- 1School of Health Sciences Research, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand
- 2Environmental, Occupational Health Sciences and NCD Research Group, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand
- 3Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand
- 4Research Center for Molecular and Cell Biology, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand
- 5Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Introduction: Burning-related air pollution is a recurrent seasonal problem in Chiang Mai, Thailand, from March to May. Exposure has been linked to pulmonary damage and oxidative stress, measurable via serum Club Cell Protein 16 (CC16) and 8-Iso-prostaglandin F2α (8-iso-PGF2α). Polycyclic aromatic hydrocarbons (PAHs), especially 1-hydroxypyrene (1-OHP), are emitted during incomplete combustion and may contribute to diabetes and hypertension through oxidative pathways. Few studies have examined how PAH exposure from seasonal air pollution affects lung function biomarkers in individuals with these conditions in this region.
Methods: A prospective cohort study was conducted in three Chiang Mai locations, following 127 participants with diabetes and/or hypertension during the before-burning (December 2023) and after-burning (May 2024) periods. Urinary 1-OHP measured PAH exposure, while serum CC16 and 8-iso-PGF2α assessed pulmonary damage and oxidative stress. Structured questionnaires captured participant characteristics and symptoms. Quantitative health-risk assessment (QHRA) converted 1-OHP to benzo[a]pyrene-equivalent doses for estimating lifetime cancer risk (LCR) and hazard quotient (HQ).
Results: From before- to after-burning, cohort-wide means increased significantly: urinary 1-OHP (0.24 ± 0.05 to 0.90 ± 1.21 μmol/mol Cre; p < 0.01), serum CC16 (63.15 ± 25.74 to 101.31 ± 48.14 ng/ml; p < 0.01), and serum 8-iso-PGF2α (47.44 ± 19.91 to 52.51 ± 20.56 ng/ml; p < 0.01). Stratified by comorbidity, hypertensive participants showed a greater 1-OHP increase (0.25 ± 0.35 to 1.31 ± 1.67; p < 0.01), while diabetic participants had larger CC16 rises (62.39 ± 18.96 to 93.23 ± 29.92; p < 0.01). Among diabetics in the after-burning period, those reporting skin irritation or shortness of breath had lower urinary 1-OHP than asymptomatic peers (0.50 vs. 1.14 and 0.48 vs. 1.08 μmol/mol Cre; p = 0.049 and 0.036, respectively). QHRA showed that during the burning season, several age–sex–disease subgroups exceeded the 1 × 10−4 LCR benchmark, notably men ≥50 years and some women >60 years, while all HQs remained < 1.
Conclusion: After burning in Chiang Mai substantially increases PAH exposure, pulmonary injury markers, and oxidative stress in individuals with diabetes and hypertension, with differential effects by comorbidity. Cancer risk thresholds were exceeded in older subgroups despite HQs below non-cancer hazard levels, highlighting the need for targeted protection strategies during burn seasons.
1 Introduction
Worldwide, environmental pollution, which damaged physical health (PH), could not be ignored (1). Particulate matter (PM2.5) was a major content because of the emission of vehicle exhaust and burning crop residues (grain maize, seed maize, and integrated farming) (2). Particulate matter (PM2.5) pollution was a major problem in Thailand, Chiangmai, because farmers burn crop residues seasonally from March to May every year in highland agricultural systems (3). Chiangmai was surrounded by mountains, and air pollution from burning crops has caused many health issues in people's lives (4). Polycyclic Aromatic Hydrocarbons (PAHs) were emitted from vehicle exhaust and burning crop residues owing to the incomplete combustion of hydrocarbons. People were exposure to PAHs through air, water, and food (5). Some studies reported that PAHs are a significant potential factor for oxidative stress, inflammatory responses, and lung disease (6). Urinary 1-Hydroxy Pyrene (1-OHP) was an intermediate marker for evaluating PAHs (7).
The inflammatory response played an important role in pulmonary damage and cancer (8). C-reactive protein (CRP) was a marker of inflammation and was produced in the liver (9). The markers of inflammation include Club Cell (farmly Clara cell) protein 16 (CC16). CC16 was a biological marker in the early period of pulmonary epithelial injury and can be detected in the alveoli of the bronchus (10). CC16 also existed in the plasma and spreads in the blood from the alveoli of the bronchus (11).
People could experience oxidative stress when exposed to a burning and smoky environment for an extended period (5). 8-Iso-prostaglandin F2α (8-iso-PGF2α) was a serum marker of oxidative stress and DNA damage (12). It was the gold standard for oxidative stress (13). When the level of oxidative stress was high, 8-iso-PGF2α was produced. It mainly existed in serum, plasma, urine, and other secretions (14).
Some studies reported that pulmonary biomarkers were associated with PM2.5. Serum CC16 levels were associated with age, smoking status, and kidney function (15). Exposure to PAHs reduced CC16 levels, and smokers with low levels of CC16 might experience more severe decline in pulmonary function (16). Exposure of alveolar injury to smoke resulted in a temporary increase in serum CC16 levels. When the concentration of 1-OHP increased, the concentration of CC16 decreased (17).
Ros-induced peroxidation of arachidonic acid produced 8-iso-PGF2α. 8-iso-PGF2α was regarded as a biomarker of pulmonary diseases and is involved in the progression of diseases such as chronic obstructive pulmonary disease, interstitial lung disease, acute lung injury, breathing disorders, and lung cancer (18–21). The concentration of 8-iso-PGF2α was high in the obese and smoking groups. However, 8-iso-PGF2α was not associated with asthma (13). As the severity of community-acquired pneumonia increased, the concentration of 8-iso-PGF2α also increased (20).
Diabetes mellitus was a common chronic metabolic disease worldwide (22). It was influenced by life and food habits, including age, smoking, and bad habits, and environmental factors, including noise, air pollution, and electromagnetic fields (23). The study found that PAHs were associated with type 2 diabetes mellitus (T2DM) for reasons including inflammation of active, resistant insulin, change in cytokine release, oxidative stress, and interference of the endocrine system (24). PAHs increased the risk of diabetes development. PAHs increased oxidative stress and secrete endocrine interference of endocrine (25).
PAHs were toxic, mutagenic, and carcinogenic (26). They influenced the function of the cardiovascular system, including blood pressure (BP) (27). One study found that 1-OHP was related to hypertension in adults (27). The study found that PAHs in particulate matter increased diastolic blood pressure and decreased systolic blood pressure (28). The cells in the coronary artery and umbilical vein endothelial cells led to oxidative stress and inflammation caused by PAHs (29). The blood pressure of males increased significantly compared with that of females in response to PAHs (30). However, few studies had focused on lung function and PAHs in participants with diabetes and hypertension. Most studies used only the cross-sectional method, which cannot exclude reversed causation.
Herein, the present study conducted a prospective cohort study to evaluate the relationship between PAHs and lung function biomarkers among participants with diabetes and hypertension in different areas of burning air pollution. This study aimed to evaluate changes in lung function before and after exposure to PAHs among metabolically disordered and oxidate stressed individuals and to formulate a protective measure for the susceptible population.
2 Materia and methods
2.1 Study design and population
This was a prospective cohort study in Ban Hua Rin, Thung Satok Subdistrict (Place 1), Ban Piang, Ban Mae Subdistrict (Place 2), and Ban Sai Mun, Mae Ka Subdistrict (Place 3), in San Pa Tong District, Chiang Mai Province during December 2023 for visit 1 of before-burning air pollution and May 2024 for visit 2 of after-burning air pollution. The study protocol was approved by the Research Ethics Committee of the Research Institute for Health Science in Chiangmai, Thailand. All procedures conformed to the Declaration of Helsinki (31). The overall sample size of 127 participants was sufficient to detect moderate effect sizes in two-group comparisons (e.g., Cohen's d ≈ 0.5) and in correlation analyses (e.g., Spearman's ρ ≈ 0.3) with adequate statistical power (≥80%). However, in subgroup analyses (e.g., stratified by diabetes or hypertension status) and three-group comparisons across the geographic regions, the sample size per group was lower (e.g., n = 28 in one region), which might have limited the ability to detect small effect sizes (e.g., d < 0.3 or ρ < 0.2).
Participants with diabetes and hypertension were recruited from local clinics in Chiang Mai. To minimize potential confounding effects of medications on biomarker levels, all participants were instructed to withhold their prescribed medications, including antihypertensive agents (e.g., ACE inhibitors, beta-blockers, angiotensin II receptor blockers) and antidiabetic agents (e.g., metformin, insulin), for at least 24 h prior to sample collection. Additionally, participants were advised to avoid strenuous physical activity, alcohol consumption, and foods rich in antioxidants for 12 h before blood and urine sampling. Individuals with poorly controlled diabetes (defined as HbA1c >10%) or those experiencing acute respiratory infections at the time of recruitment were excluded from the study to reduce potential bias.
