- 1Department of Epidemiology and Biostatistics, School of Public Health, Southeast University, Nanjing, China
- 2Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China
- 3Medical Statistics and Analysis Center, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, China
- 4Key Laboratory of Environmental Medicine Engineering, Ministry of Education, School of Public Health, Southeast University, Nanjing, China
Objectives: Since the global outbreak of SARS-CoV-2 in 2019, COVID-19 reinfection has become an increasing concern, particularly during the spread of the Omicron variant. Despite numerous international studies on COVID-19 reinfection, research focusing on healthcare workers, particularly those in primary care settings in mainland China, remains limited. This study aims to evaluate COVID-19 reinfection rates among primary healthcare workers (PHWs) in Jiangsu Province and to explore potential risk factors contributing to reinfection.
Methods: This study utilized a combination of online questionnaires and on-site surveys to conduct two waves of investigation targeting PHWs after epidemic control policy adjustment in Jiangsu Province. Differences between the infection at the baseline visit and re-infection at the follow-up visit were analyzed, and multivariate logistic regression was used to assess the factors influencing reinfection.
Results: A total of 5,541 PHWs were included in the study. At the baseline visit, the initial infection rate was 85.85% [95% confidence interval (CI): 84.93–86.77%], and the self-reported reinfection rate was 40.05% (95% CI: 38.65–41.44%). After adjustment, the reinfection rate was 29.41% (95% CI: 28.12–30.71%). The median reinfection interval between the two infections was 146 days (Interquartile range: 129–164 days). Logistic regression model revealed that female sex [odds ratio (OR) = 1.376, 95% CI: 1.190–1.592], history of fever clinic work (OR = 1.179, 95% CI: 1.045–1.330), working over 8 h per day (OR = 1.178, 95% CI: 1.040–1.336), being a nurse (OR = 1.201, 95% CI: 1.029–1.402), and a “less meat, more vegetables” diet (OR = 1.206, 95% CI: 1.020–1.426) were significant risk factors for reinfection. Additionally, regular physical exercise was found to be a protective factor (OR = 0.861, 95% CI: 0.754–0.983).
Conclusion: COVID-19 reinfection rates were relatively high among PHWs in Jiangsu Province, particularly among women, nurses, those with fever clinic experience and working over 8 h per day. This study offers valuable insights for the prevention of COVID-19 reinfection and the development of protection strategies for PHWs. It is recommended that more targeted protective measures be implemented for high-risk groups, including appropriate work arrangements, regular health monitoring, and the promotion of healthy lifestyle habits.
1 Introduction
Since December 2019, SARS-CoV-2 has caused widespread and sustained global transmission. As of the seven days leading up to 15 September 2024, more than 776 million COVID-19 cases have been reported globally, with over 7.06 million deaths, according to estimates from the World Health Organization (1). However, the actual number of infections and deaths is likely much higher than reported (2). With the continuous mutation of SARS-CoV-2, the immune evasion capabilities of the virus have significantly increased, while human immunity wanes over time (3). This has resulted in frequent reports of reinfections, even multiple infections (4–8). However, research on reinfection among healthcare workers in mainland China, particularly those in primary care settings, remains relatively limited.
Since its first detection in November 2021, the Omicron variant has demonstrated a high degree of immune evasion. By 2022, Omicron had become the dominant variant in many countries worldwide (9). Numerous studies have shown that Omicron has a higher transmission rate and greater capacity to cause reinfections, with its reinfection rate surpassing that of earlier variants (10–12). Although Omicron appears to cause milder clinical manifestations compared to earlier variants, its significantly higher re-infection rate presents new challenges for public health control efforts (13, 14).
Unlike many other regions, mainland China successfully avoided large-scale nationwide outbreaks during the early stages of the pandemic through stringent control measures. However, following the adjustment of COVID-19 control policies to “normalized management” in December 2022, China experienced a surge in COVID-19 cases, reaching its first peak by the end of that month (15). Between September 26, 2022, and July 31, 2023, all 80,531 reported domestic COVID-19 cases in mainland China were caused by the Omicron variant (16). Due to their close contact with COVID-19 patients, primary healthcare workers (PHWs) faced a significantly higher risk of infection compared to the general population (17). Studies also indicate that repeated infections may result in more severe symptoms, with the risk of severe outcomes potentially increasing with each successive infection. In the context of ongoing SARS-CoV-2 mutations and adjustments to pandemic control policies, it is crucial to track infection and reinfection risks among healthcare workers. Effective preventive measures are needed to mitigate the risk of reinfection among this vulnerable population (18, 19).
This study investigates the infection and reinfection patterns among PHWs in Jiangsu Province during two distinct infection peaks. It aims to assess the characteristics and differences between the infection at baseline visit and re-infection at follow-up visit, and seeks to identify factors that may increase the risk of reinfection.
2 Materials and methods
2.1 Study design
This study employed a combination of online questionnaires and on-site surveys to conduct two waves of investigation among PHWs in Jiangsu Province. The first wave of the survey, conducted from January 17 to February 2, 2023, involved 34,090 individuals and took place approximately 6 weeks after the adjustment of COVID-19 prevention policies. The second wave of on-site survey, conducted from July 4 to July 20, 2023, targeted 5,754 PHWs from five counties included in the first wave. Professional staff collected data on infections occurring between April 1, 2023, and the survey date. All data were collected through the Questionnaire Star platform.1
The study protocol was approved by the Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention (JSJK2023-B010-01). All participants voluntarily participated in the study and provided informed consent before completing the questionnaire.
2.2 Definitions
Some variables used in this study were defined as follows:
Individuals who self-reported infection: Defined as those meeting any of the following criteria: (1) positive nucleic acid test for SARS-CoV-2; (2) positive antigen test; (3) both nucleic acid and antigen tests positive; (4) exhibiting COVID-19-related symptoms without undergoing nucleic acid or antigen testing.
Uninfected individuals: Defined as those meeting any of the following criteria: (1) negative nucleic acid and/or antigen test; (2) the absence of COVID-19-related symptoms and no nucleic acid or antigen testing performed.
Individuals who self-reported an infection at baseline visit: Defined as those self-reported being infected with SARS-CoV-2 during the first wave of the survey.
Individuals who self-reported reinfection: Defined as those self-reported being infected during both waves of the survey.
Non-reinfected individuals: Defined as those infected during the first survey period but not confirmed to have been infected during the second survey period.
Reinfection interval (RI): The time interval between the self-reported infection at baseline visit and self-reported re-infection at the follow-up visit.
Body Mass Index (BMI): BMI was calculated by dividing weight (kg) by the square of height (m2). According to classification standards (20), BMI was categorized as follows: underweight (<18.5 kg/m2), normal weight (18.5–23.9 kg/m2), overweight (24.0–27.9 kg/m2), and obesity (≥28.0 kg/m2).
2.3 Survey questionnaire
The questionnaire used in this study was adapted from the second-round COVID-19 pneumonia questionnaire issued by the Chinese Center for Disease Control and Prevention and the Peking Union Medical College, and modified with reference to the “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 10)” (21). The information collected by the questionnaire included: (1) Basic information: Demographic characteristics, medical history, occupation, lifestyle behaviors, and habits; (2) Infection-related information: Infection dates, typical symptoms (e.g., fever, muscle aches, cough, sore throat, nasal congestion, runny nose), hospitalization status, and any newly diagnosed diseases following infection; (3) Vaccination status: Number of vaccine doses and vaccine type; (4) Work burden: Weekly working hours and whether the participant had worked in fever clinics.
2.4 Statistical analysis
The sample size calculations resulted in n = 4,489, based on a projected long COVID prevalence of 8.89% and a two-sided 95% Clopper-Pearson confidence interval (CI) with a margin of error of 0.01778 (22). Considering that the reinfection rate exceeds the prevalence of long COVID, this sample size is deemed sufficient.
