- 1Faculty of Health Sciences, University of Maribor, Maribor, Slovenia
- 2General Hospital Ptuj, Ptuj, Slovenia
- 3National Laboratory of Health, Environment and Food, Maribor, Slovenia
Objectives: The COVID-19 pandemic changed the use of antibiotics and had an impact on the development of antimicrobial resistance. The study aimed to examine the consumption of antibiotics and the occurrence of AMR infection and colonization in the selected general hospital.
Methods: Data on antibiotic consumption and data on AMR infections and colonization were monitored in the period before the COVID-19 pandemic (2018, 2019) and during the COVID-19 pandemic (2020, 2021). Descriptive statistics, the Mann–Whitney U test, and the Pearson or Spearman correlation test were used.
Results: The overall prescription of antibiotics stayed approximately the same, however, some important differences can be observed when analyzing specific groups of antibiotics (vancomycin, linezolid, piperacillin/tazobactam, meropenem, colistin). We did not observe the difference in the occurrence of AMR infections and colonizations before and during the pandemic. However, we did observe an alarming increase in CRaB, ESBL and VRE and highlighted the increase in all AMR groups between the first and second year of the pandemic.
Conclusion: The connection between antibiotic consumption and the occurrence of AMR infections and colonization was confirmed.
Introduction
On the 5th of May, 2023, after more than 3 years, the World Health Organization (WHO) declared Coronavirus disease 2019 (COVID-19) no longer a global health emergency and classified it as an established and ongoing health issue (1). Nevertheless, many studies emphasize the importance of changed or increased use of antibiotics during the pandemic and the impact on the development of antimicrobial resistance (2–8). The COVID-19 pandemic has required changes in disease prevention protocols, including the wearing of protective equipment and masks, hand washing and sanitizing, social distancing (9) and also changes in hospital guidelines, introduction of COVID-19 screening tests and patient isolation (10). On October 22nd, 2020 U. S. Food and Drug Administration approved the first drug remdesivir for use in adults and pediatric patients for the treatment of COVID-19 requiring hospitalization (11). This was followed by the development of other antiviral and anti-inflammatory drugs (12) and, finally, approving COVID-19 vaccines (13). On the other hand, patients hospitalized with COVID-19 often also receive antimicrobial treatment (7, 14, 15), which, of course, increases the threat of global antimicrobial resistance (3).
The aim of our study was to examine the consumption of antibiotics and the occurrence of antimicrobial resistance (AMR) infection and colonization in the selected general hospital in the observed period before and during the COVID-19 pandemic. We aimed to determine whether there is a causal relationship between the use of antibiotics and the increase in health care-associated infections (HCAI) and colonization with AMR, related to the COVID-19 pandemic.
Materials and methods
Study design and settings
The study was conducted in a secondary care regional hospital with 205 beds in Slovenia, which provides comprehensive hospital and specialist outpatient care at the secondary level for 110,000 inhabitants of Spodnje Podravje. During the COVID pandemic, the hospital had a COVID ward with 40 beds and an intensive care unit for the treatment of COVID-19 patients (5 beds). In 2021, the hospital also remained a COVID-19 hospital with part of its capacity dedicated to patients with COVID-19. 760 patients with COVID were treated in 2021.
Data
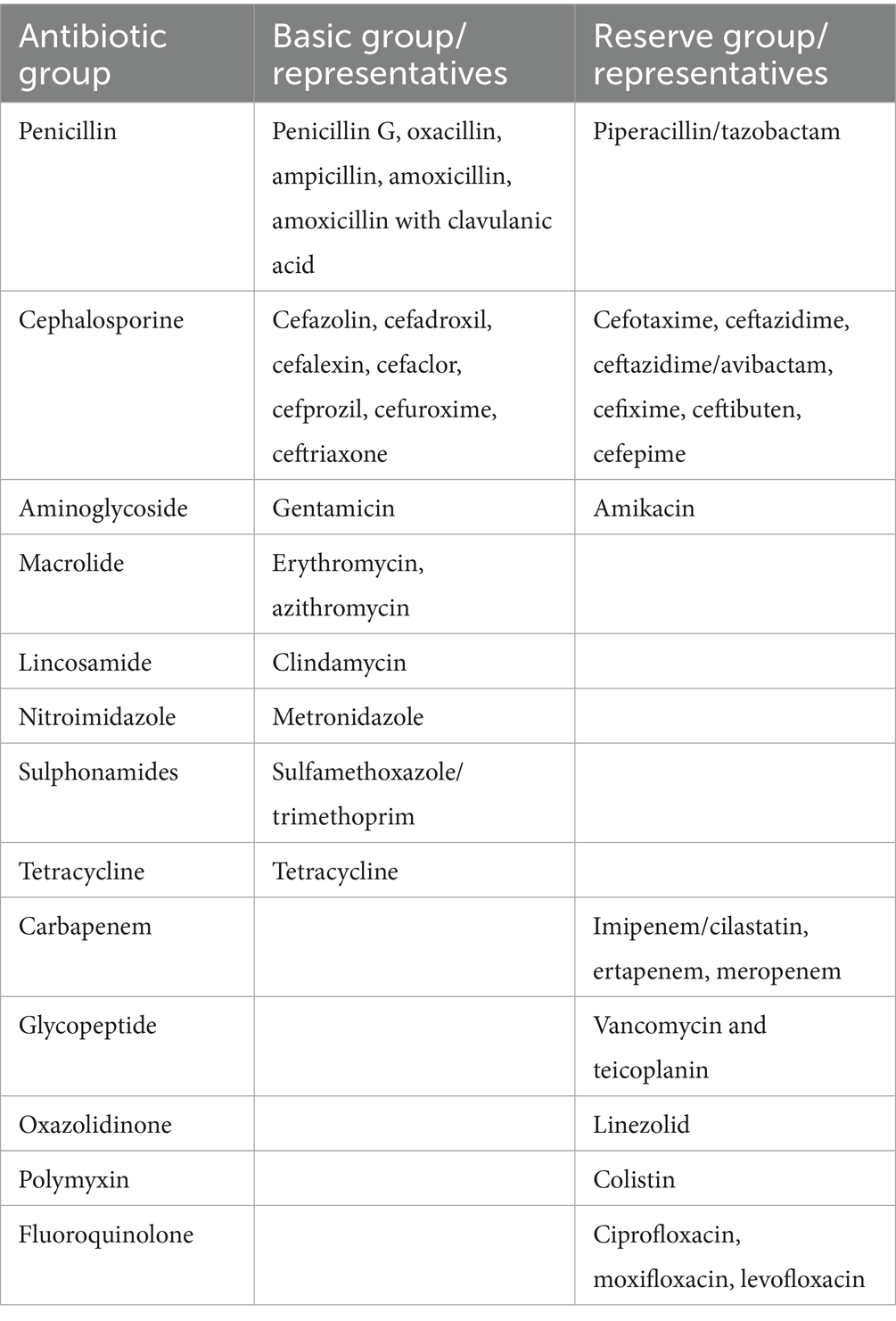
We monitored the data in the period before the COVID-19 pandemic (2018, 2019) and during the COVID-19 pandemic (2020, 2021). Data on antibiotics consumption were obtained through the hospital pharmacy divided into basic group of antibiotics (Penicilin group: penicillin G, oxacillin, ampicillin, amoxicillin with clavulanic acid; Cephalosporine group: ceftriaxone, cefuroxime, cephalothin, cefixime; Sulphonamides group: sulfamethoxazole/trimethoprim; Aminoglycoside group: gentamicin; Macrolide group: erythromycin, azithromycin; Lincosamide group: clindamycin; Tetracycline group: tetracycline; Nitroimidazole group: metronidazole and mupirocin) and the second “reserve group” of antibiotics (Penicillin group: piperacillin/tazobactam; Cephalosporine group: ceftazidime/avibactam; ceftazidime, cefepime, cefotaxime; Carbapenem group: imipenem/cilastatin, meropenem, ertapenem; Aminoglycoside group: amikacin; Fluoroquinolone group: ciprofloxacin, levofloxacin, moxifloxacin; Glycopeptide group: vancomycin, teicoplanin; Oxazolidinone group: linezolid; Polymyxin group: colistin). The exact division and representatives of the basic and reserve groups of antibiotics are shown in Table 1.
