- 1Management School, Shanxi Medical University, Taiyuan, China
- 2Department of Nephrology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
- 3Department of Urology, First Hospital of Shanxi Medical University, Taiyuan, China
- 4Department of Computer Science and Technology, Taiyuan University of Technology, Taiyuan, China
Background: Existing research has revealed that various factors, including environmental pollutants, can affect renal function. This study aims to explore the association between various synthetic Endocrine Disruptors(EDs), which are widely present in the environment, and the renal function of middle-aged and older adult populations.
Methods: Based on data from the National Health and Nutrition Examination Survey (NHANES) from 2015 to 2018, a total of 1,199 participants, with a median age of 57 years, who had CKD or at least one CKD complication (such as dyslipidemia, hypertension, diabetes, or hyperuricemia), were included. A comprehensive assessment was conducted on the relationship between 28 synthetic EDs (including perfluoro/polyfluoroalkyl compounds, phosphorus-containing pesticides, and plasticizers) and renal function. Serum concentrations of synthetic EDs were determined using mass spectrometry. Renal function was assessed through urine Albumin-to-Creatinine Ratio (ACR) measurements. The quantile regression models were employed to explore the association between synthetic EDs and renal function. The grouped regression models were used to investigate the differential associations of synthetic EDs with non-CKD and CKD populations.
Results: In this study, N-perfluorooctanoic acid, MECP phthalate, and Diethylthiophosphate were found to exert damaging effects on renal function. N-perfluorooctanoic acid and MECP phthalate were observed to exhibit differential associations with non-CKD and CKD populations. There was a significant difference in the effect of N-perfluorooctanoic acid between the non-CKD and CKD groups. N-perfluorooctanoic acid was not associated with renal function impairment in the non-CKD group, whereas it exhibited a significant adverse association in the CKD group. There was a significant difference in the effect of MECP phthalate between the non-CKD and CKD groups. MECP phthalate was associated with renal function impairment in the non-CKD group, but not in the CKD group.
Conclusion: This study elucidates the associations and differences of synthetic EDs with renal function. Based on the findings, enhanced monitoring and health education regarding environmental exposure to synthetic EDs such as N-perfluorooctanoic acid, MECP phthalate, and Diethylthiophosphate can be implemented to reduce public exposure risks.
Background
The kidney, as a crucial organ for metabolic and excretory functions in the human body, plays a vital role in maintaining systemic homeostasis. In recent years, the prevalence of Chronic Kidney Disease (CKD) and the incidence of its complications have shown a continuous upward trend, posing a significant threat to human health and quality of life (1). Recent systematic reviews by Chesnaye et al. and Francis et al. have comprehensively identified factors contributing to CKD, include age, other chronic diseases, lifestyle, and environmental pollutants (2, 3). Among these, environmental pollutants encompass both Endocrine Disruptors (EDs) such as pesticides and organophosphates, and non-EDs such as heavy metals (4, 5). EDs, which are chemical substances capable of disrupting the hormonal balance and homeostasis in organisms, can be classified into synthetic and natural EDs based on their sources (6). Synthetic EDs, such as perfluoro/polyfluoroalkyl compounds, plasticizers, and phosphorus-containing pesticides, are widely present in daily life products, including pesticides, herbicides, detergents, cosmetics, food packaging, children’s toys, and textiles, making them inevitable environmental pollutants in modern society (7). Synthetic EDs have multiple exposure routes in humans, including dietary intake, airborne inhalation, water consumption, and dermal contact. These routes increase the exposure risk and pose potential threats to human health.
Studies have shown that various synthetic EDs, such as perfluoro/polyfluoroalkyl compounds, bisphenol A, phthalates, and organophosphates, can disrupt multiple metabolic processes. These include metabolism, insulin function, stress response, and inflammation. These compounds have significantly increased the risk of various chronic diseases, including cancer, diabetes, metabolic syndrome, reproductive disorders, immune dysfunction, thyroid dysfunction, and cardiovascular diseases, many of which are associated with impaired renal function (8–12). The age-related decline in renal function and reduced metabolic clearance capacity may enhance organismal susceptibility to the toxic effects of synthetic EDs, underscoring the clinical significance of investigating the association between environmental pollutant exposure and renal impairment in middle-aged and older adult populations. Therefore, investigating the extent of organ dysfunction impacted by synthetic endocrine disruptors in middle-aged and older adult populations has become a critical research priority.
Existing research on the association between synthetic EDs and renal function has primarily focused on perfluoro/polyfluoroalkyl compounds. Epidemiological studies have shown that perfluoro/polyfluoroalkyl compounds are associated with kidney health impairment (13, 14). Stanifer et al. (15) found an association between exposure to perfluoro/polyfluoroalkyl compounds and adverse kidney outcomes, including renal function decline and kidney cancer. Furthermore, animal experiments have also demonstrated the adverse effects of perfluoro/polyfluoroalkyl compounds on kidney health, with studies revealing kidney weight gain, increased blood urea nitrogen, tubular atrophy, and tubular epithelial hyperplasia in animals exposed to these compounds (16). Although studies have shown the relationship between synthetic EDs and various chronic diseases linked to renal function impairment, research on the differential associations of plasticizers and phosphorus-containing pesticide EDs with renal function is limited. This is especially true across varying renal health states. CKD patients have impaired renal function compared to non-CKD individuals. This reduces their ability to clear exogenous and endogenous harmful substances. Therefore, in-depth exploration of the differential associations between synthetic EDs and renal function in CKD and non-CKD populations is important. It has both theoretical and applied value. Based on this, this study aims to analyze the association between synthetic EDs and renal function and their differential associations in CKD and non-CKD groups, providing an effective prevention and control strategy for clinical practice and a basis for formulating reasonable environmental protection policies.
Materials and methods
Study design and sample
To systematically evaluate the associations between diverse synthetic EDs and renal function indicators, this study utilized data from the National Health and Nutrition Examination Survey (NHANES) conducted between 2015 and 2018. Detailed data collection and quality control methods are available at https://www.cdc.gov/nchs/nhanes/. To effectively explore factors associated with renal dysfunction, this study enrolled participants with at least one of the following conditions: CKD, dyslipidemia, hypertension, diabetes, and hyperuricemia. We strictly excluded participants. These participants had missing data on sociodemographic characteristics, synthetic EDs characteristics, lifestyle characteristics, and health characteristics. This was done to ensure data integrity and reliability. A total of 1,199 individuals were ultimately included, with their detailed sociodemographic characteristics presented in Table 1. This study had received formal approval from the ethics committee of the National Center for Health Statistics. All participants voluntarily signed informed consent forms. They did so after fully understanding the study content. This ensured ethical compliance and participant rights protection.
