- 1Public Health Emergency Management Center, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
- 2Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia
- 3U.S. Centers for Disease Control and Prevention (CDC), Atlanta, GA, United States
- 4Global One Health Initiative (GOHi), Ohio State University, Columbus, OH, United States
Background: Viral respiratory pathogens have become the leading cause of acute undifferentiated febrile illness (AFI). We determined the fraction of AFI attributable to influenza and SARS-CoV-2 in Ethiopia, along with an understanding of their epidemiological characteristics.
Methods: From February 2021 to June 2022, we enrolled patients meeting an AFI case definition (age >5 years with fever ≥38°C) who presented at one of four selected sentinel hospital sites in Jimma, Harari, Addis Ababa, and Gonder. Clinical and epidemiological information was collected, Nasopharyngeal swab samples were collected and analyzed using real-time PCR for respiratory viruses (influenza and SARS-CoV-2). A quasi-binomial regression model and multivariable regression were performed to compute fractions and establish associations with the agent detected.
Result: A total of 737 AFI cases were enrolled. The overall proportion of SARS-CoV-2, influenza A, and influenza B among AFI patients were 7.8, 1.9, and 0.5 per 100,000 population, respectively. Among the enrolled AFI cases tested for SARS-CoV-2 and Influenza virus, SARS-CoV-2 was the most detected pathogen with a positivity rate of 13.7% (95% CI:11.3–16.4), followed by influenza A and influenza B, which have a positivity rate of 3.3% (95% CI: 2.2–5.1) and 0.8% (95% CI:0.3–1.8), respectively. The positivity rate of SARS-CoV-2 peaked at 37.4% in September 2021. Per the multivariable analysis, cases ≥65 years of age were three [AOR = 3.3,95% CI:(1.9–5.7)] times more likely to be positive for SARS-CoV-2.
Conclusion: SARS-CoV-2 and influenza viruses were highly prevalent among AFI cases. The proportion of SARS-CoV-2 was higher among older adults. Further study is recommended to characterize influenza subtypes, SARS-CoV-2 variants and determine their attributable fraction among a broader panel of AFI-causing pathogens that contributes for guiding the proper diagnostics, treatment and surveillance measures.
Introduction
Acute febrile illness (AFI) is one of the major reasons for outpatient visits and hospital admission among both children and adults (1, 2). AFI is typically characterized by fever without localizing manifestations, making it very challenging to confirm a diagnosis solely based on clinical history and physical examination (3). Beyond malaria, laboratory confirmation of other potential causes of AFI is expensive and complex, with unsatisfactory sensitivity and specificity (4, 5). To make things worse, resource-constrained settings have inadequate laboratory capacity to confirm suspected AFI cases hence practitioners utilize empirical management of cases (6). This approach has a far-reaching impact in exacerbating the risk of anti-microbial resistance (7).
The presence of considerable gaps in case definition, comparability of diagnostic assays, and control group to calculate attributable fractions were the major identified bottlenecks that hamper having a comparable estimate of the burden of AFI globally (8–10). Despite a significant reduction in the last two decades, malaria remains a common primary diagnosis in both malaria-endemic and non-endemic regions due to limited laboratory capacity to detect other AFI causing agents, and a lack of recognition of emerging and re-emerging etiologies as a potential differential diagnosis in consideration of local context (11–13). Among those emerging and re-emerging etiologies, viral respiratory tract infections are becoming the top cause of AFI (14).
Among viral respiratory infections, influenza A and B viruses are the most burdensome human respiratory pathogens (15). Depending on the circulating strain, annual seasonal influenza epidemics result in 290,000–650,000 deaths worldwide and affect up to 20% of the population (16). In 2020, a novel and virulent form of coronavirus emerged and was officially named by the World Health Organization (WHO) as SARS-CoV-2 (17). Due to the unprecedented speed of expansion that overwhelmed local capacity, WHO declared COVID-19 a global pandemic (18). As of 8th September 2024, over 776. 2 million confirmed cases and over 7 million deaths have been reported globally (19). Worldwide, the presence of a notable gap in case-counting makes it difficult to make a comparison across countries (20). The surveillance systems in most African countries have gaps in diagnostic capacity, shortage of staffing, and poor data handling (21, 22). Ethiopia established an influenza sentinel surveillance system in 2008 to address these gaps and optimize pandemic preparedness efforts (23).
In Ethiopia, the positivity rate of influenza among suspected Influenza-like Illness (ILI) cases is estimated at up to 20% with a predominance of Influenza A subtype along with marked seasonal variation (24, 25). On the other hand, the national positivity rate of SARS-CoV-2 ranges from 6 to 9%, with significant variation by age, sex, residence, medical condition, and background prevalence (26–29). Nevertheless, the presence of the COVID-19 pandemic in Africa severely compromised the existing surveillance system (30). The Ethiopian routine disease surveillance system was not capable of capturing COVID-19 cases due to gaps in the community and event-based surveillance (31). This study aims to determine the fraction of those selected respiratory pathogens among suspected AFI cases and, in addition, the study’s objective was to understand their clinical and epidemiological characteristics during the time of the COVID-19 pandemic. This study is expected to provide a clearer picture on the proportion of respiratory pathogens as a cause of febrile illness in the study sites.
The finding of this study contributes for a more precise mapping of viral respiratory infections with acute fever, which will be used to develop evidence-based algorithms for the management of febrile illnesses associated with influenza and SARS-CoV-2, and to inform rational surveillance efforts in the future. It also provides information to policy makers and health care workers to consider proper diagnostic approaches and treatment measures (like influenza and SARS-CoV-2 vaccination) for the public at large in the future.
Materials and methods
Setting and study period
The study was conducted in four referral hospitals (sentinel sites) in Ethiopia: namely, Jimma University Hospital (JUH, Jimma), Gonder University Hospital (GUH, Gonder), Hiwot-Fana comprehensive specialized Hospital (HFH, Harar), and St. Paul’s Millenium Medical College Hospital (SPH, Addis Ababa). The selected health facilities are estimated to serve more than 8 million people. The sites were selected using an assessment checklist with predefined criteria, including geographic location, patient volume, the capacity of the laboratory to properly collect, handle, store, and transport specimens to the testing laboratory at Ethiopian Public Health Institute, availability of sentinel surveillance system, and willingness of the hospitals to collaborate in the implementation of project activities. The study was conducted from February 2021 to June 2022.
AFI case enrollment
Inpatients and outpatients of any sex aged 5 years and above presenting at the selected facilities, and who met the case definition criteria for AFI, were eligible for enrollment in this study. The inclusion and exclusion criteria for the enrolled AFI cases were applied where the Inclusion criteria were patients aged ≥5 years old, measured axillary temperature ≥ 380c and experienced acute fever for 2–14 days. The exclusion criteria were subjects who do not consent to participate, subjects with localizing symptoms or identifiable focus of infection, subjects with chef complaint are injury or trauma; and obstetric related cases and surgical related underlying problems.
The sample size was calculated using a threshold approach (n = Z2*P*(1-P)/D2), with 95% CI, 0.05 precision value, and 20% expected prevalence (25), and the initial sample size was 480. To ensure representativeness and account for the effect of patient flow through the facilities, the sample size was doubled for each site, and cases were selected using a systematic sampling scheme where every nth case of AFI was enrolled. The nth value for each site was determined as the number of AFI cases seen by the facility divided by the maximum number of specimens that can be processed by the laboratory in a week. Samples from suspected AFI cases with inadequate specimens and incomplete data were excluded.
Clinical and demographic data collection and testing procedures
Trained data collectors were assigned to each sentinel site. They collected demographic, clinical (e.g., symptoms, signs, treatment before enrollment), and epidemiological (e.g., exposures, travel history, significant medical/social history) information from eligible and enrolled cases by interviewing the patients and guardians. Clinical, epidemiologic, and laboratory data were entered electronically onto tablets using an Open Data Kit (ODK) platform. Both electronic and paper-based data collection mechanisms were used at each study site to make one method a backup for the other.