The unit of measure was defined as daily doses (DDD)/100 bed days. Data on AMR infections and colonization were obtained through the national laboratory for microbiological analysis of clinical samples on July 1st, 2023. No ethics committee approval or participant consent was received, as no human participants were involved. Fully anonymized aggregate data on the number and type of antibiotic-resistant bacterial infections and colonization in a selected hospital were obtained over a specific period. The data were accessed for research purposes, and the authors had no access to information that could identify individual patients during or after data collection.
Statistical analysis
The data were processed in Microsoft Excel 2016 program and statistically analyzed in the IBM SPSS 20 program (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) using descriptive statistics, the Mann–Whitney U test, and Pearson or Spearman correlation test.
Results
Antibiotic consumption
In the two-year period before the COVID-19 pandemic (2018, 2019), 73.37% of the basic groups of antibiotics and 26.63% of the reserve groups of antibiotics were used. The changes during the pandemic (2020, 2021) were minimal, meaning 73.26% of the basic groups of antibiotics and 26.74% of the reserve groups of antibiotics were used.
The overall consumption of basic groups of antibiotics before and during the pandemic (Figure 1) shows the highest consumption of penicillin and cephalosporin antibiotics. The main difference between the two groups was revealed, i.e., the consumption of penicillin antibiotics decreased somewhat during the pandemic (−4.3%), while the consumption of cephalosporin antibiotics increased by +27.68%. In other basic groups of antibiotics increase during pandemic years was observed for aminoglycosides (+31.24%), macrolides (+21.08%), lincosamide (+14.86%) and nitroimidazole (+11.75%). In addition to penicillin, among the basic groups of antibiotics, consumption also decreased in the groups of sulphonamides (−12.79%) and tetracyclines (−63.27%).
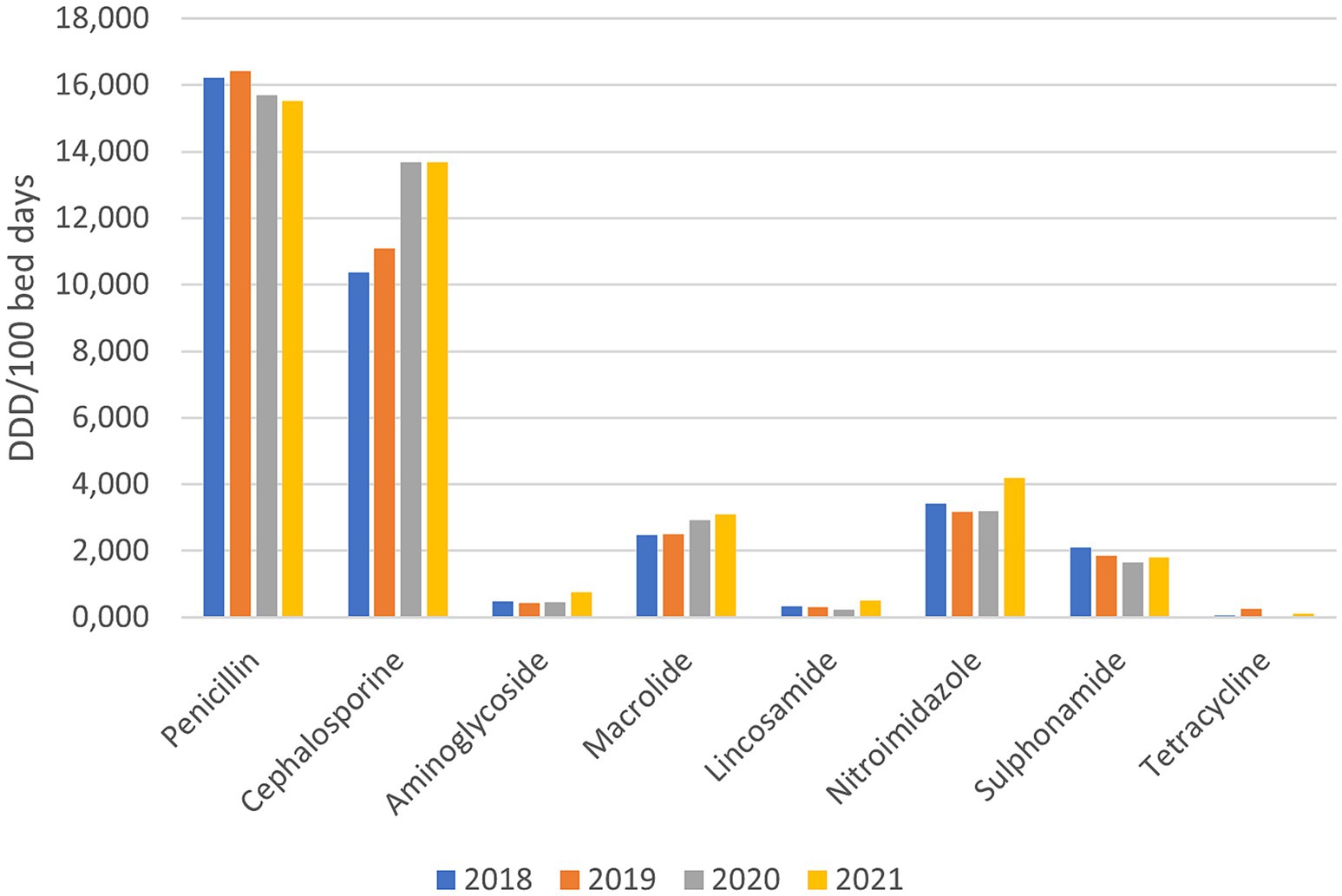
Figure 1. Consumption of basic groups of antibiotics before and during the pandemic (DDD/100 bed days).