2.1.1 Data collection
Trained staff collected data from the questionnaire. We used a structured 122-item short-form survey questionnaire (SF-122). The questionnaire included two parts: participant characteristics and physical health (PH). The characteristics of the participants included age, gender identity, occupation, education level, income, weight, height, body mass index, waist circumference, hip circumference, waist-to-hip ratio, waist-to-height ratio, and family members. Physical health (PH) included body temperature, blood oxygen concentration, blood pressure, pulse, respiratory rate, smoking status, alcohol consumption, diabetes, exercise, chronic disease, eye irritation, skin irritation, respiratory irritation, feeling short of breath, feeling dizzy, fainting, losing consciousness, and having pneumonia.
2.2 PM2.5 measurement
The air quality monitoring stations in the study area were located in three areas: San Pa Tong District, Chiang Mai Province: Ban Hua Rin, Thung Satok Subdistrict, Ban Piang, Ban Mae Subdistrict, and Ban Sai Mun, Mae Ka Subdistrict. The monthly air quality of PM2.5, measured between January 2023 and September 2024, was measured and recorded using the Northern Thailand Air Quality Health Index (NTAQHI) (32).
2.3 CC16 and 8-iso-PGF2α measurement
Venous blood (3 ml) venous blood was collected by medical laboratory technicians. A 3,000 × g centrifugal machine was used to separate the serum at 4 °C for 15 min, and the serum was stored at −80 °C. We then analyzed the serum CC16 and 8-iso-PGF2α levels. The concentrations of serum CC16 and 8-iso-PGF2α were detected using commercial human CC16 and 8-iso-PGF2α enzyme-linked immunosorbent assay (ELISA) test kits (Bio Aoruida®, Guangzhou China) according to the manufacturer's recommendations provided. After the room-temperature equilibrium for 20 min at 25 °C, the detection antibody was added to the microplates and incubated for 1 h. The microplates were read using a microplate reader at a wavelength of 450 nm. Standards and samples were added in duplicate, and the results were calculated for average data. Samples for after- and before-burning air pollution were obtained from the same analytical batch. Intra-assay coefficients of variation for serum CC16 were 5.1%−8%. The range of detectable concentration in serum was 15.1–85.5 pg/ml with R2 values was 0.982–0.985. Intra-assay coefficients of variation for serum 8-iso-PGF2α were 2.2%−8.3%. The range of detectable concentration in serum was 19.0–68.3 pg/ml with R2 values was 0.982–0.985.
2.4 One hydroxy pyrene (1-OHP) measurement
Based on a previous study (78), urinary 1-OHP levels were detected by high-performance liquid chromatography with fluorescent detection (HPLC-FLD). Urine samples from both the after- and before-burning air pollution groups were analyzed. Pool urine samples were analyzed for quality control for each batch of measurements.
2.5 Statistical analyses
The standard descriptive statistics were used to show the characteristics of the participants with after- and before-burning air pollution. One-way ANOVA was used to compare age and BMI. The t-test was used to determine sex parameters. The normality of the following variables was assessed using the Shapiro-Wilk test for normality, conducted in SPSS version 27: age, sex, smoking history, urinary 1-OHP, serum CC16, serum 8-iso-PGF2α, diabetes status, hypertension status, and PM2.5. The Shapiro-Wilk test was selected due to its suitability for medium-sized or smaller samples (n ≤ 200) (33). The results indicated that the data did not meet the assumption of normality (p < 0.05) for all variables. Normality was further evaluated visually using Q-Q plots and histograms. For comparisons between the after burning and before burning groups, the Mann–Whitney U test was used, as it was robust for comparing non-normally distributed continuous variables between two independent groups. For analysis of differences across the three regions, the Kruskal–Wallis H test was used, as it was appropriate for comparing non-normally distributed data across three or more independent groups. Post-hoc pairwise comparisons were conducted using the Dunn test to identify which specific groups differed significantly (34). Prism was used to show the results in graphs.
2.6 Quantitative health risk assessment (QHRA) framework and parameters
We estimated non-cancer hazard quotients (HQ) and lifetime cancer risk (LCR) under the U.S. EPA IRIS/RSL framework using urinary 1-hydroxypyrene (1-OHP) as a biomarker of PAH exposure (35). Creatinine-corrected 1-OHP was translated to BaP-equivalent dose by reverse dosimetry and mixture TEF/RPF conventions (36). LCR was computed as LADD × OSF_BaP and HQ as ADD ÷ RfD_BaP, using IRIS toxicity values (RfD_BaP = 3.0 × 10−4 mg/kg·day; OSF_BaP = 2 (mg/kg·day)−1; adult-only scenario: 1 (mg/kg·day)−1); the legacy OSF = 7.3 (mg/kg·day)−1 was examined in sensitivity analysis. As an inhalation cross-check, we considered IUR_BaP = 1 × 10−3 (μg/m3)−1 when air concentrations were available. Interpretation followed commonly used decision ranges (LCR 10−6−10−4; HQ < 1) (37). Default exposure inputs were IR 20 m3/day (sensitivity 18–23), BW 60 kg, EF 350 d/year, ED 30 year, and AT 70 × 365 day (cancer) or ED × 365 days (non-cancer). HQ and LCR were stratified by age ( ≤ 50, 50–60, and >60), sex, and disease status; estimates were deterministic, with uncertainty explored in sensitivity analyses varying k, Pyr:BaP ratios, TEFs/RPFs, and toxicity values.
3 Results
3.1 The characteristics of participants with diabetes and hypertension in three places
The participants (N = 127) were recruited from three locations in San Pa Tong District, Chiang Mai Province: Ban Hua Rin, Thung Satok Subdistrict (Place 1, N = 28), Ban Piang, Ban Mae Subdistrict (Place 2, N = 50), and Ban Sai Mun, Mae Ka Subdistrict (Place 3, N = 49; Figure 1), with a mean age of approximately 60 years. The overall smoking rate was 15.7% (20/127), with a markedly higher proportion of smokers in Place 1 (28.6%) compared to Place 2 (8%), and Place 3 (12.2%). The average body mass index (BMI) was 24.28, and the mean levels of urinary 1-OHP, serum CC16, and serum 8-iso-PGF2α were 0.576 μmol/mol Cre, 81.45 ng/ml, and 49.86 ng/ml, respectively. All three biomarkers were higher in the after-burning season across all areas, with the largest seasonal increases typically in Place 1 (and for CC16 also Place 3); see Table 1 (Δ = after–before burning).
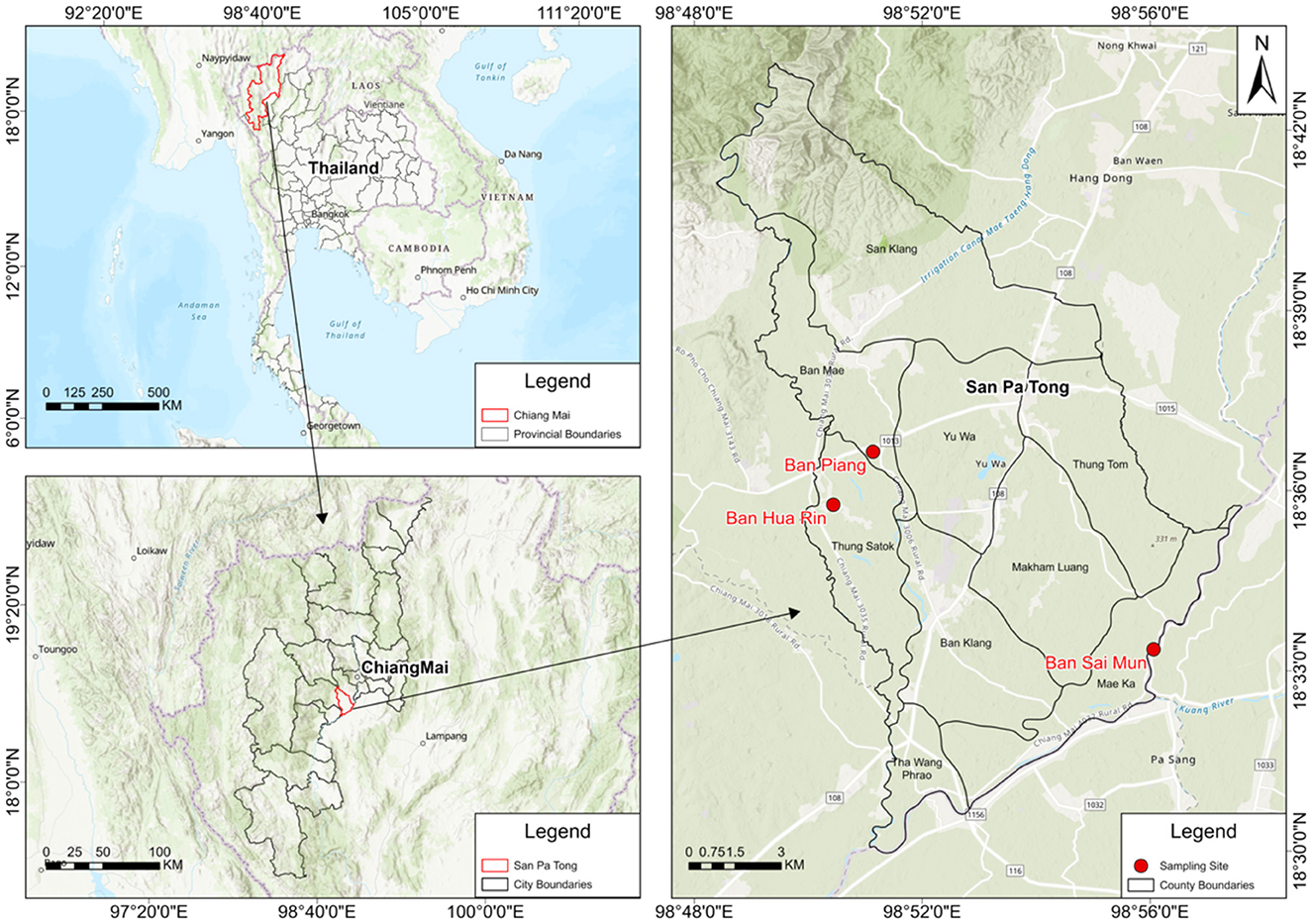
Figure 1. Study area and sampling locations. San Pa Tong District (Chiang Mai, Thailand) with the three sampling communities (Ban Hua Rin, Ban Piang, and Ban Sai Mun) indicated by red markers; administrative boundaries, north arrow, and scale bar are included for reference.
