- 1Arctech Innovation, Dagenham, United Kingdom
- 2Department of Disease Control, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 3Center for Immunology of Viral Infections, Department of Biomedicine, Aarhus University, Aarhus, Denmark
- 4Reckitt Benckiser (India) Pvt. Ltd., Gurgaon, Haryana, India
Vector-borne diseases, including dengue, threaten the health and livelihoods of over 80% of the world’s population, particularly in tropical and subtropical regions. Environmental, ecological, climatic, and socio-economic factors are expected to drive increased transmission, emphasizing the need to identify key threats and prioritize strategies for control. We examined drivers, challenges and potential solutions with global experts, using Brazil and India as case studies. Both countries face rapid population growth, unplanned urbanization and increased exposure to animal reservoirs alongside unique surveillance and control challenges. We advocate for improvements in surveillance systems and capacity, investment in sustainable vector control tools, leveraging of artificial intelligence for outbreak prediction, and fostering public-private partnerships to develop innovative interventions. A multifaceted approach, combining community-led initiatives with advanced technologies, is essential to reducing the burden of vector-borne diseases and preventing future epidemics.
Introduction
Vector-borne diseases (VBDs) account for 17% of all infectious diseases, and lead to 700,000 deaths every year (1). A review of all emerging disease events at the start of the twenty-first century revealed that almost 30% were vector-borne, and the emergence of novel pathogens has correlated significantly with environmental, ecological, climatic and socio-economic factors (2, 3). Now is a critical time to identify which diseases pose the greatest future health threats and focus on the most promising strategies to limit their impact now and in the future. We explored these issues in interviews with global experts in VBDs to understand the most pressing concerns. The thoughts of these stakeholders are presented using the countries of Brazil and India as case studies.
Burden of vector-borne diseases in Brazil and India
Brazil has the highest burden of VBDs across all Latin America and the Caribbean and has experienced a significant number of outbreaks this century. Most importantly, there has been an increase in the number of dengue epidemics and the geographical range of this disease (4, 5). In 2023 there were over 1.6 million probable cases of dengue across Brazil and over 1,000 deaths, and in 2024 those figures stood at over 6.5 million probable cases and more than 6,000 deaths, underscoring the dramatically increased risk of arbovirus infections in a short span of time (6). There were also over 260,000 probable cases of chikungunya in 2024, and over 13,800 confirmed cases of Oropouche virus infection (6).
At the start of this current rapid increase in dengue, Dr. Aline Campos, Chief of Environmental Health Surveillance Division and the State Department of Health, Brazil, told us of her state, Rio Grande do Sul: “The VBD of most concern for public health is dengue, absolutely. Dengue is the major problem here. We also have chikungunya. We also have Zika. But all the deaths this year (2022) were related to dengue.” This view was supported by Dr. Marcio Pavan, Associate Professor, Laboratório de Mosquitos Transmissores de Hematozoários, Fundação Oswaldo Cruz: “The arboviruses are the most worrisome VBDs because they are in areas of high human population density and present important sequelae. For example, Zika and microcephaly in newborns, or Guillain-Barré syndrome and chikungunya, or dengue and hemorrhagic conditions. Many infections lead to death.” Aedes-borne arboviruses – dengue, chikungunya, yellow fever and Zika – are considered the diseases most likely to cause epidemics in the future due to the anthropophilic nature of their vectors, which are widespread in tropical and sub-tropical regions (7). Brazil also has the highest number of confirmed cases of Mayaro virus infection in Latin America and the Caribbean, and other vector-borne pathogens of concern include Oropouche virus, which has been responsible for over half a million human cases in Brazil to date (8, 9).
Major VBDs circulating in India include malaria, dengue, chikungunya, Japanese encephalitis, lymphatic filariasis, visceral leishmaniasis, and plague (10–12). In 2024, the National Centre for Vector Borne Diseases Control reported 236 deaths and over 233,000 cases of dengue, as well as more than 231,000 suspected and 17,800 confirmed cases of chikungunya (13, 14). India harbors nearly 40% of all global lymphatic filariasis infections, with 619,000 lymphedema and 126,000 hydrocele cases reported as of 2023 (15). It also accounts for approximately 18% of worldwide cases of visceral leishmaniasis, known locally as kala-azar (16, 17), reporting 438 cases and 2 deaths in 2024 (18). Whilst progress has been made toward elimination, Professor Mary Cameron, Professor of Medical Entomology, the London School of Hygiene & Tropical Medicine (LSHTM), warned that the country must remain vigilant: “A significant challenge is that once elimination targets have been reached, people take the foot off the brake. So, there remains the possibility that a disease like visceral leishmaniasis can cause a huge outbreak if you do not have good surveillance systems in place.”
