- 1Department of Orthopedics, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 2Academy of Medical Sciences, Shanxi Medical University, Taiyuan, Shanxi, China
Background: The relationship between exposure to ethylene oxide (EO) and the risk of developing osteoarthritis (OA) remains unclear. We aimed to explore the association between EO exposure and OA risk among young and middle-aged adults.
Methods: We utilized data from the 2013–2018 National Health and Nutrition Examination Survey, involving 2,380 individuals aged 20–60 years. Weighted multivariable regression models, smooth curve fitting (SCF), subgroup analysis and interaction tests were employed to examine the association between EO exposure and OA risk. Furthermore, we performed variable selection via least absolute shrinkage and selection operator regression and multivariable regression analyses to construct a prediction model.
Results: Increased EO exposure was associated with increased OA risk. After full adjustment, individuals in the highest tertile of EO exposure had a significantly greater OA risk (224% increase) than did those in the lowest tertile of EO exposure (OR = 3.24; 95% CI: 1.61–6.52; p for trend = 0.002). SCF did not indicate any nonlinear associations. There was no statistically significant interaction observed in any of the subgroups (all p > 0.05). We built a prediction model visualized with a nomogram. This prediction model demonstrated good discriminatory power, excellent precision, and potential clinical benefits.
Conclusion: The findings of our research demonstrated that among middle-aged and young adults, EO exposure was positively associated with OA risk. A prediction model was developed by integrating EO exposure with other factors readily acquired from users to assist in the evaluation and management of high-risk OA groups.
Introduction
Osteoarthritis (OA) is the most common joint disease, and it is characterized by degenerative articular cartilage, altered synovium, and altered subchondral bone (1–3). OA is emerging as an increasing threat to public health. The number of Americans suffering from OA is expected to reach 67 million by 2030 (4). OA is one of the leading causes of chronic pain and prolonged disability among adults (5). Compared with the general population, OA patients have a greater propensity to experience psychological illnesses such as anxiety and depression (6–8). In addition to its impact on physical and mental health, OA also exerts a substantial economic influence on individuals and society (9–11). OA can affect individuals across all ages (6). Notably, young and middle-aged patients with OA tend to ignore their early symptoms and underestimate the serious consequences of the condition, leading to delays in diagnosis and treatment. Environmental toxins, which include heavy metals (like cadmium, lead) and air pollutants (like benzene), are likely to increase the risk of OA.
Ethylene oxide (EO), an industrial chemical, is widely employed as a sterilant for medical equipment and as an intermediate in the manufacture of other chemicals (12–14). EO exists in a gaseous state at room temperature, and inhalation is the principal route of exposure. Inhaled EO is readily absorbed into the bloodstream and can spread quickly throughout the body (15). The hemoglobin adduct of EO (HbEO) is produced by the binding of EO to Hb and has demonstrated excellent sensitivity and usefulness for evaluating EO exposure (16). People working in relevant fields are at high risk of EO exposure, while the general population may also inhale EO to a certain extent (17). Near sterilization facilities, the peak 24-h EO exposure of community inhabitants was far greater than that of individuals living in other areas (18). In addition, it is possible that EO exposure has increased with the occurrence of the COVID-19 pandemic and the increasing need for personal protective equipment (e.g., masks and gloves) sterilized with EO (19). Notably, EO is somewhat toxic, and overexposure to EO is extremely detrimental to human health. Nevertheless, the relationship between exposure to EO and OA risk remains unclear. There is increasing evidence that EO exposure is connected to inflammation and oxidative stress (20, 21). Since inflammation and oxidative stress play important roles in OA occurrence, we speculate that EO exposure may be closely correlated with OA risk.
In this study, we aimed to explore the association between EO exposure and OA risk among young and middle-aged adults.
Methods
Study population
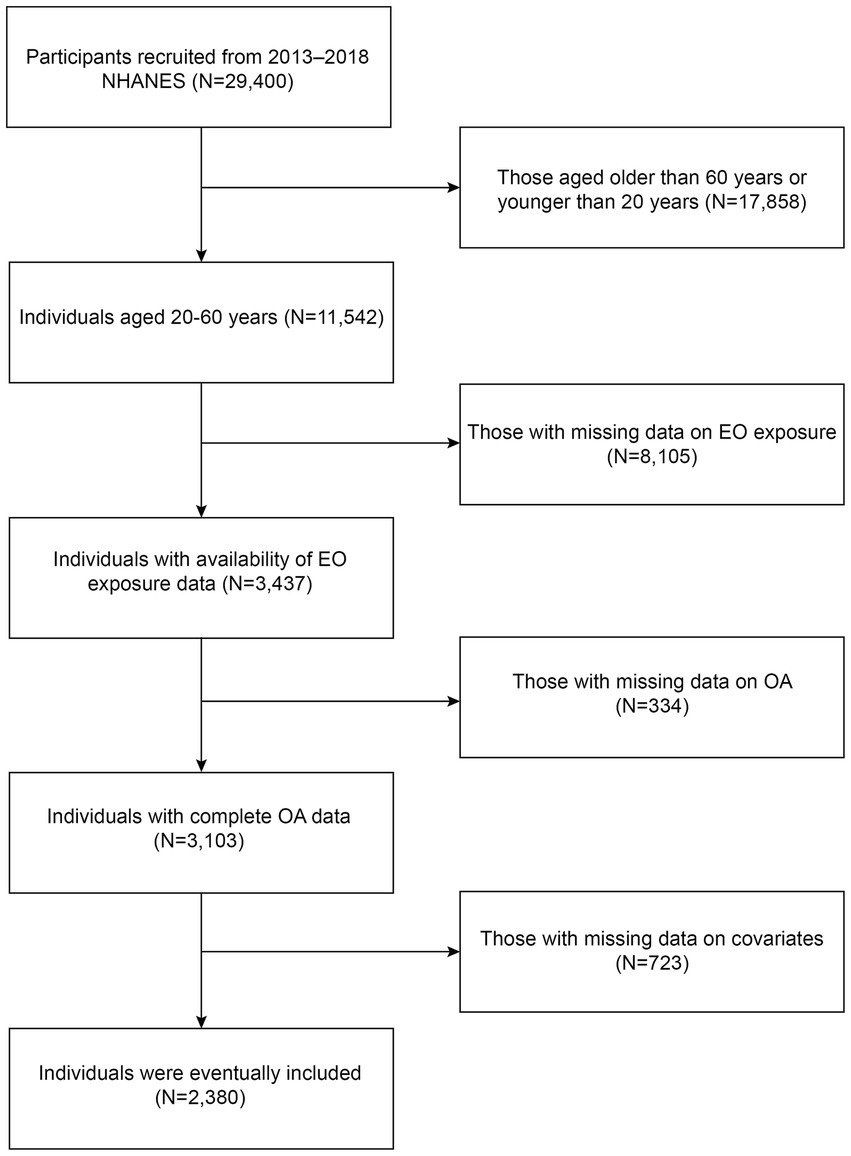
In the present study, we utilized cross-sectional data from the 2013–2018 National Health and Nutrition Examination Survey (NHANES). The NHANES is a nationwide program conducted by the National Center for Health Statistics (NCHS) to assess the nutritional and health conditions of Americans. Five types of data are included in the NHANES: demographic, examination, dietary, laboratory, and questionnaire data. The NCHS Research Ethics Review Board approved the entire program, and all participants provided written informed consent. The flowchart of the study is shown in Figure 1. Initially, a cohort of 29,400 participants from 2013 to 2018 NHANES were recruited. After excluding individuals older than 60 years or younger than 20 years (N = 17,858), those with missing data on EO exposure (N = 8,105), those with missing data on OA (N = 334), and those with missing data on covariates (N = 723), 2,380 individuals were eventually included.