Participants with diabetes (N = 87) and hypertension (N = 72) were distributed across all three locations. While no significant differences in age, sex, BMI, diabetes, or hypertension were observed among the three places, significant differences were found in urinary 1-OHP (p < 0.01), serum CC16 (p < 0.001), and serum 8-iso-PGF2α levels (p < 0.001). Place 1 exhibited consistently higher PM2.5 levels and biomarker concentrations compared to Places 2 and 3 (Table 1; Figure 2).
When stratified by smoking status, non-smokers in Place 1 still exhibited significantly higher serum 8-iso-PGF2α levels than those in Place 2, under both before-burning and after-burning conditions (p < 0.001). This trend remained even though PM2.5 concentrations were similar between Places 1 and 2. These results suggested that the elevated oxidative stress biomarker levels in Place 1 were not solely attributable to smoking, and other environmental or local factors may be contributing.
3.2 Biomarker levels (urinary 1-OHP, serum CC16, and 8-iso-PGF2α) in before- and after-burning periods stratified by age and gender
Compared to before-burning air pollution, the concentrations of serum CC16 and 8-iso-PGF2α increased in after-burning air pollution. There were significant differences (p < 0.01) among groups (p < 0.01; Table 2). As the concentration of 1-OHP increased, the concentrations of Serum CC16, and Serum 8-iso-PGF2α both increased (Figure 3).
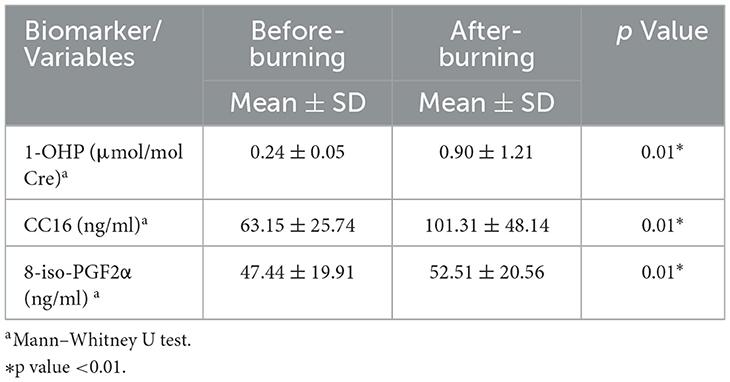
Table 2. Urinary 1-OHP, serum CC16, and 8-iso-PGF2α levels under before- and after-burning conditions.
Table 3 summarizes biomarker changes between before- and after-burning periods stratified by age group and gender. In the overall cohort (n = 127), all three biomarkers—urinary 1-OHP, serum CC16, and serum 8-iso-PGF2α–increased significantly during the after-burning period (all p < 0.001). Across age groups, significant increases in urinary 1-OHP and serum CC16 were observed in all three strata (≤50 years, 50–60 years, and >60 years), whereas serum 8-iso-PGF2α showed significant increases in the 50–60 year group (p < 0.001) but not in the ≤ 50 year group (p = 0.320) and was borderline significant in the >60 year group (p = 0.054). Gender-stratified analysis revealed that urinary 1-OHP and serum CC16 increased significantly in both males and females (all p < 0.001). Serum 8-iso-PGF2α increased significantly in females (p < 0.001) but not in males (p = 0.153).
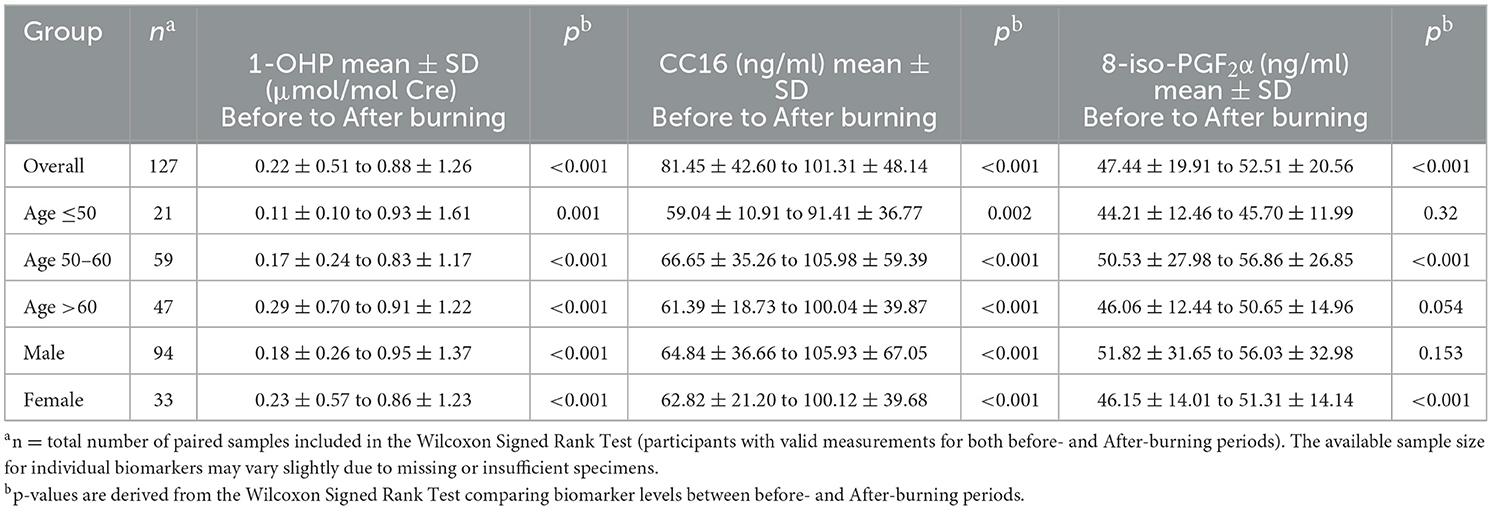
Table 3. Urinary 1-OHP, serum CC16, and 8-iso-PGF2α levels in before- and after-burning periods stratified by age and gender.
3.3 The characteristics of Urinary 1-OHP, serum CC16, and 8-iso-PGF2α among the diabetes and hypertension participants in the before-burning and after-burning air pollution
Among the participants with diabetes and hypertension, the concentrations of urinary 1-OHP, serum CC16, and 8-iso-PGF2α all increased in the after-burning air pollution group compared to the before-burning air pollution group. There were significant differences in urinary 1-OHP, serum CC16 and 8-iso-PGF2α levels in the before-burning air pollution and after-burning air pollution groups among diabetes and hypertension participants (p < 0.01; Table 4).
