- 1Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Student Research Committee, Saveh University of Medical Sciences, Saveh, Iran
- 3Department of Public Health, Saveh University of Medical Sciences, Saveh, Iran
Cervical cancer is the fourth most common cancer among women globally, claiming over 443,000 lives annually, with 98% of these deaths occurring in developing countries. Vaccination against human papillomavirus (HPV) is a preventive strategy. This review investigates the role of age at vaccination and the number of doses in determining vaccine effectiveness. Articles from 2013 to 2023 were retrieved from PubMed, Scopus, SID, and Google Scholar using keywords related to HPV, vaccine, age, and dose. The findings suggest that the highest vaccine effectiveness is observed in younger age groups (ages 9–14: 74–93%) and decreases with age. Studies indicate that while three doses provide optimal protection, a single dose may also confer significant benefits in younger populations. These findings underscore the importance of timely vaccination and adherence to dosing schedules for maximizing vaccine impact.
Introduction
HPV is part of the most prominent family of sexually transmitted viruses (STDs) that infect a very large number of people annually (1). From a virological perspective, the human papillomavirus is a small double-stranded DNA virus and belongs to the group of nonenveloped icosahedral viruses (2). More than 200 genotypes of this virus have been identified and detected (3). Some of these genotypes, such as genotypes 6, 11, 40, 42, 43, 44, 54, 61, and 72, usually cause benign lesions, such as warts in different parts of the body, such as the head, neck, and urogenital tract, which are called low-risk types (4, 5). On the other hand, types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 69 are among the common high-risk types that cause cancer (6). Among the high-risk HPV types, HPV-16 and HPV-18 are known as the etiological agents of cervical cancer, accounting for approximately 70% of the burden of this cancer (7). The virus infects both males and females, but in most cases, it is asymptomatic and does not cause any particular disease; usually, after 1–2 years, it is self-cured and eliminated from the body (8). Initially, the infection starts with damage to the mucosal and skin tissues of the desired area and causes the formation of a wound or wart at the site. This virus usually affects the epithelial tissue of the genital area, especially in women (9). If the host immune system fails to clear the HPV infection, high-risk types of, HPV can activate oncogenes in the body (10).
Cervical cancer is the third most common malignancy of the female genital tract, and according to the World Health Organization, cervical cancer is the fourth most common cancer among women (11). More than 90% of cervical cancers and precancerous lesions are associated with HPV (12). The organization also stated that by 2030, cervical cancer will affect the lives of more than 443,000 women worldwide, and 98% of these deaths will occur in developing countries (13). Cervical cancer is the second leading cause of cancer death among women in developing countries (14). The International Agency for Research on Cancer (IARC) reported that in 2020, 604,000 women worldwide were diagnosed with cervical cancer, and approximately 342,000 women died from the disease (15).
The most important way to prevent cervical cancer is to prevent HPV infection. Three types of vaccines are available to prevent this viral infection: the bivalent type is effective against types 16 and 18 of the virus. The quadrivalent type covers types 11 and 6 in addition to these two types. The 9-valent HPV vaccine (Gardasil 9), which is recommended for individuals aged 9–45 in the United States, protects against HPV types 31, 33, 45, 52, and 58 in addition to the four main types covered by earlier versions. This vaccine is approved for use in both females and males to prevent HPV-related diseases, including cervical, vulvar., vaginal, anal, and oropharyngeal cancers, as well as genital warts (16, 17). The present study aimed to investigate the effectiveness of the human papillomavirus vaccine based on the age at which it was vaccinated and the appropriate number of doses.
In this review, our goal was to provide an overview of the effectiveness of the appropriate dose of HPV vaccination and the appropriate age for receiving the vaccine, which was conducted via a narrative review method. The search process was carried out from 2013 to 2023 in PubMed, Scopus, SID, and Google Scholar search engines by entering keywords such as human papillomavirus or HPV, age, dose, papilloma vaccine (HPV vaccine), and cervical cancer. In PubMed, the subject search was performed via MeSH. In the mentioned databases, a comprehensive and accurate review of published articles on vaccination for the prevention of cervical cancer and the role of age and vaccine dose in the effectiveness of this vaccine was conducted. In this context, articles that met the inclusion criteria were selected. The inclusion criteria for this study included the following: 1. The search words and keywords were included in the title and keywords of the article. 2. Full access to the text of the articles, including tables and figures, was available. 3. The focus of the article was on the number of doses received and the age of vaccination. Incomplete articles that were off-topic and did not have full access to the text were excluded from the study.
