- 1Department of General Midwifery, School of Midwifery, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 2Department of Health Promotion and Health Behavior, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Pediatrics and Child Health Nursing, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 4Department of Health Informatics, College of Health Science, Mattu University, Mattu, Ethiopia
- 5Department of Midwifery, College of Health Science, Mattu University, Mattu, Ethiopia
- 6Department of Pediatrics and Child Health Nursing, College of Health Sciences and Referral Hospital, Ambo University, Ambo, Ethiopia
- 7University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia
Background: Globally, cervical cancer is the fourth most prevalent disease among women. It is primarily caused by persistent infections with human papillomavirus (HPV). The World Health Organization (WHO) strongly recommends HPV vaccination for girls aged 9 to 14 years. Although HPV vaccination is the most effective form of primary prevention against cervical cancer, the accessibility and uptake of the HPV vaccine remain low in developing nations, particularly in Africa. Therefore, this umbrella review aimed to determine the pooled prevalence of human papillomavirus vaccine uptake and its determinant factors in Africa.
Methods: The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under reference number CRD42024560032. Eligible systematic review and meta-analysis (SRM) studies were retrieved from PubMed, Hinari, ScienceDirect, and Google Scholar. Data were extracted using Microsoft Excel 2019 and analyzed using Stata software (version 17). The methodological quality of the included studies was examined using A Measurement Tool to Assess systematic Reviews (AMSTAR 2). Publication bias was checked using a funnel plot and Egger’s test. A random-effects model (DerSimonian–Laird method) was used to estimate the pooled prevalence of HPV vaccine uptake. The I-squared (I2) test was performed to assess statistical heterogeneity among the included studies.
Results: This umbrella review included five SRM studies conducted across Africa, encompassing a total of 707,005 study participants. The pooled prevalence of HPV vaccine uptake in Africa was 41.38% (95% CI: 34.70, 48.06). Women’s knowledge of HPV vaccination (AOR: 3.22, 95% CI: 1.64–6.33) and attitudes toward HPV immunization (AOR: 2.48, 95% CI: 2.18–2.81) were significantly associated with HPV vaccine uptake.
Conclusion: The uptake of the HPV vaccine in Africa remains significantly lower (41.38%) than the WHO’s global HPV vaccination target of 90% by 2030. Therefore, increasing vaccine uptake requires promoting women’s knowledge and attitudes toward HPV vaccination through facility-based education and counseling, planned campaigns, community-based programs, and advocacy for HPV vaccination and cervical cancer prevention using various mass media platforms.
Systematic review registration: Berihun Agegn Mengistie, Muluken Demeke, Abebaw Setegn. An Umbrella review of Human Papillomavirus (HPV) Vaccine Uptake and its predictors among females in Africa, 2024. PROSPERO 2024 Available from https://www.crd.york.ac.uk/PROSPERO/view/CRD42024560032.
Introduction
Cervical cancer is the fourth most prevalent disease among women globally, with an estimated 604,000 new cases and 342,000 deaths reported across 185 countries in 2020 (1). In low-resource nations, such as sub-Saharan Africa (SSA), the disease has the highest prevalence (84%), and 88% of deaths are associated with it (2). In 2020, 1,00,000 women were infected with human papillomavirus (HPV) in Africa alone, with approximately 70,000 deaths, accounting for 21% of all cervical cancer-related deaths worldwide (3). In general, SSA bears the highest regional burden of HPV infection, accounting for an average of 24% of global cases (4). Over 99% of cervical cancer cases are caused by chronic infections with oncogenic or high-risk sexually transmitted strains of HPV (5, 6).
The HPV vaccine has been shown to be effective in preventing HPV-related malignancies (4). The inclusion of the HPV vaccine in the National Immunization Program (NIP) is expected to alter the course and burden of cervical cancer, particularly in the SSA region. Currently, approximately 50% of African nations offer HPV vaccination through the NIP (3). However, the implementation of HPV vaccination has not been as successful as expected, particularly for the second dosage, due to various challenges (7–10). In SSA countries where vaccination programs have been implemented, the average coverage for the final dose is only 20% (3). Since 2006, the United States Food and Drug Administration (FDA) has approved three HPV vaccines, all of which are recommended by the World Health Organization (WHO), including the bivalent vaccine (Cervarix), the quadrivalent vaccine (Gardasil), and the 9-valent vaccine (Gardasil 9) (11). The WHO and other researchers recommend a one- or two-dose HPV vaccine schedule for girls aged 9–14 years, a one- or two-dose schedule for girls and women aged 15–20 years, two doses with a 6-month gap for women over 21 years, and more than two doses for immunocompromised individuals (12, 13).
Each of the vaccines offers protection against the high-risk HPV strains 16 and 18; however, Gardasil provides extra protection for the 9–14 age group against HPV strains 6 and 11, and Gardasil 9 offers protection against HPV strains 6, 11, 31, 33, 45, 52, and 58 (14). These vaccines are most effective when administered before exposed to HPV; therefore, the WHO recommends vaccinating girls between the ages of 9 and 14 years (15, 16).
Worldwide, 39.7% of women are immunized against HPV, but this proportion is 68% in nations with high-income countries and 2.7% in low- and middle-income countries (LMICs) (17). A systematic review of the population-level effects of HPV vaccination over 58 years in 14 high-income countries found that the overall prevalence of HPV strains 16 and 18 decreased by 83% among girls aged 13–19 years and by 66% among women aged 20–24 years, resulting in substantial cross-protection against HPV strains 31, 33, and 45 (18). A single dose of the HPV vaccine provides comparable protection against highly susceptible HPV strains (types 16 and 18) as two or three doses of HPV vaccination (16). Cervical cancer is highly preventable and treatable if it is diagnosed at an early stage (19). The global implementation of the HPV vaccine, combined with early screening for cervical cancer, is expected to reduce the incidence of cervical carcinoma in the next decades. The WHO has launched a global initiative to scale up prevention, screening, and therapeutic efforts aimed at eradicating cervical carcinoma as a global health issue in the 21st century (19).
In November 2020, the WHO introduced the 90–70-90 triple intervention approach, a global initiative aimed at eliminating cervical cancer as a public health concern. This global strategy aims to vaccinate 90% of girls against HPV by the age of 15 years, to screen 70% of women using a high-performance screening method twice between the ages of 35 and 45 years, and to treat at least 90% of women identified with precancerous cervical lesions or cervical cancer (19).
Over a decade of implementation programs has provided important lessons on raising vaccination coverage in various LMICs. However, compared to other parts of the globe, SSA has made only modest progress in the execution and success of national HPV vaccination programs, primarily due to challenges related to the accessibility and affordability of the vaccine (20).
Umbrella reviews involve the systematic collection and review of several systematic review and meta-analysis (SRM) studies conducted on a particular study topic. To date, several SRM studies have reported inconsistent prevalence rates of HPV vaccine uptake across Africa, ranging from 28.53 to 55% (21–25). Therefore, the aim of this umbrella review was to summarize the heterogeneous findings of existing SRM studies to generate conclusive findings regarding the coverage and determinants of HPV vaccine uptake in Africa. This comprehensive evidence will be valuable for healthcare professionals (HCPs), health managers, and policymakers in implementing evidence-based interventions to improve the uptake of the HPV vaccine in Africa. It is also considered a cost-effective primary preventive intervention for precancerous cervical lesions and cervical cancer.
Materials and methods
Study protocol
The protocol for this umbrella review was developed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) statement. Initially, a search was conducted on PROSPERO to check for the presence of a similar umbrella review, and no such studies were found to have been registered. Then, the study’s protocol was established and registered (reference number: CRD42024560032).
Searching strategy
Umbrella reviews involve the systematic collection and review of several SRM studies conducted on a particular study topic. This umbrella review aimed to systematically combine previous SRM studies on HPV vaccine uptake and its associated factors in Africa. It provides a more comprehensive and integrated understanding of the evidence regarding the HPV vaccine in Africa, allowing for the implementation of evidence-based strategies to scale up HPV vaccine uptake across the continent.
An inclusive literature search for SMR studies on HPV vaccine uptake and its associated factors in Africa was conducted using the Condition, Context, and Population (CoCoPop) framework across PubMed, Hinari, ScienceDirect, and Google Scholar. Publications were retrieved from a previous study that met the eligibility criteria. A search strategy was developed for the databases using a combination of keywords and Boolean operators (“AND” and “OR”). All systematic reviews and meta-analyses published between 1 January 2014 and 15 August 2024 were included. Furthermore, snowballing techniques were used to identify additional studies by reviewing the reference lists of the articles retrieved from the database searches. Finally, we used the following searching commands: systematic review and meta-analysis OR systematic reviews AND uptake OR use OR acceptance OR practice AND human papilloma virus vaccine OR human papillomavirus vaccination OR HPV vaccination OR HPV vaccine AND daughters OR females OR adolescent girls OR schoolgirls OR female students AND Africa OR “East Africa” OR “North Africa” OR “West Africa” OR “South Africa” OR “Sub-Saharan Africa” (Supplementary file 3).
