparations depend on correct ingredients and calculations, accurate and precise measurements, and appropriate formulation conditions and procedures (12, 17).
Therefore, this study evaluated the existing practice of prescribing and compounding extemporaneous dermatological preparations, the applicability of extemporaneous compounding guidelines, and identified the most common dermatological diseases and the most commonly prescribed extemporaneous dermatological preparations at five randomly selected hospitals in northwest Ethiopia.
Materials and methods
Study setting and design
A retrospective multicenter descriptive cross-sectional study was conducted among patients with dermatological diseases who were followed at selected hospitals in Northwest Ethiopia. The study was conducted at five comprehensive and specialized hospitals in Northwest Ethiopia from January 1st to April 30, 2023. The hospitals were the University of Gondar Comprehensive and Specialized Hospital (UoGCSH), Felege Hiwot Comprehensive and Specialized Hospital (FHCSH), Tibebe Ghion Comprehensive and Specialized Hospital (TGCSH), Debre-Markos Comprehensive and Specialized Hospital (DMCSH), and Debre-Tabor Comprehensive and Specialized Hospital (DTCSH).
The UoGCSH is located in the west of the Central Gondar Administrative Zone, which is, 747 km from, Addis Ababa, the capital city of Ethiopia, in the Northwest direction. It is the capital city of the central Gondar Zone of the Amhara Region. It provides primary and referral healthcare services for nearly 5 million people.
FHCSH is also one of the largest referral hospitals in the Amhara region, located in Bahir-Dar, 565 km, northwest Ethiopia’s capital, Addis Ababa. It serves as a comprehensive hospital a population of seven million with approxamtely 1,200 outpatient consultations daily. Two specialized dermatology clinics accept referred cases from district hospitals in the region and neighboring areas.
TGCSH is also one of the largest teaching hospitals situated in Bahir-Dar. It provides primary and referral healthcare services to more than 5.5 million people living in Bahir-Dar town and neighborhood woreda and zones.
DMCSH is the largest hospital in East Gojjam and is located 295 km northwest of Addis Ababa, the capital of Ethiopia. It provides primary and referral healthcare services to nearly 5 million people in its catchment area.
Similarly, DTCSH is 295 km from Ethiopia’s capital, Addis Ababa, and 265 km from Bahir Dar, the capital of the Amhara regional state. It provides primary and referral healthcare services for nearly 5 million people.
Sample size determination and sampling techniques
The single population proportion formula was used to calculate the required sample size by considering the following assumptions: 50% population distribution, 95% confidence level, and W = 5% margin of error (absolute level of precision).
Where n is the minimum sample size, Z is the reliability coefficient for the desired interval (CI) for 95% = 1.96, and p is the prevalence of the prescribing pattern of dermatological compounding. After considering 10% contingency for a possible incomplete response, the final sample size was 423.
A systematic random sampling technique was used to select patients prescriptions. Initial samples were selected using simple random sampling using the lottery method. Then, eligible prescriptions were included in the study using a sampling frame until the final sample size was maintained.
Eligibility criteria
All extemporaneous compounding prescriptions in the compounding pharmacy at selected Northwest hospitals were included in the study. Prescriptions with incomplete items, e.g., without a drug item, i.e., those that contain only bases, were excluded from the study.
Data collection instruments, procedures, and quality control measures
The data were collected using a semi-structured data extraction tool prepared after reviewing earlier studies. The specific types of data necessary to assess the patterns of extemporaneous preparations and dermatological problems were recorded for each patient prescription.
To ensure the quality of the data, the data collectors were trained for two days by the principal investigator. The data collection process was conducted under close supervision by the investigators, and the collected data were checked daily for completeness during the data collection period. The data collection tool was also pretested on 21 patients (5% of the calculated sample size) from the recorded prescriptions of the dermatology clinic to check the acceptability and consistency of the data collection tool 2 weeks before the actual data collection. In addition, experts in the area of face validity and approval reviewed the questionnaire.
Data entry and analysis
After collection of the data, the data were entered and analyzed using SPSS version 25. A descriptive analysis was performed to explore the patterns of extemporaneous preparations and common dermatological problems implicated in the prescription of these preparations. Frequencies and proportions were used to describe sociodemographic characteristics of the patients.
