- 1IAVI Africa, Nairobi, Kenya
- 2Ortholog, Nairobi, Kenya
Despite a significant reduction in the global HIV disease prevalence in recent years, children under 15 years of age still account for 3% of people living with HIV, 9% of new incidence, and 12% of AIDS-related deaths. Although there is increased access and use of antiretroviral drugs, children under 1 year in resource-poor settings with a high HIV disease burden remain vulnerable due to poor initiation of these critical interventions impeding progress to meet the 95–95–95 targets. There are renewed efforts to ensure that exposed children under 15 years are not left behind by scaling diagnostics and clinical management in the most affected communities. However, gaps remain in the integration of these services into maternal, child, and adolescent healthcare services within these communities, resulting in only 67% of HIV-exposed infants being tested within 2 months of birth, globally in 2023. Consequently, only 29% of all exposed children under 15 years were initiated in antiretroviral treatment before their 5th birthday in 2023. There are successes for adults aged 15 years and above, but children under 15 years risk being left behind in achieving the 95–95–95 targets. In this study, we review efforts made to reduce these substantial regional variations when comparing progress made between children under 15 years and adults and highlight gaps that might impede achievement of the 95–95–95 targets among children.
Introduction
We are at the midpoint toward the United Nations’ established goal of ending the AIDS epidemic by 2030. However, despite considerable progress in decreasing HIV incidence, challenges remain in accomplishing this goal. These challenges include diminishing funding for the HIV/AIDS response, stigma, discrimination, human rights violations, and constrained health systems, especially in resource-poor settings with a significant burden of HIV disease, that threaten success (1–3). Unlike other infectious diseases, for example malaria and TB, HIV cannot be eliminated without a cure or a vaccine, and vaccine research and development efforts have been impeded by the complex nature of the constantly evolving causative viral pathogen (4, 5). In the absence of a vaccine, antiretroviral drugs (ARVs) are used to treat HIV by reducing viral loads, decreasing the risk of disease transmission to near zero (6). This has improved longevity among people living with HIV, enabling a new span where discordant couples can have healthy children without HIV (7). However, not all of the 39.9 million people living with HIV worldwide are receiving this necessary treatment, with a disproportionate number of those not accessing treatment living in marginalized regions with high HIV disease burden (8, 9). ARV treatment programs are missing 9.3 million (23%) people who require this lifesaving treatment, with children (0–14 years) being the most affected (10). There is an urgent need to reduce HIV-related morbidity and mortality among this vulnerable population.
The UNAIDS 95–95–95 strategy
One of the strategies to end the HIV epidemic entails achieving the 95–95–95 target scheme for testing and treatment. This ensures that 95% of all people living with HIV know their HIV status; among those who know their status, 95% have sustained access to antiretroviral therapy (ART); and 95% of those receiving ART achieve viral suppression (11). This has shifted focus to HIV treatment, making it more person-centered, and expanded access to treatment as an essential public health intervention (11). The current progress made against the target for all ages is 86–89–93, with 91–91–95 among women (15 years and above), 83–86–94 among men (15 years and above), and 66–86–84 among children (0–14 years), indicating that children risk being left behind (Figure 1) (8, 10, 12). Botswana, the country with the third highest HIV prevalence in the world, has exceeded the 95–95–95 targets, highlighting variations between national HIV responses, even in regions with a disproportionate disease burden (13, 14). There is regional variation in advancements toward the 2025 95–95–95 targets in children compared to adults, and only in South Asia (children: 94–93–77 vs. adults: 74–63–62) and Eastern Europe and Central Asia (children: 75–73–63 vs. adults: 60–51–43) is there more progress in children compared to adults (12).