Climate change
Climate change and urbanization were most frequently mentioned by our experts as main contributors to the emergence and spread of VBDs. In Brazil as well as other areas of the tropics, increases in temperature, rainfall and duration of wet seasons can enhance opportunities for pathogens and vectors to reproduce at higher rates and transmit pathogens over broader geographical ranges for more months of the year. Extended wet seasons may increase opportunities for spatial and temporal overlap and interaction between vectors, pathogens and novel hosts, and this could lead to the emergence of a disease or the spillover of a pathogen to a new vector or host population (19). Simulation models project that approximately half of the global population may be exposed to Aedes aegypti by 2050, and that 60% of the world’s population will be at risk of dengue by 2080 (20, 21).
The number of months suitable for dengue transmission in India has increased over the last half-century, with Aedes population growth being enabled by more favorable climatic conditions (22). Models predict that coastal regions may experience year-round transmission of vector-borne diseases in the future, though the highest transmission potential will continue to occur during the monsoon season (23). Along the coast, rising sea levels have caused an expansion of brackish and saline water bodies. Culex tritaeniorhynchus and Cx. gelidus, which are competent vectors of Japanese encephalitis and West Nile virus, are becoming increasingly tolerant of saline conditions and can now breed in salty water bodies, which has huge implications for VBD control in India’s coastal regions (24). Public health authorities must recognize and acknowledge the impact that such adaptations can have on disease transmission, and institute appropriate surveillance and control measures.
Environmental, ecological and behavioral drivers
Other environmental and ecological factors driving the spread of VBDs were also raised, including changes in land use and increased contact between people and nature. Models exploring the spatiotemporal variation of yellow fever both inter-annually and seasonally suggest that heterogeneity in vegetation (a proxy for habitat fragmentation) and land cover could explain both the expansion of the yellow fever transmission zone as well as the highly seasonal nature of yellow fever in Brazil (25). Globally, agricultural drivers have been associated with more than 25% of emerging infectious diseases and more than 50% of emerging zoonotic diseases in humans (26). Some agricultural practices such as the expansion of habitable cropland, and other microclimatic land use changes can lead to the creation of larval habitats for vectors or increase contact between sylvatic vectors, humans and livestock (27). Dr. Emma Maynard, Research Manager, Infections and Climate, Wellcome Trust, explained: “When the natural environment is altered to facilitate a different land use, such as mining, agriculture or dams, humans are brought into closer contact with wildlife, vectors and potential infectious disease reservoirs. The local climate and environmental conditions change, which can influence vector abundance and behaviour facilitating increased disease transmission.”
Changes in land use occurring with the expansion of urban environments have been associated with the emergence and spread of those VBDs whose vectors thrive in and around human dwellings (28). In Brazil, rapid urban population growth has included informal settlements that lack the basic infrastructure and services that are important for controlling Aedes populations, such as drainage systems and waste management (29).
