- 1Behavioral Neuropharmacology and Neuroimaging Laboratory on Addictions, Department of Pharmacology and Toxicology, Clinical Research Institute on Addictions, Jacobs School of Medicine and Biosciences, University at Buffalo, Buffalo, NY, United States
- 2Department of Surgery, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY, United States
- 3Division of Addiction Research & Education, Center for Exercise Sports & Global Mental Health, Western University Health Sciences, Pomona, CA, United States
- 4Department of Molecular Biology, Adelson School of Medicine, Ariel University, Ariel, Israel
- 5Department of Psychiatry, Washington University in St. Louis, St. Louis, MO, United States
- 6Department of Natural Sciences and Mathematics, D'Youville University, Buffalo, NY, United States
- 7UBMD Pediatrics Division of Endocrinology/Diabetes, Buffalo, NY, United States
The obesity epidemic has become a global public health issue, impacting more than one billion people worldwide. 9% of the US population, or 28.8 million Americans will have an eating disorder in their lifetime. In fact, global eating disorder prevalence increased from 3.5% to 7.8% between 2000 and 2018. In spite of the fact that less than 6% of people with an eating disorder are medically underweight, it is indeed an important factor when considering issues related to obesity. This public health problem is often described as being caused by various genetic and psychosocial factors. One of the most effective strategies for treating morbid obesity and achieving significant weight loss is bariatric surgery. Recent focus on precision medicine approaches has expanded into bariatric surgery in an effort to better understand and achieve improved outcomes and reduce risk for post-operative weight regain and addiction transfers during the recovery process. Addiction transfers, including substance and non-substance addictions, are well established concerns for post-bariatric patients. This review details the genetic, molecular and psychosocial factors that can be utilized to inform and guide personalized treatment. Additionally, this review details some of the molecular mechanisms including dysregulation of catecholamine signaling as well as other neurotransmitter systems relevant to help further understand recovery science.
1 Introduction
Obesity is a growing epidemic affecting more adults each year. In 2016, 1.5 billion adults were impacted by obesity worldwide (1). This problem is projected to persist by the year 2030, with an approximated 1.35 billion overweight individuals followed by 573 million obese adults (1, 2, 159). Due to varying patterns in fat and body composition, the geographic concentration of obese individuals is greater for much of Asia, Latin America, the Middle East, and Africa.
Additionally, obesity is a risk factor for some of the most prevalent adult diseases. It was found that an increased 40 billion dollars in medical spending is required annually for obesity-related health problems (2). Insulin resistance, dyslipidemia, and high blood pressure are common comorbidities in obese patients, and there is ongoing research on possible genetic ties to these diagnoses (3). Additional comorbidities that have been associated with obesity include cardiovascular disease, some cancers, kidney disease, obstructive sleep apnea, gout, osteoarthritis, and hepatobiliary disease, many of which can shorten lifespan (4, 5). Decreasing worldwide prevalence of obesity would thus improve overall health.
While there are various FDA approved anti-obesity medications available to the public, some of these medications can present adverse effects to patients and can be costly. Bariatric surgery is an effective means of weight loss for individuals who have been unsuccessful with traditional weight loss methods (6, 7). One common failed method of weight loss includes the implementation of a restrictive diet, such as a diet low in carbohydrates. There is no significant advantage of low carbohydrate diets compared to traditional nutritionally balanced, energy restricted diets, and low carbohydrate intake can lead to further complications such as heart arrhythmias, kidney damage, osteoporosis, increased cancer risk, and more (8). Some have concluded that in comparison to dietary methods, exercise, pharmacotherapy and behavioral therapy, bariatric surgery is the most effective means of weight loss in obese patients (9).
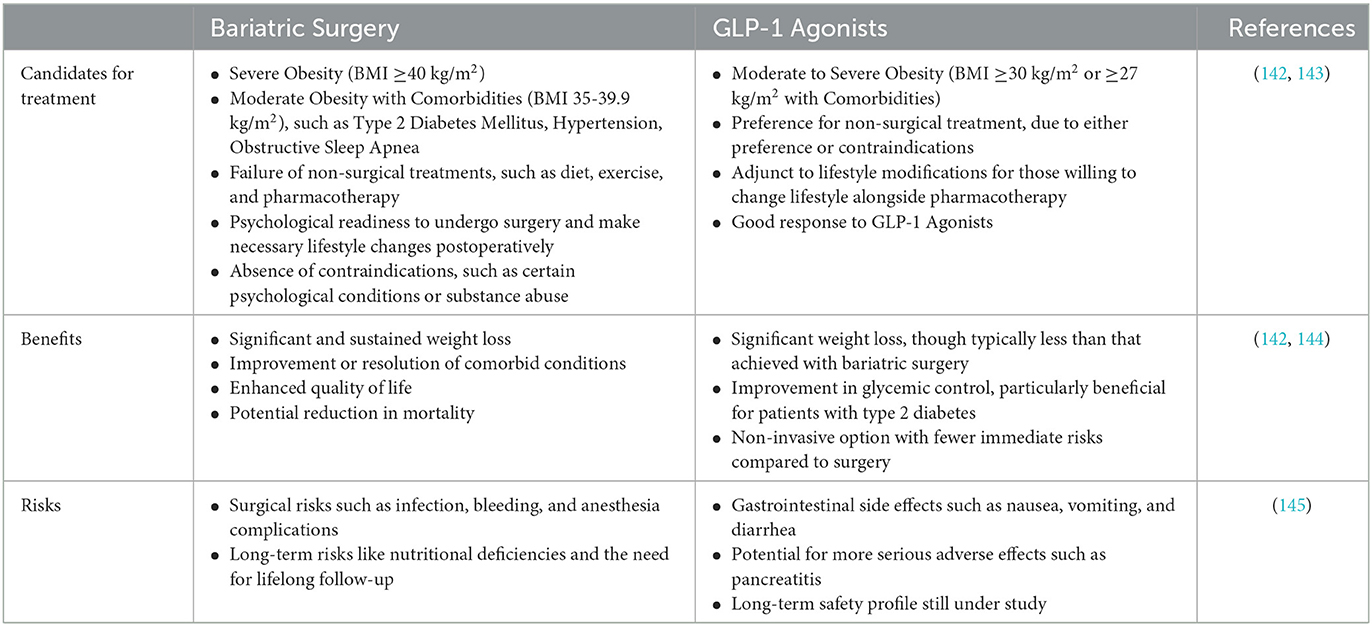
In the context of severe obesity, both bariatric surgery and GLP-1 receptor agonists (GLPs) have proven to be effective treatments. However, the decision on which treatment is appropriate for a particular individual depends on various factors, including the severity of obesity, presence of comorbidities, previous weight loss attempts, and patient preferences (see Table 1). The choice between bariatric surgery and GLP-1 receptor agonists for the treatment of severe obesity should be personalized based on individual patient characteristics, preferences, and clinical circumstances. Both options can lead to significant weight loss and improvement in obesity-related comorbidities, but they come with different risks and benefits that need to be carefully considered (6, 10–12)
Regarding bariatric surgery, the two most common types of procedures include laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (RYGB). One study reported that after 7 years, the success rates 47% weight and 55% weight loss respectively, and both surgeries improved quality of life of the patients (13). While bariatric surgery has proven to improve the lives of many individuals suffering from obesity, there are also potential risks that bariatric surgery may pose. These risks include post-operative weight regain as well as post-surgical complications including adhesions, intestinal leak, etc. In addition, several behavioral risks can be revealed after bariatric surgery. In fact, it has been found that many substance and non-substance behavioral addictions tend to increase after obesity operations (14). These risks can be assessed proactively by administering baseline psychological screenings to identify traits that patients experience before surgery. Obtaining this data can help inform psychological treatment post-surgery (6, 10–12).
This review is a thorough description of the facets of obesity and bariatric surgery (Figure 1) that crossover to addiction and reward processes. This narrative review was conducted by searching PubMed electronic databases utilizing the search terms “Obesity” “addiction”, “Dopamine”, “hypodomanergia”, “gastric bypass”, “vertical sleeve gastrectomy”, and others. In addition, genes associated with reward processing were searched in combination with variables related to obesity.
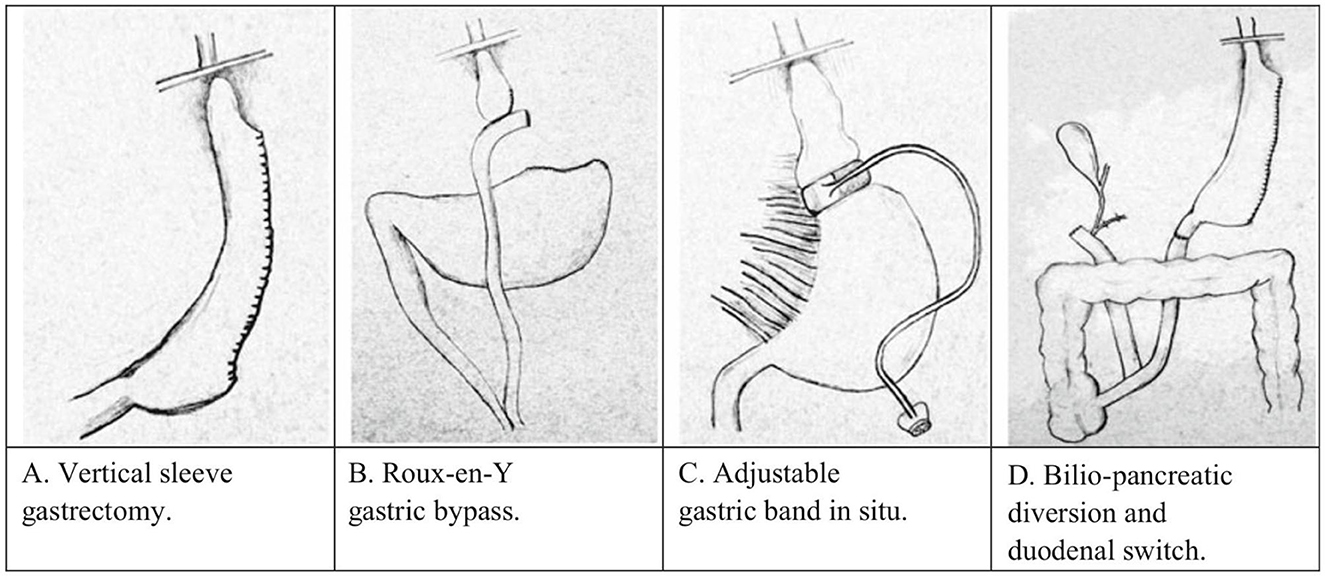
Figure 1. Types of bariatric surgery in humans as adopted from (141). (A) Vertical sleeve gastrectomy, (B) Roux-en-Y gastric bypass, (C) adjustable gastric band, and (D) bilio-pancreatic diversion and duodenal switch.
