- 1Department of Specialist Nursing, Pomeranian Medical University in Szczecin, Szczecin, Poland
- 2Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University in Szczecin, Szczecin, Poland
- 3Department of Psychiatry, Pomeranian Medical University in Szczecin, Szczecin, Poland
- 4Department of Obstetrics and Pathology of Pregnancy, Pomeranian Medical University in Szczecin, Szczecin, Poland
Objectives: The aim of this study was to analyze the impact of polymorphisms within the promoters of the MAO-A and the 5-HTT (SLC6A4) genes on the severity of anxiety and depressive disorder symptoms, and adaptation to the disease in patients with reproductive tract cancer.
Methods: This study involved a group female patients treated at the Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents of the Pomeranian Medical University in Szczecin. The inclusion criteria for the study were advanced ovarian cancer or endometrial cancer, as well as treatment in the form of cytoreductive therapy and chemotherapy. The following standardized research tools were used to collect empirical data: Beck Depression Inventory, State–Trait Anxiety Inventory and Mini-Mental Adjustment to Cancer.
Results: The study included 139 women diagnosed with endometrial cancer (63%) or ovarian cancer (37%). Assessment of the severity of anxiety and depressive symptoms in the studied group of patients depending on genotype did not show statistically significant differences. However, among patients with genotype MAO-A 4/4, the constructive style prevailed over the destructive one, and the most frequently chosen strategy was positive redefinition. In the case of patients with the 5-HTT gene polymorphism, the most frequently chosen strategies were anxious preoccupation and positive redefinition.
Conclusion: Searching for the relationship between genetic factors and the strategies adopted to cope with cancer requires intensive research. Undoubtedly, the severity of anxiety and depressive symptoms has an impact on adaptive behavior and the process of onco-logical treatment.
1 Introduction
Depression is a worldwide issue, and the one that is often accompanied by anxiety. The mechanism for the co-occurrence of these two has not yet been elucidated. Scientists debate whether anxiety and depression are two different conditions or different symptoms of one illness (1). Studies show that the emergence of depressive symptoms may be determined by numerous factors, among which comorbidities receive particular attention. A higher risk of depression may be directly or indirectly related to the biological, psychological and/or social effects of many diseases, including cancer (2–7). Depression and anxiety are serious medical problems also for oncology patients. An explanation for this phenomenon can be found in traditional concepts of psychopathology, which assume that symptoms of mental disorders reflect the underlying disease (8–11). Studies show that almost half of cancer survivors suffer from anxiety disorders, and approximately 15–25% of patients diagnosed with cancer have symptoms of depression (12). At the same time, depressive disorders occur in 56% of patients with anxiety, and anxiety is experienced by 47–58% of patients with a history of a depressive episode. Therefore, it is so important to simultaneously verify the presence of these two in cancer patients (8, 12). Undoubtedly, the great intensity of negative emotions promotes symptoms of anxiety and depression. Oncology patients are more likely to present the entire spectrum of emotions―mental distress, anxiety, depressive symptoms, fear, sadness, anger, aggression, a sense of guilt, and distrust. All of these are important indicators and may already appear during the diagnosis of cancer disease or later during treatment, and sometimes persist after its completion (13–16). Anxiety and depression are not destructive to all cancer patients. Some people, depending on individual psychological resources and psychosocial support, develop adaptive coping strategies for anxiety and depressive disorders (17). This is supported by the existing theories of evolutionary psychiatry, which assume that low-intensity depression and anxiety, under certain conditions, constitute an adaptive mechanism that allows a person to adopt an individual response strategy in a difficult situation (17). As confirmed by numerous studies, the risk of depression is determined by gene–environment interactions (18–25). Nevertheless, further research in this field is needed. Psychosocial factors most likely only predispose genetically susceptible individuals to depression. Among genetic factors, researchers mention the polymorphisms within the promoters of genes encoding the serotonin transporter (5-HTT) and monoamine oxidase A (MAO-A) (the enzyme catalyzing the oxidative degradation of monoamines) (26–28). A meta-analysis conducted by Sharpley’s team confirmed the interaction of the 5-HTTLPR polymorphism within the promoter region of the 5-HTT (SLC6A4) gene with stressful life events and depression (29). The aim of this study was to analyze the impact of polymorphisms within the promoters of the MAO-A and the 5-HTT (SLC6A4) genes on the severity of anxiety and depressive disorder symptoms, and adaptation to the disease in patients with reproductive tract cancer.
2 Methods
2.1 Project of the research
This survey-based study involved a group of 139 female patients treated at the Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents of the Pomeranian Medical University in Szczecin. Informed consent was a prerequisite for participation in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Bioethical Commission (Resolution No. KB-0012/81/18). The inclusion criteria for the study were advanced ovarian cancer or endometrial cancer (as these are the most common cancers of the reproductive tract in women), as well as treatment in the form of cytoreductive therapy and chemotherapy.
2.2 Research instruments
The study was based on a survey performed using a questionnaire technique. The following standardized research tools were used to collect empirical data:
• Beck Depression Inventory–Second Edition (BDI-II) by A. Beck, adapted by E. Łojek, J. Stańczak (30, 31),
• State–Trait Anxiety Inventory (STAI) by C. D. Spielberger, R. L. Gorsuch, R. E. Lushene, adapted by J. Strelau, M. Tysarczyk, K. Wrześniewski (32),
• Mini-Mental Adjustment to Cancer (Mini-MAC) scale by M. Watson et al., adapted by Z. Juczyński (33).
We also used an original questionnaire concerning basic sociodemographic data, i.e., age, place of residence, employment status, education, marital status and menstruation, family history of cancer, medications taken, and physical activity.
2.2.1 Beck depression inventory–second edition (BDI-II)
This questionnaire is to determine depression. It consists of 21 statements that describe the most commonly observed symptoms of depression (emotional, cognitive, motivational, and physical). Items 1–14 concern the cognitive sphere (including sadness, a feeling of being worthless, pessimism, loss of pleasure, a sense of guilt, a feeling of being punished, self-criticism, suicidal thoughts, crying, loss of interest), while items 15–21 refer to the somatic realm (including loss of energy, sleep problems, appetite problems, fatigue, loss of interest in sex). Based on this scale, depression can be ruled out (0–11) or classified as mild (12–26), moderate (27–49), or severe (50–63). It is believed that in the Polish population, a score above 11 points suggests the presence of depression and is an indication for psychiatric consultation to verify the diagnosis (30, 31).