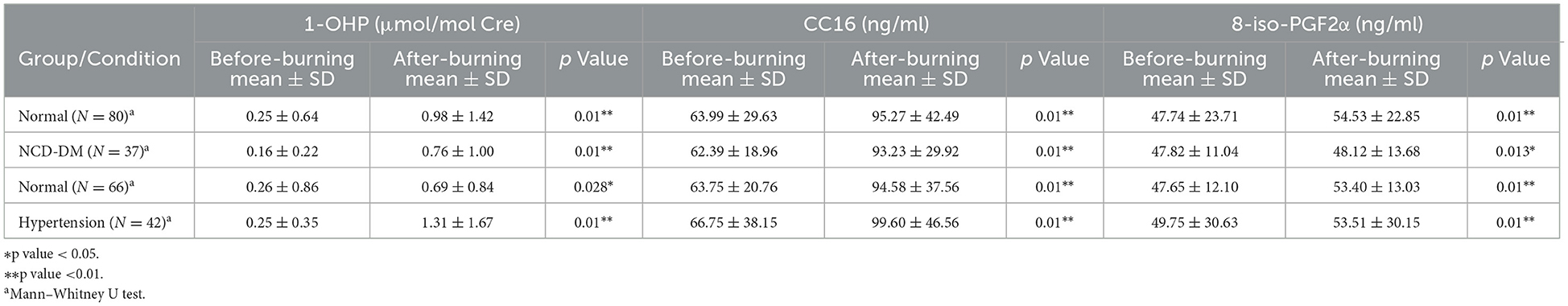
Table 4. Oxidative stress biomarkers stratified by comorbidity (diabetes and hypertension) and pollution level.
3.4 The characteristics of Urinary 1-OHP and different symptoms of participants with diabetes in the after-burning air pollution
In after-burning air pollution, the concentration of urinary 1-OHP increased when participants with Diabetes Mellitus had different symptoms, except for fainting. There were significant differences in the symptoms of skin irritation and shortness of breath (p < 0.05). For participants with symptoms of skin irritation and shortness of breath, the level of 1-OHP was lower compared with other symptoms participants (Table 5).
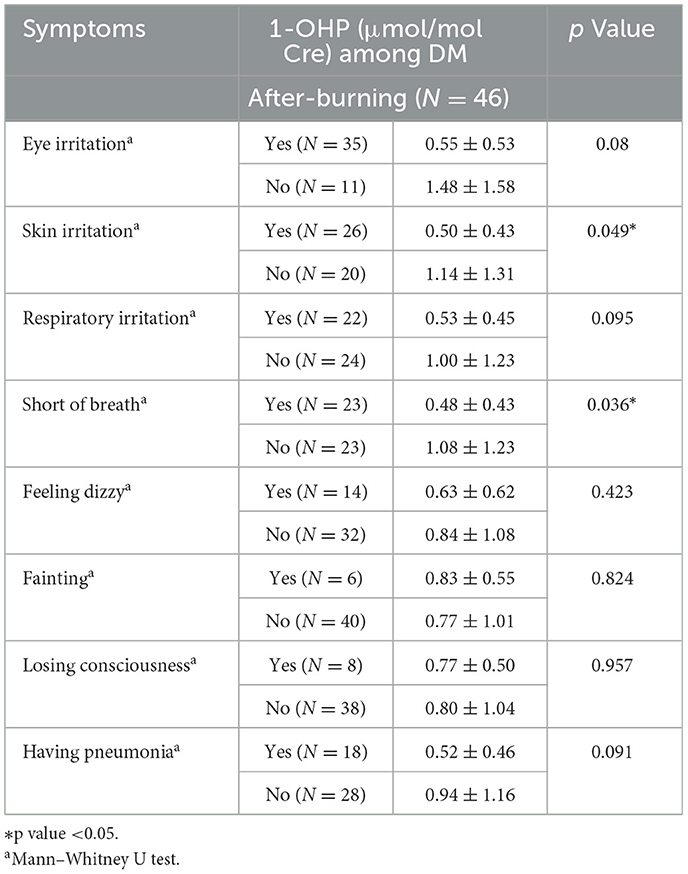
Table 5. Urinary 1-OHP levels in diabetic participants with and without self-reported symptoms during After-burning.
3.5 Health risk assessment based on urinary 1-OHP
Hazard Quotient (HQ) and lifetime cancer risk (LCR) values were calculated for each age–gender–disease subgroup under both before- and after-burning conditions (Table 6). In the before-burning period, LCR values ranged from 8.01 × 10−6 to 5.38 × 10−5, all within the acceptable risk range (10−6−10−4). Corresponding HQ values remained low (0.004–0.024), indicating negligible non-cancer risk. Across age–gender strata, subgroup comparisons showed no statistically significant differences (all p > 0.05; p ranged approximately 0.078–0.526), where estimable.
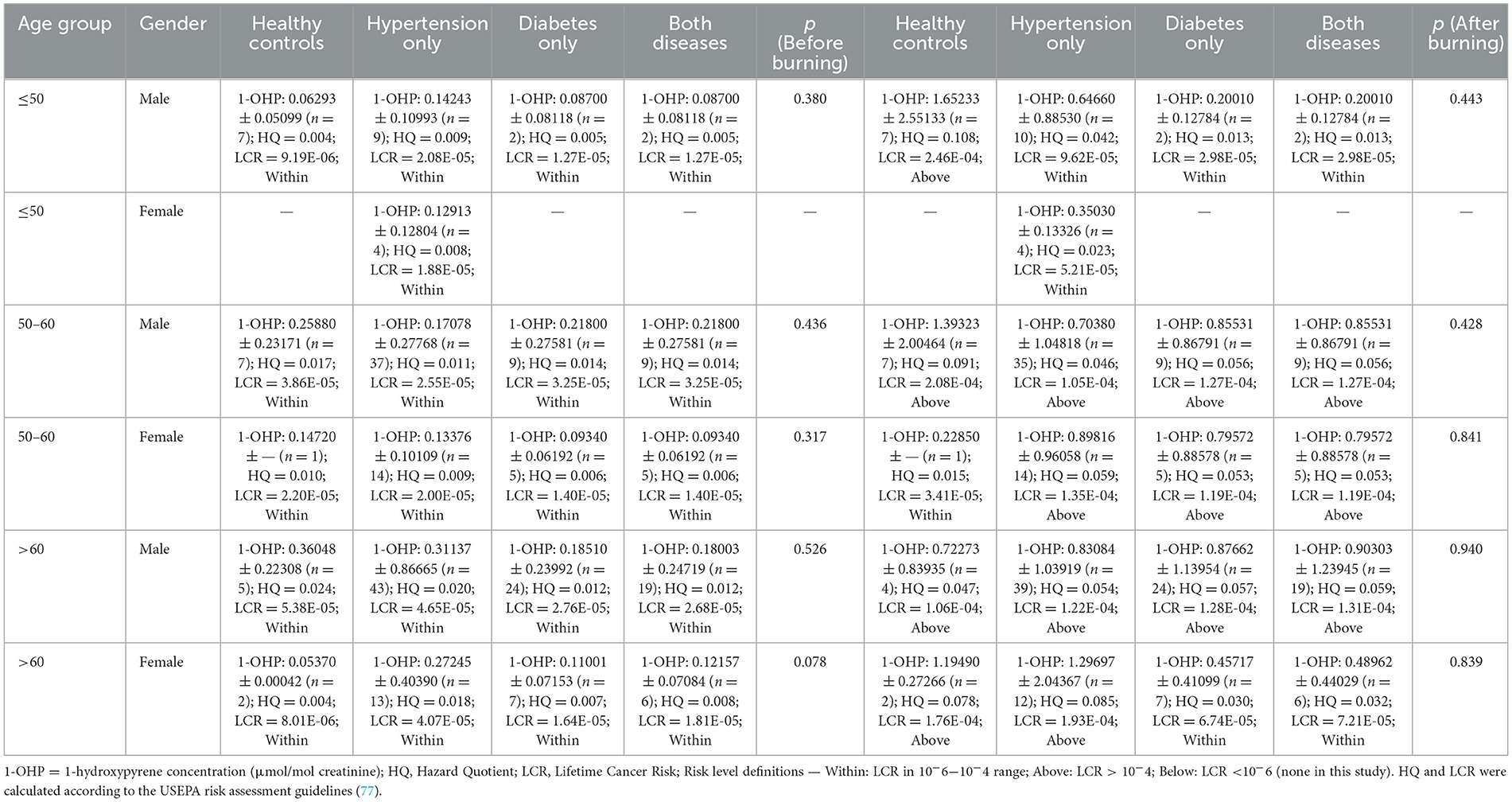
Table 6. Age- and gender-stratified urinary 1-OHP levels, hazard quotient (HQ), and lifetime cancer risk (LCR) in healthy and comorbidity groups during before- and After-burning periods.