Continuous variables were expressed as means (standard deviation, SD), while categorical variables were summarized as frequencies and percentages. The 95% CI for the reinfection rate was calculated using the Wald method. For some individuals who exhibited COVID-19 symptoms but lacked nucleic acid tests or antigen detection, the adjusted reinfection rate was estimated using a weighted approach based on data from China’s National Influenza Surveillance System (16).
Statistical differences between groups were assessed by two-sample t-tests for continuous variables. For categorical variables, Pearson’s chi-square test was applied when the expected frequency in each cell was at least 5; otherwise, Fisher’s exact test was used. For the comparison of 14 symptom rates, the Bonferroni-Hochberg method was used for multiple comparisons. Both univariate and multivariate logistic regression analyses were conducted to identify factors associated with self-reported reinfection among PHWs. All variables were included in a multivariate logistic regression analysis using a stepwise selection method, with an entry criterion of p < 0.05 and a removal criterion of p > 0.10. Odds ratio (OR) and 95%CI quantified the risk associated with reinfection. A two-sided p-value < 0.05 was considered statistically significant.
Considering that some self-reported infected individuals had not undergone virus testing, resulting in potential ambiguity in the definitions, a sensitivity analysis was conducted. Nucleic acid or antigen-positive individuals were regarded as confirmed cases, while nucleic acid/antigen-negative or untested asymptomatic individuals were categorized as uninfected. Logistic regression was performed again using these groups.
All statistical analyses were performed using R software (version 4.3.3).
3 Results
3.1 Participants and self-reported reinfection rates
In the first wave of survey, a total of 34,090 questionnaires were collected between January 17 and February 2, 2023 in Jiangsu Province, the overall infection rate among PHWs was 81.05% (95% CI: 80.61–81.48%) from December 2022 to January 2023 (23). From these, five counties/districts were selected for second wave of on-site survey: Ganyu District in Lianyungang City, Funing County, Yandu District in Yancheng City, and the county-level cities of Kunshan and Changshu in Suzhou.
The second wave of the on-site survey included 5,754 PHWs across five counties. After data verification, questionnaires with inconsistent or illogical responses were excluded, resulting in the removal of 213 questionnaires. A total of 5,541 valid questionnaires remained for analysis.
Among the 5,541 participants included in this study, 67.30% were female, with a mean age of 39.80 years (SD = 10.80). Of the 4,757 participants who self-reported an infection at baseline visit, 1,905 were self-reported reinfected, and 2,852 did not experience reinfection. The self-reported infection at baseline visit rate was 85.85% (95% CI: 84.93–86.77%), and the self-reported reinfection rate was 40.05% (95% CI: 38.65–41.44%). Among those reporting an infection at baseline visit, 6.71% exhibited COVID-19-related symptoms without having undergone nucleic acid or antigen testing. In the second wave survey, 1,253 participants had a positive nucleic acid test, positive antigen test, or both, while 652 participants (34.23%) had COVID-19-related symptoms without testing. Based on the positivity rate among influenza-like illness patients from national sentinel hospitals, we assumed that a proportion of these 652 symptomatic participants were false positives. By adding the confirmed positives to the numerator and incorporating the estimated number of false positives into the denominator, we recalculated the positivity rate, yielding an adjusted reinfection rate of 29.41% (95% CI: 28.12–30.71%).
3.2 Analysis of infection at the baseline visit and follow-up visit
3.2.1 Infection dates and time intervals
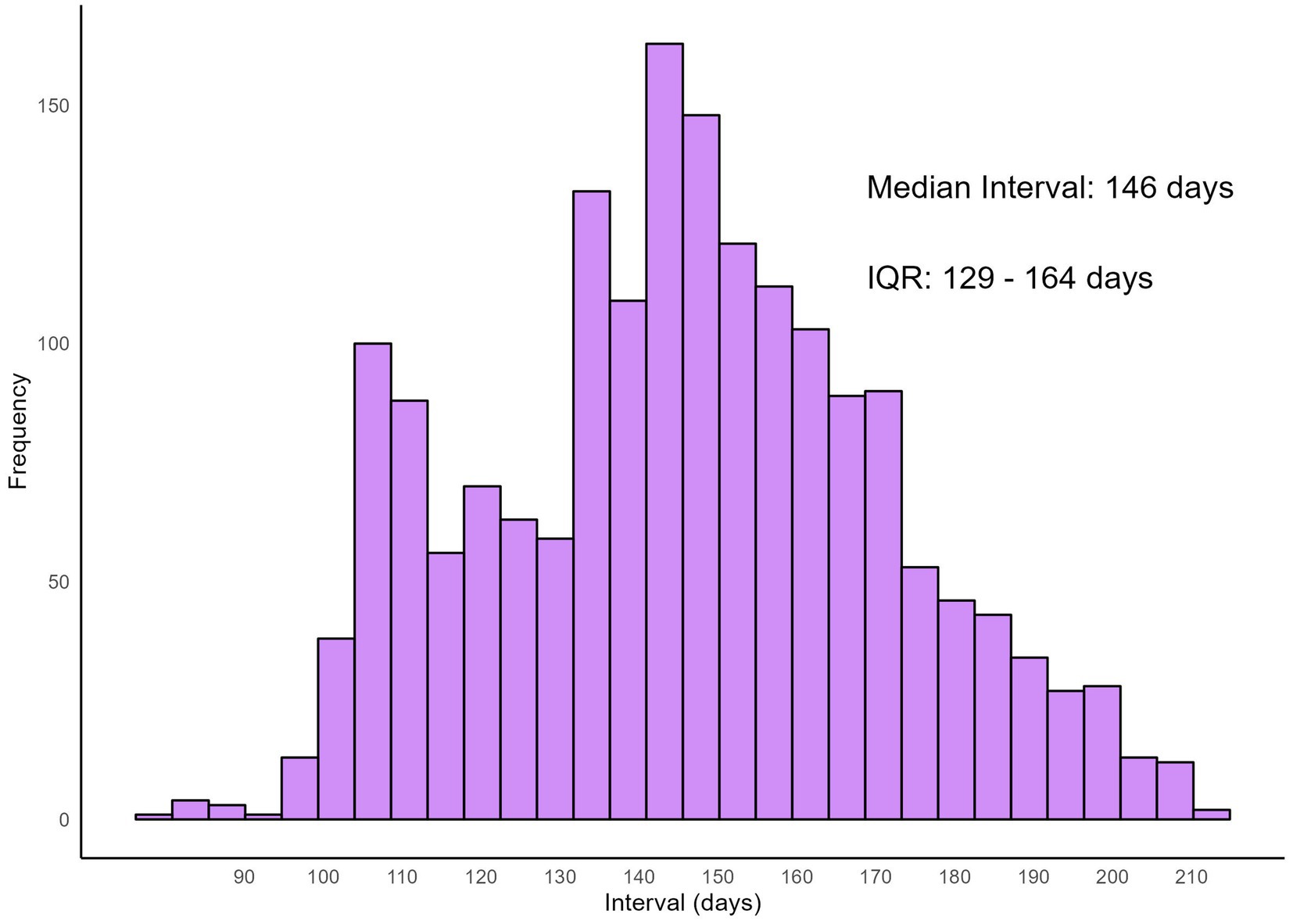
The distribution of infection dates for both self-reported infections at baseline visit and reinfections is illustrated using histograms (Figure 1). Infections at baseline visit were concentrated in late December 2022, while reinfections exhibited a more dispersed pattern, peaking in May 2023, followed by a gradual decline in infection numbers. Figure 2 presents reinfection intervals (RIs), the median RI was 146 days [Interquartile Range (IQR): 129–164 days], with the shortest and longest RIs being 77 days and 211 days, respectively.
