- 1Department of Medicine, University of Chicago, Chicago, IL, United States
- 2Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, United States
- 3Crown Family School of Social Work, Policy, and Practice, Department of Public Health Sciences, and Urban Health Lab, University of Chicago, Chicago, IL, United States
- 4Division of Community Health Sciences, School of Public Health, University of Illinois at Chicago, Chicago, IL, United States
- 5Chicago Center for HIV Elimination, Department of Medicine, University of Chicago, Chicago, IL, United States
Background: Achieving Equity in Patient Outcome Reporting for Timely Assessments of Life with HIV and Substance Use (ePORTAL HIV-S) is a research project funded by the National Institute for Drug Abuse to implement and evaluate multi-level interventions to decrease barriers to substance use screening and treatment for PLWH. At its center is a multidomain intervention addressing digital, sociocultural, and health care system environments, at individual, interpersonal, and community levels. ePORTAL HIV-S has four overall goals; this manuscript describes the protocol specifically for the randomized control trial (RCT) portion of the study. To provide additional context, we briefly describe the overall ePORTAL HIV-S project.
Methods: This project will utilize a culturally tailored approach to increase patient portal use among PLWH in our health system via a community health worker (CHW)-led initiative. This will lay the groundwork for the second aim, the focus of the current manuscript, RCT to measure the effectiveness of a population health, patient portal-based substance use screening program. Approximately 880 people will be enrolled and randomized 1:1 to intervention vs., control arms. Participants in the control arm will receive usual care (substance use screening during clinic visits), whereas the intervention arm will be invited to complete substance use screening via the patient portal as well as during clinic visits as per usual care. The primary outcome will be the percentage of people screened for substance use. ePORTAL will also implement a collaborative care model to both connect patients who screen positive for SUD to care and effectively treat PLWH. Finally, we will plan for dissemination of ePORTAL HIV-S to other sites that provide care for PLWH.
Discussion: SUD disproportionately impacts PLWH which leads to negative health outcomes. This novel approach will incorporate the privacy and convenience of patient portal screening with screening during routine clinic visits.
Clinical trial registration: clinicaltrials.gov, identifier NCT06682468.
Introduction
Substance use disorders (SUDs) and HIV are synergistic epidemics in the United States. Having a SUD is a known risk factor for the acquisition of HIV (1), and many people living with HIV (PLWH) belong to historically marginalized groups, experiencing an intersection of social stigma and structural barriers that may threaten mental health and contribute to the acquisition of SUD (2, 3). It is, therefore no surprise that SUD affects nearly half of PLWH (4–8), with accompanying high rates of polysubstance use disorders, significantly higher than the general population (4, 5, 9–13). While there is no significant difference in SUD prevalence in White or Black populations, Black populations are less likely to receive treatment than White populations (19% vs. 24%) (14). Inadequate healthcare access, stigma, and criminalization related to structural racism exacerbate disparities in HIV and SUD outcomes (15, 16).
Despite its high prevalence and adverse effect on health outcomes, SUDs are both underdiagnosed and undertreated among PLWH, especially Black PLWH. Globally, only half of HIV care and treatment sites routinely screen for SUDs and refer to substance use treatment. Rates of SUD are increasing (17), and up to three-quarters of PLWH meeting SUD criteria may not receive treatment (18). In areas with high proportion of Black residents, there were lower rates of treatment centers in the community, creating barriers to SUD treatment initiation and completion rates (18–20).
Furthermore, the current standard procedure for SUD screening relies on PLWH attending scheduled HIV clinic visits, where they are screened in the waiting area or an exam room (21, 22). Inherently, this approach poses two key structural barriers to screening. First, PLWH who have comorbid SUDs are less likely to attend clinic appointments (23–27). Second, SUD screening is usually performed by a provider during clinic visits, which may have lower validity when compared to self-reported questionnaires, particularly among racial, ethnic, and/or sexual and gender minorities (28, 29). Stigma related to substance use among PLWH may decrease disclosure of substance use during clinic visits (30, 31). Therefore, the current strategy for diagnosing SUD in PWLH is limited because it only reaches patients who both attend clinic visits and willingly disclose symptoms, disproportionately missing PLWH with the epidemiologically highest likelihood of requiring treatment for SUD at baseline.
A potential alternative that may alleviate these barriers is screening for SUD outside of clinic visits, which can be accomplished using electronic patient portals. Patient portals are secure websites, or web-based applications, that give patients access to their health information from anywhere with a web connection (32). Portals can be enabled to send questionnaires to patients to complete before clinic appointments, or even when no appointments are scheduled as part of a population health approach to screening. Moreover, portals have become increasingly available globally. Access to patient portals varies by country, with healthcare systems in developed countries often providing greater levels of access (33–36). 90% of health care systems in the United States offer online portal access to patients (37, 38). PLWH and those with SUD have high levels of access to the internet and high interest in using portals (39–41). There is also evidence that PLWH may be more likely to disclose substance use when screened using technology vs. through interviews (42). In our preliminary work, we established a system for population health to perform portal-based assessments for depression. In the primary care setting, we demonstrated higher rates of depression screening and identification of patients with moderate–severe depression when screening was performed using patient portals (43). HIV clinicians and patients have shown interest in using patient portals (39, 40, 44, 45). Additionally, patients who used patient portals have fewer no-show appointments, greater satisfaction in their care, and better engagement in care (46–52). One cross-sectional study found that patient portal utilization was associated with 80% greater odds of antiretroviral treatment adherence (53).