Inclusion and exclusion criteria
We included systematic reviews and meta-analyses of studies published in English that reported the prevalence and/or associated factors of HPV vaccine uptake in Africa. In addition, only studies published on and after 1 January 2014 were included in this umbrella review.
Articles were excluded for the following reasons: they did not measure the outcome of interest or they were narrative reviews, primary studies, qualitative reviews, expert opinions, case reports, editorials, correspondence, scoping reviews, or methodological studies.
Study selection process
Based on the inclusion and exclusion criteria, all searched studies were imported into EndNote X8, a reference management software, to remove duplicate studies. After duplicates were removed, two reviewers (EG and BAM) independently assessed the titles and abstracts of the remaining articles to identify both potentially eligible articles and any articles for which eligibility could not be determined from the title and abstract alone. Then, the full texts of the remaining articles were examined to assess their eligibility according to the criteria.
Measurement of the outcomes
The primary objective of this study was to determine the overall prevalence of HPV vaccine uptake in Africa. The prevalence was calculated by dividing the number of women who received the HPV vaccine by the total number of women in the study, then multiplying the result by 100. The second objective of this umbrella review was to identify the predictors of HPV vaccine uptake in Africa, which were evaluated using adjusted odds ratios (ORs) from previous SRM studies.
Data extraction and management
Two authors (BAM and ANY) conducted data extraction from the included SRM studies using a standardized data abstraction form developed in an Excel spreadsheet. Articles were initially screened and selected based on their title and abstract, then the full text was reviewed. In cases of disagreement, discussions were held with additional reviewers to finalize the selection of articles to include in this umbrella review. Following the comprehensive search, potentially eligible publications were imported into EndNote. Duplicate studies were removed when two or more articles shared similar features. A structured data extraction process was designed and implemented using a Microsoft Excel spreadsheet.
For each SRM study, the following data were extracted: identification data (first author’s last name and publication year), prevalence of HPV vaccine uptake, factors associated with HPV vaccine uptake, odds ratios with 95% confidence intervals, the number of primary studies included in each SRM study, study area, total sample size, publication bias assessment methods, and risk of bias assessment methods and scores.
Quality assessment
The quality of the included articles was evaluated by two separate reviewers using A Measurement Tool to Assess systematic reviews (AMSTAR 2). The new quality assessment tool builds upon the original AMSTAR. The specific quality of the included articles was assessed based on the 16 criteria of the AMSTAR 2 tool. The system categorizes the specific SMR evidence into four categories based on its quality: high, moderate, poor, and critically low. This umbrella review excluded articles with critically low-quality evidence (26) (https://amstar.ca/Amstar_Checklist.php) (Table 1).
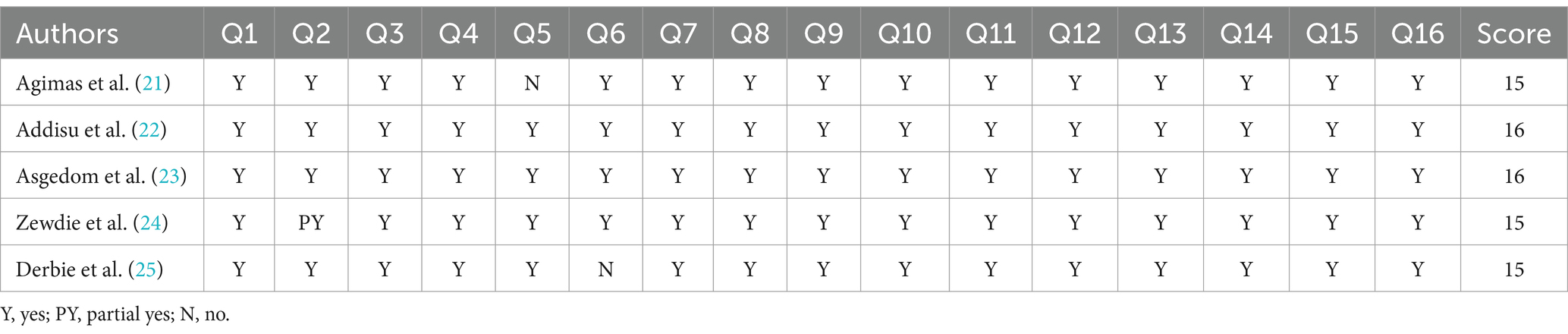
Table 1. Methodological quality assessment of the included systematic review and meta-analysis studies using AMSTAR 2.
Data synthesis and statistical analysis
After the data were extracted using a Microsoft Excel spreadsheet, they were exported to the STATA 17 statistical software, where all statistical data analyses were carried out. The extracted data were displayed using texts, tables, and forest plots. The standard error of prevalence for each SRM study was analyzed using the binomial distribution. The combined prevalence of the SRM studies was checked for heterogeneity using the I-squared (I2) test. Heterogeneity among the included studies was classified as low, moderate, and high based on I-squared values of <25, 50–75%, and 75%, respectively (27).
A random-effects meta-analysis model with DerSimonian and Laird’s method was used to determine the overall estimate of HPV vaccine uptake in Africa. Subgroup analysis was performed by country, region, and study population to identify potential causes of heterogeneity among the studies. In addition, we conducted a leave-one-out sensitivity analysis to determine the effects of individual SRM studies on the pooled estimate. Then, forest plots and tables were used to display the pooled estimates for the continent, along with their corresponding 95% confidence intervals. Publication bias or small study effects were detected using funnel plot symmetry (27). In addition, the statistical significance of publication bias was assessed using Egger’s test, with a p-value less than 0.05 indicating the presence of publication bias (28). A trim-and-fill analysis was performed if significant publication bias was detected to correct for missing studies. Finally, the meta-analysis results were presented using forest plots, along with the odds ratio (OR) and a 95% confidence interval.
Results
Study selection
A total of 7,942 studies were identified using four different search engines—PubMed, Hinari, ScienceDirect, and Google Scholar. Subsequently, 846 duplicate records were removed, leaving 7,096 studies eligible for screening. Of these, 7,034 articles were excluded based on title and abstract screening, resulting in 62 eligible studies. Among the 62 studies reviewed in full text, 57 were excluded for different reasons, including differences in study setting, irrelevance to the outcome of interest, and being qualitative systematic reviews, scoping reviews, or protocols for systematic reviews. Ultimately, five eligible studies were included in the final quantitative umbrella review (18, 22–25) (Figure 1).