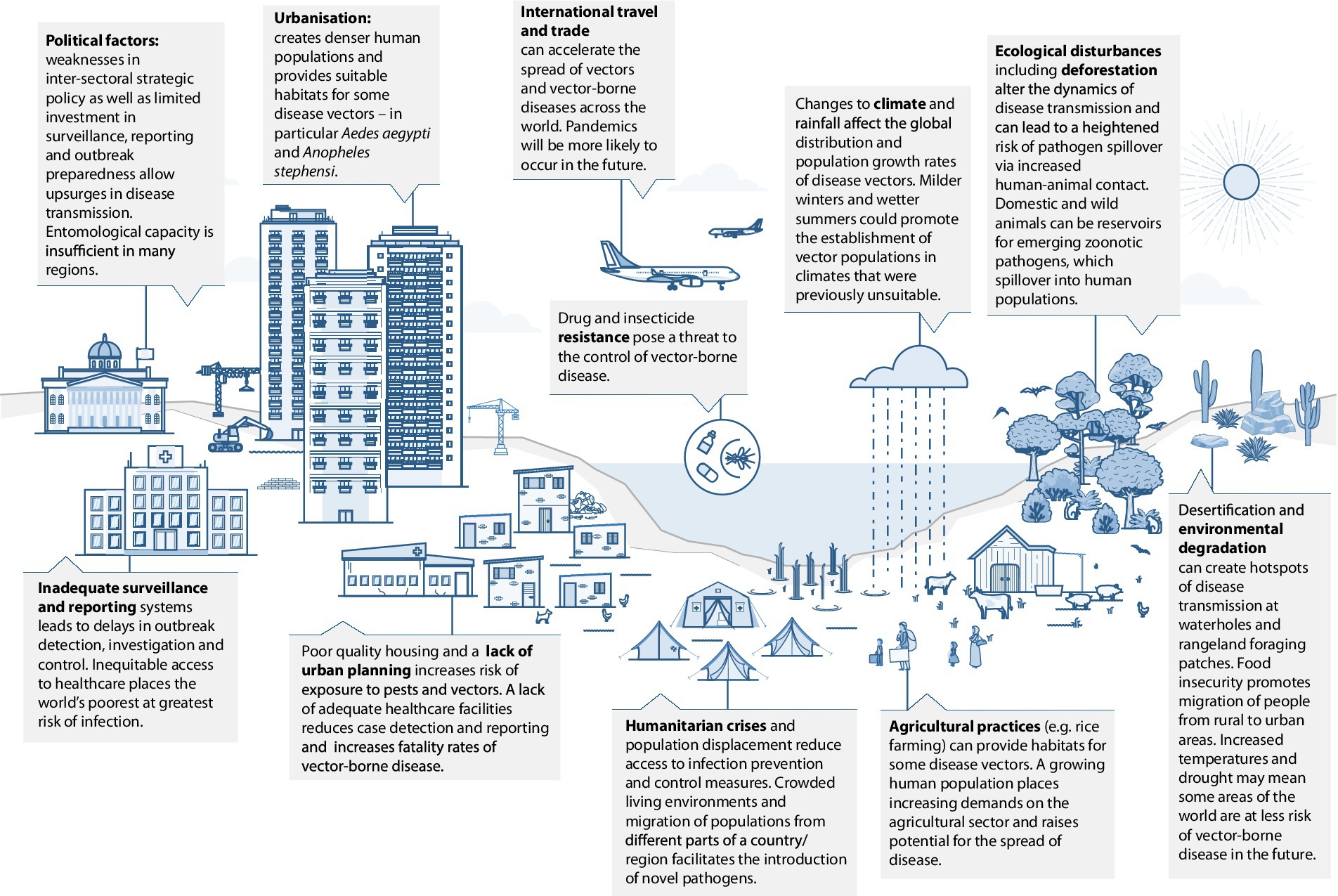
In India, human migration has been a major factor contributing to malaria transmission, especially in cities where there have been influxes of migrant workers from rural parts of the country, driven in-part by climate change and associated challenges faced by agricultural communities (30). Mobile populations may be undocumented and have limited access to health services (31), so pose a challenge to disease control and elimination. India is home to some of the largest religious and cultural mass gatherings in the world and disease outbreaks have occurred during several of these events, contributing to disease transmission in other parts of the country once visitors return home (32). It is likely that these large-scale gatherings provide platforms for exchange of genomic material and thereby evolution of pathogens, including viruses. Dr. Arun Sivan, Consultant Entomologist (Technical), National Vector Borne Disease Control Programme, Odisha, identified this among other concerns in India: “The main contributors to the emergence and spread of new and existing VBDs are rapid and unplanned urbanization, and increased migration of people for occupation, tourism, and pilgrimage” (Figure 1).
Surveillance and control
India’s Directorate of National Vector Borne Disease Control Programme (NVBDCP) is responsible for formulating technical guidance and policy-making to guide states in the implementation of disease prevention and control programs. Each state in India has a VBD control unit responsible for leading prevention, surveillance and control in their sub-districts (33), but the VBD surveillance system in India needs enhancement, especially in urban areas, and could benefit from integrated data from both private and public healthcare services (34). There is also an urgent need for human resources, including trained medical entomologists. A demand–supply analysis estimated the need for at least a thousand specialists in medical entomology in India, while annual output is typically below one hundred (35). Dr. Arun Sivan: “Shortcomings in the ability to survey and control VBDs include a lack of trained manpower, especially entomologists.”
