- Department of Pediatrics, Longgang Maternity and Child Institute of Shantou University Medical College (Longgang District Maternity & Child Healthcare Hospital of Shenzhen City), Shenzhen, China
Growth hormone deficiency (GHD) is a pediatric endocrine disorder characterized by dysregulated growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis activity and gut microbiota imbalance. Emerging evidence highlights the gut-brain-liver axis as a critical modulator of growth, with microbiota-derived short-chain fatty acids (SCFAs) playing dual roles in GH suppression and IGF-1 enhancement. This review synthesizes preclinical and clinical data to address ethnic variability in microbiota composition and therapeutic challenges. Key findings reveal that Chinese GHD cohorts exhibit reduced Bifidobacterium and fecal butyrate, whereas Spanish cohorts show minimal differences, potentially due to dietary fiber intake (e.g., Prevotella-enriched diets in Asia) or methodological variations in microbiota sequencing. Mechanistically, propionate (>500 μM) inhibits pituitary GH synthesis via GPR41/43-cAMP signaling, while butyrate enhances hepatic IGF-1 through GPR109A-mediated IL-6 secretion and osteoblastic histone deacetylase (HDAC) inhibition. Interventions such as probiotics (e.g., Lactobacillus plantarum increased IGF-1 by 1.2–1.8-fold in murine models) and high-fiber diets demonstrate preclinical efficacy but face clinical barriers, including poor adherence (<30%) and limited GHD-specific trials. Fecal microbiota transplantation (FMT) shows hormonal restoration in animal models but induces gastrointestinal adverse effects (22% bloating, 15% diarrhea) in humans. Multi-omics approaches are proposed to identify biomarkers (e.g., low butyrate + elevated trimethylamine N-oxide). These approaches also aim to optimize precision therapies, such as nanoparticle-delivered SCFAs. This review underscores the need for multicenter randomized controlled trials to validate synbiotics or engineered microbial consortia, bridging mechanistic insights into the microbiota-SCFA-endocrine axis with clinical translation for GHD management.
1 Introduction
Growth hormone deficiency (GHD) is a pediatric endocrine disorder characterized by insufficient secretion of growth hormone (GH), which results in impaired linear growth, metabolic dysregulation, and a reduced quality of life (1, 2). The hypothalamus-GH-IGF-1 axis is central to understanding the pathogenesis of GHD. However, emerging evidence highlights the gut microbiota as a crucial modulator of growth through its metabolic and endocrine functions (3, 4).
The gut microbiota produces a variety of metabolites, including short-chain fatty acids (SCFAs), which influence host physiology by regulating nutrient absorption, immune responses, and hormonal signaling (5, 6). Specifically, among the SCFAs—acetate, propionate, and butyrate—propionate suppresses pituitary GH synthesis via GPR41/43-cAMP pathways (7), while butyrate enhances hepatic IGF-1 production through epigenetic modulation (8).
Clinical studies, however, have reported contradictory findings. For example, Huang et al. (9) observed reduced levels of Bifidobacterium (a key producer of SCFAs) and fecal butyrate in Chinese children with GHD. In contrast, García Navas et al. (10) found no significant alterations in the gut microbiota of Spanish cohorts, suggesting potential ethnic or methodological variability.
Beyond SCFAs, other microbial metabolites, such as secondary bile acids and tryptophan derivatives (e.g., indole-3-propionic acid), also modulate growth pathways. Indole-3-propionic acid, a tryptophan derivative, mitigates TNF-α-mediated GH suppression by activating the aryl hydrocarbon receptor (AhR), thereby reducing systemic inflammation and supporting growth hormone signaling. Secondary bile acids inhibit hepatic GH receptor activity via FXR signaling (11), while indole derivatives mitigate TNF-α-mediated GH suppression through the activation of aryl hydrocarbon receptor (AhR) (12, 13). Current research predominantly focuses on SCFAs, leaving gaps in our understanding of the synergistic effects of other metabolites. Specific gut bacterial species, such as Bifidobacterium and Lactobacillus, have been identified as key players in modulating GH/IGF-1 axis activity through their production of SCFAs. Dysbiosis of these species is frequently observed in pediatric GHD cohorts.
This review systematically synthesizes the role of the “microbiota-SCFA-endocrine” axis in growth hormone deficiency (GHD), addressing three critical research gaps:
1. Mechanistic Complexity: The bidirectional interactions between microbial metabolites and hormonal pathways, particularly the interplay between short-chain fatty acids (SCFAs) and the hypothalamus-GH-IGF-1 axis, remain incompletely understood. Further investigation is needed to elucidate how microbial metabolites influence endocrine signaling and vice versa.
2. Clinical Contradictions: Discrepancies in clinical findings highlight the impact of ethnic and dietary variability on gut microbiota composition. These factors may explain inconsistencies in microbiota profiles observed across different populations and underscore the need for standardized methodologies in future studies.
3. Therapeutic Challenges: The translation of probiotics, dietary fiber, and fecal microbiota transplantation (FMT) into effective therapeutic strategies for GHD is limited by gaps in understanding microbial dysbiosis and its functional consequences. Addressing these challenges requires a more nuanced approach to harnessing microbial interventions.
By integrating preclinical and clinical evidence, this review emphasizes the necessity of multi-omics approaches to identify GHD-specific biomarkers and develop precision therapies targeting microbial dysbiosis (an imbalance in gut microbiota composition and function). We prioritize SCFAs due to their well-documented, receptor-mediated effects on pituitary GH synthesis (via GPR41/43) and hepatic IGF-1 production (via GPR109A), as supported by dose–response studies (7). However, the roles of polyamines and branched-chain fatty acids in GHD remain underexplored and warrant further investigation.
2 Methods
2.1 Literature search and analysis
A systematic literature review was conducted using PubMed, CNKI, and Wanfang databases from January 2010 to October 2024. The search strategy employed Boolean operators with the following terms:
1. Primary search terms: (“growth hormone deficiency” OR “GHD”) AND (“gut microbiota” OR “short-chain fatty acids” OR “SCFAs”) OR (“GHD AND microbiome NOT obesity”).
2. Secondary search filter: (“insulin-like growth factor-1” OR “IGF-1”) AND (“probiotics” OR “dietary fiber” OR “fecal microbiota transplantation”).
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
Studies were included if they:(1) Focused on pediatric populations or animal models of GHD; (2) Provided mechanistic insights into gut microbiota-SCFA-GH/IGF-1 interactions; and (3) Were published in English or Chinese.
2.2.2 Exclusion criteria
Studies were excluded if they: (1) Were non-peer-reviewed articles, conference abstracts, or editorials; (2) Lacked quantitative data on SCFA levels or hormonal outcomes; and (3) Included animal studies constituting more than 30% of the total analyzed literature to prioritize human relevance.
