- Department of General Surgery, Fourth Affiliated Hospital of Nanchang University, Nanchang, China
Background: Gallstones are the most prevalent cause of hospitalization among digestive disorders. For humans, sleep is an essential physiological function. The relationship between gallstones and sleep is well established, but the consequences of weekend catch-up sleep (WCS) on gallstones remain unclear. This research examined the connection between gallstone disease and WCS.
Methods: We included 6,957 participants from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) who met the eligibility criteria and had complete data. Logistic regression, restricted cubic spline, and subgroup analyses were employed to assess the relationship between the presence of gallstones and WCS.
Results: Our study indicated that trouble sleeping and late sleep were risk factors for gallstones in a model adjusted for all covariates. The restricted cubic spline results revealed that WCS was negatively correlated linearly with gallstone disease. Additionally, subgroup analysis showed a statistically significant relationship between WCS > 2 hours and a lower risk of gallstones, particularly among non-smokers and males.
Conclusion: Our study results demonstrate that trouble sleeping and late sleep increase the incidence of gallstones. In addition, the protective effect of WCS > 2 hours on reducing the incidence of gallstones is particularly significant in individuals who are non-smokers and males, whereas in the smoking population, WCS < 0 hours serves as a protective factor against gallstones.
1 Introduction
Gallstones have become a common digestive disorder among adults in the United States, with an incidence rate of 10–15% (1). Although nearly 80% of gallstone patients remain asymptomatic, the estimated risk of complications in asymptomatic patients is approximately 0.1–0.3% annually, whereas in individuals who have experienced their first episode of colic, the annual risk increases to between 1 and 3% (2, 3). Once symptoms occur, surgery is the primary treatment for gallstones (4). Prior research has demonstrated a significant relationship between gallstones and factors such as being female, race, increased body mass index (BMI), obesity, and vitamin C deficiency (5–8).
Sleep is a crucial physiological process that influences various metabolic and endocrine functions (9), including hormone control, cardiovascular health, energy conservation, glucose management, muscle regeneration, tissue growth, protein synthesis, and cognitive function (10–12). According to the international categorization of sleep disorders, 7–8 h is the ideal amount of sleep duration (13). Previous studies have shown that insufficient or excessive sleep duration is associated with lower bone mineral density, osteoporosis, and a higher prevalence of depression (14–17). The disparity between the duration of sleep on weekends and weekdays is referred to as weekend catch-up sleep (WCS) (18). WCS is significantly linked to obesity prevention (19), decreased incidence of cardiovascular disease (20), lower risk of hypertension (21), reduced odds of depressive symptoms (22), and reduced levels of high-sensitivity C-reactive protein (23).
A recent study has shown a close relationship between sleep and gallstones (24), but studies on the relationship between gallstones and WCS remain lacking. By examining data from the National Health and Nutrition Examination Survey (NHANES) from 2017 to March 2020, we aimed to explore the correlation between gallstones and WCS and provide new insights into the prevention of gallstone disease.
2 Methods
2.1 Research population
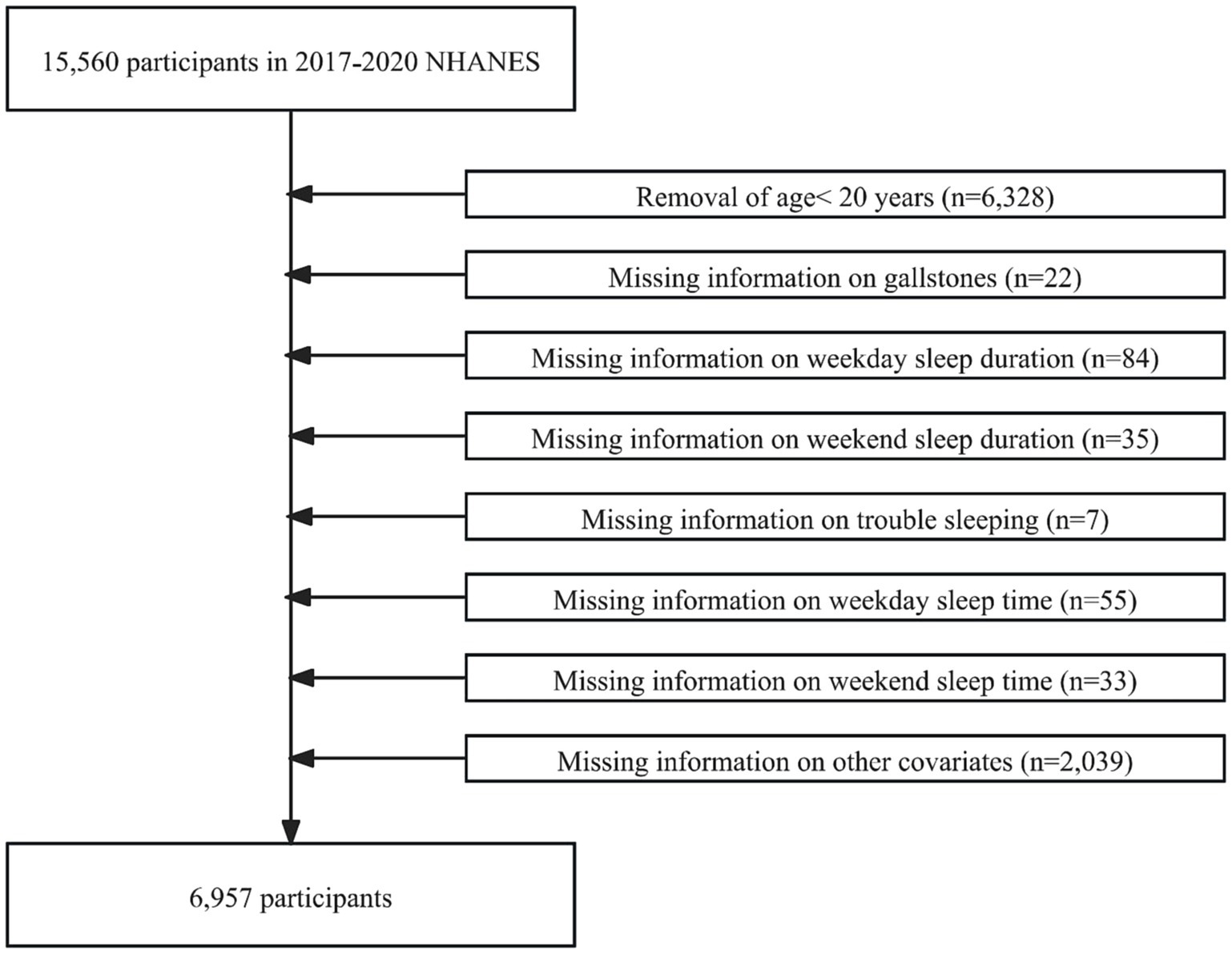
The NHANES is a representative health survey of the United States. Its comprehensive data make it a key resource for studying chronic disease, nutrition, and population health. Our study analyzed data from the 2017–2020 NHANES survey participants. In the beginning, 15,560 participants were enrolled in our study. However, we excluded participants younger than 20 years of age (n = 6,328), those with missing information about gallstones (n = 22), weekday sleep duration (n = 84), weekend sleep duration (n = 35), trouble sleeping (n = 7), weekday sleep time (n = 55), weekend sleep time (n = 33), education level (n = 13), BMI (n = 811), diabetes (n = 3), hypertension (n = 12), smoking (n = 4), and alcohol consumption (n = 1,196). Ultimately, 6,957 participants with no missing information were included in this study (Figure 1).