There are opportunities to support surveillance and control through community engagement and involvement in control activities. Dr. Melinda Rostal, Principal Scientist, Vector-Borne Diseases, EcoHealth Alliance told us: “It is critical to think about integrating social science into the development of interventions involving personal protection. This includes taking the time to understand how the community understands VBDs and the best way to present the intervention to said community and maximize uptake of the intervention.” Dr. Helen Jamet, Deputy Director, Malaria, Bill & Melinda Gates Foundation, agreed: “Most personal protection tools unfortunately rely on behavioural change and adherence, which can limit their efficacy even if the individual tool works very well.”
Community-led environmental and larval site management methods have proven to be effective methods of vector control (34). As Dr. Raman Velayudhan, of the Global Neglected Tropical Diseases Programme, World Health Organization, identified: ultimately, “a combination of tools will be needed to control VBD outbreaks in the future including multiplex diagnostics, better case management, innovative sustainable vector control tools targeting both immatures and adults, effective vaccines, therapeutics, integrated surveillance and above all community support.” Dr. Oliver Brady, Associate Professor, LSHTM told us that “new tools need to be sustainable (ideally with minimal ongoing effort), synergistic with existing interventions and have strong evidence (ideally cluster randomized trial(s)) of reducing cases of disease, not just entomological outcome(s).”
Discussion
Dengue and other Aedes-borne diseases will continue to be a significant health burden in tropical and subtropical regions of the world, and climate change, increasing urbanization, population movement and land-use change will drive the emergence and spread of VBDs. Prioritizing funding for national surveillance and data reporting systems could be an effective way to fill gaps in knowledge, enabling more accurate prediction of disease occurrence and better allocation of resources to prevent and control outbreaks. Countries should strengthen community engagement activities and education initiatives, ensuring those most affected or at greatest risk of disease are supported in adopting effective prevention measures. Governments should be open to the use of social media, citizen science and non-standard data to inform detection and early warning systems, and to public-private partnerships that can innovate and accelerate the creation of more sustainable, more efficacious tools for control, with a focus on new active ingredients and more environmentally conscious approaches to manufacturing. Industry can play a critical role in scale-up and integration of vector control tools through leveraging supply chain management and offering expertise in logistics, cost-efficiencies, and long-term planning capabilities (36). Advances in artificial intelligence and machine learning should also be embraced. These could support improvements in models and enable public health policy makers to generate outbreak preparedness plans, including effective allocation of limited health resources to tackle diseases in hotspots of transmission.
Human populations will only be free of the threat of VBDs if they can be eliminated. This is an enduring challenge, particularly in the face of resistance to chemical tools. Whilst some countries have achieved eradication of malaria or other VBDs in their territories, their citizens now, or will soon, face the risk of other infections. It is vital that we accelerate our understanding of the risks, that we push forward new ideas at pace, and take ownership of our diverse roles to create robust methods to predict, prevent and manage these diseases now and for the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AH: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing. RJ: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing. JD: Data curation, Investigation, Methodology, Resources, Writing – original draft. WD: Data curation, Investigation, Methodology, Resources, Writing – original draft. LP: Investigation, Methodology, Resources, Writing – original draft. FSp: Data curation, Investigation, Methodology, Project administration, Resources, Writing – original draft. FK: Data curation, Investigation, Writing – original draft. FSe: Data curation, Investigation, Writing – original draft. AN: Conceptualization, Funding acquisition, Writing – review & editing. AD: Conceptualization, Funding acquisition, Writing – review & editing. JL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This manuscript was developed following research that was funded by Reckitt Benckiser (India) Limited.
Acknowledgments
We thank those stakeholders who offered their expert opinion on vector-borne disease risks in their countries and globally, and for their excellent thoughts on how to address the risks.
Conflict of interest
AN and AD were employed by Reckitt Benckiser (India) Pvt. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Vector-borne diseases. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (Accessed October 2023).
2. Jones, KE, Patel, NG, Levy, MA, Storeygard, A, Balk, D, Gittleman, JL, et al. Global trends in emerging infectious diseases. Nature. (2008) 451:990–3. doi: 10.1038/nature06536
3. Chala, B, and Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: a review. Front Public Heal. (2021) 9:715759. doi: 10.3389/fpubh.2021.715759
4. Instituto Oswaldo Cruz. Recorde de óbitos por dengue chama atenção para combate ao Aedes aegypti. (2023). Available online at: https://www.ioc.fiocruz.br/noticias/recorde-de-obitos-por-dengue-chama-atencao-para-combate-ao-aedes-aegypti (Accessed October 2023).