2 Obesity and bariatric surgery (history of bariatric surgery, types, and outcomes)
The first bariatric surgery, deemed “jejuno-ileal bypass” was performed in 1954 (Kremen, Linner, and Nelson 15). Though this form of surgery introduced many risks, such as dehydration and diarrhea (15), patients with high cholesterol achieved normalized lipid levels, and resolution of diabetes/prediabetes, reduced hypertension and sleep apnea (16–23). The first form of modern bariatric surgery was performed in 1966, after it was noted that cancer patients who underwent sub-total gastrectomy lost large amounts of weight. Initially, the procedure consisted of a horizontal gastric transection with a loop ileostomy, but it was later optimized to smaller gastric pouches and stoma sizes (24). The “Roux-en-Y” loop, which diverts bile from the stomach and the esophagus, decreases bile reflux (25). This form of gastric bypass decreased the risk for diarrhea, dehydration, kidney stones and gallstones (15). The Fobi-Capella banded gastric bypass is a method designed to boost weight loss by using a ring to constrain gastric pouch enlargement and curb weight regain (26).
Performing laparoscopic RYGB presents significant technical challenges, characterized by a steep learning curve and the potential for leaks at two points of anastomosis (15). Given the technical hurdles in laparoscopic surgery for patients with Class 3 obesity, Gagner (27) proposed a staged procedure from the original Scopinaro type biliopancreatic diversion (28), initiating the process with a vertical gastrectomy (sleeve) followed by the duodenal switch (27). This stepwise approach, including sleeve gastrectomy as the first stage, had been advocated due to its effectiveness, as evidenced by significant weight loss outcomes (56%) (15).
A study by Nasser et al. detailed some of the different rationales behind performing each type of bariatric surgery (29). Sleeve gastrectomy is currently the most performed bariatric surgery. Generally, the decision to perform RYGB is based on higher BMIs and obesity comorbidities, such as diabetes mellitus, gastroesophageal reflux disease, BMI ≥ 50 kg/m2, obstructive sleep apnea, hypertension, hyperlipidemia, and American Society of Anasthesisiologists (ASA) class > 3. The ASA classification measures a patient's preoperative risk on a scale of 1 to 5 based upon physiological status and comorbid conditions (29–31). Additionally, it was found that sleeve gastrectomy was performed more often in patients who were deemed “high risk”, which included a history of smoking, steroid use, kidney disease, and chronic obstructed pulmonary disease (29). Additionally, although all bariatric surgery types pose a risk for addiction transfer and alcohol misuse, RYGB is generally understood to pose a greater risk for post-surgical alcohol consumption compared to other types of surgery (32–35).
A longitudinal study by Salminen et al. compared weight-loss outcomes between the two common procedures in 240 patients seven years after surgery at 5 years, 7 years, and 10 years (13, 36). After 5 years, The mean percentage of excess weight loss at 5 years was roughly 49% after sleeve gastrectomy and approximately 57% after gastric bypass surgery. The difference between the two groups was around 8.2 percentage units, favoring gastric bypass. These findings did not indicate equivalence between the two procedures (36). After 7 years, following sleeve gastrectomy, the mean percentage of excess weight loss (%EWL) was approximately 47% (CI 43–50%). After RYGB, the %EWL was roughly 55% (CI52%−59%). The difference between the two procedures was around 8.7 percentage units (CI 3.5–13.9 percentage units), favoring RYGB (13).
At 10 years, the median excess weight loss (%EWL) was 43.5% following LSG and 50.7% following RYGB. On average, %EWL differed between the procedures; RYGB had an 8.4% higher %EWL. There were no statistically significant differences in type 2 diabetes remission, dyslipidemia, or obstructive sleep apnea post-LSG and RYGB. However, hypertension remission was better after RYGB (8% vs. 24%; P = 0.04). Esophagitis occurred more frequently after LSG (31% vs. 7%; P < 0.001) (37).
A clinical trial by Peterli, compared the outcomes between sleeve gastrectomy and gastric bypass 5-years after the procedure (38), finding that Gastric reflux showed a higher rate of remission following RYGB (60.4%) compared to sleeve gastrectomy (25%). Conversely, gastric reflux worsened, as measured by symptoms or escalation of medical therapy, more frequently after LSG (31.8%) than after RYGB (6.3%). Reoperations or interventions were needed for 16 out of 101 patients (15.8%) after sleeve gastrectomy and 23 out of 104 patients (22.1%) after RYGB. There was no significant difference in weight loss between the two procedures in their cohort (38).
Gut microbiotia is a growing area of research in the field of bariatric surgery. Obesity leads to decreased gut microbiota diversity and increased micronutrient deficiencies, and bariatric surgery alters gut microbiota composition and impacts the synthesis of vitamins like riboflavin, folate, B12, and vitamin K2 (39). Gutiérrez-Repiso analyzed the gut microbiota of 76 patients undergoing sleeve gastrectomy, classifying them into responder and nonresponder groups based on weight loss after one year (40). It was found that the responder group had a distinct gut microbiota composition, with a higher Prevotella-to-Bacteroides ratio compared to the nonresponder group before surgery, which could potentially predict weight loss outcomes. After surgery, the responder group showed an increase in microbiota linked to beneficial metabolic effects, suggesting that preoperative gut microbiota may influence bariatric surgery success (40).
Additionally, further intervention must be considered following bariatric surgery to ensure desirable results. Currently, it has been determined that within 5 years of bariatric surgery, 50% of patients experience weight regain and comorbidity relapse. Exercise is recommended following bariatric surgery to ensure optimal outcomes; however, a systematic review of 28 studies suggests that exercise intervention is poorly conducted in patients post-bariatric surgery. It is essential that this deviation be resolved as resistance and aerobic training support healthy weight, bone and cardiometabolic health, as well as aerobic capacity following bariatric surgery (41) (see Table 2).
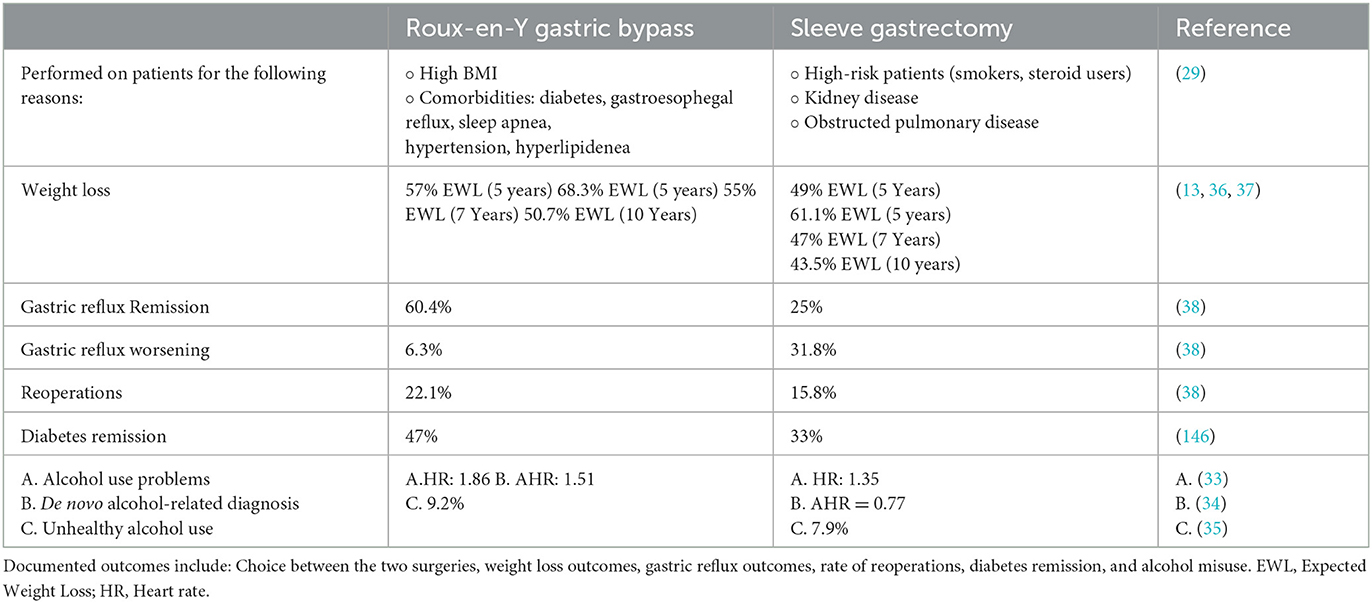
Table 2. An overview of documented outcomes comparing the two most common types of bariatric surgery: Roux-en-Y gastric bypass and sleeve gastrectomy.
3 Risk of addiction transfer
Reward Deficiency Syndrome (RDS) serves to measure the role of epigenetics and genetics in compulsive behaviors such as gambling, binge eating, alcohol and drug abuse (5). This offers a genetic descriptive of Pre-Addiction, or simply put, a predisposition to addiction behaviors (42). Further, the Genetic Addiction Risk Severity (GARS) assay is used to detect common polymorphisms related to RDS (43). A few of these polymorphisms include DRD2, DRD3, DRD4, DAT1, COMT, OPRM1, and 5HTT polymorphisms surgery, a few of which being alcohol addiction, drug addiction, and gambling (44). Alcoholism, specifically, frequently develops in patients post-bariatric surgery (45). It has been found that within 5 years of undergoing RYGB surgery, approximately 20% of patients developed alcohol use disorder (AUD) as their symptoms of food overconsumption decreased with post-surgical weight loss (45) The literature is conflicting on whether the choice of bariatric surgery procedures increases the risk of AUD. Although all bariatric surgery types pose a risk for addiction transfer and alcohol misuse, RYGB bypass is generally understood to pose a greater risk for post-surgical alcohol consumption compared to other types of surgery such as laparoscopic adjustable gastric banding (32–35, 46). Conversely, further research has suggested that there is no significant difference in the incidence of AUD following RYGB bypass compared to LSG procedures—which currently accounts for over half of all primary bariatric surgeries (47). While addiction risk does pose a serious threat to patients, bariatric surgery is still an attractive option for patients suffering from obesity as the mortality rates of obese patients post-bariatric surgery have significantly decreased (48–51).
There is, however, conflicting evidence on addiction transfer in bariatric populations (52, 53). Dickhut et al. conducted a study in 49 patients undergoing bariatric surgery (52). In this study, various measures of impulsive and compulsive behaviors were collected including those on alcohol intake, internet use, gambling, shopping, and sex addiction. In this study, no new addiction symptoms emerged and many of these scores significantly decreased at 1-year follow ups (52). Another study found that while both sleeve gastrectomy and gastric bypass significantly reduced food addiction in obesity patients, neither procedure led to cross-addiction, with no significant differences between the two surgical methods (53).
