- 1Department of Endocrinology and Metabolism, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 2Department of Endocrinology, The First People’s Hospital of Kashgar Region, Kashgar, China
- 3Department of Endocrinology, The First People’s Hospital of Zhaoqing, Guangdong, China
- 4Department of Emergency, The Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Introduction: This study elucidates the characteristics of randomized clinical trials (RCTs) related to dyslipidemia that are registered on the International Clinical Trials Registry Platform (ICTRP) to better identify research hotspots, address existing gaps, and improve clinical trial designs.
Methods: This cross-sectional study included dyslipidemia-related RCTs registered on the ICTRP up to 13 August 2024. We evaluated the relevant characteristics of these RCTs and reviewed their publication status after enrollment using PubMed.
Results: A total of 2,410 dyslipidemia-related RCTs were analyzed. The number of registered RCTs sharply increased in 2005 (N = 125). The majority of the RCTs included adults (91.4%), with a median sample size of 93 (50–229), and 92.9% of these trials had no sex-based enrollment restrictions. Few RCTs focused on participants aged ≤18 years (2.8%), those aged 19–44 years (3.4%), or exclusively women (2.8%). Medication (83.1%) was the most common type of intervention. Efficacy and safety outcomes were predominant (81.5%), while only 0.7% of the RCTs specified treatment adherence as a primary outcome. The RCTs involving adults had larger sample sizes (median 100.0 [50, 245] vs. 56.0 [27, 108], p = 0.047) and lower proportions of natural medicine and extracts (26.6% vs. 33.6%, p < 0.001) compared to age-specific RCTs. After enrollment, the 7-year cumulative publication rate was 20.8%.
Conclusions: High-quality RCTs involving younger participants, women, and adherence-related outcomes were lacking. Researchers should prioritize exploring novel therapeutic strategies to improve trial publication rates.
1 Introduction
Dyslipidemia is defined as having abnormal levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG), or any combination of these components. It is a major risk factor for cardiovascular disease (CVD) and stroke (1, 2). In 2008, the global prevalence of elevated plasma TC levels in adults was approximately 39%, and currently, approximately 4 million deaths per year are attributed to LDL-C abnormalities (3, 4). With rapid urbanization and changes in dietary habits and lifestyle, plasma cholesterol levels remain elevated. In addition, the global burden of dyslipidemia has increased over the past 30 years (5). Controlling plasma lipid levels to reduce the risk of CVD and related deaths in the coming decades warrants attention.
Randomized clinical trials (RCTs) are the gold standard for evaluating interventions and guiding clinical decision-making. To ensure standardized registration and information disclosure of clinical research, the International Committee of Medical Journal Editors proposed the registration of trials on a public platform before participant recruitment in 2004 (6). In 2005, the World Health Organization facilitated the establishment of the International Clinical Trials Registry Platform (ICTRP), which collects records of registered RCTs from various registration centers worldwide. The ICTRP integrates data from 18 WHO-endorsed primary registries across various global regions, including ClinicalTrials.gov (United States), the EU Clinical Trials Register (Europe), ChiCTR (China), CTRI (India), PACTR (Africa), ANZCTR (Australia/New Zealand), and registries from Latin America.1 Ongoing or completed clinical trials are assigned a unique registration number and standardized data within the ICTRP (7).
Understanding the characteristics of dyslipidemia-related clinical trials can guide improvements in trial design and help identify areas requiring further research. However, systematic evaluations of dyslipidemia-related RCTs are lacking. Therefore, in this study, the characteristics of dyslipidemia-related RCTs registered on the ICTRP were investigated.
2 Methods
2.1 Searching and selecting relevant registered trials
The ICTRP was searched on 13 August 2024 using the following search strategy: “dyslipidemia” OR “hyperlipidemia” OR “lipid disorders” OR “hypercholesterolemia” OR “hypertriglyceridemia” OR “blood lipid disorders.” Data were downloaded in the form of XML files and transferred into an Excel file to facilitate further data cleaning and analysis. Duplicated trials, observational trials, non-RCTs, and trials that had been withdrawn or had unknown status were excluded. LP and MS systematically removed studies unrelated to dyslipidemia.
2.2 Data extraction
The variables were independently extracted by two investigators (LP and MS) after calibrating the extraction criteria. These variables included sex, age, location, sample size, type of registration, status, masking, type of intervention, funding source, intervention mode, and primary outcome. The participants were divided into the following age groups: ≤18, 19 to 44, 45 to 64, and ≥65 years. For further data analysis, these participants were merged into “age-specific groups.” If the age range of the participants spanned two or more subgroups (19–44, 45–64, and ≥65 years), they were classified into the “adult group.” The “adult group” included participants without age restrictions. If an industry was listed as the lead funder, the trial was classified as industry-funded.
2.3 Searching publication status of the included trials
Two investigators (RH and ZH) searched PubMed by entering registration numbers in all fields. The search for publication status was updated and finalized by 30 September 2024. Publication of RCTs was confirmed by matching the brief titles and study characteristics outlined on the ICTRP with descriptions in the published articles. The earliest publication date was recorded when multiple publications existed for the same registered trial. Unpublished trials underwent a second publication search by investigators AA and Xiaochan Lin. Any disagreements or uncertainties were resolved through consensus.
2.4 Statistical analysis
The number (percentage) of categorical variables and the median (interquartile range) of continuous variables were calculated. The χ2 test was performed to compare the categorical variables. Kaplan–Meier analysis was conducted to analyze cumulative publication rates after trial enrollment. All statistical tests were conducted using SPSS version 25.0 (IBM Corporation), and a two-sided p-value of <0.05 indicated statistical significance.
