- Department of Histology and Embryology, Faculty of Medicine, Ondokuz Mayıs University, Samsun, Türkiye
Aim: The main aim of the study is to qualitatively and quantitatively evaluate the neuronal changes that occur in the hypothalamic nuclei of newborn male rats related to obesity during the intrauterine period, which was exposed to electromagnetic field (EMF). It was also investigating the expressions of obesity-related neuropeptide Y (NPY) and fat mass and obesity-associated gene (FTO) in the arcuate (ARN), ventromedial (VMN), and dorsomedial nuclei (DMN). It was also aimed to examine the role of omega-3 (ω3) and melatonin (Mel) against the side effects of EMF.
Methods: Adult Wistar albino pregnant female rats were randomly divided into seven groups: Cont, Sham, EMF, EMF-Mel, EMF-ω3, Mel, and ω3. While no treatment was applied to the control group, rats in the Sham group were kept in the cage system for 2 h per day for 21 days without exposure to EMF. EMF groups were exposed to 900 MHz EMF for 2 h per day during pregnancy. Mel-treated groups received 50 mg/kg/day melatonin, while ω3-treated animals were given 0.93 g/mL ω3 via intragastric gavage. Anxiety and locomotor behaviors were assessed. Oxidative stress parameters were analyzed. Stereological, immunohistochemical, and ultrastructural analyses were performed on the offspring rats’ hypothalamus.
Results: It was seen that serum superoxide dismutase activity was significantly higher in the ω3 group compared to the other groups (p ≤ 0.01), and serum catalase activity was significantly higher in the EMF group compared to the Cont and EMF-ω3 groups (p ≤ 0.01). The number of neurons in the ARN was significantly lower in the EMF group compared to the Sham group (p ≤ 0.05). According to the open field test, the time spent in the peripheral zone in the EMF group was longer than in the Cont group (p ≤ 0.05); In the elevated plus maze test, the number of entries into the open area in the EMF group was lower than in the Cont group (p ≤ 0.01). Stronger anti-NPY immunoreactivity was observed in the EMF group.
Conclusion: Prenatal exposure to mobile phones may have hypothalamic effects by inducing neurodegeneration and affecting FTO and NPY expressions. The possible therapeutic effects of Mel and ω3 were not apparent.
1 Introduction
The discovery of new communication technologies, such as mobile phones, has brought significant momentum to our daily lives. In addition, facilities established due to the developing telecommunications industry may lead to various health risks (1, 2). The use of 5G technology raises concerns about its effects on human health. The widespread use of wireless devices, a significant source of radiofrequency electromagnetic fields (RF-EMF), can lead to behavioral problems, especially in children and adolescents (3). Since the lifetime exposure period is more extended in children and adolescents than in adults, it has been suggested that children are prone to environmental exposure due to their developing nervous systems. In addition to this source increase, the adverse effects of exposure to electromagnetic fields (EMF) are a concern due to the widespread use of mobile phones at an early age. The possible effects of EMF on the developing nervous system have been investigated (4, 5). Evidence on the effects of EMFs emitted from sources such as microwave ovens and mobile phones on biological systems suggests that mobile phone use may also have health effects. EMF exposure is considered under two headings: low-frequency EMFs emitted from electrical and electronic devices, and radio frequency (RF) (800–2000 MHz) radiation emitted from mobile phones and cordless phones. Low-frequency EMFs have low frequency power; however, when the magnetic flux density is over 0.2 or 0.3 μT (6, 7). In addition, both EMF types are considered non-ionizing radiation (7). Epidemiological and experimental studies are noteworthy when considering the effect of EMF emitted by mobile phones on brain functions, mainly due to their use close to the brain (8–12). Acute exposure to 900 MHz EMF signals can cause molecular and cellular changes in brain cells (13).
The cognitive effects of EMF exposure in adolescence and childhood are known (11, 14, 15). It is suggested that behavioral activities increase, but memory is weakened in newborn mice exposed to RF-EMF during the prenatal period. There are also evaluations that EMF exposure may lead to hyperactivity and memory impairment (16). In addition, different studies have shown that EMF can cause many changes in brain energy metabolism and the metabolism of reactive oxygen species (ROS) (17–20). In addition to functional and morphological findings, it has been determined that EMF also affects metabolic processes in the brain (21). In the case of long-term exposure to EMF emitted from mobile phones, an increase in glucose metabolism was found in positron emission tomography scanning (21, 22). EMF exposure on glucose metabolism and endocrine effects during puberty has been discussed (23, 24). The response of different cells to stress caused by EMF under various conditions and the underlying molecular mechanisms enable the effects of EMF on disease risk to be measured (7).
Previously, the widespread use of mobile phones in society coincided with the increase in human body weight and obesity worldwide (18, 25, 26). In recent years, Wardzinski et al. (18) suggested that RF-EMF exposure triggers carbohydrate intake in humans, reporting increased cerebral high-energy phosphate metabolism after exposure. RF-EMF emitted from cell phones is mainly absorbed in the head region. Increased brain glucose metabolism has been observed with mobile phone exposure (21). Glucose-sensing neurons in the hypothalamus are critical in general energy homeostasis and feeding behavior (27). Impairments were detected in the glucose-sensing neurons of rats exposed to EMF and fructose intake, and therefore, it was suggested that there was a loss in satiety control. Fructose intake combined with exposure to RF-EMF (daily 2 h) during childhood–adolescence affects the central nervous system regions responsible for feeding behavior in adulthood (28). Similarly, Bektas et al. (29) reported that RF-EMF led to increased ghrelin and decreased nesfatin-1 levels in the brain. In this regard, they suggested that 3.5 GHz RF radiation caused changes in appetite and energy homeostasis of diabetic and healthy rats (29).
Since the developing brain is more sensitive to toxins and can absorb more RF energy, the risk is higher in juvenile rats than in adult rats. In this context, the current study aimed to examine the effects of EMF exposure in utero on the developing brain. EMF emitted from mobile phones may cause significant changes in the hypothalamus regarding neurodegenerative effects and protein expressions (30, 31). In addition, when the potential oxidative effects of RF-EMF are examined, the effects may differ depending on the frequency, amplitude, waveform, and changes in polarization of the EMF (32–34). RF-EMF can cause significant disruption in mitochondrial functions. This disruption can be observed due to increased ROS production after exposure to RF-EMF (35–37). It is known that the disorder in mitochondrial oxidative metabolism leads to neuronal dysfunction and associated neurodegeneration (38). In EMF exposure studies conducted with cellular and experimental animal models, it has been found that autophagy and mitochondrial oxidative mechanisms are activated (39).
Genetic factors contribute to the risk of developing obesity. One of them is FTO (fat mass and obesity-associated gene) and is mainly expressed in the hypothalamus, which is known as the regulatory center in appetite balance (40, 41). Therefore, it is essential in circadian rhythm, anxiety-like behaviors, energy homeostasis, and memory function. Neuropeptide Y (NPY) levels, a neuropeptide commonly found in the brain, have been observed to be reduced in conditions such as depression, chronic stress, and anxiety (42). Previously, high concentrations of obesity-related NPY levels in the hypothalamus were found in the male rats exposed to melatonin (Mel) during the prenatal period, up to 40 days of age (43). Considering the anti-obesity property of Mel, it has been suggested in recent years that it reduces the orexigenic effect by reducing NPY/Agouti-related peptide (AgRP) in the hypothalamus (44). Similarly, omega-3 (ω3) fatty acids led to an increase in orexigenic NPY expression (45).
The effects of EMF on hypothalamic FTO and NPY expression in offspring rats after prenatal exposure may provide new evidence on the predisposition to obesity. In addition, since NPY is closely related to depression and anxiety, it may lead to conclusions that EMF emitted from mobile phones triggers anxiety in infant rats. The results obtained from the interaction of Mel and ω3 with EMF in the infant rat brain, which provide data on their neuroprotective and anti-obesity effects, will provide new information on these two therapeutic agents. However, there is not enough information on the effects of Mel and ω3 on FTO expression. The results obtained from the interaction of Mel and ω3 with EMF exposure in infant rat brains, which provide data on their neuroprotective and anti-obesity effects, will reveal new information about these two therapeutic agents. Therefore, comparing the results on the interaction of Mel and ω3 with EMF exposure may provide critical information for developing treatment options. Acute or chronic exposure to 900 MHz EMF can affect brain activity regionally (13, 46). In the design of the experimental exposure model, the frequency of 900 MHz, a band widely used in mobile telephone systems, was chosen (47). Since the 915 MHz system is less energy efficient, it requires longer exposure times than, for example, the 2.4 GHz system (48).
In this study, male offspring rats were preferred because it was anticipated that hormonal changes resulting from the menstrual cycle of female rats could affect the analysis results. There are limited studies on the neuroprotective or neurogenesis-stimulating effects of Mel and ω3 against hypothalamic apoptosis and obesity development in the central nervous system. This situation requires more comprehensive experimental studies to explain the possible roles of Mel and ω3 on hypothalamic neurogenesis and the underlying mechanisms. The presented study is designed to determine whether Mel and ω3 application are protective against possible neurodegenerative effects caused by 900 MHz EMF. This study investigated the effects of 900 MHz EMF exposure in the prenatal period on the hypothalamic nuclei and hippocampus using stereological, histopathological, immunohistochemical, biochemical, and behavioral methods. Possible degenerative changes that may be caused by exposure to EMF during the prenatal period and the tendency to obesity that may occur in the postnatal period in the offspring rats were investigated.
2 Materials and methods
2.1 Animals and ethical approval
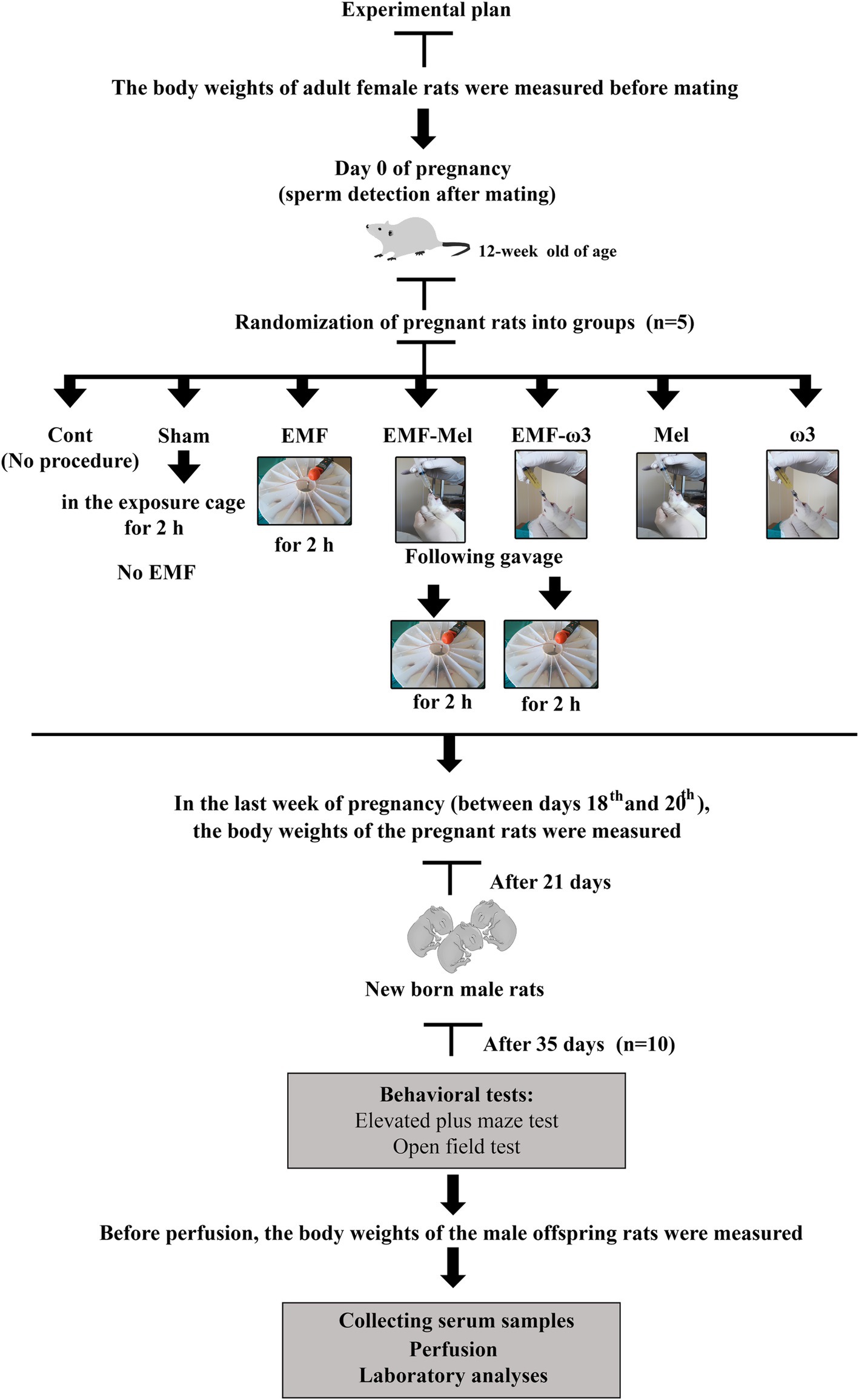
All experimental procedures conducted in this study were approved by the ethics committee decision of the Local Ethics Committee for Animal Experiments of Ondokuz Mayıs University, dated 05.04.2018 and numbered 2018/24. Power analysis was performed using Minitab (version 18.0, UK) to determine the appropriate number of animals for the groups. Following the ethics committee approval, thirty-five 10–12-week-old adult female Wistar albino rats were obtained from the Experimental Animals Application and Research Center. Adult female rats were randomly divided into seven groups, with five animals in each group. Then, these animals were allowed to mate with male rats in separate cages under appropriate conditions for one day, and the vaginal smear sample taken from the female rats was examined the next day. For the rats where sperm was detected in their samples, it was accepted as day zero of pregnancy. After the pregnancy diagnosis, rats were divided into groups and placed in separate cages. Pregnant rats were kept in a room with a humidity of 40–50% and a room temperature. The rats were kept in an environment with a 12-h dark/light cycle. During the experiment, animals had free access to standard pellet feed and water (tap water).
Animal groups consisting of adult pregnant rats that are the Cont group, the Sham group, the EMF-exposed group (EMF), the group administered 50 mg/kg/day Mel by intragastric gavage before EMF exposure (EMF-Mel), the group administered 0.93 g/mL ω3 (F8020, Sigma-Aldrich, United States) by intragastric gavage before EMF exposure (EMF-ω3), the group not exposed to EMF but administered 50 mg/kg/day Mel (Lyophilized Melatonin Powder M5250 10 g, Sigma-Aldrich, United States) (Mel) and the group not exposed to EMF but administered 0.93 g/mL ω3 (ω3). Preparation of a 50 mg/kg/day Mel (Lyophilized Melatonin Powder M5250 10 gr, Sigma Aldrich, United States) solution was done by considering the body weights of the rats. A 12.5 mg powdered Mel was weighed per animal in rats in the EMF-Mel and Mel groups. The solution was prepared fresh for 10 pregnant rats daily and given via intragastric gavage. However, 1 mL of ω3 (Sigma Aldrich, United States) prepared from a concentration of 0.93 g/mL was administered to each pregnant rat in the EMF-ω3 and ω3 groups via intragastric gavage. Fifteen pregnant rats were exposed to 900 EMF (Figure 1).
In this study, EMF exposure was created in the EMF group, and stress exposure was created in the Sham group, and antioxidants such as omega-3 and Mel were applied to the mothers of the offspring rats. The effects of such exposure were examined in the male offspring rats (35-day-old). No antioxidant or EMF application was applied to male offspring rats in the postnatal life. At the end of the gestation period, the mothers gave birth, and the offspring rats stayed with their mothers for 21 days after birth. Newborn male rats were not exposed to any treatment during postnatal life. A total of 70 rats were tested. At the end of this period, the animals were sacrificed.
