- 1Department of Epidemiology and Biostatistics, School of Public Health, College of Health Sciences, Wollo University, Dessie, Ethiopia
- 2Department of Health Informatics, School of Public Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 3Department of Environmental Health College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 4Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 5Department of Reproductive and Family Health, School of Public Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 6Nossal Institute for Global Health, Melbourne School of Population and Global Health, University of Melbourne, Parkville, VIC, Australia
- 7Department of Health Promotion, School of Public Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 8Department of Health System and Management, School of Public Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Introduction: The measles-containing vaccine (MCV) is a live attenuated vaccine that helps to develop lifelong immunity, and it prevents measles outbreak when administered at the right time in measles-endemic areas. Many infants received the initial dose of the measles vaccine later than the ideal time frame, and significant others missed the vaccination, causing a recurrent measles outbreak in Ethiopia. This study assessed the time to the first dose of a measles-containing vaccine and associated factors among infants in Ethiopia.
Methods: A cohort of 1,770 mother–infant pairs was analysed using data from the performance monitoring for action Ethiopia dataset. Cohort-2 Ethiopian data set was collected in Addis Ababa, Amhara, Oromia and SNNP regions between between November 2021 and August 2023. The key independent variables were socio-demographic characteristics, maternal health service utilization, and pregnancy intention. Multiple imputation was used to handle missing data. Survival analysis was conducted using R programming language version 4.4.1. Multicollinearity was assessed using Generalized variance inflation factors (GVIF), and model fit was evaluated using concordance index and overall model significance.
Results: Among 1,770 infants followed, only 27% were vaccinated timely, within 9–10 months of age (survival probability = 0.73), and 53.4% had not yet received MCV1 at 12 months of age. The hazard of receiving the first dose of measles vaccine (MCV1) was 35% lower among infants from pregnancies that were not desired at all (AHR = 0.65, 95% CI: 0.46-0.93) and 21% lower among those infants from pregnancies that were initially undesired but later became wanted (AHR = 0.79, 95% CI: 0.65–0.96), compared to infants from pregnancies that were desired from the beginning.
Conclusion: Despite progress in the uptake of the first dose of measles vaccine, timely vaccination in Ethiopia is still low, and many infants in Ethiopia miss the immunization. Institutional delivery, maternal intention regarding pregnancy, religion, and wealth quantile were key predictors of the timeliness of the first dose of measles vaccine. Interventions encouraging institutional deliveries, supporting unintended pregnancy, working with religious leaders, and conducting continuous outreach to immunization services are necessary to improve the timely uptake of the first dose of measles vaccine.
Introduction
Expanded Programme on Immunization (EPI) was launched in 1974 to provide children with access to life-saving vaccines around the globe. Since then, a national immunization program has started in every country in the world and has averted the deaths of 154 million children worldwide until 2024. Now, EPI has grown into what is commonly recognized as the EPI (1, 2).
Measles is the cause of morbidity and mortality for millions, despite the efforts taken, including measles vaccinations, surveillance, and outbreak investigation. The estimated number of measles cases increased by 20% worldwide, from 8,645,000 to 10,341,000, and the estimated number of measles deaths decreased by 8%, from 116,800 to 107,500, in 2023 compared to 2022. The decrease in measles deaths compared to cases is due to the increased number of cases occurring in countries with a lower risk of death (3). In Ethiopia, there were 112 measles outbreaks in 108 districts and 1,503 confirmed measles cases in 2020 (4). The first dose of measles containing-vaccine (MCV) is administered when infants reach 9 months of age in measles-endemic areas, and at 12–15 months of age in areas where measles is not common. The second dose is delivered at 15–18 months of age in endemic areas and at ages 4–6 years in non-endemic areas, such as the United States. Measles-containing vaccine (MCV) protects a vaccinated child from acquiring the measles virus and protects the community through the development of herd immunity, which is achieved when 95% of the community is vaccinated. In Ethiopia, measles-containing vaccine one (MCV1) is administered starting at 9 months, and measles-containing vaccine two (MCV2) is administered at 15 months of age (5–9).
A systematic analysis of the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) on National Trends in Routine Childhood Vaccination showed that the long-term improvement in immunization reduced between 2010 and 2019, exacerbated by COVID-19, and was unable to reach the pre-COVID state in 2023 (2). Immunization Agenda 2030 (IA2030) is set to achieve 90% coverage for DTP3, PCV3, MCV2, and HPV vaccines. Forecasts to 2030 showed that only DPT3 may reach IA2030 (2). Vaccine hesitancy in Africa is a drawback to achieving IA2030 (2, 10). The WHO–UNICEF estimates MCV1 and MCV2 coverage for the African Region were 69 and 45% in 2022, respectively. In addition, Ethiopia is one of the eight countries in the globe that comprise 50% of zero-dose children (2, 11).
The worldwide MCV1 coverage was 72% in 2000 and increased to 86% in 2019, as reported by WHO. Nevertheless, it was found that MCV1 coverage decreased to 81% in the 2022 report, after Coronavirus Disease 2019 (COVID-19); it is the lowest achievement of MCV1. It rose and reached 83% between 2022 and 2023, but is still short of achieving the WHO target of 95% MCV1 immunization coverage. As a result of the COVID-19 pandemic, there was an 18% increase in measles cases worldwide as well (5, 6, 11). Sub-Saharan Africa consists of over 52.6% of zero-dose children. Ethiopia holds the third spot with 1.7 million MCV unvaccinated infants, next to Nigeria (3 million) and the Democratic Republic of the Congo (1.8 million) among 10 countries that contributed to 52% MCV unvaccinated children under 5 years of age (2, 5). According to the 2016 Ethiopian Demographic and Health Survey (EDHS) report, MCV1 coverage was 54.3% whereas it was 59% in the 2019 mini EDHS (12–14). Many infants are at significant risk of acquiring the measles virus since they did not take the vaccine, which causes measles outbreaks. It was found that only 26.4% of children received the measles vaccine on time, leading to delayed immunity and higher vulnerability to measles (9).
Missed and untimely vaccinations compromise the child’s development of permanent immunity and ultimately lead to the development of measles outbreaks. The measles outbreak is impacting a health system that has weekend by COVID-19 pandemic and remains highly unstable due to the conflict in Ethiopia. In low-income countries, such as sub-Saharan African countries, the burden of measles continues to affect the health system as a result of low MCV coverage and poor outbreak management. Measles infection has been a persistent problem, with repeated outbreaks reported in several regions, due to untimely and missed measles vaccinations in Ethiopia. The measles virus is highly contagious, and children who do not receive MCV1 at the appropriate age are at greater risk of infection, leading to increased severe complications, such as pneumonia, encephalitis, and death, mainly among children under 5 years of age (15–20).
It has been found that several factors are associated with delayed or missed measles vaccination. Socioeconomic status, maternal education, access to immunization services such as vaccine stockouts, long distances to health facilities, rural residence, and logistical challenges such as vaccine availability, and lack of awareness about the benefit of vaccinations and vaccination schedules were found to be associated factors for timely MCV1 uptake (12, 21–27).
To ensure adequate protection, the WHO and Ethiopian Ministry of Health guidelines state that MCV1 should be administered at 9 months, followed by subsequent vaccination at 15 months of age. Strengthening routine immunization services, such as outreach immunization services for hard-to-reach areas and increasing community engagement, has paramount importance to promote timely vaccination, as stated by global and national guidelines (20, 28, 29). Sustainable Development Goal 3 (SDGs) stated that ensuring healthy lives and promoting wellbeing for all ages, targets reducing child mortality through universal immunization coverage. Expanding measles immunization is crucial to meet the SDG 3.2 goal of ending preventable deaths of newborns and children under 5 years of age (30). So far, these strategies have brought about changes in MCV coverage, even though it is not enough to achieve herd immunity and prevent recurrent measles outbreaks in Ethiopia. However, ongoing conflicts in Oromia, Amhara, and the Tigray region affects the coordination of different stakeholder immunization services in Ethiopia (9, 12, 15, 21, 31, 32).