3 Results
3.1 Distribution of dyslipidemia-related RCTs
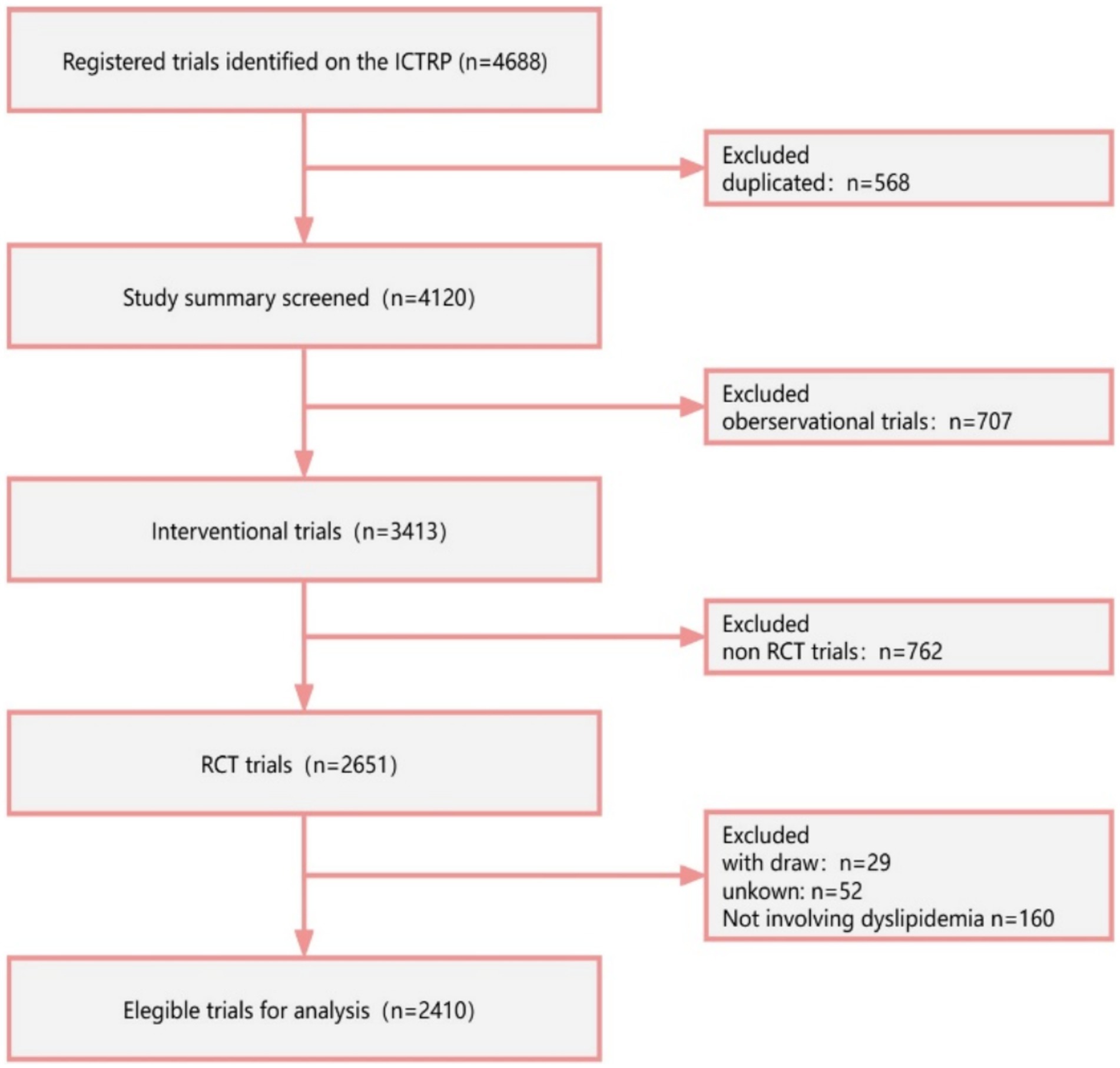
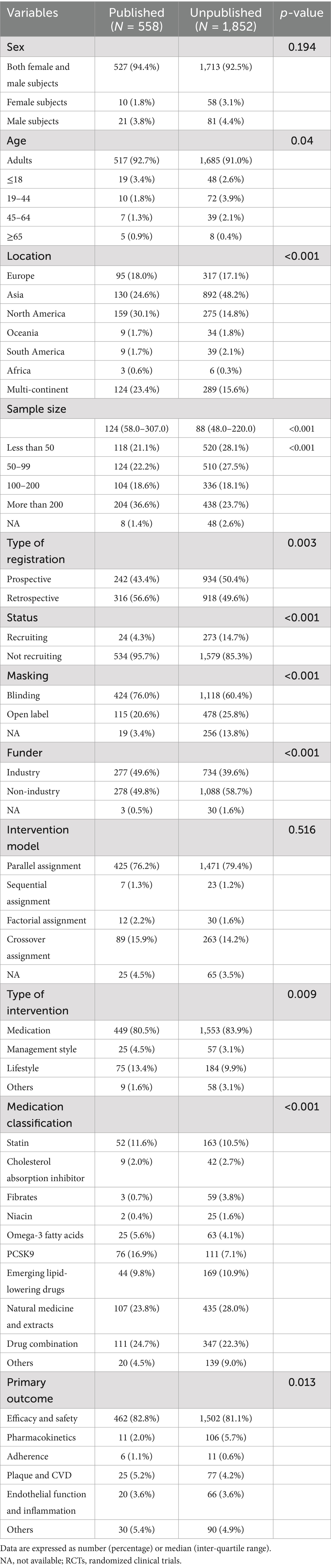
A total of 4,688 registered RCTs were retrieved from the ICTRP. Out of these, 568 duplicated trials, 707 observational trials, and 762 non-RCTs were excluded. Furthermore, trials that were withdrawn (n = 29), had unknown status (n = 52), and were irrelevant to lipids (n = 160) were excluded. Finally, 2,410 RCTs were analyzed (Figure 1). Figure 2 illustrates the distribution of RCTs by registration year from 1997 to 2024. The annual number of registrations increased from 1 in 1997 to 157 in 2020. The number of registered RCTs increased dramatically in 2005 (n = 125) (Figure 2).
