- 1Department of Pharmacy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 2Department of Nuclear Medicine/PET Image Center, The Second Xiangya Hospital of Central South University, Changsha, China
- 3School of Pharmacy, Guangdong Medical University, Dongguan, China
- 4Department of Pharmacy, The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University, Changsha, China
Objectives: Rezvilutamide, an androgen-receptor inhibitor, has been approved by the Chinese National Medical Products Administration as a first-line treatment for high-volume metastatic hormone-sensitive prostate cancer (mHSPC). This study aims to assess the cost-effectiveness of rezvilutamide plus androgen-deprivation therapy (ADT) compared to bicalutamide plus ADT for the first-line treatment of high-volume mHSPC in China.
Methods: A Markov model with three health states was developed to evaluate the health and economic outcomes of first-line treatment for high-volume mHSPC. Efficacy data were sourced from the CHART trial. Quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratios (ICERs) were calculated. To address modeling uncertainties, one-way sensitivity analysis and probabilistic sensitivity analysis were performed.
Results: Compared with bicalutamide plus ADT, rezvilutamide plus ADT resulted in an additional 2.16 QALYs, with an ICER of $39,122.16/QALY. At a willingness-to-pay (WTP) threshold of three times the gross domestic product per capita in China for 2023 ($37,256.3/QALY), the probability of cost-effectiveness for rezvilutamide plus ADT was 30%. One-way sensitivity analysis revealed that the results were most sensitive to the cost of rezvilutamide. Scenario analysis indicated that rezvilutamide could be considered cost-effective if priced below $705.46 per cycle.
Conclusion: From the perspective of Chinese payers, rezvilutamide plus ADT appears to be a less cost-effective strategy compared to bicalutamide plus ADT for the first-line treatment of high-volume mHSPC in China.
Introduction
Prostate cancer is a prevalent genitourinary malignancy, particularly among older men. According to the Global Cancer Statistics 2022, prostate cancer is the second most frequently diagnosed malignancy in men worldwide and the fifth leading cause of cancer-related deaths among men (1). In China, it accounts for 13.42% of male cancer incidence and 4.75% of cancer-related deaths (2). Although the 5-year survival rate for clinically localized prostate cancer in China increased from 53.8% to 66.4% between 2003 and 2015, it remained significantly lower compared to developed countries such as the United States, where the survival rate was close to 100% (3). However, only 30% of cases are diagnosed at an early stage, while the majority of prostate cancer cases in China are diagnosed in the intermediate to advanced stages (3). The prognosis for metastatic prostate cancer is generally poor, with a 5-year survival rate of approximately 36.6% (4, 5). As a result, innovative therapeutic approaches are critically needed to improve survival outcomes for patients with metastatic prostate cancer.
For many years, androgen deprivation therapy (ADT), which includes both surgical and chemical castration, has been the standard of care for advanced prostate cancer (6). However, most patients with metastatic hormone-sensitive prostate cancer (mHSPC) who received ADT alone are at risk of developing metastatic castration-resistant prostate cancer (mCRPC) within 2 years (7). Recent advances have revealed the considerable potential of combining docetaxel with ADT. This combination therapy has demonstrated remarkable superiority over ADT alone (8–10). Bicalutamide, a first-generation androgen receptor (AR) inhibitor approved in the United States in 1995, has been commonly used for metastatic prostate cancer. However, first-generation AR inhibitors exhibit weak affinity for the AR, leading to limited efficacy in blocking AR activity and potential drug resistance due to AR overexpression or mutation (11). Consequently, first-generation AR inhibitors in combination with ADT, as well as ADT alone, were not recommended as standard treatment options for mHSPC in the 2021 European Association of Urology Guidelines (12). Second-generation AR inhibitors, such as enzalutamide (approved in the US in 2012), apalutamide (approved in 2018), and darolutamide (approved in 2019), have been shown to effectively delay the onset of castration resistance and improve overall survival for patients (13–16). In June 2022, the Chinese National Medical Products Administration (NMPA) approved rezvilutamide for the treatment of high-volume mHSPC. This treatment approach is also recommended by the Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment of prostate cancer guideline (17).
The clinical efficacy of rezvilutamide in combination with ADT was evaluated in the CHART study (18). CHART was a randomized, open-label, phase 3 study conducted across 72 hospitals in China, Poland, the Czech Republic, and Bulgaria. The study aimed to assess the efficacy and safety of rezvilutamide plus ADT as a first-line therapy for patients with high-volume mHSPC who had not previously received chemotherapy or other localized treatments. Total of 654 patients were eligible and randomly assigned to either the rezvilutamide group (240 mg orally once daily in a 4-week cycle; n = 326) or the bicalutamide group (50 mg orally once daily in a 4-week cycle; n = 326). All patients received background therapy with either surgical ADT or luteinizing hormone-releasing hormone (LHRH) agonists or antagonists, in accordance with the package insert, throughout the study period. The co-primary endpoints of the study were radiographic progression-free survival (rPFS) and overall survival (OS). The results showed that the rezvilutamide group significantly improved rPFS compared with the bicalutamide group [not reached vs. 25.1 months; hazard ratio = 0.44, 95% confidence interval (CI) 0.33–0.58, p < 0.0001]. Furthermore, rezvilutamide significantly improved OS compared to bicalutamide (not reached vs. not reached; hazard ratio = 0.58, 95% CI 0.44–0.77, p < 0.0001).