In the after-burning period, several subgroups exceeded the acceptable upper limit for cancer risk (LCR > 10−4). The highest LCR was observed in healthy males aged ≤ 50 years (2.46 × 10−4), followed by healthy males aged 50–60 years (2.08 × 10−4) and hypertensive males aged 50–60 years (1.05 × 10−4). Elevated LCR values were also recorded in diabetic and hypertensive–diabetic subgroups aged 50–60 years and >60 years, ranging from 1.19 × 10−4 to 1.93 × 10−4. Nevertheless, subgroup comparisons within each age–gender stratum again did not reach statistical significance (all p > 0.05; p ranged approximately 0.428–0.940). In contrast, HQ values under after-burning conditions remained below 0.11 across all subgroups, suggesting no appreciable non-cancer health risk despite the elevated carcinogenic risk in certain populations. Exceedances (>10−4) were concentrated among males aged 50–60 and >60 and, in some categories, females >60, indicating priority groups for mitigation, while the single highest value occurred in healthy males ≤ 50 years.
4 Discussion
While this study focused on Chiang Mai, a region with unique geographic and agricultural characteristics (e.g., mountainous terrain and seasonal burning), the findings might not be directly generalizable to other populations or countries with different climate conditions and pollution sources. However, Chiang Mai served as a model for rural-urban transitions in Southeast Asia, and insights from this region could have informed broader environmental health policies in similar contexts.
To the best of our knowledge, the present study was the first two-stage prospective cohort study to investigate the effect of PAHs exposure on lung function in three independent locations in participants with diabetes and hypertension. One study found that the prospective cohort method had benefited from linear regression between independent variables and dependent variables (38). Another study found that the limitations of the prospective cohort method were the high cost and long-time cost (39). In this study, we found that the Mann–Whitney U test indicated that the concentrations of urinary 1-OHP, Serum CC16, and Serum 8-iso-PGF2α all increased, and there were significant differences (p < 0.01) in the period of after-burning air pollution compared with before-burning air pollution. Previous studies showed that in a short period of after-burning air pollution, the concentrations of urinary 1-OHP, Serum CC16 and Serum 8-iso- PGF2α increased (40–42).
Notably, age modifies the CC16 response: during after-burning pollution, CC16 averaged 10.01 ± 6.91 ng/ml in adults ≥60 years vs. 5.33 ± 4.24 ng/ml in those < 60 years (p < 0.05) (43). This pattern is supported by external evidence showing that urinary Σ7OH-PAHs positively correlate with 8-OHdG and 8-iso-PGF2α (r = 0.64 and 0.69; p < 0.01) (44). CC16 was the gold standard biomarker for lung function (45). Serum 8-iso- PGF2α was the gold standard biomarker of oxidative stress (46). The reason for the increase in CC16 levels was mainly that short-term exposure to particulate air pollution might have damaged the integrity of the lung epithelium and increased epithelial barrier permeability (Aghapour et al. 2022). Serum 8-iso-PGF2α was abundant because of an imbalance in redox homeostasis and reactive oxygen species (ROS) (47).
Among the participants with diabetes, 1-OHP levels all increased compared with before-burning air pollution and after-burning air pollution. One study found that exposure to PAHs accelerated the generation of reactive oxygen species (ROS), which attacked DNA, proteins, and lipids with adverse results (48). Inflammation was caused by ROS in response to activated redox-sensitive transcription factors (49). Continuous inflammation and oxidative stress caused chronic diseases, such as diabetes (50). Another study found that diabetes patients with short-time PAHs were influenced by oxidative stress (23). In this study, we found that compared to participants without hypertension or hypertension, the concentration of 1-OHP increased among hypertensive participants with after-burning air pollution compared to those with before-burning air pollution. One study reported that PAHs increased the risk of oxidative stress and inflammation in the cardiovascular system of hypertensive patients (6). We also found that the 1-OHP concentration of diabetic participants compared to non-diabetic participants decreased in the before-burning or after-burning air pollution groups. One study reported that anti-inflammatory medicines taken for diabetes led to a negative relationship between PAHs and the level of type 2 diabetes in individuals (51). Among coke-oven workers, higher urinary 4-hydroxyphenanthrene (4-OHPh) concentrations were significantly associated with increased diabetes risk (24). Individuals in the highest quartile of total urinary PAHs had higher odds of diabetes (OR 1.56, 95% CI 1.15–2.12; p = 0.005), and highest vs. lowest quartile of 2-hydroxynaphthalene was associated with stroke (OR 2.23, 95% CI 1.17–4.25; p = 0.016) and diabetes (OR 1.40, 95% CI 1.07–1.82; p = 0.015) (52).
In the study, we found that diabetes participants with skin irritation and shortness of breath symptoms were less exposed to PAHs. One study reported that diabetes people with skin irritation were inclined to have problems with infections like fungal and bacterial infections and dry skin (53). Another study found that exposure to air pollution exacerbated skin irritation for diabetes patients due to sensitivity to pollution (54). The other study reported that diabetes people had serious complications such as cardiovascular, obesity, and respiratory diseases. Diabetes people with the symptoms of shortness of breath had a higher risk when they were exposed to PAHs (55). Highest- vs. lowest-quartile exposure was associated with obesity for both urinary 2- (OR 1.46, 95% CI 1.13–1.87) and the sum of PAH metabolites (OR 1.45, 95% CI 1.13–1.87) (25).
Typically, symptoms such as skin irritation and shortness of breath were expected to occur in individuals exposed to after-burning pollution levels. However, our study found that participants with these symptoms had lower levels of 1-OHP, a biomarker for PAH exposure. This counterintuitive result was explained by behavioral modifications, such as staying indoors or using protective gear during after-burning pollution periods, which might have reduced actual pollutant exposure (56). Another possibility was that individuals with these symptoms might have exhibited altered metabolic or excretory responses to pollutants, leading to lower levels of 1-OHP despite experiencing symptoms (57). It was also possible that these participants had developed some level of tolerance or adaptation to the environmental pollution, resulting in a reduced exposure-response effect (58). Further investigation was needed to clarify whether these symptoms reflect increased sensitivity to pollutants or altered exposure-response mechanisms, including potential long-term adaptations to pollution (59).
Polycyclic aromatic hydrocarbons (PAHs) influenced oxidative stress through several pathways, primarily by being metabolized by cytochrome P450 enzymes into reactive intermediates. These intermediates generated reactive oxygen species (ROS), leading to lipid peroxidation, DNA damage, and activation of inflammatory signaling pathways (60). In individuals with diabetes and hypertension, these pathways were exacerbated due to pre-existing redox imbalances and endothelial dysfunction (61). In particular, PAHs might have worsened endothelial cell damage, contributing to impaired vasodilation, increased vascular permeability, and exacerbated blood pressure regulation (29).
The inflammatory response in these individuals might have been further amplified by PAHs, as they could have activated pathways such as NF-κB and MAPK, which were involved in chronic inflammation (62). This persistent inflammation contributed to the development of complications such as cardiovascular diseases (63). Moreover, biomarkers like CC16, secreted in response to epithelial damage, and 8-iso-PGF2α, which reflects oxidative lipid injury, were particularly sensitive to these pollutant-induced oxidative stress pathways (64). Both of these markers were elevated in individuals with pre-existing inflammatory conditions, and their levels were further amplified by systemic inflammation seen in metabolic disorders. However, further studies were needed to explore the detailed molecular mechanisms underlying these processes and their long-term implications for disease progression in polluted environments.
During the after-burning period, our data show a clear divergence between carcinogenic and non-cancer endpoints: several age–sex–disease subgroups exceeded the conventional lifetime cancer risk (LCR) benchmark of 1 × 10−4, whereas hazard quotients (HQs) remained well-below 1 (all < 0.11). This pattern was biologically and regulatorily coherent because LCR reflects a non-threshold, cumulative mechanism for PAH carcinogenicity (65), while HQ was a threshold-based construct that flags concern only when equivalent doses approach or surpass the reference dose (66). Exceedances clustered among men ≥50 years and, in some strata, women >60 years; the single highest estimate was in healthy men ≤ 50 years. Taken together, these patterns indicated that exposure intensity and behavior—rather than baseline comorbidity—are the primary risks (67). Although differences across disease categories within each age–sex stratum were not statistically significant (all p > 0.05), non-significance was not evidence of no difference (68). Because risk management was anchored to acceptability thresholds, subgroup LCR values exceeding 1 × 10−4 warrant mitigation measures (69). In practice, risk management should focus on high-burn days (70). Priority measures included targeted advisories; promoting N95/FFP2 respirators outdoors; using HEPA filtration indoors (71); limiting vigorous outdoor activity; and avoiding grilling/charred foods and tobacco smoke (72). Public health messaging should be tailored to older adults and men, while also addressing younger men experiencing high exposure (73).