Then, in the last week of pregnancy (between the 18th and 20th days), just before birth, the body weights of rats belonging to the Cont, Sham, EMF, EMF-Mel, EMF-ω3, Mel, and ω3 groups were measured again.
2.2 EMF exposure setup
After the groups were formed, pregnant mother rats were exposed to 900 MHz simultaneously every day for two hours a day for 21 days. EMF exposure was measured as signal strength: 40.5053 V/m, power density: 4.3519 W/m2. As an EMF source, an EMF exposure system with a 0–9 W output signal generator emitting 900 MHz RF waves was used (49–51) (Figure 2). The monopole antenna used to collect RF waves from a single source was placed in the middle of the 16-section Plexiglas experimental setup, 20 cm high and 25 cm in radius. EMF, EMF-Mel, and EMF-ω3 rats were exposed to 900 MHz EMF in the experimental setup. Before the EMF generator was started, the electric field value of the environment was measured. During the experiment, 20 measurements were recorded every six minutes during the two-hour EMF exposure. The distance of the cephalic region of the rats to the antenna was measured as 3.6 cm on average, the distance of the abdominal region to the antenna was 7 cm on average, and the distance of the caudal region to the antenna was 9 cm on average. An EMF meter (RF-EMF strength meter, Extech Instruments Corporation, United States) was used to measure the electric field value at the closest point from the antenna to the target tissues in the cephalic, abdominal, and caudal regions.
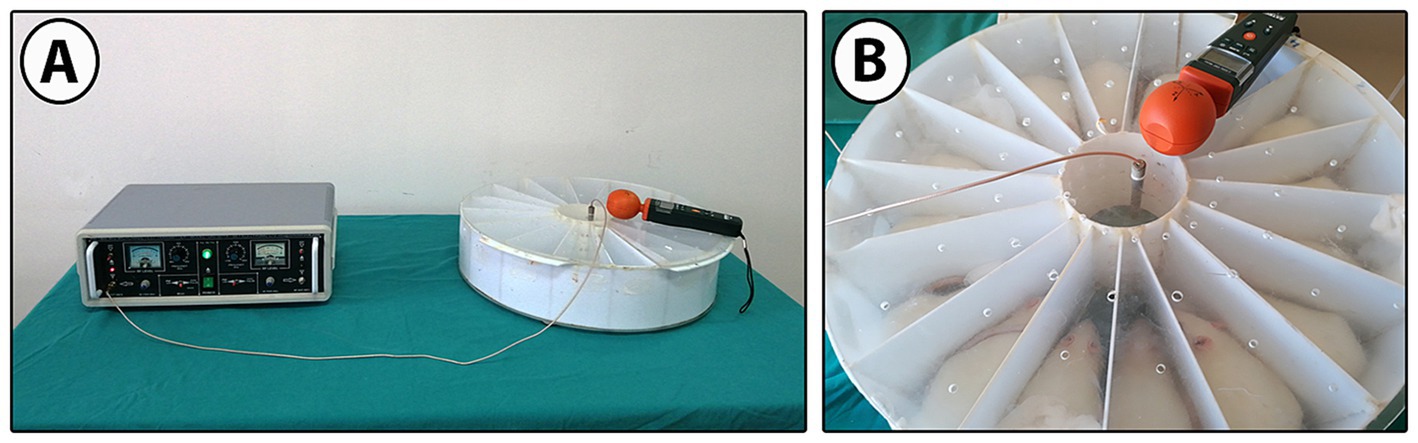
Figure 2. (A) 900 MHz EMF signal generator, EMF exposure system, and (B) EMF exposure cage with 16 equal compartments.
2.3 Behavioral tests
In this study, to investigate the emotional and locomotor effects of EMF exposure, the neuroprotective activity of Mel and ω3, as well as their possible effects on emotional and locomotor activity, 35-day-old male offspring rats were subjected to the open field test and elevated plus maze test. Behavioral tests were performed using appropriate apparatus at the Ondokuz Mayıs University Experimental Animal Application and Research Center, and the behaviors of the animals in all groups were recorded with a camera. The open field test is applied to evaluate the emotional state of male offspring rats, anxiety-like behaviors, and locomotor activity (52, 53). In the test, when rats are left in an open area for five minutes, being placed alone in a different environment triggers anxiety. Defecation and scratching behaviors within five minutes are considered when analyzing vertical and horizontal movements.
Horizontal movements refer to the rat’s transition from one frame to another; movements observed in the vertical plane are considered as the rat rising on its hind limbs (54). The study calculated the time spent by young male rats in the periphery (outside area) and center (inside area), defecation, itching numbers, and movements in horizontal and vertical planes. The test setup consists of 49 equal squares with dimensions of 100 cm x 100 cm x 30 cm. The elevated plus maze test setup, one of the tests used to examine emotional activity, is 50 cm high and consists of two open and two closed arms. Before the test, the subjects were allowed to explore the setup for five minutes. The number of times they enter open areas and the total time spent there were considered when measuring the anxiety index. High anxiety is expressed with a low index. In addition, the time spent moving from the closed area to the open area and the short time spent in the open area indicate the presence of anxiety (55).
2.4 Perfusion and serum collection
The body weights of male offspring rats were measured and recorded before perfusion. Thirty-five days after birth, male offspring rats were anesthetized for intracardiac perfusion by intraperitoneal (i.p.) injection of a ketamine (50 mg/kg)/xylazine (10 mg/kg) mixture prepared in a 5/1 ratio. Before perfusion, 1 mL blood samples taken from the hearts of each rat were centrifuged for 15 min at 2000 rpm at +4°C using a centrifuge device (Hermle Labortechnik GmbH, Germany). After centrifugation, the separated serum samples were stored at −20°C for analysis of antioxidant enzyme levels. Brain tissues of the rat that underwent intracardiac perfusion were dissected.
2.5 Biochemical analyses
To investigate the antioxidant activity of Mel and ω3 against oxidative stress mechanisms that may occur due to EMF exposure, superoxide dismutase (Cayman Chemical Company, United States) and CAT (Catalase) (Cayman Chemical Company, United States) enzyme activities were analyzed in serum samples taken from the rat at the Department of Medical Biochemistry, Faculty of Medicine, Ondokuz Mayıs University. After the serum samples were incubated in the Nuve SL 350 device for the calculation of enzyme activities, the absorbance values of the solutions reacted in the microplate were recorded on a microplate reader device (Tecan Instruments, Austria) at 450 nm for superoxide dismutase enzyme activity and at 540 nm for CAT enzyme activity.
2.6 Paraffin-embedded blocks
After intracardiac perfusion, dissected brain tissues were placed into a 4% formaldehyde (Sigma-Aldrich, United States) solution for fixation. After the fixation process, brain tissues were subjected to a routine tissue tracking procedure. A tissue tracking device was used for routine tracking of brain tissues (Thermon Shandon Citadel 2000, United States). After the tissue embedding procedure, brain tissues were embedded in paraffin in the coronal plane. All tissues were randomly numbered to ensure that all analyses in the study were done blindly.
2.7 Stereological analyses
According to the systematic random sampling (SRS) rule, serial sections of 25 μm for stereological analyses and 4 μm for immunohistochemical analyses were taken from the paraffin blocks using a Leica RM2245 microtome (Leica, Nussloch, Germany) at a 1/2 section sampling ratio. Then, the sections placed on slides were kept in an oven at 60°C overnight for deparaffinization of the sections. An average of 30 sections of 25 μm thickness obtained from each block by SRS were stained with cresyl violet. Stereological analyses were performed on these sections. The sections were stained with cresyl violet dye (C5042-10 gr, Sigma-Aldrich, United States). Neuron counting was performed using the optical fractionator method (56) in a computer-aided stereology analysis system with special software (Stereoinvestigator 9.0, MicroBrield Field; Colchester, United States) at the Department of Histology and Embryology, Faculty of Medicine, Recep Tayyip Erdoğan University. The area sampling ratio in neuron counting is 1,600 μm2/3600 μm2. In addition to neuron counting, the arcuate nucleus (ARN), the ventromedial nucleus (VMN), and the dorsomedial nucleus (DMN) sectional areas were measured using the planimetric method. The sectional areas were multiplied by the section thickness to obtain a volume of hypothalamic nuclei.
2.8 Immunohistochemical analyses
After deparaffinization of 4 μm thick sections obtained from each rat brain, immunolabelling of anti-NPY (catalog number: ab30914, dilution: 1:500, Abcam, UK), anti-FTO (catalog number:ab126605, dilution: 1/200, Abcam, UK) and anti-Ki67 (dilution: 1:500, Abcam, UK, catalog number:ab15580) primary antibodies belonging to the Control, Sham, EMF, EMF-Mel, EMF + ω3, Mel and ω3 groups were histopathologically evaluated. A mouse and rabbit-specific HRP/AEC (ABC) detection kit (catalog number: ab93705, Abcam, UK) was used for immunohistochemical analyses. Hematoxylin solution (Mayer’s modified Abcam, UK) was used as a counterstain. A dark, moist environment container was used during the procedures. In immunohistochemical evaluations, section samples taken from each paraffin block were evaluated (n = 9). After the sampled sections were stained, an average of five different areas from each section were reviewed, and representative images were used for histopathological evaluation using a microscope (Olympus, BX43, Center Valley, PA, United States) with a camera attachment (Olympus, SC5, Tokyo, Japan).
2.9 Tissue processing for electron microscopy and preparing resin-embedded tissue blocks
One brain tissue from each group was fixed in a 4% glutaraldehyde (Merck Millipore, United States) solution for electron microscopic analysis. Hypothalamic regions were dissected with a stereo microscope in a volume of approximately 2 mm3 from the brain tissues, and electron microscopic tissue processing was applied. After this process (Araldite CY212, Agar Scientific Ltd., Essex, UK), hypothalamic tissues were embedded in resin and kept in a dark environment overnight. After the centralization process, the tissues were kept in the oven at 45°C- 50°C- 55°C for 30 min and at 62°C for 48 h to ensure polymerization of the resin blocks (57, 58). Brain tissue from one subject (n = 1) from each group was embedded in resin blocks to obtain semithin and thin sections for histopathological evaluation.
Semi-thin sections (0.5 μm) were taken from resin-embedded blocks using an ultramicrotome (Thermo Scientific Shandon, United States). The collected sections were stained with 1% toluidine blue and 0.2% sodium borate solution. Then, images were taken from the ARN, VMN, and DMN in the hypothalamus for histopathological examinations using a light microscope (Olympus, BX43, Center Valley, PA, United States) with a camera attachment (Olympus, SC5, Tokyo, Japan) using the computer-aided cellSens Entry microscope software program (Olympus, Center Valley, PA, United States). In addition, 70 nm thin sections were taken with a diamond knife on an ultramicrotome (Leica ultra cut UCT, Leica Microsystems GmbH, Germany) for the examination of the ultrastructure of the cell and were placed on copper grids and contrasted with 0.5% uranyl acetate and 3% lead citrate solution. The obtained micrographs were examined under an electron microscope (JEOL JSM-7001F, JEOL Ltd., Tokyo, Japan) at the Black Sea Advanced Technical Research and Application Center.
2.10 Statistical analysis
Statistical analysis of numerical data was performed using GraphPad Prism (version 8.0, GraphPad Software, San Diego, CA, United States). All data were subjected to a normality test using the Shapiro–Wilk test. Normally distributed data were evaluated using One-way ANOVA (Post hoc: Tukey’s test), and non-normally distributed data were evaluated using the Kruskal-Wallis test (Post hoc: Dunn’s test). In data expressed as “mean ± standard deviation,” a significance value of 0.05 (p) was considered.
3 Results
3.1 Body weights
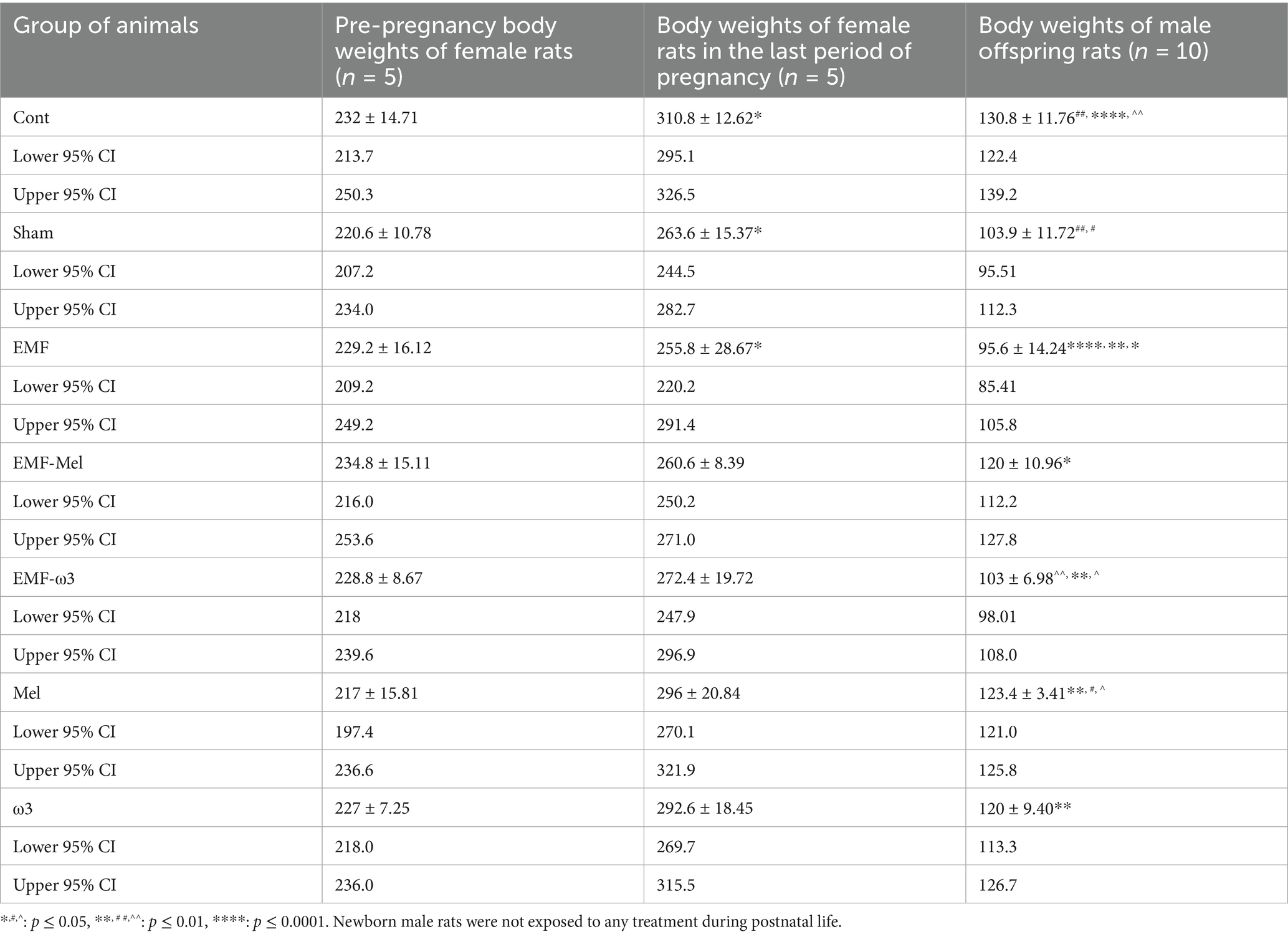
When the pre-pregnancy body weights of pregnant rats were evaluated, no difference was observed between the groups (F (6, 28) =1.143, p = 0.3639, One-way ANOVA). However, when the body weights of female rats in the last final period of pregnancy were examined, a decrease was determined in the Sham group compared to the Cont group (p = 0.047). However, there was no permanent significant difference between the body weights of female rats in the EMF and the Sham groups in the last pregnancy period (p > 0.05). A significant decrease was found when the EMF group was compared with the Cont group (p = 0.024). Similarly, a significant decrease was observed in the body weight values of the EMF-Mel group compared to the Cont group (p = 0.014). However, there was no significant difference between the EMF-ω3 and the Cont group (p > 0.05). It was observed that there was no difference in body weight performance in the ω3 and Mel-treated groups of female rats compared to the Cont group (p > 0.05) (Table 1; Figure 3).