The findings of the CHART trial formed the basis for the regulatory approval of rezvilutamide in combination with ADT for the treatment of high-volume mHSPC in China. However, clinical treatment decisions and national health policy require evidence of cost-effectiveness. Since existing economics assessment studies have used partitioned survival model and reached controversial conclusions (19, 20). The objective of this study conducted a Markov model to evaluate the cost-effectiveness of rezvilutamide plus ADT versus bicalutamide plus ADT for high-volume mHSPC, based on the data from the CHART trial, from the perspective of the Chinese healthcare system.
Methods
Model overview
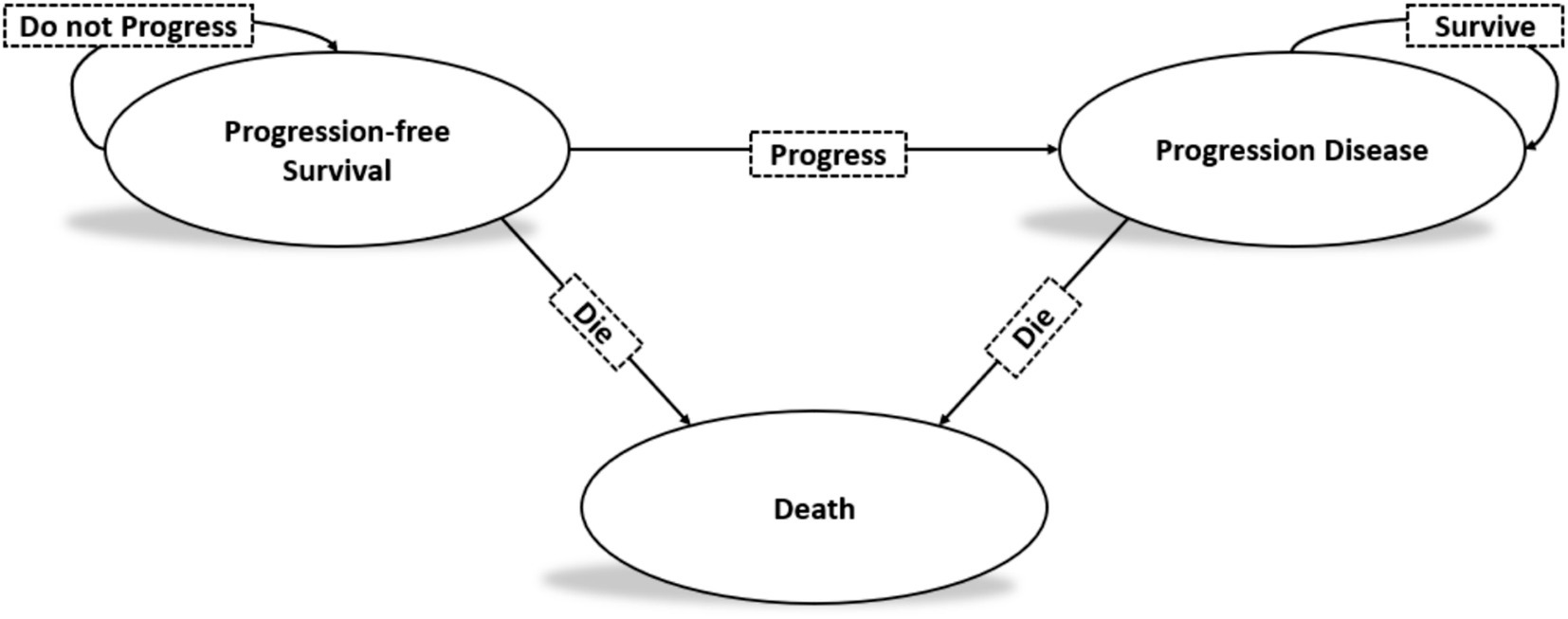
This study adhered to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guidelines (21, 22). A Markov model was developed to simulate the costs and health benefits associated with the treatment of high-volume mHSPC using rezvilutamide plus ADT versus bicalutamide plus ADT. The model included three distinct health states: progression-free survival (PFS), progressive disease (PD), and death (Figure 1). The cycle length of the Markov model was set to 28 days, aligning with the treatment periods. The average life expectancy in China in 2020 was 77.93 years (23). Given that the median age of patients in the CHART study was 69.2 years (range: 64–74 years), a 13-year time horizon was selected to better represent long-term survival of patients. All patients began in the PFS state and transitioned to either the PD state or death. Patients in the PD state could remain there or transition to death. The model’s outcomes included life years, quality-adjusted life years (QALYs), and costs. According to Chinese pharmacoeconomic evaluation guidelines, both costs and QALYs were discounted at an annual rate of 5%. Incremental cost-effectiveness ratios (ICERs) were calculated to represent the cost per additional QALY gained. The cost-effectiveness threshold in China was defined as $37,256.3, equivalent to three times the per capita gross domestic product (GDP) of China in 2023 (24, 25).
Clinical efficacy
Survival parameters were primarily derived from the Kaplan–Meier (KM) curve of the CHART trial (18). We used the Engauge Digitizer software to extract digitized data points from the PFS and OS KM curves of the CHART study. Individual patient data were then reconstructed using standard statistical methods as outlined by Guyot et al. (26). Several parametric survival functions were evaluated, including the Exponential, Weibull, Log-logistic, Gompertz, and Log-normal distributions. The model selection was based on the Akaike information criterion (AIC), Bayesian information criterion (BIC), and visual inspection of the parametric extrapolation and long-term survival estimates. The distribution with the lowest AIC or BIC values was chosen as the best-fit model (27).