5. Codeco, CT, Oliveira, SS, Ferreira, DAC, Riback, TIS, Bastos, LS, Lana, RM, et al. Fast expansion of dengue in Brazil. Lancet Reg Heal Am. (2022) 12:100274. doi: 10.1016/j.lana.2022.100274
6. Ministério da Saúde. Painel de monitoramento das arboviroses — Ministério da Saúde. (2024). Available online at: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/aedes-aegypti/monitoramento-das-arboviroses (Accessed October 2023).
7. de Carvalho, EM, Valverde, SS, and Muñoz, JAH. Aedes aegypti: the main enemy of public health in Brazil - challenges and perspective for public health In: FH Kasenga, editor. Malaria London: IntechOpen. (2019) doi: 10.5772/intechopen.79780
8. Sciancalepore, S, Schneider, MC, Kim, J, Galan, DI, and Riviere-Cinnamond, A. Presence and multi-species spatial distribution of Oropouche virus in Brazil within the one health framework. Trop Med Infect Dis. (2022) 7:111. doi: 10.3390/tropicalmed7060111
9. Sah, R, Srivastava, S, Kumar, S, Golmei, P, Rahaman, SA, Mehta, R, et al. Oropouche fever outbreak in Brazil: an emerging concern in Latin America. Lancet Microbe. (2024) 5:100904. doi: 10.1016/S2666-5247(24)00136-8
10. Kumar, P, Srivastava, S, Banerjee, A, and Banerjee, S. Prevalence and predictors of water-borne diseases among elderly people in India: evidence from longitudinal ageing study in India, 2017–18. BMC Public Health. (2022) 22:993. doi: 10.1186/s12889-022-13376-6
11. Muzembo, BA, Kitahara, K, Debnath, A, Ohno, A, Okamoto, K, and Miyoshi, SI. Cholera outbreaks in India, 2011–2020: a systematic review. Int J Environ Res Public Health. (2022) 19:5738. doi: 10.3390/ijerph19095738
12. Rajamannar, V, Govindarajan, R, Kumar, A, and Samuel, P. A review of public health important fleas (Insecta, Siphonaptera) and flea-borne diseases in India. J Vector Borne Dis. (2022) 59:12–21. doi: 10.4103/0972-9062.328977
13. National Center for Vector Borne Diseases Control India Dengue situation in India. (2025). Available online at: https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=431&lid=3715 (Accessed October 2023).
14. National Center for Vector Borne Diseases Control India. Chikungunya situation in India. (2025). Available online at: https://ncvbdc.mohfw.gov.in/index1.php?lang=1&level=2&sublinkid=5967&lid=3765 (Accessed October 2023).
15. National Center for Vector Borne Diseases Control India Filariasis magnitude of disease. (2025). Available online at: https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=455&lid=3732 (Accessed October 2023).
16. Bizhani, N, Hashemi Hafshejani, S, Mohammadi, N, Rezaei, M, and Rokni, MB. Lymphatic filariasis in Asia: a systematic review and meta-analysis. Parasitol Res. (2021) 120:411–22. doi: 10.1007/s00436-020-06991-y
17. Sundar, S, Singh, OP, and Chakravarty, J. Visceral leishmaniasis elimination targets in India, strategies for preventing resurgence. Expert Rev Anti-Infect Ther. (2018) 16:805–12. doi: 10.1080/14787210.2018.1532790
18. National Center for Vector Borne Diseases Control India. Kala-azar situation in India. (2025). Available online at: https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=467&lid=3750 (Accessed October 2023).
