- Environment and Lifestyle Epidemiology Branch, International Agency for Research on Cancer (IARC/WHO), Lyon, France
Introduction: Neuroblastoma (NB) is the most common extracranial tumor in children. Synthesizing and elucidating modifiable risk factors is fundamental to inform primary prevention of NB. The objective is to review literature and synthesize risk factors for NB.
Methods: PubMed, Web of Science, and Embase databases were searched using lists of key words and MeSH terms related to exposures and risk of NB. Studies were included if they were case-control or cohort studies of children under the age of 20 years at diagnosis and reported Relative Risks (RRs) with 95% confidence intervals (CIs). Pooled effect sizes (ES) and 95% CIs for risk factors associated with NB were estimated using random-effects models.
Results: We included 50 eligible studies from Asia, Europe, and North America, and Oceania on cases of NB diagnosed between 1964 and 2016. We observed associations for maternal occupational exposure to pesticides during preconception/pregnancy (ES 1.62, CI 1.04–2.54), high birthweight [(>4,000 g) ES 1.21, CI 1.02–1.42], and Cesarean section (ES 1.14, CI 1.00–1.30) and the risk of NB. Parental smoking showed a weak association, while breastfeeding ≥6 months (ES 0.50, CI 0.30–0.84) was inversely associated with NB. Birth characteristics such as low birthweight (<2,500 g), small and large-for-gestational age, gestation age <37 weeks and gestation age >40 weeks, and assisted reproductive technology were not associated with NB. Similarly, no associations were suggested for parental age, gestational diabetes, and pre-eclampsia. Maternal alcohol consumption during preconception/pregnancy, maternal intake of vitamin and folic acid during pregnancy, paternal occupational exposure to extremely low-frequency magnetic fields (ELF-MF), and maternal X-ray exposure during pregnancy were also not associated with the risk of NB. Paternal occupational and child's postnatal exposure to pesticides were also not associated with NB.
Discussion: This systematic review and meta-analysis suggest that maternal occupational exposure to pesticides during preconception/pregnancy, high birthweight, Cesarean section, and breastfeeding (beneficial) were associated with the risk of NB, but all associations were rather modest in strength. Synthesizing of these risk factors are needed to inform whether there are avenues for primary prevention of NB.
1 Introduction
Neuroblastoma (NB) is the most common extracranial tumor in children and the most frequent solid malignancy in children under 1 year (1). Approximately 60% of NB occur before age 2 and about 97% are diagnosed before the age of 10 years (2, 3). Globally, the incidence pattern of NB is unique among the childhood cancers and varies greatly across age groups. In developed countries, NB accounts for annually 11–13 per million in children aged <15 years and 65 per million in children <1 year but only 1 per million in children of 10–14 years (4).
Like other common childhood cancers, NB is heterogeneous, and it is classified into different risk strata such as low-risk, intermediate-risk, and high-risk groups. Survival rate varies by risk groups, and is higher than 95% in the low-risk group whereas only around 50% in the high-risk group (5). However, it is known that some of the NB patients are undergoing spontaneous regression even without any form of treatment, a more common phenomenon with NB but observed to a lesser extent in other few cancer types like renal cell carcinoma, malignant melanoma, choriocarcinoma and lymphoid malignancies (6).
While some individual epidemiological studies have suggested some risk factors associated with NB, overall its etiology remains largely unknown. These include paternal smoking, maternal alcohol consumption during the preconceptional period or pregnancy, childhood exposure to pesticides, Cesarean section (C-section), and high birthweight exceeding 4,000 g (3, 7–10). However, the evidence is inconsistent as there are also studies that have shown no associations for the same risk factors (11–15). Thus, to date no modifiable risk factor for NB has been clearly established.
NB has a variety of clinical behaviors that are mostly influenced by the biology, including unique abilities to suppress the host immune system. Chromosomal aberration is frequent in NB. For example, deletions of the short arm of chromosome 1 (1p) occur in about 70% of advanced stage. However, it is still unclear whether these events are responsible for the initiation of NB (16–18). While biology undoubtedly plays a central role, modifiable exposures could influence the timing of disease onset, immune system priming, or epigenetic regulation (19). In our study, we have been careful to avoid strong causal claims and instead frame our findings as associations that warrant further mechanistic exploration. Therefore, the aim of this systematic review and meta-analysis was to synthesize and elucidate evidence from different epidemiological studies. To give a consolidated overview of risk factors potentially associated with NB which may inform primary prevention of the disease.
2 Methods
2.1 Search strategy and study selection
This systematic review and meta-analysis was conducted according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (20) (Supplementary Table 1, p. 3). The search strategy used for article selection and methods for data extraction and analysis have been previously published (21, 22). We searched PubMed, Web of Science, and Embase databases with no restriction on publication date but selected articles are all written in English language. Identified peer reviewed articles were retrieved, imported, and screened for duplicates in EndNote version X9.3.3. The authors, FMO and RD assessed the titles, abstracts, and full text of the articles independently to determine their eligibility (Supplementary Table 2, p. 6) (23), differences arising from the independent selection process were resolved by seeking opinion of the third author, AO. Additional articles were sourced from lists of references. The search strategy was structured in line with Population, Exposure, Comparator and Outcome (PECO) components and included a list of key words and MeSH terms (Supplementary Tables 3–5, p. 7–13). The search was initially conducted in June 2022 and subsequently updated until January 2025. The studies were included if they were case-control or cohort studies of childhood NB under the age of 20 years, we reported exposure time windows, and provided estimates of Relative Risks (RRs) such as Odds Ratio (OR), Hazard Ratio (HR), Standardized Mortality Ratio (SMR), Mortality Rate Ratio (MRR), Standard Incidence Ratio (SIR), or Incidence Rate Ratio (IRR) with 95% confidence intervals (CIs). We checked publications from the same region for overlaps of their study populations. The inclusion and exclusion criteria were defined a priori (Supplementary Table 2, p. 6) (21, 22).
2.2 Data extraction
Risk factors extracted included birth and parental characteristics, environmental and occupational exposures pesticides, radiation, and lifestyle exposures. Exposure time period such as preconceptional, prenatal and postnatal were also considered. Other information extracted includes authors' name, year of publication, study location, period and age range of diagnosis, exposure assessment methods, outcome ascertainment, number of NB cases and controls or, if not available, the study population, follow-up duration, and risk estimates with their respective 95% CIs. Information regarding study design (case-control and cohort or registry-based case-control) was also extracted. Registry-based case-control studies were considered as cohort studies in the present analysis (21). Case-control studies are thereby studies requiring interaction with the study participants.
2.3 Quality assessment of eligible articles
All eligible articles underwent a quality assessment of their methodological quality using the Joanna Briggs Institute (JBI) critical appraisal tools for case-control and cohort studies (24). The appraisal checklist has 10 criteria for case-control and 11 for cohort studies. Every question answered with a “yes” received a score of 1, while a “no” scored 0, and “unclear” or “not applicable” received also 0 (Supplementary Tables 6, 7, p. 14–15). Prior to the critical appraisal of the articles, we systematically checked the articles for overlaps of their study populations and by risk factors.
2.4 Statistical analyses
We performed random-effects meta-analyses in order to estimate pooled effect sizes (ES) with their respective 95% CIs. Funnel plots and Egger's test were employed to assess potential publication bias (25). The I2 statistic was calculated to quantify the heterogeneity of the results between studies. I2 values of 0% were considered to represent “no heterogeneity”, from 1 to 35% “low heterogeneity”, from 36 to 55% as “moderate”, from 56 to 70% as “substantial” and above 71% as “considerable” heterogeneity (26). Analyses were conducted both combining case-control and cohort studies, and separately by study design (case-control vs. cohort studies). The combined analysis is presented as the primary focus, unless otherwise stated. Analyses were conducted using STATA® software, version 15.1 (College Station, TX, USA) using a nominal significance level of 0.05.
3 Results
3.1 Study characteristics
A total of 3,760 unique records were retrieved and screened, leading to the evaluation of 61 full texts. Among these, 50 studies [25 case-control and 25 cohort studies (including registry-based nested case-control studies)] met the study inclusion criteria (Figure 1 and Table 1).