There are reports that psychiatric risks including substance abuse, self-harm and even suicide are matters of concern alongside post-surgical addiction transfer, with the greatest risk occurring 1–3 years post-surgery (54). In fact, it was found that the endorsement of substance misuse was related to a lower percentage of post-surgical weight-loss (55). Post bariatric substance misuse was also associated with a family history of substance misuse and residual psychological symptoms of food addiction, including nocturnal eating and selective hunger (55). One study conducted semi-structured interviews among 24 bariatric patients in substance abuse treatment programs (56). Three-quarters of patients recognized unresolved psychological issues, while over four-fifths pinpointed addiction transfer/substitution. Additionally, more than half observed quicker onset or heightened effects from substances, and nearly half noted increased accessibility of pain medications (56). Taylored psychotherapeutic techniques such as motivational interviewing and cognitive behavioral therapy can be utilized to promote health habits, including physical exercise and healthy eating (54, 57).
4 Genetics
The brain reward cascade includes the serotonergic, GABAergic, endorphinergic, cannibinergic, glutaminergic, cholinergic, and dopaminergic pathways. Specifically, the dopaminergic pathway is endpoint for reward in the brain. Imbalanced dopamine can bring about symptoms such as anhedonia, lack of motivation, and troubles coping with stress. Since psychoactive substances and addictive behaviors induce dopamine release, these behaviors are often seen in the use of individuals exhibiting this hypodopaminergic state (58). While RDS is an indicator for compulsive eating behaviors, the presence of these alleles also accounts for compulsive behaviors such as gambling and drug addiction. It has been identified that risk of addiction can be evaluated through the presence of various polymorphisms that play a role in compulsive behaviors (such as overeating) (59, 60), vulnerability to pain (58), and behavioral/conduct disorders (61). Specifically, there is a significant risk of alcohol use disorder in the presence of MAO, DRD1, DRD2, DRD3, DRD4, DAT1, COMT, OPRM1, GABABR3, and 5HTT polymorphisms (62). Alterations in these markers also applies to RDS which establishes a framework for these epigenetic behavioral expressions (63). The GARS test was developed by Dr. Kenneth Blum's research group and assesses 10 genes and 11 risk alleles that have been associated with substance and non-substance addictions (64). Specifically, the GARS test assesses an individual for RDS. RDS can be defined as a susceptibility to pain, addiction, and related behaviors. Various polymorphisms are assessed in the GARS such as DRD1, DRD2, DRD3, DRD4, MOA-A, COMT, DAT1, OPRM1, 5HTTLLR, and GABRA3 which are factors in the vulnerability of an individual to addiction and related compulsive disorders (59). Research on these polymorphisms has revealed a role in the body's pain mechanisms as well as a link between the OPRM1 gene and heroin abuse, the DRD2 gene and a high risk of heroin dependence, and the COMT gene linked to the response of opiates and enkephalins (65). Significant associations have also been found between the DRD3, DRD4, DAT1, COMT, OPRM1, and 5HTT genes and AUD (62). One study revealed that 77% of subjects known to have AUD contained the A1 allele of the D2 receptor gene, and 72% of participants without AUD did not have the A1 allele of the D2 receptor gene (66). One allele assessed for in the GARS assay, DRD2, has a Bayesian predictive value of 74% for detecting RDS behaviors. These various SNPs have significant effects on behaviors and addictions due to their role in brain pathways. Thus, testing for correlated alleles that alter these brain pathways, such as the DRD2 allele, can aid in the planning for adverse post-surgical addiction transfer. An overview of these genes and their associated addiction risks from documented clinical studies can be found in Table 3.
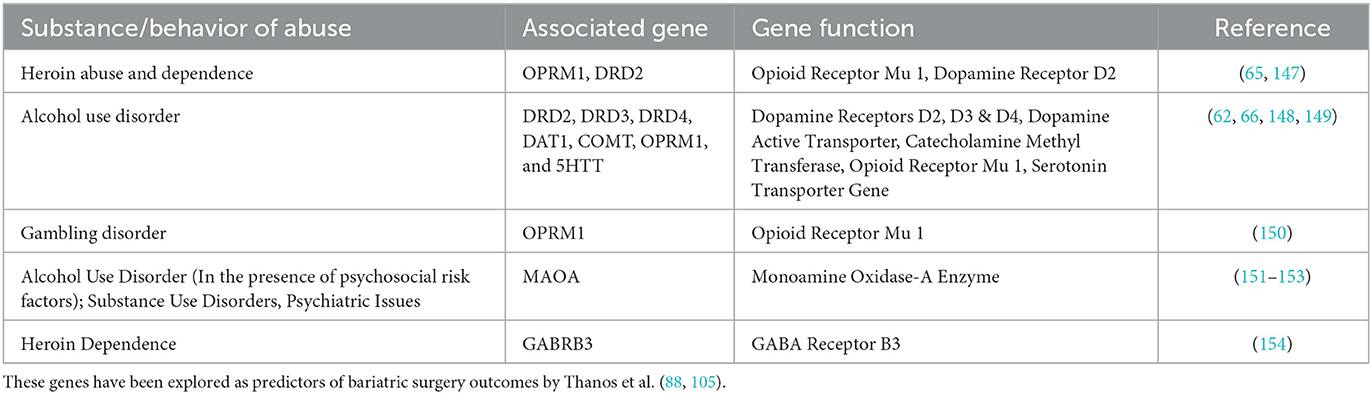
Table 3. An overview of the various addictive behaviors (substance and non-substance) associates with the SNPs as assessed by GARS.
5 Preclinical models of bariatric surgery
Several prior studies have demonstrated a causal relationship between the post-operative RYGB state and the increased risk of a variety of impulsive and compulsive behaviors. Patients who undergo RYGB often experience quicker onset, longer-lasting, and higher blood alcohol concentrations (67–69). Thanos et al. studied this phenomenon using male obese rats that underwent either RYGB or SHAM procedures (70). Both RYGB and SHAM rats were given a choice between water and varying ethanol concentrations over 32 days to assess alcohol consumption. The study found that RYGB rats consumed significantly more alcohol than obese SHAM rats. These data support that obesity is associated with hypodopaminergic signaling by way of reduced D2 and this reward deficiency in the presence of reduced food intake due to the surgery can increase risk for increased alcohol intake (70).
Polston et al. investigated whether RYGB could directly increase alcohol consumption, independent of changes in alcohol absorption and bioavailability (71). They used male obese rats which then underwent either RYGB or SHAM procedures. Both groups were trained to self-administer alcohol with RYGB rats showing greater alcohol intake and greater reinforcement of this behavior compared to SHAM rats. RYGB rats also demonstrated a greater number of responses to self-administer alcohol. These findings thus suggest that RYGB may alter brain reward pathways, increasing reward-seeking behavior rather than affecting the gastrointestinal absorption of alcohol (71).
Ghrelin, a peptide hormone produced in the stomach, is known to increase food intake in the fasting state (72). Hajnal et al. investigated how RYGB affects alcohol consumption in rats and whether changes in ghrelin activity contribute to this effect. In their study, obese male rats underwent either RYGB or SHAM procedures, and then trained to self-administer alcohol. RYGB rats showed significantly increased alcohol-seeking behavior at various ethanol concentrations compared to SHAM controls, and the ghrelin antagonist D-[Lys3]-GHRP-6 reduced ethanol intake in RYGB rats. The results suggest that RYGB may enhance sensitivity to ghrelin regulation and warrant further exploration into how ghrelin could be targeted for treating alcohol abuse in RYGB patients (72). Uchida et al. studied the impact of RYGB on ghrelin levels in obese mice (73). Both SHAM mice and control mice showed lower ghrelin levels, while RYGB obese mice had increased levels. These findings suggest a potential link between RYGB, altered ghrelin signaling, and behavioral responses, as supported by Hajnal et al. (72), who found that RYGB mice with a ghrelin antagonist showed reduced drug-seeking behavior compared to SHAM mice, indicating a complex relationship between ghrelin and reward systems (73). Orellana et al. compared the effects of vertical sleeve gastrectomy (VSG) and RYGB on ethanol intake using male rats and mice with diet-induced obesity (74), with results suggesting that the removal of ghrelin-producing cells in VSG might contribute to reduced ethanol consumption following bariatric surgery (74).
The endogenous opioid system of the body also plays a major role in regulating risk and reward behavior. McGregor et al. focused on whether RYGB affects the mu-opioid receptors of the brain and in turn, increase the risk of addictive behaviors (75). Male obese rats undergoing RYGB had decreased mu-opioid receptor levels in the central amygdala, a region known to regulate stress and anxiety response, which in turn can influence the risk of developing a substance use disorder (75).
Gamma-aminobutyric Acid (GABA) is a key inhibitory neurotransmitter implicated in alcoholism, with increased GABA-A receptors observed in post-mortem brains of individuals with alcohol use disorder due to chronic alcohol reducing natural GABA production (76, 77). It is also well known that increases in GABA-A receptors, especially in the mesolimibic circuitry of the brain, may occur by inhibiting dopamine release at the Nucleus Accumbens (78). McGregor et al. examined changes in GABA-A receptor expression in response to RYGB in male obese rats. RYGB rats exhibited increased [3H] flunitrazepam binding, a marker for GABA-A receptors, in the ectorhinal cortex and primary somatosensory cortex compared to controls. These findings suggest that RYGB surgery leads to overexpression of GABA-A receptors in specific brain regions. It is a possibility that this may contribute to a higher risk of alcohol abuse following the procedure (79).
Hamilton et al. also investigated how RYGB affects the mesolimbic dopamine (DA) system in rats, focusing on its role in eating and addictive behaviors (80). Male obese rats underwent either SHAM or RYGB surgery. After 9 additional weeks, rats were assessed for DA Type 1-like receptors (D1R), Type 2-like receptors (D2R), and DA Transporter (DAT) expression. It was found that SHAM obese rats showed reduced D1R and D2R expression and decreased DAT binding. RYGB rats, showed weight reductions but did not show significant differences in DA receptor expression compared to control rats, suggesting that RYGB may counteract the adverse effects of obesity on the dopaminergic signaling (80). These results were supported by subsequent studies (81, 82).
Thanos et al. tested the effects of RYGB on perception and anticipation of highly palatable foods compared to regular diets in obese male rats (83). Rats first underwent RYGB procedure, or a SHAM procedure. After 3 weeks post-surgery, all rats were conditioned to bacon and chow in a three-chamber Conditioned Placed Preference (CPP) Apparatus. All rats were then scanned twice throughout the duration of the experiment using in-vivo positron emission tomography (PET) to measure brain-glucose metabolism (BGluM). Results showed that Bacon CPP was only significant in RYGB rats that had stable weight loss post-procedure. Furthermore, PET of RYGB rats showcased increased BGluM in the regions of the right and midline cerebellum that are involved in subjective processes related to reward and expectation. The data suggests that anticipation of palatable foods in RYGB rats led to activation in the medial parabrachial nuclei, which is significant for gustatory processing, as well as the dorsomedial tegmental area, which is a region known to control reward, motivation, addiction, and cognition. On the other hand, bacon anticipation in control rats showed activation in the retrosplenial cortex and primary visual cortex. Thus, RYGB can lead to alterations in brain activity that influence reward expectations and sensory processing when there is anticipation of intake of palatable fatty foods (83).