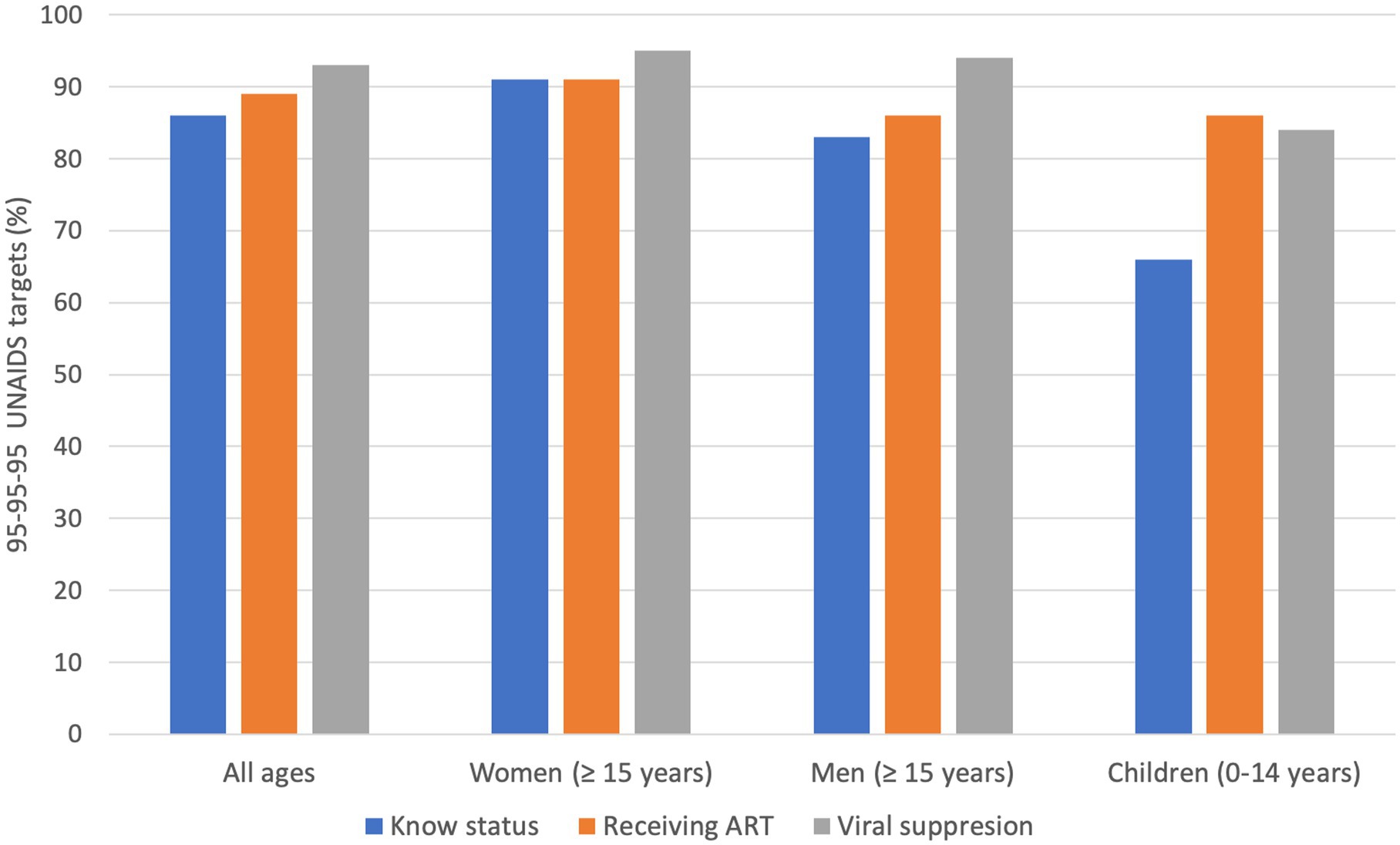
Figure 1. 95–95–95 UNAIDS targets. The achievements were made across all ages, and there is disaggregation among women, men, and children. The goals aim for 95% of all people living with HIV to know their HIV status; among these people who know their status, 95% have sustained access to antiretroviral therapy (ART); and 95% of the people receiving ART achieve viral suppression.