Measurement of synthetic endocrine disruptors
The synthetic EDs in this study included perfluoro/polyfluoroalkyl compounds, phosphorus-containing pesticides, and plasticizers, as detailed in Table 2. Perfluoro/polyfluoroalkyl compounds comprised eight types. These were Perfluorodecanoic acid (PFDE), Perfluorohexane sulfonic acid (PFHS), 2-(N-methyl-PFOSA)acetic acid (MPAH), Perfluorononanoic acid (PFNA), Perfluoroundecanoic acid (PFUA), N-perfluorooctanoic acid (NFOA), Br. perfluorooctanoic acid iso (BFOA), and N-perfluorooctane sulfonic acid (NFOS). The specific measurement methods for synthetic endocrine disruptors can be found at: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2015&_refluxos=a10. The concentrations of perfluoro/polyfluoroalkyl compounds were determined using solid-phase extraction-high-performance liquid chromatography-electrospray ionization-mass spectrometry. Phosphorus-containing pesticides included six types. These were Dimethylphosphate (OP1), Diethylphosphate (OP2), Dimethylthiophosphate (OP3), Diethylthiophosphate (OP4), Dimethyldithiophosphate (OP5), and Diethyldithiophosphate (OP6). The concentrations of phosphorus-containing pesticides were determined using solid-phase extraction (SPE) and isotope dilution ultra-high-performance liquid chromatography-mass spectrometry. Plasticizers included 14 types. These were Mono(carboxynonyl) Phthalate (CNP), Mono(carboxyoctyl) Phthalate (COP), MECP phthalate (ECP), Mono-n-butyl phthalate (MBP), Mono-(3-carboxypropyl) phthalate (MC1), MCOCH phthalate (MCOH), Mono-ethyl phthalate (MEP), Mono-3-hydroxy-n-butyl phthalate (MHBP), MHNCH (MHNC), Mono-(2-ethyl)-hexyl phthalate (MHP), Mono-isobutyl phthalate (MIB), Mono-isononyl phthalate (MNP), MEOH phthalate (MOH), and Mono-benzyl phthalate (MZP). The concentrations of plasticizers were determined using high-performance liquid chromatography-electrospray ionization-mass spectrometry.
Measurement of renal function and CKD
Currently, three well-validated biomarkers are widely used to evaluate renal function, including the estimated glomerular filtration rate (eGFR), albumin-to-creatinine ratio (ACR), and total protein-to-creatinine ratio (PCR). Among these, ACR is more advantageous in the early diagnosis of CKD due to its high sensitivity, convenience, and low cost. Therefore, considering its advantages, this study employed ACR to assess renal function. CKD was defined according to established clinical criteria. Participants needed to fulfill at least one of the following conditions: (1) persistent renal dysfunction (abnormal kidney structure or renal function) for more than 3 months; (2) ACR ≥ 30 mg/g; (3) glomerular filtration rate (eGFR) < 60 mL·min−1·(1.73 m2)−1 for more than 3 months (17–19). ACR was estimated by calculating the ratio of urine albumin to urine creatinine. eGFR was estimated using the MDRD formula based on serum creatinine (17). Urine creatinine was measured using the Roche Cobas 6,000 analyzer with an enzymatic method. Urine albumin was measured using the Sequoia-Turne 450 digital fluorometer with a fluorescence immunoassay. Serum creatinine was measured using the Beckman UniCel DxC 800 Synchron, Beckman UniCel DxC 660i Synchron with the alkaline picrate Jaffe rate method or the Roche Cobas 6,000 with an enzymatic method. The specific process is detailed at https://www.cdc.gov/nchs/nhanes/.
To comprehensively assess the association between synthetic endocrine disruptors and renal function, this study employed quantile regression analysis to examine their associations across different percentiles of the exposure distribution. In contrast to conventional linear regression that estimates average effects, quantile regression minimizes weighted absolute residuals to characterize relationships at specific points of the conditional distribution. This approach provides robust estimation of covariate effects across the entire conditional distribution, particularly at specified quantiles. We specifically analyzed the associations at the following quantiles: Q10, Q20, Q30, Q40, Q50, Q60, Q70, Q80, and Q90. This was done to capture potential differential effects across low, moderate, and high exposure levels.
Covariates
The control variables in this study included sociodemographic characteristics, lifestyle characteristics, and other health characteristics. Sociodemographic characteristics encompassed age, gender, marital status, education level, and family poverty-to-income ratio (PIR, i.e., the ratio of family income to the poverty line). Lifestyle characteristics included smoking status (whether smoking more than 100 cigarettes). They also included physical activity level, which was assessed based on the WHO-recommended Global Physical Activity Questionnaire guidelines. The threshold was 150 min of moderate-intensity activity, 75 min of high-intensity activity, or 600 metabolic equivalents (METs) per week. Additionally, they included dietary intake status, which assessed daily intake of nine types of food. This study also assessed body mass index (BMI), as well as the presence of dyslipidemia, hypertension, diabetes, and hyperuricemia as other health characteristics. Dyslipidemia was defined as fulfillment of at least one of the following conditions: (1) hypercholesterolemia (total cholesterol ≥ 5.18 mmol/L); (2) hypertriglyceridemia (triglycerides ≥ 1.70 mmol/L); (3) low-density lipoprotein cholesterol (LDL-C) ≥ 3.37 mmol/L; (4) high-density lipoprotein cholesterol (HDL-C) < 1.04 mmol/L (20). Hypertension was defined as an average systolic blood pressure > 140 mmHg or an average diastolic blood pressure > 90 mmHg, or a physician diagnosis of hypertension (21). Diabetes was defined as a fasting blood glucose level ≥ 7.0 mmol/L or a 2-h postprandial blood glucose level ≥ 11.1 mmol/L, or a physician diagnosis of diabetes (22, 23). Hyperuricemia was defined as a serum uric acid level above 420 μmol/L (7 mg/dL) for males and above 357 μmol/L (6 mg/dL) for females (24). These characteristics are detailed in Table 1.
Statistical analysis
For continuous variables exhibiting non-normal distributions, such as ACR, age, PIR, BMI, and dietary intake, P50 (P25, P75) was used for statistical descriptive analysis. For categorical variables, including gender, marital status, education level, smoking status, physical activity level, and the presence of dyslipidemia, hypertension, diabetes, and hyperuricemia, frequency (proportion) was used for statistical descriptive analysis. To determine the differences in ACR distribution across sociodemographic characteristics, Spearman tests, Mann–Whitney U tests, and Kruskal-Wallis tests were employed. Spearman tests were used for correlation analysis. Mann–Whitney U tests were used for rank sum tests between two groups, and Kruskal-Wallis H tests were used for rank sum tests among multiple groups. After controlling for sociodemographic, lifestyle, and other health characteristics, quantile regression models were used to explore the association between synthetic EDs and renal function, and grouped regression models were used to analyze the differential association between synthetic EDs and renal function in CKD and non-CKD groups. All continuous variables were transformed for normality before being included in the regression models. All tests were based on two-sided tests with a 95% confidence interval.
