- 1Choremeion Research Laboratory, 1st Department of Paediatrics, Medical School, National and Kapodistrian University of Athens, Athens, Greece
- 2Electromagnetic Field-Biophysics Research Laboratory, Athens, Greece
- 3Department of Ecology and Ecomanagement, National University of Food Technologies, Kyiv, Ukraine
- 4Reproductive Science Group, School of Environmental and Life Sciences, College of Engineering, Science and Environment, University of Newcastle, Callaghan, NSW, Australia
- 5University Research Institute of Maternal and Child Health and Precision Medicine and UNESCO Chair on Adolescent Health Care, National and Kapodistrian University of Athens, Medical School, Aghia Sophia Children’s Hospital, Athens, Greece
Exposure to anthropogenic electromagnetic fields (EMFs), especially those of wireless communications (WC) has increased tremendously. This is an unprecedented phenomenon throughout biological evolution because, all anthropogenic EMFs, being fully polarized, coherent, and, especially WC EMFs, highly variable, differ substantially from the natural EMFs. WC EMFs consist of Microwave (MW) carrier waves, modulated, by Extremely Low Frequency (ELF) signals, and included in on/off pulses repeated at various ELF rates. Moreover, they exhibit intense random variability, mainly in the Ultra Low Frequency (ULF) band. Thus, WC EMFs are a combination of MW and ELF/ULF EMFs. The combination of polarization/coherence and intense low-frequency (ELF/ULF) variability seems to be the key to EMF-bioactivity. Epidemiological and laboratory studies highlight a connection between ELF or WC EMF exposure and cancer, infertility, electro-hypersensitivity, and various other pathologies. Studies also find DNA damage and Oxidative Stress (OS) which explain these pathologies. While man-made EMFs cannot directly ionize molecules, they are capable of doing this indirectly in biological tissue, by triggering the biosynthesis of Reactive Oxygen Species (ROS) which can damage biomolecules, including DNA. The (over)production of ROS and the consequent OS are triggered by irregular gating of Voltage-Gated Ion Channels (VGICs) in the cell membranes as described by the Ion Forced Oscillation (IFO)-VGIC mechanism: Mobile ions within VGICs forced to oscillate by the applied ELF/ULF EMFs exert forces on the voltage sensors of the VGICs, similar to or greater than the forces that physiologically gate those channels, resulting in their irregular gating (dysfunction). Dysfunction of ion channels disrupts intracellular ionic concentrations. This triggers ROS overproduction and OS by the ROS-generating systems/enzymes in the cells, such as the electron transport chain (ETC) in the mitochondria, or the NADPH/NADH oxidases (NOXs), the Nitric Oxide synthases (NOS), etc. The IFO-VGIC mechanism and the consequent OS constitute a comprehensive mechanism that explains all known adverse biological and health effects reported to be induced by anthropogenic EMFs.
1 Introduction
1.1 Unique physical properties of anthropogenic and especially WC EMFs: Polarization/coherence, combination of frequency bands, modulation, pulsation, variability
All man-made electromagnetic fields (EMFs) and corresponding electromagnetic radiation (EMR) are fully polarized and coherent as they are produced by electric/electronic circuits/antennas with specific geometrical shapes. Moreover, most anthropogenic EMFs and especially those generated by Wireless Communication (WC) devices/antennas [mobile/“smart” phones and corresponding base antennas, cordless domestic (DECT: Digitally Enhanced Cordless Telecommunications) phones, “wireless fidelity” (Wi-Fi) routers for wireless Internet connection, “bluetooth” wireless connection among electronic devices etc.], are oscillating and highly variable at each moment, especially in their intensity. All types of WC EMFs consist of Microwave (MW) carrier waves (300 MHz-300 GHz), modulated, mainly by Extremely Low Frequency (ELF: 3–3,000 Hz) or Very Low Frequency (VLF: 3–30 kHz) signals, and included in on/off pulses repeated at various ELF rates. Moreover, they exhibit intense random variability in their signal amplitudes with frequencies in the Ultra Low Frequency (ULF: 0–3 Hz) band. The MW band is part of the wider Radio Frequency (RF: 300 kHz–300 GHz) band. Therefore, even though all WC EMFs are usually referred to simply as “RF” EMFs, in fact they are a combination of RF/MW, ELF and ULF EMFs (1–4). Figure 1A shows 2nd generation (2G) mobile telephony (MT) Global System for Mobile telecommunication (GSM) basic frame repetition, nominally 217 Hz, pulsations. Variability in both pulse amplitude and repetition frequency is evident as in all real-life WC signals. Newer systems 3G, 4G, 5G have basic frame repetition frequency (nominally) at 100 Hz and exhibit increasing variability in their pulsations/signals due to the increasingly higher amounts of variable information they carry (speech, images, video, Internet, etc.) (3). Figure 1B shows 100 and 200 Hz pulsations from a DECT phone.
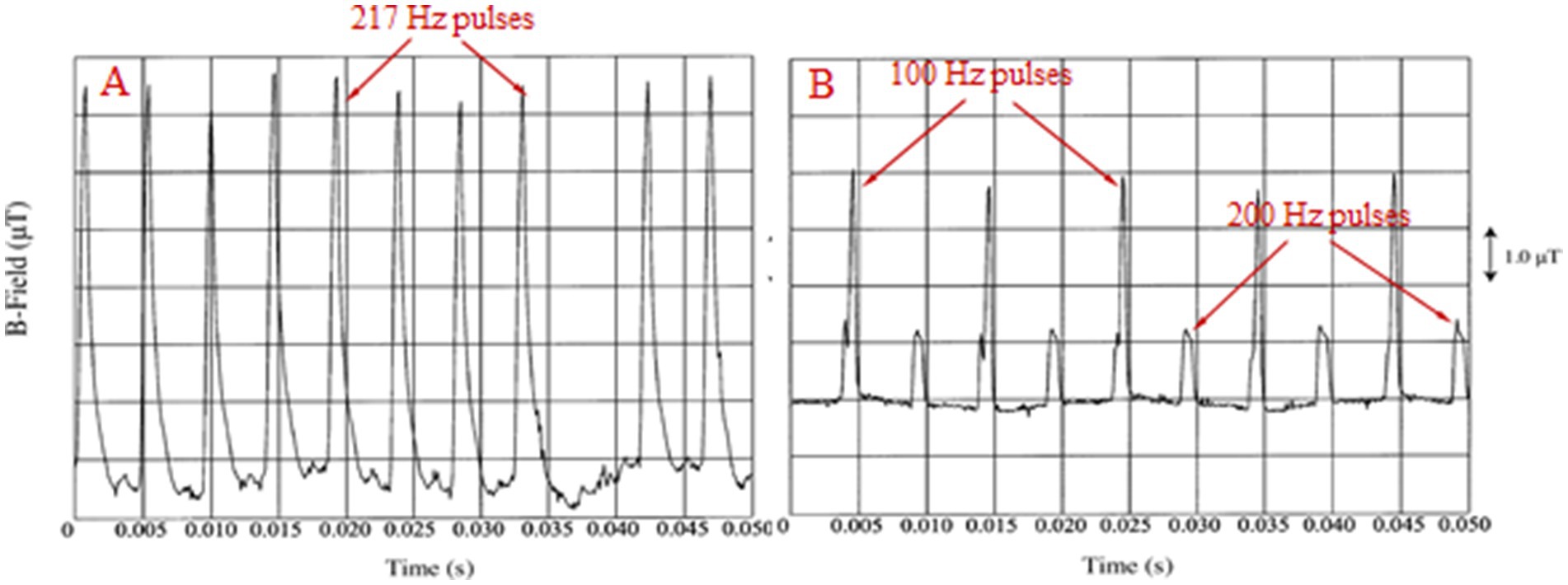
Figure 1. (A) 217 Hz (“frame repetition”) pulses from a GSM mobile phone. (B) 100 and 200 Hz pulses from a cordless domestic (DECT) phone [adapted from Pedersen (4)].
These unique features make all anthropogenic, and most of all WC EMFs very different than the natural EMFs which only in specific cases are partially polarized and/or partially coherent to a small degree (3, 5). The geomagnetic field (GMF) and geoelectric field (GEF) are significantly polarized and coherent but static, with no significant variability. During magnetic storms, approximately every 11 years, there is a variability of about 20% in their normal intensities and then there are increased rates of disease and mortality in the human/animal populations (6).
It seems that the combination of polarization/coherence and low-frequency variability is the key to EMF-bioactivity. Polarized and coherent EMFs/EMR (in contrast to, e.g., light and other types of natural EMFs/EMR) possess net electric and magnetic fields, in addition to radiation intensity, which exert forces on every electrically charged/polar particle/molecule such as the mobile ions and the charged/polar macromolecules in all biological systems. It is those unique features that make all anthropogenic EMFs, and most of all WC EMFs, significantly more adversely bioactive than natural EMFs (3, 5, 7).
It has been repeatedly documented that modulated (especially in amplitude) or pulsed RF EMFs are significantly more bioactive than non-modulated or non-pulsing fields of the same carrier frequency and the same intensity with that of the pulses (8–29). [For reviews (see 3, 30)]. In all cases, the reported effects were not accompanied by any significant heating of the exposed biological tissues, in other words they were “non-thermal.” This evidence implies that the non-thermal biological effects of WC EMFs are due to the included ELF pulsation/modulation.
In addition, ELF EMFs alone have been found independently to be bioactive, similarly to RF EMFs modulated or pulsed by ELFs, providing additional confirmation that actually the ELF pulsation and modulation EMFs are responsible for the non-thermal effects of WC EMFs and not the RF carrier EMFs (11, 18, 31–48). Again, in all cases, the described effects were non-thermal.
The evidence that the ELF/ULF and not the RF carriers of the anthropogenic/WC EMFs are those that induce the non-thermal effects, is in line with the fact that the physiological electrical activity in all forms of life is restricted to ULF/ELF EMFs. There is no physiological RF EMF in the living organisms, neither in the natural environment, despite the confusion and misinformation among the scientific community for the opposite (6, 49–52). The so called “cosmic microwaves” are actually infrared radiation reaching the Earth with a lower frequency due to the Doppler effect (50, 53). Thus, it is evident that the non-thermal biological and health effects attributed to “RF” EMFs are actually due to their included ELF pulsations, modulation, and variability. And there is practically no RF EMF in any technical application that is not combined with ELFs. All modern digital “RF” EMFs contain ELF pulsations, i.e., not only WC systems but also radars and radio/television broadcasting systems (3, 4, 7, 50, 54–56). Even though this is well documented it has escaped attention, and still authors look for different mechanisms for ELF and “RF” EMFs (57–59). Authors who report that they have found non-thermal EMF effects by non-modulated and non-pulsed RF carrier signals alone but do not provide the signal waveform [as, (e.g., 60)], either are unaware of the existence of pulsations produced by almost every existing RF generator, or the effects they report are due to the onset and offset of the RF exposure (18, 56).
1.2 Anthropogenic ELF and WC EMFs: Biological and health effects
Multiple experimental findings associate exposure of laboratory animals or cells to man-made ELF or WC EMFs/EMR with Oxidative Stress (OS) due to Reactive Oxygen Species (ROS) overproduction, genetic damage/alterations (DNA damage, chromosome damage, mutations, etc.), cell senescence (cell aging and loss of replicative capacity), cell death, and related effects [see reviews in (7, 18, 43, 50, 61–66)].
More specifically, numerous in vivo or in vitro experimental-laboratory studies have shown genetic damage and related effects induced by man-made ELF or WC EMFs on a variety of organisms/cell types under various experimental conditions, especially in recent years. Representative such studies are (15, 16, 33–38, 42, 67–126). If we add studies that found induction of OS and/or cell senescence, the list becomes much longer (62–64, 127–133).
Several of these studies have found OS, and/or DNA damage with consequent cell death in reproductive cells of various animals, resulting in decreased reproduction or embryonic death. In particular, effects of WC EMFs on the DNA of reproductive cells reported by several studies on a variety of animals display a remarkable similarity (68, 71, 72, 74, 75, 97–99, 107, 115, 117, 125, 130). The genetic damage found in reproductive cells explains other findings that connect WC EMF exposures with insect, bird, and mammalian (including human) infertility (121, 134–144), miscarriages (145), or declines in bird and insect populations (especially bees) during the past 20 years (146–150). Significant decrease in reproduction (reduced egg laying, reduced development of reproductive cells, or embryonic death) after exposure to MT radiation, was identically observed in fruit flies (97, 98, 141, 142), chicken or quail embryos (71, 125, 151), bovine oocytes (137), birds (147, 148, 150), and bees (143). Similar effects are reported for amphibians (152, 153), rats and mice (107, 121, 135, 136, 138), and human sperm (decreased number and motility of spermatozoa) (134, 144). These remarkably similar findings in various animals and humans by different research groups can be explained by the cell death in reproductive cells or embryonic death after DNA damage observed in mouse or rat sperm cells (68, 107), fruit fly ovarian cells (72, 97–99), human sperm cells (74, 75), and quail embryos (71, 125).
It is again remarkable that the effects of purely ELF EMFs on reproductive cells and reproduction are very similar to those of WC EMFs (31, 33, 36–38, 42, 45, 46), further implicating ELF EMFs as a key bioactive agent.
Apart from the laboratory findings on genetic damage and infertility, epidemiological studies increasingly link man-made ELF or WC EMF exposures with health problems, genetic damage, and cancer in human populations. More specifically, ELF EMFs from power lines and high-voltage transformers (50–60 Hz) are linked to childhood leukemia and other cancer types (154–167) for magnetic field intensities down to 2 mG (0.2 μT) (159, 161), or distances from power lines up to 600 m (157), and electric field intensities down to 10 V/m (155). WC EMFs from various antennas, especially radio broadcasting and MT antennas, have been linked to various forms of cancer (168–171) and genetic damage (113, 172, 173). During the past 15–20 years epidemiological studies find an increasing association between mobile or cordless phone use and brain tumors in humans (174–185). For a review of EMF carcinogenicity studies see Yakymenko and Tsibulin (171).
Moreover, during the past 25 years, other epidemiological studies find association between exposure to MT/WC antennas/devices with reported symptoms of un-wellness referred to as “microwave syndrome,” or “electro-hypersensitivity” (EHS). The symptoms include headaches, fatigue, sleep disorders, and various other adverse effects (169, 186–196). A high percentage (~80%) of EHS self-reporting patients were found with increased OS in their peripheral blood (197). EHS symptoms have been reported to increase dramatically among people exposed to 5G WC antennas, and the ambient RF EMF levels in cities have also been found to increase significantly during the past 2 years, after the beginning of 5G rollout (198, 199).