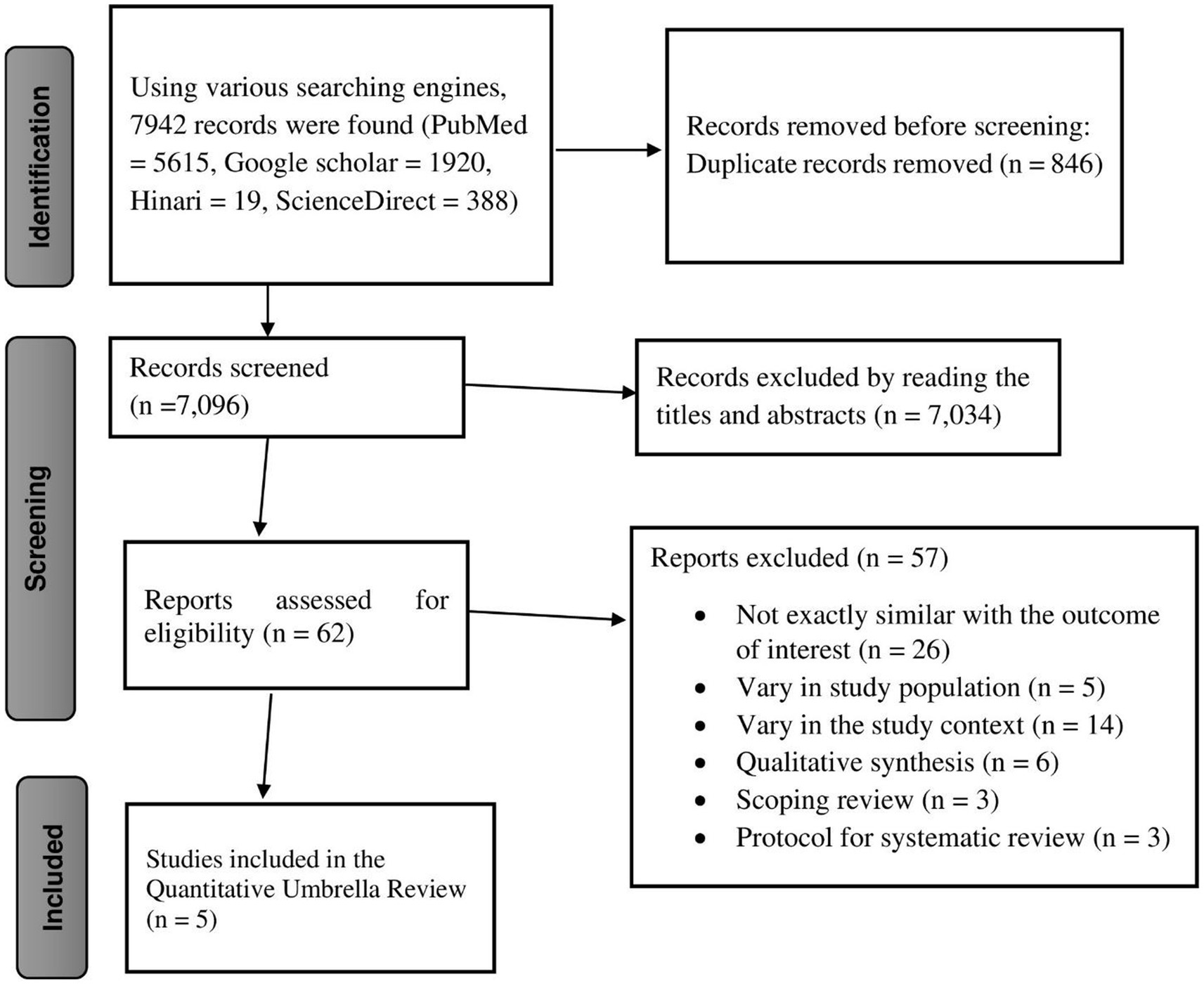
Figure 1. Prisma flow diagram showing the selection of studies for the umbrella review of HPV vaccine uptake in Africa.
Characteristics of the included studies
In this umbrella review, five eligible SRM studies, comprising a total of 707,005 study participants, were included. In addition, there were 72 primary studies included in the eligible SRM studies. In terms of the distribution of the SRM studies across Africa, three studies were conducted in Ethiopia, one in East Africa, and the remaining study in SSA. Regarding the AMSTAR 2 quality score, the quality scores of each SRM article ranged from 15 to 16 (Table 2).
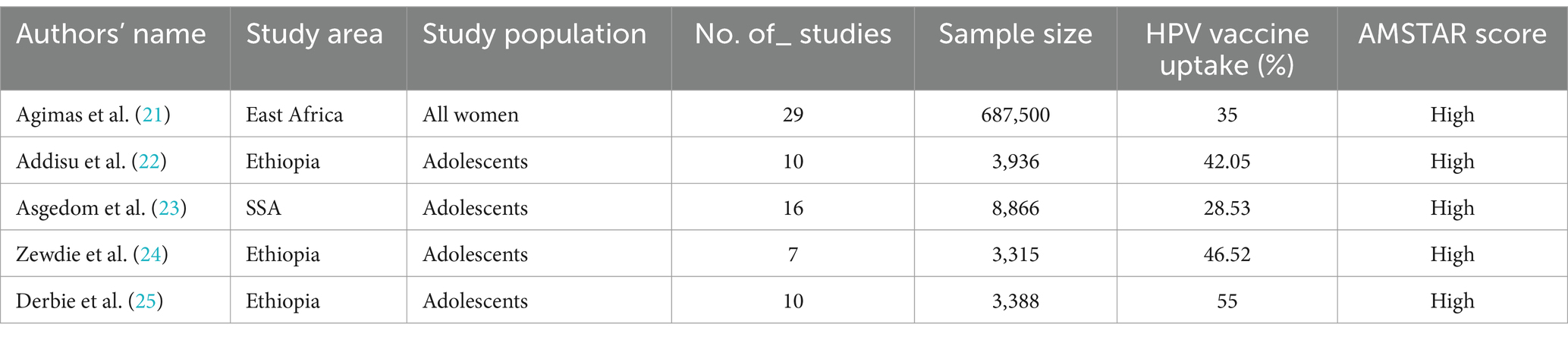
Table 2. Descriptive summary of the included systematic review and meta-analysis studies on HPV vaccine uptake among women in Africa.
Uptake of the HPV vaccine in Africa
In this umbrella review, the pooled prevalence of HPV vaccine uptake in Africa was found to be 41.38%, with a 95% confidence interval (34.70–48.06). The statistical test indicated the presence of significant heterogeneity among the included SRM studies (heterogeneity I2 = 99.59%, p = 0.00). As a result, a random-effects meta-analysis model was used (Figure 2).
Publication bias
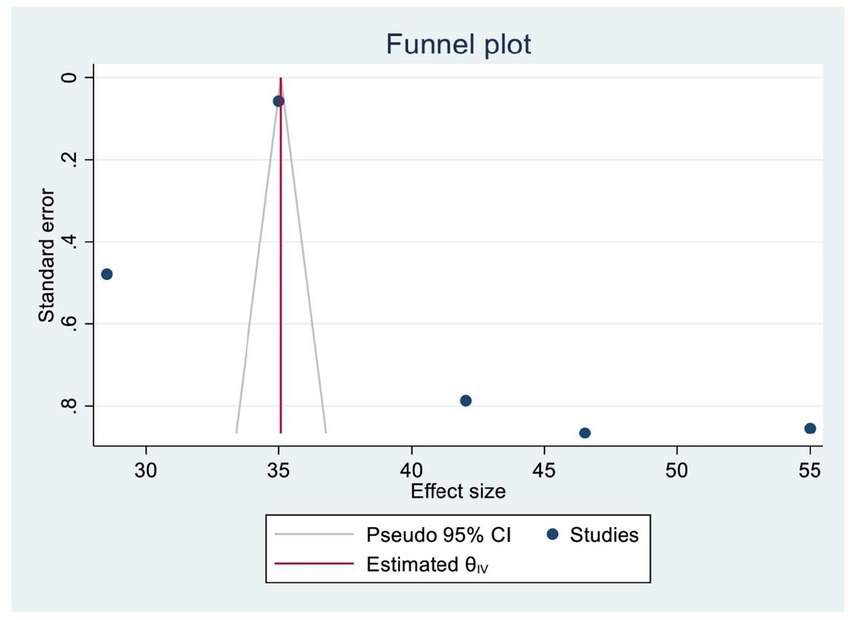
A funnel plot and Egger’s test were used to assess publication bias. A symmetrical distribution in the funnel plot indicated the absence of publishing bias. In addition, we also examined publication bias statistically using Egger’s regression test, yielding a p-value of 0.086, which confirmed the absence of significant publication bias among the included SRM studies (Figure 3).