Cost estimates
This analysis focused solely on direct medical costs, which included drug costs, costs for managing treatment-related adverse events (AEs), subsequent treatment costs, and disease management costs (outpatient visits, supportive care, nursing, laboratory tests). Drug costs were estimated based on patient dosing schedules and unit prices, calculated per treatment cycle. Unit drug costs were sourced from Hunan Public Resources Trading Service Platform (28). Costs associated with subsequent treatments, AE management, and end-of-life care were either estimated or referenced from published literature (29–31). For AE management costs, only grade ≥ 3 events from the CHART trials were included (18). According to the CHART study, the incidence of treatment-emergent AEs was 7% for hypertension and 2% for hypertriglyceridemia in the rezvilutamide plus ADT group, compared to 8% and 7%, respectively, in the bicalutamide plus ADT group. Upon disease progression, 27 and 62% of patients received subsequent treatments, including hormonal therapy, abiraterone acetate, enzalutamide, and docetaxel; the others received supportive care. All costs were adjusted to US dollars for 2024. Chinese Yuan was converted to US dollars using the exchange rate formula: 1 US $ = 7.1954 CNY (32). Key costs are shown in Table 1.
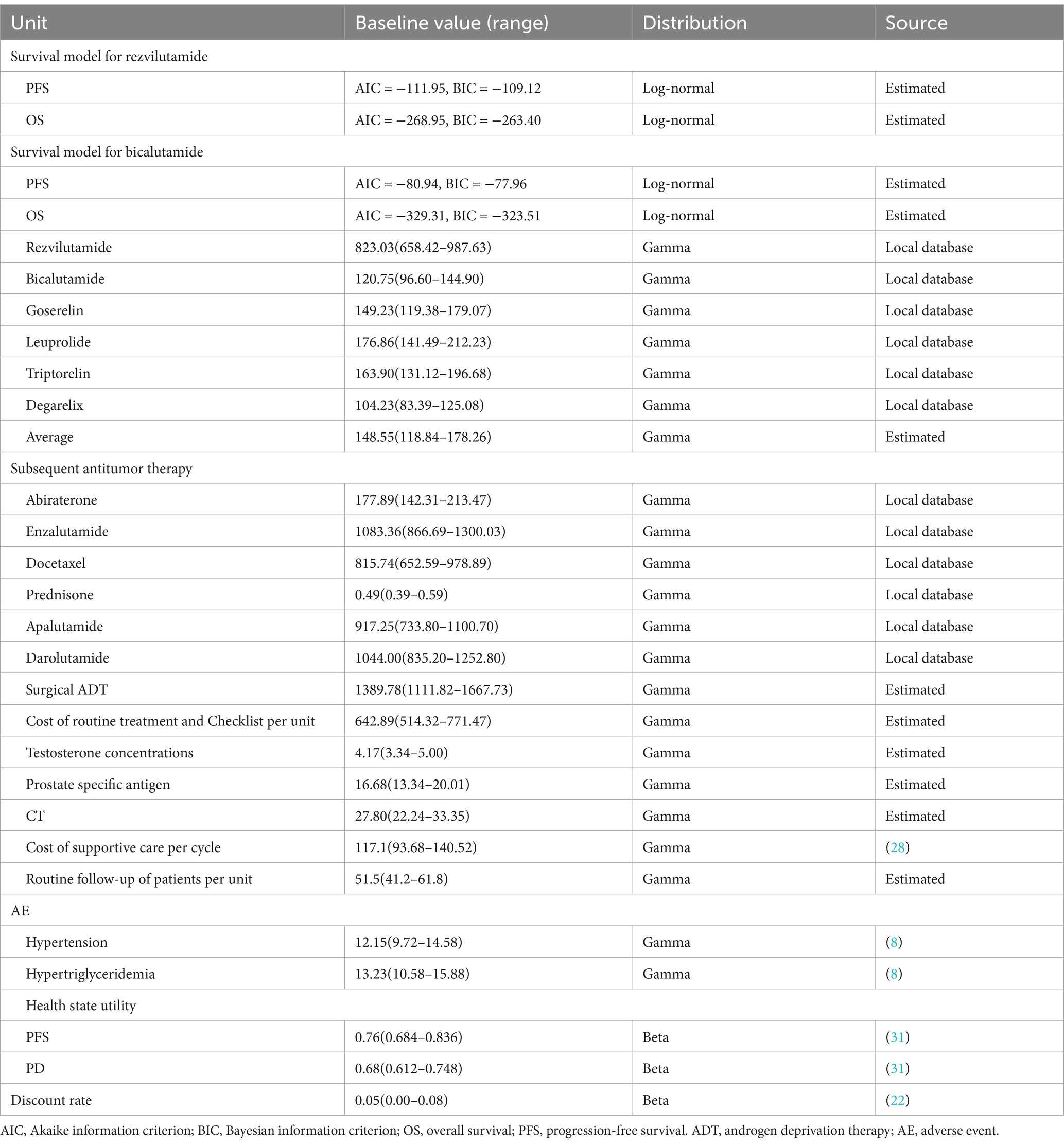
Table 1. Model parameters of clinical data, costs and utilities: baseline values, ranges, and distributions.
Utility estimates
Quality-adjusted life-years were estimated by adjusting survival time with health-related quality of life. Separate health state utility (HSU) values were applied for patients in the PFS and PD states. The HSU values for these different health states in the CHART trial population were derived from published literature (33). The values and sources for HSU were detailed in Table 1.