In this review, keywords were searched in the PubMed, Scopus, SID, and Google Scholar search engines, with a time limit of 2013–2023. The search keywords were as follows: human papillomavirus, age, dose, papilloma vaccine (HPV vaccine), and cervical cancer. The subject search was performed through MeSH. The inclusion criteria were articles focused on age and dose-specific vaccine effectiveness, full-text availability, and published in English.
Assumptions underlying vaccine efficiency analysis
The assumptions used in this review about the effectiveness of HPV vaccines are based on a number of key factors that could influence the extent to which vaccination is effective. First, it is assumed that vaccination at an earlier age, particularly before exposure to the virus (i.e., before sexual activity)—will elicit a stronger immune response, as this group is less likely to have been previously infected with HPV. This assumption is supported by the results of studies showing that 9- to 14-year-olds have higher antibody titers and greater levels of protection against HPV-related diseases than older adolescents (15–18-year-olds).
The second assumption is that completing the full vaccination schedule (two or three doses) provides optimal protection against HPV infection and related cancers. Although some studies suggest that even one dose may be sufficient for younger people, this is based on the assumption that more doses provide stronger and more lasting immunity, especially in older people or those who may have been previously exposed to the virus.
Another assumption is that vaccines such as Gardasil and Cervarix are able to provide cross-protection against different HPV types and that their effectiveness is, overall, comparable. However, some evidence suggests that the bivalent vaccine Cervarix may provide greater cross-protection against HPV types other than 16 and 18.
On the other hand, this review is based on the assumption that vaccination at an early age is not only more clinically effective but also more economically viable, as it leads to reduced future costs of treating HPV-related diseases, including cervical cancer. It is also hypothesized that single-dose vaccination strategies could simplify implementation, reduce costs, and increase vaccination coverage in resource-limited settings.
However, the review still emphasizes that completing the full vaccination regimen (two or three doses) is recommended for optimal protection, especially in older individuals, as this group may require more doses to achieve the same level of protection as younger individuals.
Finally, 17,790 records were found. A total of 992 articles were reviewed, and after irrelevant, duplicate, and noncompliant articles meeting the inclusion criteria were excluded, 28 articles were ultimately selected. Table 1 presents an overview of studies on HPV vaccine efficacy, with a particular focus on demographic factors and the development status of the countries studied. These studies show distinct differences between developed and developing countries in terms of target populations, vaccination schedules, and vaccine effects in different age groups. In developed countries, the main focus has been on the efficacy of multiple doses and age-related responses, and studies have mostly focused on adolescent girls and young women. In contrast, studies in developing countries have emphasized the potential for more cost-effective options, such as single-dose regimens, to increase vaccination coverage and reduce cervical cancer incidence. These studies suggest that demographic factors, such as age at vaccination and access to resources, play a key role in determining the best vaccination strategies. The results indicate that the effectiveness of the vaccine is greater among younger age groups and that the design of vaccination programs should be tailored to the specific characteristics and challenges of each country in terms of demographic structure and economic conditions in order to have the greatest impact on public health.