Challenges in reaching 95–95–95 targets in children (0–14 years)
Greater effort is required in many developing countries to achieve epidemic control among children. Inclusion of all demographics into HIV care, which also comprises children, and consideration of their testing, the treatment needs will not only help achieve the goal of ending the HIV epidemic as a public health threat but also have economic gains in the long term. Although some developing countries are making progress toward the 95–95–95 target, there might be children missing from these data due to loss of follow-up, especially among older children (>18 months old), underestimating the actual prevalence levels, which risks undermining the success accomplished (15).
The percentage of pregnant and breastfeeding women receiving antiretroviral therapy (84%) presently remains comparable to the levels in 2019 (10, 16, 17). ART among pregnant women is important for preventing perinatal and sexual HIV transmission. Globally, increased ART among pregnant women has resulted in a 50% reduction in perinatal infections, with infants acquiring perinatal HIV infection (48%, n = 76,800) born to mothers who are either not on ART due to lack of knowledge of their HIV status, acquired HIV during pregnancy or breastfeeding, interrupted treatment during pregnancy or breastfeeding, or did not achieve adequate viral suppression during ART (18). ART has prevented approximately 2.1 million child deaths since 2000; however, despite a marked decline in the number of new HIV infections, 120,000 new infections still occurred among children in 2023 due to vertical disease transmission (10, 16). Overall, the drop in the number of new HIV infections among children is driven by drops in eastern and southern Africa, which had 73% fewer infections in 2023 compared to 2010 (10). This contrasts findings in western and central Africa with modest (44%) declines, and these regions now account for 41% of the HIV incidence among children (10). Consequently, we are behind the target of viral suppression among 75% of children living with HIV, currently at only 48% globally (10). Poor knowledge of mother-to-child transmission of HIV, non-attendance at antenatal clinics, and inaccessible health facility delivery significantly contribute to vertical HIV transmission, and at least one HIV test is recommended for every woman during pregnancy (17).
Children (0–14 years) make up 3% of all people living with HIV, 9% of new incidences, and 12% of AIDS-related deaths (12). Children under 1 year in resource-poor settings with high HIV disease burden remain vulnerable due to poor initiation and access to treatment. In 2023, only 67% of HIV-exposed infants were tested within 2 months of birth, and consequently, only 29% of all exposed children under 15 years were initiated in antiretroviral treatment before their 5th birthday, with more older children entering ART programs (10, 12). WHO guidelines recommend the initiation of lifelong antiretroviral therapy (ART) in all children living with HIV below 5 years, notwithstanding their immune response, to improve access and disease outcome (19).
In 2023, only 48% of children living with HIV achieved viral suppression, and poor ART treatment adherence has been associated with loss of follow-up and continues to pose a great challenge, especially in developing countries (12, 20). It is, therefore, important to identify reasons for disengagement from HIV care services and remove barriers to enable re-engagement interventions tailored to the needs of children (21–26).
Children are less likely to have access to ART compared to adults, and closing this gap will ensure children living with HIV receive lifelong treatment to lead healthy lives (27). Poorer adherence to ART in children compared to adults, especially those in rural settings, can be improved by counseling targeting their parents and caregivers (27, 28). Non-adherence has been associated with poor ART outcomes that include drug resistance, treatment failure, and mortality in children and adolescents living with HIV (28–34). Moreover, children have fewer drug formulation options available and need to be considered during the development of new interventions to impede treatment failure (29, 35, 36). Limited drug formulation options make children particularly vulnerable to ART failure due to the emergence of drug-resistant HIV-1 quasispecies, which have the potential to compromise second-line therapy efficacy (37, 38). The genetic diversity of the constantly evolving HIV has been evaluated using molecular modeling approaches to understand subtype-specific drug interactions to provide insights into strategies that would optimize the limited pediatric ART regimens available. These findings show significant variation in drug-subtype binding, underscoring the importance of tailored treatment approaches, especially for children (39).
Unlike other infectious diseases, for example malaria and TB, HIV cannot be eliminated without a cure or a vaccine, and vaccine research and development efforts have been impeded by the complex nature of the constantly evolving causative viral pathogen (4, 5).