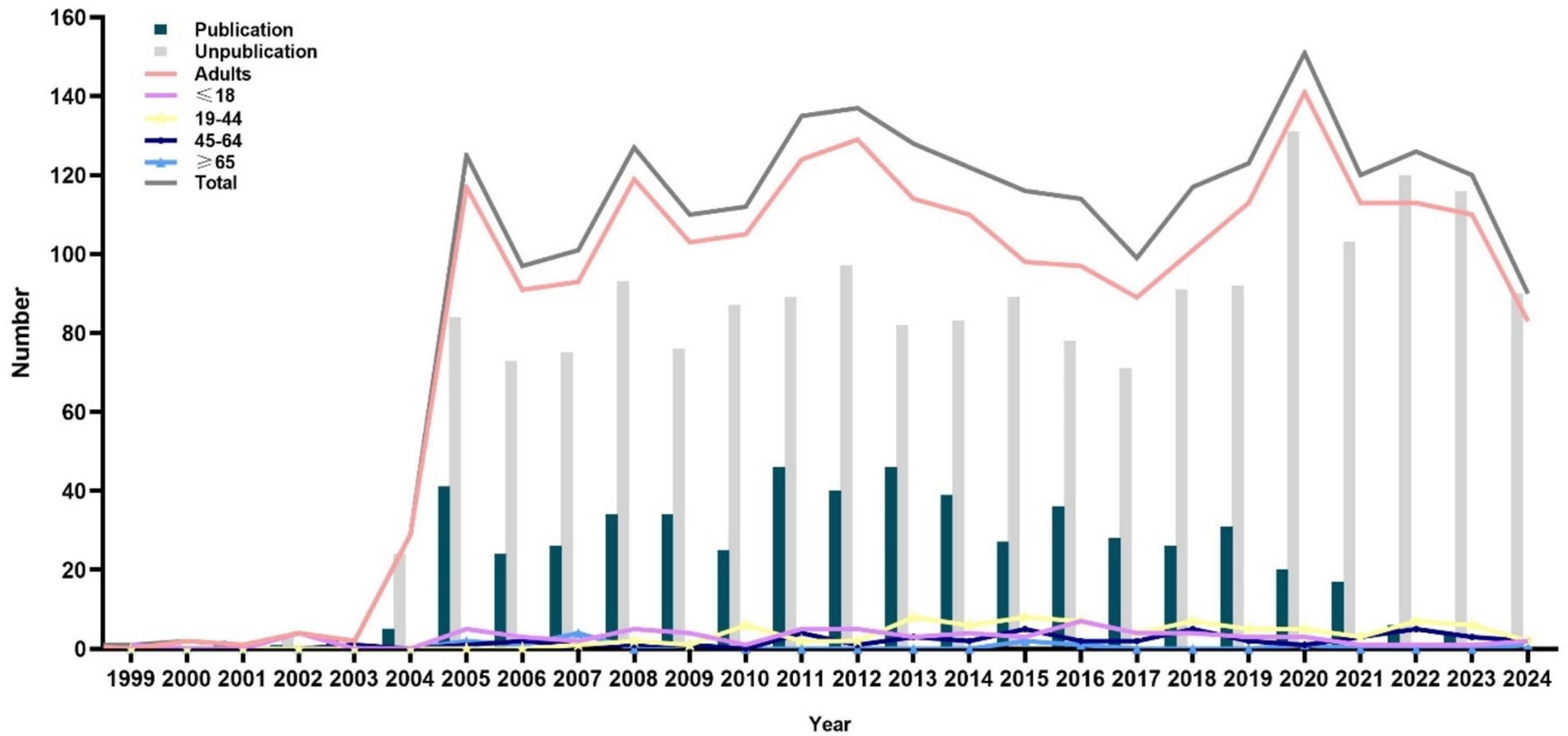
Figure 2. Distribution of dyslipidemia-related randomized clinical trials according to the registered year.
3.2 General characteristics of the included RCTs
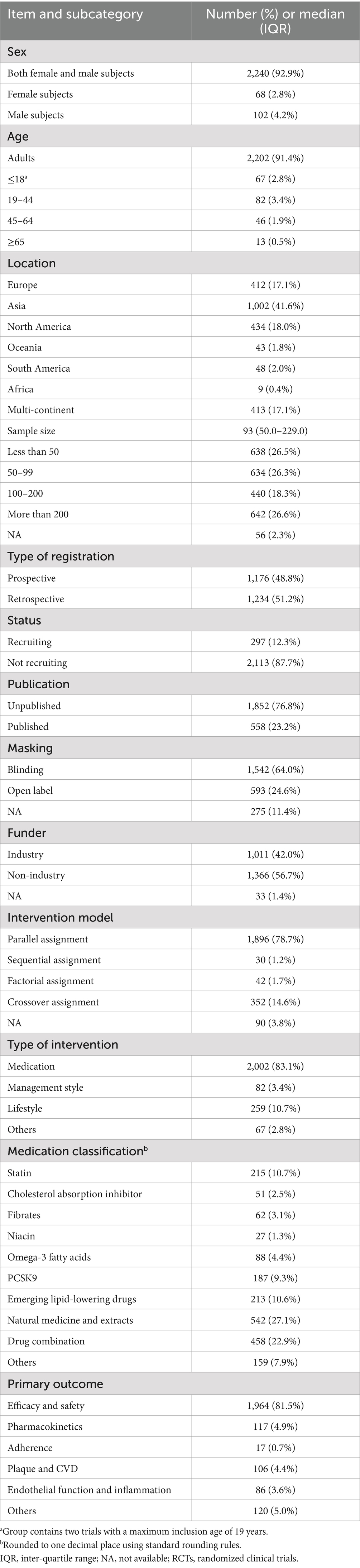
Overall, the majority of trials included adults (91.4%), with a median sample size of 93 (50–229) and no sex-based enrollment restrictions (92.9%). Few trials enrolled participants aged ≤18 years (2.8%), those aged 19 to 44 years (3.4%), or exclusively women (2.8%). Approximately half of the trials were registered after patient recruitment, and 64% utilized a blinding method in their design. The majority of the trials had a “not recruiting” status (87.7%). However, only 23.2% of the trials were published. Approximately 42% of the trials were industry-funded. The majority of the trials were conducted in Asia (41.6%), followed by North America (18.0%) and Europe (17.1%). Parallel assignment (78.7%) was the most frequently used intervention model, followed by crossover assignment (14.6%), factorial assignment (1.7%), and sequential assignment (1.2%). Medication (83.1%) was the most common type of intervention. The types of medication included natural medicine and extracts (27.1%), drug combinations (22.9%), statins (10.7%) and emerging lipid-lowering drugs (10.6%), cholesterol absorption inhibitors (2.5%), fibrates (3.1%), niacin (1.3%), omega-3 fatty acids (4.4%), proprotein convertase subtilisin/kexin type 9 (9.3%), and others (7.9%). The primary outcome predominantly focused on efficacy and safety (81.5%). The secondary outcomes included pharmacokinetics (4.9%), plaque and CVD (4.4%), endothelial function and inflammation (3.6%), adherence (0.7%), and others (5.0%) (Table 1).