Among the reserve groups of antibiotics, fluoroquinolones are the most frequently prescribed, where a slight decrease (−10.79%) was observed during the pandemic years. In all the other reserve groups of antibiotics, an increased use was observed, namely penicillin’s - piperacillin/tazobactam (+23.42%), cephalosporins (+3.19%), carbapenems (+57.21%), Polymyxin (+1,030%), glycopeptides (+66.32%), oxazolidinone (+188.46%). The consumption of aminoglycosides (amikacin), which were not prescribed before the pandemic, grew to 0.129 DDD/100 bed days in 2021 (Figure 2).
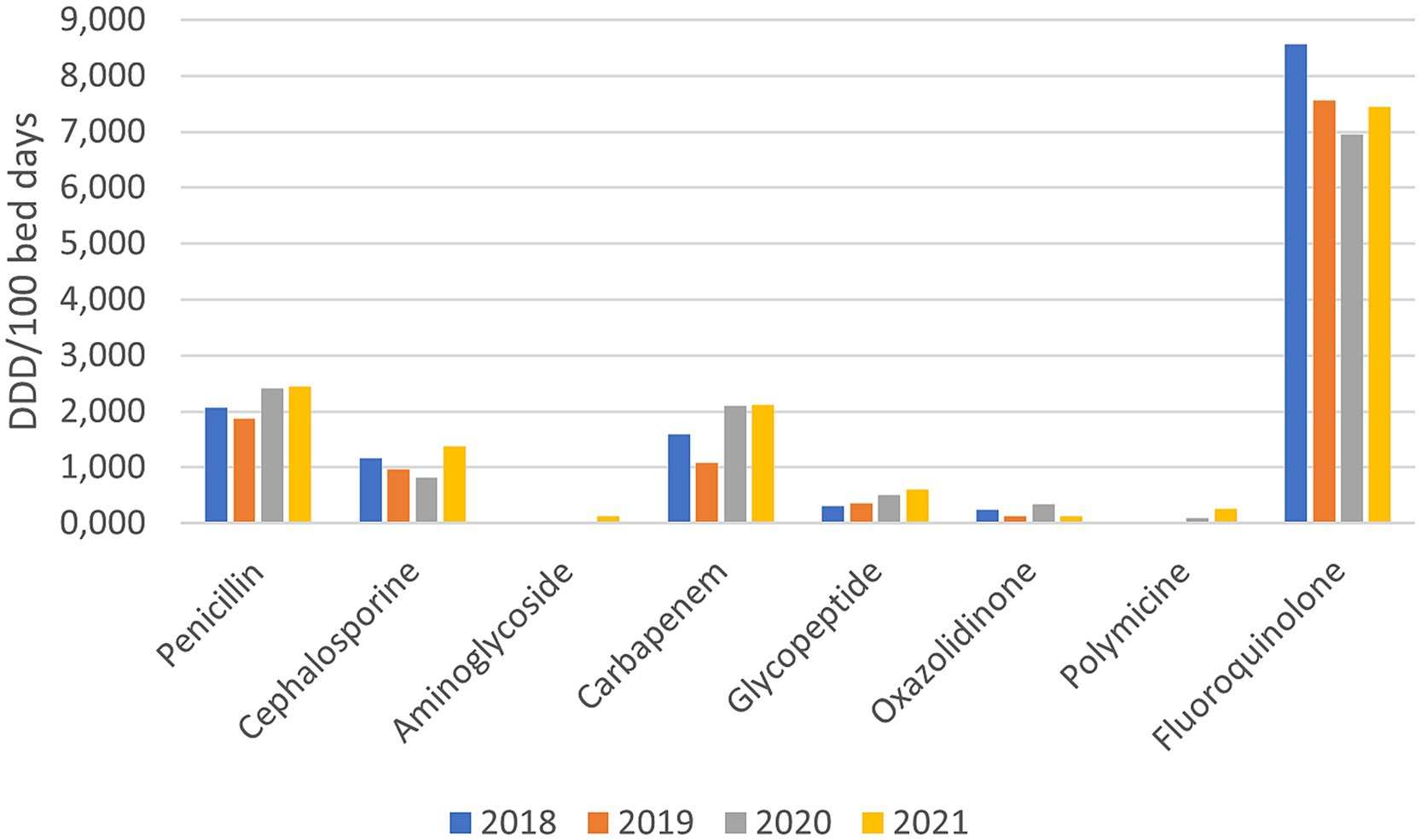
Figure 2. Consumption of reserve groups of antibiotics before and during the pandemic (DDD/100 bed days).
In Figure 3, the consumption of all antibiotics (i.e., basic and reserve group) in the observed hospital before (years 2018, 2019) and during the pandemic (years 2020, 2021) is presented. During the COVID-19 pandemic, the consumption of penicillin antibiotics decreased by 1.39%, tetracyclines by 63.27%, sulphonamides by 12.79% and fluoroquinolones by 10.79%. On the other hand, the consumption of cephalosporins increased by 25.46%, aminoglycosides by 45.47%, macrolides by 21.08%, lacosamide by 14.86%, nitroimidazole by 11.75%, carbapenems by 57.21%, Polymyxin by 1,030% (10 x increase in consumption), glycopeptides by 66.32% and oxazolidinone by 188.46%.
