- 1Center for Medical Data Science, Medical University of Vienna, Vienna, Austria
- 2Austrian Social Health Insurance Fund, Eisenstadt, Austria
- 3Clinic of Thoracic Surgery, Medical University of Vienna, Vienna, Austria
- 4Laboratory for Cardiac and Thoracic Diagnosis, Regeneration and Applied Immunology, Vienna, Austria
- 5Department of Oral and Maxillofacial Surgery, Medical University of Vienna, Vienna, Austria
- 6Clinic of Dermatology, Medical University of Vienna, Vienna, Austria
- 7Department of Internal Medicine 3, University Hospital St. Poelten, St. Poelten, Austria
- 8Department of Internal Medicine I with Cardiology and Intensive Care, St. Josef Hospital Braunau, Braunau am Inn, Austria
- 9Department of Nephrology, Hospital St. Georg Leipzig, Leipzig, Germany
Introduction: Limited data are available on long-term morbidity and mortality after hospitalization for coronavirus disease 2019 (COVID-19). In this registry-based study, we investigated long-term mortality and morbidity following hospitalization for COVID-19 and examined associations with baseline medication load.
Methods: Data were provided by the Austrian Health Insurance Funds on hospitalized COVID-19 patients in 2020 and matched controls. The primary outcome was mortality. Secondary outcomes included mortality conditional on survival of initial COVID-19 hospitalization and re-hospitalization.
Results: The median follow-up was 600 days. A total of 22,571 patients aged >18 were hospitalized in Austria in 2020 due to COVID-19. The risk of mortality was significantly higher with polypharmacy. With the exception of the youngest age group (19–40 years), patients receiving antiepileptics, antipsychotics, or iron supplements, erythropoiesis-stimulating agents, vitamin B12, or folic acid in the year before hospitalization were significantly associated with a higher risk of mortality (all p < 0.001). For patients with prescribed non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory drugs, significantly increased survival was observed (all p-values <0.001). Patients had a higher medication load than the control population. Long-term mortality and the risk of re-hospitalization for any reason were also significantly higher among patients.
Discussion: Antipsychotics and antidepressants appear to be underrecognized in identifying patients at risk for severe outcomes after hospitalization for COVID-19.
1 Introduction
The outbreak of Coronavirus Disease 2019 (COVID-19) marked the beginning of a global pandemic and has been associated with substantial morbidity and mortality. Age and sex are well-established risk factors for severe outcomes (1–5). Numerous reports have discussed how the presence of comorbidities may increase the risk of COVID-19-related deaths (1, 6–9). In addition, an increased risk of readmission and mortality after hospital discharge has been observed up to 1 year after hospitalization for COVID-19 (10–13). Patients who require hospitalization for COVID-19 have a greater comorbidity burden and are expected to have worse short-term outcomes (12). Differences in the prevalence of drug use and polypharmacy regimens have been observed when compared to the general population (14). Studies have investigated the potential associations between polypharmacy and increased morbidity and mortality among patients with COVID-19 (15, 16). Visser et al. (17) investigated the impact of polypharmacy on COVID-19-related mortality in nursing home residents and found a significant positive association between the total number of medications and 30-day COVID-19-related adjusted mortality. However, published studies focusing on the drug profiles of hospitalized COVID-19 patients and long-term outcomes are still scarce.
In this study, we present data on hospitalized patients with COVID-19 and a matched control group provided by the Austrian Health Insurance Funds, with a median follow-up of 600 days (and a maximum follow-up of 880 days). The primary aim of this study was to analyze the long-term follow-up of patients hospitalized due to COVID-19 in Austria in 2020 to re-evaluate the association between baseline medication load before hospitalization and mortality or readmission after COVID-19 hospitalization, to go beyond the classical risk factors, and to understand how to better identify and phenotype vulnerable populations of respiratory viral pathogens. We also compared the characteristics and outcomes of the hospitalized patients with an age-, sex-, and region-matched control group, presenting a real-world picture.
2 Methods
2.1 Study design and cohorts
This original research is based on a retrospective, national registry-based study that complied with the Declaration of Helsinki and was approved by the Ethics Committee of Lower Austria (GS1-EK-4/747–2021). Data on the patient and control cohorts were obtained from the Austrian Health Insurance Funds. Approximately 98% of the Austrian population is registered in the public health insurance system. Austria’s healthcare system is national, with broad access to care, is regulated by social insurance law, and is primarily financed by social insurance contributions.
Patients aged >18 years who were hospitalized in Austria with a primary diagnosis of COVID-19 (ICD-10 codes U071, U072, U049) between 1 January 2020 and 31 December 2020 were included in this study. For all patients, age, sex, region, and medication were obtained 1 year before hospitalization until the study ended.
An age-, sex-, and region-matched control group (approximately 10 controls for each patient) consisted of individuals who were not hospitalized due to COVID-19 in the year 2020 and were randomly selected from the population registered with the Austrian Health Insurance Fund, representing the Austrian population. Data on the control group were available from 1 year before the first patient was hospitalized until the study ended.
2.2 Study outcomes
The primary outcome was all-cause mortality. The secondary outcome was hospitalization for any reason. For patients, hospitalization was defined as the first readmission after the index COVID-19 hospitalization. For controls, hospitalization was defined as the first hospital admission after the index COVID-19 hospitalization of the matched patient (Supplementary Table S1). For controls, time to death was evaluated from the index COVID-19 hospital admission date of the matched patient.
2.3 Definitions and statistical analyses
For each patient, age, sex, region, and Anatomical Therapeutic Chemical (ATC) Classification Codes describing prescribed medications were available from the Austrian Health Insurance Fund. All analyses were performed separately for four age subgroups: 19–40 years, 41–64 years, 65–74 years, and ≥75 years (Supplementary Table S2). ATC codes available 1 year before hospitalization due to COVID-19 were summarized in the medication groups (Supplementary Table S3). Twenty medication groups were used for statistical modeling (MG1 to MG20, see Supplementary Table S3). For controls, a similar medication profile was generated using the drugs prescribed 1 year before the index COVID-19 hospitalization of the matched patient. These 20 medication groups were also used to define polypharmacy. COVID-19 therapies during hospitalization were not included in the definitions. Since the ATC codes were accurately documented in the Austrian Health System, the medication profile was used to describe the health status of a patient or control.
Numbers and percentages were used to summarize categorical variables, while medians and interquartile ranges were used for continuous variables.
To evaluate the associations between polypharmacy and all-cause death, simple Cox regression models were calculated while adjusting for sex, age, half-year, and polypharmacy, with region as a clustering variable. Polypharmacy was defined as the number of medication groups in which a patient received prescribed medication and was categorized into four groups (0–1, 2–5, 6–10, and >11). To evaluate the association between polypharmacy and re-hospitalization due to any reason, competing risk models (with competing risk of death) were calculated, accounting for sex, age, half-year, and polypharmacy with region as a clustering variable. Furthermore, to evaluate the association between several medication groups and all-cause death, a Cox regression model was calculated, accounting for sex, age, half-year, polypharmacy, and the 20 medication groups, with region as a clustering variable. For hospitalization, a competing risk model was calculated using the same covariables, as described in the model for all-cause mortality.
Due to underrepresentation, only 20 of the 32 medication groups were evaluated for the statistical models.