2.2.2 State–trait anxiety inventory (STAI)
This questionnaire has been developed to measure anxiety. It consists of two subscales: the first of them (STAI X-1) is used to assess anxiety as a state (transient, situationally conditioned), and the second (STAI X-2) allows analysis of anxiety as a trait (understood as a relatively permanent personality trait). Each subscale consists of 20 items. The score for each part of the inventory can range from 20 to 80 points. High scores suggest high levels of anxiety. Raw scores are converted into sten scores ranging from 1 to 10, where a score of 1–4 indicates a low level of anxiety, 5–6 means a medium level of anxiety, and 7–10 reflects a high level of anxiety (32).
2.2.3 Mini-mental adjustment to cancer (Mini-MAC)
The questionnaire enables the assessment of people’s adaptation to cancer and their ability to cope with the disease and its symptoms (pain, fatigue, malaise). This self-reported 29-item instrument measures the utilization of cancer-specific coping strategies, namely anxious preoccupation, fighting spirit, helplessness/hopelessness, and positive redefinition. These are grouped into two subscales: the constructive style (fighting spirit and positive redefinition), and the destructive style (anxious preoccupation and helplessness/hopelessness). Each statement is rated on a four-point scale: from 1 meaning ‘definitely does not apply to me’ to 4―‘definitely applies to me’. The score for each strategy is calculated separately and ranges from 7 to 28 points. The higher the score, the higher the utilization of a given coping strategy (33).
2.3 Genotyping
In accordance with the study protocol, after obtaining her consent to participate in the study each of the qualified women had venous blood collected using the Monovette closed system. DNA was isolated from the whole blood samples with the use of Invisorb Spin Blood Mini Kit (Invitec Molecular GmbH, Germany). The examined DNA regions were amplified in the PCR reaction, and then the length of the amplified fragments was analyzed using electrophoresis on 3% agarose gel with ethidium bromide staining. To analyze the 5-HTT (SLC6A4) polymorphism, the 44-bp ins-del fragment in the regulatory region of the gene was amplified with the use of forward primer 5’ GGC GTT GCC GCT CTG AAT GC 3′ and the revers primer 5′ GAG GGA CTG AGC TGG ACA ACC AC 3′. The PCR conditions were as followed: 94°C for 5 min, 30 x (94°C for 55 s, 55°C for 50 s, 72°C for 60 s), 72°C for 10 min. The VNTR polymorphism in the MAO-A promoter region was analyzed with the use of forward primer 5’ CCC AGG CTG CTC CAG AAA 3′ and the reverse primer 5′ GGA CCT GGG CAG TTG TGC 3′ and the PCR conditions: 95°C for 3 min, 34 x (94°C for 40 s, 57°C for 35 s, 72°C for 50 s), 72°C, 10 min. Four different MAO-A genotypes were identified in the study group: 3/3, 3/4, 4/4 and 4/5. However, only two women had the 4/5 genotype. Due to their small number, there was no point in conducting a separate analysis for this subgroup. Therefore, in all analyses these women were included in the group with the 4/4 genotype as a variant with a larger number of repeats compared to allele 3 carriers.
2.4 Statistical analysis
Statistical analysis was performed using the Statistica 13.3 software (TIBCO software inc.). According to the Shapiro–Wilk test, the distribution of most of the analyzed variables significantly deviated from the normal distribution, therefore non-parametric tests were used in all analyzes. For quantitative variables, group comparisons were done using the Mann Whitney U test, while for qualitative variables, a chi-square (χ2) test was used. The observed frequencies of particular 5-HTT and MAO-A genotypes were consistent with the Hardy–Weinberg equilibrium (p = 0.20 and p = 0.63, respectively). Statistical significance was set at p < 0.05, and results with p-values between 0.05 and 0.1 were considered as statistical trends (34).
3 Results
3.1 Characteristics of the study sample
The study included 139 women diagnosed with endometrial cancer (n = 87; 63%) or ovarian cancer (n = 52; 37%). The average age of the women was 61 ± 11 years (Me 62 years, IQR: 13, min: 34 years, max: 85 years). The table shows sociodemographic and clinical data of the study sample. The subgroups of patients selected according to genetic data did not differ significantly in terms of these factors, except for significant differences in the proportion of patients using and not using menopausal hormonal therapy (MHT) de-pending on the 5-HTT genotype, and significant differences in the proportion of patients with ovarian cancer and endometrial cancer depending on the MAO-A genotype (Table 1).
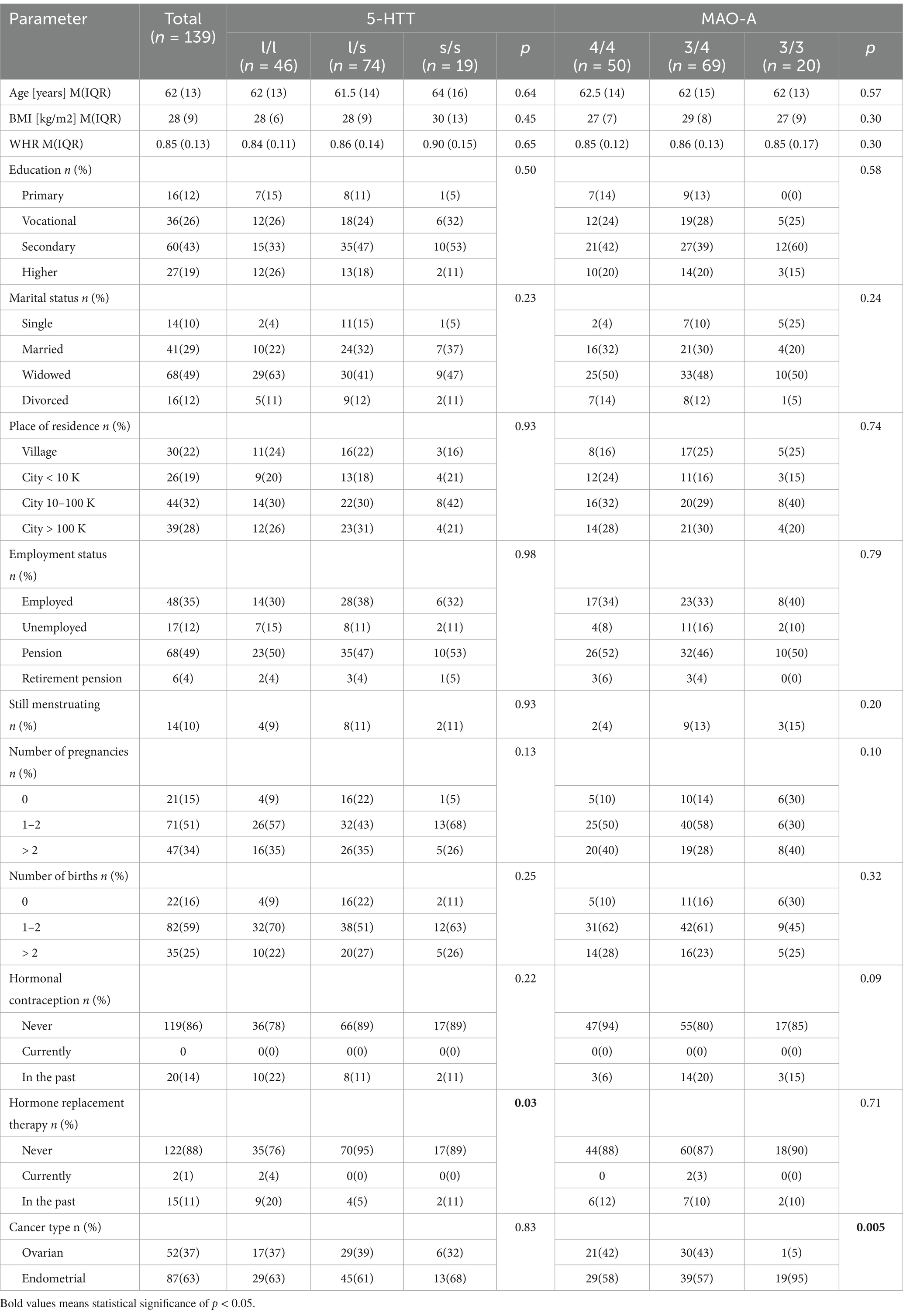
Table 1. Sociodemographic and clinical data of the study group divided by the 5-HTT (SLC6A4) and the MAO-A genotypes.