Overview of ePORTAL HIV-S
In this manuscript, we describe the protocol for a randomized controlled trial (RCT) component of a NIDA-funded project—the Achieving Equity in Patient Outcome Reporting for Timely Assessments of Life with HIV and Substance Use (ePORTAL HIV-S) study (1R01DA058965). The study seeks to achieve health equity in SUD screening and treatment among Black adults living with HIV by implementing interventions to decrease barriers to screening and treatment within a large, real-world medical home. First, we will develop a culturally tailored approach to increase patient portal use among PLWH in our health system via a community health worker (CHW)-led initiative. The CHW will train participants how to use the portal to access appointments, health histories, and test results, how to send messages, and how to respond to questionnaires. The RCT will determine the effectiveness of population health (portal+clinic visit) vs. usual (clinic visit) SUD screening among PLWH in an HIV clinic setting. Additionally, we implement a collaborative care model to connect patients who screen positive for SUD to care and effectively treat for SUD. Finally, we will disseminate ePORTAL HIV-S to other sites that provide care for PLWH. In this manuscript we focus on describing the intervention protocol for the randomized control trial (RCT) to determine the effectiveness of population health vs. usual SUD screening among PLWH in an HIV clinic. The RCT protocol paper adheres to SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) (See Supplemental material) (54). Results and protocols for the CHW-led initiative and collaborative care model are beyond the scope of the current manuscript and will be reported separately.
Methods and analysis
Overview
Study design
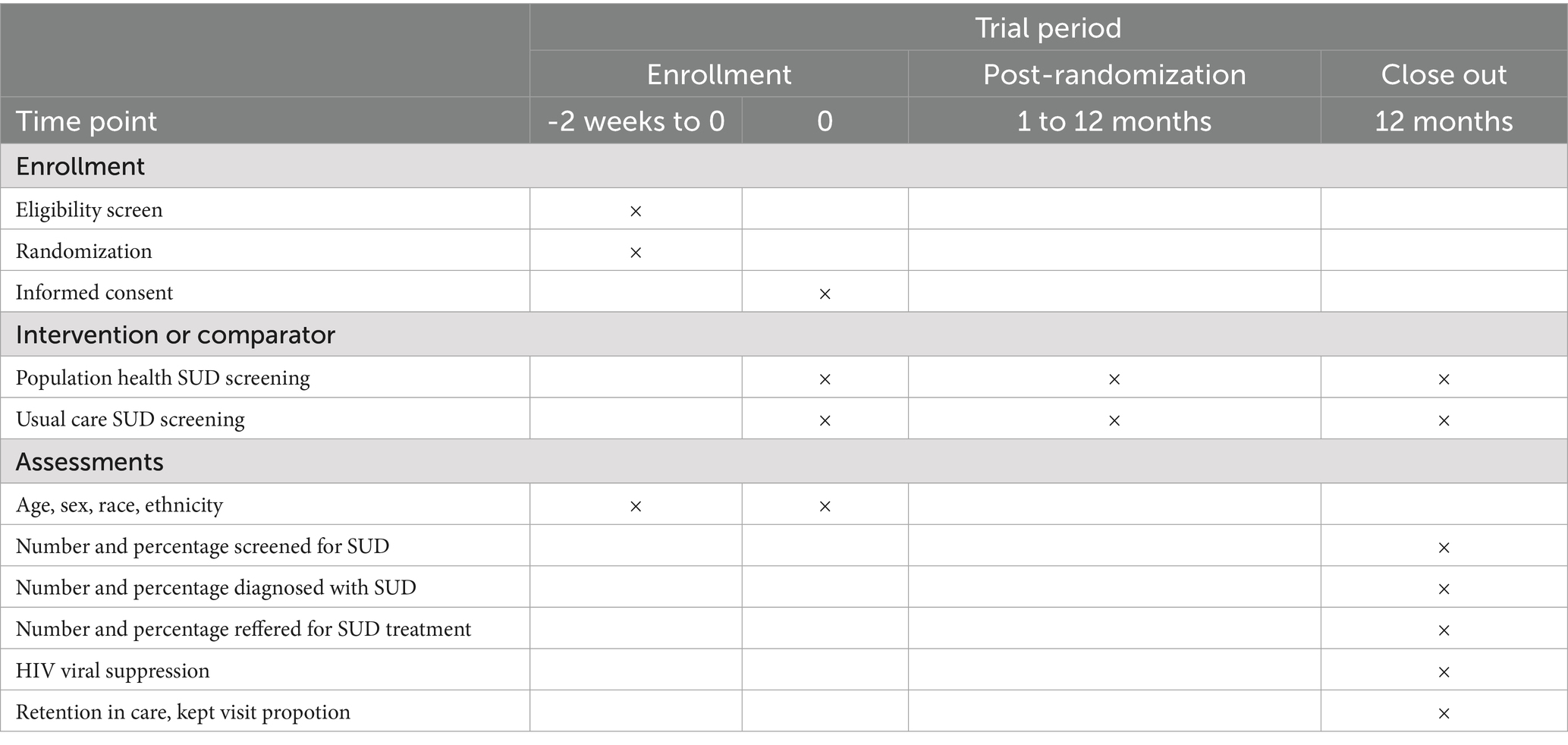
We propose an RCT, to determine whether population health, patient portal-based SUD screening will increase screening rates and identification of SUD in PLWH. We will randomize our cohort into two arms: the control arm, who will receive usual care (SUD screening during clinic visits), and the intervention arm, who will be invited to complete SUD screening via the patient portal as well as during clinic visits as per usual care. Figure 1 shows the SUD screening process for the intervention arm vs. control arm. The RCT will be a one-year study, which allows sufficient time for the usual care arm to have an HIV care clinic visit and be screened for a substance use disorder. Patients will be randomized 1:1 in groups of 100.