3.3 Comparison of RCT characteristics based on participants
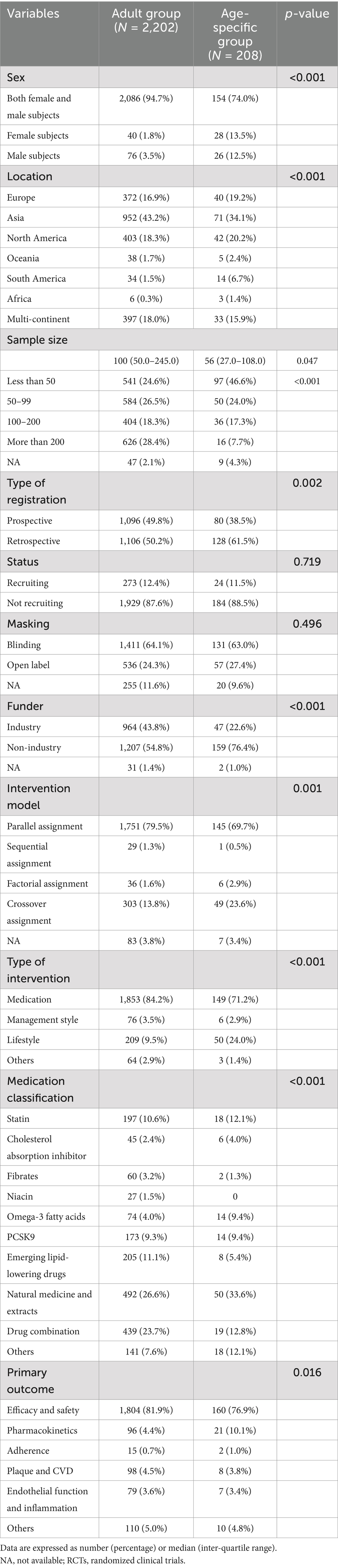
Table 2 summarizes a comparison of characteristics between the RCTs involving adults and those with age-specific participants. Recruitment status and masking did not differ between the two groups. The RCTs involving adults were more frequently conducted in Asia (43.2% vs. 34.1%), designed with parallel assignment (79.5% vs. 69.7%), multi-centered (18.0% vs. 15.9%), and involved drug combinations (23.7% vs. 12.8%). These RCTs were also more frequently funded by the industry (43.8% vs. 22.6%) and had medication interventions (84.2% vs. 71.2%), compared to the RCTs with age-specific participants (all p < 0.001). The trials involving adults had larger sample sizes than those with age-specific adults (median 100.0 [50, 245] vs. 56.0 [27, 108], p = 0.047; [sample size >200] 28.4% vs. 7.7%, p < 0.001). In addition, approximately half (46.6%) of the trials with age-specific participants had a sample size of <50, compared to only 24.6% of the trials involving adults. However, the proportion of RCTs focusing on natural medicine and extracts (33.6% vs. 26.6%, p < 0.001) and those designed with crossover assignment (23.6% vs. 13.8%) was higher in the age-specific group. The trials in the adult group focused more on efficacy and safety (81.9% vs. 76.9%, p < 0.016) than those in the age-specific group. Both the adult and age-specific groups paid minimal attention to adherence (1.0% vs. 0.7%).
Figure 3 illustrates the distribution of RCTs based on age groups: ≤18, 19 to 44, 45 to 64, and ≥65 years. Only 3.4% of the trials involved participants aged 19 to 44 years, followed by participants aged ≤18 years (2.8%) and 45 to 64 years (1.9%). Only 0.5% of the trials involved participants aged ≥65 years. In addition, only 2.07, 2.45, 1.37, and 0.29% of the RCTs focused on medication for participants aged ≤18, 19 to 44, 45 to 65, and ≥65 years, respectively. Trials were rarely (<1%) conducted on women, adherence, or management interventions within each age-specific group.
Figure 3. Comparison of the characteristics of dyslipidemia-related randomized clinical trials across different age groups: (A) sex, (B) sample size, (C) location, (D) intervention type, (E) medication, and (F) primary outcomes.
3.4 Publication status of the RCTs
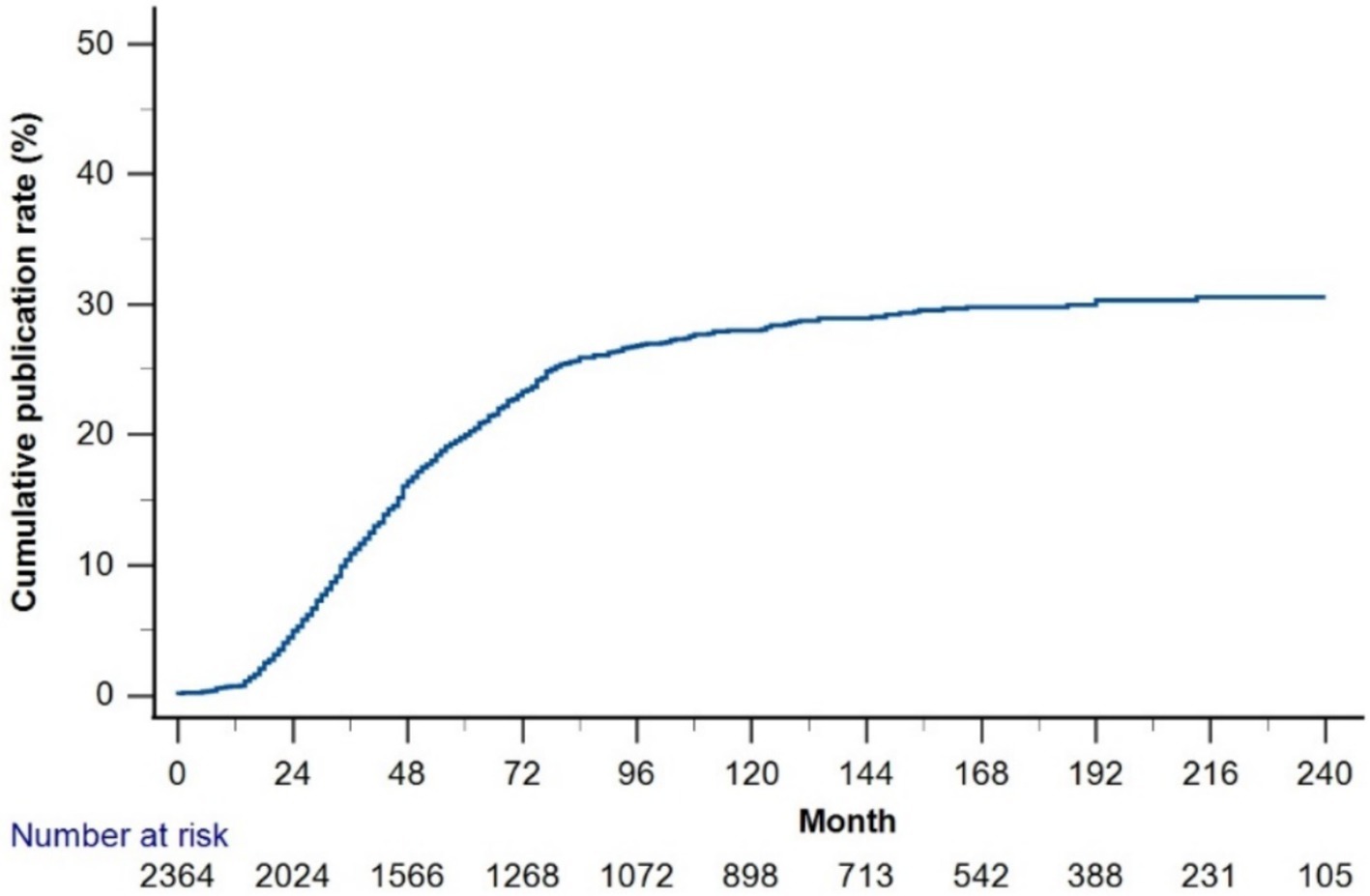
The cumulative publication rate of all trials was <30% (Figure 4). The 1-, 2-, 3-, 5-, and 7-year cumulative publication rates since enrollment were 0.6, 4.4, 9.5, 16.7, and 20.8%, respectively. Table 3 summarizes the RCT characteristics based on publication status. Compared to the unpublished RCTs, published RCTs were more frequently retrospectively registered (56.6% vs. 49.6%), funded by the industry (49.6% vs. 39.6%), and conducted in North America (30.1% vs. 14.8%) (all p < 0.001). The published RCTs included larger sample sizes than the unpublished RCTs (median 124.0 [58, 307] vs. 88 [48, 220]; [samples more than 200 groups] 36.6% vs. 23.7%, p < 0.001). Compared to the published RCTs, the unpublished RCTs focused more on medication (83.9% vs. 80.5%, p = 0.009), natural medicine and extracts (28% vs. 23.8%, p < 0.001), and pharmacokinetics (5.7% vs. 2.0%, p = 0.013).
4 Discussion
To the best of our knowledge, this is the first study to comprehensively assess the characteristics of registered dyslipidemia-related RCTs. The number of registered hyperlipidemia-related RCTs increased rapidly since 2005. These RCTs primarily focused on adults, with efficacy and safety as the primary outcomes. The interventions involved medication. Nonetheless, few RCTs focused on age-specific populations, exclusively on women, adherence, and management-style interventions. RCTs involving age-specific participants had small sample sizes and a high proportion of interventions using natural medicine and extracts. The cumulative publication rate of these RCTs was <30% after enrollment.