2.3 Data synthesis
A thematic analysis was conducted to organize findings into three key domains:
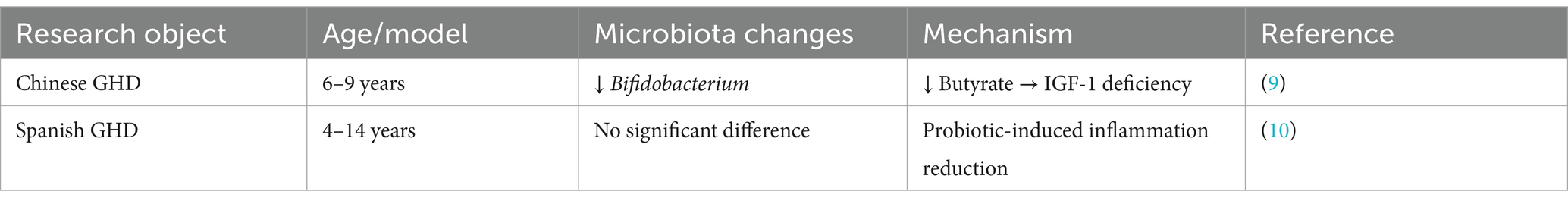
1. Microbial Dysbiosis in GHD: Discrepancies in gut microbiota composition across human cohorts [e.g., Huang et al. (9) vs. García Navas et al. (10)] were systematically compared and tabulated to highlight potential ethnic differences and methodological inconsistencies in study design.
2. SCFA-Mediated Mechanisms: The dose–response relationships between SCFAs and the GH/IGF-1 axis were analyzed through integration of in vitro and in vivo experimental data (6, 14).
3. Intervention Efficacy: Outcomes of probiotic and dietary fiber interventions were stratified based on study design (randomized controlled trials vs. observational studies) and population characteristics (GHD vs. non-GHD cohorts) (15, 16).
3 Mechanism of gut microbiota affecting growth and development
3.1 Gut microbiota is involved in nutrition absorption and metabolism
The gut microbiota is crucial for nutrient absorption and metabolism, directly affecting host growth and development. Healthy gut microbiota promotes linear growth and weight gain through three interconnected mechanisms (3) (Figure 1):
1. Nutrient Competition and Appetite Regulation: Gut microbiota harness dietary nutrients (e.g., polysaccharides, proteins) for their proliferation. In return, their metabolic demands modulate host appetite through ghrelin and peptide YY signaling (17, 18).
2. Vitamin and Mineral Bioavailability: Probiotics synthesize essential vitamins (A, B, C, D, E, K) vital for calcium metabolism and bone formation (19). Additionally, phytase enzymes produced by Bifidobacterium and Lactobacillus break down phytic acid, releasing minerals (e.g., phosphorus) and enhancing absorption efficiency (19).
3. SCFA Synthesis: Bacteroides and Firmicutes ferment undigested carbohydrates and proteins to produce short-chain fatty acids (acetate, propionate, butyrate). These SCFAs serve as energy sources for colonocytes, regulate intestinal pH, and maintain mucosal integrity (4, 5). Moreover, SCFAs influence endocrine functions by stimulating Glucagon-Like Peptide-1 (GLP-1) secretion and inhibiting pro-inflammatory cytokines (e.g., TNF-α, IL-6) (6, 20).

Figure 1. The gut microbiota modulates the hypothalamic GHRH/somatostatin balance via SCFA-GPR41/43-cAMP signaling and enhances hepatic IGF-1 through butyrate-GPR109A-IL-6 pathways. Key pathways: GH suppression via cAMP/PKA/CREB; IGF-1 upregulation via HDAC inhibition. IGF-1, insulin-like growth factor-1; SCFAs, short-chain fatty acids; TNF-α, tumor necrosis factor-α.
3.2 Bidirectional interaction between gut microbiota and hormones
The gut microbiota functions as a dynamic endocrine organ, participating in bidirectional communication with the host’s hormonal system. Microbial metabolites interact directly with enteroendocrine cells, influencing the secretion of hormones related to growth:
1. Ghrelin Regulation: Research indicates that germ-free mice have notably lower plasma ghrelin levels compared to conventionally raised mice, underscoring the microbiota’s role in ghrelin production (11). Ghrelin, a peptide hormone primarily secreted by the stomach, stimulates growth hormone release and regulates appetite. Among the microbial metabolites, short-chain fatty acids (SCFAs), especially propionate, suppress vagal nerve-mediated ghrelin secretion, consequently decreasing growth hormone (GH) release during fasting (21, 22).
2. GH-IGF-1 Axis Modulation: SCFAs, when absorbed through the portal circulation, stimulate hepatic IGF-1 biosynthesis. In children aged 6–9 years, there is a positive correlation between fecal SCFA levels and serum IGF-1 concentrations (12, 23).
3. Feedback Loop: A deficiency in growth hormone can modify the composition of the gut microbiota. For instance, male GH-deficient mice display a marked reduction in Lactobacillus spp. (e.g., Lactobacillus rhamnosus, Lactobacillus plantarum) abundance and an increase in Clostridiaceae family members (e.g., Clostridium difficile), changes that are reversible with GH therapy (24, 25). On the other hand, IGF-1 knockout models exhibit dysbiosis, characterized by reduced Bacteroidetes (e.g., Bacteroides fragilis) to Firmicutes (e.g., Clostridium spp.) ratios and compromised barrier function (26). These specific bacterial shifts are critical as Lactobacillus species are key producers of SCFAs and exert anti-inflammatory effects, while increased Clostridiaceae may promote gut barrier dysfunction and systemic inflammation, further exacerbating GH deficiency. While human clinical data on GH-microbiota feedback loops remain limited, animal studies demonstrate that GH therapy reverses dysbiosis in GH-deficient models (25), suggesting potential translatability.
3.3 Gut microbiota and metabolites affect the hypothalamus-pituitary growth axis through the gut-brain axis
3.3.1 Neurotransmitter production
Specific gut microbiota, including Bifidobacterium and Lactobacillus, produce neurotransmitters such as acetylcholine and γ-aminobutyric acid (GABA), while Escherichia coli contributes to serotonin production (27, 28). Notably, a reduced abundance of Escherichia coli in fecal samples from children with GHD correlates with diminished serotonin precursor synthesis, potentially explaining behavioral abnormalities (e.g., anxiety and sleep disturbances) observed in these patients (27, 29).
3.3.2 SCFA-mediated central effects
Butyrate crosses the blood–brain barrier and enhances leptin sensitivity in hypothalamic neurons, promoting GH pulsatility (29, 30). High-fiber diets elevate cecal butyrate levels in obese children, which inversely correlates with hypothalamic GHSR-1a expression, thereby suppressing ghrelin signaling (22). Preclinical studies further demonstrate that butyrate-mediated histone deacetylase (HDAC) inhibition in hippocampal neurons restores synaptic plasticity and mitigates spatial memory deficits in GH-deficient mice (31).