Nasopharyngeal swabs were collected from enrolled cases using a sterile COPAN brand universal transport medium containing 1–3 mL Viral Transport Media. The collected sample was vortexed and aliquoted in two cryovials for molecular testing at the National Influenza Center (NIC), Ethiopian Public Health Institute (EPHI). A specimen requisition form was filled out with basic demographic information, and the collected specimens were triple packaged and sent weekly with completed forms. Study sites were equipped with freezers or refrigerators throughout the study to temporarily store samples at 4°C until transported by trained postal service officers to the NIC at EPHI. EPHI’s NIC (laboratory) identified respiratory viruses from nasopharyngeal swabs. Nucleic acid was extracted from the swabs using the MagaBio plus Virus RNA Purification Kit II by MGISP-NE32 automated extractor. Real-time PCR was conducted on an ABI 7500FAST system (Life Technologies, Carlsbad, CA USA) using primers provided by CDC International Reagent Resource (CDC-IRR), a biological reagent repository established to provide better access to laboratory reagents.
Statistical analysis
Statistical analyses were performed using R Studio version 4.2.2 and Stata version 17. Descriptive statistics were presented as medians, ranges, and interquartile ranges (IQR) for continuous variables and as proportions and charts for categorical variables. The proportion and positivity rate of respiratory infections associated with SARS-CoV-2 and influenza were determined to understand the epidemiology of the selected pathogens (73, 74). The proportion was computed by taking confirmed respiratory infection as a numerator, and the total outpatient visits and inpatient admissions of AFI cases as a denominator, whereas, in the case of positivity rate (PR) calculation, the denominator was changed to total AFI cases enrolled in the study while the numerator remained unchanged. The proportion was presented per 100,000 cases, while the PR was presented by percentage.
A quasi-binomial regression model was used to compute the fraction of SARS-CoV-2 and Influenza that was associated with AFI. Finally, the association between clinical and epidemiologic characteristics and testing positive for selected pathogens were further evaluated using a multivariable logistic regression approach.
Ethical clearance
Ethical clearance for this study was obtained from EPHI’s Scientific and Ethical Review Office (SERO) and Addis Ababa University (EPHI-IRB-254-2020; MM No. 065). Written informed consent was obtained from each participant or guardian (for pediatric patients aged 5–12 years old) prior to the enrollment in the study for an interview and sample collection. Participants were identified by coded study numbers in all data collection forms and electronic databases. No individual identifiers were used in any reports.
Result
Patient screening and enrollment
During the study period, a total of 737 eligible cases were enrolled, among them, 29.3, 26.8, 24.6, and 19.1% were enrolled from Addis Ababa, Gonder, Jimma, and Harari, respectively. Most enrolled participants were male (61.7%) and inpatient cases (66.3%). The median age of enrolled cases was 37 years, with a minimum age of 5 years to a maximum of 95 years (Table 1). Furthermore, only 19.0% of cases took fever-reducing medication before seeking care, and 48% of the cases had had a recorded temperature >38°C at the time of initial diagnosis.
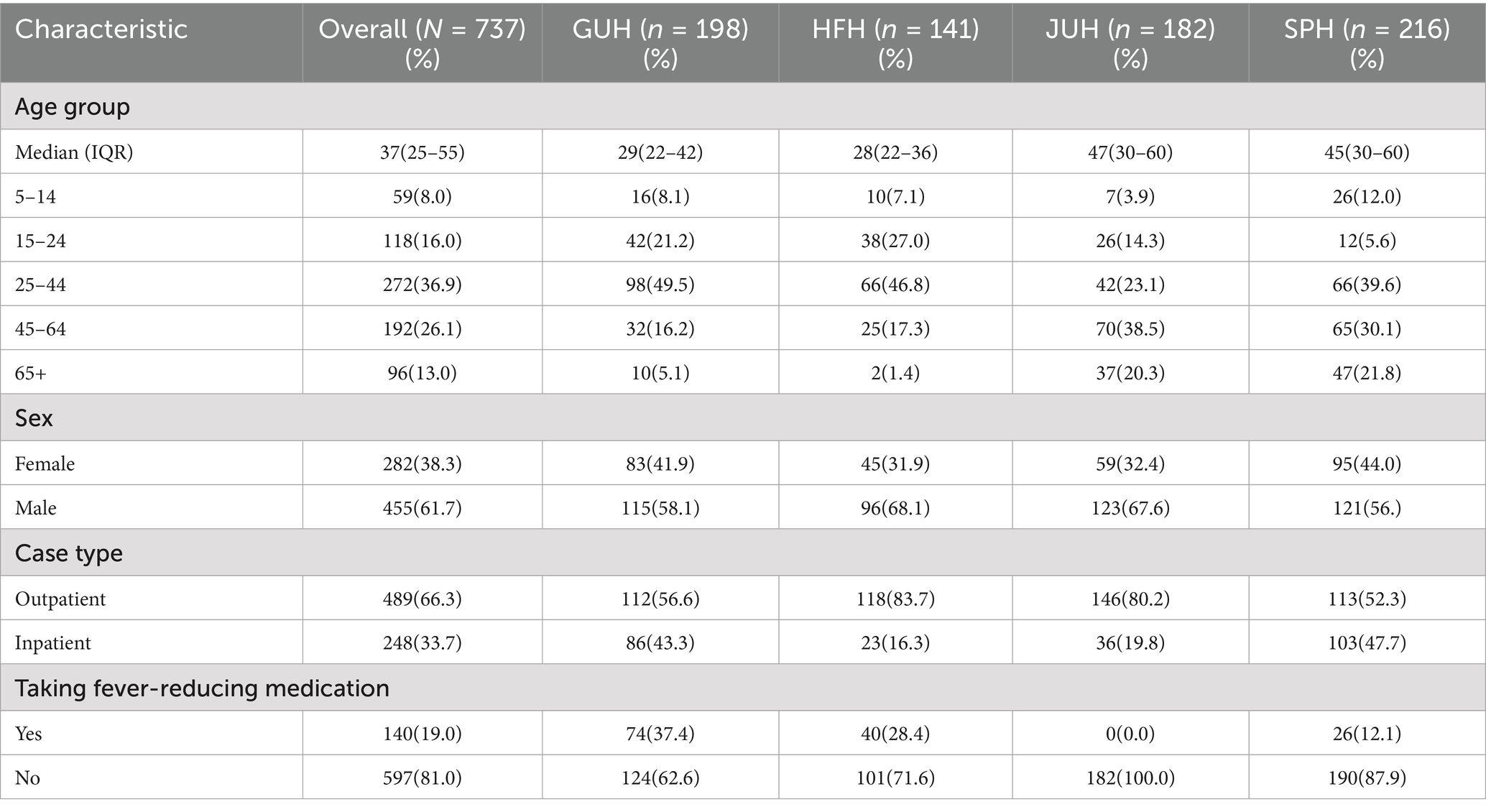
Table 1. Demographic and clinical characteristics of enrolled cases included in the study by site in Ethiopia.
