- 1CDUTCM-KEELE Joint Health and Medical Sciences Institute, School of Acupuncture and Tuina, School of Basic Medical Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Meditation has gained increasing recognition as a simple, cost-effective, and non-invasive therapeutic approach for older adults with subjective cognitive decline (SCD), mild cognitive impairment (MCI), and Alzheimer’s disease (AD). This meta-analysis aimed to systematically evaluate its effectiveness on this population.
Methods: A comprehensive search across nine databases was performed from inception to April 1, 2024, to identify eligible randomized controlled trials (RCTs). The primary outcome was global cognitive performance measured by the Mini-Mental State Examination (MMSE), while the secondary outcomes included sleep quality estimated through the Pittsburgh Sleep Quality Index (PSQI), health status assessed using the 36-Item Short Form Health Survey (SF-36), and depression evaluated with the Geriatric Depression Scale (GDS). This meta-analysis utilized R 4.3.1 software and adhered to the Cochrane Handbook and PRISMA reporting guidelines.
Results: A total of 25 RCTs published between 2013 and 2024 involving 2,095 participants were included in this study. The pooled findings demonstrated that meditation significantly improved global cognitive performance (MD 2.22, 95% CI: 0.83–3.62, p = 0.002), sleep quality (MD −1.40, 95% CI: −2.52 to −0.27, p = 0.015), and health status (MD 3.50, 95% CI, 0.45–6.56, p = 0.020). However, no significant effect was observed on depression compared to the control group (SMD −0.16, 95% CI: −0.63 to 0.31, p = 0.514).
Conclusion: This meta-analysis suggests that meditation is an effective adjunct therapy for improving global cognitive performance, sleep quality, and health status in older adults with SCD, MCI, and AD. However, given the heterogeneity and limited sample sizes, these findings should be interpreted with caution. More large-scale and high-quality RCTs are needed to further substantiate these effects.
Introduction
With the global rise in population aging, mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) have emerged as major contributors to disability worldwide representing a continuum of cognitive disorders associated with aging and neurodegeneration, particularly under the broader spectrum of AD progression (1). Within this continuum, subjective cognitive decline (SCD), characterized by self-reported cognitive complaints in the absence of objective impairment, is increasingly recognized as the earliest clinical manifestation of AD pathology. SCD is associated with amyloid-β (Aβ) accumulation biomarkers and neurodegeneration, conferring an elevated risk of progression to MCI and subsequent dementia. Epidemiologically, cognitive decline has been rising dramatically in recent years and is estimated to reach 115 million cases by 2050, and 30–50% of individuals with SCD may progress to MCI within 4–5 years with a substantial proportion eventually advancing to AD (2, 3). Given the socioeconomic and public health burden imposed by these conditions, the development and implementation of evidence-based therapeutic strategies are urgently warranted (4).
Current therapeutic options for cognitive decline remain limited. Although pharmacotherapies may delay cognitive decline by 6–12 months in about 50% of AD patients (3),1 their clinical utility is constrained by transient efficacy and numerous adverse events. Furthermore, no disease-modifying therapies are available, necessitating a paradigm integrating non-pharmacological interventions to alleviate this pressing public health challenge. Among these, meditation, encompassing modalities such as focused attention meditation (FAM) and dynamic mindfulness meditation (DMM), demonstrates significant potential to improve cognitive performance, concentration, and long-term memory in older adults (5–7), while concurrently reducing sleep disturbances, enhancing mental health, and improving overall quality of life (8). Additionally, its cost-effectiveness and safety also facilitate its scalability in clinical and community settings for widespread use, including nursing homes (7, 9, 10).
Emerging evidence suggests that meditation may mitigate neurocognitive decline by regulating neuroinflammation and promoting neuroplasticity. Studies in individuals with MCI and AD have revealed that sustained meditation practice induces structural and functional brain adaptations, including increased cortical thickness and gray matter volumes in regions critical for executive function, memory consolidation, and emotional regulation (11, 12). Physiologically, meditation attenuates the sympathetic nervous system, modulates the hypothalamic–pituitary–adrenal axis, and enhances parasympathetic nervous system activity, contributing to a neuroprotective state of relaxation and enhancement of patient wellbeing (13). Biomarker studies further indicate that meditation may improve plasma Aβ levels and modulate telomere length and attrition, which correlate with enhanced cognitive performance, sleep quality, mood, and overall quality of life (QOL) (14, 15). These findings collectively suggest that meditation may yield a promising non-pharmacological intervention to enhance brain structure and function, regulate neurodegenerative-related biomarkers, and improve cognitive and overall wellbeing in the aging population.
In recent years, randomized controlled trials (RCTs) investigating the therapeutic potential of meditation in individuals with cognitive decline have been steadily increased. Although pervious reviews have provided valuable insights into this topic, their scope has been primarily focused on cognitive outcomes and are constrained by their reliance on older studies (3, 7). To advance an updated and comprehensive synthesis of the evidence, we conducted a systematic review and meta-analysis integrating the “Reach,” “Effectiveness,” and “Implementation” dimensions of the RE-AIM framework into the research design (16, 17). In this study, the impact of meditation was assessed on global cognitive performance, sleep quality, health status, and depression across the spectrum of SCD, MCI, and AD. Hopefully, these findings may yield an empirical foundation for developing public health strategies to integrate meditation into clinical and community-based care paradigms for aging populations.
Methods
This systematic review was conducted in adherence to the PRISMA reporting guidelines (18). The study protocol was registered with PROSPERO (CRD42019145932) and published2 prior to the initiation of the research (19). No ethical approval was required, as it was a secondary analysis of de-identified data.
Changes to the study protocol
Before conducting the study, the protocol was modified as follows: (1) The study population was expanded to include individuals with SCD in addition to MCI and AD. This adjustment aligned with emerging research since 2020 highlighting SCD as a critical precursor to MCI and AD, thereby enhancing the public health significance of this study. (2) Outcome measures were streamlined to prioritize consistently available and representative assessments, as certain measures in the original protocol (e.g., Alzheimer’s Disease Assessment Scale-Cognitive Subscale, Activities of Daily Living Scale, Trail Making Test, Stroop Test, Digit Span, Hopkins Verbal Learning Test, Rey Auditory Verbal Learning Test, California Verbal Learning Test II, Rey Complex Figure Test, Clock-Drawing Task, Lowenstein Occupational Therapy Cognitive Assessment, Boston Naming Test, improvements in biomarkers, and effective rates) were inconsistently or insufficiently reported across RCTs. This refinement was to strengthen the rigor and feasibility of this study.
Data source
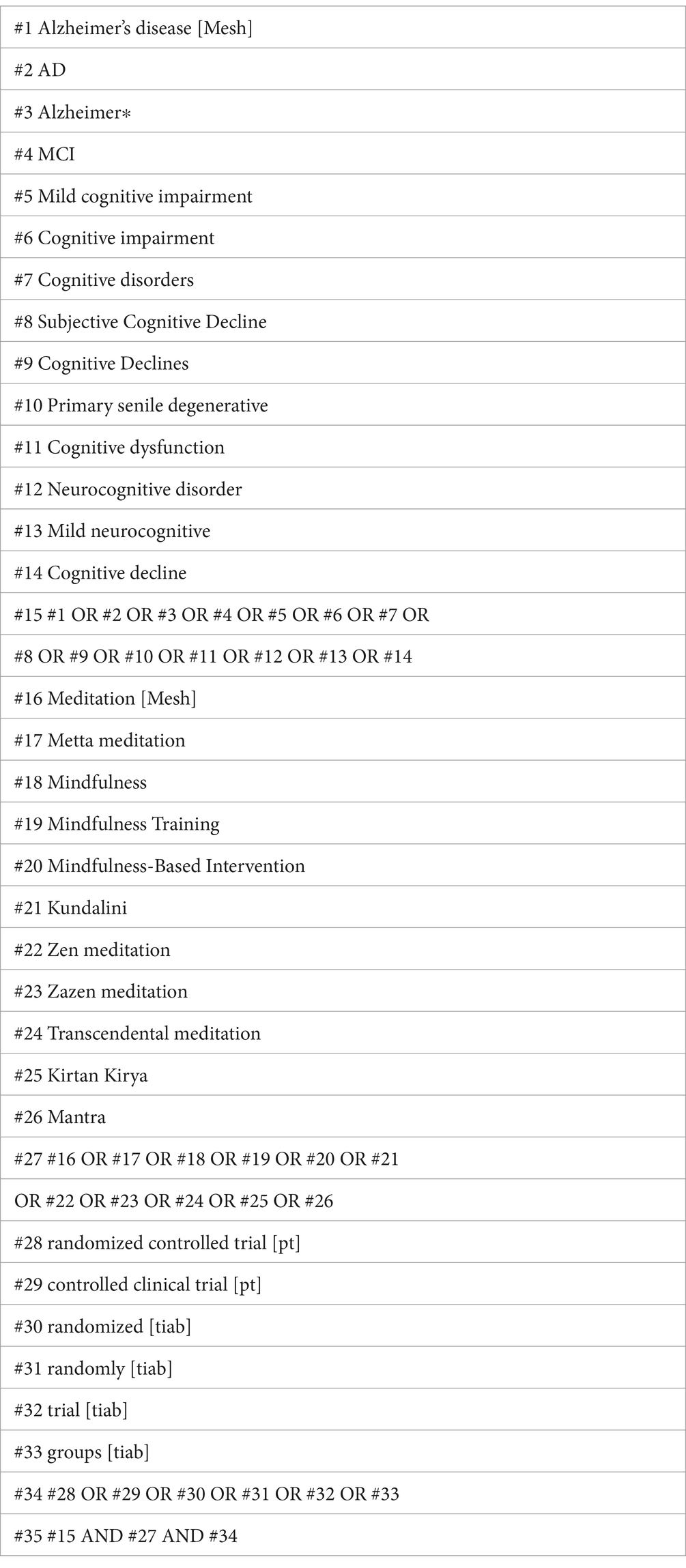
Comprehensive searches without language restriction were implemented across nine databases, including PubMed, EMBASE, Web of Science (WoS), Cochrane Central Register of Controlled Trials (CENTRAL), World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), China National Knowledge Infrastructure (CNKI), China Biology Medicine (CBM), China Science and Technology Journal Database (VIP), and Chinese Clinical Trial Registry (ChiCTR) from the inception to April 1, 2024. The detailed search strategy is presented in Table 1, with appropriate amendments applied to other databases as needed.