As patients hospitalized due to COVID-19 potentially had more serious comorbidities compared to the general Austrian population, we attempted to account for this imbalance by performing propensity score matching (PSM) for age, sex, region, and medication groups MG1 to MG20. To evaluate the difference between patients and controls in the risk of all-cause death, a Cox regression model was calculated, adjusting for group, sex, age, half-year, polypharmacy, and the 20 medication groups, with region as a clustering variable. For hospitalization due to any reason, a competing risk model was calculated using the same covariables, as described in the model for all-cause death.
Schönfeld residuals were used to evaluate the proportional hazard assumption, and variance inflation factors were used to evaluate multicollinearity. For all models, hazard ratios (HRs) and confidence intervals (CIs) are provided. Due to the large sample size and the large number of covariables investigated (i.e., 25), a p-value <0.002 (=0.05/25 applying conservative Bonferroni correction) was considered significant. All analyses were performed using R software, release 4.2.2 (18). A detailed statistical analysis statement, including definitions, can be found in the supplemental material, Section 3.
3 Results
3.1 Characteristics of the patient and control cohorts
The study population included 22,571 COVID-19-hospitalized patients (Table 1 and Figure 1A). The median follow-up time varied from 594 to 615 days across the four age groups. The age-, sex-, and region-matched control groups included 217,295 controls (Table 1).
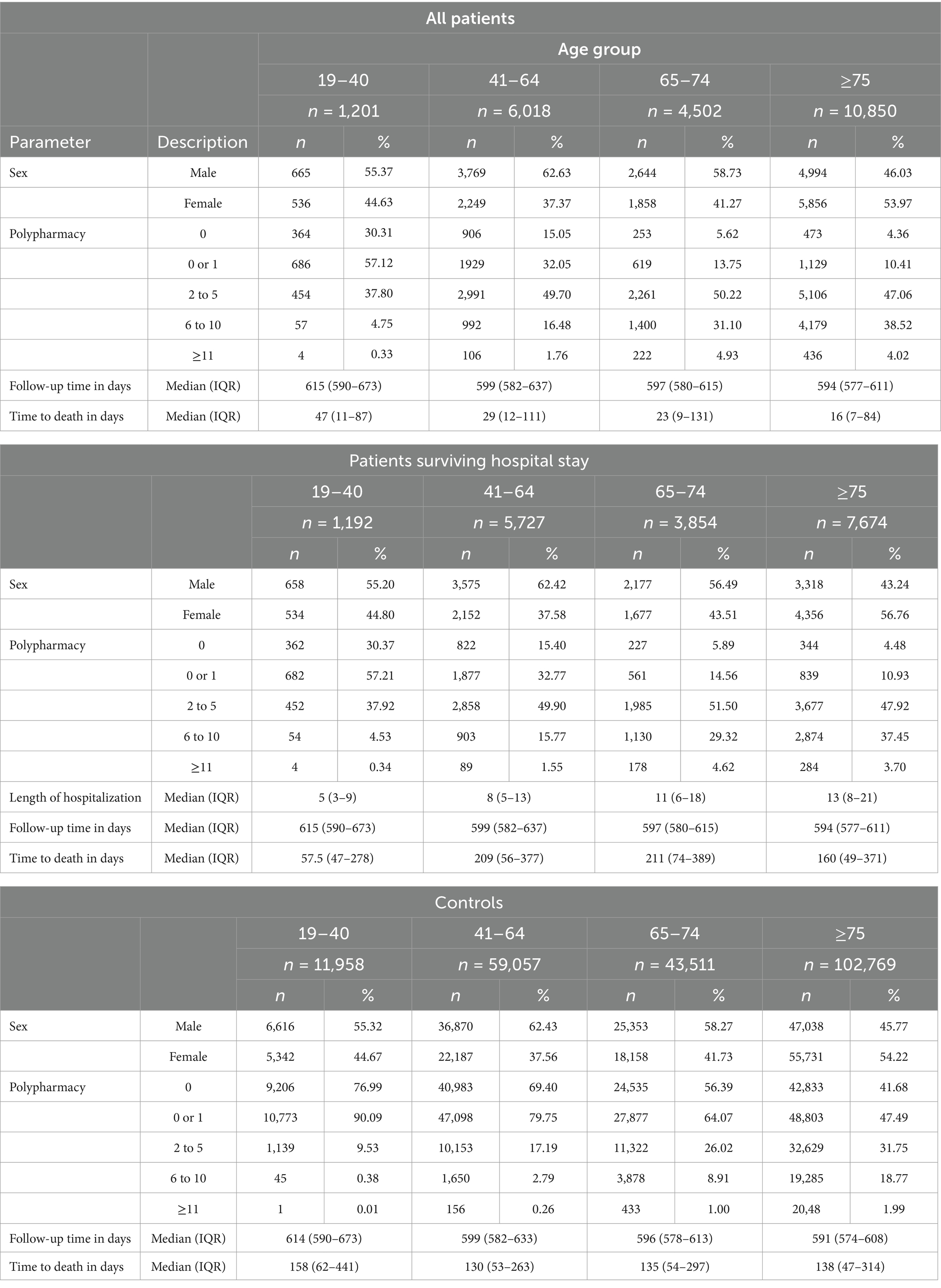
Table 1. Demographic data for hospitalized patients with COVID-19 in 2020 in Austria and corresponding age-, sex-, and region-matched controls.
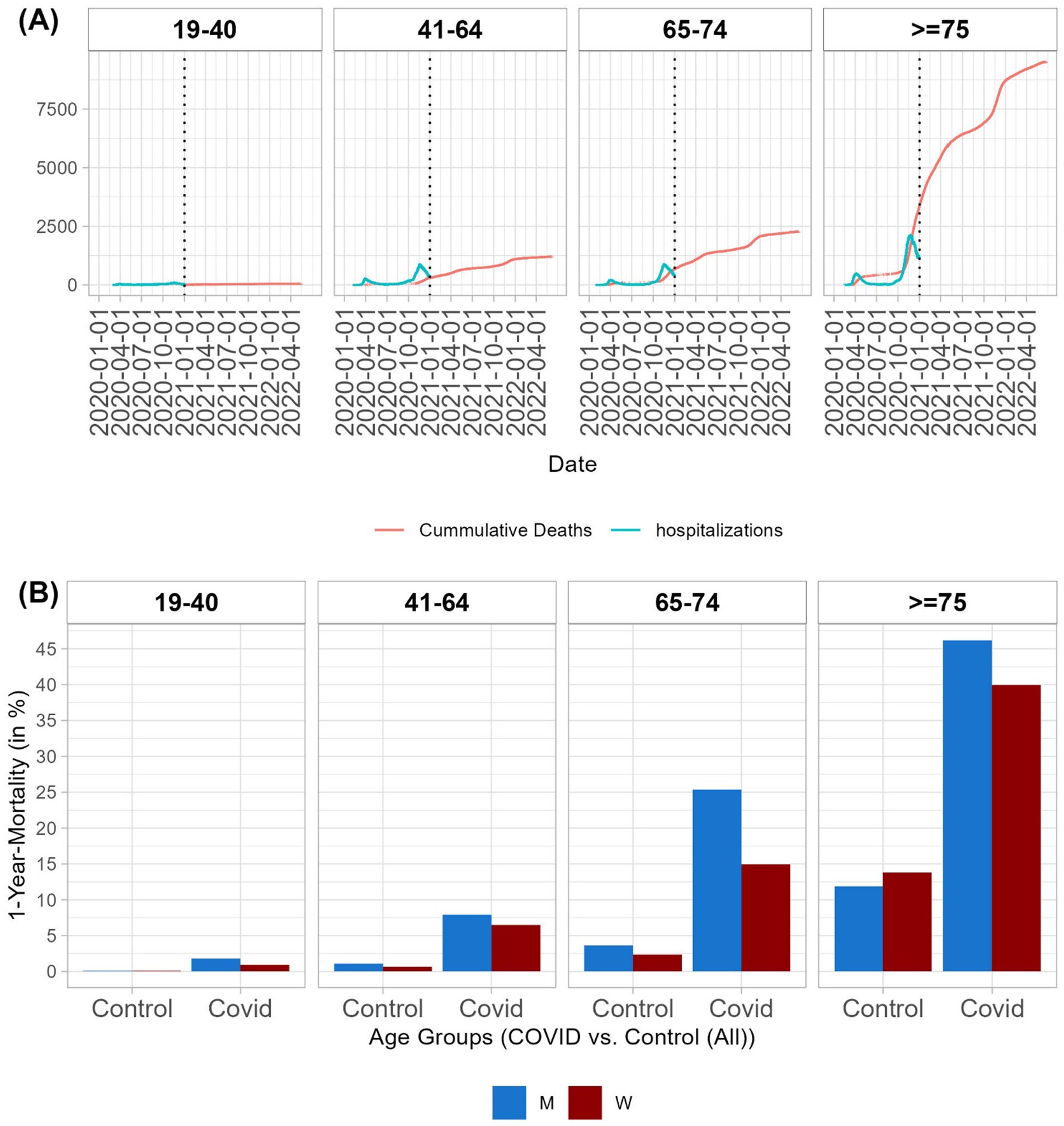
Figure 1. Mortality by age group. (A) Timeline of hospitalizations due to COVID-19 in 2020 (hospitalizations per day in blue) and timeline of deaths (cumulative number of deaths in red) of hospitalized patients with COVID-19. (B) One-year mortality rates by age group and sex for hospitalized patients due to COVID-19 and controls. For details on mortality rates for patients and controls, along with patients who survived the hospitalization with and without propensity score matching, see Supplementary Tables S8,S9.