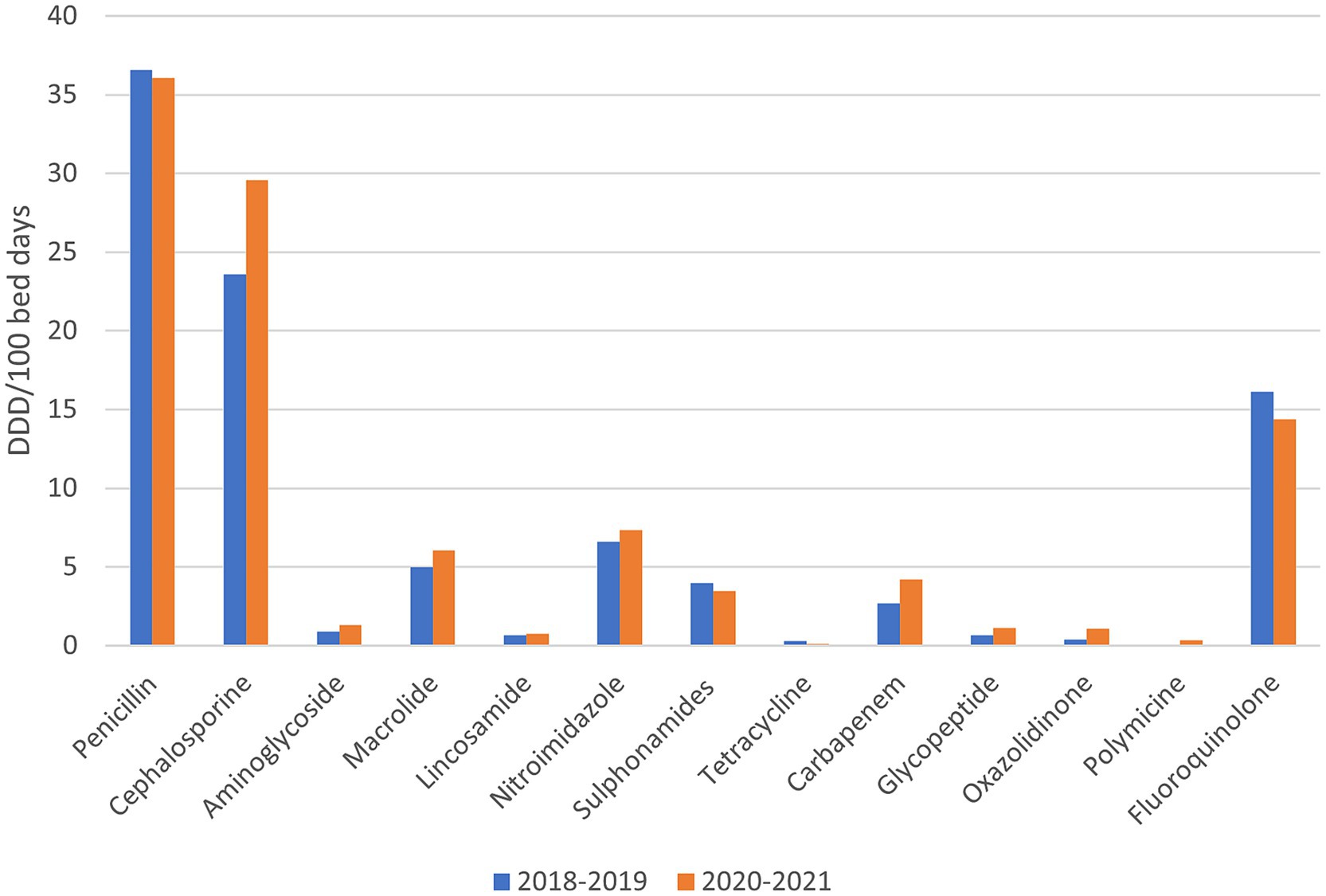
Figure 3. The consumption of all antibiotics (i.e., basic and reserve group) in the observed hospital before and during the pandemic.
The data analysis using the Mann–Whitney U Test did not show a statistically significant difference in total antibiotic consumption before and during the pandemic (p = 0.626).
AMR infections and colonizations
The total occurrence of nosocomial infections and colonization with AMR bacteria in the observed hospital by individual years are shown in Table 2. All first cases (N) of newly discovered nosocomial infections and/or colonization in an individual patient, identified from clinical isolates and/or surveillance samples, are covered.
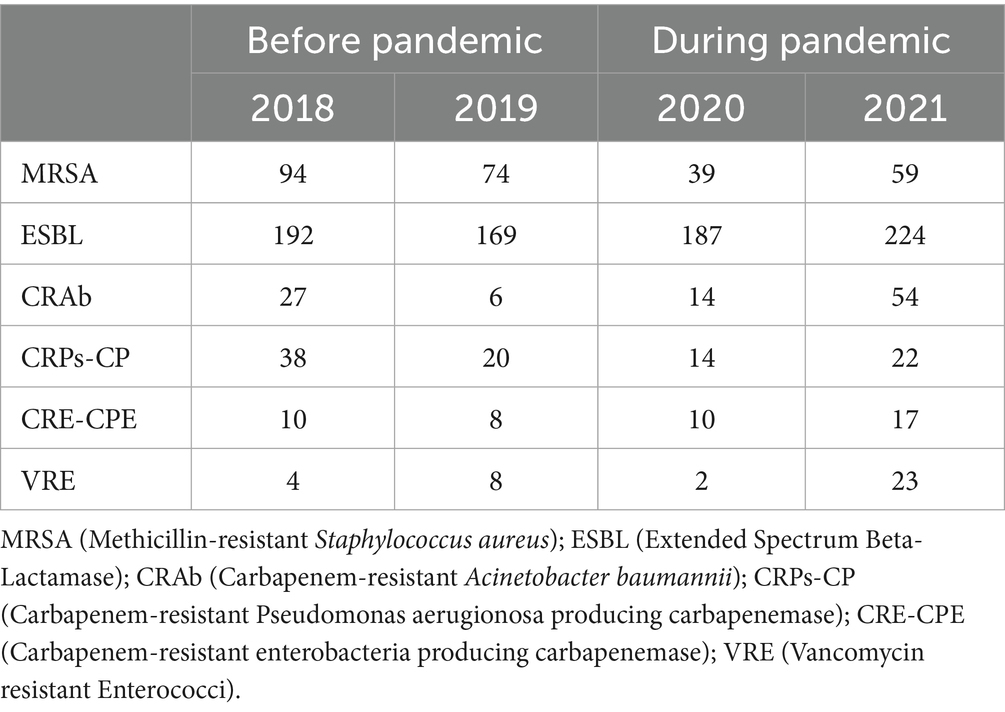
Table 2. Total nosocomial infections and colonization with AMR bacteria in the observed hospital by individual years.
During the pandemic, the occurrence of Methicillin-resistant Staphylococcus aureus (MRSA) decreased by 41.67% and Carbapenem-resistant Pseudomonas aerugionosa producing carbapenemase (CRPs-CP) by 37.93%, despite the high consumption of penicillin, cephalosporins and higher consumption of carbapenems. This resulted in an increased occurrence of ESBL by 13.85%, Carbapenem-resistant Acinetobacter baumannii (CRAb) by 106.06%, Carbapenem-resistant enterobacteria producing carbapenemase (CRE-CPE) by 50% and Vancomycin resistant Enterococci (VRE) by 108.33% compared to the period before the COVID-19 pandemic.
The data analysis using the Mann–Whitney U Test did not show a statistically significant difference in the occurrence of AMR infections and colonizations before and during the pandemic (p = 0.631).
Using a Spearman correlation test, the connection between total antibiotic consumption and the occurrence of AMR infections and colonization was confirmed (p = 0.028).
Next, a correlation analysis of the consumption of specific antibiotics versus the occurrence of specific nosocomial infections and colonization with AMR bacteria in the observed hospital by individual years was performed and is shown in Table 3.