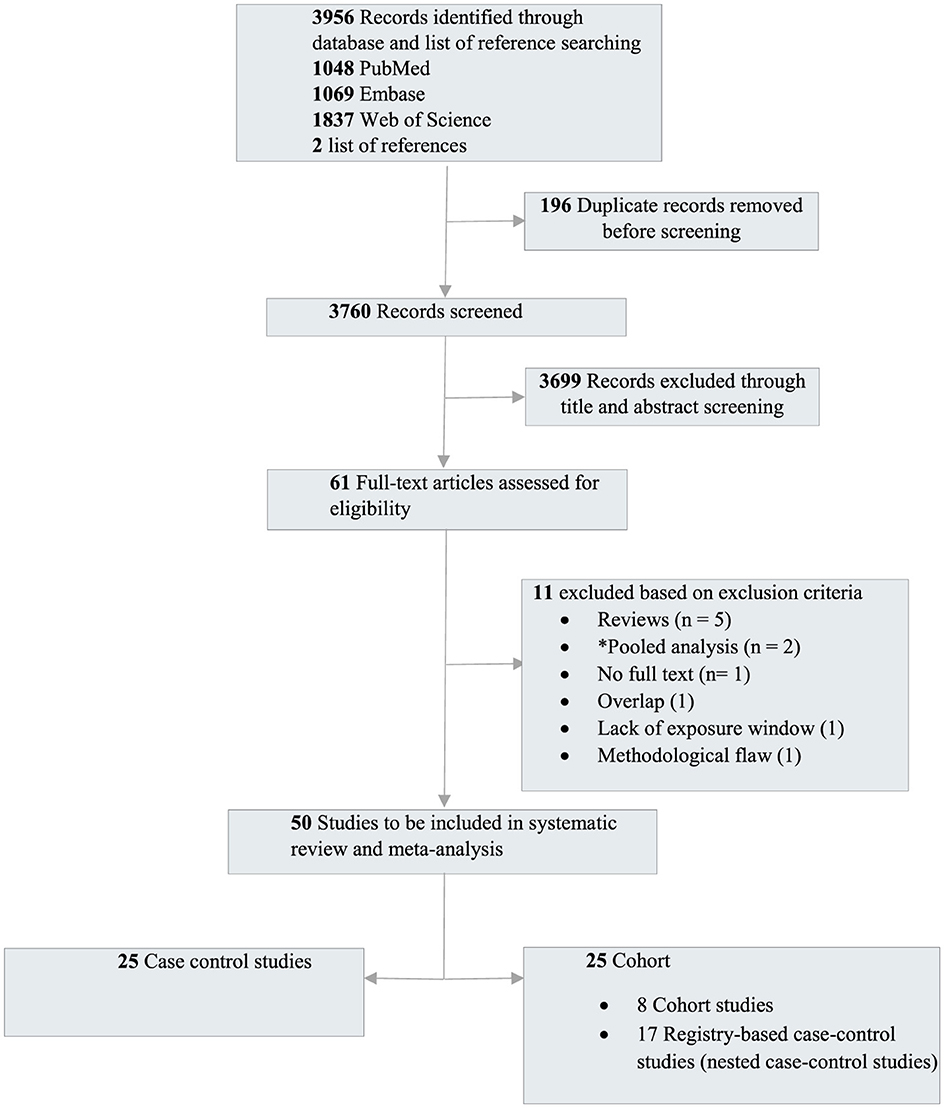
Figure 1. Preferred reporting items for systematic reviews and meta-analyses flow diagram outlining the study selection. *The pooled studies were excluded as the primary studies were already published and included in our meta-analysis.
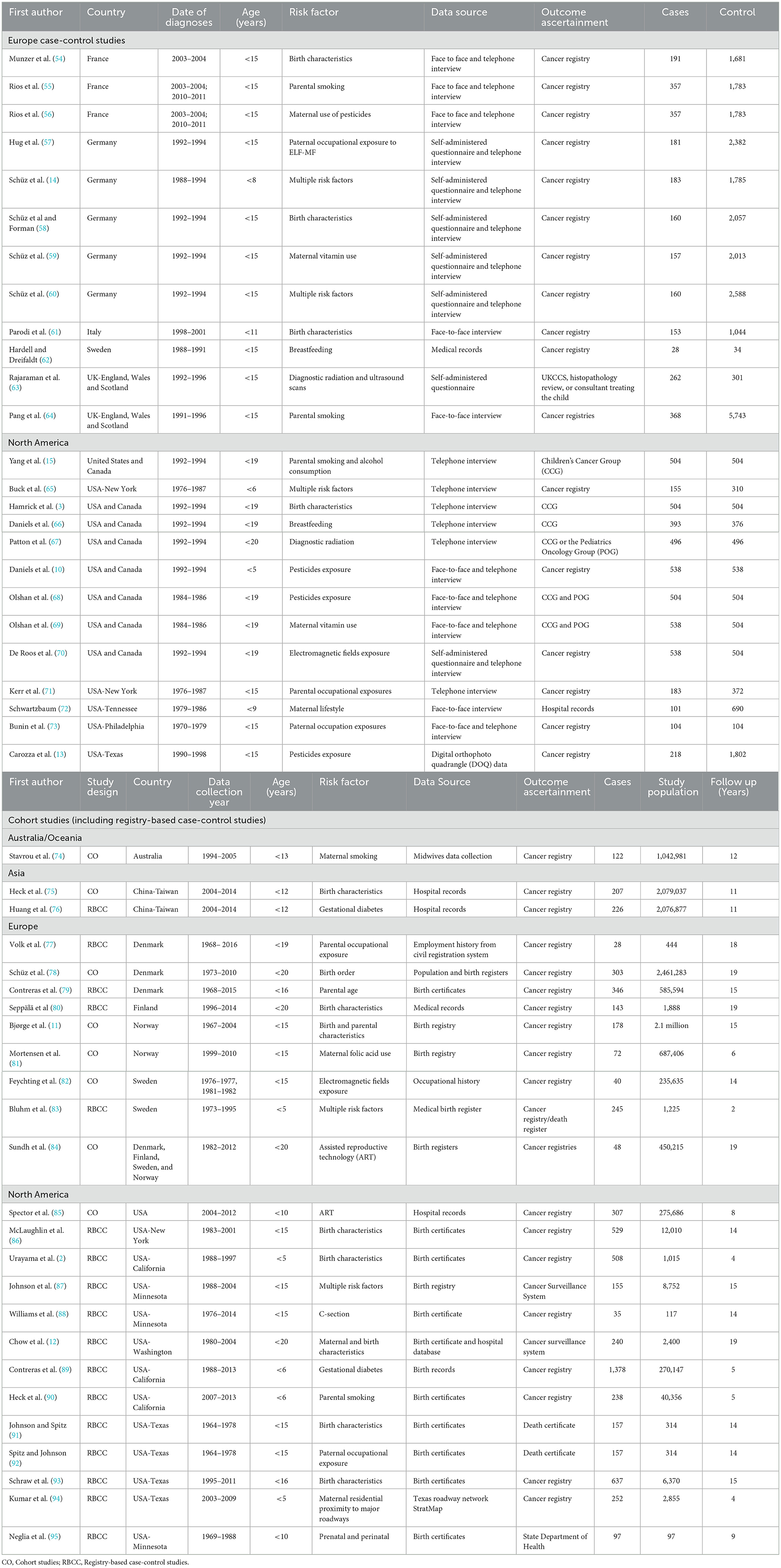
Table 1. Characteristics of the 50 studies included in the systematic review and meta-analysis, sorted by country.
Among all the studies that met the criteria, 52% (n = 26) were carried out in North America. Europe came next with 42% (n = 21), followed by Asia with 4% (n = 2), and Oceania with 2% (n = 1). There were no eligible studies in Latin America and Africa.
3.2 Study bias and quality assessment
The 50 articles critically appraised for quality using the JBI tools were generally of good quality (86%). The least ranked case-control study scored 7 out of 10 points, while for cohort study, it was 7 out of 11 points. Thus, all screened articles appraised were included in the final analysis (Supplementary Tables 6, 7, p. 14–15).
3.3 Birth and parental characteristics
C-section was a suggestive risk factor of NB (ES 1.14, CI 1.00–1.30) with Eggers p-value of 0.57 and moderate heterogeneity across studies. This outcome is based on 8 cohort studies and one case-control study. There was no association observed between gestational age <37 weeks or > 40 weeks and NB risk. Analyses relating to small and large for gestational age were also not suggestive of association with NB, though the ES for large gestational age (LGA) was slightly elevated with confidence intervals including 1 (ES 1.23, CI 0.89–1.70) based on 1 cohort and 1 case control study. Assisted reproductive technology (ART) and hormonal/infertility treatment did not show an association with NB (Figure 2).