Cancer in experimental animals after chronic exposure to MT/WC EMFs has also been reported (200, 201). A study of the US National Toxicology Program (NTP) found that exposure of rats to simulated 2G or 3G MT emissions (2 years, 9 h per day) induced brain cancer (glioma) and heart cancer (malignant schwannoma) for both lower and higher radiation levels than the officially accepted limits (202). The study also found significantly increased DNA damage (strand breaks) in the brains of exposed animals (124) confirming the tight link between DNA damage and carcinogenesis. An Italian life-span exposure study of rats to a simulated 2G MT EMF also found induction of heart schwannomas and brain glial tumors, confirming the results of the NTP study (203).
Other studies have reported no effects of ELF or RF/WC EMFs in all of the above end points [see reviews in (3, 18, 42, 43, 61–65, 141, 171, 204–213)], especially studies that employed simulated MT/WC exposures from generators with invariable parameters and no modulation. By contrast, more than 95% of the studies that employed real-life MT/WC exposures, from commercially available devices (mobile/cordless phones, Wi-Fi, etc.) with high signal variability, find effects (3, 7, 206, 209, 210, 214–216).
Regardless of real-life or simulated exposures, the majority of experimental studies (approximately 70%) either with “RF” (combined with ELF) or purely ELF EMFs do find effects (62–64, 206, 208). Jagetia (64) did an extensive review of laboratory studies addressing genotoxic effects of either ELF or RF/WC EMFs in a variety of biological systems, and found that among 207 studies, 144 (69.6%) found statistically significant genotoxic effects. The vast majority of reported effects were non-thermal, and the vast majority of employed EMFs contained ELF/ULF components.
The recorded human and animal carcinogenicity, the DNA/genetic damage, the OS findings, and the reproductive declines due to DNA damage in ovarian or sperm cells or embryonic death, all point toward the same direction: Man-made EMFs induce OS and DNA damage, infertility, cancer, and other related pathologies. The reason why the same effects are observed in a wide variety of animals such as mammals, birds, insects, etc., and humans, is that all biological and health effects initiate in cells and all cells are essentially identical in all animals, humans, and even plants. They have identical membranes, ions, ion channels and pumps, biomolecules such as DNA, RNA, proteins, etc., water, ROS, identical cellular organelles such as nuclei, mitochondria, ribosomes, endoplasmic reticulum, etc., and very similar metabolic processes and regulatory mechanisms. These similarities at the cellular level between all animals and humans are much more fundamental than differences in volume, mass, shape, macroscopic functions, intelligence, etc. As a result, any effect induced by EMFs in animal cells such as OS, DNA damage, etc., is expected to be induced also in the human cells, and vice-versa (7, 66).
The exposure levels in the vast majority of all the aforementioned studies were significantly below the officially accepted exposure limits for ELF and RF EMFs, which are recommended by a private organization called the International Commission on Non-Ionizing Radiation Protection (ICNIRP) to prevent discharges on humans in the case of ELF and acute heating of living tissues in the case of RF/WC EMFs. It is remarkable that this organization arbitrarily ignores the overwhelming evidence of non-thermal effects which constitute the vast majority of effects of anthropogenic EMFs, and yet, governments adopt its recommendations instead of following the Precautionary Principle which dictates the obvious, that no new technology should be applied unless those who promote it have proven its safety beyond any doubt (50, 217–226).
The International Agency for Research on Cancer (IARC) branch of the World Health Organization (WHO), has, since long time, classified both ELF and “RF” (in fact WC) EMFs as possibly carcinogenic to humans (Group 2B) (204, 205, 227). Based on additional scientific evidence after the 2011 IARC classification for “RF” EMFs, several studies have argued that “RF”/WC EMFs should be re-evaluated and classified as probably carcinogenic (Group 2A) or carcinogenic (Group 1) to humans (50, 63, 64, 66, 118–120, 171, 179, 182, 183, 218, 219). Moreover, studies have asked for the urgent application of the Precautionary Principle, stricter exposure limits, especially for WC EMFs, and a moratorium on 5G rollout (50, 215, 217, 218, 225, 228).
1.3 Ionization in living tissue by “non-ionizing” EMFs
As indicated by the long list of laboratory and epidemiological studies, man-made EMF exposures are linked to OS, genetic damage, infertility, EHS, and cancer. Damage to DNA or other biological molecules involves breakage of chemical bonds and chemical alterations, in other words ionization. Man-made EMFs with frequencies up to the low limit of infrared (0–3 × 1011 Hz) examined here cannot directly break chemical bonds and cause ionization, except for very strong field intensities (≥106 V/m) (229, 230). Such field intensities are rarely present in the environment, apart from very close proximity to high-voltage power lines and transformers, or very close to atmospheric discharges (lightning). How are then man-made EMFs at environmental intensities capable of ionizing DNA and other biological molecules? What is the unique property that makes man-made EMFs capable of inducing adverse biological/health effects in contrast to natural EMFs including light? It has been shown that this unique property is polarization and coherence combined with low frequency (ULF/ELF) variability (2, 3, 5, 7).
In the present work we provide an updated description of how man-made EMFs at non-thermal levels are capable of inducing dysfunction of Voltage-Gated Ion Channels (VGICs) in cell membranes, triggering ROS overproduction and OS, which in turn is responsible for most, if not all, known adverse biological/health effects including DNA damage and related pathologies. Thus, ionization of biological molecules occurs indirectly after man-made EMF exposure, mediated by the produced ROS in the cells (2, 210).
2 Anthropogenic ELF or WC EMFs and OS: Experimental evidence
Yakymenko et al. (62) reviewed 100 published experimental studies that examined OS in living cells from a wide variety of organisms (humans, rats, mice, rabbits, quail embryos, plants etc.) exposed in vitro or in vivo to RF/WC EMFs. From those studies, 93 found increased OS expressed as activation of key pathways generating ROS overproduction, peroxidation, oxidative damage of DNA, changes in activity of antioxidant enzymes, etc. In a more recent update, Yakymenko and Tsibulin (63) found that among 131 published peer-reviewed studies looking for oxidative effects of RF/WC EMFs at non-thermal intensities, in most cases pulsed/modulated by ELF EMFs, 124 (95%) confirmed statistically significant oxidative effects on various types of biological systems. And among 39 published studies on oxidative effects of purely ELF EMFs, 36 of them (92%) also found significant oxidative effects induced by the exposure. Therefore, it is well-documented that anthropogenic EMF exposures cause ROS overproduction and OS in living cells, which in turn is responsible for the observed DNA damage, infertility, cancer, and other related pathologies.
Even though ROS at sub-toxic levels in the cells act as signaling molecules involved in various physiological cellular processes, they can also damage biological molecules (such as lipids, proteins, and nucleic acids) causing various diseases when they are in excess (231–234). Most ROS are free radicals. Free radicals are extremely unstable and reactive molecules containing an unpaired electron denoted by a dot (•) in their chemical formula. They possess a very strong tendency to chemically react with other molecules and/or with each other in order to couple their unpaired electron, balance electron spins, and become stable. This extreme reactivity is the reason why they have extremely short lifetimes. Most ROS react rapidly with surrounding biomolecules, causing chemical alterations (235, 236).
Two important initial free radical ROS found in cells after exposure to man-made EMFs are the superoxide anion (O2•−) and the nitric oxide (NO•) (62, 63, 125). The superoxide anion free radical may be converted into hydroxyl radical (OH•), or react with nitric oxide to form peroxynitrite (ONOO−). Both products (especially the hydroxyl radical) are very reactive ROS with biological molecules, especially DNA (62, 125, 237, 238).
3 Biochemistry of ROS
3.1 ROS sources in the cells: Identity and function: Dependence on ion concentrations
3.1.1 Mitochondria
A major source of ROS in all cells is the Electron Transport Chain (ETC) in the inner membrane of the mitochondria (62, 65, 234, 235, 239), likely contributing 50–80%, or even 90% of total cellular ROS production under normal conditions (240). Electron leakage from Complexes I and III of the ETC is a significant source of ROS, especially superoxide anion free radical (O2•−) after adherence to molecular oxygen (241):
Certain cell types such as neurons or spermatozoa have high energy demands and thus high mitochondrial activity, making them particularly susceptible to OS from mitochondrial ROS production (65, 242).
The large amounts of energy (in the form of Adenosine Triphosphate-ATP) required for the maintenance of a cell’s aerobic life are generated predominantly by oxidative phosphorylation in the mitochondria at the expense of molecular oxygen, which is reduced to water by 2 electrons per water molecule terminating in complex IV of the ETC [2H+ + 1/2O2 + 2e− → H2O] (65). However, a fraction of the electrons will ‘leak’ from the ETC and partially reduce oxygen (Equation 1). The result is that a small amount (~2%) of oxygen during this process is converted to superoxide anion radical, hydrogen peroxide and related ROS. Cells and tissues reliant on oxidative phosphorylation have evolved effective antioxidant measures to keep these modest ROS levels in check and inhibit OS. Nevertheless, perturbation of electron flow and/or the homeostasis of the mitochondrial environment can dramatically elevate the risk of OS. This is exemplified in spermatozoa, where minimal cytoplasm and abundant substrates for oxidative chemistry offer a platform for run-away levels of ROS with minimal perturbation of their mitochondrial environment (65, 243). Electron leakage from the ETC in spermatozoa under WC EMF exposure was recorded to be sourced from complex III, as this was tested in parallel with the use of complex III-inhibitors (115). See Miller et al. (65) for a more detailed view on mitochondrial ETC and ROS production.
When considering the mitochondrial origins of OS, Ca2+ plays a key role. Physiological increases in mitochondrial Ca2+ can stimulate ATP production when energy demands are high, however this can also result in elevated ROS generation. Excessive Ca2+ accumulation can lead to mitochondrial dysfunction and a drop of ATP production, but importantly, further elevates ROS production and apoptotic factors (244). Ca2+ can act upon the mitochondria through its regulatory activity on key mitochondrial specific dehydrogenases, such as pyruvate dehydrogenase, which is an intrinsic mediator of electron flow through the ETC and influences ROS production (245). Therefore, careful control of Ca2+ levels in the mitochondria is a key factor in ROS homestasis, with any dysfunction of Ca2+ channels induced by EMFs potentially leading to ROS overproduction.
Voltage-gated anion channels in the outer membrane of the mitochondria regulate Ca2+ entry into the intermembrane space and the matrix. While increased levels of Ca2+ in the mitochondria stimulate O2•− synthesis by the ETC, the presence of the other “initial” ROS, NO•, inhibits complex IV of the ETC causing additional electron leakage and increased O2•− production (235, 244). Thus, the two important primary ROS (NO• and O2•−) act in synergy in the mitochondria, and increased levels of NO• stimulate further production of O2•−.
ROS overproduction in the mitochondria may damage DNA both in the mitochondria and the nucleus, and may initiate a signaling cascade leading to apoptosis. In turn, excessive apoptosis induced by increased ROS levels, has been linked to inflammatory diseases and cancer (246).
3.1.2 NADPH/NADH oxidases (NOXs)
These plasma membrane enzymes, found in abundance in all cells, normally generate ROS for the elimination of invading microorganisms. NAD(P)H oxidase is an enzyme exhibiting different affinities for its two substrates; Nicotinamide Adenine Dinucleotide Phosphate (NADPH), and Nicotinamide Adenine Dinucleotide (NADH), with the NADPH substrate being the most common (247–251). The NOXs catalyze the production of superoxide anion free radical by transferring electrons to oxygen from NAD(P)H according to the reaction:
Thus, they also generate an ETC for the reduction of extracellular O2 to O2•−. The activity of NOX is intimately connected with H+ channels and the enzyme may even act as a H+ VGIC itself, due to its gp91phox transmembrane subunit (252–254).
NOXs are also activated by cytosolic Ca2+ and possess a Ca2+-binding site, apart from their H+ voltage-gated channel (gp91phox domain) (251). Thus, perturbation of intracellular concentrations of either H+ or Ca2+, after irregular gating of their VGICs, can affect NOX function and trigger ROS (over)production.
NOXs may contribute 10–30% of total ROS production in neurons under basal conditions. However, their contribution can increase significantly during neuronal activation or inflammation. NOXs, particularly NOX2 and NOX4, are expressed in neurons and play roles in synaptic plasticity, neuronal signaling, and neuroinflammation. Their activity can be upregulated in response to various stimuli, leading to increased ROS production (255).
Some NOX isoforms, such as NOX2 in phagocytes, are directly activated by Ca2+ through interactions with regulatory subunits like p47phox and p67phox, and increased intracellular Ca2+ levels can promote ROS production. Ca2+ can also indirectly regulate NOX activity through various signaling pathways, such as those involving protein kinase C and other kinases (256).
The NOXs have been identified as a key target for man-made EMFs. Friedman et al. (257) found rapid ROS production by the NADH oxidase in cultured cells after a few min exposure to simulated mobile phone EMF.
A common function in the NOXs and the mitochondria is the generated ETC. In both cases, the primary ROS produced is superoxide anion radical (O2•−), which may finally convert to hydroxyl radical (OH•), or peroxynitrite (ONOO−) as shown below (Equations 3–7).
3.1.3 Nitric oxide synthases (NOS)
These are specific enzymes found in various locations in all animal and plant cells, and as denoted by their name, produce nitric oxide free radicals (NO•). Several NOS enzymes have been well described, such as the neuronal NOS (nNOS), or the endothelial NOS (eNOS) which are plasma membrane enzymes. Their activation seems to be dependent on intracellular calcium levels and calmodulin. Increased levels of intracellular Ca2+ stimulate NO• synthesis by the NOS. NO• is ubiquitous in cells and tissues in all vertebrates as an intercellular messenger and certain studies suggest that it is not particularly toxic by itself, but it may easily be converted to peroxynitrite which is particularly toxic (237, 258). Increases in Ca2+ and NO• levels in cells have been found to be triggered very rapidly (within a few seconds) by EMF exposure (259), with the induction of DNA damage by peroxynitrite blocked by NOS inhibitors (260) and antioxidants (238, 261, 262). While nNOS and eNOS, are Ca2+-dependent enzymes activated by increased intracellular Ca2+ levels, and promoting NO• production, under certain conditions NOS can become “uncoupled” due to substrate depletion or cofactor deficiency, triggering the cell machinery to produce O2•− instead of NO• by other ROS sourses/enzymes (263).
3.1.4 Xanthine oxidase (XO)
Xanthine Oxidase (XO) is another important source of ROS in the cytoplasm of living cells. XO catalyzes the oxidation of hypoxanthine to xanthine and then to uric acid, producing superoxide anion radical (O2•−) and hydrogen peroxide (H2O2) as byproducts. XO is involved in purine metabolism and can be a significant source of ROS under certain conditions, such as ischemia–reperfusion injury (264).
3.1.5 Other ROS-generating/promoting enzymes
Such enzymes in cells are, e.g., cytochrome P450 (CYP), lipoxygenases, cyclooxygenases, myeloperoxidases and others (240). They may contribute to ROS generation differently under different physiological conditions. For example, CYP is localized in the endoplasmic reticulum and is crucial for the metabolism of various endogenous and exogenous compounds, including drugs, steroids, and fatty acids. CYP reactions can generate ROS as byproducts, particularly during the catalytic cycle when oxygen is activated (265).
