Abstract
Animal manure is applied in agriculture to improve soil fertility and crop yield. Nonetheless, manure can also carry Escherichia coli (E. coli), including antibiotic-resistant strains. Therefore, it may pose a risk for environmental contamination. This review includes 50 studies which were identified from the search terms related to the transmission of E. coli through manure. The review outlines the potential routes of E. coli transmission from manure to soil, water and crops and which factors most critically determine persistence and contamination. The persistence of E. coli in soil is highly variable, ranging from <30 days for composted manures to more than 200 days in cooler conditions. These differences depend on the type of manure used, the environmental conditions and the treatment employed. While crops can be contaminated directly through application of manure, contaminated irrigation water may be a more important pathway. The foremost cause of surface water contamination seems to be rainfall runoff, whereas groundwater contamination is rather uncommon, mainly happening in areas with specific soil conditions. Composting and adherence to pre-harvest intervals are very effective mitigation strategies that can greatly reduce contamination risks. Overall, this review identifies research gaps on water contamination pathways and the persistence of resistant strains. Moreover, it sets up the basis for the development of robust risk assessments and evidence-informed approaches to address the contamination risks that are linked to animal manure.
Introduction
While animal manure is an effective fertilizer that enhances soil fertility and crop yields (1) it can serve as a source of microbial contamination, including Escherichia coli (E. coli). These bacteria may migrate from animal manure to the environment, as for example into soil (2), water (3), and crops (4, 5), thereby posing potential risks for environmental contamination. Furthermore, once established in the environment, they may affect human health through different pathways, such as fresh vegetable consumption (6), recreational swimming (7) or direct contact with grazing animals (8). Organic fertilizers derived from livestock manure can also contain various antimicrobial resistance genes (ARGs) (9, 10), which may be introduced into soil and water systems (11). To create effective mitigation strategies, it is essential to identify major contamination pathways and understand how E. coli survives in various environmental settings.
One of the main goals of the ENVIRE project (http://www.envire-project.de) is investigating how broiler chicken manure might facilitate the dissemination of resistant E. coli into the environment, ultimately affecting human health. To address these challenges, the project adopts various on-farm interventions and manure treatments. In parallel, the project implements a Quantitative Microbial Risk Assessment (QMRA) model to evaluate human exposure from different pathways and the effectiveness of several interventions (12).
While various bacteria can indicate fecal contamination, this review focuses solely on Escherichia coli. That choice reflects the ENVIRE project's mandate and the need to harmonize with our project partners. We excluded Polymerase chain reaction (PCR)-only ARG studies, because our QMRA model requires counts of E. coli colony-forming-units (CFUs) rather than gene presence alone, since no dose-response relationship exists for genes.
This review aims to (i) identify non-negligible pathways for the transfer of E. coli from animal manure to the environment, relevant to the QMRA; (ii) compile existing quantitative data (e.g., survival and concentration of E. coli) across these pathways to inform the QMRA; (iii) outline effective interventions that mitigate manure-derived E. coli contamination; and (iv) pinpoint research gaps where further data are needed. By mapping these pathways, the review seeks to strengthen the evidence base for subsequent risk modeling and guide policy and practical interventions to minimize contamination risks.
Materials and methods
Literature search strategy
A literature search was conducted using PubMed and Google Scholar. Search terms included combinations of the following keywords:
-
“E. coli” OR “Escherichia coli”
-
“antibiotic-resistant E. coli”
-
“manure”
-
“soil contamination”
-
“water contamination”
-
“agricultural crops”
-
“environmental pathways”
The search focused on studies published from 2000 onwards to ensure relevance to contemporary agricultural practices and public health concerns.
Selection criteria
Studies were included if they satisfied at least one of these criteria:
-
Focused on E. coli in manure-amended agricultural contexts, highlighting the potential for contamination of soil, water, or crops.
-
Provided insights into the persistence, transmission, or mitigation of E. coli in agricultural contexts.
-
Discussed agricultural management practices relevant to manure application.
Exclusion criteria included studies focused solely on non-agricultural environments or lacking explicit relevance to E. coli.
Data synthesis
Data from the selected studies was collected and synthesized to identify common themes and patterns. The review was organized according to three environmental pathways:
-
Soil contamination
-
Crops contamination
-
Water contamination
Within each pathway, key findings on E. coli persistence, factors influencing survival, and potential mitigation measures were extracted.
We applied a qualitative environmental-contamination risk-ranking to summarize how relevant each pathway is for the persistence and transfer of E. coli in environmental compartments, based on the strength of evidence found. Each was assigned a risk level: low, medium, or high. This ranking reflects expert judgement and follows the approach described in FAO/WHO (13). While our primary focus is on environmental contamination, these rankings also inform potential human-exposure risks in downstream assessments.
Results and discussion
Description of the studies
In total 50 studies were included in the synopsis. Geographically, 25 of them were from North America, 15 from Europe, four from Asia, three from Africa, two from South America, and one from Oceania. Regarding study type, 27 were field studies, 14 were lab studies, five were modeling studies, and four were reviews (Table 1). The manure investigated in these studies came from cattle, poultry, swine, or horse.