One limitation of this study was the lack of data on medication use, such as antidiabetics, antihypertensives, and corticosteroids, which might have influenced biomarkers like CC16 and 8-iso-PGF2α due to their effects on inflammatory and oxidative processes (74). These medications could have acted as confounding factors, potentially impacting observed associations with air pollution. In addition, environmental variables like temperature and humidity, which varied seasonally in Chiang Mai, might influenced pollutant levels and participants' physiological responses (75). Future studies should account for both medication use and seasonal environmental factors to improve the accuracy and generalizability of findings. Beyond medication use and meteorology, our translation from urinary 1-OHP to mixture-level risk assumes stable PAH composition and extrapolates short-term peaks; thus, LCR exceedances are best interpreted as upper bounds (76). Creatinine-only correction and unmeasured behaviors/co-exposures may influence subgroup patterns, while parameter/route choices were not explored probabilistically. Limited stratum sizes, lack of concurrent PAH speciation, and the after-burning context may constrain precision and generalizability.
5 Conclusions
Seasonal burning in Chiang Mai is linked to higher PAH exposure and early evidence of airway injury and oxidative stress, with CC16 and 8-iso-PGF2α serving as sensitive indicators. Quantitative risk assessment based on urinary 1-OHP showed that during the high-burn period several age–sex–disease strata exceeded the conventional lifetime cancer-risk benchmark (while all hazard quotients remained < 1). In practice, burn-season measures should prioritize high-risk subgroups with targeted alerts, consistent N95/FFP2 use outdoors, indoor HEPA filtration, and integration of seasonal 1-OHP with CC16/8-iso-PGF2α checks into diabetes and hypertension care. At the policy level, authorities should replace open burning with supported residue-management programs, provide clean-air rooms or purifier subsidies in heavily affected areas, and tailor risk communication to high-risk groups. Future work should link these biomarkers to clinical endpoints and sharpen exposure assessment through PAH speciation and personal monitoring.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of the Associate Medical Sciences, Chiang Mai University (protocol code AMSEC-66EX-062). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CX: Data curation, Formal analysis, Methodology, Writing – original draft. SY: Conceptualization, Data curation, Methodology, Writing – review & editing. PT: Formal analysis, Investigation, Writing – review & editing. SP: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. WK: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – original draft. KC: Conceptualization, Project administration, Validation, Writing – review & editing. SY: Conceptualization, Methodology, Resources, Writing – review & editing. AW: Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing. KK: Investigation, Methodology, Writing – review & editing. NK: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. SH: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Chiang Mai University (No. PM10/2566).
Acknowledgments
Authors would like to thank the School of Health Sciences Research, Research Institute for Health Sciences, and Chiang Mai University for the student research fund and for facilitating the research work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fuller R, Landrigan PJ, Balakrishnan K, Bathan G, Bose-O'Reilly S, Brauer M, et al. Pollution and health: a progress update. Lancet Planet Health. (2022) 6:e535–47. doi: 10.1016/S2542-5196(22)00090-0
2. Das S, Pal D, Sarkar A. Particulate matter pollution and global agricultural productivity. In:Singh R, Kumar Singh V, Lichtfouse E, , editors. Sustainable Agriculture Reviews 50: Emerging Contaminants in Agriculture. Cham, Switzerland: Springer (2021). p. 79–107. doi: 10.1007/978-3-030-63249-6_4
3. Suriyawong P, Chuetor S, Samae H, Piriyakarnsakul S, Amin M, Furuuchi M, et al. Airborne particulate matter from biomass burning in Thailand: recent issues, challenges, and options. Heliyon. (2023) 9:e14261. doi: 10.1016/j.heliyon.2023.e14261
4. Saengas T, Harnkiattiwong T. Haze problems in Samoeng district Chiang-mai, Thailand. EQA-Int J Environ Qual. (2025) 68:21–7. doi: 10.6092/issn.2281-4485/21157
5. Ravanbakhsh M, Yousefi H, Lak E, Ansari MJ, Suksatan W, Qasim QA, et al. Effect of polycyclic aromatic hydrocarbons (PAHs) on respiratory diseases and the risk factors related to cancer. Polycycl Aromat Comp. (2023) 43:8371–87. doi: 10.1080/10406638.2022.2149569
6. Wu X, Cao X, Lintelmann J, Peters A, Koenig W, Zimmermann R, et al. Assessment of the association of exposure to polycyclic aromatic hydrocarbons, oxidative stress, and inflammation: a cross-sectional study in Augsburg, Germany. Int J Hygiene Environ Health. (2022) 244:113993. doi: 10.1016/j.ijheh.2022.113993
7. Zhang H, Liu R, Yang L, Cheng H, Wang S, Zhang B, et al. Exposure to polycyclic aromatic hydrocarbons (PAHs) in outdoor air and respiratory health, inflammation and oxidative stress biomarkers: a panel study in healthy young adults. Sci Total Environ. (2023) 899:165582. doi: 10.1016/j.scitotenv.2023.165582
8. Ahmad S, Manzoor S, Siddiqui S, Mariappan N, Zafar I, Ahmad A, et al. Epigenetic underpinnings of inflammation: connecting the dots between pulmonary diseases, lung cancer and COVID-19. Semin Cancer Biol. (2022) 83:384–98. doi: 10.1016/j.semcancer.2021.01.003
9. Stanimirovic J, Radovanovic J, Banjac K, Obradovic M, Essack M, Zafirovic S, et al. Role of C-reactive protein in diabetic inflammation. Mediators Inflamm. (2022) 2022:3706508. doi: 10.1155/2022/3706508
10. Almuntashiri S, Zhu Y, Han Y, Wang X, Somanath PR, Zhang D. Club cell secreted protein CC16: potential applications in prognosis and therapy for pulmonary diseases. J Clin Med. (2020) 9:4039. doi: 10.3390/jcm9124039
11. Pingle S, Sherekar P, Thakkar L, Tumane R, Barde S, Jawade A, et al. Goblet, club and alveolar cells: front-line defenders of the airways in chronic obstructive pulmonary disease, a most common lung disease in miners. In:Randive KR, Shubhangi P, Anupam A, , editors. Medical Geology in Mining: Health Hazards Due to Metal Toxicity. Cham, Switzerland: Springer (2022). p. 83–100. doi: 10.1007/978-3-030-99495-2_4
12. Nour Eldin EEM, Nour Eldein MM, El-Readi MZ, Mirza AA, Fatani SH, Al-Amodi HS, et al. Evaluation of the diagnostic and predicative values of 8-iso-prostaglandin F2α as a biomarker of breast cancer. Oncol Res Treatment. (2020) 43:506–17. doi: 10.1159/000509671
13. Woo S-D, Park HS, Yang E-M, Ban G-Y, Park H-S. 8-Iso-prostaglandin F2α as a biomarker of type 2 low airway inflammation and remodeling in adult asthma. Ann Allergy Asthma Immunol. (2024) 133:73–80.e2 doi: 10.1016/j.anai.2024.04.007
14. Boldeanu L, Văduva C-C, Caragea DC, Novac MB, Manasia M, Siloşi I, et al. Association between serum 8-iso-prostaglandin F2α as an oxidative stress marker and immunological markers in a cohort of preeclampsia patients. Life. (2023) 13:2242. doi: 10.3390/life13122242
15. Neumann S, Casjens S, Hoffmeyer F, Rühle K, Gamrad-Streubel L, Haase L-M, et al. Club cell protein (CC16) in serum as an effect marker for small airway epithelial damage caused by diesel exhaust and blasting fumes in potash mining. Int Archiv Occup Environ Health. (2024) 97:121–132. doi: 10.1007/s00420-023-02035-x
16. Wei J, Wang Y, Kong H, Wu J, Jiang L, Pan B, et al. Association between plasma CC16 levels and lung function changes in coke oven workers: a cohort study from 2014 to 2023. Ecotoxicol Environ Saf. (2024) 284:117002. doi: 10.1016/j.ecoenv.2024.117002
17. Wang Y, Duan H, Meng T, Shen M, Ji Q, Xing J, et al. Reduced serum club cell protein as a pulmonary damage marker for chronic fine particulate matter exposure in Chinese population. Environ Int. (2018) 112:207–17. doi: 10.1016/j.envint.2017.12.024
18. Wang S, Liu C. Clinical significance of the level of 8-iso-prostaglandin F2α, serum ferritin, superoxide dismutase in serum from children with severe mycoplasma pneumoniae pneumonia. Clin Med China. (2014) 991–5.