This study assessed the timing of MCV1 vaccination and its associated factors among infants in Ethiopia using data from the Performance Monitoring for Action (PMA). This study gives valuable evidence for policymakers and health professionals who are engaged in interventions on timely vaccination and coverage, ultimately reducing the burden of the measles outbreak in Ethiopia through identifying predictors for timely immunization of MCV1 to address the ongoing challenges of childhood vaccination in Ethiopia and ensuring that all children receive their vaccinations on time.
Methods
Study area and period
The Performance Monitoring for Action (PMA) survey is a major source of data on reproductive, maternal, and newborn health (RMNH) for decision-making. It has been conducted in many countries, such as Ethiopia. The project conducts cross-sectional and cohort surveys to fill data gaps not addressed by other major surveys, such as RMNH care services and the factors influencing their delivery across Amhara, Oromia, SNNP, and Addis Ababa regions. PMA Cohort-2 data were collected from November 2021 to August 2023, which consisted of baseline data on socio-demographic characteristics, pregnancy intentions, and antenatal care services from pregnant and 5–9 weeks postpartum. Eligible women were followed at 6 weeks, 6 months, and 1 year after childbirth. This study includes those mother-infant pairs who participated and completed the 1-year follow-up period (33–38).
Study design
A cohort study was conducted. At baseline, a cross-sectional design was conducted to enroll eligible women and collect initial data.
Population
Source population
The source population includes all infant-mother pairs residing in the selected regions of Ethiopia (2021–2023).
Study population
The study population consisted of all infant-mother pairs residing in the selected regions of Ethiopia with infants who were either 9 weeks of age by November 2021 or born between November 2021 and October 2022. All infants were required to survive to at least 9 months of age.
Eligibility criteria
Inclusion criteria
The inclusion criteria consisted of all infants aged 9 months and older from cohort 2 of the PMA data (2021–2023).
Exclusion criteria
Children who received MCV1 before 9 months (270 days) of age.
Sample size determination
Sample size determination PMA
PMA Ethiopia determined the sample size using data from previous PMA2020 surveys to estimate the modern contraceptive prevalence rate, account for the design effect, and anticipate non-response. A total of 217 enumeration areas (EAs) were selected to achieve a 5% margin of error for modern contraceptive prevalence rate estimates in each panel region, with an additional 81 EAs included for non-panel regions. Based on fertility rates, approximately 2,800 women were expected to enroll in the panel for cohort-1. The cohort-2 was updated from this cohort-1 as Tigray, Afar, and the eight EAs in SNNP were removed in cohort-2. Finally, the final EA sample size for cohort-2 was 162 EAs (38).
The sample size included in this study
PMA cohort 2 included 10,389 women in the baseline survey. Among these, only 2,297 were eligible for the follow-up survey, which comprised women who were pregnant (1,796), 0–4 weeks postpartum (228), and 5–9 weeks postpartum (273). A total of 8,092 women were not enrolled into the cohort-2 as they were neither pregnant nor postpartum at baseline. Finally, 1,990 were followed up on until the 1-year follow-up period, and 1,859 consented and completed the 1-year follow-up survey. After excluding infants who received MCV1 before 9 months of age, 1,770 mother-infant pairs were included in the analysis.
Sampling technique and procedure
A multistage stratified cluster sampling method was utilized for PMA data. The enumeration areas (EAs) were chosen with probability proportional to size within the strata. In Amhara, Oromia, and SNNP, the strata were defined by urban/rural residence, while no strata applied to Addis ababa. Within the panel regions, census of households was conducted, all women aged 15–49 who were regular household members were identified. Eligible participants for follow-up were those who were pregnant or had given birth in the past 9 weeks (38).