3.2 Correlations of the 5-HTT and the MAO-A genotypes with the severity of depressive and anxiety symptoms, and with coping strategies
The analysis did not confirm significant differences in the severity of anxiety as measured by the STAI X-1 and STAI X-2 scales depending on the 5-HTT and the MAO-A genotypes (Table 2).
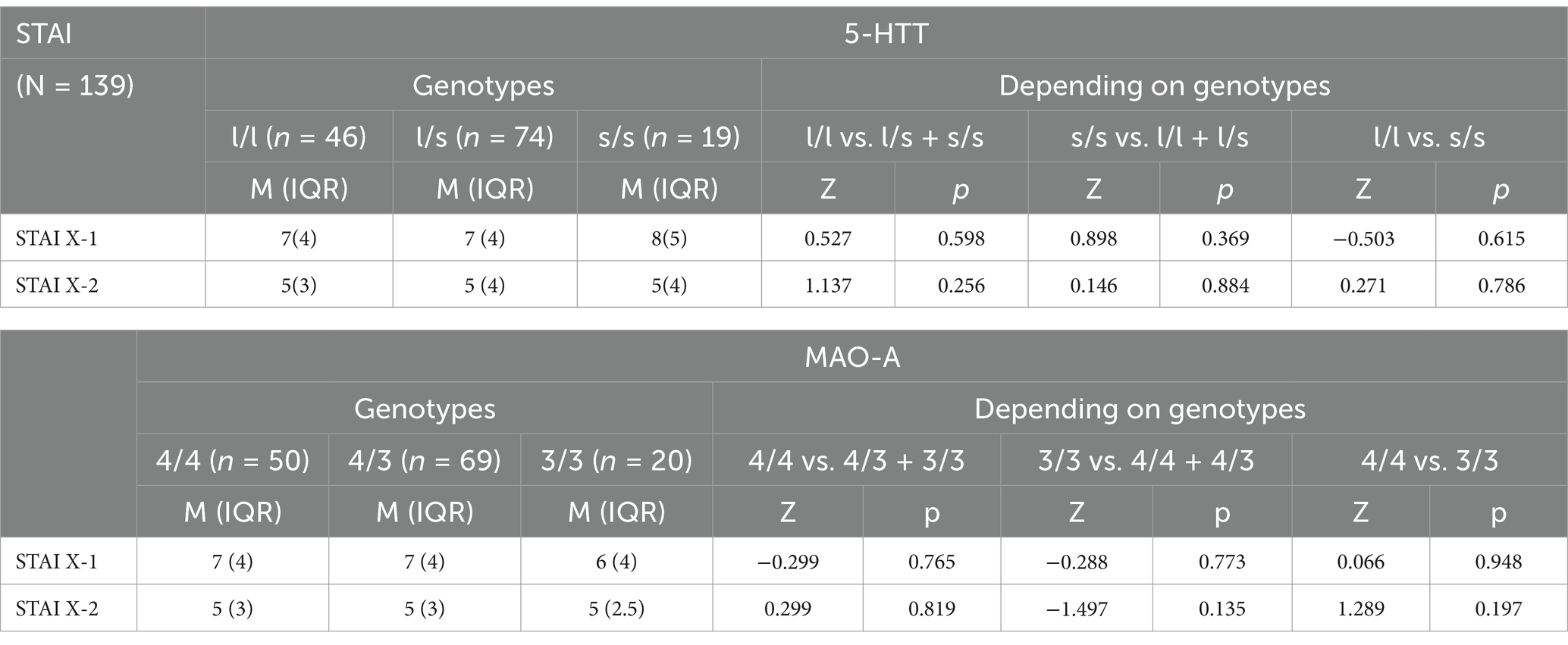
Table 2. Associations of the 5-HTT (SLC6A4) and the MAO-A genotypes with the severity of anxiety symptoms as measured by the STAI.
There were no significant differences in the severity of depressive symptoms as measured by the BDI-II depending on the 5-HTT and the MAO-A genotypes (neither in the quantitative analysis―the number of points obtained, nor in the qualitative analysis―the presence of depressive symptoms vs. their absence) (Table 3).