Results
Patterns of participants’ dermatological disorders
Analyses were performed on 423 prescriptions that contained dermatological products for compounding. Most patients (46.3%) were female (82.1%) and between the ages of 30–64. The four top skin conditions for which compounded remedies were prescribed were Melasoma (81.9%), Acne vulgaris (68.6%), Acute dermatitis (63.27%), and Rosacea (61.54%). Among the 423 reported skin diseases, melasma was found to predominantly affect female patients (82.9%) in the age group of 30–64 years (46.7%). The age range of 18–29 years was the main demographic impacted by acne vulgaris, which was the second most prevalent dermatological issue among females (68.6%). Table 1 presents the thorough diagnosis and information on patient education.
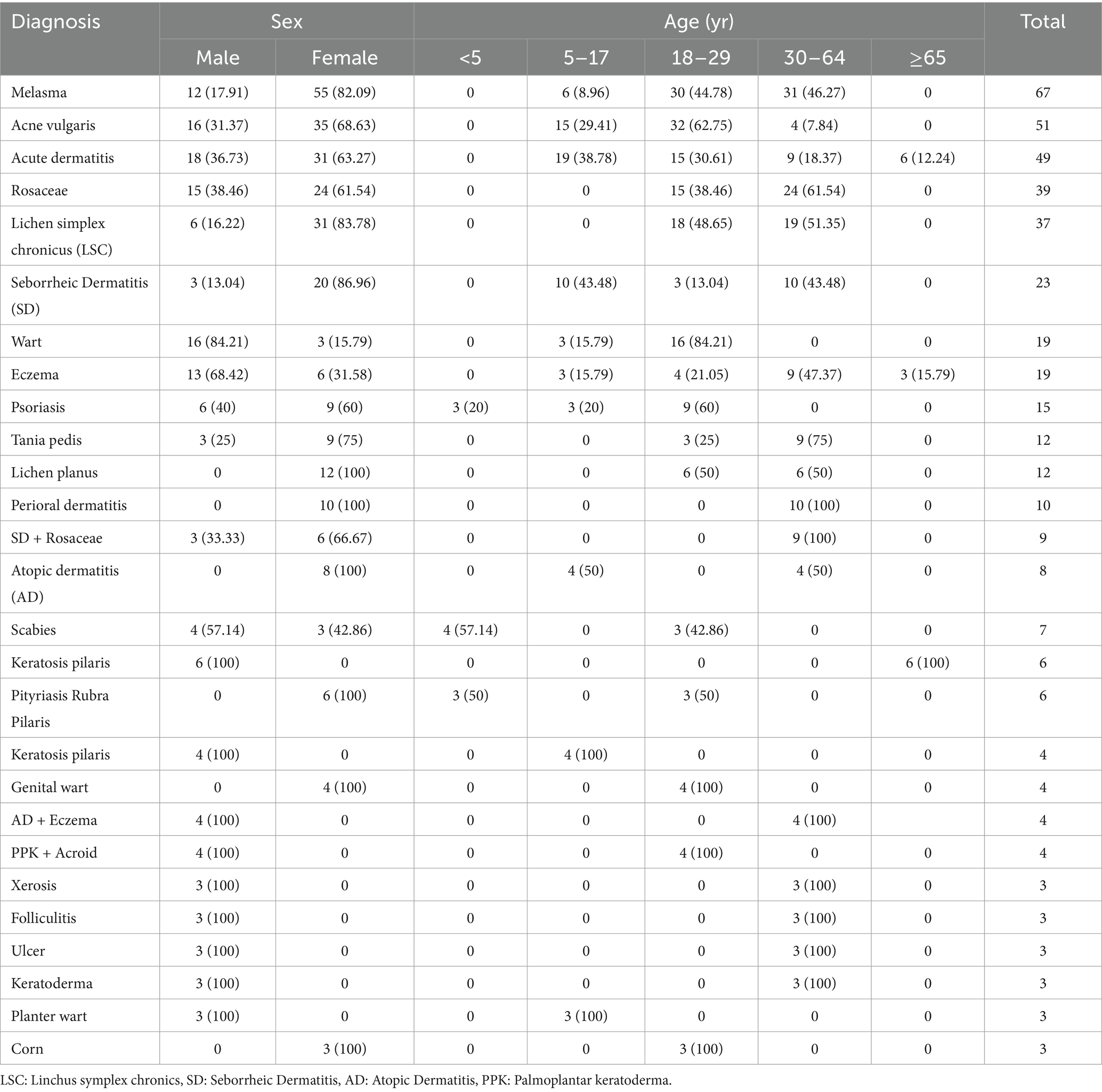
Table 1. Distribution of the top ten dermatological conditions for which compounded medications were prescribed by age and sex at selected hospitals in Northwest Ethiopia, 2023 (N = 423).