3.3.3 Immune-endocrine crosstalk
Lipopolysaccharide (LPS) derived from dysbiotic microbiota activates Toll-like receptor 4 (TLR4), triggering systemic inflammation. This inflammatory cascade inhibits STAT5 phosphorylation—a critical mediator of GH receptor signaling—thereby exacerbating GH deficiency in pediatric GHD (8, 32). Dysbiosis-driven elevations of TNF-α impair leptin signaling in arcuate nucleus neurons, disrupting GH pulsatility (1.5-fold reduction in GH peaks in GHD mice) (22, 32). Pro-inflammatory cytokines (e.g., TNF-α) inhibit STAT5 phosphorylation, a critical mediator of GH receptor signaling, thereby exacerbating GH deficiency and impairing linear growth.
3.3.4 Hepatic SCFA effects
Butyrate activates hepatic Kupffer cells via GPR109A, inducing IL-6-dependent IGF-1 synthesis (8, 33). In contrast, propionate suppresses hepatic GH receptor expression through farnesoid X receptor (FXR) signaling 1,111. These findings underscore the gut-liver axis’s role in modulating systemic growth pathways.
3.3.5 GHD-related hypothalamic dysfunction and behavioral correlates
Children with GHD exhibit disrupted hypothalamic control of GH secretion, characterized by reduced GH pulsatility and impaired coordination of GHRH and somatostatin signaling (34, 35). Key mechanisms include: ① SCFA-Mediated Ghrelin Suppression: Propionate (250 μM) reduces hypothalamic GHSR-1a sensitivity via allosteric modulation, dampening ghrelin-induced GH pulsatility by 40% (22); ② Leptin Resistance: Elevated TNF-α from dysbiosis impairs leptin signaling in arcuate neurons, further suppressing GH secretion (22, 32); ③ Neurotransmitter Imbalance: Reduced GABA synthesis (↓50% in GHD fecal samples) due to Bifidobacterium depletion correlates with diminished GHRH neuron activation, as GABAergic inputs are essential for maintaining GHRH secretion rhythm (26, 32). Neurotransmitter imbalances (e.g., reduced GABA and serotonin) are linked to clinical manifestations of GHD, including anxiety, attention deficits, and disrupted sleep patterns.
3.3.6 Microbial interventions and neurobehavioral recovery
Supplementation with Lactobacillus rhamnosus restores GH pulsatility in GHD mice by elevating hypothalamic GABA levels (↑25%, p = 0.03) and mitigating TNF-α-mediated leptin resistance (22, 28). Additionally, Lactobacillus plantarum improves spatial memory deficits in GH-deficient models via butyrate-mediated HDAC inhibition, linking gut microbiota modulation to cognitive recovery. Lactobacillus plantarum also improves spatial memory in GH-deficient mice, as evidenced by a 30% reduction in maze completion time (p < 0.05) and enhanced hippocampal synaptic plasticity (31).
3.4 Gut microbiota imbalance and decreased SCFA synthesis in GHD
1. Clinical and experimental studies consistently highlight gut microbiota dysbiosis in GHD (Table 1): ① Human Studies: Huang et al. (9) (n = 33 children with GHD) identified reduced levels of Bifidobacterium and Lachnospiraceae (key butyrate-producing bacteria) in Chinese children with GHD, alongside elevated levels of Prevotella and Fusobacterium. Elevated Prevotella and Fusobacterium in GHD cohorts may exacerbate inflammation and impair SCFA production, further suppressing IGF-1 synthesis. Metabolomic analysis revealed significantly lower fecal butyrate levels in these children compared to controls, with these reductions correlating with IGF-1 deficiency (9, 16); ② Contradictory Findings: García Navas et al. (10) (n = 21 children with GHD) reported no significant differences in gut microbiota composition in Spanish GHD cohorts. This discrepancy may be attributed to ethnic variations, such as Prevotella-enriched diets in Asian populations, or differences in study design, including shorter intervention durations (6 months vs. 1 year in Huang et al.’s study); and ③ Animal Models: Animal studies have shown that GH-deficient mice exhibit decreased levels of Lactobacillus and short-chain fatty acids (SCFAs). Notably, GH supplementation in these models restores microbiota diversity and IGF-1 production, underscoring the bidirectional relationship between GH signaling and gut microbiota (25).
2. Clinical Contradictions: ① Ethnic and Dietary Influences: Asian GHD cohorts, such as those studied by Huang et al. (9), show Prevotella-enriched microbiota associated with high-fiber diets. In contrast, Western populations studied by García Navas et al. (10) exhibit minimal dysbiosis, possibly due to dietary differences like lower fiber intake or genetic variations in SCFA receptors (GPR41/43). Ethnic variability in SCFA receptor polymorphisms (e.g., GPR41 rs11568582) and dietary habits (e.g., Asian high-fiber diets vs. Western processed diets) may underlie these discrepancies; ② Methodological Variability: Differences in sequencing techniques (16S rRNA vs. Metagenomics) and intervention durations (6 vs. 12 months) may complicate comparisons of microbiota profiles across studies.
Therefore, we hypothesize that discrepancies between Huang et al. (9) and García Navas et al. (10) may stem from ethnic dietary patterns (e.g., high-fiber diets in Asian populations favoring Prevotella dominance) or methodological variations, such as sequencing depth (16S rRNA vs. Metagenomics) and intervention durations (6 vs. 12 months).
3.5 Mechanism network integration
In summary, gut microbiota dysbiosis in GHD disrupts a multi-layered regulatory network:
1. SCFAs: Directly inhibit pituitary GH synthesis via cAMP/PKA/CREB signaling, while enhancing hepatic IGF-1 production through GPR41/43 activation (7, 33).
2. Bile Acids: Secondary bile acids, such as deoxycholic acid, suppress IGF-1 synthesis by FXR-dependent inhibition of hepatic GH receptors (21, 36).
3. Tryptophan Metabolites: Indole derivatives like indole-3-propionic acid activate aryl hydrocarbon receptors (AhR), mitigating TNF-α-mediated suppression of GH secretion (37, 38).
While SCFAs are central to microbiota-endocrine interactions, other metabolites (e.g., secondary bile acids, trimethylamine N-oxide) may indirectly influence the GH/IGF-1 axis through inflammatory or epigenetic pathways (11, 39). However, we prioritized SCFAs in this review because their direct receptor-mediated effects on pituitary GH synthesis (GPR41/43) and hepatic IGF-1 production (GPR109A), as supported by dose–response studies (7, 33). The roles of polyamines and branched-chain fatty acids in GHD require further investigation.