2.2 Defining gallstone disease
We used the question “Has a doctor or other health professional ever told you that you had gallstones?” to determine whether participants had gallstones. If the participant answered “Yes,” it meant the presence of gallstones; if “No,” it meant the absence of gallstones.
2.3 Definition of WCS and other sleep factors
Weekday and weekend sleep durations were obtained from the questions on “Number of hours usually slept on weekdays” and “Number of hours usually slept on weekends,” respectively. We categorized both weekday sleep duration and weekend sleep duration into three groups: normal (<7 h per night), short (7–9 h per night), and long (>9 h per night) (25). WCS was defined as the difference between weekend sleep duration and weekday sleep duration. We categorize WCS into four groups: <0 h, =0 h, 0–2 h, and >2 h (20). Weekday sleep time and weekend sleep time were obtained from the questions “What time do you usually fall asleep on weekends?” and “What time do you usually fall asleep on weekends?.” We categorized both weekday sleep duration and weekend sleep duration into three groups: normal sleep time (20:00–23:00), late sleep (>23:00), and abnormal sleep time (>03:00). Trouble sleeping was determined by the question “Have you ever told a doctor or other health professional that you have trouble sleeping?.” If the participant answered “Yes,” it meant the presence of trouble sleeping; if “No,” it meant the absence of trouble sleeping.
2.4 Identification of covariates
In our study, the following variables were employed as covariates: gender, age, BMI, race, alcohol consumption, education level, smoking status, hypertension, and diabetes. We divided the ages into four groups: <35 years, 35–49 years, 50–64 years, and ≥65 years. We categorized the races into five groups: Mexican American, Non-Hispanic White, Other Hispanic, Non-Hispanic Black, and Other Race. We categorized education levels into three groups: <High school (below the 12th grade and those who did not obtain a high school diploma), High school [high school graduate/general educational development (GED) or equivalent], and >High school (some college or associate’s degree, college graduate or above). BMI was stratified into two groups: <30 kg/m2 and ≥30 kg/m2. The questions “Doctor told you had hypertension” and “Doctor told you had diabetes” were used to determine whether a participant had hypertension or diabetes. Smoking status was determined by the question “Have you smoked at least 100 cigarettes in your entire life?.” The responses “Yes” and “No” identified smokers and non-smokers, respectively. Alcohol consumption status was determined based on the question “Ever drink 4/5 cups or more a day?.” The responses “Yes” and “No” identified drinkers and non-drinkers, respectively.
2.5 Statistical analyses
Categorical variables were presented as numbers (n) and percentages (%). For categorical variables, the chi-square test was used to compare the characteristics between participants with and without gallstones. Our study utilized logistic regression to calculate the odds ratios (OR) and corresponding 95% confidence intervals (CI) for the association between WCS, other sleep factors, and gallstones. Three logistic regression models were constructed to further explore the potential links between them. Model 1 was unadjusted for covariates. Model 2 race, age, and gender were adjusted. Model 3 race, age, gender, BMI, education level, diabetes, hypertension, smoking, and alcohol consumption status were adjusted. Additionally, to find any potential non-linear dose–response relationship between WCS and gallstone disease, we employed a restricted cubic spline (RCS) model. Covariates, namely gender, race, education level, BMI, diabetes, smoking, and alcohol consumption, were adjusted when constructing this model. Finally, we performed subgroup analyses to examine the association between WCS and gallstones in the different groups. The R program (4.4.2) was utilized for our study, and p < 0.05 was defined as statistically significant.