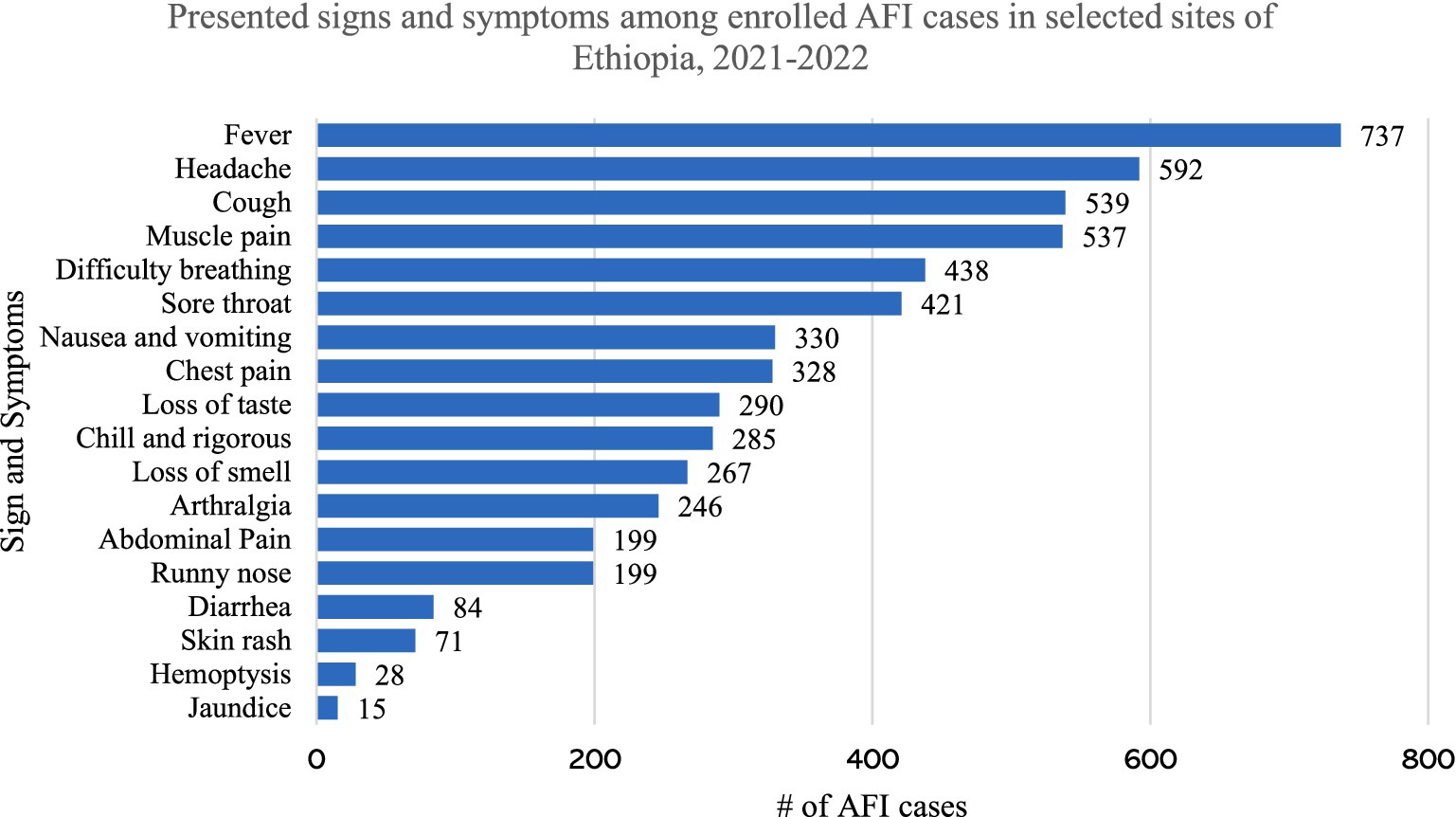
The most common presenting complaints, other than fever, were headache (80.3), cough (73.1%), muscle and joint pain (72.9%), and difficulty of breathing (59.4%). The least common presenting complaints were diarrhea (11.4%), skin rash (9.6%), hemoptysis (3.8%) and jaundice (2.0%) (Figure 1).
When examined by study site, 92.4 and 88.9% of cases from GUH presented with headache and muscle pain, while 84.1% of enrolled cases from JUH presented with cough (Table 2). Furthermore, 61.2, 17.6, and 7.6% of enrolled cases were primarily diagnosed as respiratory tract infections, malaria, and gastrointestinal tract infections, respectively. Geographically, 79.2% of cases from SPH and 73.6% of the cases from JUH were primarily diagnosed as respiratory tract infections, whereas 53.5% of cases from GUH were diagnosed as malaria. With respect to possible risk factors, 21.9, 5.3, and 5.2% of cases had a comorbid illness, contact with a dead animal, and travel history, respectively (Table 2).
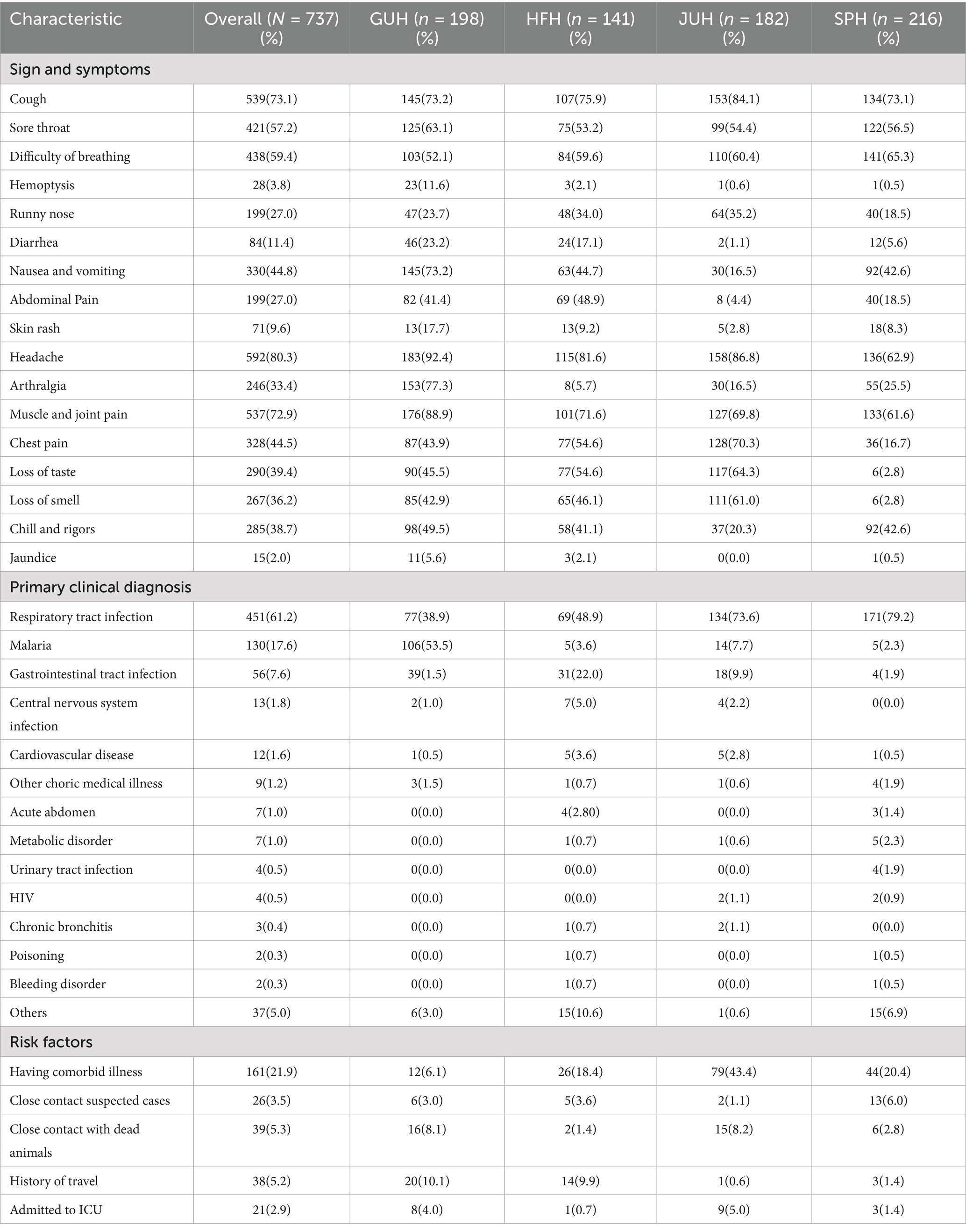
Table 2. Clinical manifestations, tentative diagnosis, and possible risk factor of enrolled cases by the site in Ethiopia.