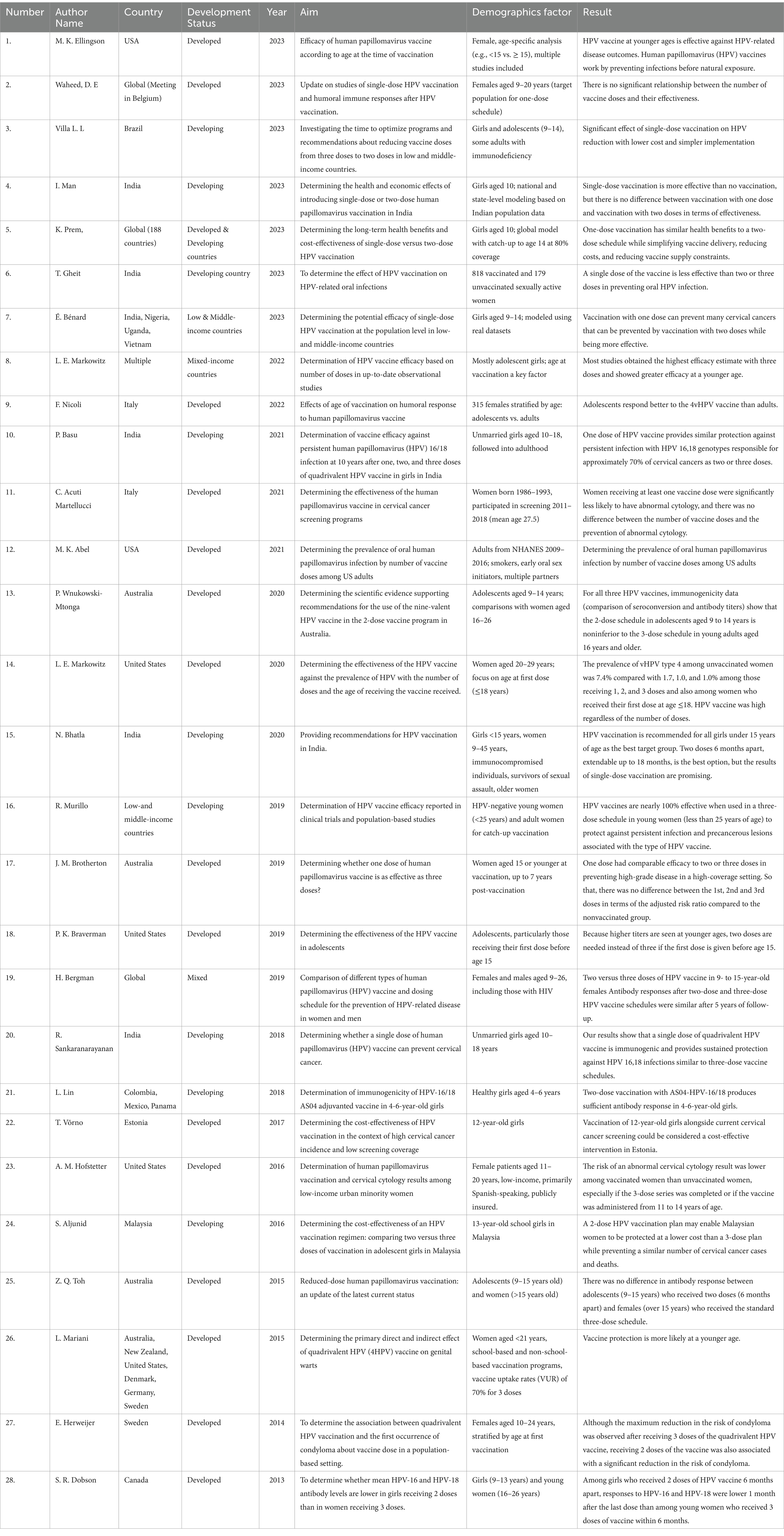
Table 1. Purpose, outcome, and demographic distribution considerations in HPV vaccine effectiveness studies based on the number of vaccine doses and development status.
Effectiveness of the vaccine based on the number of doses received
Several studies have shown a relationship between the number of vaccine doses and their effectiveness and immunogenicity, and the responses from a single dose of HPV vaccination result in lower antibody titers in the serum (18). A single vaccine dose may be less effective than two or three doses in preventing oral HPV infection (19), and the maximum effectiveness of the vaccine is achieved by receiving three doses (20).
On the other hand, some studies have shown no difference between the number of vaccine doses and the vaccine effectiveness (21–24). A single dose of the HPV vaccine provides similar protection against persistent infection from HPV genotypes 16 and 18, which are responsible for nearly 70% of cervical cancers, as two or three doses (25). Single-dose HPV vaccination can significantly reduce the incidence of precancerous and cervical cancers attributed to HPV, with reduced costs for vaccine delivery and simpler implementation, allowing more countries to introduce HPV vaccination or increase compliance in the target population. Although it does not differ in its ability to induce immunity at two doses, it is more effective (26, 27). Global introduction of one dose of HPV at 10 years of age has been presented as a cost-saving policy, particularly in the low-and middle-income context where vaccine cost and availability are a huge consideration (28). Evidence is such that the immune response provided by one dose is found to be extremely intense and long-lasting, yet any marginal value created by increasing doses from one may not be a reason for an added logistical expense and burden incurred (29). Single-dose vaccination with Gardasil 9 (9-valent) has similar health benefits to two-dose vaccination while simplifying vaccine delivery, reducing costs, and alleviating vaccine supply constraints. The second dose may be cost-effective if there is a shorter duration of protection from one dose, a cheaper vaccine, vaccination delivery strategies, and a high burden of cervical cancer (30).