3 Results
3.1 Baseline characteristics of participants
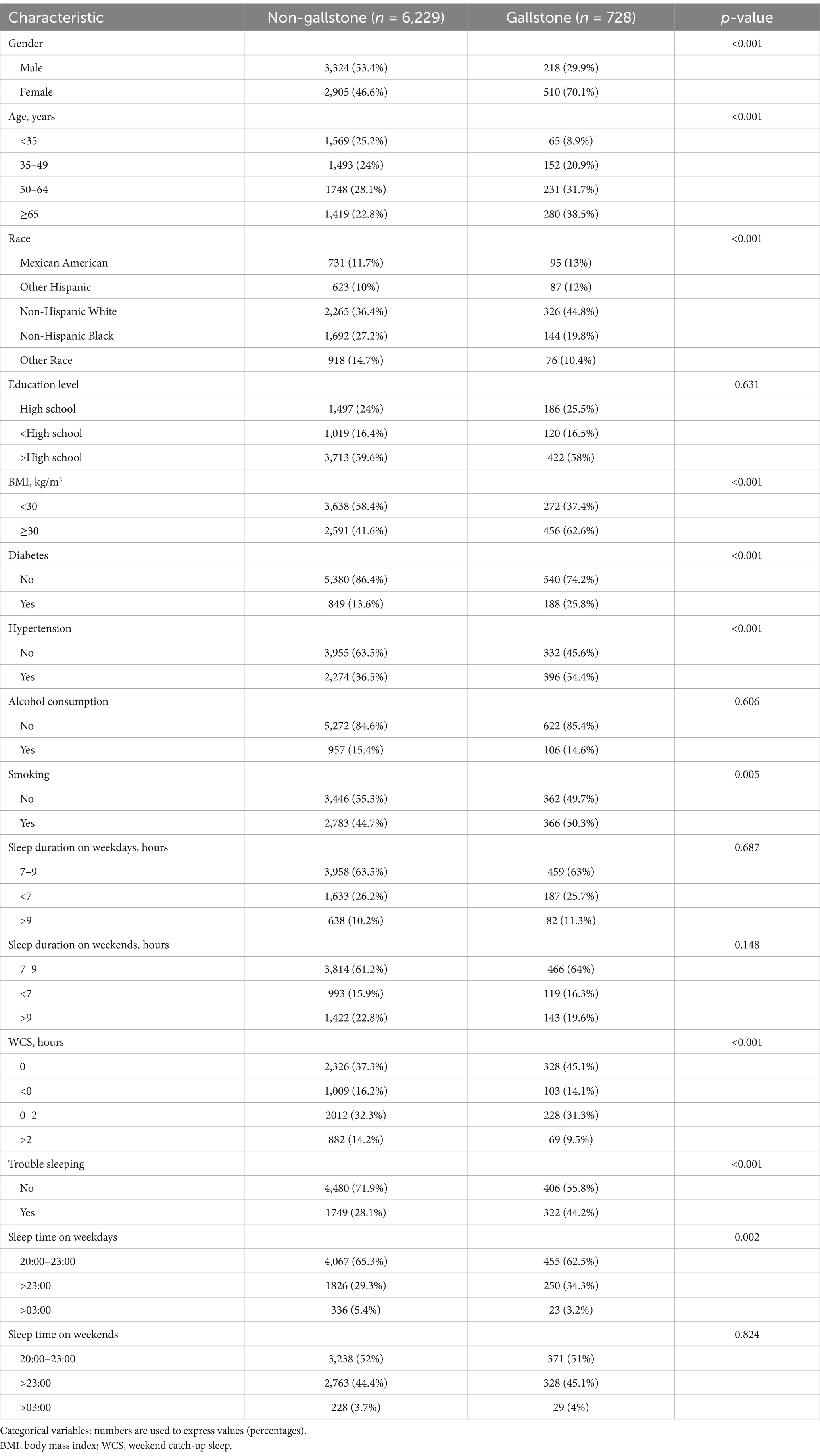
The characteristics of the variables involved in this study are summarized in Table 1. After applying our exclusion criteria, there were 6,957 participants in the study, including 728 participants with gallstones and 6,229 participants without gallstones. The participants with gallstones tended to be female (p < 0.001), older (p < 0.001), Non-Hispanic White (p < 0.001), have a BMI ≥ 30 kg/m2 (p < 0.001), smokers (p = 0.005), have trouble sleeping (p < 0.001), WCS = 0 (p < 0.001), sleep time >23:00 on weekdays (p = 0.002), have diabetes (p < 0.001) and hypertension (p < 0.001) compared to participants without gallstones.
3.2 Association between gallstones and WCS and other sleep factors
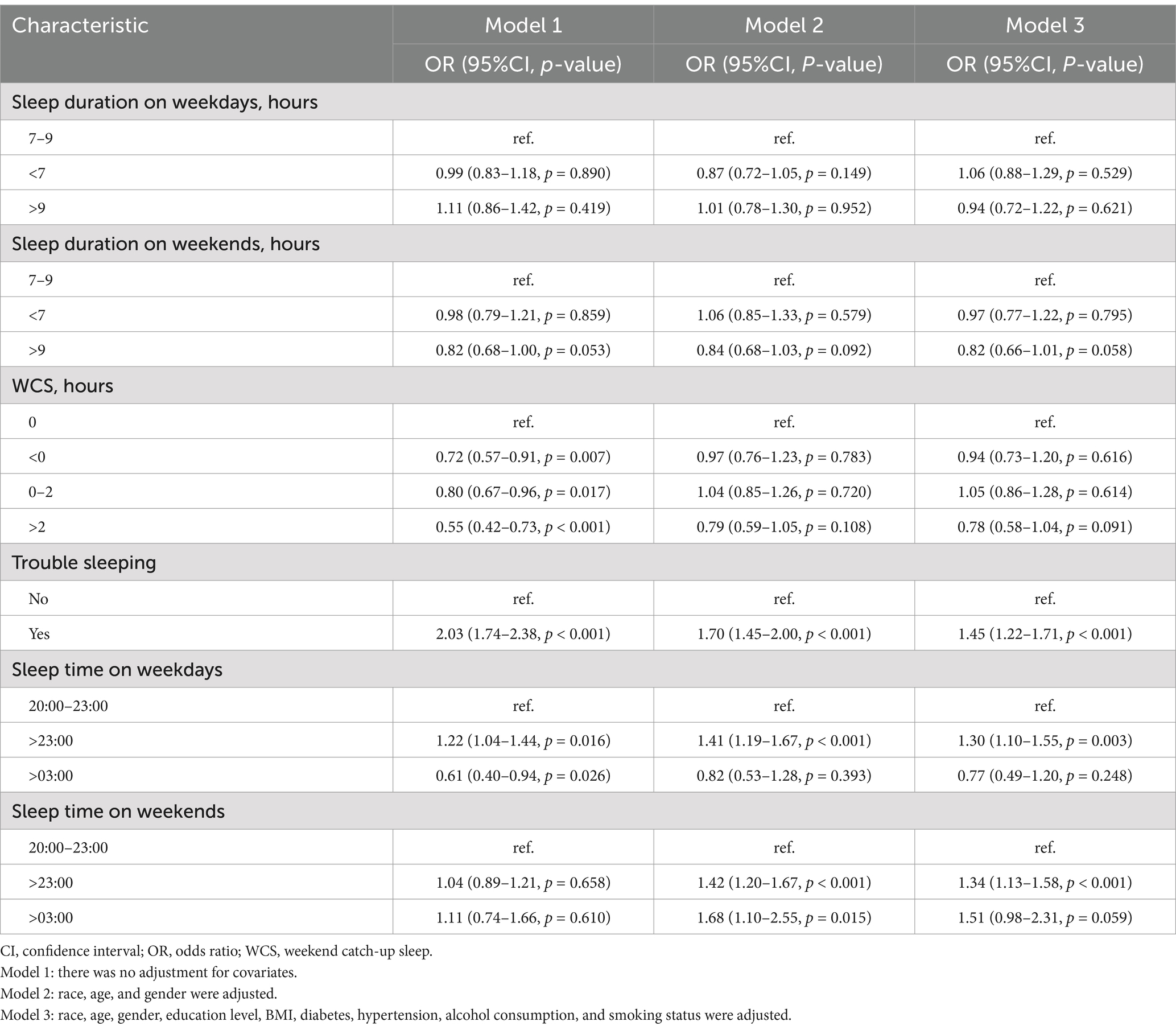
Table 2 shows the relationship between gallstones and WCS and other sleep factors. In Model 1, when comparing with the reference group of WCS = 0 h, WCS < 0 h (OR = 0.72; 95% CI: 0.57–0.91), WCS 0–2 h (OR = 0.80; 95% CI: 0.67–0.96), and WCS > 2 h (OR = 0.55; 95% CI: 0.42–0.73) were identified as protective factors for gallstones. In Model 2, gender, age, and race were adjusted. When comparing participants with sleep time from 20:00 to 23:00 as the reference group, sleep time > 23:00 on weekdays showed 1.41 times higher risk of gallstones, as well as sleep time > 23:00 and > 03:00 on weekends, showed 1.42 times and 1.68 times higher risk of gallstones, respectively. In Model 3, all covariates were adjusted; participants with trouble sleeping showed a 1.45 times higher risk of gallstones. Furthermore, participants with sleep time > 23:00 on both weekdays (OR = 1.30; 95% CI: 1.10–1.55) and weekends (OR = 1.34; 95% CI: 1.13–1.58) exhibited a higher probability of developing gallstone disease.