Eligibility criteria
Only RCTs were included in this review, while observational and longitudinal studies were excluded. The study population comprised SCD, MCI, and AD patients diagnosed with internationally recognized guidelines (20). The meditation interventions encompassed various meditation practices, such as mindfulness-based stress reduction (MBSR), Metta, Mantra, Zen, Kirtan Kriya, Kundalini, and Tibetan Sound Meditation. Control groups included active and non-active comparators, such as usual care, cognitive rehabilitation therapy (CRT), Tai Chi Chuan, aerobic exercise, health education, and psychoeducation. The primary outcome was the effect of meditation on global cognitive performance measured by the Mini-Mental State Examination (MMSE) (21). Secondary outcomes included sleep quality measured by the Pittsburgh Sleep Quality Index (PSQI) (22), health status assessed using the 36-Item Short Form Health Survey (SF-36) (23), and depression evaluated with the Geriatric Depression Scale (GDS). Studies were excluded if they were reviews, reports, abstracts, conference presentations, and empirical studies failing to provide adequate methodological description or accessibility to the full text and complete dataset.
Selection process
The literature was imported into EndNote version 20 (Clarivate Analytics), and duplicated records were eliminated. Two independent investigators (HT and JWW) screened the titles and abstracts of the retrieved literature, followed by a thorough full-text review based on the predefined eligibility criteria. Any discrepancies between reviewers were resolved through consultation with a third investigator (YHC). Cohen’s Kappa (κ) coefficient was calculated to evaluate inter-rater reliability for full-text screening, and the strength of agreement was interpreted using Altman’s criteria (24). The inter-rater agreement between the two reviewers (HT and WWJ) for full-text selection was measured to be excellent (κ = 0.862).
Data extraction
The initial data extraction sheet, piloted by two reviewers (HT and JWW) on non-included articles, was used for data extraction from the selected studies. Both reviewers independently performed the extraction to ensure accuracy and minimize bias. Extracted data included first author, publication year, sample size, study design, participant characteristics, intervention parameters, and outcomes. Discrepancies were resolved by consulting a third reviewer (YHC). All reviewers cross-verified the extracted data sheets before initiating the quality assessment.
Risk of bias assessment
Two independent authors (JXS and YX) evaluated the risk of bias in individual studies using the Cochrane Risk of Bias 2 (RoB2) tool (25). This tool assesses the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. Each study was graded as having a high, low, or unclear risk of bias. Discrepancies between investigators were resolved through consultation with a third reviewer (YHC).
GRADE certainty assessment
The GRADE system was utilized to rate the certainty of evidence for each outcome. The overall certainty of evidence was categorized into high, moderate, or low levels based on the five key domains of risk of bias, inconsistency, indirectness, imprecision, and other considerations (26).
Statistical analysis