Number of active ingredients used in extemporaneous dermatological preparations
A total of 13 Active pharmaceutical Ingredients (API) were prescribed to be developed as extemporaneous dermatological preparations. Majority of the prescriptions, 238 (56.26%) contained two drugs (Table 2).
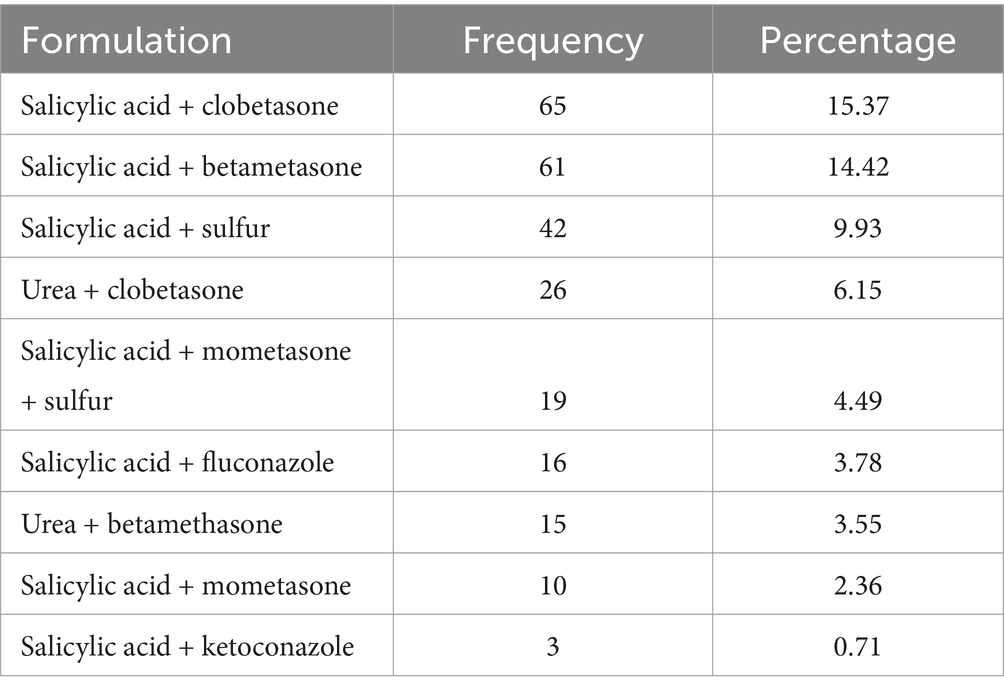
Table 2. Commonly prescribed combination of active ingredients for dermatological preparation at selected hospitals in Northwest Ethiopia, 2023 (N = 423).
Dosage forms used in extemporaneous dermatological preparations
Of the total 423 formulations prepared extemporaneously, 406 (95.98%) were semisolid preparations, while the remaining 17 (4.02%) were liquids (Table 3).
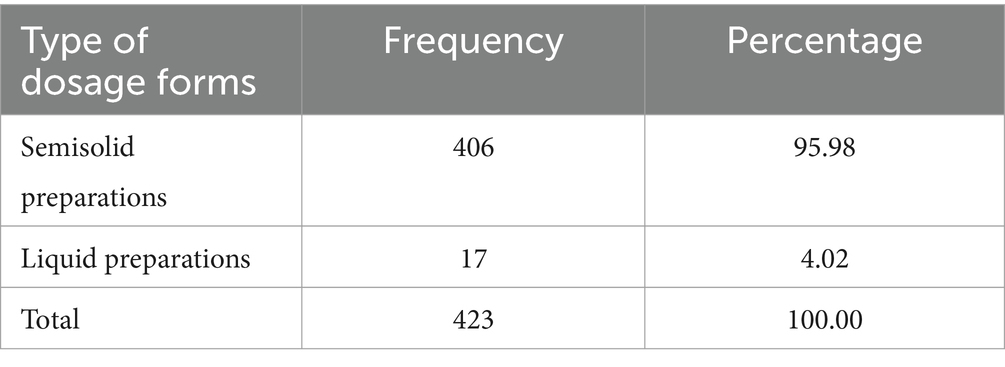
Table 3. Type of dosage forms used for extemporaneous dermatological preparation at selected hospitals in Northwest Ethiopia, 2023 (N = 423).