As illustrated in Figure 2, after adjusting for covariates, namely gender, race, education level, BMI, diabetes, alcohol consumption, and smoking status, the RCS results showed that the p-value of the overall effect test (p-overall) was 0.045, indicating a significant association between WCS and gallstones. However, the p-value of the non-linear effect test (p-non-linear) was 0.337, suggesting that no significant non-linear relationship was found. Therefore, it can be concluded that the relationship between WCS and gallstones is linear.
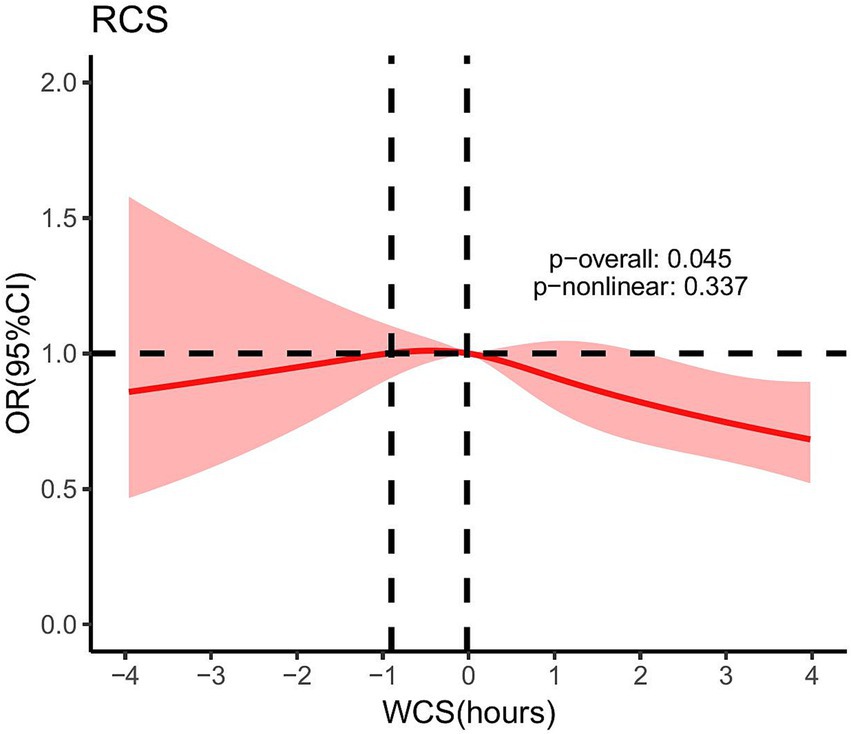
Figure 2. After adjusting for gender, race, education level, BMI, diabetes, alcohol consumption, and smoking status, the RCS for the association between WCS and gallstones. Dotted lines mean: At WCS values of −0.90 h and −0.02 h, the OR was equal to 1. WCS, weekend catch-up sleep; OR, odds ratio; RCS, restricted cubic spline.
3.3 Subgroup analyses
To further examine the relationship between WCS and gallstone disease, we categorized individuals based on gender, BMI, hypertension, diabetes, and smoking status, followed by a multivariable logistic regression analysis (Figure 3). There were significant interaction effects between gender (p for interaction = 0.003), smoking status (p for interaction = 0.022), and WCS on the risk of gallstones. In addition, we found that in the male, non-smoking participants, WCS > 2 h is a protective factor against gallstones, whereas in the smoking participants, WCS < 0 h served as a protective factor against gallstones.