3.2.2 Newly diagnosed diseases and symptom comparison between infection at baseline visit and re-infection at follow-up visit
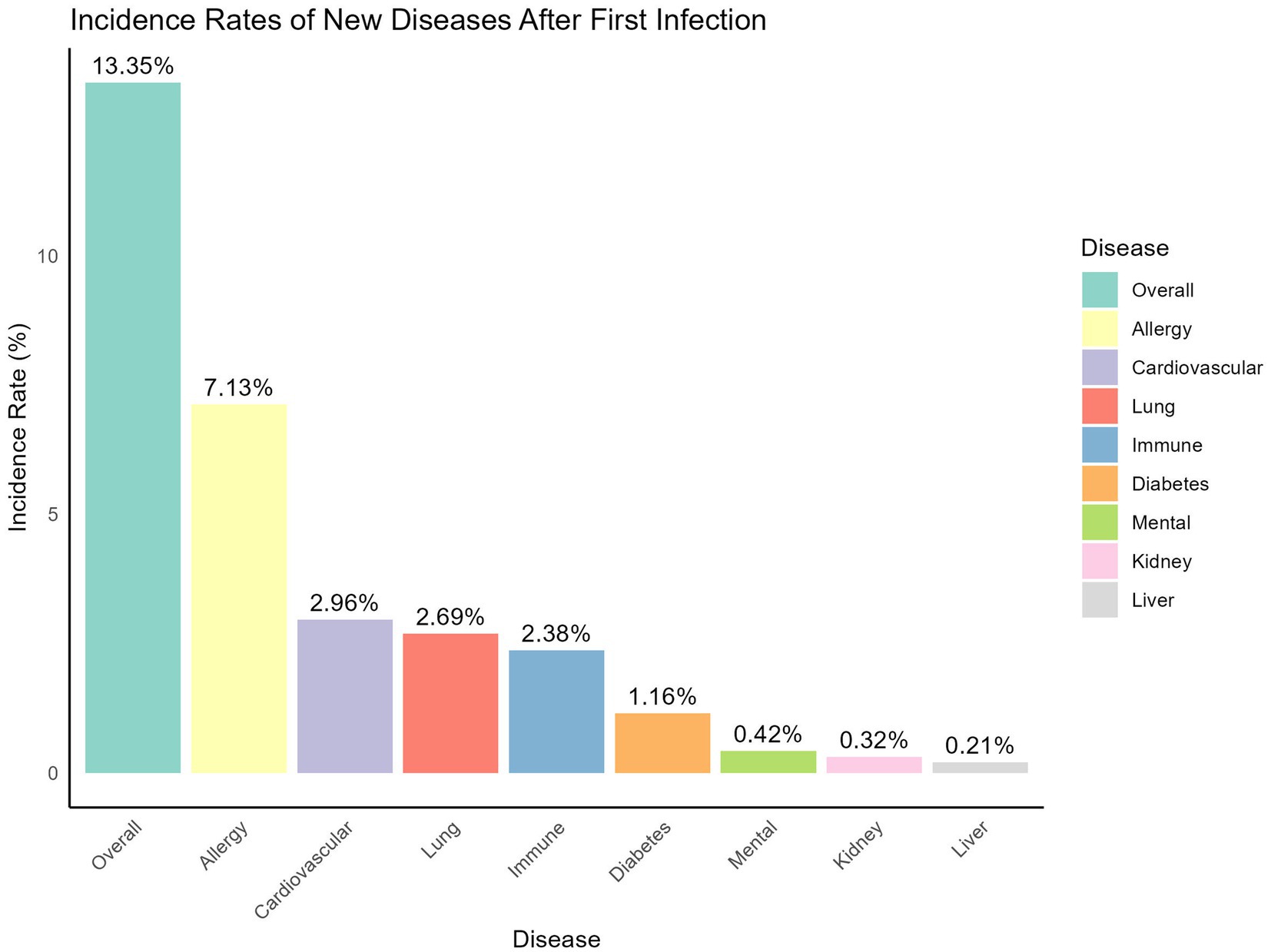
As shown in Figure 3, 13.35% of participants who experienced an infection at baseline visit reported developing one or more newly diagnosed diseases afterward. The most frequently reported condition was allergic diseases, such as asthma and rhinitis, affecting 7.13% of participants. This was followed by cardiovascular disease (2.96%), lung disease (2.69%), immune system disorders (2.38%), diabetes (1.16%), mental health issues (0.42%), kidney disease (0.32%), and liver disease (0.21%).
When comparing symptom frequencies between the infection at baseline visit and re-infection at Follow-up Visit (Figure 4), the main symptoms during the infection at baseline visit were cough (87.81%), fatigue (82.83%), fever (82.38%), sore throat/dry throat (73.45%), muscle pain (72.73%), and nasal congestion/runny nose (69.77%). During the reinfection, the main symptoms included fatigue (80.37%), sore throat/dry throat (78.74%), nasal congestion/runny nose (72.60%), cough (71.23%), dizziness/headache (69.45%), and fever (67.14%). In comparison, cough, diarrhea, fatigue, fever, loss of taste/smell, muscle pain, and nausea/vomiting were significantly more frequent during the infection at baseline visit, while nasal congestion/runny nose, rash, eczema and other dermatological manifestations, and sore throat/dry throat were significantly more frequent during the reinfection. All comparisons among the symptoms were performed using chi-square tests.
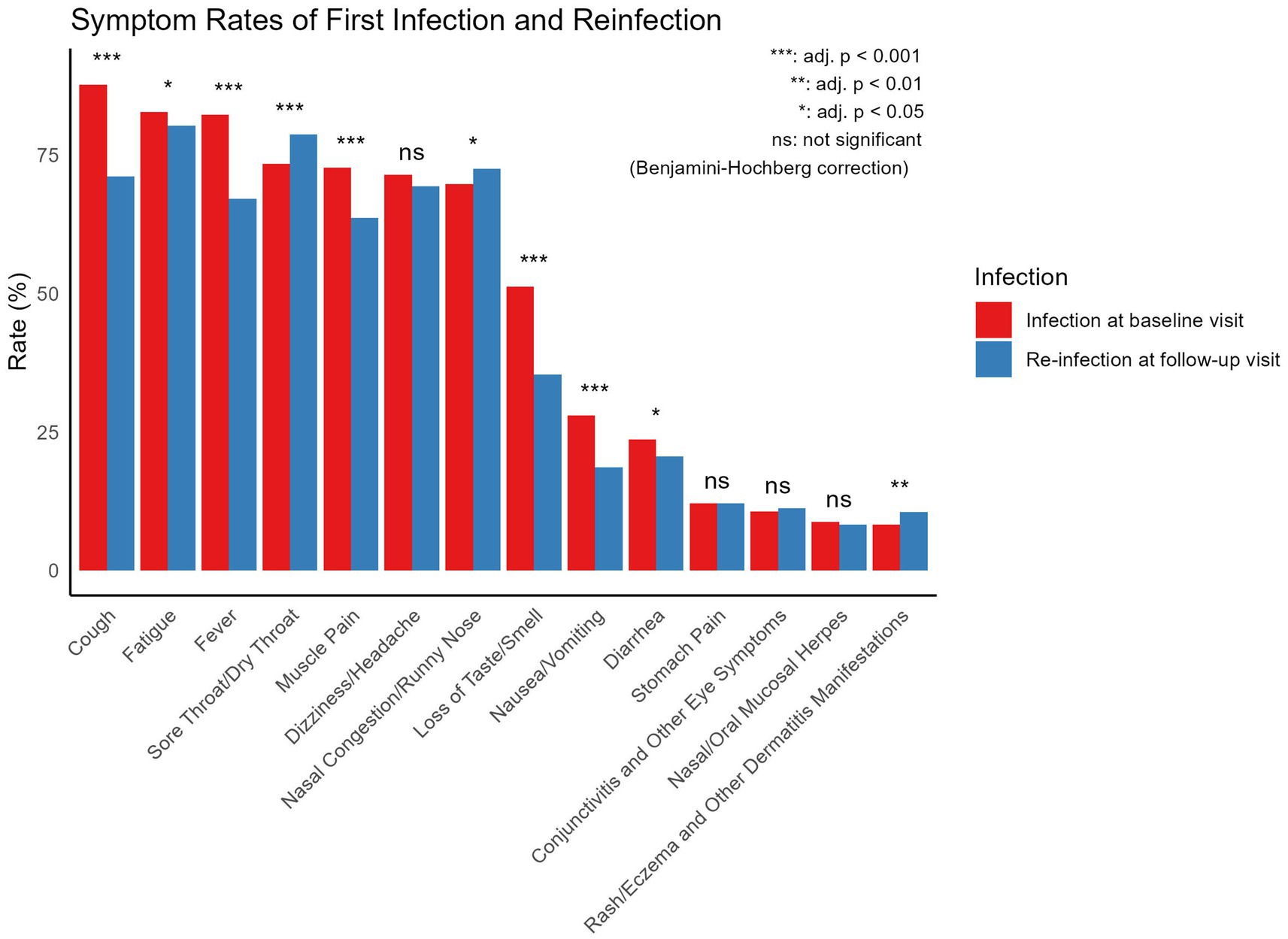
Figure 4. Comparison of symptom frequencies between infection at baseline visit and self-reported re-infection at follow-up visit. All symptom comparisons were performed using Chi-square tests.