Heterogeneity and subgroup analysis
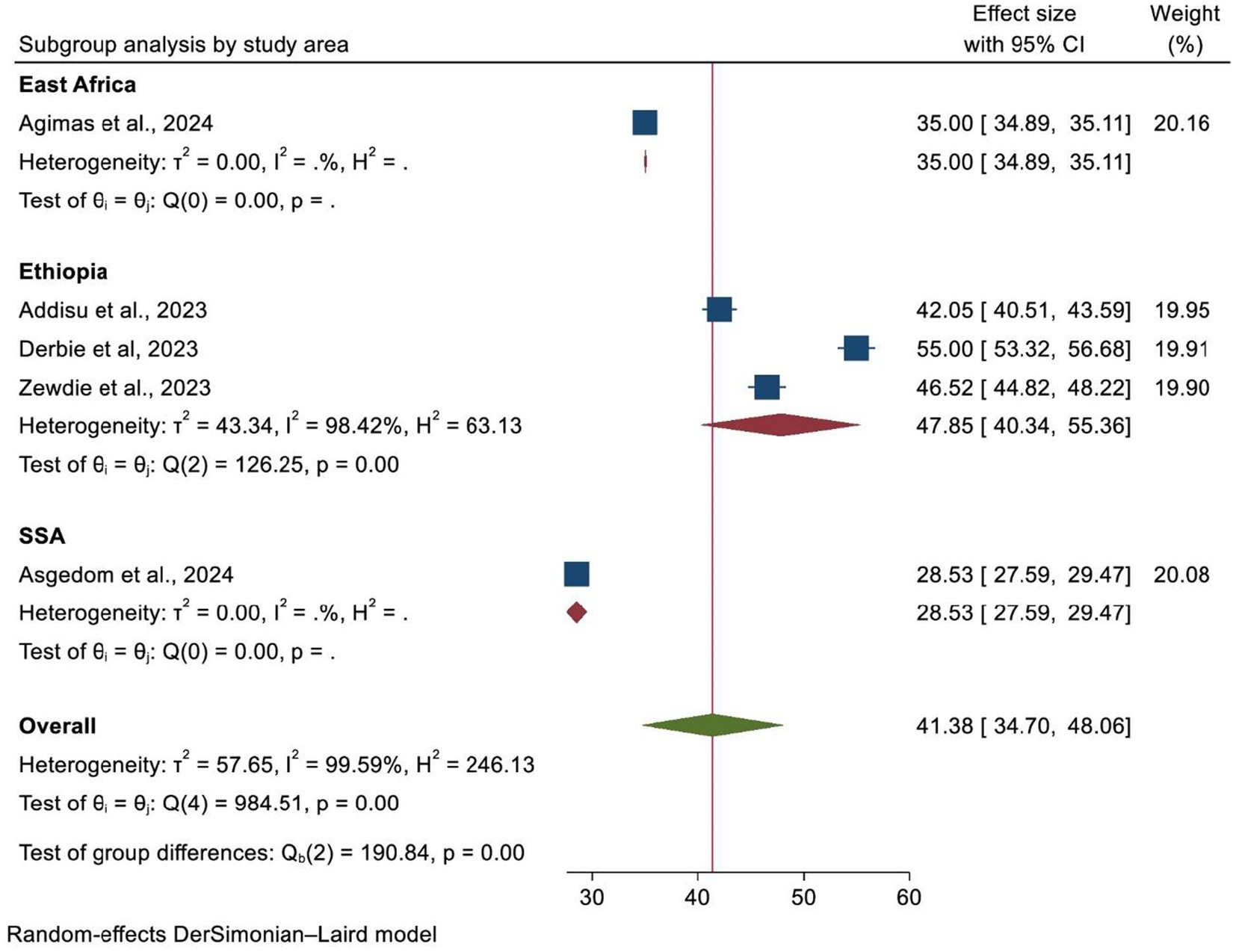
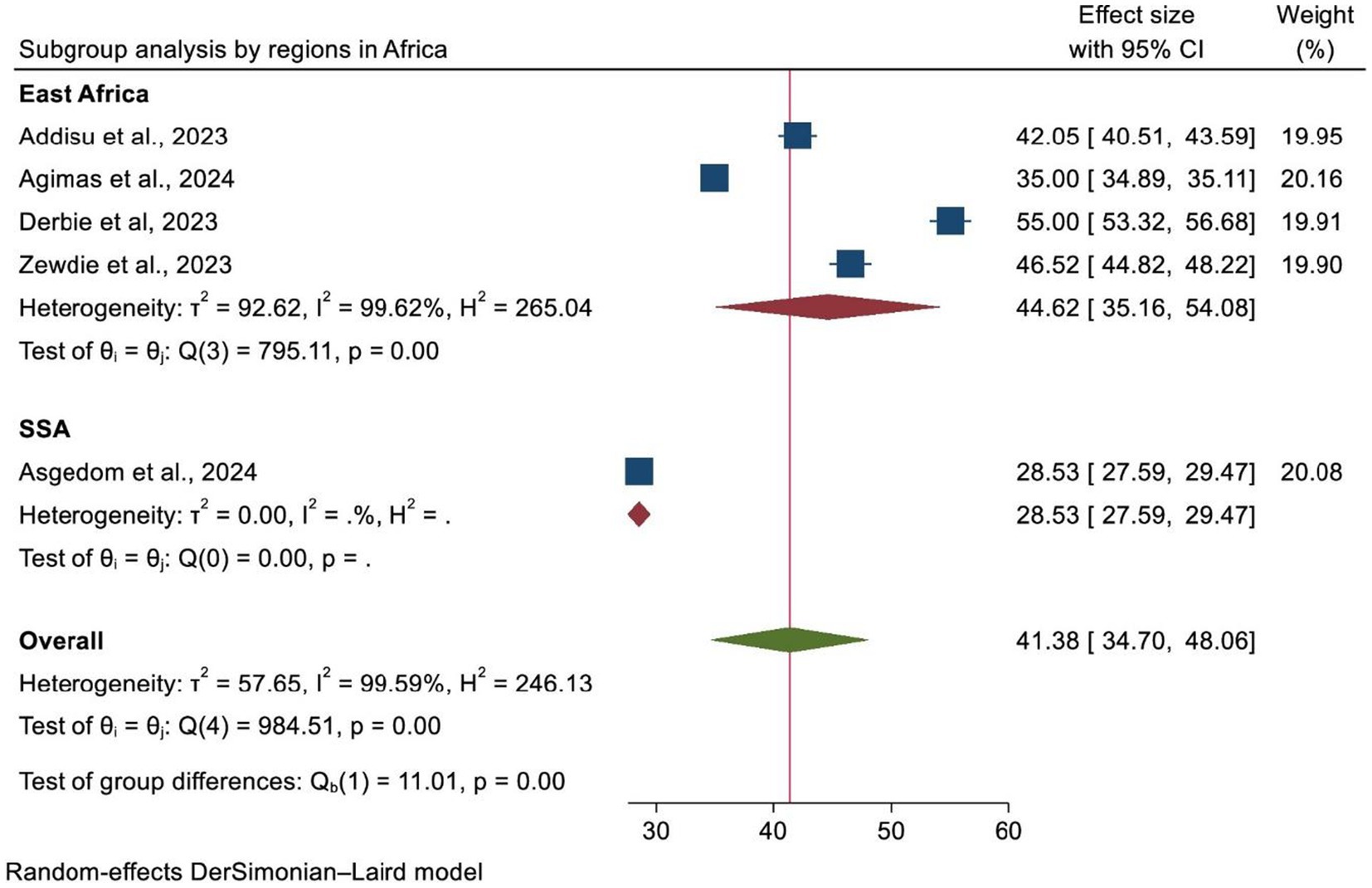
A subgroup analysis was carried out according to the country where the study was performed, the region of Africa, and the number of primary studies included in each SRM. As a result, Ethiopia and SSA had the highest and lowest overall prevalence of HPV vaccination at 47.85% (95% CI: 40.34–55.36) and 35.00% (95% CI: 34.89, 35.11), respectively (Figure 4). In addition, the highest prevalence of HPV vaccination was observed in East Africa at 44.62% (95% CI: 35.16–54.08). However, the lowest HPV vaccination coverage was observed in SSA, with a prevalence of 28.53% (95% CI: 27.59–29.47) (Figure 5). Lastly, a subgroup analysis based on the study population showed a higher pooled prevalence of HPV vaccination among adolescent schoolgirls, at 43% (95% CI: 30.70–55.32) (Figure 6). Despite performing subgroup analysis using the aforementioned criteria, there was no significant improvement in the variability of the overall prevalence of HPV vaccination.