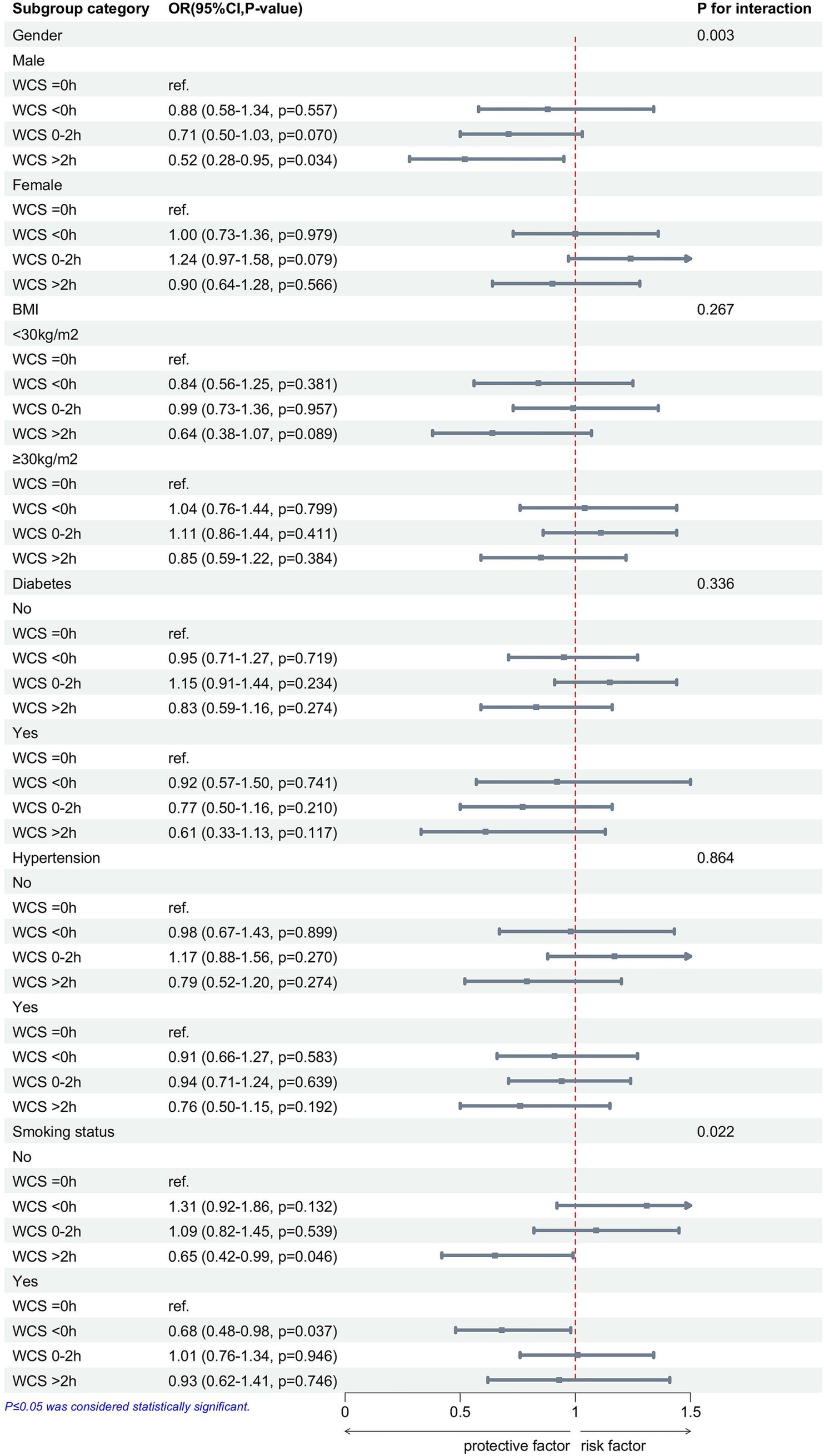
Figure 3. After adjusting for race, age, gender, BMI, education level, diabetes, hypertension, smoking status, and alcohol consumption, subgroup analyses show the associations between WCS and gallstones. WCS, weekend catch-up sleep; BMI, body mass index; OR, odds ratio; CI, confidence interval.
4 Discussion
This cross-sectional study used NHANES data from 2017 to 2020 to examine the relationship between WCS, other sleep factors, and gallstones. The results of this study demonstrated that trouble sleeping, sleep time on weekdays and weekends, and WCS are closely connected with gallstones. In Model 1, unadjusted for any variables, both WCS < 0 h and WCS > 0 h were identified as protective factors against gallstones. In model 3, adjusted for all covariates, trouble sleeping and late sleeping were risk factors for gallstones. In addition, the RCS demonstrated a negative linear correlation between WCS and gallstones. In subgroup analyses adjusted for all covariates, WCS > 2 h was found to be a protective factor for gallstones in the male and non-smokers, but WCS < 0 h was a protective factor for gallstones in the smokers.