Besides the dosing number, the formulation of the HPV vaccine used may contribute to the modulation of the immune response and vaccine efficacy. Other studies have pointed out that the bivalent vaccine (Cervarix) is more likely to induce a better and more durable antibody response against HPV 16 and HPV 18 than are the quadrivalent and 9-valent vaccines, particularly after single-dose regimens, due to reasons presumably owing to its adjuvant system (31, 32). However, the 9-valent vaccine (Gardasil-9) has broader coverage by targeting an additional five oncogenic HPV types, potentially for use in the presence of more disseminated circulating genotypes (33). Although few direct comparisons have occurred, evidence on hand suggests vaccine type choice will moderately influence immune response in regimens using one dose, though more research must be done in order to render absolute judgments.
In general, women who receive one dose do not differ in terms of abnormal cervical cytology from women who receive two or three doses (34), and individuals who receive one dose of the HPV vaccine may have a similar prevalence of oral HPV6, 11, 16, and 18 infections as those who receive additional doses (35).
Additionally, other studies have shown that a two-dose vaccination program does not have lower immunogenicity than a three-dose vaccination does (36–38), and two doses with a 6-month interval, which can be increased to 18 months, are the best option (39). Vaccination with two doses induces a sufficient antibody response (40) and prevents a similar number of cervical cancers and deaths at a lower cost than three doses (41). However, some studies have shown that among girls who received two doses of the HPV vaccine 6 months apart, the response to HPV-16 and HPV-18 one month after the last dose was lower than that in young women who received three doses of the vaccine within 6 months (42).
Effectiveness of the vaccine based on age at vaccination
Most studies have shown greater effectiveness when individuals are vaccinated at a younger age (20–28, 30, 34–43). The younger the subjects are at the time of vaccination, the less likely they are to have been exposed to HPV; therefore, they are more likely to be protected by preventive vaccines such as the HPV4 vaccine (39). In other words, higher titers are produced at younger ages, and if the first dose is given before the age of 15, one dose of the vaccine is needed instead of three doses (44). Based on a systematic review including 21 individual studies, the reported vaccine effectiveness ranged from approximately 74–93% among adolescents aged 9–14 years, and from 12 to 90% among those aged 15–18 years. These wide ranges reflect substantial variability in study populations, methodologies, and outcome definitions. These results suggest that the HPV vaccine is more effective against HPV-related disease outcomes when administered at younger ages and emphasizes the importance of timely vaccination (45).
On the other hand, the percentage of naive B and CD4 + T cells was significantly greater in adolescents, and the latter correlated directly with IgG titers against 3 of the 4 HPV types. HPV-specific IgGs, but not memory B cells, are induced and maintained at relatively high levels in individuals vaccinated during adolescence (46). Among women who received their first dose under the age of 18, the estimated effectiveness of the HPV vaccine was high, regardless of the number of doses (21). The target group under 15 and 11–14 years of age is the best target group for receiving the vaccine (39, 47). In general, vaccination with 3 doses in women under 25 years of age has an effectiveness close to 100% in protecting against persistent infection (48), and vaccinating 12-year-old girls, along with screening, can be considered an effective intervention (49). Some studies have also shown that for all three HPV vaccines, the immunogenicity data (comparison of seroconversion and antibody titers) indicate that they are not lower in adolescents aged 9–14 years than in young adults aged 16 years and older (36).
The present study investigated the effectiveness of the human papillomavirus vaccine on the basis of the number of doses and the appropriate age at vaccination. The presence of papillomaviruses can lead to cervical cancer (12). Cervical cancer can be controlled through screening, vaccination, and safe sexual relationships. To date, on the basis of the existing serotypes, three types of vaccines have been developed against this virus: Cervarix (bivalent), Gardasil (quadrivalent), and Gardasil-9 (9-valent) are the three vaccines developed against HPV (16, 17). Cervarix is a vaccine capable of protecting against types 16 and 18, which cause 91% of cervical cancers, 91% of anal cancers, and 61% of vaginal cancers (50). The Gardasil vaccine protects against four HPV serotypes, namely, 6, 11, 16, and 18. The Gardasil-9 vaccine contains particles of serotypes 6, 11, 16, 18, 31, 33, 45, 52, and 58 and induces immunity against these serotypes (51). Despite being bivalent, the efficacy and coverage of the Cervarix vaccine are significantly greater than those of the quadrivalent Gardasil vaccine, demonstrating the high potential of the Cervarix vaccine in providing cross-protection (50).