Results
Participant characteristics
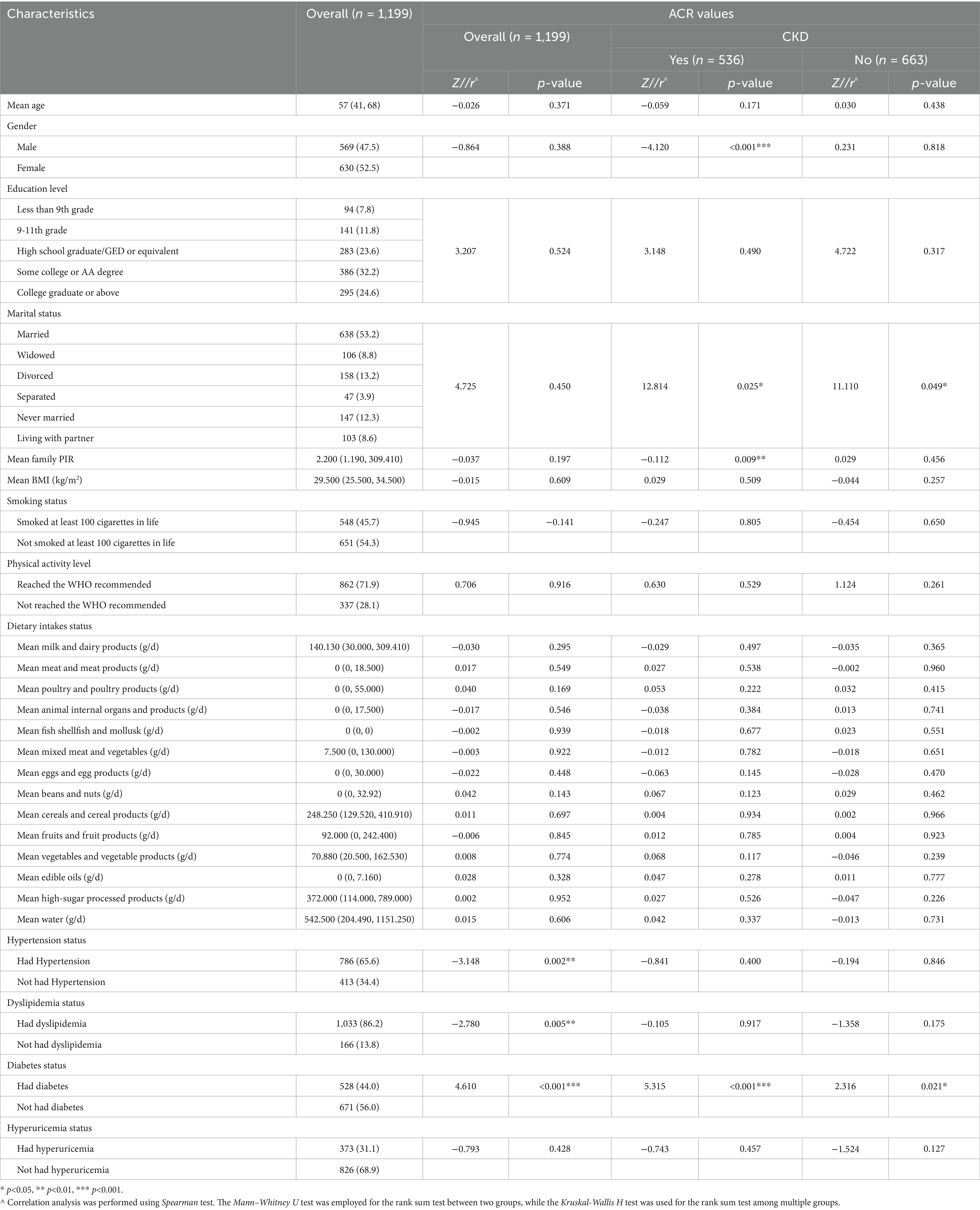
The basic characteristics of the participants are shown in Table 1. About half were female (52.5%), 24.6% had a college or associate degree or higher, 53.2% were married, 45.7% had smoked at least 100 cigarettes in their lifetime, and 71.9% met the WHO-recommended physical activity level. The median age was 57 years (P50), with P25 at 41 and P75 at 68 years. The median PIR was 2.200 (P50), with P25 at 1.190 and P75 at 309.410. Additionally, the median BMI was 29.5 (P50), with P25 at 25.5 and P75 at 34.5. Over half had hypertension (65.6%), 86.2% had dyslipidemia, 44.0% had diabetes, 31.1% had hyperuricemia, and 44.7% had CKD. The median ACR was 8.160 (P50), with P25 at 5.040 and P75 at 20.170.
Table 1 provides a detailed distribution of ACR values among participants. The study demonstrated significant correlations between the prevalence of hypertension, dyslipidemia, and diabetes mellitus and renal function decline, as