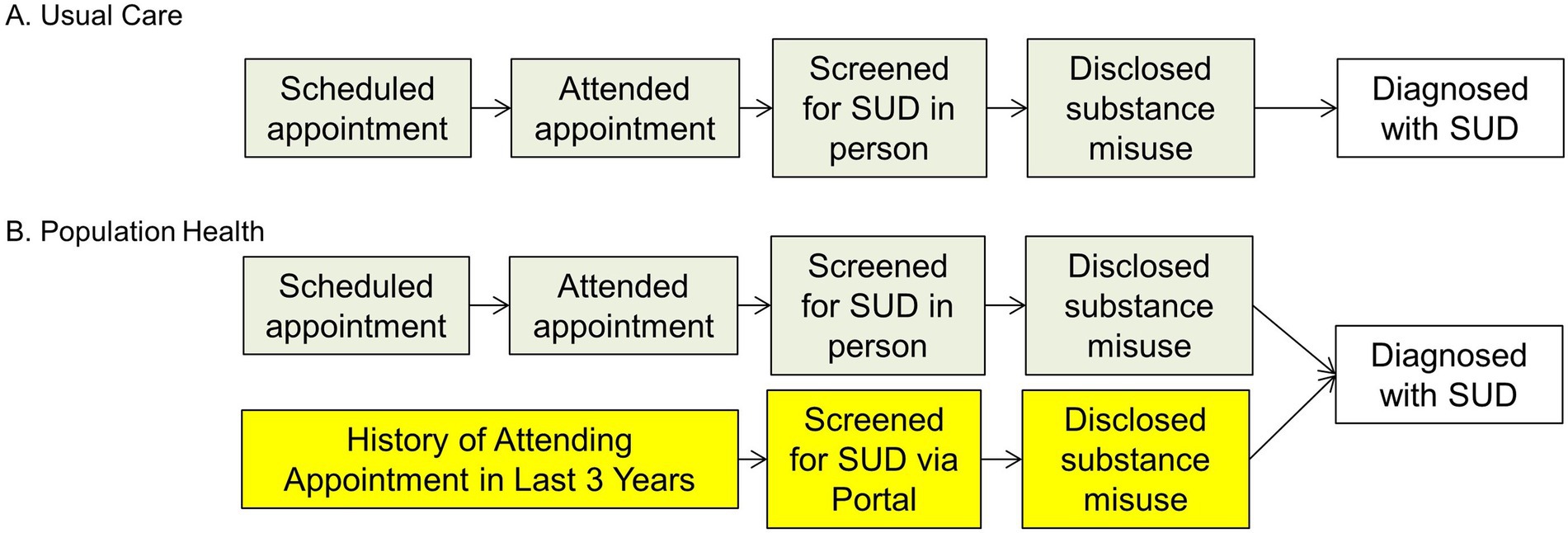
Figure 1. Clinic based screening vs. Population health screening. (A) Clinal based screening (current practice) and (B) Population health screening (new practice). SUD, substance use disorder.
Selection/treatment of subjects
This study will be conducted at the University of Chicago Medicine (UCM) Ryan White Adult HIV Care Program. The UCM Ryan White clinic is the lead site for the Chicago Department of Public Health-funded South Side Health Home (S2H2), a major provider of HIV prevention and care services for residents of Chicago’s South Side, one of the epicenters of the United States HIV epidemic (55). It is currently staffed by 15 physicians, 8 fellows, 1 nurse practitioner, 1 licensed practical nurse, 2 pharmacists, and 2 licensed social workers. Eligibility criteria include adult (>18) patients living with HIV who receive care at the UCM Ryan White Adult HIV Care Program, and appear on the HIV care patient registry, who have not completed SUD screening in the prior 12 months, and who have an active portal account not managed by a proxy.
Sample size
There are ~880 patients who will meet eligibility criteria; therefore, ~440 PLWH will be randomized to each arm. There is an approximately 80% retention in care rate in our clinic. For the usual care arm, based on the rates of depression screening at our clinic, we estimate the rate of in-clinic screening will be about 60%. For the intervention arm, we estimate that 40% of people will complete the portal screener, based on our data from the PORTAL-Depression study (56). Therefore, we anticipate the screening rates will be 48% in usual care and 69% in the intervention arm (Figure 2). Using a two-sided chi-square test to detect a difference in proportion between two independent samples, with an alpha of 0.05 we will have 99% power to detect this difference.
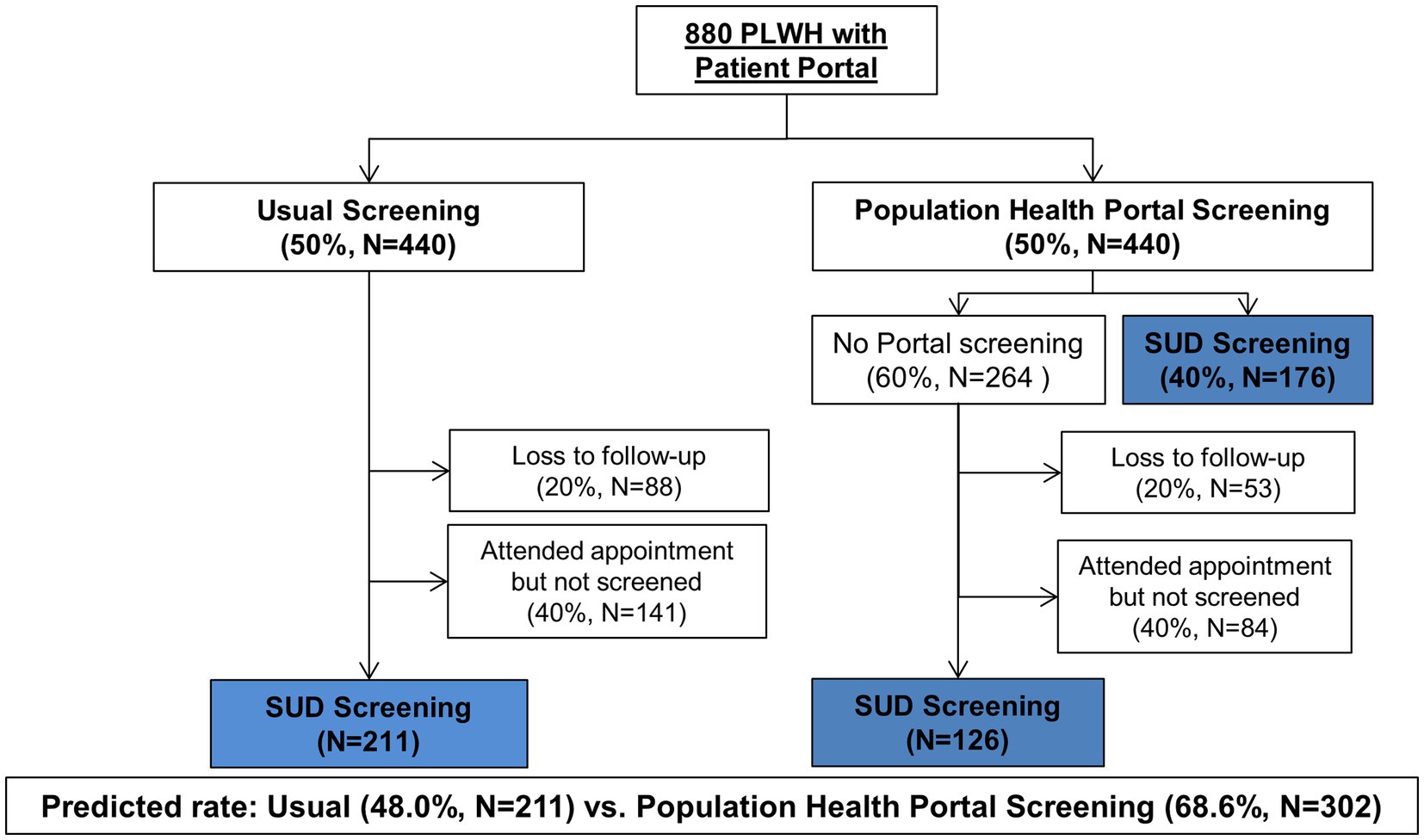
Figure 2. Flow diagram of planned participant enrollment and follow-up. PLWH, people living with human immunodeficiency virus; SUD, substance use disorder.
Intervention
Development of ePORTAL HIV-S
ePORTAL HIV-S was developed by evaluating and adapting previously implemented successful methods of electronic mental and behavioral health screening and applying these to our specific patient population of PLWH. We first built on the PORTAL-Depression study (56), which developed a system for population health mental health assessments via the patient portal. We chose to use the NIDA Quick Screen V1.0 to screen for SUD in order to provide HIV providers with a thorough, evidence-based screener for patients (45). These studies led to adaptations of the PORTAL-Depression study for this intervention.
Screening protocol
Patients randomized to population health screening–the intervention arm–will receive an email invitation and/or mobile app message (depending on their preferences) to log into their portal account and complete the screener. Patients in this arm will receive invitations regardless of whether they have a scheduled appointment. The language in the email invitation/mobile app message is standard to all portal-based messages from UCM, encouraging patients to log in to their portal account to read a message. Once the message is opened, patients will be invited to complete the SUD screener. The SUD screener opens with a brief paragraph that serves to normalize and de-stigmatize screening by explaining that these questions are a part of standard clinical practice in the HIV clinic, and that a research study is being conducted regarding screening via the electronic patient portal. Electronic informed consent is obtained. If patients consent, they can complete