Sleeve gastrectome (SG) has also been utilized in preclinical studies. Ding et al. found that compared with the sham operation group, SG rats showed improvements in a number of measures (84). These included improved metabolic and body weight measures, cognitive functions measured by Morris water maze and Y maze, and changes in the hippocampus related to cognitive decline including inhibition of hippocampal apoptosis and decreased phosphorylation of Tau at Ser 404 and Ser396 sites (84). Additional data suggests metabolism is improved in rats after SG, where improvements in insulin sensitivity was observed in both obesity prone and non-obesity probe rats (85). This study also observed decreased body weight, food intake, increased rectal temperature and upregulated brown adipose tissue Ucp-1 protein expression levels (85). Cardiovascular improvements, as well as reductions in bodyweight and increases in excess weight loss, have also been observed in SG treated rats (86). Multiple studies show how compared to RYGB and sham controls, rats who underwent SG consumed less alcohol (74, 87).
A summary of the various behavioral and neurochemical effects of bariatric surgery from preclinical studies is summarized in Table 4.
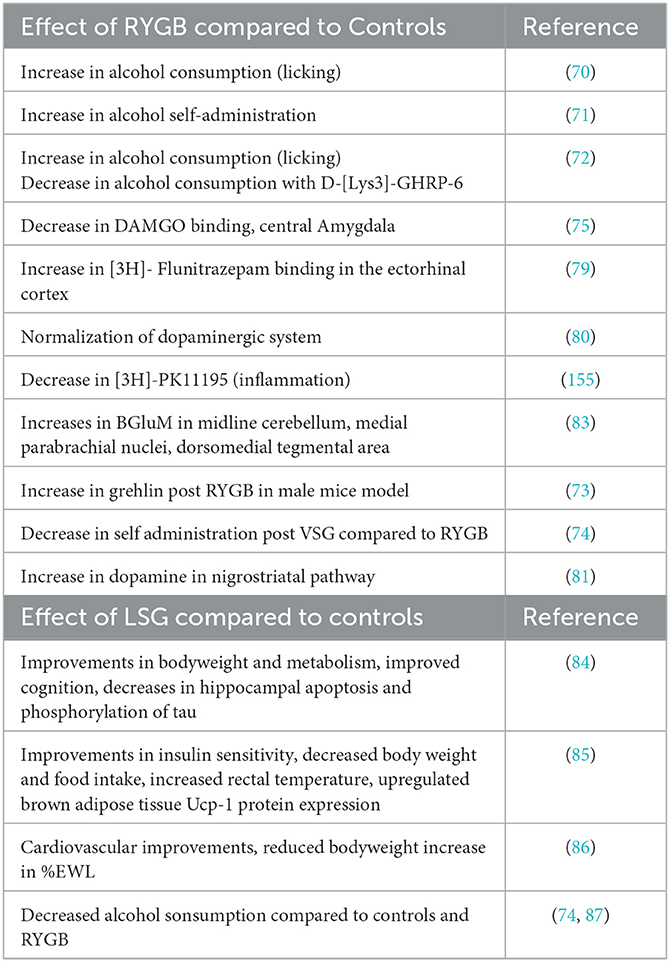
Table 4. Summary effects of bariatric surgery on behavior and neurochemistry in preclinical studies.
6 Genetics as a predictor of bariatric surgery outcomes
Various studies have been conducted to investigate the role of genetics in bariatric surgery outcomes. Though the relationship between obesity and However, the genetics associated with addiction risk have been underexplored in this domain. Thanos' group conducted exploratory research on this topic and evaluated GARS and psychosocial data as a means of predicting bariatric surgery outcomes at various time points post-surgery (88). Thirty four patients scheduled to undergo bariatric surgery underwent genetic testing using the GARS assay to evaluate for the presence of the 11 polymorphisms associated with motivation and reward using PCR amplification (43). Patients also submitted presurgical psychological data to evaluate nutritional habits, food addiction, binge eating disorder symptoms, chronic stress and life quality, and sleep. 6-month after the operation, several correlations were identified between various psychosocial questionnaire scores, weight change, and individual risk alleles (88).
The most prevalent homozygote alleles within this study were of the DRD2 and MAO genes detected among 38% and 47% of subjects, respectively. The GARS assay also revealed that 76% of participants fell into the high-risk category for alcoholism (score of 7 or greater). In response to psychosocial questionnaires, many subjects revealed symptoms of depression, trouble with sleep, as well as food cravings.
It was found that the DRD4 risk allele showed significant correlation with change in weight and change in BMI. Receptors for D4 are in several brain regions, with a range of functions such as regulating attention, decision making, reinforcing properties of food, and inhibitory control (89, 90). Scores from the Difficulties in Emotional Regulation Scale (DERS) (91) were positively correlated with the OPRM1 allele. This gene (G allele) has been associated with increased mood disturbances as well as decreased emotional regulation which may result in an increased sensitivity to stressors (92–94). While zero patients in this study were homozygous carriers of the A11G polymorphism, those who were heterozygous for the G allele did display these associated symptoms. DERS results were also positively correlated with pre- and post-surgery BMI, suggesting levels of emotional regulation may have significant correlation with obesity within an individual (95–97). A significant negative correlation was found between the COMT allele, the Eating Expectancies Inventory questionnaire (EEI) (98–100), and the Pittsburgh Sleep Quality Index (PSQI) (101–103). The presence of the COMT risk allele may play a role in the impulsivity that influences binge eating disorder (104).
Thanos et al. also conducted a follow-up with the same group of participants 1 year after bariatric surgery (105). 1-year BMI of subjects revealed a significant negative correlation with the OPRM1 allele and DRD2 alleles. DRD2 was also positively correlated with change in weight and positively correlated with %EWL. ANOVA discovered a significant difference in change of BMI between expressions of the MAOA risk allele. GARS scores were found to be correlated with %EWL, change in weight, and change in BMI. Finally, FCQ scores were revealed to have negative correlation with %EWL and 1-year post-surgical weight loss.
The A1 allelic presence has long been associated with different forms of obesity, including parental/hereditary obesity (106). D2 striatal receptor availability can decrease with obesity and overeating (107–110). The listed findings suggest that surgery may alter D2 sensitivity/activity and the associated reward mechanisms. Additionally, there is evidence supporting an upregulation of D2 receptors after bariatric surgery (80, 107, 111–113). Preclinical research from Thanos et al. showed how obese rats displays reduced D2Rs (158), and how these can be regulated via bariatric surgery (80). In a clinical study, women with obesity showed decreased baseline striatal D2 and D3 expression, which increased with improved body weight 2 years after surgery (113).
The mu-Opioid Receptor is known to modulate reward processing, motivation, and hedonic behaviors (114). Expressions of this receptor have been negatively related to obesity and food cravings (115–118) OPRM1 cerebral availability has been inversely related to external eating behaviors (116). Additionally, when compared to controls, 13 women with obesity showed significantly decreased availability of OPRM1 in the ventral striatum, insula, and thalamus detected with [11(C)]carfentanil PET scans (118).
The MAOA gene encodes for enzymes that breaking down monoamines (119, 120). Polymorphisms of this gene can effect dopamine levels (121). This gene has been correlated with facets of obesity such as weight, BMI, and body fat in Portuguese men (122) as well as female Caucasian twins (120).
A summary of the post-operative results, including bodyweight and genetic data, can be viewed in Table 5.
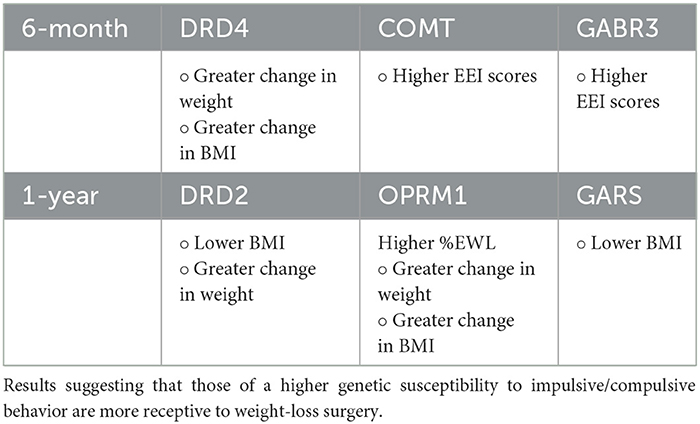
Table 5. Summarizing the bodyweight and psychosocial results of patient data 6-month and 1-year after bariatric surgery.
7 Psychosocial risk factors related to epigenetics in predicting bariatric surgery outcomes
The following psychosocial questionnaires have been utilized to measure obesity-related behaviors. The Eating Attitudes Test-26 (EAT-26), Food Cravings Questionnaire-Trait Reduced (FCQ-TR) and Eating Expectancies Inventory (EEI) questionnaires serve as a measure of nutritional habits (98–100). In one study, 262 women completed the EEI before undergoing bariatric surgery. The results of this study suggest that the EEI is a reliable and valid indicator of eating patterns/pathology and postsurgical weight loss outcomes (123). Additionally, the modified Yale Food Addiction Scale 2.0 (mYFAS 2.0) assesses food addiction (124). This questionnaire has been used as a measure of cravings in bariatric surgery patients. Similarly, the Weight Influenced Self-Esteem Questionnaire (WISE-Q) measures binge eating disorder symptoms (125). The DERS and Center for Epidemiologic Studies Depression Scale (CESDS) questionnaires assess anxiety/depression within participants (91, 126). One study utilized the DERS questionnaire among both obese and control adult female participants. This study revealed that DERS scores were higher in the obese group which is likely due to individuals with higher BMI exhibiting less effective inhibition in the amygdala during reappraisal. This alteration likely contributes to an increased difficulty in regulating emotions in obese women (127). Finally, chronic stress and life quality and sleep can be measured through the Chronic Stress Index (CSI) and Pittsburgh Sleep Quality Index (PSQI), respectively (101–103). A cross-sectional study measuring the association between sleep quality and obesity in Korean adults was completed which utilized the PSQI, associating obesity with insufficient sleep in women (160). A meta-analysis revealed that shorter sleep duration is significantly associated with obesity (128). Administering these questionnaires will help in aiding psychosocial concerns following bariatric surgery that have been seen to be linked to these various sociodemographic factors.
8 Bariatric surgery: implications for personalized medicine
Here we have detailed some of the neurogenetic and molecular mechanisms that may be associated with genotypical receptivity to bariatric surgery. Results from Thanos et al., generally detail which genotypes are more receptive to treatment based on their individual GARS scores (88, 105). Further studies could target nutrigenomics, correlating this concept with post-surgery weight loss. Nutrigenomics, specific nutrition plans targeting genotypical deficiencies, is an important factor for personalized medicine (129). This concept has been explored by Blum's group and others especially in the realms of targeted nutritional replacement therapies, notably amino acid therapies, for addiction treatment and induction of dopamine homeostasis (130).