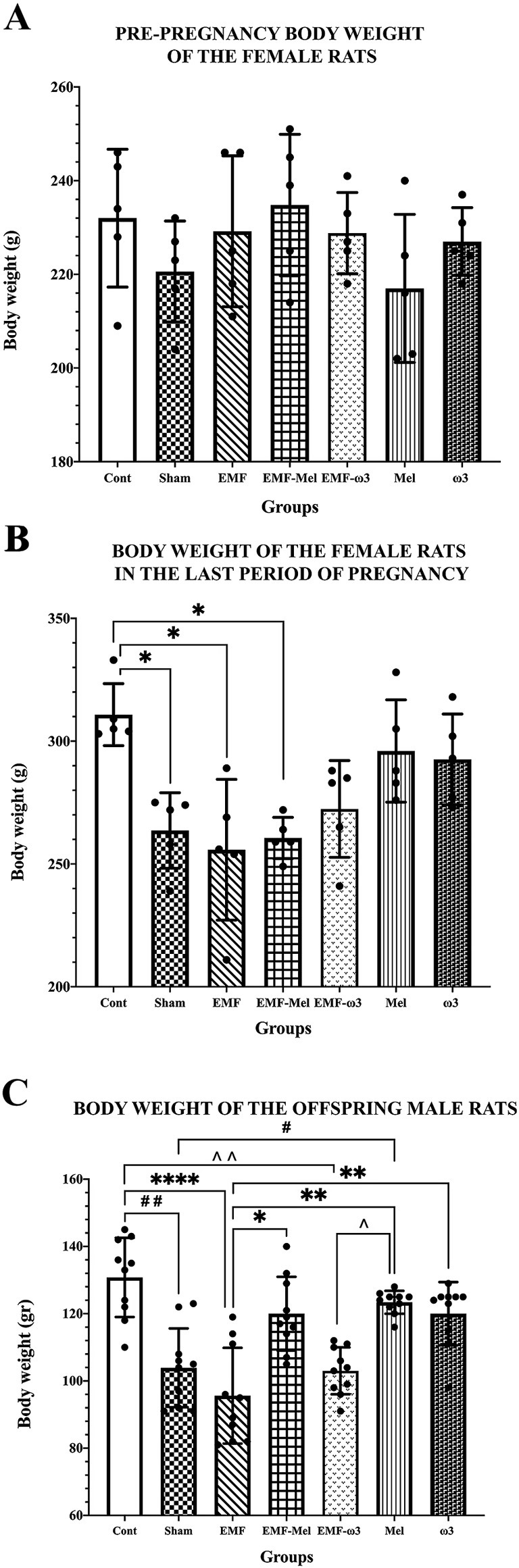
Figure 3. (A) Pre-pregnancy body weight values of female rats, (B) Body weight values of female rats in the last period of pregnancy, (C) Body weight values of 35-day-old male offspring rats are given. (A–C) Body weight values of groups are expressed as “mean ± standard deviation.” In the graphs, statistically significant differences at the level of p ≤ 0.05 (*, #, ^^), significant differences at the level of p ≤ 0.01 (**, ##, ^^), and significant differences at the level of p ≤ 0.0001 (****) are shown.
The body weights of male offspring rats were measured before perfusion. It was found that there was a statistically significant decrease in the body weight of male offspring rats in the EMF group compared to the Cont group (p = 0.0001). When the Sham group was compared to the Cont group, a highly significant decrease was seen in this group (p = 0.003). There was no significant difference between the EMF-ω3 and EMF groups (p > 0.05). However, A significant increase in body weight was found in the offspring rats of the EMF-Mel group compared to those of the EMF group (p = 0.036). Also, it was seen that there was a significant decrease in the body weight of the rat in the EMF-ω3 group compared to the Cont group (p = 0.0011). The body weight of the rat in the Mel group was higher than that of the rats in the Sham group (p = 0.032). In addition, the weights of animals belonging to the Mel and ω3 groups were higher than those of the EMF group (respectively, p = 0.0013, p = 0.0097). However, there is no significant difference between the Cont, Mel, and ω3 groups (p > 0.05) (Table 1; Figure 3).
3.2 Antioxidant enzyme levels
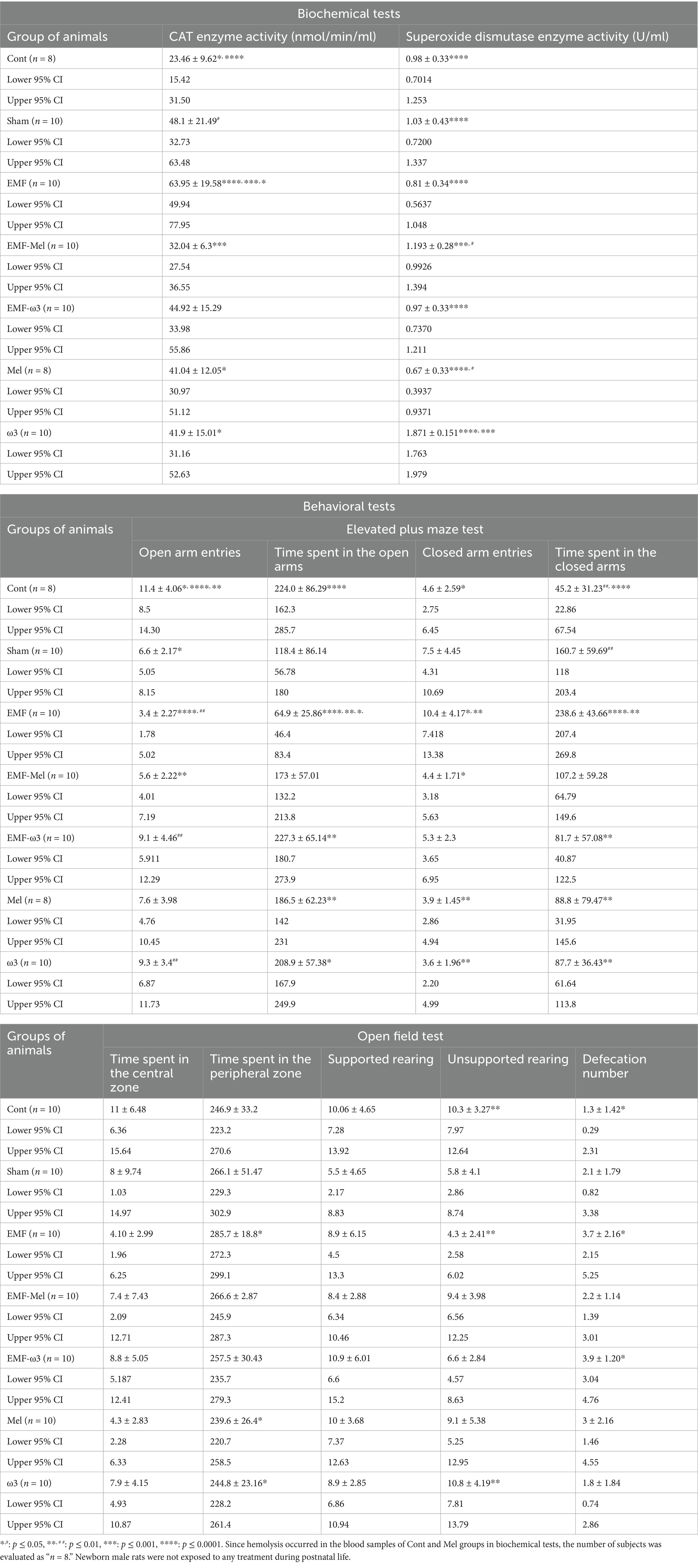
Statistical differences between the groups were compared in terms of the CAT enzyme (F (6, 59) =6.415, p ≤ 0.0001, One-way ANOVA). When all groups’ CAT enzyme activity measurements were evaluated, a significant increase was found in the EMF group compared to the control group (p = 0.0001). Similarly, a significant increase was observed in the Sham group regarding CAT enzyme activity (nmol/min/ml) compared to the Cont group (p = 0.0196). A statistically significant decrease in CAT enzyme activity was observed in the EMF-Mel group compared to the EMF group (p = 0.0003). Additionally, a significant increase was found in the EMF group compared to the Mel and ω3 groups (respectively, p = 0.038, p = 0.032). In addition, statistical differences between the groups were evaluated in terms of the SOD enzyme (F (6, 59) =14.05, p ≤ 0.0001, One-way ANOVA). When the superoxide dismutase enzyme activity (U/ml) measurements of all groups were examined, a significant increase was found in the ω3 group compared to the Cont, Sham, EMF, EMF-ω3, and Mel groups (p = 0.0001). Also, the ω3 group had a higher superoxide dismutase (SOD) enzyme level than the EMF-Mel group (p = 0.0003). A significant increase was observed in the SOD enzyme activity in the EMF-Mel group compared to the Mel group (p = 0.017). No statistical difference was found between the other groups (p > 0.05) (Table 2; Figure 4).
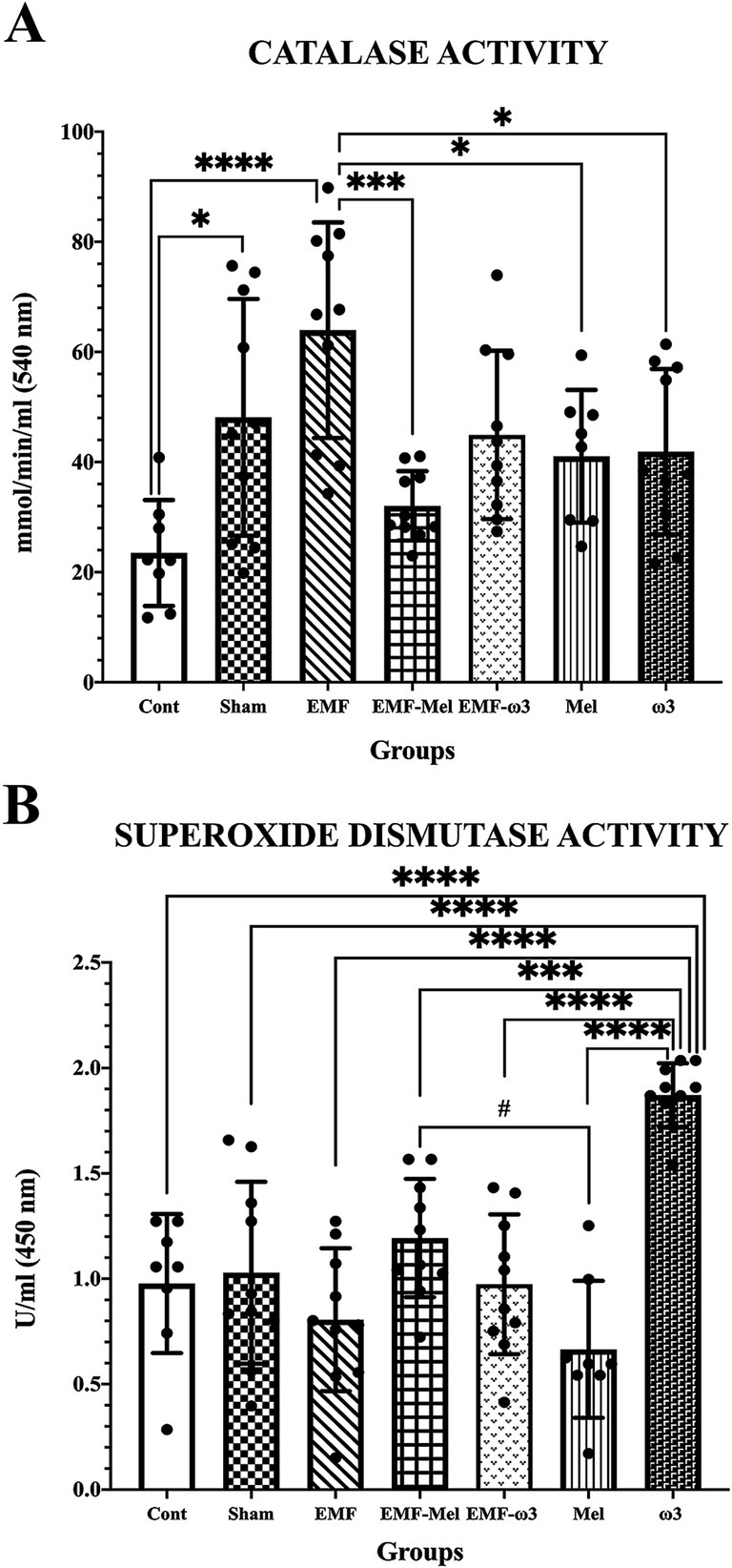
Figure 4. (A,B) CAT enzyme activity (nmol/min/ml) and superoxide dismutase enzyme activity (U/ml) values of all groups are shown in the graphs. Differences that are statistically significant at p ≤ 0.05 are shown with (*, #), differences that are significant at p ≤ 0.001 and p ≤ 0.0001 are shown with (respectively ***, ****).
3.3 Open field test
Anxiety and locomotor activity were evaluated regarding the time spent in the central zone; it was observed that there was no significant difference between all groups (p > 0.05). However, it was found that the time spent in the peripheral zone by the animals in the EMF group was longer than in the Mel and ω3 groups (respectively, p = 0.015, p = 0.038). On the other hand, when the time spent in the peripheral zone was compared, there was no significant difference between other groups (p > 0.05). When the number of unsupported rearing of the offspring rats in the open field test was examined, a significant decrease was found in the EMF group compared to the Cont and ω3 groups (p = 0.006, p = 0.007). No difference was observed between the other groups (p > 0.05). However, when the values of all groups were statistically examined in terms of supported rearing, no difference was found between the groups (F (6, 63) =1.57, p = 0.1708, One-way ANOVA). In the open field test, the defecation numbers of the groups were compared statistically (F (6, 63) =3.473, p ≤ 0.05, One-way ANOVA). A significant increase was found in the EMF group compared to the Cont group (p = 0.0032). Similarly, when the EMF-ω3 group was compared with the Cont group, a significant increase in the number of defecations was found (p = 0.015). However, no difference was found between the other groups (p > 0.05) (Table 2; Figure 5).