19. van't Erve TJ, Lih FB, Kadiiska MB, Deterding LJ, Mason RP. Elevated plasma 8-iso-prostaglandin F2α levels in human smokers originate primarily from enzymatic instead of non-enzymatic lipid peroxidation. Free Rad Biol Med. (2018) 115:105–12. doi: 10.1016/j.freeradbiomed.2017.11.008
20. Zheng L, Fei J, Feng C-M, Xu Z, Fu L, Zhao H. Serum 8-iso-PGF2α predicts the severity and prognosis in patients with community-acquired pneumonia: a retrospective cohort study. Front Med. (2021) 8:633442. doi: 10.3389/fmed.2021.633442
21. Ma L, Sun D, Xiu G, Lazarus P, Vachani A, Penning TM, et al. Quantification of plasma 8-isoprostane by high-performance liquid chromatography with tandem mass spectrometry in a case-control study of lung cancer. Int J Environ Res Public Health. (2022) 19:12488. doi: 10.3390/ijerph191912488
22. Liu J, Ren Z-H, Qiang H, Wu J, Shen M, Zhang L, et al. Trends in the incidence of diabetes mellitus: results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health. (2020) 20:1–12. doi: 10.1186/s12889-020-09502-x
23. Mallah MA, Basnet TB, Ali M, Xie F, Li X, Feng F, et al. Association between urinary polycyclic aromatic hydrocarbon metabolites and diabetes mellitus among the US population: a cross-sectional study. Int Health. (2023) 15:161–70. doi: 10.1093/inthealth/ihac029
24. Wang X, Li A, Xu Q. The association between urinary polycyclic aromatic hydrocarbons metabolites and Type 2 diabetes mellitus. Int J Environ Res Public Health. (2022) 19:7605. doi: 10.3390/ijerph19137605
25. Lee I, Park H, Kim MJ, Kim S, Choi S, Park J, et al. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds is associated with a risk of obesity and diabetes mellitus among Korean adults: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Int J Hygiene Environ Health. (2022) 240:113886. doi: 10.1016/j.ijheh.2021.113886
26. Maciejczyk M, Tyrpień-Golder K, Janoszka B, Gierat B, Muzyka R. Mutagenic and carcinogenic polycyclic aromatic hydrocarbons (PAHs) in food–occurrence, human health effects, and assessment methods of exposure. Environ Med. (2023) 26:8–15. doi: 10.26444/ms/168971
27. Kim JH, Hong Y-C. (2024) Associations among urinary 1-hydroxypyrene level, oxidative stress, and high blood pressure: a panel study among elderly Koreans. Chemosphere. 368:143693. doi: 10.1016/j.chemosphere.2024.143693
28. Chen X, Dong L, Yang L, Yang Y, Yang L, Han S. Prenatal exposure to polycyclic aromatic hydrocarbons and blood pressure in the early life of children. Ecotoxicol Environ Saf. (2025) 291:117830. doi: 10.1016/j.ecoenv.2025.117830
29. He J, Pang Q, Huang C, Xie J, Hu J, Wang L, et al. Environmental dose of 16 priority-controlled PAHs mixture induce damages of vascular endothelial cells involved in oxidative stress and inflammation. Toxicol Vitro. (2022) 79:105296. doi: 10.1016/j.tiv.2021.105296
30. Mirzababaei A, Daneshzad E, Moradi S, Abaj F, Mehranfar S, Asbaghi O, et al. The association between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases and blood pressure: a systematic review and meta-analysis of observational studies. Environ Sci Pollut Res Int. (2022) 29:1712–28. doi: 10.1007/s11356-021-17091-4
31. Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human participants. JAMA. (2025) 333:71–4. doi: 10.1001/jama.2024.21972
32. Kausar S, Tongchai P, Yadoung S, Sabir S, Pata S, Khamduang W, et al. Impact of fine particulate matter (PM2. 5) on ocular health among people living in Chiang Mai, Thailand. Sci Rep. (2024) 14:26479. doi: 10.1038/s41598-024-77288-8
33. Yagin FH, Yagin B, Pinar A. Normality distributions commonly used in sport and health sciences. J Exerc Sci Phys Activ Rev. (2024) 2: 124–31.
34. Agbangba CE, Aide ES, Honfo H, Kakai RG. On the use of post-hoc tests in environmental and biological sciences: a critical review. Heliyon. (2024) 10:e25131. doi: 10.1016/j.heliyon.2024.e25131
35. Piotrowski M. Multi-media Health Risk Assessment for Southeast Chicago Residents Using Environmental Monitoring Data, University of Illinois at Chicago (2025).
36. Clauzel A, Persoons R, Maître A, Balducci F, Petit P. Review of environmental airborne pyrene/benzo [a] pyrene levels from industrial emissions for the improvement of 1-hydroxypyrene biomonitoring interpretation. J Toxicol Environ Health Part B. (2024) 27:212–32. doi: 10.1080/10937404.2024.2362632
37. Foroughi P, Golbabaei F, Sadeghi-Yarandi M, Yaseri M, Fooladi M, Kalantary S. Occupational exposure, carcinogenic and non-carcinogenic risk assessment of formaldehyde in the pathology labs of hospitals in Iran. Sci Rep. (2024) 14:12006. doi: 10.1038/s41598-024-62133-9
38. Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health. (2018) 3:e419–28. doi: 10.1016/S2468-2667(18)30135-X
39. Knebel C, Menzemer J, Pohlig F, Herschbach P, Burgkart R, Obermeier A, et al. Peri-prosthetic joint infection of the knee causes high levels of psychosocial distress: a prospective cohort study. Surg Infect. (2020) 21:877–83. doi: 10.1089/sur.2019.368
40. Provost EB, Chaumont A, Kicinski M, Cox B, Fierens F, Bernard A, et al. Serum levels of club cell secretory protein (Clara) and short-and long-term exposure to particulate air pollution in adolescents. Environ Int. (2014) 68:66–70. doi: 10.1016/j.envint.2014.03.011
41. Naksen W, Kawichai S, Srinual N, Salrasee W, Prapamontol T. First evidence of high urinary 1-hydroxypyrene level among rural school children during smoke haze episode in Chiang Mai Province, Thailand. Atmos Pollut Res. (2017) 8:418–27. doi: 10.1016/j.apr.2016.11.002
42. Hu W, Wang Y, Wang T, Ji Q, Jia Q, Meng T, et al. Ambient particulate matter compositions and increased oxidative stress: exposure-response analysis among high-level exposed population. Environ Int. (2021) 147:106341. doi: 10.1016/j.envint.2020.106341
43. Nangola S, Thongtip S, Saoin S, Kloypan C, Pimonsree S, Tantrakarnapa K. Factors related to club cell protein 16 (CC16) and quality of life in Northern Thailand. Environ Asia. (2023) 16:169–83.
44. Chen J-J, Liu S-Q, Li M, Hao K-l, Zhang Y-l, Li W-h, et al. Relationship between polycyclic aromatic hydrocarbons internal exposure and lung function change among healthy college students (2023).
45. Chen M, Xu K, He Y, Jin J, Mao R, Gao L, et al. CC16 as an inflammatory biomarker in induced sputum reflects chronic obstructive pulmonary disease (COPD) severity. Int J Chronic Obst Pulmon Dis. (2023) 18:705–17. doi: 10.2147/COPD.S400999
46. Morsi HK, Ismail MM. The value of 8-iso prostaglandin F2 alpha and superoxide dismutase activity as a clinical indicator of oxidative stress in type II diabetes mellitus. J Clin Diagn Res. (2018) 12:BC10–14. doi: 10.7860/JCDR/2018/35505.12255
47. Cammisotto V, Nocella C, Bartimoccia S, Sanguigni V, Francomano D, Sciarretta S, et al. The role of antioxidants supplementation in clinical practice: focus on cardiovascular risk factors. Antioxidants. (2021) 10:146. doi: 10.3390/antiox10020146
48. Molina L, Segura A. Biochemical and metabolic plant responses toward polycyclic aromatic hydrocarbons and heavy metals present in atmospheric pollution. Plants. (2021) 10:2305. doi: 10.3390/plants10112305
49. Zuo L, Wijegunawardana D. Redox role of ROS and inflammation in pulmonary diseases. Adv Exp Med Biol. (2021) 1304:187–204. doi: 10.1007/978-3-030-68748-9_11
50. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. (2019) 11:45–63.