Data analysis was conducted using R 4.3.1 software. Mean differences (MD) were used for MMSE, PSQI, and QOL scales, while standardized mean difference (SMD) was employed for GDS due to variations in measurement scales across studies. Pre- and post-treatment measurements from all included studies were analyzed. SMD was derived from between-group comparisons of within-group changes from post- to pre-measurements. When the standard deviation (SD) of change scores was unavailable, missing values were calculated using available information, such as the correlation between pre- and post-measurements. If estimations were not feasible, study authors were contacted. Studies were excluded if no response was received within 2 weeks and data remained unobtainable (27, 28). Pooled estimates were evaluated with 95% confidence intervals (CIs), and statistical heterogeneity was assessed using the Chi2 test and I2 statistic. Statistical significance was deemed as p < 0.05.
According to the Cochrane Handbook for Systematic Reviews, a fixed-effects model is appropriate only when assuming all effect estimates reflect the same underlying intervention effect (28). Given the substantial differences in intervention designs and control conditions across studies, a random-effects model was utilized when this assumption was unlikely to hold. Multi-arm studies were analyzed separately for each intervention. Subgroup analyses were executed based on intervention duration, meditation type, and different comparators. Publication bias was assessed using funnel plots, complemented by Egger’s (linear regression method) and Begg’s tests (rank correlation method) (29, 30). Sensitivity analyses were performed by excluding studies with high concerns for bias and recalculating pooled estimates to evaluate the robustness of the findings.
Results
Characteristics of included studies
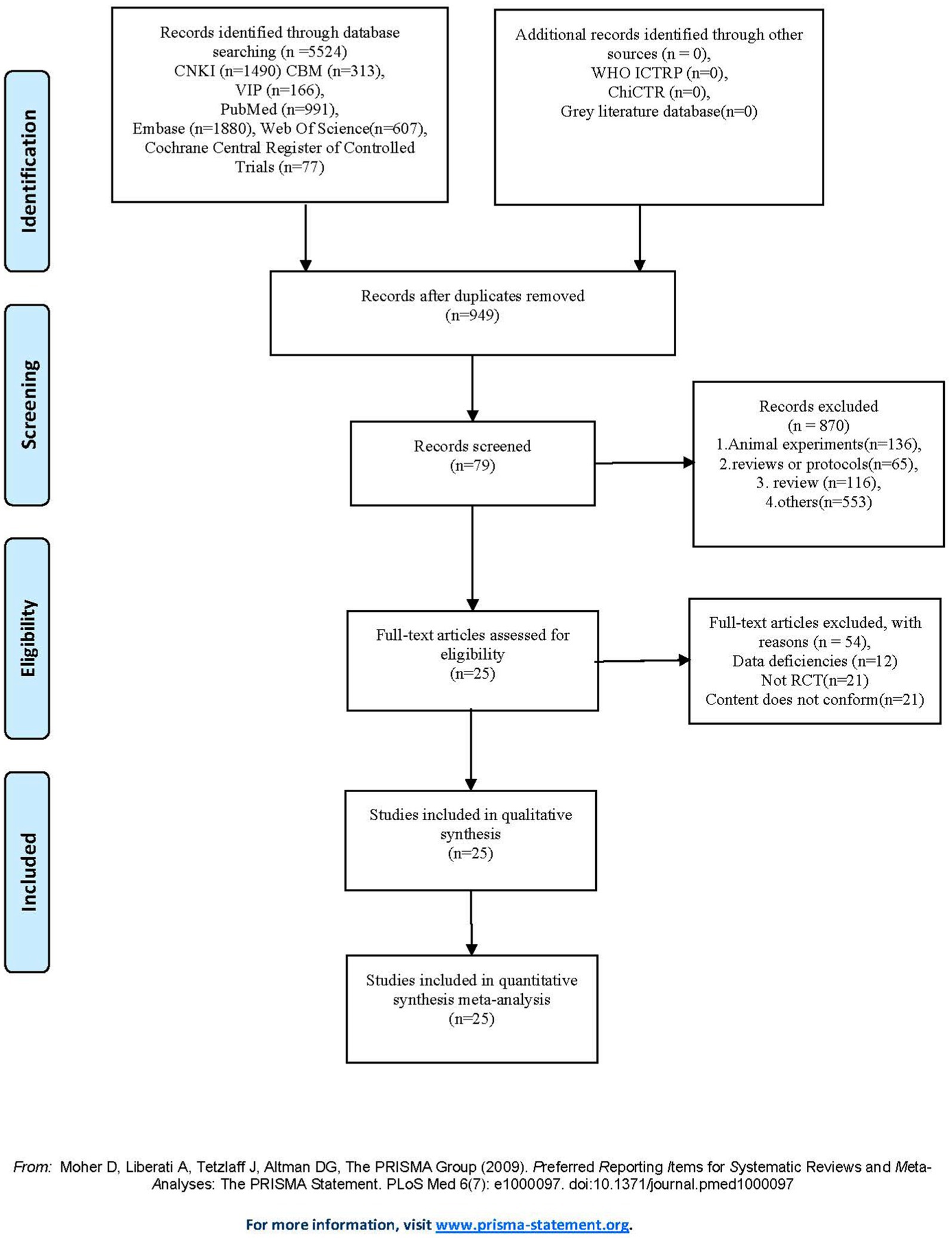
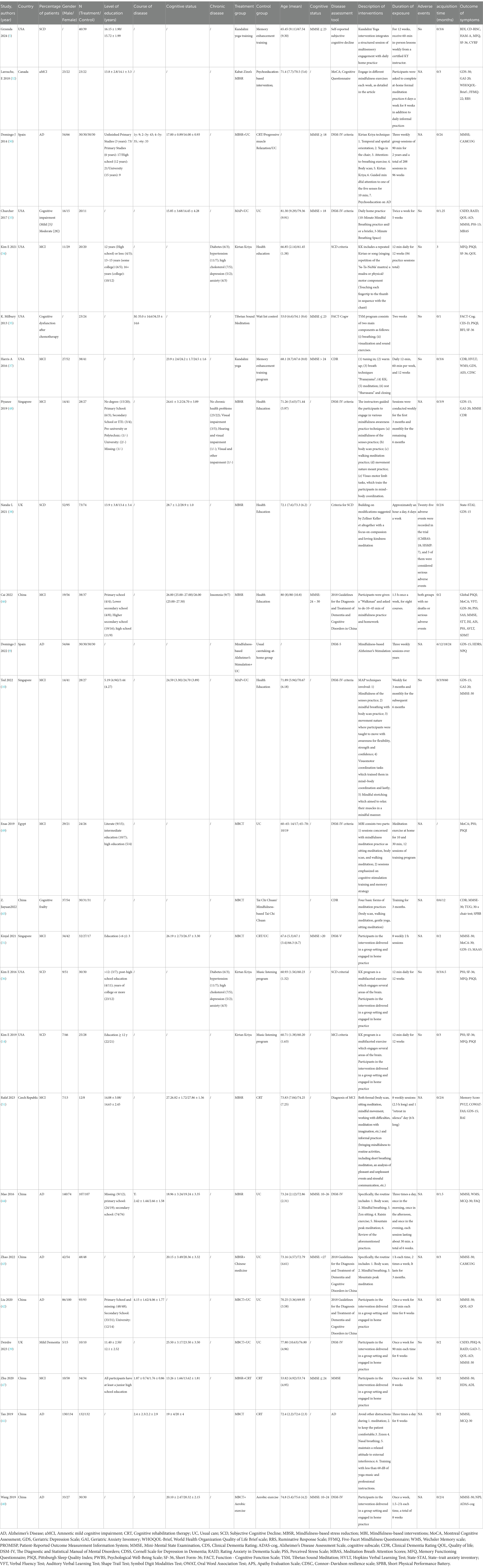
A total of 5,523 records were screened, of which 25 RCTs with 2,095 participants were included. Reasons for exclusion at the full-text stage are presented in Figure 1. All included RCTs were parallel-group trials, except for one three-arm study (31). All studies were published between 2013 and 2024 and conducted in multiple countries: one in Canada (32), seven in the United States (5, 14, 33–37), two in the United Kingdom (38, 39), eight in China (40–47), three in Singapore (10, 31, 48), one in Egypt (49), two in Spain (9, 50), and one in the Czech Republic (51). The ages of participants ranged from 53 to 80 years. The meditation interventions were broadly categorized into FAM and DMM. FAM was examined in 19 studies (9, 10, 14, 32–40, 42, 44, 47–51), and DMM was assessed in six studies (5, 35, 41, 43, 45, 46). Specific meditation programs included mindfulness-based cognitive therapy (MBCT) in eight studies (31, 39–42, 45, 47, 49), MBSR in eight studies (9, 38, 43, 44, 46, 48, 50, 51), mindful awareness practice (MAP) in two studies (10, 33), Kabat-Zinn’s in one study (32), Kirtan Kriya in three studies (14, 34, 36), Kundalini yoga in two study (5, 37), and Tibetan Sound Meditation in one study (35). Among the outcomes, global cognitive performance was reported in 14 studies (10, 31, 33, 39–48, 50), sleep quality