3.2.3 Severity of infections
Among self-reported reinfected participants, 73.65% reported that their symptoms during the infection at baseline visit were more severe, 14.5% indicated that the severity of symptoms were similar between the two waves, and only 11.9% perceived that their symptoms were more severe during the reinfection. However, the hospitalization rate (1.10%) during reinfection was significantly higher compared to the hospitalization rate (0.48%) during the infection at baseline visit (p < 0.05, Chi-squared test). The ICU admission rates during the follow-up visit and at the baseline visit were 0.21 and 0.04%, respectively. The difference between the two groups was not statistically significant (p = 0.059, Fisher’s exact test), as shown in Figure 5.
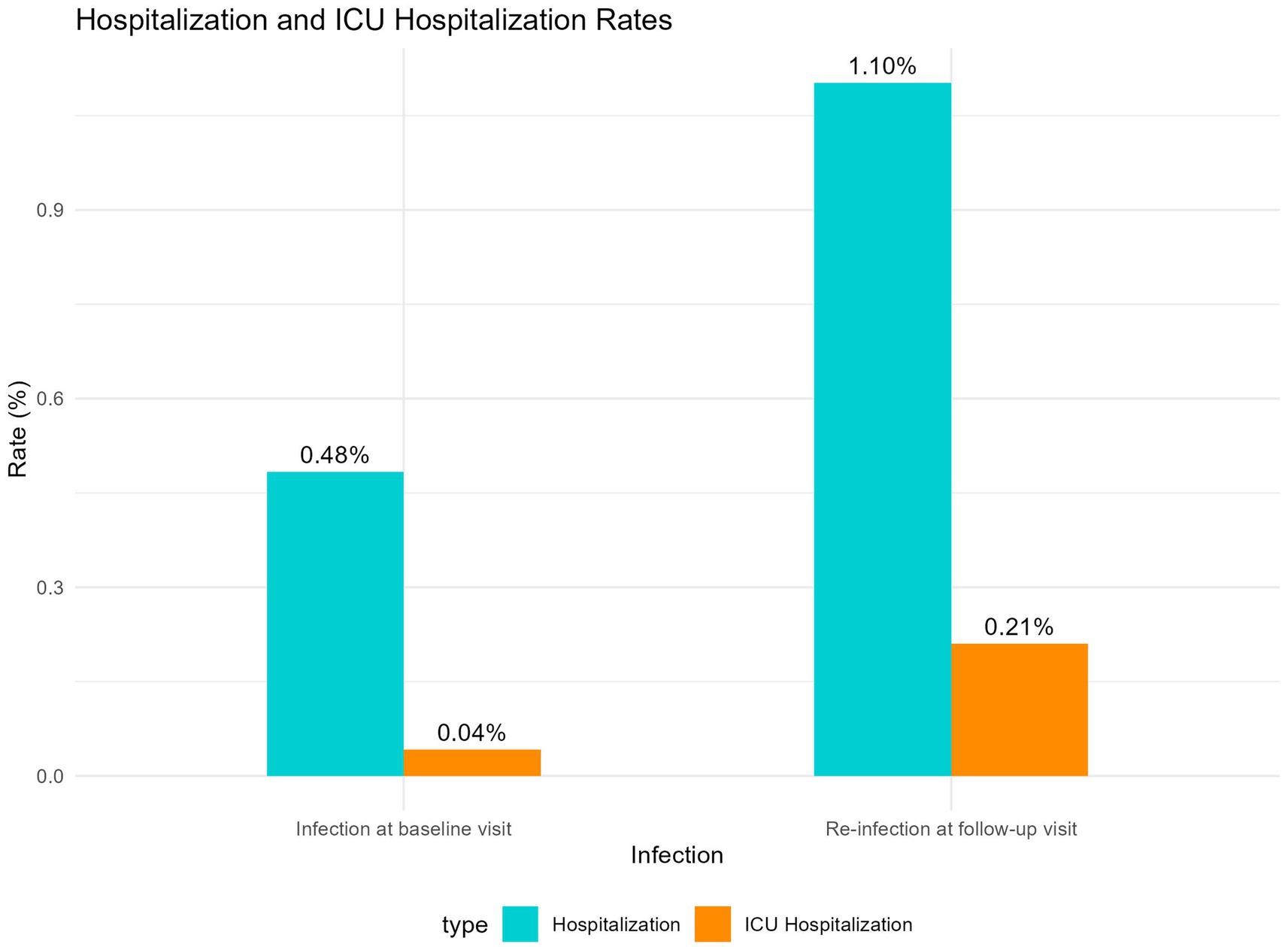
Figure 5. Comparison of hospitalization and ICU admission rates between infection at baseline visit and re-infection at follow-up visit.
