- 1Department of Infectious Diseases, Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Medical Affairs, Shaanxi Provincial people’s Hospital, Xi’an, China
Background: Patients with Alzheimer’s disease or dementia are at increased risk for COVID-19 hospitalization and mortality. However, no study has examined whether memory function is associated with COVID-19 outcomes in general older adults.
Methods: Data were obtained from SHARE (the Survey of Health, Ageing and Retirement in Europe), HRS (the Health and Retirement Study), and ELSA (the English Longitudinal Study of Ageing), three prospective and representative cohorts of non-institutionalized adults aged 50 years and older in 25 European countries plus Israel, the United States, and the United Kingdom, respectively. Memory function was measured with immediate and delayed 10-words recall tests. Associations of 10-words recall with COVID-19 hospitalization and mortality were assessed using logistic models adjusted for age, sex, race, body mass index, smoking, physical activity, household income, education level, and chronic conditions.
Results: A total of 4,062 participants with COVID-19 infection from SHARE, 1349 from HRS, and 278 from ELSA were included in the analysis. 610 (15.0%) in SHARE, 142 (10.5%) in HRS, and 39 (14.0%) in ELSA were hospitalized, and 102 (2.5%) died of COVID-19 or related complications in SHARE. The adjusted odds ratios (aORs) for COVID-19 hospitalization were 1.15 (95% CI, 1.09–1.22) in SHARE, 1.07 (95% CI, 0.94–1.21) in HRS, and 1.34 (95% CI, 1.02–1.77) in ELSA, per word decrease in immediate 10-words recall. For delayed 10-words recall, the corresponding aORs were 1.11 (95% CI, 1.06–1.17), 1.12 (95% CI, 1.01–1.24), and 1.25 (95% CI, 1.01–1.55), respectively. The aORs for COVID-19 mortality were 1.06 (95% CI, 0.93–1.20) and 1.14 (95% CI, 1.01–1.28) per word decrease in immediate and delayed 10-words recall in SHARE, respectively. Results were relatively robust to missing data of covariates, exclusion of cases based on symptoms alone, or exclusion of cases with Alzheimer’s disease or dementia.
Conclusion: This study shows that low memory performance, as measured by 10-words recall, is independently associated with an increased risk of COVID-19 hospitalization and mortality in adults aged 50 years and older.
Introduction
Coronavirus disease 2019 (COVID-19) has posed a significant global public health threat. As of March 2023, more than 760 million confirmed cases and over 6.8 million deaths have been reported worldwide (1). The clinical manifestations of COVID-19 range from asymptomatic cases to severe illness and death. Older adults are particularly at risk, with the majority of severe and fatal cases occurring in individuals aged 50 years and older (2). Hospitalization and mortality rates among this population are 3 to 15 times and 25 to 350 times higher, respectively, compared to younger individuals (2). Understanding the risk factors for severe and fatal COVID-19 outcomes in this vulnerable group is critical for identifying high-risk patients who may benefit from early intervention to prevent disease progression and adverse outcomes.
Established risk factors for severe COVID-19 include male sex, low socioeconomic status, poor physical fitness, and a range of chronic underlying conditions, such as cardiovascular disease, respiratory disease, cancer, obesity, diabetes, and dementia (3–5). However, despite these known factors, other potential contributors to severe COVID-19 outcomes remain to be fully understood.
One such potential risk factor is memory function, a key aspect of cognitive health that plays a central role in various other cognitive processes. Individuals with impaired memory function may struggle with complex tasks necessary for self-care, which could influence their ability to manage symptoms and seek timely medical intervention. Impaired memory has been linked to higher mortality in both the general population and in individuals with chronic illnesses, such as cancer and end-stage renal disease (6–8). While patients with Alzheimer’s disease or dementia have been shown to have an increased risk of COVID-19 hospitalization and mortality (9–12), it remains unclear whether poor memory performance in the general older adult population is similarly associated with adverse COVID-19 outcomes.
This study aims to fill this gap by examining the associations between pre-pandemic memory function and COVID-19 hospitalization and mortality in adults aged 50 years and older across three prospective cohorts. By investigating this hypothesized relationship, we seek to determine whether memory performance can serve as an additional risk factor for severe COVID-19 outcomes, which could have important implications for early risk identification and patient management.
Methods
Data were obtained from SHARE (the Survey of Health, Ageing and Retirement in Europe) (13), HRS (the Health and Retirement Study) (14), and ELSA (the English Longitudinal Study of Ageing) (15), three prospective and representative cohorts of non-institutionalized adults aged 50 years and older in 25 European countries plus Israel, the United States, and the United Kingdom, respectively. Details of the design and methods of the three cohorts can be found elsewhere (13–15). After the COVID-19 outbreak, participants in the three cohorts participated in surveys on COVID-19 infection and changes in life during the pandemic (two rounds from 2020 to 2021 in SHARE, two rounds from 2020 to 2022 in SHARE, and two rounds during 2020 in ELSA). SHARE, HRS, and ELSA were approved by the Ethics Council of the Max Planck Society, the Institutional Review Board of the University of Michigan, and the South Central Berkshire Research Ethics Committee, respectively.
We used data from wave 7 (2017) in SHARE, wave 14 (2018) in HRS, and wave 9 (2018/2019) in ELSA. These waves were considered baselines because they had the most recent memory tests before the COVID-19 outbreak. COVID-19 infection was defined if participants experienced COVID-19 symptoms, were tested positive for coronavirus, were told by a health care provider that they had the disease, attended an emergency department, or were hospitalized for COVID-19, or died of COVID-19 or related complications (Appendix 1). Participants were excluded if they did not have 10-words recall tests at baseline, did not have COVID-19 infection, were younger than 50 years in 2020, or had missing values for covariates.
COVID-19 hospitalization
In SHARE, participants were asked, “Who was hospitalized due to an infection from the Coronavirus?” Participants who reported being hospitalized were included in the COVID-19 hospitalization analysis. In HRS and ELSA, COVID-19 hospitalization was identified by the question “Were you admitted to the hospital because of the virus?” and “Have you had to stay in hospital for treatment due to coronavirus?”
COVID-19 mortality
SHARE asked interviewers to verify a participant’s death by contacting a proxy respondent. If the participant was deceased, an end-of-life interview was undertaken to collect information related to the death. The proxy respondent was selected from the deceased participant’s immediate social network, including family/household members, neighbors, or other close acquaintances. Participants who died of COVID-19 or related complications were included in the COVID-19 mortality analysis. These participants also constituted the COVID-19 hospitalization sample, as they typically died in healthcare facilities. HRS and ELSA were not analyzed for COVID-19 mortality because their end-of-life data are not currently available.