well as ACR values. The Z-values were −3.148, −2.780, and 4.610, respectively (p < 0.01). In the CKD group, multivariate analysis revealed significant associations between gender, marital status, family PIR, diabetes status, and ACR values (p < 0.05). In non-CKD participants, only marital status and diabetes were significantly associated with ACR levels (p < 0.05).
Association between synthetic endocrine disruptors and renal function
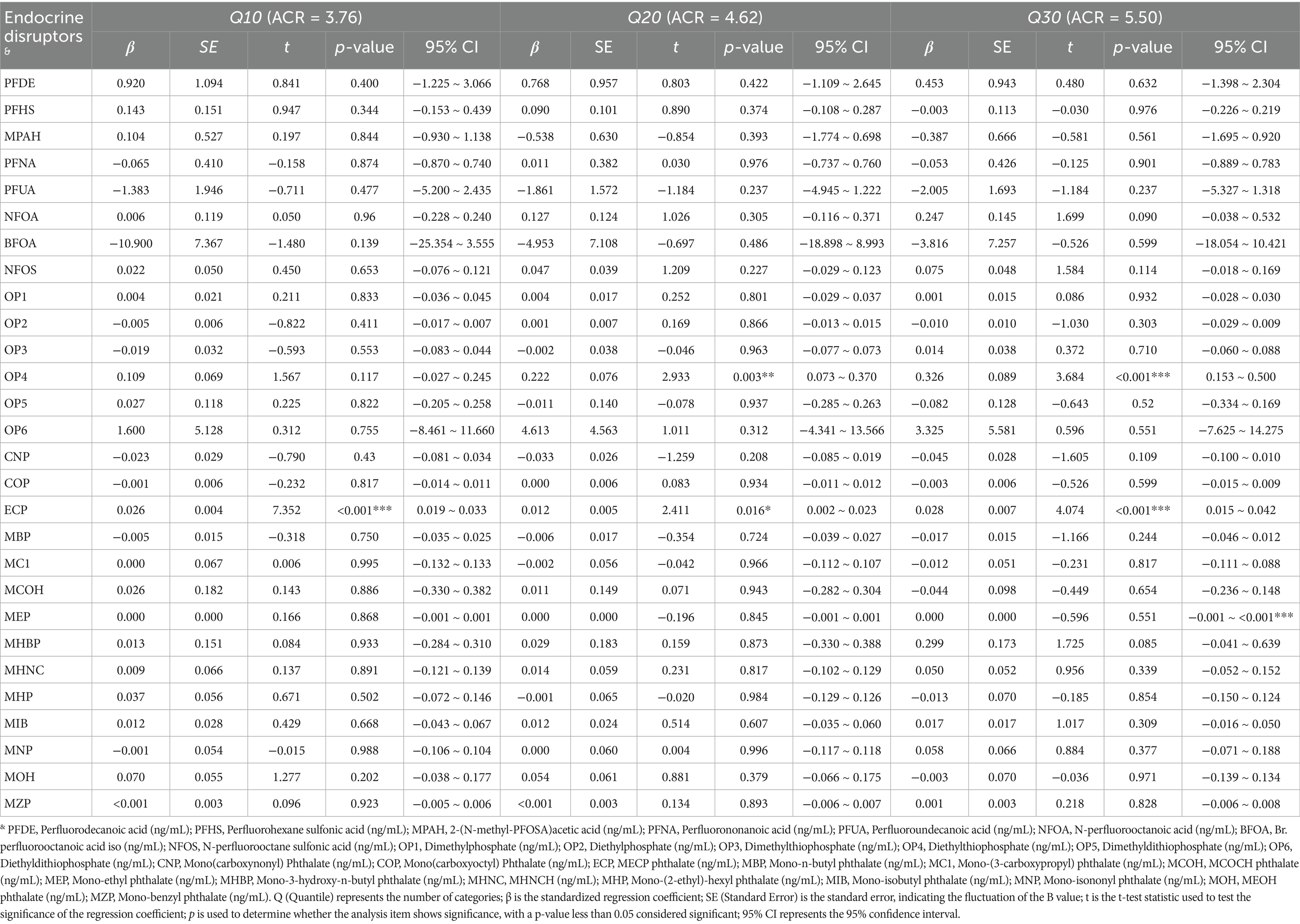
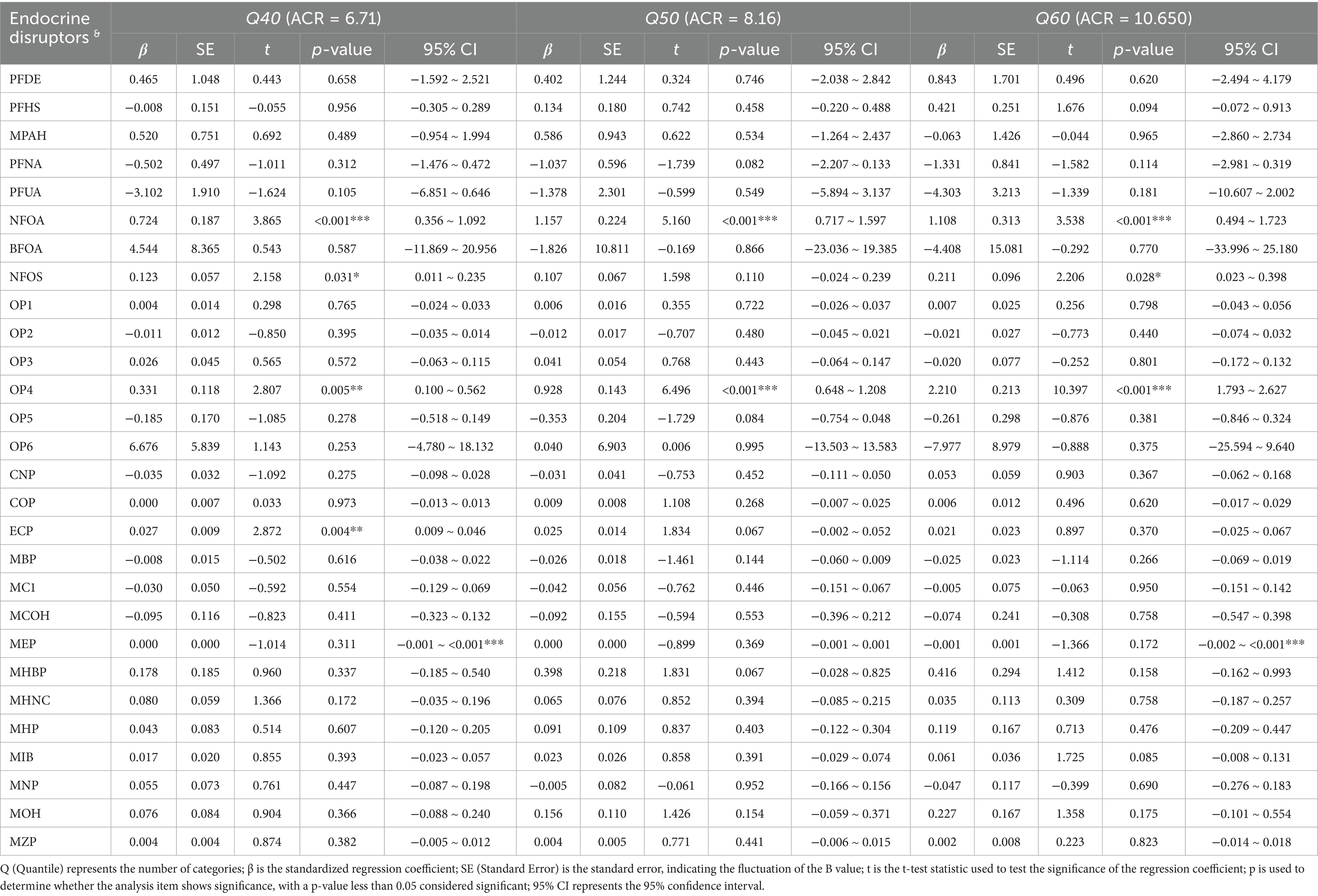
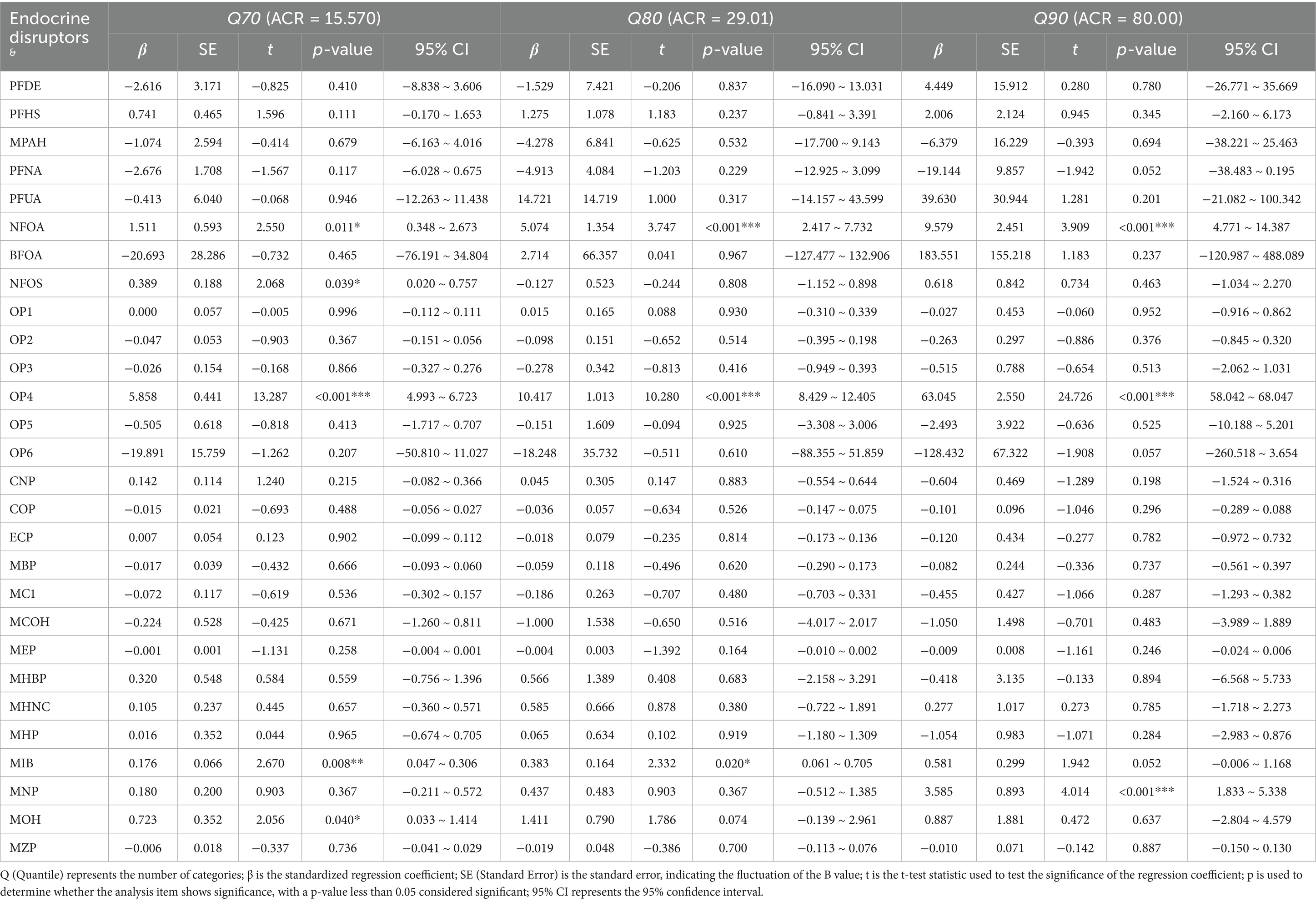
The comprehensive association between synthetic EDs and renal function is presented in Tables 2–4. Tables 2–4 show that N-perfluorooctanoic acid and N-perfluorooctane sulfonic acid among perfluoro/polyfluoroalkyl compounds, Diethylthiophosphate among phosphorus-containing pesticides, and MECP phthalate, Mono-isobutyl phthalate, Mono-isononyl phthalate, and MEOH phthalate among plasticizers were associated with renal function impairment (p < 0.05).