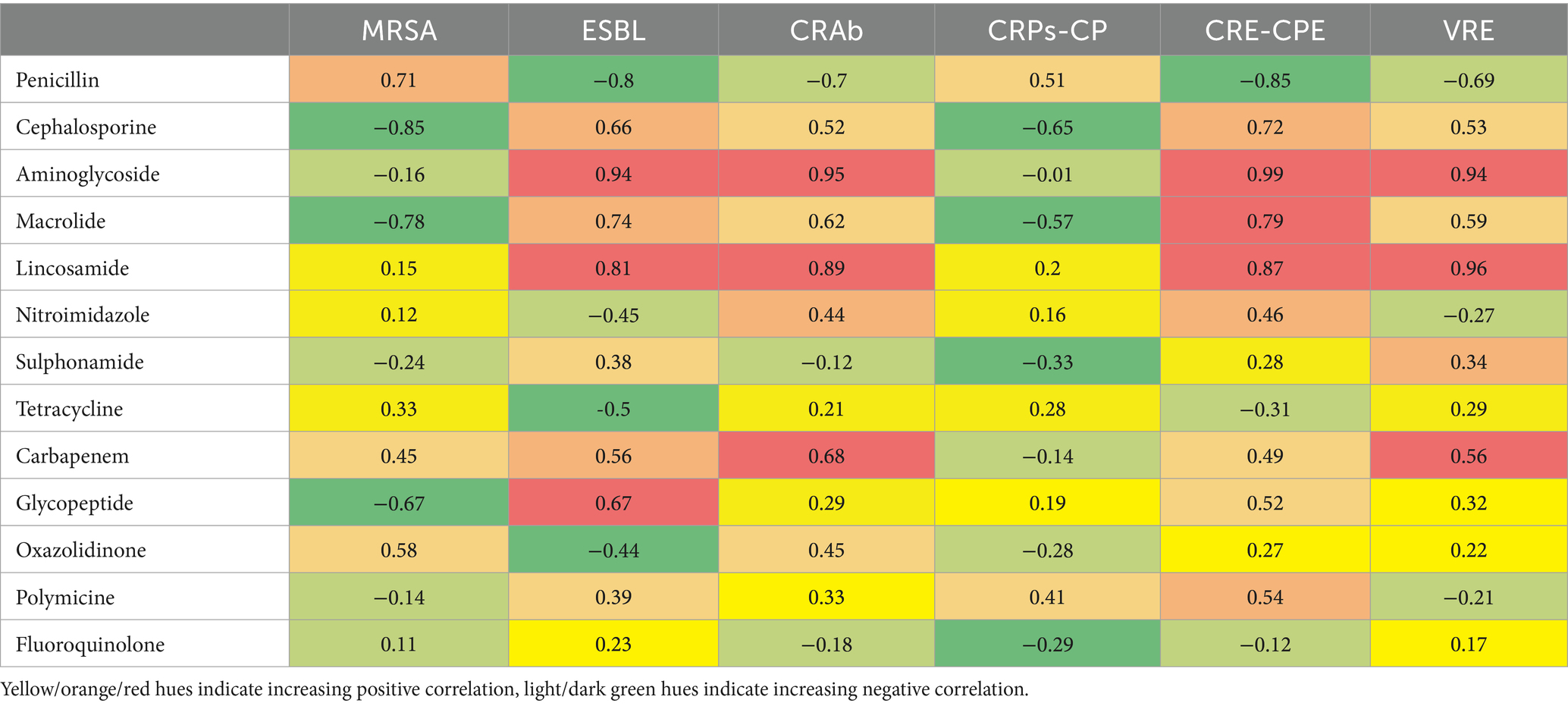
Table 3. Correlation coefficients (r) of specific antibiotics consumed versus occurrence of specific nosocomial infections and colonization with AMR bacteria.
Aminoglycoside and lincosamide consumption correlated strongly (0.94 ≤ r ≤ 0.99) with multiple resistance types, including Extended Spectrum Beta-Lactamase (ESBL), CRAb, CRE-CPE, and VRE, suggesting a higher risk of resistance associated with their use. Carbapenem and glycopeptide consumption showed moderate positive correlations (0.52 ≤ r ≤ 0.68) with resistance, particularly with CRE-CPE, CRAb, and VRE. Penicillin and fluoroquinolone consumption generally displayed weaker or negative correlations (−0.80 ≤ r ≤ −0.12) with resistance types like ESBL and CRE-CPE, suggesting a lower association with resistance. The data showed mixed correlations with occurrences of MRSA, with some antibiotic use (e.g., penicillin and macrolide) correlating negatively, while other usage (e.g., carbapenem, oxazolidinone) showed weak positive correlations.
Discussion
Misuse and overuse of antibiotics led to complex global public health challenges (16) way before the COVID-19 pandemic appeared. In the late 1990s and 2000, the WHO organized various meetings to address the public health threat of antimicrobial resistance, leading to the publication of the Global Strategy for Containment of Antimicrobial Resistance in 2001 (17). With the emergence of the COVID-19 pandemic, antibiotic misuse and AMR renewed their attention (6). It is estimated that 75% of patients with COVID-19 worldwide receive antibiotic prescriptions, although less than 10% of hospitalized and community-based patients are diagnosed with secondary bacterial infection requiring antibiotics (2, 14, 18–21).
Analyzing the data on antibiotic consumption in a selected Slovenian general hospital, the overall prescription of antibiotics stayed approximately the same, although some other studies report of increase in overall hospital antibiotic consumption in 2020 compared with that in 2019 (8, 22–26). However, some important differences can be observed when analyzing specific groups of antibiotics.
Penicillin
Among penicillin antibiotics, basic group representatives’ consumption decreased, while reserve penicillin antibiotics (i.e., piperacillin/tazobactam) increased by as much as 23.42%. This somehow correlates with other research (8, 27) reporting piperacillin/tazobactam as one of the most frequently prescribed antibiotics during the COVID-19 pandemic, being a broad-spectrum antibiotic promising in treating very sick intensive care unit (ICU) admitted patients often requiring ventilatory support (28).
Cephalosporin
Another group of antibiotics with observed increased consumption were cephalosporins (+25.46%) being repurposed during the pandemic (8, 19, 29). Despite increased consumption of penicillin and cephalosporin antibiotics, the occurrence of nosocomial infections and colonization with MRSA decreased by 41.67%. However, a statistically significant decrease in MRSA cases in EU countries was already observed between 2015 and 2019 (30), which is the consequence of improved infection prevention strategies and control (31) and prudent antimicrobial use (32).
Macrolide
In our study, the consumption of macrolides increased by 21.08%. Nandi et al. (6) reported that the sales of macrolide antibiotics increased by 1.5% when the COVID-19 cases increased by 10% in the month since azithromycin was the most common antimicrobial agent used while treating COVID-19 (33). Although, at the beginning of the pandemic, azithromycin was believed to have some action against the SARS-COV-2 virus (34), subsequent research disproves this assumption (35). Cephalosporins and macrolides are being reported as the most common classes of antibiotics used during COVID-19 pandemic (28, 36–39), known to be effective against opportunistic pathogens that could cause secondary infection in COVID-19 (28).