Polypharmacy involving more than six medication groups was observed more frequently in the older age groups (65–74 years and ≥75 years). Among these older age groups, 5.62% of patients aged 65–74 years and 4.36% of patients aged ≥75 years, and, among controls, 56.39% of patients aged 65–74 years and 41.68% of patients aged ≥75 years did not receive any medication from the investigated medication groups (Table 1). It should be noted that polypharmacy may be interpreted as an indicator for patients with several underlying diseases and thus potentially identifies risk groups. Patients hospitalized due to COVID-19 are expected to have more comorbidities and, consequently, a higher medication burden than the general population. This pattern was also observed in our Austrian population. Patients received more medications in the year before hospitalization in all investigated medication groups (Table 2 and Supplementary Tables S4–S7) across all age groups compared to controls.
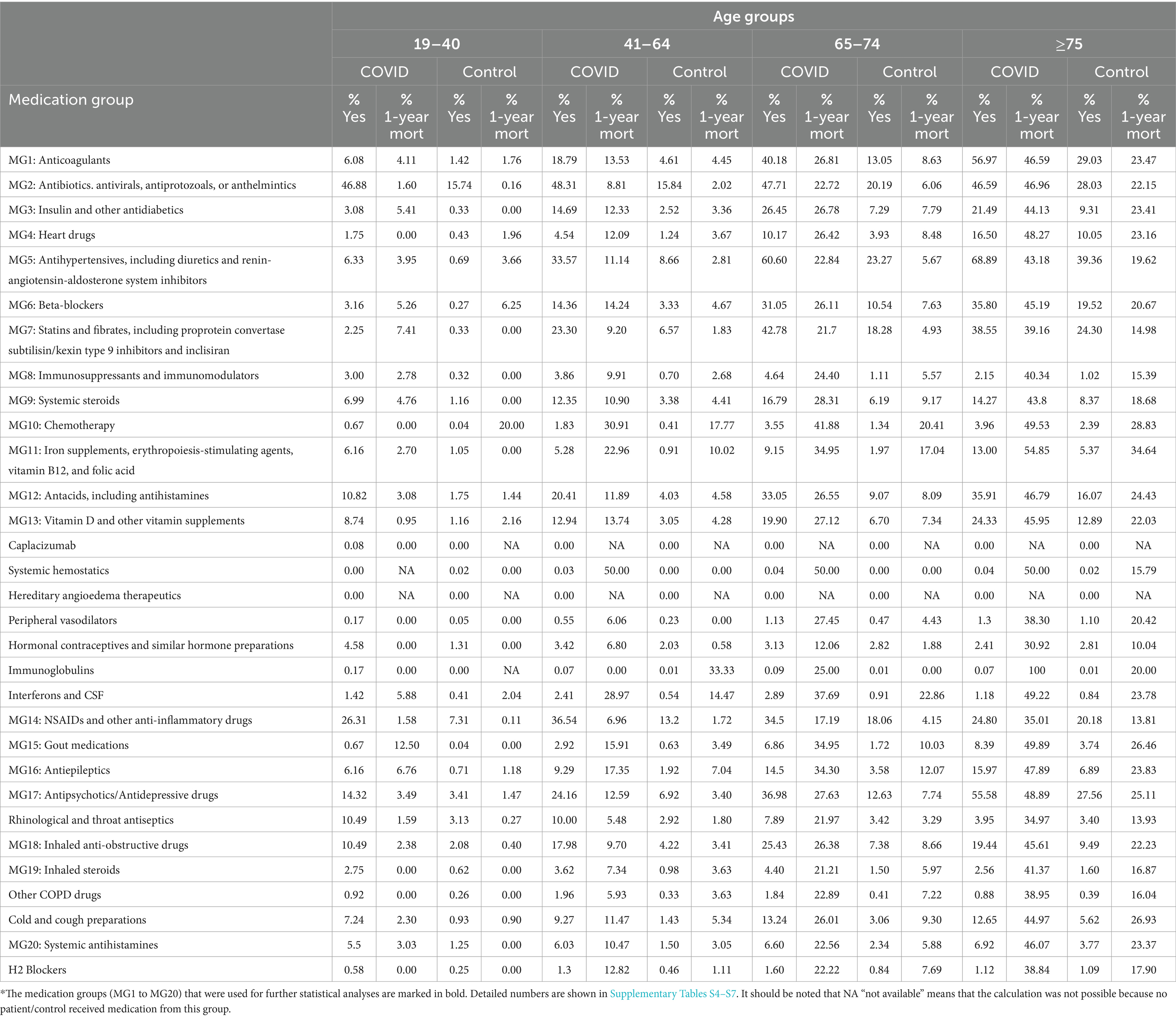
Table 2. Percentages of patients hospitalized with COVID-19 and controls who received at least one medication from the investigated medication group 1 year before COVID-19 hospitalization and the corresponding 1-year mortality rates.
Interestingly, we observed that 24.2% of patients aged 41–64 years, 37% of patients aged 65–74 years, and 55.6% of patients aged ≥75 years received antipsychotic/antidepressant drugs before COVID-19 hospitalization. In the control group, the rate of prescribed antipsychotic/antidepressant drugs was 6.9% for patients aged 41–64 years, 12.6% for patients aged 65–74 years, and 27.6% for patients aged ≥75 years.