Assessment of OA status
The OA status of the participants was evaluated through a questionnaire. The participants were asked “Has a doctor or other health professional ever told you that you have arthritis?” Participants who answered “no” were considered not to have OA, and those who answered “yes” were then asked the following question: “What type of arthritis?” Participants who responded “osteoarthritis” were considered to have OA.
Measurement of EO
HbEO has been established as a dependable biomarker for evaluating the level of EO exposure, since the half-life of HbEO in vivo is longer than that of EO. The participants’ morning blood samples were processed and preserved at −30 °C until they were sent to the National Center for Environmental Health for assessment. The quantity of HbEO was determined through a modified Edman reaction and high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). The outcomes of the measurements are presented as pmol/g Hb. Owing to the skewed distribution of HbEO levels, they were log2-transformed.
Covariates
According to biological plausibility, prior related studies, and NHANES guidelines, the following covariate data were gathered: (1) demographic data: age (years), sex (male/female), race (Hispanic/non-Hispanic white/non-Hispanic black/other), education level (less than high school/high school/more than high school), and marital status (live with others/live alone); (2) examination data: body mass index (BMI, kg/m2); (3) questionnaire data: diabetes status (yes or borderline/no), hypertension status (yes/no), smoking status (smoked at least 100 cigarettes in life, yes/no), moderate activity status (at least 10 consecutive minutes of exercise or fitness or recreational activity during a typical week leading to a slight increase in respiration or heart rate, yes/no), and alcohol consumption status (drinking alcohol at least once per month, yes/no); and (4) laboratory data: albumin (g/dL), alanine transaminase (U/L), aspartate aminotransferase (U/L), blood urea nitrogen (mg/dL), total calcium (mg/dL), total protein (g/dL), high-density lipoprotein cholesterol (mg/dL), and total cholesterol (mg/dL).
Statistical analysis
Statistical analysis was conducted utilizing appropriate NHANES sampling weights, accounting for the intricate multistage cluster survey. Continuous variables are represented as survey-weighted means [95% confidence intervals (CIs)], whereas categorical variables are displayed as survey-weighted percentages (95% CIs). We utilized weighted multivariable regression models to examine the association between EO exposure and OA risk. Model 1 remained unadjusted; Model 2 was adjusted for age, sex, and race; and Model 3 was adjusted for all covariates. The results are presented as odds ratios (ORs) and 95% CIs. We also employed smooth curve fitting (SCF) to explore the potential nonlinear associations between EO exposure and OA risk. Additionally, subgroup analysis and interaction tests were performed to assess the consistency of the associations across different subgroups. The stratification was based on age, sex, race, education level, marital status, diabetes status, hypertension status, moderate activity status, and alcohol consumption status.
We then selected the variables for constructing a prediction model via least absolute shrinkage and selection operator (LASSO) regression and multivariable regression analysis. The prediction model was visualized with a nomogram and evaluated via receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA). All analyses were conducted in R version 4.4.01 and EmpowerStats.2 p < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
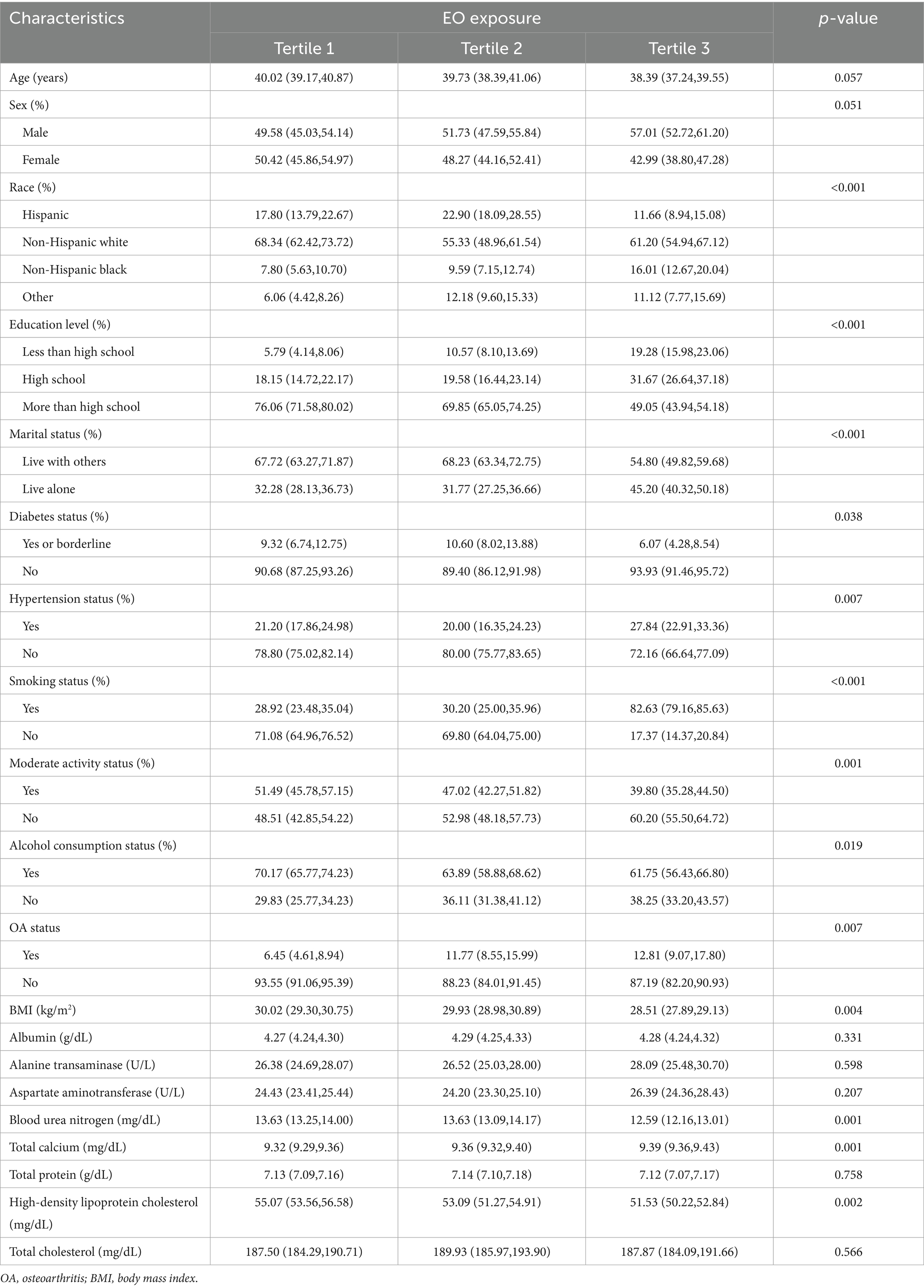
The weighted demographic baseline characteristics of the study participants are depicted in Table 1. The participants were categorized into three groups according to the tertiles of EO exposure. Significant differences in race, education level, marital status, diabetes status, hypertension status, smoking status, moderate activity status, alcohol consumption status, OA status, BMI, blood urea nitrogen concentrations, total calcium concentrations, and high-density lipoprotein cholesterol concentrations (p < 0.05) were detected among the three groups.