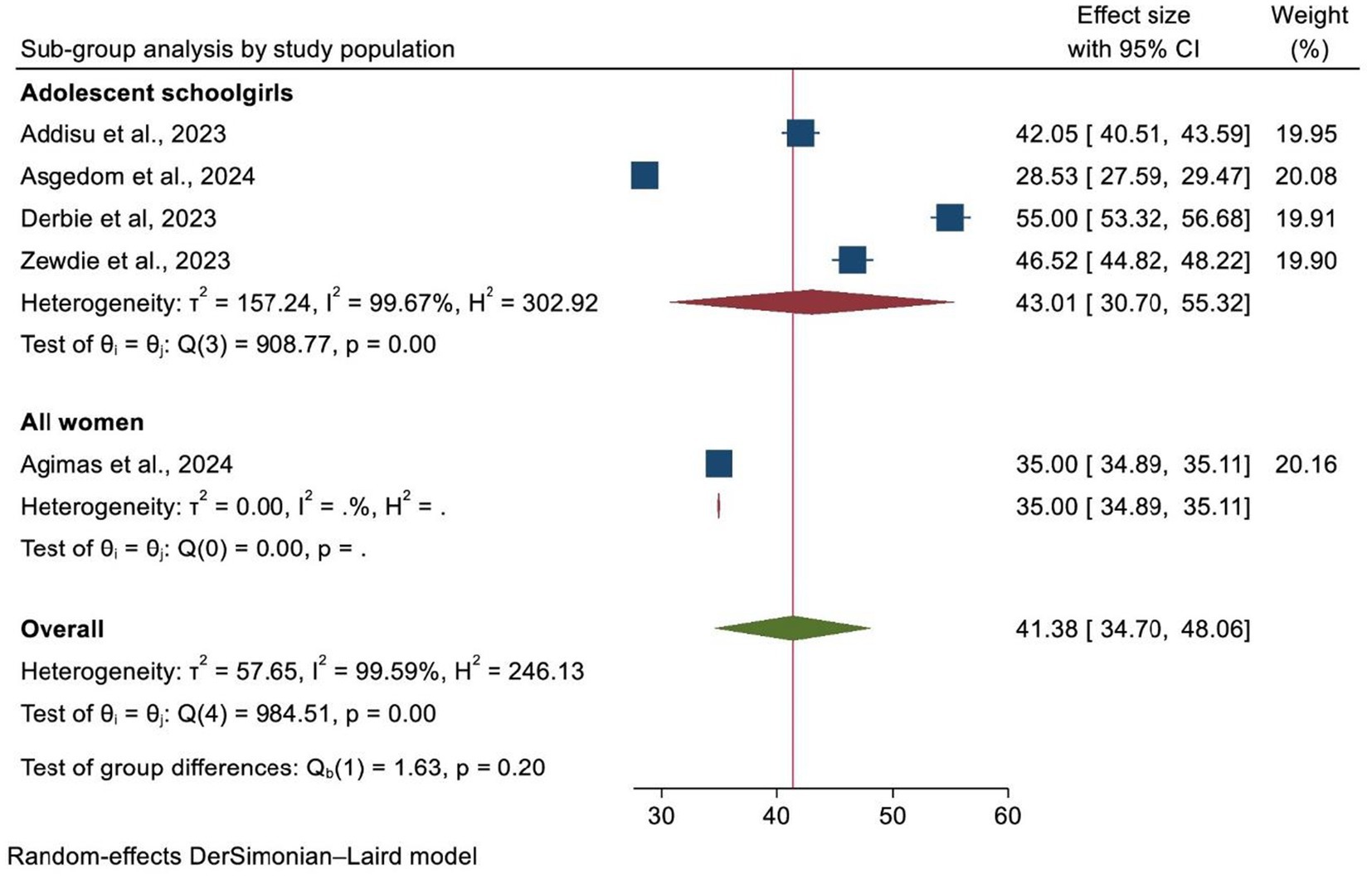
Figure 6. A forest plot showing the sub-group analysis of HPV vaccine uptake by the study population.
A leave-one-out sensitivity analysis
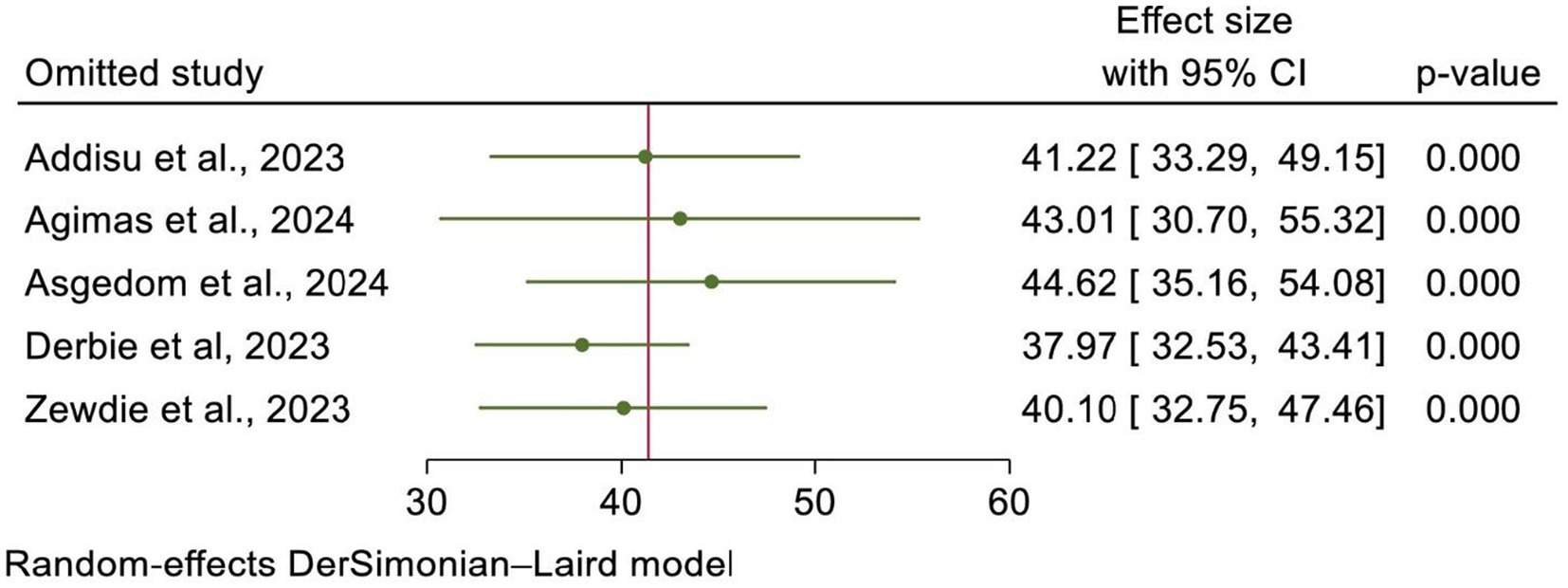
Using the random-effects model, a leave-one-out sensitivity analysis was conducted to assess the effect of each individual SRM study on the effect size. The results indicated that no single study significantly influenced the pooled estimate, and the point estimate from its omitted analysis fell within the confidence interval of the combined analysis. This indicated the robustness of the overall estimate of HPV vaccine uptake in Africa (Figure 7).
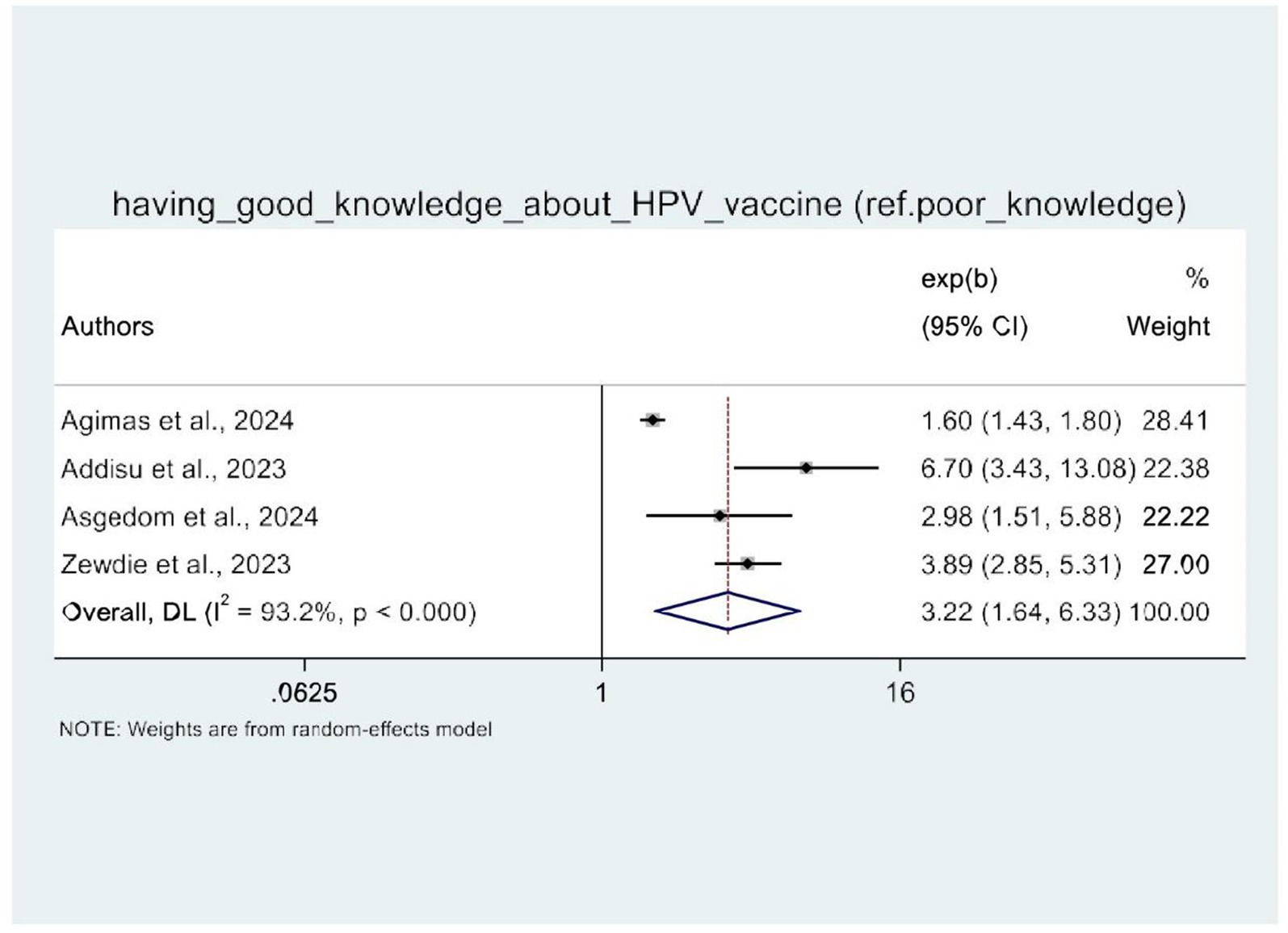
Meta-analysis of the factors associated with HPV vaccine uptake in Africa
In this umbrella review, four of the five SRM studies were examined for factors influencing HPV vaccine uptake (21–24). The random-effects model demonstrated that women’s knowledge of HPV vaccination and attitudes toward the HPV vaccine were significantly associated with HPV vaccine uptake. Therefore, four SRM studies showed the statistical significance of women’s knowledge about HPV vaccination. Women with good knowledge of the HPV vaccine (AOR: 3.22, 95% CI: 1.64–6.33) were three times more likely to be vaccinated against HPV as compared to their counterparts (Figure 8). Similarly, women with a positive attitude toward the HPV vaccine were three times more likely to receive the HPV vaccine (AOR: 2.48, 95% CI: 2.18–2.81) than those with a negative attitude toward the HPV vaccine (Figure 9).