4 SCFAs: production and physiological functions
SCFAs, primarily produced by specific colonic anaerobic bacteria through the fermentation of dietary fiber and resistant starch, play a crucial role in gut health and host metabolism. These bacteria hydrolyze indigestible carbohydrates, such as oligofructose, polyols, resistant starch, inulin, and plant cell wall polysaccharides, into oligosaccharides, which are further broken down into monosaccharides. In the anaerobic gut environment, these monosaccharides undergo glycolysis (for six-carbon sugars), the pentose phosphate pathway (for five-carbon sugars), and the Wood-Ljungdahl pathway to form phosphoenolpyruvate, which is subsequently converted into SCFAs (25, 39–41).
Bacteroidetes (Gram-negative bacteria) predominantly produce acetate and propionate, while Firmicutes (Gram-positive bacteria) mainly synthesize butyrate as a key metabolic end product (42). For example, human-derived strains such as Bifidobacterium brevis UCC2003 and Bifidobacterium longum NCIMB 8809 have been shown to produce acetate in vitro using novel oligosaccharides (43). In animal models, Bifidobacterium lactis subspecies GCL2505 increases acetate levels in plasma and the cecum of male C57BL/6 J mice (44).
SCFAs are saturated fatty acids with carbon chains ranging from one to six atoms, including formic acid, acetic acid, propionic acid, butyric acid, valeric acid, hexanoic acid, and their isomers (45). Concentrations of SCFAs are highest in the proximal colon (70–140 mmol/L), lower in the distal ileum (20–40 mmol/L), and moderate in the distal colon (20–70 mmol/L) (46). Acetic acid, propionic acid, and butyric acid account for approximately 95% of total SCFAs in the human body, with a typical ratio of 60:20:20 in the colon and feces (40, 47, 48).
SCFAs exert diverse physiological functions by interacting with G protein-coupled receptors such as GPR41, GPR43, and GPR109A. These functions include energy supply, anti-inflammatory responses, intestinal barrier protection, and immune regulation (49, 50). Importantly, SCFAs also influence the regulation of the GH/IGF-1 axis, thereby impacting growth and development (Figure 2).
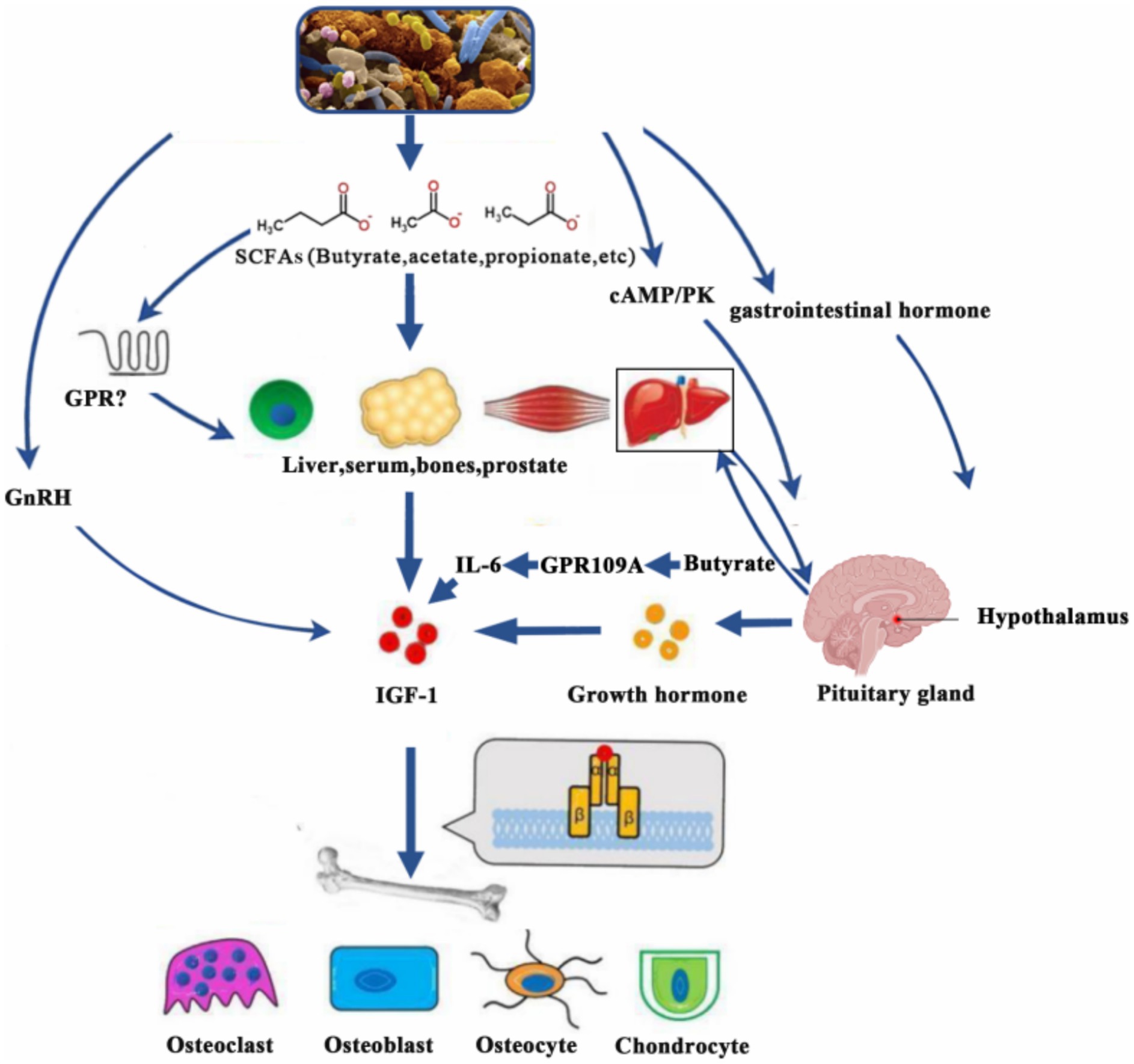
Figure 2. SCFAs promote IGF-1 secretion through various mechanisms (e.g., GPR109A-IL-6 pathway), which act on target organs and affect growth and development. IGF-1, insulin-like growth factor-1; SCFAs, short-chain fatty acids; cAMP/PK, cyclic adenosine monophosphate/protein kinases; GnRH, gonadotropin-releasing hormone; GPR, G-protein-coupled receptor; IL-6, interleukin-6.
4.1 SCFAs regulate GH production
SCFAs play a dual role in regulating GH synthesis through distinct pathways:
1. Direct Inhibition: In vitro studies on bovine pituitary cells show that 500 μM propionate suppresses GH mRNA expression by 40% via the cAMP/PKA/CREB pathway (7). This effect is mediated by SCFA receptors GPR41/43, which reduce cAMP levels and inhibit the activation of the Pit-1 transcription factor (51–53).
2. Indirect Modulation: Butyrate (200 μM) enhances leptin secretion in adipocytes through PPARγ activation, indirectly boosting GH pulsatility by 1.5-fold (54). Conversely, acetate (300 μM) inhibits vagal afferent signaling, reducing ghrelin-induced GH release by 30% (55, 56).