Memory function
Memory was assessed using the 10-words recall tests (16), which were conducted in a similar manner across the three cohorts. Participants were presented with a list of 10 words, one at a time. After the list was completed, participants were asked to recall and either type or speak as many words as they could remember, in any order (immediate 10-word recall). Approximately 5 min later, they were asked to recall the words again (delayed 10-word recall). The words used in the test were simple, everyday terms, such as “hotel,” “river,” “tree,” “skin,” “gold,” “market,” “paper,” “child,” “king,” and “book.” Scores on both the immediate and delayed recall tests ranged from 0 to 10, representing the number of words successfully remembered by the participants.
Covariates
Potential confounders collected in all three cohorts were included in the analyses: age in 2020, sex, race (HRS and ELSA only), body mass index, smoking status, physical activity, household income, education, and underlying health conditions. These factors were previously reported to be related to COVID-19 outcomes (3–5). Body mass index was calculated as weight/height (2), and height and weight were self-reported. Participants were asked how many days per week they did moderate and vigorous physical activity. If participants answered “rarely or never,” they were considered to be physical inactivity. Smoking status was divided into two categories: ever or current smoker and never smoker. In SHARE, educational attainment was coded based on the International Standard Classification of Education-97 (ISCED-97) and classified as low (no education or codes 1 and 2), medium (codes 3 and 4), and high (codes 5 and 6). In HRS and ELSA, low, medium, and high levels of education were less than high school, high school, and more than high school, respectively. Household income was categorized into country-specific quartiles. The following health conditions were asked whether participants had ever been diagnosed with or had at the time of the baseline interview: respiratory disease (such as emphysema or chronic bronchitis), cardiovascular disease (heart attack, congestive heart failure, or any other heart problem, and stroke or cerebral vascular disease), diabetes, cancer, and dementia or Alzheimer’s disease.
Statistical analyses
Continuous variables were presented as the median and interquartile range (IQR). Categorical variables were presented as numbers and percentages. Differences in characteristics among the three cohorts were tested using the Kruskal-Wallis test or the chi-square test. Based on the results of these comparisons, we assessed the heterogeneity of the three cohorts and the suitability of pooling the results.
Four logistic regression models were fitted to test the associations of immediate and delayed 10-words recall with COVID-19 hospitalization and mortality. Model 0 was unadjusted. Model 1 adjusted for age, sex, and race (HRS and ELSA only). Model 2 additionally adjusted for body mass index, smoking status, physical activity, household income, and educational attainment. Finally, Model 3 further adjusted for underlying chronic diseases, including respiratory disease, cardiovascular disease, diabetes, cancer, and dementia or Alzheimer’s disease. Linear associations between continuous independent variables and COVID-19 hospitalization and mortality were tested using the Box-Tidwell test. As no evidence of deviation from linearity was found (Appendix 2), the 10-words recall was treated as a continuous variable in the aforementioned logistic models, and odds ratios (ORs) per word decrease were calculated.
Three separate sensitivity analyses were performed. In the first sensitivity analysis, participants were excluded if the diagnosis of COVID-19 infection was based on symptoms alone. Thus, the included participants were those who were tested positive for coronavirus, were hospitalized due to COVID-19, or died of COVID-19 or related complications. In the second sensitivity analysis, participants were excluded if they had ever been diagnosed with or had dementia or Alzheimer’s disease at the time of the baseline interview. In the third sensitivity analysis, missing values of covariates were imputed (Appendix 3). Due to the small sample size, the above sensitivity analyses were not performed for ELSA.
ORs were reported along with their 95% confidence intervals (95% CI). Statistically significant was defined as p < 0.05 on both sides. StataSE 15 statistical software (StataCorp) was used for all analyses.
Results
Study population
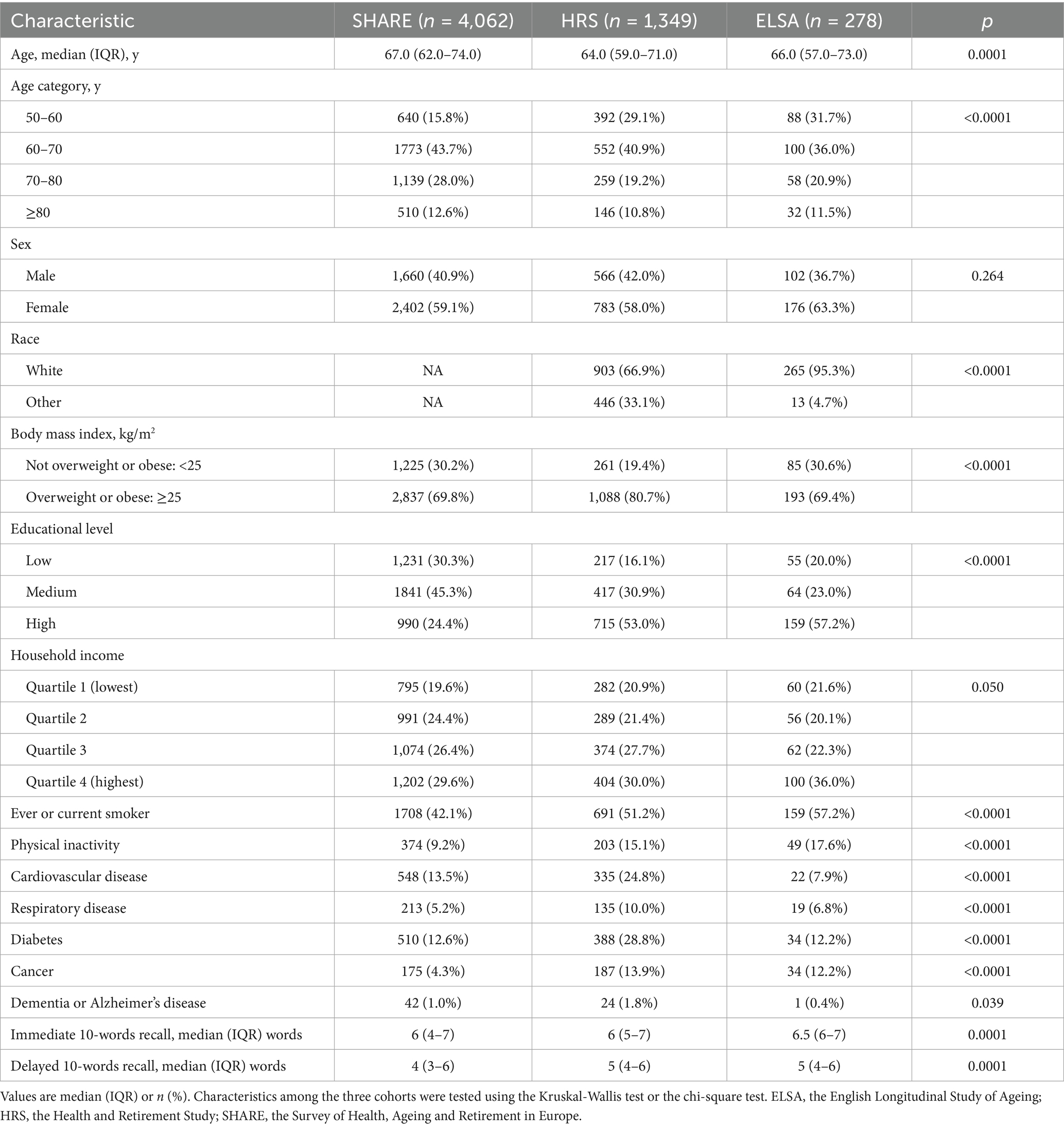
A total of 4,062 participants with COVID-19 infection from SHARE (25 European countries and Israel), 1,349 from HRS (the United States), and 278 from ELSA (the United Kingdom) were included in the analysis. Figure 1 shows the detailed participant selection process. Across all three cohorts, the participants included in the study were generally younger, had higher levels of education, and reported higher household incomes. Additionally, in the SHARE and HRS cohorts, participants were more likely to be female, overweight or obese, and less likely to engage in physical inactivity or have cardiovascular disease compared to those who were excluded.