Table 1
| Authors | Pathway | Region | Type of study |
|---|---|---|---|
| Agga et al. (2024) | Soil contamination | USA | Field study |
| Alegbeleye et al. (2020) | Water contamination | Brazil | Review |
| Amato et al. (2020) | Water contamination | USA | Field study |
| Arnaud et al. (2015) | Soil contamination | Canada | Field study |
| Atanasova et al. (2025) | Soil contamination | Germany | Lab study |
| Avery et al. (2004) | Crops contamination | UK | Field study |
| Black et al. (2021) | Soil contamination | Northern Ireland | Review |
| Çekiç et al. (2017) | Soil contamination | USA | Lab study |
| Chapman et al. (2018) | Water contamination | Canada | Modeling |
| Chuwku et al. (2023) | Soil contamination | Nigeria | Modeling |
| Cook et al. (2011) | Water contamination | USA | Lab study |
| Darkazanli and Kiseleva (2019) | Crops contamination | Russia | Lab study |
| Detert et al. (2021) | Soil contamination | Germany | Lab study |
| Ekman et al. (2021) | Soil contamination | Australia | Field study |
| Entry et al. (2004) | Soil contamination | USA | Field study |
| Fatoba et al. (2022) | Soil contamination | South Africa | Field study |
| Forslund et al. (2011) | Water contamination | Denmark | Field study |
| Franz et al. (2008) | Soil contamination | Netherlands | Lab study |
| Gagliardi and Karns (2000) | Soil contamination | USA | Lab study |
| Habteselassie et al. (2010) | Crops contamination | USA | Lab study |
| Holvoet et al. (2013) | Crops contamination | Belgium | Field study |
| Howard et al. (2016) | Soil contamination | USA | Field study |
| Hubbard et al. (2020) | Water contamination | USA | Field study |
| Ingham et al. (2004) | Crops contamination | USA | Field study |
| Islam et al. (2004) | Crops contamination | USA | Field study |
| Islam et al. (2005) | Crops contamination | USA | Field study |
| Iwu and Okoh (2019) | Water contamination | South Africa | Review |
| Jacobs et al. (2019) | Water contamination | USA | Field study |
| Jensen et al. (2013) | Crops contamination | Denmark | Field study |
| Kljujev et al. (2015) | Crops contamination | Serbia | Field study |
| Marutescu et al. (2022) | Soil contamination | Romania | Review |
| Merchant et al. (2012) | Soil contamination | Canada | Field study |
| Mootian et al. (2009) | Crops contamination | USA | Lab study |
| Mügler et al. (2021) | Water contamination | Laos | Field study |
| Okada et al. (2024) | Soil contamination | Argentina | Field study |
| Pang et al. (2020) | Soil contamination | USA | Modeling |
| Sharma et al. (2019) | Soil contamination | USA | Field study |
| Sheng et al. (2019) | Soil contamination | USA | Lab study |
| Siller et al. (2019) | Soil contamination | Germany | Field study |
| Solomon et al. (2002) | Crops contamination | USA | Modeling |
| Sowah et al. (2020) | Water contamination | Canada | Field study |
| Subirats et al. (2021) | Soil contamination | USA | Lab study |
| Sun et al. (2021) | Crops contamination | Japan | Field study |
| Suzuki et al. (2024) | Crops contamination | Canada | Field study |
| Thomas et al. (2024) | Soil contamination | Germany | Field study |
| Tien et al. (2017) | Water contamination | Netherlands | Modeling |
| van Overbeek et al. (2021) | Crops contamination | China | Field study |
| Wang et al. (2021) | Soil contamination | USA | Lab study |
| Weller et al. (2017) | Soil contamination | China | Lab study |
| Yao et al. (2013) | Soil contamination | Poland | Lab study |
List of studies included.
Soil contamination
Duration of persistence
This section explores E. coli survival in soils amended with both fresh and treated manure, focusing on the role of environmental and management factors (Table 2).
Table 2
| Authors | Last detection | Sampling times | Type of manure | Composted/fresh |
|---|---|---|---|---|
| Avery et al. (2004) | 120 days (sheep), 162 days (cattle, pig) | Day 0, 2, 4, 6, 16, 23, 49, 63, 78, and every 14 days until day 218 | Cattle, sheep, swine manure | Fresh |
| Detert et al. (2021) | 42 days (22°C), 84 days (4°C) | Days 0, 21, 42, 63, 84 | Cattle manure | Fresh |
| Ekman et al. (2021) | 50 days | Days 0, 7, 12, 19, 28, 35, 42, 50 | Poultry litter, cow manure | Fresh |
| Ekman et al. (2021) | Up to 50 (both) | Days 0, 6, 16, 27, 38, 49 (soil); days 42, 49 (lettuce for pathogens). | Poultry litter, cattle manure | Fresh |
| Entry et al. (2005) | E. coli 1 day, enteroccoccae 294 days | Days −1, 1, 7, 14, 28, 179, and 297 | Cattle manure | Composted |
| Fatoba et al. (2022) | 42 days | Days 0, 7, 14, 21, and 42 | Poultry litter | Fresh |
| Franz et al. (2008) | 54–105 days | 6 samplings | Cattle manure | Fresh |
| Habteselassie et al. (2010) | Up to 41 (end of experiment) | Days 15, 27, 32, 41, and 50 | Cattle manure | Fresh |
| Ingham et al. (2004) | 168 days | Biweekly intervals up to 168 days | Cattle manure | Fresh |
| Islam et al. (2004, 2005) | 154 days (alkaline-stabilized dairy manure compost), 196 days (poultry manure compost, dairy manure compost) | Days 0, 7, 14, 21, 35, 42, 49, 70, 84, 91, 105, 112, 126, 140, 154, 168, 182, 196 | Poultry litter and cattle manure | Composted |
| Merchant et al. (2012) | Up to 210 days | August (day 0), September, October, November, December, January, and March | Poultry litter | Fresh |
| Mootian et al. (2009) | Up to 9 days | Days 3, 6, and 9 | Cattle manure | Fresh |
| Pang et al. (2020) | Modeled survival up to 150 days (dairy manure), up to 120 days (poultry litter), and up to 90 days (horse manure) | Biweekly samplings over 12 trials at varying intervals | Cattle manure, poultry litter, horse manure | Fresh |
| Sharma et al. (2019) | 90 days (poultry litter), 60 days (dairy manure), 45 days (horse manure) | Days 0, 1, 3, 7, 14, 28, 56, 90, 120, and 150 | Poultry litter, horse manure, cattle solids | Fresh |
| Sheng et al. (2019) | 60 days | Periodic samplings over 12 trials across three seasons (specific intervals not provided) | Cattle manure | Composted and fresh |
| Solomon et al. (2002) | Up to 9 days | Days 1, 3, and 5 post-inoculation (lettuce tissues); days 3, 6, and 9 for soil | Cattle manure | Fresh |
| Subirats et al. (2021) | <30 days | Days 0, 7, 30, and at vegetable harvest | Poultry litter | Composted and fresh |
| Suzuki et al. (2024) | 60 days | Days 0, 7, 60 | Cattle manure | Composted |
| van Overbeek et al. (2021) | 272 days | At planting (day 0), 39 days (lettuce), 90 days (leek 2018), 272 days (leek 2019). | Cattle manure | Fresh |
Studies including data regarding persistence of E. coli in agricultural soil.