the screener through the patient portal. Once completed, screening results will be automatically uploaded into a flowsheet in the electronic health record (EHR). Patients may choose to ignore screening invitations, leave the study by notifying their provider, or only partially complete the tool. Additionally, some of these patients may have clinic visits during the study period and could receive screening as part of their usual clinical care. Patients in the usual care arm will be offered SUD screening during clinic visits using the NIDA Quick Screen V1.0. Medical Assistants (MAs) will offer SUD screening to PLWH as a routine practice after directing them to a clinic room. Medical providers may also administer the screening if MAs do not do so. A best practice advisory in the electronic health record alerts MAs and other providers if a patient is due for screening, i.e., has not completed SUD screening in the prior 12 months.
For both the intervention and control arms, if a person has a NIDA Quick Screen score >3, indicating moderate to high risk of SUD, the EHR will automatically send a message to our collaborating CHW, infectious disease social worker, and the patient’s HIV clinician informing them of this result. The CHW will then contact patients who screen positive either in person during clinic visits or by telephone to follow-up and link patients to treatment. If they cannot reach patients by phone, they will send portal messages to engage patients in care.
ePORTAL HIV-S training/education
Prior to implementation of ePORTAL HIV-S, all medical staff, including clinicians, social workers, CHW and MAs will be informed of the initiative and the contents and administration of NIDA Quick Screen V1.0 via in-person training sessions (including faculty meetings and clinical operations meetings). The ePORTAL HIV-S team will monitor screening and linkage to care rates to provide dynamic assistance throughout the project and to ensure fidelity to study protocols.
Randomization
We will randomize patients at a 1:1 ratio to either population health or usual SUD screening (Figure 2). Randomization will be performed via computer-generated simple randomization. Concealment will be maintained via an electronic system. A staff member in the Center for Research Informatics at the University of Chicago who is not a member of the research team will be responsible for randomization and enrollment. Patients randomized to the population health screening arm will not be blinded to the study, since they will receive an email notification to complete a questionnaire in their portal account. Patients randomized to usual screening will not receive email notification, and thus, will be blinded to trial assignment. Clinicians will not be informed of the randomization; however, some patients may inform them of their assignment. If patients screen positive for a SUD, clinicians will receive notification of these positive results, which could lead to unblinding. However, the study data analysts who will be assessing outcomes will remain blinded.
Implementation plan
We will inform all HIV clinicians (attending physicians, fellows, and advanced practice nurses) in the UCM Ryan White clinic about the study via email and at regular clinic meetings. Clinicians can opt-in to the intervention via email. Clinicians who do not provide assent will have their patients excluded from the RCT. Once launched, the ePORTAL HIV-S staff will meet regularly to monitor screening rates, discuss patient engagement, data collection, and identify issues related to workflow. We will provide feedback to clinical leadership and MAs regarding rates of screening and linkage to care (Table 1).