Sensitivity analysis
The robustness of the results was tested with a series of one-way sensitivity analysis on several parameters. In the one-way sensitivity analysis, the cost parameters ranged between −20% and +20% and the utility parameters were variable at 10% efficiency, as detailed in Table 1. The results of the one-way sensitivity analysis are presented as tornado diagrams. Moreover, probabilistic sensitivity analysis were conducted using Monte Carlo simulations with 10,000 replicated outcomes. The results of these are presented as cost-effectiveness acceptability curves. For the model, different distribution types were applied to each parameter: Gamma distributions for cost inputs and Beta distributions for utility values.
Results
Base-case analysis
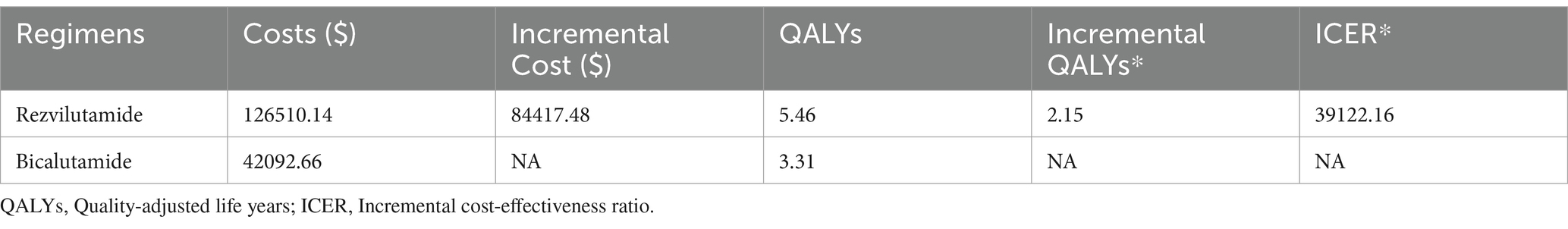
The results of the deterministic analysis are showed in Table 2. In patients with high-volume mHSPC, compared to bicalutamide plus ADT, rezvilutamide plus ADT yielded an additional 2.16 QALYs (5.46 QALYs vs. 3.31 QALYs), corresponding to an incremental cost of $84,417.48. The calculated ICER was $39,122.16/QALY gained, which exceeded the WTP threshold of three times the GDP per capita in China.
Sensitivity analysis
According to the one-way deterministic sensitivity analysis (DSA), the model was most sensitive to the cost of rezvilutamide (Figure 2). In the probabilistic sensitivity analysis (PSA), the cost-effectiveness plane illustrated the results of 10,000 Monte Carlo simulations (Figure 3). Comparing rezvilutamide plus ADT with bicalutamide plus ADT the cost-effectiveness acceptability curve indicated a nearly 30% probability of cost-effectiveness at the WTP threshold of $37,256.30, consistent with the base-case analysis results (Figure 4).
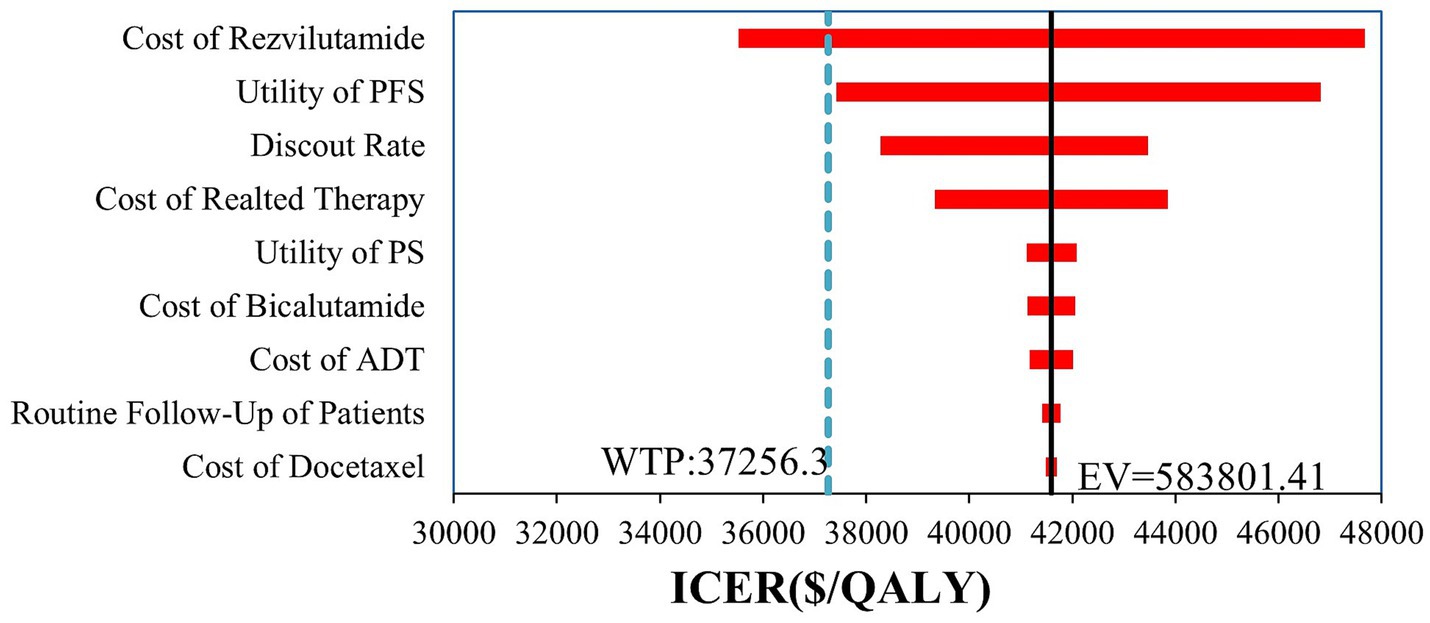
Figure 2. Tornado plot generated in the one-way deterministic sensitivity analysis. Only the top 9 most influential parameters are presented. PFS, progression-free survival; PS, progression survival; ADT, androgen deprivation therapy; WTP, willingness-to-pay; QALY, quality-adjusted life-year; ICER incremental cost-effectiveness ratio; EV, expect value.