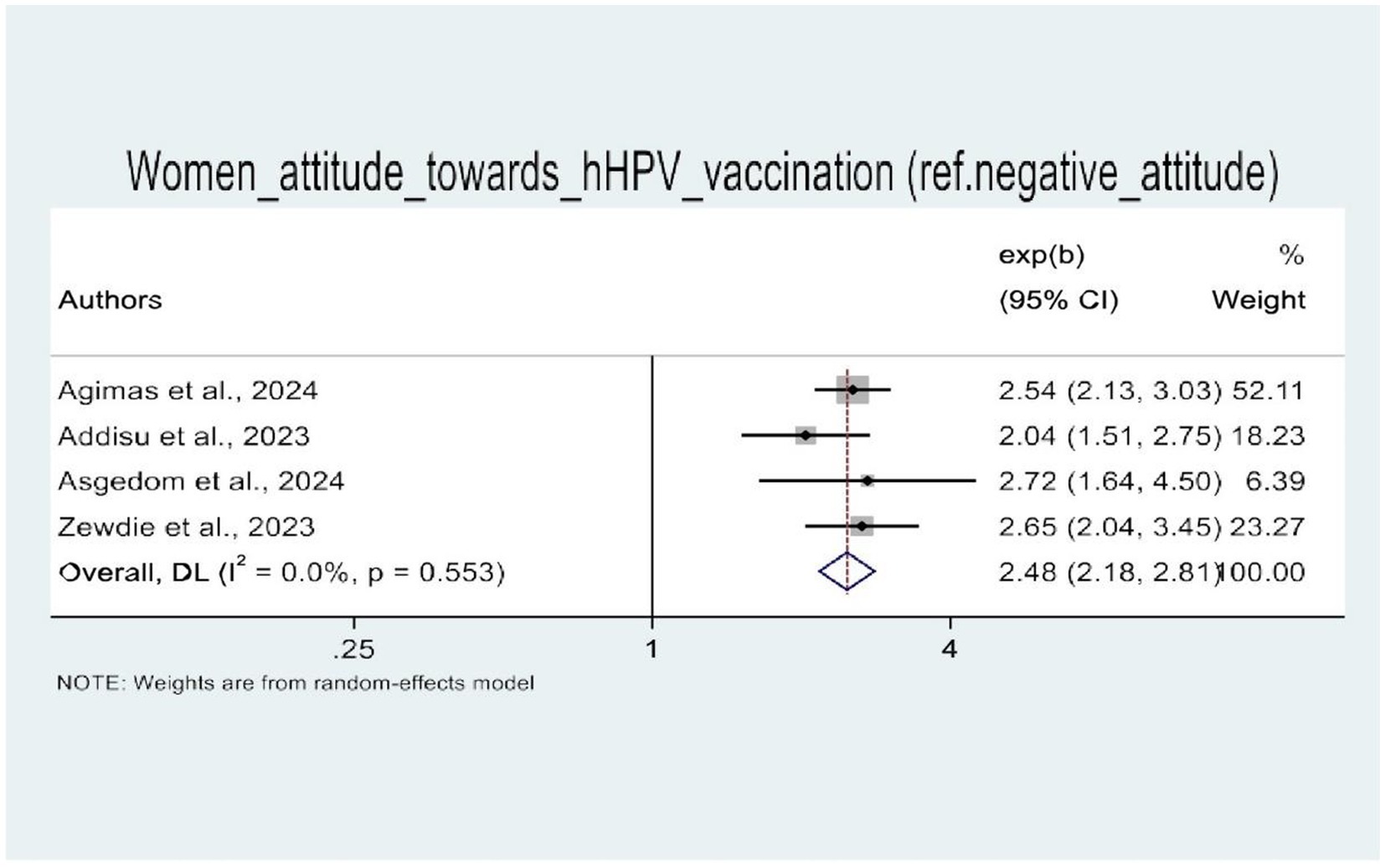
Figure 9. A forest plot that shows the pooled odds ratio of women’s attitude toward uptake of HPV vaccine in Africa.
Discussion
Cervical cancer, caused by infection with HPV, is a significant threat to public health, particularly in low- and middle-income countries (LMICs) (9). However, it is a preventable type of cancer through the prevention of HPV infection (29). This can be accomplished via the highly effective and cost-efficient preventative technique employing the HPV vaccine, which protects against the most common strains of high-risk and low-risk HPV infections (30, 31). As of 2020, over 50% of WHO member nations had implemented HPV vaccination programs to support the 2030 Sustainable Development Goal (SDG) of reaching a 90% vaccination target (9). This umbrella review of systematic reviews and meta-analyses aimed to assess the pooled prevalence of HPV vaccination and its associated factors in Africa.
In this study, the pooled prevalence of HPV vaccination in Africa was 41.38% (95% CI: 34.70–48.06). This is in agreement with findings from China (32) and LMICs, where the prevalence was 38 and 45.48%, respectively (11). However, the finding of this umbrella review is much lower than the 90% target set by the WHO Global Strategic Plan for HPV vaccination by 2030 (18), the global estimate of 77% HPV vaccine coverage (9), and national estimates such as 66% in India (33), 62.8% in the USA (34), and 55.92% in Canada (35). This discrepancy might be due to differences in the accessibility of the HPV vaccine and disparities in socioeconomic status and healthcare systems across countries. The current study combined SRM studies conducted in African countries, which are classified as LMICs, while the remaining studies were conducted in developed nations with well-structured healthcare systems, extensive immunization programs, and higher levels of awareness among women regarding cervical cancer and HPV vaccination.
To achieve the WHO cervical cancer eradication goals, efforts must be made to boost HPV vaccine access and coverage in low- and middle-income countries (36). HPV immunization is a highly cost-effective form of primary prevention, and it has been commercially available for more than 10 years. However, the coverage of HPV vaccination remains low, and the burden of cervical cancer remains unacceptably high in Africa (11, 37). Adolescents who have not yet engaged in sexual activity can effectively prevent cervical cancer by receiving preventive HPV vaccines. However, in regions with limited resources, vaccine supply and accessibility continue to be significant challenges (38). As of 2023, 29 of 54 African countries have integrated HPV vaccination into their national immunization programs (38).
On this continent, vaccination program initiatives encounter a variety of institutional and individual barriers (39, 40). Challenges related to HPV vaccine inaccessibility and logistical problems include maintaining the cold chain, disruptions caused by the COVID-19 pandemic, delayed transportation, a shortage of healthcare professionals, and a lack of monitoring and evaluation (11, 41–43). Individual-level factors, such as vaccine hesitancy, poor uptake, and missing vaccinations for different reasons (financial issues, inaccessible health facilities, lack of awareness, socio-cultural factors, and misconceptions about HPV vaccination), collectively serve as barriers to HPV vaccination in the region (42, 44, 45). Furthermore, academic institutions are at the forefront of conducting investigations and producing important information required by policymakers and other stakeholders to guide the implementation of HPV vaccination programs (46, 47).
Furthermore, a subgroup analysis by study population also revealed that the uptake of the HPV vaccine was higher among adolescents (43.01%) as compared to other age groups. This might be associated with the fact that the majority of countries focus their efforts on vaccinating adolescent girls (9–14 years). It can also relate to the fact that countries have attempted to expand school-based immunization campaigns among adolescents, which might be a possible justification for the increase in HPV vaccination rates in this group.