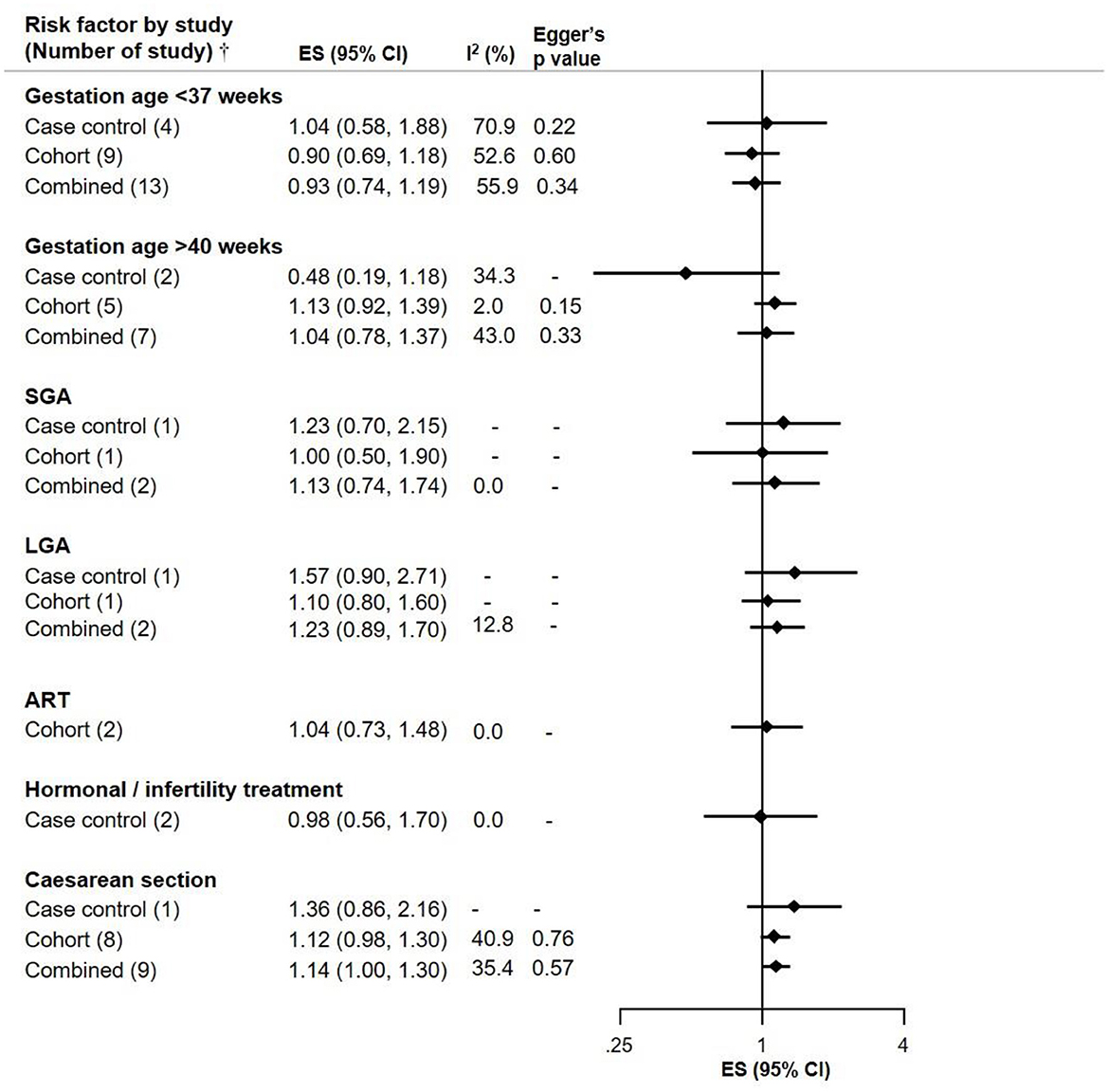
Figure 2. Meta-analysis of pooled effect sizes (ES) of exposure to birth characteristics [Gestation Age <37 weeks, >40 weeks; Small for Gestation Age (SGA), Large for Gestation Age (LGA); assisted reproductive technology (ART); Hormonal/Infertility treatment and C-section] for the risk of NB and heterogeneity (I2) with Eggers p-value by study design.†Where only one study was identified, it is referred to as RR and not ES.
Low birthweight (<2,500 g) was not associated with NB, but an association was seen between high birthweight (>4,000 g) and the risk of NB (ES 1.21, CI 1.02–1.42). Breastfeeding appeared to be inversely associated with NB in an exposure-response manner, as shown in children breastfed for ≥6 months (ES 0.50, CI 0.30–0.84). Birth order (2 and ≥3) was not associated with NB risk. While the mother's parity ≥3 showed an increased risk of NB based on three cohort studies (ES 1.50, CI 1.13–1.99), this was attenuated when combined with 2 case control studies, and there was moderate heterogeneity across studies (Figure 3).
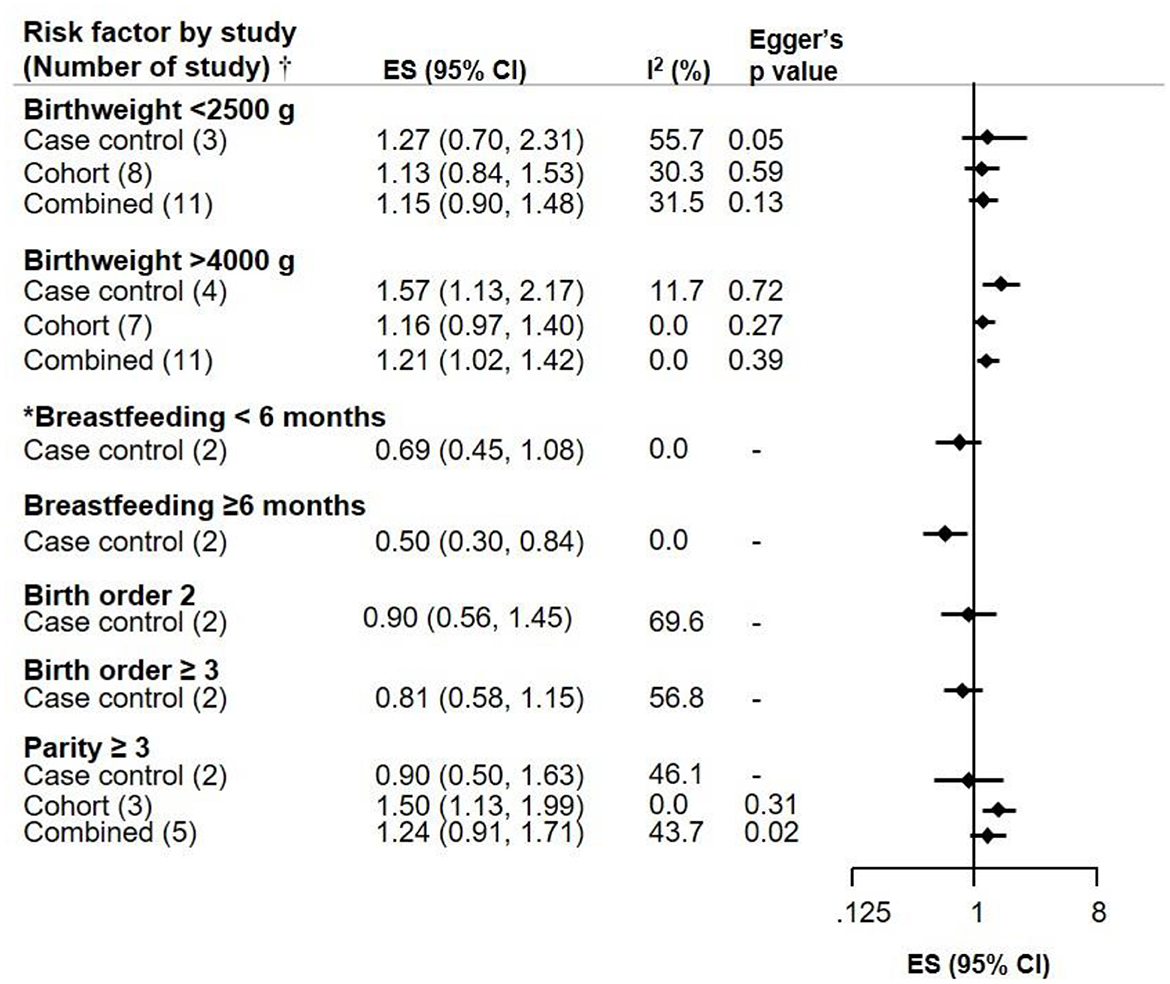
Figure 3. Meta-analysis of pooled effect sizes (ES) of exposure to birth characteristics (Birthweight, Breastfeeding, Birth order 2 and ≥3, and Parity ≥3) for the risk of NB and heterogeneity (I2) with Eggers p-value, by study design.†Where only one study was identified, it is referred to as RR and not ES. *Breastfeeding <6 months does not include 0 months.