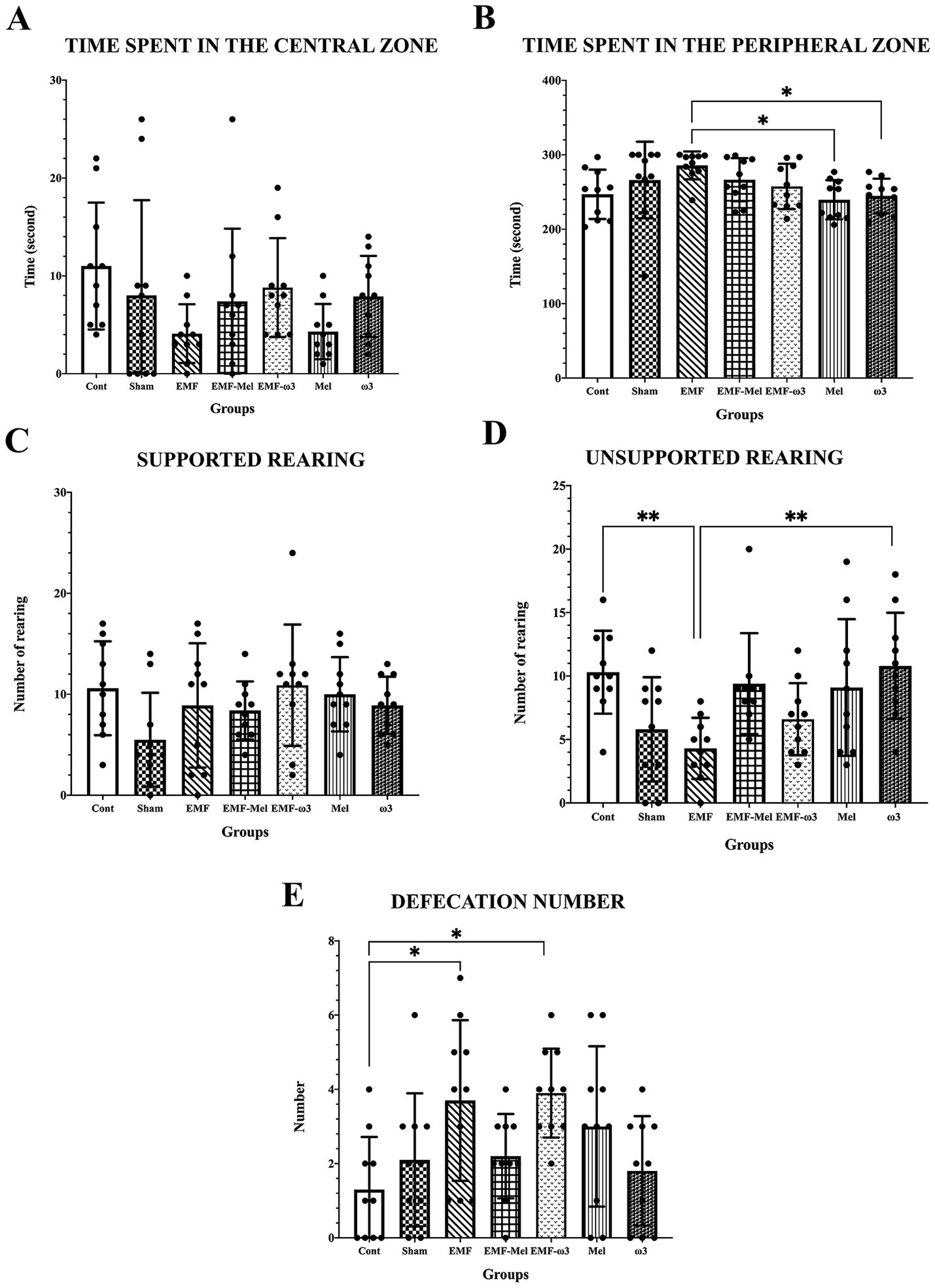
Figure 5. (A–E) Graphs for the parameters of the open field test present a statistical comparison of the open field test parameters of all groups: (A) the time spent in the center, (B) the time spent in the peripheral area, (C) supported rearing, (D) unsporting rearing, (E) the number of defecations. (A–E) Data for the groups are expressed as “mean ± standard deviation.” Statistically significant differences at the level of p ≤ 0.05 between the groups are shown with (*), and differences at the level of p ≤ 0.01 are shown with (**).
3.4 Elevated plus maze test
When elevated plus maze test findings were evaluated, statistical differences between the groups were evaluated in terms of the number of entries into the open area (F (6, 63) =6.269, p ≤ 0.0001, One-way ANOVA). A significant decrease was observed in the number of entries into the open area in the Sham group compared to the Cont group (p = 0.033). Additionally, a significant decrease was found in the number of entries into the open area of the EMF group compared to the Cont group (p < 0.0001). A significant increase was found when the results of the EMF-ω3, ω3 groups were compared to the EMF group (respectively, p = 0.006, p = 0.004). In addition, a significant decrease in the EMF-Mel group was compared to the Cont group (p = 0.005). No statistically significant difference was found between the other groups (p > 0.05). When the test findings were evaluated regarding the time spent in the open arms, a significant decrease was found in the EMF group compared to the Cont group (p = 0.0005). A significant increase was observed in the EMF-ω3 group compared to the EMF group (p = 0.0003). Similarly, a significant increase was observed in the Mel and ω3 groups compared to the EMF group (respectively, p = 0.03, p = 0.004). No difference was seen between the other groups (p > 0.05). When the number of entries into the closed arms was compared, there was a significant increase in the EMF group than in the Cont group (p = 0.0038). It was observed that the number of entries into the closed arms in the EMF-Mel group decreased significantly compared to the EMF group (p = 0.0038). Additionally, the number of entries to the closed arms in the Mel and ω3 groups was higher than in the EMF group (respectively, p = 0.007, p = 0.0012). No significant difference was found between the other groups regarding the number of entries into the closed arms (p > 0.05). When the elevated plus maze test was evaluated regarding the time spent in the closed arms, it was found that the Sham rats stayed longer than the Cont group rats (p = 0.004). A significant increase was observed in the EMF group compared to the Cont group (p = 0.0001). When the EMF-ω3, Mel, ω3 groups were compared with the EMF group regarding the time spent in the closed arms, a significant decrease was found (respectively, p = 0.002, p = 0.002, p = 0.015). There was no significant difference in the duration of stay in the closed area between the other groups (p > 0.05) (Table 2; Figure 6).
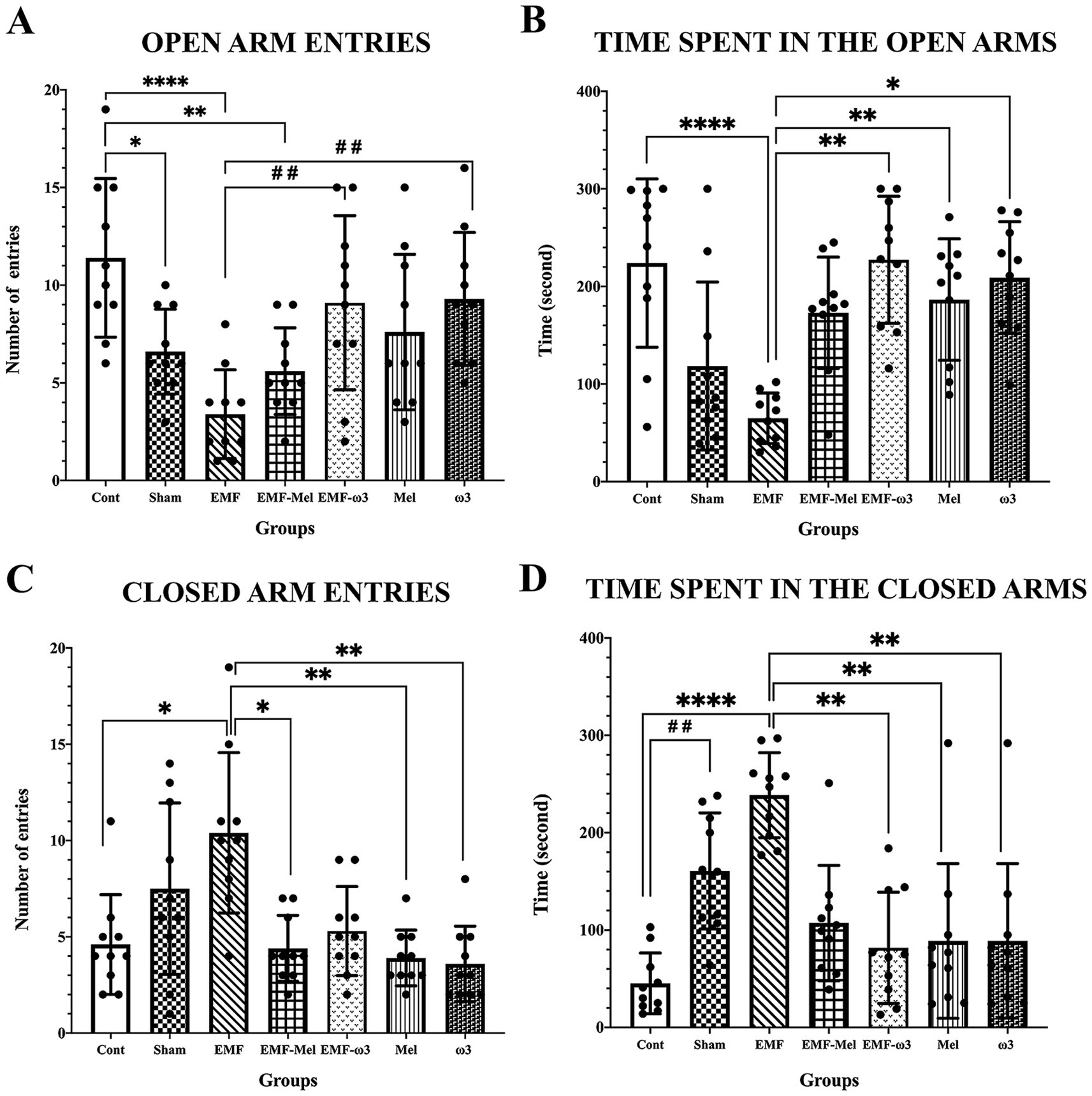
Figure 6. (A–D) Graphs for the parameters of the elevated plus maze test is presented: (A) number of open area entries, (B) duration of stay in the open area, (C) number of closed area entries, (D) duration of stay in the closed area, and statistical comparison between groups. Values belonging to groups are expressed as “mean ± standard deviation.” Statistically significant differences at the p ≤ 0.05 level between groups are shown with (*). Significant differences at the p ≤ 0.01 level are shown with (**, # #), and significant differences at the p ≤ 0.0001 level are shown with (****).
3.5 Stereological data
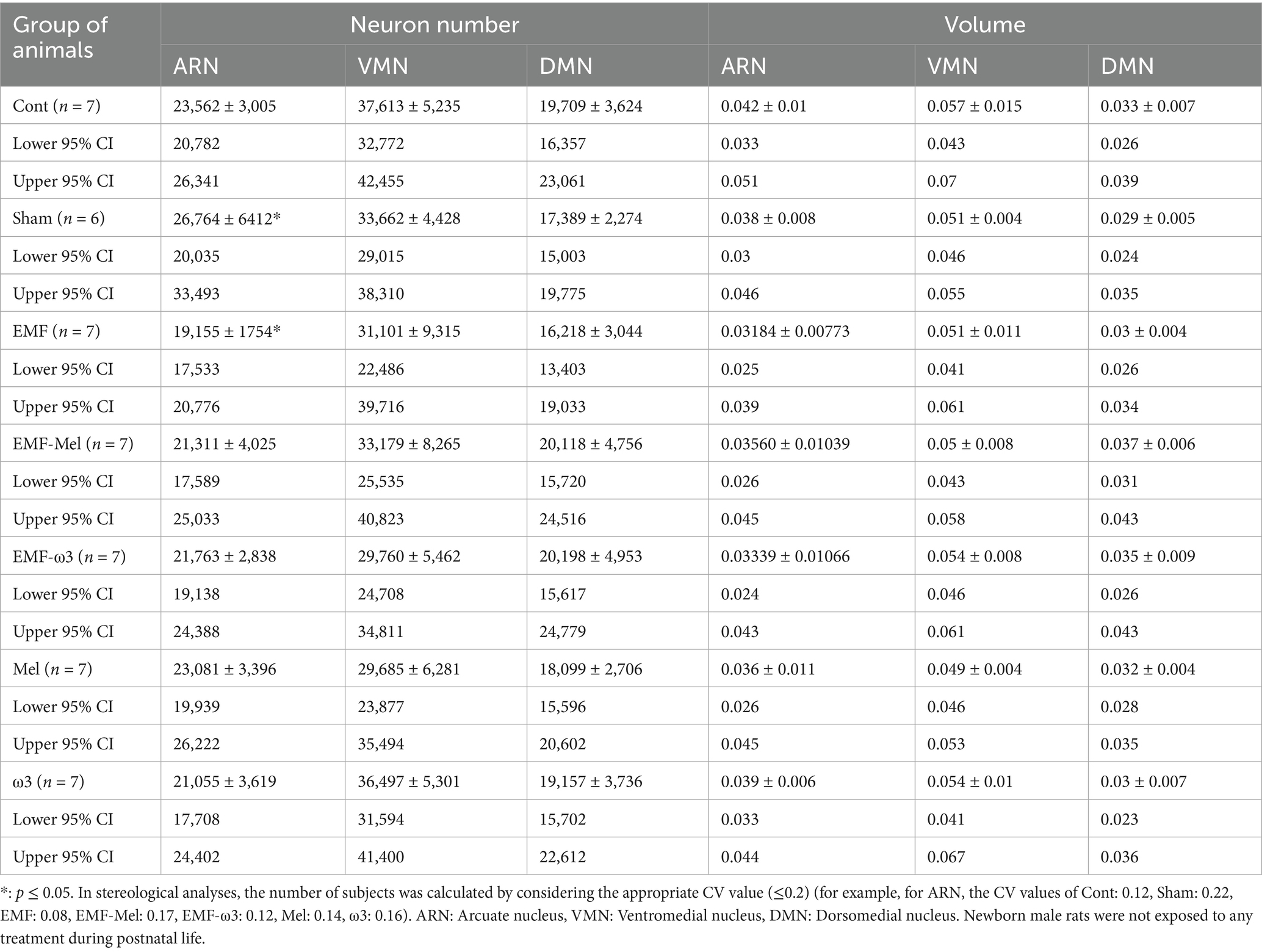
Statistical differences between the neuron number in the ARN of groups were compared (F (6, 41) =2.677, p ≤ 0.05, One-way ANOVA). As a result of the statistical evaluation of the total neuron number in the ARN region of the hypothalamus, a highly significant decrease was observed in the total neuron number of the EMF group compared to the Sham group (p = 0.012). No significant difference was observed between the other groups regarding neuron number (p > 0.05). There was no difference between the groups in terms of the number of neurons in the DMN (F (6, 41) =1.129, p = 0.3629, One-way ANOVA). Similarly, there was no difference between the groups in terms of neuron number in the VMN. When the ARN, VMN, and DMN volume values of the hypothalamus were compared statistically, no difference was observed between the groups (p > 0.05). Similarly, in terms of the total number of neurons in the VMN and DMN, no significant difference was observed between the groups (p > 0.05) (Table 3; Figure 7).