N-perfluorooctanoic acid was associated with renal function impairment when ACR values were at Q40 or above (p < 0.05). The damaging effect increased with increasing ACR values. Specifically, at ACR Q40, β = 0.724 (95% CI: 0.356, 1.092). At ACR Q50, β = 1.157 (95% CI: 0.717, 1.597). At ACR Q60, β = 1.108 (95% CI: 0.494, 1.723). At ACR Q70, β = 1.511 (95% CI: 0.348, 2.673). At ACR Q80, β = 5.074 (95% CI: 2.417, 7.732). At ACR Q90, β = 9.579 (95% CI: 4.771, 14.387). Diethylthiophosphate was also associated with renal function impairment when ACR values were above Q20 (p < 0.05). The damaging effect increased with increasing ACR values. Specifically, at ACR Q20, β = 0.222 (95% CI: 0.073, 0.370). At ACR Q30, β = 0.326 (95% CI: 0.153, 0.500). At ACR Q40, β = 0.331 (95% CI: 0.100, 0.562). At ACR Q50, β = 0.928 (95% CI: 0.648, 1.208). At ACR Q60, β = 2.210 (95% CI: 1.793, 2.627). At ACR Q70, β = 5.858 (95% CI: 4.993, 6.723). At ACR Q80, β = 10.417 (95% CI: 8.429, 12.405). And at ACR Q90, β = 63.045 (95% CI: 58.042, 68.047). Both N-perfluorooctanoic acid and Diethylthiophosphate exhibited gradually increasing damaging effects on renal function with increasing ACR values.
MECP phthalate was associated with renal function impairment when ACR values were below Q40 (p < 0.05). Specifically, at ACR Q10, β = 0.026 (95% CI: 0.019, 0.033). At ACR Q20, β = 0.012 (95% CI: 0.002, 0.023). At ACR Q30, β = 0.028 (95% CI: 0.015, 0.042). At ACR Q40, β = 0.027 (95% CI: 0.009, 0.046).
Additionally, N-perfluorooctane sulfonic acid was associated with renal function impairment in individuals with ACR values at Q40, Q60, and Q70 (p < 0.05). Specifically, at ACR Q40, β = 0.123 (95% CI: 0.011, 0.235). At ACR Q60, β = 0.211 (95% CI: −0.023, 0.398). And at ACR Q70, β = 0.389 (95% CI: 0.020, 0.757). Mono-isobutyl phthalate was associated with renal function impairment in individuals with ACR values at Q70 and Q80 (p < 0.05). Specifically, at ACR Q70, β = 0.176 (95% CI: 0.047, 0.306). At ACR Q80, β = 0.383 (95% CI: 0.061, 0.705). Mono-isononyl phthalate was associated with renal function impairment in individuals with ACR values at Q90 (p < 0.05), with β = 3.585 (95% CI: 1.833, 5.338). MEOH phthalate was associated with renal function impairment in individuals with ACR values at Q70 (p < 0.05), with β = 0.723 (95% CI: 0.033, 1.414).
The experimental results of this study demonstrate that certain per- and polyfluoroalkyl substances (PFAS) and multiple plasticizers can adversely affect renal function. PFAS may impair kidney function partly because of their high chemical stability, which facilitates accumulation within renal tissues. This bioaccumulation can disrupt critical signaling pathways and metabolic processes, such as those involving fatty acid-binding proteins, peroxisome proliferator-activated receptors (PPARs) and estrogen receptors. Plasticizers, which are known to cause endocrine disruption and hepatotoxicity while potentially promoting diabetes and hyperuricemia, may indirectly influence renal function through complex physiological mechanisms. Additionally, some organophosphate pesticides were found to be nephrotoxic, likely due to their effects on cholinergic signaling, membrane toxicity, and pro-oxidative pathways, all of which may contribute to renal dysfunction.
Differential association between synthetic endocrine disruptors and renal function: non-CKD vs. CKD
This study further explored the differential association between synthetic EDs and renal function in non-CKD and CKD groups. The details are shown in Table 5. The association between synthetic EDs and renal function differed between non-CKD and CKD groups, with Chow Test results showing F = 3.258, p < 0.001. As shown in Table 5, N-perfluorooctanoic acid had different associations with renal function in non-CKD and CKD groups. The difference was B1-B2 = −3.307, p = 0.004. Specifically, in the non-CKD group, N-perfluorooctanoic acid showed no association with renal function impairment in the non-CKD group (β = 0.158, p = 0.029). However, in the CKD group, it was associated with impairment in the CKD group (β = 0.007, p = 0.899). MECP phthalate also had a differential association with renal function in non-CKD and CKD groups. The difference was B1-B2 = 0.176, p = 0.002. Specifically, in the non-CKD group, MECP phthalate was associated with renal function impairment in the non-CKD group (β = 0.163, p = 0.006). In the CKD group, it was not associated with renal function (β = −0.024, p = 0.852). The damaging effects of N-perfluorooctanoic acid in perfluoro/polyfluoroalkyl compounds and diethylthiophosphate in organophosphate pesticides on renal function exhibit a progressively increasing trend. In contrast, MECP phthalate from plasticizers primarily exerts certain negative effects on individuals whose renal function has not yet suffered severe impairment. N-perfluorooctanoic acid is especially harmful to the renal function of chronic CKD patients. In contrast, MECP phthalate more readily impairs the renal function of non-CKD individuals.
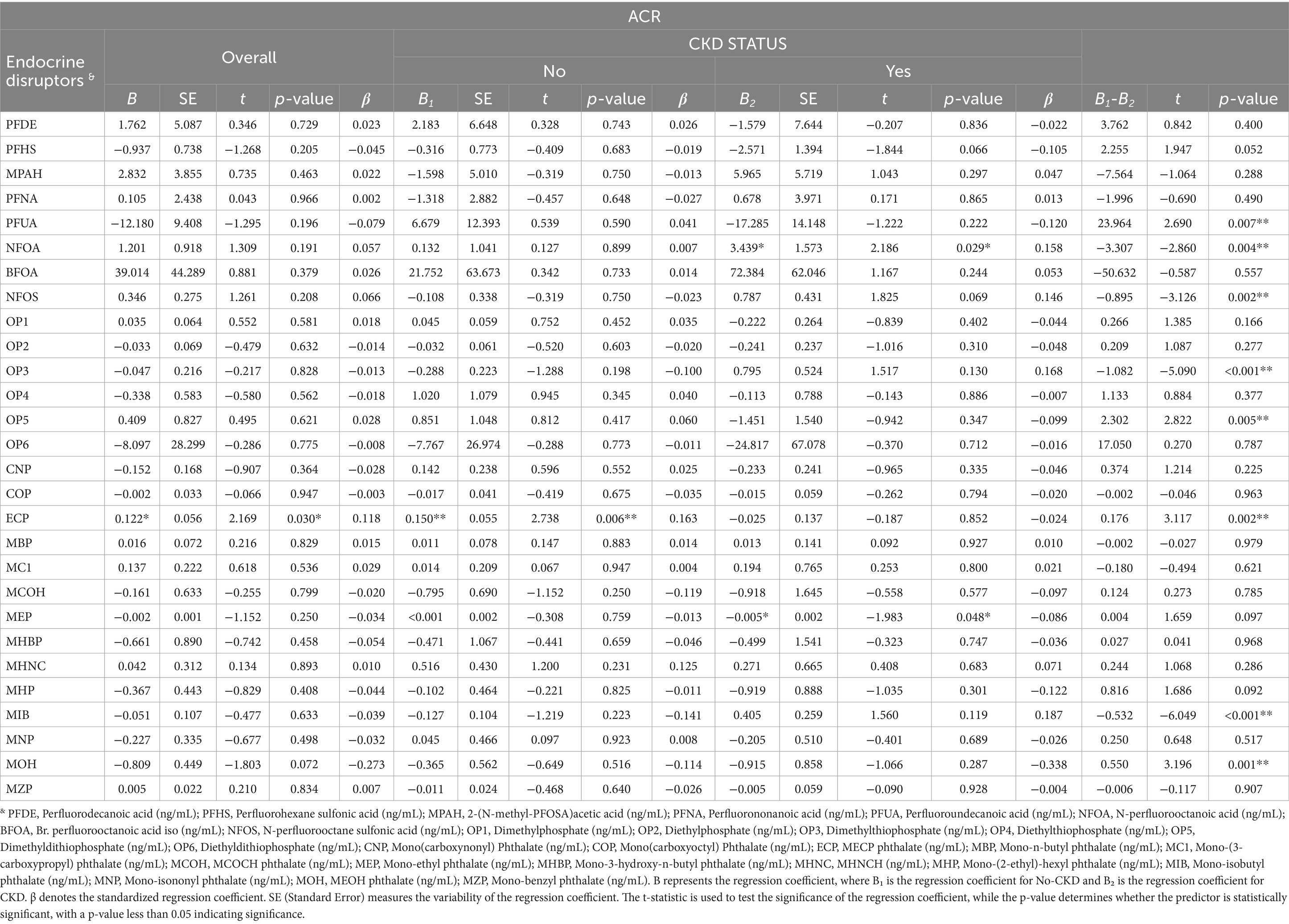
Table 5. Synthetic endocrine disruptors association with renal function: non-CKD vs. CKD differences.