Active ingredients used in extemporaneous dermatological preparations
The two extemporaneous medications that were prescribed the most commonly were Salicylic acid (35.39%) and Clobetasone (13.03%), respectively (Table 4).
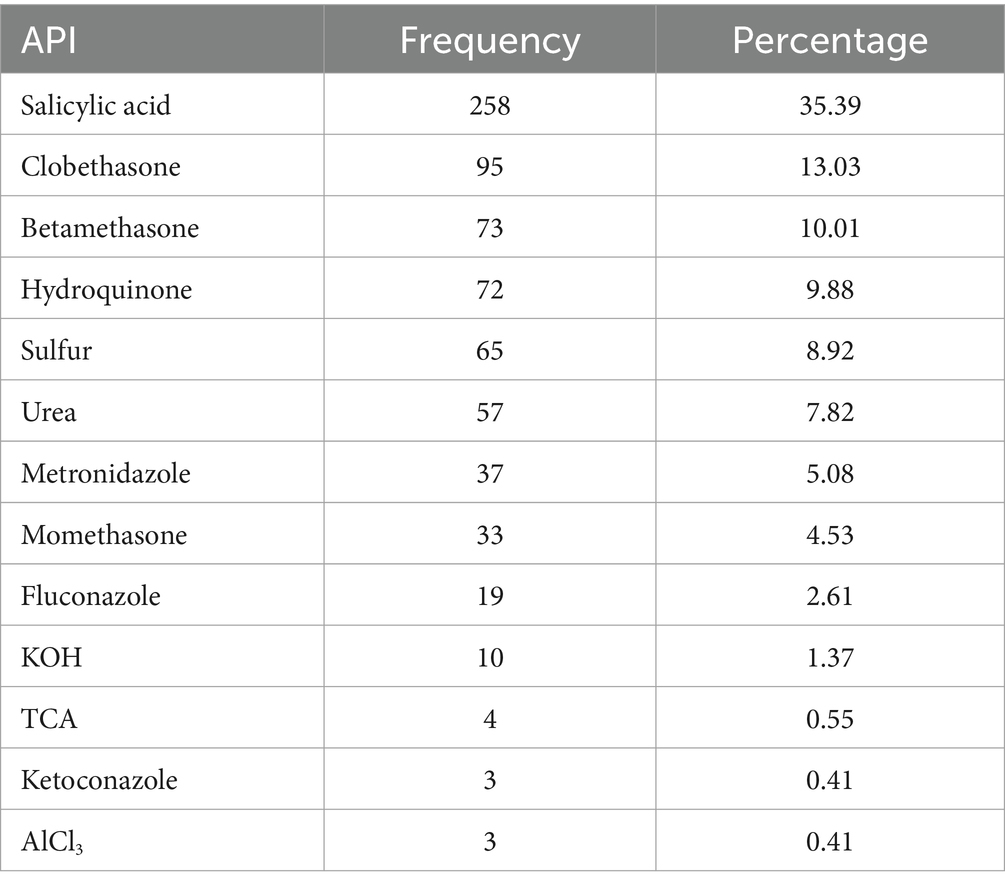
Table 4. Number of APIs per encounter of dermatological preparations at selected hospitals in Northwest Ethiopia, 2023 (N = 423).
Excipients used in extemporaneous dermatological preparations
Among the frequently utilized excipients for the compounding of dermatological preparations, Vaseline 200 (47.6%), Nivea cream 186 (44.3%), followed by absolute alcohol and liquid paraffin 17 (4.05%), respectively (Figure 1).

Figure 1. Excipients used for the compounding of dermatological preparations at selected hospitals in Northwest Ethiopia between January and April 2023 (N = 423).
Characteristics and composition the active ingredients most frequently used in dermatological preparations
Hydroquinone (4%) with Nivea cream (30gm) (17.0%), followed by salicylic acid (5%) + Betamethasone (75 g) + Vaseline (20 g) (10.6%) were most commonly prescribed dermatological formulations in the study area (Table 5).