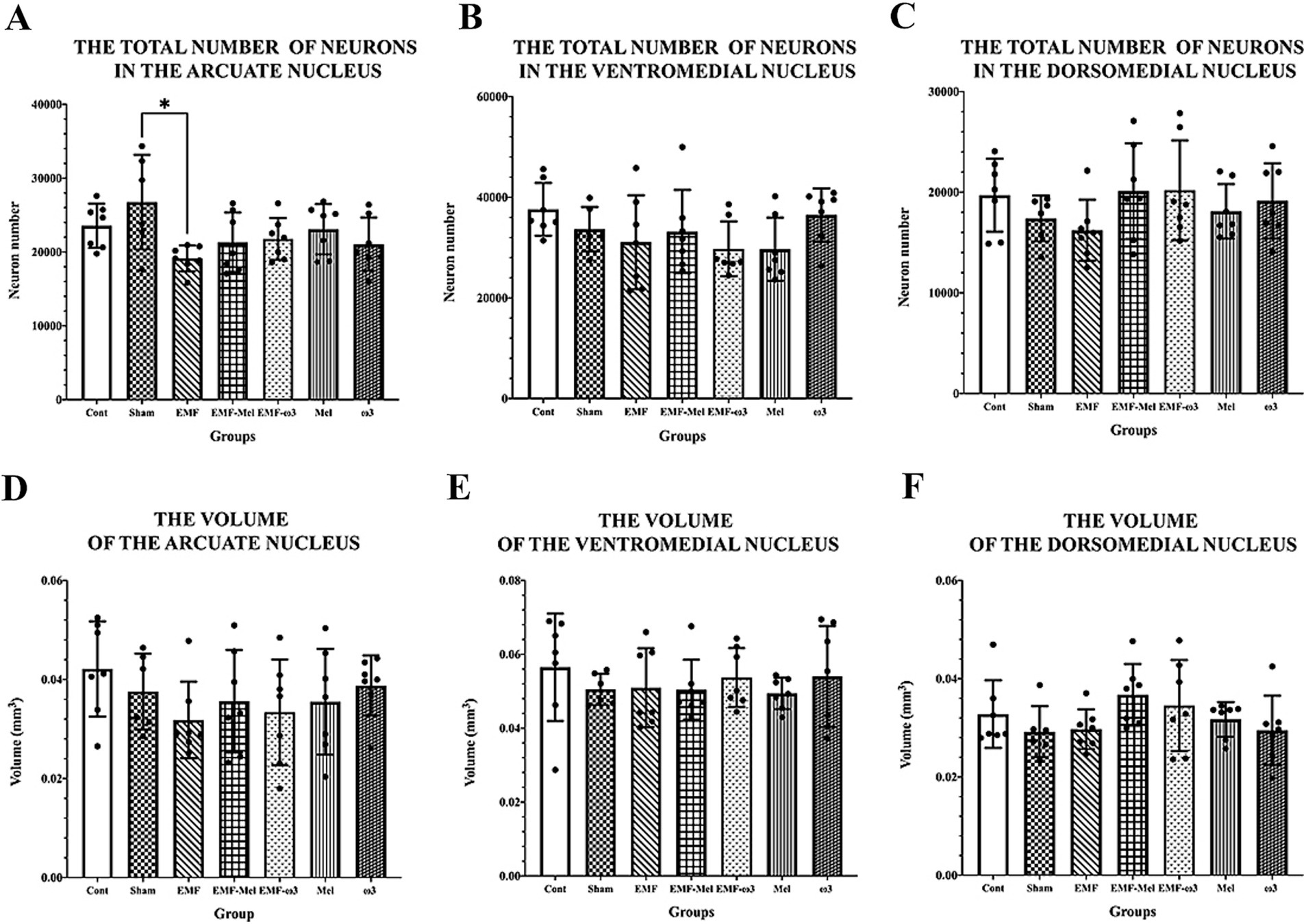
Figure 7. (A–F) The graph shows neuronal number and volumetric values of ARN, VMN, and DMN. Neuron numbers and volumes are expressed as mean ± standard deviation. (A) A statistically significant difference (*) is seen between the EMF and Sham at the level of p ≤ 0.05.
3.6 Light microscopic findings
When the images of the hypothalamic region obtained from the brain tissue of the Cont group were examined, it was observed that the cells and other tissue elements were healthy. Euchromatic nuclei and nucleoli were evident in the neurons. It was observed that the cytoplasm of some cells in the region was darkly stained due to the abundance of granular endoplasmic reticulum. There were many healthy neuroglia cells and blood vessels between the neurons. The nucleus and nucleolus borders of the neurons were evident, and it was observed that the nuclear membranes of the cells in this region had a high degree of invagination. Neurons and neuroglia in the hypothalamic region of the Sham group had normal morphology. Deep invaginations in the nuclei of some neurons were noticeable. Degenerated cells, thought to have lost their function, were also found among the neurons.
Neurons and neuroglia cells of different diameters are observed in the hypothalamic region of the EMF-Mel and EMF-ω3 groups. It was observed that the nucleolus diameters were large and located close to the nuclear membrane in most neurons of the EMF-Mel group. The presence of cells with darkly stained cytoplasm among healthy neurons was striking. Also, in the EMF-ω3 group, there were deep invaginations in the membranes of the nuclei in neurons with a spindle-shaped oval nucleus. When the hypothalamic region of the Mel group was examined, it was observed that the nuclear borders of the neurons with normal morphology were distinct. In addition, it was observed that the neurons of different diameters and the myelinated nerve fibers around them of the ω3 group had normal morphology and that the neuron nuclei were stained euchromatically. Darkly stained glial cells were encountered (Figure 8).
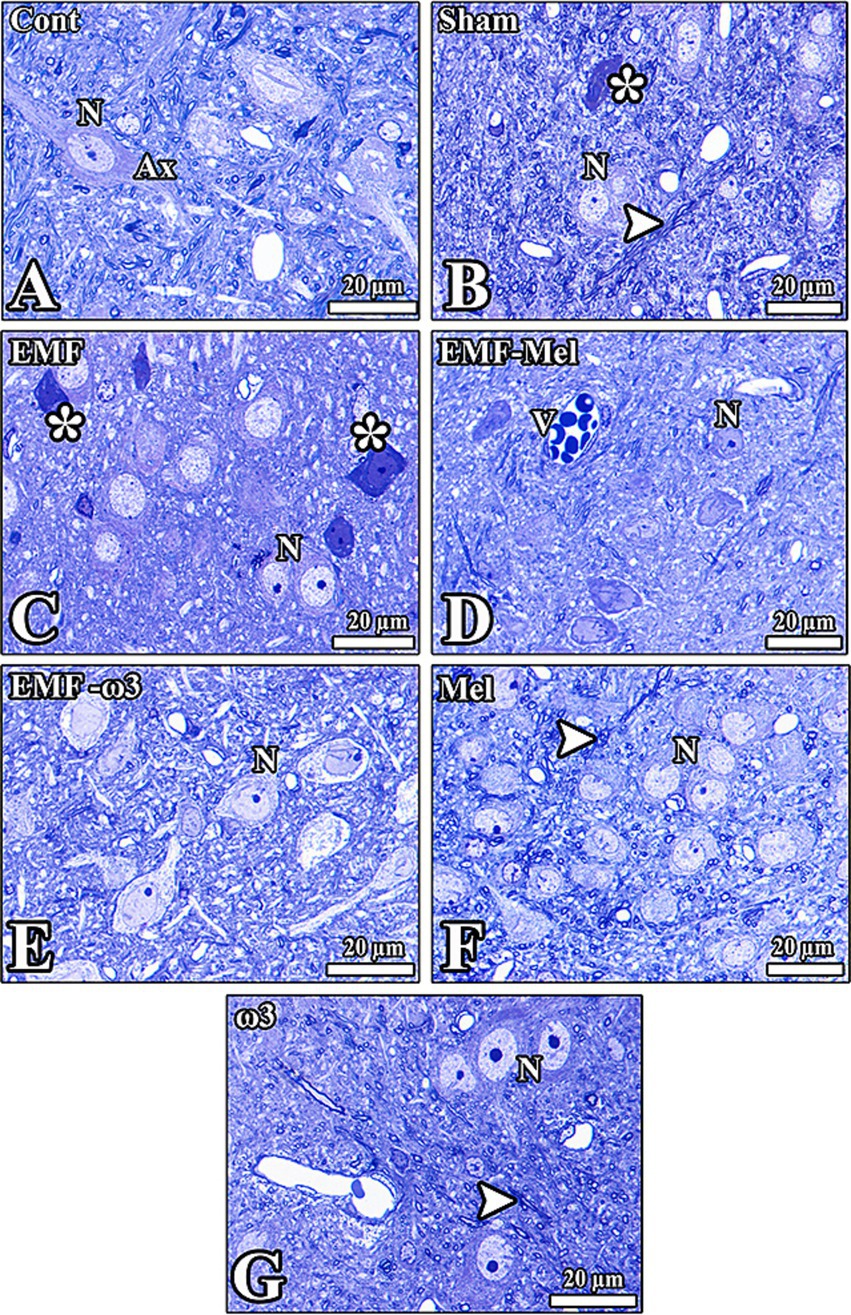
Figure 8. (A–G) Histological images from the groups were seen. (C) However, healthy neurons (N) and nerve fibers (Ax) in the hypothalamic region of all groups are observed except the EMF-exposed group. The morphology of neurons was seriously impaired (*). V: Vessels in the tissue, degenerated cells (*), and axons (arrowhead) are seen. Semithin sections (0.5 μm) obtained from the resin block were stained with toluidine blue.
3.7 Electron microscopic findings
It was observed that the neurons and myelinated nerve fibers in the hypothalamic region of the Cont group had a standard structure. Most of the neuron nuclei were centrally located, and the integrity of the organelles in the cell was well preserved. The borders of the cytoplasm and nuclei of the neurons in the hypothalamus of the Sham group were apparent. Darkly stained nucleoli were noticeable in euchromatic nuclei. It was observed that the cells could not maintain their integrity with the surrounding tissue. Deep invaginations were observed in the nuclear membranes. It was observed that most of the neurons and neuroglia cells in the hypothalamic region in the EMF group degenerated. It was noticed that vacuoles formed around the degenerated neuron. Euchromatic stained nuclei and dark-stained nucleoli of neurons in the hypothalamus of the EMF-Mel group were observed. Deep invagination in the nuclei of neurons in the region and well-developed granular endoplasmic reticulum sacs inside the cells were seen. Neurons in the hypothalamic region of the EMF-ω3 group are healthy. It is noticed that the nucleus and nucleolus structures of the neurons and neuroglia cells are healthy. It was noted that the nucleolus diameters in the nucleus were large. It was observed that there were many myelinated nerve fiber sections in the transverse and longitudinal planes around the neurons. The nuclear borders of the neurons of the Mel group were distinct; the nucleolus was close to the nuclear membrane. The large-diameter nucleolus in the euchromatic nucleus was stained darkly. Organelles were distributed homogeneously in the neurons. The neurons and neuroglia cells in the hypothalamus of the ω3 group were healthy. There were also neuroglia cells with normal morphology around the neuron with an euchromatic-stained nucleus. Large invaginations in the nucleus were striking (Figure 9).
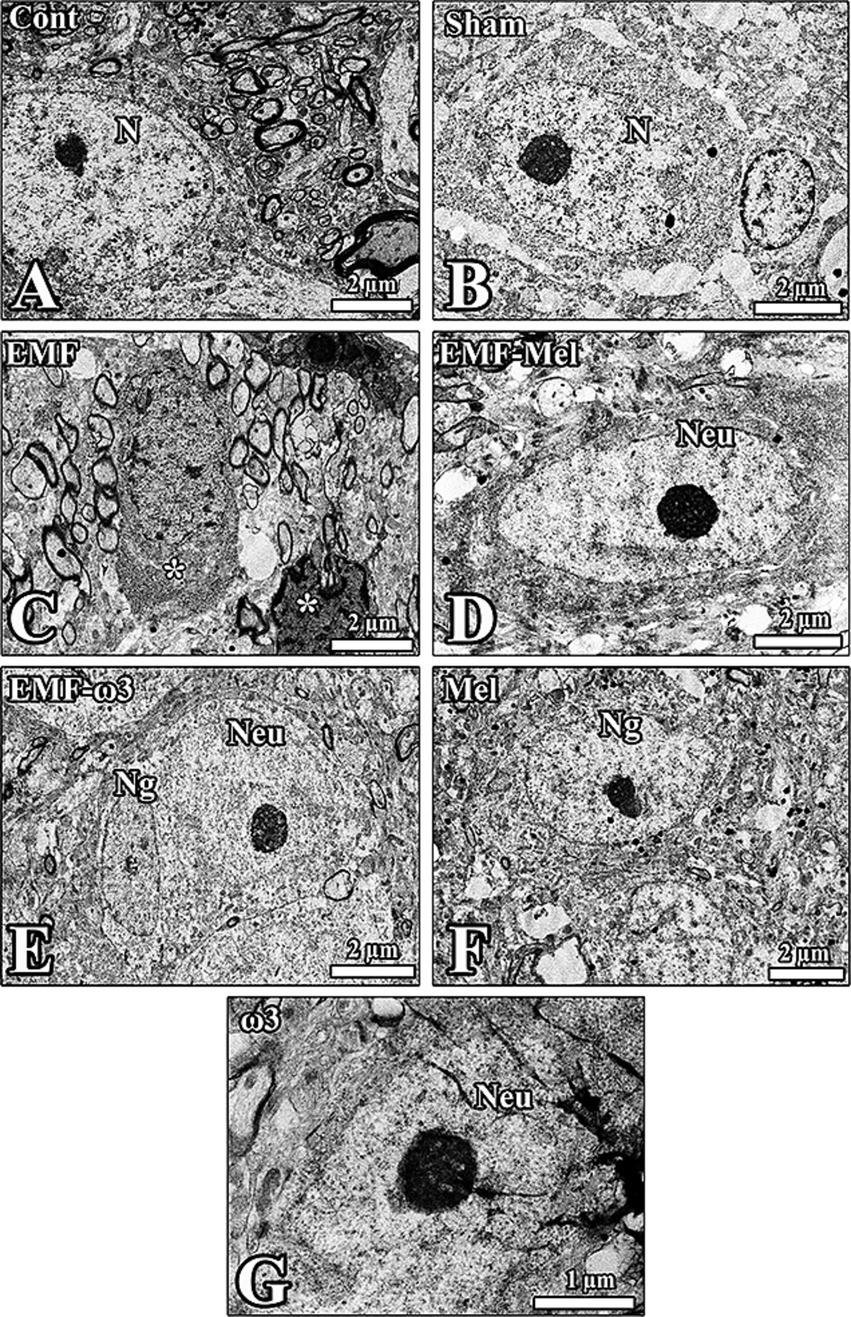
Figure 9. (A–G) Electron microscopic images taken from the groups are shown. Neuron (Neu) and neuroglia (Ng) cells in the hypothalamus of all groups are seen. (C) The ultrastructural structure of the nucleus (N) of the neurons from all groups is observed, as seen in the degenerated cells in the EMF-exposed groups, which are apparent (*). The impaired shape of neurons and neuroglia was prominent in this group.
3.8 Anti-FTO immunolabelling
In the rats of the Cont group, anti-FTO (+) activity was observed in neurons of the ARN region and ependymal cells forming the third ventricle wall. In addition, positively stained areas were seen extensively large in the ARN region, especially in the intercellular medium. In the sham group rats, no anti-FTO (+) staining was observed in the ARN and DMN regions of the hypothalamus. In contrast, anti-FTO (+) staining was observed in ependymal cells located in the wall of the third ventricle. Anti-FTO staining was observed in rats in the EMF group, and low-intensity anti-FTO (+) staining was observed in a small number of neurons in the hypothalamus. While a positive reaction was observed in the cytoplasm in some of the neurons, it was observed in the nucleus in others. In the EMF-Mel group, anti-FTO expression was observed in the intercellular medium around the third ventricle.
In contrast, anti-FTO (+) staining was not observed in ependymal cells and neurons. No anti-FTO (+) staining was observed in the neurons of the rats in the EMF + ω3 group. However, low-intensity anti-FTO staining was observed in the intercellular medium. In the Mel group, anti-FTO (+) staining was evident in the ependymal cells’ microvilli in the third ventricle’s wall. In contrast, no positive staining was observed in the neurons in the hypothalamus. No anti-FTO (+) staining was observed in the nuclei of the ependymal cells in the wall of the third ventricle in the hypothalamus of the ω3 group. In contrast, intense positive staining was observed in the intercellular medium. In addition, it was noticed that the neurons in the periventricular hypothalamic nucleus and the intercellular space were positively stained (Figure 10).