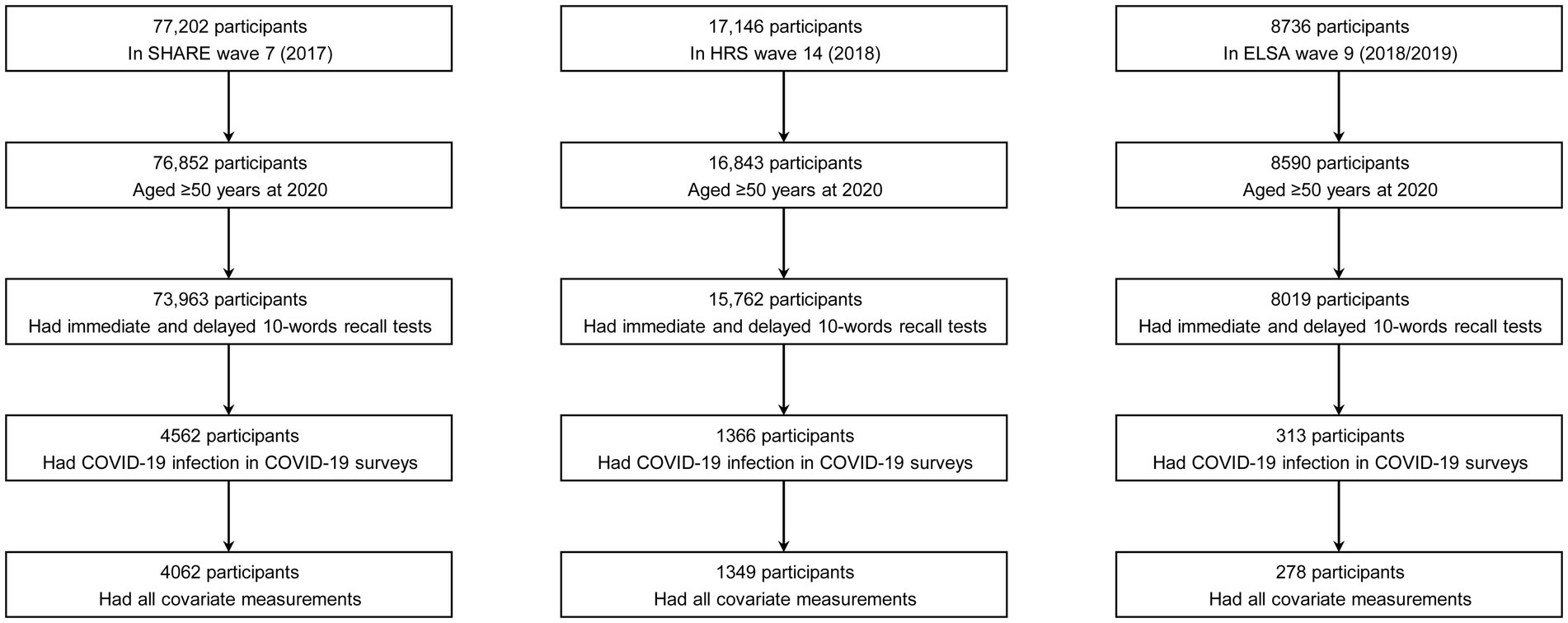
Figure 1. Determination of the study sample. COVID-19, coronavirus disease 2019; ELSA, the English Longitudinal Study of Ageing; HRS, the Health and Retirement Study; SHARE, the Survey of Health, Ageing and Retirement in Europe.
Median age of included participants was 67.0 (IQR: 62.0–74.0) years in SHARE, 64.0 (IQR: 59.0–71.0) years in HRS, and 66.0 (IQR: 57.0–73.0) years in ELSA. Men accounted for 40.9%, 42.0%, and 36.7% of the cohorts, respectively. The distributions of immediate and delayed 10-words recall test scores in the three cohorts are shown in Figure 2. The median number of words for immediate 10-words recall was 6 (IQR: 4–7) words in SHARE, 6 (IQR: 5–7) words in HRS, and 6.5 (IQR: 6–7) words in ELSA. For delayed 10-words recall, the corresponding median word counts were 4 (IQR: 3–6) words, 5 (IQR: 4–6) words, and 5 (IQR: 4–6) words, respectively. Most baseline characteristics differed among the three cohorts, as summarized in Table 1. Given the observed heterogeneity among the three cohorts, a pooled analysis was not performed.