in six studies (14, 34–36, 46, 49), health status in five studies (5, 14, 34–36), and depression scores in nine studies (9, 10, 31, 32, 37, 38, 46, 49, 51). The duration of interventions varied widely across studies, ranging from 2 to 96 weeks. Most studies reported no significant meditation-related adverse events. Only a few studies mentioned minor adverse events that did not compromise the overall safety and feasibility of the interventions. The characteristics of the studies included in this review are presented in Table 2.
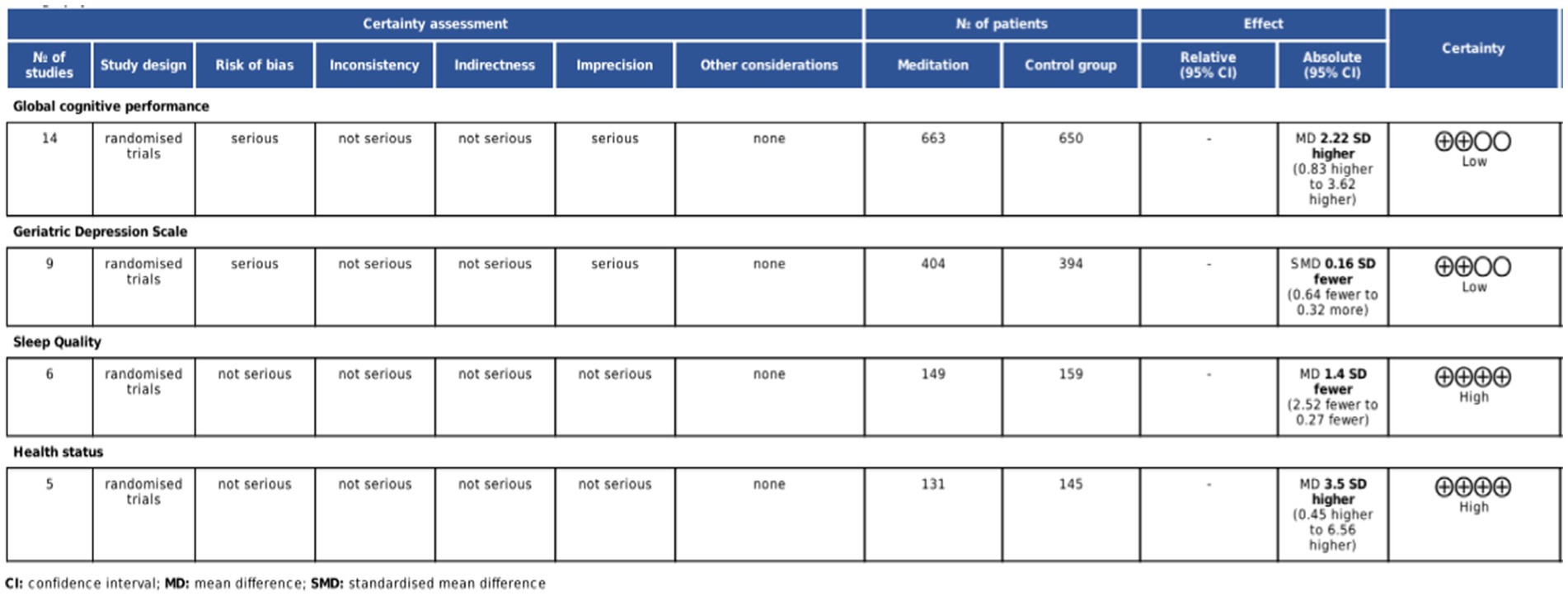
Risk of bias assessment and GRADE quality rating
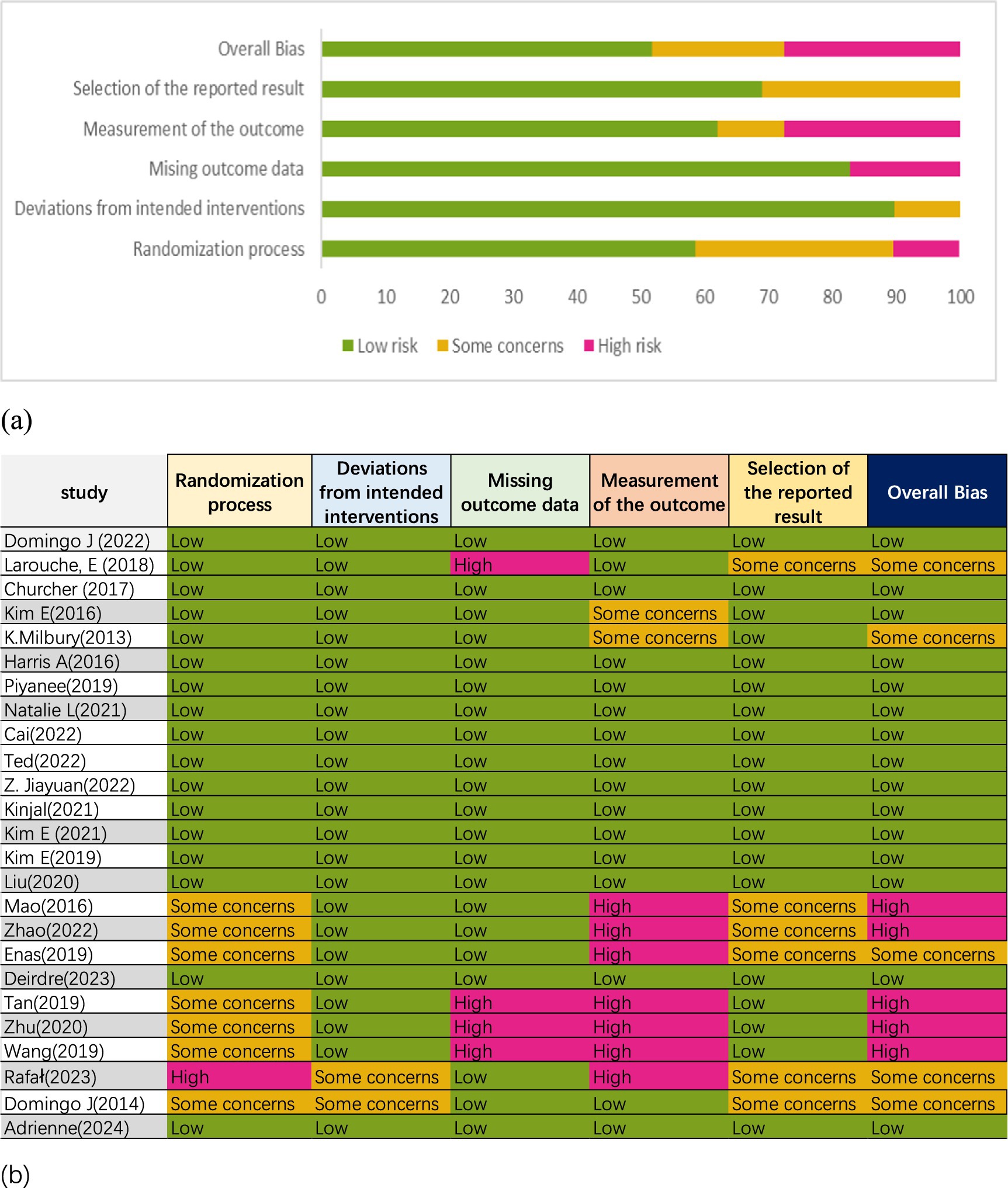
Seventeen studies described the randomization process (5, 9, 10, 14, 31–39, 42, 45, 46, 48), two studies raised concerns about deviation from intended intervention (50, 51), four studies exhibited a high risk of missing outcome data (32, 40, 41, 47), seven studies presented a high risk in outcome measurement (40, 41, 43, 44, 47, 49, 51), and two had relative risks (31, 36). Six studies had some concerns about the selection of reported results (32, 39, 43, 44, 49, 51). Overall, five studies were classified as high risk (40, 41, 43, 44, 47), five studies had some concerns (32, 35, 49–51), and 15 studies were deemed low risk (5, 9, 10, 14, 31, 33, 34, 36–39, 42, 45, 46, 48). The risk of bias assessment is illustrated in Figure 2. According to the GRADE assessment, the quality of evidence for global cognitive performance and depression was rated as low, whereas the evidence for sleep quality and health status was measured to be of high certainty (Figure 3).
Primary outcome
Global cognitive performance
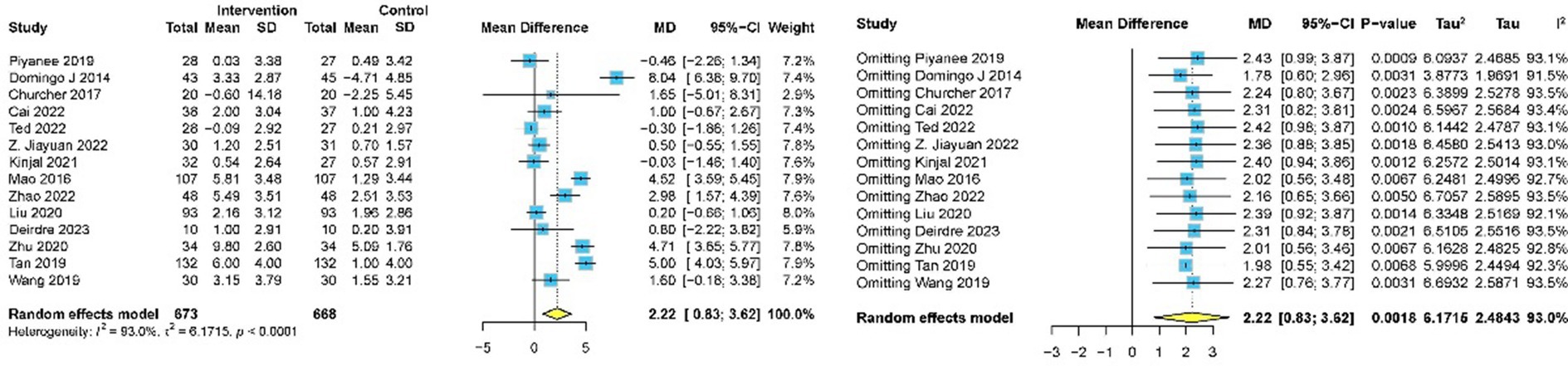
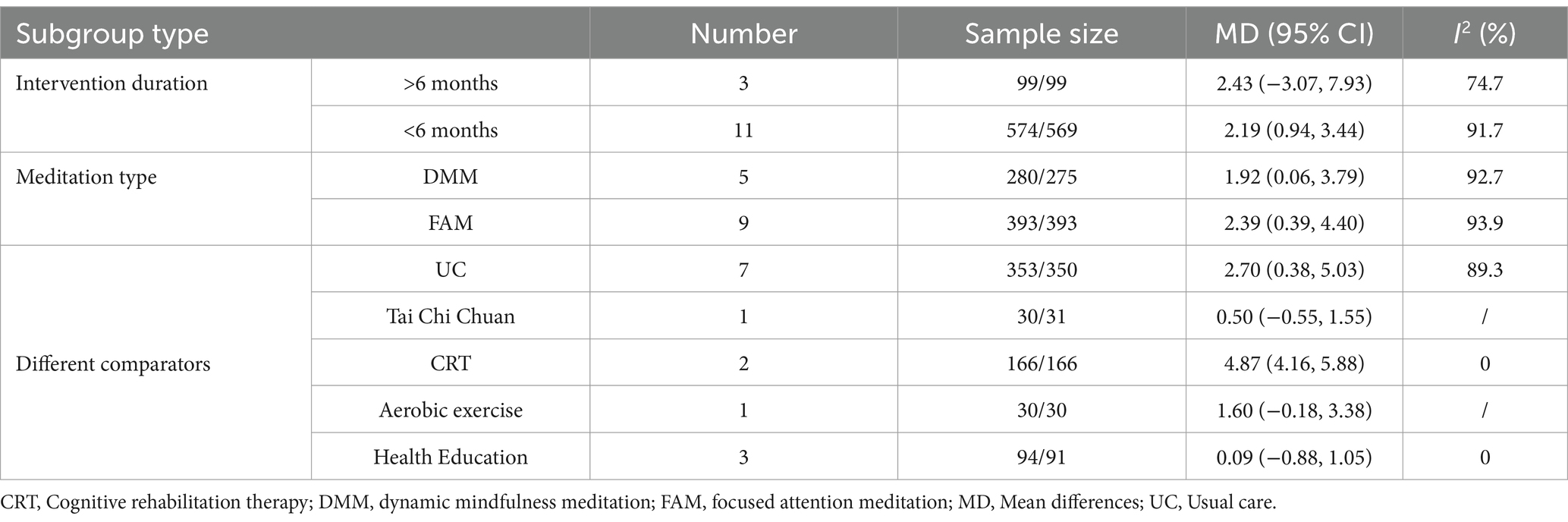
Fourteen studies involving 1,341 participants assessed global cognitive performance using MMSE (10, 31, 33, 39–48, 50). Meta-analysis of the pooled data demonstrated that meditation exhibited a statistically significant improvement in cognitive performance compared to the control group (MD 2.22, 95% CI: 0.83–3.62, p = 0.002) (Figure 4). Subgroup analyses were implemented based on intervention duration, meditation type, and different comparators. Statistically significant improvements in global cognitive performance were observed in 11 studies with intervention lasting less than 6 months (31–33, 39–46) (MD 2.19, 95% CI: 0.94–3.44, p < 0.001), while no significant effect was found in three studies with intervention lasting more than 6 months (10, 48, 50) (MD 2.43, 95% CI: −3.07 to 7.93, p = 0.387). Subgroup analysis by meditation type revealed statistically significant improvements in both FAM (10, 33, 39, 40, 42, 44, 47, 48, 50) (MD 2.39, 95% CI: 0.39–4.40, p = 0.019) and DMM (31, 41, 43, 45, 46) (MD 1.92, 95% CI: 0.06–3.79, p = 0.043). For different comparators, meditation was superior to usual care (31, 33, 39, 42–44, 50) (MD 2.70, 95% CI: 0.38–5.03, p = 0.023) and CRT (41, 47) (MD 4.87, 95% CI: 4.16–5.58, p < 0.001). No significant difference was observed when meditation was compared with aerobic exercise (40) (MD 1.60, 95% CI: −0.18 to 3.38, p = 0.080), health education (10, 46, 48) (MD 0.85, 95% CI: −0.87 to 1.05, p = 0.862), and Tai Chi Chuan (45) (MD 0.50, 95% CI: −0.55 to 1.55, p = 0.350) (Table 3).
