- 1School of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2School of Public Health, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Background: The term severe asthma with fungal sensitization (SAFS) has been coined due to the non-negligible role of fungal sensitization in the development of asthma. These patients typically exhibit poorer lung function, worse clinical prognosis, and a significantly elevated risk of life-threatening asthma exacerbations.
Methods: We conducted electronic searches in three databases as of October 31, 2024. Two evaluators independently screened titles and abstracts to identify studies for full-text review. For studies meeting inclusion and exclusion criteria, the investigators used the JBI Critical Appraisal Checklist and the Newcastle-Ottawa Scale (NOS) to evaluate the quality of cross-sectional studies and a case–control study, respectively, followed by data extraction from included studies.
Results: Among the 10 fungal genera examined, sensitization to Aspergillus, Penicillium, and Cladosporium spp. was significantly associated with an increased risk of severe asthma, with pooled odds ratios (ORs) and 95% confidence intervals (CIs) of 2.36 (1.29–4.31), 1.75 (1.02–3.03), and 2.63 (1.76–3.92), respectively. Within the Aspergillus spp., Aspergillus fumigatus-specific sensitization demonstrated a stronger association with severe asthma (OR = 2.98, 95% CI: 1.32–6.75). Subgroup analyses further revealed that Aspergillus (A. fumigatus) sensitization was more strongly linked to severe asthma risk in younger and male populations: ORs with 95% CIs were 2.55 (1.35–4.83) in the ≥40 years subgroup, 3.04 (1.01–9.12) in the <40 years subgroup, and 2.77 (1.16–6.62) in the female-majority subgroup.
Conclusion: In this study, we quantified the risk of sensitization to distinct fungal genera/species, aiming to provide a scientific rationale for screening high-risk fungal sensitization, early detection of severe asthma risk, and personalized health management for patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier [CRD42024620737].
1 Introduction
Asthma is a complex airway inflammatory disorder characterized by airflow limitation and airway hyperresponsiveness (1). While the majority of asthmatic patients can achieve effective disease control with low-dose medications, approximately 3–8% exhibit severe asthma. These individuals experience significant limitations in daily activities and may even encounter life-threatening clinical events (2).
Dampness and mold make significant contributions to the increase and exacerbation of asthma. One study estimate that 21% of asthma cases in the United States may be attributable to residential dampness and mold (3). Fungi not only harm respiratory health, but also increase socioeconomic burden. A study estimated that the economic burden of asthma onset due to moisture and mold in the United States alone is $15.1 billion (4).
However, the mechanisms of fungal influence on asthma severity are highly complex, involving multiple pathways such as immune modulation (5), antigen release (6), and airway remodeling (7). Fungal sensitization is particularly prevalent in patients with severe asthma (8). This process is primarily mediated by the adaptive immune system, especially through T helper 2 cells (TH2 cells). These cells drive the production of fungal antigen–specific immunoglobulin E (IgE) antibodies involved in allergic and inflammatory response (6). Meanwhile, exposure to fungal allergens and the production of chemokines trigger the recruitment of various immune cells—including eosinophils, neutrophils, and lymphocytes—to the airways. Eosinophils play a crucial role in the inflammatory response to fungal sensitization (7). They release toxic signaling molecules, such as major basic protein, eosinophil peroxidase, and eosinophil cationic protein, which contribute to tissue damage and inflammation. Eosinophils also secrete cytokines and chemokines that regulate asthma severity by interacting with other immune cells (e.g., neutrophils, macrophages, mast cells) (9). Compared with non-sensitized individuals, patients with fungal sensitization have a higher incidence of early-onset asthma and elevated serum interleukin-33 levels (10). Those with severe sensitization tend to have poorer lung function, a poorer prognosis, and a notably increased risk of developing life-threatening asthma exacerbations (11).
Given the role of fungal sensitization in asthma development, the medical community coined the term “Severe Asthma with Fungal Sensitization (SAFS)” to emphasize the important role and clinical significance of fungi in severe asthma (12).
In recent years, there has been a surge in studies on asthma with fungal sensitization. Although prior studies have demonstrated a link between fungal sensitization and severe asthma, many gaps remain in understanding the risks of sensitization to specific fungal genera and species. Epidemiological research has reported a high prevalence of fungal sensitization among asthma patients, with genera such as Aspergillus and Alternaria frequently implicated. However, extensive quantitative risk assessments for sensitization to these specific fungi are still lacking (13, 14).
Multiple fungal genera have been shown to induce IgE-mediated type I hypersensitivity in susceptible populations. These fungi belong to three main phyla: Ascomycetes, Basidiomycota, and Saprophytes (15). Within the Ascomycota phylum, several genera—including Aspergillus, Penicillium, Alternaria, and Cladosporium—are abundant in both indoor and outdoor environments (16) and pose a potential hazard to asthma progression.
Our research focuses on 10 common fungal genera, including Aspergillus, Penicillium, Alternaria, Cladosporium, Helminthosporium, Epicoccum, Fusarium, Candida, Mucor, and Trichophyton species, to investigate their contributions to asthma development. Using a rigorous epidemiological approach, we conducted a systematic study. On one hand, we aim to quantitatively evaluate fungal sensitization at the genus level and accurately compare how different fungal sensitizations impact asthma severity; on the other hand, our in-depth analysis of the intrinsic characteristics of patients with Aspergillus-triggered severe asthma aids in the investigation of the potential mechanisms and correlates, providing a scientific basis for precision prevention and treatment.
2 Methods
2.1 Search strategy
We conducted electronic searches in three databases (PUBMED, WEB OF SCIENCE, and EMBASE) on October 31, 2024, following the protocol (PROSPERO reference: CRD42024620737).
To ensure the sensitivity and specificity of the search strategy, our overall search strategy is structured as “severe asthma + fungi + sensitization.” For the concept of “severe asthma,” we employed the terminology recommended by the GINA guidelines (“severe asthma”) alongside multiple synonymous terms (e.g., refractory asthma, difficult-to-control asthma, poorly controlled asthma, irreversible asthma, life-threatening asthma, fatal asthma) to capture relevant literature comprehensively (17). The detailed search strategy is documented in the Supplementary file.
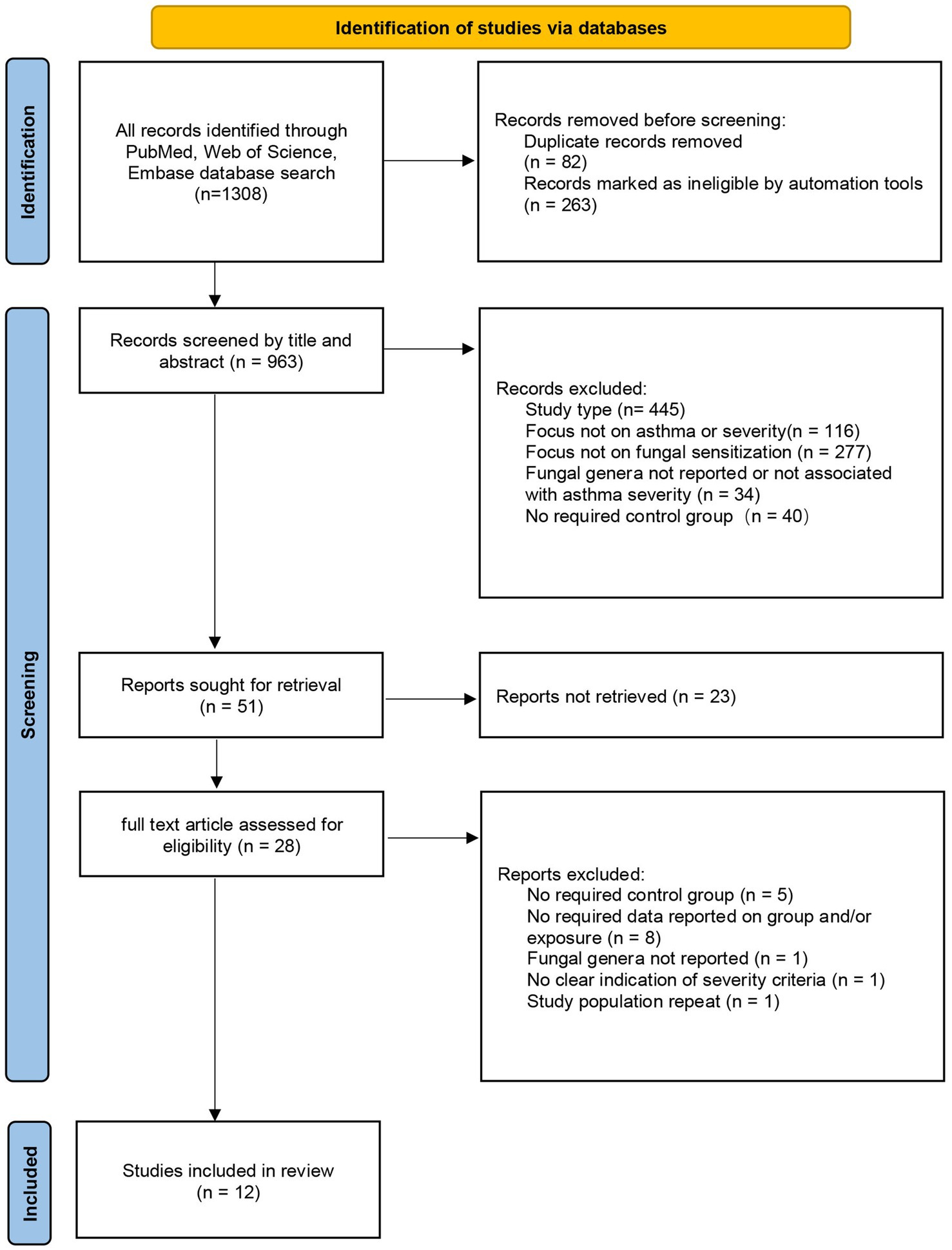
A full search strategy was applied across all three databases to identify eligible articles. We used Endnote version X9 for article management, removing duplicates and selecting studies for full-text screening based on title or abstract; the screening process was documented in a PRISMA flowchart. Two team members independently assessed and screened articles. In cases of disagreement, a third evaluator was consulted, and problems were resolved through discussion.