In this study, it was found that women’s knowledge about the HPV vaccine and their attitude toward HPV vaccination are the two predictors that are significantly associated with the uptake of the vaccine. Accordingly, having good knowledge of cervical cancer and HPV vaccination is positively associated with increased HPV vaccine uptake. This finding is consistent with the findings of SRM studies in adolescents (48), in Italy (49), and in China (32, 50).
This could be explained by women’s strong understanding of cervical cancer and HPV vaccination, which enables them to make informed decisions, resolve concerns, and actively seek out the HPV vaccine, resulting in increased HPV vaccination coverage and improved public health.
In addition, women’s attitude toward HPV vaccine use was an important determining factor. Therefore, women with a good attitude toward the HPV vaccine were more likely to receive it than those with a negative attitude toward it. This pooled effect is consistent with findings from other systematic review and meta-analysis studies (48). The most plausible explanation for this is that a good attitude toward the HPV vaccine shows favorable views, reduced misconceptions, and greater motivation, all of which contribute to a higher likelihood of vaccine uptake among women.
Overcoming negative attitudes and fostering positive knowledge about the HPV vaccine is crucial for increasing vaccination rates and ultimately improving public health. Moreover, women with a positive attitude toward the vaccine are more likely to seek out information, understand the importance of vaccination, and make a well-informed decision to get vaccinated.
Finally, the findings of this umbrella review highlight that the current overall HPV vaccination coverage in Africa is far from meeting the WHO’s cervical cancer elimination strategy by 2030. Therefore, it is essential to advocate for and expand HPV vaccination programs in all countries, promoting collaborations and partnerships to make the HPV vaccine accessible, affordable, and available in low-income countries. In addition, encouraging community participation, implementing school-based campaigns, ensuring ongoing financial support, and, most importantly, conducting regular monitoring and evaluation of the program are some of the key interventions to scale up HPV vaccine uptake.
Conclusion
In summary, the overall prevalence of HPV vaccine uptake among African women remains below half (41.38%) of the WHO’s target of 90% HPV vaccination coverage. Having good knowledge and a favorable attitude were found to be significantly associated with increased HPV vaccine uptake. Therefore, it is strongly recommended to promote awareness and understanding to increase knowledge about HPV vaccination and its benefits. In addition, addressing any unfavorable attitudes or misconceptions that may hinder the uptake of the HPV vaccine, as well as making the HPV vaccine more accessible and affordable for women in Africa, is crucial. This can be achieved through facility-based education and vaccination programs (school-based, institutional-based), planned campaigns, community-based advocacy, and the promotion of HPV vaccination and cervical cancer prevention measures via various mass media.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
BM: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. AY: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. GL: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. GM: Formal analysis, Writing – original draft, Writing – review & editing. AS: Methodology, Writing – original draft, Writing – review & editing. AC: Methodology, Writing – original draft, Writing – review & editing. GK: Software, Writing – original draft, Writing – review & editing. WW: Software, Writing – original draft, Writing – review & editing. AA: Methodology, Writing – original draft, Writing – review & editing. EG: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their heartfelt gratitude to all the authors of the systematic review and meta-analysis studies and of the primary studies included in this umbrella review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1537250/full#supplementary-material
Abbreviations
AOR, Adjusted odds ratio; CI, Confidence interval; HPV, Human papillomavirus; SSA, sub-Saharan Africa; SRM, Systematic review and meta-analysis; WHO, World Health Organization.
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Ba, DM, Ssentongo, P, Musa, J, Agbese, E, Diakite, B, Traore, CB, et al. Prevalence and determinants of cervical cancer screening in five sub-Saharan African countries: a population-based study. Cancer Epidemiol. (2021) 72:101930. doi: 10.1016/j.canep.2021.101930
3. Adeyanju, G, Essoh, T, and Sidibe, A. Human papillomavirus vaccination acceleration and introduction in sub-saharan Africa: a multi-country cohort analysis. Vaccine. (2024) 12:489. doi: 10.3390/vaccines12050489
4. Kutz, J-M, Rausche, P, Gheit, T, Puradiredja, DI, and Fusco, D. Barriers and facilitators of HPV vaccination in sub-saharan Africa: a systematic review. BMC Public Health. (2023) 23:974. doi: 10.1186/s12889-023-15842-1
5. De Martel, C, Plummer, M, Vignat, J, and Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. (2017) 141:664–70. doi: 10.1002/ijc.30716
6. Okunade, KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. (2020) 40:602–8. doi: 10.1080/01443615.2019.1634030
7. Adeyanju, GC, Sprengholz, P, Betsch, C, and Essoh, T-A. Caregivers’ willingness to vaccinate their children against childhood diseases and human papillomavirus: a cross-sectional study on vaccine hesitancy in Malawi. Vaccine. (2021) 9:1231. doi: 10.3390/vaccines9111231
8. Essoh, T-A, Adeyanju, GC, Adamu, AA, Tall, H, Aplogan, A, and Tabu, C. Exploring the factors contributing to low vaccination uptake for nationally recommended routine childhood and adolescent vaccines in Kenya. BMC Public Health. (2023) 23:912. doi: 10.1186/s12889-023-15855-w
9. Bruni, L, Saura-Lázaro, A, Montoliu, A, Brotons, M, Alemany, L, Diallo, MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. (2021) 144:106399. doi: 10.1016/j.ypmed.2020.106399
10. Nhumba, N, and Sunguya, B. Low uptake of the second dose of human papillomavirus vaccine in Dar Es Salaam, Tanzania. Vaccine. (2022) 10:1919. doi: 10.3390/vaccines10111919
11. Dorji, T, Nopsopon, T, Tamang, ST, and Pongpirul, K. Human papillomavirus vaccination uptake in low-and middle-income countries: a meta-analysis. EClinicalMedicine. (2021) 34:100836. doi: 10.1016/j.eclinm.2021.100836
12. Whitworth, HS, Gallagher, KE, Howard, N, Mounier-Jack, S, Mbwanji, G, Kreimer, AR, et al. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: a systematic review of evidence from clinical trials. Vaccine. (2020) 38:1302–14. doi: 10.1016/j.vaccine.2019.12.017
13. Schwarz, TF, Huang, LM, Valencia, A, Panzer, F, Chiu, CH, Decreux, A, et al. A ten-year study of immunogenicity and safety of the AS04-HPV-16/18 vaccine in adolescent girls aged 10-14 years. Hum Vaccin Immunother. (2019) 15:1970–9. doi: 10.1080/21645515.2019.1625644
14. Castle, P, and Maza, M. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect. (2016) 144:449–68. doi: 10.1017/S0950268815002198
15. WHO (2020). World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. World Health Organization.
16. Basu, P, Malvi, SG, Joshi, S, Bhatla, N, Muwonge, R, Lucas, E, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. (2021) 22:1518–29. doi: 10.1016/S1470-2045(21)00453-8
17. Bruni, L, Diaz, M, Barrionuevo-Rosas, L, Herrero, R, Bray, F, Bosch, FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. (2016) 4:e453–63. doi: 10.1016/S2214-109X(16)30099-7
18. Drolet, M, Bénard, É, Pérez, N, Brisson, M, Ali, H, Boily, M-C, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. (2019) 394:497–509. doi: 10.1016/S0140-6736(19)30298-3
19. WHO. (2024). WHO. Cervical cancer. Available at: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (Accessed March 5, 2024).