The reviewed studies consistently show that HPV vaccination is more effective when it is administered at younger ages, typically before exposure to HPV and the initiation of sexual activity (43, 45). Vaccination during preadolescence and early adolescence (e.g., ages 9–14) resulted in the highest rates of seroconversion, antibody titers, and protection against HPV infection and related diseases (39). Vaccination in older adolescents and young adults (e.g., ages 15 to 26) is still beneficial but has slightly lower effectiveness than in younger individuals (48).
These data indicate that a complete series of 2 or 3 doses of HPV vaccines is required to achieve optimal protection (24, 38). Receiving fewer than recommended number of doses was associated with decreased antibody levels and lower effectiveness against HPV infection and related diseases (38). However, some studies have shown that even a single dose of the HPV vaccine provides meaningful protection, particularly in younger age groups (22, 25). A study by Aljunid et al. (41) revealed that the two-and three-dose regimens of the HPV vaccine had similar effects on controlling cervical cancer, and using the two-dose regimen resulted in lower costs and was more cost-effective (41).
This review highlights that younger age at vaccination and adherence to dosing schedules are critical for maximizing HPV vaccine effectiveness. These findings align with global recommendations advocating vaccination before exposure to HPV, typically before the onset of sexual activity.
Despite the strengths of this study, its limitations include the following: Heterogeneity: Variability in study designs, populations, and endpoints limits direct comparisons of findings. Data gaps: Inadequate data on the long-term effectiveness and immunity in single-dose recipients call for additional studies. A review of published research from 2013 to 2023 indicates that protection against single-dose immune response and single-dose protection have persisted for up to 10 years. However, additional follow-up studies are necessary to ascertain if such protection can persist over a decade.
Conclusion
Since cervical cancer is a common cancer, preventing it is considered important. Accordingly, preventing HPV infection forms the basis and foundation of recent research. Cumulative evidence emphasizes the vital role of the HPV vaccine in preventing HPV infections and related diseases, especially cervical cancer. With the help of vaccination at an appropriate age, many precancerous and cancerous lesions of the female genital area can be prevented. HPV vaccination is a pivotal strategy for reducing the incidence of cervical cancer, particularly in resource-limited settings. Early administration and appropriate dosing maximize immunogenicity and cost-effectiveness. Public health campaigns should emphasize timely vaccination and adherence to recommended schedules. Even in developing or underdeveloped countries, appropriate vaccination doses against this virus can be administered cost-effectively. On the other hand, educating and raising awareness among individuals in society will play a clear and crucial role in the successful and timely (appropriate age) administration of vaccination. By adhering to the recommended number of doses on the basis of age groups and initiating vaccination at younger ages, healthcare providers can maximize the effectiveness of the vaccine in providing long-term protection against HPV-related health risks.
Author contributions
MR: Writing – original draft, Writing – review & editing. ZK: Writing – original draft. HZ: Investigation, Writing – original draft. PH: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HPV, Human papillomavirus; WHO, World Health Organization; STDs, Sexually Transmitted Viruses; IARC, International Agency for Research on Cancer.