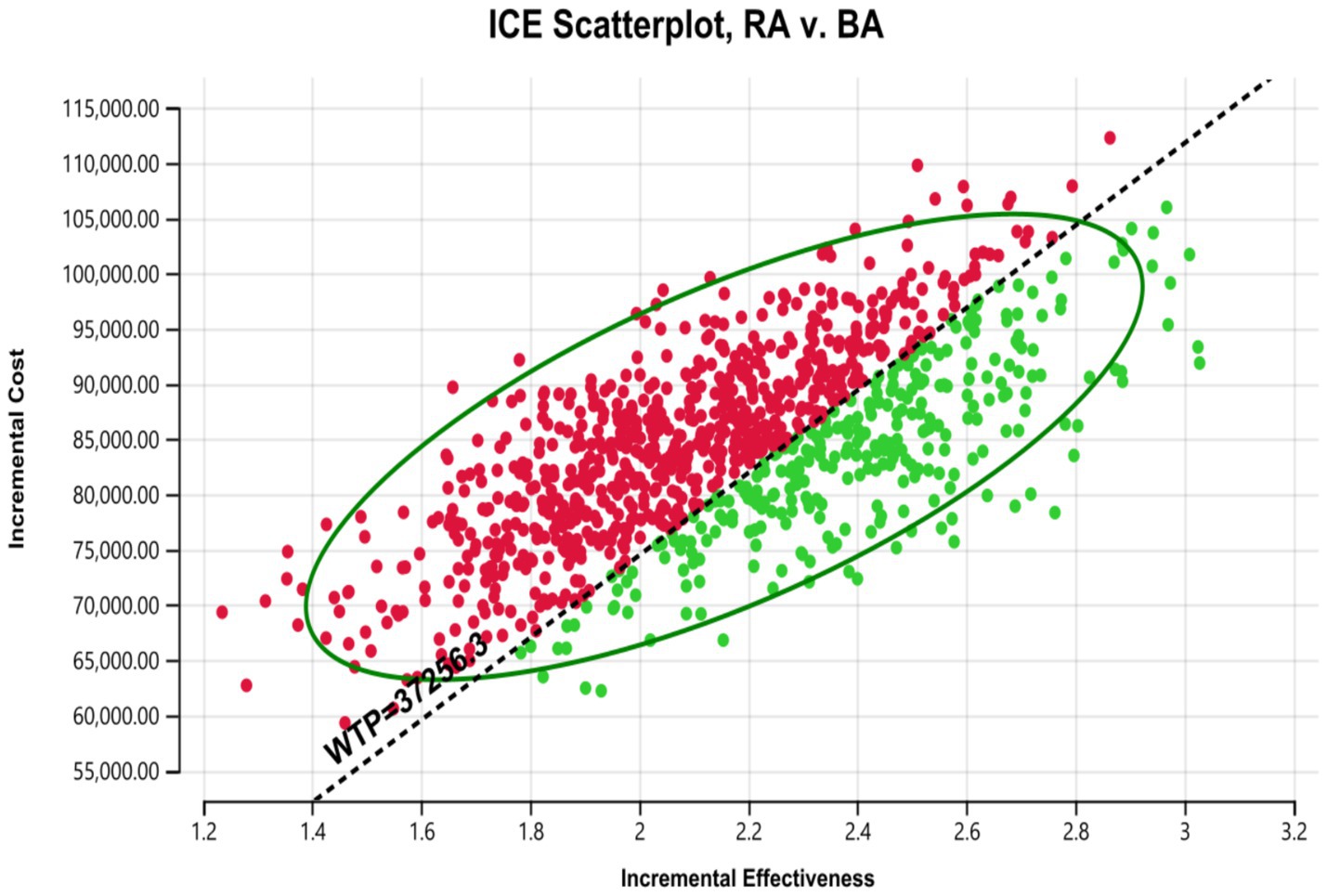
Figure 3. Cost-effectiveness plane generated in the probabilistic sensitivity analysis. WTP, willingness to pay; RA, rezvilutamide; BA, bicalutamide. Red: ICER value greater than WTP. Green: ICER value less than WTP.