Carbapenems, polymyxin, glycopeptides, oxazolidinone
Regarding other reserve groups of antibiotics (i.e., carbapenems, polymyxin, glycopeptides, oxazolidinone), increased consumptions were observed, the most significant in 10 x increase consumption for polymyxin antibiotics, similar to some other studies (8, 40, 41). The increased use of reserve groups of antibiotics raises concerns (41) since those are meant to be used for treating infections with high-priority pathogens on the WHO list (42) or infections in the ICU (43, 44). Another concern associated with the increased use of carbapenem antibiotics is the rise of carbapenem-resistant and/or carbapenemase-producing isolates (i.e., ESBL, CRAb, CRPs-CP, CRE-CPE), being observed in our study and also reported by others (45, 46). The most significant increase was observed for CRAb, also demonstrated in other studies (47–53), which may be due to higher use of immunosuppressive therapy, invasive devices like ventilators and catheters, and more ICU admissions (54, 55). A similar observation was found for VRE infections and colonization being correlated to the increased use of vancomycin, but this could also be a continuation of the rising trend of VRE infections before the pandemic (14, 30, 56).
Correlation analysis of antibiotic use and resistance
Our analysis found strong correlations between several antibiotics and resistance types. Aminoglycosides demonstrated high positive correlations with ESBL, CRAb, CRE-CPE, and VRE, indicating that increased aminoglycoside consumption may contribute to resistance in these organisms (57, 58). Similarly, lincosamides strongly correlated with VRE, suggesting a link between lincosamide use and resistance development (59). Carbapenems exhibited moderate to strong correlations with CRAb, CRE-CPE, and VRE, consistent with previous research associating carbapenem use with carbapenem-resistant bacteria (60, 61). Glycopeptides showed moderate positive correlations with ESBL and CRE-CPE, though to a lesser extent than aminoglycosides.
In contrast, penicillins and fluoroquinolones displayed weak to negative correlations with resistance, particularly with ESBL and CRE-CPE (62–64). This may suggest that these antibiotics pose a lower resistance risk in this context, though caution remains warranted given their potential resistance effects in other settings (35). The research’s limitations are that the data were collected in only one hospital, so we cannot generalize them more widely. Also, the data on prescribed antibiotics was collected, which does not necessarily reflect actual consumption. We should take into account that in 2020/2021, there were several waves of COVID-19, and the hospital situation changed every month, so perhaps certain effects of the epidemic are lost in the review of results on an annual basis.
Conclusion
Although there are differences between antibiotic use and the occurrence of AMR bacteria both before and during the COVID-19 pandemic, the Centers for Disease Control and Prevention (CDC) reported that the pandemic caused a loss of progress in combating AMR (65) and the latest WHO evidence shows that the extensive overuse of antibiotics during COVID-19 pandemic worldwide may have worsened the spread of AMR (66). Such observations were also found in the Romanian hospital, where, despite a lower number of patients, an increased use of antibiotics was observed in 2021 (67). Even though we did not detect an increase in the overall consumption of antibiotics, there remains a worrying increase in certain important groups of antibiotics (the so-called representatives of the last line of defense: vancomycin, linezolid, piperacillin/tazobactam, meropenem, colistin). Similar was observed in the Romanian hospital where they also noticed a decrease in the proportion of antibiotics from the Watch and Reserve class and an increase in the proportion of antibiotics from the Access class (67). In our research, we did not observe the difference in the occurrence of AMR infections and colonizations before and during the pandemic. But we did observe an alarming increase in CRaB, ESBL and VRE and, in particular, highlight the increase in all AMR groups between the first and second year of the pandemic (2020, 2021). If in the first year of the pandemic (2020), the impact of the long-term effort to limit AMR may still be present, in the second year of the pandemic (2021), an extraordinary spread of AMR is already visible, which may also be the result of pandemic fatigue among employees. Nevertheless, the connection between antibiotic consumption and the occurrence of AMR infections and colonization was confirmed (p = 0.028).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
UR: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. KK: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AŠ: Conceptualization, Data curation, Supervision, Writing – review & editing. UK: Conceptualization, Data curation, Supervision, Writing – review & editing. DV: Methodology, Supervision, Writing – review & editing. ML: Data curation, Methodology, Software, Visualization, Writing – review & editing. ST: Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was financially supported by a National Research programme (P2-0118).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. (2023) Statement on the fifteenth meeting of the IHR (2005) emergency committee on the COVID-19 pandemic 2023. Available online at: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (Accessed December 5, 2024).
2. Fukushige, M, Ngo, N-H, Lukmanto, D, Fukuda, S, and Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: a systematic review comparing 2019 and 2020 data. Front Public Health. (2022) 10:946077. doi: 10.3389/fpubh.2022.946077
3. Ghosh, S, Bornman, C, and Zafer, MM. Antimicrobial resistance threats in the emerging COVID-19 pandemic: where do we stand? J Infect Public Health. (2021) 14:555–60. doi: 10.1016/j.jiph.2021.02.011
4. Malik, SS, and Mundra, S. Increasing consumption of antibiotics during the COVID-19 pandemic: implications for patient health and emerging anti-microbial resistance. Antibiotics. (2022) 12:45. doi: 10.3390/antibiotics12010045
5. Monnet, DL, and Harbarth, S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Euro Surveill. (2020) 25:25 (45). doi: 10.2807/1560-7917.Es.2020.25.45.2001886
6. Nandi, A, Pecetta, S, and Bloom, DE. Global antibiotic use during the COVID-19 pandemic: analysis of pharmaceutical sales data from 71 countries, 2020-2022. eClinicalMedicine. (2023) 57:101848. doi: 10.1016/j.eclinm.2023.101848
7. Rehman, S. A parallel and silent emerging pandemic: antimicrobial resistance (AMR) amid COVID-19 pandemic. J Infect Public Health. (2023) 16:611–7. doi: 10.1016/j.jiph.2023.02.021
8. Silva, AR, Salgado, DR, Lopes, LP, Castanheira, D, Emmerick, IC, and Lima, EC. Increased use of antibiotics in the intensive care unit during coronavirus disease (COVID-19) pandemic in a Brazilian hospital. Front Pharmacol. (2021) 12:778386. doi: 10.3389/fphar.2021.778386
9. Dadras, O, Alinaghi, SAS, Karimi, A, Mohsseni Pour, M, Barzegary, A, Vahedi, F, et al. Effects of COVID-19 prevention procedures on other common infections: a systematic review. Eur J Med Res. (2021) 26:67. doi: 10.1186/s40001-021-00539-1
10. Jeong, YD, Ejima, K, Kim, KS, Joohyeon, W, Iwanami, S, Fujita, Y, et al. Designing isolation guidelines for COVID-19 patients with rapid antigen tests. Nat Commun. (2022) 13:4910. doi: 10.1038/s41467-022-32663-9
11. Rubin, D, Chan-Tack, K, Farley, J, and Sherwat, A. FDA approval of Remdesivir — a step in the right direction. N Engl J Med. (2020) 383:2598–600. doi: 10.1056/NEJMp2032369
12. Kim, S. COVID-19 Drug Development. J Microbiol Biotechnol. (2022) 32:1–5. doi: 10.4014/jmb.2110.10029
13. Ndwandwe, D, and Wiysonge, CS. COVID-19 vaccines. Curr Opin Immunol. (2021) 71:111–6. doi: 10.1016/j.coi.2021.07.003
14. Langford, BJ, So, M, Raybardhan, S, Leung, V, Soucy, JR, Westwood, D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. (2021) 27:520–31. doi: 10.1016/j.cmi.2020.12.018
15. Nori, P, Cowman, K, Chen, V, Bartash, R, Szymczak, W, Madaline, T, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the new York City pandemic surge. Infect Control Hosp Epidemiol. (2021) 42:84–8. doi: 10.1017/ice.2020.368
16. WHO. Global antimicrobial resistance and use surveillance system (GLASS) report 2022. Geneva: World Health Organization (2022).