A personalized approach can be important for patient education. Genetic counseling can lead to lifestyle changes that promote health and wellbeing (131). The genotypes discussed in this review typically deal with psychiatric neurogenetics. Other investigators in the field of obesity genetics have delved into the genetics of metabolic networks, adiposity, and hormone receptors (132–134).
Adiposity genetics was observed as a predictor of post-bariatric surgery weight loss. In this study, 13 SNPs related to adiposity were associated with post bariatric weight loss (132). These included SNPs in the following genes: PKHD1, ST8SIA2/SLCO3A1, PRKD1, NUP54/SCARB2, GBE1, AGBL4, BCDIN3D, NLRC3, TCF7L2, BCL2, MEIS1, RSPO3, GDF5, CCDC92, DNM3-PIGC. Patients genetically predisposed to low body mass index had lower weight loss after bariatric surgery. In 2013, two genome-wide association studies (GWASs) exploring weight reduction following bariatric surgery, each comprising 1,143 and 1,018 participants, respectively, identified significant associations between surgery-induced weight loss and the SNPs rs728996 (located in PKHD1) and rs17702901 (found in ST8SIA2) (135, 136).
Measures such as genetic considerations provide a framework for implementing personalized medicine approaches in clinics such as Bariatric Surgery centers. This suggests a more intricate level of tailoring, encompassing diagnostic, screening, treatment, and management strategies rooted in genomics and other pertinent factors, along with a methodical integration of this personalized approach into healthcare delivery (137). The benefit of a test like GARS is that it can pinpoint individuals at elevated risk and offering tailored interventions to mitigate risks, thus preempting the onset of disease symptoms (137–139). Genetic testing in personalized and precision medicine encompasses a wide range of disorders, including schizophrenia, bipolar disorder, posttraumatic stress disorder, cardiac disease, metabolic disease, renal disease, and substance abuse (137, 140) and among specific sub populations such as veterans (140).
9 Conclusion
The obesity epidemic has become a global issue, impacting more than one billion people worldwide. This public health problem is often described as being caused by various genetic, psychosocial factors. One of the most effective strategies for treating morbid obesity and achieving significant weight loss is bariatric surgery. Recent focus on precision medicine approaches have expanded into bariatric surgery in an effort to better understand and achieve improved outcomes and reduce risk for post-operative weight regain and addiction transfers. Addiction transfers, including substance and non-substance addictions, are well established concerns for post-bariatric patients. Genetic data, psychological data, and other data (156, 157) can be utilized to guide and inform clinical decisions in bariatric surgery.
Author contributions
CH: Writing – original draft, Writing – review & editing. FC: Writing – original draft, Writing – review & editing. SC: Writing – original draft, Writing – review & editing. AP: Writing – review & editing. JB: Writing – review & editing. KB: Writing – review & editing. MG: Writing – review & editing. LG: Writing – review & editing. LM: Writing – review & editing. TQ: Writing – review & editing. PT: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Blue Sky Foundation to PT, TQ, and LM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Carpaij OA, van den Berge M. The asthma-obesity relationship: underlying mechanisms and treatment implications. Curr Opin Pulm Med. (2018) 24:42–9. doi: 10.1097/MCP.0000000000000446
2. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. (2016) 22:s176–85.
3. Caballero B. (2019). Humans against obesity: who will win? Adv Nutr. 10:S4–s9. doi: 10.1093/advances/nmy055
4. Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. (2018) 39:79–132. doi: 10.1210/er.2017-00253
5. Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. (2014) 5:919. doi: 10.3389/fpsyg.2014.00919
6. Wolfe BM, Kvach E, Eckel RH. Treatment of obesity. Circ Res. (2016) 118:1844–55. doi: 10.1161/CIRCRESAHA.116.307591
7. Alberga AS, Sigal RJ, Sweet SN, Doucette S, Russell-Mayhew S, Tulloch H, et al. Understanding low adherence to an exercise program for adolescents with obesity: the HEARTY trial. Obes Sci Pract. (2019) 5:437–48. doi: 10.1002/osp4.357
8. Bilsborough SA, Crowe TC. Low-carbohydrate diets: what are the potential short- and long-term health implications? Asia Pac J Clin Nutr. (2003) 12:396–404.
9. Panteliou E, Miras AD. What is the role of bariatric surgery in the management of obesity? Climacteric. (2017) 20:97–102. doi: 10.1080/13697137.2017.1262638
10. Sarwer DB, Allison KC, Wadden TA, Ashare R, Spitzer JC, McCuen-Wurst C, et al. Psychopathology, disordered eating, and impulsivity as predictors of outcomes of bariatric surgery. Surg Obesity Related Dis. (2019) 15:650–5. doi: 10.1016/j.soard.2019.01.029
11. König IR, Fuchs O, Hansen G, von Mutius E, Kopp MV. What is precision medicine? Eur Respirat J. (2017) 50:1700391. doi: 10.1183/13993003.00391-2017
12. Belligoli A, Bettini S, Segato G, Busetto L. Predicting responses to bariatric and metabolic surgery. Curr Obes Rep. (2020) 9:373–9. doi: 10.1007/s13679-020-00390-1
13. Grönroos S, Helmiö M, Juuti A, Tiusanen R, Hurme S, Löyttyniemi E, et al. Effect of laparoscopic sleeve gastrectomy vs roux-en-y gastric bypass on weight loss and quality of life at 7 years in patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. (2021) 156:137–46. doi: 10.1001/jamasurg.2020.5666
14. Eryilmaz G. Gambling disorder following bariatric surgery. Curr Addict Res. (2018) 2:62–3. doi: 10.5455/car.105-1553261039
15. Faria GR. A brief history of bariatric surgery. Porto Biomed J. (2017) 2:90–2. doi: 10.1016/j.pbj.2017.01.008
16. Buchwald H, Varco RL. Ileal bypass in lowering high cholesterol levels. Surg Forum. (1964) 15:289–91.
17. Buchwald H, Varco RL. Ileal bypass in patients with hypercholesterolemia and atherosclerosis: preliminary report on therapeutic potential. JAMA. (1966) 196:627–30. doi: 10.1001/jama.1966.03100200067021
18. Affinati AH, Esfandiari NH, Oral EA, Kraftson AT. Bariatric surgery in the treatment of type 2 diabetes. Curr Diab Rep. (2019) 19:156. doi: 10.1007/s11892-019-1269-4
19. Liu T, Zou X, Ruze R, Xu Q. Bariatric surgery: targeting pancreatic β cells to treat type II diabetes. Front Endocrinol. (2023) 14:1031610. doi: 10.3389/fendo.2023.1031610
20. Owen JG, Yazdi F, Reisin E. Bariatric surgery and hypertension. Am J Hypertens. (2017) 31:11–7. doi: 10.1093/ajh/hpx112
21. Al Oweidat K, Toubasi AA, Tawileh RBA, Tawileh HBA, Hasuneh MM. Bariatric surgery and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. (2023) 27:2283–94. doi: 10.1007/s11325-023-02840-1
22. Quintas-Neves M, Preto J, Drummond M. Assessment of bariatric surgery efficacy on Obstructive Sleep Apnea (OSA). Rev Port Pneumol. (2016) 22:331–6. doi: 10.1016/j.rppnen.2016.05.006
23. Pontiroli AE, Benetti A, Folini L, Merlotti C, Frigè F. Other aspects of bariatric surgery: liver steatosis, ferritin and cholesterol metabolism. Nutr Hosp. (2013) 28:104–108. doi: 10.3305/nh.2013.28.sup2.6720
24. Mason EE, Printen KJ, Hartford CE, Boyd WC. Optimizing results of gastric bypass. Ann Surg. (1975) 182:405–14. doi: 10.1097/00000658-197510000-00006
25. Griffen W, Young VL, Stevenson C. A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg. (1977) 186:500–9. doi: 10.1097/00000658-197710000-00012
26. Fobi MA, Fleming AW. Vertical banded gastroplasty vs gastric bypass in the treatment of obesity. J Natl Med Assoc. (1986) 78:1091–8.
27. Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg. (2000) 10:514–23. doi: 10.1381/096089200321593715
28. Benaiges D, Más-Lorenzo A, Goday A, Ramon JM, Chillarón JJ, Pedro-Botet J, et al. Laparoscopic sleeve gastrectomy: more than a restrictive bariatric surgery procedure? World J Gastroenterol. (2015) 21:11804–14. doi: 10.3748/wjg.v21.i41.11804
29. Nasser H, Ivanics T, Carlin AM. Factors influencing the choice between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc. (2021) 35:4691–9. doi: 10.1007/s00464-020-07933-6
30. Li G, Walco JP, Mueller DA, Wanderer JP, Freundlich RE. Reliability of the ASA physical status classification system in predicting surgical morbidity: a retrospective analysis. J Med Syst. (2021) 45:83. doi: 10.1007/s10916-021-01758-z
31. Doyle DJ, Hendrix JM, Garmon EH. American Society of Anesthesiologists Classification. In: StatPearls. StatPearls Publishing LLC: Treasure Island (FL). (2024).