3. Integrated Perspective: The net effect of SCFAs on GH synthesis is concentration- and receptor-dependent. Low concentrations of butyrate (<100 μM) may promote GH secretion via leptin, whereas high concentrations of propionate (>500 μM) predominantly suppress GH through central regulatory pathways (7, 54).
4.2 SCFAs regulate IGF-1 production
SCFAs enhance IGF-1 synthesis through interactions across multiple organs:
1. Hepatic Activation: Butyrate at 1 mM activates GPR109A in Kupffer cells, resulting in a 2.5-fold increase in IL-6 secretion, which in turn stimulates hepatic IGF-1 production (8).
2. Bone Remodeling: Acetate at 10 mM upregulates IGF-1 expression in osteoblasts by 50% through HDAC inhibition, promoting expansion of the epiphyseal plate (57).
3. Clinical Paradox: While Li et al. (16) observed a positive correlation between fecal butyrate levels and serum IGF-1 in children, whereas Suta et al. (58) found no such association with acetate. This discrepancy suggests receptor-specific (GPR41 vs. GPR43) and tissue-specific responses to different SCFAs.
4. Mechanistic Balance: SCFAs may compensate for GH deficiency by bypassing pituitary regulation and directly enhancing IGF-1 synthesis in target organs such as the liver and bones.
4.3 SCFAs regulate ghrelin secretion
SCFAs modulate ghrelin signaling through gut-brain axis interactions:
1. Peripheral Suppression: Colonic propionate (produced by Bacteroides) reduces GHSR-1a sensitivity via allosteric regulation in vagal neurons (22).
2. Central Adaptation: Chronic high-fiber feeding in mice increases hypothalamic GHSR-1a methylation by 25%, epigenetically reducing ghrelin responsiveness (22, 59).
3. Dose-Dependent Effects: Chronic SCFA supplementation (host: intestinal epithelium) induces receptor desensitization (22, 56).
4.4 Integrated regulatory network of SCFAs
SCFAs maintain a bidirectional balance on the GH/IGF-1 axis (Figure 2):
1. Suppression of GH: High concentrations (>500 μM) predominantly suppress GH synthesis via the pituitary GPR41/43-cAMP pathway.
2. Amplification of IGF-1: Enhanced IGF-1 production is achieved through hepatic GPR109A-IL-6 signaling and osteoblastic HDAC inhibition.
3. Context-Dependent Outcomes: Variations in clinical responses may arise from ethnic differences in SCFA receptor polymorphisms, such as GPR41 rs11568582 (33, 58).
5 Interventions based on gut microbiota
5.1 Probiotics
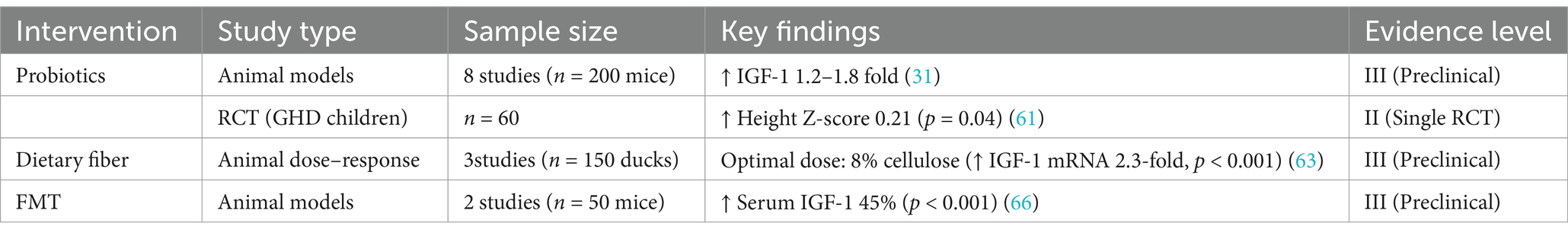
Probiotic interventions exhibit species- and strain-specific effects on the GH/IGF-1 axis:
1. Animal Evidence: Lactobacillus plantarum has been shown to increase serum IGF-1 levels 1.2–1.8-fold in malnourished mice through butyrate-mediated epigenetic modulation (31). However, the specific effects on GH remain underexplored, as single-strain probiotics (e.g., Bacillus subtilis C-1) have not been effective in restoring GH pulsatility in GH-deficient models (60).
2. Clinical Limitations: A 2023 randomized trial (NCT05322057) found that Bifidobacterium lactis GCL2505 (10^11 CFU/day for 6 months) improved height Z-scores by 0.21 in stunted children (p = 0.04) but did not elevate GH levels (61). Challenges in translating these findings to clinical practice include discrepancies in dose scaling from mice to humans and the lack of standardized probiotic formulations for GHD populations (15, 62).
5.1.1 Future directions
The development of engineered microbial consortia (e.g., combining Akkermansia muciniphila with Bifidobacterium longum) or CRISPR-edited strains designed to produce SCFAs may offer more precise targeting of the GH/IGF-1 axis, potentially enhancing therapeutic efficacy in GHD.
5.2 Dietary fiber
The production of SCFAs induced by fiber shows dose-dependent therapeutic potential:
1. Dose–Response Complexity: In ducks, 8% cellulose raised cecal butyrate by 60% and hepatic IGF-1 mRNA by 2.3-fold. However, over 12% fiber hampered growth due to competition for nutrients (63).
2. Ethnic Variability: High-fiber diets boost Bacteroides-driven butyrate synthesis in Asian children (IGF-1 ↑18%, p = 0.01) but not in Western groups with Prevotella-dominant microbiota (16, 64).
3. Practical Barriers: Long-term adherence to high-fiber diets is low (<30% compliance at 6 months), highlighting the need for palatable synbiotic formulations (e.g., fiber combined with Lactobacillus rhamnosus) (65).
5.2.1 Future directions
Personalized fiber blends, tailored to regional microbiota and SCFA receptor polymorphisms (e.g., GPR41 rs11568582), could enhance compliance and efficacy.
5.3 Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) remains experimental but shows mechanistic promise (Table 2):
1. Preclinical Evidence: In sickle cell mouse models, FMT restored IGF-1 levels by 45% through butyrate-GPR41 signaling (p < 0.001) (66).
2. Clinical Caution: Pediatric FMT trials have reported adverse effects such as bloating (22%) and diarrhea (15%), with long-term risks remaining unclear (67). To date, no human studies have directly targeted GHD.
5.3.1 Future directions
Techniques such as washed microbiota transplantation (WMT) or virome-depleted FMT may reduce side effects while preserving SCFA-producing bacterial taxa, offering a safer approach for potential therapeutic applications (68).