Epidemiological description of confirmed cases
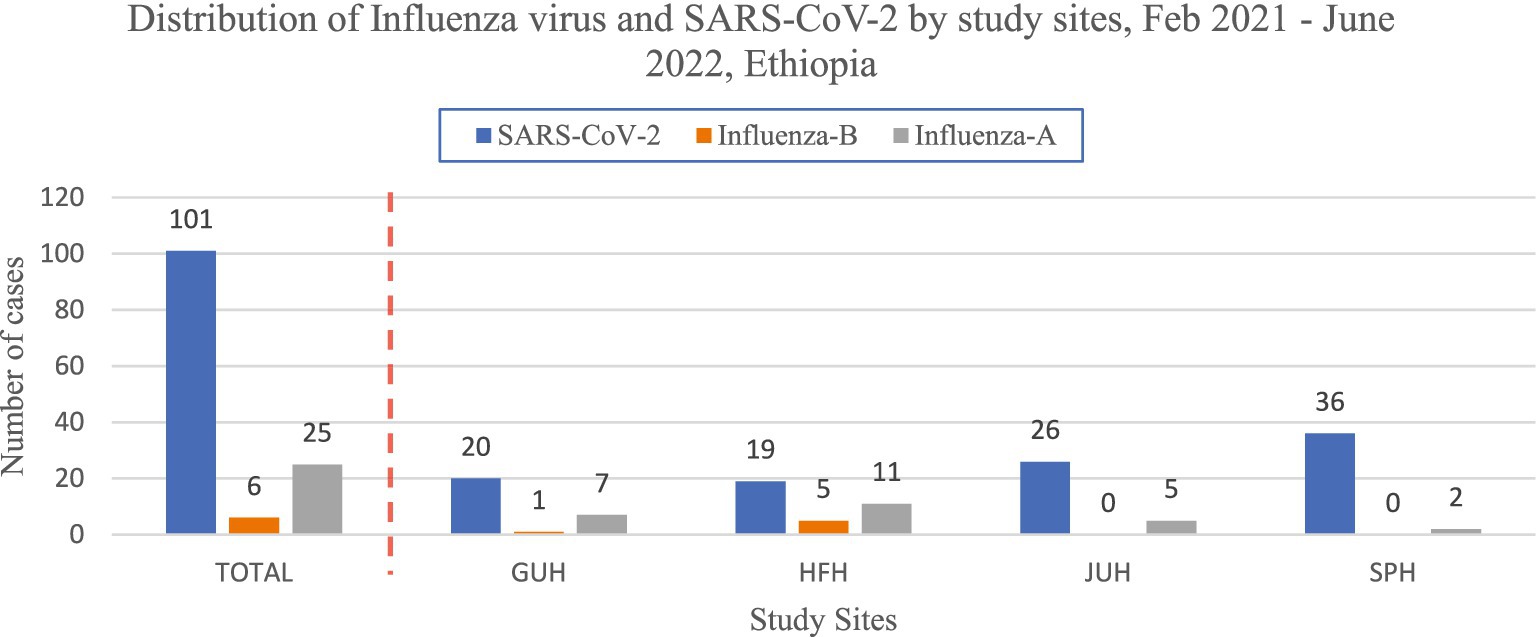
A total of 132(17.9%) cases were positive for Influenza and SARS-CoV-2 respiratory viruses (Figure 2).
The most prevalent respiratory pathogen was SARS-CoV-2 with a positivity rate of 13.7% followed by influenza A and influenza B, which had positivity rates of 3.3 and 0.8%, respectively. The positivity rate of SARS-CoV-2 was higher among cases from St. Paul Hospital (SPH) in Addis Ababa (16.7%), males (15.2%), and age >65 years (30.2%). Furthermore, influenza A virus was higher among cases from Hiwot-Fana Hospital (HFH) in Harar (7.8%), females (5.3%), and age 15–24 years (6.8%). With respect to proportion of influenza and SARS-CoV-2, the rate was higher among those ≥ 65 years (17.1 per 100,000 population), males (9.8 per 100,000 population), and Harar site (13.3 per 100,000 population) as compared to their respective categories (Table 3).
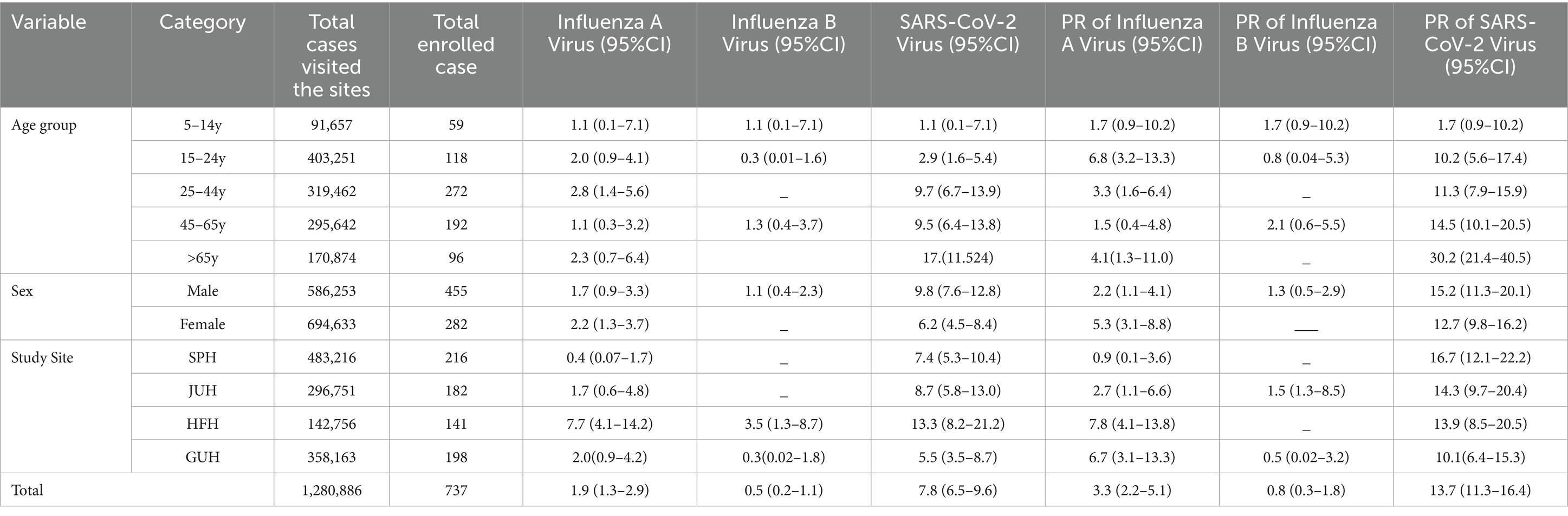
Table 3. Proportion and positivity rate of influenzas and SARS_CoV-2 by age, sex, and site in Ethiopia.
The positivity rates of SARS_CoV-2 markedly varied across months of the year, the positivity rate peaked during 2021 in September (37.9%), July (34.4%), and April (20.0%). The positivity rate of influenza A sharply increased in 2022, from 2.3% in April to 25% in June (Figure 3).
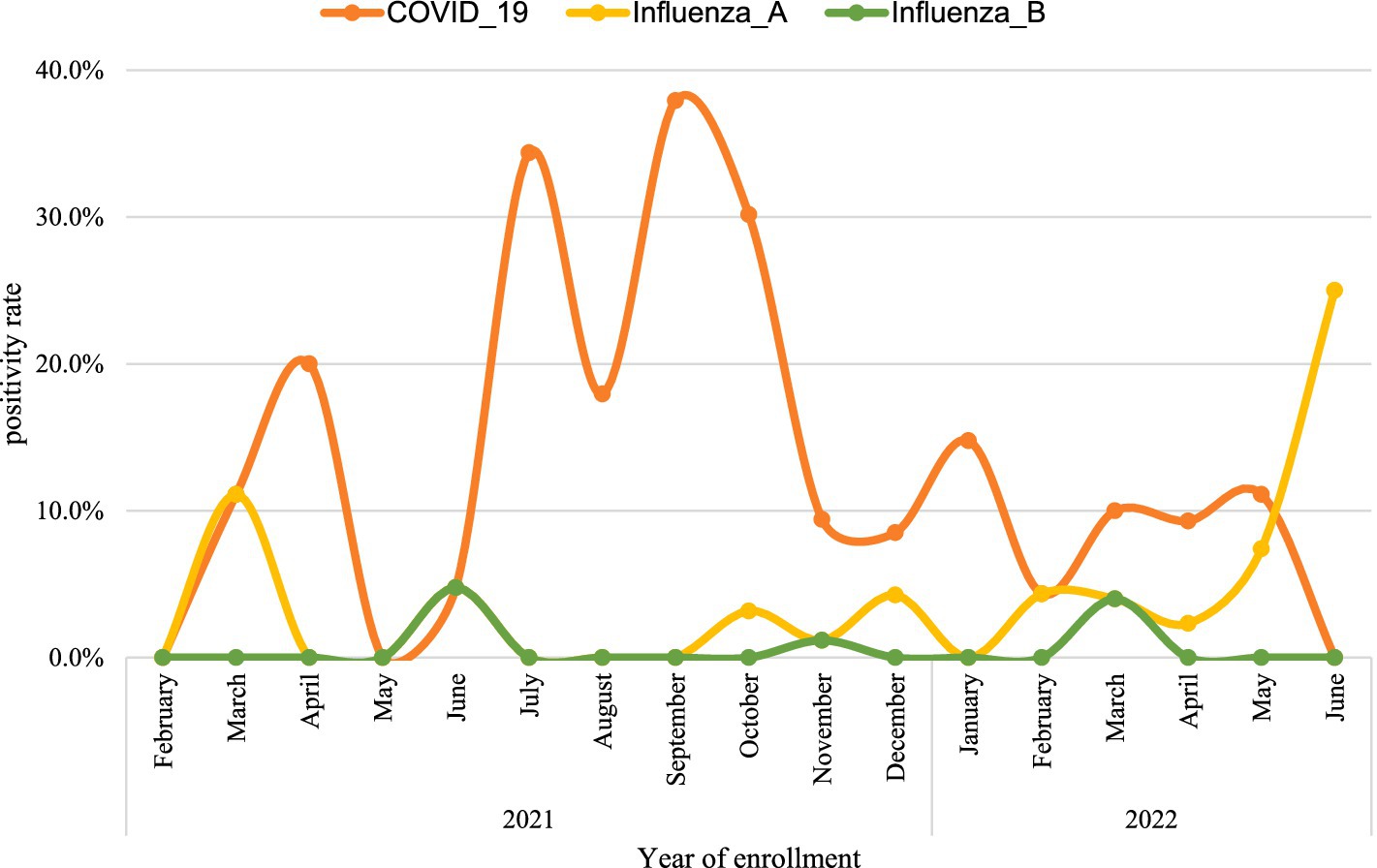
Figure 3. The trend of the positivity rate of influenza and SARS_COV-2 from 2021 to 2022 in Ethiopia.