Association between EO exposure and OA risk
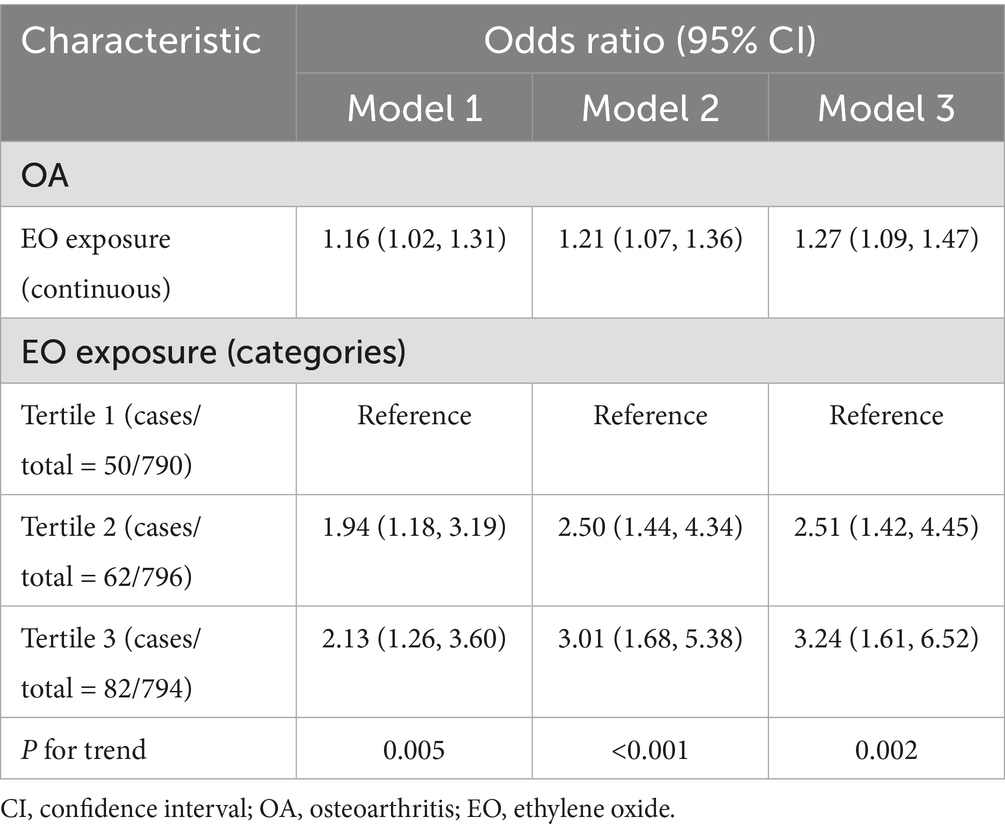
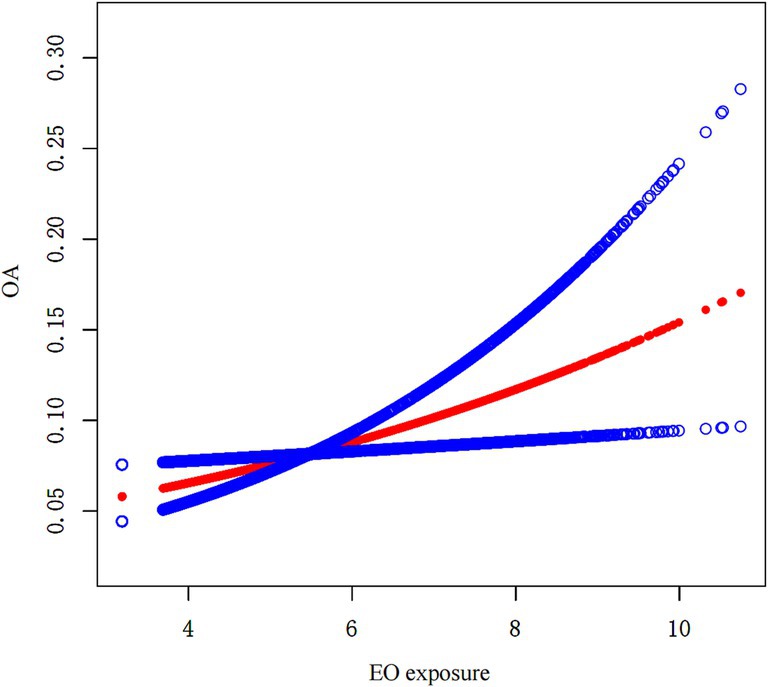
The association between EO exposure and OA risk is shown in Table 2. These findings indicated that increased EO exposure was related to increased OA risk. In both the crude and partially/fully adjusted models, a positive association was found between EO exposure and OA risk. After full adjustment, individuals with a one-unit rise in EO exposure had a 27% greater likelihood of developing OA (OR = 1.27; 95% CI: 1.09–1.47). Continuous EO exposure was then converted to a categorical variable on the basis of tertiles for sensitivity analysis. Compared with those in the lowest tertile of EO exposure, individuals in the highest tertile of EO exposure had a significantly greater OA risk, with a 224% increase (OR = 3.24; 95% CI: 1.61–6.52; p for trend = 0.002). Furthermore, the outcomes of SCF revealed that there was no nonlinear association between EO exposure and OA risk (Figure 2).
Subgroup analysis
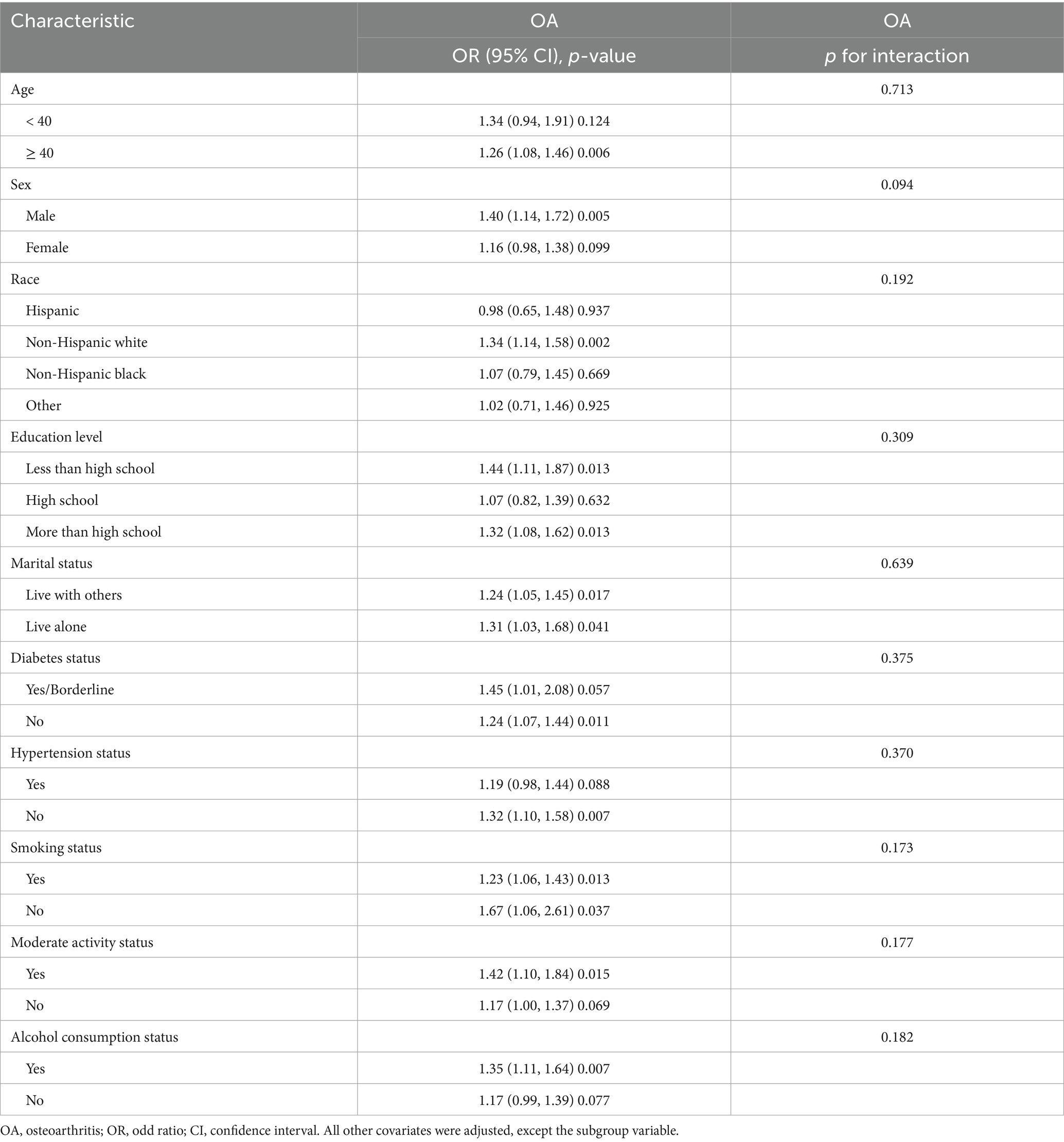
The results of the subgroup analysis and interaction tests are presented in Table 3. There was no statistically significant interaction observed in any of the subgroups (all p > 0.05), suggesting that the positive association between EO exposure and OA risk remained strong and consistent across different subpopulations.