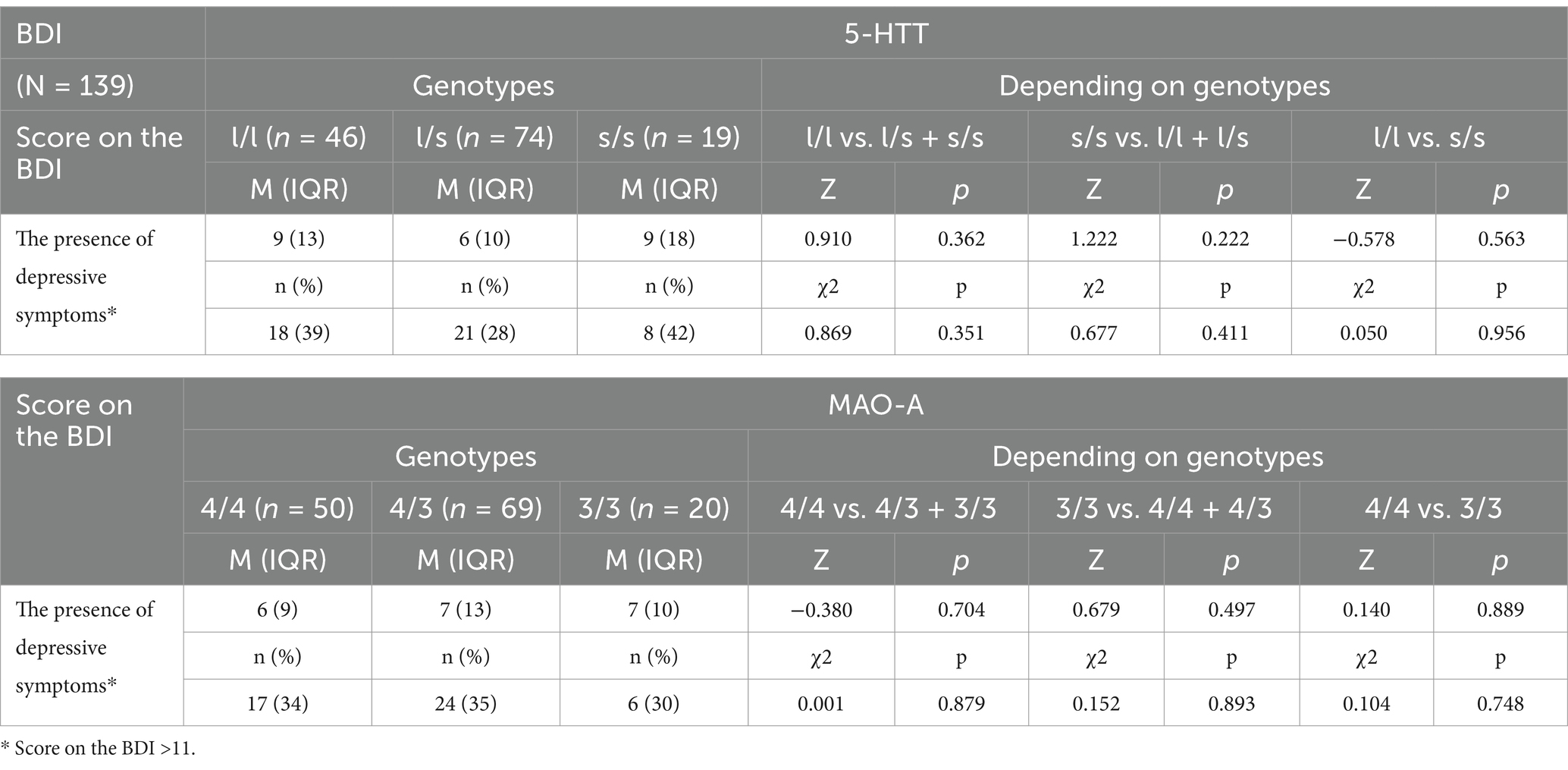
Table 3. Associations of the 5-HTT (SLC6A4) and the MAO-A genotypes with the severity of depressive symptoms as measured by the BDI-II.
The combined analysis of both factors MAO-A and 5-HTT with the use of two-way ANOVA, with interactions did not show any significant impact of those genetic factors on depression or anxiety symptoms.
Patients with the MAO-A 4/4 genotype scored significantly higher for the strategy based on positive redefinition compared to the other patients (Table 4). They also obtained higher scores―both raw scores (Z = 2.06, p = 0.039) and sten scores―for the constructive style (Table 4, Figure 1). In the case of the 5-HTT gene polymorphism, patients with the s/s genotype scored higher―close to the level of statistical significance (p < 0.1)―for anxious preoccupation and positive redefinition than the other patients.
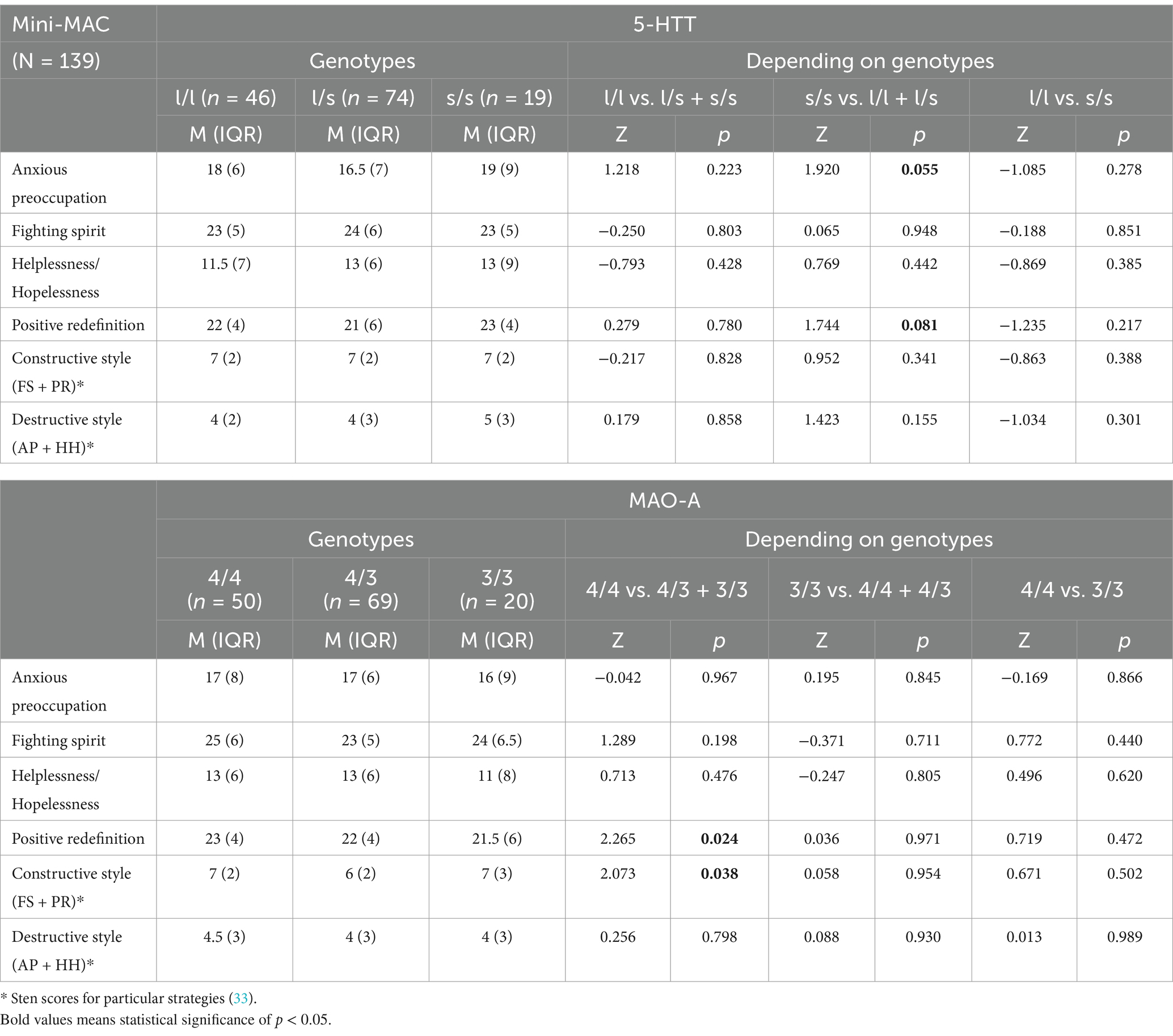
Table 4. Associations of the 5-HTT (SLC6A4) and the MAO-A genotypes with the utilization of particular coping strategies according to the Mini-MAC.