3.2 All-cause mortality for COVID-19 patients
In the 19–40 age group, 1.05% of men and 0.37% of women died in the hospital. One-year mortality rates in this age group were 1.8% for men and 0.93% for women. In older age groups, higher mortality rates were observed. In the 41–64 age group, 5.15% of men and 4.31% of women died during hospitalization. The one-year mortality rate in this age group was 7.91% for men and 6.49% for women. In the 65–74 age group, 17.66% of men and 9.97% of women died during the hospitalization. Within 1 year of hospital admission, 25.38% of men and 14.96% of women died. Furthermore, 33.56% of men and 25.61% of women aged ≥75 years died during the hospitalization. The one-year mortality rate in this age group was 46.2% for men and 39.94% for women (Supplementary Table S8).
In all age groups, we found a trend of a higher risk of death for men than women, which was significant in the older age groups (age >65 years) after multiplicity correction (Supplementary Table S10). The number of hospital admissions per day and the cumulative number of deaths are shown in Figure 1A. The one-year mortality rates are presented in Figure 1B.
A significantly higher risk of all-cause death was evaluated for patients with a larger number of prescribed medication groups compared to patients receiving drugs from none or only one of the investigated medication groups. No significant relationship was observed for patients aged 19–41 years (Figures 2A–D and Supplementary Figure S1).
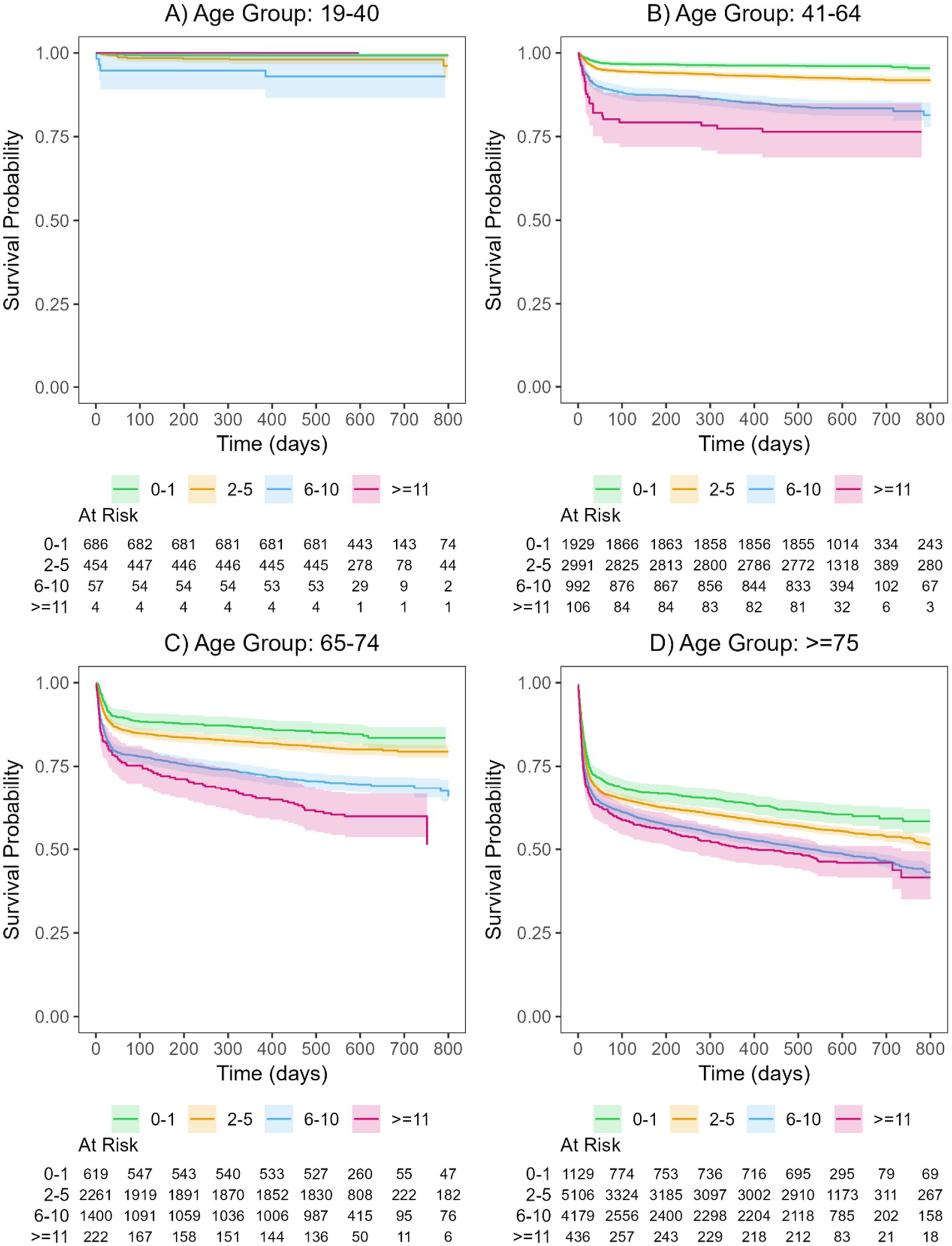
Figure 2. Kaplan-Meier curves and their corresponding 95% confidence intervals for the polypharmacy groups of patients hospitalized with COVID-19 for the four age groups (A: 19-40, B: 41-64, C: 65-74, D: >75). For details on hazard ratios, confidence intervals, and p-values see Supplementary Table S10. The curves are shown separately for patients with drugs in 0-1 medication group (green), 2-5 medication groups (orange), 6-10 medication groups (blue), and >=11 medication groups (magenta).
Due to the large number of evaluated medication groups and their potential interactions, polypharmacy may represent only one factor in the complex system used to evaluate the associations between medication load and underlying diseases with all-cause death after a hospitalization with COVID-19. Therefore, we additionally included all 20 medication groups in the Cox regression models. Because of the small number of events in the youngest age group (19–40 years), not all 20 medication groups could be included in the model for this age group.
Figures 3AD summarizes the results of the Cox regression model of all-cause mortality when including the 20 medication groups (Supplementary Table S11). In the youngest group (19–40 years), only prescribed vitamin D supplements and other vitamins (p < 0.001) and systemic antihistamines (p = 0.002) were significantly associated with survival. For patients receiving vitamin supplements in the year before hospitalization due to COVID-19, we observed a significantly lower risk of death. In addition, systemic antihistamines were significantly associated with poor survival. The other medication groups did not show significant results. However, due to the small number of events in this age group, these results should be interpreted with caution.