When we looked at age group and clinical manifestation, cases aged 65 and above, were 3 times [AOR = 3.3,95%CI (1.9–5.7)] more likely to be positive for SARS-CoV-2 as compared to cases aged between 25 to 44. Moreover, chest pain [AOR = 0.6,95%CI (0.3–0.9)] and chill and rigorous [AOR = 0.5,95%CI (0.3–0.8)] were negatively associated with SARS-CoV-2 (Table 4).
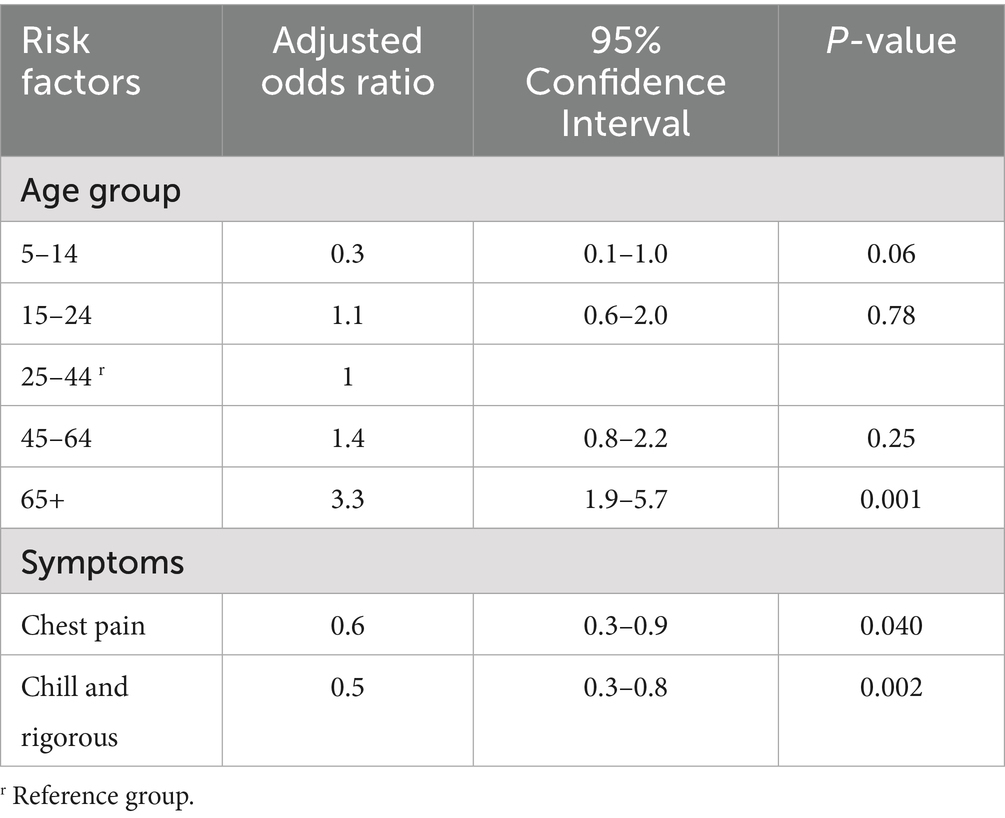
Table 4. Association of age group and clinical features of overall study participants with SARS_CoV-2.
Discussion
This study provides a deeper insight into the epidemiological and clinical manifestation of selected respiratory pathogens among AFI patients across four hospitals in Ethiopia. A standardized case definition of AFI were used for the enrolled cases with an inclusion and exclusion criteria and the case definition were not specific for respiratory cases. The proportion and positivity rate of SARS-CoV-2 was four-fold higher than influenza A and nearly 10 times higher than influenza B. Furthermore, marked variation in the positivity rate was observed in terms of sex, age group, site, and season.
The overall positivity rate of SARS-CoV-2 was 13.7%. The finding was comparable with studies conducted in India (32), and Pakistan (33); however, the positivity rate was much lower as compared to studies conducted in Tanzania (34), Kenya (35), Uganda (36) and Madagascar (37). The variation in the SARS-CoV-2 positivity rate may be explained by the method of laboratory investigation, study population, changes in the COVID-19 pandemic due to the introduction of new variants and vaccination as well as the level of preventive measures put forward in a country. Overall, tracking positivity rate is critical in monitoring the implemented mitigation measures along with paving the way for taking additional interventions to contain the pandemic (36).
This study elucidated that the proportion and positivity rate of SARS-CoV-2 was slightly higher among males as compared to females. The finding was parallel with studies conducted in other countries (38–41). This could be explained since males have lower health seeking behavior, so only present for care when sicker and more likely to test positive. Furthermore, their occupation and social engagements makes them more vulnerable to having a higher viral load resulting in disease severity, and mortality (42). Males are also thought to adhere to prescribed preventive measures less frequently than females (43). Thus, targeted communication to higher risk groups should be adopted and put in place for the proper implementation of the designed interventions.
The study revealed that the positivity rate of SARS-CoV-2 was much higher among old-aged cases (age ≥65 years) when compared with other age groups. This finding is corroborated by studies conducted elsewhere (44–47). Further exploring the risk of age with SARS-CoV-2 infection might be necessary to identify the exact correlation. Perhaps, older people are usually attached to low socioeconomic status, which has a role in contracting the illness due to a lack of hygienic measures like frequent hand washing and keeping physical distance (48). However, the preventive measures put in place to control the pandemic have resulted in untoward consequence on the health of the people (i.e., physical, mental, emotional, and social) and the economy of the world (49). Overall, control measures should be in place by evaluating the untoward consequences of those measures among the older population vis-à-vis outweighing the severity of the illness.
The positivity rate of SARS-CoV-2 reached its peak between July and September 2021. This finding concurred with other studies conducted (50, 51). Different geographic arrangements could account for different changes in the pandemic’s dynamics. For example, in African countries, the rapid rise in the positivity rate is linked to the emergence of new variants, such as the Beta and Delta variants (52). In summary, the findings imply a need for regular monitoring of the positivity rate of SARS-CoV-2 and influenza virus through the routine surveillance system where the surveillance data and specimens collected on a regular manner and reported via the Integrated Disease Surveillance System (IDSR) accompanied by ad hoc surveillance to take timely measures.
In this study, chills and rigor as well as chest pain were not indicative symptoms for the diagnosis of SARS-CoV-2. This finding agreed with studies conducted elsewhere (53–55). Chest pain is commonly attached to major adverse cardiac events such as coronary artery disease (56–58), while chills and rigor are usually indicative diagnosis of arboviral and other acute respiratory tract infections (59–62). Thus, health professionals should be vigilant enough to consider other diagnoses, other than SARS-CoV-2, when they encounter chills, rigor, and chest pain while taking a clinical history of cases.