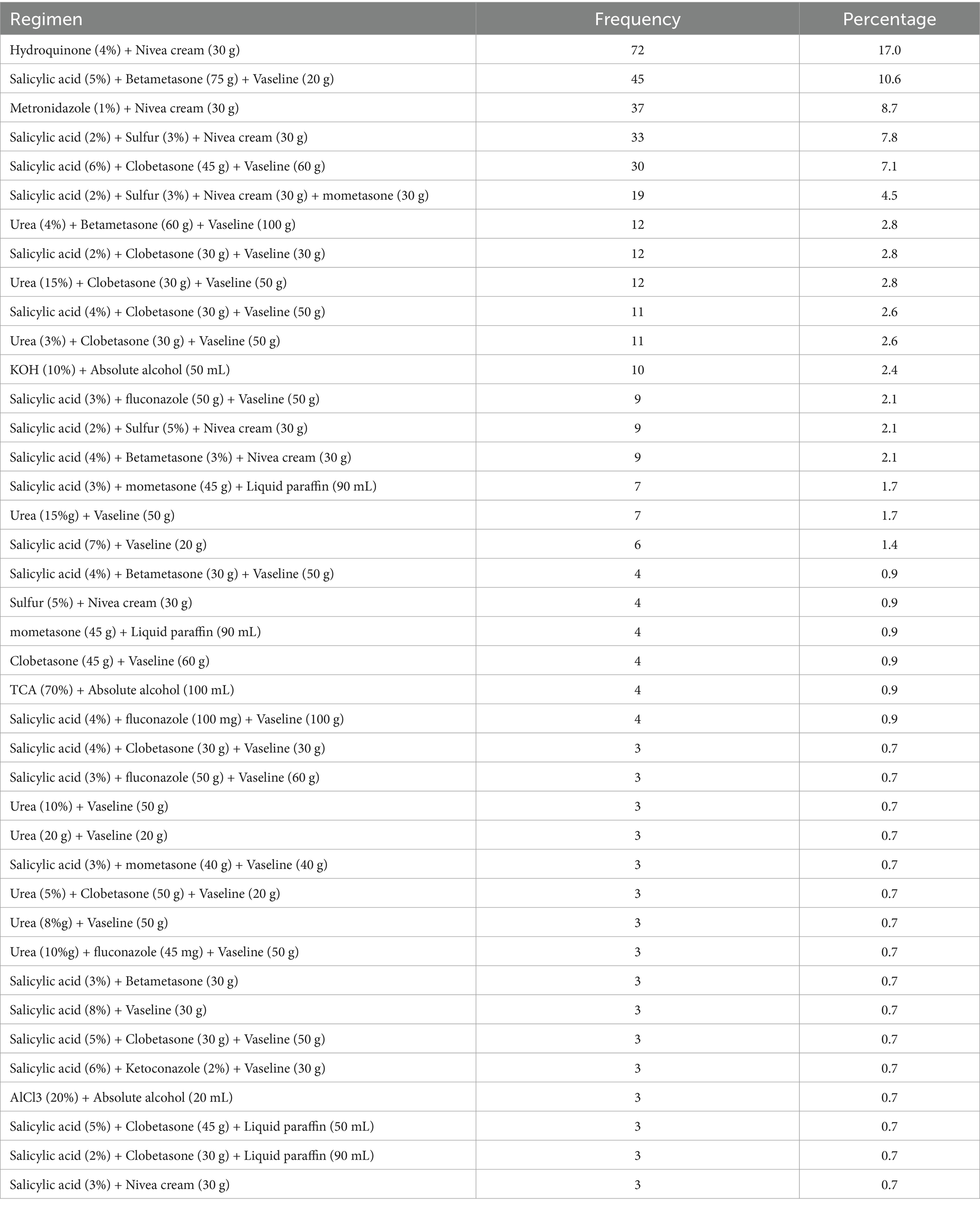
Table 5. Compositions of formulations prescribed for dermatological preparation at selected hospitals in Northwest Ethiopia, 2023 (N = 423).
Discussion
Most patients in this study were female, and females were more likely to suffer from autoimmune and allergy diseases as well as skin conditions such as psychosomatic issues, pigmentary disorders, specific hair diseases, and psychosomatic problems. Although differences in skin structure and physiology due to the impact of sex hormones may influence gender disparities in skin diseases, the processes behind these differences remain largely unclear (18).