2.2 Eligibility criteria and study selection
The included studies all reported an association between sensitization to common fungal genera and asthma severity.
Inclusion criteria:
a. Original peer-reviewed articles reporting primary data;
b. Cross-sectional studies, case–control studies, or cohort studies;
c. Studies with populations of asthma patients stratified by severity using exact standards and reference guidelines;
d. Studies assessing individual sensitization to fungal genera/species, with sensitization diagnostic methods including skin prick tests (SPT) or measurement of specific immunoglobulin E (sIgE) levels;
e. Studies reporting and comparing sensitization to fungal genera/species across asthma severity levels (severe vs. non-severe asthma);
f. Studies presenting raw data and/or odds ratios (ORs) with confidence intervals (CIs).
Exclusion criteria:
a. Duplicated studies or overlapping study populations;
b. Studies of the following types: reviews, conference abstracts, meta-analyses, case reports, case studies, and animal experiments;
c. Studies lacking required controls (severe vs. non-severe asthma comparison).
2.3 Data extraction
The following information was extracted from the studies that meeting the criteria: (1) authors; (2) publication year; (3) publication source; (4) duration of the study; (5) geographic region; (6) study design; (7) sample size; (8) participants’ age, gender, and course of disease; (9) asthma severity classification criteria; (10) diagnostic method of fungal sensitization; (11) counts and/or odds ratios (ORs) with confidence intervals (CIs) for sensitization to fungal genera/species across asthma severity levels.
2.4 Quality assessment
The 11 cross-sectional studies and 1 case–control research were evaluated independently by two team members using the JBI Critical Appraisal Checklist and the Newcastle-Ottawa Scale (NOS) (18) (quality assessment of individual studies in the Supplementary file). The JBI scale assigns scores based on the degree of conformity to its items: 0 indicates non-compliance; 1 point signifies mention without detailed description; 2 points denote a complete and comprehensive explanation; an overall score exceeding 70% of the total indicates a low risk of bias. The NOS scale evaluates study quality across three domains—study selection, comparability, and exposure definition—with a maximum score of 9, where higher scores reflect better quality. Two team members independently scored the included literature, and final scores were determined by consensus.
2.5 Statistical analysis
All statistical analyses were performed using Review Manager 5.4 software. In this study, we used original data to calculate the unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) for fungal sensitization as an exposure factor between the severe asthma group and the non-severe asthma group (control group). In addition, we directly incorporated ORs from a few studies that reported unadjusted OR values for subsequent calculations.
After completing data collection and organization, we merged data using the random effects model and applied the inverse variance method for weighting. To compute the pooled effect estimates, all ORs and 95% CIs were log-transformed. Specifically, in RevMan’s random-effects model, the Generic Inverse Variance method by default adopts the DerSimonian-Laird estimator. This estimator calculates between-study heterogeneity variance (Tau2) and dynamically adjusts the weights of individual study effect sizes to reflect the variations among studies, thus achieving a robust estimation of the pooled effect size.
We generated forest plots to directly visualize the study outcomes. In the forest plots, red squares represented the weighted contribution of each study, and p < 0.05 was considered statistically significant. The I2 statistic was used for the outcome test to assess the degree of variation between studies, and heterogeneity was considered to be significant when I2 was greater than 50%. At last, to ensure the reliability and robustness of the findings, we conducted sensitivity analyses by altering the pooling model and assessed publication bias via funnel plot, respectively.
3 Results
3.1 Study design characteristics of included studies
After screening and evaluation, 12 literatures were finally included in this study. Among them, 11 were cross-sectional studies and 1 was a case–control study: Beeh, K. M. 2001 (19), Cazzoletti, L. 2010 (20), Chopra, V. 2017 (21), Gupta, A. 2015 (22), Hayes, D. 2013 (23), Kwizera, R. 2021 (24), Niedoszytko, M. 2007 (25), Ogawa, H. 2011 (26), Saxena, P. 2021 (27), Tanaka, A. 2016 (28), Yeğit, O. O. 2023 (29), Vincent, M. 2018 (30). The flowchart of literature screening is shown in Figure 1. We used the JBI scale and the NOS scale to evaluate the quality of cross-sectional studies and a case–control study, respectively. For the JBI scale, a study was classified as having low bias risk when its score exceeded 70% of the total. Among the 11 studies evaluated with this scale, only one had a score equaling 70%, while the remaining had higher scores. The single study assessed using the NOS scale scored 6 out of 9, indicating moderate quality (detailed in the Supplementary file).
3.2 Participant characteristics of included studies
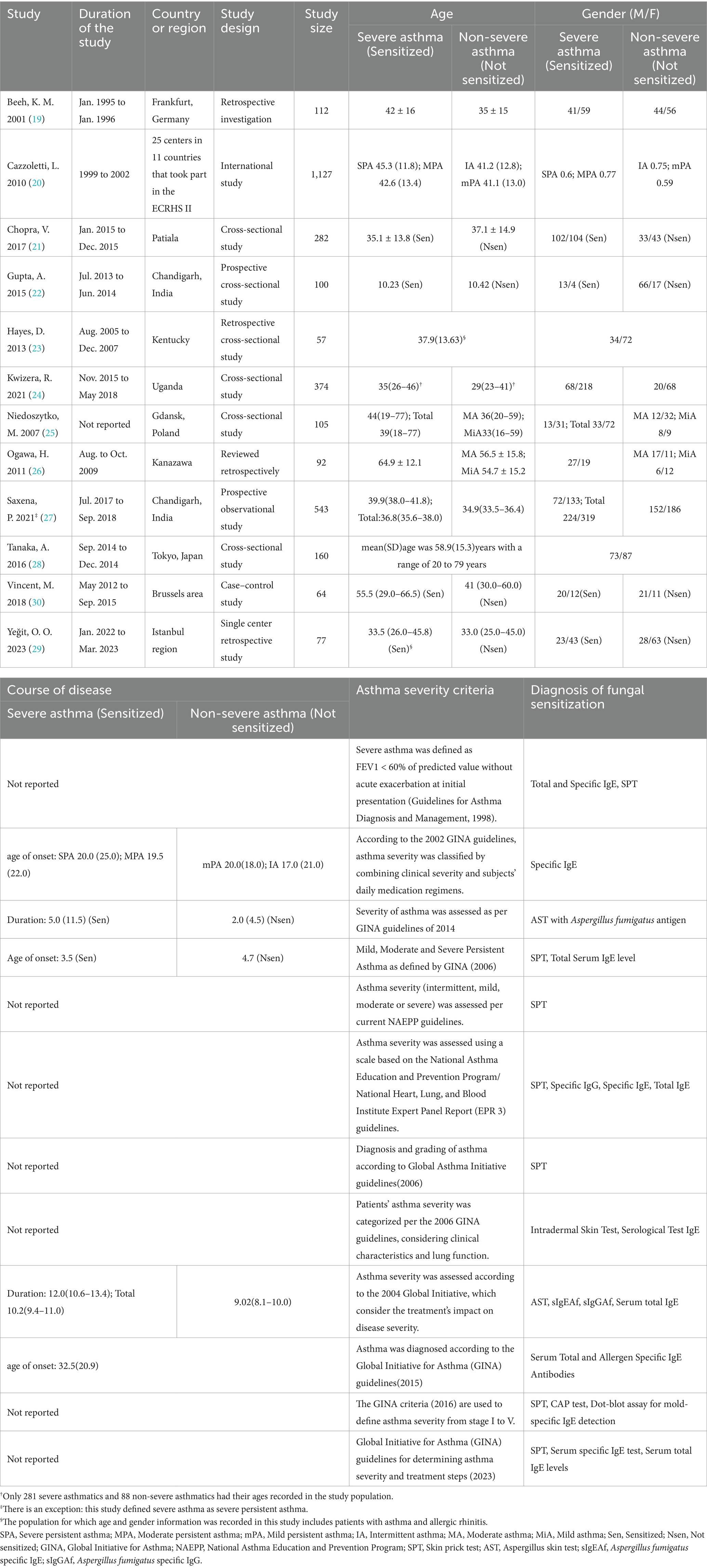
The included studies originated from multiple countries and regions, with geographically diverse settings. Four studies were conducted in Europe: one utilized data from ECRHS II, which included participants from 11 European countries; one was from Germany, one from Poland, and one from Belgium. Of the remaining eight studies, three were from India, two from Japan, and the other three from the United States, Turkey, and Uganda. Based on pooled results (Table 1), all but one study—focusing on children (mean age around10 years)—included participants with a mean/median age over 18 years. Among these, seven studies had participants with a mean/median age under 40, while four reported a mean/median age over 40. Three studies included more male than female participants, and nine had more female than male. Six studies reported age at onset or disease duration.