Fresh manure
Field trials have shown that fresh manure often sustains E. coli for weeks to months under real-world conditions. Fresh manure may support the extended survival of E. coli in soil due to its nutrient-rich composition. Fatoba (14), with E. coli persisting up to 42 days in soils amended with fresh chicken manure Likewise, Ekman (16) reported the survival of E. coli in soils fertilized with fresh poultry litter and cow manure for up to 50 days in Australia. In another research, E. coli persisted in soils amended with cattle, sheep, and swine manure for up to 19 weeks, with swine manure supporting the longest survival (17). A long persistence was also observed by Ingham (18), 168 days in soils treated with non-composted bovine manure, and by van Overbeek (19), up to 272 days in soils treated with fresh cow manure in the rhizosphere of leek crops in the Netherlands. Merchant et al. observed long survival, with E. coli detected for up to 210 days. However, a genotype analysis revealed that only a small portion of E. coli originated from the manure (20).
Under controlled conditions, lab experiments have detailed how temperature and moisture shape persistence. For example, Habteselassie (15) observed up to 41 days of survival in soils amended with fresh dairy manure. Çekiç et al. (21) demonstrated that environmental conditions strongly influence E. coli survival, with significantly longer persistence during cooler fall conditions (up to 280 days) compared to warmer summer conditions, where survival durations ranged from 84 to 112 days depending on the site. Furthermore, in a study by Detert and Schmidt, longer survival was observed at 4°C (84 days) compared to 22°C (42 days) (22). In a research performed in the USA (23), no E. coli were detected after 60 days with both raw and composted manure. However, the authors did not take intermediate sampling, making it impossible to determine which type of manure application led to shorter E. coli survival. Similarly, Franz et al. (24) observed survival durations of between 54 and 105 days in soil mixed with cattle manure, kept at 16°C in experimental setting.
Pang (25) modeled persistence for up to 90 days under optimal moisture conditions in soils treated with raw manure, pointing up the impact of environmental factors. Poultry litter supported shorter survival durations compared to cow manure, spotlighting the role of manure type. In contrast, in a modeling study by Sharma et al. (26) the longest survival was observed with poultry litter (90 days), followed by dairy manure (60 days), and horse manure (45 days).
Treated manure
Well-composted manure has been shown to promote microbial degradation processes, further reducing bacterial survival (27).
Several field studies reported shorter E. coli survival durations highlighting the effectiveness of composting. For instance, composted cattle manure restricted E. coli persistence for up to 60 days in soils under experimental conditions in Japan (28). Even shorter durations, consistently below 30 days, were observed with composted chicken manure, accompanied by significant reductions in E. coli populations and associated antibiotic-resistance genes, both in field and laboratory settings (5).
In another field study, fecal coliforms and enterococci persisted for at least 42 weeks in soils amended with dairy manure, while E. coli populations dropped below detection levels within a day (29).
From other field studies, it appears that composting primarily impacts E. coli survival by reducing initial concentrations rather than significantly shortening persistence durations. For example, E. coli O157:H7 persisted for 154 to 217 days in soils treated with poultry or dairy manure composts, with poultry manure generally supporting longer survival, potentially due to its higher nitrogen content (30, 31).
Interestingly, while some studies highlight shorter durations with composted manure, experiments conducted in laboratory report extended persistence, suggesting that factors beyond manure treatment, such as soil composition and environmental conditions, may play a more dominant role (21, 32).
Manure treatments
Composting is one of the most common treatments for reducing E. coli populations in manure. A study by Thomas (33) evaluated different composting configurations, including uncovered static piles, covered static piles, and periodically turned piles. Their results showed that E. coli was undetectable within 24 h in all configurations. A key factor was the temperature, that exceeding 50°C, caused the total pathogen inactivation. Proper composting techniques for poultry litter are crucial for reducing antibiotic-resistant E. coli (AREc) and associated antibiotic residues. For example, mechanically aerated piles with optimized carbon-to-nitrogen ratios (e.g., C:N = 30) significantly decrease AREc levels, providing dual benefits of improving manure quality and reducing the spread of antimicrobial resistance (AMR) (34).
Similarly, in a model by Tien et al. it was observed that composted dairy manure contained lower levels of antibiotic resistance genes compared to raw or digested manure. According to the study, composting effectively reduces the initial abundance of resistant bacteria and ARGs, although persistence in soil post-application remains dependent on environmental conditions and manure composition (35).
Anaerobic digestion (AD) of chicken manure achieves rapid pathogen inactivation under mesophilic conditions. In experimental trials, total and antibiotic-resistant E. coli concentrations fell below detection limits in just 14 days at 30°C and 7 days at 37°C (36). However, Weibull-model simulations indicate that residual E. coli cells after AD may persist in soil longer than those from composted manure, suggesting that a brief post-AD composting step would optimize both log-reduction efficacy and environmental decay rates (37).
Broiler litter short-term storage is another practical measure for decreasing E. coli concentrations. Extended-spectrum beta-lactamase (ESBL)-producing E. coli levels may decline by more than 2 log10 within 72 h during summer storage, primarily due to elevated temperatures in deeper litter layers. However, reductions may be less consistent during winter, due to the significant role of environmental conditions. To address this, longer storage periods have been suggested for colder climates to achieve greater reductions (38).
Dairy manure management systems have also evidenced significant efficacy in reducing E. coli levels. Howard et al. highlighted that tiered management practices, such as separating solid from liquid waste, were particularly effective, achieving up to a 3-log reduction in E. coli concentrations. These systems reduce E. coli populations by exposing the bacteria to stress-inducing environments, such as drying beds and lagoons. Advanced separation techniques not only reduce bacterial concentrations but also impact the diversity of E. coli populations, limiting the presence of potentially pathogenic strains (39).
Collectively, these findings underscore the complex relation between manure type, treatment status, and environmental conditions in shaping E. coli persistence in soil environments. Treated manure primarily serves to reduce contamination risks, though it does not universally limit pathogen survival durations. Persistence levels depend on environmental factors and manure management practices. Proper composting and soil incorporation generally lead to significant bacterial decay, so we assigned those pathways a low or medium environmental contamination risk-ranking. However, in specific scenarios, such as loamy soils or environments with limited microbial competition, E. coli can survive for longer periods (5, 23, 25).