3.3 Comparison between reinfected and non-reinfected groups
3.3.1 Univariate analysis
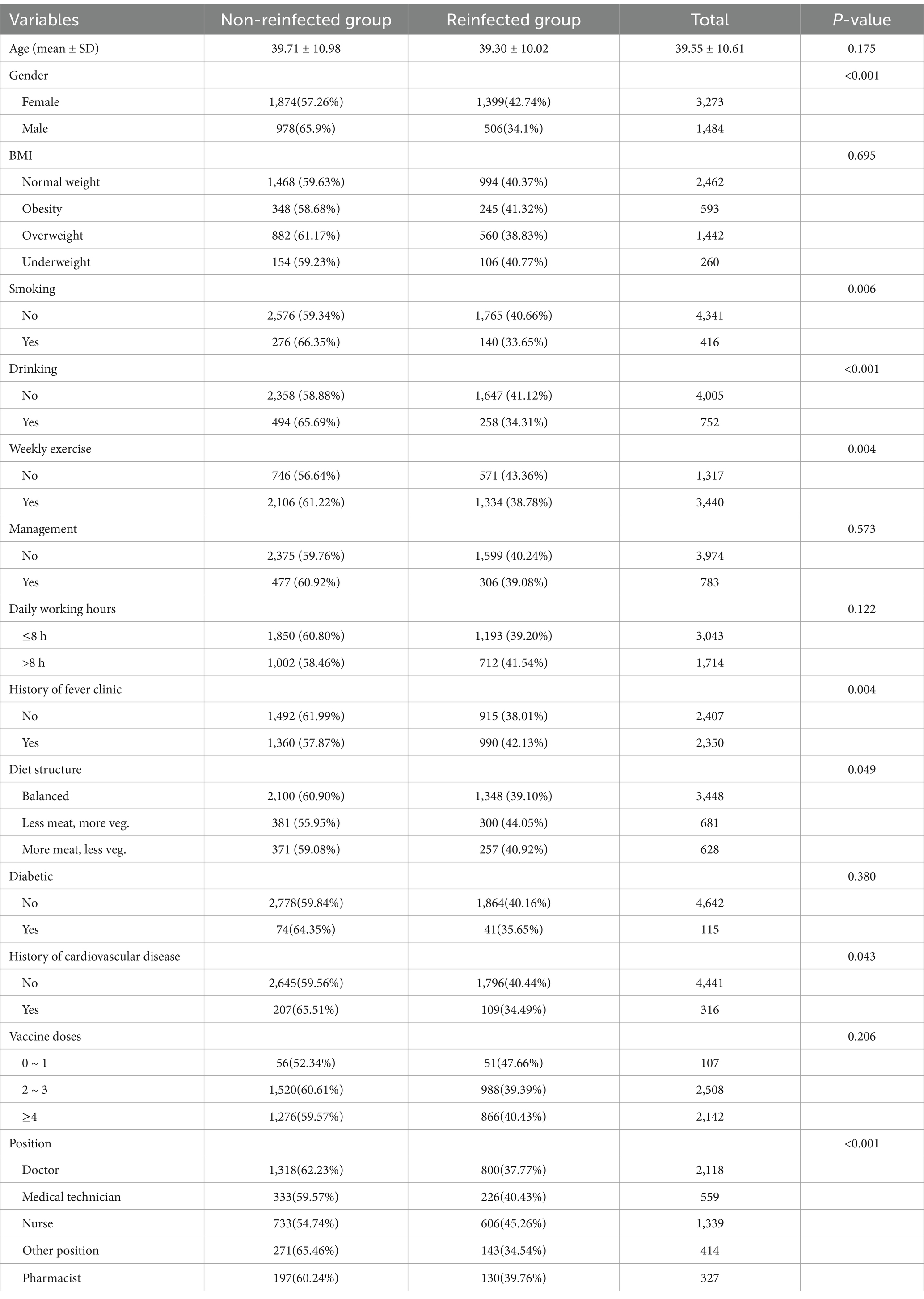
Univariate analysis was performed to compare the baseline characteristics of 1,905 reinfected individuals and 2,852 non-reinfected individuals, as shown in Table 1. Females had a significantly higher reinfection rate (42.74%) compared to males (34.10%), indicating that females may face a higher risk of reinfection. In addition, nurses had the highest reinfection rate, followed by medical technicians and pharmacists. Regarding vaccination status, those who had received 0–1 doses of the vaccine had the highest reinfection rate (47.66%). The reinfection rate decreased as the number of vaccine doses increased, and the differences between the various dose groups were not statistically significant. Chi-square test was used in all univariate analyses, statistically significant differences were observed among the variables gender, smoking, drinking, weekly exercise, history of fever clinic, diet structure and position.
3.3.2 Multivariate logistic regression model
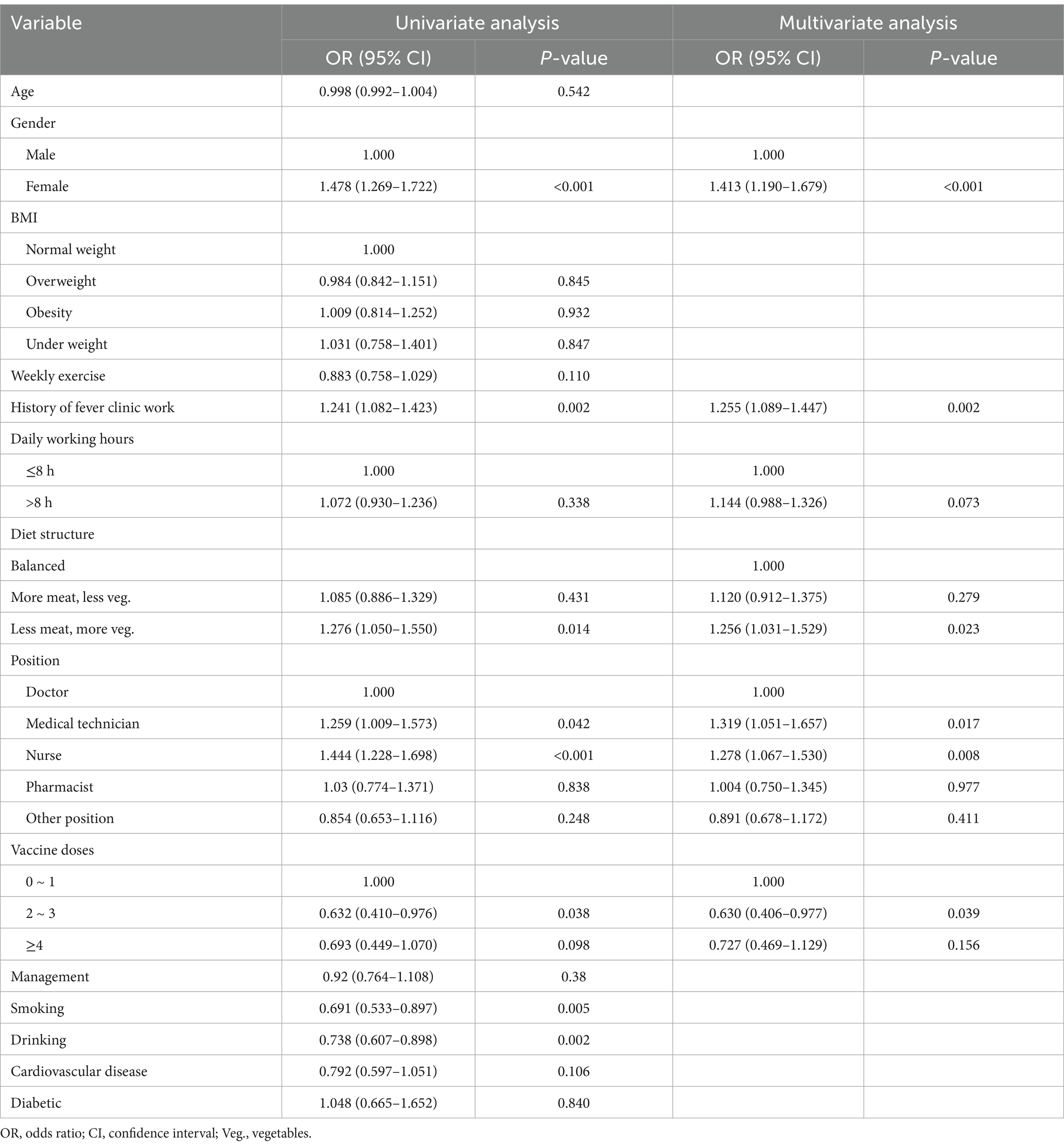
The final model (Table 2) indicated that female gender (OR = 1.376, 95% CI: 1.190–1.592), history of fever clinic work (OR = 1.179, 95% CI: 1.045–1.330), working over 8 h per day (OR = 1.178, 95% CI: 1.040–1.336), and a “less meat, more vegetables” diet (OR = 1.206, 95% CI: 1.020–1.426), along with being a nurse (OR = 1.201, 95% CI: 1.029–1.402), were significant risk factors for reinfection. Regular weekly exercise was identified as a protective factor (OR = 0.861, 95% CI: 0.754–0.983).