Regarding asthma severity classification, only three of the 12 studies cited external guidelines, while the remaining nine used the Global Initiative for Asthma (GINA) guidelines from various years. Fungal sensitization was diagnosed using skin prick tests (SPT) and measurement of fungal antigen–specific immunoglobulin E (sIgE) levels (6). The diagnostic methods of sensitization corresponding to the sensitization data of the studies we eventually documented were SPT in six studies, sIgE test in three studies, and a combination of SPT and sIgE test in three studies (Table 2).
3.3 Synthesis
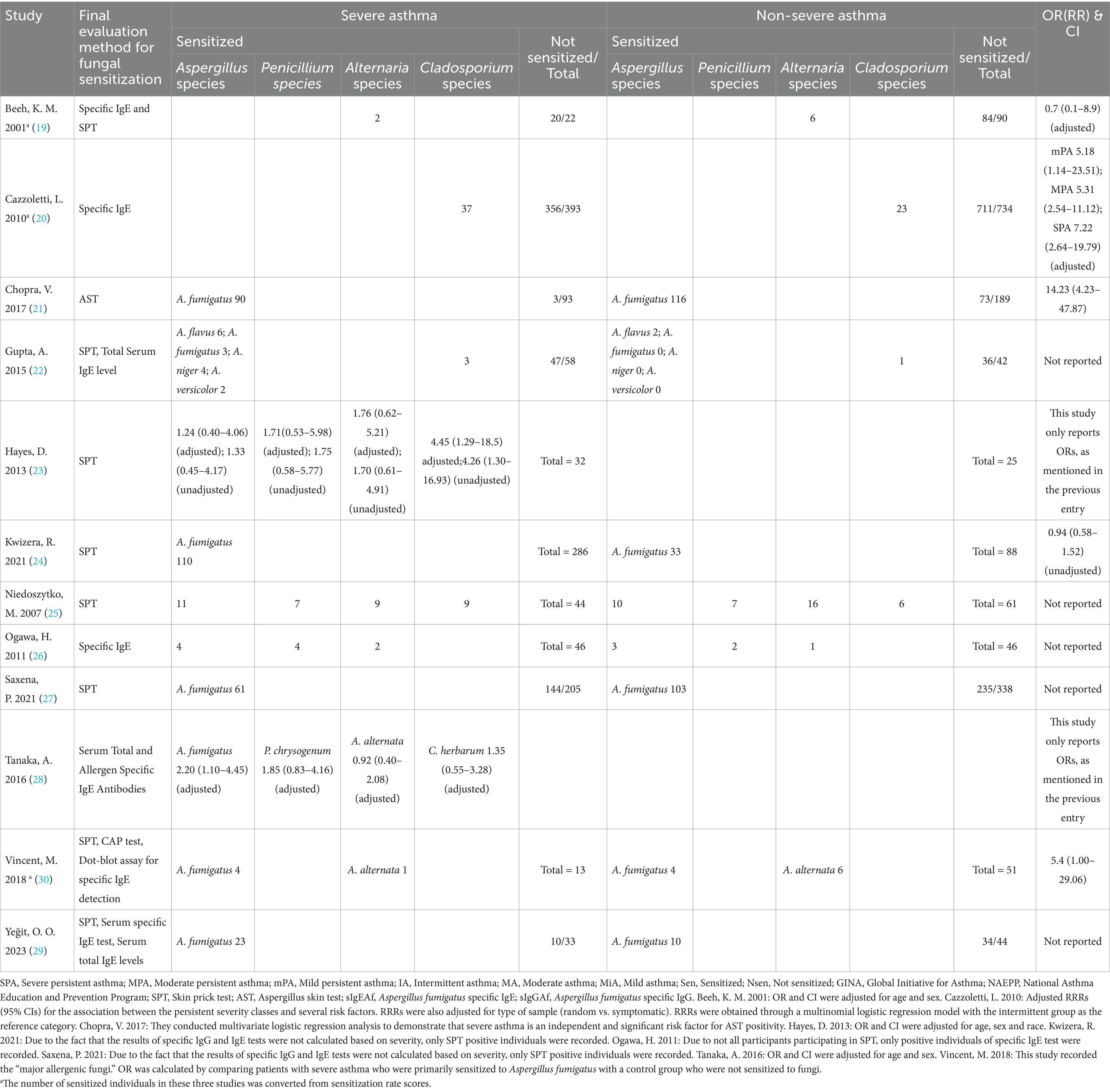
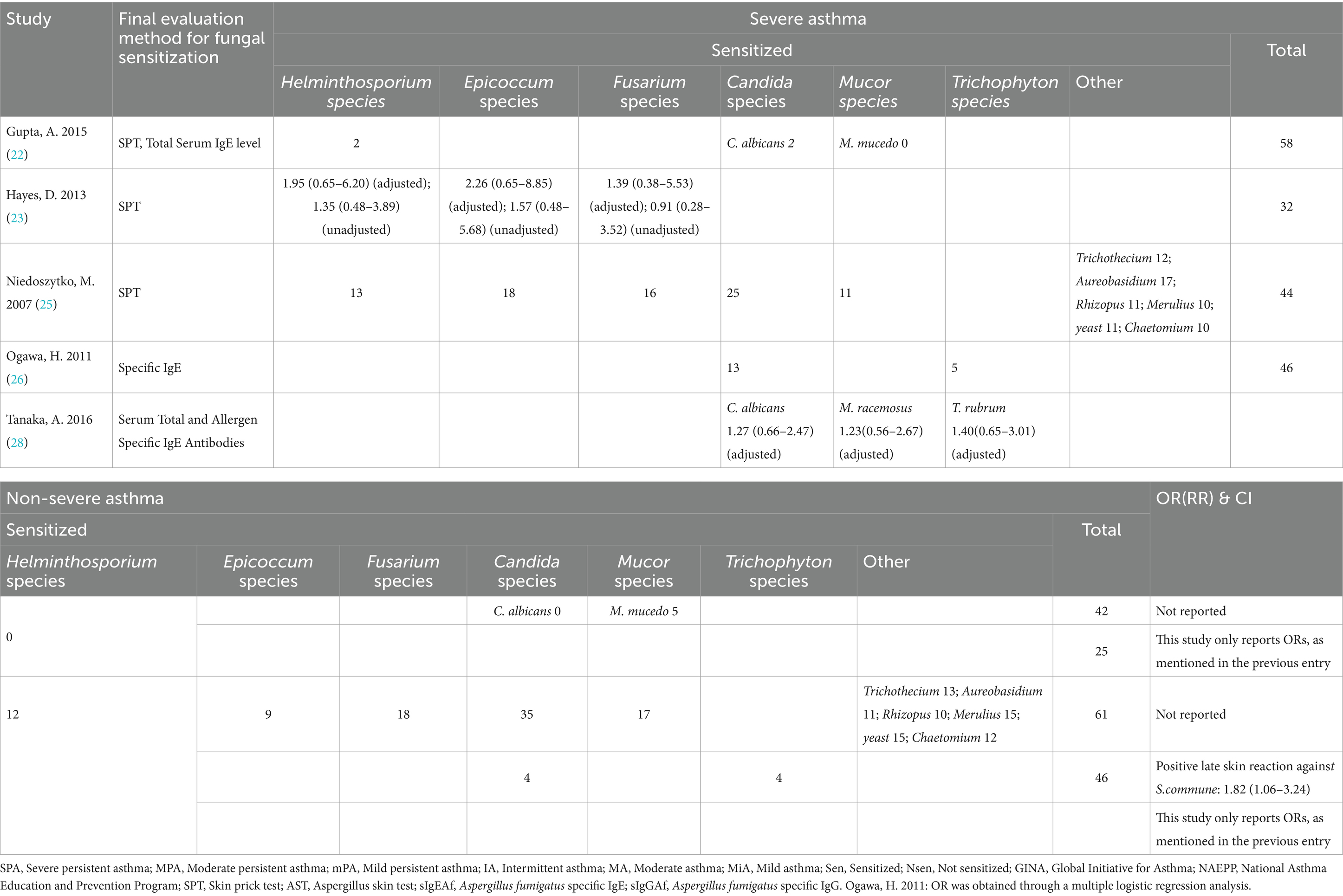
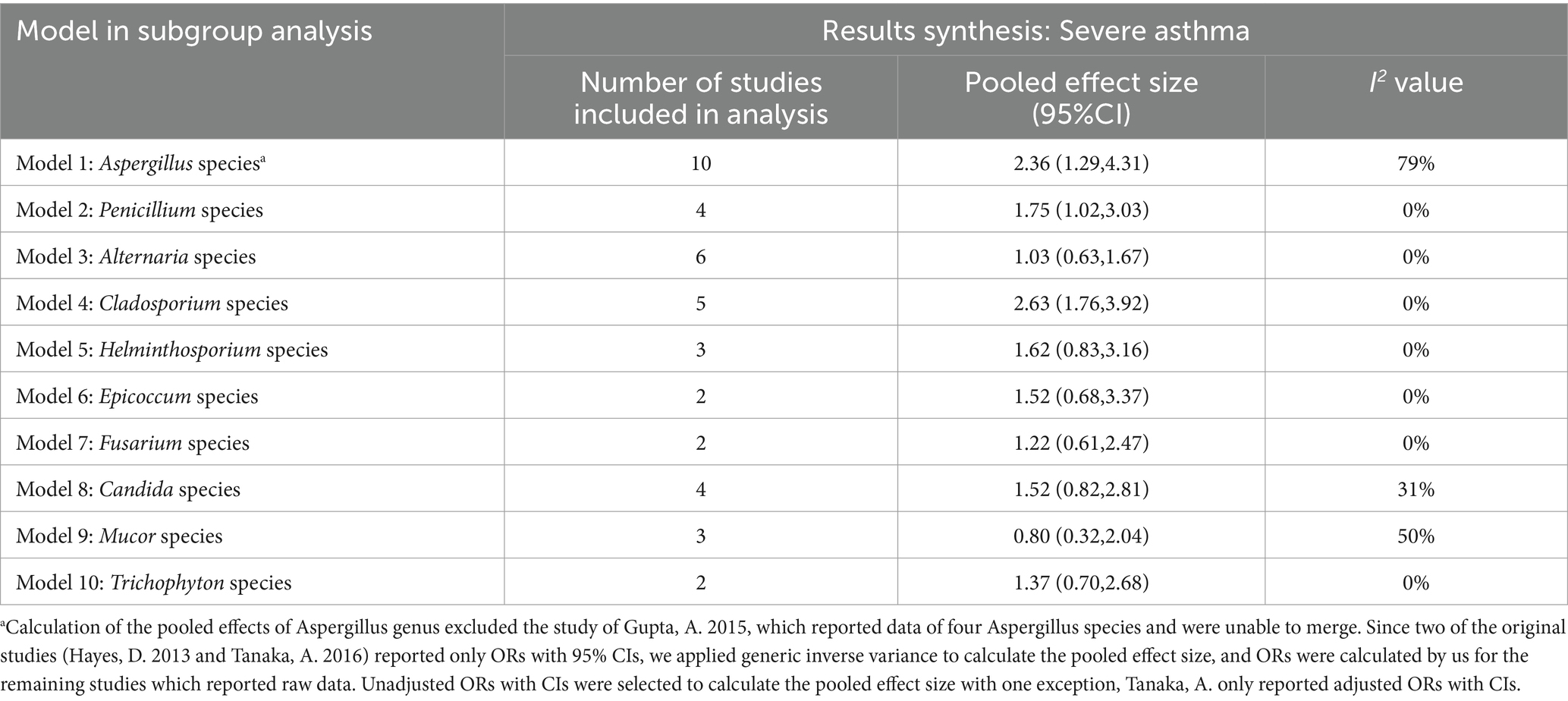
We ultimately identified 12 eligible studies meeting the inclusion and exclusion criteria for meta-analysis, examining the association between fungal sensitization—stratified by genus—and severe asthma. Fungi are widely distributed in both indoor and outdoor environments, and they are correlated, albeit species and concentrations differ (31). We focused on the risk of severe asthma due to sensitization to four fungal genera (Aspergillus, Penicillium, Cladosporium, and Alternaria species), which are common in both indoor and outdoor environments, and also assessed the risk of six other fungal genera (Helminthosporium, Epicoccum, Fusarium, Candida, Mucor, and Trichophyton species; Tables 2, 3).
The link between Aspergillus spp. and severe asthma has been of great interest (8). We not only quantitatively assessed the strength of this epidemiological association, but also explored the intrinsic characteristics of patients with severe asthma due to Aspergillus sensitization through subgroup analyses, with the goal of providing more comprehensive and in-depth insights into this field of study.