HIV and TB are both infectious diseases of pressing global health concern. In children, co-infection with TB and HIV may delay diagnosis due to atypical presentation in patients, and treatment requires consideration of potential TB and HIV drug-to-drug interactions and delays initiating ART during TB treatment, especially in children with advanced immunosuppression (40). Therefore, a cohesive approach is required when managing pediatric patients with comorbidity, especially in resource-constrained settings (41).
Discovery medicine often does not prioritize diseases affecting low- and middle-income countries (LMICs), and there are fewer product development pipelines and subsequent clinical trials inclined toward diseases in these countries. This poses a challenge to access to treatment products primarily because they are not registered or licensed in LMICs to expand product access to lifesaving treatments. An example is the growing concern over access to the pre-exposure prophylaxis (PrEP) injectable drug, lenacapavir, given every 6 months that has been shown to have 100% efficacy by vulnerable and marginalized populations in developing countries (42–44).
HIV vaccines remain important strategies that are still under development for use alongside ART to ensure an AIDS-free generation. However, children have largely been left out of HIV vaccine research, and inclusion in immune-based clinical trials is essential to evaluate safe vaccines providing perinatal protection administered passively to pregnant women or actively to infants at birth to prevent vertical HIV transmission (45, 46). The huge costs of vaccine manufacturing and weak or outmoded regulatory processes in developing countries, which are not optimized to support novel discovery medicine vaccines and treatment interventions critical for the protection of vulnerable children, also compound the hurdles to disease prevention (47).
There is now an emphasis on devolving pediatric diagnosis and clinical management of children exposed to or living with HIV (12, 48). This will enable integration with maternal and child health services at facilities to be more easily accessed by communities at the sub-national level, reducing barriers to the HIV response among children (11, 49–51).
Efforts to achieve the goal of ending the AIDS epidemic by 2030 require financial resources and investments on a global scale. In 2023, there was a $358 million decrease in donor government funding compared to a total of $8.22 billion available in 2022 (52). A total of $19.8 billion was available for the AIDS response in low- and middle-income countries against $29.3 billion required annually to end the epidemic by 2030 (8, 10). Targeted interventions and further investment are required to reach the 95–95–95 targets, without which the AIDS response will be reversed, and the current progress will diminish as incidence and mortality begin to overtake the response (50). At present, the HIV epidemic response requires continued and increased investment to ensure no child has AIDS (53).
Armed conflict is a unique challenge that ravages healthcare infrastructure and systems, leading to an increased HIV incidence and prevalence (54). A sustained HIV response, difficult as it is, is necessary in conflict and post-conflict to ensure preventing new infections. An example is the end of the civil war in Liberia, which resulted in a decline in HIV prevalence among children and women attributed to the establishment of a National AIDS Control Program. The success in Liberia can be attributed to the program that provided counseling and voluntary testing, prevention of mother-to-child transmission, and an expanded ART program (55).
Conclusion
In conclusion, addressing the unique challenges faced by children living with HIV is essential to ensure they are not left behind in achieving the 95–95–95 target. Improved access to life-saving ART, adherence support during treatment, inclusion in new intervention and vaccine development, better integration of pediatric HIV diagnosis and clinical management, and continuous resource mobilization are some of the concerted efforts that are important for bridging the gap between children and adults. This will enable the realization of a future where children living with HIV can lead long, healthy lives, ending AIDS as a public health threat.
Author contributions
BK: Conceptualization, Writing – original draft, Writing – review & editing. CW: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wesonga, CA, and Kulohoma, B. Prioritising health systems to achieve SDGs in Africa: a review of scientific evidence In: M Ramutsindela and D Mickler, editors. Africa and the sustainable development goals. Sustainable development goals series. Cham: Springer (2020). 113–21.