Study variables
Dependent variables
Time to first dose of MCV (vaccinated or event = 1, censor = 0).
Independent variables
Socio-demographic characteristics: religion, women’s age group, strata, education status of women, residence, wealth quintile, living together after marriage, and marital status.
Reproductive variables: desired pregnancy, parity, delivery place, and PNC vaccination counseling.
Operational definition
Survival time: Time from 9 months (269 days) to 1-year follow-up. The vaccination date was obtained from the child’s immunization card. For those who were censored, survival time was calculated from the date of the last follow-up interview. For individuals without an immunization card, survival time was estimated using multiple imputation techniques (34, 35). Time zero was defined at 269 days of age, and the event was considered to occur at 270 days of age (9 months) to assess timely vaccination during the follow-up period. Children who received MCV1 before 270 days of age were excluded from the analysis. This prevents the misclassification of early vaccinations, which are affected by maternal antibodies, as a good outcome.
Event: When the infant took the MCV1 vaccine, starting from 270 days till the follow-up period ends.
Timely MCV1 vaccination: When the infant received MCV1 between 9–10 completed months of age (4, 9, 39).
Data extraction tools and procedure
During the census, enumerators screened women to identify those who were pregnant or had given birth within the past 9 weeks. During the initial interview, data were collected on various socio-demographic characteristics. Women who consented to participate were enrolled in the follow-up survey and asked about maternal and child health until 1-year follow-up. Data were collected at each visit only for children who were still alive. Since the study focused on time to MCV1, only women whose children were alive and who completed the 1-year follow-up cohort were included in the analysis (38).
Data quality management
To ensure data quality, data collectors and supervisors received training and ongoing monitoring throughout the data collection process. Daily data checks were conducted, and the field teams received feedback to address any discrepancies. Additionally, data validation rules were integrated into the ODK system to minimize entry errors and enhance the reliability of the collected data (38).
Data processing and analysis
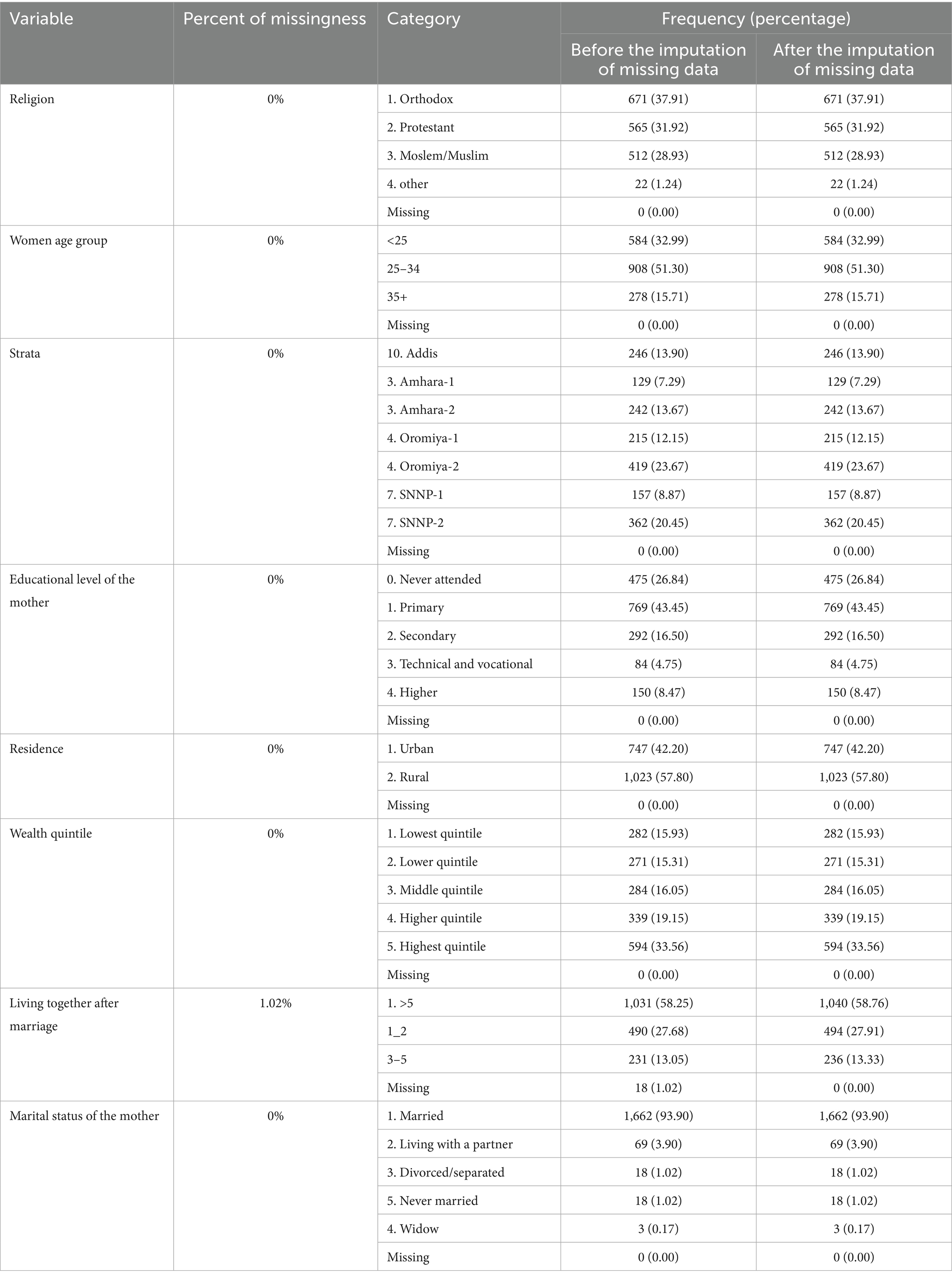
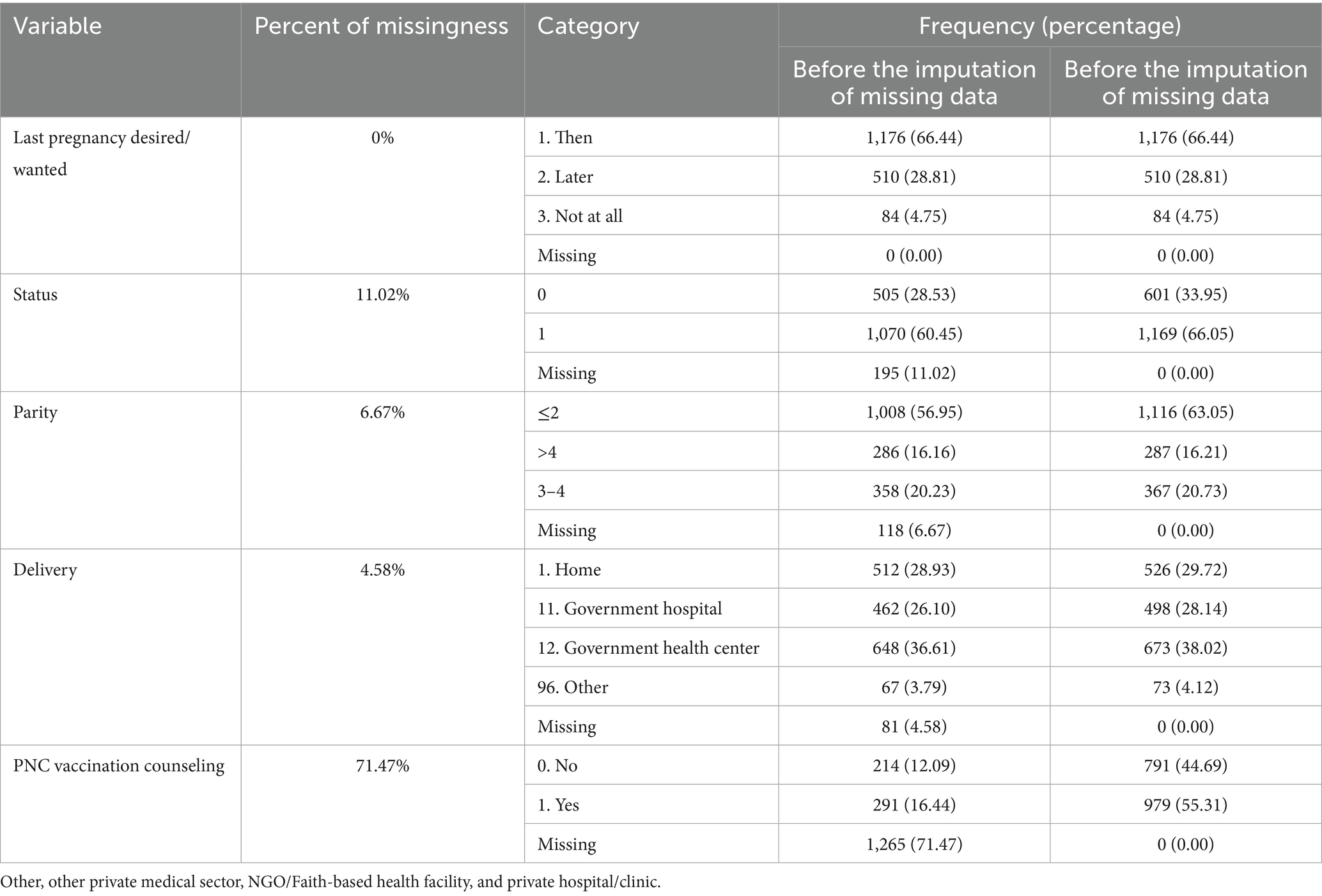
Data were cleaned, processed, and analyzed using R statistical software. Descriptive statistics were calculated to summarize key indicators, and multiple imputations were applied to address missing values in the variables. Sensitivity analysis was conducted, and the results were pooled using Rubin’s rule. Survival analysis was conducted using the survey package, which accounts for complex survey design in R (version 4.4.1) to account for the clustering, strata, and weighting effects inherent in the survey design. The proportional hazards assumption held for all variables. Multicollinearity was checked. Variable selection was conducted based on the likelihood significance test. Model fitness was assessed using the concordance index value (concordance index = 0.74), and the overall model significance test.
Ethical approval
The dataset was obtained from the PMA website via email after presenting the study’s objective and overall purpose.
Results
Socio-demographic variables
Out of 1,770 participants, the largest proportion, 419 (23.67%), was from Oromia-2, followed by SNNP-2 with 362 (20.45%), and the least from Amhara-1 with 129 (7.29%). Regarding residence, 1,023 (57.80%) of respondents were from rural areas. The lowest, lower, middle, higher, and highest wealth quintiles accounted for 282 (15.93%), 271 (15.31%), 284 (16.05%), 339 (19.15%), and 594 (33.56%) participants, respectively (Table 1).
Maternal and reproductive health factors
Desired pregnancy comprises 1,176 (66.44%) of the participants. Home delivery was accountable for 526 (29.72%), while institutional deliveries, government hospitals (28.14%), government health centers (38.02%), and other institutional deliveries (4.12%) comprise the rest (Table 2).