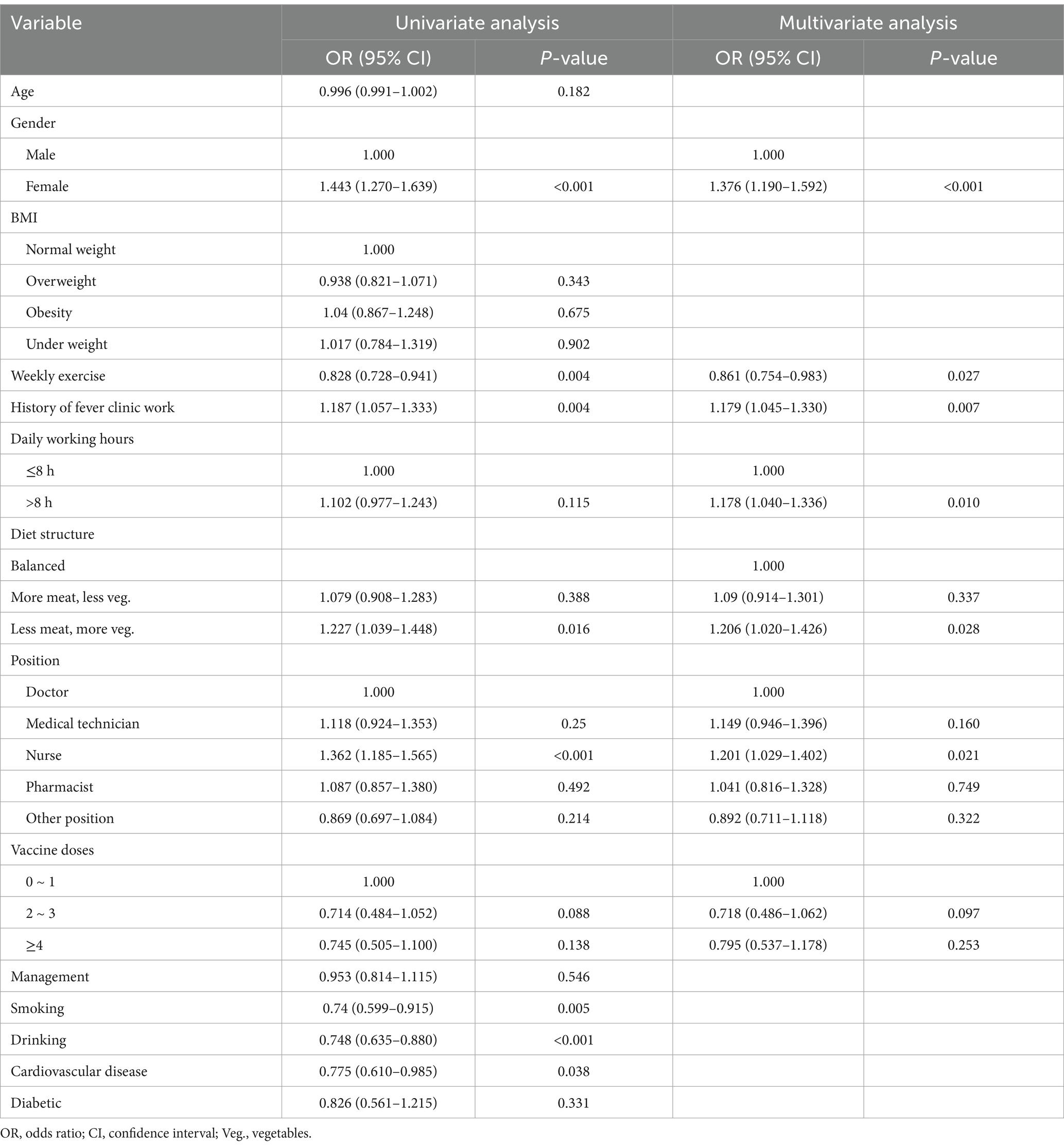
Table 2. Factors associated with Self-reported reinfection in univariate and multivariate logistic regression.
3.3.3 Sensitivity analysis
The sensitivity analysis results (Table 3) were highly consistent with the main analysis, with female gender (OR = 1.413, 95% CI: 1.190–1.679), a “less meat, more vegetables” diet (OR = 1.256, 95% CI: 1.031–1.529), fever clinic work (OR = 1.255, 95% CI: 1.089–1.447), and being a nurse (OR = 1.278, 95% CI: 1.067–1.530) remaining significant risk factors for reinfection. Medical technician (OR = 1.319, 95% CI: 1.051–1.657) was identified as a risk factor only in the sensitivity analysis. Vaccine doses of 2 ~ 3 was identified as a protective factor (OR = 0.630, 95% CI: 0.406–0.977). These findings demonstrate that regardless of the infection definition used, certain risk factors consistently contribute to reinfection, further confirming the robustness of the results.
4 Discussion
In this study, we analyzed the reinfection characteristics and potential risk factors among PHWs during the Omicron variant wave. Female PHWs, nurses, and individuals with a history of fever clinic work were found to have a significantly higher risk of re-infection. Additionally, working more than 8 h per day and having a “less meat, more vegetables” diet were identified as risk factors, while regular weekly exercise was a protective factor. Vaccine doses of 2 ~ 3 was identified as a protective factor against reinfection in the sensitivity analysis. These findings highlight important occupational and behavioral factors associated with COVID-19 reinfection, underscoring the need for targeted protective measures for high-risk healthcare workers.
The results indicate that after adjustments to epidemic control policies, the two major waves of Omicron infections in Jiangsu occurred at the end of 2022 and mid-2023, with a median reinfection interval of 146 days (IQR: 129–164 days). Moreover, first-time COVID-19 infections may be associated with the onset of various diseases, particularly allergic conditions (e.g., asthma, rhinitis), with 7.13% of patients reporting new allergic conditions after infection. Although these comorbidities may not be directly caused by COVID-19—such as the development of diabetes potentially due to natural aging—the higher rates of allergies, immune system, pulmonary, and cardiovascular conditions suggest that COVID-19 could have a broader impact on these systems, providing a direction for future research into long-COVID effects (24, 25). The study also observed that primary infections tended to present more systemic symptoms (e.g., fever, cough, fatigue), while reinfections predominantly involved upper respiratory symptoms (e.g., sore throat, nasal congestion). This pattern may reflect the immune system’s response to reinfection. During the first infection, the immune system is in its initial response phase, resulting in a stronger systemic reaction.
In terms of reinfection rates, the study found that the primary infection rate among frontline healthcare workers in Jiangsu was 85.85% (95% CI: 84.93–86.77%), and the reinfection rate was 40.05% (95% CI: 38.65–41.44%). After adjusting for sentinel hospital influenza-like illness SARS-CoV-2 positivity rates, the recalculated reinfection rate was 29.41% (95% CI: 28.12–30.71%). Flacco et al.’s meta-analysis from June 2022 found that reinfection rates before the Omicron wave were 0.97%, rising to 3.31% in the first 3 months of Omicron’s spread (26). Similarly, research by Fonseca et al. in Brazil found a reinfection rate of up to 8%, which increased over time (27). Wei et al., utilizing data from the UK Office for National Statistics (ONS) COVID-19 Infection Survey, reported that reinfection rates rose from 10–11% to 14–16% (28). In China, a retrospective study by Zhang et al. in Shanxi Province found that the reinfection rate could reach as high as 25.1% (29). Additionally, research by Cai et al. in Guangdong Province indicated a reinfection rate of 18.4% following an initial Omicron infection, with an overall reinfection rate of 28.3% (30). The reinfection rates observed in these studies are consistent with our findings on the Chinese population. The relatively high reinfection rates in our study may be attributed to concentrated infection clusters during the adjustment of national control policies, potentially resulting in a higher reinfection rate compared to other countries.