17. WHO. WHO global strategy for containment of antimicrobial resistance. Geneva: World Health Organization (2001).
18. Alshaikh, FS, Godman, B, Sindi, ON, Seaton, RA, and Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: a systematic review and meta-analysis. PLoS One. (2022) 17:e0272375. doi: 10.1371/journal.pone.0272375
19. Karami, Z, Knoop, BT, Dofferhoff, ASM, Blaauw, MJT, Janssen, NA, van Apeldoorn, M, et al. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in the Netherlands. Infect Dis. (2021) 53:102–10. doi: 10.1080/23744235.2020.1839672
20. Rose, AN, Baggs, J, Wolford, H, Neuhauser, MM, Srinivasan, A, Gundlapalli, AV, et al. Trends in antibiotic use in United States hospitals during the coronavirus disease 2019 pandemic. Open Forum Infect Dis. (2021) 8:ofab236. doi: 10.1093/ofid/ofab236
21. Russell, CD, Fairfield, CJ, Drake, TM, Turtle, L, Seaton, RA, Wootton, DG, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. (2021) 2:e354–65. doi: 10.1016/s2666-5247(21)00090-2
22. Andrews, A, Budd, EL, Hendrick, A, Ashiru-Oredope, D, Beech, E, Hopkins, S, et al. Surveillance of antibacterial usage during the COVID-19 pandemic in England, 2020. Antibiotics. (2021) 10:841. doi: 10.3390/antibiotics10070841
23. Grau, S, Hernández, S, Echeverría-Esnal, D, Almendral, A, Ferrer, R, Limón, E, et al. Antimicrobial consumption among 66 acute care hospitals in Catalonia: impact of the COVID-19 pandemic. Antibiotics. (2021) 10:943. doi: 10.3390/antibiotics10080943
24. Macera, M, Onorato, L, Calò, F, Monari, C, Annibale, R, Signoriello, G, et al. The impact of the SARS-Cov 2 pandemic on a persuasive educational antimicrobial stewardship program in a university hospital in southern Italy: a pre-post study. Antibiotics. (2021) 10:1405. doi: 10.3390/antibiotics10111405
25. Murgadella-Sancho, A, Coloma-Conde, A, and Oriol-Bermúdez, I. Impact of the strategies implemented by an antimicrobial stewardship program on the antibiotic consumption in the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. (2022) 43:1292–3. doi: 10.1017/ice.2021.237
26. Padhan, STP, Chandrakar, R, Galhotra, A, and Borkar, NB. Assessment of the impact of COVID-19 on drug store Management in a Tertiary Care Teaching Hospital of Central India. Cureus. (2021) 13:e19723. doi: 10.7759/cureus.19723
27. Tenforde, MW, Kim, SS, Lindsell, CJ, Billig Rose, E, Shapiro, NI, Files, DC, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, march–June 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:993–8. doi: 10.15585/mmwr.mm6930e1
28. Thapa, B, Pathak, SB, Jha, N, Sijapati, MJ, and Shankar, PR. Antibiotics use in hospitalised COVID-19 patients in a tertiary care Centre: a descriptive cross-sectional study. JNMA J Nepal Med Assoc. (2022) 60:625–30. doi: 10.31729/jnma.7394
29. Durojaiye, AB, Clarke, JD, Stamatiades, GA, and Wang, C. Repurposing cefuroxime for treatment of COVID-19: a scoping review of in silico studies. J Biomol Struct Dyn. (2021) 39:4547–54. doi: 10.1080/07391102.2020.1777904
30. ECDC. Surveillance of antimicrobial resistance in Europe 2017. Stockholm: European Centre for Disease Prevention and Control (2018).
31. Wee, LEI, Conceicao, EP, Tan, JY, Magesparan, KD, Amin, IBM, Ismail, BBS, et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am J Infect Control. (2021) 49:469–77. doi: 10.1016/j.ajic.2020.10.019
32. ECDC. (2024). Directory of online resources for prevention and control of antimicrobial resistance (AMR) and healthcare-associated infections (HAI). Available online at: https://www.ecdc.europa.eu/en/publications-data/directory-online-resources-prevention-and-control-antimicrobial-resistance-amr (Accessed December 5, 2024).
33. Cangini, A, Fortinguerra, F, Di Filippo, A, Pierantozzi, A, Da Cas, R, Villa, F, et al. Monitoring the community use of antibiotics in Italy within the National Action Plan on antimicrobial resistance. Br J Clin Pharmacol. (2021) 87:1033–42. doi: 10.1111/bcp.14461
34. Echeverría-Esnal, D, Martin-Ontiyuelo, C, Navarrete-Rouco, ME, De-Antonio Cuscó, M, Ferrández, O, Horcajada, JP, et al. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti-Infect Ther. (2021) 19:147–63. doi: 10.1080/14787210.2020.1813024
35. Butler, CC, Dorward, J, Yu, LM, Gbinigie, O, Hayward, G, Saville, BR, et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. (2021) 397:1063–74. doi: 10.1016/S0140-6736(21)00461-X
36. Goncalves Mendes Neto, A, Lo, KB, Wattoo, A, Salacup, G, Pelayo, J, DeJoy, R III, et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol. (2021) 93:1489–95. doi: 10.1002/jmv.26441
37. Mustafa, L, Tolaj, I, Baftiu, N, and Fejza, H. Use of antibiotics in COVID-19 ICU patients. J Infect Dev Ctries. (2021) 15:501–5. doi: 10.3855/jidc.14404
38. Wei, W, Ortwine, JK, Mang, NS, Joseph, C, Hall, BC, and Prokesch, BC. (2020). Limited role for antibiotics in COVID-19: scarce evidence of bacterial coinfection. Available online at: https://www.medrxiv.org/content/10.1101/2020.06.16.20133181v1.