20. Denny, L. HPV vaccine introduction and implementation in low-and middle-income countries. Vaccine. (2022) 40 Suppl 1:A1. doi: 10.1016/j.vaccine.2022.02.074
21. Agimas, MC, Adugna, DG, Derseh, NM, Kassaw, A, Kassie, YT, Abate, HK, et al. Uptake of human papilloma virus vaccine and its determinants among females in East Africa: a systematic review and meta-analysis. BMC Public Health. (2024) 24:842. doi: 10.1186/s12889-024-18141-5
22. Addisu, D, Gebeyehu, NA, and Belachew, YY. Knowledge, attitude, and uptake of human papillomavirus vaccine among adolescent schoolgirls in Ethiopia: a systematic review and meta-analysis. BMC Womens Health. (2023) 23:279. doi: 10.1186/s12905-023-02412-1
23. Asgedom, YS, Kebede, TM, Seifu, BL, Mare, KU, Asmare, ZA, Asebe, HA, et al. Human papillomavirus vaccination uptake and determinant factors among adolescent schoolgirls in sub-Saharan Africa: a systematic review and meta-analysis. Hum Vaccin Immunother. (2024) 20:2326295. doi: 10.1080/21645515.2024.2326295
24. Zewdie, A, Kasahun, AW, Habtie, A, Gashaw, A, and Ayele, M. Human papillomavirus vaccine acceptance among adolescent girls in Ethiopia: a systematic review and meta-analysis. BMC Public Health. (2023) 23:1369. doi: 10.1186/s12889-023-16305-3
25. Derbie, A, Mekonnen, D, Misgan, E, Maier, M, Woldeamanuel, Y, and Abebe, T. Acceptance of human papillomavirus vaccination and parents’ willingness to vaccinate their adolescents in Ethiopia: a systematic review and meta-analysis. Infectious Agents Cancer. (2023) 18:59. doi: 10.1186/s13027-023-00535-6
26. Shea, BJ, Reeves, BC, Wells, G, Thuku, M, Hamel, C, Moran, J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ Clin Res Ed. (2017) 358, 1–9. doi: 10.1136/bmj.j4008
27. Ioannidis, JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. (2008) 14:951–7. doi: 10.1111/j.1365-2753.2008.00986.x
28. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
29. Cheng, L, Wang, Y, and Du, J. Human papillomavirus vaccines: an updated review. Vaccine. (2020) 8. doi: 10.3390/vaccines8030391
30. Gervais, F, Dunton, K, Jiang, Y, and Largeron, N. Systematic review of cost-effectiveness analyses for combinations of prevention strategies against human papillomavirus (HPV) infection: a general trend. BMC Public Health. (2017) 17:283. doi: 10.1186/s12889-017-4076-3
31. Tran, PT, Riaz, M, Chen, Z, Truong, CB, and Diaby, V. An umbrella review of the cost effectiveness of human papillomavirus vaccines. Clin Drug Investig. (2022) 42:377–90. doi: 10.1007/s40261-022-01155-5
32. Chan, DNS, Li, C, Law, BMH, Choi, KC, Lee, PPK, and So, WKW. Factors affecting HPV vaccine uptake among ethnic minority adolescent girls: a systematic review and meta-analysis. Asia Pac J Oncol Nurs. (2023) 10:100279. doi: 10.1016/j.apjon.2023.100279
33. Swarnapriya, K, Kavitha, D, and Reddy, GM. Knowledge, attitude and practices regarding HPV vaccination among medical and Para medical in students, India a cross sectional study. Asian Pac J Cancer Prev. (2015) 16:8473–7. doi: 10.7314/apjcp.2015.16.18.8473
34. Wilson, AR, Hashibe, M, Bodson, J, Gren, LH, Taylor, BA, Greenwood, J, et al. Factors related to HPV vaccine uptake and 3-dose completion among women in a low vaccination region of the USA: an observational study. BMC Womens Health. (2016) 16:41. doi: 10.1186/s12905-016-0323-5
35. Bird, Y, Obidiya, O, Mahmood, R, Nwankwo, C, and Moraros, J. Human papillomavirus vaccination uptake in Canada: a systematic review and Meta-analysis. Int J Prev Med. (2017) 8:1–9. doi: 10.4103/ijpvm.IJPVM_49_17
36. Guillaume, D, Waheed, D-e-N, Schleiff, M, Muralidharan, KK, Vorsters, A, and Limaye, RJ. Global perspectives of determinants influencing HPV vaccine introduction and scale-up in low-and middle-income countries. PLoS One. (2024) 19:e0291990. doi: 10.1371/journal.pone.0291990
37. Cunningham, MS, Davison, C, and Aronson, KJ. HPV vaccine acceptability in Africa: a systematic review. Prev Med. (2014) 69:274–9. doi: 10.1016/j.ypmed.2014.08.035
38. Asempah, E, and Ikpebe, E. Accelerating HPV vaccination in Africa for health equity. Global Health Research Policy. (2024) 9:37. doi: 10.1186/s41256-024-00380-z
39. Mavundza, EJ, Cooper, S, and Wiysonge, CS. A systematic review of factors that influence parents' views and practices around routine childhood vaccination in Africa: a qualitative evidence synthesis. Vaccines. (2023) 11. doi: 10.3390/vaccines11030563
40. Oketch, SY, Ochomo, EO, Orwa, JA, Mayieka, LM, and Abdullahi, LH. Communication strategies to improve human papillomavirus (HPV) immunisation uptake among adolescents in sub-Saharan Africa: a systematic review and meta-analysis. BMJ Open. (2023) 13:e067164. doi: 10.1136/bmjopen-2022-067164
41. Ekezie, W, Igein, B, Varughese, J, Butt, A, Ukoha-Kalu, BO, Ikhile, I, et al. Vaccination communication strategies and uptake in Africa: a systematic review. Vaccine. (2024) 12:1333. doi: 10.3390/vaccines12121333
42. Janusz, CB, Frye, M, Mutua, MK, Wagner, AL, Banerjee, M, and Boulton, ML. Vaccine delay and its association with undervaccination in children in sub-Saharan Africa. Am J Prev Med. (2021) 60:S53–64. doi: 10.1016/j.amepre.2020.10.003
43. Ackah, BB, Woo, M, Stallwood, L, Fazal, ZA, Okpani, A, Ukah, UV, et al. COVID-19 vaccine hesitancy in Africa: a scoping review. Glob Health Res Policy. (2022) 7:21. doi: 10.1186/s41256-022-00255-1
44. Mbonigaba, E, Nderu, D, Chen, S, Denkinger, C, Geldsetzer, P, McMahon, S, et al. Childhood vaccine uptake in Africa: threats, challenges, and opportunities. J Glob Health Rep. (2021) 5:e2021080. doi: 10.29392/001c.26312
45. Razai, MS, Oakeshott, P, Esmail, A, Wiysonge, CS, Viswanath, K, and Mills, MC. COVID-19 vaccine hesitancy: the five Cs to tackle behavioural and sociodemographic factors. J R Soc Med. (2021) 114:295–8. doi: 10.1177/01410768211018951
46. Guillaume, D, Meyer, D, Waheed, D-e-N, Schlieff, M, Muralidharan, K, Chou, VB, et al. Factors influencing the prioritization of vaccines by policymakers in low-and middle-income countries: a scoping review. Health Policy Plan. (2023) 38:1–30. doi: 10.1093/heapol/czac092
47. Guillaume, D, Waheed, D-e-N, Schlieff, M, Muralidharan, K, Vorsters, A, and Limaye, R. Key decision-making factors for human papillomavirus (HPV) vaccine program introduction in low-and-middle-income-countries: global and national stakeholder perspectives. Hum Vaccin Immunother. (2022) 18:2150454. doi: 10.1080/21645515.2022.2150454
48. Kessels, SJ, Marshall, HS, Watson, M, Braunack-Mayer, AJ, Reuzel, R, and Tooher, RL. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. (2012) 30:3546–56. doi: 10.1016/j.vaccine.2012.03.063
49. Cocchio, S, Bertoncello, C, Baldovin, T, Fonzo, M, Bennici, SE, Buja, A, et al. Awareness of HPV and drivers of HPV vaccine uptake among university students: a quantitative, cross-sectional study. Health Soc Care Community. (2020) 28:1514–24. doi: 10.1111/hsc.12974
Keywords: Africa, human papillomavirus vaccine, HPV vaccine, umbrella review, uptake
Citation: Mengistie BA, Yirsaw AN, Lakew G, Mekonnen GB, Shibabaw AA, Chereka AA, Kitil GW, Wondie WT, Abuhay AE and Getachew E (2025) Human papillomavirus vaccine uptake and its determinants among women in Africa: an umbrella review. Front. Public Health. 13:1537250. doi: 10.3389/fpubh.2025.1537250
Edited by:
Harris Onywera, Africa Centres for Disease Control and Prevention, EthiopiaReviewed by:
Eiman Alawadhi, Kuwait University, KuwaitDebkumar Pal, All India Institute of Medical Sciences, India
Copyright © 2025 Mengistie, Yirsaw, Lakew, Mekonnen, Shibabaw, Chereka, Kitil, Wondie, Abuhay and Getachew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Berihun Agegn Mengistie, YmVyaWh1bmFnZWduMjFAZ21haWwuY29t
 Berihun Agegn Mengistie
Berihun Agegn Mengistie Amlaku Nigusie Yirsaw
Amlaku Nigusie Yirsaw Gebeyehu Lakew
Gebeyehu Lakew Gebrehiwot Berie Mekonnen
Gebrehiwot Berie Mekonnen Adamu Ambachew Shibabaw
Adamu Ambachew Shibabaw Alex Ayenew Chereka
Alex Ayenew Chereka Gemeda Wakgari Kitil
Gemeda Wakgari Kitil Wubet Tazeb Wondie
Wubet Tazeb Wondie Alemken Eyayu Abuhay
Alemken Eyayu Abuhay Eyob Getachew
Eyob Getachew