In this study, both PR and proportion of SARS-CoV-2 were higher in HFH and SPH as compared to other sites. A similar finding was also reported from studies conducted in Spain and Kenya, which indicated that the burden of SARS-CoV-2 was much higher in major cities (63, 64). Major cities have unique characteristics explained by high population density and road connectivity, which are fertile ground for rapid transmission of the pandemic due to the increased chance of physical contact (65, 66). For effective containment of SARS-CoV-2, measures should be taken to raise public awareness of physical distancing in public places.
With respect to the overall proportion and positivity rate of influenza virus, influenza A was predominant over influenza B. However, in Ethiopia, the positivity rate observed during this study period was five folds lower than when compared before the pandemic, which reached up to 20% (24, 25). This could be explained by alterations in the epidemiology of the virus during the course of the pandemic (67). Non-pharmaceutical Interventions (NPI) implemented to curb the burden of the pandemic have resulted in a reduction in hospitalization and mortality due to Non-SARS-CoV-2 (68–70). Although the burden of influenza notably reduced, the reduction during the pandemic has raised a tone of questions that need to be addressed related to seasonality and preventative measures (71). On top of this, the pandemic has impacted the health system (72). The combination of all these could result in the rebound of influenza and could left the world in a precarious public health position that warrants weighing potential pandemic risks more seriously.
This study and its results are subject to the following limitations. RT-PCR misses the detection of people with SARS-CoV-2 infection unless cases present during the acute phase of illness when viremia is high and detectable by RT-PCR assay. This hinders to enroll the actual sample size and could introduce a substantial risk of bias by underestimating the positivity rate. In addition, case enrollment across all months/season were not consistent. As a result, seasonal variations in the occurrence of influenza and SARS-CoV-2; and other AFI causing pathogens were not ruled out by laboratory testing in this study.
Conclusion
SARS-CoV-2 and influenza viruses were highly prevalent in AFI cases in Ethiopia. The proportion of SARS-CoV-2 was highest among persons aged over 65 years of age. A further study is recommended to explore influenza subtypes and SARS-CoV-2 variants among cases and determine their influence on disease severity, signs and symptoms, and on the fraction of broader pathogens causing AFI in Ethiopia so as to provide evidence-based information that guides a proper diagnosis, clinical care and surveillance approaches.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethiopian Public Health Institute IRB AND Addis Ababa University’s Aklilu Lemma Institute of Pathobiology IRB. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DS: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. AT: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. ZM: Conceptualization, Methodology, Writing – review & editing. NT: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. AyA: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. AG: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. WS: Data curation, Investigation, Methodology, Writing – review & editing. MG: Data curation, Investigation, Methodology, Writing – review & editing. AdA: Data curation, Investigation, Methodology, Writing – review & editing. AsH: Investigation, Methodology, Writing – review & editing. YA: Investigation, Methodology, Writing – review & editing. MW: Project administration, Supervision, Validation, Writing – review & editing. AbH: Data curation, Investigation, Methodology, Writing – review & editing, Formal analysis, Validation. PM: Validation, Writing – review & editing. RS: Writing – review & editing. HD: Methodology, Validation, Writing – review & editing. LB: Validation, Writing – review & editing. TK: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. NK: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The publication was supported by the Cooperative Agreement Number, NU2HGH000069 funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Acknowledgments
We would like to express our gratitude to all AFI sentinel surveillance sites and the personnel that operate there for their crucial contribution. We are grateful to the United States Centers for Disease Control and Prevention (CDC) for their technical support and provision of lab supplies and reagents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fpubh.2025.1664179.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
References
1. Prasad, N, Sharples, KJ, Murdoch, DR, and Crump, JA. Community prevalence of fever and relationship with malaria among infants and children in low-resource areas. Am J Tropical Med Hygiene. (2015) 93:178–80. doi: 10.4269/ajtmh.14-0646
2. Crump, JA, and Kirk, MD. Estimating the burden of febrile illnesses. PLoS Negl Trop Dis. (2015) 9:e0004040. doi: 10.1371/journal.pntd.0004040
3. Snow, RW, Eckert, E, and Teklehaimanot, A. Estimating the needs for artesunate-based combination therapy for malaria case management in Africa. Trends Parasitol. (2003) 19:363–9. doi: 10.1016/s1471-4922(03)00168-5
4. Peeling, RW, and Fongwen, N. Solving the enigma of acute febrile illness. Lancet Infect Dis. (2022) 22:1261–2. doi: 10.1016/S1473-3099(22)00313-9
5. Crump, JA, Gove, S, and Parry, CM. Management of adolescents and adults with febrile illness in resource-limited areas. BMJ. (2011) 343:d4847. doi: 10.1136/bmj.d4847
6. Hercik, C, Cosmas, L, Mogeni, OD, Wamola, N, Kohi, W, Omballa, V, et al. A diagnostic and epidemiologic investigation of acute febrile illness (AFI) in Kilombero, Tanzania. PLoS One. (2017) 12:e0189712. doi: 10.1371/journal.pone.0189712
7. Murray, CJ, Ikuta, KS, Sharara, F, Swetschinski, L, Aguilar, GR, Gray, A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
8. Rhee, C, Kharod, GA, Schaad, N, Furukawa, NW, Vora, NM, Blaney, DD, et al. Global knowledge gaps in acute febrile illness etiologic investigations: a scoping review. PLoS Negl Trop Dis. (2019) 13:e0007792. doi: 10.1371/journal.pntd.0007792
9. Hopkins, H, Bruxvoort, KJ, Cairns, ME, Chandler, CI, Leurent, B, Ansah, EK, et al. Impact of introduction of rapid diagnostic tests for malaria on antibiotic prescribing: analysis of observational and randomized studies in public and private healthcare settings. BMJ. (2017) 356:j1054. doi: 10.1136/bmj.j1054
10. Bressan, CD, Teixeira, MD, Gouvêa, MI, de Pina-Costa, A, Santos, HF, Calvet, GA, et al. Challenges of acute febrile illness diagnosis in a national infectious diseases center in Rio de Janeiro: 16-year experience of syndromic surveillance. PLoS Negl Trop Dis. (2023) 17:e0011232. doi: 10.1371/journal.pntd.0011232
11. Bhowmick, IP, Pandey, A, Subbarao, SK, Pebam, R, Majumder, T, Nath, A, et al. Diagnosis of indigenous non-malarial vector-borne infections from malaria negative samples from community and rural hospital surveillance in Dhalai District, Tripura, north-East India. Diagnostics. (2022) 12:362. doi: 10.3390/diagnostics12020362
12. Iroh Tam, PY, Obaro, SK, and Storch, G. Challenges in the etiology and diagnosis of acute febrile illness in children in low-and middle-income countries. J Pediatr Infect Dis Soc. (2016) 5:190–205. doi: 10.1093/jpids/piw016
13. Crump, JA, Morrissey, AB, Nicholson, WL, Massung, RF, Stoddard, RA, Galloway, RL, et al. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. (2013) 7:e2324. doi: 10.1371/journal.pntd.0002324
14. Kaboré, B, Post, A, Lompo, P, Bognini, JD, Diallo, S, Kam, BT, et al. Aetiology of acute febrile illness in children in a high malaria transmission area in West Africa. Clin Microbiol Infect. (2021) 27:590–6. doi: 10.1016/j.cmi.2020.05.029
15. Sharma, Y, Horwood, C, Hakendorf, P, and Thompson, C. Clinical characteristics and outcomes of influenza A and B virus infection in adult Australian hospitalized patients. BMC Infect Dis. (2020) 20:1–9. doi: 10.1186/s12879-020-05670-8
16. Tyrrell, CS, Allen, JL, and Gkrania-Klotsas, E. Influenza: epidemiology and hospital management. Medicine. (2021) 49:797–804. doi: 10.1016/j.mpmed.2021.09.015
17. Huang, C, Wang, Y, Li, X, Ren, L, Zhao, J, Hu, Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
18. Cucinotta, D, and Vanelli, M. WHO declares COVID-19 a pandemic. Acta Bio Medica Atenei Parmensis. (2020) 91:157. doi: 10.23750/abm.v91i1.9397
19. World Health Organization. (2023). COVID-19 weekly epidemiological update, edition 171, 2024. World Health Organization. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (Accessed December 22, 2024).