For young mothers (<20 years) and older fathers (≥35 years) the ES were slightly elevated in two case-control studies (ES 1.54, CI 0.81–2.93 and ES 1.40, CI 0.80–2.60), but not in the more numerous cohort studies; the potential selection bias leading to spurious associations with young parental age in childhood cancer has been noted before (27). Gestational diabetes, and pre-eclampsia were not associated with NB risk. However, Egger's p-value was 0.01 for the cohort and combined studies on gestational diabetes, suggesting potential publication bias (Figure 4).
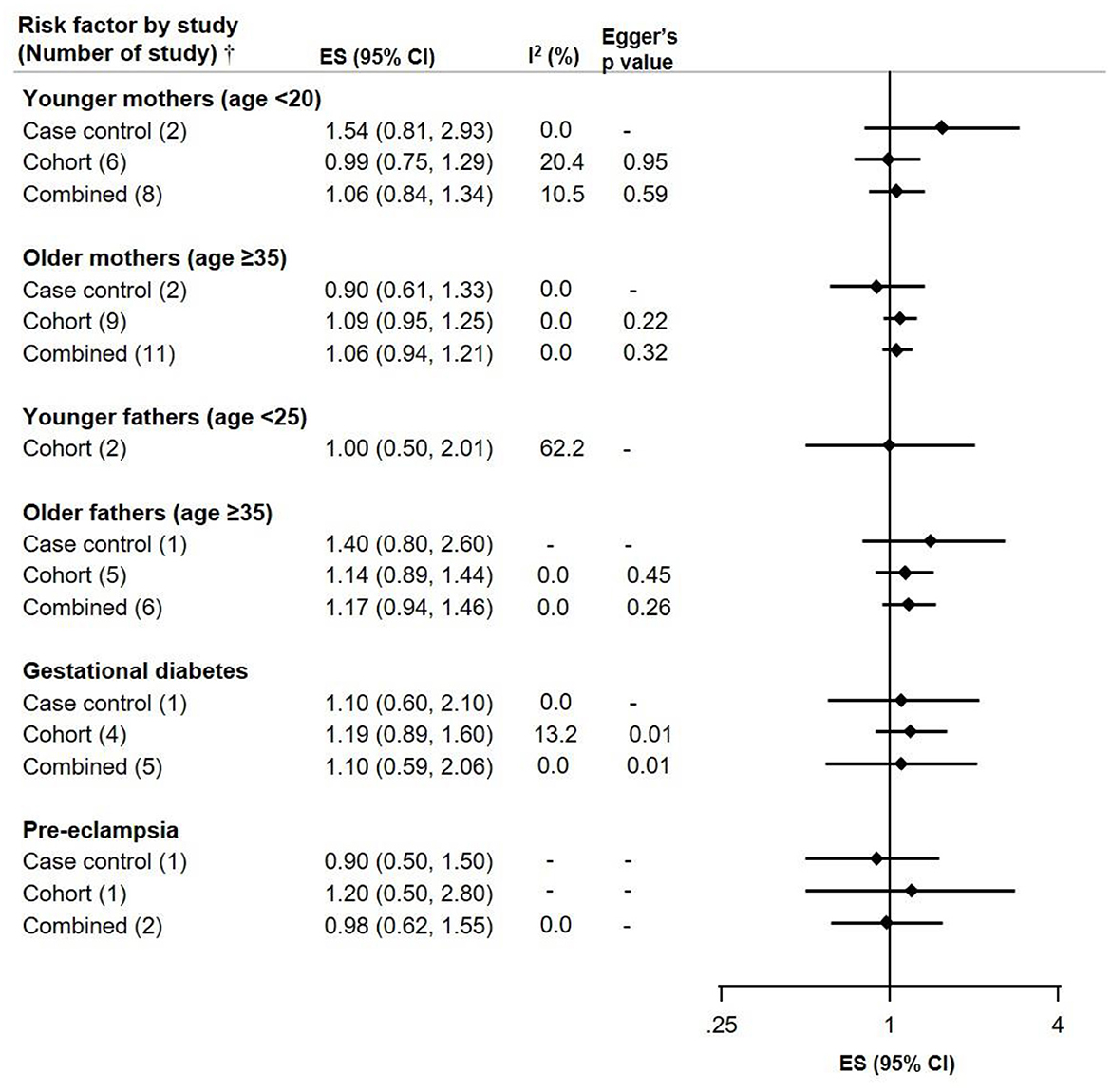
Figure 4. Meta-analysis of pooled effect sizes (ES) of exposure to parental characteristics [Younger mothers (age <20) and fathers (age <25)], Older mothers and fathers (age ≥35), Gestational diabetes and Pre-eclampsia for the risk of NB and heterogeneity (I2) with Eggers p-value, by study design.†Where only one study was identified, it is referred to as RR and not ES.
3.4 Lifestyle
Maternal smoking during pregnancy (ever smokers) showed a weak association for the risk of NB. Similarly, there was a weak association for mothers who smoked 1–10 cigarettes per/day in case-control studies (ES 1.35, CI 1.00–1.83), but it was attenuated when combined with the one cohort study on the topic (ES 0.85, CI 0.50–1.47; Table 2). In the same vein, paternal smoking during preconception/prenatal showed weak association with NB (ES 1.12, CI 0.97–1.30, p = 0.44), based on 4 case-control studies with no heterogeneity across studies. Maternal consumption of alcohol during preconception/pregnancy did not show an association with NB risk. Likewise, no association was observed for maternal intake of vitamin and folic acid during preconception/pregnancy and the risk of NB (ES 0.98, CI 0.53–1.84, 2 case-control and 1 cohort studies), although with substantial heterogeneity.