Figure 1. Histogram showing the distribution of the patients by sten scores for the use of the constructive style depending on the MAO-A genotype.
The higher sten scores obtained by patients with the MAO-A 4/4 genotype for the constructive style are shown in Figure 1.
Analysis of sociodemographic and clinical factors potentially affecting the use of the constructive style (sten scores) revealed only one significant relationship―with place of residence (H = 18.17, p = 0.0004). Patients living in cities with up to 10,000 inhabitants were characterized by higher sten values for the constructive style than the other groups (Figure 2).
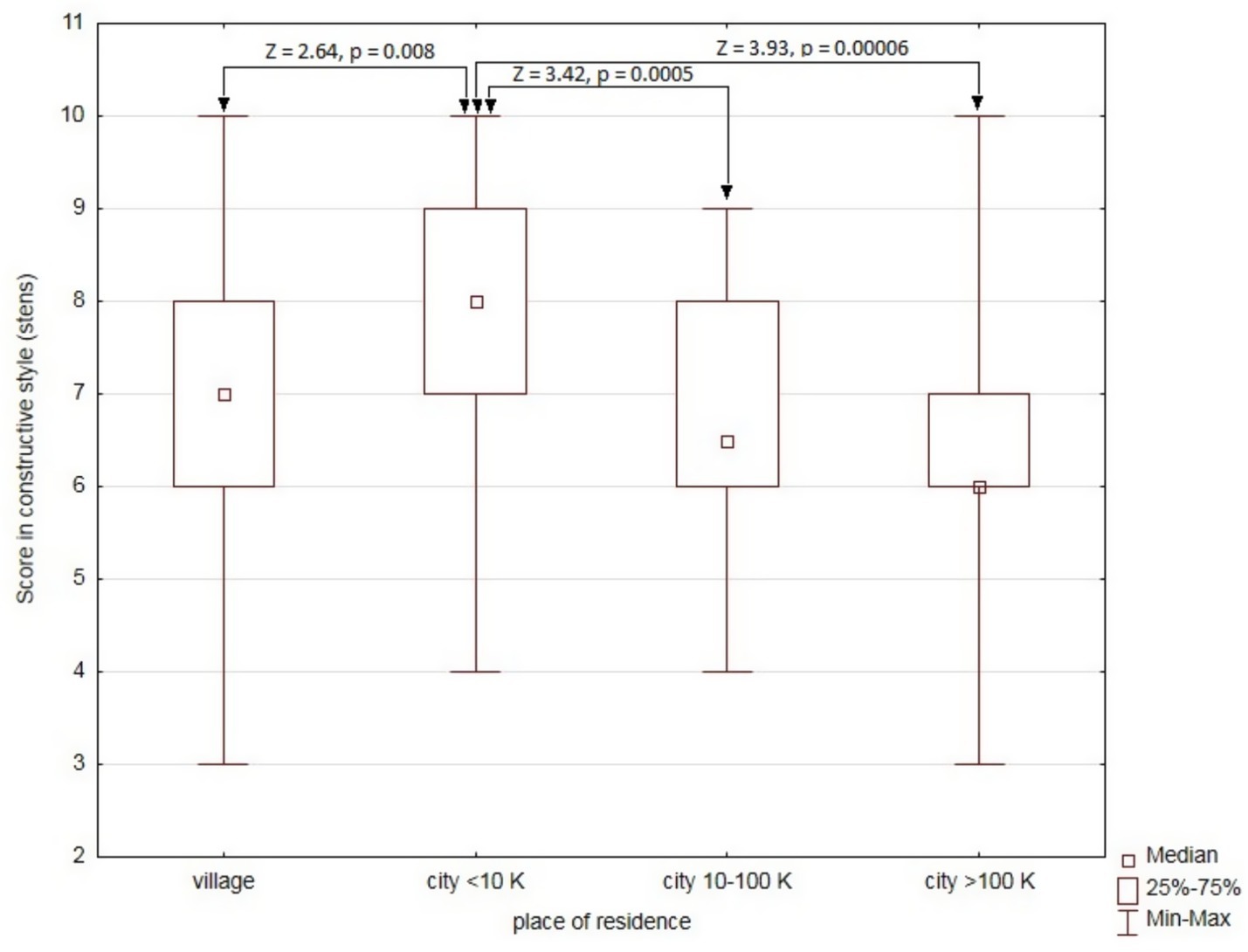
Figure 2. Comparison of sten scores for the use of the constructive style depending on the place of residence.
A two-way ANOVA was performed to analyze the effect of the MAO-A 4/4 genotype and place of residence on the score for the use of the constructive style. The analysis did not reveal a statistically significant influence of the interaction between the MAO-A 4/4 genotype and place of residence (F (3) = 0.48, p = 0.70). However, a simple analysis of main effects showed that both the MAO-A 4/4 genotype and place of residence had a statistically significant independent effect on the score for the constructive style (F (1) = 4.13, p = 0.044 and F (3) = 12.51, p = 0.0006, respectively).