2. Nyblade, L, Mingkwan, P, and Stockton, MA. Stigma reduction: an essential ingredient to ending AIDS by 2030. Lancet HIV. (2021) 8:e106–13. doi: 10.1016/S2352-3018(20)30309-X
3. Jurgens, R, Csete, J, Amon, JJ, Baral, S, and Beyrer, C. People who use drugs, HIV, and human rights. Lancet. (2010) 376:475–85. doi: 10.1016/S0140-6736(10)60830-6
4. Andrews, SM, and Rowland-Jones, S. Recent advances in understanding HIV evolution. F1000Res. (2017) 6:597. doi: 10.12688/f1000research.10876.1
5. Rose, R, Lamers, SL, Nolan, DJ, Maidji, E, Faria, NR, Pybus, OG, et al. HIV maintains an evolving and dispersed population in multiple tissues during suppressive combined antiretroviral therapy in individuals with cancer. J Virol. (2016) 90:8984–93. doi: 10.1128/JVI.00684-16
6. Bekker, L-G, Das, M, Abdool Karim, Q, Ahmed, K, Batting, J, Brumskine, W, et al. Twice-yearly lenacapavir or daily F/TAF for HIV prevention in cisgender women. N Engl J Med. (2024) 391:1179–92. doi: 10.1056/NEJMoa2407001
7. Baza, MB, Jerónimo, A, Río, I, Rodriguez, C, Vera, M, Hernando, V, et al. Natural conception is safe for HIV-Serodiscordant couples with persistent suppressive antiretroviral therapy for the infected partner. J Women’s Health. (2019) 28:1555–62. doi: 10.1089/jwh.2018.7485
8. UNAIDS, FACT Sheet. (2024). Available online at: https://www.unaids.org/en/resources/documents/2024/UNAIDS_FactSheet (Accessed January 20, 2025).
9. van Schalkwyk, C, Mahy, M, Johnson, LF, and Imai-Eaton, JW. Updated data and methods for the 2023 UNAIDS HIV estimates. JAIDS J Acquir Immune Deficiency Syndromes. 95:e1–4.
10. UNAIDS. global AIDS report — the urgency of now: AIDS at a crossroads. (2024). Available online at: https://www.unaids.org/sites/default/files/media_asset/2024-unaids-global-aids-update_en.pdf (Accessed January 20, 2025).
11. Ambrose, Z, Frescura, L, Godfrey-Faussett, P, Feizzadeh, A, El-Sadr, W, Syarif, O, et al. Achieving the 95 95 95 targets for all: a pathway to ending AIDS. PLoS One. (2022) 17:e0272405. doi: 10.1371/journal.pone.0272405
12. UNICEF, slightly more than half of children under 15 years of age living with HIV are on antiretroviral medications. Available online at: https://data.unicef.org/topic/hivaids/paediatric-treatment-and-care/ (Accessed January 20, 2025).
13. Mine, M, Stafford, KA, Laws, RL, Marima, R, Lekone, P, Ramaabya, D, et al. Progress towards the UNAIDS 95-95-95 targets in the fifth Botswana AIDS impact survey (BAIS V 2021): a nationally representative survey. Lancet HIV. (2024) 11:e245–54doi: 10.1016/s2352-3018(24)00003-1
14. Musuka, G, Moyo, E, Cuadros, D, Herrera, H, and Dzinamarira, T. Redefining HIV care: a path toward sustainability post-UNAIDS 95-95-95 targets. Front Public Health. (2023) 11:1273720. doi: 10.3389/fpubh.2023.1273720
15. Chandiwana, N, Sawry, S, Chersich, M, Kachingwe, E, Makhathini, B, and Fairlie, L. High loss to follow-up of children on antiretroviral treatment in a primary care HIV clinic in Johannesburg, South Africa. Medicine. (2018) 97. doi: 10.1097/MD.0000000000010901
16. UNICEF. Progress in reducing new HIV infections among children has stagnated in recent years. (2024). Available online at: https://data.unicef.org/topic/hivaids/emtct/ (Accessed January 20, 2025).