39. Yacouba, A, Olowo-okere, A, and Yunusa, I. Repurposing of antibiotics for clinical management of COVID-19: a narrative review. Ann Clin Microbiol Antimicrob. (2021) 20:37. doi: 10.1186/s12941-021-00444-9
40. Erdem, I, Ardic, E, Turker, E, Kardan, ME, and Javarova Demirkapu, M. Comparison of antibiotic use in the COVID-19 pandemic with the pre-pandemic period in a university hospital. Arch Med Sci. (2022) 18:1392–4. doi: 10.5114/aoms/152752
41. Mah-E-Muneer, S, Hassan, MZ, Biswas, MAAJ, Rahman, F, Akhtar, Z, Das, P, et al. Use of antimicrobials among suspected COVID-19 patients at selected hospitals, Bangladesh: findings from the first wave of COVID-19 pandemic. Antibiotics. (2021) 10:738. doi: 10.3390/antibiotics10060738
42. Tacconelli, E, Carrara, E, Savoldi, A, Harbarth, S, Mendelson, M, Monnet, DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. (2018) 18:318–27. doi: 10.1016/S1473-3099(17)30753-3
43. Doi, Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis. (2019) 69:S565–75. doi: 10.1093/cid/ciz830
44. Karaiskos, I, Lagou, S, Pontikis, K, Rapti, V, and Poulakou, G. The "old" and the "new" antibiotics for MDR gram-negative pathogens: for whom, when, and How. Front Public Health. (2019) 7:151. doi: 10.3389/fpubh.2019.00151
45. Castro, MG, Ubiergo, L, Vicino, M, Cuevas, G, and Argarañá, F. Rising incidence of carbapenem resistant isolates: an Argentinian hospital’s experience. More trouble in the aftermath of the COVID-19 pandemic. Iberoam J Med. (2022) 4:92–9. doi: 10.53986/ibjm.2022.0020
46. Ramette, A, Gasser, M, Nordmann, P, Zbinden, R, Schrenzel, J, Perisa, D, et al. Temporal and regional incidence of carbapenemase-producing Enterobacterales, Switzerland, 2013 to 2018. Euro Surveill. (2021) 26:1900760. doi: 10.2807/1560-7917.Es.2021.26.15.1900760
47. de Carvalho Hessel Dias, VM, Tuon, F, de Jesus Capelo, P, Telles, JP, Fortaleza, C, and Pellegrino Baena, C. Trend analysis of carbapenem-resistant gram-negative bacteria and antimicrobial consumption in the post-COVID-19 era: an extra challenge for healthcare institutions. J Hosp Infect. (2022) 120:43–7. doi: 10.1016/j.jhin.2021.11.011
48. Lai, CC, Chen, SY, Ko, WC, and Hsueh, PR. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. (2021) 57:106324. doi: 10.1016/j.ijantimicag.2021.106324
49. Pascale, R, Bussini, L, Gaibani, P, Bovo, F, Fornaro, G, Lombardo, D, et al. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: a multicenter before-and-after cross-sectional study. Infect Control Hosp Epidemiol. (2022) 43:461–6. doi: 10.1017/ice.2021.144
50. Polemis, M, Mandilara, G, Pappa, O, Argyropoulou, A, Perivolioti, E, Koudoumnakis, N, et al. COVID-19 and antimicrobial resistance: data from the Greek electronic system for the surveillance of antimicrobial resistance-WHONET-Greece (January 2018-march 2021). Life. (2021) 11:996. doi: 10.3390/life11100996
51. Polly, M, de Almeida, BL, Lennon, RP, Cortês, MF, Costa, SF, and Guimarães, T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am J Infect Control. (2022) 50:32–8. doi: 10.1016/j.ajic.2021.09.018
52. Shbaklo, N, Corcione, S, Vicentini, C, Giordano, S, Fiorentino, D, Bianco, G, et al. An observational study of MDR hospital-acquired infections and antibiotic use during COVID-19 pandemic: a call for antimicrobial stewardship programs. Antibiotics. (2022) 11:695. doi: 10.3390/antibiotics11050695
53. Sinto, R, Lie, KC, Setiati, S, Suwarto, S, Nelwan, EJ, Djumaryo, DH, et al. Blood culture utilization and epidemiology of antimicrobial-resistant bloodstream infections before and during the COVID-19 pandemic in the Indonesian national referral hospital. Antimicrob Resist Infect Control. (2022) 11:73. doi: 10.1186/s13756-022-01114-x
54. Kim, YJ, Kim, SI, Kim, YR, Hong, KW, Wie, SH, Park, YJ, et al. Carbapenem-resistant Acinetobacter baumannii: diversity of resistant mechanisms and risk factors for infection. Epidemiol Infect. (2012) 140:137–45. doi: 10.1017/s0950268811000744
55. Sheng, WH, Liao, CH, Lauderdale, TL, Ko, WC, Chen, YS, Liu, JW, et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis. (2010) 14:e764–9. doi: 10.1016/j.ijid.2010.02.2254
56. Cassini, A, Högberg, LD, Plachouras, D, Quattrocchi, A, Hoxha, A, Simonsen, GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect Dis. (2019) 19:56–66. doi: 10.1016/s1473-3099(18)30605-4
57. Garneau-Tsodikova, S, and Labby, KJ. Mechanisms of resistance to aminoglycoside antibiotics: overview and perspectives. Medchemcomm. (2016) 7:11–27. doi: 10.1039/c5md00344j
58. WHO. WHO report on surveillance of antibiotic consumption: 2016–2018 early implementation. Geneva: WHO (2018).
59. Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin Infect Dis. (2002) 34:482–92. doi: 10.1086/324626
61. Nordmann, P, Naas, T, and Poirel, L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. (2011) 17:1791–8. doi: 10.3201/eid1710.110655
62. Dalhoff, A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip Perspect Infect Dis. (2012) 2012:976273. doi: 10.1155/2012/976273
63. Garcia-Bustos, V, Cabañero-Navalón, MD, and Salavert Lletí, M. Resistance to beta-lactams in gram-negative bacilli: relevance and potential therapeutic alternatives. Rev Esp Quimioter. (2022) 35:1–15. doi: 10.37201/req/s02.01.2022
64. Pana, ZD, and Zaoutis, T. Treatment of extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLs) infections: what have we learned until now? F1000Res. (2018) 7:1347. doi: 10.12688/f1000research.14822.1
65. CDC. COVID-19: U.S. impact on antimicrobial resistance, special report 2022. Atlanta, GA: CDC (2022).
66. WHO. WHO reports widespread overuse of antibiotics in patients hospitalized with COVID-19. Geneva: WHO (2024).
Keywords: COVID-19, antibiotics, AMR, general hospital, Slovenia
Citation: Rozman U, Kranjec K, Šeruga A, Kramar U, Vrbnjak D, Lavrič M and Turk SŠ (2025) Impact of the COVID-19 pandemic on the consumption of antibiotics and the emergence of AMR: case study in a general hospital. Front. Public Health. 13:1584574. doi: 10.3389/fpubh.2025.1584574
Edited by:
German Matias Traglia, Universidad de La Republica, Uruguay, UruguayReviewed by:
Dana Carmen Zaha, University of Oradea, RomaniaEvelyn Yayra Bonney, University of Ghana, Ghana
Copyright © 2025 Rozman, Kranjec, Šeruga, Kramar, Vrbnjak, Lavrič and Turk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Urška Rozman, dXJza2Eucm96bWFuQHVtLnNp
 Urška Rozman
Urška Rozman Konrad Kranjec1
Konrad Kranjec1 Dominika Vrbnjak
Dominika Vrbnjak Miha Lavrič
Miha Lavrič