The ionic balance within a cell (also called electrochemical balance) is most crucial for ROS production, and both ion channels and pumps can trigger ROS production by the above described ROS sources. The ion pumps (active ion transporters) such as the Na+/K+ pump (ATPase), in coordination with the ion channels (passive ion transporters) determine the membrane voltage, the electrochemical balance, the cell’s homeostasis, the redox status (concentrations of reducing and oxidizing molecules), and the cell’s volume among other functions. Ca2+ and K+ channels are involved in cell proliferation, apoptosis, and carcinogenesis (266, 267). In addition, they are both involved in iron entry into the cells (268–270). Iron catalyzes the production of OH• via the Fenton reaction and thus, impaired function of these channels can promote cellular toxicity. Dysfunction of Na+, K+, Mg2+, and Ca2+ VGICs will affect the function of the Na+/K+ pump (ATPase), and Ca2+ pumps in the plasma membranes of all cells.
In addition to its role as an ion pump, Na+/K+-ATPase operates as a signal regulator, transducing signals from the plasma membrane to the intracellular organelles and acting as a normalizer of the Na+/K+ balance in cells after, e.g., VGIC dysfunction (231, 234, 271). It has long been shown that the activity of the Na+/K+-ATPase is affected by ELF EMFs (44, 272, 273), and that changes in its activity are linked to ROS production by the mitochondria and in turn, increased mitochondrial ROS production stimulates the signaling function of the enzyme forming a positive amplification feedback loop (274, 275). Thus, changes in the activity of the Na+/K+-ATPase due to EMF-induced dysfunction of VGICs can stimulate ROS production by the mitochondria and the process can be amplified by increasing ROS levels (2, 210).
3.2 ROS action on DNA and other biological molecules
DNA damage induced by OS/ROS leading to mutations and disease has been well documented since long time. The effects of ROS on DNA commonly result in altering the nucleotide bases which directly affects their paring elevating mutational load, altering a sugar (deoxyribose), breaking a covalent bond between deoxyribose and nucleotide base, and causing single- and double-strand breaks, further increasing the repair burden on the affected cells and tissues (2, 210, 243, 276–279).
We have described the evidence regarding the generation of primary ROS, NO• and O2•−, by the ROS sources in the cells after anthropogenic ELF or WC EMF exposure (section 2). These initial ROS are converted to other even more potent ROS, hydroxyl radical and peroxynitrite, which we call “final” ROS, and are those that mainly damage DNA and other biological molecules.
3.2.1 Peroxynitrite
Increased concentrations of NO• and O2•− within a cell lead to peroxynitrite (ONOO−) overproduction after reaction among the two initial ROS, as follows:
Peroxynitrite is a strong non-free radical ROS which can damage critical molecules including DNA (280). Both nitric oxide and peroxynitrite can diffuse anywhere within the cell and, thus, act directly on DNA or other molecules. The effects of peroxynitrite on DNA include base and sugar oxidative modifications, and DNA single-strand breaks (258, 281). Of the four DNA bases, guanine is the most vulnerable to peroxynitrite (282). DNA single-strand breaks caused by peroxynitrite is a well-documented effect which can be prevented by use of calcium channel blockers and antioxidants (238, 258, 261, 262, 283). Pall (238) noted a connection between EMF-induced dysfunction of Voltage-Gated Calcium Channels (VGCCs) and NO•/ONOO− overproduction. Peroxynitrite can also decompose easily in the presence of H+ to form OH• and NO2• (284, 285):
3.2.2 Hydroxyl radical
The superoxide anion radical (O2•−) produced by the mitochondria or the NOXs, is catalyzed by superoxide dismutase (SOD) in the cytosol or the mitochondria converting to hydrogen peroxide (H2O2) (248, 280, 286):
H2O2 can move to any cellular site, including the nucleus, where it can be converted to the most potent hydroxyl radical (OH•) which can damage any biological molecule, including DNA (287–291).
OH• is considered the most potent oxidant of DNA. It is mainly produced by iron-catalyzed conversion of H2O2 via the Fenton reaction (292): Fe2+ is oxidized by H2O2 to Fe3+, producing a OH• radical and a hydroxide ion (OH−). Fe3+ is then reduced back to Fe2+ by another H2O2 molecule, producing a hydroperoxyl radical (HOO•) and a proton. The net effect is the conversion of two hydrogen peroxide molecules to produce OH• and HOO•, with water (H+ + OH−) as a byproduct (2, 210):
Another way for OH• production is the Haber-Weiss reaction (293):
The OH• radical can break biological macromolecules (R-R or R-H) in its immediate environment or subtract atoms from them (such as the various hydrogen atoms of the deoxyribose molecule) by breakage of covalent bonds. This results in chemical alterations in the macromolecules and production of new free radicals such as R• or RO• (2, 210, 294, 295).
The new free radicals will further react with other molecules and with each other resulting in additional chemical alterations.
A more specific example is the action of OH• on DNA that results in the breakage of the DNA chain (single- or double-strand breaks). The backbone of each strand of the DNA is formed by phosphodiester bonds between two successive deoxyribose (DOX) molecules and a phosphate (–PO4–) between them. For a strand breakage, the phosphodiester bond needs to break. The double-strand breaks (breakage of both strands of the double helix at the same point) are the most severe and in most cases irreparable damages that lead to DNA fragmentation, mutations, cell death etc. The breakage of the phosphodiester bond by two hydroxyl radicals can be written as:
By (b) we denote the DNA bases connected to the DOXi and DOXi + 1 molecules. (3΄) and (5΄) are the carbon atoms in successive deoxyribose molecules that form the phosphodiester bond with the phosphate. In the products of the reaction, PO3 is separated from the DNA molecule and is not anymore part of the phosphodiester bond that kept the strand intact. The second base is oxidized by the hydroxyl radical as well (to form O-b) in addition to the breakage of the strand (296). The breakage forms OH at the terminals of the broken strands. The TUNEL (Terminal deoxynucleotide transferase dUTP Nick End Labeling) assay used in biology to detect fragmented DNA, specifically detects the free OH terminals of the broken strands (42, 97, 98).
4 Anthropogenic EMFs and VGICs
4.1 VGICs: Most sensitive electromagnetic sensors in living organisms
Previous studies have hypothesized the existence of specific electro/magneto-sensor organs/cells in animals/humans in order to explain the biological effects of EMFs [see review in (297)]. This is not necessary, as all cells in all animals including humans and even plants are equipped with the most sensitive EMF-sensors which are no other than the VGICs, the most abundant class of ion channels in all cell membranes (231, 234, 271, 297, 298).
Normally VGICs convert between open and closed states by membrane voltage changes dV ≥ 30 mV which exert forces on their voltage-sensors. More specifically VGICs respond to changes between ⁓30 and ⁓100 mV. The voltage sensors of the VGICs are four symmetrically arranged, transmembrane, positively charged parallel α-helices (subunits), each one named S4. They occupy the 4th position in a group of 6 parallel α-helices (S1-S6), and are the closest helices to the pore apart from the S5-S6 helices which form the pore walls. The channel consists of four identical such groups (main units I-IV) in symmetrical positions around the pore of the channel (Figure 2). The sensors are positive Lys and Arg amino-acids in the S4 helices. The effective (net) charge on each S4 has been calculated to be q = 1.7qe, where qe is the elementary charge. The positive charges of the S4 sensors are paired with negative charges from adjacent helices so that the net charge on the walls of the pore is zero. The ions pass dehydrated and in single file through the channel gate, the narrowest part of the pore (Figure 2). At least four dehydrated mobile ions are very close to the S4 sensors at a distance of less than 1 nm, as, except for the ion(s) that may be passing through the gate any moment or is just outside the gate ready to pass, at least three more are bound in specific ion-binding sites very close to the gate (234, 297, 299–304).
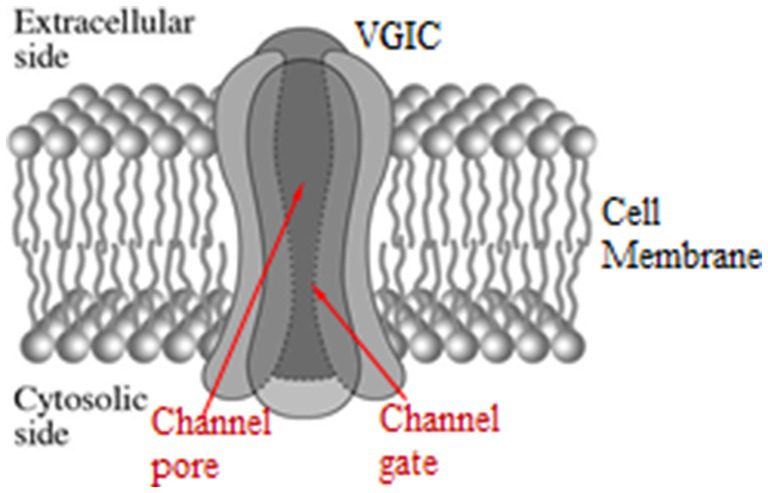
Figure 2. Schematic representation of a VGIC with its four main units, the pore, and the gate in opened state. Each main unit consists of six parallel α-helices (S1–S6). The voltage-sensors of the VGIC are the four S4 helices, one in each main unit, in symmetrical positions around the pore [adapted from Panagopoulos et al. (297)].
4.2 Anthropogenic EMFs and VGIC dysfunction: The IFO-VGIC mechanism
A biophysical mechanism for EMF-induced biological effects has been described in Panagopoulos et al. (2, 5, 297, 305, 306), and Panagopoulos (302). It explains, in standard physics and biology, how polarized, coherent and slow-varying (ULF/ELF/VLF) EMFs, even at very low field intensities, can cause irregular gating (opening/closing) of VGICs in cell membranes with consequent disruption of the cell’s electrochemical balance, redox state and homeostasis. As ELF/ULF/VLF EMFs are basic components of the WC EMFs, this mechanism accounts for the biological effects of the vast majority of all man-made (polarized, coherent, and varying) EMFs.
While VGICs are normally gated by ⁓30–100 mV voltage changes in the very strong transmembrane field, in other words respond to field changes between 3 × 106 and 107 V/m, they may also respond to very weak polarized, coherent, and slow-varying EMFs down to ⁓10−5 V/m via the forced-oscillation such EMFs induce on mobile ions in close proximity (<1 nm) to the sensors (ion forced-oscillation: IFO). This occurs because the force exerted on the S4 sensors by oscillating ions in close proximity, depending upon the inverse third power of the distance between charges (see Equation 12 below), is much greater than a direct force from an externally applied EMF which depends upon the first power of the applied field (2, 5, 297). The aforementioned (at least) four ions close to the pore gate, once forced to oscillate in parallel and in phase, exert constructive coordinated forces on the S4 sensors able to gate the channel (Figure 3).
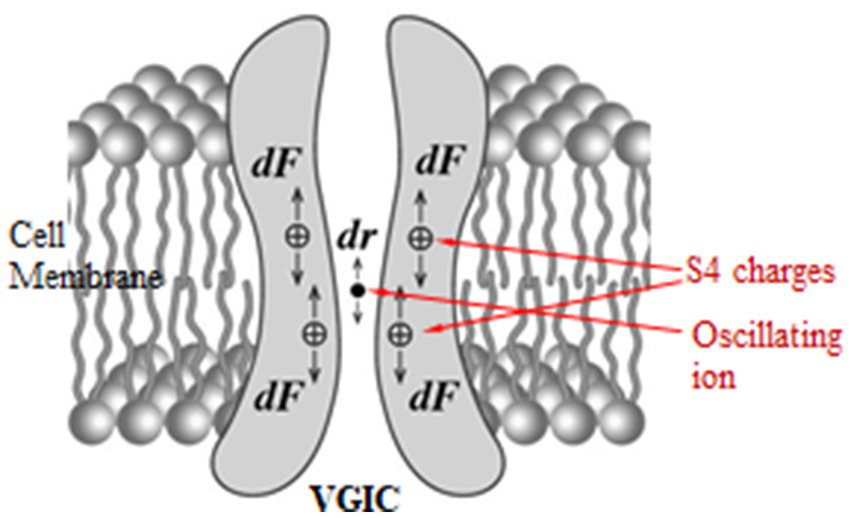
Figure 3. Forces dF exerted on the S4 positive charges in a VGIC due to the displacement dr of an oscillating ion in the channel pore [adapted from Panagopoulos et al. (297)].
Forces exerted by an external polarized EMF on any mobile ion within any VGIC, cause a displacement dr of the ion from its “initial” position which in turn exerts an additional Coulomb force dF on the S4 voltage-sensors of the VGIC which can result in the opening/closing (gating) of the channel (2, 5, 297, 305, 306) (Figure 3). This additional Coulomb force on each S4 sensor due to the ion displacement dr is given by the equation:
with q = 1.7 qe the effective (net) charge of the S4 sensor, zqe the mobile ion charge with z the ion valence (e.g., z = 1 for K+, Na+ or z = 2 for Ca2+ ions), qe = 1.6 × 10−19C the elementary charge, εo = 8.854 × 10−12 N−1m−2C2 the vacuum permittivity, ε ⁓ 4 the relative permittivity of the ion channel, and r = 1 nm the “initial” distance between the two charges (2, 299, 305–308).
In the simplest case of a harmonically oscillating applied EMF, the maximal ion displacement in one direction during its oscillation is 2A (A the amplitude of the forced oscillation). By solving the corresponding differential equation [(see 302, 305)] we get that for an applied electric field,
where Eo is the intensity amplitude (maximal value) of the applied field, ω = 2πν (ν the frequency of the applied field), and β the damping coefficient during the ion oscillation (found to be within channels ≅ 6.4z × 10−12kg/s, with Em ~ 107 V/m the transmembrane electric field, and uo = 0.25 m/s the ion maximal velocity through an open channel).
When the VGIC is gated physiologically by membrane voltage changes dV ≥ 30 mV, the minimum force on each voltage sensor that causes gating is calculated to be dF = 8.16 × 10−13 N, which, according to Equation 12, corresponds to a minimum coordinated displacement dr of four z-valence ions within the channel at ~1 nm distance from the sensors (2, 297), dr = 10−12/z (in m), and thus in order for an applied electric field to be able to gate the VGIC, the max ion displacement (2A) must satisfy the condition:
For double-valence cations (z = 2) (e.g., Ca+2), this condition finally becomes:
This is the condition for electric fields in order to be bioactive with respect to field frequencies, and is represented in logarithmic scales (logΕo, logν) by the area above the line (including the line) in Figure 4 (“bioactive region”). The intensity-frequency combinations of all known anthropogenic EMF sources linked to adverse biological/health effects are within the predicted bioactive region. As the frequency of the applied EMF increases from ULF/ELF to VLF/LF (Low Frequency: 30–300 kHz) the required minimum field intensity in order to be able to induce effects increases considerably, and purely RF/MW EMFs need to have very high intensities (hundreds or thousands of V/m) in order to be bioactive.