Crop contamination: is manure application a significant threat?
The extent to which manure application may be a risk for crops contamination depends on multiple factors. It may act as a source of E. coli under certain circumstances, though it may not always be the primary vector compared to other crops contamination sources.
Regarding the direct manure-crops contamination, it has been observed that E. coli O157:H7 can persist on the surfaces of lettuce and parsley for up to 77 days following the application of raw manure before planting (30). Similarly, E. coli were detected on up to 54% of lettuce grown in soils amended with slurry, with splash events during irrigation or rainfall identified as key mechanisms for transferring bacteria to crop surfaces (4). In addition, in experimental settings, it appears that also the lowest bacteria concentration inoculated in manure and water (104 CFU/g) can lead to the colonization of plant surfaces and internal tissues (40). However, the results are heterogenius, and in another experiment E. coli persisted in both rhizosphere and bulk soil but was not detected on the lettuce phyllosphere after day 27 post-fresh manure application (15). Similarly, in further experiment, it has been found that E. coli can survive in the root zones of lettuce and leek for over 200 days. However, minimal transfer to edible portions suggests that while manure may introduce E. coli into the soil, its direct impact on crops is often limited (19). Moreover, it has been demonstrated that applying dairy manure at least 4 months before raspberry harvest results in no detectable E. coli on fruits, even when raw manure is used (23).
In a model by Solomon et al. E. coli O157:H7 could be transmitted from manure and contaminated irrigation water to lettuce plants, entering through the root system and migrating to internal plant tissues. This internalization makes the bacteria inaccessible to surface sanitizing treatments, such as chlorine rinses, which are typically used to reduce microbial contamination (41). Oppositely, on the field, Ekman et al. (16) observed that, even applying fresh manure, crop contamination was minimal, with no pathogens detected on mature lettuce at harvest.
Similarly, Suzuki et al. (28) detected ARG-bearing coliforms in the root and stem zones, but no E. coli was identified on the edible portions of the corn, provided the manure was fully composted. A study by Ingham et al. showed that vegetables grown in soil fertilized with non-composted manure could harbor E. coli. While indigenous E. coli levels in soil decreased by about 3 log CFU/g within 90 days, low concentrations (0.9–1.6 CFU/g) persisted for over 100 days and were detected up to 168 days post-manure application. Sporadic contamination was observed on washed carrots and lettuce, with occasional positive results even beyond the 120-day harvest interval (18).
However, proper manure management can significantly reduce these risks Additionally, fully composted manure has been shown to eliminate detectable E. coli on corn crops, effectively mitigating contamination risks (28).
Furthermore, environmental factors such as sunlight and humidity play an important role in reducing bacterial loads on crop surfaces. For example, die-off rates of 0.52 log most-probable-number/day were observed for E. coli on lettuce under field conditions (42).
Alternative contamination sources often surpass manure in directly contributing to E. coli presence on crops. For instance, irrigation water contaminated with animal fecal matter has been identified as a major risk factor (43, 44). Similarly, wildlife activity in agricultural fields may introduce E. coli, as underlined by Merchant (20), especially in areas with high wildlife density. Greenhouse cultivation systems further complicate the issue, as such environments create conditions favorable for prolonged E. coli persistence (45). Avery et al. (17) highlighted the influence of environmental factors, such as rainfall, on the transfer of pathogens from manure to crops. E. coli from livestock feces could survive on grass for up to 6 months under conducive conditions, thereby increasing the risk of contamination through runoff or splash effects during heavy rainfall.
Research by Sun et al. demonstrated that the primary pathway for the transmission of manure-borne microbes and ARGs to lettuce is from surface soil to the leaf episphere. The study found that ~81% of the microbes and 62% of the ARGs present in the lettuce episphere originated from surface soil, pointing up the significant role of splashing during irrigation and direct contact in facilitating contamination from manure-amended soils to leafy greens. Notably, manure had limited effects on the rhizosphere microbiome and associated resistant genes. This suggests that horizontal transfer (the physical movement of manure-associated bacteria or free DNA, via splash or irrigation) from surface soil rather than uptake through roots, represents the dominant pathway for contamination of crops by manure-borne ARGs (46).
The effects of poultry litter soil amendments on E. coli and antibiotic-resistant strains have also been explored. For instance, an increase in tetracycline- and third-generation cephalosporin-resistant E. coli populations was observed during the first 28 days following application, with levels returning to baseline by day 70. These results suggest that while raw poultry litter may transiently enrich antibiotic-resistant E. coli, its long-term impact is minimal in managed cropping systems. Additionally, cover cropping has been shown to further reduce tetracycline-resistant E. coli levels, offering an effective supplemental mitigation strategy (47).
Manure management practices such as composting and adherence to pre-harvest intervals are essential for mitigating contamination risks (48). Manure-related E. coli contamination of crops depends on pathways such as surface splash, irrigation, and internalization via roots. While contamination risks are reduced by proper manure management and pre-harvest intervals, practices like raw manure application carries a low to medium risk-ranking, with increased probability when environmental conditions promote bacterial transfer.
Water contamination
Surface water
Manure application significantly contributes to E. coli contamination in surface waters through runoff during precipitation events and improper manure management. There are evidences that demonstrates that raindrop impact enhances E. coli detachment from manure-amended soils, leading to increased bacterial runoff into adjacent water bodies (3). Field experiments indicated that mitigating raindrop impacts, such as by maintaining vegetative cover, substantially reduced E. coli transport during runoff events (49). A study by Amato et al. observed that poultry litter applied to croplands contributed to increased presence of cephalosporin-resistant E. coli in streams within the Chesapeake Bay area in the USA. The study found that the density of poultry barns was positively correlated with increased resistance in E. coli between 6.2 and 18.9%, indicating that manure from these operations is a critical source of nutrient pollution and antibiotic-resistant bacteria in nearby water bodies (2). Alegbeleye et al. (50) and Jacobs et al. (51) further underscore that hydrological drivers such as storms and heavy rainfall significantly influence the transport of manure-borne pathogens into water bodies.