19. Ishtiaq, F. Ecology and evolution of avian malaria: implications of land use changes and climate change on disease dynamics. J Indian Inst Sci. (2021) 101:213–25. doi: 10.1007/s41745-021-00235-3
20. Kraemer, MUG, Reiner, RC, Brady, OJ, Messina, JP, Gilbert, M, Pigott, DM, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. (2019) 4:854–63. doi: 10.1038/s41564-019-0376-y
21. Messina, JP, Brady, OJ, Golding, N, Kraemer, MUG, Wint, GRW, Ray, SE, et al. The current and future global distribution and population at risk of dengue. Nat Microbiol. (2019) 4:1508–15. doi: 10.1038/s41564-019-0476-8
22. Kumar, S, Singh, M, and Chakraborty, A. Review article climatic imbalance and their effect on prevalence of dengue fever in India. Int J Curr Microbiol AppSci. (2015) 4:185–91. Available at: https://www.ijcmas.com/vol-4-11/Sanjay%20Kumar%2C%20et%20al.pdf
23. Kakarla, SG, Bhimala, KR, Kadiri, MR, Kumaraswamy, S, and Mutheneni, SR. Dengue situation in India: suitability and transmission potential model for present and projected climate change scenarios. Sci Total Environ. (2020) 739:140336. doi: 10.1016/j.scitotenv.2020.140336
24. Balasubramanian, R, Nadh, V, and Sahina, S. Ecology of breeding habitats of mosquito population and screening for virus of Japanese encephalitis and West Nile in the coastal area of Kerala, India. J Vector Borne Dis. (2021) 58:232–9. doi: 10.4103/0972-9062.318307
25. Hamlet, A, Gaythorpe, KAM, Garske, T, and Ferguson, NM. Seasonal and inter-annual drivers of yellow fever transmission in South America. PLoS Negl Trop Dis. (2021) 15:e0008974. doi: 10.1371/journal.pntd.0008974
26. Rohr, JR, Barrett, CB, Civitello, DJ, Craft, ME, Delius, B, DeLeo, GA, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. (2019) 2:445–56. doi: 10.1038/s41893-019-0293-3
27. Faust, CL, McCallum, HI, Bloomfield, LSP, Gottdenker, NL, Gillespie, TR, Torney, CJ, et al. Pathogen spillover during land conversion. Ecol Lett. (2018) 21:471–83. doi: 10.1111/ele.12904
28. Kolimenakis, A, Heinz, S, Wilson, ML, Winkler, V, Yakob, L, Michaelakis, A, et al. The role of urbanisation in the spread of Aedes mosquitoes and the diseases they transmit—a systematic review. PLoS Negl Trop Dis. (2021) 15:e0009631. doi: 10.1371/journal.pntd.0009631
29. Charlesworth, SM, Kligerman, DC, Blackett, M, and Warwick, F. The potential to address disease vectors in favelas in Brazil using sustainable drainage systems: Zika, drainage and greywater management. Int J Environ Res Public Heal. (2022) 19:2860. doi: 10.3390/ijerph19052860
30. Adsul, BB, Laad, PS, Howal, PV, and Chaturvedi, RM. Health problems among migrant construction workers: a unique public-private partnership project. Indian J Occup Environ Med. (2011) 15:29–32. doi: 10.4103/0019-5278.83001
32. Koul, PA, Mir, H, Saha, S, Chadha, MS, Potdar, V, Widdowson, MA, et al. Respiratory viruses in returning Hajj & Umrah pilgrims with acute respiratory illness in 2014-2015. Indian J Med Res. (2018) 148:329–33. doi: 10.4103/ijmr.IJMR_890_17
33. India Directorate General of Health Services. History of malaria control in India. (2017). Available online at: https://dghs.gov.in/content/1364_3_NationalVectorBorneDiseaseControlProgramme.aspx. (Accessed October 2023).
34. Paradkar, PN, Sahasrabudhe, PR, Sawant, MG, Mukherjee, S, and Blasdell, KR. Towards integrated management of dengue in Mumbai. Viruses. (2021) 13:2436. doi: 10.3390/v13122436
35. Pandey, A, Zodpey, S, and Kumar, R. Demand-supply gaps in human resources to combat vector-borne disease in India: capacity-building measures in medical entomology. WHO South East Asia J Public Heal. (2015) 4:92. doi: 10.4103/2224-3151.206627
Keywords: vector-borne disease, climate, Brazil, India, surveillance
Citation: Hiscox A, Jones RT, Dennehy J, Dyall W, Paris L, Spencer FI, Keating F, Seelig F, Narendran A, Das A and Logan JG (2025) An exploration of current and future vector-borne disease threats and opportunities for change. Front. Public Health. 13:1585412. doi: 10.3389/fpubh.2025.1585412
Edited by:
André Ricardo Ribas Freitas, São Leopoldo Mandic School, BrazilReviewed by:
Tatjana Pustahija, Institute of Public Health of Vojvodina, SerbiaCopyright © 2025 Hiscox, Jones, Dennehy, Dyall, Paris, Spencer, Keating, Seelig, Narendran, Das and Logan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica Dennehy, SmVzc2ljYS5EZW5uZWh5QGFyY3RlY2hpbm5vdmF0aW9uLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Alexandra Hiscox1†
Alexandra Hiscox1† Robert T. Jones
Robert T. Jones Jessica Dennehy
Jessica Dennehy Frederik Seelig
Frederik Seelig