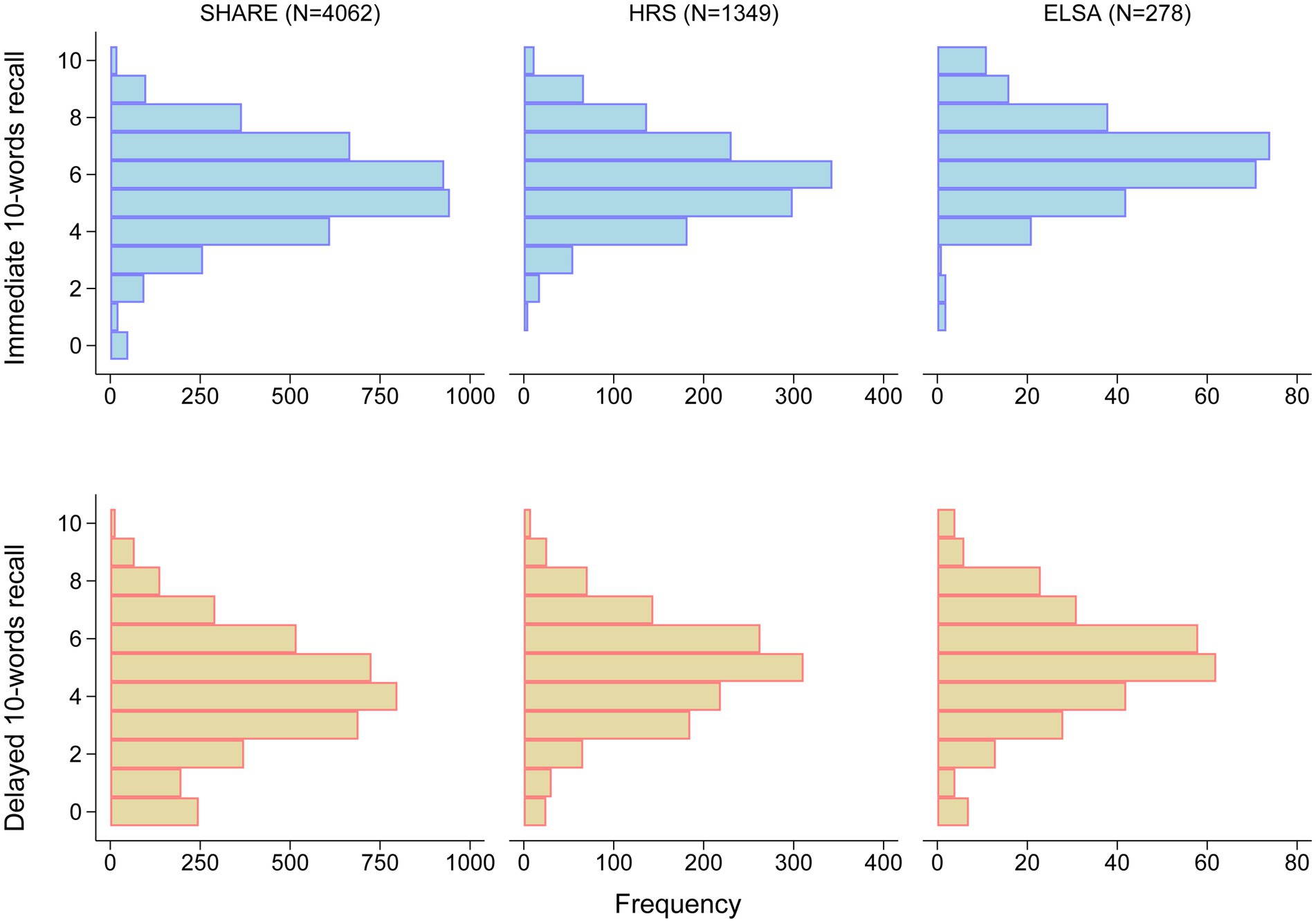
Figure 2. Distribution of scores on immediate and delayed 10-words recall tests. ELSA, the English Longitudinal Study of Ageing; HRS, the Health and Retirement Study; SHARE, the Survey of Health, Ageing and Retirement in Europe.
Associations of 10-word recall with COVID-19 hospitalization
Among participants with COVID-19 infection, 610 out of 4,062 (15.0%) in SHARE, 142 out of 1,349 (10.5%) in HRS, and 39 out of 278 (14.0%) in ELSA were hospitalized. Hospitalized participants recalled fewer words in the immediate 10-words recall test performed prior to the COVID-19 outbreak, compared with those who were not hospitalized, in each of the three cohorts ([median (IQR)]: 5 (4–6) words vs. 6 (5–7) words, in SHARE; 5 (4–7) words vs. 6 (5–7) words, in HRS; 6 (5–6) words vs. 7 (6–8) words, in ELSA; Supplementary Figure S1). The results were similar for the delayed 10-words recall test ([median (IQR)]: 3 (2–5) words vs. 4 (3–6) words, in SHARE; 4 (3–6) words vs. 5 (4–6) words, in HRS; 4 (3–5) words vs. 5 (4–7) words, in ELSA; Supplementary Figure S1).
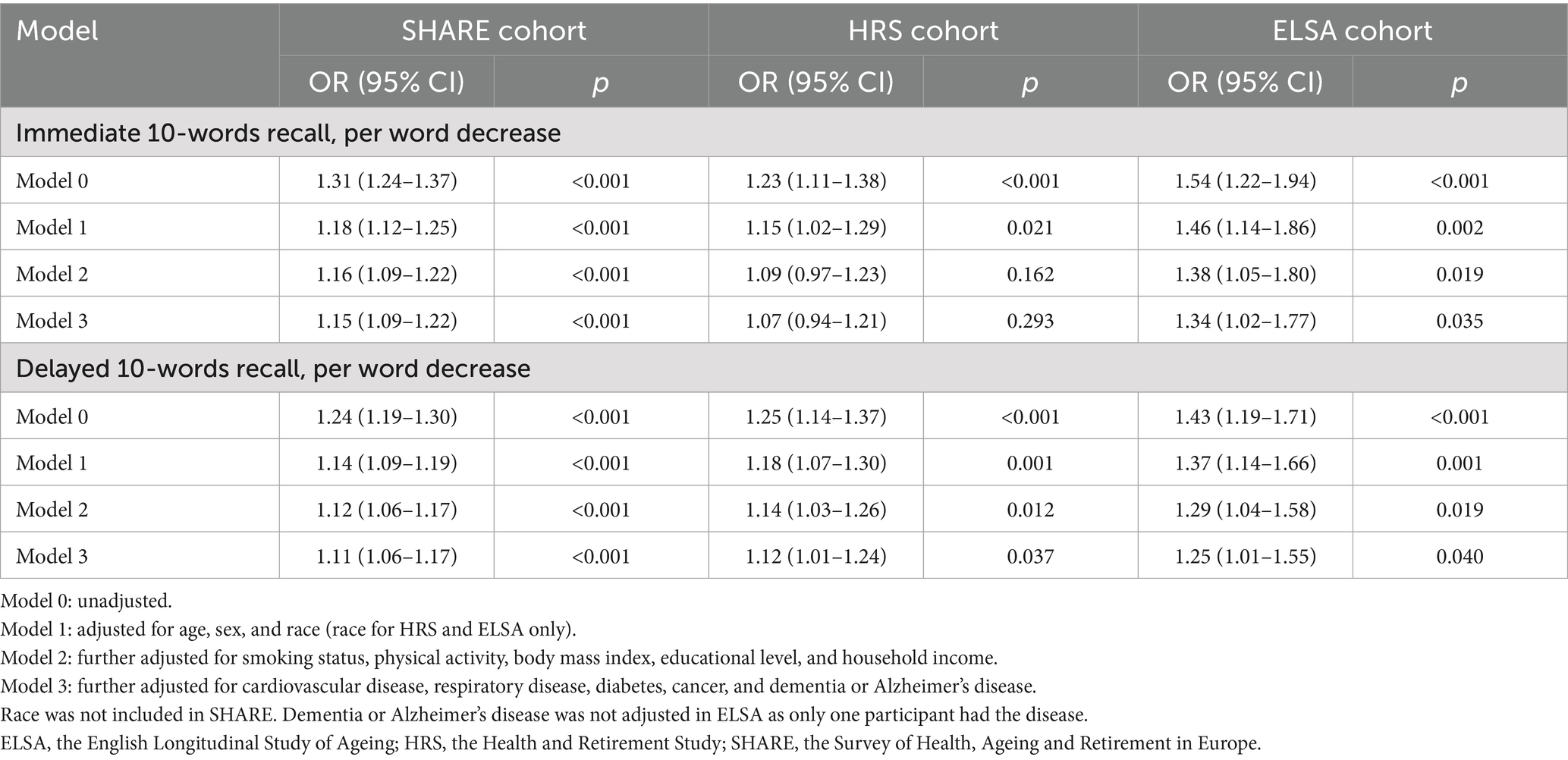
As shown in Table 2, in Model 0, the crude ORs of hospitalization per word decrease in immediate 10-words recall were 1.31 (95% CI, 1.24–1.37) in SHARE, 1.23 (95% CI, 1.11–1.38) in HRS, and 1.54 (95% CI, 1.22–1.94) in ELSA. For delayed 10-words recall, the corresponding crude ORs per word decrease were 1.24 (95% CI, 1.19–1.30), 1.25 (95% CI, 1.14–1.37), and 1.43 (95% CI, 1.19–1.71), respectively. These ORs were dwarfed in each cohort, after adjustment for age, sex, and race in Model 1; after further adjustment, the magnitude of the ORs were slightly attenuated in Model 2 and Model 3. In the fully adjusted model (Model 3), the ORs for hospitalization were 1.15 (95% CI, 1.09–1.22) in SHARE, 1.07 (95% CI, 0.94–1.21) in HRS, and 1.34 (95% CI, 1.02–1.77) in ELSA, per word decrease in