Gallstones are a major public health issue with high prevalence rates in the United States and Europe, where they are the most frequently encountered gastrointestinal disorders leading to hospital admissions (1, 26, 27). Sleep is a critical physiological process that modulates various metabolic and endocrine functions (9). Additionally, WCS has been linked to some health conditions (20, 22). In Table 1, we found that weekday sleep time was significantly associated with gallbladder stones, whereas weekend sleep time was not. One possibility is that weekday routines are more consistent, while weekend behaviors are influenced by diet (28). This may lead to a non-significant association between weekend sleep time and gallstones. In Table 2, we found that both WCS < 0 h and WCS > 0 h were protective factors for gallstones compared to WCS = 0 h. WCS < 0 h indicates that weekend sleep duration is shorter than weekday sleep duration, which typically corresponds to earlier waking times and earlier breakfast consumption on weekends. This may promote earlier bile secretion, preventing bile stasis and thereby reducing the risk of gallstone formation (29). It may also represent relatively longer daytime activity and increased exercise on weekends, thus reducing the incidence of gallstones (30). WCS > 0 h means weekend sleep duration is greater than weekday sleep duration. Previous studies have indicated that for each hour increase in WCS, BMI decreases by 0.12 kg/m2 (19), and WCS > 1 h can reduce the risk of developing hypertension (21). Obesity and hypertension are risk factors associated with an increased incidence of gallstones (6, 31), suggesting that WCS may reduce the prevalence of gallstones. In addition, the study by Han et al. found that WCS can reduce high-sensitivity C-reactive protein levels (23). C-reactive protein is an acute-phase protein secreted by the liver in response to infection, inflammation, or tissue injury, induced by pro-inflammatory cytokines (32). An inflammatory response in gallbladder epithelial cells may be triggered by elevated levels of circulating inflammatory proteins and cytokines, leading to reduced contractility, wall fibrosis, and epithelial damage (33–35). This dysmotility impairs gallbladder contraction and bile expulsion, creating conditions conducive to gallstone formation (36, 37). In the study by Jiang et al. (38), higher Log high-sensitivity C-reactive protein levels were associated with an increased risk of gallstones, particularly in younger individuals. This suggests that WCS may reduce gallstone formation by lowering high-sensitivity C-reactive protein levels. In our study, we also found that sleep time on weekends and weekdays is similarly connected with the presence of gallstones. All covariates were adjusted, and we found that late sleep and abnormal sleep time are correlated with a higher incidence of developing gallstones. This is consistent with the findings of Zhuang et al. (24), who reported that late sleep increases the prevalence of gallstones. One study demonstrated that circadian rhythm disruption leads to impairments in hepatic lipid metabolism and dysbiosis of gut microbiota in mice (39). Disruptions in lipid metabolism and gut microbiota are risk factors for gallstone formation, promoting the development of gallstones (1). Late sleep is often associated with prolonged exposure to light at night (40), as indoor light exposure before bedtime inhibits melatonin production and reduces its duration (41). Research indicates that melatonin can reduce gallstone formation by decreasing intestinal cholesterol absorption and enhancing gallbladder motility (42). In our study, we discovered that trouble sleeping is connected with a higher prevalence of gallstone disease, which aligns with the findings of Zhuang et al. (24). Trouble sleeping is associated with hypertension, obesity, and reduced vitamin C (43–45). These conditions, in turn, are linked to an increased risk of gallstone formation (6, 7, 31). Zhang et al. (24) identified short sleep duration as a risk factor for gallstones; however, our study found no significant correlation between either long or short sleep duration and gallstone incidence. We require large-scale prospective cohort studies to delineate the associations between them more comprehensively.
Subgroup analysis showed a significant association between WCS and gallstones, especially in the gender and smoking groups. Gender and smoking were strongly associated with gallstones, and male and non-smokers were protective factors for gallstones (5, 46). There is a close association between gender and sleep, with females exhibiting longer sleep duration but poorer sleep quality (47). This may cause WCS to be influenced by both sleep quality and sleep duration, resulting in gender differences in the protective effect of WCS against gallstones. Studies have shown a significant link between smoking and sleep, with smoking disrupting sleep structure, reducing sleep quality, and affecting sleep-related complications (48). WCS < 0 h usually implies an early breakfast, which may promote bile excretion and avoid cholestasis (29). It may also reflect differences in smokers’ lifestyle habits, and further biological and behavioral studies are needed to verify this. In addition, prospective studies and mechanistic experiments are needed to verify the interactive effects of gender, smoking, and WCS on gallstone formation.
Our research presents several notable advantages. First, the individuals in the NHANES study comprise an adequate representation sample of the U.S. population, meticulously adhering to a rigorously designed protocol and subjected to stringent quality control measures, thereby ensuring the validity of our findings. Second, we performed a comprehensive analysis of the relationships between WCS and other sleep-related factors with gallstone disease. Finally, through subgroup analyses, we further investigated the connection between WCS and gallstone disease among several demographic categories.
Nonetheless, our research also has inherent restrictions. First, because it is a cross-sectional study, the causality between WCS and gallstones remains to be clarified. Second, the NHANES data are derived from self-reported questionnaires, which may introduce recall bias. Despite these limitations, this study offers novel perspectives on the relationship between WCS and the prevalence of gallstone disease.