References
1. Preston, SM, and Darrow, WW. Improving human papillomavirus-related knowledge and attitudes among ethnically diverse young adults. Health Equity. (2019) 3:254–63. doi: 10.1089/heq.2018.0091
2. Liu, M, Zhang, X, Guo, L, Sun, W, and Jiang, X. HPV prevalence and genotype distribution among 38 056 women in Weifang, China: a cross-sectional study. BMJ Open. (2023) 13:e073332. doi: 10.1136/bmjopen-2023-073332
3. Wang, L, Wu, B, Li, J, and Chen, L. Prevalence of human papillomavirus and its genotype among 1336 invasive cervical cancer patients in Hunan province, central South China. J Med Virol. (2015) 87:516–21. doi: 10.1002/jmv.24094
4. Jalilian, S, Izadi, B, Madani, SH, and Mohajeri, P. The prevalence and genotype distribution of human papillomavirus types in the general female population in west of Iran. Jundishapur J Microbiol. (2017) 10:e40855. doi: 10.5812/jjm.40855
5. Baddal, B, Oktay, MN, Bostanci, A, and Yenen, MC. Prevalence and genotype screening of human papillomavirus among women attending a private hospital in northern Cyprus: an 11-year retrospective study. BMC Womens Health. (2023) 23:1–8. doi: 10.1186/s12905-023-02451-8
6. Katirachi, SK, Grønlund, MP, Jakobsen, KK, Grønhøj, C, and von Buchwald, C. The prevalence of HPV in oral cavity squamous cell carcinoma. Viruses. (2023) 15:451. doi: 10.3390/v15020451
7. Moniri Javadhesari, S, Pourseif, S, and Khakpour, K. Nucleic acid vaccines for human papillomavirus; prevention or treatment. Iran J Obstetr Gynecol Infert. (2019) 22:77–88. doi: 10.22038/ijogi.2019.13821
8. de Sanjose, S, Brotons, M, and Pavon, MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. (2018) 47:2–13. doi: 10.1016/j.bpobgyn.2017.08.015
9. Javanmard, D, Namaei, MH, Haghighi, F, Ziaee, M, Behravan, M, Mirzaei, J, et al. The frequency and typing of human papilloma virus among women with normal and abnormal cytology in southern Khorasan, eastern Iran. Jundishapur J Microbiol. (2017) 10:e43213. doi: 10.5812/jjm.43213
10. Moniri Javadhesari, S, Khakpour, K, Pourseif, S, and Mozaffari, H. Common genotypes of human papillomavirus in East Azerbaijan population using HPV direct flow CHIP kit. Iran J Obstetr Gynecol Infert. (2020) 23:18–25. doi: 10.22038/ijogi.2020.16610
12. Shalchimanesh, Z, Ghane, M, and Kalantar, E. Prevalence of human papillomavirus genotypes in Tehran. Iran J Res Health Sci. (2022) 22:e00553. doi: 10.34172/jrhs.2022.88
13. Mboumba Bouassa, R-S, Nodjikouambaye, ZA, Sadjoli, D, Adawaye, C, Péré, H, Veyer, D, et al. High prevalence of cervical high-risk human papillomavirus infection mostly covered by Gardasil-9 prophylactic vaccine in adult women living in N’Djamena, Chad. PLoS One. (2019) 14:e0217486. doi: 10.1371/journal.pone.0217486
14. Pahlevan, S, Sepehr, V, Rezaei, A, and Pardehshenas, F: Frequency of high risk human papillomavirus (HPV) serotypes in cervicovaginal smear of wives of men with genital warts and its relation with pap smear. Iran. J. Obstet. Gynecol. Infertil. (2022) 25:46–52.
15. Cervical Cancer Available online at: https://www.iarc.who.int/cancer-type/cervical-cancer/ (Accessed September 19, 2024).
16. Yavari, P. Epidemiology textbook of prevalent disease in Iran, vol. 3. 2nd ed. Gap: Tehran, Iran. (2021).
17. Seddiq, S, Khalili, F, Abdoli, A, Azarkish, F, and Abdolmohammadi, K: Human papilloma virus (HPV) vaccine, prevention of cervical cancer: a review article. Tehran Univ. Med. J. (2022) 80:161–167.
18. Waheed, DE, Burdier, FR, Eklund, C, Baussano, I, Mariz, FC, Téblick, L, et al. An update on one-dose HPV vaccine studies, immunobridging and humoral immune responses - a meeting report. Prev Med Rep. (2023) 35:102368. doi: 10.1016/j.pmedr.2023.102368
19. Gheit, T, Muwonge, R, Lucas, E, Galati, L, Anantharaman, D, McKay-Chopin, S, et al. Impact of HPV vaccination on HPV-related oral infections. Oral Oncol. (2023) 136:106244. doi: 10.1016/j.oraloncology.2022.106244