The majority (91.4%) of the RCTs enrolled adults, with a median sample size of 100 [50, 245]. In contrast, the RCTs that included age-specific participants had smaller sample sizes than those involving adults (median 56 [27, 108] vs.100 [50, 245], p = 0.047). Small sample sizes are unreliable for assessing the effect size and may even yield spurious results. Although our analysis identified 125 large-scale RCTs with over 1,000 participants, these RCTs represent only approximately 5% of the total 2,410 RCTs included in our study. While some RCTs had large sample sizes, demographic variable-based stratification often yielded inadequate sample sizes for each subgroup (8). A small sample size has no universal definition and remains controversial. Sample size calculations for clinical trials should be based on robust parameter assumptions, such as the power of a test (β) and significance level (α) (9), which were neglected in the RCTs on the ICTRP. Therefore, sample size calculations should be comprehensively described during registration. Modern evidence-based medicine, particularly RCTs, encounters challenges when studying older populations, which are often underrepresented in individual trials. This limitation may help explain why only 2.6% of the dyslipidemia-related RCTs on the ICTRP focused on participants aged ≥65 years.
Concerning lipid levels, studies have strongly emphasized the implication of “the earlier, the better” (10). Lipid disorders have been associated with subclinical atherosclerosis and CVD across the lifespan, affecting not only older adults but also children and young adults (2, 11–13). In China, trends in serum lipid levels have worsened among children and adolescents, while favorable trends have been reported in the US (11, 14). Despite these improvements among US adolescents, the incidence of lipid disorders remains high, with rates ranging from 19 to 25% (11). Childhood lipid disorders often persist into adulthood (13); however, only 2.8% of the RCTs involved children and adolescents in the present study. Future research on dyslipidemia must include more diverse populations, with an increased focus on younger demographics, to provide evidence-based foundations for precision prevention strategies.
The impact of dyslipidemia in early life on long-term health extends beyond adolescence and is also applicable to young adults (15). A South Korean study reported that even modest increases in lipid levels were associated with an increased risk of CVD among young adults aged 20–39 years (16). Similar results were reported in the Framingham Offspring Study involving adults aged 40–50 years who did not have CVD at the age of 55. Notably, the association between hyperlipidemia in young adulthood and the risk of CVD remained significant even after adjusting for non-HDL-C levels at the age of 55 (13). In young adults with a low risk of CVD, lowering LDL-C levels effectively reduced the occurrence of major cardiovascular events, showing results comparable to those seen in older adults (17). However, current guidelines recommend quantitative risk assessments using 10-year risk equations starting at age 40, with limited recommendations for apparently healthy young adults aged 20 to 39 years (18). Coincidently, cardiovascular mortality rates in young adults, particularly among women, have plateaued, despite a marked decrease in overall cardiovascular mortality in recent decades (19). Lipid levels in women are more atherogenic and are influenced by transitional life stages, such as the menstrual cycle, pregnancy, breastfeeding, and menopause (20). For example, 38.5% of women with a history of gestational diabetes mellitus develop dyslipidemia in the early postpartum period (21). Despite this, only <1% of the RCTs involved women aged 19–44 years, according to the data on the ICTRP. The next era of CVD prevention should focus on prioritizing trials in younger populations and developing precision medication management (15). There is a critical need for more high-quality RCTs involving younger demographics, particularly women, to address the existing gaps in research.