Variable selection
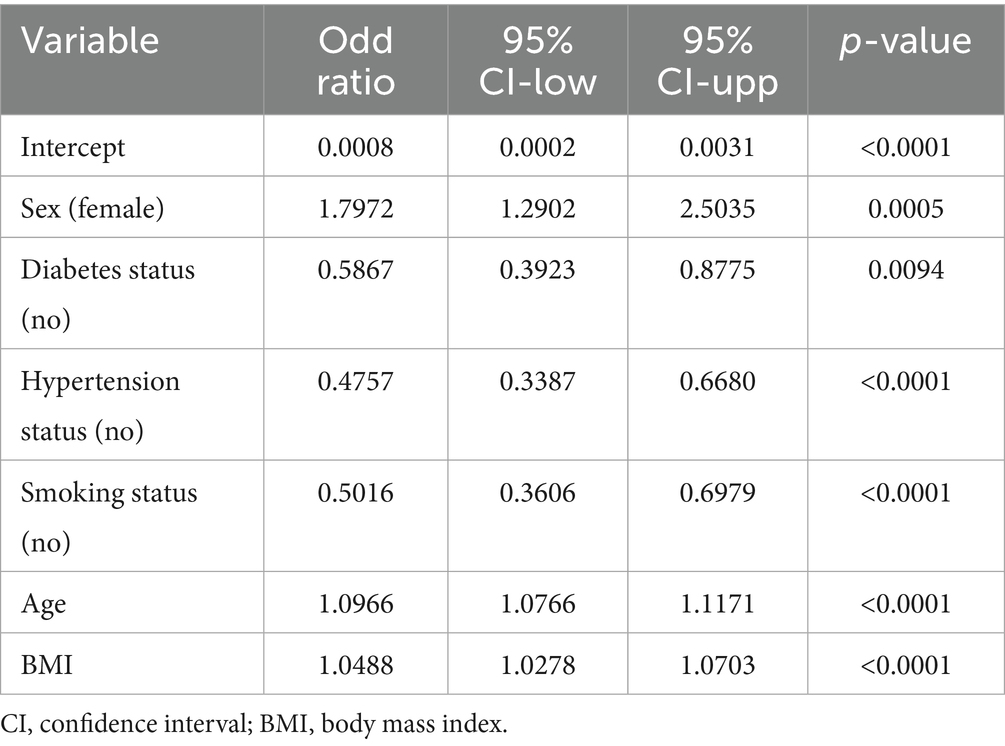
The prediction model initially considered candidate predictors that included indicators from the baseline characteristics in addition to EO exposure. Six key variables, including sex, diabetes status, hypertension status, smoking status, age, and BMI were selected through LASSO regression (Figure 3). The six selected variables were subsequently included in multivariable regression analysis to further screen the variables. The variables in that analysis with p values less than 0.05 were included in the prediction model (Table 4).
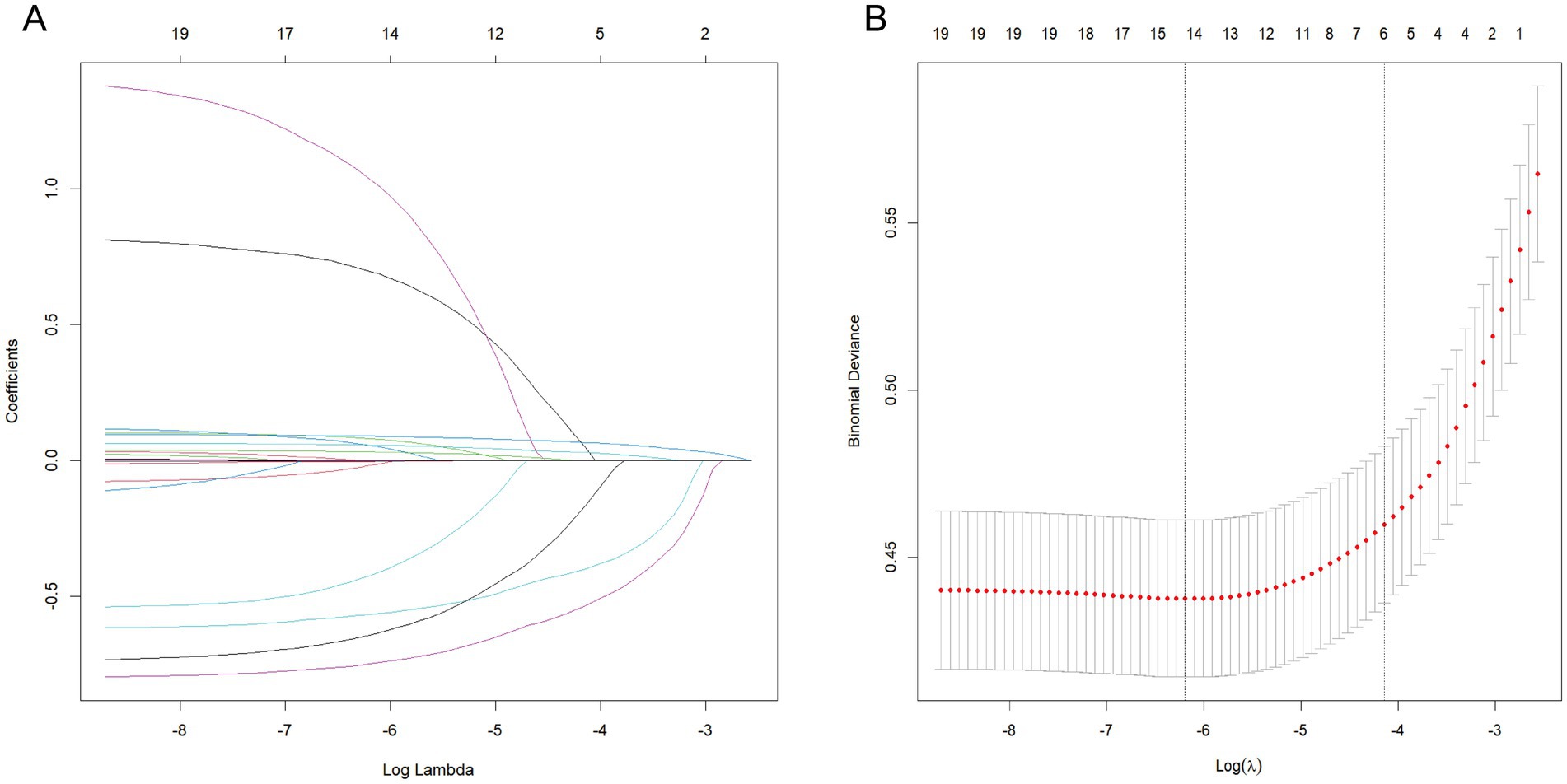
Figure 3. LASSO regression for related variables. (A) coefficient path plot. (B) cross-validation plot. The dashed lines on the left and right represent lambda.min and lambda.1se, respectively. We selected lambda.1se. The reason for choosing lambda.1se is that it leads to a more severe penalty and a lower number of variables than lambda.min does.