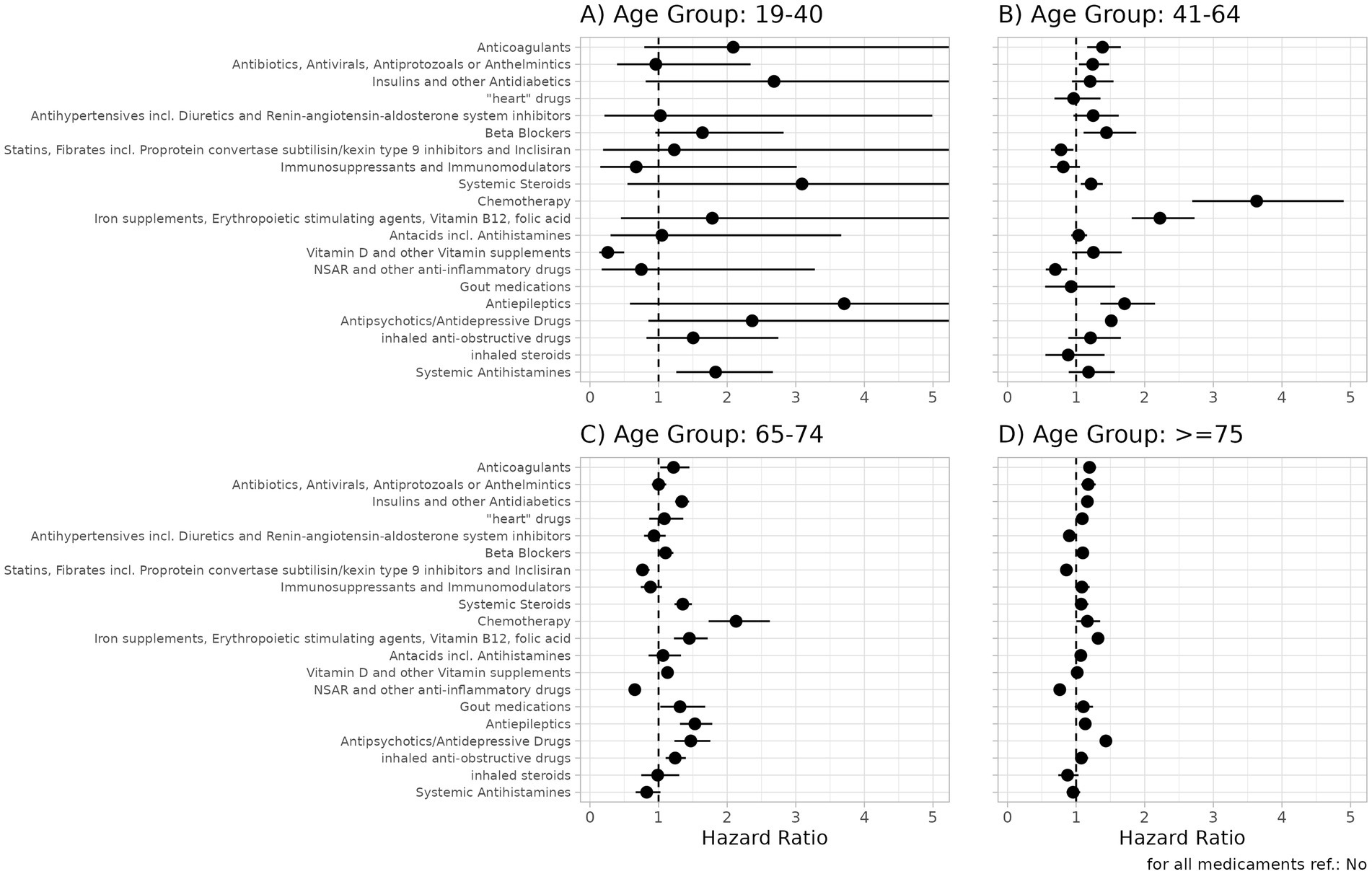
Figure 3. Hazard ratios and 95% confidence intervals for medication groups for the outcome all-cause-mortality in patients hospitalized with COVID-19 for the four age groups (A: 19-40, B: 41-64, C: 65-74, D: >75). For details, see Supplementary Table S11. A hazard ratio >1 indicates a higher risk of all-cause death for patients receiving a drug in the corresponding medication group.
For several medication groups, the results were significantly different between the age groups. Anticoagulants (p < 0.001 for 41–64 years and ≥75 years); antibiotics, antivirals, antiprotozoals, or anthelmintics (p < 0.001 for ≥75 years); insulin and other antidiabetics (p < 0.001 for age groups ≥65 years); heart drugs (p = 0.001 for ≥75 years); beta-blockers (p < 0.001 for ≥75 years); systemic steroids (p < 0.001 for 65–74 years); chemotherapy (p < 0.001 for 41–74 years); antacids (p < 0.001 for ≥75 years); vitamin D or other vitamin supplements (p = 0.001 for 64–74 years); and inhaled anti-obstructive drugs (p < 0.001 for 64–74 years) were significantly associated with poor survival. Statins and fibrates were significantly associated with a lower risk of death in patients aged >64 years.
In the three age groups >40 years, a significant association was observed between prescribed antiepileptics (all p < 0.001), antipsychotics/antidepressants (all p < 0.001), and iron supplements, erythropoiesis-stimulating agents (ESAs), vitamin B12 (B12), and folic acid (FA) (all p < 0.001) and a higher risk of all-cause death. For patients receiving non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory drugs in the year before hospitalization due to COVID-19, a significantly larger probability for survival was observed (all age groups>40: p < 0.001). Figures 4AF presents the Kaplan–Meier curves for key prescribed medication groups associated with poor survival, while Supplementary Figure S6 shows the Kaplan–Meier curves for the medication groups associated with poor survival in the control cohort.
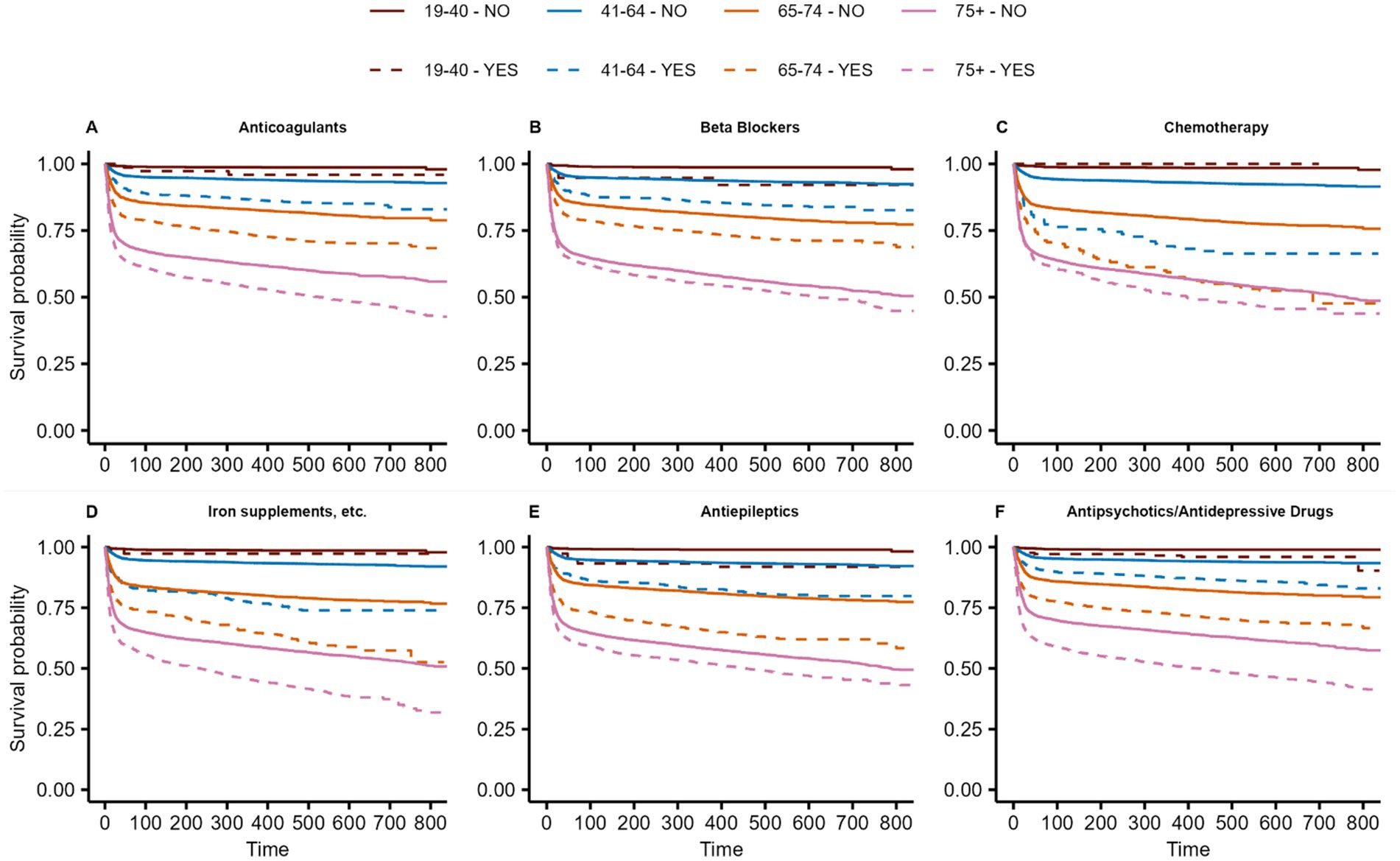
Figure 4. Kaplan-Meier curves of Anticoagulants (A), Beta Blockers (B), Chemotherapy (C), Iron supplements, etc. (D), Antiepileptics (E) and Antipsychotics/Antidepressive Drugs associated with poor survival among patients hospitalized with COVID-19. Curves are given for the 4 age groups (brown: 19–40, blue: 41–64, orange: 65–74, magenta: >75) as well for patients with (dashed lines) and without (solid line) the prescribed medication groups. For details on hazard ratios, confidence intervals, and p-values, see Supplementary Table S11.