immediate 10-words recall (Supplementary Figure S2). For delayed 10-words recall in Model 3, the corresponding adjusted ORs per word decrease were 1.11 (95% CI, 1.06–1.17), 1.12 (95% CI, 1.01–1.24), and 1.25 (95% CI, 1.01–1.55), respectively (Supplementary Figure S3). Other factors associated with COVID-19 hospitalization were older age (SHARE and HRS), male sex (SHARE and HRS), non-white race (HRS), being overweight or obese (SHARE and HRS), physical inactivity (SHARE and HRS), lower levels of income (HRS and ELSA), lower levels of education (SHARE), diabetes (SHARE), and dementia or Alzheimer’s disease (HRS) (Supplementary Figures S2, S3).
Associations of 10-word recall with COVID-19 mortality
Only SHARE had end-of-life data to allow analysis of the COVID-19 mortality. Of the 4,062 participants with COVID-19 infection in SHARE, 102 (2.5%) died of COVID-19 or related complications. Participants who died of COVID-19 or related complications recalled fewer words in both immediate and delayed recall tests performed before the COVID-19 outbreak compared with those who did not (immediate 10-words recall [median (IQR)]: 4.5 (3–6) words vs. 6 (5–7) words; delayed 10-words recall [median (IQR)]: 3 (1–4) words vs. 4 (3–6) words; Supplementary Figure S4).
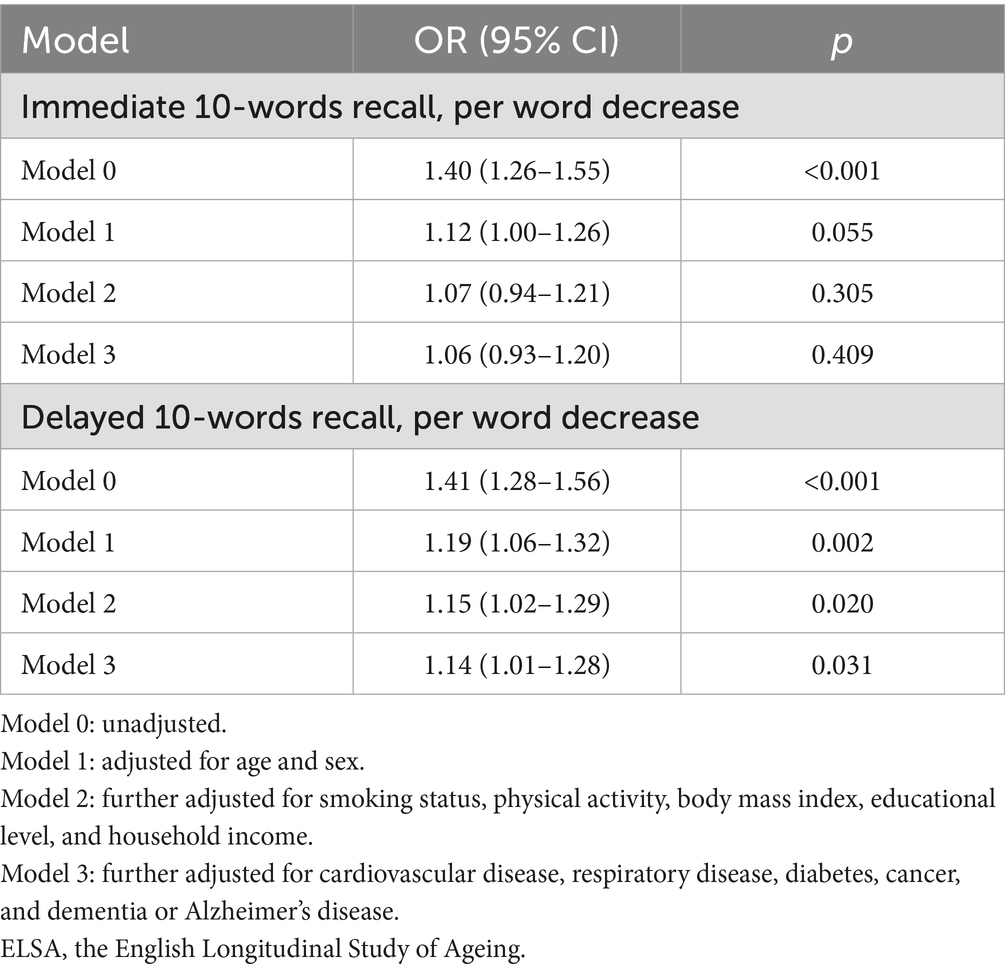
As shown in Table 3, the crude ORs for COVID-19 mortality per word decrease were 1.40 (95% CI, 1.26–1.55) for immediate 10-words recall and 1.41 (95% CI, 1.28–1.56) for delayed 10-words recall. After full adjustment, the association with COVID-19 mortality was still statistically significant for delayed 10-words recall (OR, 1.14; 95% CI, 1.01–1.28; Supplementary Figure S5) but not for immediate 10-words recall (OR, 1.06; 95% CI, 0.93–1.20; Supplementary Figure S6). Other factors associated with COVID-19 mortality in SHARE were older age, male sex, physical inactivity, and diabetes (Supplementary Figures S5, S6).