32. Mahmud N, Panchal S, Abu-Gazala S, Serper M, Lewis JD, Kaplan DE, et al. Association between bariatric surgery and alcohol use-related hospitalization and all-cause mortality in a veterans affairs cohort. JAMA Surg. (2023) 158:162–71. doi: 10.1001/jamasurg.2022.6410
33. Mellinger JL, Shedden K, Winder GS, Fernandez AC, Lee BP, Waljee J, et al. Bariatric surgery and the risk of alcohol-related cirrhosis and alcohol misuse. Liver Int. (2021) 41:1012–9. doi: 10.1111/liv.14805
34. Kim HP, Jiang Y, Farrell TM, Peat CM, Hayashi PH, Barritt AS. Roux-en-Y gastric bypass is associated with increased hazard for de novo alcohol-related complications and liver disease. J Clin Gastroenterol. (2022) 56:181–5. doi: 10.1097/MCG.0000000000001506
35. Maciejewski ML, Smith VA, Berkowitz TSZ, Arterburn DE, Mitchell JE, Olsen MK, et al. Association of bariatric surgical procedures with changes in unhealthy alcohol use among US veterans. JAMA Netw Open. (2020) 3:e2028117. doi: 10.1001/jamanetworkopen.2020.28117
36. Salminen P, Helmiö M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. (2018) 319:241–54. doi: 10.1001/jama.2017.20313
37. Salminen P, Grönroos S, Helmiö M, Hurme S, Juuti A, Juusela R, et al. Effect of laparoscopic sleeve gastrectomy vs Roux-en-Y gastric bypass on weight loss, comorbidities, and reflux at 10 years in adult patients with obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. (2022) 157:656–66. doi: 10.1001/jamasurg.2022.2229
38. Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. (2018) 319:255–65. doi: 10.1001/jama.2017.20897
39. Ciobârcă D, Cătoi AF, Copăescu C, Miere D, Crişan G. Bariatric surgery in obesity: effects on gut microbiota and micronutrient status. Nutrients. (2020) 12:235. doi: 10.3390/nu12010235
40. Gutiérrez-Repiso C, Garrido-Sánchez L, Alcaide-Torres J, Cornejo-Pareja I, Ocaña-Wilhelmi L, García-Fuentes E, et al. Predictive role of gut microbiota in weight loss achievement after bariatric surgery. J Am Coll Surg. (2022) 234:861–71. doi: 10.1097/XCS.0000000000000145
41. Baena-Raya A, Martínez-Rosales E, Ruiz-González D, Hernández-Martínez A, López-Sánchez L, Ferrer-Márquez M, et al. Exercise interventions following bariatric surgery are poorly reported: a systematic review and a call for action. Obesity Rev. (13758). doi: 10.1111/obr.13758
42. McLellan AT, Koob GF, Volkow ND. Preaddiction-a missing concept for treating substance use disorders. JAMA Psychiatry. (2022) 79:749–51. doi: 10.1001/jamapsychiatry.2022.1652
43. Blum K, Bowirrat A, Baron D, Lott L, Ponce JV, Brewer R, et al. Biotechnical development of genetic addiction risk score (GARS) and selective evidence for inclusion of polymorphic allelic risk in substance use disorder (SUD). J Syst Integr Neurosci. (2020) 6:1000221. doi: 10.15761/JSIN.1000221
44. Blum K, Bailey J, Gonzalez AM, Oscar-Berman M, Liu Y, Giordano J, et al. Neuro-Genetics of Reward Deficiency Syndrome (RDS) as the root cause of “addiction transfer”: a new phenomenon common after bariatric surgery. J Genet Syndr Gene Ther. (2012) 2012:S2-001. doi: 10.4172/2157-7412.S2-001
45. Steffen KJ, Engel SG, Wonderlich JA, Pollert GA, Sondag C. Alcohol other addictive disorders following bariatric surgery: prevalence, risk factors and possible etiologies. Eur Eat Disord Rev. (2015) 23:442–50. doi: 10.1002/erv.2399
46. Thanos PK, Gold MS, Blum K, Badgaiyan RD, Avena NM. Bariatric surgery: Potential post-operative heightened sensitivity to substances or behaviors. J Syst Integr Neurosci. (2021) 8:1.
47. Ibrahim N, Alameddine M, Brennan J, Sessine M, Holliday C, Ghaferi AA, et al. New onset alcohol use disorder following bariatric surgery. Surg Endosc. (2019) 33:2521–30. doi: 10.1007/s00464-018-6545-x
48. Chiles C, van Wattum PJ. Psychiatric aspects of the obesity crisis. In: Psychiatric Times (2010). p. 27.
49. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. (2007) 357:741–52. doi: 10.1056/NEJMoa066254
50. Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. (2007) 357:753–61. doi: 10.1056/NEJMoa066603
51. O'Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. (2006) 144:625–33. doi: 10.7326/0003-4819-144-9-200605020-00005
52. Dickhut C, Hase C, Gruner-Labitzke K, Mall JW, Köhler H, Zwaan M, et al. No addiction transfer from preoperative food addiction to other addictive behaviors during the first year after bariatric surgery. Eur Eat Disord Rev. (2021) 29:924–36. doi: 10.1002/erv.2857
53. Chiappetta S, Stier C, Hadid MA, Malo N, Theodoridou S, Weiner R, et al. Remission of food addiction does not induce cross-addiction after sleeve gastrectomy and gastric bypass: a prospective cohort study. Obes Facts. (2020) 13:307–20. doi: 10.1159/000506838
54. Koball AM, Ames G, Goetze RE. Addiction transfer and other behavioral changes following bariatric surgery. Surg Clin North Am. (2021) 101:323–33. doi: 10.1016/j.suc.2020.12.005
55. Reslan S, Saules KK, Greenwald MK, Schuh LM. Substance misuse following Roux-en-Y gastric bypass surgery. Subst Use Misuse. (2014) 49:405–17. doi: 10.3109/10826084.2013.841249
56. Ivezaj V, Saules KK, Wiedemann AA. “I didn't see this coming.”: why are postbariatric patients in substance abuse treatment? Patients' perceptions of etiology and future recommendations. Obes Surg. (2012) 22:1308–14. doi: 10.1007/s11695-012-0668-2
57. Ames GE, Koball AM, Clark MM. Behavioral interventions to attenuate driven overeating and weight regain after bariatric surgery. Front Endocrinol. (2022) 13:934680. doi: 10.3389/fendo.2022.934680
58. Moran M, Blum K, Ponce JV, Lott L, Gondré-Lewis MC, Badgaiyan S, et al. High genetic addiction risk score (GARS) in chronically prescribed severe chronic opioid probands attending multi-pain clinics: an open clinical pilot trial. Mol Neurobiol. (2021) 58:3335–46. doi: 10.1007/s12035-021-02312-1
59. Blum K, Modestino EJ, Gondre-Lewis M, Chapman EJ, Neary J, Siwicki D, et al. The benefits of genetic addiction risk score (GARS(™)) testing in substance use disorder (SUD). Int J Genom Data Min. (2018) 12:1772. doi: 10.29011/2577-0616.000115
60. Fried L, Modestino EJ, Siwicki D, Lott L, Thanos PK, Baron D, et al. Hypodopaminergia and “precision behavioral management”(PBM): it is a generational family affair. Curr Pharm Biotechnol. (2020) 21:528–41. doi: 10.2174/1389201021666191210112108
61. Modestino EJ, Blum K, Dennen CA, Downs BW, Bagchi D, Llanos-Gomez L, et al. Theorizing the role of dopaminergic polymorphic risk alleles with intermittent explosive disorder (IED), violent/aggressive behavior and addiction: justification of genetic addiction risk severity (GARS) testing. J Pers Med. (2022) 12:1946. doi: 10.3390/jpm12121946
62. Blum K, Han D, Gupta A, Baron D, Braverman ER, Dennen CA, et al. Statistical validation of risk alleles in genetic addiction risk severity (GARS) test: early identification of risk for alcohol use disorder (AUD) in 74,566 case-control subjects. J Pers Med. (2022) 12:1385. doi: 10.3390/jpm12091385
63. Blum K, Dennen CA, Elman I, Bowirrat A, Thanos PK, Badgaiyan RD, et al. Should reward deficiency syndrome (RDS) be considered an umbrella disorder for mental illness and associated genetic and epigenetic induced dysregulation of brain reward circuitry? J Personalized Med. (2022) 12:1719. doi: 10.3390/jpm12101719
64. Blum K, Han D, Bowirrat A, Downs BW, Bagchi D, Thanos PK, et al. Genetic addiction risk and psychological profiling analyses for “preaddiction” severity index. J Pers Med. (2022) 12:1772. doi: 10.3390/jpm12111772
65. Blum K, Chen ALC, Thanos PK, Febo M, Demetrovics Z, Dushaj K III, et al. Genetic addiction risk score (GARS) ™, a predictor of vulnerability to opioid dependence. Front Biosci. (2018) 10:175–96. doi: 10.2741/e816
66. Blum K, Bowirrat A, Elman I, Baron D, Thanos PK, Gold MS, et al. Evidence for the DRD2 Gene as a determinant of reward deficiency syndrome (RDS). Clin Exp Psychol. (2023) 9:8–11.