In models that included all investigated medication groups, the polypharmacy variable was not significant, indicating that polypharmacy alone may not be a good predictor of all-cause death. Specific medication groups representing patients with certain underlying diseases may be more critical factors in determining outcomes for hospitalized patients with COVID-19.
3.3 All-cause death after COVID-19 hospital survival
Among patients who survived a hospitalization for COVID-19, 0.76% of men and 0.56% of women aged 19–40 years died within the first year after hospital discharge. The 1-year mortality rates were 2.91 and 2.28% for men and women aged 41–64 years, 9.37 and 5.78% for men and women aged 65–74 years, and 19.02 and 19.26% for men and women aged ≥75 years, respectively (Supplementary Table S9).
In the age groups >40 years, we found significant associations with a higher risk of death after surviving a hospitalization for COVID-19 among patients receiving chemotherapy (all p-values <0.001) and among those receiving iron supplements, erythropoiesis-stimulating agents, B12, and FA (all p-values <0,001). For patients receiving antiepileptics, a significantly higher risk of all-cause death was found in the 41–64 and 65–74 age groups, with an observed trend in the oldest age group (p = 0.016). Moreover, patients receiving antipsychotics/antidepressants in the year before hospitalization were significantly associated with poor survival in all age groups >40 years (all p-values <0.001).
For NSAIDs and other anti-inflammatory drugs, a significant association with a lower risk of all-cause death after hospital survival was observed in the age group >40 years (p-value of <0.001) and for statins and fibrates in the two age groups >65 years (both p-values <0.001). Detailed results are shown in Supplementary Table S12 and Supplementary Figure S2.
3.4 Re-hospitalization after COVID-19 hospital survival
In the age groups >40 years, a significantly higher risk of readmission was observed due to any cause for patients receiving anticoagulants (all p < 0.001), antiepileptics (all p < 0.001), systemic steroids (all p < 0.001), and chemotherapy (all p < 0.008).
We observed a trend toward a higher risk of re-hospitalization for patients receiving antipsychotics/antidepressants (p < 0.003 for 19–64 years and ≥75 years; p = 0.006 for 65–74 years). Detailed results are shown in Supplementary Table S13 and Supplementary Figure S3.
3.5 Comparison of outcomes between COVID-19 patients and controls
The age-, sex-, and region-matched control population received fewer medications than the patient population before hospitalization. Interestingly, in controls without the observed medication groups, remarkably good survival was observed even in the older age groups (Supplementary Figures S4,S5), whereas a steep decrease in survival after hospital admission was observed in patients with COVID-19 (Supplementary Figure S1).
The difference in all-cause mortality between hospitalized patients with COVID-19 and the Austrian control population could not be evaluated in the 19–40 age group due to the small number of events.
For all other subgroups, a significant difference in the risk of all-cause mortality was found between COVID-19-hospitalized patients and PSM controls (all p < 0.001). For the subgroup of patients surviving the COVID-19 hospitalization, reduced hazard ratios were observed compared to the PSM controls. The difference between patients and controls remained significant in the age groups 41–64 years (p < 0.001) and 65–74 years (p < 0.001), but not in the oldest age group (p = 0.078). The Kaplan–Meier curves for propensity score-matched COVID-19 patients and controls are shown in Supplementary Figure S7.
Regarding hospitalization for any reason, in all four age groups, a significantly higher probability of re-hospitalization was observed among COVID-19-hospitalized patients compared to controls (all p < 0.001). These results suggest that polypharmacy may not completely explain the adverse outcomes in patients with severe COVID-19 (Supplementary Table S14 and Supplementary Figure S3).
4 Discussion
In this retrospective study, we evaluated whether baseline medication profiles, which may be interpreted as an indicator for underlying diseases, are associated with survival or hospitalization for any reason after a COVID-19-related hospitalization in an Austrian population. Hospitalized patients with a diagnosis of COVID-19 had a higher medication load before hospitalization and increased long-term mortality, especially patients aged >75 years. Pre-COVID-19 prescriptions of antipsychotic/antidepressant drugs, antiepileptic drugs, chemotherapy, iron/FA/B12, beta-blockers, and anticoagulants were significantly associated with increased mortality, whereas patients who had been prescribed NSAIDs and other anti-inflammatory drugs before hospitalization due to COVID-19 had a significantly lower risk of all-cause death. Due to our study design, we were able to present the “real world” prescription and mortality rate of patients hospitalized with the diagnosis of COVID-19 in Austria, and in a matched control population that was followed from 2020 for up to a maximum of 880 days.
Polypharmacy frequently occurs in patients with COVID-19 and may be associated with increased mortality (15, 16). Analyzing the prescription data of the control population, we detected no drug prescription of investigated medications in 77% (19–40 years), 69% (41–64 years), 56% (65–74 years), and 41% (≥75 years) of controls. In contrast, 30% (19–40 years), 15% (41–64 years), 6% (65–74 years), and 4% (≥75 years) of COVID-19-hospitalized patients had no drug prescriptions.
We found that patients who had been prescribed antipsychotic/antidepressant drugs 1 year before hospitalization had a significantly increased risk of death. Among patients aged ≥75 years in the medication group, the estimated mortality for patients with prescribed antipsychotic/antidepressant drugs was 56.3% (CI: 54.7–57.9) within a 2-year follow-up period as compared to 40.83% (CI: 42.5–39.1) for patients not receiving antipsychotic/antidepressant drugs. Among patients aged ≥75 years in the control group, the mortality rate with prescribed antipsychotic/antidepressant drugs was 32.9% (CI: 32.1–33.7) within a 2-year follow-up as compared to patients not receiving these drugs (11.4%, CI: 11.7–11.1).