6 Summary and outlook
6.1 Summary
Gut microbiota dysbiosis in GHD disrupts a complex regulatory network, with SCFAs exhibiting concentration-dependent effects. High concentrations of propionate directly inhibit pituitary GH synthesis via GPR41/43-cAMP pathways, while butyrate enhances IGF-1 production through hepatic GPR109A and osteoblastic HDAC inhibition. Clinical discrepancies, such as divergent findings between Huang et al. (9) and García Navas et al. (10), may stem from ethnic dietary patterns (e.g., Prevotella-enriched diets in Asian populations) or variations in intervention durations. Non-SCFA metabolites, including bile acids and indole derivatives, also modulate growth pathways but require further validation in GHD cohorts. Preclinical interventions, such as probiotics and FMT, show promise—e.g., Lactobacillus plantarum increased IGF-1 by 15% in malnourished mice, and FMT restored IGF-1 levels in sickle cell models via butyrate-GPR41 activation. However, human trials face challenges, including low adherence to high-fiber diets (<30% compliance) and limited GHD-specific data.
6.2 Future research directions-multi-omics profiling
1. Metabolomics: Identify GHD-specific biomarkers, such as low butyrate combined with elevated TMAO, to enable patient stratification for personalized therapies (39, 47).
2. Integrated Multi-omics: Combine metagenomics, metabolomics, and host transcriptomics to delineate the “microbiota-SCFA-endocrine” axis. For example, link Bifidobacterium depletion to reduced acetate and downstream IGF-1 suppression (8, 43).
3. Precision Interventions: Develop SCFA-targeted delivery systems (e.g., butyrate-loaded nanoparticles) or CRISPR-Cas9-based editing of SCFA-producing bacteria (Bacteroides, Firmicutes) to restore microbial balance (68).
Future studies should integrate Metagenomics (species-level dysbiosis), metabolomics (SCFA/TMAO ratios), and single-cell transcriptomics (hypothalamic neuron activity) to fully characterize the microbiota-endocrine axis.
6.3 Clinical translation
1. Multicenter RCTs: Prioritize large-scale, multicenter randomized controlled trials to validate the efficacy of high-fiber diets (e.g., 8–12% cellulose) or probiotic blends (Lactobacillus plantarum + Bifidobacterium lactis) in children with GHD (31, 63).
2. Compliance Strategies: Address adherence challenges by developing palatable fiber formulations or synbiotics (prebiotic + probiotic combinations) (65).
Future clinical studies should standardize microbiota profiling methods and control for dietary variables to resolve ethnic disparities and enhance translational potential.
Author contributions
DM: Conceptualization, Funding acquisition, Writing – review & editing. WK: Formal analysis, Software, Supervision, Writing – review & editing. SC: Visualization, Writing – review & editing. HL: Visualization, Writing – review & editing. CH: Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work has been strongly supported by the Research Initiation Fund of Longgang District Maternity and Child Healthcare Hospital in Shenzhen City (Grant No. Y2024011), the Longgang District Science and Technology Innovation Bureau (LGWJ2022-48 and LGWJ2024-59).
Acknowledgments
We are very grateful to all the medical staff members for their enormous efforts in this study. The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Henry, RK. Childhood growth hormone deficiency, a diagnosis in evolution: the intersection of growth hormone history and ethics. Growth Hormon IGF Res. (2020) 55:101358. doi: 10.1016/j.ghir.2020.101358
2. Silva, N, Bullinger, M, Sommer, R, Rohenkohl, A, Witt, S, and Quitmann, J. Children's psychosocial functioning and parents' quality of life in paediatric short stature: the mediating role of caregiving stress. Clin Psychol Psychother. (2018) 25:e107–18. doi: 10.1002/cpp.2146
3. Jensen, EA, Young, JA, Mathes, SC, List, EO, Carroll, RK, Kuhn, J, et al. Crosstalk between the growth hormone/insulin-like growth factor-1 axis and the gut microbiome: a new frontier for microbial endocrinology. Growth Hormon IGF Res. (2020) 53–54:101333. doi: 10.1016/j.ghir.2020.101333
4. Rastelli, M, Cani, PD, and Knauf, C. The gut microbiome influences host endocrine functions. Endocr Rev. (2019) 40:1271–84. doi: 10.1210/er.2018-00280
5. Markowiak-Kopeć, P, and Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. (2020) 12:1107. doi: 10.3390/nu12041107
6. Marrs, T, Jo, JH, Perkin, MR, Rivett, DW, Witney, AA, Bruce, KD, et al. Gut microbiota development during infancy: impact of introducing allergenic foods. J Allergy Clin Immunol. (2021) 147:613–621.e9. doi: 10.1016/j.jaci.2020.09.042
7. Wang, JF, Fu, SP, Li, SN, Hu, ZM, Xue, WJ, Li, ZQ, et al. Short-chain fatty acids inhibit growth hormone and prolactin gene transcription via cAMP/PKA/CREB signaling pathway in dairy cow anterior pituitary cells. Int J Mol Sci. (2013) 14:21474–88. doi: 10.3390/ijms141121474
8. Yan, J, Herzog, JW, Tsang, K, Brennan, CA, Bower, MA, Garrett, WS, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA. (2016) 113:E7554–63. doi: 10.1073/pnas.1607235113
9. Huang, C, Meng, D, Li, Y, Lu, S, Yang, W, Wu, B, et al. Gut microbiota composition alteration analysis and functional categorization in children with growth hormone deficiency. Front Pediatr. (2023) 11:1133258. doi: 10.3389/fped.2023.1133258
10. García Navas, P, Ruíz Del Prado, MY, Villoslada Blanco, P, Recio Fernández, E, Ruíz Del Campo, M, and Pérez, MP. Composition of the microbiota in patients with growth hormone deficiency before and after treatment with growth hormone. An Pediatr (Engl Ed). (2024) 100:404–11. doi: 10.1016/j.anpede.2024.05.004
11. Frye, RE, Rose, S, Slattery, J, and MacFabe, DF. Gastrointestinal dysfunction in autism spectrum disorder:the role of the mitochondria and the enteric microbiome. Microb Ecol Health Dis. (2015) 26:27458. doi: 10.3402/mehd.v26.27458
12. Tu, Y, Kuang, X, Zhang, L, and Xu, X. The associations of gut microbiota, endocrine system and bone metabolism. Front Microbiol. (2023) 14:1124945. doi: 10.3389/fmicb.2023.1124945