Measles first dose
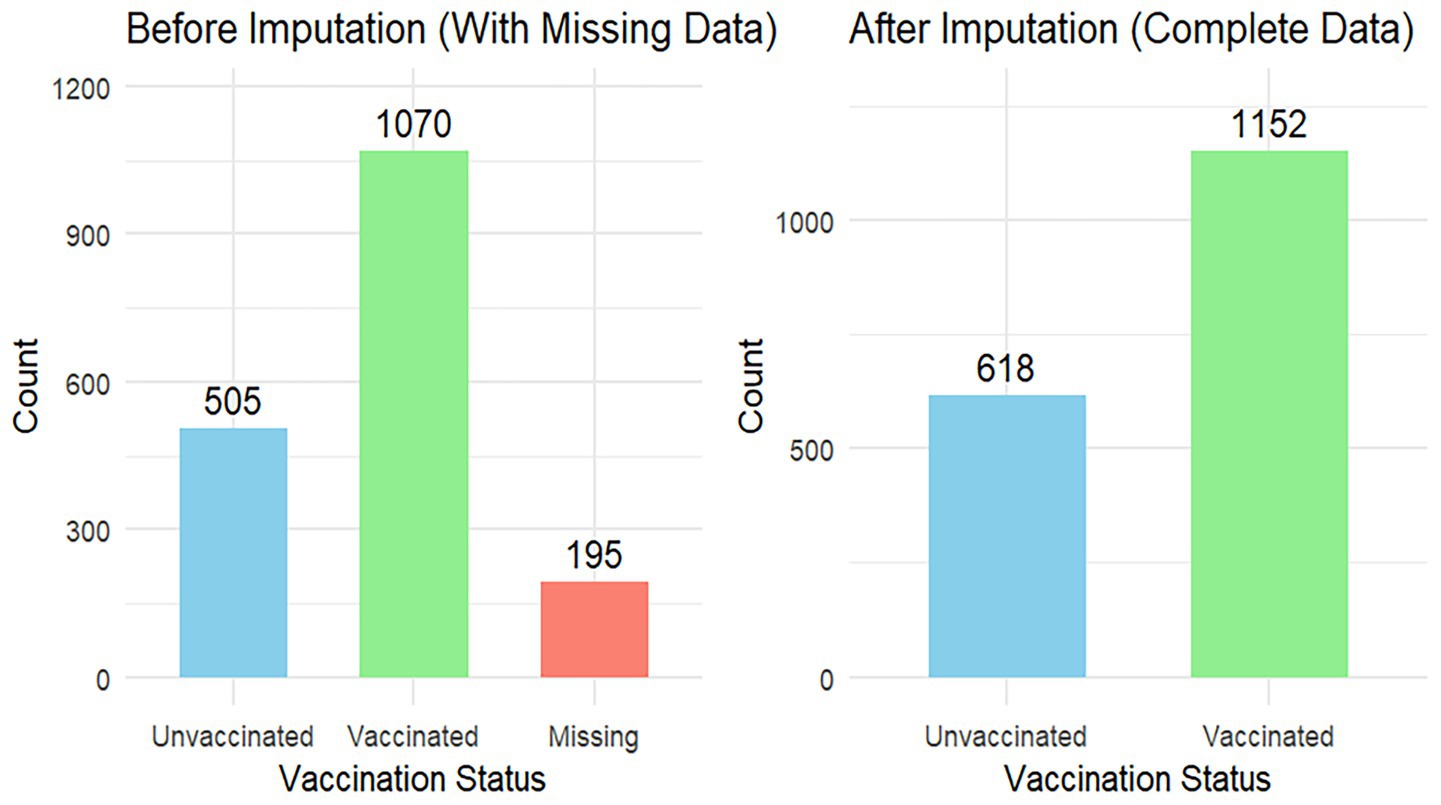
Among 1,770 participants, 618 (65.08%) developed an event of interest with a maximum follow-up time of 146 days. The incidence density was 12 per 1,000 person-days of follow-up (Figure 1).
Survival probability
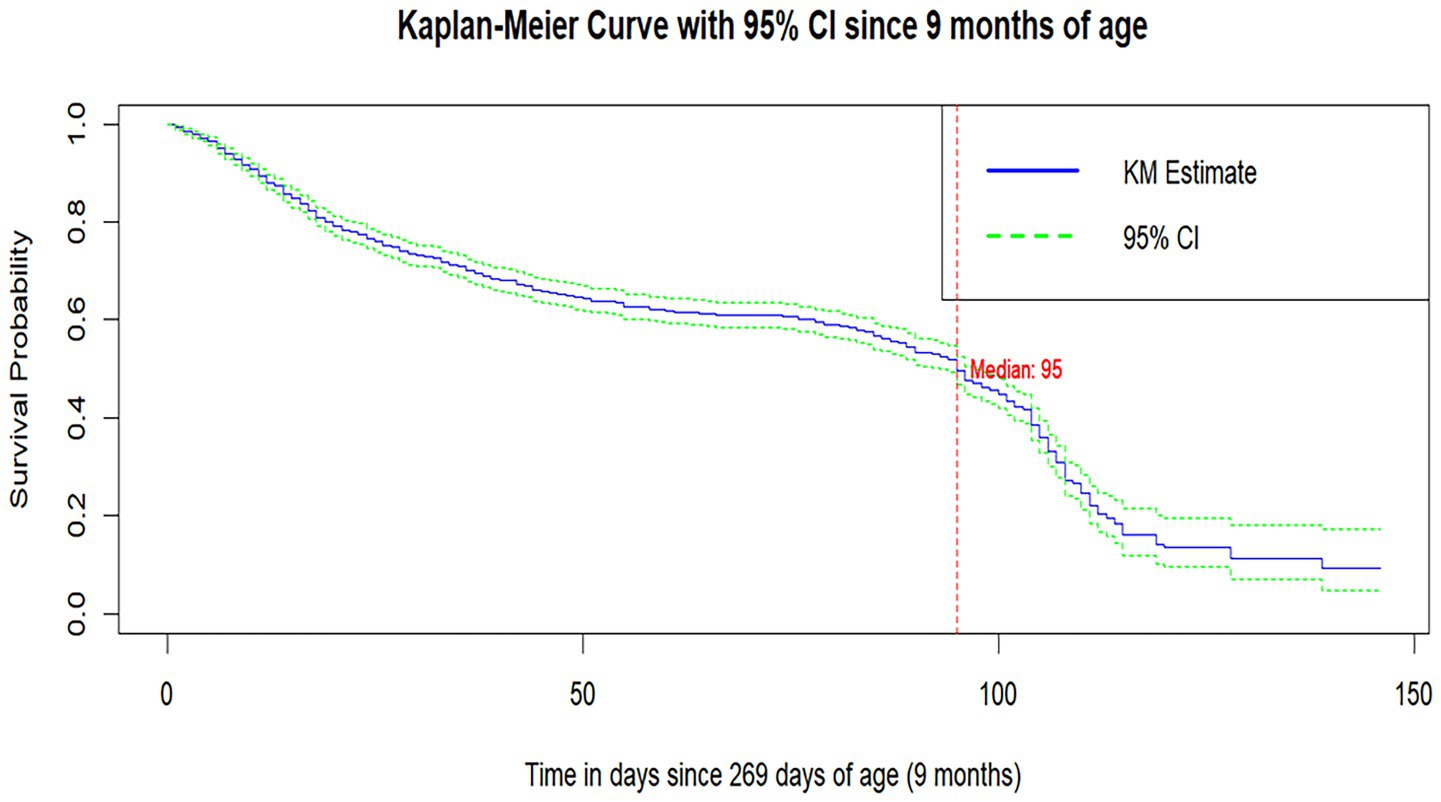
The median survival time to MCV1 vaccination was 95 days after the child turned 9 months old. The survival probability of MCV1 at 10 months of age since the infant completed 9 months of age was 0.73 (95%CI: 0.708,0.75), which indicates that 27% of infants took MCV1 at 10 months of age. The survival probability of MCV1 at 1 year of age was 0.534 (95%CI: 0.507, 0.562), which indicates that 46.6% of them received MCV1 on their first birthday (Figure 2).