The severity of reinfection remains a contentious issue, with no clear consensus in the literature (31). Some studies suggest that reinfection leads to milder symptoms, with lower hospitalization and mortality rates (32). However, other studies report an increased risk of hospitalization and death during reinfection, with cumulative risks and burdens rising with each subsequent infection (33). Nguyen et al. found no significant difference in symptom severity between primary and secondary infections (19). In our study, most participants perceived their reinfection symptoms as milder than their initial infection, while the hospitalization and ICU admission rates for reinfection were significantly higher. This discrepancy may be related to strained healthcare resources during the first wave of infections, such as shortages of hospital bed, which led to lower hospitalization rates during primary infections. Hadley et al. found a statistically significant association between the severity of the first and subsequent infections, with individuals experiencing severe first infections showing a higher risk of hospitalization and death upon reinfection (34). These findings underscore the importance of studying the severity of COVID-19 reinfection, which is crucial for assessing disease burden and shaping future public health policies.
The multivariate logistic regression analysis indicated that being female, having a history of working in fever clinics, and being a nurse were significant risk factors for reinfection. The higher reinfection risks for females and nurses might be related to their higher occupational exposure frequency, particularly nurses who often work in high-risk environments on the frontlines of care. Furthermore, experience working in fever clinics increases exposure risks, highlighting the need for strengthened occupational protections for healthcare workers, particularly for high-risk groups. Only in the sensitivity analysis, Vaccine doses of 2~3 was identified as a protective factor against reinfection. Although the OR values for both 2~3 and ≥4 doses were less than 1.000, most of the p-values were greater than 0.05. This may be due to the small number of individuals with Vaccine doses of 0~1, as only 2.25% of PHWs received one or fewer doses. This may also suggest that maintaining high immunity levels through vaccine boosters is critical in the context of ongoing SARS-CoV-2 mutations. Future vaccination strategies should consider the waning efficacy of vaccines over time, and regular booster doses may be an effective way to reduce reinfection risks (35).
Increasing the supply of personal protective equipment, rationalizing work hours, implementing regular health monitoring, and prioritizing vaccination for these groups could effectively reduce infection risks.
However, this study has several limitations. First, data collection involved a combination of online and offline methods, with online questionnaires used during the first wave and on-site during the second. This discrepancy may have led to inconsistencies in the accuracy of responses. Second, due to the extensive scale of the outbreak, it was not feasible to test all participants for SARS-CoV-2 using nucleic acid or antigen tests. Consequently, individuals who were not tested but exhibited symptoms were classified as infected, which may have overestimated infection and reinfection rates. To mitigate these limitations, we adjusted the reinfection rate using data from sentinel hospitals, and conducted a sensitivity analysis to validate the robustness of the results. However, some residual errors may still remain.
In conclusion, this study identified higher reinfection risks for female healthcare workers, nurses, “less meat, more vegetables” diet and those with fever clinic experience. It also highlighted significant differences in the symptoms and severity of primary and secondary infections. These findings provide important insights into the risk factors for reinfection and strategies for its prevention.
5 Conclusion
This study examined the characteristics of COVID-19 primary infections and reinfections among PHWs during the Omicron wave in Jiangsu Province and identified potential risk factors for reinfection. The results showed a primary infection rate of 85.85% and a reinfection rate of 40.05%, with an adjusted reinfection rate of 29.41%. The study also highlighted differences in symptomatology and severity between primary infections and reinfections. While primary infections were more systemic in nature, reinfections predominantly involved upper respiratory symptoms. Although most participants perceived their reinfection symptoms as milder, the hospitalization and ICU admission rates were significantly higher during reinfection compared to primary infections, possibly due to healthcare resource constraints during the first infection wave.
Female healthcare workers, nurses, and individuals with a history of working in fever clinics were found to have a significantly higher risk of reinfection. Additionally, working more than 8 h per day and following a “less meat, more vegetables” diet were associated with increased reinfection risk, while regular weekly exercise was identified as a protective factor. These findings underscore the need for targeted protective measures for high-risk healthcare workers, particularly females, nurses, and those with fever clinic experience. Enhancing personal protective equipment supplies, rationalizing work hours, and implementing regular health monitoring for these high-risk groups may effectively reduce the risk of reinfection. By improving workplace protections and health measures for healthcare workers, the risk of reinfection can be mitigated, further safeguarding this critical population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Jiangsu Provincial Center for Disease Prevention and Control. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. HC: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Resources, Writing – review & editing. YQ: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. YZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. HG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. YS: Conceptualization, Formal analysis, Investigation, Project administration, Writing – review & editing. BX: Conceptualization, Formal analysis, Methodology, Writing – review & editing. WH: Conceptualization, Data curation, Methodology, Writing – review & editing. HZ: Conceptualization, Data curation, Methodology, Writing – review & editing. JX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. BC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Health Commission of Nanjing, China (ZKX22019) and the 2022 Annual Open Project of Jiangsu Provincial Primary Health Development and General Practice Medical Education Research Center (No. 2022A02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Generative AI, ChatGPT (GPT-4, OpenAI, https://openai.com) was used to help polish this manuscript, followed by multiple rounds of human proofreading and revision to ensure accuracy and clarity.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PHWs, primary healthcare workers; RIs, reinfection intervals; CI, confidence interval; HCWs, healthcare workers; BMI, body mass index; OR, odds ratio; IQR, Interquartile range.
Footnotes
References
1. World Health Organization. WHO COVID-19 dashboard: global case data 2024. Available online at: https://data.who.int/dashboards/covid19/cases?m49=001&n=c.
2. Wang, H, Paulson, KR, Pease, SA, Watson, S, Comfort, H, Zheng, P, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. (2022) 399:1513–36. doi: 10.1016/S0140-6736(21)02796-3
3. Goldberg, Y, Mandel, M, Bar-On, YM, Bodenheimer, O, Freedman, LS, Ash, N, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. (2022) 386:2201–12. doi: 10.1056/NEJMoa2118946
4. To, KK-W, Hung, IF-N, Ip, JD, Chu, AW-H, Chan, W-M, Tam, AR, et al. Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis. (2020) 73:e2946–51. doi: 10.1093/cid/ciaa1275
5. Sabino, EC, Buss, LF, Carvalho, MPS, Prete, CA Jr, Crispim, MAE, Fraiji, NA, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. (2021) 397:452–5. doi: 10.1016/S0140-6736(21)00183-5
6. Falahi, S, and Kenarkoohi, A. COVID-19 reinfection: prolonged shedding or true reinfection? New Microb New Infect. (2020) 38:100812. doi: 10.1016/j.nmni.2020.100812
7. Pinto, LM, Nanda, V, Sunavala, A, and Rodriques, C. Reinfection in COVID-19: a scoping review. Med J Armed Forces India. (2021) 77:S257–63. doi: 10.1016/j.mjafi.2021.02.010
8. Hadley, E, Yoo, YJ, Patel, S, Zhou, A, Laraway, B, Wong, R, et al. Insights from an N3C RECOVER EHR-based cohort study characterizing SARS-CoV-2 reinfections and long COVID. Commun Med. (2024) 4:129. doi: 10.1038/s43856-024-00539-2
9. Rathinasamy, M, and Kandhasamy, S. An exploratory study on the propagation of SARS-CoV-2 variants: omicron is the most predominant variant. J Med Virol. (2022) 94:2414–21. doi: 10.1002/jmv.27634
10. Chen, Y, Zhu, W, Han, X, Chen, M, Li, X, Huang, H, et al. How does the SARS-CoV-2 reinfection rate change over time? The global evidence from systematic review and meta-analysis. BMC Infect Dis. (2024) 24:339. doi: 10.1186/s12879-024-09225-z
11. Guedes, AR, Oliveira, MS, Tavares, BM, Luna-Muschi, A, Lazari, CS, Montal, AC, et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci Rep. (2023) 13:712. doi: 10.1038/s41598-022-25908-6
12. Bastard, J, Taisne, B, Figoni, J, Mailles, A, Durand, J, Fayad, M, et al. Impact of the omicron variant on SARS-CoV-2 reinfections in France, march 2021 to February 2022. Euro Surveill. (2022) 27:2200247. doi: 10.2807/1560-7917.ES.2022.27.13.2200247
13. Rana, R, Kant, R, Huirem, RS, Bohra, D, and Ganguly, NK. Omicron variant: current insights and future directions. Microbiol Res. (2022) 265:127204. doi: 10.1016/j.micres.2022.127204
14. Tian, D, Sun, Y, Xu, H, and Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 omicron variant. J Med Virol. (2022) 94:2376–83. doi: 10.1002/jmv.27643
15. Luo, M, Gong, F, Sun, J, and Gong, Z. For COVID-19, what are the priorities of normalized prevention and control strategies? Biosci Trends. (2023) 17:63–7. doi: 10.5582/bst.2023.01005
16. Prevention CCfDCa. National situation of novel coronavirus infection. (2024). Available online at https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202301/t20230125_263519.html (Accessed August 20, 2024).