Intervention outcomes
The primary outcome is the percentage of PLWH who have had a clinic visit in the last two years in the Ryan White Clinic and who were screened for SUDs. Secondary outcomes include the number and percentage of PLWH diagnosed with SUDs, and the number and percentage of PLWH referred for SUD treatment. Secondary HIV care outcomes will include (1) retention in care measured as the kept visit proportion (the number of clinic visits attended divided by the number of clinic visits scheduled) in the 12 months after screening, and (2) HIV viral suppression, defined as a quantitative viral load <200 copies/mL in the year post-screening.
Data collection
Data will be stored in the electronic health record. To protect participant confidentiality, only limited data (deidentified except for dates) will be used for analysis. Data will be exported and deidentified by the Center for Research Informatics before it is securely transferred to the research team for analysis.
Ethics statement and dissemination
This study was approved by the University of Chicago Biological Sciences Division Institutional Review Board (IRB24-0684). The study was registered on 2024-09-27 with clinicaltrials.gov (NCT06682468). Patients will be consented for participation. Data will not be shared because it includes dates and is not completely deidentified, in accordance with institutional policy. Results from the study will be disseminated through conference presentations, peer-reviewed publications, and community reporting.
Data safety and monitoring
A Data Safety and Monitoring Board (DSMB) will be formed to protect the safety of participants. The DSMB will evaluate the implementation of the trial, retention rates, patient safety, and maintenance of the integrity of data collection and management during this study. Any and all serious adverse events will be forwarded to the DSMB within 48 h of being recognized by study staff. The DSMB will have authority to recommend modifications to the clinical investigations or to stop these investigations if there are concerns with patient safety or the integrity of the study. The DSMB will meet every six months for the duration of the clinical trial.
Data analysis
The intention to treat principle will be applied to all analyses (57). For all data we will use descriptive statistics to characterize and describe both the control and intervention arms. To confirm successful randomization, study arms will be compared with regards to age, sex and race/ethnicity distribution. Additionally, we will confirm that differences between the two arms do not exist for type of insurance and major comorbidities. If data is missing for variables under comparison to determine successful randomization, we will report the number of missing responses for each and show differences in between individuals with complete data and those with incomplete data across intervention arms, multiple imputation using the chained equations (MICE) method. Finally, logistic regression models will be used to adjust for baseline imbalances between groups. Comparisons will be made for categorical variables using a chi-square test or Fisher’s exact test, and for continuous variables, a two-sample t-test or Wilcoxon rank-sum test. We will use Bonferroni correction during our examination of baseline differences between treatment and control groups if analyses suggest differences between the groups, we will present adjusted model results rather than unadjusted results.
For primary and secondary outcomes that are proportions or binomial outcomes (percentage screened for SUD, number diagnosed with SUD, percentage diagnosed with SUD, number referred for SUD treatment, percentage referred for SUD treatment, HIV viral suppression, retention in care and kept visit proportion) a chi-square test or Fisher’s exact test and beta regression models will be used to compare the proportional outcomes in the trial arms. All significance testing will be two sided and use a level of 0.05.
Discussion
PLWH are disproportionately affected by comorbid SUDs, which leads to negative health outcomes, potentiated by the fact that SUDs are underdiagnosed and often not treated among PLWH. Currently, limited evidence exists describing the optimal screening strategy for SUD among PLWH. Our approach will be one of the first to incorporate the privacy and convenience of a patient portal-based SUD screener compared to the current standard of care: in-person screening during routine clinic visits. Our method allows patients to self-report data, which then is automatically relayed to the patient’s primary HIV clinician, thus potentially increasing sensitivity of screening and both standardizing and improving documentation of screening results. Furthermore, we are screening for SUD among a population of PLWH who have historically received care at the clinic, as a population health approach, which differs from the reactive approach of screening during visits. This will allow the opportunity to reach PLWH who may not attend clinic visits. Also, because ePORTAL HIV-S is being implemented pragmatically within a large, real-world medical home, it allows us to test our intervention’s effectiveness and implementation strategy within the context of existing clinical practice. SUD remains one of the biggest challenges facing healthcare today, across diverse populations (15, 58, 59). We believe that the results of this study–and the fundamentals of ePORTAL HIV-S, including confidential, convenient online screening and incorporation of a CHW–will be potentially applicable to broader populations at risk for SUD. It will provide crucial information regarding the effectiveness of portal-based screening for SUD. Our findings may also have implications for screening for other behavioral or mental health conditions, or other conditions that may be associated with stigma.
Limitations
Our findings may not be generalizable in settings where there are significant differences in cultural mores related to substance use, HIV, and stigma compared to ours. Additionally, our findings are contingent on patients’ access to private personal electronic devices with internet access. Finally, our health system has access to behavioral health services, a CHW, and clinician collaboration, which may not generalize to other settings, particularly ones without access to EHR technology or low-resource medical settings, including other countries.