67. Klockhoff H, Näslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. (2002) 54:587–91. doi: 10.1046/j.1365-2125.2002.01698.x
68. Hagedorn JC, Encarnacion B, Brat GA, Morton JM. Does gastric bypass alter alcohol metabolism? Surg Obes Relat Dis. 3:543–8. doi: 10.1016/j.soard.2007.07.003
69. Woodard GA, Downey J, Hernandez-Boussard T, Morton JM. Impaired alcohol metabolism after gastric bypass surgery: a case-crossover trial. J Am Coll Surg. (2011) 212:209–14. doi: 10.1016/j.jamcollsurg.2010.09.020
70. Thanos PK, Subrize M, Delis F, Cooney RN, Culnan D, Sun M, et al. Gastric bypass increases ethanol and water consumption in diet-induced obese rats. Obes Surg. (2012) 22:1884–92. doi: 10.1007/s11695-012-0749-2
71. Polston JE, Pritchett CE, Tomasko JM, Rogers AM, Leggio L, Thanos PK, et al. Roux-en-Y gastric bypass increases intravenous ethanol self-administration in dietary obese rats. PLoS ONE. (2013) 8:e83741. doi: 10.1371/journal.pone.0083741
72. Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, et al. Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS ONE. (2012) 7:e49121. doi: 10.1371/journal.pone.0049121
73. Uchida A, Zechner JF, Mani BK, Park WM, Aguirre V, Zigman JM, et al. Altered ghrelin secretion in mice in response to diet-induced obesity and Roux-en-Y gastric bypass. Mol Metab. (2014) 3:717–30. doi: 10.1016/j.molmet.2014.07.009
74. Orellana ER, Jamis C, Horvath N, Hajnal A. Effect of vertical sleeve gastrectomy on alcohol consumption and preferences in dietary obese rats and mice: a plausible role for altered ghrelin signaling. Brain Res Bull. (2018) 138:26–36. doi: 10.1016/j.brainresbull.2017.08.004
75. McGregor M, Hamilton J, Hajnal A, Thanos PK. Roux-en-Y gastric bypass in rat reduces mu-opioid receptor levels in brain regions associated with stress and energy regulation. PLoS ONE. (2019) 14:e0218680. doi: 10.1371/journal.pone.0218680
76. Ticku MK, Kulkarni SK. Molecular interactions of ethanol with GABAergic system and potential of RO15–4513 as an ethanol antagonist. Pharmacol Biochemist Behav. (1988) 30:501–10. doi: 10.1016/0091-3057(88)90487-X
77. Tran VT, Snyder SH, Major LF, Hawley RJ. GABA receptors are increased in brains of alcoholics. Ann Neurol. (1981) 9:289–92. doi: 10.1002/ana.410090312
78. Du Y, Zhou S, Ma C, Chen H, Du A, Deng G, et al. Dopamine release and negative valence gated by inhibitory neurons in the laterodorsal tegmental nucleus. Neuron. (2023) 111:3102–18.e7. doi: 10.1016/j.neuron.2023.06.021
79. McGregor M, Hamilton J, Hajnal A, Thanos PK. Roux-en-Y gastric bypass increases GABA-A receptor levels in regions of the rat brain involved in object recognition memory and perceptual acuity. Physiol Behav. (2020) 224:113053. doi: 10.1016/j.physbeh.2020.113053
80. Hamilton J, Swenson S, Hajnal A, Thanos PK. Roux-en-Y gastric bypass surgery normalizes dopamine D1, D2, DAT levels. Synapse. (2018). doi: 10.1002/syn.22058
81. Reddy IA, Wasserman DH, Ayala JE, Hasty AH, Abumrad NN, Galli A, et al. Striatal dopamine homeostasis is altered in mice following Roux-en-Y Gastric bypass surgery. ACS Chem Neurosci. (2014) 5:943–51. doi: 10.1021/cn500137d
82. Blum K, Baron D, McLaughlin T, Thanos PK, Dennen C, Ceccanti M, et al. Summary document research on RDS anti-addiction modeling: annotated bibliography. J Addict Psychiatry. (2024) 8:1–33. doi: 10.17756/jap.2024-043
83. Thanos PK, Michaelides M, Subrize M, Miller ML, Bellezza R, Cooney RN, et al. Roux-en-Y gastric bypass alters brain activity in regions that underlie reward and taste perception. PLoS ONE. (2015) 10:e0125570. doi: 10.1371/journal.pone.0125570
84. Ding H, Liu C, Zhang S, Li B, Xu Q, Shi B, et al. Sleeve gastrectomy attenuated diabetes-related cognitive decline in diabetic rats. Front Endocrinol. (2022) 13:1015819. doi: 10.3389/fendo.2022.1015819
85. Moncada R, Becerril S, Rodríguez A, Méndez-Giménez L, Ramírez B, Catalán V, et al. Sleeve gastrectomy reduces body weight and improves metabolic profile also in obesity-prone rats. Obes Surg. (2016) 26:1537–48. doi: 10.1007/s11695-015-1915-0
86. Rodríguez A, Becerril S, Valentí V, Ramírez B, Martín M, Méndez-Giménez L, et al. Sleeve gastrectomy reduces blood pressure in obese (fa/fa) Zucker rats. Obes Surg. 22:309–315. doi: 10.1007/s11695-011-0562-3
87. Orellana ER, Piscura MK, Horvath N, Hajnal A. Differential response in ethanol behaviors of female rats given various weight loss surgeries. Alcohol Alcohol. (2021) 56:599–604. doi: 10.1093/alcalc/agab054
88. Thanos PK, Hanna C, Mihalkovic A, Hoffman AB, Posner AR, Busch J, et al. The first exploratory personalized medicine approach to improve bariatric surgery outcomes utilizing psychosocial and genetic risk assessments: encouraging clinical research. J Pers Med. (2023) 13:1164. doi: 10.3390/jpm13071164
89. Patte KA, Davis CA, Levitan RD, Kaplan AS, Carter-Major J, Kennedy JL, et al. A behavioral genetic model of the mechanisms underlying the link between obesity and symptoms of ADHD. J Atten Disord. (2020) 24:1425–36. doi: 10.1177/1087054715618793
90. Ariza M, Garolera M, Jurado MA, Garcia-Garcia I, Hernan I, Sánchez-Garre C, et al. Dopamine genes (DRD2/ANKK1-TaqA1 and DRD4-7R) and executive function: their interaction with obesity. PLoS ONE. (2012) 7:e41482. doi: 10.1371/journal.pone.0041482
91. Kaufman EA, Xia M, Fosco G, Yaptangco M, Skidmore CR, Crowell SE, et al. The Difficulties in Emotion Regulation Scale Short Form (DERS-SF): Validation and Replication in Adolescent and Adult Samples. J Psychopathol Behav Assess. (2016) 38:443–55. doi: 10.1007/s10862-015-9529-3
92. Cimino S, Carola V, Cerniglia L, Bussone S, Bevilacqua A, Tambelli R, et al. The μ-opioid receptor gene A118G polymorphism is associated with insecure attachment in children with disruptive mood regulation disorder and their mothers. Brain Behav. (2020) 10:e01659. doi: 10.1002/brb3.1659
93. Daniel AM, Rushing BG, Tapia Menchaca KY. Variation of the human mu-opioid receptor (OPRM1) gene predicts vulnerability to frustration. Sci Rep. (2020) 10:21840. doi: 10.1038/s41598-020-78783-4
94. Peciña M, Love T, Stohler CS, Goldman D, Zubiet JK. Effects of the Mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology. (2015) 40:957–65. doi: 10.1038/npp.2014.272
95. Benzerouk F, Djerada Z, Bertin E, Barrière S, Gierski F, Kaladjian A, et al. Contributions of emotional overload, emotion dysregulation, and impulsivity to eating patterns in obese patients with binge eating disorder and seeking bariatric surgery. Nutrients. (2020) 12:99. doi: 10.3390/nu12103099
96. Casagrande M, Boncompagni I, Forte G, Guarino A, Favieri F. Emotion overeating behavior: effects of alexithymia and emotional regulation on overweight and obesity. Eat Weight Disord. (2020) 25:1333–45. doi: 10.1007/s40519-019-00767-9
97. Fereidouni F, Atef-Vahid MK, Fathali Lavasani F, Jamshidi Orak R, Klonsky ED, Pazooki A, et al. Are Iranian obese women candidate for bariatric surgery different cognitively. Emotionally and behaviorally from their normal weight counterparts? Eat Weight Disord. (2015) 20:397–403. doi: 10.1007/s40519-014-0168-6
98. Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. (1982) 12:871–8. doi: 10.1017/S0033291700049163
99. Meule A, Hermann T, Kübler A. A short version of the Food Cravings Questionnaire-Trait: the FCQ-T-reduced. Front Psychol. (2014) 5:190. doi: 10.3389/fpsyg.2014.00190
100. Fitzsimmons-Craft EE, Keatts DA, Bardone-Cone AM. Eating expectancies in relation to eating disorder recovery. Cognit Ther Res. (2013) 37:1041–7. doi: 10.1007/s10608-013-9522-7
101. Schulz AJ, Israel BA, Zenk SN, Parker EA, Lichtenstein R, Shellman-Weir S, et al. Psychosocial stress and social support as mediators of relationships between income, length of residence and depressive symptoms among African American women on Detroit's eastside. Soc Sci Med. (2006) 62:510–22. doi: 10.1016/j.socscimed.2005.06.028
102. Schulz AJ, Mentz G, Lachance L, Johnson J, Gaines C, Israel BA, et al. Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. Am J Public Health. (2012) 102:1706–14. doi: 10.2105/AJPH.2011.300412
103. Buysse DJ, Reynolds C. F. III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiat Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
104. Leehr EJ, Schag K, Brückmann C, Plewnia C, Zipfel S, Nieratschker V, et al. A putative association of COMT Val(108/158)Met with impulsivity in binge eating disorder. Eur Eat Disord Rev. (2016) 24:169–73. doi: 10.1002/erv.2421
105. Thanos PK, Hanna C, Mihalkovic A, Hoffman A, Posner A, Butsch J, et al. Genetic correlates as a predictor of bariatric surgery outcomes after 1 year. Biomedicines. (2023) 11:2644. doi: 10.3390/biomedicines11102644
106. Noble EP, Noble RE, Ritchie T, Syndulko K, Bohlman MC, Noble LA, et al. D2 dopamine receptor gene and obesity. Int J Eat Disord. (1994) 15:205–17.
107. Ribeiro G, Maia A, Cotovio G, Oliveira FPM, Costa DC, Oliveira-Maia AJ, et al. Striatal dopamine D2-like receptors availability in obesity and its modulation by bariatric surgery: a systematic review and meta-analysis. Sci Rep. (2023) 13:4959. doi: 10.1038/s41598-023-31250-2
108. Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. (2005) 8:555–560. doi: 10.1038/nn1452
109. Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. (2008) 363:3191–200. doi: 10.1098/rstb.2008.0107
110. Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. (2011) 15:37–46. doi: 10.1016/j.tics.2010.11.001
111. Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. (2010) 20:369–74. doi: 10.1007/s11695-009-0015-4
112. van der Zwaal EM, Weijer BA, van de Giessen EM, Janssen I, Berends FJ, van de Laar A, et al. (2016). Striatal dopamine D2/3 receptor availability increases after long-term bariatric surgery-induced weight loss. Eur Neuropsychopharmacol. 26:1190–1200. doi: 10.1016/j.euroneuro.2016.04.009
113. Weijer S, la Fleur E, Booij J, Serlie MJ. Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity. Diabetologia. (2014) 57:1078–80. doi: 10.1007/s00125-014-3178-z
114. Nummenmaa L, Saanijoki T, Tuominen L, Hirvonen J, Tuulari JJ, Nuutila P, et al. μ-opioid receptor system mediates reward processing in humans. Nat Commun. (2018) 9:1500. doi: 10.1038/s41467-018-03848-y
115. Joutsa J, Karlsson HK, Majuri J, Nuutila P, Helin S, Kaasinen V, et al. Binge eating disorder and morbid obesity are associated with lowered mu-opioid receptor availability in the brain. Psychiatry Res Neuroimaging. (2018) 276:41–5. doi: 10.1016/j.pscychresns.2018.03.006
116. Kantonen T, Karjalainen T, Pekkarinen L, Isojärvi J, Kalliokoski K, Kaasinen V, et al. Cerebral μ-opioid and CB(1) receptor systems have distinct roles in human feeding behavior. Transl Psychiatry. (2021) 11:442. doi: 10.1038/s41398-021-01559-5
117. Kantonen T, Pekkarinen L, Karjalainen T, Bucci M, Kalliokoski K, Haaparanta-Solin M, et al. Obesity risk is associated with altered cerebral glucose metabolism and decreased μ-opioid and CB(1) receptor availability. Int J Obes. (2022) 46:400–7. doi: 10.1038/s41366-021-00996-y
118. Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. (2015) 35:3959–65. doi: 10.1523/JNEUROSCI.4744-14.2015
119. Brunner HG. (1996). MAOA deficiency and abnormal behaviour: perspectives on an association. Ciba Found Symp. 194:155–64.