Sensitivity analyses
After imputation for missing data of covariates, the association of delayed 10-words recall with COVID-19 mortality was no longer statistically significant in SHARE (OR, 1.09; 95% CI, 0.98–1.22). In other sensitivity analyses, there was little change in the magnitude of the associations with either immediate or delayed 10-word recall in both SHARE and HRS (Supplementary Tables S1–S5).
Discussion
The main finding of this study is that low pre-pandemic memory performance was associated with an increased risk of COVID-19-related hospitalization among individuals aged 50 years and older, based on data from three longitudinal cohorts across 25 European countries and Israel, the United States, and the United Kingdom. In addition, low pre-pandemic memory performance was linked to a higher risk of COVID-19 mortality in individuals from 25 European countries and Israel. These associations remained robust even after adjusting for established risk factors for severe COVID-19 outcomes, including older age, male sex, non-white race, low socioeconomic status, physical inactivity, and pre-existing chronic conditions such as cardiovascular disease, respiratory disease, cancer, diabetes, and obesity. Notably, the strongest association with hospitalization was observed in the delayed 10-word recall test, with consistent results across all three cohorts. In contrast, the immediate 10-word recall appeared less relevant, as its association with hospitalization was only observed in the SHARE and ELSA cohorts, but not in the HRS cohort.
To our best knowledge, rare study examined the association between memory function and COVID-19 outcomes in general older adults. Previous studies have focused primarily on patients with Alzheimer’s disease or dementia, which has been shown to be an independent risk factor for COVID-19 hospitalization and mortality (9–12). In this study, we also found that individuals with Alzheimer’s disease or dementia tend to be hospitalized or die when infected with COVID-19, especially for hospitalization in the HRS cohort. However, in our study, the samples were from community-dwelling populations. Alzheimer’s disease or dementia accounted for only a very small proportion of the study samples (<2%); the vast majority were older adults without dementia. Furthermore, we performed adjusted analyses and sensitivity analyses excluding Alzheimer’s disease or dementia. Therefore, we found the associations not only in general older adults, but also in the non-dementia older adults.
Recommendations for the management of COVID-19 are highly dependent on the risk of a patient progressing from a milder state to the next more severe state (17). The identification of risk factors for severe COVID-19 is of great importance to clinicians and to public health strategies for the management of this disease. In this study, we identified low memory performance, measured as 10-words recall test, as a potential risk factor of such. The test can be easily performed, and the respondent burden is low. Moreover, the test has been demonstrated good validity and reliability and now has been widely adopted, particularly in large surveys of aging populations (13–15). Its utility for COVID-19 prognosis and treatment guidance, especially in combination with established risk factors, is worthy of evaluation in both community and clinical settings.
Behavioral pathways and socioeconomic status are thought to mediate, in part, the association between memory function and clinical outcomes. For example, individuals with low memory performance are less likely to engage in healthy behaviors (e.g., healthy eating, physical activity, reduced alcohol consumption, and smoking cessation) and to adhere to their medication regimens (18, 19). One study found that older adults with low memory performance were less likely to adhere to COVID-19 protective behaviors (20). The association between low socioeconomic status and predementia and dementia syndromes is supported by the majority of studies (19). In the current study, the ORs for COVID-19 hospitalization and mortality were attenuated after adjustment for smoking status, physical activity, body mass index, educational level, and household income (model 2), suggesting that health behaviors and socioeconomic status contribute to some extent to their associations.
Common factors, such as comorbidities, gut microbes, environmental factors, and inflammatory cytokines, can cause both low memory performance and severe disease in COVID-19. More than 100 diseases cause memory impairment (21). In this study, the ORs for COVID-19 hospitalization and mortality were attenuated after adjustment for underlying diseases, including cardiovascular disease, respiratory disease, diabetes, cancer, and obesity, suggesting that comorbidities contribute in part to the observed associations. Gut microbiota may alter brain integrity and cognitive function (22). One study showed that COVID-19 patients with lower gut microbiota diversity were more likely to require ICU admission (23). Adverse environments, such as air pollution, are associated with impaired cognition (24). A meta-analysis showed that 127 out of 139 studies reported an association between air pollution and unfavorable COVID-19 outcomes (25). Increased levels of inflammation, such as interleukin-1β, interleukin-6, and C-reactive protein, which can potentially cause or aggravate biological aging and cellular aging, are involved in memory loss and other cognitive functions (26). These inflammatory factors mediate COVID-19 progression and are well-defined markers of severe COVID-19 (27).
In addition to low memory performance, this study identified several factors associated with COVID-19 hospitalization. These include older age, male sex, non-white race, overweight or obesity, lower levels of education and household income, physical inactivity, and underlying conditions such as diabetes and dementia or Alzheimer’s disease. Furthermore, older age, male sex, physical inactivity, and diabetes were found to be associated with COVID-19 mortality. These are well-established risk factors for COVID-19 severity and death. However, other commonly recognized risk factors, such as smoking, cardiovascular disease, and chronic respiratory disease, were not found to be significant in this study. It is important to note that prior research has predominantly focused on individuals at the highest risk, often those already hospitalized (3–5, 9–12). As a result, the findings of previous studies may not be generalizable to the broader population, as is the case in the present study.
The main strength of the study is the longitudinal design of SHARE, HRS, and ELSA, and their good representation of European, American, and British populations aged 50 years and older. The association of low memory performance with COVID-19 hospitalization was robust in our study. First, the association was observed in each of the three cohorts, even though there was great heterogeneity among the three cohorts. Delayed recall represents a more cognitively demanding task than immediate recall, requiring successful completion of three distinct neurocognitive processes: encoding, consolidation, and retrieval. In contrast, immediate recall primarily assesses encoding capacity. This cognitive complexity likely explains why delayed recall demonstrates greater sensitivity in detecting mild cognitive impairment (28), consistent with our findings of stronger predictive validity for delayed than immediate recall measures. Second, the association was still observed after full adjustment for demographics, socioeconomic status, lifestyle, and underlying diseases. Third, the ORs for COVID-19 hospitalization remained largely unchanged in the three sensitivity analyses. Fourth, our findings are consistent with prepandemic research demonstrating that baseline mild cognitive impairment serves as a significant predictor of both hospitalization and readmission in older adults (29).