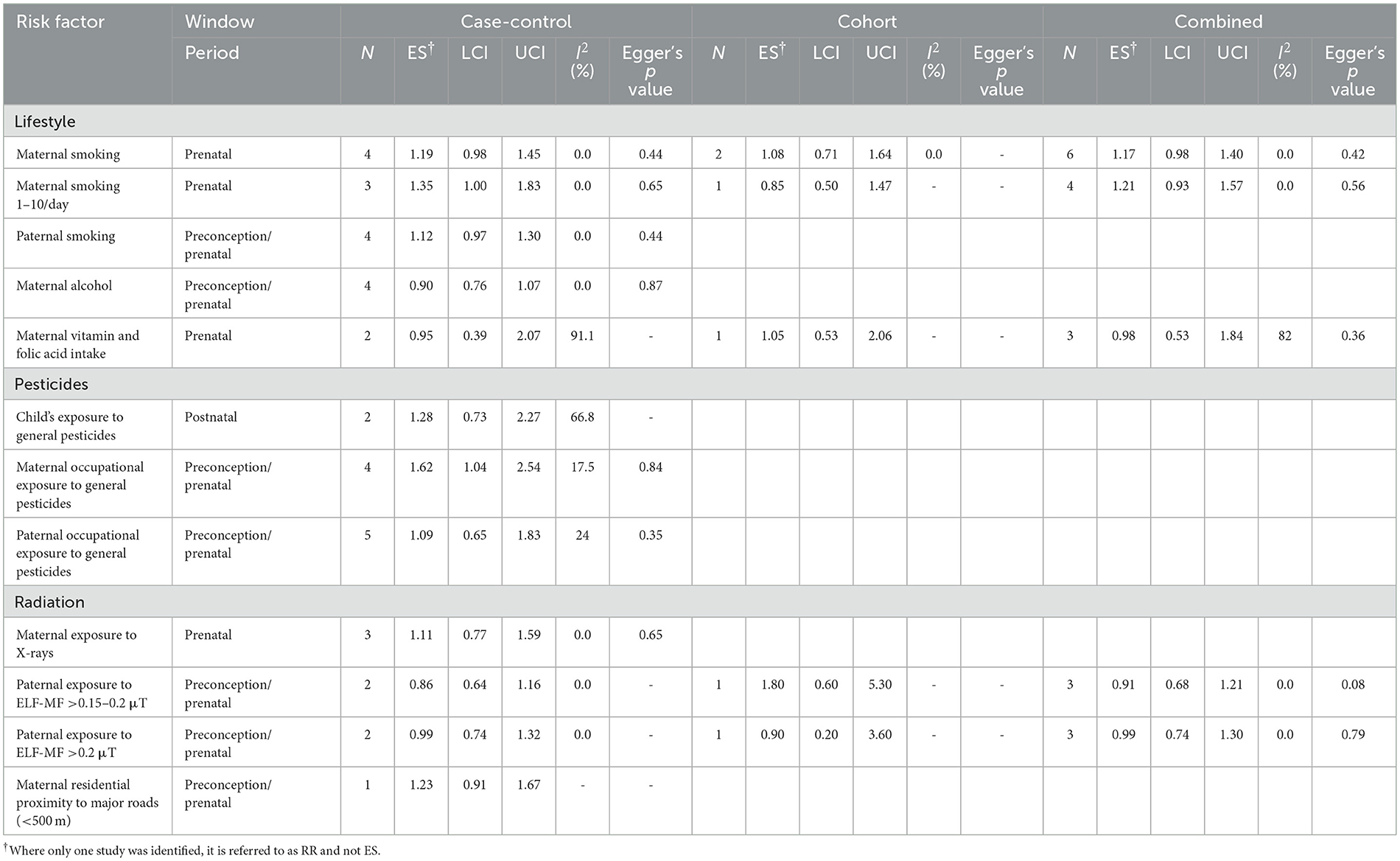
Table 2. Meta-analysis of pooled effect sizes (ES) of exposure to lifestyle, pesticides, and radiation for the risk of NB and heterogeneity (I2) between studies, by study design.
3.5 Chemicals and radiations
Exposure to general pesticides during childhood and the risk of NB, showed a slightly elevated ES but with wide confidence intervals (ES 1.28, CI 0.73–2.27) based on 2 case-control studies (Table 2). On the other hand, we observed an association for maternal exposure to general pesticides during preconception/pregnancy and the risk of NB (ES 1.62, CI 1.04–2.54; four case-control studies). We did not observe an association for paternal exposure to general pesticides during preconception/pregnancy and the risk of NB (ES 0.86. CI 0.51–1.45; four case-control studies) with low heterogeneity and Eggers p-value 0.35.
Maternal exposure to X-ray during pregnancy was not associated with NB risk based on three case-control studies (ES 1.11, CI 0.77–1.59, p = 0.65), with no heterogeneity across studies. Likewise, there were no associations observed between paternal occupational exposure to ELF-MF and the risk of NB (Table 2). Maternal residential proximity of <500 m to major roads was elevated (RR 1.23, CI 0.91–1.67, 1 cohort study) when compared to those living ≥500 m away from major roads; but this is based on only one study with a wide confidence interval.
4 Discussion
In this systematic review and meta-analysis including 50 epidemiological studies with an approximate total of 14,000 cases of NB. We synthesized the evidence of factors that have been studied in relation to NB in children. Breastfeeding was beneficial with longer duration (≥6 months). Maternal occupational exposure to pesticides during preconception/pregnancy was associated with an increased risk of NB. High birthweight (>4,000 g) showed a slightly elevated ES with borderline significance, as well as for C-section. Associations seen with parental smoking (for paternal in case control studies with self-reported information only) are weak and partly inconsistent, not allowing to draw clear conclusions, but suggesting very modest associations if any. The remaining studied risk factors including gestational age and size, ART and hormonal/infertility treatment, birth order (2 and ≥3), gestational diabetes and pre-eclampsia, maternal alcohol consumption and exposure to X-ray during pregnancy, paternal occupational exposure to pesticides and ELF-MF at the levels studied were not associated with NB risk.
The protective effect of breastfeeding ≥6 months in our study is consistent with other reviews, were the authors reported 39% (28) and 46 % (29) lower risks of NB for longest breastfeeding vs. shortest breastfeeding. Similar findings on the protective effect of breastfeeding ≥6 months have also been reported for other childhood cancer types like leukemia and Wilms tumor (22, 30). The mechanism by which breast milk can reduce the risk of NB is not fully understood. However, breast milk has been reported to contain immunologically active components and multifactorial anti-inflammatory defense mechanisms that influence the development of the immune system of the breastfed infants. Tumor necrosis factor (TNF) -related apoptosis-inducing ligand (TRAIL) in breast milk can control apoptosis and cell proliferation in various organs and tissues (28, 29, 31).
We reported that C-section was suggestive for the risk of NB. Elective C-section is gradually becoming more frequent especially in high socioeconomic status populations, and due to improved surgical procedures in develop countries (32). Systematic reviews and meta-analyses have reported associations between C-sections and childhood leukemia (30, 33) and Wilms tumor (22, 34). C-section has been hypothesized to negatively impact on the function of the developing immune system. The mechanisms for the association between C-section and increased risk of childhood cancer is thought to be due the fact that these neonates do not undergo the essential stress during vaginal delivery that activates the hypothalamic–pituitary–adrenal axis and prime the immune system for future function. Hence creating a permissive environment for malignancies to develop (35–37).
The association we reported for high birthweight (>4,000 g) and increased risk of NB in the present systematic review and meta-analyses was driven by studies published before 2010 and those conducted in North America. Our finding is in agreement with the meta-analysis conducted by Harder et al. (38) who also found an association with slightly lower magnitude (1.19) compared to the present systematic review and meta-analysis (1.21) including subsequent studies, and excluding those with substantial overlaps. Birthweights may affect C-section delivery rates, as small and large new-borns have more C-section deliveries than those of average weight (39). Underlying genetic and epigenetic mechanisms play significant role in high birthweight and childhood cancer. There are several known susceptibility genes, pediatric overgrowth disorders and factors that may influence the association between high birthweight and childhood cancers such as Beckwith–Wiedemann syndrome (BWS), Weaver syndrome, CLOVES, Proteus syndrome, Simpson–Golabi–Behmel syndrome, kaposiform hemangioendothelioma, macrosomia, and organomegaly. About 5%−10% of children with BWS may develop childhood cancer especially Wilms tumor (40–42).
The association we observed for maternal exposure to pesticides during preconception/pregnancy in the main study, was only elevated in sub analysis but with consistent magnitude across decades and in Europe and North America where the studies were conducted. This results are consistent with the findings of Khan et al. (43) who found an association for prenatal pesticides exposure with the same magnitude of association as those reported in the present systematic review and meta-analysis. The mechanisms underlying the associations of pesticides with childhood cancer may differ depending on the type and composition of pesticides. For example, pyrethroids used for pest control on fruits, vegetables as well as for household insecticides, have been reported to induce multiple biological effects (genotoxic and non-genotoxic effects) and initiation the development of childhood cancer (44–46). These insecticides may pass through the feto-placental barrier and thus expose the fetus (47–49). During intrauterine life, there are immunological adaptations to ensure optimal fetal development (50). However, exposure to pesticides induces modifications in the immune system according to the specific pesticide altering the well-regulated immune responses to tumor and microbial antigens, and potentially increasing susceptibility, and development of cancers (51–53). Clear interpretation is hampered by the fact that there are no data on specific active ingredients in pesticides.
4.1 Strengths and limitations
This systematic review and meta-analysis was limited by the number of eligible articles which was small for most risk factors, hence, results should be interpreted with caution. Others include potential information and selection biases inherent in the studies, crude exposure assessment methods and exposure misclassification, most likely non-differential, may also have influenced the results. Majority of the studies were conducted in Europe and North America.