Survival probabilities for independent variables
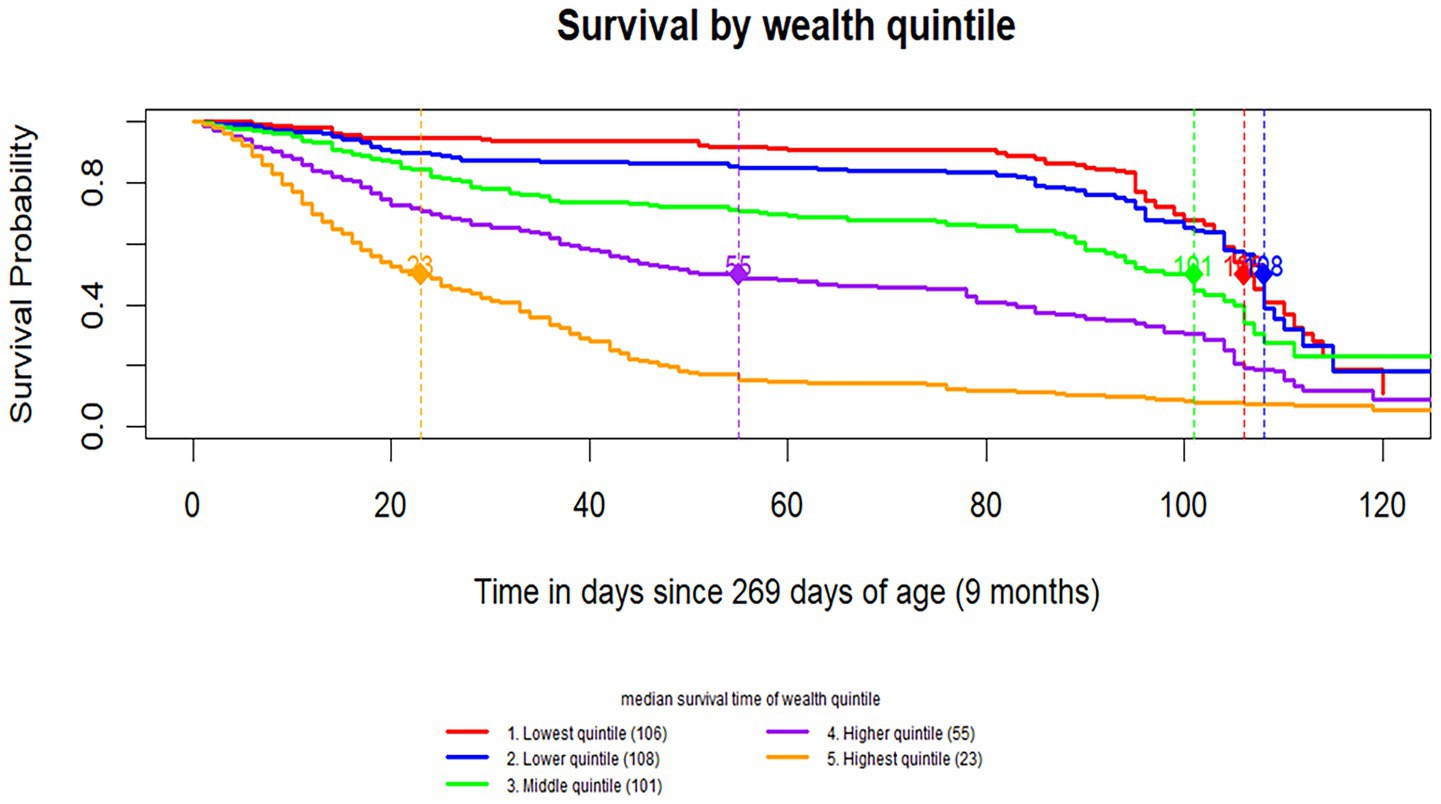
The survival probability varied across wealth quintile groups. The poorest groups showed noticeably higher survival probabilities compared to the wealthier groups. The median survival time was longest in the lower wealth quintiles (106 days for the lowest quintile and 108 days for the lower quintile) and progressively shorter in higher wealth groups, with the highest quintile showing the shortest median survival time, at 23 days (Figure 3).
Multivariable cox proportional hazards model
The hazard of receiving MCV1 was 35% lower for pregnancies, which were not desired at all compared to pregnancies desired from the beginning. The hazard of receiving MCV1 was 21% lower among pregnancies that were initially undesired but later became wanted compared with those pregnancies that were desired from the beginning. The children from the highest wealth quintile had 4.35 times the hazard of MCV1 vaccination (95% CI: 3.10–6.11) compared with the lowest quintile. Protestant Christian (AHR = 0.63, 95% CI: 0.49–0.80) and Muslim (AHR = 0.60, 95% CI: 0.47–0.78) mothers were less likely to vaccinate their children timely than Orthodox Christian mothers. Institutional deliveries (hospitals/health centers) were associated with 62–68% timely vaccination uptake compared with home births (Table 3).
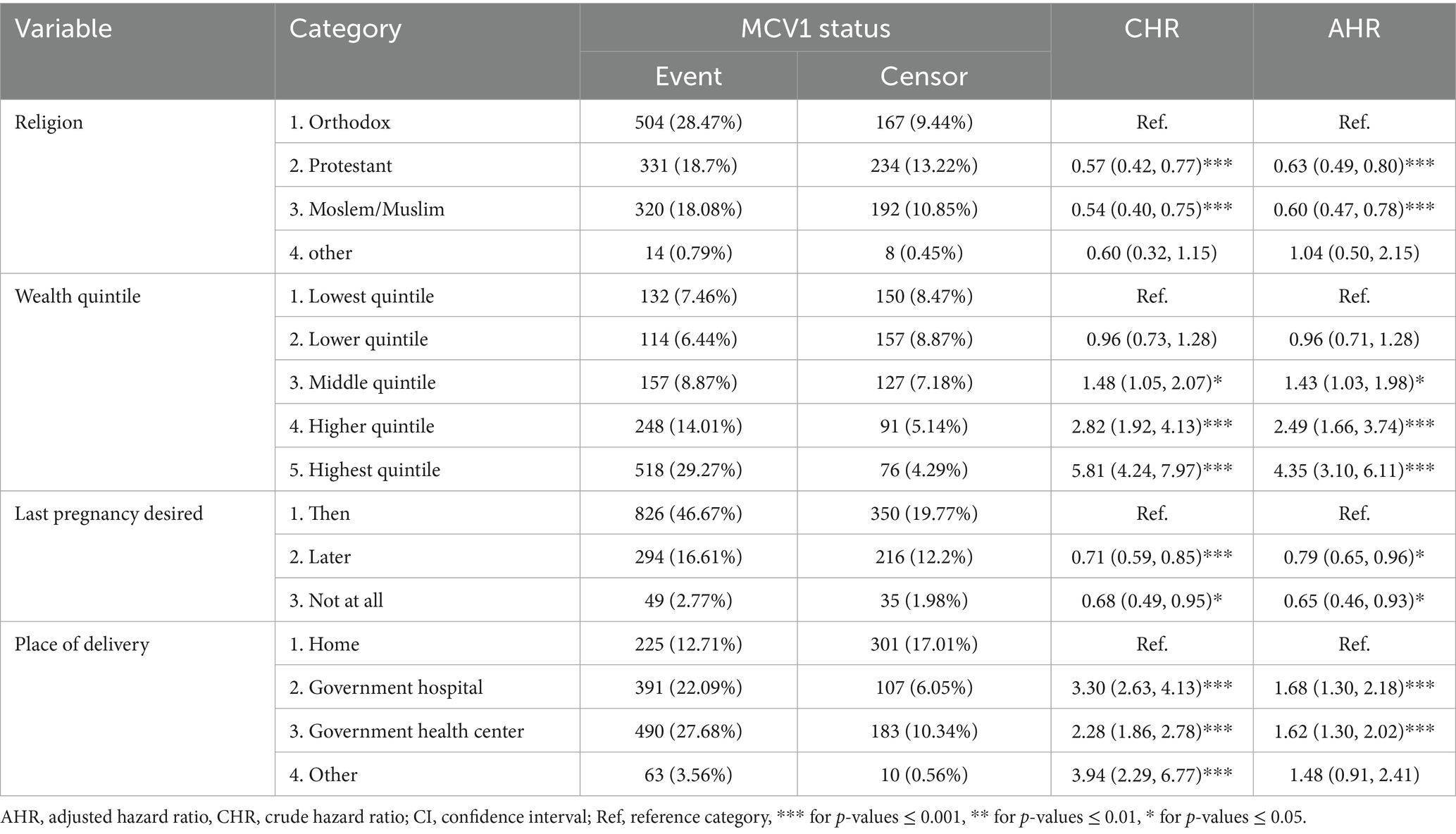
Table 3. Final multivariable model of time to first dose of measles-containing vaccine and associated factors.
Discussion
The study included those children who were eligible for MCV1 at 9 months of age. The result showed that 27% of respondents received the MCV1 vaccination at the age of 9 to 10 months, indicating that more than two-third of the infants did not receive the MCV1 vaccination on time. Approximately 53.4% did not take MCV1 at 1 year of age, increasing the risk of the measles epidemic before complete immunity is achieved. This study’s findings were consistent with previous research findings conducted in the North Shoa zone of Ethiopia, and the Worabe data from EDHS (9, 40). The reason behind this low vaccination timing may be due to limited access to immunization services as a result of hard-to-reach areas, availability of MCV1 vaccines as a result of logistics barriers, such as vaccine carriers, fridge shortage, transportation, and misunderstandings about the ideal vaccination age in rural areas (21, 22, 41).
On the contrary, the findings of MCV1 timely vaccination rates were higher than previous study findings conducted in Ethiopia, such as the Somalia region, North Shoa, Oromia region, Gondar City, Wolaita Zone, and West Shewa Zone (39, 42–45). This PMA data is a national-level data that covers a large geographical region of the country, and it may include hard-to-reach areas for immunization that may not be included in these specific area studies. This may reflect differences in immunization access, differences in awareness about immunization benefits, and cultural attitudes toward vaccination in different areas of Ethiopia. The other inconsistency may be due to sample size and modeling differences; this study used national data and advanced statistical modeling (12, 22, 41).