Discussion
This study delved into the complex relationship between three types of synthetic EDs and renal function. It showed how the association between synthetic EDs and renal function varies with different levels of kidney impairment. This helps us better understand kidney toxicity caused by synthetic EDs. The study found that some chemicals were linked to kidney function damage. These include N-perfluorooctanoic acid and N-perfluorooctane sulfonic acid (from perfluoro/polyfluoroalkyl compounds), Diethylthiophosphate (from phosphorus-containing pesticides), and MECP phthalate, Mono-isobutyl phthalate, Mono-isononyl phthalate, and MEOH phthalate (from plasticizers). The damage levels varied. Further analysis revealed significant differences in the damaging effects between non-CKD and CKD patients. N-perfluorooctanoic acid was significantly associated with renal function impairment in CKD patients. MECP phthalate showed a stronger association with renal dysfunction in non-CKD individuals. This study found that certain perfluoro/polyfluoroalkyl compounds and various plasticizers were associated with renal function impairment. This finding is consistent with previous epidemiological and animal studies, which have observed harmful effects of perfluoro/polyfluoroalkyl compounds on renal function (13–16). These compounds may also be indirectly associated with renal function through a series of complex physiological mechanisms (25–28).
Epidemiological studies have shown that once absorbed into the human body, these compounds primarily accumulate in the serum, liver, and kidney. Previous studies have found that perfluoroalkyl compounds are more likely to accumulate in the kidney than in other tissues (29). The link between perfluoro/polyfluoroalkyl substances and kidney function damage may be because they are very stable chemically. This stability makes them build up in kidney tissues. This accumulation can interfere with various critical signaling pathways and metabolic processes, including fatty acid-binding proteins, peroxisome proliferator-activated receptors and estrogen receptors. These disruptions can cause abnormal fat metabolism, more oxidative stress, and worse inflammation in kidney cells. This can damage kidney tissue structure and affect how the kidneys filter blood and reabsorb substances, leading to renal function decline or failure (30–34). The way plasticizers may be linked to kidney function damage could be by activating biochemical processes. These include problems with mitochondria, DNA damage, abnormal cell growth, and abnormal protein structure. This can damage kidney structure and function, leading to kidney tissue damage and reduced function (35).
Furthermore, our study identified an association between specific organophosphorus pesticides and renal function impairment. This finding aligns with existing evidence demonstrating that exposure to organophosphorus compounds may induce both acute and chronic nephrotoxicity, potentially progressing to renal dysfunction or even end-stage renal disease (36, 37). The possible reasons for this include the ability of phosphorus-containing pesticides to interfere with normal serum albumin metabolism, induce hypoxia, trigger oxidative stress, accumulate lipid peroxidation products, disrupt enzyme system functions, and abnormally activate MAPK signaling pathways. They do this through biological pathways like cholinergic reactions, membrane toxicity, and pro-oxidant reactions. This can ultimately cause kidney tube cell death and kidney function damage (38).
This study revealed significant differences in the damaging effects of various types of synthetic EDs on renal function. Specifically, as the degree of kidney impairment increased, the damaging effects of N-perfluorooctanoic acid among perfluoro/polyfluoroalkyl compounds and Diethylthiophosphate among phosphorus-containing pesticides on renal function exhibited a gradually increasing trend. In contrast, MECP phthalate among plasticizers was primarily associated with adverse outcomes in individuals with less severe renal function impairment. N-perfluorooctanoic acid was especially harmful to the renal function of patients with CKD. While MECP phthalate was more likely to impair the renal function of individuals without CKD (non-CKD).
This difference in effect may be because N-perfluorooctanoic acid has a similar structure to fatty acids. It is also very stable and tends to build up in organs with a lot of fat, like the kidney. This can interfere with important enzymes or receptors in fat metabolism pathways. N-perfluorooctanoic acid affects energy metabolism and the stability of cell membranes in kidney cells. This leads to a lack of energy for the cells and damage to the cell membrane structure. Ultimately, it harms normal kidney function. When kidney cell damage is mild, N-perfluorooctanoic acid builds up less and its effect is weaker. The body’s ability to repair itself is strong. But when kidney cell damage gets worse, N-perfluorooctanoic acid builds up more and the body’s ability to regenerate declines. This makes the harmful effects worse (39). In contrast, MECP phthalate has a relatively stable structure. It mainly affects the normal functions of kidney cells by influencing cell signaling pathways or gene expression. But it builds up less in the kidneys. This leads to a different pattern of kidney toxicity compared to N-perfluorooctanoic acid.
This study has several limitations in exploring the potential association between synthetic EDs and renal function. Firstly, the assessment of synthetic EDs exposure primarily relies on retrospective data or surrogate biomarkers (such as urine markers). Due to the lack of comprehensive exposure history and time-related data, these methods may not accurately capture an individual’s long-term exposure levels. This makes it hard to precisely quantify the dose–response relationship between synthetic EDs exposure and renal function. Secondly, since this is a cross-sectional study, it cannot prove a definite cause-and-effect relationship between synthetic EDs exposure and the observed kidney function damage in CKD and its related conditions. It also cannot completely rule out the possibility of reverse causality or other influencing factors. To elucidate this potential causal relationship, future studies should employ longitudinal cohort studies or randomized controlled trials. Thirdly, the study population may not fully represent the CKD patient group, particularly in terms of demographic characteristics, socioeconomic status, and lifestyle. To address this, future studies should expand the sample size, include more diverse populations, and adequately adjust for potential confounding factors. Finally, this study did not delve into the potential synergistic or antagonistic effects of synthetic EDs on renal function. Future research should fully consider how synthetic EDs interact with each other and how they together affect kidney function outcomes. It should also explore the biological mechanisms behind this.