17. Misganie, YG, Andargie, BA, Lealem, EB, and Angaw, DA. Trend, spatial distribution, and factors associated with HIV testing uptake among pregnant women in Ethiopia, based on 2005–2016 Ethiopia demographic and health survey: a multivariate decomposition analysis and geographically weighted regression. PLoS One. (2024) 19:e0308167. doi: 10.1371/journal.pone.0308167
18. Eke, AC, Lockman, S, and Mofenson, LM. Antiretroviral treatment of HIV/AIDS during pregnancy. JAMA. (2023) 329:1308–9. doi: 10.1001/jama.2023.5076
19. Collins, IJ, Judd, A, and Gibb, DM. Immediate antiretroviral therapy in young HIV-infected children. Curr Opin HIV AIDS. (2014) 9:87–94. doi: 10.1097/COH.0000000000000027
20. Dememew, ZG, Girma, D, Abita, Z, Lemu, LG, Asmelash, D, Bambo, GM, et al. Incidence of lost to follow up among HIV-positive children on antiretroviral therapy in Ethiopia: systematic review and meta-analysis. PLoS One. (2024) 19:e0304239. doi: 10.1371/journal.pone.0304239
21. Humphrey, J, Kipchumba, B, Alera, M, Sang, E, Musick, B, Muli, L, et al. Outcomes after loss to follow-up for pregnant and postpartum women living with HIV and their children in Kenya: a prospective cohort study. J Acq Immune Deficiency Syndromes. (2024) 97:242–52. doi: 10.1097/QAI.0000000000003487
22. Togun, TO, Suffrin, JCD, Rosenthal, A, Kamtsendero, L, Kachimanga, C, Munyaneza, F, et al. Re-engagement and retention in HIV care after preventive default tracking in a cohort of HIV-infected patients in rural Malawi: a mixed-methods study. PLoS Global Public Health. (2024) 4:e0002437. doi: 10.1371/journal.pgph.0002437
23. Oluoch, T, Cornet, R, Muthusi, J, Katana, A, Kimanga, D, Kwaro, D, et al. A clinical decision support system is associated with reduced loss to follow-up among patients receiving HIV treatment in Kenya: a cluster randomized trial. BMC Med Inform Decis Mak. (2021) 21:357. doi: 10.1186/s12911-021-01718-0
24. Hémono, R, Kelly, NK, Fahey, CA, Hassan, K, Msasa, J, Mfaume, RS, et al. Financial incentives to improve re-engagement in HIV care: results from a randomized pilot study. AIDS Care. (2022) 35:935–41. doi: 10.1080/09540121.2022.2041164
25. Fernández-Luis, S, Nhampossa, T, Fuente-Soro, L, Augusto, O, Casellas, A, Bernardo, E, et al. Pediatric HIV care cascade in southern Mozambique: missed opportunities for early ART and re-engagement in care. Pediatr Infect Dis J. (2020) 39:429–34. doi: 10.1097/INF.0000000000002612
26. WHO. Supporting re-engagement in HIV treatment services: policy brief. (2024). Available online at: https://iris.who.int/bitstream/handle/10665/378179/9789240097339-eng.pdf?sequence=1 (Accessed January 20, 2025).
27. Mengesha, MM, Embibel, M, Gobena, T, Tunje, A, Jerene, D, and Hallström, IK. Antiretroviral therapy non-adherence among children living with HIV in Dire Dawa, Eastern Ethiopia: a case-control study. BMC Pediatr. (2022) 22:653. doi: 10.1186/s12887-022-03697-1
28. Gemechu, GB, Hebo, H, and Kura, Z. Children’s adherence to antiretroviral therapy and associated factors: multicenter cross-sectional study. HIV/AIDS Res Palliative Care. (2023) 15:423–34. doi: 10.2147/HIV.S407105
29. Tsikhutsu, I, Bii, M, Dear, N, Ganesan, K, Kasembeli, A, Sing’oei, V, et al. Prevalence and correlates of viral load suppression and human immunodeficiency virus (HIV) drug resistance among children and adolescents in south Rift Valley and Kisumu, Kenya. Clin Infect Dis. (2022) 75:936–44. doi: 10.1093/cid/ciac059