20. Alvarez, E, Bielska, IA, Hopkins, S, Belal, AA, Goldstein, DM, Slick, J, et al. Limitations of COVID-19 testing and case data for evidence-informed health policy and practice. Health Res Policy Syst. (2023) 21:11. doi: 10.1186/s12961-023-00963-1
21. Adebisi, YA, Rabe, A, and Lucero-Prisno, DE III. COVID-19 surveillance systems in African countries. Health Promotion Perspectives. (2021) 11:382–92. doi: 10.34172/hpp.2021.49
22. Fawole, OI, Bello, S, Adebowale, AS, Bamgboye, EA, Salawu, MM, Afolabi, RF, et al. COVID-19 surveillance in Democratic Republic of Congo, Nigeria, Senegal and Uganda: strengths, weaknesses, and key lessons. BMC Public Health. (2023) 23:1–5. doi: 10.1186/s12889-023-15708-6
23. Ayele, W, Demissie, G, Kassa, W, Zemelak, E, Afework, A, Amare, B, et al. Challenges of establishing routine influenza sentinel surveillance in Ethiopia, 2008–2010. J Infect Dis. (2012) 206:S41–5. doi: 10.1093/infdis/jis531
24. Woyessa, AB, Mengesha, M, Belay, D, Tayachew, A, Ayele, W, Beyene, B, et al. Epidemiology of influenza in Ethiopia: findings from influenza sentinel surveillance and respiratory infection outbreak investigations, 2009–2015. BMC Infect Dis. (2018) 18:1–11. doi: 10.1186/s12879-018-3365-5
25. Tadesse, M, Mengesha, M, Tayachew, A, Belay, D, Hassen, A, Woyessa, AB, et al. Burden and seasonality of medically attended influenza like illness (ILI) in Ethiopia, 2012 to 2017. BMC Infect Dis. (2020) 20:1–3. doi: 10.1186/s12879-020-4827-0
26. Gudina, EK, Gobena, D, Debela, T, Yilma, D, Girma, T, Mekonnen, Z, et al. COVID-19 in Oromia region of Ethiopia: a review of the first 6 months’ surveillance data. BMJ Open. (2021) 11:e046764. doi: 10.1136/bmjopen-2020-046764
27. Gedefie, A, Tilahun, M, Fiseha, M, Alemayehu, E, Shibabaw, A, Bisetegn, H, et al. Epidemiology of SARS-CoV-2 infection in Ethiopia: a systematic review and meta-analysis. COVID. (2023) 3:703–14. doi: 10.3390/covid3050052
28. Muleta, D, Simieneh, A, Duguma, T, Tekalign, E, Worku, T, Ayele, G, et al. SARS-CoV-2 infections, clinical characteristics, and related risk factors: the first 8 months surveillance study conducted in Southwest Ethiopia. Inquiry. (2023) 60:00469580231166794. doi: 10.1177/00469580231166794
29. Adane, T, Adugna, Y, and Aynalem, M. Prevalence of COVID-19 in West Gondar zone, Northwest Ethiopia: a population-based retrospective study. Disaster Med Public Health Prep. (2023) 17:e156. doi: 10.1017/dmp.2022.72
30. Aborode, AT, Hasan, MM, Jain, S, Okereke, M, Adedeji, OJ, Karra-Aly, A, et al. Impact of poor disease surveillance system on COVID-19 response in Africa: time to rethink and rebuilt. Clin Epidemiol Global Health. (2021) 12:100841. doi: 10.1016/j.cegh.2021.100841
31. Tamiru, A, Regassa, B, Alemu, T, and Begna, Z. The performance of COVID-19 surveillance system as timely containment strategy in Western Oromia, Ethiopia. BMC Public Health. (2021) 21:1–4. doi: 10.1186/s12889-021-12380-6
32. Inbaraj, LR, George, CE, and Chandrasingh, S. Seroprevalence of COVID-19 infection in a rural district of South India: a population-based sero epidemiological study. PLoS One. (2021) 16:e0249247. doi: 10.1371/journal.pone.0249247
33. Ahmad, AM, Shahzad, K, Masood, M, Umar, M, Abbasi, F, and Hafeez, A. COVID-19 seroprevalence in Pakistan: a cross-sectional study. BMJ Open. (2022) 12:e055381. doi: 10.1136/bmjopen-2021-055381
34. Nyawale, HA, Moremi, N, Mohamed, M, Njwalila, J, Silago, V, Krone, M, et al. High Seroprevalence of SARS-CoV-2 in Mwanza, northwestern Tanzania: a population-based survey. Int J Environ Res Public Health. (2022) 19:11664. doi: 10.3390/ijerph191811664
35. Etyang, AO, Adetifa, I, Omore, R, Misore, T, Ziraba, AK, Ng’oda, MA, et al. SARS-CoV-2 seroprevalence in three Kenyan health and demographic surveillance sites, December 2020-May 2021. PLoS Glob Public Health. (2022) 2:e0000883. doi: 10.1371/journal.pgph.0000883
36. Al Dallal, A, AlDallal, U, and Al Dallal, J. Positivity rate: an indicator for the spread of COVID-19. Curr Med Res Opin. (2021) 37:2067–76. doi: 10.1080/03007995.2021.1980868
37. Schoenhals, M, Rabenindrina, N, Rakotondramanga, JM, Dussart, P, Randremanana, R, Heraud, JM, et al. SARS-CoV-2 antibody seroprevalence follow-up in Malagasy blood donors during the 2020 COVID-19 epidemic. EBioMedicine. (2021) 68:103419. doi: 10.1016/j.ebiom.2021.103419
38. Bassi, F, Arbia, G, and Falorsi, PD. Observed and estimated prevalence of COVID-19 in Italy: how to estimate the total cases from medical swabs data. Sci Total Environ. (2021) 764:142799. doi: 10.1016/j.scitotenv.2020.142799
39. Javed, W, Abidi, SH, and Baqar, JB. Seroprevalence and characteristics of coronavirus disease (COVID-19) in workers with non-specific disease symptoms. BMC Infect Dis. (2022) 22:1–8. doi: 10.1186/s12879-022-07461-9
40. Abate, BB, Kassie, AM, Kassaw, MW, Aragie, TG, and Masresha, SA. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. (2020) 10:e040129. doi: 10.1136/bmjopen-2020-040129
41. Tunheim, G, Rø, GØ, Tran, T, Kran, AM, Andersen, JT, Vaage, EB, et al. Trends in seroprevalence of SARS-CoV-2 and infection fatality rate in the Norwegian population through the first year of the COVID-19 pandemic. Influenza Other Respir Viruses. (2022) 16:204–12. doi: 10.1111/irv.12932
42. Pradhan, A, and Olsson, PE. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ. (2020) 11:1. doi: 10.1186/s13293-020-00330-7
43. Bwire, GM. Coronavirus: why men are more vulnerable to Covid-19 than women? SN Comprehens Clin Med. (2020) 2:874–6. doi: 10.1007/s42399-020-00341-w
44. Herlinda, O, Bella, A, Kusnadi, G, Swasthika Nurshadrina, D, Thoriq Akbar, M, Nida, S, et al. Seroprevalence of antibodies against SARS-Cov-2 in the high impacted sub-district in Jakarta, Indonesia. PLoS One. (2021) 16:e0261931. doi: 10.1371/journal.pone.0261931
45. Abul, Y, Leeder, C, and Gravenstein, S. Epidemiology and clinical presentation of COVID-19 in older adults. Infect Dis Clin N Am. (2023) 37:1–26. doi: 10.1016/j.idc.2022.11.001
46. Setiadi, W, Rozi, IE, Safari, D, Daningrat, WO, Johar, E, Yohan, B, et al. Prevalence and epidemiological characteristics of COVID-19 after one year of pandemic in Jakarta and neighbouring areas, Indonesia: a single center study. PLoS One. (2022) 17:e0268241. doi: 10.1371/journal.pone.0268241
47. Damayanthi, HD, Prabani, KI, and Weerasekara, I. Factors associated for mortality of older people with COVID-19: a systematic review and meta-analysis. Gerontol Geriatr Med. (2021) 7:23337214211057392. doi: 10.1177/23337214211057392
48. Crimmins, EM. Age-related vulnerability to coronavirus disease 2019 (COVID-19): biological, contextual, and policy-related factors. Public Policy Aging Report. (2020) 30:142–6. doi: 10.1093/ppar/praa023
49. Cocuzzo, B, Wrench, A, and O’Malley, C. Effects of COVID-19 on older adults: physical, mental, emotional, social, and financial problems seen and unseen. Cureus. (2022) 14:3–7. doi: 10.7759/cureus.29493