Nomogram development for risk prediction
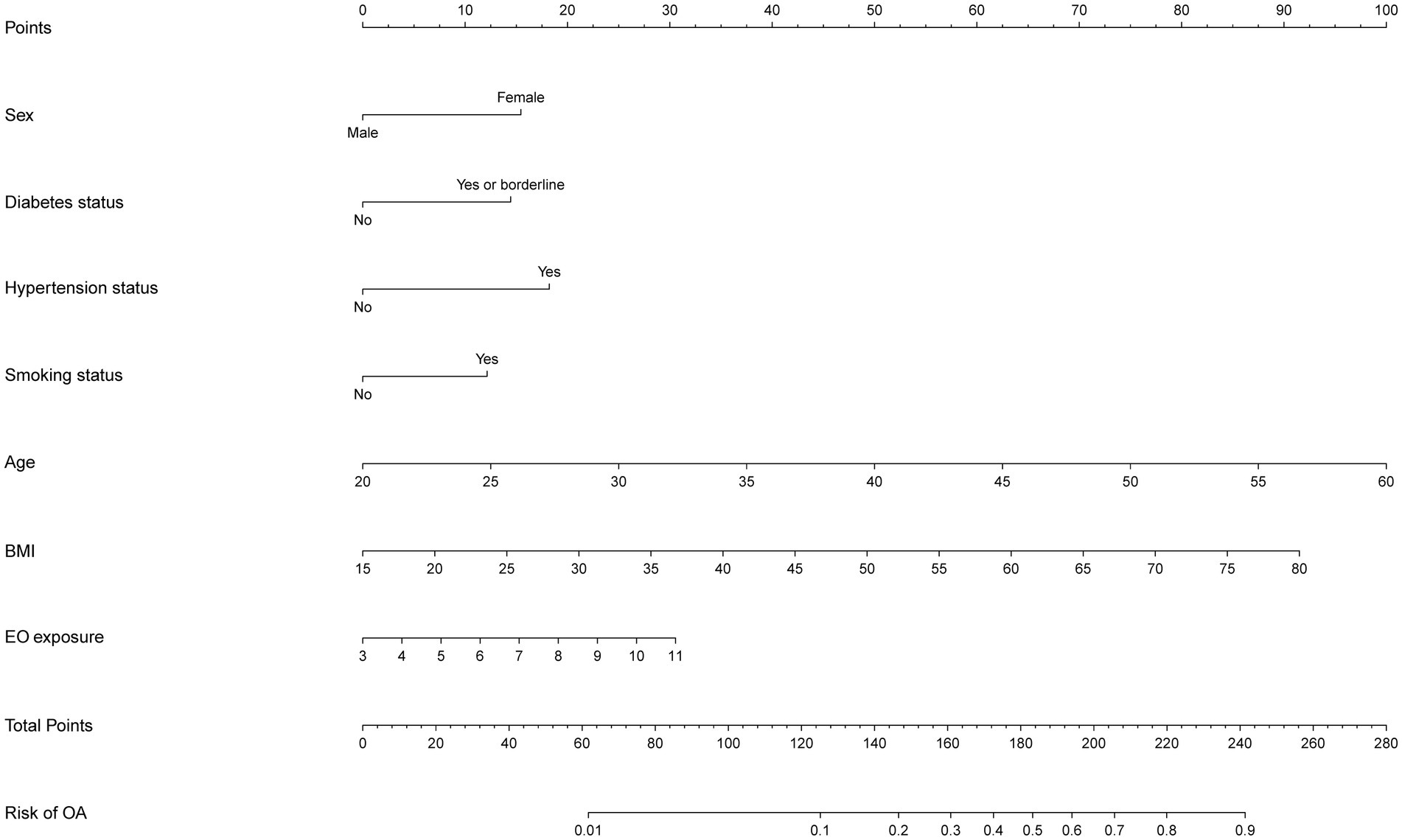
After two stages of variable selection (LASSO regression and multivariable regression analysis), we built a prediction model that was visualized with a nomogram (Figure 4). The final prediction model incorporated seven predictors (EO exposure, sex, diabetes status, hypertension status, smoking status, age, and BMI). The nomogram consisted of 10 axes, where axes two through eight corresponded to every predictor included in the prediction model. Every predictor was given its own score in the nomogram. Axes nine and 10 showed that as the overall score increased, the OA risk increased accordingly.
Assessment of the prediction model
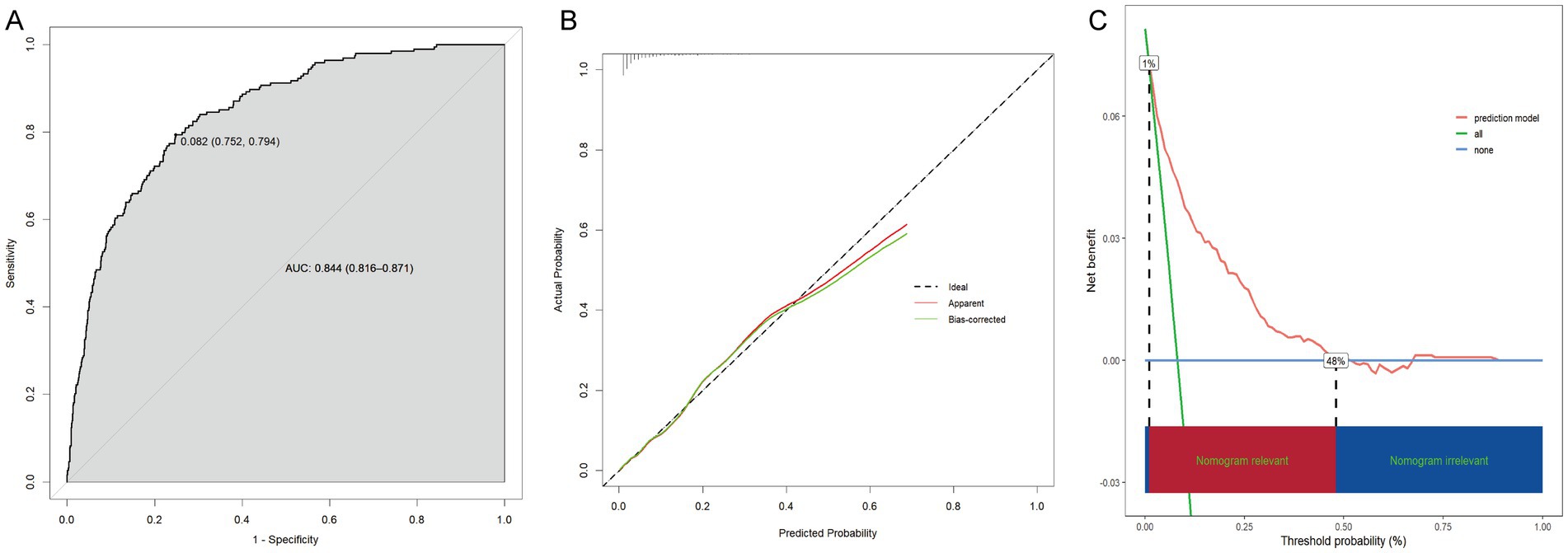
With an area under the ROC curve of 0.844 (95% CI: 0.816–0.871), the model clearly had good discriminatory power (Figure 5A). As illustrated in the calibration curve, the predicted probabilities were highly consistent with the actual probabilities, which indicates a high level of precision in the prediction model (Figure 5B). According to the DCA results, the prediction model was beneficial when the risk threshold was below 0.48 (Figure 5C).
Discussion
According to the 2013–2018 NHANES, a total of 2,380 eligible young and middle-aged adults were recruited for our cross-sectional study. The main outcomes of this research were as follows. (1) EO exposure was shown to be positively correlated with OA risk. (2) The positive association between EO exposure and OA risk remained strong and consistent across different subpopulations. (3) We constructed a prediction model for OA risk by combining variables that could be readily acquired from users, including EO exposure, sex, diabetes status, hypertension status, smoking status, age, and BMI. (4) This prediction model demonstrated good discriminatory power, excellent precision, and potential clinical benefits. The above findings suggest that this model has the potential to be a straightforward and user-friendly instrument for assessing OA risk.