Cook (52) reported high genetic diversity of E. coli in agriculturally impacted streams, suggesting multiple contamination sources, including manure runoff and wildlife contributions. The role of poultry litter in surface water contamination is further underlined by Hubbard (53) who detected E. coli in streams near large-scale poultry operations. It was further highlighted that contaminated irrigation water can serve as a vector for E. coli transport, leading to internalization into lettuce tissues (6, 54).
Groundwater
Groundwater contamination by E. coli is less frequent but poses significant risks in regions with porous soils and high manure application rates. E. coli contamination was detected in groundwater following livestock manure applications, even with a 12-meter-thick unsaturated zone comprising coarse and heterogeneous glacial sediments (55).
Further supporting this another study, investigated the leaching of E. coli and other pathogens through intact soil cores following surface application and injection of slurry. The study found that under natural weather conditions, microorganisms from manure could be transported through the soil into groundwater, posing a risk to water quality (56).
There are also indications that soil type, tillage practice, and the presence of manure influence the leaching of E. coli O157:H7, suggesting that different soil conditions and agricultural practices significantly impact the vertical movement of pathogens (57).
For manure to water systems, the contamination risk increases significantly, especially for surface waters and was assigned as high environmental contamination risk-ranking. Sowah and Hubbard highlight that manure runoff during rainfall events is a primary contributor to E. coli and resistant bacteria presence in streams, particularly near confined feeding operations. This contamination risk is exacerbated by poor manure storage and runoff controls (3, 53). Additionally, groundwater contamination, although categorized as a lower risk compared to surface waters, remains significant in regions with porous soils and high manure application rates (55, 58).
By identifying non-negligible pathways of E. coli transfer (Objective i), our map of soil, crop and water routes can serve as the structural backbone for QMRA risk pathways. Second, the quantitative persistence and concentration data compiled (Objective ii) provide the parameter values needed for the exposure assessment. Furthermore, the interventions we outline (Objective iii) such as composting, anaerobic digestion of manure, short-term poultry litter storage and pre-harvest intervals can be directly incorporated in QMRA as mitigation options to compare reduction in human exposure. Finally, by highlighting research gaps (Objective iv), we flag where the QMRA will be most uncertain and where future experiments should focus to increase model robustness.
Limitations
This work was conducted as an exploratory narrative review and the number of studies screened, excluded and included in this work, was not systematically recorded. We opted for an exploratory because the available studies differed widely, making a single, unified protocol impossible. Furthermore, the literature selection and screening process was performed by a single person. The manure treatments section provides only a broad overview of the current state of research and does not analyse in depth all possible treatment methods. Although our search terms did not restrict by region, the preponderance of North American and European studies reflects where research has been published, not our methodology. We searched both PubMed and Google Scholar without geographical filters, so the observed bias stems from the literature itself.
Research gaps
This review identifies several critical gaps in the literature on the environmental transfer of E. coli from manure:
-
Focus on resistant strains: research predominantly examines general E. coli, with limited attention to antibiotic-resistant strains and their persistence.
-
Limited water contamination data: studies on manure's impact on E. coli contamination of water systems, especially groundwater, are scarce compared to soil and crops research.
-
Heterogeneity in study design: variability in experimental conditions, manure types, and methodologies complicates data synthesis and application to specific agricultural contexts.
-
Geographical bias: research is predominantly concentrated in specific regions, with most studies originating from North America, followed by Europe. Other regions, such as Africa, Asia, South America, and Oceania, are significantly underrepresented. This imbalance underscores the need for more studies in these areas to ensure the global applicability of our findings and to address region-specific challenges.
Conclusion
The transfer of E. coli from manure to soil, water, and crops poses significant environmental and public health challenge, with contamination risk depending on the pathway (Figure 1). Key findings include:
-
Soil contamination: persistence of E. coli is influenced by manure type, treatment, and environmental factors with soil serving as a primary reservoir. Treated manure decreases the contamination risks but may not effectively reduce E. coli persistence length.
-
Crop contamination: while it is possible for crops to be contaminated directly through manure application, other pathways, such as contaminated irrigation water and wildlife, may have a greater influence. These risks can be reduced substantially, however, through appropriate manure management and adherence to pre-harvest intervals.
-
Water contamination: surface water contamination arises primarily from runoff during precipitation events. Groundwater contamination is less frequent but poses serious risks in vulnerable regions with porous soils.
Figure 1
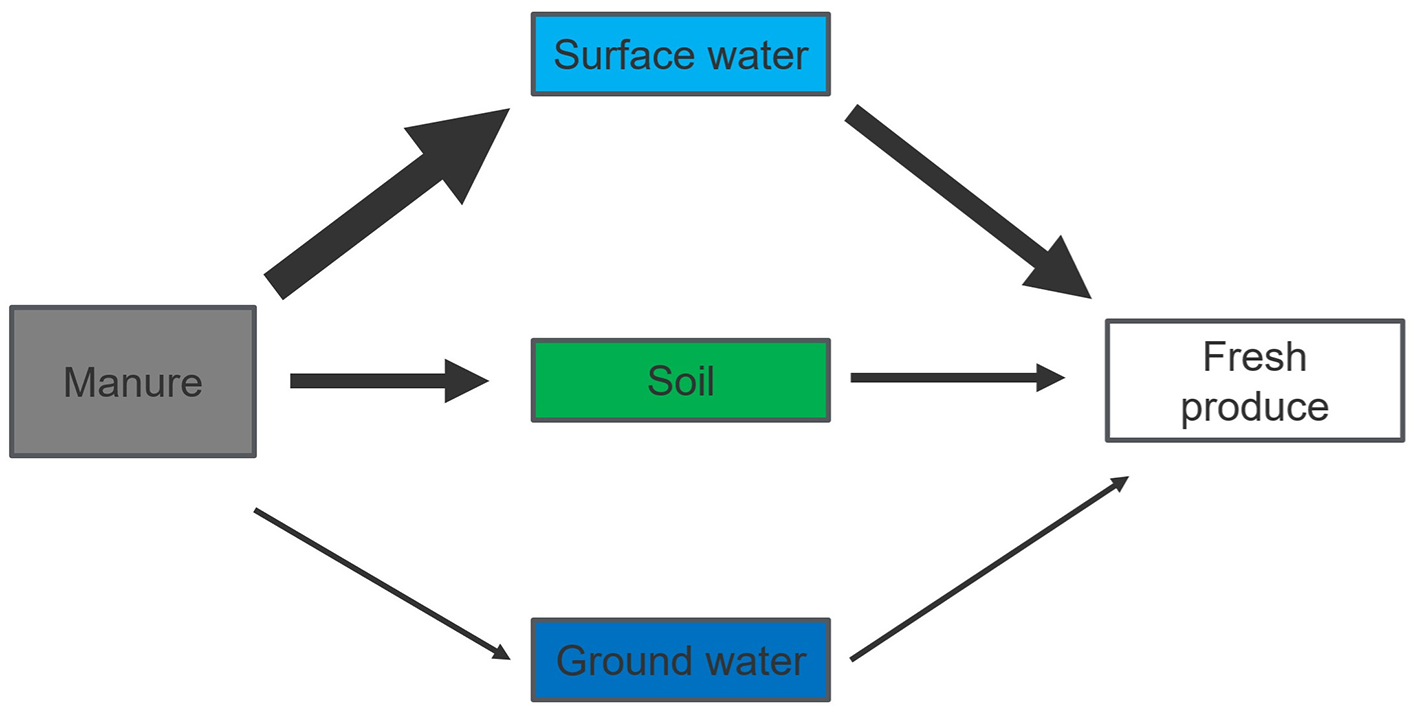
Relative contribution of selected risk pathways to E. coli human exposure via fresh produce consumption. Thicker arrow indicates higher risk, thinner arrow indicates lower risk or no evidences.