Our study also has some strengths, including well-structured search strategy, separation of case-control and cohort/registry-based case-control studies in the meta-analysis. Group of persons exposed (paternal, maternal and childhood) and exposure time window (preconception, prenatal and postnatal) were also separated.
5 Conclusion
The present systematic review and meta-analysis suggests that breastfeeding reduces the risk of NB, while maternal occupational pesticides exposure, high birthweight and C-section show a modest association with NB. Improved exposure assessment is needed in further studies including stratification by risk groups, to obtain solid evidence of modifiable risk factors of NB.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FO: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. RD: Data curation, Investigation, Methodology, Software, Visualization, Writing – review & editing. AO: Investigation, Methodology, Supervision, Validation, Writing – review & editing. LB: Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. JS: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by a grant from the French National Cancer Institute (INCa:15670; PEDIAC consortium).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1576101/full#supplementary-material
References
1. Nong J, Su C, Li C, Wang C, Li W, Li Y, et al. Global, regional, and national epidemiology of childhood neuroblastoma (1990-2021): a statistical analysis of incidence, mortality, and DALYs. EClinicalMedicine. (2025) 79:102964. doi: 10.1016/j.eclinm.2024.102964
2. Urayama KY, Von Behren J, Reynolds P. Birth characteristics and risk of neuroblastoma in young children. Am J Epidemiol. (2007) 165:486–95. doi: 10.1093/aje/kwk041
3. Hamrick SE, Olshan AF, Neglia JP, Pollock BH. Association of pregnancy history and birth characteristics with neuroblastoma: a report from the Children's Cancer Group and the Pediatric Oncology Group. Paediatr Perinat Epidemiol. (2001) 15:328–37. doi: 10.1046/j.1365-3016.2001.0376a.x
4. Tas ML, Reedijk AMJ, Karim-Kos HE, Kremer LCM, van de Ven CP, Dierselhuis MP, et al. Neuroblastoma between 1990 and 2014 in the Netherlands: increased incidence and improved survival of high-risk neuroblastoma. Eur J Cancer. (2020) 124:47–55. doi: 10.1016/j.ejca.2019.09.025
5. Irwin MS, Naranjo A, Zhang FF, Cohn SL, London WB, Gastier-Foster JM, et al. Revised neuroblastoma risk classification system: a report from the Children's Oncology Group. J Clin Oncol. (2021) 39:3229–41. doi: 10.1200/JCO.21.00278
6. Brodeur GM. Spontaneous regression of neuroblastoma. Cell Tissue Res. (2018) 372:277–86. doi: 10.1007/s00441-017-2761-2
7. Sorahan T, Lancashire RJ, Hulten MA, Peck I, Stewart AM. Childhood cancer and parental use of tobacco: deaths from 1953 to 1955. Br J Cancer. (1997) 75:134–8. doi: 10.1038/bjc.1997.22
8. Tsai J, Kaye WE, Bove FJ. Wilms' tumor and exposures to residential and occupational hazardous chemicals. Int J Hyg Environ Health. (2006) 209:57–64. doi: 10.1016/j.ijheh.2005.09.003
9. Yeazel MW, Ross JA, Buckley JD, Woods WG, Ruccione K, Robison LL. High birth weight and risk of specific childhood cancers: a report from the Children's Cancer Group. J Pediatr. (1997) 131:671–7. doi: 10.1016/S0022-3476(97)70091-X
10. Daniels JL, Olshan AF, Teschke K, Hertz-Picciotto I, Savitz DA, Blatt J, et al. Residential pesticide exposure and neuroblastoma. Epidemiology. (2001) 12:20–7. doi: 10.1097/00001648-200101000-00005
11. Bjørge T, Engeland A, Tretli S, Heuch I. Birth and parental characteristics and risk of neuroblastoma in a population-based norwegian cohort study. Br J Cancer. (2008) 99:1165–9. doi: 10.1038/sj.bjc.6604646
12. Chow EJ, Friedman DL, Mueller BA. Maternal and perinatal characteristics in relation to neuroblastoma. Cancer. (2007) 109:983–92. doi: 10.1002/cncr.22486
13. Carozza SE Li B, Wang Q, Horel S, Cooper S. Agricultural pesticides and risk of childhood cancers. Int J Hyg Environ Health. (2009) 212:186–95. doi: 10.1016/j.ijheh.2008.06.002
14. Schüz J, Kaletsch U, Meinert R, Kaatsch P, Spix C, Michaelis J. Risk factors for neuroblastoma at different stages of disease. Results from a population-based case-control study in Germany. J Clin Epidemiol. (2001) 54:702–9. doi: 10.1016/S0895-4356(00)00339-5
15. Yang Q, Olshan AF, Bondy ML, Shah NR, Pollock BH, Seeger RC, et al. Parental smoking and alcohol consumption and risk of neuroblastoma. Cancer Epidemiol Biomarkers Prev. (2000) 9:967–72.
16. Rivera Z, Escutia C, Madonna MB, Gupta KH. Biological insight and recent advancement in the treatment of neuroblastoma. Int J Mol Sci. (2023) 24:8470. doi: 10.3390/ijms24108470
17. Davidoff AM. Neuroblastoma. Semin Pediatr Surg. (2012) 21:2–14. doi: 10.1053/j.sempedsurg.2011.10.009
18. Tsubota S, Kadomatsu K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res. (2018) 372:211–21. doi: 10.1007/s00441-018-2796-z
19. Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics. (2011) 3:267–77. doi: 10.2217/epi.11.22
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
21. Onyije FM, Dolatkhah R, Olsson A, Bouaoun L, Deltour I, Erdmann F, et al. Risk factors for childhood brain tumours: a systematic review and meta-analysis of observational studies from 1976 to 2022. Cancer Epidemiol. (2024) 88:102510. doi: 10.1016/j.canep.2023.102510
22. Onyije FM, Dolatkhah R, Olsson A, Bouaoun L, Schüz J. Environmental risk factors of wilms tumour: a systematic review and meta-analysis. EJC Paediatr Oncol. (2024) 4:100178. doi: 10.1016/j.ejcped.2024.100178
23. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (eds.). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4.. Chichester: John Wiley & Sons (2023).
24. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk (2020). In: Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, , editors. JBI Manual for Evidence Synthesis. JBI (2024). doi: 10.46658/JBIMES-24-06
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Lin L, Shi L, Chu H, Murad MH. The magnitude of small-study effects in the cochrane database of systematic reviews: an empirical study of nearly 30 000 meta-analyses. BMJ Evid Based Med. (2020) 25:27–32. doi: 10.1136/bmjebm-2019-111191
27. Schüz J. Non-response bias as a likely cause of the association between young maternal age at the time of delivery and the risk of cancer in the offspring. Paediatr Perinat Epidemiol. (2003) 17:106–12. doi: 10.1046/j.1365-3016.2003.00460.x
28. Su Q, Sun X, Zhu L, Yan Q, Zheng P, Mao Y, et al. Breastfeeding and the risk of childhood cancer: a systematic review and dose-response meta-analysis. BMC Med. (2021) 19:90. doi: 10.1186/s12916-021-01950-5
29. Martin RM, Gunnell D, Owen CG, Smith GD. Breast-feeding and childhood cancer: a systematic review with metaanalysis. Int J Cancer. (2005) 117:1020–31. doi: 10.1002/ijc.21274
30. Onyije FM, Olsson A, Baaken D, Erdmann F, Stanulla M, Wollschlaeger D, et al. Environmental risk factors for childhood acute lymphoblastic leukemia: an umbrella review. Cancers. (2022) 14:382. doi: 10.3390/cancers14020382
31. Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. (2016) 387:475–90. doi: 10.1016/S0140-6736(15)01024-7
32. Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PLoS ONE. (2016) 11:e0148343. doi: 10.1371/journal.pone.0148343
33. Jiang L-L, Gao Y-Y, He W-B, Gan T, Shan H-Q, Han X-M. Cesarean section and risk of childhood leukemia: a systematic review and meta-analysis. World J Pediatr. (2020) 16:471–9. doi: 10.1007/s12519-020-00338-4
34. Han MA, Storman D, Al-Rammahy H, Tang S, Hao Q, Leung G, et al. Impact of maternal reproductive factors on cancer risks of offspring: a systematic review and meta-analysis of cohort studies. PLoS ONE. (2020) 15:e0230721. doi: 10.1371/journal.pone.0230721
35. Williams LA, Richardson M, Spector LG, Marcotte EL. Cesarean section is associated with an increased risk of acute lymphoblastic leukemia and hepatoblastoma in children from minnesota. Cancer Epidemiol Biomarkers Prev. (2021) 30:736–42. doi: 10.1158/1055-9965.EPI-20-1406
36. Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. (2013) 208:249–54. doi: 10.1016/j.ajog.2012.08.009
37. Fritz J, Lamadrid-Figueroa H, Muñoz-Rocha TV, Huerta-García Y, Martínez-Silva G, Trejo-Valdivia B, et al. Cesarean birth is associated with lower motor and language development scores during early childhood: a longitudinal analysis of two cohorts. Sci Rep. (2024) 14:23438. doi: 10.1038/s41598-024-73914-7
38. Harder T, Plagemann A, Harder A. Birth weight and risk of neuroblastoma: a meta-analysis. Int J Epidemiol. (2010) 39:746–56. doi: 10.1093/ije/dyq040
39. Poma PA. Correlation of birth weights with cesarean rates. Int J Gynaecol Obstet. (1999) 65:117–23. doi: 10.1016/S0020-7292(98)00261-6
40. Connolly GK, Harris RD, Shumate C, Rednam SP, Canfield MA, Plon SE, et al. Pediatric cancer incidence among individuals with overgrowth syndromes and overgrowth features: a population-based assessment in seven million children. Cancer. (2024) 130:467–75. doi: 10.1002/cncr.35041
41. Manor J, Lalani SR. Overgrowth syndromes-evaluation, diagnosis, and management. Front Pediatr. (2020) 8:574857. doi: 10.3389/fped.2020.574857
42. MacFarland SP, Duffy KA, Bhatti TR, Bagatell R, Balamuth NJ, Brodeur GM, et al. Diagnosis of beckwith-wiedemann syndrome in children presenting with wilms tumor. Pediatr Blood Cancer. (2018) 65:e27296. doi: 10.1002/pbc.27296
43. Khan A, Feulefack J, Sergi CM. Pre-conceptional and prenatal exposure to pesticides and pediatric neuroblastoma. A meta-analysis of nine studies. Environ Toxicol Pharmacol. (2022) 90:103790. doi: 10.1016/j.etap.2021.103790
44. Navarrete-Meneses MDP, Salas-Labadía C, Gómez-Chávez F, Pérez-Vera P. Environmental pollution and risk of childhood cancer: a scoping review of evidence from the last decade. Int J Mol Sci. (2024) 25:3284. doi: 10.3390/ijms25063284
45. Navarrete-Meneses MDP, Pérez-Vera P. Pyrethroid pesticide exposure and hematological cancer: epidemiological, biological and molecular evidence. Rev Environ Health. (2019) 34:197-210. doi: 10.1515/reveh-2018-0070
46. Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L, et al. Carcinogenicity of lindane, Ddt, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. (2015) 16:891–2. doi: 10.1016/S1470-2045(15)00081-9
47. Rager JE, Bangma J, Carberry C, Chao A, Grossman J, Lu K, et al. Review of the environmental prenatal exposome and its relationship to maternal and fetal health. Reprod Toxicol. (2020) 98:1–12. doi: 10.1016/j.reprotox.2020.02.004
48. Mathiesen L, Buerki-Thurnherr T, Pastuschek J, Aengenheister L, Knudsen LE. Fetal exposure to environmental chemicals; insights from placental perfusion studies. Placenta. (2021) 106:58–66. doi: 10.1016/j.placenta.2021.01.025
49. Ventura-Miranda MI, Fernández-Medina IM, Guillén-Romera E, Ortíz-Amo R, Ruíz-Fernández MD. Effect of gestational pesticide exposure on the child's respiratory system: a narrative review. Int J Environ Res Public Health. (2022) 19:15418. doi: 10.3390/ijerph192215418
50. Woods Rebecca M, Lorusso Jarred M, Fletcher J, ElTaher H, McEwan F, Harris I, et al. Maternal immune activation and role of placenta in the prenatal programming of neurodevelopmental disorders. Neuronal Signal. (2023) 7:NS20220064. doi: 10.1042/NS20220064
51. Gangemi S, Gofita E, Costa C, Teodoro M, Briguglio G, Nikitovic D, et al. Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases (review). Int J Mol Med. (2016) 38:1012–20. doi: 10.3892/ijmm.2016.2728
52. Colosio C, Corsini E, Barcellini W, Maroni M. Immune parameters in biological monitoring of pesticide exposure: current knowledge and perspectives. Toxicol Lett. (1999) 108:285–95. doi: 10.1016/S0378-4274(99)00100-9
53. Lee G-H, Choi K-C. Adverse effects of pesticides on the functions of immune system. Comp Biochem Physiol C Toxicol Pharmacol. (2020) 235:108789. doi: 10.1016/j.cbpc.2020.108789
54. Munzer C, Menegaux F, Lacour B, Valteau-Couanet D, Michon J, Coze C, et al. Birth-related characteristics, congenital malformation, maternal reproductive history and neuroblastoma: the ESCALE study (SFCE). Int J Cancer. (2008) 122:2315–21. doi: 10.1002/ijc.23301
55. Rios P, Bailey HD, Poulalhon C, Valteau-Couanet D, Schleiermacher G, Bergeron C, et al. Parental smoking, maternal alcohol consumption during pregnancy and the risk of neuroblastoma in children. A pooled analysis of the ESCALE and ESTELLE French studies. Int J Cancer. (2019) 145:2907–16. doi: 10.1002/ijc.32161
56. Rios P, Bailey HD, Lacour B, Valteau-Couanet D, Michon J, Bergeron C, et al. Maternal use of household pesticides during pregnancy and risk of neuroblastoma in offspring. A pooled analysis of the ESTELLE and ESCALE French studies (SFCE). Cancer Causes Control. (2017) 28:1125–32. doi: 10.1007/s10552-017-0944-5
57. Hug K, Grize L, Seidler A, Kaatsch P, Schüz J. Parental occupational exposure to extremely low frequency magnetic fields and childhood cancer: a german case-control study. Am J Epidemiol. (2010) 171:27–35. doi: 10.1093/aje/kwp339
58. Schüz J, Forman MR. Birthweight by gestational age and childhood cancer. Cancer Causes Control. (2007) 18:655–63. doi: 10.1007/s10552-007-9011-y
59. Schüz J, Weihkopf T, Kaatsch P. Medication use during pregnancy and the risk of childhood cancer in the offspring. Eur J Pediatr. (2007) 166:433–41. doi: 10.1007/s00431-006-0401-z
60. Schüz J, Kaatsch P, Kaletsch U, Meinert R, Michaelis J. Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol. (1999) 28:631–9. doi: 10.1093/ije/28.4.631