OA represents a slowly advancing condition impacting the human joint system (22). It can impact any synovial joint, with the hip, knee, hand, foot, and spine being the most often influenced locations (23). In addition to being painful, OA may also contribute to disability and reduce one’s life expectancy (24). Currently, the complicated etiology of OA is not completely known, but there is a growing consensus that nonignorable environmental factors may be responsible for this condition (25, 26). EO is widely present in the environment (13). It has a molecular weight of 44.05, is highly soluble in both water and organic solvents, and has the ability to react with a wide range of chemicals (27). The yearly output of EO is approximately 35 million metric tons, and the worldwide demand for EO is projected to increase the yearly output rate by a minimum of 2% by 2025 (28). EO can be emitted into the atmosphere in substantial amounts through industrial facilities, cigarette smoke, and automobile emissions, thereby contributing to environmental pollution (29–31). EO in the atmosphere can undergo degradation by reacting with photochemically generated hydroxyl radicals, but this process is slow (32). Industrial discharges of EO have been monitored by the U. S. Environmental Protection Agency since 1987 because of the potential adverse effects of EO exposure on human health (33).
The mutagenicity and carcinogenicity of EO have long been known. It has been proposed that EO induces genetic injury by the formation of DNA adducts, which leads to genetic mutations and chromosomal aberrations in both animals and humans (34). Multiple investigations have shown that prolonged EO exposure increases the likelihood of developing malignant neoplasms (such as leukemia, lymphoma, breast cancer, and gastric cancer) (15, 33, 35, 36). Prior studies have also examined the associations of EO with a variety of other illnesses or potentially unhealthy conditions (37–41). For example, Wu et al. reported that among nonsmokers, increased EO exposure was closely associated with elevated chronic kidney disease incidence and worse chronic kidney disease outcomes (37). Liu et al. reported that increased EO exposure was related to a decrease in cognitive function among older individuals (38). Additionally, a cross-sectional study involving 6,016 individuals revealed that elevated EO exposure was linked to increased susceptibility to depression, particularly in women, drinkers and smokers (39). In this study, EO exposure was positively related to OA risk. According to the sensitivity analysis, the OA risk was much greater for individuals in the highest tertile of EO exposure than for those in the lowest tertile. We observed a consistent trend across different subpopulations.
The pathogenesis of OA involves oxidative stress and inflammation (42, 43). Oxidative stress occurs when the formation of reactive oxygen species (ROS) exceeds the ability of the antioxidant defense system to remove them (44, 45). Oxidative stress is increased in OA chondrocytes and is a major cause of chronic inflammation (46–48). The levels of inflammatory mediators (such as IL-1β, TNF-α, and IL-6) are substantially elevated in OA joints, contributing to ROS generation (48). In other words, inflammation and oxidative stress are mutually dependent. They can activate signaling pathways in cartilage, generating phenotypic alterations marked by the failure of chondrocytes to maintain tissue homeostasis, thereby leading to OA (49–51). At present, the mechanism linking EO exposure to OA risk remains unknown. According to multiple studies, prolonged exposure to EO could result in diminished glutathione reductase activity and increased hepatic lipid peroxidation, both of which are linked to oxidative stress in vivo (52, 53). Chronic exposure to EO can also contribute to inflammatory lesions in rodent organs (54). EO exposure has been reported to be strongly linked to the onset of depressive symptoms, with inflammation serving as a crucial mediator (55). In addition, there is evidence that inflammation is involved in the occurrence of EO-associated periodontitis (56). Hence, it is plausible that increased EO exposure may increase OA risk via inflammation and oxidative stress.
Overall, our research revealed a positive association between EO exposure and OA risk among middle-aged and young adults. Inflammation and oxidative stress may be the mechanisms underlying this association, but additional verification is needed in subsequent investigations. Additionally, other factors, such as age, undoubtedly influence the occurrence of OA, as demonstrated by our multivariable regression analysis. Therefore, we included these readily obtainable factors related to the occurrence of OA in our prediction model to make it more effective and accurate, thereby contributing to the recognition and management of high-risk populations. Environmental factors are among the modifiable risk factors for OA. We advise that workers in related industries minimize EO exposure as much as possible or adopt appropriate safeguards to decrease professional risk. If an EO factory is surrounded by a heavily-populated community, it is advisable to relocate the factory to a less-populated area. Given the increased utilization of personal protective equipment in recent years, it is necessary to create a wholly innocuous sterilization substance to replace EO. For high-risk OA patients, especially those with high exposure to EO, anti-inflammatory therapies may be considered to prevent OA.
Notably, in the sex-stratified analysis, the association between EO exposure and OA risk was significant in males but not in females, although the p for interaction did not reach statistical significance. Regarding the potential mechanisms underlying subgroup differences, the researchers proposed the following explanations. Changes in enzyme activity because of sex differences may allow female systems to detoxify EO more rapidly (57). Furthermore, estrogen levels in females have a protective effect on cartilage, whereas males lack this effect (58, 59).
However, several limitations of this research must be acknowledged. Initially, although the concordance between self-reported and clinically diagnosed OA cases was high (81%) (60), determining whether a participant has OA based on the questionnaire may still lead to bias. Secondly, establishing a causal relationship between EO exposure and OA risk with a cross-sectional design was infeasible, necessitating further confirmation through a prospective cohort study. Similar to other epidemiological analyses, including each relevant covariate that may influence EO exposure or OA risk is difficult. Additional covariates, although absent from our current model, are potentially predictive and require further examination. Moreover, the prediction model requires validation in a larger external cohort. Finally, given that EO exposure can vary dynamically, a single measurement may fail to accurately represent the cumulative exposure and its effects on the risk of developing OA. Subsequent research should involve data from multiple measurements taken at different times to investigate the cumulative impacts of EO exposure on the risk of developing OA.
Conclusion
The findings of our research demonstrated that among middle-aged and young adults, EO exposure was positively associated with OA risk. A prediction model was developed by integrating EO exposure with other factors that are readily acquired from users to assist in the evaluation and management of high-risk OA groups. There is no doubt that people should reduce their exposure to EO as much as possible. For high-risk OA patients, especially those with high exposure to EO, anti-inflammatory therapies may be considered to prevent the development of OA. Additional prospective research is needed to corroborate our findings.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: National Health and Nutrition Examination Surveys database (https://www.cdc.gov/nchs/nhanes/).
Ethics statement
The studies involving humans were approved by Research Ethics Reviewer Board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. MW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ZG: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. CX: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 81972075) and the Central Guided Local Science and Technology Development Funds (No. YDZJSX20231A062).