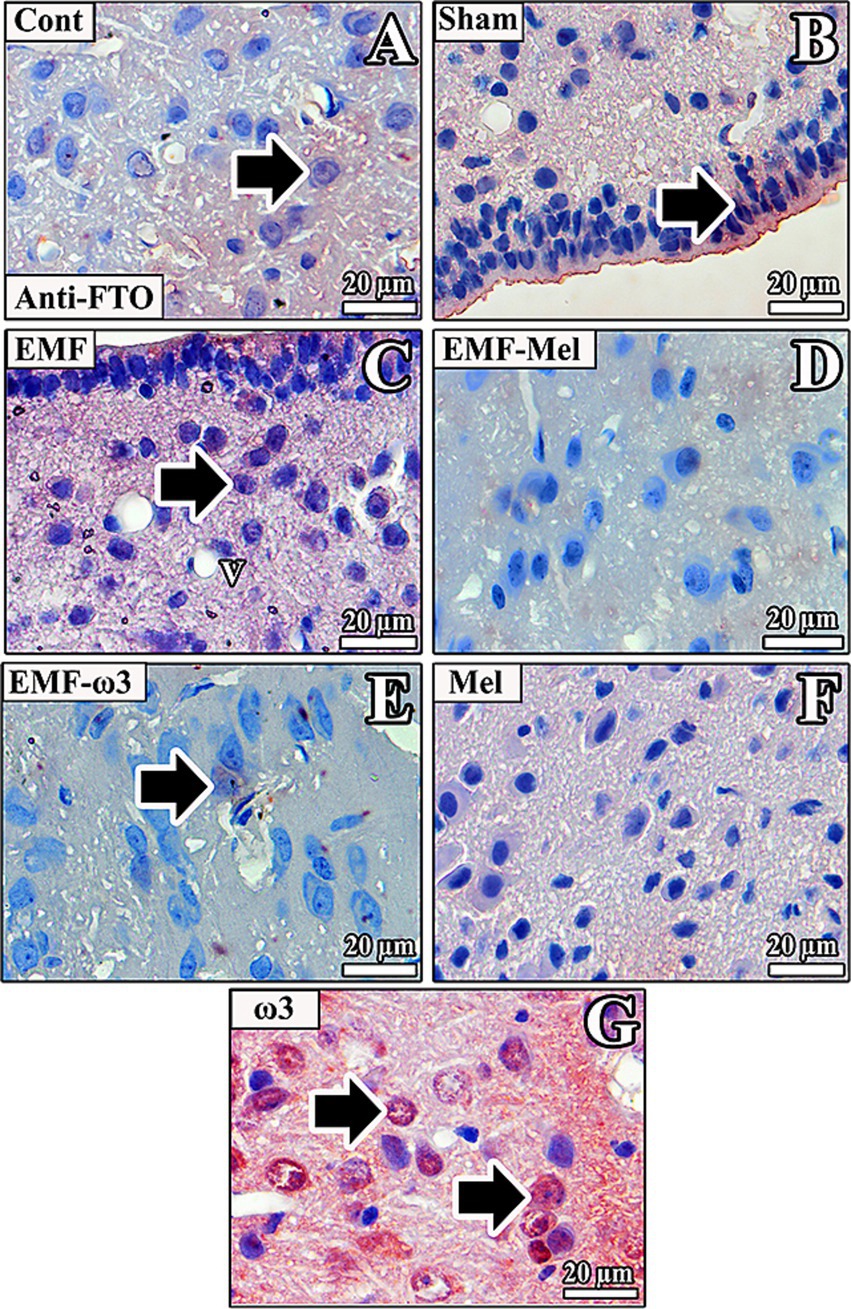
Figure 10. (A–G) Neurons in the hypothalamus and ependymal cells of the third ventricle of all groups are observed. Arrows indicate anti-FTO (+) staining. Counterstaining was performed in the sections using Mayer’s hematoxylin.
3.9 Anti-NPY immunolabelling
No anti-NPY (+) staining neurons were observed in the hypothalamus nuclei in the Cont group. It was noticed that there were rare anti-NPY (+) areas in the intercellular medium. In the ARN of the Sham group, anti-NPY (+) staining was observed in the intercellular medium. It was observed that anti-NPY (+) staining was incredibly intense in the intercellular medium. While anti-NPY (+) staining was not commonly observed in the nuclei of neurons and ependymal cells, staining was widespread in the intercellular medium in the subependymal region.
In the EMF group, anti-NPY (+) staining was incredibly intense in the intercellular area. In addition, while the anti-NPY (+) reaction was not observed in the ependymal cells in the wall of the third ventricle, it was especially intense in the intercellular medium in the periventricular hypothalamic nucleus region of the hypothalamus.
In the EMF-Mel group, neurons had low anti-NPY (+) staining intensity. Anti-NPY (+) staining was evident in some neurons in the VMN region of the hypothalamus. In contrast, no positive staining was observed in the ependymal cells in the third ventricle wall.
In the rats in the EMF + ω3 group, high-density anti-NPY (+) staining was observed in the nuclei of neuroglia cells. Positive staining was also observed in the environment around the nerve cells. In addition, a similar anti-NPY (+) staining was observed in the microvilli of the ependymal cells in the third ventricle wall.
In the rats of the Mel group, strong anti-NPY (+) staining was observed in interneuron areas. Anti-NPY (+) staining was more evident in the intercellular environment. No staining was observed in the hypothalamus of the ω3 group. However, a low-density anti-NPY (+) staining was observed in the regions close to the third ventricle wall, intercellular environment, and cytoplasm of some neurons in this group (Figure 11).
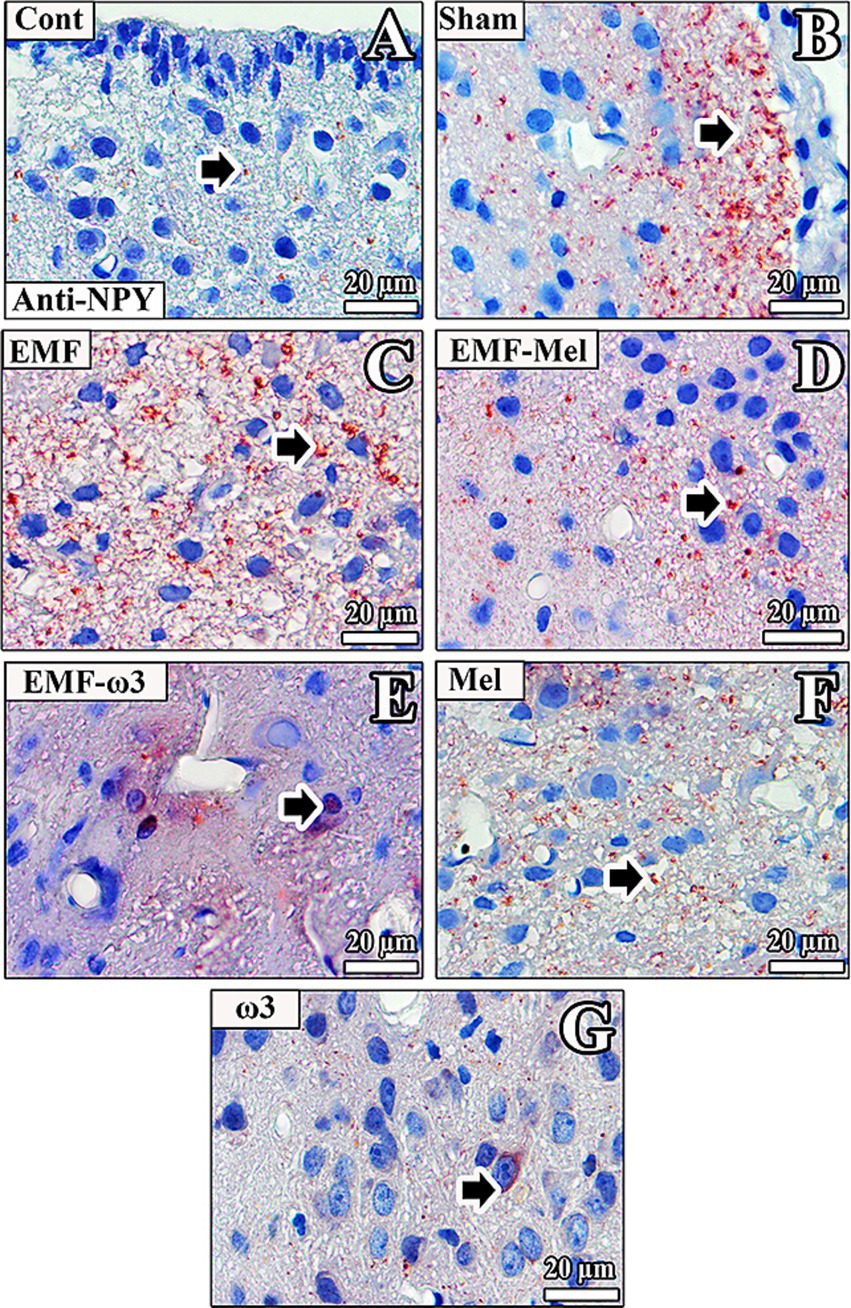
Figure 11. (A–G) Neurons in the hypothalamus and ependymal cells of the third ventricle of all groups are observed. Arrows indicate anti-NPY (+) staining. Counterstaining was performed in the sections using Mayer’s hematoxylin.
3.10 Anti-Ki67 immunolabelling
In the rats of the Cont group, anti-Ki67 (+) staining was observed to be incredibly intense in the third ventricle wall and regions close to the ventricle wall. Anti-Ki67 (+) staining was observed in the ependymal cell nucleus and the microvilli on the apical surface. In addition, there was anti-Ki67 (+) staining in some neurons in the ARN and VMN. In the Sham group rats, anti-Ki67 (+) staining was not observed in the neurons of the ARN, VMN, and DMN regions. A low-intensity anti-Ki67 (+) staining was observed in the neurons, neuroglia cells, and ependymal cells in the third ventricle wall of the EMF group. In the EMF-Mel group, no anti-Ki67 staining was observed in the intercellular medium. However, low-intensity positive staining was observed in the intercellular medium but not in the cells. Anti-Ki67 (+) staining was not observed in the ependymal cells. Similarly, there was no anti-Ki67 (+) staining in the nuclei of neurons belonging to the EMF-ω3 group, whereas high-density staining was observed in the intercellular space. Positive staining was observed in the nuclei of some ependymal cells in the third ventricle wall and the low-density microvilli of the cells. Anti-Ki67 (+) staining was noticeable in the third ventricle wall of the Mel group. High staining levels were observed, especially in the microvilli of ependymal cells.
On the other hand, no staining was observed in neurons in the ARN, VMN, and DMN regions of the hypothalamus. In the ω3 group, anti-Ki67 (+) staining was observed in the cytoplasm of ependymal cells in the third ventricle wall. At the same time, it was noticed that the nuclei of very few ependymal cells were positively stained (Figure 12).
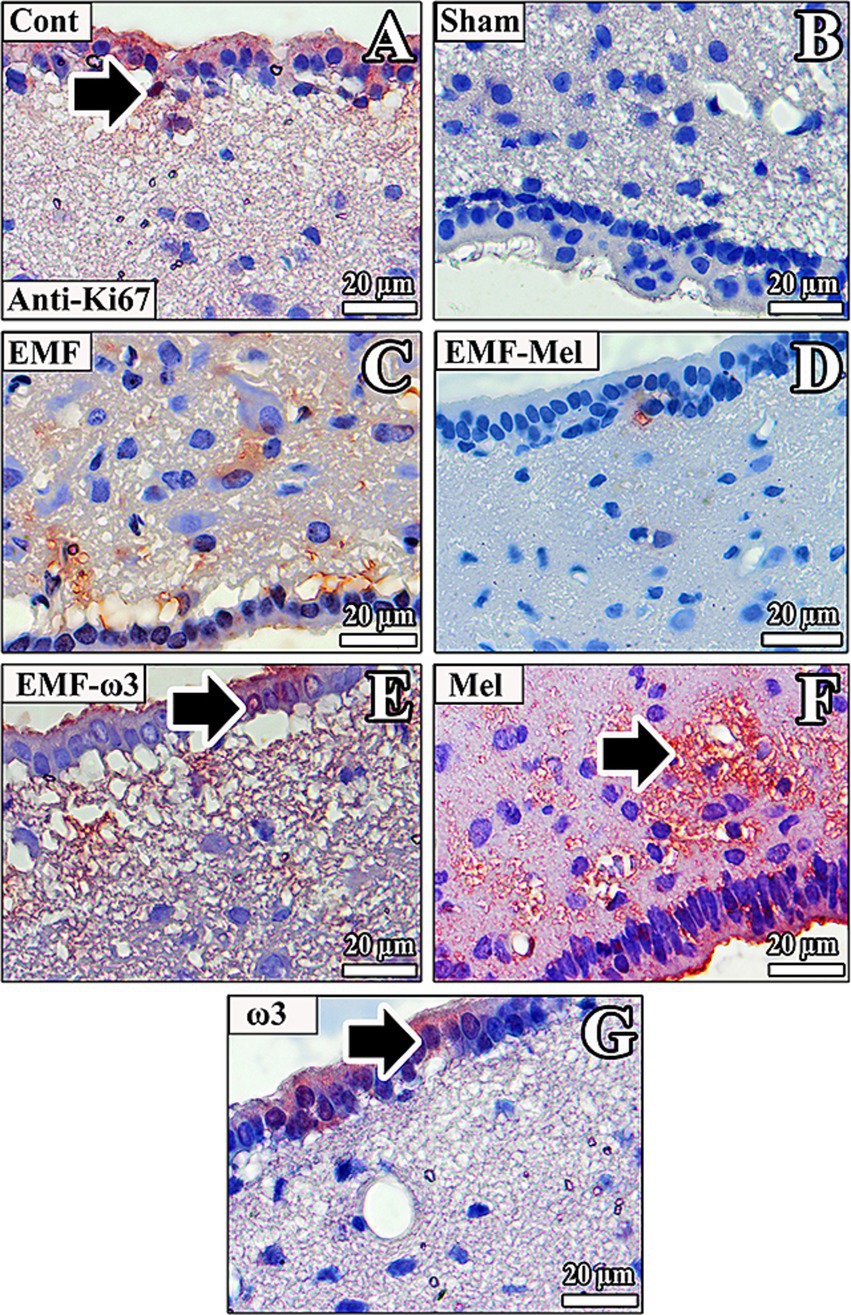
Figure 12. (A–G) Neurons in the hypothalamus and ependymal cells of the third ventricle of all groups are observed. Anti-Ki67 (+) staining is indicated by arrows. Counterstaining was performed in the sections using Mayer’s hematoxylin.
4 Discussion
4.1 Expression of the obesity-related genes
In the current study, the hypothalamic effects of EMF emitted from mobile phones were examined in terms of whether they trigger obesity predisposition. The fact that EMF increases the expression of FTO and NPY in the hypothalamus may be evidence that it increases the risk of obesity in infant rats. Considering the open field and elevated plus maze test findings, it can be suggested that EMF may cause stress-induced anxiety. However, more comprehensive studies are needed on this subject. When FTO expression was examined, it was observed that EMF increased expression in the hypothalamus. When EMF was considered a risk factor in hypothalamic obesity predisposition, it was observed that the body weights of offspring in the EMF group decreased compared to the Cont group. Similarly, a decrease in body weight in offspring rats in the EMF group is consistent with that of Dast Amooz et al. (59). They also suggested low brain-derived neurotrophic factor (BDNF) levels were observed after 2,400 MHz EMF exposure in the prenatal period. Long-term epidemiological studies are needed to determine the effect of EMF on hypothalamic obesity.
4.2 Association of body weight with obesity-related proteins and genes
When the body weight measurements of mother and offspring rats were evaluated, no difference was observed in the body weight measurements of mother rats belonging to the EMF-ω3 and ω3 groups before pregnancy. However, a decrease in body weight was observed in the EMF-ω3 group compared to the Cont group in the offspring rats. It is seen that Mel and ω3 applied after EMF exposure were not effective in the offspring rats. On the other hand, when only Mel was applied due to the increase in FTO expression, a decrease in the body weight of the offspring rats compared to the Cont group was found, while no difference was observed in the ω3 group compared to the Cont group. It is suggested that ω3 fatty acids do not cause an increase in body weight; it is thought that they may influence body weight due to the calories in the fat (60). The effects of EMF on FTO and NPY expression were observed to be inhibited by Mel and ω3 application. Regarding the effects of mobile phones on FTO, a comprehensive investigation is necessary on the effects of EMF at different frequencies and exposure times on the expression of FTO, the obesity-prone gene.