In contrast, this study shows that MCV1 vaccination rates were lower in previous research findings from Ethiopian DHS data (46), suggesting that the interventions implemented in recent times may be more effective or better executed. This inconsistency may be explained by improved access to immunization services compared to previous years, strengthened community outreach programs, increased government investment in immunization, better coordination through health development armies, greater caregiver awareness of the benefits of timely vaccination, and improved communication about vaccine side effects (47, 48).
Institutional delivery was associated with timely receipt of MCV1. It is supported by previous study findings (24, 27, 39, 41, 49, 50). The reason behind this association is that maybe mothers who give birth at health facilities are more likely to receive comprehensive postnatal care, such as guidance on vaccination timelines and follow-up appointments. In addition, institutional delivery increases healthcare seeking behavior, which enhances adherence to recommended vaccination schedules (51–53).
Unintended pregnancy was associated with delayed receipt of MCV1. It is supported by previous studies (9, 54). Due to its potential impact on maternal and household dynamics, women who did not desire their pregnancies might experience reduced support from their partners or family members, which can affect their ability to access immunization services. This reduced support may also contribute to psychosocial stress and lower prioritization of immunization services, resulting in delays in taking their children to vaccinations. Furthermore, non-desired pregnancies might result in limited healthcare utilization during pregnancy and postpartum periods, leading to less awareness of vaccination schedules (55–58).
These findings suggest that children born to Protestant Christian and Muslim mothers were less likely to receive timely measles vaccination compared to those born to Orthodox Christian mothers. This disparity may reflect differences in beliefs toward vaccination, trust in the vaccination, or may reflect the belief that their faith protects children from disease (59, 60).
Children from wealthier households were associated with timely MCV1 uptake than those from poorer households. This association may be due to the role of socioeconomic inequality in access to timely vaccination. Wealthier families may have better access to health facilities for vaccination, higher health literacy, and fewer barriers to transportation or opportunity costs to immunization sites. In contrast, families in the lowest wealth quintile live in remote areas and may face transportation and lack of access to immunization sites, even when vaccines are provided free of charge (61–63).
Strengths and limitations
This study has several strengths, such as a large, representative sample and the use of longitudinal data from the PMA Ethiopia cohort, which enhances validity. Advanced statistical methods using survey design survival analysis was used to control the collinearity of cluster effects, strata and survey weighting. The missing data was handled using multiple imputations. In addition, this study explicitly assessed the timeliness of MCV1 and its associated variables. However, this study did not consider the overall MCV1 coverage. The data were collected from four highly populated regions of the country, and other areas were not included, which may restrict generalizability to the country, Ethiopia. Moreover, the major limitation of the study is the lack of a comparison group, so that participants may have altered their behavior since similar questions were asked at each follow-up. This could affect the reliability of the findings. Potential unmeasured variables from this secondary dataset, such as maternal health beliefs, may influence vaccination behavior. There was a lack of convergence to the PNC counseling variable during multiple imputation since 71% of the data were missing, which may affect the validity of the variable significance.
Conclusion
The findings showed that the timely MCV1 uptake was low. Place of delivery, maternal pregnancy intention, religion, and wealth quantiles were associated with the time to measles first dose. Infants born in health facilities were more likely to receive timely vaccinations. Unwanted pregnancies, poor wealth quantile, protestant and Muslim religion, and home of delivery were associated with delayed MCV1 uptake.
Implications of the study
The findings of this study have several important implications for MCV1 uptake and immunization programs in Ethiopia. This study emphasizes the role of institutional deliveries in timely vaccination. Encouraging mothers to deliver in health facilities provides an opportunity to counsel them about timely vaccination and creates good caregiver and healthcare provider relationships. Health care providers are encouraged to integrate facility delivery with immunization, which in turn contributes to improved vaccination adherence.
Unwanted pregnancies lead to delayed vaccination. Healthcare providers, health system planners, and other stakeholders are advised to support unintended pregnancies, and educating mothers on the importance of vaccination and adhering to vaccination schedules can enhance timely immunization.
The health authorities need to collaborate more closely with religious leaders to build trust toward vaccination and tailor vaccine messaging to be respectful and relevant to religious beliefs. Implementing a continuous outreach immunization service helps low-income individuals obtain timely vaccination.
Future research are recommended to conducted longitudinal studies to assess how adherence to the MCV1 immunization contributes to sustained herd immunity and outbreak prevention.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the data is accessible only when the objective of the study is stated to the performance monitoring for action (PMA) data set owners. Requests to access these datasets should be directed to https://www.pmadata.org/countries/ethiopia.
Ethics statement
Ethical approval was not required for the studies involving humans because it is a secondary data set, obtained from performance monitoring for action project (PMA). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
EA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EE: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AE: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We extend our sincere gratitude to the PMA data owners for granting us access to the data. Their support was invaluable in enabling our research and facilitating analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
COVID-19, Coronavirus disease 2019; EAs, Enumeration areas; EDHS, Ethiopian Demographic Health Survey; IA2030, Immunization Agenda 2030; MCV1, Measles-containing vaccine first dose; PMA, Performance Monitoring for Action; RMNH, Reproductive, Maternal, and Newborn Health; PNC, Postnatal Care, Maternal, and Newborn Health; SDG, Sustainable Development Goal; SNNP, Southern Nations, Nationalities, and Peoples’ (Region); WHO, World Health Organization.
References
1. WHO. Essential Programme on Immunization. (n.d.) Available online at: https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/ (Accessed August 2025).
2. Haeuser, E, Byrne, S, Nguyen, J, Raggi, C, McLaughlin, SA, Bisignano, C, et al. Global, regional, and national trends in routine childhood vaccination coverage from 1980 to 2023 with forecasts to 2030: a systematic analysis for the global burden of disease study 2023. Lancet. (2025) 406:235–60. doi: 10.1016/S0140-6736(25)01037-2
3. Minta, AA, Ferrari, M, Antoni, S, Portnoy, A, Sbarra, A, Lambert, B, et al. Progress toward regional measles elimination—worldwide, 2000-2021. MMWR Morb Mortal Wkly Rep. (2022) 71:1489–95. doi: 10.15585/mmwr.mm7147a1
4. Federal Ministry of Health. Ethiopia National Expanded Program on immunization. Addis Ababa: Federal Ministry of Health (2021).
5. Minta, AA. Progress toward measles elimination—worldwide, 2000–2022. MMWR Morb Mortal Wkly Rep. (2023) 72:1262–8. doi: 10.15585/mmwr.mm7246a3
6. World Health Organization Measles. (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/measles (Accessed May 2025).
7. World Health Organization History of measles vaccination. (2024). Available online at: https://www.who.int/news-room/spotlight/history-of-vaccination/history-of-measles-vaccination (Accessed September 2024).
8. Ibrahim, HA, Wariyo, A, Asefa, EM, Cheru, A, Abebe Lonsako, A, and Dirirsa, G. Measles second dose vaccine uptake and associated factors among under-five children in Jigjiga City, Somali region, eastern Ethiopia: a community-based cross-sectional study. Front Public Health. (2024) 12:802. doi: 10.3389/fpubh.2024.1395802
9. Marefiaw, TA, Yenesew, MA, and Mihirete, KM. Age-appropriate vaccination coverage and its associated factors for pentavalent 1-3 and measles vaccine doses, in Northeast Ethiopia: a community-based cross-sectional study. PLoS One. (2019) 14:e0218470. doi: 10.1371/journal.pone.0218470
10. Anjorin, AA, Odetokun, IA, Abioye, AI, Elnadi, H, Umoren, MV, Damaris, BF, et al. Will Africans take COVID-19 vaccination? PLoS One. (2021) 16:e0260575. doi: 10.1371/journal.pone.0260575
11. Masresha, BG, Wiysonge, CS, Katsande, R, O’Connor, PM, Lebo, E, and Perry, RT. Tracking measles and rubella elimination Progress—World Health Organization African region, 2022–2023. Vaccine. (2024) 12:949. doi: 10.3390/vaccines12080949
12. Geremew, TT, Gezie, LD, and Abejie, AN. Geographical variation and associated factors of childhood measles vaccination in Ethiopia: a spatial and multilevel analysis. BMC Public Health. (2019) 19:1–15. doi: 10.1186/s12889-019-7529-z
13. Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. Ethiopia Mini demographic and health survey 2019: Key indicators. Rockville, Maryland, USA: EPHI and ICF (2019).