There is a critical lack of studies on antibiotic-resistant E. coli and how long they survive in the environment compared to non-resistant strains. Future research should focus on standardized methodologies, regional challenges and interventions to address AMR risks in agriculture.
Statements
Author contributions
NS: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SB: Conceptualization, Visualization, Writing – review & editing. LC: Conceptualization, Supervision, Writing – review & editing. RM: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was part of the European project ENVIRE funded by the JPIAMR program of the European Union and the German Federal Ministry for Research and Education (Support Code: 01KI2202A), and supported by the Open Access Funds of Freie Universität Berlin.
Acknowledgments
The authors would like to thank all the members of the ENVIRE project consortium for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Cai A Xu M Wang B Zhang W Liang G Hou E et al . Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. (2019) 189:168–75. 10.1016/j.still.2018.12.022
2.
Amato HK Wong NM Pelc C Taylor K Price LB Altabet M et al . Effects of concentrated poultry operations and cropland manure application on antibiotic resistant Escherichia coli and nutrient pollution in Chesapeake Bay watersheds. Sci Total Environ. (2020) 735:139401. 10.1016/j.scitotenv.2020.139401
3.
Sowah R Bradshaw K Snyder B Spidle D Molina M . Evaluation of the soil and water assessment tool (SWAT) for simulating E. coli concentrations at the watershed-scale. Sci Total Environ. (2020) 746:140669. 10.1016/j.scitotenv.2020.140669
4.
Jensen AN Storm C Forslund A Baggesen DL Dalsgaard A . Escherichia coli contamination of lettuce grown in soils amended with animal slurry. J Food Prot. (2013) 76:1137–44. 10.4315/0362-028X.JFP-13-011
5.
Subirats J Murray R Yin X Zhang T Topp E . Impact of chicken litter pre-application treatment on the abundance, field persistence, and transfer of antibiotic resistant bacteria and antibiotic resistance genes to vegetables. Sci Total Environ. (2021) 801:149718. 10.1016/j.scitotenv.2021.149718
6.
O'Flaherty E Solimini AG Pantanella F de Giusti M Cummins E . Human exposure to antibiotic resistant-Escherichia coli through irrigated lettuce. Environ Int. (2019) 122:270–80. 10.1016/j.envint.2018.11.022
7.
O'Flaherty E Solimini A Pantanella F Cummins E . The potential human exposure to antibiotic resistant-Escherichia coli through recreational water. Sci Total Environ. (2018) 650:786–95. 10.1016/j.scitotenv.2018.09.018
8.
Chapman B Pintar K Smith BA . Multi-exposure pathway model to compare Escherichia coli O157 risks and interventions. Risk Anal. (2018) 38:392–409. 10.1111/risa.12826
9.
Xu Y Li H Shi R Lv J Li B Yang F et al . Antibiotic resistance genes in different animal manures and their derived organic fertilizer. Environ Sci Eur. (2020) 32:102. 10.1186/s12302-020-00381-y
10.
Zhang Y-J Hu H-W Chen Q-L Singh BK Yan H Chen D et al . Transfer of antibiotic resistance from manure-amended soils to vegetable microbiomes. Environ Int. (2019) 130:104912. 10.1016/j.envint.2019.104912
11.
Zalewska M Błażejewska A Czapko A Popowska M . Antibiotics and antibiotic resistance genes in animal manure – consequences of its application in agriculture. Front Microbiol. (2021) 12:610656. 10.3389/fmicb.2021.610656
12.
Sarnino N Friese A Kabelitz T Collineau L Malakauskas M Kuźmińska-Bajor M et al . ENVIRE: Interventions to Control the Dynamics of Antimicrobial Resistance From Chickens Through the Environment. Society for Veterinary Epidemiology and Preventive Medicine (SVEPM) (2023).
13.
World Health Organization Food and Agriculture Organization of the United Nations . Risk Characterization of Microbiological Hazards in Food: Guidelines no. 17. Rome: World Health Organization (2009). Available online at: https://iris.who.int/handle/10665/44224
14.
Fatoba D Amoako D Abia A Essack S . Transmission of antibiotic-resistant Escherichia coli from chicken litter to agricultural soil. Front Environ Sci. (2022) 9. 10.3389/fenvs.2021.751732
15.
Habteselassie M Gray M Applegate B Reuhs B Turco R . Understanding the role of agricultural practices in the potential colonization and contamination by Escherichia coli in the rhizospheres of fresh produce. J Food Prot. (2010) 73:2001–9. 10.4315/0362-028X-73.11.2001
16.