4.3 Assessing the anxiety-related behavioral tests
The increase in the number of defecations in the EMF group in the open plus maze test, the decrease in the number of free standing, as well as the increasing in the number of times in the EMF group entered the dark area and the duration of their stay in the dark area in the elevated plus maze test indicate that prenatal exposure to 900 MHz EMF triggers anxiety in the offspring rats. The increase in the duration of stay in the open area in the Sham group compared to the EMF group provides evidence that the anxiety is not only due to stress. A recent study observed anxiety-like behaviors in rats exposed to ELF-EMF before birth (61).
4.4 Stereological estimations and mitotic activity
When the stereological data obtained from the study are evaluated, a decrease in the number of neurons in the ARN after EMF exposure is quite evident compared to the Sham group. Therefore, the EMF effect induced by stress in the behavioral model can be considered. Still, it can be said that the neurodegenerative effect is only due to EMF exposure. The fact that no difference was observed between the Sham and EMF groups in some behavioral test parameters can be associated with the insufficient number of animals in the behavioral tests. With an increase in sample size, the anxiety-triggering effects of EMF can be observed more clearly. The neurodegenerative effect of EMF is more severe in the presence of stress. In addition to the neurodegenerative effect, a low-density anti-Ki67 expression in the Sham, EMF, and EMF-Mel groups compared to the Cont group is also noteworthy. In this context, our study investigated the effect of prenatal EMF exposure in the hypothalamic regions with immunohistochemical markers. Ki67 immunoreactivity was observed prominently in the Cont group, especially in the third ventricle wall and in neurons and neuroglia cells close to the ventricle in the hypothalamus. In the EMF group, Ki67 immunoreactivity was observed prominently in some ependymal cells in the third ventricle wall, compared to the Sham group. It was noticed that Ki67 expression was lower in the EMF group compared to the Cont group. This may be related to the neurodegeneration and mitotic activity-suppressing effect of EMF. The images in the Sham group may be evidence that maternal stress also suppresses mitotic activity in the brain of young rats.
4.5 Oxidative stress effects on serum samples
It was observed that 900 MHz EMF exposure increased the level of the CAT enzyme, one of the oxidative stress parameters. When the CAT enzyme level of the EMF exposure group was compared to the control and Sham groups, the level of CAT enzyme increased. In the current study, it was remarkable that the CAT enzyme activity was very high in eliminating increased superoxide radicals in the EMF group. However, the lack of any difference between the SOD enzyme level in the EMF group and the Cont group may be related to the EMF exposure dose and duration of application. It was observed that Mel administered before EMF exposure caused a decrease in CAT enzyme activity. In addition, the absence of any difference between the CAT enzyme activity in the EMF-Mel group and the Cont group shows a positive effect of Mel on CAT enzyme activity. Mel plays an active role in protecting the cell from oxidative stress. However, when our biochemical results were evaluated in terms of superoxide dismutase enzyme activity, it was noticed that there was a difference between the Mel and EMF-Mel groups. It was noticed that there was a low SOD activity in the Mel group. The fact that there was no difference between the Mel and ω3 groups and the Cont group regarding CAT enzyme activity shows that these agents do not cause toxic effects when applied alone. It was observed that the oxidative stress that occurred after EMF exposure did not change with the application of ω3. When the group where ω3 was applied alone was examined, it was seen that there was a significant increase in the SOD enzyme activity in the ω3 group compared to the other groups.
A recent study found that there was a decrease in body weight gain in juvenile rats exposed to RF-EMF in 5G technology. It has been suggested that this effect is insignificant in adult rats exposed to RF-EMF. The decrease in body weight observed in EMF-exposed juvenile rats in our study is consistent with that of Krivova et al. (62). It has been suggested that the RF-EMF exposure in early life has important effects on brain programming and control of eating behavior and body weight (63). The disorder in NPY expression is one of the neurochemical similarities between obesity and depression. NPY-expressing cells were increased after six hours of stress exposure and returned to control levels after 24 h. The increase in the number of NPY-expressing neurons in the ARN was evaluated to be glucocorticoid-induced (64, 65). In addition to evaluating NPY’s excessive hypothalamic expression resulting from EMF at the level of stress response, it is conceivable that it may cause obesity in later life stages due to its orexigenic effects. Overexpression of NPY may lead to obesity. It has previously been suggested that obesity is not caused by hyperphagia, but that the lipogenic effects of NPY play a role in the obesity process (66). In the current study, it can be suggested that EMF triggers anxiety and increases NPY expression, which may trigger a tendency to obesity in male offspring rats. There are not enough studies on the direct effects of EMF on NPY protein. New studies are needed to see the effects of EMF on NPY at the molecular level and elucidate this mechanism.
Accordingly, considering the sensitivity of the fetus to prenatal effects, it is critical to investigate in detail the effects of EMF exposure during the prenatal period on the development of the central nervous system. Another study examining the relationship between prenatal EMF exposure and obesity provided evidence that RF-EMF during embryogenesis may adversely affect molecular mechanisms related to adipogenesis and insulin resistance in zebrafish (67). Future studies are needed to show whether the effects of EMF exposure during the embryonic and prenatal periods may lead to obesity in postnatal life. In an epidemiological study, it was reported that prenatal exposure to high-dose EMF emitted from mobile phones increases the risk of childhood obesity. The growth patterns of children exposed to EMF during pregnancy were investigated (68). Since pregnancy is a highly sensitive period to environmental effects, prenatal EMF exposure can trigger childhood obesity by damaging the development of metabolic and endocrine systems (68, 69). In addition to the hormonal and metabolic factors underlying persistent obesity observed in childhood, it should not be ignored that morphological changes occurring in hypothalamic neurons may increase the risk of predisposition to obesity.
There is insufficient information on the possible effects of EMF on the hypothalamus (23, 70, 71). Any change in neurotransmission observed in the hypothalamic region also significantly affects neurological functions. In this context, 835 MHz EMF exposure affects the energy balance of the hypothalamus and food intake. It has been reported that 835 MHz EMF at 4 W/kg SAR for five hours daily reduces hypothalamic neurotransmission (70). It is suggested that the autophagy response in the cell after 12 weeks of EMF exposure shows a neuroprotective function by preventing neuronal cell death. It is stated that autophagy and apoptosis can coincide in the cell due to their different sensitivity thresholds (71). In our study, an increase in the number of darkly stained neurons in the EMF group was observed in the hypothalamic region. It is thought that these neurons are involved in the apoptosis process. Compared to the Sham group, a higher number of degenerated neurons in the EMF group may indicate the adverse effects of EMF exposure in the hypothalamus that was exposed to EMF during the prenatal period. Apoptosis in hypothalamic neurons plays an essential role in developing and maintaining obesity. It is known that neurogenesis has a restorative effect in the hypothalamic region as well as in the hippocampal region (72). In this context, we think that ω3 protects against the apoptotic effects of EMF exposure. It was observed that the protective effect of ω3 was more pronounced in hippocampal neurons than in Mel’s neuroprotective effects.
The apoptotic effect in the hypothalamus after EMF exposure can lead to obesity. In this context, the neuroprotective effect of ω3 in the hypothalamus can be considered in terms of preventing obesity. On the other hand, observing hypothalamic Ki67 immunoreactivity, especially in the ependymal cells in the hypothalamus and the subependymal region, suggests the neurogenesis-inducing effect of ω3. In a previous study, the subependymal region has been reported to be a niche area in neural stem cell proliferation (73). It can be suggested that ω3 can be used as a therapeutic agent against hypothalamic obesity. Similarly, when ω3 was applied alone, it was observed to inhibit NPY expression in the hypothalamus.
In addition to neurogenesis observed in the subventricular and subgranular regions of the dentate gyrus in the brain, the subependymal niche in the hypothalamus is considered a neurogenic region (73, 74). Additionally, the stress caused by EMF exposure may inhibit mitotic activity. Mel application induces the proliferation of neural stem cells, stimulating the differentiation of stem cells into mature neurons by providing neural differentiation (75). An intense Ki67 immunoreactivity in ependymal cells in the third ventricle wall of the hypothalamus in the Mel group may be due to neurogenesis activity in the subependymal region. However, intense mitotic activity was not observed in the hypothalamic regions in the EMF-Mel group.
5 Conclusion
In conclusion, in addition to the effects at the protein level, morphological changes in the cell’s ultrastructure should also be considered for the predisposition to obesity. Our study found neurodegenerative effects in hypothalamic nuclei following EMF exposure. When stereological data were considered, it was observed that the number of neurons decreased because of EMF exposure, especially in the ARN of the hypothalamus. The degenerative effect of EMF exposure was observed in the ARN.
There is no study whose results are supported by qualitative and quantitative evaluations on the effects of EMF exposure during the prenatal period. Studies are needed to examine protein values at the tissue level after EMF exposure and explain the underlying mechanisms. Prenatal exposure to EMF may create a risk of predisposition to obesity. More studies are needed on the protective and healing effects of Mel and ω3 on the prenatally EMF-exposed subjects.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Local Ethics Committee for Animal Experiments of Ondokuz Mayıs University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. SK: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was funded by project number 119S115 of the Turkish Scientific and Technical Research Council (TUBITAK).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jalilian, H, Eeftens, M, Ziaei, M, and Roosli, M. Public exposure to radiofrequency electromagnetic fields in everyday microenvironments: An updated systematic review for Europe. Environ Res. (2019) 176:108517. doi: 10.1016/j.envres.2019.05.048
2. Meo, SA, Almahmoud, M, Alsultan, Q, Alotaibi, N, Alnajashi, I, and Hajjar, WM. Mobile phone base station tower settings adjacent to school buildings: impact on students' cognitive health. Am J Mens Health. (2019) 13:1557988318816914. doi: 10.1177/1557988318816914
3. Ajmal, A, Yamazaki, K, Tamura, N, Bamai, YA, Yoshikawa, T, Hikage, T, et al. Link between Wi-fi, cordless devices, mobile phone usage patterns, and behavioral problems among Japanese children: a prospective cohort study. Environ Res. (2024) 261:119715. doi: 10.1016/j.envres.2024.119715
4. Kaplan, S, Deniz, OG, Onger, ME, Turkmen, AP, Yurt, KK, Aydin, I, et al. Electromagnetic field and brain development. J Chem Neuroanat. (2016) 75:52–61. doi: 10.1016/j.jchemneu.2015.11.005
5. Warille, AA, Onger, ME, Turkmen, AP, Deniz, OG, Altun, G, Yurt, KK, et al. Controversies on electromagnetic field exposure and the nervous systems of children. Histol Histopathol. (2016) 31:461–8. doi: 10.14670/HH-11-707
6. Zagar, T, Valic, B, Kotnik, T, Korat, S, Tomsic, S, Zadnik, V, et al. Estimating exposure to extremely low frequency magnetic fields near high-voltage power lines and assessment of possible increased cancer risk among Slovenian children and adolescents. Radiol Oncol. (2023) 57:59–69. doi: 10.2478/raon-2023-0002
7. Hardell, L, and Sage, C. Biological effects from electromagnetic field exposure and public exposure standards. Biomed Pharmacother. (2008) 62:104–9. doi: 10.1016/j.biopha.2007.12.004
8. Odaci, E, Bas, O, and Kaplan, S. Effects of prenatal exposure to a 900 MHz electromagnetic field on the dentate gyrus of rats: a stereological and histopathological study. Brain Res. (2008) 1238:224–9. doi: 10.1016/j.brainres.2008.08.013
9. Odaci, E, Hanci, H, Ikinci, A, Sonmez, OFAslan, A, Sahin, A, et al. Maternal exposure to a continuous 900-MHz electromagnetic field provokes neuronal loss and pathological changes in cerebellum of 32-day-old female rat offspring. J Chem Neuroanat. (2016) 75:105–10. doi: 10.1016/j.jchemneu.2015.09.002
10. Nirwane, A, Sridhar, V, and Majumdar, A. Neurobehavioural changes and brain oxidative stress induced by acute exposure to GSM900 mobile phone radiations in zebrafish (Danio rerio). Toxicol Res. (2016) 32:123–32. doi: 10.5487/Tr.2016.32.2.123
11. Erdem Koc, G, Kaplan, S, Altun, G, Gumus, H, Gulsum Deniz, O, Aydin, I, et al. Neuroprotective effects of melatonin and omega-3 on hippocampal cells prenatally exposed to 900 MHz electromagnetic fields. Int J Radiat Biol. (2016) 92:590–5. doi: 10.1080/09553002.2016.1206223
12. Foerster, M, Thielens, A, Joseph, W, Eeftens, M, and Roosli, M. A prospective cohort study of adolescents' memory performance and individual brain dose of microwave radiation from wireless communication. Environ Health Perspect. (2018) 126:077007. doi: 10.1289/EHP2427
13. Bontempi, B, Lévêque, P, Dubreuil, D, Jay, TM, and Edeline, JM. Effects of head-only exposure to 900 MHz GSM electromagnetic fields in rats: changes in neuronal activity as revealed by c-fos imaging without concomitant cognitive impairments. Biomedicines. (2024) 12:1954. doi: 10.3390/biomedicines12091954
14. Lee, TM, Ho, SM, Tsang, LY, Yang, SH, Li, LS, Chan, CC, et al. Effect on human attention of exposure to the electromagnetic field emitted by mobile phones. Neuroreport. (2001) 12:729–31. doi: 10.1097/00001756-200103260-00023
15. Krause, CM, Bjornberg, CH, Pesonen, M, Hulten, A, Liesivuori, T, Koivisto, M, et al. Mobile phone effects on children's event-related oscillatory EEG during an auditory memory task. Int J Radiat Biol. (2006) 82:443–50. doi: 10.1080/09553000600840922
16. Aldad, TS, Gan, G, Gao, XB, and Taylor, HS. Fetal radiofrequency radiation exposure from 800-1900 mhz-rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep. (2012) 2:312. doi: 10.1038/srep00312
17. Kwon, MS, Vorobyev, V, Kännälä, S, Laine, M, Rinne, JO, Toivonen, T, et al. GSM mobile phone radiation suppresses brain glucose metabolism. J Cereb Blood Flow Metab. (2011) 31:2293–301. doi: 10.1038/jcbfm.2011.128
18. Wardzinski, EK, Jauch-Chara, K, Haars, S, Melchert, UH, Scholand-Engler, HG, and Oltmanns, KM. Mobile phone radiation deflects brain energy homeostasis and prompts human food ingestion. Nutrients. (2022) 14:339. doi: 10.3390/nu14020339
19. Meral, I, Mert, H, Mert, N, Deger, Y, Yoruk, I, Yetkin, A, et al. Effects of 900-MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. Brain Res. (2007) 1169:120–4. doi: 10.1016/j.brainres.2007.07.015
20. Furtado-Filho, OV, Borba, JB, Maraschin, T, Souza, LM, Henriques, JA, Moreira, JC, et al. Effects of chronic exposure to 950 MHz ultra-high-frequency electromagnetic radiation on reactive oxygen species metabolism in the right and left cerebral cortex of young rats of different ages. Int J Radiat Biol. (2015) 91:891–7. doi: 10.3109/09553002.2015.1083629
21. Volkow, ND, Tomasi, D, Wang, GJ, Vaska, P, Fowler, JS, Telang, F, et al. Effects of cell phone radiofrequency signal exposure on brain glucose metabolism. JAMA. (2011) 305:808–13. doi: 10.1001/jama.2011.186
22. Son, Y, Kim, JS, Jeong, YJ, Jeong, YK, Kwon, JH, Choi, HD, et al. Long-term RF exposure on behavior and cerebral glucose metabolism in 5xFAD mice. Neurosci Lett. (2018) 666:64–9. doi: 10.1016/j.neulet.2017.12.042
23. Sangun, O, Dundar, B, Comlekci, S, and Buyukgebiz, A. The effects of electromagnetic field on the endocrine system in children and adolescents. Pediatr Endocrinol Rev. (2015) 13:531–45.