14. Tesfaye, L, Forzy, T, Getnet, F, Misganaw, A, Woldekidan, MA, and Wolde, AA. Estimating immunization coverage at the district level: a case study of measles and diphtheria-pertussis-tetanus-Hib-HepB vaccines in Ethiopia. PLoS Glob Public Health. (2024) 4:e0003404. doi: 10.1371/journal.pgph.0003404
15. Lo, NC, and Hotez, PJ. Public health and economic consequences of vaccine hesitancy for measles in the United States. JAMA Pediatr. (2017) 171:887–92. doi: 10.1001/jamapediatrics.2017.1695
16. Cataldi, JR. The many costs of measles outbreaks and Undervaccination: why we need to invest in public health. Pediatrics. (2021) 147:303. doi: 10.1542/peds.2020-035303
17. Perrone, O, and Meissner, HC. The importance of MMR immunization in the United States. Pediatrics. (2020) 146:251. doi: 10.1542/peds.2020-0251
18. Eshetu, D, Tosisa, W, Regassa, BT, Hundie, GB, and Mulu, A. Epidemiology of measles outbreaks, incidence and associated risk factors in Ethiopia from 2000 to 2023: a systematic review and meta-analysis. BMC Infect Dis. (2024) 24:914. doi: 10.1186/s12879-024-09828-6
19. Tariku, MK, Worede, DT, Belete, AH, Bante, SA, and Misikir, SW. Attack rate, case fatality rate and determinants of measles infection during a measles outbreak in Ethiopia: systematic review and meta-analysis. BMC Infect Dis. (2023) 23:756. doi: 10.1186/s12879-023-08757-0
20. World Health Organization Disease outbreak news; measles—Ethiopia. (2023). Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON460 (Accessed September 2024).
21. Addis, M, Mekonnen, W, and Estifanos, AS. Health system barriers to the first dose of measles immunization in Ethiopia: a qualitative study. BMC Public Health. (2024) 24:665. doi: 10.1186/s12889-024-18132-6
22. Bangura, JB, Xiao, S, Qiu, D, Ouyang, F, and Chen, L. Barriers to childhood immunization in sub-Saharan Africa: a systematic review. BMC Public Health. (2020) 20:1108. doi: 10.1186/s12889-020-09169-4
23. Wagner, AL, Zhang, Y, Montgomery, JP, Ding, Y, Carlson, BF, and Boulton, ML. Timely measles vaccination in Tianjin, China: a cross-sectional study of immunization records and mothers. BMC Public Health. (2014) 14:1–7. doi: 10.1186/1471-2458-14-888
24. Hu, Y, Wang, Y, Chen, Y, Liang, H, and Chen, Z. Measles vaccination coverage, determinants of delayed vaccination and reasons for non-vaccination among children aged 24–35 months in Zhejiang province, China. BMC Public Health. (2018) 18:1–8. doi: 10.1186/s12889-018-6226-7
25. Panda, BK, Mishra, S, and Awofeso, N. Socio-demographic correlates of first dose of measles (MCV1) vaccination coverage in India. BMC Public Health. (2020) 20:1221. doi: 10.1186/s12889-020-09321-0
26. Tesfa, GA, Demeke, AD, Hailegebreal, S, Amede, ES, Ngusie, HS, Kasie, MN, et al. Spatial distribution and associated factors of measles vaccination among children aged 12–23 months in Ethiopia. A spatial and multilevel analysis. Hum Vaccin Immunother. (2022) 18:558. doi: 10.1080/21645515.2022.2035558
27. Woyessa, AB, Shah, MP, Azmeraye, BM, Pan, J, Lisanwork, L, Yimer, G, et al. Factors associated with uptake of routine measles-containing vaccine doses among young children, Oromia regional state, Ethiopia, 2021. Vaccine. (2024) 12:762. doi: 10.3390/vaccines12070762
28. WHO Africa. Ethiopia Launches Measles Vaccine Second Dose (MCV2) Introduction: Over 3.3 million children will receive the vaccine annually. (2019). Available from: https://www.afro.who.int/news/ethiopia-launches-measles-vaccine-second-dose-mcv2-introduction-over-33-million-children-will (Accessed August 2024).
29. Animaw, W, Taye, W, Merdekios, B, Tilahun, M, and Ayele, G. Expanded program of immunization coverage and associated factors among children age 12 – 23 months in Arba Minch town and Zuria District, southern Ethiopia, 2013. BMC Public Health. (2014) 14:464. doi: 10.1186/1471-2458-14-464
30. Sadoh, AE, and Eregie, CO. Timeliness and completion rate of immunization among Nigerian children attending a clinic-based immunization service. J Health Popul Nutr. (2009) 27:391–5. doi: 10.3329/jhpn.v27i3.3381
31. Sullivan, MC, Tegegn, A, Tessema, F, Galea, S, and Hadley, C. Minding the immunization gap: family characteristics associated with completion rates in rural Ethiopia. J Community Health. (2010) 35:53–9. doi: 10.1007/s10900-009-9192-2
33. Anglès-Acedo, S, Ros-Cerro, C, Escura-Sancho, S, Elías-Santo-Domingo, N, Palau-Pascual, MJ, and Espuña-Pons, M. Coital resumption after delivery among OASIS patients: differences between instrumental and spontaneous delivery. BMC Womens Health. (2019) 19:154. doi: 10.1186/s12905-019-0845-8
34. Addis Ababa University School of Public Health; and the Bill & Melinda Gates Institute for Population and Reproductive Health at the Johns Hopkins Bloomberg School of Public Health. Performance Monitoring for Action Ethiopia (PMA-ET) Panel: Cohort 2 Household and Female Baseline Survey (Version #), PMAET-Panel-C2-BL-HQFQ. (2021). Ethiopia and Baltimore, Maryland, USA.
35. Addis Ababa University School of Public Health and The Bill & Melinda Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health. Performance Monitoring for Action Ethiopia (PMA-ET) Panel: Cohort 2—Six-Week Follow-up Survey (Version #), PMAET-Panel-C2-6wkFU. (2022). Ethiopia and Baltimore, Maryland, USA.
36. Addis Ababa University School of Public Health and The Bill & Melinda Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health. Performance Monitoring for Action Ethiopia (PMA-ET) Panel: Cohort 2—Six-Month Follow-up Survey (Version #), PMAET-Panel-C2-6moFU. (2023). Ethiopia and Baltimore, Maryland, USA.
37. Addis Ababa University School of Public Health and the William H. Gates Sr. Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health. Performance Monitoring for Action Ethiopia (PMA-ET) Panel: Cohort 2—one-Year Follow-up Survey (Version 1.0), PMAET-Panel-C2-1yrFU. (2023). Ethiopia and Baltimore, Maryland, USA.
38. Addis Ababa University School of Public Health and The William H. Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health. Performance monitoring for action Ethiopia (PMA-ET) cohort two baseline maternal newborn health technical report. (2021). Ethiopia and Baltimore, Maryland, USA.