Lipid-lowering therapies (LLTs), including LDL-C, TG, and lipoprotein(a), effectively reduce major vascular events (18). In this study, more than 80% of dyslipidemia-related RCTs focused on medication interventions and the efficacy and safety of LLTs. However, parallel assignment (78.7%) was the most frequently used intervention model, while only 14.6% of dyslipidemia-related RCTs were designed with a crossover assignment. Statins—first-line drugs for CVD prevention and treatment—accounted for 10.7% of the RCTs. TG-lowering drugs, such as fibrates and omega-3 fatty acids, accounted for 4.4 and 3.1% of RCTs, respectively. Despite the importance of achieving target LDL-C levels and adhering to LLTs, suboptimal outcomes continue to be a concern (22). Guidelines recommend drug combination therapy for patients who are statin-intolerant or do not meet their target LDL-C levels (18). Approximately 22.9% of RCTs focused on drug combination therapy. In addition, emerging lipid-lowering drugs accounted for only 10.6% of RCTs. The development of novel treatment modalities and therapeutic targets holds promise for reducing the risk of adverse cardiovascular events, despite the limited data available (23). Continuous investigation into emerging lipid-lowering drugs is essential to identify novel molecular targets, and researchers should prioritize diverse intervention models (such as parallel-group, crossover, cluster, or other types) in RCT research.
Interestingly, natural medicine and extracts (27.1%) accounted for the largest proportion of medication-related RCTs among adults. This trend was also observed in the age-specific trials. Nutraceuticals that are certified for their lipid-lowering effects have garnered research interest (24). However, the cardiovascular benefits of nutraceuticals have not yet been reported; therefore, they cannot replace traditional lipid-lowering drugs (25). Chinese herbal medicine represents another important category within natural medicine and extracts. Chinese herbal remedies include single herbs, Chinese patent medicines, and compound formulas; all grounded in over 2,000 years of experiential knowledge in treating diseases (26). The effective components in Chinese herbal remedies can delay the formation of atherosclerotic plaque by protecting endothelial cells, inhibiting inflammatory reactions and lipid deposition, regulating gut microbiota, lowering antioxidants, reducing foam cell formation in macrophages, and decreasing lipid peroxidation reactions (27, 28). Multiple active ingredients in Chinese herbs offer cardiovascular benefits and are used in the treatment of CVD (29). However, the limitations of these remedies include single administration methods, poor water solubility, low bioavailability, and weak targeting capabilities (30). The combination of modern scientific technologies, such as nanotechnology, and Chinese herbal medicine provides a more scientific approach to addressing these shortcomings in clinical applications (27, 30). Traditional Chinese medicine—one of the oldest healing systems—encompasses herbal medicine, acupuncture, moxibustion, massage, food therapy, and physical exercise such as Tai Chi (26). Acupuncture—categorized as “other intervention” in the present trials—positively regulates lipid metabolism (31). Novel molecular targets and therapeutic strategies for dyslipidemia and CVD may emerge with advancements in modern scientific techniques, combined with the expanding scope of Chinese herbal medicine research.
Patient non-adherence to LLTs contributes to the failure to achieve LDL-C goals (32). Low adherence to treatment is a major public health problem, adversely affecting morbidity, mortality, and healthcare costs (33). Real-world studies have observed high non-persistence rates and poor adherence across all LLT regimens (32). However, only 1% of the dyslipidemia-related RCTs examined adherence as the primary outcome both in adult and age-specific groups. These data highlight the inadequate attention given to adherence. Multifactorial contributors to poor adherence often involve both patient and physician factors, in addition to therapy-related factors. Common barriers include insufficient knowledge about LLTs, limited implementation strategies, and high costs (32). Management style—a group of interventions—encompasses implementation strategies such as shared decision-making, decision tools, digital tools, physician education programs, and pharmacy-based programs. However, management style-related RCTs accounted for only 3.4, 3.5, and 2.9% of dyslipidemia-related RCTs in the total population, adult group, and age-specific group, respectively. This finding highlights the need for more RCTs focused on management styles and adherence. Researchers should prioritize implementing novel strategies to improve treatment adherence.
In this study, the cumulative publication rate of dyslipidemia-related RCTs after enrollment was <30%. This result is similar to or marginally lower than the findings from other RCTs, such as those on osteoporosis (30.5%) and type 1 diabetes (less than 40%) (34, 35). This discrepancy may be attributed to the inclusion of incomplete RCTs that were still in the recruitment stage. Selective publication bias and discrepancies between expected and actual results have been recognized as key barriers to the dissemination of trial findings (34). Blumenthal et al. (36) found that 20% of researchers withheld results due to financial or reputational concerns.
Investigators have the moral and ethical responsibility to register and publish the results of all clinical trials (37). Publishing clinical trial results provides a reliable basis for evidence-based medicine, facilitates the establishment of health policies, aids clinicians in decision-making, and promotes the development of public health and clinical medicine (38). Failure to publish results undermines the individual contributions of research participants and reduces public trust in clinical science (39). Non-publication of RCTs represents a waste of human and financial resources, leads to biased evidence, and violates the ethical obligation to share results and reduce harm (40). Trial registration increases mandatory transparency in research; however, it remains insufficient to reduce publication bias. Funders, journals, and ethics committees should prioritize enhancing mandatory transparency and avoiding publication bias. To address this issue, we emphasize the need for stricter adherence to prospective registration mandates and the enforcement of results submission on the ICTRP. We further propose the inclusion of mandatory fields in the ICTRP, including “Post-trial completion publication status” and “Reasons for non-publication,” to enhance transparency. Other innovative strategies should also be explored to address low publication rates in the future.