24. Sangun, O, Dundar, B, Darici, H, Comlekci, S, Doguc, DK, and Celik, S. The effects of long-term exposure to a 2450 MHz electromagnetic field on growth and pubertal development in female Wistar rats. Electromagn Biol Med. (2015) 34:63–71. doi: 10.3109/15368378.2013.871619
25. Swinburn, BA, Sacks, G, Hall, KD, McPherson, K, Finegood, DT, Moodie, ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. (2011) 378:804–14. doi: 10.1016/S0140-6736(11)60813-1
26. Collaborators GBDRF. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study (2019). Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
27. Williams, RH, Alexopoulos, H, Jensen, LT, Fugger, L, and Burdakov, D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci USA. (2008) 105:11975–80. doi: 10.1073/pnas.0802687105
28. Tripathi, R, Banerjee, SK, Nirala, JP, and Mathur, R. Simultaneous exposure to electromagnetic field from mobile phone and unimpeded fructose drinking during pre-, peri-, and post-pubertal stages perturbs the hypothalamic and hepatic regulation of energy homeostasis by early adulthood: experimental evidence. Environ Sci Pollut Res Int. (2022) 29:7438–51. doi: 10.1007/s11356-021-15841-y
29. Bektas, H, Algul, S, Altindag, F, Yegin, K, Akdag, MZ, and Dasdag, S. Effects of 3.5 GHz radiofrequency radiation on ghrelin, nesfatin-1, and irisin level in diabetic and healthy brains. J Chem Neuroanat. (2022) 126:102168. doi: 10.1016/j.jchemneu.2022.102168
30. Hardell, L. Effects of Mobile phones on children's and adolescents' health: a commentary. Child Dev. (2018) 89:137–40. doi: 10.1111/cdev.12831
31. Gandhi, OP, Morgan, LL, de Salles, AA, Han, YY, Herberman, RB, and Davis, DL. Exposure limits: the underestimation of absorbed cell phone radiation, especially in children. Electromagn Biol Med. (2012) 31:34–51. doi: 10.3109/15368378.2011.622827
32. Consales, C, Merla, C, Marino, C, and Benassi, B. Electromagnetic fields, oxidative stress, and neurodegeneration. Int J Cell Biol. (2012) 2012:683897. doi: 10.1155/2012/683897
33. Irmak, MK, Fadillioglu, E, Gulec, M, Erdogan, H, Yagmurca, M, and Akyol, O. Effects of electromagnetic radiation from a cellular telephone on the oxidant and antioxidant levels in rabbits. Cell Biochem Funct. (2002) 20:279–83. doi: 10.1002/cbf.976
34. Xu, S, Zhou, Z, Zhang, L, Yu, Z, Zhang, W, Wang, Y, et al. Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons. Brain Res. (2010) 1311:189–96. doi: 10.1016/j.brainres.2009.10.062
35. von Niederhäusern, N, Ducray, A, Zielinski, J, Murbach, M, and Mevissen, M. Effects of radiofrequency electromagnetic field exposure on neuronal differentiation and mitochondrial function in SH-SY5Y cells. Toxicol In Vitro. (2019) 61:104609. doi: 10.1016/j.tiv.2019.104609
36. Luukkonen, J, Hakulinen, P, Maki-Paakkanen, J, Juutilainen, J, and Naarala, J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat Res. (2009) 662:54–8. doi: 10.1016/j.mrfmmm.2008.12.005
37. Ertilav, K, Uslusoy, F, Ataizi, S, and Naziroglu, M. Long term exposure to cell phone frequencies (900 and 1800 MHz) induces apoptosis, mitochondrial oxidative stress and TRPV1 channel activation in the hippocampus and dorsal root ganglion of rats. Metab Brain Dis. (2018) 33:753–63. doi: 10.1007/s11011-017-0180-4
38. Schon, EA, and Manfredi, G. Neuronal degeneration and mitochondrial dysfunction. J Clin Invest. (2003) 111:303–12. doi: 10.1172/JCI17741
39. Jiang, DP, Li, JH, Zhang, J, Xu, SL, Kuang, F, Lang, HY, et al. Long-term electromagnetic pulse exposure induces Abeta deposition and cognitive dysfunction through oxidative stress and overexpression of APP and BACE1. Brain Res. (2016) 1642:10–9. doi: 10.1016/j.brainres.2016.02.053
40. Sideratou, T, Atkinson, F, Campbell, GJ, Petocz, P, Bell-Anderson, KS, and Brand-Miller, J. Glycaemic index of maternal dietary carbohydrate differentially alters Fto and Lep expression in offspring in C57BL/6 mice. Nutrients. (2018) 10:1342. doi: 10.3390/nu10101342
41. Vohra, MS, Benchoula, K, Serpell, CJ, and Hwa, WE. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur J Pharmacol. (2022) 915:174611. doi: 10.1016/j.ejphar.2021.174611
42. Carboni, L, El Khoury, A, Beiderbeck, DI, Neumann, ID, and Mathé, AA. Neuropeptide Y, calcitonin gene-related peptide, and neurokinin a in brain regions of HAB rats correlate with anxiety-like behaviours. Eur Neuropsychopharmacol. (2022) 57:1–14. doi: 10.1016/j.euroneuro.2021.12.011
43. Díaz, E, Debeljuk, L, Arce, A, Esquifino, A, and Díaz, B. Prenatal melatonin exposure affects luteinizing hormone and hypothalamic and striatal neuropeptide Y in the male rat offspring. Neurosci Lett. (2000) 292:143–6. doi: 10.1016/s0304-3940(00)01425-7
44. Suriagandhi, V, and Nachiappan, V. Protective effects of melatonin against obesity-induced by leptin resistance. Behav Brain Res. (2022) 417:113598. doi: 10.1016/j.bbr.2021.113598
45. Roy, J, Larroquet, L, Surget, A, Lanuque, A, Sandres, F, Terrier, F, et al. Impact on cerebral function in rainbow trout fed with plant based omega-3 long chain polyunsaturated fatty acids enriched with DHA and EPA. Fish Shellfish Immunol. (2020) 103:409–20. doi: 10.1016/j.fsi.2020.05.044
46. Mausset-Bonnefont, AL, Hirbec, H, Bonnefont, X, Privat, A, Vignon, J, and de Sèze, R. Acute exposure to GSM 900-MHz electromagnetic fields induces glial reactivity and biochemical modifications in the rat brain. Neurobiol Dis. (2004) 17:445–54. doi: 10.1016/j.nbd.2004.07.004
47. Migdal, P, Bieńkowski, P, Cebrat, M, Berbeć, E, Plotnik, M, Murawska, A, et al. Exposure to a 900 MHz electromagnetic field induces a response of the honey bee organism on the level of enzyme activity and the expression of stress-related genes. PLoS One. (2023) 18:e0285522. doi: 10.1371/journal.pone.0285522
48. Simo, KA, Tsirline, VB, Sindram, D, McMillan, MT, Thompson, KJ, Swan, RZ, et al. Microwave ablation using 915-MHz and 2.45-GHz systems: what are the differences? HPB (Oxf). (2013) 15:991–6. doi: 10.1111/hpb.12081
49. Deniz, ÖG, and Kaplan, S. The effects of different herbals on the rat hippocampus exposed to electromagnetic field for one hour during the prenatal period. J Chem Neuroanat. (2022) 119:102043. doi: 10.1016/j.jchemneu.2021.102043
50. Elamin, AAE, Deniz, OG, and Kaplan, S. The effects of gum Arabic, curcumin (Curcuma longa) and on the rat hippocampus after electromagnetic field exposure: a stereological and histological study. J Chem Neuroanat. (2022) 120:102060. doi: 10.1016/j.jchemneu.2021.102060
51. Mohamed, H, Deniz, OG, and Kaplan, S. The neuroprotective effects of baobab and black seed on the rat hippocampus exposed to a 900-MHz electromagnetic field. J Chem Neuroanat. (2024) 137:102405. doi: 10.1016/j.jchemneu.2024.102405
52. Kuniishi, H, Ichisaka, S, Yamamoto, M, Ikubo, N, Matsuda, S, Futora, E, et al. Early deprivation increases high-leaning behavior, a novel anxiety-like behavior, in the open field test in rats. Neurosci Res. (2017) 123:27–35. doi: 10.1016/j.neures.2017.04.012
53. Phillips, KM. Effects of time and administration of ethanol on open field behavior in hamsters. Physiol Behav. (1982) 29:785–7. doi: 10.1016/0031-9384(82)90325-0
54. Prut, L, and Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. (2003) 463:3–33. doi: 10.1016/s0014-2999(03)01272-x
55. Moragrega, I, Carrasco, MC, Vicens, P, and Redolat, R. Spatial learning in male mice with different levels of aggressiveness: effects of housing conditions and nicotine administration. Behav Brain Res. (2003) 147:1–8. doi: 10.1016/s0166-4328(03)00112-8
56. Mayhew, TM, and Gundersen, HJ. If you assume, you can make an ass out of u and me': a decade of the disector for stereological counting of particles in 3D space. J Anat. (1996) 188:1–15.
57. Delibas, B, and Kaplan, S. The histomorphological and stereological assessment of rat dorsal root ganglion tissues after various types of sciatic nerve injury. Histochem Cell Biol. (2024) 161:145–63. doi: 10.1007/s00418-023-02242-0
58. Aktaş, A, Yiğit, F, Delibaş, B, Kaplan, AA, Hamour, HM, Marangoz, AH, et al. The effects of Garcinia kola and curcumin on the dorsal root ganglion of the diabetic rat after peripheral nerve transection injury. J Chem Neuroanat. (2024) 136:102395. doi: 10.1016/j.jchemneu.2024.102395
59. Dast Amooz, S, Broujeni, ST, and Sarahian, N. A primary study on rat fetal development and brain-derived neurotrophic factor levels under the control of electromagnetic fields. J Public Health Afr. (2023) 14:2347. doi: 10.4081/jphia.2023.2347
60. Lenox, CE, and Bauer, JE. Potential adverse effects of omega-3 fatty acids in dogs and cats. J Vet Intern Med. (2013) 27:217–26. doi: 10.1111/jvim.12033
61. Hosseini, E, and Kianifard, D. Effect of prenatal stress and extremely low-frequency electromagnetic fields on anxiety-like behavior in female rats: with an emphasis on prefrontal cortex and hippocampus. Brain Behav. (2023) 13:e2949. doi: 10.1002/brb3.2949
62. Krivova, NA, Kudabaeva, MS, Zaeva, OB, Borodina, SV, Lepekhina, TB, Pavlenko, OA, et al. The effect of exposure to RF-EMF from the laboratory simulator of 5G NR base station on physiological parameters and cognitive abilities of male wistar rats of different ages. Sci Rep. (2024) 14:10283. doi: 10.1038/s41598-024-60862-5
63. de Lima, RMS, Dos Santos Bento, LV, di Marcello Valladão Lugon, M, Barauna, VG, Bittencourt, AS, Dalmaz, C, et al. Early life stress and the programming of eating behavior and anxiety: sex-specific relationships with serotonergic activity and hypothalamic neuropeptides. Behav Brain Res. (2020) 379:112399. doi: 10.1016/j.bbr.2019.112399
64. Conrad, CD, and McEwen, BS. Acute stress increases neuropeptide Y mRNA within the arcuate nucleus and hilus of the dentate gyrus. Brain Res Mol Brain Res. (2000) 79:102–9. doi: 10.1016/s0169-328x(00)00105-4
65. Watanabe, Y, Akabayashi, A, and Mcewen, BS. Adrenal-steroid regulation of neuropeptide Y (NPY) messenger-RNA—differences between dentate hilus and locus-coeruleus and arcuate nucleus. Mol Brain Res. (1995) 28:135–40. doi: 10.1016/0169-328x(94)00192-H
66. Vähätalo, LH, Ruohonen, ST, Mäkelä, S, Kovalainen, M, Huotari, A, Mäkelä, KA, et al. Neuropeptide Y in the noradrenergic neurones induces obesity and inhibits sympathetic tone in mice. Acta Physiol Oxf. (2015) 213:902–19. doi: 10.1111/apha.12436
67. Koc, IY, Beler, M, Unal, I, Paker, S, Emekli-Alturfan, E, Alturfan, AA, et al. Investigating the effect of radiofrequency electromagnetic field exposure on molecular pathways related to insulin resistance and adipogenesis in zebrafish embryos—a pilot study without quantitative exposure metrics. Sci Total Environ. (2024) 954:176038. doi: 10.1016/j.scitotenv.2024.176038
68. Li, DK, Ferber, JR, Odouli, R, and Quesenberry, CP. A prospective study of in-utero exposure to magnetic fields and the risk of childhood obesity. Sci Rep. (2012) 2:540. doi: 10.1038/srep00540
69. Newbold, RR, Padilla-Banks, E, Snyder, RJ, and Jefferson, WN. Perinatal exposure to environmental estrogens and the development of obesity. Mol Nutr Food Res. (2007) 51:912–7. doi: 10.1002/mnfr.200600259
70. Kim, JH, Huh, YH, and Kim, HR. Trafficking of synaptic vesicles is changed at the hypothalamus by exposure to an 835 MHz radiofrequency electromagnetic field. Gen Physiol Biophys. (2019) 38:379–88. doi: 10.4149/gpb_2019020
71. Kim, JH, Huh, YH, and Kim, HR. Induction of autophagy in the striatum and hypothalamus of mice after 835 MHz radiofrequency exposure. PLoS One. (2016) 11:e0153308. doi: 10.1371/journal.pone.0153308
72. Nascimento, LF, Souza, GF, Morari, J, Barbosa, GO, Solon, C, Moura, RF, et al. N-3 fatty acids induce neurogenesis of predominantly POMC-expressing cells in the hypothalamus. Diabetes. (2016) 65:673–86. doi: 10.2337/db15-0008
73. Rojczyk-Golebiewska, E, Palasz, A, and Wiaderkiewicz, R. Hypothalamic subependymal niche: a novel site of the adult neurogenesis. Cell Mol Neurobiol. (2014) 34:631–42. doi: 10.1007/s10571-014-0058-5
74. He, Z, Cui, L, Paule, MG, and Ferguson, SA. Estrogen selectively mobilizes neural stem cells in the third ventricle stem cell niche of postnatal day 21 rats. Mol Neurobiol. (2015) 52:927–33. doi: 10.1007/s12035-015-9244-9
Keywords: electromagnetic field, childhood obesity, hypothalamic nuclei, melatonin, omega-3, optical fractionator
Citation: Altun G and Kaplan S (2025) Possible effects of melatonin and omega-3 on the obesity-related hypothalamic nuclei of the electromagnetic field-exposed offspring rats: a stereological and immunohistochemical analysis. Front. Public Health. 13:1583097. doi: 10.3389/fpubh.2025.1583097
Edited by:
Hugh Taylor, Yale University, United StatesReviewed by:
Xiao-Bing Gao, Yale University, United StatesRasha A. Al-Lami, Yale University, United States
Nimisha Gawde, Yale University, United States
Copyright © 2025 Altun and Kaplan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Süleyman Kaplan, c2thcGxhbm9tdUB5YWhvby5jb20=; c2thcGxhbkBvbXUuZWQudHI=
 Gamze Altun
Gamze Altun Süleyman Kaplan
Süleyman Kaplan