39. Dejene, H, Girma, D, Geleta, LA, and Legesse, E. Vaccination timeliness and associated factors among children aged 12–23 months in Debre Libanos district of north Shewa zone, Oromia regional state, Ethiopia. Front Pediatr. (2022) 10:867846. doi: 10.3389/fped.2022.867846
40. Wagner, AL, Tefera, YA, Gillespie, BW, Carlson, BF, and Boulton, ML. Vaccine coverage, timeliness and delay estimated from regional and national cross-sectional surveys in Ethiopia, 2016. Pan Afr Med J. (2021) 39:205. doi: 10.11604/pamj.2021.39.205.22777
41. Gelagay, AA, Worku, AG, Bashah, DT, Tebeje, NB, Gebrie, MH, Yeshita, HY, et al. Complete childhood vaccination and associated factors among children aged 12–23 months in Dabat demographic and health survey site, Ethiopia, 2022. BMC Public Health. (2023) 23:802. doi: 10.1186/s12889-023-15681-0
42. Dirirsa, K, Makuria, M, Mulu, E, and Deriba, BS. Assessment of vaccination timeliness and associated factors among children in toke Kutaye district, Central Ethiopia: a mixed study. PLoS One. (2022) 17:e0262320. doi: 10.1371/journal.pone.0262320
43. Mekonnen, ZA, Gelaye, KA, Were, MC, and Tilahun, B. Timely completion of vaccination and its determinants among children in northwest, Ethiopia: a multilevel analysis. BMC Public Health. (2020) 20:908. doi: 10.1186/s12889-020-08935-8
44. Geta Hardido, T, Atinafu Ataro, B, and Elifios, E. Timely vaccination and its associated factors among parents with children aged from 0 to 23 months in Wolaita zone public hospitals, southern Ethiopia, 2024: a facility-based cross-sectional study. Glob Pediatr Health. (2024) 11:2333794x241297034. doi: 10.1177/2333794X241297034
45. Marine, BT, Mengistie, DT, Zewde, MG, Kassie, MZ, Dimore, AL, Duftu, KB, et al. Predictors of timely administration of measles immunization among children aged 12–23 months in aw-bare Woreda, Ethiopia. Discover Med. (2025) 2:218. doi: 10.1007/s44337-025-00471-x
46. Boulton, ML, Carlson, BF, Wagner, AL, Porth, JM, Gebremeskel, B, and Abeje, Y. Vaccination timeliness among newborns and infants in Ethiopia. PLoS One. (2019) 14:e0212408. doi: 10.1371/journal.pone.0212408
47. Baptiste, AEJ, Masresha, B, Wagai, J, Luce, R, Oteri, J, Dieng, B, et al. Trends in measles incidence and measles vaccination coverage in Nigeria, 2008–2018. Vaccine. (2021) 39:C89–95. doi: 10.1016/j.vaccine.2021.03.095
48. Wang, R, Jing, W, Liu, M, and Liu, J. Trends of the global, regional, and national incidence of measles, vaccine coverage, and risk factors in 204 countries from 1990 to 2019. Front Med. (2022) 8:798031. doi: 10.3389/fmed.2021.798031
49. Moyer, CA, Tadesse, L, and Fisseha, S. The relationship between facility delivery and infant immunization in Ethiopia. Int J Gynecol Obstet. (2013) 123:217–20. doi: 10.1016/j.ijgo.2013.06.030
50. Budu, E, Seidu, A-A, Agbaglo, E, Armah-Ansah, EK, Dickson, KS, Hormenu, T, et al. Maternal healthcare utilization and full immunization coverage among 12–23 months children in Benin: a cross sectional study using population-based data. Arch Public Health. (2021) 79:1–12. doi: 10.1186/s13690-021-00554-y
51. Darega, B, Dida, N, Tafese, F, and Ololo, S. Institutional delivery and postnatal care services utilizations in Abuna Gindeberet District, west Shewa, Oromiya region, Central Ethiopia: a community-based cross sectional study. BMC Pregnancy Childbirth. (2016) 16:1–7. doi: 10.1186/s12884-016-0940-x
52. Dahiru, T, and Oche, OM. Determinants of antenatal care, institutional delivery and postnatal care services utilization in Nigeria. Pan Afr Med J. (2015) 21:527. doi: 10.11604/pamj.2015.21.321.6527
53. Geda, NR, Feng, CX, Whiting, SJ, Lepnurm, R, Henry, CJ, and Janzen, B. Disparities in mothers’ healthcare seeking behavior for common childhood morbidities in Ethiopia: based on nationally representative data. BMC Health Serv Res. (2021) 21:1–11. doi: 10.1186/s12913-021-06704-w
54. Singh, A, Singh, A, and Mahapatra, B. The consequences of unintended pregnancy for maternal and child health in rural India: evidence from prospective data. Matern Child Health J. (2013) 17:493–500. doi: 10.1007/s10995-012-1023-x
55. Wado, YD, Afework, MF, and Hindin, MJ. Unintended pregnancies and the use of maternal health services in southwestern Ethiopia. BMC Int Health Hum Rights. (2013) 13:36. doi: 10.1186/1472-698X-13-36
56. Khan, MN, Harris, ML, Shifti, DM, Laar, AS, and Loxton, D. Effects of unintended pregnancy on maternal healthcare services utilization in low- and lower-middle-income countries: systematic review and meta-analysis. Int J Public Health. (2019) 64:743–54. doi: 10.1007/s00038-019-01238-9
57. Martin-de-Las-Heras, S, Velasco, C, de Dios Luna, J, and Martin, A. Unintended pregnancy and intimate partner violence around pregnancy in a population-based study. Women Birth. (2015) 28:101–5. doi: 10.1016/j.wombi.2015.01.003
58. Lewinsohn, R, Crankshaw, T, Tomlinson, M, Gibbs, A, Butler, L, and Smit, J. This baby came up and then he said,“I give up!”: the interplay between unintended pregnancy, sexual partnership dynamics and social support and the impact on women's well-being in KwaZulu-Natal, South Africa. Midwifery. (2018) 62:29–35. doi: 10.1016/j.midw.2018.03.001
59. Tiwana, MH, and Smith, J. Faith and vaccination: a scoping review of the relationships between religious beliefs and vaccine hesitancy. BMC Public Health. (2024) 24:1806. doi: 10.1186/s12889-024-18873-4
60. Santos, TM, Cata-Preta, BO, Wendt, A, Arroyave, L, Hogan, DR, Mengistu, T, et al. Religious affiliation as a driver of immunization coverage: analyses of zero-dose vaccine prevalence in 66 low- and middle-income countries. Front Public Health. (2022) 10:977512. doi: 10.3389/fpubh.2022.977512
61. Patenaude, BN, Sriudomporn, S, Odihi, D, Mak, J, and de Broucker, G. Comparing multivariate with wealth-based inequity in vaccination coverage in 56 countries: toward a better measure of equity in vaccination coverage. Vaccine. (2023) 11:536. doi: 10.3390/vaccines11030536
62. Ali, HA, Hartner, A-M, Echeverria-Londono, S, Roth, J, Li, X, Abbas, K, et al. Vaccine equity in low and middle income countries: a systematic review and meta-analysis. Int J Equity Health. (2022) 21:82. doi: 10.1186/s12939-022-01678-5
Keywords: measles-containing vaccine, timeliness of measles first dose, child health, measles immunization, survey design survival analysis, MCV1
Citation: Abeje ET, Enyew EB, Daba C, Asmare L, Bayou FD, Arefaynie M, Mohammed A, Tareke AA, Keleb A, Kebede N, Tsega Y, Endawkie A, Kebede SD and Abera KM (2025) Time to first dose of measles-containing vaccine and associated factors among infants in Ethiopia: a survival analysis from performance monitoring for action data. Front. Public Health. 13:1521602. doi: 10.3389/fpubh.2025.1521602
Edited by:
Effua Usuf, Medical Research Council The Gambia Unit (MRC), GambiaReviewed by:
Horst von Bernuth, Charité University Medicine Berlin, GermanyKamal A. Kadhim, Ministry of Health, Iraq
Copyright © 2025 Abeje, Enyew, Daba, Asmare, Bayou, Arefaynie, Mohammed, Tareke, Keleb, Kebede, Tsega, Endawkie, Kebede and Abera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eyob Tilahun Abeje, ZXlvYnQ1MjUxNTJAZ21haWwuY29t
 Eyob Tilahun Abeje
Eyob Tilahun Abeje Ermias Bekele Enyew
Ermias Bekele Enyew Chala Daba
Chala Daba Lakew Asmare
Lakew Asmare Fekade Demeke Bayou
Fekade Demeke Bayou Mastewal Arefaynie
Mastewal Arefaynie Anissa Mohammed
Anissa Mohammed Abiyu Abadi Tareke
Abiyu Abadi Tareke Awoke Keleb
Awoke Keleb Natnael Kebede
Natnael Kebede Yawkal Tsega
Yawkal Tsega Abel Endawkie
Abel Endawkie Shimels Derso Kebede
Shimels Derso Kebede Kaleab Mesfin Abera
Kaleab Mesfin Abera