61. Parodi S, Merlo DF, Ranucci A, Miligi L, Benvenuti A, Rondelli R, et al. Risk of neuroblastoma, maternal characteristics and perinatal exposures: the setil study. Cancer Epidemiol. (2014) 38:686–94. doi: 10.1016/j.canep.2014.09.007
62. Hardell L, Dreifaldt AC. Breast-feeding duration and the risk of malignant diseases in childhood in Sweden. Eur J Clin Nutr. (2001) 55:179–85. doi: 10.1038/sj.ejcn.1601142
63. Rajaraman P, Simpson J, Neta G, Berrington de. Gonzalez A, Ansell P, Linet MS, et al. Early life exposure to diagnostic radiation and ultrasound scans and risk of childhood cancer: case-control study. BMJ. (2011) 342:d472. doi: 10.1136/bmj.d472
64. Pang D, McNally R, Birch JM, Investig UKCCS. Parental smoking and childhood cancer: results from the United Kingdom childhood cancer study. Br J Cancer. (2003) 88:373–81. doi: 10.1038/sj.bjc.6600774
65. Buck GM, Michalek AM, Chen CJ, Nasca PC, Baptiste MS. Perinatal factors and risk of neuroblastoma. Paediatr Perinat Epidemiol. (2001) 15:47–53. doi: 10.1046/j.1365-3016.2001.00307.x
66. Daniels JL, Olshan AF, Pollock BH, Shah NR, Stram DO. Breast-feeding and neuroblastoma, USA and Canada. Cancer Causes Control. (2002) 13:401–5. doi: 10.1023/A:1015746701922
67. Patton T, Olshan AF, Neglia JP, Castleberry RP, Smith J. Parental exposure to medical radiation and neuroblastoma in offspring. Paediatr Perinat Epidemiol. (2004) 18:178–85. doi: 10.1111/j.1365-3016.2004.00554.x
68. Olshan AF, De Roos AJ, Teschke K, Neglia JP, Stram DO, Pollock BH, et al. Neuroblastoma and parental occupation. Cancer Causes Control. (1999) 10:539–49. doi: 10.1023/A:1008998925889
69. Olshan AF, Smith JC, Bondy ML, Neglia JP, Pollock BH. Maternal vitamin use and reduced risk of neuroblastoma. Epidemiology. (2002) 13:575–80. doi: 10.1097/00001648-200209000-00014
70. De Roos AJ, Teschke K, Savitz DA, Poole C, Grufferman S, Pollock BH, et al. Parental occupational exposures to electromagnetic fields and radiation and the incidence of neuroblastoma in offspring. Epidemiology. (2001) 12:508–17. doi: 10.1097/00001648-200109000-00008
71. Kerr MA, Nasca PC, Mundt KA, Michalek AM, Baptiste MS, Mahoney MC. Parental occupational exposures and risk of neuroblastoma: a case-control study (United States). Cancer Causes Control. (2000) 11:635–43. doi: 10.1023/A:1008951632482
72. Schwartzbaum JA. Influence of the mothers prenatal drug consumption on risk of neuroblastoma in the child. Am J Epidemiol. (1992) 135:1358–67. doi: 10.1093/oxfordjournals.aje.a116247
73. Bunin GR, Ward E, Kramer S, Rhee CA, Meadows AT. Neuroblastoma and parental occupation. Am J Epidemiol. (1990) 131:776–80. doi: 10.1093/oxfordjournals.aje.a115568
74. Stavrou EP, Baker DF, Bishop JF. Maternal smoking during pregnancy and childhood cancer in New South Wales: a record linkage investigation. Cancer Causes Control. (2009) 20:1551–8. doi: 10.1007/s10552-009-9400-5
75. Heck JE, Lee PC, Wu CK, Tsai HY, Ritz B, Arah OA, et al. Gestational risk factors and childhood cancers: a cohort study in Taiwan. Int J Cancer. (2020) 147:1343–53. doi: 10.1002/ijc.32905
76. Huang X, Hansen J, Lee P-C, Wu C-K, Federman N, Arah OA, et al. Maternal diabetes and childhood cancer risks in offspring: two population-based studies. Br J Cancer. (2022). doi: 10.1038/s41416-022-01961-w
77. Volk J, Heck JE, Schmiegelow K, Hansen J. Parental occupational organic dust exposure and selected childhood cancers in Denmark 1968-2016. Cancer Epidemiol. (2020) 65:101667. doi: 10.1016/j.canep.2020.101667
78. Schüz J, Luta G, Erdmann F, Ferro G, Bautz A, Simony SB, et al. Birth order and risk of childhood cancer in the danish birth cohort of 1973-2010. Cancer Causes Control. (2015) 26:1575–82. doi: 10.1007/s10552-015-0651-z
79. Contreras ZA, Hansen J, Ritz B, Olsen J, Yu F, Heck JE. Parental age and childhood cancer risk: a danish population-based registry study. Cancer Epidemiol. (2017) 49:202–15. doi: 10.1016/j.canep.2017.06.010
80. Seppälä LK, Vettenranta K, Leinonen MK, Tommiska V, Madanat-Harjuoja LM. Preterm birth, neonatal therapies and the risk of childhood cancer. Int J Cancer. (2021) 148:2139–47. doi: 10.1002/ijc.33376
81. Mortensen JHS, Øyen N, Fomina T, Melbye M, Tretli S, Vollset SE, et al. Supplemental folic acid in pregnancy and childhood cancer risk. Br J Cancer. (2016) 114:71–5. doi: 10.1038/bjc.2015.446
82. Feychting M, Floderus B, Ahlbom A. Parental occupational exposure to magnetic fields and childhood cancer (Sweden). Cancer Causes Control. (2000) 11:151–6. doi: 10.1023/A:1008922016813
83. Bluhm E, McNeil DE, Cnattingius S, Gridley G, El Ghormli L, Fraumeni JF Jr. Prenatal and perinatal risk factors for neuroblastoma. Int J Cancer. (2008) 123:2885–90. doi: 10.1002/ijc.23847
84. Sundh KJ, Henningsen AK, Källen K, Bergh C, Romundstad LB, Gissler M, et al. Cancer in children and young adults born after assisted reproductive technology: a nordic cohort study from the committee of nordic art and safety (conartas). Hum Reprod. (2014) 29:2050–7. doi: 10.1093/humrep/deu143
85. Spector LG, Brown MB, Wantman E, Letterie GS, Toner JP, Doody K, et al. Association of in vitro fertilization with childhood cancer in the United States. JAMA Pediatr. (2019) 173:e190392-e. doi: 10.1001/jamapediatrics.2019.0392
86. McLaughlin CC, Baptiste MS, Schymura MJ, Zdeb MS, Nasca PC. Perinatal risk factors for neuroblastoma. Cancer Causes Control. (2009) 20:289–301. doi: 10.1007/s10552-008-9243-5
87. Johnson KJ, Puumala SE, Soler JT, Spector LG. Perinatal characteristics and risk of neuroblastoma. Int J Cancer. (2008) 123:1166–72. doi: 10.1002/ijc.23645
88. Williams LA, Sample J, McLaughlin CC, Mueller BA, Chow EJ, Carozza SE, et al. Sex differences in associations between birth characteristics and childhood cancers: a five-state registry-linkage study. Cancer Causes Control. (2021) 32:1289–98. doi: 10.1007/s10552-021-01479-1
89. Contreras ZA, Ritz B, Virk J, Cockburn M, Heck JE. Maternal pre-pregnancy and gestational diabetes, obesity, gestational weight gain, and risk of cancer in young children: a population-based study in California. Cancer Causes Control. (2016) 27:1273–85. doi: 10.1007/s10552-016-0807-5
90. Heck JE, Contreras ZA, Park AS, Davidson TB, Cockburn M, Ritz B. Smoking in pregnancy and risk of cancer among young children: a population-based study. Int J Cancer. (2016) 139:613–6. doi: 10.1002/ijc.30111
91. Johnson CC, Spitz MR. Neuroblastoma: case-control analysis of birth characteristics. J Natl Cancer Inst. (1985) 74:789–92.
92. Spitz MR, Johnson CC. Neuroblastoma and paternal occupation. A case-control analysis. Am J Epidemiol. (1985) 121:924–9. doi: 10.1093/oxfordjournals.aje.a114062
93. Schraw JM, Rodriguez KB, Scheurer ME, Foster JH, Lupo PJ. Associations of demographic and perinatal factors with childhood neuroblastoma in texas, 1995–2011. Cancer Epidemiol. (2022) 78:102165. doi: 10.1016/j.canep.2022.102165
94. Kumar SV, Lupo PJ, Pompeii LA, Danysh HE. Maternal residential proximity to major roadways and pediatric embryonal tumors in offspring. Int J Environ Res Public Health. (2018) 15:505. doi: 10.3390/ijerph15030505
Keywords: neuroblastoma, high birthweight, Cesarean section, breastfeeding, pesticides, systematic review and meta-analysis
Citation: Onyije FM, Dolatkhah R, Olsson A, Bouaoun L and Schüz J (2025) Risk factors of neuroblastoma: a systematic review and meta-analysis. Front. Public Health 13:1576101. doi: 10.3389/fpubh.2025.1576101
Received: 13 February 2025; Accepted: 05 June 2025;
Published: 25 June 2025.
Edited by:
Tim S. Nawrot, University of Hasselt, BelgiumReviewed by:
Naonori Kawakubo, Kyushu University, JapanMaite Gorostegui, Sant Joan de Déu Hospital, Spain
Copyright © 2025 Onyije, Dolatkhah, Olsson, Bouaoun and Schüz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Felix M. Onyije, b255aWplZkBpYXJjLndoby5pbnQ=
 Felix M. Onyije
Felix M. Onyije Roya Dolatkhah
Roya Dolatkhah Ann Olsson
Ann Olsson Joachim Schüz
Joachim Schüz