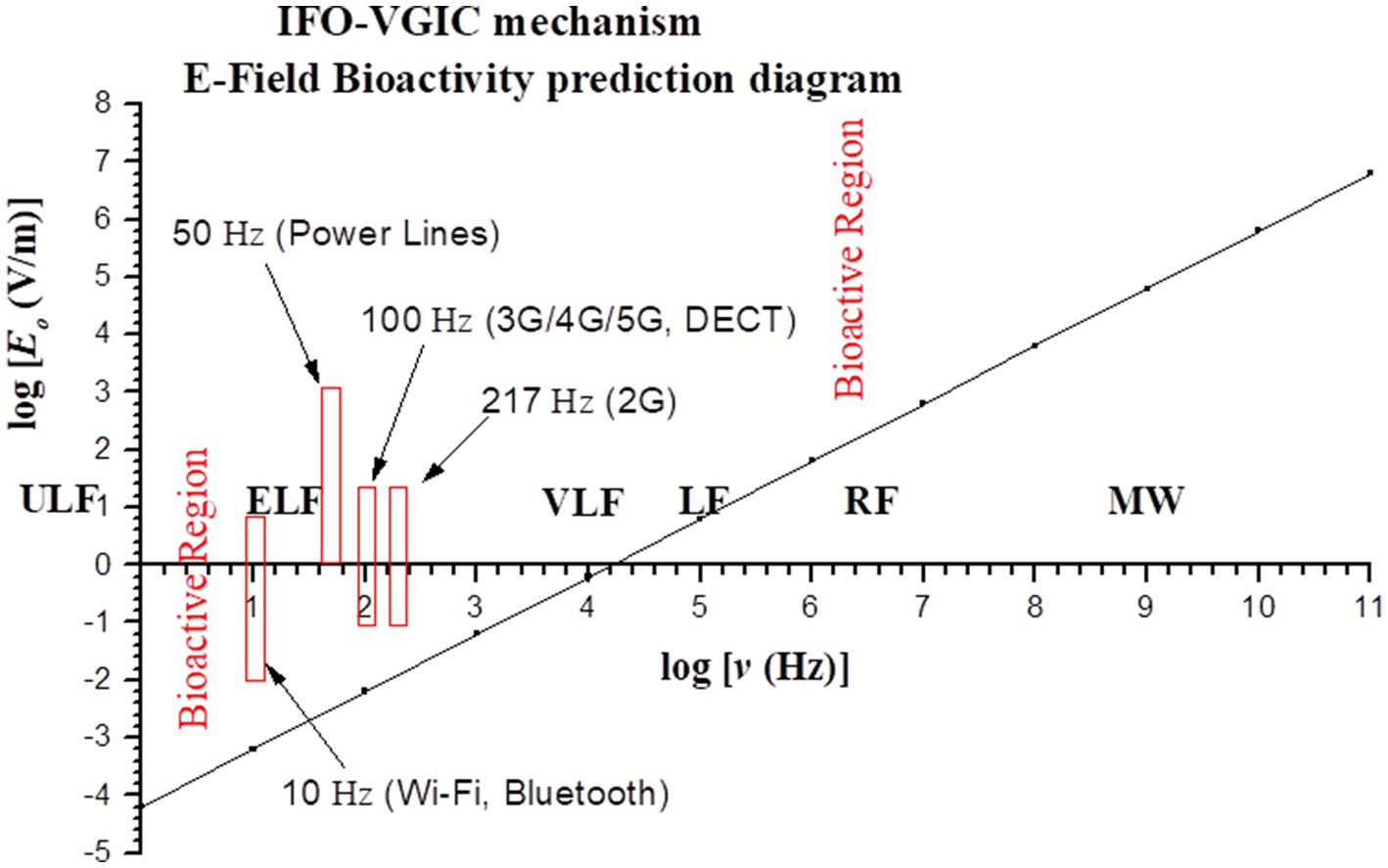
Figure 4. Graph (in logarithmic scales) showing the bioactive combinations of EMF intensity and frequency (above line) as predicted by the IFO-VGIC mechanism. The intensity-frequency combinations of all known anthropogenic EMF sources linked to adverse bioeffects are within the predicted bioactive region (2, 302).
The maximal ion displacement (2A) expresses the potential of the applied EMF to cause VGIC gating and initiate biological effects, in other words it represents the biological activity (or bioactivity) of the applied EMF:
Thus, the IFO-VGIC mechanism finds that the biological activity of an EMF is proportional to its maximum intensity and inversely proportional to its frequency, meaning that the reported effects in the literature are induced by low-frequency (ULF/ELF/VLF), and not high frequency (purely RF/MW), EMFs. Moreover, it finds that pulsing EMFs are significantly more bioactive than corresponding continuous-wave (non-pulsing) EMFs (see analysis in (2, 297, 302)).
According to the IFO-VGIC mechanism, VGICs respond to changes of polarized, coherent and slow-varying electric fields down to ⁓10−5–10−4 V/m, which is in impressive agreement with the threshold intensities of ELF man-made EMFs reported to induce biological effects in cell/tissue cultures (309, 310), and be sensed by electrosensitive animals (311, 312).
Apart from the electric fields, in fast moving animals/humans, the magnetic fields become increasingly bioactive with increasing velocities, and the same mechanism has explained animal orientation and navigation in the GMF (297).
It is known that living organisms are not particularly affected by static electric or magnetic fields but mostly by oscillating/varying (and polarized) ones. This is consistent with the IFO-VGIC mechanism, as VGICs are not gated by the normal static voltage/electric field across the cell membrane, but only by membrane voltage changes of the order of 30% in this voltage/field that cause membrane depolarization. In other words, VGICs do not respond simply to the presence of an invariable (static) electric field, otherwise they would open/close chaotically all the time and no life could be maintained. The same holds for the static magnetic fields, which can become bioactive with a variable animal velocity (297). This is the reason why the GMF and the GEF are not particularly bioactive under normal conditions but they become bioactive when ⁓20% changes in their normal intensities occur during magnetic storms (3, 6, 7, 297).
Thus, the IFO-VGIC mechanism predicts that polarized/coherent and slow-varying EMFs cause VGIC dysfunction (irregular gating), and this is today verified by many experimental studies [(see 313–317), and reviews (44, 210, 238, 298, 318)].
5 VGIC dysfunction leading to OS: Connecting the dots for a comprehensive mechanism of EMF-induced biological and health effects
How can the initial ROS (O2•− and NO•) generated after EMF exposure be produced by VGIC dysfunction? This was a missing link until recently when we specifically looked for such evidence (2, 210). We realized that even though plenty of data connecting impaired ion channel function and induction of cell death or cancer had been available for a long time (266, 267), and even though most ion channels, especially cation channels, are VGICs, the connection between VGIC dysfunction induced by EMF exposure and OS (2, 210, 238, 319–322) leading to DNA/cellular damage, had escaped the necessary attention.
Many studies have found a connection between Ca2+, K+, Na+, and Cl− VGIC dysfunction with OS and related pathologies (238, 319, 321, 322). It is repeatedly shown that VGIC dysfunction induced by anthropogenic EMFs can trigger immediate ROS generation in the cells with this effect significantly diminished by the use of specific ion channel blockers (238, 259, 314, 316). Recent research further confirms the connection between VGIC dysfunction and ROS (over)production. For example, ROS overproduction through the activation of NADPH oxidase by extracellular tau-protein in co-cultures of neurons and astrocytes was reduced in the presence of nifedipine, inhibitor of Ca2+ VGIC (323). Epithelial cell death associated with elevation in ROS was prevented by lidocaine, a well-known Na+ VGIC inhibitor with antioxidant effects (324). Induced ROS production in murine microglia was inhibited in a dose-dependent manner by K+ VGIC blockage, and to a more limited degree, by Cl− channel blockage (325).
Various pathological conditions, including neurodegenerative diseases, termed “channelopathies”, were discovered to be caused by ion channel dysfunction. Impairment of either voltage- or ligand-gated ion channels has been identified as a cause of neurological diseases. The ion channels involved include Ca2+, K+, and Na+ VGICs (318, 326). Multiple studies have documented the connection between Ca2+, K+, Na+, and Cl− channel dysfunction and the development of OS-related pathologies (321). Ion channel dysfunction leading to OS is also a common cause of degenerative Central Nervous System (CNS) diseases of various genetic etiologies, and is a common factor in neurological disorders. The role of ion channels in neurodegenerative disorders associated with OS has now been recognized, as the ion channels undergo functional adjustments in such conditions (318, 327).
It is evident that the function of ion pumps and channels controls the intracellular concentrations of mobile ions, and in turn the function of the cellular systems/enzymes that produce ROS (2, 210). Any dysfunction in ion channels will affect the otherwise carefully controlled intracellular ionic concentrations, disrupting the cell’s electrochemical balance and homeostasis, including the intracellular redox status which is an index of the ROS content in the cell. From the evidence highlighted here, it follows that disturbance of ion homeostasis can trigger OS by ROS overproduction and subsequent DNA damage. Inversely, the intracellular redox status can alter the gating properties of ion channels and trigger opening or closing of Ca2+, Na+, and K+ channels in order to reinstate homeostasis (318, 331). Ion channels are thus gate keepers of redox status, and the cell’s electrochemical balance and homeostasis (305, 328).
For example, Ca2+ is a critical signaling factor, regulating many cell functions including cell proliferation, differentiation, and apoptosis (233, 244, 267, 329). Alterations in intracellular Ca2+ levels are decoded by Ca2+-sensors, which initiate signaling for various physiological processes (330). Alterations in Ca2+ homoeostasis and signaling are often associated with various pathological conditions, including cancer. The ROS regulatory system is closely linked to the Ca2+ signaling system which operates by changes in intracellular Ca2+ concentrations. Dysfunction of Ca2+ channels in the plasma or the mitochondrial membrane will disrupt the signaling system and increase ROS levels in any cell, potentiating harmful effects including cytotoxicity and resulting in pathogenesis of various disorders (44, 244, 321, 322, 328, 330, 331). Inversely, ROS can significantly affect calcium concentration in the cell by modifying the function of Ca2+ channels (233).
Increased levels of intracellular Ca2+ in some cases are associated with increased apoptosis, probably due to activation of Ca2+ dependent DNase I (332). This may be an alternative pathway for DNA damage and related pathologies. Changes in normal Ca2+ levels in the mitochondria can induce release of cytochrome C, a mitochondrial protein which is a signaling molecule for apoptosis in the cytoplasm, which then goes on to initiate apoptosis in the cell, and activation of nucleases which will cause DNA damage (330).
The effect of man-made EMFs, especially ELF, or RF pulsed or amplitude-modulated by ELF signals, on calcium concentrations in exposed cells and the unique role of the calcium VGICs or VGCCs in EMF-induced bioeffects have been well-documented for long time (8–10, 18, 22, 44, 238, 298, 314–316, 333–341), and explained by the IFO-VGIC mechanism (2, 5, 297, 305, 306). Dysfunction of VGCCs will cause alterations in the intracellular calcium concentrations, impairment of the Ca2+ signaling system and consequent ROS overproduction.
Walleczek (44) reviewed many studies showing effects of ELF EMFs on cells of the immune system revealing the critical role of intracellular calcium. But until that time, the site of interaction of EMFs with cells was unknown, even though the facts were pointing toward the calcium ion channels in the cell membranes as a most reasonable explanation. At the same time, Liburdy (315) in a series of pioneering experiments showed that calcium influx in lymphocytes which occurred within minutes after the onset of ELF EMF exposures, was due to an effect on the calcium channels in the cell plasma membranes (most of which are voltage-gated), and not due to release from intracellular stores.
Apart from the effect of EMFs on Ca2+, Na+, K+, etc. VGICs, proton (H+) VGICs will be similarly affected (342, 343). This in turn will disturb the function of NOXs triggering ROS generation (section 3.1.2). Thus, not only VGCCs, but all VGICs are the sites where the effects of man-made EMFs on cells take place (2, 210).
Besides many other adverse effects, ROS can also affect ion channels themselves. For example, many ion channels contain cysteine residues with highly reactive thiol (SH) groups. These are particularly susceptible to oxidation by ROS. Oxidation of cysteine residues can lead to formation of disulfide bonds. This can alter the channel conformation and affect channel gating. Another effect of ROS on VGICs can be the formation of sulfenic, sulfinic, or sulfonic acids: These modifications can change the channel’s structure and function, potentially leading to channel inactivation or altered ion permeability (318). Oxidation of K+ channels by ROS is a common event in the aging brain (344). Therefore, ion channel dysfunction leads to ROS overproduction, and ROS further amplify ion channel dysfunction. Obviously, we have a vicious circle here, where VGIC dysfunction leads to OS in cells, and this in turn disrupts the ion channels even more, leading to even more pronounced OS.
The balance of the various mobile ions in a cell is closely linked to and, in fact, determines the cell’s homeostasis. ROS production in all cells initiates after imbalance of ion concentrations. Dysfunction of ion channels or pumps due to any reason, including EMF-exposure, can readily cause ionic imbalance, ROS overproduction and OS. Figure 5 shows the biochemical processes related with OS, and initiated after EMF-induced dysfunction of VGICs and imbalance of ion concentrations.

Figure 5. Schematic diagram of the OS processes initiated in living cells after anthropogenic EMF exposure and dysfunction of VGICs. Genetic/cell damage is executed by the generated “final” ROS [adapted from Panagopoulos et al. (210)].
It is thus well documented that ion channel dysfunction causes OS, and here we make the case that the OS found after anthropogenic and especially WC EMF exposure is induced after VGIC dysfunction. We have a clear sequence of events starting from irregular gating of VGICs by man-made EMFs up to OS, cell/DNA damage and related pathologies including infertility and carcinogenesis. Therefore, a comprehensive mechanism of EMF-induced bioeffects can be clearly delineated, with a biophysical stage causing VGIC dysfunction and ionic imbalance, and a subsequent biochemical stage resulting in OS-related pathogenesis. Figure 6 shows a schematic representation of the described comprehensive mechanism initiated by EMF-induced dysfunction of VGICs, and resulting in OS and cellular damage.
6 Discussion
We have reviewed experimental and epidemiological studies referring to the biological and health impacts of anthropogenic ELF and WC EMF exposures. We find once again that it is well documented that both purely ELF and WC/RF (containing ELF) man-made EMFs induce OS and genetic damage, which can lead to related pathologies, such as infertility and cancer in both humans and animals.
We have documented that all anthropogenic EMFs referred to as “RF,” especially WC EMFs, apart from their RF emissions (carrier waves), emit ELF/ULF/VLF EMFs in the form of modulation, pulsation, and variability, and thus, in fact, are a combination of RF and ELF/ULF/VLF EMFs.