5 Study strengths and limitations
The strength of this study is that it presents the first comprehensive overview of registered dyslipidemia-related RCTs. However, there was a notable lack of trials specifically designed for age-specific populations, women, adherence, and management style. Furthermore, researchers should focus on improving publication rates. This study has several limitations. First, the trial data obtained from the ICTRP were often incomplete and outdated, which might have resulted in the omission of protocols or critical information in the analysis. Second, this study provides only an overview of the characteristics of registered dyslipidemia-related RCTs. The advantages and disadvantages of the research could not be investigated owing to insufficient information. Although the ICTRP provides comprehensive coverage through its primary registries, there might be some nuances or additional details that could be obtained by accessing these registries directly. Finally, since the ICTRP data were provided by researchers, they cannot be further verified and validated by the platform.
6 Conclusion
In conclusion, to the best of our knowledge, this is the first study to present a comprehensive overview of registered dyslipidemia-related RCTs. High-quality RCTs should prioritize younger and older populations, especially women, and incorporate diverse intervention models. The majority of dyslipidemia-related RCTs lack published results and insufficiently address adherence. To address the issue of low publication rates, it is essential to enforce mandatory trial registration, ensure transparent reporting, and implement innovative strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LP: Writing – original draft, Funding acquisition. MS: Writing – original draft. RY: Data curation, Writing – original draft. ZH: Data curation, Writing – original draft. AA: Data curation, Writing – original draft. XCL: Data curation, Writing – original draft. HZ: Formal analysis, Writing – original draft. GA: Formal analysis, Writing – original draft. AS: Supervision, Writing – review & editing. XLL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Science and Technology Projects in Guangzhou (Grant number: 2024A04J4096), the Fundamental Research Funds for the Central Universities (Grant number: 21623306), Clinical research Fund of Guangdong Medical Association (Grant number: A202301035), and Administration of Traditional Chinese Medicine of Guangdong Province, China (Grant number: 20241069).
Acknowledgments
We would like to thank the participants of the ICTRP databases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
RCTs, Randomized clinical trials; ICTRP, International Clinical Trials Registry Platform; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; CVD, cardiovascular disease; LLT, Lipid-lowering therapies.
Footnotes
References
1. Berberich, AJ, and Hegele, RA. A modern approach to dyslipidemia. Endocr Rev. (2022) 43:611–53. doi: 10.1210/endrev/bnab037
2. Arvanitis, M, and Lowenstein, CJ. Dyslipidemia. Ann Intern Med. (2023) 176:ITC81–96. doi: 10.7326/AITC202306200
3. World Health Organization. Noncommunicable diseases: risk factors. The Global Health Observatory. (2021). Available online at: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-risk-factors
4. Banach, M, Surma, S, and Toth, PPEndorsed by the International Lipid Expert Panel (ILEP). 2023: the year in cardiovascular disease - the year of new and prospective lipid lowering therapies. Can we render dyslipidemia a rare disease by 2024? Arch Med Sci. (2023) 19:1602–15. doi: 10.5114/aoms/174743
5. Pirillo, A, Casula, M, Olmastroni, E, Norata, GD, and Catapano, AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. (2021) 18:689–700. doi: 10.1038/s41569-021-00541-4
6. De Angelis, C, Drazen, JM, Frizelle, FA, Haug, C, Hoey, J, Horton, R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. (2004) 351:1250–1. doi: 10.1056/NEJMe048225
7. Kannan, S, and Gowri, S. Clinical trials in allied medical fields: a cross-sectional analysis of World Health Organization international clinical trial registry platform. J Ayurveda Integr Med. (2016) 7:48–52. doi: 10.1016/j.jaim.2015.09.003
8. Piovesana, A, and Senior, G. How small is big: sample size and skewness. Assessment. (2018) 25:793–800. doi: 10.1177/1073191116669784
9. Kang, H. Sample size determination and power analysis using the G*power software. J Educ Eval Health Prof. (2021) 18:17. doi: 10.3352/jeehp.2021.18.17
10. Banach, M, Reiner, Ž, Surma, S, Bajraktari, G, Bielecka-Dabrowa, A, Bunc, M, et al. 2024 recommendations on the optimal use of lipid-lowering therapy in established atherosclerotic cardiovascular disease and following acute coronary syndromes: a position paper of the international lipid expert panel (ILEP). Drugs. (2024) 84:1541–1577. doi: 10.1007/s40265-024-02105-5
11. Perak, AM, Ning, H, Kit, BK, de Ferranti, SD, van Horn, LV, Wilkins, JT, et al. Trends in levels of lipids and apolipoprotein B in US youths aged 6 to 19 years, 1999-2016. JAMA. (2019) 321:1895–905. doi: 10.1001/jama.2019.4984
12. Navar-Boggan, AM, Peterson, ED, D'Agostino, RB Sr, Neely, B, Sniderman, AD, and Pencina, MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation. (2015) 131:451–8. doi: 10.1161/CIRCULATIONAHA.114.012477
13. Yan, Y, Li, S, Liu, Y, Guo, Y, Fernandez, C, Bazzano, L, et al. Associations between life-course lipid trajectories and subclinical atherosclerosis in midlife. JAMA Netw Open. (2022) 5:e2234862. doi: 10.1001/jamanetworkopen.2022.34862
14. Ding, W, Cheng, H, Yan, Y, Zhao, X, Chen, F, Huang, G, et al. 10-year trends in serum lipid levels and dyslipidemia among children and adolescents from several schools in Beijing, China. J Epidemiol. (2016) 26:637–45. doi: 10.2188/jea.JE20140252
15. Gidding, SS, and Robinson, J. It is now time to focus on risk before age 40. J Am Coll Cardiol. (2019) 74:342–5. doi: 10.1016/j.jacc.2019.04.064