50. Bergeri, I, Whelan, MG, Ware, H, Subissi, L, Nardone, A, Lewis, HC, et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: a systematic review and meta-analysis of standardized population-based studies. PLoS Med. (2022) 19:e1004107. doi: 10.1371/journal.pmed.1004107
51. Lyimo, E, Fougeroux, C, Malabeja, A, Mbwana, J, Hayuma, PM, Liheluka, E, et al. Seroprevalence of SARS-CoV-2 antibodies among children and adolescents recruited in a malariometric survey in North-Eastern Tanzania July 2021. BMC Infect Dis. (2022) 22:846. doi: 10.1186/s12879-022-07820-6
52. Lewis, HC, Ware, H, Whelan, M, Subissi, L, Li, Z, Ma, X, et al. SARS-CoV-2 infection in Africa: a systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021. BMJ Glob Health. (2022) 7:e008793. doi: 10.1136/bmjgh-2022-008793
53. Grande, M, Bjørnsen, LP, Næss-Pleym, LE, Laugsand, LE, and Grenne, B. Observational study on chest pain during the Covid-19 pandemic: changes and characteristics of visits to a Norwegian emergency department during the lockdown. BMC Emerg Med. (2022) 22:1–11. doi: 10.1186/s12873-022-00612-w
54. Sinkeldam, M, Buenen, AG, Celiker, E, van Diepen, M, and de Vos, AM. Characteristics of chest pain in COVID-19 patients in the emergency department. Neth Hear J. (2022) 30:526–32. doi: 10.1007/s12471-022-01730-7
55. Islam, MA, Kundu, S, Alam, SS, Hossan, T, Kamal, MA, and Hassan, R. Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of 17515 patients. PLoS One. (2021) 16:e0249788. doi: 10.1371/journal.pone.0249788
56. Zhang, PI, Hsu, CC, Kao, Y, Chen, CJ, Kuo, YW, Hsu, SL, et al. Real-time AI prediction for major adverse cardiac events in emergency department patients with chest pain. Scand J Trauma Resusc Emerg Med. (2020) 28:1–7. doi: 10.1186/s13049-020-00786-x
57. Aerts, M, Minalu, G, Bösner, S, Buntinx, F, Burnand, B, Haasenritter, J, et al. Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J Clin Epidemiol. (2017) 81:120–8. doi: 10.1016/j.jclinepi.2016.09.011
58. Gencer, B, Vaucher, P, Herzig, L, Verdon, F, Ruffieux, C, Bösner, S., et al. Ruling out coronary heart disease in primary care patients with chest pain: a clinical prediction score. BMC Med. (2010) 8:9. doi: 10.1186/1741-7015-8-9
59. Forshey, BM, Guevara, C, Laguna-Torres, VA, Cespedes, M, Vargas, J, Gianella, A, et al. Arboviral etiologies of acute febrile illnesses in western South America, 2000–2007. PLoS Negl Trop Dis. (2010) 4:e787. doi: 10.1371/journal.pntd.0000787
60. Reller, ME, Wunder, EA Jr, Miles, JJ, Flom, JE, Mayorga, O, Woods, CW, et al. Unsuspected leptospirosis is a cause of acute febrile illness in Nicaragua. PLoS Negl Trop Dis. (2014) 8:e2941. doi: 10.1371/journal.pntd.0002941
61. Assiri, A, Al-Tawfiq, JA, Al-Rabeeah, AA, Al-Rabiah, FA, Al-Hajjar, S, Al-Barrak, A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. (2013) 13:752–61. doi: 10.1016/S1473-3099(13)70204-4
62. Cheng, VC, Chan, JF, To, KK, and Yuen, KY. Clinical management and infection control of SARS: lessons learned. Antivir Res. (2013) 100:407–19. doi: 10.1016/j.antiviral.2013.08.016
63. Pollán, M, Pérez-Gómez, B, Pastor-Barriuso, R, Oteo, J, Hernán, MA, Pérez-Olmeda, M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. (2020) 396:535–44. doi: 10.1016/S0140-6736(20)31483-5
64. Uyoga, S, Adetifa, IM, Karanja, HK, Nyagwange, J, Tuju, J, Wanjiku, P, et al. Seroprevalence of anti–SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science. (2021) 371:79–82. doi: 10.1126/science.abe1916
65. Kadi, N, and Khelfaoui, M. Population density, a factor in the spread of COVID-19 in Algeria: statistic study. Bull Natl Res Cent. (2020) 44:1–7. doi: 10.1186/s42269-020-00393-x
66. Khavarian-Garmsir, AR, Sharifi, A, and Moradpour, N. Are high-density districts more vulnerable to the COVID-19 pandemic? Sustain Cities Soc. (2021) 70:102911. doi: 10.1016/j.scs.2021.102911
67. Agca, H, Akalin, H, Saglik, I, Hacimustafaoglu, M, Celebi, S, and Ener, B. Changing epidemiology of influenza and other respiratory viruses in the first year of COVID-19 pandemic. J Infect Public Health. (2021) 14:1186–90. doi: 10.1016/j.jiph.2021.08.004
68. Chow, EJ, Uyeki, TM, and Chu, HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. (2023) 21:195–210. doi: 10.1038/s41579-022-00807-9
69. Groves, HE, Papenburg, J, Mehta, K, Bettinger, JA, Sadarangani, M, Halperin, SA, et al. The effect of the COVID-19 pandemic on influenza-related hospitalization, intensive care admission and mortality in children in Canada: a population-based study. Lancet Regional Health Am. (2022) 7:100132. doi: 10.1016/j.lana.2021.100132
70. Takeuchi, H, and Kawashima, R. Disappearance and re-emergence of influenza during the COVID-19 pandemic: association with infection control measures. Viruses. (2023) 15:223. doi: 10.3390/v15010223
71. Lee, SS, Viboud, C, and Petersen, E. Understanding the rebound of influenza in the post COVID-19 pandemic period holds important clues for epidemiology and control. Int J Infect Dis. (2022) 122:1002–4. doi: 10.1016/j.ijid.2022.08.002
72. Cheng, K, Wu, C, Gu, S, Lu, Y, Wu, H, and Li, C. WHO declares end of COVID-19 global health emergency: lessons and recommendations from the perspective of ChatGPT/GPT-4. Int J Surg. (2023) 29:10–97. doi: 10.1097/JS9.0000000000000521
73. Gupta, A, Siddiqui, F, Purwar, S, Joshi, R, and Mukhopadhyay, C. Is it always COVID-19 in acute febrile illness in the tropics during the pandemic? PLoS Negl Trop Dis. (2022) 16:2–4. doi: 10.1371/journal.pntd.0010891
Keywords: AFI, influenza virus, SARS-CoV-2, Ethiopia, proportion
Citation: Chekol MT, Sugerman D, Tayachew A, Mekuria Z, Tesfay N, Alemu A, Gashu A, Shura W, Gonta M, Agune A, Hailemariam A, Assefa Y, Wossen M, Hassen A, Michele P, Silver R, Delelegn H, Briana L, Kasa T and Kebede N (2025) Clinical and epidemiological characteristics of influenza and SARS-CoV-2 virus among patients with acute febrile illness in selected sites of Ethiopia 2021–2022. Front. Public Health. 13:1549159. doi: 10.3389/fpubh.2025.1549159
Edited by:
Ramendra Pati Pandey, SRM University (Delhi-NCR), IndiaReviewed by:
Himadri Nath, Indian Institute of Chemical Biology (CSIR), IndiaGiovanni Rezza, Ministry of Health, Italy
Copyright © 2025 Chekol, Sugerman, Tayachew, Mekuria, Tesfay, Alemu, Gashu, Shura, Gonta, Agune, Hailemariam, Assefa, Wossen, Hassen, Michele, Silver, Delelegn, Briana, Kasa and Kebede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Musse Tadesse Chekol, bGVtdXNzZTdAZ21haWwuY29t
 Musse Tadesse Chekol
Musse Tadesse Chekol David Sugerman3
David Sugerman3 Zelalem Mekuria
Zelalem Mekuria Neamin Tesfay
Neamin Tesfay Aynalem Alemu
Aynalem Alemu Andargachew Gashu
Andargachew Gashu Yonas Assefa
Yonas Assefa Mesfin Wossen
Mesfin Wossen Hulemenaw Delelegn
Hulemenaw Delelegn Nigatu Kebede
Nigatu Kebede