The association between anti-inflammatory medications, such as NSAIDs, and lower mortality could be attributed to the pathophysiology of COVID-19, which involves a high pro-inflammatory state in the second phase of the disease, also referred to as the cytokine storm phase (19, 20), which could be attributed to anti-inflammatory medications. In patients with a high pro-inflammatory state in need of oxygen therapy and SARS-CoV-2-associated lung infiltration, anti-inflammatory therapy (e.g., dexamethasone) was the only pharmacological intervention to reduce mortality (21). However, we cannot exclude the possibility of indication bias with patients using NSAIDs regularly potentially being healthier, since patients with more comorbidities (e.g., chronic kidney disease, diabetes, cardiovascular disease, or heart failure) are regularly advised to avoid NSAIDs.
The pronounced association of antipsychotic medication with higher mortality is likely related to the comorbidities present in a population with high prescription rates of such medication, especially at older ages. Antipsychotic/antidepressant drugs are associated with severe COVID-19 morbidity and mortality (16). Antipsychotic medication is primarily prescribed to patients in residential or nursing homes with dementia or behavioral disorders (22, 23). The number of antipsychotic prescriptions is higher in nursing homes (57.1%) than in residential homes (29.5%) (24), emphasizing more frequent prescriptions in a more morbid population. Therefore, chronic use of antipsychotic medications at older ages is most likely indicative of patients with severe impairments (25–27). Antipsychotics are also associated with impaired swallowing, drug-induced dysphagia, and choking, which can lead to detrimental outcomes (28).
Among nursing home residents, a significant positive association was observed between the total number of medications and 30-day, COVID-19-related, adjusted mortality (17). After additional adjustment for dementia and the use of proton pump inhibitors (PPI), vitamin D, antipsychotics/antidepressants, and antithrombotics, this effect was no longer significant, suggesting that polypharmacy itself may not be the issue; rather, the type of medication may be. In our analyses, polypharmacy did not remain significant after correcting for several medication groups.
In patients who were discharged alive after a COVID-19-related hospitalization, the risk of post-discharge death within 180 days among patients aged >64 years was nearly twice as high as that observed in historical controls admitted to the hospital with influenza (12). Furthermore, crude differences in drug use between COVID-19 patients and the general population were identified, particularly in the use of antithrombotic agents, antiepileptics, anti-gout preparations, and cardiac therapy (14).
Scant data are available on clinical outcomes in patients discharged alive from COVID-19 hospitalization. Data from a large study with patients discharged after COVID-19 reported an increased risk of readmission and mortality during a follow-up of 140 days (10). In a German cohort of hospitalized COVID-19 patients, 30-day all-cause mortality was 23.9%, and 180-day all-cause mortality was 29.6%. Another study, after COVID-19 hospitalization of patients in Italy, reported an 8% age-related overall relative increase in all-cause death after 6 months of follow-up (13). Another report investigated the 12-month mortality following recovery from an initial episode of COVID-19 and found a significantly higher adjusted all-cause mortality risk over a 12-month period in patients with severe COVID-19 compared to both COVID-19-negative patients and mild COVID-19 patients. A large study indicated that the overall 2-year mortality risk was worse by day 180 among those infected with COVID-19 compared to matched uninfected comparators (29). In our study, we observed increased long-term mortality and an increased risk of hospitalization for any reason following surviving a COVID-19 hospitalization. Moreover, mortality was more pronounced within the first 50 days after index hospitalization.
The main strength of this study is its use of a large, representative, real-world national database. The retrospective design, however, is a limitation, which we sought to mitigate by including several potential confounding factors in the statistical models and performing propensity score matching to support meaningful comparisons. However, despite the large sample size and long-term follow-up, unmeasured confounding and bias are still possible. The study population was drawn exclusively from the Austrian Health Insurance Fund in 2020, raising potential concerns about the generalizability of these findings to broader patient populations or to later COVID-19 variants. The information used in this study is based on billing records and ATC codes that describe medication load, representing certain underlying comorbidities. However, the contribution of the medication itself or a combination of medications cannot be completely ruled out. Furthermore, we rely on accurate coding across the country, which we cannot verify or correct retrospectively. No information was available from the Austrian Insurance Fund on causes of death or use of intensive care in hospitals.
In conclusion, this large cohort of hospitalized patients with COVID-19 showed an increased short-and long-term risk of mortality. Patients had a higher medication load (polypharmacy). Antipsychotics/antidepressants were significantly associated with poor survival in patients aged >40 years. Our findings may help identify the most vulnerable patients at higher risk of mortality after a COVID-19-related hospital discharge, regardless of age, by screening the prescribed medication groups, with implications for preventive measures. Antipsychotics and antidepressants are assumed to be an underrecognized medication group to identify patients at risk for severe outcomes after hospitalization for COVID-19.
Data availability statement
The datasets presented in this article are not readily available because of data protection. Data that support the findings of this study are available upon reasonable request from the corresponding author. Requests to access the datasets should be directed to Hendrik Jan Ankersmit, aGVuZHJpay5hbmtlcnNtaXRAbWVkdW5pd2llbi5hYy5hdA==.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Lower Austria (GS1-EK-4/747–2021). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the study design of a retrospective, anonymous registry-based study.
Author contributions
AG: Conceptualization, Formal analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BR: Conceptualization, Data curation, Methodology, Software, Writing – review & editing. CW: Formal analysis, Methodology, Software, Validation, Visualization, Writing – review & editing. PK: Formal analysis, Methodology, Software, Writing – review & editing. DT-W: Investigation, Methodology, Validation, Writing – review & editing. MM: Investigation, Methodology, Validation, Writing – review & editing. JM: Conceptualization, Data curation, Methodology, Validation, Writing – review & editing. CA: Methodology, Validation, Writing – review & editing. JA: Investigation, Methodology, Validation, Writing – review & editing. RW: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HA: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the ARGE Ankersmit of the surgical research laboratory.