13. Huang, ZB, Hu, Z, Lu, CX, Luo, SD, Chen, Y, Zhou, ZP, et al. Gut microbiota-derived indole 3-propionic acid partially activates aryl hydrocarbon receptor to promote macrophage phagocytosis and attenuate septic injury. Front Cell Infect Microbiol. (2022) 12:1015386. doi: 10.3389/fcimb.2022.1015386
14. Bokulich, NA, Chung, J, Battaglia, T, Henderson, N, Jay, M, Li, H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. (2016) 8:343ra82. doi: 10.1126/scitranslmed.aad7121
15. Izuddin, WI, Loh, TC, Samsudin, AA, Foo, HL, Humam, AM, and Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Vet Res. (2019) 15:315. doi: 10.1186/s12917-019-2064-9
16. Li, L, Chen, L, Yang, Y, Wang, J, Guo, L, An, J, et al. Characteristics of gut microbiome and its metabolites, short-chain fatty acids, in children with idiopathic short stature. Front Endocrinol (Lausanne). (2022) 13:890200. doi: 10.3389/fendo.2022.890200
17. Grosso, G. Nutritional psychiatry: how diet affects brain through gut microbiota. Nutrients. (2021) 13:1282. doi: 10.3390/nu13041282
18. Cox, NJ, Bowyer, RCE, Ni Lochlainn, M, Wells, PM, Roberts, HC, and Steves, CJ. The composition of the gut microbiome differs among community dwelling older people with good and poor appetite. J Cachexia Sarcopenia Muscle. (2021) 12:368–77. doi: 10.1002/jcsm.12683
19. Wang, J, and Ji, H. Influence of probiotics on dietary protein digestion and utilization in the gastrointestinal tract. Curr Protein Pept Sci. (2019) 20:125–31. doi: 10.2174/1389203719666180517100339
20. Heianza, Y, Sun, D, Li, X, DiDonato, JA, Bray, GA, Sacks, FM, et al. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: the POUNDS lost trial. Gut. (2019) 68:263–70. doi: 10.1136/gutjnl-2018-316155
21. Burokas, A, Moloney, RD, Dinan, TG, and Cryan, JF. Microbiota regulaTion of the mammalian gut-brain axis. Adv Appl Microbiol. (2015) 91:1–62. doi: 10.1016/bs.aambs.2015.02.001
22. Torres-Fuentes, C, Golubeva, AV, Zhdanov, AV, Wallace, S, Arboleya, S, Papkovsky, DB, et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. (2019) 33:13546–59. doi: 10.1096/fj.201901433R
23. Piñeiro-Ramos, JD, Parra-Llorca, A, Ten-Doménech, I, Gormaz, M, Ramón-Beltrán, A, Cernada, M, et al. Effect of donor human milk on host-gut microbiota and metabolic interactions in preterm infants. Clin Nutr. (2021) 40:1296–309. doi: 10.1016/j.clnu.2020.08.013
24. Wiesenborn, DS, Galvez, EJC, Spinel, L, Victoria, B, Allen, B, Schneider, A, et al. The role of Ames dwarfism and calorie restriction on gut microbiota. J Gerontol A Biol Sci Med Sci. (2020) 75:e1–8. doi: 10.1093/gerona/glz236
25. Jensen, EA, Young, JA, Jackson, Z, Busken, J, List, EO, Carroll, RK, et al. Growth hormone deficiency and excess alter the gut microbiome in adult male mice. Endocrinology. (2020) 161:bqaa026. doi: 10.1210/endocr/bqaa026
26. Zheng, Y, Song, Y, Han, Q, Liu, W, Xu, J, Yu, Z, et al. Intestinal epithelial cell-specific IGF1 promotes the expansion of intestinal stem cells during epithelial regeneration and functions on the intestinal immune homeostasis. Am J Physiol Endocrinol Metab. (2018) 315:E638–49. doi: 10.1152/ajpendo.00022.2018
27. Cryan, JF, and Dinan, TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
28. Lyte, M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. (2004) 12:14–20. doi: 10.1016/j.tim.2003.11.004
29. Clarke, G, Grenham, S, Scully, P, Fitzgerald, P, Moloney, RD, Shanahan, F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. (2013) 18:666–73. doi: 10.1038/mp.2012.77
30. Wikoff, WR, Anfora, AT, Liu, J, Schultz, PG, Lesley, SA, Peters, EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. (2009) 106:3698–703. doi: 10.1073/pnas.0812874106
31. Schwarzer, M, Makki, K, Storelli, G, Machuca-Gayet, I, Srutkova, D, Hermanova, P, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. (2016) 351:854–7. doi: 10.1126/science.aad8588
32. Yakar, S, and Isaksson, O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: lessons from mouse models. Growth Hormon IGF Res. (2016) 28:26–42. doi: 10.1016/j.ghir.2015.09.004
33. Tolhurst, G, Heffron, H, Lam, YS, Parker, HE, Habib, AM, Diakogiannaki, E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. (2012) 61:364–71. doi: 10.2337/db11-1019
34. Soriano-Guillén, L, Barrios, V, Campos-Barros, A, and Argente, J. Ghrelin levels in obesity and anorexia nervosa: effect of weight reduction or recuperation. J Pediatr. (2004) 144:36–42. doi: 10.1016/j.jpeds.2003.10.036
35. Müller, EE, Locatelli, V, Ghigo, E, Cella, SG, Loche, S, Pintor, C, et al. Involvement of brain catecholamines and acetylcholine in growth hormone deficiency states. Drugs. (1991) 41:161–77. doi: 10.2165/00003495-199141020-00002
36. Wahlström, A, Sayin, SI, Marschall, HU, and Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. (2016) 24:41–50. doi: 10.1016/j.cmet.2016.05.005
37. Rothhammer, V, Mascanfroni, ID, Bunse, L, Takenaka, MC, Kenison, JE, Mayo, L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. (2016) 22:586–97. doi: 10.1038/nm.4106
38. Cervantes-Barragan, L, Chai, JN, Tianero, MD, di Luccia, B, Ahern, PP, Merriman, J, et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science. (2017) 357:806–10. doi: 10.1126/science.aah5825
39. Nogal, A, Valdes, AM, and Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. (2021) 13:1–24. doi: 10.1080/19490976.2021.1897212
40. den Besten, G, van Eunen, K, Groen, AK, Venema, K, Reijngoud, DJ, and Bakker, BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012
41. Miller, TL, and Wolin, MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial Flora. Appl Environ Microbiol. (1996) 62:1589–92. doi: 10.1128/aem.62.5.1589-1592.1996
42. Macfarlane, S, and Macfarlane, GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. (2003) 62:67–72. doi: 10.1079/PNS2002207
43. Ruiz-Aceituno, L, Esteban-Torres, M, James, K, Moreno, FJ, and Sinderen, DV. Metabolism of biosynthetic oligosaccharides by human-derived Bifidobacterium Breve Ucc2003 and Bifidobacterium Longum Ncimb 8809. Int J Food Microbiol. (2020) 316:108476. doi: 10.1016/j.ijfoodmicro.2019.108476