Author contributions
ER: Writing – review & editing. DZ: Writing – review & editing. JG: Writing – review & editing. HP: Writing – review & editing. BB: Writing – review & editing. JS: Writing – review & editing. EF: Writing – review & editing. JP-B: Writing – review & editing. JR: Writing – review & editing. NL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Institute of Drug Abuse of the National Institutes of Health under Award Number 1R01DA058965. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1583546/full#supplementary-material
References
1. Gras, J, Pillet, M, Antoni, G, Cua, E, Charreau, I, Raffi, F, et al. Risk factors for HIV infection among men who have sex with men in the ANRS IPERGAY PrEP trial. Sex Transm Infect. (2022) 98:383–6. doi: 10.1136/sextrans-2021-055199
2. Murphy, PJ, Garrido-Hernansaiz, H, Mulcahy, F, and Hevey, D. HIV-related stigma and optimism as predictors of anxiety and depression among HIV-positive men who have sex with men in the United Kingdom and Ireland. AIDS Care. (2018) 30:1173–9. doi: 10.1080/09540121.2018.1445827
3. Crockett, KB, Kalichman, SC, Kalichman, MO, Cruess, DG, and Katner, HP. Experiences of HIV-related discrimination and consequences for internalised stigma, depression and alcohol use. Psychol Health. (2019) 34:796–810. doi: 10.1080/08870446.2019.1572143
4. Duko, B, Ayalew, M, and Ayano, G. The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy. (2019) 14:52. doi: 10.1186/s13011-019-0240-3
5. DeLorenze, GN, Satre, DD, Quesenberry, CP, Tsai, A-L, and Weisner, CM. Mortality after diagnosis of psychiatric disorders and co-occurring substance use disorders among HIV-infected patients. AIDS Patient Care STDs. (2010) 24:705–12. doi: 10.1089/apc.2010.0139
6. Tsui, JI, Akosile, MA, Lapham, GT, Boudreau, DM, Johnson, EA, Bobb, JF, et al. Prevalence and medication treatment of opioid use disorder among primary care patients with hepatitis C and HIV. J Gen Intern Med. (2021) 36:930–7. doi: 10.1007/s11606-020-06389-7
7. Doshi, RK, Byrne, M, Levy, M, Varga, L, Kuo, I, Horberg, MA, et al. Association of Substance use Disorders with engagement in care and mortality among a clinical cohort of people with HIV in Washington, DC. AIDS Behav. (2021) 25:2289–300. doi: 10.1007/s10461-021-03157-4
8. Hartzler, B, Dombrowski, JC, Crane, HM, Eron, JJ, Geng, EH, Christopher Mathews, W, et al. Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS Behav. (2017) 21:1138–48. doi: 10.1007/s10461-016-1584-6
9. Atkinson, JH. Prevalence of psychiatric disorders among men infected with human immunodeficiency virus: a controlled study. Arch Gen Psychiatry. (1988) 45:859. doi: 10.1001/archpsyc.1988.01800330091011
10. Kilbourne, AM, Justice, AC, Rabeneck, L, Rodriguez-Barradas, M, and Weissman, S. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. J Clin Epidemiol. (2001) 54:S22–8. doi: 10.1016/S0895-4356(01)00443-7
11. Bing, EG, Burnam, MA, Longshore, D, Fleishman, JA, Sherbourne, CD, London, AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. (2001) 58:721–8. doi: 10.1001/archpsyc.58.8.721
12. Samet, JH, Phillips, SJ, Horton, NJ, Traphagen, ET, and Freedberg, KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retrovir. (2004) 20:151–5. doi: 10.1089/088922204773004860
13. Pence, BW, Miller, WC, Whetten, K, Eron, JJ, and Gaynes, BN. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the southeastern United States. J Acquir Immune Defic Syndr. (2006) 42:298–306. doi: 10.1097/01.qai.0000219773.82055.aa
14. Center for Behavioral Health Statistics and Quality. Racial/ethnic differences in substance use, substance use disorders, and substance use treatment utilization among people aged 12 or older (2015-2019). Rockville, MD: Substance Abuse and Mental Health Services Administration (2021).
15. Batchelder, AW, Foley, JD, Wirtz, MR, Mayer, K, and O'Cleirigh, C. Substance use stigma, avoidance coping, and missed HIV appointments among MSM who use substances. AIDS Behav. (2021) 25:1454–63. doi: 10.1007/s10461-020-02982-3
16. Rowell-Cunsolo, TL, and Bellerose, M. Utilization of substance use treatment among criminal justice-involved individuals in the United States. J Subst Abus Treat. (2021) 125:108423. doi: 10.1016/j.jsat.2021.108423
17. Parcesepe, AM, Lancaster, K, Edelman, EJ, DeBoni, R, Ross, J, Atwoli, L, et al. Substance use service availability in HIV treatment programs: data from the global IeDEA consortium, 2014-2015 and 2017. PLoS One. (2020) 15:e0237772. doi: 10.1371/journal.pone.0237772
18. Korthuis, PT, Josephs, JS, Fleishman, JA, Hellinger, J, Himelhoch, S, Chander, G, et al. Substance abuse treatment in human immunodeficiency virus: the role of patient-provider discussions. J Subst Abus Treat. (2008) 35:294–303. doi: 10.1016/j.jsat.2007.11.005
19. Krawczyk, N, Feder, KA, Fingerhood, MI, and Saloner, B. Racial and ethnic differences in opioid agonist treatment for opioid use disorder in a U.S. national sample. Drug Alcohol Depend. (2017) 178:512–8. doi: 10.1016/j.drugalcdep.2017.06.009