Ekman J Goldwater A Bradbury M Matthews J Rogers G . Persistence of human pathogens in manure-amended Australian soils used for production of leafy vegetables. Agriculture. (2021) 11:14. 10.3390/agriculture11010014
17.
Avery SM Moore A Hutchison ML . Fate of Escherichia coli originating from livestock faeces deposited directly onto pasture. Lett Appl Microbiol. (2004) 38:355–9. 10.1111/j.1472-765X.2004.01501.x
18.
Ingham S Losinski J Andrews M Breuer J Breuer J Wood T et al . Escherichia coli contamination of vegetables grown in soils fertilized with noncomposted bovine manure: garden-scale studies. Appl Environ Microbiol. (2004) 70:6420–7. 10.1128/AEM.70.11.6420-6427.2004
19.
van Overbeek L Duhamel M Aanstoot S van der Plas CL Nijhuis E Poleij L et al . Transmission of Escherichia coli from manure to root zones of field-grown lettuce and leek plants. Microorganisms. (2021) 9:2289. 10.3390/microorganisms9112289
20.
Merchant LE Rempel H Forge T Kannangara T Bittman S Delaquis P et al . Characterization of antibiotic-resistant and potentially pathogenic Escherichia coli from soil fertilized with litter of broiler chickens fed antimicrobial-supplemented diets. Can J Microbiol. (2012) 58:1084–98. 10.1139/w2012-082
21.
Çekiç SK De J Jubair M Schneider KR . Persistence of indigenous Escherichia coli in raw bovine manure-amended soil. J Food Prot. (2017) 80:1562–73. 10.4315/0362-028X.JFP-17-033
22.
Detert K Schmidt H . Survival of Enterohemorrhagic Escherichia coli O104:H4 strain C227/11Φcu in agricultural soils depends on rpoS and environmental factors. Pathogens. (2021) 10:1443. 10.3390/pathogens10111443
23.
Sheng L Shen X Benedict C Su Y Tsai H-C Schacht E et al . Microbial safety of dairy manure fertilizer application in raspberry production. Front Microbiol. (2019) 10:2276. 10.3389/fmicb.2019.02276
24.
Franz E Semenov A Termorshuizen A de Vos OJ Bokhorst J van Bruggen A . Manure-amended soil characteristics affecting the survival of E. coli O157:H7 in 36 Dutch soils. Environ Microbiol. (2008) 10:313–27. 10.1111/j.1462-2920.2007.01453.x
25.
Pang H Mokhtari A Chen Y Oryang D Ingram DT Sharma M et al . A predictive model for survival of Escherichia coli O157:H7 and generic E. coli in soil amended with untreated animal manure. Risk Anal. (2020) 40:1367–82. 10.1111/risa.13491
26.
Sharma M Millner PD Hashem F Vinyard BT East CL Handy ET et al . Survival of Escherichia coli in manure-amended soils is affected by spatiotemporal, agricultural, and weather factors in the mid-Atlantic United States. Appl Environ Microbiol. (2019) 85:e02392-18. 10.1128/AEM.02392-18
27.
Marutescu LG Jaga M Postolache C Barbuceanu F Milita N Romascu LM et al . Insights into the impact of manure on the environmental antibiotic residues and resistance pool. Front Microbiol. (2022) 13:965132. 10.3389/fmicb.2022.965132
28.
Suzuki Y Horita T Nishimura E Xie H Tamai S Kobayashi I et al . Crop contamination evaluation by antimicrobial-resistant bacteria via livestock waste compost-fertilized field soil. J Hazard Mater. (2024) 480:135987. 10.1016/j.jhazmat.2024.135987
29.
Entry JA Leytem AB Verwey S . Influence of solid dairy manure and compost with and without alum on survival of indicator bacteria in soil and on potato. Environ Pollut. (2005) 138:212–8. 10.1016/j.envpol.2005.04.002
30.
Islam M Doyle M Phatak S Millner P Jiang X . Persistence of Enterohemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. J Food Prot. (2004) 67:1365–70. 10.4315/0362-028X-67.7.1365
31.
Islam M Doyle M Phatak S Millner P Jiang X . Survival of Escherichia coli O157:H7 in soil and on carrots and onions grown in fields treated with contaminated manure composts or irrigation water. Food Microbiol. (2005) 22:63–70. 10.1016/j.fm.2004.04.007
32.
Darkazanli M Kiseleva I . The transmission of Escherichia coli from irrigation water to barley the transmission of Escherichia coli from irrigation water to barley. AIP Conf Proc. (2019) 2063:030004. 10.1063/1.5087312
33.
Thomas C Idler C Ammon C Amon T . Applied research note: survival of Escherichia coli and temperature development during composting of chicken manure with a typically low carbon/nitrogen ratio and moisture content. J Appl Poult Res. (2024) 33:100402. 10.1016/j.japr.2024.100402
34.
Okada E Young BJ Pérez DJ Pellegrini MC Carciochi WD Lavallén CM et al . Effect of on-farm poultry litter composting processes on physicochemical, biological, and toxicological parameters and reduction of antibiotics and antibiotic-resistant Escherichia coli. Waste Manag. (2024) 174:310–9. 10.1016/j.wasman.2023.12.005
35.
Tien Y-C Li B Zhang T Scott A Murray R Sabourin L et al . Impact of dairy manure pre-application treatment on manure composition, soil dynamics of antibiotic resistance genes, and abundance of antibiotic-resistance genes on vegetables at harvest. Sci Total Environ. (2017) 581–582:32–9. 10.1016/j.scitotenv.2016.12.138
36.
Atanasova A Amon T Friese A Rösler U Merle R Herrmann C et al . Effects of carbon–to–nitrogen ratio and temperature on the survival of antibiotic-resistant and non-resistant Escherichia coli during chicken manure anaerobic digestion. Poultry. (2025) 4:9. 10.3390/poultry4010009
37.
Chukwu VA Smith JU Strachan NJ Avery LM . Modelling the deactivation of Escherichia coli in Nigerian soils amended with differently treated manures. J Appl Microbiol. (2023) 134:lxad098. 10.1093/jambio/lxad098
38.