Historically, asthma severity has been classified into two major systems. The current standard classification defines severity based on treatment intensity required for control: mild (MiA), moderate (MA), and severe asthma (SA) (17). By contrast, earlier guidelines categorized severity into four tiers—intermittent (IA), mild persistent (mPA), moderate persistent (MPA), severe persistent (SPA)—based on symptom severity, airflow limitation, and lung function variability (32).
For the latter classification criteria, we defined severe asthma in principle as moderate persistent and severe persistent asthma (Table 1). Given the variability in reported data across studies, we derived unadjusted odds ratios (ORs) from raw data or extracted them directly from the literature for subsequent pooled effect estimates.
3.4 Results of four common indoor and outdoor fungal genera
Based on the pooled effects of the four most prevalent indoor and outdoor fungal genera, the results of the three subgroups of Aspergillus, Penicillium, and Cladosporium spp. were statistically significant (Table 4; Figure 2). Sensitization to Aspergillus spp. was associated with a 2.36-fold increased risk of severe asthma (95% CI: 1.29–4.31); sensitization to Penicillium spp. had an OR of 1.75 (1.02–3.03); and sensitization to Cladosporium spp. showed an OR of 2.63 (1.76–3.92). Heterogeneity assessed via I2 indicated high variability among Aspergillus-related studies (I2 = 79%), whereas the remaining genera—including Alternaria spp., which showed no significant association—had I2 values of 0%, indicating low heterogeneity.
3.5 Results of six other fungal genera
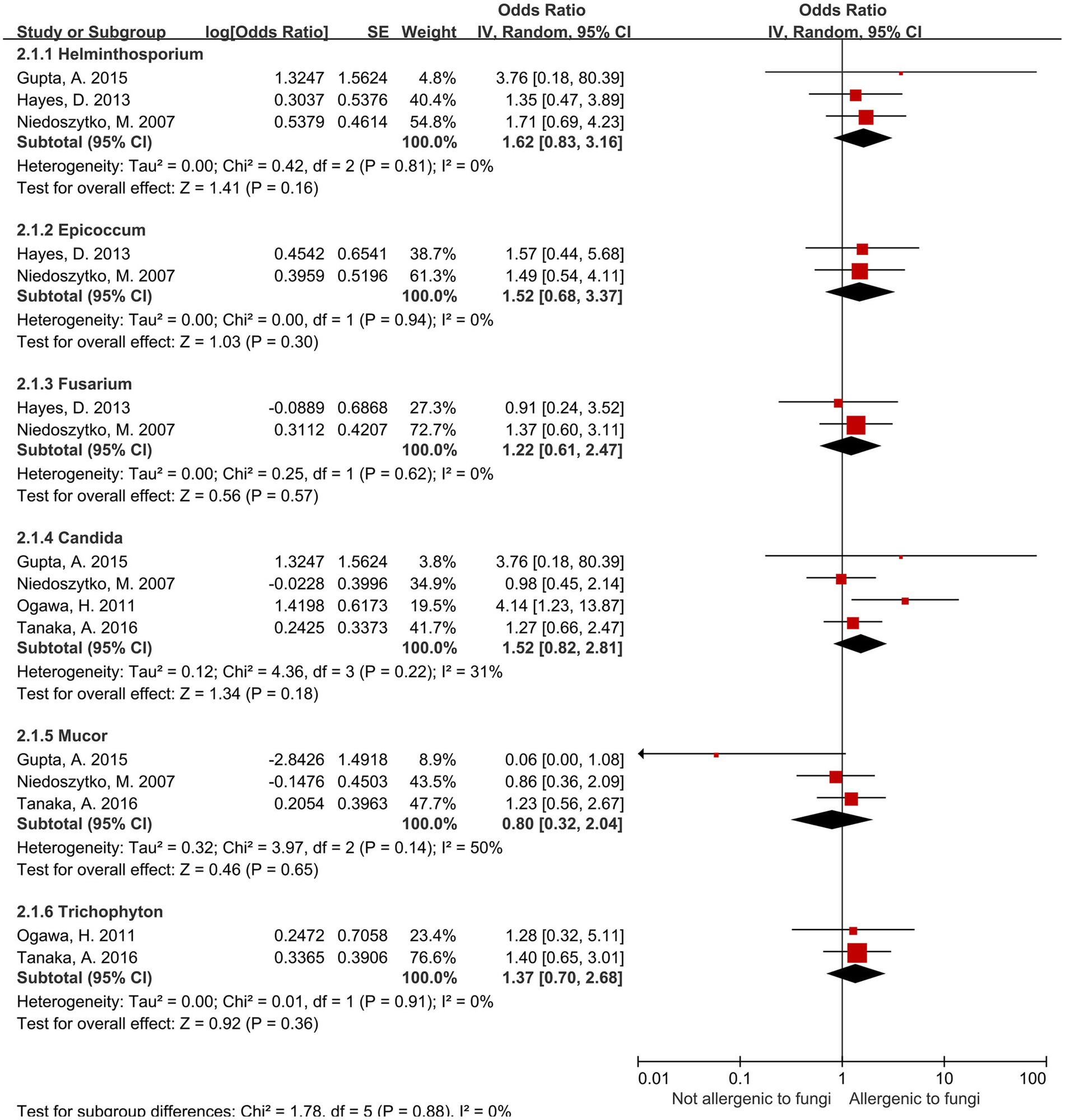
For the present being, no significant association was observed between sensitization to the remaining six fungal genera—including Candida spp.—and increased risk of severe asthma (Figure 3). Heterogeneity assessed via I2 showed four genera had I2 values of 0%, while the other two—Candida spp. (I2 = 31%) and Mucor spp. (I2 = 50%)—exhibited moderate heterogeneity.