Acknowledgments
We thank the Pharmaco-economics Advisory Council of the Austrian Sickness Funds for providing the data, especially Karin Allmer for ensuring the quality of the database query, and Ludwig Weissengruber for his organizational support in data generation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1565677/full#supplementary-material
References
1. Williamson, EJ, Walker, AJ, Bhaskaran, K, Bacon, S, Bate, X, Morton, CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. (2020) 584:430–6. doi: 10.1038/s41586-020-2521-4
2. Posch, M, Bauer, P, Posch, A, and Koenig, F. Analysis of Austrian COVID-19 deaths by age and sex. Wien Klin Wochenschr. (2020) 132:685–9. doi: 10.1007/s00508-020-01707-9
3. Bauer, P, Brugger, J, Koenig, F, and Posch, M. An international comparison of age and sex dependency of COVID-19 deaths in 2020: a descriptive analysis. Sci Rep. (2021) 11:19143. doi: 10.1038/s41598-021-97711-8
4. Kautzky-Willer, A, Kaleta, M, Lindner, SD, Leutner, M, Thurner, S, and Klimek, P. Sex Differences in Clinical Characteristics and Outcomes of Patients with SARS-CoV-2 Infection Admitted to Intensive Care Units in Austria. JPM. (2022) 12:4. doi: 10.3390/jpm12040517
5. Zajic, P, Hiesmayr, M, Bauer, P, Baron, DM, Gruber, A, Joannidis, M, et al. Nationwide analysis of hospital admissions and outcomes of patients with SARS-CoV-2 infection in Austria in 2020 and 2021. Sci Rep. (2023) 13:8548. doi: 10.1038/s41598-023-35349-4
6. Sze, S, Pan, D, Nevill, CR, Gray, LJ, Martin, CA, Nazareth, J, et al. Ethnicity and clinical outcomes in COVID-19: A systematic review and meta-analysis. Eclinical Med. (2020) 29-30:100630. doi: 10.1016/j.eclinm.2020.100630
7. Zheng, Z, Peng, F, Xu, B, Zhao, J, Liu, H, Peng, J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. (2020) 81:e16–25. doi: 10.1016/j.jinf.2020.04.021
8. Ssentongo, P, Ssentongo, AE, Heilbrunn, ES, Ba, DM, and Chinchilli, VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One. (2020) 15:e0238215. doi: 10.1371/journal.pone.0238215
9. Liu, S, Cao, Y, Du, T, and Zhi, Y. Prevalence of Comorbid Asthma and Related Outcomes in COVID-19: A Systematic Review and Meta-Analysis. J Allerg Clin Immunol Pract. (2020) 9:693–701. doi: 10.1016/j.jaip.2020.11.054
10. Ayoubkhani, D, Khunti, K, Nafilyan, V, Maddox, T, Humberstone, B, Diamond, I, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: a retrospective cohort study. British Journal of Medicine. (2021) 372:n693. doi: 10.1136/bmj.n693
11. Mainous, AG III, Rooks, BJ, Wu, V, and Orlando, FA. COVID-19 Post-acute Sequelae among Adults: 12 Month Mortality Risk. Front Med. (2020) 8:778434. doi: 10.3389/fmed.2021.778434
12. Oseran, AS, Song, Y, Xu, J, Dahareh, IJ, Wadhera, RK, Lemos, JA, et al. Long term risk of death and readmission after hospital admission with covid-19 among older adults: retrospective cohort study. BMJ. (2023) 382:e076222. doi: 10.1136/bmj-2023-076222
13. Renda, G, Ricci, F, Spinoni, EG, Grisafi, L, D’Ardes, D, Mennuni, M, et al. Predictors of Mortality and Cardiovascular Outcome at 6 Months after Hospitalization for COVID-19. J Clin Med. (2022) 11:729. doi: 10.3390/jcm11030729
14. Orlando, V, Coscioni, E, Guarino, I, Mucherino, S, Perrella, A, Trama, U, et al. Menditto E Drug-utilisation profiles and COVID-19. Sci Rep. (2021) 11:8913. doi: 10.1038/s41598-021-88398-y
15. Ghasemi, H, Darvishi, N, Salari, N, Hosseinian-Far, A, Akbari, H, and Mohammadi, M. Global prevalence of polypharmacy among the COVID-19 patients: a comprehensive systematic review and meta-analysis of observational studies. Trop Med Heal. (2022) 50:60. doi: 10.1186/s41182-022-00456-x
16. Iloanusi, S, Mgbere, O, and Essien, EJ. Polypharmacy among COVID-19 patients: A systematic review. J Am Pharm Assoc. (2021) 61:e14–25. doi: 10.1016/j.japh.2021.05.006
17. Visser, AGR, Winkens, B, Schols, JMGA, Janknegt, R, and Spaetgens, B. The impact of polypharmacy on 30-day COVID-related mortality in nursing home residents: a multicenter retrospective cohort study. Eur Geriatr Med. (2023) 14:51–7. doi: 10.1007/s41999-022-00723-4
18. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2021) https://www.R-project.org.
19. Fajgenbaum, DC, and June, CH. Cytokine Storm. N Engl J Med. (2020) 383:2255–73. doi: 10.1056/NEJMra2026131
20. Wendt, R, Lingitz, MT, Laggner, M, Mildner, M, Traxler, D, Graf, A, et al. Clinical Relevance of Elevated Soluble ST2, HSP27 and 20S Proteasome at Hospital Admission in Patients with COVID-19. Biology. (2021) 10:1186. doi: 10.3390/biology10111186
21. Horby, P, Lim, WS, Emberson, J, Mafham, M, Bell, JL, Linsell, L, et al. Dexamethasone in Hospitalized Patients with COVID-19. N Engl J Med. (2020) 384:693–704. doi: 10.1056/NEJMoa202143
22. Ivers, NM, Taljaard, M, Giannakeas, V, Reis, C, Williams, E, and Bronskill, S. Public reporting of antipsychotic prescribing in nursing homes: population-based interrupted time series analyses. BMJ Qual Saf. (2019) 28:121–31. doi: 10.1136/bmjqs-2018-007840
23. Rochon, PA, Stukel, TA, Bronskill, SE, Gomes, T, Sykora, K, Wodchis, WP, et al. Variation in Nursing Home Antipsychotic Prescribing Rates. Arch Intern Med. (2007) 167:676–83. doi: 10.1001/archinte.167.7.676
24. Chakraborty, A, and Linton, CR. Antipsychotic prescribing in dementia patients in care homes: proactive in-reach service improved quality of care. Int J Geriatr Psychiatry. (2012) 27:1097–8. doi: 10.1002/gps.2827
25. Lövheim, H, Sandman, PO, Kallin, K, Karlsson, S, and Gustafson, Y. Relationship between antipsychotic drug use and behavioral and psychological symptoms of dementia in old people with cognitive impairment living in geriatric care. Int Psychogeriatr. (2006) 18:713–26. doi: 10.1017/S1041610206003930
26. Gauthier, S, Cummings, J, Ballard, C, Brodaty, H, Grossberg, G, Robert, P, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. (2010) 22:346–72. doi: 10.1017/S1041610209991505
27. Coon, JT, Abbott, R, Rogers, M, Whear, R, Pearson, S, Lang, I, et al. Interventions to Reduce Inappropriate Prescribing of Antipsychotic Medications in People With Dementia Resident in Care Homes: A Systematic Review. J Am Méd Dir Assoc. (2014) 15:706–18. doi: 10.1016/j.jamda.2014.06.012
28. Feola, A, Ciamarra, P, Cavezza, A, Carfora, A, and Campobasso, CP. Choking in patients with neurological disorders and role of drug-induced dysphagia. Case Reports. (2024) 69:102339. doi: 10.1016/j.legalmed.2023.102339
Keywords: COVID-19 hospitalization, all-cause mortality, polypharmacy, baseline medication load, readmission, registry-based observational study
Citation: Graf A, Reichardt B, Wagenlechner C, Krotka P, Traxler-Weidenauer D, Mildner M, Mascherbauer J, Aigner C, Auer J, Wendt R and Ankersmit HJ (2025) Baseline medication load and long-term outcomes in COVID-19-hospitalized patients: results of the AUTCOVSTUDY. Front. Public Health. 13:1565677. doi: 10.3389/fpubh.2025.1565677
Edited by:
Severino Jefferson Ribeiro Da Silva, University of Toronto, CanadaReviewed by:
Carlo Pietro Campobasso, University of Campania Luigi Vanvitelli, ItalyEustachio Cuscianna, University of Bari Aldo Moro, Italy
Copyright © 2025 Graf, Reichardt, Wagenlechner, Krotka, Traxler-Weidenauer, Mildner, Mascherbauer, Aigner, Auer, Wendt and Ankersmit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hendrik J. Ankersmit, aGVuZHJpay5hbmtlcnNtaXRAbWVkdW5pd2llbi5hYy5hdA==
†These authors have contributed equally to this work
 Alexandra Graf
Alexandra Graf Berthold Reichardt2
Berthold Reichardt2 Michael Mildner
Michael Mildner Clemens Aigner
Clemens Aigner Hendrik J. Ankersmit
Hendrik J. Ankersmit