Some authors confuse “pulsation” with exposure intermittence. Zahumenska et al. (373) applied an intermittent exposure (6 × 10 min) to a continuous-wave LF EMF (87–207 kHz), with 10-min pauses between exposure periods, and claimed they studied effects of pulsed EMF, finding no significant difference from the absence of effects with an uninterrupted exposure (1 × 60 min). This is not the case. In a “pulsing field” the on/off pulsations are inherent in the signal and occur at ELF/VLF rates usually of the order of hundreds/thousands pulsations per second, whereas in the “intermittent” exposure as in this case, the field/signal is interrupted externally by a timer, or even manually by use of a switch. While pulsed EMFs are, in almost all cases, found to produce significantly greater effects than continuous-wave (non-pulsed) EMFs, an intermittent exposure to any EMF may produce smaller effects than an uninterrupted exposure to the same EMF when the intermittence interval is long enough (e.g., ≥10 min) to allow the exposed organism repair damages and/or adapt to the stressor (72). By confusing pulsation with intermittence one may draw completely misleading conclusions. Zahumenska et al. (373) actually found no effect by use of a continuous-wave LF EMF, which was expected, but they claimed the found no effect with a “pulsed” EMF. A definition of the various physical parameters of EMFs can be found in Panagopoulos and Margaritis (345) and Panagopoulos et al. (3).
We have explained that all man-made EMFs are fully polarized and coherent, with low-frequency (ELF/ULF/VLF) intensity variations in the vast majority of cases, meaning they possess net electric and magnetic fields oscillating (at ELF/ULF/VLF rates) in single directions and in phase. This condition induces parallel and coherent low-frequency forced oscillations of mobile ions and other charged/polar molecules in living tissues. The IFO-VGIC mechanism has described how such oscillations induce dysfunction of VGICs in the membranes of all cells resulting in altered intracellular ionic concentrations (2, 5, 297, 305, 306).
According to the IFO-VGIC mechanism, the non-thermal biological/health effects reported in the literature, are induced specifically by the low-frequency (ULF/ELF/VLF), and not the high-frequency (purely RF/MW), EMFs. This explains why, in the absence of low-frequency modulation/pulsation/variability, the non-thermal effects, attributed before to “RF” EMFs, disappear. It follows that purely RF/MW EMFs can only induce heating of biological tissues at adequately high intensities and frequencies approaching infrared (2, 3). An overview of VGIC structure and function, and the IFO-VGIC mechanism has been provided in section 4.
It is important to note that VGICs are not gated by direct forces on their S4 sensors by externally applied EMFs. That would require applied fields of the order of the transmembrane fields (~107 V/m) (56). The reason why even very weak (down to 10−5–10−4 V/m) ULF/ELF anthropogenic fields can gate VGICs is that due to their polarized and coherent character combined with low frequency variability, they can induce parallel and coordinated low frequency forced oscillations of mobile ions within the channels. And the forces exerted on the S4 sensors by several oscillating ions in close proximity (≤1 nm), depending upon the inverse third power of the distance (Equation 12), are much greater than direct forces from externally applied EMFs. In other words, due to the IFO phenomenon in close proximity to the VGIC-sensors, the forces are enormously amplified. This is a key point in understanding the IFO-VGIC mechanism.
It is thus, polarization and coherence combined with low frequency variability that make anthropogenic EMFs able to irregularly gate (open or close) VGICs, the most sensitive EMF sensors and the most abundant class of ion channels in all cell membranes of all living organisms. This causes perturbation of ionic concentrations in the cells which in turn triggers (over)production of ROS. ROS can readily cause ionization/chemical alterations in living tissue, i.e., breakage of chemical bonds, and DNA damage.
We described biochemical processes initiated in living cells by dysfunction of VGICs due to man-made EMF-exposure, leading to altered intracellular concentrations of critical ions such as Ca2+, Na+, K+, H+, etc., and disruption of the cell’s electrochemical balance, redox state, and homeostasis. This leads to immediate production of the two initial ROS, superoxide anion (O2•−) and nitric oxide (NO•), which can then be easily converted to the powerful “final” ROS peroxynitrite (ONOO−) and/or hydroxyl radical (OH•), which can damage DNA or any other biological molecule.
It is remarkable that the same “final” ROS that ultimately cause biological damage in the case of EMFs (“non-ionizing” radiations), hydroxyl radical and peroxynitrite, are also found in the case of exposure to ionizing radiations. It is estimated that about 2/3 of the DNA damages caused by ionizing radiation are due to OH• (276, 294, 295, 346–348). This provides an answer to claims that “non-ionizing” anthropogenic EMFs cannot possibly cause biological damage. It comes that the same ROS that actually execute the biological damage in most cases, are produced by either ionizing radiation or “non-ionizing” EMFs/EMR. This is related with the fact that in most cases the action of radiation in biological tissue is indirect. The external agent causes impairment of cell homeostasis and in response, the cell generates ROS which execute the damage.
ROS sources in cells are the ETC in the mitochondria, the ETC in the NOXs in the plasma membrane, the NOS enzymes at various locations in the cell, and various other secondary sources (described in section 3.1). All ROS sources/promoters are affected by the intracellular concentrations of cations like Ca2+, K+, Na+, H+, with most cation channels being voltage-gated (VGICs) (231, 234, 271). Therefore, all ROS sources in cells can be affected by man-made EMFs. It is remarkable that in all cases reported so far in the literature, anthropogenic EMF exposures increase and not decrease ROS/OS in cells. This is an additional indication that the cells perceive anthropogenic EMFs as a disturbance.
Even though many of the details of the ion signaling that triggers ROS generation by the above sources are still unexplored, we do know that the triggering involves changes in the intracellular ionic concentrations. Since man-made EMFs have the ability to cause dysfunction of VGICs, the basic parts of the entire process leading to DNA damage and related pathologies are already identified, and the dots are already connected revealing the complete EMF-induced bioeffects mechanism.
Several questions still need to be addressed. For example, we did not discuss the state of the antioxidant system (AOS) under the condition of chronic OS due to long-term EMF exposure. As the production of ROS at physiological levels is an essential part in any cell’s life, the role of the AOS is to limit the level of ROS under the OS threshold where damage would ensue. Moreover, the AOS controls the activity of repair enzymes. Cells/organisms with compromised antioxidant capacity or high energy demands are particularly vulnerable to OS, and, subsequently, to man-made EMFs. Many studies have revealed significant changes in activity of key antioxidant enzymes under modulated and/or pulsed RF/WC EMF exposure [see reviews in (62–64)]. And while in many cases the changes in activities of antioxidant enzymes may be induced by ROS overproduction in the exposed cells, they may also be affected by ionic imbalances related to VGIC dysfunction. For example, Ca2+ can influence the activity of transcription factors like NF-κB and Nrf2, which regulate the expression of antioxidant genes (349). Further, disruption of Na+/K+ gradients can indirectly affect Ca2+ homeostasis through the Na+/ Ca2+ exchanger, which can operate in both directions depending on the ion gradients (350).
When overproduction of ROS in a cell exceeds the capacity of its AOS, the cell/organism is under OS. A sustained or repeated such condition leads to DNA/cellular damage. Intracellular ions, particularly Ca2+, affect the activity of AOS and DNA repair enzymes. For example, some DNA repair pathways are Ca2+-dependent. Disruption of Ca2+ homeostasis can therefore impair DNA repair capacity, making cells more susceptible to DNA damage (351). Unrepaired/misrepaired DNA lesions such as strand breaks, covalent bond breakage, or nucleotide base and sugar damages, can lead to cell senescence, cell death, or mutations, and related pathologies such as aging, infertility, neurodegenerative diseases, and cancer (2, 61, 210, 233, 280, 290).
The processes initiated in living cells due to VGIC dysfunction in their cell membranes resulting in OS, genetic damage, and related pathologies provide an explanation for the plethora of biological and health effects reviewed in the Introduction (section 1.2). Moreover, the dysfunction of VGICs caused by man-made EMF exposure and leading to OS can also explain EHS, as EHS is accompanied by OS (197, 352), and in fact it is likely due to chronic OS. The pathophysiological changes in the CNS observed to accompany EHS [(see 190)] can be explained by the fact that neurons have higher percentages of VGICs, as VGICs (specifically Na+ and K+ VGICs) are the mediators for the transmission of the nerve impulses (231, 271).
Several studies have found that ELF EMFs induce epigenetic changes in cells, commonly resulting in altered gene expression. Such changes include methylation/de-methylation of genes via activation/deactivation of methyltransferase enzymes, post-translational modification of histone proteins, and alteration of microRNA expression (353–355). Epigenetic changes can induce significant alterations in cell function and consequently the health of an organism. Since ROS affect cell signaling (232) related also with epigenetic changes (320, 354), the reported epigenetic effects induced by anthropogenic ELF EMFs can be due to ROS signaling, and the presented mechanism of EMF-induced ROS (over)production provides an explanation for this. For example, EMF-induced ROS may interfere with DNA or histone methyltransferases and histone deacetylases, resulting in modifications of the epigenome at various regions, including the promoter regions of tumor suppressor genes, resulting in their silencing/inactivation, and leading to cancer promotion (356, 357).
Like us, Blank and Goodman (57, 58) also noted that both ELF and “RF” (actually WC) EMFs produce similar effects, especially in inducing synthesis of stress proteins in cells very rapidly (within a few min). For us, an apparent explanation of the common ELF and RF/WC EMF effects that escaped attention, is that “RF” EMFs affect cells not by their carrier (RF) components, but by their ELF components of pulsing and modulation. As this study shows, actually only the ELF EMFs are those that induce the non-thermal biological effects, and they do not act directly on DNA, but indirectly through VGIC dysfunction and consequent induction of OS (2, 210). Further, it follows that purely RF EMFs can only induce heating at adequately high intensities and frequencies (3).
As documented here, anthropogenic EMFs at environmentally existing levels can ionize living tissue through the action of the generated ROS/OS. It is through the action of ROS the damage found in the DNA after anthropogenic and especially WC EMF exposures. There is a tight link between anthropogenic EMF-exposures, VGIC dysfunction, OS, and DNA/cellular damage.
For cells with irreparably damaged genomic DNA, possible outcomes are, cell senescence or cell death (which may result in aging, organic/neurodegenerative diseases, and/or reproductive difficulties), cancer, or mutated offspring (Figure 7), depending on cell type, the specific biological/environmental conditions, and the state of the organism (2, 210). Thus, DNA damage induced by OS explains the pathologies linked to chronic exposure to anthropogenic EMFs, such as infertility and cancer.
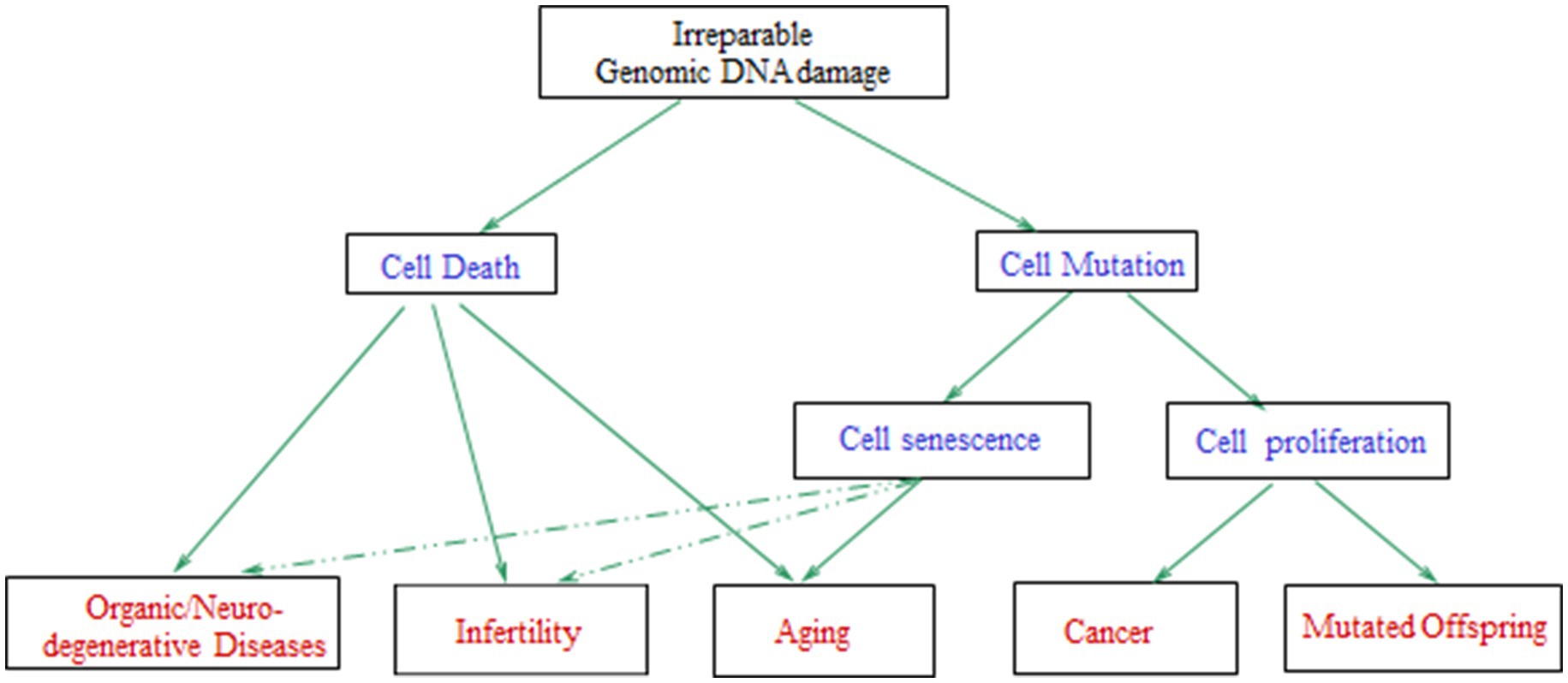
Figure 7. Schematic diagram with the possible consequences of irreparable genomic DNA damage in the cells of a living organism. The arrows with dashed lines mean that the effects can be induced in a lesser degree [adapted from Panagopoulos et al. (210)].
Man-made EMFs, and especially the most detrimental ones from WC antennas/devices and high-voltage electric power lines, have become a new reality in modern life exposing billions of people on a daily basis (7, 50). Even though they are significantly less cytotoxic than radioactivity or certain toxic chemicals, they represent an evolutionary novel and most persistent daily cytotoxic agent, against which, existing repair mechanisms may not be efficient enough. Especially in individuals who are already genetically or epigenetically compromised.