Conclusion
This study explored the association between synthetic EDs, particularly perfluoro/polyfluoroalkyl compounds (such as N-perfluorooctanoic acid and N-perfluorooctane sulfonic acid), phosphorus-containing pesticides (such as Diethylthiophosphate), and various plasticizers (including MECP phthalate, Mono-isobutyl phthalate, Mono-isononyl phthalate, and MEOH phthalate), with individual renal function. The results indicate that these synthetic EDs have varying degrees of association with renal function, with distinct differences in their effects. Our analysis revealed that distinct patterns of N-perfluorooctanoic exposure were significantly associated with renal impairment in CKD patients. In contrast, MECP showed associations with renal dysfunction in non-CKD individuals. Therefore, for CKD patients, to prevent further kidney damage from harmful substances like N-perfluorooctanoic acid, we should take comprehensive measures. This includes better medical monitoring of CKD patients, assessing their risk of exposure to products with N-perfluorooctanoic acid, and promoting health education to raise their awareness of harmful substances. This will help them actively avoid or reduce exposure to these substances and protect their kidney health.
This study systematically examined the specific link between various synthetic EDs and individual renal function. These synthetic EDs include perfluoro/polyfluoroalkyl compounds (like N-perfluorooctanoic acid and N-perfluorooctane sulfonic acid), phosphorus-containing pesticides (like Diethylthiophosphate), and various plasticizers (like MECP phthalate, Mono-isobutyl phthalate, Mono-isononyl phthalate, and MEOH phthalate). Our findings demonstrate significant but heterogeneous associations between these synthetic EDs and renal function parameters. Specifically, N-perfluorooctanoic acid was primarily associated with renal function impairment in CKD patients, while MECP phthalate was primarily associated with renal function impairment in non-CKD individuals. In light of this, for CKD patient groups, to alleviate the further harm associated with N-perfluorooctanoic acid to renal function, a comprehensive assessment of CKD patients should be strengthened, meticulously analyzing their risk of exposure to products containing N-perfluorooctanoic acid is needed. Through systematic health education, we should increase the self-protection awareness of CKD patients. This will help them actively avoid or reduce exposure to these substances and effectively protect their kidney health.
In summary, to reduce the potential harm of synthetic endocrine disruptors like MECP phthalate to kidney health, we should widely spread knowledge about the dangers of harmful substances like MECP phthalate through media and the internet. This will raise public awareness of self-protection. Simultaneously, relevant authorities should strengthen market supervision, particularly over daily plastic products that are likely to contain MECP phthalate, ensuring that products on the market comply with safety standards to safeguard consumer health. Additionally, promoting an eco-friendly and safe lifestyle is important. We should encourage the public to use fewer disposable plastic products that may contain harmful plasticizers and choose environmentally friendly alternatives. This will reduce exposure risks to hazardous substances like MECP phthalate at the source and collectively protect kidney health.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistic. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JM: Funding acquisition, Writing – original draft, Writing – review & editing. YL: Writing – review & editing. LH: Writing – review & editing. MM: Writing – original draft. MZ: Writing – original draft. XW: Supervision, Writing – review & editing. DM: Conceptualization, Funding acquisition, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was also supported by the Scientific and Technologial Innovation Programs of Higher Education Institutions in Shanxi (2023L065), Scientific and Technologial Innovation Programs of Higher Education Institutions in Shanxi (2023L064), Fundamental Research Program of Shanxi Province (202303021222162), Fundamental Research Program of Shanxi Province (202403021222233), Philosophy and Social Sciences Planning Project of Shanxi Province (2024QN048), the Postdoctoral Research Fund of Shanxi Bethune Hospital (388172).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ammirati, AL. Chronic renal disease. Rev Assoc Med Bras. (2020) 66:s03–9. doi: 10.1590/1806-9282.66.S1.3
2. Chesnaye, NC, Ortiz, A, Zoccali, C, Stel, VS, and Jager, KJ. The impact of population ageing on the burden of chronic renal disease. Nat Rev Nephrol. (2024) 20:569–85. doi: 10.1038/s41581-024-00863-9
3. Francis, A, Harhay, MN, Ong, ACM, Tummalapalli, SL, Ortiz, A, Fogo, AB, et al. Chronic renal disease and the global public health agenda: an international consensus. Nat Rev Nephrol. (2024) 20:473–85. doi: 10.1038/s41581-024-00820-6
4. Parameswaran, S, Rinu, PK, Kar, SS, Harichandrakumar, KT, James, TD, Priyamvada, PSP, et al. A newly recognized endemic region of CKD of undetermined etiology (CKDu) in South India Tondaimandalam nephropathy. Kidney Int Rep. (2020) 5:2066–73. doi: 10.1016/j.ekir.2020.08.032
5. John, O, Gummudi, B, Jha, A, Gopalakrishnan, N, Kalra, OP, Kaur, P, et al. Chronic renal disease of unknown etiology in India: what do we know and where we need to go. Kidney Int Rep. (2021) 6:2743–51. doi: 10.1016/j.ekir.2021.07.031
6. Autrup, H, Barile, FA, Berry, SC, Blaauboer, BJ, Boobis, A, Bolt, H, et al. Human exposure to synthetic endocrine disrupting chemicals (S-endocrine disruptors) is generally negligible as compared to natural compounds with higher or comparable endocrine activity. How to evaluate the risk of the S-endocrine disruptors? Environ Toxicol Pharmacol. (2020) 78:103396. doi: 10.1016/j.etap.2020.103396
7. Cano, R, Pérez, JL, Dávila, LA, Ortega, Á, Gómez, Y, Valero-Cedeño, NJ, et al. Role of endocrine-disrupting chemicals in the pathogenesis of non-alcoholic fatty liver disease: a comprehensive review. Int J Mol Sci. (2021) 22:4807. doi: 10.3390/ijms22094807
8. Kabir, ER, Rahman, MS, and Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol. (2015) 40:241–58. doi: 10.1016/j.etap.2015.06.009
9. Alabdulmohsen, DM, AlDeaiji, LA, Abdul Hai, UA, Ghazwani, MY, Alsulaim, KM, Alanazi, RH, et al. Lifestyle and chemicals: exploring behavioral habits related to endocrine disruptor exposure among the general population of Saudi Arabia. Cureus. (2024) 16:e64392. doi: 10.7759/cureus.64392
10. Mitra, T, Gulati, R, Ramachandran, K, Rajiv, R, Enninga, EAL, Pierret, CK, et al. Endocrine disrupting chemicals: gestational diabetes and beyond. Diabetol Metab Syndr. (2024) 16:95. doi: 10.1186/s13098-024-01317-9
11. Di Credico, A, Gaggi, G, Bucci, I, Ghinassi, B, and Di Baldassarre, A. The effects of combined exposure to bisphenols and Perfluoroalkyls on human perinatal stem cells and the potential implications for health outcomes. Int J Mol Sci. (2023) 24:15018. doi: 10.3390/ijms241915018
12. Rahimlou, M, Mousavi, MA, Chiti, H, Peyda, M, and Mousavi, SN. Association of maternal exposure to endocrine disruptor chemicals with cardio-metabolic risk factors in children during childhood: a systematic review and meta-analysis of cohort studies. Diabetol Metab Syndr. (2024) 16:82. doi: 10.1186/s13098-024-01320-0
13. Blake, BE, Pinney, SM, Hines, EP, Fenton, SE, and Ferguson, KK. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, renal function, and body mass index in the Fernald community cohort. Environ Pollut. (2018) 242:894–904. doi: 10.1016/j.envpol.2018.07.042
14. Jain, RB, and Ducatman, A. Perfluoroalkyl acids serum concentrations and their relationship to biomarkers of renal failure: serum and urine albumin, creatinine, and albumin creatinine ratios across the spectrum of glomerular function among US adults. Environ Res. (2019) 174:143–51. doi: 10.1016/j.envres.2019.04.034
15. Stanifer, JW, Stapleton, HM, Souma, T, Wittmer, A, Zhao, X, and Boulware, LE. Perfluorinated chemicals as emerging environmental threats to renal health: a scoping review. Clin J Am Soc Nephrol. (2018) 13:1479–92. doi: 10.2215/CJN.04670418
16. Blake, BE, and Fenton, SE. Early life exposure to per- and polyfluoroalkyl substances (FAS) and latent health outcomes: a review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology. (2020) 443:152565. doi: 10.1016/j.tox.2020.152565
17. KDIGO. (2023). Renal disease improving global outcomes guidelines. Available online at: www.kdigo.org/2023. (Accessed November 1, 2024).