3.6 Stratification analysis of the aspergillus group
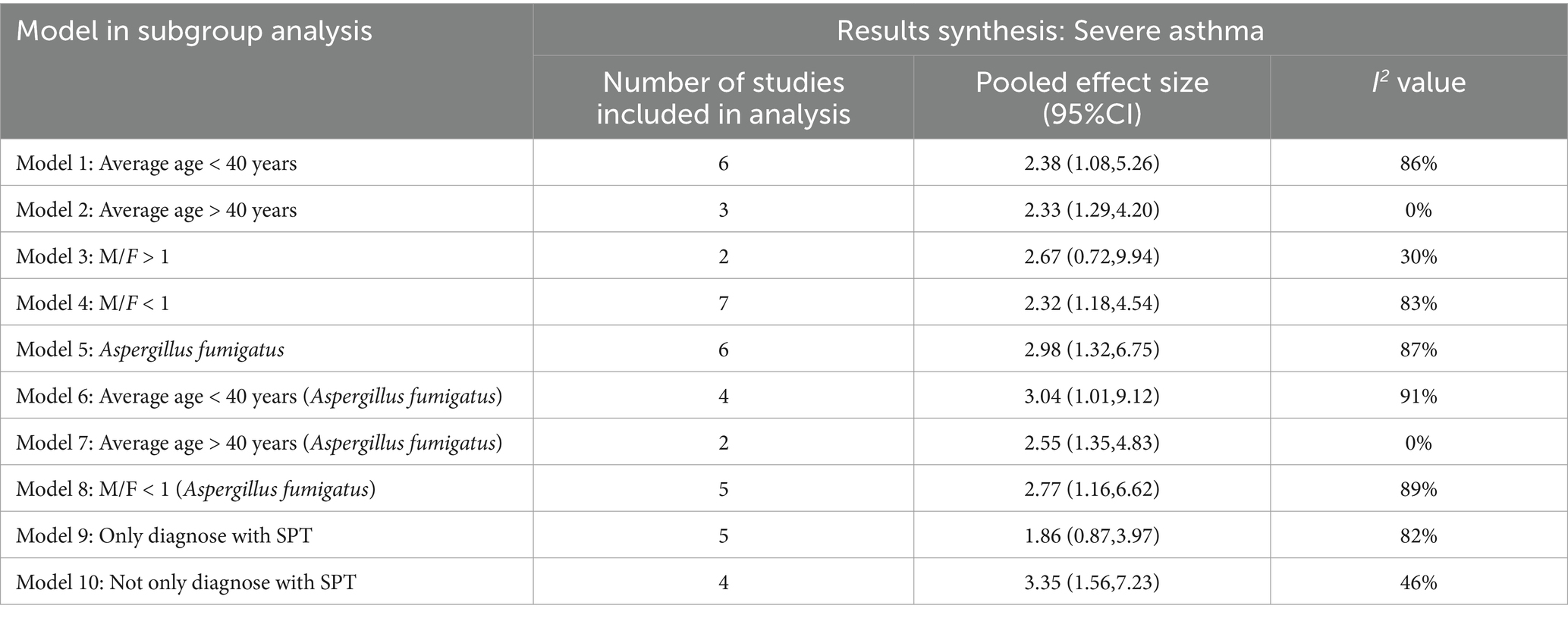
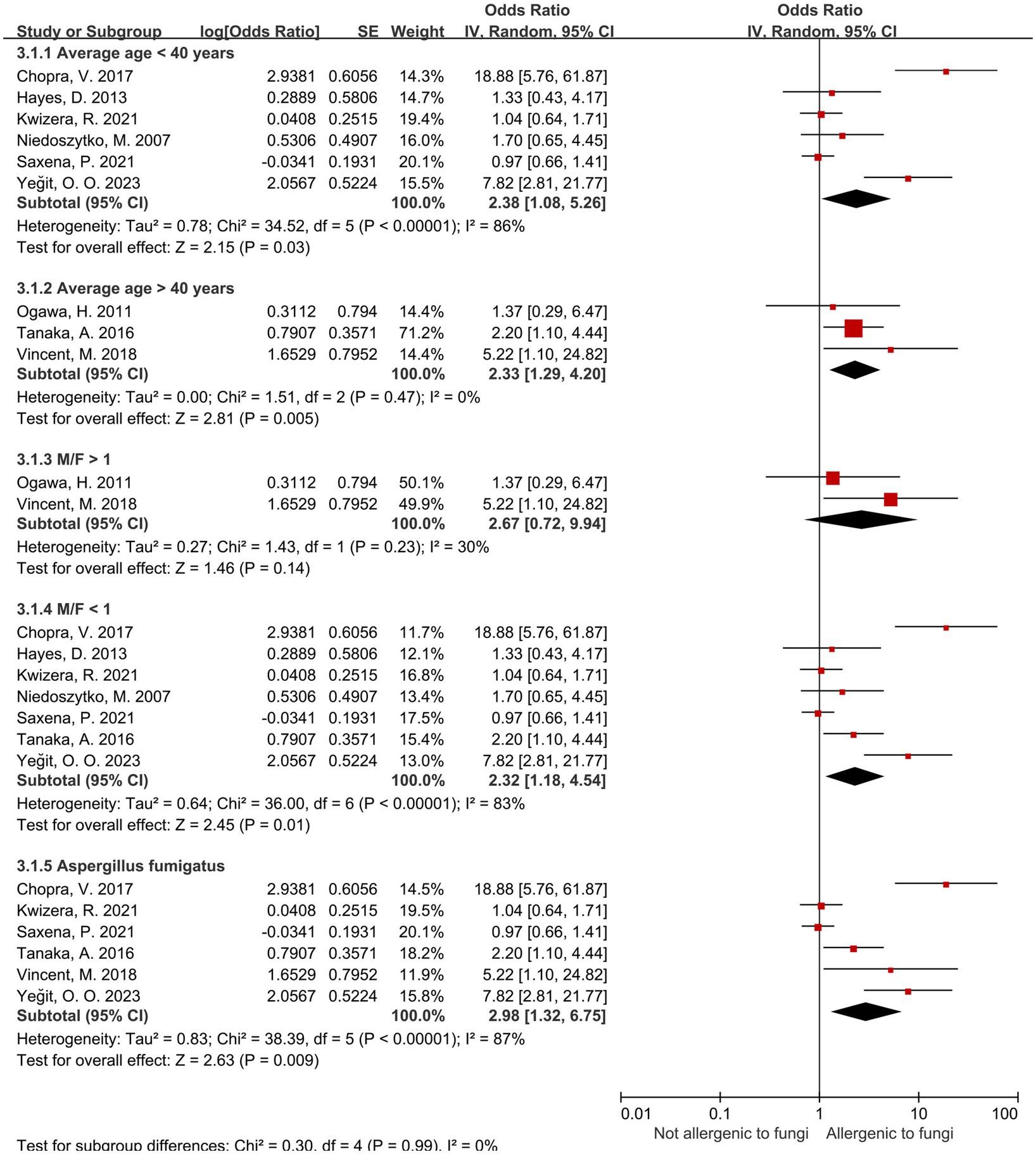
Regarding the Aspergillus spp. group, we conduct subgroup analyses (Table 5) from the perspectives of age, male-to-female ratio, Aspergillus fumigatus, age and sex under Aspergillus fumigatus, and diagnostic modality of sensitization (Figures 4, 5). In the age-stratified analysis, the OR for the ≥ 40 years group decreased slightly compared to the overall OR, while the <40 years group showed a marginal increase; however, neither change reached statistical significance. By gender, the OR was slightly reduced in female-dominated subgroups, whereas male-dominated subgroups showed no significant association, likely owing to limited sample size. Notably, Aspergillus fumigatus-specific sensitization demonstrated a stronger association with severe asthma (OR = 2.98, 95% CI: 1.32–6.75) compared to the broader Aspergillus spp. analysis. Following that, we examined the Aspergillus fumigatus sensitization group in terms of age and gender individually. The findings for the age-sex subgroup of Aspergillus fumigatus were the same as before (age-sex subgroup of Aspergillus spp.), but with more significant changes: the ORs with 95% CIs were 2.55 (1.35–4.83) for ≥40 years, 3.04 (1.01–9.12) for <40 years, and 2.77 (1.16–6.62) for female-dominated groups.