20. Markowitz, LE, Drolet, M, Lewis, RM, Lemieux-Mellouki, P, Pérez, N, Jit, M, et al. Human papillomavirus vaccine effectiveness by number of doses: updated systematic review of data from national immunization programs. Vaccine. (2022) 40:5413–32. doi: 10.1016/j.vaccine.2022.06.065
21. Markowitz, LE, Naleway, AL, Klein, NP, Lewis, RM, Crane, B, Querec, TD, et al. Human papillomavirus vaccine effectiveness against HPV infection: evaluation of one, two, and three doses. J Infect Dis. (2020) 221:910–8. doi: 10.1093/infdis/jiz555
22. Brotherton, JM, Budd, A, Rompotis, C, Bartlett, N, Malloy, MJ, Andersen, RL, et al. Is one dose of human papillomavirus vaccine as effective as three?: a national cohort analysis. Papillomavirus Res. (2019) 8:100177. doi: 10.1016/j.pvr.2019.100177
23. Bergman, H, Buckley, BS, Villanueva, G, Petkovic, J, Garritty, C, Lutje, V, et al. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst Rev. (2019) 2019:CD013479. doi: 10.1002/14651858.CD013479
24. Sankaranarayanan, R, Joshi, S, Muwonge, R, Esmy, PO, Basu, P, Prabhu, P, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. (2018) 36:4783–91. doi: 10.1016/j.vaccine.2018.02.087
25. Basu, P, Malvi, SG, Joshi, S, Bhatla, N, Muwonge, R, Lucas, E, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. (2021) 22:1518–29. doi: 10.1016/S1470-2045(21)00453-8
26. Villa, LL, and Richtmann, R. HPV vaccination programs in LMIC: is it time to optimize schedules and recommendations? J Pediatr. (2023) 99:S57–s61. doi: 10.1016/j.jped.2022.11.012
27. Bénard, É, Drolet, M, Laprise, JF, Gingras, G, Jit, M, Boily, MC, et al. Potential population-level effectiveness of one-dose HPV vaccination in low-income and middle-income countries: a mathematical modelling analysis. Lancet Public Health. (2023) 8:e788–99. doi: 10.1016/S2468-2667(23)00180-9
28. MdC, T, Man, I, Georges, D, Saraswati, LR, Bhandari, P, Kataria, I, et al. Health and economic effects of introducing single-dose or two-dose human papillomavirus vaccination in India. BMJ Glob Health. (2023) 8:e012580. doi: 10.1136/bmjgh-2023-012580
29. Joshi, S, Anantharaman, D, Muwonge, R, Bhatla, N, Panicker, G, Butt, J, et al. Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination. Vaccine. (2023) 41:236–45. doi: 10.1016/j.vaccine.2022.11.044
30. Prem, K, Choi, YH, Bénard, É, Burger, EA, Hadley, L, Laprise, JF, et al. Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: a comparative modelling analysis. BMC Med. (2023) 21:313. doi: 10.1186/s12916-023-02988-3
31. Kreimer, AR, Herrero, R, Sampson, JN, Porras, C, Lowy, DR, Schiller, JT, et al. Evidence for single-dose protection by the bivalent HPV vaccine-review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. (2018) 36:4774–82. doi: 10.1016/j.vaccine.2017.12.078
32. Herrero, R, González, P, and Markowitz, LE. Present status of human papillomavirus vaccine development and implementation. Lancet Oncol. (2015) 16:e206–16. doi: 10.1016/S1470-2045(14)70481-4
33. Joura, EA, Giuliano, AR, Iversen, OE, Bouchard, C, Mao, C, Mehlsen, J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. (2015) 372:711–23. doi: 10.1056/NEJMoa1405044
34. Acuti Martellucci, C, Nomura, S, Yoneoka, D, Ueda, P, Brotherton, J, Canfell, K, et al. Human papillomavirus vaccine effectiveness within a cervical cancer screening programme: cohort study. BJOG. (2021) 128:532–9. doi: 10.1111/1471-0528.16429
35. Abel, MK, Mann, AK, Sonawane, K, Kapp, DS, Deshmukh, AA, and Chan, JK. Prevalence of Oral human papillomavirus infection by number of vaccine doses among US adults. JNCI Cancer Spectr. (2021) 5:pkab086. doi: 10.1093/jncics/pkab086
36. Wnukowski-Mtonga, P, Jayasinghe, S, Chiu, C, Macartney, K, Brotherton, J, Donovan, B, et al. Scientific evidence supporting recommendations on the use of the 9-valent HPV vaccine in a 2-dose vaccine schedule in Australia. Commun Dis Intell. (2018) 44:44. doi: 10.33321/cdi.2020.44.33