18. Chen, TK, Knicely, DH, and Grams, ME. Chronic renal disease diagnosis and management: a review. JAMA. (2019) 322:1294–304. doi: 10.1001/jama.2019.14745
19. Pethő, ÁG, Tapolyai, M, Csongrádi, É, and Orosz, P. Management of chronic renal disease: the current novel and forgotten therapies. J Clin Transl Endocrinol. (2024) 36:100354. doi: 10.1016/j.jcte.2024.100354
20. Ba, Y, Guo, Q, Meng, S, Tong, G, He, Y, Guan, Y, et al. Association of exposures to serum terpenes with the prevalence of dyslipidemia: a population-based analysis. Environ Sci Pollut Res Int. (2023) 30:115295–309. doi: 10.1007/s11356-023-30546-0
21. Hein, M, Lanquart, JP, Loas, G, Hubain, P, and Linkowski, P. Risk of high blood pressure associated with objective insomnia and self-reported insomnia complaints in major depression: a study on 703 individuals. Clin Exp Hypertens. (2019) 41:538–47. doi: 10.1080/10641963.2018.1516775
22. Memish, ZA, Chang, JL, Saeedi, MY, Al Hamid, MA, Abid, O, and Ali, MK. Screening for type 2 diabetes and Dysglycemia in Saudi Arabia: development and validation of risk scores. Diabetes Technol Ther. (2015) 17:693–700. doi: 10.1089/dia.2014.0267
23. Li, W, Jiao, Y, Wang, L, Wang, S, Hao, L, Wang, Z, et al. Association of Serum Magnesium with insulin resistance and type 2 diabetes among adults in China. Nutrients. (2022) 14:1799. doi: 10.3390/nu14091799
24. Rong, J, Fang, C, Chen, X, Hong, C, and Huang, L. Association of serum uric acid with prognosis in patients with myocardial infarction: an update systematic review and meta-analysis. BMC Cardiovasc Disord. (2023) 23:512. doi: 10.1186/s12872-023-03523-1
25. Routti, H, Diot, B, Panti, C, Duale, N, Fossi, MC, Harju, M, et al. Contaminants in Atlantic walruses in Svalbard part 2: relationships with endocrine and immune systems. Environ Pollut. (2019) 246:658–67. doi: 10.1016/j.envpol.2018.11.097
26. Zeng, XW, Bloom, MS, Dharmage, SC, Lodge, CJ, Chen, D, Li, S, et al. Prenatal exposure to perfluoroalkyl substances is associated with lower hand, foot and mouth disease viruses antibody response in infancy: findings from the Guangzhou birth cohort study. Sci Total Environ. (2019) 663:60–7. doi: 10.1016/j.scitotenv.2019.01.325
27. Wang, G, Sun, S, Wu, X, Yang, S, Wu, Y, Zhao, J, et al. Intestinal environmental disorders associate with the tissue damages induced by perfluorooctane sulfonate exposure. Ecotoxicol Environ Saf. (2020) 197:110590. doi: 10.1016/j.ecoenv.2020.110590
28. Maddela, NR, Kakarla, D, Venkateswarlu, K, and Megharaj, M. Additives of plastics: entry into the environment and potential risks to human and ecological health. J Environ Manag. (2023) 348:119364. doi: 10.1016/j.jenvman.2023.119364
29. Pérez, F, Nadal, M, Navarro-Ortega, A, Fàbrega, F, Domingo, JL, Barceló, D, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. (2013) 59:354–62. doi: 10.1016/j.envint.2013.06.004
30. Guo, S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. (2014) 220:T1–T23. doi: 10.1530/JOE-13-0327
31. Genser, L, Casella Mariolo, JR, Castagneto-Gissey, L, Panagiotopoulos, S, and Rubino, F. Obesity, type 2 diabetes, and the metabolic syndrome: pathophysiologic relationships and guidelines for surgical intervention. Surg Clin North Am. (2016) 96:681–701. doi: 10.1016/j.suc.2016.03.013
32. Lilienthal, H, Dieter, HH, Hölzer, J, and Wilhelm, M. Recent experimental results of effects of perfluoroalkyl substances in laboratory animals - relation to current regulations and guidance values. Int J Hyg Environ Health. (2017) 220:766–75. doi: 10.1016/j.ijheh.2017.03.001
33. Kvandová, M, Majzúnová, M, and Dovinová, I. The role of PPARgamma in cardiovascular diseases. Physiol Res. (2016) 65:S343–63. doi: 10.33549/physiolres.933439
34. Kersten, S, and Stienstra, R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie. (2017) 136:75–84. doi: 10.1016/j.biochi.2016.12.019
35. Yadav, R, Kumar, D, Singh, J, and Jangra, A. Environmental toxicants and nephrotoxicity: implications on mechanisms and therapeutic strategies. Toxicology. (2024) 504:153784. doi: 10.1016/j.tox.2024.153784
36. Cavari, Y, Landau, D, Sofer, S, Leibson, T, and Lazar, I. Organophosphate poisoning-induced acute renal failure. Ped Emerg Care. (2013) 29:646–7. doi: 10.1097/PEC.0b013e31828e9e45
37. Zafar, R, Munawar, K, Nasrullah, A, Haq, S, Ghazanfar, H, Sheikh, AB, et al. Renal failure due to organophosphatepoisoning: a case report. Cureus. (2017) 9:e1523. doi: 10.7759/cureus.1523
38. Sobolev, VE, Sokolova, MO, Jenkins, RO, and Goncharov, NV. Molecular mechanisms of acute organophosphate nephrotoxicity. Int J Mol Sci. (2022) 23:8855. doi: 10.3390/ijms23168855
Keywords: synthetic endocrine disruptors, renal function, perfluoro/polyfluoroalkyl compounds, phosphorus-containing pesticides, plasticizers
Citation: Mu J, Li Y, He L, Ma M, Zhang M, Wang X and Mao D (2025) Differential associations between synthetic endocrine disruptors and renal function impairment across varying degrees in middle-aged and older adult populations. Front. Public Health. 13:1526070. doi: 10.3389/fpubh.2025.1526070
Edited by:
Jiao Lu, Xi’an Jiaotong University, ChinaReviewed by:
Brigitte Le Magueresse-Battistoni, INSERM U1060 Laboratoire de Recherche en Cardiovasculaire, Métabolisme, Diabétologie et Nutrition, FranceImran Tipu, University of Management and Technology, Pakistan
Copyright © 2025 Mu, Li, He, Ma, Zhang, Wang and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wang, V2FuZ3hpbjk4NEAxNjMuY29t; Danhui Mao, Nzg0NTgxMjIzQHFxLmNvbQ==
 Junfang Mu1
Junfang Mu1 Danhui Mao
Danhui Mao