Therapeutic effects of man-made EMFs have also been reported in the literature, especially of pulsing ELF EMFs and specifically in bone fracture healing (238, 358–363). Altered intracellular calcium levels have also been reported to accompany such effects, and the same biophysical mechanism of induced VGIC gating seems to be involved in both the detrimental and therapeutic effects of man-made EMFs (238, 359, 363). Several authors speak of therapeutic effects of pulsing ELF EMFs without specifying or discussing which parameter of the EMF exposure might be the therapeutic one (364–366). This might lead to the false impression that any EMF with ELF/ULF pulsations may be therapeutic, which, of course, is not the case, as, e.g., all WC EMFs consist of such pulsations, and yet are the most detrimental. Some other authors suggest that there are specific “beneficial” or “detrimental” frequencies in the ELF range (367) without considering the IFO-VGIC mechanism published for almost 25 years (and already referenced by more than a thousand other publications), which clearly shows according to generally accepted mathematics, physics, and biology, that the bioactivity of polarized, coherent, and oscillating EMFs, is proportional to field intensity and inversely proportional to field frequency, which makes all ULF/ELF frequencies very bioactive rather than only some specific ones (2, 5, 297, 305, 306). Thus, the basis of EMF bioactivity is not some specific frequencies, but polarization and coherence combined with low frequency variability (at any ULF/ELF frequency), with the lower the frequency the more bioactive the field (Equation 16). Once an EMF is polarized, coherent, and slow varying, we cannot exclude the possibility of resonance phenomena taking place at specific physiological ULF/ELF frequencies. However, we would not expect such phenomena, if they occur, to be particularly intense, especially under actual damping conditions within cells and ion channels (368).
In our opinion, a condition for an applied EMF in order to have a therapeutic action is, to simulate natural EMFs or physiological endogenous cellular signals. Once we know that the most bioactive polarized and coherent EMFs are the ULF/ELF ones, the critical issue for an applied ULF/ELF EMF is whether its included frequencies (and other parameters such as waveform, polarity, etc.) reinforce or cancel the endogenous physiological electrical activity of the cells which is responsible for the specific therapeutic action (49, 210, 369). The basic frequency of the natural atmospheric “Schumann” electromagnetic resonances (7.83 Hz) and its harmonics are detected in the human/animal brain activity, and the physical parameters of electromagnetic brain activity and atmospheric lightning display remarkable similarities (369–371). Thus, we have suggested (210) that the therapeutic effects of pulsed EMFs are expected to be optimal at pulsing frequencies coinciding with the Schumann frequencies, or the endogenous ionic oscillations in cells (49). Indeed, Yan et al. (372) found that pulses at an ELF repetition rate coinciding with the basic Schumann frequency 7.83 Hz inhibit proliferation and induce apoptosis of cancer cells while this does not occur with normal cells. This needs to be further verified and certainly, there are important limitations: All anthropogenic EMFs are fully polarized and coherent something that does not occur with the natural EMFs which are only partially polarized on certain occasions (5). This seems to be the reason why the vast majority of effects of anthropogenic EMFs are detrimental, whereas the vast majority of natural EMFs can be beneficial.
In conclusion, the IFO-VGIC mechanism that explains VGIC dysfunction, and the subsequent OS, provide a comprehensive biophysical/biochemical mechanism explaining the plethora of experimental and epidemiological findings connecting anthropogenic EMF exposures with OS, DNA/cellular damage and related pathologies such as poor health, EHS, infertility, organic/neurodegenerative diseases, cancer, etc. Even though the mechanistic details of how exactly the ionic perturbations stimulate ROS production by their sources need to be further explored, the basic scheme of the complete EMF-bioeffects mechanism is revealed already. The long existing experimental and epidemiological findings connecting exposure to man-made EMFs and DNA damage, infertility, and cancer, are now explained by the presented comprehensive mechanism. We hope this provides a better understanding of the involved science, a basis for future research, and the establishment of biologically relevant EMF exposure guidelines for effective protection of public health and the environment.
Author contributions
DP: Conceptualization, Writing – original draft. IY: Writing – review & editing. GI: Writing – review & editing. GC: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Special Account for Research Grants of the National Kapodistrian University of Athens (grant number 16599).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Misek, J, Jakus, J, Hamza Sladicekova, K, Zastko, L, Veternik, M, Jakusova, V, et al. Extremely low frequency magnetic fields emitted by cell phones. Front Phys. (2023) 11:1094921. doi: 10.3389/fphy.2023.1094921
2. Panagopoulos, DJ, Karabarbounis, A, Yakymenko, I, and Chrousos, GP. Human-made electromagnetic fields: ion forced-oscillation and voltage-gated ion channel dysfunction, oxidative stress and DNA damage. Int J Oncol. (2021) 59:92. doi: 10.3892/ijo.2021.5272
3. Panagopoulos, DJ, Karabarbounis, A, and Lioliousis, C. Defining wireless communication (WC) electromagnetic fields (EMFs): A. Polarization is a principal property of all man-made EMFs; B. Modulation, pulsation, and variability are inherent parameters of WC EMFs; C. Most man-made EMF exposures are non-thermal; D. Measuring incident EMFs is more relevant than specific absorption rate (SAR); E. All man-made EMFs emit continuous waves, not photons; F. Differences from natural EMFs. Interaction with matter In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: Biological and health effects. Boca Raton: CRC Press (2022)
4. Pedersen, GF. Amplitude modulated RF fields stemming from a GSM/DCS-1800 phone. Wirel Netw. (1997) 3:489–98. doi: 10.1023/A:1019158712657
5. Panagopoulos, DJ, Johansson, O, and Carlo, GL. Polarization: a key difference between man-made and natural electromagnetic fields, in regard to biological activity. Sci Rep. (2015) 5:14914. doi: 10.1038/srep14914
7. Panagopoulos, DJ. Comparing DNA damage induced by Mobile telephony and other types of man-made electromagnetic fields. Mutation Res Rev. (2019) 781:53–62. doi: 10.1016/j.mrrev.2019.03.003
8. Bawin, SM, Kaczmarek, LK, and Adey, WR. Effects of modulated VHF fields, on the central nervous system. Ann NY Acad Sci. (1975) 247:74–81.
9. Bawin, SM, Adey, WR, and Sabbot, IM. Ionic factors in release of 45Ca2+ from chick cerebral tissue by electromagnetic fields. Proc Natl Acad Sci USA. (1978) 75:6314–8.
10. Blackman, CF, Benane, SG, Elder, JA, House, DE, Lampe, JA, and Faulk, JM. Induction of calcium-ion efflux from brain tissue by radiofrequency radiation: effect of sample number and modulation frequency on the power-density window. Bioelectromagnetics. (1980) 1:35–43. doi: 10.1002/bem.2250010104
11. Blackman, CF, Benane, SG, Kinney, LS, Joines, WT, and House, DE. Effects of ELF fields on calcium-ion efflux from brain tissue in vitro. Radiat Res. (1982) 92:510–20.
12. Bolshakov, MA, and Alekseev, SI. Bursting responses of Lymnea neurons to microwave radiation. Bioelectromagnetics. (1992) 13:119–29. doi: 10.1002/bem.2250130206
13. Byus, CV, Lundak, RL, Fletcher, RM, and Adey, WR. Alterations in protein kinase activity following exposure of cultured lymphocytes to modulated microwave fields. Bioelectromagnetics. (1984) 5:341–51. doi: 10.1002/bem.2250050307
14. Byus, CV, Kartum, K, Pieper, SE, and Adey, WR. Ornithine decarboxylase activity in liver cells is enhanced by low-level amplitude modulated microwave fields. Cancer Res. (1988) 48:4222–6.
15. Campisi, A, Gulino, M, Acquaviva, R, Bellia, P, Raciti, G, Grasso, R, et al. Reactive oxygen species levels and DNA fragmentation on astrocytes in primary culture after acute exposure to low intensity microwave electromagnetic field. Neurosci Lett. (2010) 473:52–5. doi: 10.1016/j.neulet.2010.02.018
16. Franzellitti, S, Valbonesi, P, Ciancaglini, N, Biondi, C, Contin, A, Bersani, F, et al. Transient DNA damage induced by high-frequency electromagnetic fields (GSM 1.8 GHz) in the human trophoblast HTR-8/SVneo cell line evaluated with the alkaline comet assay. Mutat Res. (2010) 683:35–42. doi: 10.1016/j.mrfmmm.2009.10.004
17. Frei, M, Jauchem, J, and Heinmets, F. Physiological effects of 2.8 GHz radio-frequency radiation: a comparison of pulsed and continuous-wave radiation. J Microw Power Electromagn Energy. (1988) 23:2.
18. Goodman, EM, Greenebaum, B, and Marron, MT. Effects of electro- magnetic fields on molecules and cells. Int Rev Cytologia. (1995) 158:279–338.
19. Hinrikus, H, Bachmann, M, Lass, J, Tomson, R, and Tuulik, V. Effect of 7, 14 and 21 Hz modulated 450 MHz microwave radiation on human electroencephalographic rhythms. Int J Radiat Biol. (2008) 84:69–79. doi: 10.1080/09553000701691679
20. Höytö, A, Luukkonen, J, Juutilainen, J, and Naarala, J. Proliferation, oxidative stress and cell death in cells exposed to 872 MHz radiofrequency radiation and oxidants. Radiat Res. (2008) 170:235–43. doi: 10.1667/RR1322.1
21. Huber, R, Treyer, V, Borbely, AA, Schuderer, J, Gottselig, JM, Landolt, HP, et al. Electromagnetic fields, such as those from mobile phones, alter regional cerebral blood flow and sleep and waking EEG. J Sleep Res. (2002) 11:289–95. doi: 10.1046/j.1365-2869.2002.00314.x
22. Lin-Liu, S, and Adey, WR. Low frequency amplitude modulated microwave fields change calcium efflux rates from synaptosomes. Bioelectromagnetics. (1982) 3:309–22. doi: 10.1002/bem.2250030303
23. Litovitz, TA, Krause, D, Penafiel, M, Elson, EC, and Mullins, JM. The role of coherence time in the effect of microwaves on ornithine decarboxylase activity. Bioelectromagnetics. (1993) 14:395–403. doi: 10.1002/bem.2250140502
24. Mohammed, HS, Fahmy, HM, Radwan, NM, and Elsayed, AA. Non-thermal continuous and modulated electromagnetic radiation fields effects on sleep EEG of rats. J Adv Res. (2013) 4:181–7. doi: 10.1016/j.jare.2012.05.005
25. Penafiel, LM, Litovitz, T, Krause, D, Desta, A, and Mullins, MJ. Role of modulation on the effect of microwaves on ornithine decarboxylase activity in L929 cells. Bioelectromagnetics. (1997) 18:132–41. doi: 10.1002/(SICI)1521-186X(1997)18:2<132::AID-BEM6>3.0.CO;2-3
26. Somosy, Z, Thuroczy, G, Kubasova, T, Kovacs, J, and Szabo, LD. Effects of modulated and continuous microwave irradiation on the morphology and cell surface negative charge of 3T3 fibroblasts. Scanning Microsc. (1991) 5:1145–55.
27. Thuroczy, G, Kubinyi, G, Bodo, M, Bakos, J, and Szabo, LD. Simultaneous response of brain electrical activity (EEG) and cerebral circulation (REG) to microwave exposure in rats. Rev Environ Health. (1994) 10:135–48. doi: 10.1515/REVEH.1994.10.2.135
28. Van der Meer, JN, Eisma, YB, Meester, R, Jacobs, M, and Nederveen, AJ. Effects of mobile phone electromagnetic fields on brain waves in healthy volunteers. Sci Rep. (2023) 13:21758. doi: 10.1038/s41598-023-48561-z
29. Veyret, B, Bouthet, C, Deschaux, P, de Seze, R, Geffard, M, Joussot-Dubien, J, et al. Antibody responses of mice exposed to low-power microwaves under combined, pulse-and-amplitude modulation. Bioelectromagnetics. (1991) 12:47–56. doi: 10.1002/bem.2250120107
30. Mohammed, HS. Effects of wireless communication electromagnetic fields on human and animal brain activity In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC Press (2022)
31. Aydin, M, Turk, G, Yuksel, M, Cevik, A, Apaydin, A, and Yilmaz, S. Effect of electromagnetic field on the sperm characteristics and histopathological status of testis in rats. Med Weter. (2007) 63:178–83.
32. Azanza, MJ, Perez Bruzon, RN, Lederer, D, et al. Reversibility of the effects induced on the spontaneous bioelectric activity of neurons under exposure to 8.3 and 217.0 Hz low intensity magnetic fields. 2nd Int. Workshop Biol Effects of EMFs, Rhodes, Greece. (2002). p. 651–659.
33. Hong, R, Zhang, Y, Liu, Y, and Weng, EQ. Effects of extremely low frequency electromagnetic fields on DNA of testicular cells and sperm chromatin structure in mice. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. (2005) 23:414–7.
34. Ivancsits, S, Diem, E, Pilger, A, Rüdiger, HW, and Jahn, O. Induction of DNA strand breaks by intermittent exposure to extremely-low-frequency electromagnetic fields in human diploid fibroblasts. Mutat Res. (2002) 519:1–13. doi: 10.1016/S1383-5718(02)00109-2
35. Ivancsits, S, Diem, E, Jahn, O, and Rüdiger, HW. Intermittent extremely low frequency electromagnetic fields cause DNA damage in a dose-dependent way. Int Arch Occup Environ Health. (2003) 76:431–6. doi: 10.1007/s00420-003-0446-5
36. Lee, JS, Ahn, SS, Jung, KC, Kim, YW, and Lee, SK. Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J Androl. (2004) 6:29–34.
37. Lee, SK, Park, S, Gimm, YM, and Kim, YW. Extremely low frequency magnetic fields induce spermatogenic germ cell apoptosis: possible mechanism. Biomed Res Int. (2014) 2014:567183:1–8. doi: 10.1155/2014/567183
38. Ma, TH, and Chu, KC. Effect of the extremely low frequency (ELF) electromagnetic field (EMF) on developing embryos of the fruit fly (Drosophila melanogaster L.). Mutat Res. (1993) 303:35–9. doi: 10.1016/0165-7992(93)90006-H
39. Moghadam, MK, Firoozabadi, SM, and Janahmadi, M. 50 Hz alternating extremely low frequency magnetic fields affect excitability, firing and action potential shape through interaction with ionic channels in snail neurones. Environmentalist. (2008) 28:341–7. doi: 10.1007/s10669-007-9143-3
40. Moghadam, MK, Firoozabadi, SM, and Janahmadi, M. Reduction of F1 neuronal excitability by exposure to 217 Hz magnetic fields from GSM 900 mobile phone. Cell J. (2009) 11:176–83.