37. Toh, ZQ, Licciardi, PV, Fong, J, Garland, SM, Tabrizi, SN, Russell, FM, et al. Reduced dose human papillomavirus vaccination: an update of the current state-of-the-art. Vaccine. (2015) 33:5042–50. doi: 10.1016/j.vaccine.2015.07.102
38. Herweijer, E, Leval, A, Ploner, A, Eloranta, S, Simard, JF, Dillner, J, et al. Association of varying number of doses of quadrivalent human papillomavirus vaccine with incidence of condyloma. JAMA. (2014) 311:597–603. doi: 10.1001/jama.2014.95
39. Bhatla, N, Meena, J, Gupta, K, Pal, B, Divakar, H, Bhalerao, S, et al. Human papillomavirus vaccination: good clinical practice recommendations from the Federation of Obstetric and Gynecological Societies of India. J Obstet Gynaecol Res. (2020) 46:1651–60. doi: 10.1111/jog.14345
40. Lin, L, Parra, MM, Sierra, VY, Cespedes, AS, Granados, MA, Luque, A, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in 4-6-year-old girls: results to month 12 from a randomized trial. Pediatr Infect Dis J. (2018) 37:e93–e102. doi: 10.1097/INF.0000000000001871
41. Aljunid, S, Maimaiti, N, Nur, AM, Noor, MRM, and Wan Puteh, SE. Cost-effectiveness of HPV vaccination regime: comparing twice versus thrice vaccinations dose regime among adolescent girls in Malaysia. BMC Public Health. (2016) 16:71. doi: 10.1186/s12889-016-2754-1
42. Dobson, SR, McNeil, S, Dionne, M, Dawar, M, Ogilvie, G, Krajden, M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. (2013) 309:1793–802. doi: 10.1001/jama.2013.1625
43. Mariani, L, Vici, P, Suligoi, B, Checcucci-Lisi, G, and Drury, R. Early direct and indirect impact of quadrivalent HPV (4HPV) vaccine on genital warts: a systematic review. Adv Ther. (2015) 32:10–30. doi: 10.1007/s12325-015-0178-4
44. Braverman, PK. HPV Vaccine in Adolescents. Pediatr Ann. (2019) 48:e71–7. doi: 10.3928/19382359-20190118-02
45. Ellingson, MK, Sheikha, H, Nyhan, K, Oliveira, CR, and Niccolai, LM. Human papillomavirus vaccine effectiveness by age at vaccination: a systematic review. Hum Vaccin Immunother. (2023) 19:2239085. doi: 10.1080/21645515.2023.2239085
46. Nicoli, F, Mantelli, B, Gallerani, E, Telatin, V, Squarzon, L, Masiero, S, et al. Effects of the age of vaccination on the humoral responses to a human papillomavirus vaccine. Npj Vac. (2022) 7:37. doi: 10.1038/s41541-022-00458-0
47. Hofstetter, AM, Ompad, DC, Stockwell, MS, Rosenthal, SL, and Soren, K. Human papillomavirus vaccination and cervical cytology outcomes among urban Low-income minority females. JAMA Pediatr. (2016) 170:445–52. doi: 10.1001/jamapediatrics.2015.3926
48. Murillo, R, and Ordóñez-Reyes, C. Human papillomavirus (HPV) vaccination: from clinical studies to immunization programs. Int J Gynecol Cancer. (2019) 29:1317–26. doi: 10.1136/ijgc-2019-000582
49. Võrno, T, Lutsar, K, Uusküla, A, Padrik, L, Raud, T, Reile, R, et al. Cost-effectiveness of HPV vaccination in the context of high cervical cancer incidence and low screening coverage. Vaccine. (2017) 35:6329–35. doi: 10.1016/j.vaccine.2017.08.083
50. Mohammadian, H, and Nateghi, MR. The human papillomavirus (HPV) and its vaccines: a narrative review. Sarem J Med Res. (2022) 7:231–41. doi: 10.61186/sjrm.7.4.231
Keywords: vaccine efficacy, dose optimization, public health, HPV, cervical cancer
Citation: Rostami Varnousfaderani M, Khoshnazar Z, Zeratie H and Hosseini Koukamari P (2025) Optimizing HPV vaccine effectiveness: impact of vaccination age and dose schedule on immunogenicity and cervical cancer prevention. Front. Public Health. 13:1544220. doi: 10.3389/fpubh.2025.1544220
Edited by:
Abdu A. Adamu, South African Medical Research Council, South AfricaCopyright © 2025 Rostami Varnousfaderani, Khoshnazar, Zeratie and Hosseini Koukamari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parisa Hosseini Koukamari, cC5ob3NzZWluaWtAZ21haWwuY29t
 Mehran Rostami Varnousfaderani
Mehran Rostami Varnousfaderani Zahedeh Khoshnazar
Zahedeh Khoshnazar Hamidreza Zeratie2
Hamidreza Zeratie2 Parisa Hosseini Koukamari
Parisa Hosseini Koukamari