Acknowledgments
We thank the participants of the NHANES databases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Glyn-Jones, S, Palmer, AJ, Agricola, R, Price, AJ, Vincent, TL, Weinans, H, et al. Osteoarthritis. Lancet. (2015) 386:376–87. doi: 10.1016/S0140-6736(14)60802-3
2. Pritzker, KP, Gay, S, Jimenez, SA, Ostergaard, K, Pelletier, JP, Revell, PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. (2006) 14:13–29. doi: 10.1016/j.joca.2005.07.014
3. Gao, K, Zhang, C, Zhang, Y, Zhang, L, Xu, J, Xue, H, et al. Is chronic kidney disease associated with osteoarthritis? The United States national health and nutrition examination survey 2011-2020. BMC Nephrol. (2024) 25:236. doi: 10.1186/s12882-024-03672-1
4. Cisternas, MG, Murphy, L, Sacks, JJ, Solomon, DH, Pasta, DJ, and Helmick, CG. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care Res (Hoboken). (2016) 68:574–80. doi: 10.1002/acr.22721
5. Rice, D, McNair, P, Huysmans, E, Letzen, J, and Finan, P. Best evidence rehabilitation for chronic pain part 5: osteoarthritis. J Clin Med. (2019) 8:1769. doi: 10.3390/jcm8111769
6. Marshall, DA, Liu, X, Barnabe, C, Yee, K, Faris, PD, Barber, C, et al. Existing comorbidities in people with osteoarthritis: a retrospective analysis of a population-based cohort in Alberta, Canada. BMJ Open. (2019) 9:e033334. doi: 10.1136/bmjopen-2019-033334
7. Ravi, A, DeMarco, EC, Gebauer, S, Poirier, MP, and Hinyard, LJ. Prevalence and predictors of depression in women with osteoarthritis: cross-sectional analysis of nationally representative survey data. Healthcare (Basel). (2024) 12:502. doi: 10.3390/healthcare12050502
8. Stubbs, B, Aluko, Y, Myint, PK, and Smith, TO. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing. (2016) 45:228–35. doi: 10.1093/ageing/afw001
9. Jin, X, Liang, W, Zhang, L, Cao, S, Yang, L, and Xie, F. Economic and humanistic burden of osteoarthritis: an updated systematic review of large sample studies. PharmacoEconomics. (2023) 41:1453–67. doi: 10.1007/s40273-023-01296-1
10. Puig-Junoy, J, and Ruiz Zamora, A. Socio-economic costs of osteoarthritis: a systematic review of cost-of-illness studies. Semin Arthritis Rheum. (2015) 44:531–41. doi: 10.1016/j.semarthrit.2014.10.012
11. Dieleman, JL, Baral, R, Birger, M, Bui, AL, Bulchis, A, Chapin, A, et al. US spending on personal health care and public health, 1996-2013. JAMA. (2016) 316:2627–46. doi: 10.1001/jama.2016.16885
12. Shintani, H. Ethylene oxide gas sterilization of medical devices. Biocontrol Sci. (2017) 22:1–16. doi: 10.4265/bio.22.1
13. Kirman, CR, Li, AA, Sheehan, PJ, Bus, JS, Lewis, RC, and Hays, SM. Ethylene oxide review: characterization of total exposure via endogenous and exogenous pathways and their implications to risk assessment and risk management. J Toxicol Environ Health B Crit Rev. (2021) 24:1–29. doi: 10.1080/10937404.2020.1852988
14. Robinson, ES, Tehrani, MW, Yassine, A, Agarwal, S, Nault, BA, Gigot, C, et al. Ethylene oxide in southeastern Louisiana's petrochemical corridor: high spatial resolution Mobile monitoring during HAP-MAP. Environ Sci Technol. (2024) 58:11084–95. doi: 10.1021/acs.est.3c10579
15. Jinot, J, Fritz, JM, Vulimiri, SV, and Keshava, N. Carcinogenicity of ethylene oxide: key findings and scientific issues. Toxicol Mech Methods. (2018) 28:386–96. doi: 10.1080/15376516.2017.1414343
16. Ogawa, M, Oyama, T, Isse, T, Yamaguchi, T, Murakami, T, Endo, Y, et al. Hemoglobin adducts as a marker of exposure to chemical substances, especially PRTR class i designated chemical substances. J Occup Health. (2006) 48:314–28. doi: 10.1539/joh.48.314
17. Jain, RB. Associations between observed concentrations of ethylene oxide in whole blood and smoking, exposure to environmental tobacco smoke, and cancers including breast cancer: data for US children, adolescents, and adults. Environ Sci Pollut Res Int. (2020) 27:20912–9. doi: 10.1007/s11356-020-08564-z
18. Olaguer, EP, Robinson, A, Kilmer, S, Haywood, J, and Lehner, D. Ethylene oxide exposure attribution and emissions quantification based on ambient air measurements near a sterilization facility. Int J Environ Res Public Health. (2019) 17:42. doi: 10.3390/ijerph17010042
19. Zhu, X, Kong, X, Chen, M, Shi, S, Cheang, I, Zhu, Q, et al. Blood ethylene oxide, systemic inflammation, and serum lipid profiles: results from NHANES 2013-2016. Chemosphere. (2022) 299:134336. doi: 10.1016/j.chemosphere.2022.134336
20. Rasool, M, Malik, A, Abdul Basit Ashraf, M, Mubbin, R, Ayyaz, U, Waquar, S, et al. Phytochemical analysis and protective effects of Vaccinium macrocarpon (cranberry) in rats (Rattus norvegicus) following ethylene oxide-induced oxidative insult. Bioengineered. (2021) 12:4593–604. doi: 10.1080/21655979.2021.1955528
21. Zhou, C, Wang, S, Ju, L, Zhang, R, Yang, Y, and Liu, Y. Positive association between blood ethylene oxide levels and metabolic syndrome: NHANES 2013-2020. Front Endocrinol (Lausanne). (2024) 15:1365658. doi: 10.3389/fendo.2024.1365658
22. Poole, AR. Osteoarthritis as a whole joint disease. HSS J. (2012) 8:4–6. doi: 10.1007/s11420-011-9248-6
23. O’Neill, TW, McCabe, PS, and McBeth, J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract Res Clin Rheumatol. (2018) 32:312–26. doi: 10.1016/j.berh.2018.10.007
24. Ma, VY, Chan, L, and Carruthers, KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. (2014) 95:986–995.e1. doi: 10.1016/j.apmr.2013.10.032
25. Chen, L, Zhao, Y, Liu, F, Chen, H, Tan, T, Yao, P, et al. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. (2022) 20:207. doi: 10.1186/s12916-022-02403-3
26. Liang, Z, Sun, X, Lan, J, Guo, R, Tian, Y, Liu, Y, et al. Association between pyrethroid exposure and osteoarthritis: a national population-based cross-sectional study in the US. BMC Public Health. (2023) 23:1521. doi: 10.1186/s12889-023-16225-2
27. Gimeno, P, Auguste, ML, Handlos, V, Nielsen, AM, Schmidt, S, Lassu, N, et al. Identification and quantification of ethylene oxide in sterilized medical devices using multiple headspace GC/MS measurement. J Pharm Biomed Anal. (2018) 158:119–27. doi: 10.1016/j.jpba.2018.05.035
28. Urbiztondo, M, Ramirez, A, Hueso, JL, Santamaria, J, Ruiz-Salvador, AR, and Hamad, S. Unravelling the key factors in the chlorine-promoted epoxidation of ethylene over a silver-copper oxide nanocatalyst. Nanoscale. (2022) 14:7332–40. doi: 10.1039/D2NR00702A
29. Lynch, HN, Kozal, JS, Russell, AJ, Thompson, WJ, Divis, HR, Freid, RD, et al. Systematic review of the scientific evidence on ethylene oxide as a human carcinogen. Chem Biol Interact. (2022) 364:110031. doi: 10.1016/j.cbi.2022.110031
30. Zhou, W, Zhao, Y, Jin, J, Cheng, M, Bai, Y, and Xu, J. The association of hemoglobin ethylene oxide levels with albuminuria in US adults: analysis of NHANES 2013-2016. Environ Sci Pollut Res Int. (2024) 31:4130–9. doi: 10.1007/s11356-023-31083-6
31. Thoma, ED, Gitipour, A, George, I, Kariher, P, MacDonald, M, Queiroz, G, et al. Assessment of chemical facility ethylene oxide emissions using Mobile and multipoint monitoring. Atmos Environ X. (2023) 18:1–11. doi: 10.1016/j.aeaoa.2023.100214
32. Agency for Toxic Substances and Disease Registry. Toxicological profiles. In: Toxicological profile for ethylene oxide. Atlanta, GA: Agency for Toxic Substances and Disease Registry (US) (2022).