41. Moghadam, MK, Firoozabadi, M, and Janahmadi, M. Effects of weak environmental magnetic fields on the spontaneous bioelectrical activity of snail neurons. J Membr Biol. (2011) 240:63–71. doi: 10.1007/s00232-011-9344-z
42. Panagopoulos, DJ, Karabarbounis, A, and Lioliousis, C. ELF alternating magnetic field decreases reproduction by DNA damage induction. Cell Biochem Biophys. (2013) 67:703–16. doi: 10.1007/s12013-013-9560-5
43. Santini, MT, Ferrante, A, Rainaldi, G, Indovina, P, and Indovina, PL. Extremely low frequency (ELF) magnetic fields and apoptosis: a review. Int J Radiat Biol. (2005) 81:1–11. doi: 10.1080/09553000400029502
44. Walleczek, J. Electromagnetic field effects on cells of the immune system: the role of calcium signaling. FASEB J. (1992) 6:3177–85. doi: 10.1096/fasebj.6.13.1397839
45. Górski, R, Kotwicka, M, Skibińska, I, Jendraszak, M, and Wosiński, S. Effect of low-frequency electric field screening on motility of human sperm. Ann Agric Environ Med. (2020) 27:427–34. doi: 10.26444/aaem/116019
46. Karbalay-Doust, S, Darabyan, M, Sisakht, M, Haddadi, G, Sotoudeh, N, Haghani, M, et al. Extremely low frequency-electromagnetic fields (ELF-EMF) can decrease spermatocyte count and motility and change testicular tissue. J Biomed Phys Eng. (2023) 13:135–46. doi: 10.31661/jbpe.v0i0.2011-1234
47. Mahna, A, Firoozabadi, SM, and Atashi, A. Cell phone and breast cancer: the cell phone-generated pulsed 217Hz ELF magnetic field increases angiogenesis. Iran J Med Phys. (2021) 18:421–9. doi: 10.22038/IJMP.2020.52303.1859
48. Mansourian, M, Firoozabadi, M, and Hassan, ZM. The role of 217-Hz ELF magnetic fields emitted from GSM mobile phones on electrochemotherapy mechanisms. Electromagn Biol Med. (2020) 39:239–49. doi: 10.1080/15368378.2020.1762635
49. Panagopoulos, DJ. Electromagnetic interaction between environmental fields and living systems determines health and well-being In: Electromagnetic fields: principles, engineering applications and biophysical effects. K Myung-Hee and Y Sang-Ook (editors). New York, USA: Nova Science Publishers (2013)
50. Panagopoulos, DJ. Electromagnetic fields of wireless communications: biological and health effects. 1st ed. Boca Raton: CRC Press (2023).
52. Wever, R. The circadian system of man: results of experiments under temporal isolation. New York: Springer-Verlag (1979).
53. Panagopoulos, DJ. Man-made electromagnetic radiation is not quantized In: A Reimer, editor. Horizons in world physics, vol. 296. New York: Nova Science Publishers (2018). 1–57.
54. Puranen, L, and Jokela, K. Radiation hazard assessment of pulsed microwave radars. J Microw Power Electromagn Energy. (1996) 31:165–77. doi: 10.1080/08327823.1996.11688307
55. Pirard, W, and Vatovez, B. Study of pulsed character of radiation emitted by wireless telecommunication systems. Liège, Belgium: Institut scientifique de service public. (Available in: https://www.issep.be/wp-content/uploads/7IWSBEEMF_B-Vatovez_W-Pirard.pdf)
56. Panagopoulos, DJ. Comments on Pall’s “millimeter (MM) wave and microwave frequency radiation produce deeply penetrating effects: the biology and the physics”. Rev Environ Health. (2021) 37:295–7. doi: 10.1515/reveh-2021-0090
57. Blank, M, and Goodman, R. Electromagnetic fields stress living cells. Pathophysiology. (2009) 16:71–8. doi: 10.1016/j.pathophys.2009.01.006
58. Blank, M, and Goodman, R. DNA is a fractal antenna in electromagnetic fields. Int J Radiat Biol. (2011) 87:409–15. doi: 10.3109/09553002.2011.538130
59. Henshaw, DL, and Philips, A. A mechanistic understanding of human magnetoreception validates the phenomenon of electromagnetic hypersensitivity (EHS). Int J Radiat Biol. (2024):1–19.
60. Roux, D, Vian, A, Girard, S, Bonnet, P, Paladian, F, Davies, E, et al. Electromagnetic fields (900MHz) evoke consistent molecular responses in tomato plants. Physiol Plant. (2006) 128:283–8. doi: 10.1111/j.1399-3054.2006.00740.x
61. Phillips, JL, Singh, NP, and Lai, H. Electromagnetic fields and DNA damage. Pathophysiology. (2009) 16:79–88. doi: 10.1016/j.pathophys.2008.11.005
62. Yakymenko, I, Tsybulin, O, Sidorik, E, Henshel, D, Kyrylenko, O, and Kyrylenko, S. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn Biol Med. (2016) 35:186–202. doi: 10.3109/15368378.2015.1043557
63. Yakymenko, I, and Tsybulin, O. Oxidative stress induced by wireless communication electromagnetic fields In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC Press (2022)
64. Jagetia, GC. Genotoxic effects of wireless communication electromagnetic fields In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC Press, Taylor and Francis (2022)
65. Miller, K, Harrison, K, Martin, JH, Nixon, B, and De Iuliis, GN. The impacts of wireless communication electromagnetic fields on human reproductive biology In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC press, Taylor and Francis (2022)
66. Panagopoulos, DJ. DNA and chromosome damage in human and animal cells induced by mobile telephony electromagnetic fields and other stressors In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC Press (2022)
67. Agarwal, A, Desai, NR, Makker, K, Varghese, A, Mouradi, R, Sabanegh, E, et al. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil Steril. (2009) 92:1318–25. doi: 10.1016/j.fertnstert.2008.08.022
68. Aitken, RJ, Bennetts, LE, Sawyer, D, Wiklendt, AM, and King, BV. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int J Androl. (2005) 28:171–9. doi: 10.1111/j.1365-2605.2005.00531.x
69. Banerjee, S, Singh, NN, Sreedhar, G, and Mukherjee, S. Analysis of the genotoxic effects of Mobile phone radiation using buccal micronucleus assay: a comparative evaluation. J Clin Diagn Res. (2016) 10:ZC82–5. doi: 10.7860/JCDR/2016/17592.7505
70. Belyaev, IY, Hillert, L, Protopopova, M, Tamm, C, Malmgren, LOG, Persson, BRR, et al. 915 MHz microwaves and 50 Hz magnetic field affect chromatin conformation and 53BP1 foci in human lymphocytes from hypersensitive and healthy persons. Bioelectromagnetics. (2005) 26:173–84. doi: 10.1002/bem.20103
71. Burlaka, A, Tsybulin, O, Sidorik, E, Lukin, S, Polishuk, V, Tsehmistrenko, S, et al. Overproduction of free radical species in embryonic cells exposed to low intensity radiofrequency radiation. Exp Oncol. (2013) 35:219–25.
72. Chavdoula, ED, Panagopoulos, DJ, and Margaritis, LH. Comparison of biological effects between continuous and intermittent exposure to GSM-900 MHz mobile phone radiation. Detection of apoptotic cell death features. Mutation Res. (2010) 700:51–61. doi: 10.1016/j.mrgentox.2010.05.008
73. Daroit, NB, Visioli, F, Magnusson, AS, Vieira, GR, and Rados, PV. Cell phone radiation effects on cytogenetic abnormalities of oral mucosal cells. Braz Oral Res. (2015) 29:1–8. doi: 10.1590/1807-3107BOR-2015.vol29.0114
74. De Iuliis, GN, Newey, RJ, King, BV, and Aitken, RJ. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One. (2009) 4:e6446. doi: 10.1371/journal.pone.0006446
75. De Iuliis, GN, Thomson, LK, Mitchell, LA, Finnie, JM, Koppers, AJ, et al. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol Reprod. (2009) 81:517–24. doi: 10.1095/biolreprod.109.076836
76. Delgado, JMR. Biological effects of extremely low frequency electromagnetic fields. J Bioelectr. (1985) 4:75–92. doi: 10.3109/15368378509040362
77. Diem, E, Schwarz, C, Adlkofer, F, Jahn, O, and Rudiger, H. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. (2005) 583:178–83. doi: 10.1016/j.mrgentox.2005.03.006
78. Ferreira, AR, Knakievicz, T, de Bittencourt Pasquali, MA, Gelain, DP, Dal-Pizzol, F, Fernández, CER, et al. Ultra high frequency-electromagnetic field irradiation during pregnancy leads to an increase in erythrocytes micronuclei incidence in rat offspring. Life Sci. (2006) 80:43–8050. doi: 10.1016/j.lfs.2006.08.018
79. Garaj-Vrhovac, V, Horvat, D, and Koren, Z. The effect of microwave radiation on the cell genome. Mutat Res. (1990) 243:87–93. doi: 10.1016/0165-7992(90)90028-I
80. Garaj-Vrhovac, V, Horvat, D, and Koren, Z. The relationship between colony-forming ability, chromosome aberrations and incidence of micronuclei in V79 Chinese hamster cells exposed to microwave radiation. Mutat Res. (1991) 263:143–9. doi: 10.1016/0165-7992(91)90054-8
81. Garaj-Vrhovac, V, Fucić, A, and Horvat, D. The correlation between the frequency of micronuclei and specific chromosome aberrations in human lymphocytes exposed to microwave radiation in vitro. Mutat Res. (1992) 281:181–6. doi: 10.1016/0165-7992(92)90006-4
82. Guler, G, Tomruk, A, Ozgur, E, and Seyhan, N. The effect of radiofrequency radiation on DNA and lipid damage in non-pregnant and pregnant rabbits and their newborns. Gen Physiol Biophys. (2010) 29:59–60. doi: 10.4149/gpb_2010_01_59
83. Ji, S, Oh, E, Sul, D, Choi, JW, Park, H, and Lee, E. DNA damage of lymphocytes in volunteers after 4 hours use of Mobile phone. J Prev Med Public Health. (2004) 37:373–80.
84. Koana, T, Okada, MO, Takashima, Y, Ikehata, M, and Miyakoshi, J. Involvement of eddy currents in the mutagenicity of ELF magnetic fields. Mutat Res. (2001) 476:55–62. doi: 10.1016/S0027-5107(01)00082-3
85. Lai, H, and Singh, NP. Acute low-intensity microwave exposure increases DNA single-strand breaks in rat brain cells. Bioelectromagnetics. (1995) 16:207–10. doi: 10.1002/bem.2250160309
86. Lai, H, and Singh, NP. Single- and double-strand DNA breaks in rat brain cells after acute exposure to radiofrequency electromagnetic radiation. Int J Radiat Biol. (1996) 69:513–21. doi: 10.1080/095530096145814
87. Lai, H, and Singh, NP. Acute exposure to a 60 Hz magnetic field increases DNA strand breaks in rat brain cells. Bioelectromagnetics. (1997) 18:156–65. doi: 10.1002/(SICI)1521-186X(1997)18:2<156::AID-BEM8>3.0.CO;2-1
88. Lee, KS, Choi, JS, Hong, SY, Son, TH, and Yu, K. Mobile phone electromagnetic radiation activates MAPK signaling and regulates viability in Drosophila. Bioelectromagnetics. (2008) 29:371–9. doi: 10.1002/bem.20395
89. Liu, C, Gao, P, Xu, SC, Wang, Y, Chen, CH, He, MD, et al. Mobile phone radiation induces mode-dependent DNA damage in a mouse spermatocyte-derived cell line: a protective role of melatonin. Int J Radiat Biol. (2013) 89:993–1001. doi: 10.3109/09553002.2013.811309
90. Lixia, S, Yao, K, Kaijun, W, Deqiang, L, Huajun, H, Xiangwei, G, et al. Effects of 1.8 GHz radiofrequency field on DNA damage and expression of heat shock protein 70 in human lens epithelial cells. Mutat Res. (2006) 602:135–42. doi: 10.1016/j.mrfmmm.2006.08.010
91. Luukkonen, J, Hakulinen, P, Mäki-Paakkanen, J, Juutilainen, J, and Naarala, J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat Res. (2009) 662:54–8. doi: 10.1016/j.mrfmmm.2008.12.005
92. Mailankot, M, Kunnath, AP, Jayalekshmi, H, Koduru, B, and Valsalan, R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics. (2009) 64:561–5. doi: 10.1590/S1807-59322009000600011
93. Markova, E, Hillert, L, Malmgren, L, Persson, BR, and Belyaev, IY. Microwaves from GSM mobile telephones affect 53BP1 and gamma-H2AX foci in human lymphocytes from hypersensitive and healthy persons. Environ Health Perspect. (2005) 113:1172–7. doi: 10.1289/ehp.7561
94. Mausset-Bonnefont, AL, et al. Acute exposure to GSM 900-MHz electromagnetic fields induces glial reactivity and biochemical modifications in the rat brain. Neurobiol Dis. (2004) 17:445–54. doi: 10.1016/j.nbd.2004.07.004
95. Mihai, CT, Rotinberg, P, Brinza, F, and Vochita, G. Extremely low-frequency electromagneticfields cause DNA strand breaks in normal cells. J Environ Health Sci Eng. (2014) 12:15. doi: 10.1186/2052-336X-12-15
96. Nikolova, T, Czyz, J, Rolletschek, A, Blyszczuk, P, Fuchs, J, Jovtchev, G, et al. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J. (2005) 19:1686–8. doi: 10.1096/fj.04-3549fje
97. Panagopoulos, DJ, Chavdoula, ED, Nezis, IP, and Margaritis, LH. Cell death induced by GSM 900MHz and DCS 1800MHz Mobile telephony radiation. Mutat Res. (2007) 626:69–78. doi: 10.1016/j.mrgentox.2006.08.008
98. Panagopoulos, DJ, Chavdoula, ED, and Margaritis, LH. Bioeffects of Mobile telephony radiation in relation to its intensity or distance from the antenna. Int J Radiat Biol. (2010) 86:345–57. doi: 10.3109/09553000903567961
99. Panagopoulos, DJ. Effect of microwave exposure on the ovarian development of Drosophila melanogaster. Cell Biochem Biophys. (2012) 63:121–32. doi: 10.1007/s12013-012-9347-0
100. Pesnya, DS, and Romanovsky, AV. Comparison of cytotoxic and genotoxic effects of plutonium-239 alpha particles and mobile phone GSM 900 radiation in the Allium cepa test. Mutat Res. (2013) 750:27–33. doi: 10.1016/j.mrgentox.2012.08.010
101. Sarkar, S, Ali, S, and Behari, J. Effect of low power microwave on the mouse genome: a direct DNA analysis. Mutat Res. (1994) 320:141–7. doi: 10.1016/0165-1218(94)90066-3
102. Sokolovic, D, et al. Melatonin reduces oxidative stress induced by chronic exposure of microwave radiation from Mobile phones in rat brain. J Radiat Res. (2008) 49:579–86. doi: 10.1269/jrr.07077
103. Svedenstal, BM, Johanson, KJ, and Mild, KH. DNA damage induced in brain cells of CBA mice exposed to magnetic fields. In Vivo. (1999) 13:551–2.