Publication bias for global cognitive performance was evaluated using funnel plots (Figure 5), and no evidence of bias was indicated by Egger’s test (p = 0·619) and Begg’s test (p = 0·869). Sensitivity analysis for global cognitive performance confirmed high heterogeneity (I2 = 95%). Sequential exclusion of individual studies revealed minimal impact on the overall effect size and heterogeneity. The 14 study-specific MD ranged from 2.22 (95% CI: 0.83–3.62) to 1.78 (95% CI: 0.60–2.96), with heterogeneity slightly decreasing to I2 = 91.5%. Overall, the effect size remained statistically significant (p < 0.05), indicating the robustness of the findings.
Secondary outcomes
Sleep quality
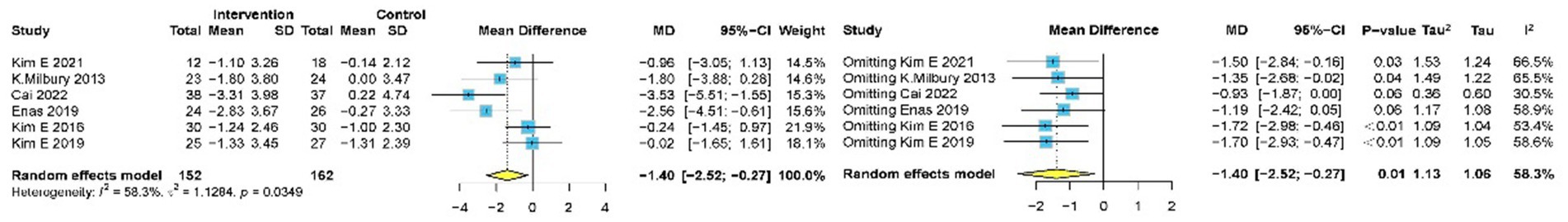
Six studies (14, 34–36, 46, 49) with 316 participants assessed sleep quality using PSQI. Meta-analysis of the pooled data demonstrated that meditation significantly improved the quality of sleep, as evident by the decreased PSQI (MD −1.40, 95% CI: −2.52 to −0.27, p = 0.015) (Figure 6).
Sensitivity analysis confirmed high heterogeneity (I2 = 58.3%), and sequential exclusion of individual studies showed minimal impact on the overall effect size and heterogeneity. The six study-specific MD ranged from −1.40 (95% CI: −2.52 to −0.27) to −0.93 (95% CI: −1.87 to 0.00), with heterogeneity slightly decreasing to I2 = 30.5%.
Health status
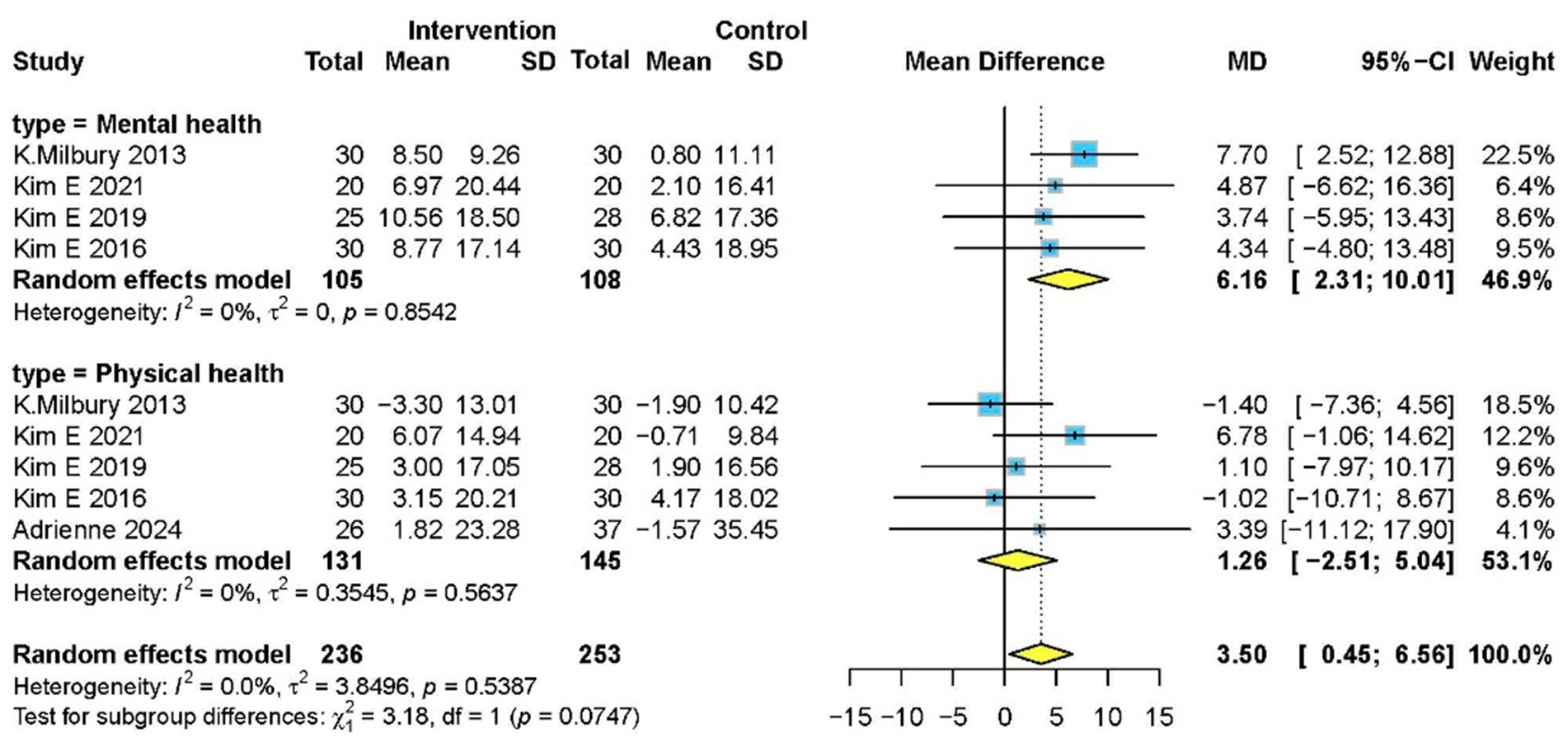
Five studies (5, 14, 34–36) with 276 participants evaluated health status using SF-36. Meditation significantly improved overall health status (MD 3.50, 95% CI: 0.45–6.56, p = 0.020) and mental health subdomain (MD 6.16, 95% CI: 2.31–10.01, p = 0.010), though no significant effect was observed in physical health subdomain (Figure 7).