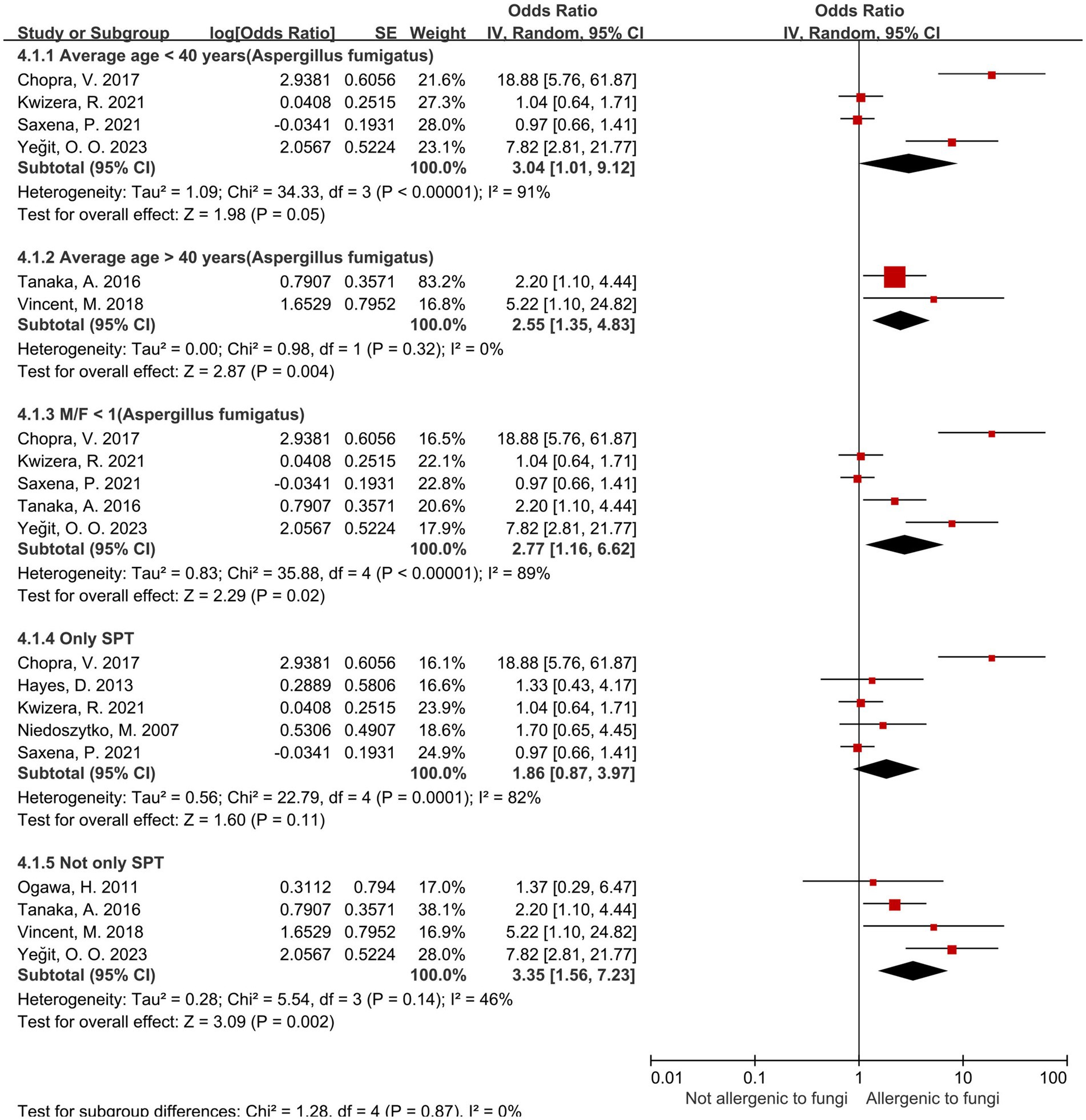
Figure 5. Subgroup analysis of Aspergillus spp. in terms of sensitization diagnosis and sex, age under Aspergillus fumigatus.
3.7 Risk of bias of individual studies
We evaluated included cross-sectional and case–control studies with the JBI and NOS scales, respectively (detailed in the Supplementary file), finding generally low bias and comparable quality across studies. Since we ultimately calculated the unadjusted odds ratio (OR) without controlling for confounding factors, there is potential for bias in the results. Additionally, the study by Tanaka (28) only presented the adjusted OR, and we included it in our calculation process. This approach may also lead to the occurrence of bias. Funnel plots were used to assess publication bias for five fungal genera (Aspergillus, Penicillium, Cladosporium, Alternaria, and Candida spp.), as fewer than four studies existed for the remaining genera, precluding funnel plot analysis. The funnel plot revealed some publication bias in studies of Aspergillus, Alternaria, and Candida spp. (in the Supplementary file). Moreover, sensitivity analyses, conducted by changing the data pooling model, showed consistent conclusions in nature between fixed- and random-effects models, suggesting the robustness of our findings.
4 Discussion
During the evaluation of the studies on fungal sensitization associated with severe asthma, we carried out a structured systematic evaluation in strict accordance with a pre-published protocol. Our meta-analysis of the association between sensitization to specific fungal genera and severe asthma revealed that, of the 10 fungal genera involved in the study, sensitization to Aspergillus, Penicillium, and Cladosporium spp. significantly increased the risk of severe asthma, among which sensitization to Aspergillus and Cladosporium spp. conferred relatively higher risks. When comparing the ORs, sensitization to Cladosporium spp. was no less essential for the risk of severe asthma than the more widely studied sensitization to Aspergillus spp.; within the Aspergillus spp., Aspergillus fumigatus showed a particularly strong association with increased risk. Subgroup analyses further highlighted population-specific patterns, such as Aspergillus (A. fumigatus) sensitization being more strongly linked to severe asthma risk in younger and male populations. In addition to the primary conclusions listed above, the included studies yielded some intriguing observations. Cazzoletti (20) reported a dose–response relationship: increasing asthma severity correlated with higher Aspergillus sensitization, reinforcing the likelihood of a causal link (Table 2). This finding implied that effective control of fungal sensitization is important in asthma management. The study by Ogawa (26) identified another fungus that may enhance the risk of severe asthma after sensitization (S. commune: 1.82 [1.06–3.24]; Table 3).
Sensitization to fungi is significantly associated with severe asthma, which frequently provokes undesirable clinical consequences such as increased ICU admissions and mechanical ventilation requirements (11). However, the effects of different fungal allergens on asthma vary, highlighting notable species-specific impacts (33). In this study, we quantified the risk magnitude of sensitization to distinct fungal genera/species, aiming to provide a theoretical foundation for both screening high-risk fungal sensitization and early identification of severe asthma risk. Secondly, for patients with poorly controlled or severe asthma, this study points in the direction of optimizing asthma management and developing targeted interventions, with the expectation of informing clinical practice and enhancing patient outcomes and quality of life (34). Current therapeutic approaches for severe asthma with confirmed fungal sensitization have advanced into clinical research and application phases. Antifungal treatments, such as Itraconazole (35) and Omalizumab (36), have demonstrated efficacy in improving disease control for these patients. Biological therapies targeting eosinophilic inflammation, including Mepolizumab or Benralizumab (37), have also shown clinical promise. In parallel with the above implications for clinical practice, the study proposes tailored disease management strategies and patient-centered recommendations to enhance quality of life through personalized self-health management. Aspergillus, Penicillium, and Cladosporium spp. are common fungi in both indoor and outdoor environments (16), with their concentrations influenced by environmental (38), seasonal, and humidity conditions. Fungi proliferate more rapidly in autumn (39) and winter (40), as well as in humid (41, 42) conditions, leading to increased production of spores, mycelium, and fragments (43), also increase. The fungi listed above are not only ubiquitously present in air but also deeply intertwined with human activities. Aspergillus oryzae and Aspergillus niger are widely used in food fermentation and the industrial production of organic acids (citric acid) (44); Cladosporium can produce a variety of beneficial enzymes, which play an important role in the field of microbial chemistry and plant science (45); Penicillium chrysogenum serves as a key penicillin-producing strain and synthesizes bioactive secondary metabolites, which show excellent biosynthesis potential (46). For workers in the above industries, exposure to bioaerosols, including fungal spores, represents a significant occupational hazard that cannot be ignored. Therefore, in order to prevent the asthma deterioration, asthmatics may need to minimize exposure to humid environments and avoid activities with high-risk fungal contact.