120. Need AC, Ahmadi KR, Spector TD, Goldstein DB. Obesity is associated with genetic variants that alter dopamine availability. Ann Hum Genet. (2006) 70:293–303. doi: 10.1111/j.1529-8817.2005.00228.x
121. Avsar O, Kuskucu A, Sancak S, Genc E. Are dopaminergic genotypes risk factors for eating behavior and obesity in adults? Neurosci Lett. (2017) 654:28–32. doi: 10.1016/j.neulet.2017.06.023
122. Dias H, Muc M, Padez C, Manco L. Association of polymorphisms in 5-HTT (SLC6A4) and MAOA genes with measures of obesity in young adults of Portuguese origin. Arch Physiol Biochem. (2016) 122:8–13. doi: 10.3109/13813455.2015.1111390
123. Williams-Kerver GA, Schaefer LM, Hawkins MAW, Crowther JH, Duncan J. Eating expectancies before bariatric surgery: assessment and associations with weight loss trajectories. Surg Obes Relat Dis. (2019) 15:1793–9. doi: 10.1016/j.soard.2019.07.028
124. Gearhardt AN, Corbin WR, Brownell KD. Development of the yale food addiction scale version 2.0′. Psychol Addict Behav. (2016) 30:113. doi: 10.1037/adb0000136
125. Trottier K, McFarlane T, Olmsted MP, McCabe RE. The weight influenced self-esteem questionnaire (WISE-Q): factor structure and psychometric properties. Body Image. (2013) 10:112–20. doi: 10.1016/j.bodyim.2012.08.008
126. Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck depression Inventory-II (BDI-II), center for epidemiologic studies depression scale (CES-D), geriatric depression scale (GDS), hospital anxiety and depression scale (HADS), and patient health Questionnaire-9 (PHQ-9). Arthr Care Res. (2011) 63:S454–S66. doi: 10.1002/acr.20556
127. Maturana-Quijada P, Steward T, Vilarrasa N, Miranda-Olivos R, Jiménez-Murcia S, Carey HJ, et al. Dynamic fronto-amygdalar interactions underlying emotion-regulation deficits in women at higher weight. Obesity. (2023) 31:2283–93. doi: 10.1002/oby.23830
128. Bacaro V, Ballesio A, Cerolini S, Vacca M, Poggiogalle E, Donini LM, et al. Sleep duration and obesity in adulthood: An updated systematic review and meta-analysis. Obes Res Clin Pract. (2020) 14:301–9. doi: 10.1016/j.orcp.2020.03.004
129. Fallaize R, Macready AL, Butler LT, Ellis JA, Lovegrove JA. An insight into the public acceptance of nutrigenomic-based personalised nutrition. Nutr Res Rev. (2013) 26:39–48. doi: 10.1017/S0954422413000024
130. Blum K, Gold MS, Llanos-Gomez L, Jalali R, Thanos PK, Bowirrat A, et al. Hypothesizing nutrigenomic-based precision anti-obesity treatment and prophylaxis: should we be targeting sarcopenia induced brain dysfunction? Int J Environ Res Public Health. (2021) 18:9774. doi: 10.3390/ijerph18189774
131. Maher B. Nature readers flirt with personal genomics. Nature. (2011) 478:19. doi: 10.1038/478019a
132. Aasbrenn M, Schnurr TM, Have CT, Svendstrup M, Hansen DL, Worm D, et al. Genetic determinants of weight loss after bariatric surgery. Obes Surg. (2019) 29:2554–61. doi: 10.1007/s11695-019-03878-5
133. Galyean S, Sawant D, Shin AC. Personalized nutrition for management of micronutrient deficiency-literature review in non-bariatric populations and possible utility in bariatric cohort. Obes Surg. (2020) 30:3570–82. doi: 10.1007/s11695-020-04762-3
134. Guclu-Geyik F, Coban N, Can G, Erginel-Unaltuna N. The rs2175898 polymorphism in the ESR1 gene has a significant sex-specific effect on obesity. Biochem Genet. (2020) 58:935–52. doi: 10.1007/s10528-020-09987-6
135. Rinella ES, Still C, Shao Y, Wood GC, Chu X, Salerno B, et al. Genome-wide association of single-nucleotide polymorphisms with weight loss outcomes after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. (2013) 98:E1131–6. doi: 10.1210/jc.2012-3421
136. Hatoum IJ, Greenawalt DM, Cotsapas C, Daly MJ, Reitman ML, Kaplan LM, et al. Weight loss after gastric bypass is associated with a variant at 15q26.1′. Am J Hum Genet. (2013) 92:827–34. doi: 10.1016/j.ajhg.2013.04.009
137. Ramos E. Genetic counseling, personalized medicine, precision health. Cold Spring Harb Perspect Med. (2020) 10. doi: 10.1101/cshperspect.a036699
138. Blum K, Bowirrat A, Thanos PK, Hanna C, Khalsa J, Baron D, et al. Evidence based clinical analytics supporting the genetic addiction risk severity (GARS) assessment to early identify probands in preaddiction. EC Psychol Psychiatr. (2024) 13:1–3.
139. Blum K, Lott L, Siwicki D, Fried L, Hauser M, Simpatico T, et al. Genetic addiction risk score (GARS(™)) as a predictor of substance use disorder: identifying predisposition not diagnosis. Curr Trends Med Diagn Methods. (2018) 1:555556. doi: 10.19080/GJARM.2017.01.555556
140. Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, et al. Million veteran program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. (2016) 70:214–23. doi: 10.1016/j.jclinepi.2015.09.016
141. Thanos PK, Lamoshi A, Nubelo A, Hamilton J, Chernoguz A, Hoffman AB, et al. Clinical and Preclinical Bariatric Surgery Approaches to Studying Obesity. In: Nicole MA, editor. Animal Models of Eating Disorders. Springer US: New York, NY. (2021).
142. Wang JY, Wang QW, Yang XY, Yang W, Li DR, Jin JY, et al. GLP-1 receptor agonists for the treatment of obesity: Role as a promising approach. Front Endocrinol. (2023) 14:1085799. doi: 10.3389/fendo.2023.1085799
143. Zenter A. Clinical assessment to determine a patient's suitability for bariatric surgery. BC Med J. (2018) 60:151–5.
144. Gulinac M, Miteva DG, Peshevska-Sekulovska M, Novakov IP, Antovic S, Peruhova M, et al. Long-term effectiveness, outcomes and complications of bariatric surgery. World J Clin Cases. (2023) 11:4504–12. doi: 10.12998/wjcc.v11.i19.4504
145. Gorgojo-Martínez JJ, Mezquita-Raya P, Carretero-Gómez J, Castro A, Cebrián-Cuenca A, Torres-Sánchez A, et al. Clinical recommendations to manage gastrointestinal adverse events in patients treated with Glp-1 receptor agonists: a multidisciplinary expert consensus. J Clin Med. (2022) 12:145. doi: 10.3390/jcm12010145
146. Murphy R, Plank LD, Clarke MG, Evennett NJ, Tan J, Kim DDW, et al. Effect of banded Roux-en-Y gastric bypass versus sleeve gastrectomy on diabetes remission at 5 years among patients with obesity and type 2 diabetes: a blinded randomized clinical trial. Diabetes Care. (2022) 45:1503–11. doi: 10.2337/dc21-2498
147. Gondré-Lewis MC, Elman I, Alim T, Chapman E, Settles-Reaves B, Galvao C, et al. Frequency of the dopamine receptor D3 (rs6280) vs. opioid receptor μ1 (rs1799971) polymorphic risk alleles in patients with opioid use disorder: a preponderance of dopaminergic mechanisms? Biomedicines. (2022) 10:0870. doi: 10.3390/biomedicines10040870
148. Blum K, Thanos PK, Hanna C, Gold MS, Baron D, Elman I, et al. “TO BE OR NOT TO BE” GWAS ends the controversy about the DRD2 gene as a determinant of reward deficiency syndrome (RDS). Psychol Res Behav Manag. (2023) 16:4287–91. doi: 10.2147/PRBM.S428841
149. Uhl G, Blum K, Noble E, Smith S. Substance abuse vulnerability and D2 receptor genes. Trends Neurosci. (1993) 16:83–8. doi: 10.1016/0166-2236(93)90128-9
150. Kim KM, Choi SW, Kim D, Lee J, Kim JW. Associations among the opioid receptor gene (OPRM1) A118G polymorphism, psychiatric symptoms, and quantitative EEG in Korean males with gambling disorder: a pilot study. J Behav Addict. (2019) 8:463–70. doi: 10.1556/2006.8.2019.41
151. Ziegler C, Domschke K. Epigenetic signature of MAOA and MAOB genes in mental disorders. J Neural Transm. (2018) 125:1581–8. doi: 10.1007/s00702-018-1929-6
152. Nilsson KW, Comasco E, Åslund C, Nordquist N, Leppert J., Oreland L, et al. MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addict Biol. (2011) 16:347–355. doi: 10.1111/j.1369-1600.2010.00238.x
153. Zalsman G, Patya M, Frisch A, Ofek H, Schapir L, Blum I, et al. Association of polymorphisms of the serotonergic pathways with clinical traits of impulsive-aggression and suicidality in adolescents: a multi-center study. World J Biol Psychiatry. (2011) 12:33–41. doi: 10.3109/15622975.2010.518628
154. Chen CH, Huang CC, Liao DL. Association analysis of GABRB3 promoter variants with heroin dependence. PLoS ONE. (2014) 9:e102227. doi: 10.1371/journal.pone.0102227
155. Hamilton J, Nguyen C, McAvoy M, Roeder N, Richardson B, Quattrin T, et al. Calorie restriction, but not Roux-en-Y gastric bypass surgery, increases [(3) H] PK11195 binding in a rat model of obesity. Synapse. (2023) 77:e22258. doi: 10.1002/syn.22258
156. Lim R, Beekley A, Johnson DC, Davis KA. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. (2018) 3:e000219. doi: 10.1136/tsaco-2018-000219
157. Kremen AJ, Linner JH, Nelson CH. An experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann Surg. (1954) 140:439. doi: 10.1097/00000658-195409000-00018
158. Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. (2008) 62:50–61. doi: 10.1002/syn.20468
159. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. (2008) 32:1431–7. doi: 10.1038/ijo.2008.102
Keywords: obesity, drug abuse, addiction, reward deficiency, hypodomanergia, gastric bypass, vertical sleeve gastrectomy, personalized medicine
Citation: Hanna C, Comstock F, Chatrath S, Posner A, Butsch J, Blum K, Gold MS, Georger L, Mastrandrea LD, Quattrin T and Thanos PK (2025) Utilization of a precision medicine genetic and psychosocial approach in outcome assessment of bariatric weight loss surgery: a narrative review. Front. Public Health 13:1516122. doi: 10.3389/fpubh.2025.1516122
Received: 23 October 2024; Accepted: 07 April 2025;
Published: 01 May 2025.
Edited by:
Bart Torensma, Leiden University Medical Center (LUMC), NetherlandsReviewed by:
Ashuin Kammar-García, Instituto Nacional de Geriatría, MexicoAriana Vargas Castillo, Dana–Farber Cancer Institute, United States
Copyright © 2025 Hanna, Comstock, Chatrath, Posner, Butsch, Blum, Gold, Georger, Mastrandrea, Quattrin and Thanos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark S. Gold, ZHJtYXJrc2dvbGRAZ21haWwuY29t; Panayotis K. Thanos, dGhhbm9zQGJ1ZmZhbG8uZWR1
 Colin Hanna
Colin Hanna Fiona Comstock1
Fiona Comstock1 Shtakshe Chatrath
Shtakshe Chatrath Kenneth Blum
Kenneth Blum Mark S. Gold
Mark S. Gold Panayotis K. Thanos
Panayotis K. Thanos