Melasma was found to predominantly affect female patients (82.9%) in the age group of 30–64 years (46.7%), this result is similar to previous studies as epidemiological review showed a clear female predominance was observed in the reports of the disease, ranging from 9 or 10 to 1 (19). This could be because female sex hormones are well-known risk factors for the development of melasma, and their preponderance in fertile women reinforces this hypothesis (20). In contrast to earlier research among Nigerian undergraduates, where there was a substantial difference between the severity of acne in males and females, acne vulgaris was the second most prevalent dermatological issue among females (68.6%), and mostly affected the age period of 18–29 years (68.6%) (21, 22). The female predominance in this study could be because stress is a significant factor in acne and females are more likely than males to suffer from stress-related depressive disorders and anxiety (23).
For most skin conditions in this study, extemporaneous dermatological products were prescribed for patients above the age of 18 years, and this result is similar to that of a previous study. For majority of skin problems, compounded dermatological products were meant for adults and older adult patients. The possible reason is dosage modifications or combining commercial products, which is considered in the study’s finding that the majority (56.3%) of prescriptions carry the combined drugs (5). This finding is opposite to other studies in which children under 12 years of age received relatively more extemporaneous compounded dermatological medicines than other age groups, which could be due to the need for the patient to be fashioned or fitted to resemble a tailor’s therapy for children (24–27).
The majority (60.76%) of compounded prescriptions carry two or more drugs, which is supported by other similar studies in ALERT hospital, were the majority of prescriptions (65%) carry more than one active pharmaceutical substance (5). However, the rate was greater than in our study in the compounded prescription pattern study carried out in Jogjakarta Province hospital (28) because of the nature of some skin disorders that could respond to a single active medicinal ingredient. A survey of Australian general practitioners mentioned these as the major reasons for prescribing certain extemporaneous formulations (29).
Among prescribed drugs, salicylic acid (35.39%) and clobetasone (13.03%) were the most commonly used active pharmaceutical substances in the hospital for extemporaneous compounded products, which is similar to a previous study in which salicylic acid (38.0%) was the most commonly used ingredients (2). Therefore, their continued availability is necssary to satisfy the specific needs of patients. In contrast to clobetasone, which was purchased as a cream from the market and used for compounding, salicylic acid is available in the hospital in powder form.
Ointments and creams comprised the majority of dose forms from the compounded products in the study area, which is consistent with other similar studies (26, 30, 31). Dermatological treatments offered in the form of these dosage forms may be due to the type of skin lesions (dry or leaking moisture) found on patients and the ease of accessibility to vehicle agents.
Several semisolid formulations are available for topical applications intended to address the type and location of skin problems. Ointments are substances used on the skin to soothe or heal wounds, burns, rashes, scrapes, and other skin problems. Also called unguent. The possible reason for the preference of these semisolid dosage forms is their ease of application, rapid formulation, and ability to topically deliver a wide variety of drug molecules compared with the application site in liquid preparations (18, 23).
Strength and limitations
A strength of the current study is its multicenter nature, which increases the generalizability of the findings. Despite the findings of this study, we acknowledge that it has limitations. Drugs that were not available in the hospital are excluded and extemporaneous products from community pharmacies or outside the hospital were not included.