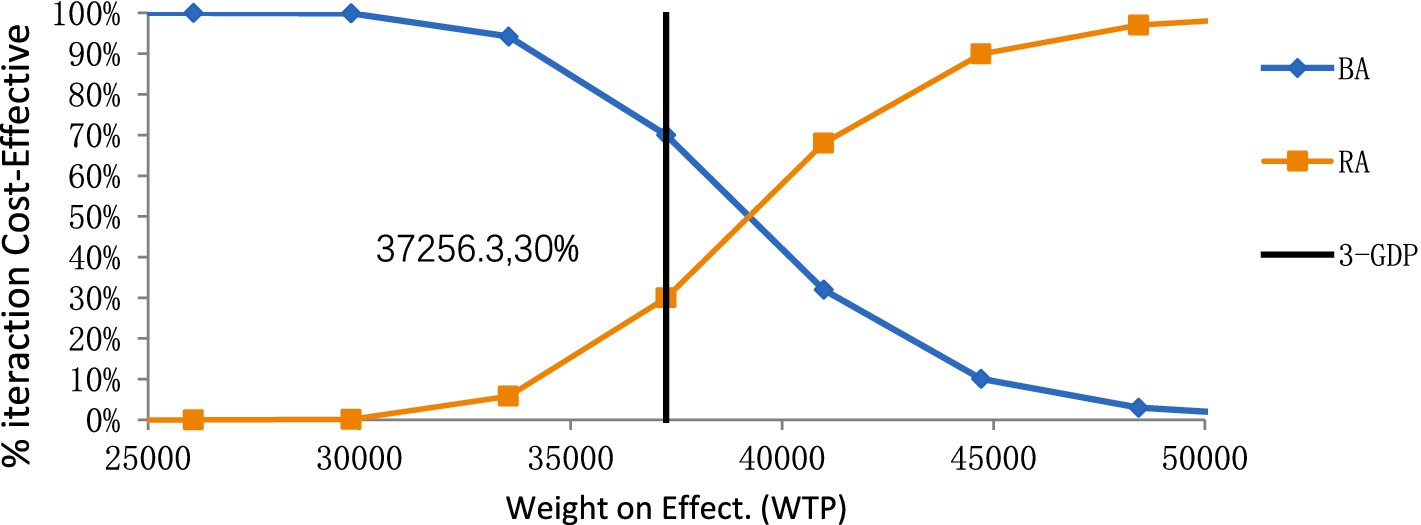
Figure 4. The cost-effectiveness acceptability curve for rezvilutamide versus bicalutamide. BA, bicalutamide; RA, rezvilutamide; WTP, willingness-to-pay; GDP, gross domestic product.
Discussion
The introduction of second-generation AR inhibitors has brought a new ray of hope to the treatment of high-volume mHSPC. The combination of AR inhibitors with ADT has been recommended as a first-line treatment in the guideline of the CSCO for prostate cancer (17). The recommended first-line treatment for patients with high-volume mHSPC includes ADT combined with an antiandrogen, such as bicalutamide, rezvilutamide, abiraterone, enzalutamide, or apalutamide. Despite these positive developments, many of these second-generation AR inhibitors were imported medications, and the clinical studies that have been registered primarily focused on Western populations. The CHART trial demonstrated that rezvilutamide, in combination with ADT, significantly improved the prognosis of patients with high-volume mHSPC when compared with bicalutamide plus ADT (18).
The purpose of this study, which employed a Markov model, was to assess the cost-effectiveness of rezvilutamide plus ADT compared with bicalutamide plus ADT in the treatment of high-volume mHSPC. The results suggest that, compared with bicalutamide plus ADT, rezvilutamide plus ADT was associated with an incremental survival of 2.16 QALYs and an incremental cost of $84,417.28. The calculated ICER was $39,122.16 per QALY. The cost-effectiveness acceptability curve revealed that rezvilutamide plus ADT was not cost-effective when the WTP threshold was set at $37,256.3 per QALY. However, these findings should not be used as a basis for limiting the use of rezvilutamide, as this may result in missed opportunities for beneficial treatment options. Instead, they should be regarded as economic considerations for informing the implementation of China’s national pricing negotiation policies. To address the issue of high drug prices, promote patient access, and ensure the sustainability of the medical insurance fund, China formally launched national reimbursement-linked price negotiations in 2017 (34, 35). Such a policy could significantly improve the cost-effectiveness of rezvilutamide.
The results of the one-way sensitivity analysis showed that the cost of rezvilutamide was the most sensitive factor affecting the ICER. The analysis revealed that the cost-effectiveness of rezvilutamide plus ADT compared to bicalutamide plus ADT could be influenced by the price of rezvilutamide. Rezvilutamide can be considered cost-effective only when priced below $705.46 per cycle at a WTP threshold of $37,256.3 per QALY. The results of both the one-way sensitivity analysis and probabilistic sensitivity analysis demonstrate the robustness of these findings. These results provide important insights for China’s health insurance policymakers when determining the price of rezvilutamide following its launch.
Several studies have assessed the cost-effectiveness of first-line treatments for high-volume mHSPC, including two Chinese studies focusing on the cost-effectiveness of rezvilutamide. As a novel therapy, rezvilutamide was associated with a high economic burden, highlighting the need for pharmacoeconomic research based on the CHART trial to evaluate its cost-effectiveness (19, 20, 36, 37). Ding et al. (20) previously conducted a partitioned survival model to evaluate the cost-effectiveness of rezvilutamide combined with ADT for high-volume mHSPC in China. Their study demonstrated that rezvilutamide plus ADT was more cost-effective compared to bicalutamide plus ADT as the first-line treatment for high-volume mHSPC from the perspective of the Chinese healthcare system (20). In contrast, Wu et al. (19) conducted a similar study using a partitioned survival model and concluded that rezvilutamide plus ADT is unlikely to be cost-effective for most adults when compared to bicalutamide plus ADT, considering a WTP threshold of $38,223.3 per QALY from the perspective of the Chinese healthcare system. However, they suggested that a promising economic advantage could be achieved if rezvilutamide were included in the National Reimbursement Drug List (NRDL) with a 10% price reduction (19). Due to the discrepancies between the findings of these two studies, we constructed a Markov model to assess the cost-effectiveness of rezvilutamide plus ADT versus bicalutamide plus ADT as a first-line treatment for high-volume mHSPC from the perspective of the Chinese healthcare system. Our results indicated that rezvilutamide treatment regimen would be considered cost-effective only when priced below $705.46 per cycle at a WTP threshold of $37,256.3 per QALY, yielding findings consistent with those of Wu et al. (19). This provided new evidence to inform clinical decision-making regarding antiandrogen drugs for Chinese patients with high-volume mHSPC based a Markov model cost-effectiveness comparison.