Depression
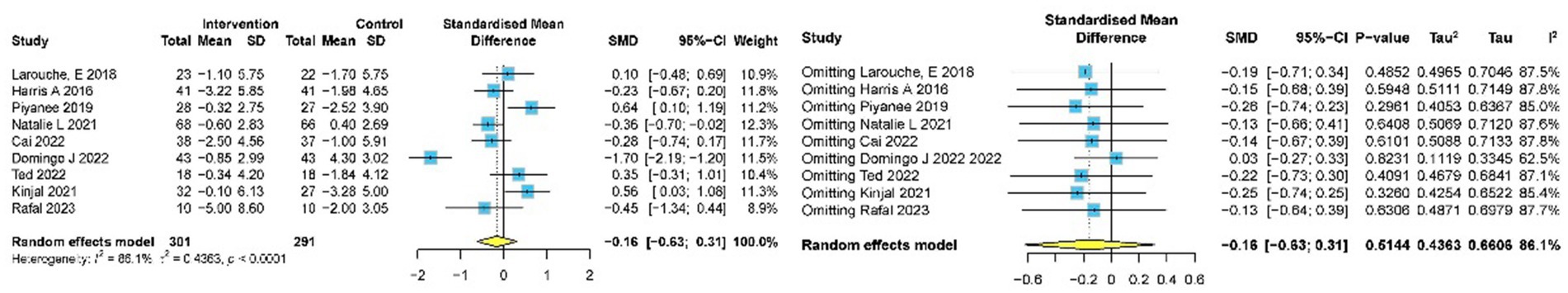
Nine studies (9, 10, 31, 32, 37, 38, 46, 48, 51) involving 592 participants assessed the impact of meditation on depression using GDS. Pooled data indicated no significant reduction in depression (SMD −0.16, 95% CI: −0.63 to 0.31, p = 0.514) (Figure 8). Sensitivity analysis reduced heterogeneity from I2 = 86.1% to I2 = 62.5%, with an adjusted effect size of SMD = 0.03 (95% CI: −0.27 to 0.33). No publication bias was detected by Egger’s test (p = 0.374) and Begg’s test (p = 1.000).
Discussion
Meditation has emerged as a scalable and cost-effective non-pharmacological therapeutic intervention, extending beyond clinical settings into community-based and older adult care frameworks (15, 44). This meta-analysis systematically evaluated the effectiveness of meditation across multiple domains, such as global cognitive performance, sleep quality, health status, and depression, in older adults with SCD, MCI, and AD. The findings demonstrated that meditation significantly improved global cognitive performance, sleep quality, and health status outcomes in this population. These results are consistent with prior cohort studies (52, 53), underscoring the potential of integrative public health approaches in mitigating cognitive decline. By synthesizing contemporary evidence and incorporating multidimensional outcome assessments, this study further highlighted the utility of meditation as a pragmatic and adjunctive therapeutic strategy for addressing cognitive decline in aging populations.
MMSE, a commonly used cognitive assessment scale, was employed in this study to evaluate the effectiveness of meditation on global cognitive performance. Pooled analysis showed that meditation significantly improved cognitive performance among individuals with SCD, MCI, and AD, consistent with previous studies demonstrating substantial cognitive enhancement from meditative practices (54). Subgroup analysis based on intervention duration suggested that meditation lasting less than 6 months yielded positive outcomes, which may provide insights for optimizing treatment duration in future practice. When stratified by meditation type, both FAM and DMM demonstrated notable memory-related benefits. FAM has been reported to foster mental focus and stability, improve attention, and reduce distractions, while DMM incorporates physical movement and may enhance physical function and alleviate stress through increased body awareness. Patients may benefit from selecting meditation modalities in accordance with their individual needs to maximize the desired outcomes (6). Additionally, subgroup analysis of comparators indicated that meditation outperformed both usual care and CRT in improving global cognitive performance. However, given the heterogeneity and limited sample sizes across included studies, these findings might be interpreted with cautious.
This study also provided robust evidence that meditation significantly enhanced sleep quality measured by PSQI and mental health subdomains of SF-36 in patients with SCD, MCI, and AD. These benefits may be attributed to the capacity of meditation to reduce ruminative thinking and enhance emotional regulation (55). Although no statistically significant improvements were observed in the physical health subdomain of SF-36, the mental health gains aligned with the growing recognition of meditation as a complementary therapy for insomnia and psychosocial wellbeing (8, 56). In the present study, depression scores showed no statistically significant differences between meditation and control groups. This may be associated with subclinical baseline depression levels in the included RCTs, potentially limiting the measurable impact of interventions. Future studies targeting cognitive decline populations with moderate-to-severe depression may help further elucidate the potential benefits of meditation in this domain (38, 46, 48).
While this study highlights the potential benefits of meditation for older adults with SCD, MCI, and AD, further explorations are still warranted from the perspective of the RE-AIM framework. The promotion of interventions in public health settings depends on their ‘Reach’ and ‘Implementation’ quality. The implementation quality of meditation may vary significantly across diverse populations, particularly among older adults, where such challenges as limited accessibility, variable adherence, and the need for contextual adaptability may arise (16). Additionally, the absence of long-term follow-up data in most studies underscores the need for future research to incorporate regular assessments and supportive measures to evaluate sustained effectiveness over extended periods further (57). Addressing these issues may facilitate the applicability of meditation in clinical and community settings and ensure its sustained effectiveness.
Strengths and limitations
The strengths of this study are as follows: (1) it covers the neurodegenerative continuum of SCD, MCI, and AD, while incorporating the most recent evidence to provide a contemporary synthesis of the therapeutic potential of meditation; (2) the systematic search strategy was designed to include both English and Chinese language databases, mitigating linguistic bias and enhancing the global applicability of findings; (3) the study employed multidimensional outcome assessments, yielding a more comprehensive evaluation of meditation impact that transcends the narrow cognitive focus of earlier reviews; and (4) the application of the GRADE ensured transparent and standardized appraisal of evidence certainty and might reinforce the robustness and reliability of outcomes.
However, several limitations warrant consideration: (1) although the search strategy prioritized database comprehensiveness, the exclusion of gray literature and manual reference searches might have introduced selection bias; (2) small sample sizes in some included studies might limit statistical power, though sensitivity analyses confirmed that small-sample effects did not drive heterogeneity; (3) while MMSE is widely used and highly accepted among health professionals, it has limited responsiveness over time and may carry a high risk of bias in detecting subtle cognitive changes (58, 59); (4) substantial heterogeneity remained persistently evident in MMSE-measured global cognitive performance, which might limit the reliability of pooled estimates and undermine the interpretability of the findings; (5) heterogeneous outcome assessment tools employed across the included RCTs necessitated focusing on certain selected subset, which might result in incomplete findings by excluding relevant data captured by alternative instruments; and (6) technology-enhanced meditation interventions have shown emerging promise for populations at risk of cognitive decline (60, 61), their absence in this study may limit the generalizability of the findings.
Implications for further research
For future research, further investigation into the effects of meditation on patients with cognitive decline remains warranted. Conducting multi-center, large-sample, high-quality clinical trials is crucial to further validate the positive effects of meditation and assess its applicability in patients with cognitive decline. Special attention should be paid to how meditation may impact patients at various stages of cognitive decline, as the effects may differ depending on disease progression. To better understand the long-term benefits of meditation, future studies should incorporate extended follow-up periods to evaluate its sustained effects. Moreover, given the complex nature of cognitive health, interdisciplinary collaboration will be pivotal. Future research should foster cooperation across fields, such as psychology, neurology, and clinical medicine, to decipher the biological mechanisms underlying meditation effects and enhance its clinical applications in public health.
Conclusion
This study highlights the potential of meditation as an effective adjunct approach for older adults with SCD, MCI, and AD, yielding significant improvements in global cognitive performance, sleep quality, and health status. However, given the limitation in evidence quality, heterogeneity, and sample sizes across studies, these findings should be interpreted with caution. More large-scale and well-designed high-quality RCTs with long-term follow-ups are warranted to validate the effectiveness of meditation further and decipher its underlying mechanisms.
Data availability statement
All datasets generated for this study are included in the article/supplementary material.