Conclusion
Dermatological disease is more prevalent in the study area. Females are mostly affected. The four top prevalent dermatological diseases are: melasma, acne vulgaris, acute dermatitis, and rosacea. Extemporaneous topical semisolid preparations containing two or more active ingredients are the most widely compounded and prescribed products for pharmaceutical care. Salicylic acid, clobetasone, and betametasone are widely used active pharmaceutical ingredients. Vaseline and Nivea cream are the most commonly used vehicles for compounding preparations. Hydroquinone (4%) with Nivea cream (30gm), Salicylic acid (5%) + Betametasone (75 g) + Vaseline (20 g) were the most commonly prescribed dermatological formulations. There are concerns related to the applicability of a country regulatory requirements and good manufacturing practices, and their efficacy, quality, and safety. So, this study suggests that application of good manufacturing practices and compounding guidelines for extemporaneous compounding of dermatological formulations to ensure efficacy, quality, and safety.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by a letter of ethical approval received from Debre Markos University’s ethical review committee with reference number SPH01/10/19. Oral consent, from all study participants was obtained before data collection, and the data collection was started after getting permission and provision. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
ED: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AS: Conceptualization, Data curation, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. WT: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft. BM: Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft. MG: Conceptualization, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors want to thank the hospital administration for their positive cooperation during the study. We would also like to forward our gratitude to the data collectors and study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fpubh.2025.1685055.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hay, RJ, Johns, NE, Williams, HC, Bolliger, IW, Dellavalle, RP, Margolis, DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. (2014) 134:1527–34. doi: 10.1038/jid.2013.446
2. Giesey, RL, Mehrmal, S, Uppal, P, and Delost, GR. The global burden of skin and subcutaneous disease: A longitudinal analysis from the global burden of disease study from 1990-2017. Ski J Cutan Med. (2021) 5:125–36. doi: 10.25251/skin.5.2.7
3. Flohr, C, and Hay, R. Putting the burden of skin diseases on the global map. Br J Dermatol. (2021) 184:189–90. doi: 10.1111/bjd.19704
4. Seth, D, Cheldize, K, Brown, D, and Freeman, EE. Global burden of skin disease: inequities and innovations. Curr Dermatol Rep. (2017) 6:204–10. doi: 10.1007/s13671-017-0192-7
5. Selam, MN, Ababu, A, Bayisa, R, Abdella, M, Diriba, E, Wale, M, et al. Prescribing pattern of dermatological compounding in Ethiopia: the case of ALERT hospital. Integr Pharm Res Pract. (2022) 11:1–8. doi: 10.2147/IPRP.S346395
6. Barankin, B, and DeKoven, J. Psychosocial effect of common skin diseases. Can Fam Physician. (2002) 48:712–6. Available at: https://pmc.ncbi.nlm.nih.gov/articles/instance/2214020/pdf/12046366.pdf
7. Watson, CJ, Whitledge, JD, Siani, AM, and Burns, MM. Pharmaceutical compounding: a history, regulatory overview, and systematic review of compounding errors. J Med Toxicol. (2021) 17:197–217. doi: 10.1007/s13181-020-00814-3
8. Mohiuddin A, K. Extemporaneous compounding: cautions, controversies and convenience. IP Int J Compr Adv Pharmacol. (2020) 3:124–37. doi: 10.18231/2456-9542.2018.0028
9. Minghetti, P, Pantano, D, Gennari, CGM, and Casiraghi, A. Regulatory framework of pharmaceutical compounding and actual developments of legislation in Europe. Health Policy (New York). (2014) 117:328–33. doi: 10.1016/j.healthpol.2014.07.010
10. Yusuff, KB. Extent of extemporaneous compounding and pattern of prescribing and use of extemporaneous medicines in a developing setting. J Pharm Heal Serv Res. (2019) 10:255–60. doi: 10.1111/jphs.12297
11. Helm, MF, Farah, JB, Carvalho, M, Farah, FS, and Farah, RS. Compounded topical medications for diseases of the skin: A long tradition still relevant today. North Am J Med Sci. (2017) 10:116–8. doi: 10.7156/najms.2017.1003116
12. Ethiopian Food and Drug Authority. Guideline for registration of low-risk medicines; (2020). Available online at: https://www.efda.gov.et/publication/guideline-for-registration-of-low-risk-medicines/ (Accessed October 22, 2020).
13. Elder, DL. A practical guide to contemporary pharmacy practice and compounding. 4th ed. Wolters Kluwer Health: Philadelphia, PA, USA (2018).
14. Allen, LV. The art, science, and Technology of Pharmaceutical Compounding. 5th ed. American Pharmacists Association: Washington, DC, USA (2016).
15. Ministry of Health-Ethiopia. National guideline for compounding of dermatological preparations ; (2020). Available online at: https://www.researchgate.net/publication/359414292_Final_FMOH_National_Guideline_for_Compounding_of_Dermatological_2
16. Ababu and Selam. Extemporaneous compounding practice for dermatologic preparations in Ethiopian public hospitals: regulatory requirements and quality concerns. Risk Manag Healthc Policy. (2021) 14:1933–8. doi: 10.2147/RMHP.S300906
17. USP (795). Pharmaceutical compounding—non sterile preparations; (2019). Available online at: https://www.usp.org/compounding/general-chapter-795 (Accessed October 24, 2020).