30. Kerkhoff, AD, Villiera, JB, Katsabola, H, Bvumbwe, M, Mhango, J, Khosa, J, et al. Factors associated with antiretroviral therapy adherence among adolescents living with HIV in the era of isoniazid preventive therapy as part of HIV care. PLoS Global Public Health. (2022) 2:e0000418. doi: 10.1371/journal.pgph.0000418
31. Fetzer, BC, Mupenda, B, Lusiama, J, Kitetele, F, Golin, C, and Behets, F. Barriers to and facilitators of adherence to pediatric antiretroviral therapy in a sub-Saharan setting: insights from a qualitative study. AIDS Patient Care STDs. (2011) 25:611–21. doi: 10.1089/apc.2011.0083
32. Isaakidis, P, Kamau, SG, Akatusasira, R, Namatovu, A, Kibet, E, Ssekitto, JM, et al. The level of antiretroviral therapy (ART) adherence among orphan children and adolescents living with HIV/AIDS: a systematic review and meta-analysis. PLoS One. (2024) 19:e0295227. doi: 10.1371/journal.pone.0295227
33. Arage, G, Tessema, GA, and Kassa, H. Adherence to antiretroviral therapy and its associated factors among children at south Wollo zone hospitals, Northeast Ethiopia: a cross-sectional study. BMC Public Health. (2014) 14:365. doi: 10.1186/1471-2458-14-365
34. Charles, J, Exavery, A, Ally, A, Mseya, R, Mbwambo, T, Barankena, A, et al. Rates and determinants of retention on ART among orphans and vulnerable children living with HIV in Tanzania. Front Public Health. (2022) 10:934412. doi: 10.3389/fpubh.2022.934412
35. Schlatter, AF, Deathe, AR, and Vreeman, RC. The need for pediatric formulations to treat children with HIV. AIDS Res Treatment. (2016) 2016:1–8. doi: 10.1155/2016/1654938
36. Lee, C, Sapasap, J, LaRochelle, J, Smith, RO, and Badowski, ME. Antiretroviral therapy in children and adolescents: a look into modern single tablet regimens. J Pediatr Pharmacol Ther. (2021) 26:783–94. doi: 10.5863/1551-6776-26.8.783
37. Hackett, S, Teasdale, CA, Pals, S, Muttiti, A, Mogashoa, M, Chang, J, et al. Drug resistance mutations among south African children living with HIV on WHO-recommended ART regimens. Clin Infect Dis. (2021) 73:e2217–25. doi: 10.1093/cid/ciaa1068
38. Obasa, AE, Ambikan, AT, Gupta, S, Neogi, U, and Jacobs, GB. Increased acquired protease inhibitor drug resistance mutations in minor HIV-1 quasispecies from infected patients suspected of failing on national second-line therapy in South Africa. BMC Infect Dis. (2021) 21:214. doi: 10.1186/s12879-021-05905-2
39. Isaacs, D, Mikasi, SG, Obasa, AE, Ikomey, GM, Shityakov, S, Cloete, R, et al. Structural comparison of diverse HIV-1 subtypes using molecular modelling and docking analyses of integrase inhibitors. Viruses. (2020) 12:936. doi: 10.3390/v12090936
40. Patel, A, Pundkar, A, Agarwal, A, Gadkari, C, Nagpal, AK, and Kuttan, N. A comprehensive review of HIV-associated tuberculosis: clinical challenges and advances in management. Cureus. (2024) 16:e68784. doi: 10.7759/cureus.68784
41. Frigati, LJ, Ameyan, W, Cotton, MF, Gregson, CL, Hoare, J, Jao, J, et al. Chronic comorbidities in children and adolescents with perinatally acquired HIV infection in sub-Saharan Africa in the era of antiretroviral therapy. Lancet Child Adolescent Health. (2020) 4:688–98. doi: 10.1016/S2352-4642(20)30037-7
42. The Lancet HIV. Lenacapavir licenses will not deliver on all opportunities. Lancet HIV. (2024) 11:e717. doi: 10.1016/S2352-3018(24)00273-X