20. Cummings, JR, Wen, H, Ko, M, and Druss, BG. Race/ethnicity and geographic access to Medicaid substance use disorder treatment facilities in the United States. JAMA Psychiatry. (2014) 71:190–6. doi: 10.1001/jamapsychiatry.2013.3575
21. Satre, DD, Anderson, AN, Leibowitz, AS, Levine-Hall, T, Slome, S, Flamm, J, et al. Implementing electronic substance use disorder and depression and anxiety screening and behavioral interventions in primary care clinics serving people with HIV: protocol for the promoting access to care engagement (PACE) trial. Contemp Clin Trials. (2019) 84:105833. doi: 10.1016/j.cct.2019.105833
22. Fredericksen, R, Crane, PK, Tufano, J, Ralston, J, Schmidt, S, Brown, T, et al. Integrating a web-based, patient-administered assessment into primary care for HIV-infected adults. J AIDS HIV Research. (2012) 4:47–55. doi: 10.5897/JAHR11.046
23. Myers, K, Li, T, Baum, M, Ibanez, G, and Fennie, K. The individual, interactive, and syndemic effect of substance use, depression, education, and ethnicity on retention in HIV care. Int J STD AIDS. (2021) 32:184–93. doi: 10.1177/0956462419890727
24. Vetrova, MV, Cheng, DM, Bendiks, S, Gnatienko, N, Lloyd-Travaglini, C, Jiang, W, et al. HIV and substance use stigma, Intersectional Stigma and Healthcare Among HIV-Positive PWID in Russia. AIDS Behavior. (2021) 25:2815–26. doi: 10.1007/s10461-021-03172-5
25. Rebeiro, P, Althoff, KN, Buchacz, K, Gill, J, Horberg, M, Krentz, H, et al. Retention among north American HIV-infected persons in clinical care, 2000-2008. J Acquir Immune Defic Syndr. (2013) 62:356–62. doi: 10.1097/QAI.0b013e31827f578a
26. Bockting, W, MacCrate, C, Israel, H, Mantell, JE, and Remien, RH. Engagement and retention in HIV Care for Transgender Women: perspectives of medical and social service providers in new York City. AIDS Patient Care STDs. (2020) 34:16–26. doi: 10.1089/apc.2019.0067
27. Kipp, AM, Rebeiro, PF, Shepherd, BE, Brinkley-Rubinstein, L, Turner, M, Bebawy, S, et al. Daily marijuana use is associated with missed clinic appointments among HIV-infected persons engaged in HIV care. AIDS Behav. (2017) 21:1996–2004. doi: 10.1007/s10461-017-1716-7
28. McGinnis, KA, Tate, JP, Williams, EC, Skanderson, M, Bryant, KJ, Gordon, AJ, et al. Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend. (2016) 168:196–202. doi: 10.1016/j.drugalcdep.2016.09.015
29. Williams, EC, Achtmeyer, CE, Thomas, RM, Grossbard, JR, Lapham, GT, Chavez, LJ, et al. Factors underlying quality problems with alcohol screening prompted by a clinical reminder in primary care: a multi-site qualitative study. J Gen Intern Med. (2015) 30:1125–32. doi: 10.1007/s11606-015-3248-z
30. Batchelder, AW, Foley, JD, Kim, J, Thiim, A, Kelly, J, Mayer, K, et al. Intersecting internalized stigmas and HIV self-care among men who have sex with men and who use substances. Soc Sci Med. (2021) 275:113824. doi: 10.1016/j.socscimed.2021.113824
31. Earnshaw, VA, Smith, LR, Cunningham, CO, and Copenhaver, MM. Intersectionality of internalized HIV stigma and internalized substance use stigma: implications for depressive symptoms. J Health Psychol. (2015) 20:1083–9. doi: 10.1177/1359105313507964
32. Byrne, JM, Elliott, S, and Firek, A. Initial experience with patient-clinician secure messaging at a VA medical center. J Am Med Inform Assoc. (2009) 16:267–70. doi: 10.1197/jamia.M2835
33. Goldberg, N, Herrmann, C, Di Gion, P, Hautsch, V, Hefter, K, Langebartels, G, et al. Sociodemographic and socioeconomic determinants for the usage of digital patient portals in hospitals: systematic review and Meta-analysis on the digital divide. J Med Internet Res. (2025) 27:e68091. doi: 10.2196/68091
34. Essén, A, Scandurra, I, Gerrits, R, Humphrey, G, Johansen, MA, Kierkegaard, P, et al. Patient access to electronic health records: differences across ten countries. Health Policy Technology. (2018) 7:44–56. doi: 10.1016/j.hlpt.2017.11.003
35. Honein-AbouHaidar, GN, Antoun, J, Badr, K, Hlais, S, and Nazaretian, H. Users' acceptance of electronic patient portals in Lebanon. BMC Med Inform Decis Mak. (2020) 20:31. doi: 10.1186/s12911-020-1047-x
36. El-Jardali, F, Bou-Karroum, L, Jabbour, M, Bou-Karroum, K, Aoun, A, Salameh, S, et al. Digital health in fragile states in the Middle East and North Africa (MENA) region: a scoping review of the literature. PLoS One. (2023) 18:e0285226. doi: 10.1371/journal.pone.0285226
37. Lyles, CR, Nelson, EC, Frampton, S, Dykes, PC, Cemballi, AG, and Sarkar, U. Using electronic health record portals to improve patient engagement: research priorities and best practices. Ann Intern Med. (2020) 172:S123–9. doi: 10.7326/M19-0876
38. Medical Group Management Association (MGMA). MGMA Stat: Most practices offer a patient portal (2018) Available at: https://www.mgma.com/news-insights/quality-patient-experience/mgma-stat-most-practices-offer-a-patient-portal.