4 Discussion
Depression and anxiety are common comorbidities in cancer patients. Undoubtedly, they exacerbate emotional stress, which reduces the quality of such patients’ lives. Therefore, emotional distress is considered an important indicator in the treatment of cancer (35). Negative emotions are experienced by patients already at the time of cancer diagnosis as well as during treatment. It has been observed that the intensity of negative emotions is associated with the frequency of experiencing symptoms of anxiety and depression (13–16). The severity of these symptoms, on the other hand, may depend on many factors, including age, sex, education, type of oncological disease, its stage, time of diagnosis, treatment methods (radiotherapy or chemotherapy), and prognosis (36). Other factors that determine the experience of anxiety and depression in this group of patients are emotional and social support, coping strategies, and the availability of therapy and information about the disease (37). In the long run, the coexistence of depressive and anxiety disorders affects the results of the therapy, and causes that disease symptoms are felt more strongly (38). It has been indicated for many years that psychosocial factors play a key role in the etiopathogenesis of depression. According to Landowski, the emergence of depressive symptoms is a result of stress and the interaction of environmental and genetic factors (39). Thus, combination of psychosocial and genetic risk factors predispose to depression. Interactions between 5-HTTLPR and stressful events experienced both in the past and recently are an important factor in depressive disorders (40, 41). The importance of the interaction between genetic and environmental factors that increase susceptibility to the disease has been confirmed by Caspi et al. (26) and Kendler et al. (42). According to these researchers, increased sensitivity to stress may be associated with the 5-HTTLPR polymorphism (26, 42). The occurrence of depressive symptoms has also been found to be influenced by the MAO-A polymorphism and by environmental factors. According to Cicchetti et al. (27) the severity of depression in the group of traumatized patients correlated with low MAO-A activity, while those with high MAO-A activity were characterized by better-developed stress coping mechanisms and less severe depressive symptoms (27).
The results of numerous studies show that individual response to stress is determined by genetic susceptibility. In the case of the 5-HTTLPR gene, significantly more severe depressive symptoms were observed in people with the short ‘s’ allele (SS or SL genotypes) than in those with the long ‘l’ allele (LL genotype) (26, 43, 44). Stein et al. (45) studied the link between the presence of the 5-HTTLPR polymorphism, depressive symptoms as measured by the BDI, and the subjects’ personality. The results did not reveal a statistically significant relationship between these variables, but suggested that the 5-HTTLPR polymorphism had an impact on the subjects’ psychological resilience (as it is directly related to the risk of anxiety and mood disorders)―people with the ‘s’ allele of this gene had higher levels of anxiety (45). In our study, an attempt to assess the level of anxiety and depressive symptoms depending on the genotype did not confirm statistically significant differences. Perhaps this was due to the fact that our study sample was too small to detect weak relationships or that different parameters were analyzed. Undoubtedly, expanding the sample size by including additional genotypes and parameters would increase the importance of the research.
Undoubtedly, coping strategies have a significant impact on the treatment process in cancer (46, 47). A large proportion of cancer patients at various stages of disease progression are diagnosed with depressive disorders, which hinder psychological adjustment to cancer (48, 49). The patient’s adoption of the constructive or destructive coping strategies may affect both their quality of life and the distant effects of the treatment. While the attitudes of fighting spirit and denial of the disease may contribute to higher survival rates, the attitudes of stoic acceptance or helplessness/hopelessness may impede the fight against the disease and disrupt defense mechanisms (47, 50, 51). Schillani’s team studied the correlation between the 5-HTTLPR polymorphism and psychological adjustment to breast cancer. The results indicated that in patients with early breast cancer, the strategies of helplessness/hopelessness and anxious preoccupation significantly correlated with depression, while avoidance correlated with anxiety. Patients with advanced cancer showed similar correlation results, and a negative correlation of depression with fighting spirit and avoidance. A significant correlation was found between helplessness/hopelessness and depression in early-stage breast cancer patients with the long L/L allele. Thus, the 5-HTTLPR polymorphism determines coping strategies and the occurrence of depression, which may have implications for further treatment (52). In our study, correlation analysis between the utilization of individual stress coping strategies and the 5-HTTLPR polymorphism showed no significant differences, except for higher values (close to statistical significance) for the strategies of anxious preoccupation and positive redefinition in patients with the s/s genotype. Patients with the MAO-A 4/4 genotype scored considerably higher on the positive redefinition strategy compared to other patients, and scored higher for the constructive style. These results indicate a potential link between genetic factors and strategies, but require confirmation on a larger group of patients.
The next stage of our research was an attempt to assess the influence of sociodemographic and medical variables on the choice of a specific strategy of adaptation to the disease. The analysis revealed only one significant association, namely the influence of place of residence on the choice of the constructive style by the respondents. It was observed that both the presence of the MAO-A 4/4 genotype and place of residence had a statistically significant independent impact on the score for the use of the constructive style. This style consists of the strategies of fighting spirit and positive redefinition, which determine treating the disease as a challenge, and thus taking action to combat it (53). An attempt to evaluate the influence of selected sociodemographic and medical variables on the degree of adaptation to the disease in a group of women treated for gynecological cancer was made by Kupcewicz’s team. The results showed that the women tended to adopt constructive ways of coping with cancer, and the age of the subjects was a determining factor (54). The literature lacks analyses of the relationship between coping strategies and place of residence. This topic seems interesting, but requires further investigation.
Oncological disease has a number of consequences, both in terms of physical and emotional functioning. Among them, the most common conditions are anxiety and depression, which are most often destructive. Therefore, it is worth taking steps to prevent and effectively treat these conditions in cancer patients.
The premise in our research was the long-term follow-up of patients with endometrial cancer and ovarian cancer. However, during the collection of the material, we encountered some limitations due to the SARS-CoV2 pandemic and subsequent sanitary-epidemiological restrictions, which reduced the size of the study sample and thus the statistical power of the results obtained. Therefore, we see the need for further research in this area with longitudinal studies conducted on a larger sample with additional genotypes and assessing the interaction between genes and other determinants of anxiety and depression.
5 Conclusion
To sum up, although no association was found between the 5-HTT and the MAO-A genotypes and the severity of anxiety and depressive symptoms in oncology patients, our findings showed that the dominant style among patients with the MAO-A 4/4 genotype was the constructive style, and the dominant strategy was positive redefinition.
Data availability statement
The original contributions presented in the study are included in the article further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Bioethics Committee Pomeranian Medical University in Szczecin (Resolution no. KB-0012/81/18). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AJ: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. AC-G: Supervision, Validation, Writing – review & editing. AM: Formal analysis, Investigation, Validation, Writing – review & editing. DĆ: Writing – review & editing. JO: Investigation, Resources, Software, Writing – review & editing. SW-H: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project is financed from the program of the Minister of Science and Higher Education under the name “Regional Initiative of Excellence” in 2019–2022; project number 002/RID/2018/19; amount of financing 12,000,000 PLN.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gołota, S, Białczyk, K, Wyszkowska, Z, Popiołek, A, Krajnik, M, and Borkowska, A. Anxiety and depression in cancer patients — what do we lose? Medycyna Paliatywna w Praktyce. (2017) 11:111–7.