17. van der Plaat, DA, Madan, I, Coggon, D, van Tongeren, M, Edge, R, Muiry, R, et al. Risks of COVID-19 by occupation in NHS workers in England. Occup Environ Med. (2022) 79:176–83. doi: 10.1136/oemed-2021-107628
18. Gholami, M, Fawad, I, Shadan, S, Rowaiee, R, Ghanem, H, Hassan Khamis, A, et al. COVID-19 and healthcare workers: a systematic review and meta-analysis. Int J Infect Dis. (2021) 104:335–46. doi: 10.1016/j.ijid.2021.01.013
19. Bowe, B, Xie, Y, and Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. (2022) 28:2398–405. doi: 10.1038/s41591-022-02051-3
20. Zhou, BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96.
21. Commission GOotNH. Diagnosis and treatment protocol for novel coronavirus infection (trial version 10). (2023). Available online at: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_11815/202301/t20230107_263257.html (Accessed August 24, 2024).
22. Chen, H, Qian, Y, Lu, B, Ma, R, Miao, P, Fu, M, et al. Prevalence and factors influencing long COVID among primary healthcare workers after epidemic control policy adjustment in Jiangsu, China. BMC Infect Dis. (2024) 24:964. doi: 10.1186/s12879-024-09764-5
23. Lu, B, Ma, R, Xu, J, Zhang, Y, Guo, H, Chen, H, et al. Primary healthcare workers’ COVID-19 infection status following implementation of adjusted epidemic prevention and control strategies: a cross-sectional study in Jiangsu, China. Front Public Health. (2023) 11:1297770. doi: 10.3389/fpubh.2023.1297770
24. Boufidou, F, Medić, S, Lampropoulou, V, Siafakas, N, Tsakris, A, and Anastassopoulou, C. SARS-CoV-2 reinfections and long COVID in the post-omicron phase of the pandemic (2023) 24:12962. doi: 10.3390/ijms241612962,
25. Lippi, G, Sanchis-Gomar, F, and Henry, BM. COVID-19 and its long-term sequelae: what do we know in 2023? Polish Arch Internal Med. (2023) 133:16402. doi: 10.20452/pamw.16402
26. Flacco, ME, Acuti, MC, Baccolini, V, De Vito, C, Renzi, E, Villari, P, et al. Risk of reinfection and disease after SARS-CoV-2 primary infection: Meta-analysis. Eur J Clin Investig. (2022) 52:e13845. doi: 10.1111/eci.13845
27. Fonseca, PLC, Malta, FSV, Braga-Paz, I, do Prado Silva, J, de Souza, CSA, de Aguiar, RS, et al. SARS-CoV-2 reinfection rate before and after VOC omicron emergence: a retrospective study in Brazil. Braz J Microbiol. (2024) 55:3959–64. doi: 10.1007/s42770-024-01467-y
28. Wei, J, Stoesser, N, Matthews, PC, Khera, T, Gethings, O, Diamond, I, et al. Risk of SARS-CoV-2 reinfection during multiple omicron variant waves in the UK general population. Nat Commun. (2024) 15:1008. doi: 10.1038/s41467-024-44973-1
29. Zhang, M, Cao, L, Zhang, L, Li, X, Chen, S, and Zhang, Y. SARS-CoV-2 reinfection with omicron variant in Shaanxi Province, China: December 2022 to February 2023. BMC Public Health. (2024) 24:496. doi: 10.1186/s12889-024-17902-6
30. Chunsheng Cai, YL, Hu, T, Liang, R, Wang, K, Guo, C, Li, Y, et al. The associated factors of SARS-CoV-2 reinfection by omicron variant—Guangdong Province, China, December 2022 to January 2023. China CDC Weekly. (2023) 5:391–6. doi: 10.46234/ccdcw2023.075
31. Chen, Y-H, Lee, C-Y, Cheng, H-Y, Chen, C-M, Cheuh, Y-N, Lee, C-L, et al. Risk factors and mortality of SARS-CoV-2 reinfection during the omicron era in Taiwan: a nationwide population-based cohort study. J Microbiol Immunol Infect. (2024) 57:30–7. doi: 10.1016/j.jmii.2023.10.013
32. Medić, S, Anastassopoulou, C, Lozanov-Crvenković, Z, Vuković, V, Dragnić, N, Petrović, V, et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, Serbia: a population-level observational study (2022) 20:100453. doi: 10.1016/j.lanepe.2022.100453,
33. Mensah, AA, Lacy, J, Stowe, J, Seghezzo, G, Sachdeva, R, Simmons, R, et al. Disease severity during SARS-COV-2 reinfection: a nationwide study. J Infect. (2022) 84:542–50. doi: 10.1016/j.jinf.2022.01.012
34. Nguyen, NN, Houhamdi, L, Hoang, VT, Delerce, J, Delorme, L, Colson, P, et al. SARS-CoV-2 reinfection and COVID-19 severity. Emerg Microb Infect. (2022) 11:894–901. doi: 10.1080/22221751.2022.2052358
Keywords: COVID-19, COVID-19 reinfection, SARS-CoV-2, primary healthcare workers, omicron variant
Citation: Fu M, Chen H, Qian Y, Zhang Y, Guo H, Shen Y, Xu B, Han W, Zhou H, Xu J and Chen B (2025) Analysis of COVID-19 reinfection and its influencing factors among primary healthcare workers in Jiangsu Province: a study based on the omicron variant epidemic. Front. Public Health. 13:1521658. doi: 10.3389/fpubh.2025.1521658
Edited by:
Faris Lami, University of Baghdad, IraqReviewed by:
Gamze Kalin Unuvar, Erciyes University, TürkiyeDmitry Bulaev, Luxembourg Institute of Health, Luxembourg
Copyright © 2025 Fu, Chen, Qian, Zhang, Guo, Shen, Xu, Han, Zhou, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingwei Chen, ZHJjaGVuYndAMTI2LmNvbQ== Jinshui Xu, MzUzMTEyMzU0QHFxLmNvbQ==
 Mingwang Fu
Mingwang Fu Hualing Chen
Hualing Chen Yongkang Qian
Yongkang Qian Yongjie Zhang
Yongjie Zhang Haijian Guo
Haijian Guo Ya Shen
Ya Shen Biyun Xu
Biyun Xu Wantong Han
Wantong Han Haoran Zhou
Haoran Zhou Jinshui Xu
Jinshui Xu Bingwei Chen
Bingwei Chen