51. Zhang H, Han Y, Qiu X, Wang Y, Li W, Liu J, et al. Association of internal exposure to polycyclic aromatic hydrocarbons with inflammation and oxidative stress in prediabetic and healthy individuals. Chemosphere. (2020) 253:126748. doi: 10.1016/j.chemosphere.2020.126748
52. Zhang A, Zhang H, Mi L, Ding L, Jiang Z, Yu F, et al. Diabetes: a potential mediator of associations between polycyclic aromatic hydrocarbon exposure and stroke. Environ Sci Pollut Res. (2024) 1–11. doi: 10.1007/s11356-024-32324-y
53. David P, Singh S, Ankar R. A comprehensive overview of skin complications in diabetes and their prevention. Cureus. (2023) 15:e38961. doi: 10.7759/cureus.38961
54. Passeron T, Krutmann J, Andersen M, Katta R, Zouboulis C. Clinical and biological impact of the exposome on the skin. J Eur Acad Dermatol Venereol. (2020) 34:4–25. doi: 10.1111/jdv.16614
55. Nwaozuzu CC, Partick-Iwuanyanwu KC, Abah SO. Systematic review of exposure to polycyclic aromatic hydrocarbons and obstructive lung disease. J Health Pollut. (2021) 11:210903. doi: 10.5696/2156-9614-11.31.210903
56. Carlsten C, Salvi S, Wong GW, Chung KF. Personal strategies to minimise effects of air pollution on respiratory health: advice for providers, patients and the public. Eur Respir J. (2020) 55:1902056. doi: 10.1183/13993003.02056-2019
57. Hisamuddin NH, Jalaludin J. Children's exposure to polycyclic aromatic hydrocarbon (PAHs): a review on urinary 1-hydroxypyrene and associated health effects. Rev Environ Health. (2023) 38:151–68. doi: 10.1515/reveh-2021-0013
58. Loria A, Cristescu ME, Gonzalez A. Mixed evidence for adaptation to environmental pollution. Evolution Appl. (2019) 12:1259–73. doi: 10.1111/eva.12782
59. Mousavi SE, Delgado-Saborit JM, Adivi A, Pauwels S, Godderis L. Air pollution and endocrine disruptors induce human microbiome imbalances: a systematic review of recent evidence and possible biological mechanisms. Sci Total Environ. (2022) 816:151654. doi: 10.1016/j.scitotenv.2021.151654
60. Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. (2021) 22:4642. doi: 10.3390/ijms22094642
61. Liu Z, Lu J, Sha W, Lei T. Comprehensive treatment of diabetic endothelial dysfunction based on pathophysiological mechanism. Front Med. (2025) 12:1509884. doi: 10.3389/fmed.2025.1509884
62. Kunnumakkara AB, Shabnam B, Girisa S, Harsha C, Banik K, Devi TB, et al. Inflammation, NF-κB, and chronic diseases: how are they linked? Crit Rev Immunol. (2020) 40:1–39. doi: 10.1615/CritRevImmunol.2020033210
63. Henein MY, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. (2022) 23:12906. doi: 10.3390/ijms232112906
64. Yang L, Cai X, Li R. Ferroptosis induced by pollutants: an emerging mechanism in environmental toxicology. Environ Sci Technol. (2024) 58:2166–84. doi: 10.1021/acs.est.3c06127
65. Blum K, FitzGerald R, Wilks MF, Barle EL, Hopf NB. Use of the benchmark-dose (BMD) approach to derive occupational exposure limits (OELs) for genotoxic carcinogens: N-nitrosamines. J Appl Toxicol. (2023) 43:1183–200. doi: 10.1002/jat.4455
66. Ouyang X, Chen J, Cao L. Threshold effect of ecosystem services in response to human activity in China's urban agglomeration: a perspective on quantifying ecological resilience. Environ Sci Pollut Res. (2024) 31:9671–84. doi: 10.1007/s11356-024-31865-6
67. Tartaglione AM, Racca A, Ricceri L. Developmental exposure to polycyclic aromatic hydrocarbons (PAHs): focus on benzo [a] pyrene neurotoxicity. Reprod Toxicol. (2023) 119:108394. doi: 10.1016/j.reprotox.2023.108394
68. Hopkins WG. Replacing statistical significance and non-significance with better approaches to sampling uncertainty. Front Physiol. (2022) 13:962132. doi: 10.3389/fphys.2022.962132
69. Verma D, Patel KS, Pandey PK, Wakhle B, Sharma S, Petrović M, et al. Environmental and Health Implications of Land Pollution in Ambagarh Chowki, India. J Hazard Toxic Radio Waste. (2025) 29:05025002. doi: 10.1061/JHTRBP.HZENG-1531
70. Güngöroglu C, Ismailoglu I, Kapukaya B, Özcan O, Yanalak M, Musaoglu N. Comparison between post-fire analysis and pre-fire risk assessment according to various geospatial data. Sustainability. (2024) 16:1569. doi: 10.3390/su16041569
71. Dheda K, Charalambous S, Karat AS, von Delft A, Lalloo UG, van Zyl Smit R, et al. A position statement and practical guide to the use of particulate filtering facepiece (N95, FFP2, or equivalent) respirators for South African health care workers exposed to respiratory pathogens including M. tuberculosis and SARS-CoV-2. Afr J Thorac Crit Care Med. (2021) 27:177–86. doi: 10.31730/osf.io/zp9wn
72. Shen M, Liu G, Zhou L, Yin H, Arif M, Leung KMY. Spatial distribution, driving factors and health risks of fine particle-bound polycyclic aromatic hydrocarbons (PAHs) from indoors and outdoors in Hefei, China. Sci Total Environ. (2022) 851:158148. doi: 10.1016/j.scitotenv.2022.158148
73. Burgio KL, Cunningham SD, Newman DK, Low LK, Nodora J, Lipman TH, et al. Preferences for public health messaging related to bladder health in adolescent and adult women. J Women Health. (2023) 32:1120–35. doi: 10.1089/jwh.2022.0463
74. Yang C, Hou L, Sharif HMA, Wang Y, Cai Y, Li C, et al. Tailored anthraquinone-based covalent organic frameworks boosted atrazine degradation through peroxymonosulfate activation driven by visible light. Sep Purif Technol. (2024) 350:127941. doi: 10.1016/j.seppur.2024.127941
75. Jainonthee C, Wang Y-L, Chen CW, Jainontee K. Air pollution-related respiratory diseases and associated environmental factors in Chiang Mai, Thailand, in 2011–2020. Tropic Med Infect Dis. (2022) 7:341. doi: 10.3390/tropicalmed7110341
76. Comnea-Stancu IR, van Staden JKF, Stefan-van Staden R-I. Trends in recent developments in electrochemical sensors for the determination of polycyclic aromatic hydrocarbons from water resources and catchment areas. J Electrochem Soc. (2021) 168:047504. doi: 10.1149/1945-7111/abf260
77. Williams AJ, Lambert JC, Thayer K, Dorne J-LC. Sourcing data on chemical properties and hazard data from the US-EPA CompTox chemicals dashboard: a practical guide for human risk assessment. Environ Int. (2021) 154:106566. doi: 10.1016/j.envint.2021.106566
Keywords: PAHs, diabetes mellitus, hypertension, lung function biomarkers, PM2.5 pollution
Citation: Xianfeng C, Yadoung S, Tongchai P, Pata S, Khamduang W, Chawansuntati K, Yodkeeree S, Wongta A, Kulprachakarn K, Kosashunhanan N and Hongsibsong S (2025) Biomarker changes before and after the 2024 peak burning period in healthy, diabetic, and hypertensive residents of Chiang Mai, Thailand. Front. Public Health 13:1535448. doi: 10.3389/fpubh.2025.1535448
Received: 27 November 2024; Accepted: 25 September 2025;
Published: 13 October 2025.
Edited by:
Tongjian Cai, Army Medical University, ChinaReviewed by:
Qiuxia (Lisa) Li, University of California, Los Angeles, Los Angeles, United StatesRabia Aslam, Government College University, Lahore, Pakistan
Copyright © 2025 Xianfeng, Yadoung, Tongchai, Pata, Khamduang, Chawansuntati, Yodkeeree, Wongta, Kulprachakarn, Kosashunhanan and Hongsibsong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Surat Hongsibsong, c3VyYXQuaG9uZ3NpYnNvbmdAY211LmFjLnRo; Natthapol Kosashunhanan, bmF0dGhhcG9sLmtvQGNtdS5hYy50aA==
†These authors have contributed equally to this work
 Cao Xianfeng
Cao Xianfeng Sumed Yadoung
Sumed Yadoung Phannika Tongchai
Phannika Tongchai Supansa Pata
Supansa Pata Woottichai Khamduang3
Woottichai Khamduang3 Anurak Wongta
Anurak Wongta Kanokwan Kulprachakarn
Kanokwan Kulprachakarn Surat Hongsibsong
Surat Hongsibsong