Siller P Daehre K Thiel N Nübel U Roesler U . Impact of short-term storage on the quantity of extended-spectrum beta-lactamase–producing Escherichia coli in broiler litter under practical conditions. Poult Sci. (2020) 99:2125–35. 10.1016/j.psj.2019.11.043
39.
Howard KJ Martin E Gentry T Feagley S Karthikeyan R . Effects of dairy manure management practices on E. coli concentration and diversity. Water Air Soil Pollu. (2016) 228:4. 10.1007/s11270-016-3182-7
40.
Mootian G Wu W-H Matthews KR . Transfer of Escherichia coli O157:H7 from soil, water, and manure contaminated with low numbers of the pathogen to lettuce plants. J Food Prot. (2009) 72:2308–12. 10.4315/0362-028X-72.11.2308
41.
Solomon E Yaron S Matthews K . Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl Environ Microbiol. (2002) 68:397–400. 10.1128/AEM.68.1.397-400.2002
42.
Weller DL Kovac J Roof S Kent DJ Tokman JI Kowalcyk B et al . Survival of Escherichia coli on lettuce under field conditions encountered in the Northeastern United States. J Food Prot. (2017) 80:1214–21. 10.4315/0362-028X.JFP-16-419
43.
Holvoet K Sampers I Callens B Dewulf J Uyttendaele M . Moderate prevalence of antimicrobial resistance in Escherichia coli isolates from lettuce, irrigation water, and soil. Appl Environ Microbiol. (2013) 79:6677–83. 10.1128/AEM.01995-13
44.
Iwu CD Okoh AI . Preharvest transmission routes of fresh produce associated bacterial pathogens with outbreak potentials: a review. Int J Environ Res Public Health. (2019) 16:4407. 10.3390/ijerph16224407
45.
Yao Z Wei G Wang H Wu L Wu J Xu J . Survival of Escherichia coli O157:H7 in soils from vegetable fields with different cultivation patterns. Appl Environ Microbiol. (2013) 79:1755–6. 10.1128/AEM.03605-12
46.
Sun Y Snow D Walia H Li X . Transmission routes of the microbiome and resistome from manure to soil and lettuce. Environ Sci Technol. (2021) 55:11102–12. 10.1021/acs.est.1c02985
47.
Agga GE Durso LM Sistani KR . Effect of poultry litter soil amendment on antibiotic-resistant Escherichia coli. J Environ Qual. (2024) 53:300–13. 10.1002/jeq2.20560
48.
Black Z Balta I Black L Naughton PJ Dooley JS Corcionivoschi N . The fate of foodborne pathogens in manure treated soil. Front Microbiol. (2021) 12:781357. 10.3389/fmicb.2021.781357
49.
Mügler C Ribolzi O Viguier M Janeau J-L Jardé E Latsachack K et al . Experimental and modelling evidence of splash effects on manure borne Escherichia coli washoff. Environmen Sci Pollut Res. (2021) 28:33009–20. 10.1007/s11356-021-13011-8
50.
Alegbeleye OO Sant'Ana AS . Manure-borne pathogens as an important source of water contamination: an update on the dynamics of pathogen survival/transport as well as practical risk mitigation strategies. Int J Hyg Environ Health. (2020) 227:113524. 10.1016/j.ijheh.2020.113524
51.
Jacobs K Wind L Krometis L-A Hession WC Pruden A . Fecal indicator bacteria and antibiotic resistance genes in storm runoff from dairy manure and compost-amended vegetable plots. J Environ Qual. (2019) 48:1038–46. 10.2134/jeq2018.12.0441
52.
Cook KL Bolster CH Ayers KA Reynolds DN . Escherichia coli diversity in livestock manures and agriculturally impacted stream waters. Curr Microbiol. (2011) 63:439–49. 10.1007/s00284-011-0002-6
53.
Hubbard LE Givens CE Griffin DW Iwanowicz LR Meyer MT Kolpin DW . Poultry litter as potential source of pathogens and other contaminants in groundwater and surface water proximal to large-scale confined poultry feeding operations. Sci Total Environ. (2020) 735:139459. 10.1016/j.scitotenv.2020.139459
54.
Kljujev I Raicevic V Andrews S Jackson R Lalevic B Dorati F . Transmission of E. coli from contaminated irrigation water and soil to plant tissue. J Hyg Eng Design. (2012) 1. 10.5555/20133175028
55.
Arnaud E Best A Parker B Aravena R Dunfield K . Transport of through a thick vadose zone. J Environ Qual. (2015) 44:1424–34. 10.2134/jeq2015.02.0067
56.
Forslund A Markussen B Toenner-Klank L Bech T Jacobsen O Dalsgaard A . Leaching of Cryptosporidium parvum oocysts, Escherichia coli, and a Salmonella enterica serovar typhimurium bacteriophage through intact soil cores following surface application and injection of slurry. Appl Environ Microbiol. (2011) 77:8129–38. 10.1128/AEM.05675-11
57.
Gagliardi J Karns J . Leaching of Escherichia coli O157:H7 in diverse soils under various agricultural management practices. Appl Environ Microbiol. (2000) 66:877–83. 10.1128/AEM.66.3.877-883.2000
58.
Wang Y Pan F Chang J Wu R Tibamba M Lu X et al . Effect and risk assessment of animal manure pollution on Huaihe River Basin, China. Chin Geogr Sci. (2021) 31:751–64. 10.1007/s11769-021-1222-8
Summary
Keywords
Escherichia coli , environmental contamination, animal manure, crops contamination, water contamination, soil contamination
Citation
Sarnino N, Basak S, Collineau L and Merle R (2025) Pathways of Escherichia coli transfer from animal manure: risks and mitigation in agriculture. Front. Public Health 13:1568621. doi: 10.3389/fpubh.2025.1568621
Received
30 January 2025
Accepted
22 May 2025
Published
06 June 2025
Volume
13 - 2025
Edited by
Luminita Marutescu, University of Bucharest, Romania
Reviewed by
Getahun E. Agga, Agricultural Research Service (USDA), United States
Updates
Copyright
© 2025 Sarnino, Basak, Collineau and Merle.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nunzio Sarnino Nunzio.Sarnino@fu-berlin.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.