16. Kim, MK, Han, K, Kim, HS, Yoon, KH, and Lee, SH. Lipid cutoffs for increased cardiovascular disease risk in non-diabetic young people. Eur J Prev Cardiol. (2022) 29:1866–77. doi: 10.1093/eurjpc/zwac139
17. Wang, N, Fulcher, J, Abeysuriya, N, Park, L, Kumar, S, di Tanna, GL, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. (2020) 8:36–49. doi: 10.1016/S2213-8587(19)30388-2
18. Arnett, DK, Blumenthal, RS, Albert, MA, Buroker, AB, Goldberger, ZD, Hahn, EJ, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 140:e649–50. doi: 10.1161/CIR.0000000000000678
19. Wilmot, KA, O'Flaherty, M, Capewell, S, Ford, ES, and Vaccarino, V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. (2015) 132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293
20. Holven, KB, and Roeters van Lennep, J. Sex differences in lipids: a life course approach. Atherosclerosis. (2023) 384:117270. doi: 10.1016/j.atherosclerosis.2023.117270
21. Pei, L, Xiao, H, Lai, F, Li, Z, Li, Z, Yue, S, et al. Early postpartum dyslipidemia and its potential predictors during pregnancy in women with a history of gestational diabetes mellitus. Lipids Health Dis. (2020) 19:220. doi: 10.1186/s12944-020-01398-1
22. Ray, KK, Molemans, B, Schoonen, WM, Giovas, P, Bray, S, Kiru, G, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. (2021) 28:1279–89. doi: 10.1093/eurjpc/zwaa047
23. Michaeli, DT, Michaeli, JC, Albers, S, Boch, T, and Michaeli, T. Established and emerging lipid-lowering drugs for primary and secondary cardiovascular prevention. Am J Cardiovasc Drugs. (2023) 23:477–95. doi: 10.1007/s40256-023-00594-5
24. Derosa, G, Colletti, A, Maffioli, P, D’Angelo, A, Lupi, A, Zito, GB, et al. Lipid-lowering nutraceuticals update on scientific evidence. J Cardiovasc Med. (2020) 21:845–59. doi: 10.2459/JCM.0000000000000970
25. Cicero, AFG, Fogacci, F, Stoian, AP, Vrablik, M, al Rasadi, K, Banach, M, et al. Nutraceuticals in the Management of Dyslipidemia: which, when, and for whom? Could nutraceuticals help low-risk individuals with non-optimal lipid levels? Curr Atheroscler Rep. (2021) 23:57. doi: 10.1007/s11883-021-00955-y
26. Tang, JL, Liu, BY, and Ma, KW. Traditional Chinese medicine. Lancet. (2008) 372:1938–40. doi: 10.1016/S0140-6736(08)61354-9
27. Xuan, X, Zhang, J, Fan, J, and Zhang, S. Research progress of traditional Chinese medicine (TCM) in targeting inflammation and lipid metabolism disorder for arteriosclerosis intervention: a review. Medicine. (2023) 102:e33748. doi: 10.1097/MD.0000000000033748
28. Zhang, HY, Tian, JX, Lian, FM, Li, M, Liu, WK, Zhen, Z, et al. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed Pharmacother. (2021) 133:110857. doi: 10.1016/j.biopha.2020.110857
29. Jiang, Y, Zhao, Q, Li, L, Huang, S, Yi, S, and Hu, Z. Effect of traditional Chinese medicine on the cardiovascular diseases. Front Pharmacol. (2022) 13:806300. doi: 10.3389/fphar.2022.806300
30. Wei, D, Yang, H, Zhang, Y, Zhang, X, Wang, J, Wu, X, et al. Nano-traditional Chinese medicine: a promising strategy and its recent advances. J Mater Chem B. (2022) 10:2973–94. doi: 10.1039/D2TB00225F
31. Han, J, Guo, X, Meng, XJ, Zhang, J, Yamaguchi, R, Motoo, Y, et al. Acupuncture improved lipid metabolism by regulating intestinal absorption in mice. World J Gastroenterol. (2020) 26:5118–29. doi: 10.3748/wjg.v26.i34.5118
32. Desai, NR, Farbaniec, M, and Karalis, DG. Nonadherence to lipid-lowering therapy and strategies to improve adherence in patients with atherosclerotic cardiovascular disease. Clin Cardiol. (2023) 46:13–21. doi: 10.1002/clc.23935
33. Vonbank, A, Agewall, S, Kjeldsen, KP, Lewis, BS, Torp-Pedersen, C, Ceconi, C, et al. Comprehensive efforts to increase adherence to statin therapy. Eur Heart J. (2017) 38:2473–9. doi: 10.1093/eurheartj/ehw628
34. Lai, F, Pei, L, Chen, X, and Li, J. Osteoporosis-related randomized clinical trials with middle-aged and older adults registered on the international clinical trials registry platform. Front Endocrinol. (2021) 12:702261. doi: 10.3389/fendo.2021.702261
35. Liang, R, Long, J, Zheng, Q, Yuan, G, Chen, X, Xin, Z, et al. Current landscape of type 1 diabetes mellitus-related interventional clinical trials registered on ClinicalTrials.gov: a cross-sectional study. Acta Diabetol. (2021) 58:723–33. doi: 10.1007/s00592-020-01627-0
36. Blumenthal, D, Campbell, EG, Anderson, MS, Causino, N, and Louis, KS. Withholding research results in academic life science. Evidence from a National survey of faculty. JAMA. (1997) 277:1224–8. doi: 10.1001/jama.277.15.1224
37. O'Connor, CM. Why should we publish all clinical trials? JACC Heart Fail. (2019) 7:730. doi: 10.1016/j.jchf.2019.06.009
38. Doi, Y. Moving toward the reduction of publication/reporting biases in clinical trials using a new international standard. Nihon Koshu Eisei Zasshi. (2016) 63:599–605.
39. Fernandez, CV, Kodish, E, and Weijer, C. Informing study participants of research results: an ethical imperative. IRB. (2003) 25:12–9. doi: 10.2307/3564300
Keywords: dyslipidemia, randomized clinical trials, publication, International Clinical Trials Registry Platform, adherence
Citation: Pei L, Su M, Yakeya R, Hu Z, Abudurexiti A, Lin X, Zhao H, Abudourexiti G, Sidike A and Li X (2025) Current landscape of dyslipidemia-related randomized clinical trials registered on the International Clinical Trials Registry Platform. Front. Public Health. 13:1554858. doi: 10.3389/fpubh.2025.1554858
Edited by:
Federica Fogacci, University of Bologna, ItalyReviewed by:
Lokman Hekim Tanriverdi, İnönü University, TürkiyeRafael Zubiran, National Heart, Lung, and Blood Institute (NIH), United States
Copyright © 2025 Pei, Su, Yakeya, Hu, Abudurexiti, Lin, Zhao, Abudourexiti, Sidike and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abudulimu Sidike, MzEzMzg0MjQwNkBxcS5jb20=; Xiaoli Li, bGl4aWFvbGk4ODhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Ling Pei
Ling Pei Muzhen Su3†
Muzhen Su3†