This study had several limitations. On the one hand, the number of included studies was relatively small and the evidence grade low (11 cross-sectional studies and 1 case–control study), which somewhat obscured potential associations between sensitization to other fungal genera and severe asthma and limited demographic-based stratification. Moreover, after the calculation and combination of the data, what we eventually obtained is the unadjusted odds ratio (OR). This methodology presents a risk of result bias, which is consistent with that observed in other studies of a similar nature. On the other hand, the analysis of I2 revealed high heterogeneity among the nine Aspergillus spp.-related studies. In order to explore the source of heterogeneity and to investigate the intrinsic characteristics of patients with Aspergillus-sensitized severe asthma, we conducted subgroup analyses across dimensions such as age and gender. Sensitization diagnostic methods provided clues to heterogeneity (Table 5). Studies using only skin prick tests (SPT) showed high heterogeneity and non-significant pooled effects, whereas subgroups using sIgE tests (alone or combined with SPT) had significantly lower heterogeneity. SPT and the sIgE test are both fundamental diagnostic methods for sensitization, each with distinct advantages and limitations that make them non-interchangeable (47). SPT is often influenced by the quality of the reagent and the experience of the operator, which may explain the large heterogeneity among studies that used SPT alone to diagnose Aspergillus sensitization. Although subgroup analyses revealed some intrinsic characteristics of disease groups, such as sensitization to Aspergillus spp. (Aspergillus fumigatus) being more likely to pose a risk of severe asthma in younger age groups and in the male population, unresolved heterogeneity hinders definitive conclusions. Besides, it is also worth pointing out that potential covariates were not considered in this study. For example, other allergens such as dust mites also contribute to asthma exacerbation, for which the effects of these factors on the strength and direction of the association between sensitization to distinct fungal genera/species and severe asthma remain unclear. Looking ahead, more comprehensive diagnostic approaches for sensitization (e.g., SPT combined with sIgE tests), large-sample multicenter studies, and more robust evidence of causality may help us to uncover more quantitative associations between sensitization to identified genera/species of fungi and severe asthma, as well as their specific impact on diverse populations.
Fungal sensitization is a key mechanism underlying the increased risk of severe asthma following fungal exposure. However, the effects of fungal exposure on respiratory health are complex and multifaceted, with distinct fungal communities at varying concentrations exerting differential impacts (48). It has even been proposed that exposure to species-rich fungal environments may inversely reduce asthma risk (49). As molecular biology advances, our understanding of fungal sensitization mechanisms in asthma deepens, yet multiple perspectives are still needed to precisely clarify how fungi contribute to asthma development and severity exacerbation. This requires deeper insights into the role of fungal metabolites and fragments in these processes, as well as the detailed immunoinflammatory response mechanisms (50, 51). It is of great significance to investigate these mechanisms. On the one hand, it can fulfill the gaps in our knowledge about fungi’s biological features; on the other hand, it can pave the path for the development of targeted therapies and bring new therapeutic hope to asthma patients. Given fungi’s pivotal role in asthma, it will undoubtedly become a key research direction in the future to elucidate the roles and underlying mechanisms of biologics and antifungal therapies in the management of fungal sensitization in asthma through animal experiments as well as randomized clinical trials (6). Furthermore, emerging evidence suggests genetic susceptibility may influence individual vulnerability to fungal-associated asthma (52, 53). Nevertheless, this area remains poorly understood, and more in-depth studies are urgently needed to fully characterize the gene–environment interactions driving fungal-associated asthma. We anticipate that more extensive studies will be conducted in the future to further explore and examine the underlying mechanisms, clarify the role of relevant genetic factors, develop targeted therapies, and implement effective controls to improve patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft. YH: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. HB: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. Our author’s native language is not English, so while independently writing the English paper, we use two software (web version of DeepL & Quillbot) to assist in translating, polishing, and grammar checking, and carefully check the content.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1582643/full#supplementary-material
Abbreviations
SPA, Severe persistent asthma; MPA, Moderate persistent asthma; mPA, Mild persistent asthma; IA, Intermittent asthma; MA, Moderate asthma; MiA, Mild asthma; Sen, Sensitized; Nsen, Not sensitized.
References
1. Lemanske, RFJr, and Busse, WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. (2010) 125:S95–S102. doi: 10.1016/j.jaci.2009.10.047
2. Ten Have, L, Meulmeester, FL, de Jong, K, and Ten Brinke, A. Patient-Centred outcomes in severe asthma: fatigue, sleep, physical activity and work. Eur Respir Rev. (2025) 34:p.1. doi: 10.1183/16000617.0122-2024
3. Who Guidelines Approved by the Guidelines Review Committee. Who guidelines for indoor air quality: Dampness and Mould. Geneva: World Health Organization (2009). 77 p.
4. Caillaud, D, Leynaert, B, Keirsbulck, M, and Nadif, R. Indoor Mould exposure, asthma and rhinitis: findings from systematic reviews and recent longitudinal studies. Eur Respir Rev. (2018) 27:148. doi: 10.1183/16000617.0137-2017
5. Zhang, Z, Biagini Myers, JM, Brandt, EB, Ryan, PH, Lindsey, M, Mintz-Cole, RA, et al. Β-glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant T(H)2/T(H)17 responses. J Allergy Clin Immunol. (2017) 139:54–65.e8. doi: 10.1016/j.jaci.2016.02.031
6. Kao, CC, Hanania, NA, and Parulekar, AD. The impact of fungal allergic sensitization on asthma. Curr Opin Pulm Med. (2021) 27:3–8. doi: 10.1097/mcp.0000000000000740
7. Ghosh, S, Hoselton, SA, Dorsam, GP, and Schuh, JM. Eosinophils in fungus-associated allergic pulmonary disease. Front Pharmacol. (2013) 4:8. doi: 10.3389/fphar.2013.00008
8. Agarwal, R, Muthu, V, and Sehgal, IS. Relationship between aspergillus and asthma. Allergol Int. (2023) 72:507–20. doi: 10.1016/j.alit.2023.08.004
9. Pritam, P, Manna, S, Sahu, A, Swain, SS, Ramchandani, S, Bissoyi, S, et al. Eosinophil: a central player in modulating pathological complexity in asthma. Allergol Immunopathol (Madr). (2021) 49:191–207. doi: 10.15586/aei.v49i2.50
10. Masaki, K, Fukunaga, K, Matsusaka, M, Kabata, H, Tanosaki, T, Mochimaru, T, et al. Characteristics of severe asthma with fungal sensitization. Ann Allergy Asthma Immunol. (2017) 119:253–7. doi: 10.1016/j.anai.2017.07.008
11. Medrek, SK, Kao, CC, Yang, DH, Hanania, NA, and Parulekar, AD. Fungal sensitization is associated with increased risk of life-threatening asthma. J Allergy Clin Immunol Pract. (2017) 5:1025–31.e2. doi: 10.1016/j.jaip.2016.11.015
12. Denning, DW, O'Driscoll, BR, Hogaboam, CM, Bowyer, P, and Niven, RM. The link between Fungi and severe asthma: a summary of the evidence. Eur Respir J. (2006) 27:615–26. doi: 10.1183/09031936.06.00074705
13. Bush, A. Kids, difficult asthma and fungus. J Fungi (Basel). (2020) 6:2. doi: 10.3390/jof6020055
14. Oliveira, M, Oliveira, D, Lisboa, C, Boechat, JL, and Delgado, L. Clinical manifestations of human exposure to Fungi. J Fungi (Basel). (2023) 9:p.2. doi: 10.3390/jof9030381
15. Simon-Nobbe, B, Denk, U, Pöll, V, Rid, R, and Breitenbach, M. The Spectrum of fungal allergy. Int Arch Allergy Immunol. (2008) 145:58–86. doi: 10.1159/000107578
16. Oliveira, M, Ribeiro, H, Delgado, JL, and Abreu, I. Aeromycological profile of indoor and outdoor environments. J Environ Monit. (2009) 11:1360–7. doi: 10.1039/b820736d
17. Global Initiative for Asthma. Global strategy for asthma management and prevention. (2024). Available online at: https://ginasthma.org/2024-report/ [Accessed 04/14/2025]
18. GA Wells, BS, O'Connell, D, Peterson, J, Welch, V, Losos, M, and Tugwell, P. The Newcastle-Ottawa scale (Nos) for assessing the quality of nonrandomised studies in Meta-analyses. (2021). Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed February 2, 2025).