This study has several limitations. First, information on COVID-19 infection, hospitalization, mortality, and covariates was collected via questionnaires. This may introduce bias at various stages of questionnaire design and administration. Direct measurements or data linked to medical records and mortality registries would likely provide more accurate estimates of the associations. Second, although adjustments were made for a range of demographic, lifestyle, socioeconomic, and clinical factors, the potential influence of unmeasured variables (e.g., unadjusted comorbidities, gut microbiota, environmental factors, and inflammatory cytokines) cannot be entirely ruled out. Factors that were not available across all three datasets or that were measured using inconsistent methods, such as loneliness and social isolation (30), were also not adjusted. Advance care planning decisions (e.g., “do not resuscitate” or “do not intubate”)—factors known to affect severity and death—were not collected in the SHARE cohort, and their inclusion might have weakened or negated the observed associations. Third, the lack of end-of-life data from HRS and ELSA prevented the analysis of COVID-19 mortality in these two cohorts. Although SHARE provided mortality data, the number of COVID-19-related deaths was relatively small, and the results were sensitive to imputations for missing covariates. While SHARE had the largest sample size for hospitalization analysis, ELSA included only 39 hospitalized individuals. Older participants recalled fewer words across all three cohorts (data not shown), but due to the limited sample size in each age group, age-stratified analyses were not conducted. The imbalance between hospitalized and non-hospitalized participants may also have biased the results. Therefore, cohorts with larger sample sizes are needed to validate our findings. Fourth, there was a lack of data on COVID-19 vaccination status and viral strains, both of which are critical determinants of disease severity (31). The impact of these factors on the observed associations remains unclear, and updated data will be necessary as vaccination coverage increases and the virus continues to evolve.
Conclusion
Our study shows that low memory performance, as measured by 10-words recall test, is independently associated with an increased risk of COVID-19 hospitalization and mortality in adults aged 50 years and older. Further studies are warranted to validate these associations in recent omicron waves of the COVID-19 pandemic and in clinical settings, and to elucidate the underlying mechanisms in order to improve the management of COVID-19.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: SHARE: http://www.shareproject.org/data-access.html; HRS: https://hrs.isr.umich.edu/; ELSA: https://www.elsa-project.ac.uk/.
Ethics statement
The studies involving humans were approved by the Ethics Council of the Max Planck Society, the Institutional Review Board of the University of Michigan, and the South Central Berkshire Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JS: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. XS: Validation, Writing – original draft, Writing – review & editing. YT: Validation, Writing – original draft, Writing – review & editing. RL: Writing – review & editing. JL: Writing – review & editing. XG: Writing – review & editing. SZ: Writing – review & editing. XJ: Writing – review & editing. FJ: Writing – review & editing. SD: Supervision, Writing – review & editing. WW: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This paper uses data from SHARE. The SHARE data collection has been funded by the European Commission, DG RTD through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006-062193, COMPARE: CIT5-CT-2005-028857, SHARELIFE: CIT4-CT-2006-028812), FP7 (SHARE-PREP: no. 211909, SHARE-LEAP: no. 227822, SHARE M4: no. 261982, DASISH: no. 283646) and Horizon 2020 (SHARE-DEV3: no. 676536, SHARE-COHESION: no. 870628, SERISS: no. 654221, SSHOC: no. 823782, SHARE-COVID19: no. 101015924) and by DG Employment, Social Affairs & Inclusion through VS 2015/0195, VS 2016/0135, VS 2018/0285, VS 2019/0332, and VS 2020/0313. Additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C, RAG052527A) and from various national funding sources is gratefully acknowledged (see www.share-project.org). This paper uses data from HRS, sponsored by the National Institute on Aging (National Institutes of Health, Bethesda, MD; grant number NIA U01AG009740) and conducted by the University of Michigan (Ann Arbor, MI). This paper uses data from ELSA Wave 9, and COVID-19 wave 1 and 2. ELSA is funded by the National Institute on Aging (R01AG017644), and by UK Government Departments coordinated by the National Institute for Health and Care Research (NIHR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1577334/full#supplementary-material
Abbreviations
CI, confidence intervals; COVID-19, Coronavirus disease 2019; ELSA, the English Longitudinal Study of Ageing; HRS, the Health and Retirement Study; IQR, interquartile range; ISCED-97, International Standard Classification of Education-97; OR, odds ratio; SHARE, the Survey of Health, Ageing and Retirement in Europe.
References
1. World Heath Organization. Available online at:https://covid19.who.int/. (2023).
2. Centers for Disease Control and Prevention. Available online at:https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Accessed March 22, 2023.
3. Jordan, RE, Adab, P, and Cheng, KK. Covid-19: risk factors for severe disease and death. BMJ. (2020) 368:m1198. doi: 10.1136/bmj.m1198
4. Nieman, DC, and Sakaguchi, CA. Physical activity lowers the risk for acute respiratory infections: time for recognition. J Sport Health Sci. (2022) 11:648–55. doi: 10.1016/j.jshs.2022.08.002
5. Centers for Disease Control and Prevention. Available online at:https://www.cdc.gov/covid/hcp/clinical-care/underlying-conditions.html#cdc_generic_section_3-demographic-factors. Accessed March 22, 2023.