The present study has several limitations. First, the pharmacoeconomic evaluation was based on the CHART trial, which unfortunately had a limited follow-up period. As a result, we obtained progression-free survival PFS and OS data by fitting parameter distributions. Although extrapolation could obtain relevant data outside the follow-up period of the CHART trial, this would increase model uncertainty. To mitigate this, we conducted a comparative analysis using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) to select the best-fitting distribution, and performed sensitivity analysis to assess the robustness of the model results. Second, the study only focused on the two most serious AEs related to treatment, neglecting other potential AEs. As a result, there may be some degree of bias between the calculated costs and those in real-world settings. Third, several key parameters in the analysis, including utility scores, were obtained from the literature. However, a comprehensive search of existing studies did not identify utility scores specific to the Chinese population, which may impact the accuracy of the model results. Finally, this analysis was conducted while Rezvilutamide was still under patent protection. The pricing of generic equivalents may influence the cost-effectiveness analysis in future studies.
Conclusion
In conclusion, our analysis indicates that rezvilutamide plus ADT is not cost-effective at the current price compared to bicalutamide plus ADT. However, it becomes cost-effective if the price of rezvilutamide is reduced by 14.29%.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JH: Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft. XZ: Formal analysis, Methodology, Validation, Writing – original draft. WC: Data curation, Investigation, Writing – original draft. ZZ: Data curation, Investigation, Writing – original draft. YH: Data curation, Investigation, Writing – original draft. JM: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. LO: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Shenzhen High-level Hospital Construction Fund and the Natural Science Foundation of Hunan Province (2021JJ80080).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Zheng, RS, Chen, R, Han, BF, Wang, SM, Li, L, Sun, KX, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. (2024) 46:221–31. doi: 10.3760/cma.j.cn112152-20240119-00035
3. He, J, Chen, WQ, Li, N, Cao, W, Ye, DW, Ma, JH, et al. China guideline for the screening and early detection of prostate cancer (2022, Beijing). Zhonghua Zhong Liu Za Zhi. (2022) 44:29–53. doi: 10.3760/cma.j.cn112152-20211226-00975
4. Zeng, H, Chen, W, Zheng, R, Zhang, S, Ji, JS, Zou, X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. (2018) 6:e555–67. doi: 10.1016/S2214-109X(18)30127-X
5. National Cancer Institute Surveillance, Epidemiology, and end results (SEER) program. Cancer Stat Facts Prostate Cancer. Available online at: https://seer.cancer.gov/statfacts/html/prost.html (Accessed October 20, 2024).
6. Virgo, KS, Rumble, RB, and Talcott, J. Initial Management of Noncastrate Advanced, recurrent, or metastatic prostate Cancer: ASCO guideline update. J Clin Oncol. (2023) 41:3652–6. doi: 10.1200/JCO.23.00155
7. Lin, TT, Chen, YH, Wu, YP, Chen, SZ, Li, XD, Lin, YZ, et al. Risk factors for progression to castration-resistant prostate cancer in metastatic prostate cancer patients. J Cancer. (2019) 10:5608–13. doi: 10.7150/jca.30731
8. Kyriakopoulos, CE, Chen, YH, Carducci, MA, Liu, G, Jarrard, DF, Hahn, NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate Cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. (2018) 36:1080–7. doi: 10.1200/JCO.2017.75.3657
9. James, ND, Sydes, MR, Clarke, NW, Mason, MD, Dearnaley, DP, Spears, MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. (2016) 387:1163–77. doi: 10.1016/S0140-6736(15)01037-5
10. Gravis, G, Fizazi, K, Joly, F, Oudard, S, Priou, F, Esterni, B, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. (2013) 14:149–58. doi: 10.1016/S1470-2045(12)70560-0
11. Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. (2000) 355:1491–8. doi: 10.1016/S0140-6736(00)02163-2
12. Cornford, P, van den Bergh, RCN, Briers, E, Van den Broeck, T, Cumberbatch, MG, De Santis, M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer. Part II-2020 update: treatment of relapsing and metastatic prostate Cancer. Eur Urol. (2021) 79:263–82. doi: 10.1016/j.eururo.2020.09.046
13. Smith, MR, Hussain, M, Saad, F, Fizazi, K, Sternberg, CN, Crawford, ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate Cancer. N Engl J Med. (2022) 386:1132–42. doi: 10.1056/NEJMoa2119115
14. Hussain, M, Tombal, B, Saad, F, Fizazi, K, Sternberg, CN, Crawford, ED, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate Cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. (2023) 41:3595–607. doi: 10.1200/JCO.23.00041
15. Chi, KN, Chowdhury, S, Bjartell, A, Chung, BH, de Santana, P, Gomes, AJ, et al. Apalutamide in patients with metastatic castration-sensitive prostate Cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. (2021) 39:2294–303. doi: 10.1200/JCO.20.03488
16. Armstrong, AJ, and Iguchi, AAT. Final overall survival (OS) analysis from ARCHES: a phase III, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) + androgen deprivation therapy (ADT) in men with metastatic hormone-sensitive prostate cancer (mHSPC). Ann Oncol. (2021) 32:S1300–1. doi: 10.1016/j.annonc.2021.08.2101
17. CSCO. Guidelines of Chinese society of clinical oncology (CSCO) diagnosis and treatment of prostate cancer guideline. People’s Medical Publishing House Press. (2024).