19. Beeh, KM, Micke, P, Ksoll, M, and Buhl, R. Cigarette smoking, but not sensitization to Alternaria, is associated with severe asthma in urban patients. J Asthma. (2001) 38:41–9. doi: 10.1081/jas-100000020
20. Cazzoletti, L, Marcon, A, Corsico, A, Janson, C, Jarvis, D, Pin, I, et al. Asthma severity according to global initiative for asthma and its determinants: an international study. Int Arch Allergy Immunol. (2010) 151:70–9. doi: 10.1159/000232572
21. Chopra, V, Jain, H, Goel, AD, Chopra, S, Chahal, AS, Garg, N, et al. Correlation of aspergillus skin hypersensitivity with the duration and severity of asthma. Monaldi Arch Chest Dis. (2017) 87:826. doi: 10.4081/monaldi.2017.826
22. Gupta, A, Singh, M, Chakrabarti, A, Mathew, JL, and Rawat, A. Correlation between fungal sensitisation in childhood persistent asthma and disease severity. Mycoses. (2018) 61:195–200. doi: 10.1111/myc.12726
23. Hayes, DJr, Jhaveri, MA, Mannino, DM, Strawbridge, H, and Temprano, J. The effect of Mold sensitization and humidity upon allergic asthma. Clin Respir J. (2013) 7:135–44. doi: 10.1111/j.1752-699X.2012.00294.x
24. Kwizera, R, Bongomin, F, Olum, R, Meya, DB, Worodria, W, Bwanga, F, et al. Fungal asthma among Ugandan adult asthmatics. Med Mycol. (2021) 59:923–33. doi: 10.1093/mmy/myab023
25. Niedoszytko, M, Chełmińska, M, Jassem, E, and Czestochowska, E. Association between sensitization to Aureobasidium pullulans (Pullularia Sp) and severity of asthma. Ann Allergy Asthma Immunol. (2007) 98:153–6. doi: 10.1016/s1081-1206(10)60688-6
26. Ogawa, H, Fujimura, M, Takeuchi, Y, and Makimura, K. The influence of Schizophyllum commune on asthma severity. Lung. (2011) 189:485–92. doi: 10.1007/s00408-011-9320-5
27. Saxena, P, Choudhary, H, Muthu, V, Sehgal, IS, Dhooria, S, Prasad, KT, et al. Which are the optimal criteria for the diagnosis of allergic bronchopulmonary aspergillosis? A latent class analysis. J Allergy Clin Immunol Pract. (2021) 9:328–35.e1. doi: 10.1016/j.jaip.2020.08.043
28. Tanaka, A, Fujiwara, A, Uchida, Y, Yamaguchi, M, Ohta, S, Homma, T, et al. Evaluation of the association between sensitization to common inhalant Fungi and poor asthma control. Ann Allergy Asthma Immunol. (2016) 117:163–8.e1. doi: 10.1016/j.anai.2016.06.001
29. Yeğit, OO, and Özdedeoğlu, Ö. Impact of Aspergillus Fumigatus allergen sensitivity on allergic rhinitis and asthma: a single center retrospective study. Alergologia Polska - Polish J Allergol. (2023) 10:167–72. doi: 10.5114/pja.2023.131705
30. Vincent, M, Corazza, F, Chasseur, C, Bladt, S, Romano, M, Huygen, K, et al. Relationship between Mold exposure, specific Ige sensitization, and clinical asthma: a case-control study. Ann Allergy Asthma Immunol. (2018) 121:333–9. doi: 10.1016/j.anai.2018.06.016
31. Martin-Sanchez, PM, Estensmo, EF, Morgado, LN, Maurice, S, Engh, IB, Skrede, I, et al. Analysing indoor Mycobiomes through a large-scale citizen science study in Norway. Mol Ecol. (2021) 30:2689–705. doi: 10.1111/mec.15916
32. Bateman, E, Hurd, S, Barnes, P, Bousquet, J, Drazen, J, Fitzgerald, JM, et al. Global strategy for asthma management and prevention: Gina executive summary. European Respiratory J: Official J European Society for Clin Respiratory Physiol. (2008) 31:143–78. doi: 10.1183/09031936.00138707
33. Fukutomi, Y, and Taniguchi, M. Sensitization to fungal allergens: resolved and unresolved issues. Allergol Int. (2015) 64:321–31. doi: 10.1016/j.alit.2015.05.007
34. Mistry, H, Ajsivinac Soberanis, HM, Kyyaly, MA, Azim, A, Barber, C, Knight, D, et al. The clinical implications of Aspergillus Fumigatus sensitization in difficult-to-treat asthma patients. J Allergy Clin Immunol Pract. (2021) 9:4254–67.e10. doi: 10.1016/j.jaip.2021.08.038
35. Agarwal, R, and Gupta, D. Severe asthma and Fungi: current evidence. Med Mycol. (2011) 49:S150–7. doi: 10.3109/13693786.2010.504752
36. Di Stefano, F, Cinti, B, and Antonicelli, L. Efficacy of Omalizumab in severe asthma with fungal sensitisation: a case report. Eur Ann Allergy Clin Immunol. (2014) 46:56–9. doi: 10.1136/bcr-2012-008462
37. Dhariwal, J, Hearn, AP, Kavanagh, JE, d'Ancona, G, Green, L, Fernandes, M, et al. Real-world effectiveness of anti-Il-5/5r therapy in severe atopic eosinophilic asthma with fungal sensitization. J Allergy Clin Immunol Pract. (2021) 9:2315–20.e1. doi: 10.1016/j.jaip.2021.02.048
38. Weikl, F, Tischer, C, Probst, AJ, Heinrich, J, Markevych, I, Jochner, S, et al. Fungal and bacterial communities in indoor dust follow different environmental determinants. PLoS One. (2016) 11:e0154131. doi: 10.1371/journal.pone.0154131
39. Lee, T, Grinshpun, SA, Martuzevicius, D, Adhikari, A, Crawford, CM, and Reponen, T. Culturability and concentration of indoor and outdoor airborne Fungi in six single-family homes. Atmos Environ. (2006) 40:2902–10. doi: 10.1016/j.atmosenv.2006.01.011
40. Gonçalves, FL, Bauer, H, Cardoso, MR, Pukinskas, S, Matos, D, Melhem, M, et al. Indoor and outdoor atmospheric fungal spores in the São Paulo metropolitan area (Brazil): species and numeric concentrations. Int J Biometeorol. (2010) 54:347–55. doi: 10.1007/s00484-009-0284-6
41. Hegarty, B, Dannemiller, KC, and Peccia, J. Gene expression of indoor fungal communities under damp building conditions: implications for human health. Indoor Air. (2018) 28:548–58. doi: 10.1111/ina.12459
42. van Laarhoven, KA, Huinink, HP, Segers, FJ, Dijksterhuis, J, and Adan, OC. Separate effects of moisture content and water activity on the hyphal extension of Penicillium Rubens on porous media. Environ Microbiol. (2015) 17:5089–99. doi: 10.1111/1462-2920.13012
43. Reponen, T, Seo, SC, Grimsley, F, Lee, T, Crawford, C, and Grinshpun, SA. Fungal fragments in moldy houses: a field study in homes in New Orleans and southern Ohio. Atmos Environ. (2007) 41:8140–9. doi: 10.1016/j.atmosenv.2007.06.027
44. Park, HS, Jun, SC, Han, KH, Hong, SB, and Yu, JH. Diversity, application, and synthetic biology of industrially important aspergillus Fungi. Adv Appl Microbiol. (2017) 100:161–202. doi: 10.1016/bs.aambs.2017.03.001
45. Chaibub, AA, Sousa, TP, Araújo, LG, and Filippi, MCC. Molecular and morphological characterization of Rice Phylloplane Fungi and determination of the antagonistic activity against Rice pathogens. Microbiol Res. (2020) 231:126353. doi: 10.1016/j.micres.2019.126353
46. Fierro, F, Vaca, I, Castillo, NI, García-Rico, RO, and Chávez, R. Penicillium Chrysogenum, a vintage model with a cutting-edge profile in biotechnology. Microorganisms. (2022) 10:1–3. doi: 10.3390/microorganisms10030573
47. Ansotegui, IJ, Melioli, G, Canonica, GW, Caraballo, L, Villa, E, Ebisawa, M, et al. Ige allergy diagnostics and other relevant tests in allergy, a world allergy organization position paper. World Allergy Organ J. (2020) 13:100080. doi: 10.1016/j.waojou.2019.100080
48. Sharpe, RA, Bearman, N, Thornton, CR, Husk, K, and Osborne, NJ. Indoor fungal diversity and asthma: a Meta-analysis and systematic review of risk factors. J Allergy Clin Immunol. (2015) 135:110–22. doi: 10.1016/j.jaci.2014.07.002
49. Gern, JE. Barnyard microbes and childhood asthma. N Engl J Med. (2011) 364:769–70. doi: 10.1056/NEJMe1013713
50. Jeong, JS, Kim, SR, and Lee, YC. Can controlling endoplasmic reticulum dysfunction treat allergic inflammation in severe asthma with fungal sensitization? Allergy, Asthma Immunol Res. (2018) 10:106–20. doi: 10.4168/aair.2018.10.2.106
51. Bartemes, KR, and Kita, H. Innate and adaptive immune responses to Fungi in the airway. J Allergy Clin Immunol. (2018) 142:353–63. doi: 10.1016/j.jaci.2018.06.015
52. Vicencio, AG, Chupp, GL, Tsirilakis, K, He, X, Kessel, A, Nandalike, K, et al. Chit1 mutations: genetic risk factor for severe asthma with fungal sensitization? Pediatrics. (2010) 126:e982–5. doi: 10.1542/peds.2010-0321
Keywords: meta-analysis, sensitization to fungi, fungal genera, aspergillus spp., severe asthma
Citation: Chen Z, He Y and Bu H (2025) Association between sensitization to common fungi and severe asthma. Front. Public Health. 13:1582643. doi: 10.3389/fpubh.2025.1582643
Edited by:
Atin Adhikari, Georgia Southern University, United StatesReviewed by:
João Cavaleiro Rufo, University Porto, PortugalJayanta Gupta, Florida Gulf Coast University, United States
Copyright © 2025 Chen, He and Bu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaien Bu, YnVodWFpZW5AMTYzLmNvbQ==
 Ziqiu Chen1
Ziqiu Chen1 Huaien Bu
Huaien Bu