In conclusion, our research indicates that WCS can lower the incidence of gallstones, especially when WCS > 2 h, while trouble sleeping and late sleep can increase this incidence. In addition, the protective effect of WCS > 2 h on reducing the incidence of gallstones is particularly significant in individuals who are non-smokers and males, whereas in the smoking population, WCS < 0 h serves as a protective factor against gallstones.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Institutional Review Board and Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JC: Data curation, Methodology, Writing – original draft, Software, Validation, Writing – review & editing. WZ: Investigation, Software, Writing – original draft. JY: Investigation, Software, Writing – original draft. YZ: Investigation, Software, Writing – original draft. XT: Formal analysis, Writing – original draft. XZ: Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are grateful to the NHANES program’s founders and participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Di Ciaula, A, Wang, DQ, and Portincasa, P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. (2018) 34:71–80. doi: 10.1097/MOG.0000000000000423
2. Innes, K, Hudson, J, Banister, K, Croal, B, Ramsay, C, Ahmed, I, et al. Core outcome set for symptomatic uncomplicated gallstone disease. Br J Surg. (2022) 109:539–44. doi: 10.1093/bjs/znac095
3. European Association for the Study of the Liver (EASL). EASL clinical practice guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. (2016) 65:146–81. doi: 10.1016/j.jhep.2016.03.005
4. Gutt, C, Schläfer, S, and Lammert, F. The treatment of gallstone disease. Dtsch Arzteblatt Int. (2020) 117:148–58. doi: 10.3238/arztebl.2020.0148
5. Figueiredo, JC, Haiman, C, Porcel, J, Buxbaum, J, Stram, D, Tambe, N, et al. Sex and ethnic/racial-specific risk factors for gallbladder disease. BMC Gastroenterol. (2017) 17:153. doi: 10.1186/s12876-017-0678-6
6. Stender, S, Nordestgaard, BG, and Tybjaerg-Hansen, A. Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a mendelian randomization study. Hepatol Baltim Md. (2013) 58:2133–41. doi: 10.1002/hep.26563
7. Simon, JA, and Hudes, ES. Serum ascorbic acid and gallbladder disease prevalence among US adults: the third national health and nutrition examination survey (NHANES III). Arch Intern Med. (2000) 160:931–6. doi: 10.1001/archinte.160.7.931
8. Friedman, GD, Kannel, WB, and Dawber, TR. The epidemiology of gallbladder disease: observations in the Framingham study. J Chronic Dis. (1966) 19:273–92. doi: 10.1016/0021-9681(66)90132-9
9. Sharma, S, and Kavuru, M. Sleep and metabolism: An overview. Int J Endocrinol. (2010) 2010:270832. doi: 10.1155/2010/270832
10. Song, X, Mitnitski, A, and Rockwood, K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. (2010) 58:681–7. doi: 10.1111/j.1532-5415.2010.02764.x
11. Van Cauter, E, Spiegel, K, Tasali, E, and Leproult, R. Metabolic consequences of sleep and sleep loss. Sleep Med. (2008) 9:S23–8. doi: 10.1016/S1389-9457(08)70013-3
12. Zielinski, MR, McKenna, JT, and McCarley, RW. Functions and mechanisms of sleep. AIMS Neurosci. (2016) 3:67–104. doi: 10.3934/Neuroscience.2016.1.67
13. Watson, NF, Badr, MS, Belenky, G, Bliwise, DL, Buxton, OM, Buysse, D, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep. (2015) 38:843–4. doi: 10.5665/sleep.4716
14. Fu, X, Zhao, X, Lu, H, Jiang, F, Ma, X, and Zhu, S. Association between sleep duration and bone mineral density in Chinese women. Bone. (2011) 49:1062–6. doi: 10.1016/j.bone.2011.08.008
15. Cunningham, TD, and Di Pace, BS. Is self-reported sleep duration associated with osteoporosis? Data from a 4-year aggregated analysis from the national health and nutrition examination survey. J Am Geriatr Soc. (2015) 63:1401–6. doi: 10.1111/jgs.13477
16. Ochs-Balcom, HM, Hovey, KM, Andrews, C, Cauley, JA, Hale, L, Li, W, et al. Short sleep is associated with low bone mineral density and osteoporosis in the women’s health initiative. J Bone Miner Res Off J Am Soc Bone Miner Res. (2020) 35:261–8. doi: 10.1002/jbmr.3879
17. Chunnan, L, Shaomei, S, and Wannian, L. The association between sleep and depressive symptoms in US adults: data from the NHANES (2007-2014). Epidemiol Psychiatr Sci. (2022) 31:e63. doi: 10.1017/S2045796022000452
18. Kang, SG, Lee, YJ, Kim, SJ, Lim, W, Lee, HJ, Park, YM, et al. Weekend catch-up sleep is independently associated with suicide attempts and self-injury in Korean adolescents. Compr Psychiatry. (2014) 55:319–25. doi: 10.1016/j.comppsych.2013.08.023
19. Im, HJ, Baek, SH, Chu, MK, Yang, KI, Kim, WJ, Park, SH, et al. Association between weekend catch-up sleep and lower body mass: population-based study. Sleep. (2017) 40:89. doi: 10.1093/sleep/zsx089
20. Zhu, H, Qin, S, and Wu, M. Association between weekend catch-up sleep and cardiovascular disease: evidence from the national health and nutrition examination surveys 2017-2018. Sleep Health. (2024) 10:98–103. doi: 10.1016/j.sleh.2023.09.006
21. Hwangbo, Y, Kim, WJ, Chu, MK, Yun, CH, and Yang, KI. Association between weekend catch-up sleep duration and hypertension in Korean adults. Sleep Med. (2013) 14:549–54. doi: 10.1016/j.sleep.2013.02.009
22. Luo, Z, Wang, T, Wu, W, Yan, S, and Chen, L. Association between weekend catch-up sleep and depressive symptoms in American adults: finding from NHANES 2017-2020. J Affect Disord. (2024) 354:36–43. doi: 10.1016/j.jad.2024.03.008
23. Han, KM, Lee, HJ, Kim, L, and Yoon, HK. Association between weekend catch-up sleep and high-sensitivity C-reactive protein levels in adults: a population-based study. Sleep. (2020) 43:zsaa010. doi: 10.1093/sleep/zsaa010
24. Zhuang, Q, Cheng, J, Wu, S, Shen, S, Huang, D, Ning, M, et al. Association between sleep and gallstone disease in United States adults: a cross-sectional study. BMC Public Health. (2024) 24:3291. doi: 10.1186/s12889-024-20824-y