Author contributions
JXS: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. HT: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. JWW: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. WHX: Investigation, Writing – original draft, Writing – review & editing. QL: Validation, Writing – original draft, Writing – review & editing. JP: Validation, Writing – original draft, Writing – review & editing. JX: Validation, Writing – original draft, Writing – review & editing. WYH: Validation, Writing – original draft, Writing – review & editing. YX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. YHC: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the International Cooperation and Exchange Project of Science & Technology Department of Sichuan Province (grant numbers 2023YFH0100, 2017HH0004); the Sichuan Provincial Administration of Traditional Chinese Medicine (grant number 2021MS464); the National Natural Science Foundation of China (grant number 81860840) and the National Natural Science Foundation of China (grant number 81603537). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. (2024) 20:3708–3821. doi: 10.1002/alz.13809
2. Wang, X, Huang, W, Su, L, Xing, Y, Jessen, F, Sun, Y, et al. Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer's disease. Mol Neurodegener. (2020) 15:1–27. doi: 10.1186/s13024-020-00395-3
3. Russell-Williams, J, Jaroudi, W, Perich, T, Hoscheidt, S, El Haj, M, and Moustafa, AA. Mindfulness and meditation: treating cognitive impairment and reducing stress in dementia. Rev Neurosci. (2018) 29:791–804. doi: 10.1515/revneuro-2017-0066
4. Rajendra, A, Bondonno, NP, Rainey-Smith, SR, Gardener, SL, Hodgson, JM, and Bondonno, CP. Potential role of dietary nitrate in relation to cardiovascular and cerebrovascular health, cognition, cognitive decline and dementia: a review. Food Funct. (2022) 13:12572–89. doi: 10.1039/d2fo02427f
5. Grzenda, A, Siddarth, P, Milillo, MM, Aguilar-Faustino, Y, Khalsa, DS, and Lavretsky, H. Cognitive and immunological effects of yoga compared to memory training in older women at risk for Alzheimer's disease. Transl Psychiatry. (2024) 14:96. doi: 10.1038/s41398-024-02807-0
6. D'Andrea, A, Croce, P, O'Byrne, J, Jerbi, K, Pascarella, A, Raffone, A, et al. Mindfulness meditation styles differently modulate source-level MEG microstate dynamics and complexity. Front Neurosci. (2024) 18:1295615. doi: 10.3389/fnins.2024.1295615
7. Mirabito, G, and Verhaeghen, P. The effects of mindfulness interventions on older adults' cognition: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. (2023) 78:394–408. doi: 10.1093/geronb/gbac143
8. Rusch, HL, Rosario, M, Levison, LM, Olivera, A, Livingston, WS, Wu, T, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. (2019) 1445:5–16. doi: 10.1111/nyas.13996
9. Quintana-Hernandez, DJ, Rojas-Hernandez, J, Santana-Del Pino, A, Céspedes Suárez, C, Pellejero Silva, M, Miró-Barrachina, MT, et al. Mindfulness prevents depression and psychopathology in elderly people with mild to moderate Alzheimer's disease: a randomized clinical trial. J Alzheimers Dis. (2023) 91:471–81. doi: 10.3233/JAD-220889
10. Ng, TKS, Tan, XR, Todd, M, Chen, ACC, Feng, L, Lu, Y, et al. Effects of mindful awareness practice (MAP) on subclinical depressive and anxiety symptoms and general cognitive function in older adults with mild cognitive impairment: a 5-year follow-up of the MAP-randomized controlled trial. J Alzheimers Dis. (2022) 90:1677–88. doi: 10.3233/JAD-220641
11. Dwivedi, M, Dubey, N, Pansari, AJ, Bapi, RS, das, M, Guha, M, et al. Effects of meditation on structural changes of the brain in patients with mild cognitive impairment or Alzheimer's disease dementia. Front Hum Neurosci. (2021) 15:728993. doi: 10.3389/fnhum.2021.728993
12. Leow, Y, Rashid, N, Klainin-Yobas, P, Zhang, Z, and Wu, XV. Effectiveness of mindfulness-based interventions on mental, cognitive outcomes and neuroplastic changes in older adults with mild cognitive impairment: a systematic review and meta-analysis. J Adv Nurs. (2023) 79:4489–505. doi: 10.1111/jan.15720
13. Pascoe, MC, Thompson, DR, and Ski, CF. Meditation and endocrine health and wellbeing. Trends Endocrinol Metab. (2020) 31:469–77. doi: 10.1016/j.tem.2020.01.012
14. Innes, KE, Selfe, TK, Brundage, K, Montgomery, C, Wen, S, Kandati, S, et al. Effects of meditation and music-listening on blood biomarkers of cellular aging and Alzheimer's disease in adults with subjective cognitive decline: an exploratory randomized clinical trial. J Alzheimers Dis. (2018) 66:947–70. doi: 10.3233/JAD-180164
15. Jamil, A, Gutlapalli, SD, Ali, M, Oble, MJP, Sonia, SN, George, S, et al. Meditation and its mental and physical health benefits in 2023. Cureus. (2023) 15:e40650. doi: 10.7759/cureus.40650
16. Harden, SM, Smith, ML, Ory, MG, Smith-Ray, RL, Estabrooks, PA, and Glasgow, RE. RE-AIM in clinical, community, and corporate settings: perspectives, strategies, and recommendations to enhance public health impact. Front Public Health. (2018) 6:71. doi: 10.3389/fpubh.2018.00071
17. Glasgow, RE, Harden, SM, Gaglio, B, Rabin, B, Smith, ML, Porter, GC, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. (2019) 7:64. doi: 10.3389/fpubh.2019.00064
18. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
19. Chen, Y, Zhang, J, Zhang, T, Cao, L, You, Y, Zhang, C, et al. Meditation treatment of Alzheimer disease and mild cognitive impairment: a protocol for systematic review. Medicine (Baltimore). (2020) 99:e19313. doi: 10.1097/MD.0000000000019313
20. Frisoni, GB, Winblad, B, and O'Brien, JT. Revised NIA-AA criteria for the diagnosis of Alzheimer's disease: a step forward but not yet ready for widespread clinical use. Int Psychogeriatr. (2011) 23:1191–6. doi: 10.1017/S1041610211001220
21. Creavin, ST, Wisniewski, S, Noel-Storr, AH, Trevelyan, CM, Hampton, T, Rayment, D, et al. Mini-mental state examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev. (2016) 2016:CD011145. doi: 10.1002/14651858.CD011145.pub2
22. Harlies, CM, and Friedlander, W. Sleep quality of adult psychiatric outpatients at Chris Hani Baragwanath academic hospital. S Afr J Psychiatry. (2023) 29:2113. doi: 10.4102/sajpsychiatry.v29i0.2113
23. Jenkinson, C, Wright, L, and Coulter, A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. (1994) 3:7–12. doi: 10.1007/BF00647843
24. Rothwell, PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet. (2005) 365:82–93. doi: 10.1016/S0140-6736(04)17670-8
25. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
27. Budhiraja, P, Kalot, MA, Alayli, AE, Dimassi, A, Kaplan, B, Chakkera, HA, et al. Reporting and handling of missing participant data in systematic reviews of kidney transplant studies. Transplantation. (2021) 105:1708–17. doi: 10.1097/TP.0000000000003503
28. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. eds. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons (2019).
29. Irwig, L, Macaskill, P, Berry, G, and Glasziou, P. Bias in meta-analysis detected by a simple, graphical test. Graphical test is itself biased. BMJ. (1998) 316:470; author reply 470-1.
30. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
31. Doshi, K, Henderson, SL, Fan, Q, Wong, KF, and Lim, J. Mindfulness-based training does not improve neuropsychological outcomes in mild cognitive impairment more than spontaneous reversion rates: a randomized controlled trial. J Alzheimers Dis. (2021) 84:449–58. doi: 10.3233/JAD-215035
32. Larouche, E, Hudon, C, and Goulet, S. Mindfulness mechanisms and psychological effects for aMCI patients: a comparison with psychoeducation. Complement Ther Clin Pract. (2019) 34:93–104. doi: 10.1016/j.ctcp.2018.11.008
33. Churcher Clarke, A, Chan, JMY, Stott, J, Royan, L, and Spector, A. An adapted mindfulness intervention for people with dementia in care homes: feasibility pilot study. Int J Geriatr Psychiatry. (2017) 32:e123–31. doi: 10.1002/gps.4669
34. Innes, KE, Montgomery, C, Selfe, TK, Wen, S, Khalsa, DS, and Flick, M. Incorporating a usual care comparator into a study of meditation and music listening for older adults with subjective cognitive decline: a randomized feasibility trial. J Alzheimers Dis Rep. (2021) 5:187–206. doi: 10.3233/ADR-200249
35. Milbury, K, Chaoul, A, Biegler, K, Wangyal, T, Spelman, A, Meyers, CA, et al. Tibetan sound meditation for cognitive dysfunction: results of a randomized controlled pilot trial. Psychooncology. (2013) 22:2354–63. doi: 10.1002/pon.3296
36. Innes, KE, Selfe, TK, Khalsa, DS, and Kandati, S. Effects of meditation versus music listening on perceived stress, mood, sleep, and quality of life in adults with early memory loss: a pilot randomized controlled trial. J Alzheimers Dis. (2016) 52:1277–98. doi: 10.3233/JAD-151106
37. Eyre, HA, Siddarth, P, Acevedo, B, van Dyk, K, Paholpak, P, Ercoli, L, et al. A randomized controlled trial of kundalini yoga in mild cognitive impairment. Int Psychogeriatr. (2017) 29:557–67. doi: 10.1017/S1041610216002155
38. Marchant, NL, Barnhofer, T, Coueron, R, Wirth, M, Lutz, A, Arenaza-Urquijo, EM, et al. Effects of a mindfulness-based intervention versus health self-management on subclinical anxiety in older adults with subjective cognitive decline: the SCD-well randomized superiority trial. Psychother Psychosom. (2021) 90:341–50. doi: 10.1159/000515669
39. Noone, D, Payne, J, Stott, J, Aguirre, E, Patel-Palfreman, MM, Stoner, C, et al. The feasibility of a mindfulness intervention for depression in people with mild dementia: a pilot randomized controlled trial. Clin Gerontol. (2023) 46:346–58. doi: 10.1080/07317115.2022.2094741
40. Wang, L, Bai, QY, Xu, CP, and Wang, Y. Effects of aerobic exercise combined with positive thinking training on cognitive function in elderly patients with cognitive impairment. Chinese Pharm Sci. (2019) 9:18–22.
41. HongZhu, T. Effects of positive meditation on cognitive ability and metacognitive levels in patients with Alzheimer's disease. Shanxi Med J. (2019) 48:1515–7. doi: 10.3969/j.issn.0253-9926.2019.12.049
42. Liu Ying, GJ, ChunYan, H, XiuChun, L, Hao, W, and Qing, Z. Positive thought cognitive therapy on cognitive function and quality of life in patients with Alzheimer's disease quality of life in patients with Alzheimer's disease. Chinese J Modern Nurs. (2020) 26:2903–8.
43. Zhao, X, and Wei, J. Therapeutic effect of meditation training plus the Gulu Erxian decoction on mild Alzheimer’s disease. Clin J Chinese Med. (2022) 14:57–9. doi: 10.3969/j.issn.1674-7860.2022.09.021
44. Mao, DF. Effect research of meditation intervention on cognitive impairment in elderly patinets with Alzheimers disease. J Nurs Res. (2018) 32:1382–6. doi: 10.1186/s13195-024-01482-z
45. Jiayuan, Z, Xiang-Zi, J, Li-Na, M, Jin-Wei, Y, and Xue, Y. Effects of mindfulness-based Tai Chi Chuan on physical performance and cognitive function among cognitive frailty older adults: a six-month follow-up of a randomized controlled trial. J Prev Alzheimers Dis. (2022) 9:104–12. doi: 10.14283/jpad.2021.40
46. Cai, ZZ, Lin, R, Wang, XX, Yan, YJ, and Li, H. Effects of mindfulness in patients with mild cognitive impairment with insomnia: a double-blind randomized controlled trial. Geriatr Nurs. (2022) 47:239–46. doi: 10.1016/j.gerinurse.2022.08.001
47. Zhu, BZ, Rong, JC, Gao, YH, Tan, YJ, and Liang, SF. Positive thinking training on cognitive function and patients with cognitive impairment after ischemic stroke daily living ability in patients with post-ischemic stroke cognitive impairment. Chinese J Pract Med. (2020) 15:188–91.
48. Klainin-Yobas, P, Kowitlawakul, Y, Lopez, V, Tang, CT, Hoek, KE, Gan, GL, et al. The effects of mindfulness and health education programs on the emotional state and cognitive function of elderly individuals with mild cognitive impairment: a randomized controlled trial. J Clin Neurosci. (2019) 68:211–7. doi: 10.1016/j.jocn.2019.05.031
49. Abdelaziz, E, Elsharkawy, N, Reshia, F, and Ahmed, M. The effects of mindfulness-cognitive based therapy on cognition and sleep quality among older adults with mild cognitive impairment. Sylwan. 163:518–45. doi: 10.3233/JAD-143009
50. Quintana-Hernandez, DJ, Miro-Barrachina, MT, Ibanez-Fernandez, IJ, Pino, AS, Quintana-Montesdeoca, MP, Rodriguez-de Vera, B, et al. Mindfulness in the maintenance of cognitive capacities in Alzheimer's disease: a randomized clinical trial. J Alzheimers Dis. (2016) 50:217–32.
51. Marciniak, R, Sumec, R, Vyhnalek, M, Bendickova, K, Laznickova, P, Forte, G, et al. The effect of mindfulness-based stress reduction (MBSR) on depression, cognition, and immunity in mild cognitive impairment: a pilot feasibility study. Clin Interv Aging. (2020) 15:1365–81. doi: 10.2147/CIA.S249196
52. Matthews, FE, Arthur, A, Barnes, LE, Bond, J, Jagger, C, Robinson, L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the cognitive function and ageing study I and II. Lancet. (2013) 382:1405–12. doi: 10.1016/S0140-6736(13)61570-6
53. Christensen, K, Thinggaard, M, Oksuzyan, A, Steenstrup, T, Andersen-Ranberg, K, Jeune, B, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet. (2013) 382:1507–13. doi: 10.1016/S0140-6736(13)60777-1
54. Watt, JA, Veroniki, AA, Tricco, AC, and Straus, SE. Using a distribution-based approach and systematic review methods to derive minimum clinically important differences. BMC Med Res Methodol. (2021) 21:41. doi: 10.1186/s12874-021-01228-7
55. van Vugt, MK, Hitchcock, P, Shahar, B, and Britton, W. The effects of mindfulness-based cognitive therapy on affective memory recall dynamics in depression: a mechanistic model of rumination. Front Hum Neurosci. (2012) 6:257. doi: 10.3389/fnhum.2012.00257
56. Tsui, MCF, JCN, T, and Lee, ATC. Mindfulness meditation, mental health, and health-related quality of life in Chinese Buddhist monastics. East Asian Arch Psychiatr. (2020) 30:67–72. doi: 10.12809/eaap1949
57. Estabrooks, PA, and Glasgow, RE. Developing a dissemination and implementation research agenda for aging and public health: the what, when, how, and why? Front Public Health. (2023) 11:1123349. doi: 10.3389/fpubh.2023.1123349
58. Pellegrino, LD, Peters, ME, Lyketsos, CG, and Marano, CM. Depression in cognitive impairment. Curr Psychiatry Rep. (2013) 15:384. doi: 10.1007/s11920-013-0384-1
59. Arevalo-Rodriguez, I, Smailagic, N, Roque-Figuls, M, Ciapponi, A, Sanchez-Perez, E, Giannakou, A, et al. Mini-mental state examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. (2021) 2021:CD010783. doi: 10.1002/14651858.CD010783.pub3
60. Zaccaro, A, Piarulli, A, Laurino, M, Garbella, E, Menicucci, D, Neri, B, et al. How breath-control can change your life: a systematic review on psycho-physiological correlates of slow breathing. Front Hum Neurosci. (2018) 12:353. doi: 10.3389/fnhum.2018.00353
Keywords: meditation, subjective cognitive decline, mild cognitive impairment, Alzheimer’s disease, meta-analysis
Citation: Shi J, Tian H, Wei J, Xu W, Luo Q, Peng J, Xia J, Huai W, Xiong Y and Chen Y (2025) Meditation for subjective cognitive decline, mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Front. Public Health. 13:1524898. doi: 10.3389/fpubh.2025.1524898
Edited by:
Matthew Lee Smith, Texas A and M University, United StatesReviewed by:
Natalia Sharashkina, Pirogov Russian National Research Medical University, RussiaPatrick Manser, Karolinska Institutet (KI), Sweden
Copyright © 2025 Shi, Tian, Wei, Xu, Luo, Peng, Xia, Huai, Xiong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xiong, eGlvbmd5aW5nQHdjaHNjdS5jbg==; Yunhui Chen, Y2hlbnl1bmh1aUBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Jiaxin Shi1†
Jiaxin Shi1† Hao Tian
Hao Tian Qin Luo
Qin Luo Jun Xia
Jun Xia Yunhui Chen
Yunhui Chen