39. Chu, D, Schuster, T, Lessard, D, Mate, K, Engler, K, Ma, Y, et al. Acceptability of a patient portal (opal) in HIV clinical care: a feasibility study. J Personalized Medicine. (2021) 11:134. doi: 10.3390/jpm11020134
40. Lee, SB, and Valerius, J. mHealth interventions to promote anti-retroviral adherence in HIV: narrative review. JMIR Mhealth Uhealth. (2020) 8:e14739. doi: 10.2196/14739
41. Luque, AE, van Keken, A, Winters, P, Keefer, MC, Sanders, M, and Fiscella, K. Barriers and facilitators of online patient portals to personal health records among persons living with HIV: formative research. JMIR Research Protocols. (2013) 2:e8. doi: 10.2196/resprot.2302
42. Delker, E, Aharonovich, E, and Hasin, D. Interviewer-administered TLFB vs. self-administered computerized (A-CASI) drug use frequency questions: a comparison in HIV-infected drug users. Drug Alcohol Depend. (2016) 161:29–35. doi: 10.1016/j.drugalcdep.2016.01.007
43. Franco, MI, Staab, EM, Zhu, M, Knitter, A, Wan, W, Gibbons, R, et al. Pragmatic clinical trial of population health, portal-based depression screening: the PORTAL-depression study. J Gen Intern Med. (2023) 38:857–64. doi: 10.1007/s11606-022-07779-9
44. Zimmer, D, Staab, EM, Ridgway, JP, Schmitt, J, Franco, M, Hunter, SJ, et al. Population-level portal-based anxiety and depression screening perspectives in HIV care clinicians: qualitative study using the consolidated framework for implementation research. JMIR Formative Research. (2024) 8:e48935. doi: 10.2196/48935
45. Walker, JA, Staab, EM, Ridgway, JP, Schmitt, J, Franco, MI, Hunter, S, et al. Patient perspectives on portal-based anxiety and depression screening in HIV care: a qualitative study using the consolidated framework for implementation research. Int J Environ Res Public Health. (2024) 21:692. doi: 10.3390/ijerph21060692
46. Nijland, N, van Gemert-Pijnen, JE, Kelders, SM, Brandenburg, BJ, and Seydel, ER. Factors influencing the use of a web-based application for supporting the self-care of patients with type 2 diabetes: a longitudinal study. J Med Internet Res. (2011) 13:e71. doi: 10.2196/jmir.1603
47. Zhong, X, Liang, M, Sanchez, R, Yu, M, Budd, PR, Sprague, JL, et al. On the effect of electronic patient portal on primary care utilization and appointment adherence. BMC Med Inform Decis Mak. (2018) 18:84. doi: 10.1186/s12911-018-0669-8
48. Nazi, KM, Hogan, TP, McInnes, DK, Woods, SS, and Graham, G. Evaluating patient access to electronic health records: results from a survey of veterans. Med Care. (2013) 51:S52–6. doi: 10.1097/MLR.0b013e31827808db
49. Ketterer, T, West, DW, Sanders, VP, Hossain, J, Kondo, MC, and Sharif, I. Correlates of patient portal enrollment and activation in primary care pediatrics. Acad Pediatr. (2013) 13:264–71. doi: 10.1016/j.acap.2013.02.002
50. Lam, R, Lin, VS, Senelick, WS, Tran, HP, Moore, AA, and Koretz, B. Older adult consumers' attitudes and preferences on electronic patient-physician messaging. Am J Manag Care. (2013) 19:eSP7–eSP11.
51. Reed, ME, Huang, J, Brand, RJ, Neugebauer, R, Graetz, I, Hsu, J, et al. Patients with complex chronic conditions: health care use and clinical events associated with access to a patient portal. PLoS One. (2019) 14:e0217636. doi: 10.1371/journal.pone.0217636
52. Neuner, J, Fedders, M, Caravella, M, Bradford, L, and Schapira, M. Meaningful use and the patient portal: patient enrollment, use, and satisfaction with patient portals at a later-adopting center. Am J Med Qual. (2015) 30:105–13. doi: 10.1177/1062860614523488
53. Keith McInnes, D, Shimada, SL, Rao, SR, Quill, A, Duggal, M, Gifford, AL, et al. Personal health record use and its association with antiretroviral adherence: survey and medical record data from 1871 US veterans infected with HIV. AIDS Behav. (2013) 17:3091–100. doi: 10.1007/s10461-012-0399-3
54. Chan, A-W, Boutron, I, Hopewell, S, Moher, D, Schulz, KF, Collins, GS, et al. SPIRIT 2025 statement: updated guideline for protocols of randomised trials. BMJ. (2025) 389:e081477. doi: 10.1136/bmj-2024-081477
56. Franco, MI, Staab, EM, Zhu, M, Deehan, W, Moses, J, Gibbons, R, et al. Implementation of an EHR-integrated web-based depression assessment in primary care: PORTAL-depression. JAMIA Open. (2024) 7. doi: 10.1093/jamiaopen/ooae094
57. Fisher, L, Dixon, D, Herson, J, Frankowski, RMH, and Peace, M. Statistical issues in drug research and development. New York: Marcel Dekker (1990).
58. Alegria, M, Carson, NJ, Goncalves, M, and Keefe, K. Disparities in treatment for substance use disorders and co-occurring disorders for ethnic/racial minority youth. J Am Acad Child Adolesc Psychiatry. (2011) 50:22–31. doi: 10.1016/j.jaac.2010.10.005
59. Cleland, CM, Gwadz, M, Collins, LM, Wilton, L, Sherpa, D, Dorsen, C, et al. African American/black and Latino adults with detectable HIV viral load evidence substantial risk for polysubstance substance use and co-occurring problems: a latent class analysis. AIDS Behav. (2021) 25:2501–16. doi: 10.1007/s10461-021-03212-0
Keywords: substance use disorder, HIV, population health, patient portal screening, Comorbidity
Citation: Roessler E, Zimmer D, Grant J, Pollack H, Boodram B, Schmitt J, Friedman E, Pagkas-Bather J, Brewer RA, Ridgway J and Laiteerapong N (2025) Protocol for a randomized controlled trial of patient–portal–based screening for substance use among people with HIV. Front. Public Health. 13:1583546. doi: 10.3389/fpubh.2025.1583546
Edited by:
Hannah Knudsen, University of Kentucky, United StatesReviewed by:
Rezvan Hosseinzadeh, Babol University of Medical Sciences, IranAmelia Dias Teixeira, Federal University of Rio Grande do Sul, Brazil
Copyright © 2025 Roessler, Zimmer, Grant, Pollack, Boodram, Schmitt, Friedman, Pagkas-Bather, Brewer, Ridgway and Laiteerapong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neda Laiteerapong, bmxhaXRlZXJAYnNkLnVjaGljYWdvLmVkdQ==
 Eric Roessler
Eric Roessler Daniela Zimmer
Daniela Zimmer Jon Grant
Jon Grant Harold Pollack
Harold Pollack Basmattee Boodram
Basmattee Boodram Jessica Schmitt1,5
Jessica Schmitt1,5 Jade Pagkas-Bather
Jade Pagkas-Bather Jessica Ridgway
Jessica Ridgway Neda Laiteerapong
Neda Laiteerapong