2. Ciesla, JA, and Roberts, JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. (2001) 158:725–30. doi: 10.1176/appi.ajp.158.5.725
3. Pohjasvaara, T, Leppavuori, A, Siira, I, Vataja, R, Kaste, M, and Erkinjuntti, T. Frequency and clinical determinants of poststroke epression. Stroke. (1998) 29:2311–7. doi: 10.1161/01.STR.29.11.2311
4. Carney, RM, and Freedland, KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. (2003) 54:241–7. doi: 10.1016/S0006-3223(03)00111-2
5. Frasure-Smith, N, Lesperance, F, and Talajic, M. Depression following myocardial infarction: impact on 6-month survival. JAMA. (1993) 270:1819–25. doi: 10.1001/jama.1993.03510150053029
7. Polsky, D, Doshi, JA, Marcus, S, Oslin, D, Rothbard, A, Thomas, N, et al. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med. (2005) 165:1260–6. doi: 10.1001/archinte.165.11.1260
8. Beard, C, Millner, AJ, Forgeard, MJ, Fried, EI, Hsu, KJ, Treadway, MT, et al. Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychol Med. (2016) 46:3359–69. doi: 10.1017/S0033291716002300
9. Borsboom, D. Psychometric perspectives on diagnostic systems. J Clin Psychol. (2008) 64:1089–108. doi: 10.1002/jclp.20503
10. Schmittmann, VD, Cramer, AO, Waldorp, LJ, Epskamp, S, Kievit, RA, and Borsboom, D. Deconstructing the construct: a network perspective on psychological phenomena. New Ideas Psychol. (2013) 31:43–53. doi: 10.1016/j.newideapsych.2011.02.007
11. Di Matteo, MR, Lepper, HS, and Croghan, TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. (2000) 160:2101–7. doi: 10.1001/archinte.160.14.2101
12. Mello, S, Tan, ASL, Armstrong, K, Schwartz, JS, and Hornik, RC. Anxiety and depression among cancer survivors: the role of engagement with sources of emotional support information. Health Commun. (2013) 28:389–96. doi: 10.1080/10410236.2012.690329
13. Hewitt, M, Greenfield, SH, and Stovall, E. From Cancer patient to Cancer survivor: Lost in transition. Washington, DC: The National Academies Press (2005).
14. Zaorsky, NG, Zhang, Y, Tuanquin, L, Bleuthmann, SM, Pork, HS, and Chinchilli, VM. Suicide among cancer patients. Nat Commun. (2019) 10:1–7. doi: 10.1038/s41467-018-08170-1
15. Spiegel, D, and Riba, MB. Psychological issues in Cancer In: VT DeVita, TS Lawrence, and SA Rosenberg, editors. Cancer: Principles & practice of oncology. Philadelphia: Wolters Kluwer Health, Lippincott Williams & Wilkins (2011). 2467–76.
16. Mesárošová, M, and Ostró, A. Psychological responses to cancer among female patients with malignant and benign breast disease. Stud Psychol. (2005) 47:91–102.
17. Nesse, R. Emotional disorders in evolutionary perspective. Br J Med Psychol. (2011) 71:397–415. doi: 10.1111/j.2044-8341.1998.tb01000.x
18. Munafò, MR. Understanding the candidate gene environment interaction debate: epistemological or evidential divide? Int J Epidemiol. (2015) 44:1130–2. doi: 10.1093/ije/dyv056
19. Zammit, S, Owen, MJ, and Lewis, G. Misconceptions about gene– environment interactions in psychiatry. Evid Based Ment Health. (2010) 13:65–8. doi: 10.1136/ebmh1056
20. Taylor, AE, and Munafò, MR. Triangulating meta-analyses: the example of the serotonin transporter gene, stressful life events and major depression. BMC Psychol. (2016) 4:23. doi: 10.1186/s40359-016-0129-0
21. Rutter, M, Thapar, A, and Pickles, A. Gene–environment interactions: biologically valid pathway or artifact? Arch Gen Psychiatry. (2009) 66:1287–9. doi: 10.1001/archgenpsychiatry.2009.167
22. Uher, R. Gene–environment interactions in common mental disorders: an update and strategy for a genome-wide search. Soc Psych Psych Epid. (2014) 49:3–14. doi: 10.1007/s00127-013-0801-0
23. Krishnan, V, and Nestler, EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. (2010) 167:1305–20. doi: 10.1176/appi.ajp.2009.10030434
24. Dunn, EC, Brown, RC, Dai, Y, Rosand, J, Nugent, NR, Amstadter, AB, et al. Genetic determinants of depression: recent findings and future directions. Harv Rev Psychiatry. (2015) 23:1–18. doi: 10.1097/HRP.0000000000000054
25. Suppli, NP, Bukh, JD, Moffitt, TE, Caspi, A, Johansen, C, Tjønneland, A, et al. Genetic variants in 5-HTTLPR, BDNF, HTR1A, COMT, and FKBP5 and risk for treated depression after cancer diagnosis. Depress Anxiety. (2017) 34:845–55. doi: 10.1002/da.22660
26. Caspi, A, Sudgen, K, Moffitt, TE, Taylor, A, Craig, IW, Harrington, H, et al. Influence of live stress on depression: moderation y a polymorphism in the 5-HTT gene. Science. (2003) 301:386–9. doi: 10.1126/science.1083968
27. Cicchetti, D, Rogosh, FA, and Sturge-Apple, ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase a polymorphisms: depressive symptomatology among adolescent from low socioeconomic status backgrounds. Dev Psychopathol. (2007) 19:1161–80. doi: 10.1017/S0954579407000600
28. Kendler, KS, Thornton, LM, and Gardner, CO. Stressful life events and previous episodes in the etiology of major depression in women: an evolution of the kindling hypothesis. Am J Psychiatry. (2000) 157:1243–51. doi: 10.1176/appi.ajp.157.8.1243
29. Sharpley, CF, Palanisamy, SKA, Glyde, NS, Dillingham, PW, and Agnew, LL. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav Brain Res. (2014) 273:89–105. doi: 10.1016/j.bbr.2014.07.030
30. Beck, AT, Steer, AR, Brown, GK, Łojek, E, Stańczak, J, and Jaworowska, A. BDI-II Inwentarz depresji Becka. 2nd ed. Wydawnictwo Pracownia Testów PTP: Warszawa, Poland (2019).
31. Beck, AT, Steet, RA, Ball, R, and Ranieri, WF. Comparison of Beck depression inventories-IA and II in psychiatric outpatients. J Pers Assess. (1996) 67:588–97. doi: 10.1207/s15327752jpa6703_13
32. Wrześniewski, K, and Sosnowski, T. Inwentarz Stanu i Cechy Lęku Polska adaptacja STAI. Warszawa: Wydawnictwo Pracownia Testów Psychologicznych PTP (2011).
33. Juczyński, Z. Narzędzia Pomiaru w Promocji i Psychologii Zdrowia. 2nd ed. Warszawa: Pracownia Testów Psychologicznych PTP (2012).