6. Connors, MH, Sachdev, PS, Kochan, NA, Xu, J, Draper, B, and Brodaty, H. Cognition and mortality in older people: the Sydney memory and ageing study. Age Ageing. (2015) 44:1049–54. doi: 10.1093/ageing/afv139
7. Drew, DA, Weiner, DE, Tighiouart, H, Scott, T, Lou, K, Kantor, A, et al. Cognitive function and all-cause mortality in maintenance hemodialysis patients. Am J Kidney Dis. (2015) 65:303–11. doi: 10.1053/j.ajkd.2014.07.009
8. Hshieh, TT, Jung, WF, Grande, LJ, Chen, J, Stone, RM, Soiffer, RJ, et al. Prevalence of cognitive impairment and association with survival among older patients with hematologic cancers. JAMA Oncol. (2018) 4:686–93. doi: 10.1001/jamaoncol.2017.5674
9. Gilstrap, L, Zhou, W, Alsan, M, Nanda, A, and Skinner, JS. Trends in mortality rates among Medicare enrollees with Alzheimer disease and related dementias before and during the early phase of the COVID-19 pandemic. JAMA Neurol. (2022) 79:342–8. doi: 10.1001/jamaneurol.2022.0010
10. Tahira, AC, Verjovski-Almeida, S, and Ferreira, ST. Dementia is an age-independent risk factor for severity and death in COVID-19 inpatients. Alzheimers Dement. (2021) 17:1818–31. doi: 10.1002/alz.12352
11. Wang, Q, Davis, PB, Gurney, ME, and Xu, R. COVID-19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. (2021) 17:1297–306. doi: 10.1002/alz.12296
12. Wang, Y, Li, M, Kazis, LE, and Xia, W. Clinical outcomes of COVID-19 infection among patients with Alzheimer's disease or mild cognitive impairment. Alzheimers Dement. (2022) 18:911–23. doi: 10.1002/alz.12665
13. Börsch-Supan, A, Brandt, M, Hunkler, C, Kneip, T, Korbmacher, J, Malter, F, et al. Data resource profile: the survey of health, ageing and retirement in Europe (SHARE). Int J Epidemiol. (2013) 42:992–1001. doi: 10.1093/ije/dyt088
14. Sonnega, A, Faul, JD, Ofstedal, MB, Langa, KM, Phillips, JW, and Weir, DR. Cohort profile: the health and retirement study (HRS). Int J Epidemiol. (2014) 43:576–85. doi: 10.1093/ije/dyu067
15. Steptoe, A, Breeze, E, Banks, J, and Nazroo, J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. (2013) 42:1640–8. doi: 10.1093/ije/dys168
16. Chaves, MLF, and Camozzato, AL. How many items from a word list can Alzheimer's disease patients and normal controls recall? Do they recall in a similar way? Dement Neuropsychol. (2007) 1:52–8. doi: 10.1590/S1980-57642008DN10100009
17. National Institutes of Health. Coronavirus disease 2019 treatment guidelines. Available online at:www.covid19treatmentguidelines.nih.gov. Accessed March 22, 2023.
18. Solfrizzi, V, Capurso, C, D'Introno, A, Colacicco, AM, Santamato, A, Ranieri, M, et al. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev Neurother. (2008) 8:133–58. doi: 10.1586/14737175.8.1.133
19. Stilley, CS, Bender, CM, Dunbar-Jacob, J, Sereika, S, and Ryan, CM. The impact of cognitive function on medication management: three studies. Health Psychol. (2010) 29:50–5. doi: 10.1037/a0016940
20. O'Shea, DM, Davis, JD, and Tremont, G. Verbal memory is associated with adherence to COVID-19 protective behaviors in community dwelling older adults. Aging Clin Exp Res. (2021) 33:2043–51. doi: 10.1007/s40520-021-01905-z
21. Vijayan, M, and Reddy, PH. Stroke, vascular dementia, and Alzheimer's disease: molecular links. J Alzheimers Dis. (2016) 54:427–43. doi: 10.3233/JAD-160527
22. Ettinger, S. Diet, gut microbiome, and cognitive decline. Curr Nutr Rep. (2022) 11:643–52. doi: 10.1007/s13668-022-00435-y
23. Trøseid, M, Holter, JC, Holm, K, Vestad, B, Sazonova, T, Granerud, BK, et al. Gut microbiota composition during hospitalization is associated with 60-day mortality after severe COVID-19. Crit Care. (2023) 27:69. doi: 10.1186/s13054-023-04356-2
24. Thompson, R, Smith, RB, Karim, YB, Shen, C, Drummond, K, Teng, C, et al. Air pollution and human cognition: a systematic review and meta-analysis. Sci Total Environ. (2023) 859:160234. doi: 10.1016/j.scitotenv.2022.160234
25. Bhaskar, A, Chandra, J, Hashemi, H, Butler, K, Bennett, L, Cellini, J, et al. A literature review of the effects of air pollution on COVID-19 health outcomes worldwide: statistical challenges and data visualization. Annu Rev Public Health. (2023) 44:1–20. doi: 10.1146/annurev-publhealth-071521-120424
26. Tangestani Fard, M, and Stough, C. A review and hypothesized model of the mechanisms that underpin the relationship between inflammation and cognition in the elderly. Front Aging Neurosci. (2019) 11:56. doi: 10.3389/fnagi.2019.00056
27. Gao, YD, Ding, M, Dong, X, Zhang, JJ, Kursat Azkur, A, Azkur, D, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. (2021) 76:428–55. doi: 10.1111/all.14657
28. Brumer, N, Elkins, E, Parada, J, Hillyer, J, and Parbery-Clark, A. Examining delayedrecall in cochlear implant users using the Montreal cognitive assessment, California verbal learning test, third edition, and item specific deficit approach: preliminary results. Front Psychol. (2021) 12:749045. doi: 10.3389/fpsyg.2021.749045
29. Callahan, KE, Lovato, JF, Miller, ME, Easterling, D, Snitz, B, and Williamson, JD. Associations between mild cognitive impairment and hospitalization and readmission. J Am Geriatr Soc. (2015) 63:1880–5. doi: 10.1111/jgs.13593
30. Barnes, TL, Ahuja, M, MacLeod, S, Tkatch, R, Albright, L, Schaeffer, JA, et al. Loneliness, social isolation, and all-cause mortality in a large sample of older adults. J Aging Health. (2022) 34:883–92. doi: 10.1177/08982643221074857
Keywords: COVID-19, SARS-CoV-2, risk factor, memory function, severity
Citation: Shi J, Shen X, Tian Y, Lu R, Li J, Geng X, Zhai S, Jia X, Ji F, Dang S and Wang W (2025) Association of memory function with COVID-19 outcomes in adults aged 50 years and older: analysis of three prospective cohorts. Front. Public Health. 13:1577334. doi: 10.3389/fpubh.2025.1577334
Edited by:
Qiang Zhang, University of Iowa Hospitals and Clinics, United StatesReviewed by:
Kapil Goel, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaDeema Fattal, The University of Iowa, United States
Copyright © 2025 Shi, Shen, Tian, Lu, Li, Geng, Zhai, Jia, Ji, Dang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangsuo Dang, ZGFuZ3NodWFuZ3N1bzEyM0B4anR1LmVkdS5jbg==; Wenjun Wang, d2VuanVuX3dhbmdAeGp0dS5lZHUuY24=
†These authors have contributed equally to this work
 Juanjuan Shi
Juanjuan Shi Xin Shen2†
Xin Shen2† Xiaozhen Geng
Xiaozhen Geng Xiaoli Jia
Xiaoli Jia Fanpu Ji
Fanpu Ji Shuangsuo Dang
Shuangsuo Dang Wenjun Wang
Wenjun Wang