18. Gu, W, Han, W, Luo, H, Zhou, F, He, D, Ma, L, et al. Rezvilutamide versus bicalutamide in combination with androgen-deprivation therapy in patients with high-volume, metastatic, hormone-sensitive prostate cancer (CHART): a randomised, open-label, phase 3 trial. Lancet Oncol. (2022) 23:1249–60. doi: 10.1016/S1470-2045(22)00507-1
19. Wu, H, Sun, L, Feng, R, Zhang, H, Tang, K, Wang, S, et al. Cost-effectiveness of rezvilutamide versus bicalutamide and androgen-deprivation therapy in patients with highvolume, metastatic, hormone-sensitive prostate cancer. Front Pharmacol. (2023) 14:1269129. doi: 10.3389/fphar.2023.1269129
20. Ding, H, Li, S, Xu, X, Xu, W, He, C, Xin, W, et al. Cost-effectiveness analysis of rezvilutamide versus bicalutamide in the treatment of metastatic hormone-sensitive prostate cancer. BMJ Open. (2024) 14:e073170. doi: 10.1136/bmjopen-2023-073170
21. Husereau, D, Drummond, M, Petrou, S, Carswell, C, Moher, D, Greenberg, D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Eur J Health Econ. (2013) 14:367–72. doi: 10.1007/s10198-013-0471-6
22. Husereau, D, Drummond, M, Augustovski, F, de Bekker-Grob, E, Briggs, AH, Carswell, C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. (2022) 20:23. doi: 10.1186/s12916-021-02204-0
23. National Health Commission. China health statistics yearbook. China: Union Medical University Press (2022).
25. National Bureau of Statistics of China. Available online at: https://data.stats.gov.cn/search.htms=GDP (accessed October 20, 2024)
26. Guyot, P, Ades, AE, Ouwens, MJ, and Welton, NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
27. Latimer, NR. Survival analysis for economic evaluations alongside clinical trials extrapolation with patient-level data National Institute for Health and Care Excellence (NICE). (2013).
28. Hunan Public Resources Trading Service Platform. Available online at: https://jyjy.hnsggzy.com/ (accessed October 20, 2024).
29. Zhao, J, Jiang, K, Li, Q, Zhang, Y, Cheng, Y, Lin, Z, et al. Cost-effectiveness of olanzapine in the first-line treatment of schizophrenia in China. J Med Econ. (2019) 22:439–46. doi: 10.1080/13696998.2019.1580714
30. Zhang, PF, Xie, D, and Li, Q. Adding enzalutamide to first-line treatment for metastatic hormone-sensitive prostate Cancer: a cost-effectiveness analysis. Front Public Health. (2021) 9:608375. doi: 10.3389/fpubh.2021.608375
31. Ming, J, Wu, Y, Han, R, Xu, X, Waldeck, R, and Hu, S. Cost-utility analysis of Darolutamide combined with androgen deprivation therapy for patients with high-risk non-metastatic castration-resistant prostate Cancer in China. Adv Ther. (2023) 40:1087–103. doi: 10.1007/s12325-022-02389-7
32. Bank of China. Foreign exchange rate Available online at: https://www.boc.cn/sourcedb/whpj/ (accessed October 20, 2024)
33. Liu, M, Qu, S, Liu, Y, Yao, X, and Jiang, W. Comparative clinical effects and cost-effectiveness of maximum androgen blockade, docetaxel with androgen deprivation therapy and ADT alone for the treatment of mHSPC in China. J Comp Eff Res. (2019) 8:865–77. doi: 10.2217/cer-2018-0133
34. Zhou, J, Lan, T, Lu, H, and Pan, J. Price negotiation and pricing of anticancer drugs in China: an observational study. PLoS Med. (2024) 21:e1004332. doi: 10.1371/journal.pmed.1004332
35. Liu, GG, Wu, J, He, X, and Jiang, Y. Policy updates on access to and affordability of innovative medicines in China. Value Health Reg Issues. (2022) 30:59–66. doi: 10.1016/j.vhri.2021.12.003
36. Yoo, M, Nelson, RE, Haaland, B, Dougherty, M, Cutshall, ZA, Kohli, R, et al. Cost-effectiveness analysis of 7 treatments in metastatic hormone-sensitive prostate cancer: a public-payer perspective. J Natl Cancer Inst. (2023) 115:1374–82. doi: 10.1093/jnci/djad135
Keywords: cost-effectiveness, rezvilutamide, bicalutamide, high-volume mHSPC, Markov model
Citation: Hong J, Zeng X, Chen W, Zhou Z, He Y, Meng J and Ouyang L (2025) Cost-effectiveness analysis of rezvilutamide versus bicalutamide and androgen-deprivation therapy in patients with high-volume, metastatic, hormone-sensitive prostate cancer: a Markov’s model-based evaluation. Front. Public Health. 13:1574780. doi: 10.3389/fpubh.2025.1574780
Edited by:
Jing Yuan, Fudan University, ChinaReviewed by:
Davi Rumel, Retired, São Paulo, BrazilAleksandra Kovačević, Military Medical Academy, Serbia
Copyright © 2025 Hong, Zeng, Chen, Zhou, He, Meng and Ouyang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihui Ouyang, eGlhbmd5dWV4aWFuZzEyM0AxNjMuY29t; Jun Meng, NjU3MTU2NDUxQHFxLmNvbQ==
 Juan Hong
Juan Hong Xiaohui Zeng
Xiaohui Zeng Wenjun Chen1
Wenjun Chen1 Lihui Ouyang
Lihui Ouyang