34. Thiese, MS, Ronna, B, and Ott, U. P value interpretations and considerations. J Thorac Dis. (2016) 8:E928–31. doi: 10.21037/jtd.2016.08.16
35. Bultz, BD, and Carlson, LE. Emotional distress: the sixth vital sign in cancer care. J Clin Oncol. (2005) 23:6440–1. doi: 10.1200/JCO.2005.02.3259
36. Hinz, A, Krauss, O, Hauss, JP, Höckel, M, Kortmann, RD, Stolzenburg, JU, et al. Anxiety and depression in cancer patients compared with the general population. Eur J Cancer Care. (2010) 19:522–9. doi: 10.1111/j.1365-2354.2009.01088.x
37. Beekers, N, Husson, O, Mols, F, Van Eenbergen, M, and Van de Poll-Franse, LV. Symptoms of anxiety and depression are associated with satisfaction with information provision and internet use among 3080: Cancer survivors results of the PROFILES registry. Cancer Nurs. (2015) 38:335–42. doi: 10.1097/NCC.0000000000000184
38. Van Esch, L, Roukema, JA, Ernst, MF, Nieuwenhuijzen, GAP, and De Vries, J. Combined anxiety and depressive symptoms before diagnosis of breast cancer. J Affect Disord. (2012) 136:895–901. doi: 10.1016/j.jad.2011.09.012
39. Landowski, J. Badania laboratoryjne w psychiatrii In: A Bilikiewicz, S Pużyński, and J Rybakowski, editors. Psychiatria. Tom I. Podstawy Psychiatrii. Wrocław: Wydawnictwo Medyczne Urban & Partner (2003)
40. Simonyte, S, Grabauskyte, I, Macijauskiene, J, Lesauskaite, V, Lesauskaite, V, Kvaal, KS, et al. Associations of the serotonin transporter gene polymorphism, 5-HTTLPR, and adverse life events with late life depression in the elderly Lithuanian population. Sci Rep. (2023) 13:12920. doi: 10.1038/s41598-023-40215-4
41. Juhasz, G, Gonda, X, Hullam, G, Eszlari, N, Kovacs, D, Lazary, J, et al. Variability in the effect of 5-HTTLPR on depression in a large european population: the role of age, symptom profile, type and intensity of life stressors. PLoS One. (2015) 10:e0116316. doi: 10.1371/journal.pone.0116316
42. Kendler, KS, Kuhn, JW, Vittum, J, Prescott, CA, and Riley, B. The interaction of stressful live events and a serotonin transporter polymorphism in the prediction of episodes of major depression. Arch Gen Psychiatry. (2005) 62:529–35. doi: 10.1001/archpsyc.62.5.529
43. Wurtman, RJ. Genes, stress and depression. Metabolism. (2005) 54:16–9. doi: 10.1016/j.metabol.2005.01.007
44. Tennant, C. Life events, stress and depression: a review of recent findings. Aust N Z J Psychiatry. (2002) 36:173–82. doi: 10.1046/j.1440-1614.2002.01007.x
45. Stein, MB, Campbell-Sills, L, and Gelernter, J. Genetic variation in 5HTTLPR is associated with emotional resilience. Am J Med Genet B Neuropsychiatr Genet. (2009) 150B:900–6. doi: 10.1002/ajmg.b.30916
46. Asghari, A, and Nicholas, MK. Pain self-efficacy beliefs and pain behavior. A prospective study. Pain. (2001) 94:85–100. doi: 10.1016/S0304-3959(01)00344-X
47. Syriala, KL, Jensen, MP, Mendoza, ME, Yi, JC, Fisher, HM, and Keefe, FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. (2014) 32:1703–11. doi: 10.1200/JCO.2013.54.4825
48. Grassi, L, Buda, P, Cavana, L, Annunziata, MA, Torta, R, and Varetto, A. Styles of coping with cancer: the Italian version of the Mini-mental adjustment to Cancer (Mini-Mac) scale. Psychooncology. (2004) 14:115–24. doi: 10.1002/pon.826
49. Torta, R, Siri, I, and Caldera, P. Sertraline effectiveness and safety in depressed oncological patients. Support Care Cancer. (2008) 16:83–91. doi: 10.1007/s00520-007-0269-0
50. Lõhmussaar, K, Boretto, M, and Clevers, H. Human-derived model Systems in Gynecological Cancer Research. Trends Cancer. (2020) 6:1031–43. doi: 10.1016/j.trecan.2020.07.007
51. Malicka, I, Szczepańska, J, Anioł, K, Rymaszewska, J, and Woźniewski, M. Zaburzenia nastroju i strategie przystosowania do choroby u kobiet leczonych operacyjnie z powodu nowotworu piersi i narządów rodnych. Współczesna Onkol. (2009) 13:41–6.
52. Schillani, G, Martinis, E, Capozzo, MA, Era, D, Cristante, T, Mustacchi, G, et al. Psychological response to cancer: role of 5-HTTLPR genetic polymorphism of serotonin transporter. Anticancer Res. (2010) 30:3823–6.
53. Rogala, D, Mazur, A, Maślińska, M, Koper, K, and Staniszewska, M. Psychological adaptation to cancer and strategies for coping with pain in patients with cervical cancer. Arch Perinatal Med. (2016) 22:1–7.
Keywords: women, gynecological cancer, adaptation, depression, anxiety
Citation: Jurczak A, Chudecka-Głaz A, Michalczyk A, Ćwiek D, Owsianowska J and Wieder-Huszla S (2025) The influence of genetic factors on the severity of anxiety and depressive symptoms and the choice of coping strategies in reproductive tract cancer―a preliminary study. Front. Public Health. 13:1543696. doi: 10.3389/fpubh.2025.1543696
Edited by:
Wulf Rössler, Charité University Medicine Berlin, GermanyReviewed by:
Jerry Lorren Dominic, Jackson Memorial Hospital, United StatesTao Ren, First Affiliated Hospital of Chengdu Medical College, China
Copyright © 2025 Jurczak, Chudecka-Głaz, Michalczyk, Ćwiek, Owsianowska and Wieder-Huszla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylwia Wieder-Huszla, c3lsd2lhLndpZWRlci5odXN6bGFAcHVtLmVkdS5wbA==
†ORCID: Anna Jurczak, https://orcid.org/0000-0003-1935-5285
Anita Chudecka-Głaz, https://orcid.org/0000-0003-2784-7968
Anna Michalczyk, https://orcid.org/0000-0001-7433-161X
Dorota Ćwiek, https://orcid.org/0000-0002-4908-9056
Joanna Owsianowska, https://orcid.org/0000-0001-9096-8477
Sylwia Wieder-Huszla, https://orcid.org/0000-0002-6084-5780
 Anna Jurczak1†
Anna Jurczak1† Sylwia Wieder-Huszla
Sylwia Wieder-Huszla