33. Jones, RR, Fisher, JA, Medgyesi, DN, Buller, ID, Liao, LM, Gierach, G, et al. Ethylene oxide emissions and incident breast cancer and non-Hodgkin lymphoma in a US cohort. J Natl Cancer Inst. (2023) 115:405–12. doi: 10.1093/jnci/djad004
34. Ghosh, M, and Godderis, L. Genotoxicity of ethylene oxide: a review of micronucleus assay results in human population. Mutat Res Rev Mutat Res. (2016) 770:84–91. doi: 10.1016/j.mrrev.2016.05.002
35. Steenland, K, Whelan, E, Deddens, J, Stayner, L, and Ward, E. Ethylene oxide and breast cancer incidence in a cohort study of 7576 women (United States). Cancer Causes Control. (2003) 14:531–9. doi: 10.1023/A:1024891529592
36. Kirman, CR, Sweeney, LM, Teta, MJ, Sielken, RL, Valdez-Flores, C, Albertini, RJ, et al. Addressing nonlinearity in the exposure-response relationship for a genotoxic carcinogen: cancer potency estimates for ethylene oxide. Risk Anal. (2004) 24:1165–83. doi: 10.1111/j.0272-4332.2004.00517.x
37. Wu, S, Yang, YM, Zhu, J, Wang, LL, Xu, W, Lyu, SQ, et al. Impact of hemoglobin adducts of ethylene oxide on the prevalence and prognosis of chronic kidney disease in US adults: an analysis from NHANES 2013-2016. Environ Sci Pollut Res Int. (2024) 31:2802–12. doi: 10.1007/s11356-023-30712-4
38. Liu, S, Li, J, Wang, L, Zhang, Y, Wei, B, and Li, Y. Association between ethylene oxide exposure and cognitive function in US older adults: NHANES 2013-2014. J Alzheimer's Dis. (2024) 101:951–9. doi: 10.3233/JAD-240662
39. Jiang, S, Wang, Y, Wang, M, Xu, Y, Zhang, W, Zhou, X, et al. Sex difference in the non-linear relationship between ethylene oxide exposure and depressive symptoms: a cross-sectional study. J Affect Disord. (2024) 345:386–93. doi: 10.1016/j.jad.2023.10.147
40. Li, S, Wang, J, Lei, D, Peng, D, Zong, K, Li, K, et al. Associations between ethylene oxide exposure and liver function in the US adult population. Toxics. (2024) 12:12. doi: 10.3390/toxics12080551
41. Le, L, Lan, Z, and Chen, C. Positive association of ethylene oxide levels with young stroke: a population-based study. Front Aging Neurosci. (2024) 16:1391176. doi: 10.3389/fnagi.2024.1391176
42. Xie, J, Lin, J, Wei, M, Teng, Y, He, Q, Yang, G, et al. Sustained Akt signaling in articular chondrocytes causes osteoarthritis via oxidative stress-induced senescence in mice. Bone Res. (2019) 7:23. doi: 10.1038/s41413-019-0062-y
43. Yang, J, Luo, J, Tian, X, Zhao, Y, Li, Y, and Wu, X. Progress in understanding oxidative stress, aging, and aging-related diseases. Antioxidants (Basel). (2024) 13:394. doi: 10.3390/antiox13040394
44. Bolduc, JA, Collins, JA, and Loeser, RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. (2019) 132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038
45. Li, XL, Yu, XY, Tao, YA, Xu, YZ, Li, X, Wang, JD, et al. Polyphenols as potential antioxidants for the treatment of intervertebral disc degeneration. Curr Med Chem. (2024) 32:3405–22. doi: 10.2174/0109298673287391231228081400
46. Li, D, Ni, S, Miao, KS, and Zhuang, C. PI3K/Akt and caspase pathways mediate oxidative stress-induced chondrocyte apoptosis. Cell Stress Chaperones. (2019) 24:195–202. doi: 10.1007/s12192-018-0956-4
47. Adam, MS, Zhuang, H, Ren, X, Zhang, Y, and Zhou, P. The metabolic characteristics and changes of chondrocytes in vivo and in vitro in osteoarthritis. Front Endocrinol (Lausanne). (2024) 15:1393550. doi: 10.3389/fendo.2024.1393550
48. Ansari, MY, Ahmad, N, and Haqqi, TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. (2020) 129:110452. doi: 10.1016/j.biopha.2020.110452
49. Minguzzi, M, Cetrullo, S, D'Adamo, S, Silvestri, Y, Flamigni, F, and Borzì, RM. Emerging players at the intersection of chondrocyte loss of maturational arrest, oxidative stress, senescence and low-grade inflammation in osteoarthritis. Oxidative Med Cell Longev. (2018) 2018:3075293. doi: 10.1155/2018/3075293
50. Olivotto, E, Otero, M, Marcu, KB, and Goldring, MB. Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open. (2015) 1:e000061. doi: 10.1136/rmdopen-2015-000061
51. Tudorachi, NB, Totu, EE, Fifere, A, Ardeleanu, V, Mocanu, V, Mircea, C, et al. The implication of reactive oxygen species and antioxidants in knee osteoarthritis. Antioxidants (Basel). (2021) 10:985. doi: 10.3390/antiox10060985
52. Katoh, T, Higashi, K, Inoue, N, and Tanaka, I. Effects of chronic inhalation of ethylene oxide on lipid peroxidation and glutathione redox cycle in rat liver. Res Commun Chem Pathol Pharmacol. (1988) 61:281–4.
53. Mori, K, Inoue, N, Fujishiro, K, Kikuchi, M, and Chiba, S. Biochemical changes in rat erythrocytes caused by ethylene oxide exposure. Fundam Appl Toxicol. (1990) 15:441–7. doi: 10.1016/0272-0590(90)90030-N
54. Lynch, DW, Lewis, TR, Moorman, WJ, Burg, JR, Groth, DH, Khan, A, et al. Carcinogenic and toxicologic effects of inhaled ethylene oxide and propylene oxide in F344 rats. Toxicol Appl Pharmacol. (1984) 76:69–84. doi: 10.1016/0041-008x(84)90030-9
55. Wang, H, Chen, X, Lin, F, Zheng, J, Chen, K, Wang, X, et al. Association between ethylene oxide levels and depressive symptoms: a cross-sectional study based on NHANES 2013-2018 database. J Affect Disord. (2024) 348:135–42. doi: 10.1016/j.jad.2023.12.050
56. Liu, Y, Liu, N, Xiong, W, and Wang, R. Association between blood ethylene oxide levels and periodontitis risk: a population-based study. Front Public Health. (2024) 12:1338319. doi: 10.3389/fpubh.2024.1338319
57. Wu, W, Wu, J, Hou, Z, Yan, Q, Qin, K, Zhao, Y, et al. Association between ethylene oxide exposure and serum sex hormone levels measured in a reference sample of the US general population. Front Endocrinol (Lausanne). (2025) 16:1533516. doi: 10.3389/fendo.2025.1533516
58. Liang, Y, Duan, L, Xiong, J, Zhu, W, Liu, Q, Wang, D, et al. E2 regulates MMP-13 via targeting miR-140 in IL-1β-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther. (2016) 18:105. doi: 10.1186/s13075-016-0997-y
59. Hutchinson, JL, Hutchinson, AJ, Feng, J, and Séguin, CA. The role of sex hormones in cartilaginous tissues: a scoping review. JOR Spine. (2025) 8:e70072. doi: 10.1002/jsp2.70072
Keywords: osteoarthritis, ethylene oxide, middle-aged and young adults, cross-sectional study, national health and nutrition examination survey
Citation: Wang X, Wang M, Guo Z and Xiang C (2025) Association between ethylene oxide exposure and osteoarthritis risk among middle-aged and young adults: a cross-sectional study. Front. Public Health. 13:1550456. doi: 10.3389/fpubh.2025.1550456
Edited by:
Zhilin Zeng, Huazhong University of Science and Technology, ChinaReviewed by:
Lai Xuefeng, Huazhong University of Science and Technology, ChinaYe Li, Southern Medical University, China
Copyright © 2025 Wang, Wang, Guo and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Xiang, Y2h1YW54aWFuZ0BzeG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Xudong Wang1†
Xudong Wang1† Chuan Xiang
Chuan Xiang