43. Wu, L, Kaftan, D, Wittenauer, R, Arrouzet, C, Patel, N, Saravis, AL, et al. Health impact, budget impact, and price threshold for cost-effectiveness of lenacapavir for HIV pre-exposure prophylaxis in eastern and southern Africa: a modelling analysis. Lancet HIV. (2024) 11:e765–73. doi: 10.1016/S2352-3018(24)00239-X
44. Yamey, G, and Machingaidze, S. Lenacapavir: a giant step forward in HIV prevention—but a missed opportunity for achieving equity and access. BMJ. (2024) 387:q2254. doi: 10.1136/bmj.q2254
45. Sheppard, DC, Goswami, R, Berendam, SJ, Li, SH, Nelson, AN, De Paris, K, et al. Harnessing early life immunity to develop a pediatric HIV vaccine that can protect through adolescence. PLoS Pathog. (2020) 16:e1008983. doi: 10.1371/journal.ppat.1008983
46. Fouda, GG, Cunningham, CK, and Permar, SR. Infant HIV-1 Vaccines. JAMA. (2015) 313:1513–4. doi: 10.1001/jama.2015.1382
47. Cohen, J. Children are left behind in Congo’s mpox vaccination drive. Science. (2024) 386:954. doi: 10.1126/science.adu8279
48. Kulohoma, BW, Marriage, F, Vasieva, O, Mankhambo, L, Nguyen, K, Molyneux, ME, et al. Peripheral blood RNA gene expression in children with pneumococcal meningitis: a prospective case-control study. BMJ Paediatr Open. (2017) 1:e000092. doi: 10.1136/bmjpo-2017-000092
49. Mwaura, HM, Kamanu, TK, and Kulohoma, BW. Bridging data gaps: predicting sub-national maternal mortality rates in Kenyan using machine learning models. Cureus. (2024) 16:e72476. doi: 10.7759/cureus.72476
50. Mwaura, HM, Kamanu, TK, and Kulohoma, BW. Sub-National Disparities in indicators of maternal mortality in Kenya: insights from demographic health surveys towards attaining SDG 3. J Women's Health Dev. (2024) 7:29–40. doi: 10.26502/fjwhd.2644-288400118
51. Ifeanyi Obeagu, E, and Uzoma Obeagu, G. Strengthening laboratory systems for ensuring accurate diagnoses in mother-to-child transmission (MTCT) prevention programs in Uganda: a narrative review. Ann Med Surg. (2024) 86:5256–65. doi: 10.1097/MS9.0000000000002154
52. Wexler, A., Kates, J., Oum, S., and Lief, E., Donor government funding for HIV in low- and middle-income countries in 2023. Global Health Policy. (2024). Available online at: https://www.kff.org/global-health-policy/report/donor-government-funding-for-hiv-in-low-and-middle-income-countries-in-2023/ (Accessed January 20, 2025).
53. Mugabe, JO, Kulohoma, BW, Matoke-Muhia, D, Ubalijoro, E, Fagbamigbe, FA, Mwaura, G, et al. Securing Africa’s health sovereignty: why investing in science and innovation matters. AAS Open Res. (2020) 3:52. doi: 10.21955/aasopenres.1115135.1
54. Daw, MA, El-Bouzedi, AH, and Ahmed, MO. The impact of armed conflict on the prevalence and transmission dynamics of HIV infection in Libya. Front Public Health. (2022) 10:779778. doi: 10.3389/fpubh.2022.779778
Keywords: children, HIV, 95–95–95 HIV/AIDS goals, challenges, Africa
Citation: Kulohoma BW and Wesonga CSA (2025) Considerable variation in the 95-95-95 targets accomplishment between children and adults might delay achievement of set targets. Front. Public Health. 13:1565242. doi: 10.3389/fpubh.2025.1565242
Edited by:
Raphael Zozimus Sangeda, Muhimbili University of Health and Allied Sciences, TanzaniaReviewed by:
Adetayo Emmanuel Obasa, Stellenbosch University, South AfricaCopyright © 2025 Kulohoma and Wesonga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Colette S. A. Wesonga, Y29idW5nYUB5YWhvby5jb20=; Benard W. Kulohoma, a3Vsb2hvbWFAZ21haWwuY29t
 Benard W. Kulohoma
Benard W. Kulohoma Colette S. A. Wesonga2*
Colette S. A. Wesonga2*