25. Chaput, JP, Dutil, C, and Sampasa-Kanyinga, H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep. (2018) 10:421–30. doi: 10.2147/NSS.S163071
26. Farthing, M, Roberts, SE, Samuel, DG, Williams, JG, Thorne, K, Morrison-Rees, S, et al. Survey of digestive health across europe: final report. Part 1: the burden of gastrointestinal diseases and the organisation and delivery of gastroenterology services across europe. United Eur Gastroenterol J. (2014) 2:539–43. doi: 10.1177/2050640614554154
27. Unalp-Arida, A, and Ruhl, CE. Burden of gallstone disease in the United States population: Prepandemic rates and trends. World J Gastrointest Surg. (2024) 16:1130–48. doi: 10.4240/wjgs.v16.i4.1130
28. An, R. Weekend-weekday differences in diet among U.S. adults, 2003-2012. Ann Epidemiol. (2016) 26:57–65. doi: 10.1016/j.annepidem.2015.10.010
29. Zhang, H, Xu, C, Zhu, X, Zhang, J, Yin, J, Yao, N, et al. Associations between temporal eating patterns and energy distribution patterns with gallstones: a cross-sectional study based on NHANES 2017-2018. BMC Public Health. (2024) 24:2994. doi: 10.1186/s12889-024-20512-x
30. Zhang, Y-P, Zhao, Y-L, Sun, Y-L, Zhu, R-T, Wang, W-J, and Li, J. Physical activity and the risk of gallstone disease: a systematic review and meta-analysis. J Clin Gastroenterol. (2017) 51:857–68. doi: 10.1097/MCG.0000000000000571
31. Zhang, Y, Sun, L, Wang, X, and Chen, Z. The association between hypertension and the risk of gallstone disease: a cross-sectional study. BMC Gastroenterol. (2022) 22:138. doi: 10.1186/s12876-022-02149-5
32. Han, E, Fritzer-Szekeres, M, Szekeres, T, Gehrig, T, Gyöngyösi, M, and Bergler-Klein, J. Comparison of high-sensitivity C-reactive protein vs. C-reactive protein for cardiovascular risk prediction in chronic cardiac disease. J Appl Lab Med. (2022) 7:1259–71. doi: 10.1093/jalm/jfac069
33. Rege, RV. Inflammatory cytokines alter human gallbladder epithelial cell absorption/secretion. J Gastrointest Surg Off J Soc Surg Aliment Tract. (2000) 4:185–92. doi: 10.1016/s1091-255x(00)80055-4
34. Maurer, KJ, Carey, MC, and Fox, JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. (2009) 136:425–40. doi: 10.1053/j.gastro.2008.12.031
35. van Erpecum, KJ, Wang, DQ, Moschetta, A, Ferri, D, Svelto, M, Portincasa, P, et al. Gallbladder histopathology during murine gallstone formation: relation to motility and concentrating function. J Lipid Res. (2006) 47:32–41. doi: 10.1194/jlr.M500180-JLR200
36. Portincasa, P, Di Ciaula, A, and vanBerge-Henegouwen, GP. Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep. (2004) 6:151–62. doi: 10.1007/s11894-004-0043-0
37. Lavoie, B, Nausch, B, Zane, EA, Leonard, MR, Balemba, OB, Bartoo, AC, et al. Disruption of gallbladder smooth muscle function is an early feature in the development of cholesterol gallstone disease. Neurogastroenterol Motil. (2012) 24:e313–24. doi: 10.1111/j.1365-2982.2012.01935.x
38. Jiang, Z, Jiang, H, Zhu, X, Zhao, D, and Su, F. The relationship between high-sensitivity C-reactive protein and gallstones: a cross-sectional analysis. Front Med. (2024) 11:1453129. doi: 10.3389/fmed.2024.1453129
39. He, C, Shen, W, Chen, C, Wang, Q, Lu, Q, Shao, W, et al. Circadian rhythm disruption influenced hepatic lipid metabolism, gut microbiota and promoted cholesterol gallstone formation in mice. Front Endocrinol. (2021) 12:723918. doi: 10.3389/fendo.2021.723918
40. Fonken, LK, and Nelson, RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. (2014) 35:648–70. doi: 10.1210/er.2013-1051
41. Gooley, JJ, Chamberlain, K, Smith, KA, Khalsa, SB, Rajaratnam, SM, Van Reen, E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. (2011) 96:E463–72. doi: 10.1210/jc.2010-2098
42. Koppisetti, S, Jenigiri, B, Terron, MP, Tengattini, S, Tamura, H, Flores, LJ, et al. Reactive oxygen species and the hypomotility of the gall bladder as targets for the treatment of gallstones with melatonin: a review. Dig Dis Sci. (2008) 53:2592–603. doi: 10.1007/s10620-007-0195-5
43. Cai, Y, Chen, M, Zhai, W, and Wang, C. Interaction between trouble sleeping and depression on hypertension in the NHANES 2005-2018. BMC Public Health. (2022) 22:481. doi: 10.1186/s12889-022-12942-2
44. Wang, L, Sun, Y, Li, Y, He, L, Niu, Y, and Yan, N. The association between trouble sleeping and obesity among the U.S. elderly from NHANES 2011-2014: a moderated mediation model of depressive symptoms and cognitive function. J Affect Disord. (2024) 350:58–64. doi: 10.1016/j.jad.2024.01.112
45. Wang, S, Lai, F, Zhao, L, Zhou, J, Kong, D, Yu, H, et al. Association between vitamin C in serum and trouble sleeping based on NHANES 2017-2018. Sci Rep. (2024) 14:9727. doi: 10.1038/s41598-024-56703-0
46. Larsson, SC, and Burgess, S. Appraising the causal role of smoking in multiple diseases: a systematic review and meta-analysis of Mendelian randomization studies. EBioMedicine. (2022) 82:104154. doi: 10.1016/j.ebiom.2022.104154
47. Burgard, SA, and Ailshire, JA. Gender and time for sleep among U.S. adults. Am Sociol Rev. (2013) 78:51–69. doi: 10.1177/0003122412472048
Keywords: NHANES, weekend catch-up sleep, sleep, gallstones, smoking
Citation: Cao J, Zhang W, Yi J, Zhang Y, Tong X and Zhu X (2025) Association between weekend catch-up sleep and gallstone disease in US adults: a cross-sectional study from NHANES 2017–2020. Front. Public Health. 13:1573858. doi: 10.3389/fpubh.2025.1573858
Edited by:
Allen C. Meadors, Independent Researcher, Seven Lakes, NC, United StatesReviewed by:
Jeff Bolles, Francis Marion University, United StatesHarshi Weerakoon, Rajarata University of Sri Lanka, Sri Lanka
Copyright © 2025 Cao, Zhang, Yi, Zhang, Tong and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangnan Zhu, enhuMTM4MDc5MTg0MjBAMTYzLmNvbQ==
 Jianwei Cao
Jianwei Cao Weishuai Zhang
Weishuai Zhang Xiangnan Zhu
Xiangnan Zhu