44. Horiuchi, H, Kamikado, K, Aoki, R, Suganuma, N, Nishijima, T, Nakatani, A, et al. Bifidobacterium animalis subsp. lactis GCL2505 modulates host energy metabolism via the short-chain fatty acid receptor GPR43. Sci Rep. (2020) 10:4158. doi: 10.1038/s41598-020-60984-6
45. David, LA, Maurice, CF, Carmody, RN, Gootenberg, DB, Button, JE, Wolfe, BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505:559–63. doi: 10.1038/nature12820
46. Wong, JM, de Souza, R, Kendall, CW, Emam, A, and Jenkins, DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. (2006) 40:235–43. doi: 10.1097/00004836-200603000-00015
47. Huda-Faujan, N, Abdulamir, AS, Fatimah, AB, Anas, OM, Shuhaimi, M, Yazid, AM, et al. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J. (2010) 4:53–8. doi: 10.2174/1874091X01004010053
48. Gonçalves, P, and Martel, F. Regulation of colonic epithelial butyrate transport: focus on colorectal Cancer. Porto Biomed J. (2016) 1:83–91. doi: 10.1016/j.pbj.2016.04.004
49. Hu, J, Lin, S, Zheng, B, and Cheung, PCK. Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr. (2018) 58:1243–9. doi: 10.1080/10408398.2016.1245650
50. He, J, Zhang, P, Shen, L, Niu, L, Tan, Y, Chen, L, et al. Short-chain fatty acids and their association with Signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. (2020) 21:6356. doi: 10.3390/ijms21176356
51. Mishra, SP, Karunakar, P, Taraphder, S, and Yadav, H. Free fatty acid receptors 2 and 3 as microbial metabolite sensors to shape host health: Pharmacophysiological view. Biomedicines. (2020) 8:154. doi: 10.3390/biomedicines8060154
52. Brown, AJ, Goldsworthy, SM, Barnes, AA, Eilert, MM, Tcheang, LL, Daniels, D, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. (2003) 278:11312–9. doi: 10.1074/jbc.M211609200
53. Wu, Y, Wang, CZ, Wan, JY, Yao, HQ, and Yuan, CS. Dissecting the interplay mechanism between epigenetics and gut microbiota: health maintenance and disease prevention. Int J Mol Sci. (2021) 22:6933. doi: 10.3390/ijms22136933
54. Soliman, MM, Ahmed, MM, Salah-Eldin, AE, and Abdel-Aal, AAA. Butyrate regulates leptin expression through different signaling pathways in adipocytes. J Vet Sci. (2011) 12:319–23. doi: 10.4142/jvs.2011.12.4.319
55. Fukumori, R, Sugino, T, Hasegawa, Y, Kojima, M, Kangawa, K, Obitsu, T, et al. Plasma ghrelin concentration is decreased by short chain fatty acids in wethers. Domest Anim Endocrinol. (2011) 41:50–5. doi: 10.1016/j.domaniend.2011.04.001
56. Lal, S, Kirkup, AJ, Brunsden, AM, Thompson, DG, and Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. (2001) 281:G907–15. doi: 10.1152/ajpgi.2001.281.4.G907
57. Yan, J, Takakura, A, Zandi-Nejad, K, and Charles, JF. Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes. (2018) 9:84–92. doi: 10.1080/19490976.2017.1371893
58. Suta, S, Ophakas, S, Manosan, T, Honwichit, O, Charoensiddhi, S, Surawit, A, et al. Influence of prolonged whole egg supplementation on insulin-like growth factor 1 and short-chain fatty acids product: implications for human health and gut microbiota. Nutrients. (2023) 15:4804. doi: 10.3390/nu15224804
59. Graham, C, Mullen, A, and Whelan, K. Obesity and the gastrointestinal microbiota:a review of associations and mechanisms. Nutr Rev. (2015) 73:376–85. doi: 10.1093/nutrit/nuv004
60. Du, R, Jiao, S, Dai, Y, An, J, Lv, J, Yan, X, et al. Probiotic Bacillus amyloliquefaciens C-1 improves growth performance, stimulates GH/IGF-1, and regulates the gut microbiota of growth-retarded beef calves. Front Microbiol. (2018) 9:2006. doi: 10.3389/fmicb.2018.02006
61. Li, L, Wang, Y, Huang, Y, Lu, Y, Wang, W, Chen, X, et al. Impact of different growth hormone levels on gut microbiota and metabolism in short stature. Pediatr Res. (2024) 96:115–23. doi: 10.1038/s41390-024-03140-4
62. Kareem, KY, Loh, TC, Foo, HL, Akit, H, and Samsudin, AA. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet Res. (2016) 12:163. doi: 10.1186/s12917-016-0790-9
63. Hao, Y, Ji, Z, Shen, Z, Xue, Y, Zhang, B, Yu, D, et al. Increase dietary Fiber intake ameliorates Cecal morphology and drives Cecal species-specific of short-chain fatty acids in white Pekin ducks. Front Microbiol. (2022) 13:853797. doi: 10.3389/fmicb.2022.853797
64. Koh, A, de Vadder, F, Kovatcheva-Datchary, P, and Bäckhed, F. From dietary Fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
65. Fordjour, L, D'Souza, A, Cai, C, Ahmad, A, Valencia, G, Kumar, D, et al. Comparative effects of probiotics, prebiotics, and synbiotics on growth factors in the large bowel in a rat model of formula-induced bowel inflammation. J Pediatr Gastroenterol Nutr. (2010) 51:507–13. doi: 10.1097/MPG.0b013e3181df5ff2
66. Xiao, L, Zhou, Y, Bokoliya, S, Lin, Q, and Hurley, M. Bone loss is ameliorated by fecal microbiota transplantation through SCFA/GPR41/ IGF1 pathway in sickle cell disease mice. Sci Rep. (2022) 12:20638. doi: 10.1038/s41598-022-25244-9
67. Jess, AT, Eskander, GH, Vu, MH, and Michail, S. Short-chain fatty acid levels after fecal microbiota transplantation in a pediatric cohort with recurrent Clostridioides difficile infection. Meta. (2023) 13:1039. doi: 10.3390/metabo13101039
Keywords: growth hormone deficiency, gut microbiota, short-chain fatty acids, gut-brain axis, insulin-like growth factor-1, therapeutic interventions
Citation: Meng D, Kong W, Cheng S, Liu H and Huang C (2025) Gut-brain-liver axis in growth hormone deficiency: role of microbiota-derived short-chain fatty acids in ethnic variability and therapeutic development. Front. Public Health. 13:1541654. doi: 10.3389/fpubh.2025.1541654
Edited by:
Sukanta Das, University of Illinois Chicago, United StatesReviewed by:
Meghan Lynne Ruebel, United States Department of Agriculture, United StatesSiddhartha Sengupta, University of Illinois Chicago, United States
Copyright © 2025 Meng, Kong, Cheng, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Congfu Huang, NzgzMzM3NTVAcXEuY29t
†These authors have contributed equally to this work
 Dongming Meng†
Dongming Meng† Congfu Huang
Congfu Huang