18. Chen, W, Mempel, M, Traidl-Hofmann, C, Al Khusaei, S, and Ring, J. Gender aspects in skin diseases. J Eur Acad Dermatology Venereol. (2010) 24:1378–85. doi: 10.1111/j.1468-3083.2010.03668.x
19. Handel, AC, Miot, LDB, and Miot, HA. Melasma: A clinical and epidemiological review. An Bras Dermatol. (2014) 89:771–82. doi: 10.1590/abd1806-4841.20143063
20. Espósito, ACC, Cassiano, DP, da Silva, CN, Lima, PB, Dias, JAF, Hassun, K, et al. Update on Melasma—part I: pathogenesis. Dermatol Ther (Heidelb). (2022) 12:1967–88. doi: 10.1007/s13555-022-00779-x
21. Ayanlowo, O, Ariyo, M, and Adekanmbi, A. Acne vulgaris in an undergraduate population in Nigeria. West Afr J Med. (2020) 37:62–6.
22. Ashok Kumar, M, Noushad, PP, Shailaja, K, Jayasutha, J, and Ramasamy, C. A study on drug prescribing pattern and use of corticosteroids in dermatological conditions at a tertiary care teaching hospital. Int J Pharm Sci Rev Res. (2011) 9:132–5.
23. Dreno, B, Bagatin, E, Blume-Peytavi, U, Rocha, M, and Gollnick, H. Female type of adult acne: physiological and psychological considerations and management. JDDG-J Ger Soc Dermatology. (2018) 16:1185–94. doi: 10.1111/ddg.13664
24. Brion, F, Nunn, AJ, and Rieutord, A. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatr Int J Paediatr. (2003) 92:486–90. doi: 10.1111/j.1651-2227.2003.tb00583.x
25. Collier, CN, Harper, JC, Cantrell, WC, Wang, W, Foster, KW, and Elewski, BE. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. (2008) 58:56–9. doi: 10.1016/j.jaad.2007.06.045
26. Kairuz, T, Chhim, S, Hasan, F, Kumar, K, Lal, A, Patel, R, et al. Extemporaneous compounding in a sample of New Zealand hospitals: a retrospective survey. N Z Med J. (2007) 120:1–9.
27. Ohrem, HL, Schornick, E, Kalivoda, A, and Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm Dev Technol. (2014) 19:257–62. doi: 10.3109/10837450.2013.775154
28. Wiedyaningsih, C, Widyaswari, R, Hasani, M, and Dhani, W. Compounding prescription patterns: factors influencing the physicians to prescribe compounded medicines for pediatric outpatients. Res Soc Adm Pharm. (2012) 8:e29. doi: 10.1016/j.sapharm.2012.08.067
29. Pappas, A, Mac Pherson, R, and Stewart, K. Extemporaneous prescribing: whatever happened to it? A survey of australian general practitioners. J Pharm Pract Res. (2002) 32:310–4. doi: 10.1002/jppr2002324310
30. Euphenia, MM, Fatima, S, and Thirumala, G. Investigating extemporaneous compounding practices in the Polokwane tertiary hospital pharmacies in South Africa—a pilot study. African J Pharm Pharmacol. (2015) 9:1099–105. doi: 10.5897/AJPP2015.4282
Keywords: compounding, extemporaneous preparation, dermatological disease, skin preparations, Ethiopia
Citation: Dagnew EM, Sendekie AK, Tsega W, Mekonnen BA and Getachew M (2025) Extemporaneous dermatological compounding in hospital pharmacies, Northwest Ethiopia. Front. Public Health. 13:1486936. doi: 10.3389/fpubh.2025.1486936
Edited by:
Eman Leung, The Chinese University of Hong Kong, ChinaReviewed by:
Abraham Getachew Kelbore, Wolaita Sodo University, EthiopiaMarc Marie Dooms, University Hospitals Leuven, Belgium
Copyright © 2025 Dagnew, Sendekie, Tsega, Mekonnen and Getachew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ephrem Mebratu Dagnew, bWVicmF0dWVwaHJlbTIwMTNAZ21haWwuY29t
†ORCID: Biset Asrade Mekonnen, orcid.org/0000-0001-8799-7146
 Ephrem Mebratu Dagnew
Ephrem Mebratu Dagnew Ashenafi Kibret Sendekie
Ashenafi Kibret Sendekie Wondale Tsega
Wondale Tsega Biset Asrade Mekonnen
Biset Asrade Mekonnen Melese Getachew
Melese Getachew