104. Tomruk, A, Guler, G, and Dincel, AS. The influence of 1800 MHz GSM-like signals on hepatic oxidative DNA and lipid damage in nonpregnant, pregnant, and newly born rabbits. Cell Biochem Biophys. (2010) 56:39–47. doi: 10.1007/s12013-009-9068-1
105. Winker, R, Ivancsits, S, Pilger, A, Adlkofer, F, and Rüdiger, HW. Chromosomal damage in human diploid fibroblasts by intermittent exposure to extremely low-frequency electromagnetic fields. Mutat Res. (2005) 585:43–9. doi: 10.1016/j.mrgentox.2005.04.013
106. Yadav, AS, and Sharma, MK. Increased frequency of micronucleated exfoliated cells among humans exposed in vivo to mobile telephone radiations. Mutat Res. (2008) 650:175–80. doi: 10.1016/j.mrgentox.2007.11.005
107. Yan, JG, Agresti, M, Bruce, T, Yan, YH, Granlund, A, and Matloub, HS. Effects of cellular phone emissions on sperm motility in rats. Fertil Steril. (2007) 88:957–64. doi: 10.1016/j.fertnstert.2006.12.022
108. Yao, K, et al. Electromagnetic noise inhibits radiofrequency radiation-induced DNA damage and reactive oxygen species increase in human lens epithelial cells. Mol Vis. (2008) 19:964–9.
109. Zhang, DY, Xu, ZP, Chiang, H, Lu, DQ, and Zeng, QL. Effects of GSM 1800 MHz radiofrequency electromagnetic fields on DNA damage in Chinese hamster lung cells. Zhonghua Yu Fang Yi Xue Za Zhi. (2006) 40:149–52.
110. Zhang, G, Yan, H, Chen, Q, Liu, K, Ling, X, Sun, L, et al. Effects of cell phone use on semen parameters: results from the MARHCS cohort study in Chongqing, China. Environ Int. (2016) 91:116–21. doi: 10.1016/j.envint.2016.02.028
111. D'Silva, MH, Swer, RT, Anbalagan, J, and Rajesh, B. Effect of radiofrequency radiation emitted from 2G and 3G cell phone on developing liver of Chick embryo - a comparative study. J Clin Diagn Res. (2017) 11:5–9. doi: 10.7860/JCDR/2017/26360.10275
112. D’Silva, MH, Swer, RT, Anbalagan, J, and Bhargavan, R. Assessment of DNA damage in chick embryo brains exposed to 2G and 3G cell phone radiation using alkaline comet assay technique. J Clin Diagn Res. (2021) 15:AC01–4. doi: 10.7860/JCDR/2021/47115.14441
113. Gulati, S, Mosgoeller, W, Moldan, D, Kosik, P, Durdik, M, Jakl, L, et al. Evaluation of oxidative stress and genetic instability among residents near mobile phone base stations in Germany. Ecotoxicol Environ Saf. (2024) 279:116486. doi: 10.1016/j.ecoenv.2024.116486
114. Gunes, M, Ates, K, Yalcin, B, Akkurt, S, Ozen, S, and Kaya, B. An evaluation of the genotoxic effects of electromagnetic radiation at 900 MHz, 1800 MHz, and 2100 MHz frequencies with a SMART assay in Drosophila melanogaster. Electromagn Biol Med. (2021) 40:254–63. doi: 10.1080/15368378.2021.1878210
115. Houston, BJ, Nixon, B, King, BV, Aitken, RJ, and De Iuliis, GN. Probing the origins of 1,800 MHz radio frequency electromagnetic radiation induced damage in mouse immortalized germ cells and spermatozoa in vitro. Front Public Health. (2018) 6:270. doi: 10.3389/fpubh.2018.00270
116. Kesari, KK, Agarwal, A, and Henkel, R. Radiations and male fertility. Reprod Biol Endocrinol. (2018) 16:118. doi: 10.1186/s12958-018-0431-1
117. Keskin, I, Karabulut, S, Kaplan, AA, Alagöz, M, Akdeniz, M, Tüfekci, KK, et al. Preliminary study on the impact of 900 MHz radiation on human sperm: an in vitro molecular approach. Reprod Toxicol. (2024) 130:108744. doi: 10.1016/j.reprotox.2024.108744
118. Panagopoulos, DJ. Chromosome damage in human cells induced by UMTS Mobile telephony radiation. Gen Physiol Biophys. (2019) 38:445–54. doi: 10.4149/gpb_2019032
119. Panagopoulos, DJ. Comparing chromosome damage induced by Mobile telephony radiation and a high caffeine dose. Effect of combination and exposure duration. Gen Physiol Biophys. (2020) 39:531–44. doi: 10.4149/gpb_2020036
120. Panagopoulos, DJ. Mobile telephony radiation exerts genotoxic action and significantly enhances the effects of gamma radiation in human cells. Gen Physiol Biophys. (2024) 43:103–20. doi: 10.4149/gpb_2023036
121. Shahin, S, Singh, SP, and Chaturvedi, CM. Mobile phone (1800MHz) radiation impairs female reproduction in mice, Mus musculus, through stress induced inhibition of ovarian and uterine activity. Reprod Toxicol. (2017) 73:41–60. doi: 10.1016/j.reprotox.2017.08.001
122. Sharma, A, Shrivastava, S, and Shukla, S. Exposure of radiofrequency electromagnetic radiation on biochemical and pathological alterations. Neurol India. (2020) 68:1092–100. doi: 10.4103/0028-3886.294554
123. Singh, KV, Prakash, C, Nirala, JP, Nanda, RK, and Rajamani, P. Acute radiofrequency electromagnetic radiation exposure impairs neurogenesis and causes neuronal DNA damage in the young rat brain. Neurotoxicology. (2023) 94:46–58. doi: 10.1016/j.neuro.2022.11.001
124. Smith-Roe, SL, Wyde, ME, Stout, MD, Winters, JW, Hobbs, CA, Shepard, KG, et al. Evaluation of the genotoxicity of cell phone radiofrequency radiation in male and female rats and mice following subchronic exposure. Environ Mol Mutagen. (2019) 1:276–90.
125. Yakymenko, I, Burlaka, A, Tsybulin, I, Brieieva, I, Buchynska, L, Tsehmistrenko, S, et al. Oxidative and mutagenic effects of low intensity GSM 1800 MHz microwave radiation. Exp Oncol. (2018) 40:282–7. doi: 10.31768/2312-8852.2018.40
126. Yuan, LQ, Wang, C, Lu, DF, Zhao, XD, Tan, LH, and Chen, X. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging. (2020) 12:3662–81. doi: 10.18632/aging.102836
127. Cappucci, U, Casale, AM, Proietti, M, Marinelli, F, Giuliani, L, and Piacentini, L. WiFi related radiofrequency electromagnetic fields promote transposable element dysregulation and genomic instability in Drosophila melanogaster. Cells. (2022) 11:4036. doi: 10.3390/cells11244036
128. Choi, J, Min, K, Jeon, S, Kim, N, Pack, JK, and Song, K. Continuous exposure to 1.7 GHz LTE electromagnetic fields increases intracellular reactive oxygen species to decrease human cell proliferation and induce senescence. Sci Rep. (2020) 10:9238. doi: 10.1038/s41598-020-65732-4
129. Kim, JH, Jeon, S, Choi, HD, Lee, JH, Bae, JS, Kim, N, et al. Exposure to long-term evolution radiofrequency electromagnetic fields decreases neuroblastoma cell proliferation via Akt/mTOR-mediated cellular senescence. J Toxic Environ Health A. (2021) 84:846–57. doi: 10.1080/15287394.2021.1944944
130. Shahin, S, Singh, VP, Shukla, RK, Dhawan, A, Gangwar, RK, Singh, SP, et al. 2.45 GHz microwave irradiation-induced oxidative stress affects implantation or pregnancy in mice, Mus musculus. Appl Biochem Biotechnol. (2013) 169:1727–51. doi: 10.1007/s12010-012-0079-9
131. Czerwiński, M, Vian, A, Woodcock, BA, Goliński, P, Virto, LR, and Januszkiewicz, Ł. Do electromagnetic fields used in telecommunications affect wild plant species? A control impact study conducted in the field. Ecol Indic. (2023) 150:110267. doi: 10.1016/j.ecolind.2023.110267
132. Deena, K, Maadurshni, GB, Manivannan, J, and Sivasamy, R. Short-term exposure of 2.4 GHz electromagnetic radiation on cellular ROS generation and apoptosis in SH-SY5Y cell line and impact on developing chick embryo brain tissue. Mol Biol Rep. (2025) 52:144. doi: 10.1007/s11033-025-10217-8
133. Özen, G, Kahvecioğlu, D, Bulut, İ, Erel, Ö, Neşelioğlu, S, Üstün, Y, et al. Effect of Mobile phone usage during pregnancy on total oxidant and antioxidant levels in cord blood. J Behcet Uz Children's Hosp. (2023) 13:177–84. doi: 10.4274/jbuch.galenos.2023
134. Agarwal, A, Deepinder, F, Sharma, RK, Ranga, G, and Li, J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. (2008) 89:124–8. doi: 10.1016/j.fertnstert.2007.01.166
135. Bakacak, M, Bostancı, MS, Attar, R, Yıldırım, ÖK, Yıldırım, G, Bakacak, Z, et al. The effects of electromagnetic fields on the number of ovarian primordial follicles: an experimental study. Kaohsiung J Med Sci. (2015) 31:287–92. doi: 10.1016/j.kjms.2015.03.004
136. Gul, A, Celebi, H, and Uğraş, S. The effects of microwave emitted by cellular phones on ovarian follicles in rats. Arch Gynecol Obstet. (2009) 280:729–33. doi: 10.1007/s00404-009-0972-9
137. Guzey, YZ, and Onal, AG. Effects of chronic exposure to 2G/3G cell phone radiation on in vitro maturation of bovine oocytes. Indian J Anim Res. (2018) 52:523–6.
138. Gye, MC, and Park, CJ. Effect of electromagnetic field exposure on the reproductive system. Clin Exp Reprod Med. (2012) 39:1–9. doi: 10.5653/cerm.2012.39.1.1
139. La Vignera, S, Condorelli, RA, Vicardi, E, D’Agata, R, and Calogero, AE. Effects of the exposure to Mobile phones on male reproduction: a review of the literature. J Androl. (2012) 33:350–6. doi: 10.2164/jandrol.111.014373
140. Magras, IN, and Xenos, TD. RF radiation-induced changes in the prenatal development of mice. Bioelectromagnetics. (1997) 18:455–61. doi: 10.1002/(SICI)1521-186X(1997)18:6<455::AID-BEM8>3.0.CO;2-1
141. Panagopoulos, DJ, Karabarbounis, A, and Margaritis, LH. Effect of GSM 900-MHz Mobile phone radiation on the reproductive capacity of Drosophila melanogaster. Electromagn Biol Med. (2004) 23:29–43. doi: 10.1081/JBC-120039350
142. Panagopoulos, DJ, Chavdoula, ED, Karabarbounis, A, and Margaritis, LH. Comparison of bioactivity between GSM 900 MHz and DCS 1800 MHz Mobile telephony radiation. Electromagn Biol Med. (2007) 26:33–44. doi: 10.1080/15368370701205644
143. Sharma, VP, and Kumar, NR. Changes in honeybee behaviour and biology under the influence of cellphone radiations. Curr Sci. (2010) 98:1376–8.
144. Wdowiak, A, Wdowiak, L, and Wiktor, H. Evaluation of the effect of using mobile phones on male fertility. Ann Agric Environ Med. (2007) 14:169–72.
145. Irani, M, Aradmehr, M, Ghorbani, M, and Baghani, R. Electromagnetic field exposure and abortion in pregnant women: a systematic review and meta-analysis. Malays J Med Sci. (2023) 30:70–80. doi: 10.21315/mjms2023.30.5.6
146. Bacandritsos, N, Granato, A, Budge, G, Papanastasiou, I, Roinioti, E, Caldon, M, et al. Sudden deaths and colony population decline in Greek honey bee colonies. J Invertebr Pathol. (2010) 105:335–40. doi: 10.1016/j.jip.2010.08.004
147. Balmori, A. Possible effects of electromagnetic fields from phone masts on a population of White stork (Ciconia ciconia). Electromagn Biol Med. (2005) 24:109–19. doi: 10.1080/15368370500205472
148. Balmori, A, and Hallberg, O. The urban decline of the house sparrow (Passer domesticus): a possible link with electromagnetic radiation. Electromagn Biol Med. (2007) 26:141–51. doi: 10.1080/15368370701410558
149. Cucurachi, S, Tamis, WLM, Vijver, MG, Peijnenburg, WJGM, Bolte, JFB, and de Snoo, GR. A review of the ecological effects of radiofrequency electromagnetic fields (RF-EMF). Environ Int. (2013) 51:116–40. doi: 10.1016/j.envint.2012.10.009
150. Everaert, J, and Bauwens, D. A possible effect of electromagnetic radiation from mobile phone base stations on the number of breeding house sparrows (Passer domesticus). Electromagn Biol Med. (2007) 26:63–72. doi: 10.1080/15368370701205693
151. Batellier, F, Couty, I, Picard, D, and Brillard, JP. Effects of exposing chicken eggs to a cell phone in "call" position over the entire incubation period. Theriogenology. (2008) 69:737–45. doi: 10.1016/j.theriogenology.2007.12.006
152. Balmori, A. The incidence of electromagnetic pollution on the amphibian decline: is this an important piece of the puzzle? Toxicol Environ Chem. (2006) 88:287–99. doi: 10.1080/02772240600687200
153. Balmori, A. Mobile phone mast effects on common frog (Rana temporaria) tadpoles: the city turned into a laboratory. Electromagn Biol Med. (2010) 29:31–5. doi: 10.3109/15368371003685363
154. Ahlbom, A, Day, N, Feychting, M, Roman, E, Skinner, J, Dockerty, J, et al. A pooled analysis of magnetic fields and childhood leukaemia. Br J Cancer. (2000) 83:692–8. doi: 10.1054/bjoc.2000.1376
155. Coghill, RW, Steward, J, and Philips, A. Extra low frequency electric and magnetic fields in the bed place of children diagnosed with leukaemia: a case-control study. Eur J Cancer Prev. (1996) 5:153–8. doi: 10.1097/00008469-199606000-00002
156. Coleman, MP, Bell, CM, Taylor, HL, and Primic-Zakelj, M. Leukaemia and residence near electricity transmission equipment: a case-control study. Br J Cancer. (1989) 60:793–8. doi: 10.1038/bjc.1989.362
157. Draper, G, Vincent, T, Kroll, ME, and Swanson, J. Childhood cancer in relation to distance from high voltage power lines in England and Wales: a case-control study. BMJ. (2005) 330:1290. doi: 10.1136/bmj.330.7503.1290
158. Feychting, M, and Ahlbom, A. Magnetic fields and cancer in children residing near Swedish high - voltage power lines. Am J Epidemiol. (1993) 138:467–81. doi: 10.1093/oxfordjournals.aje.a116881
159. Feychting, M, and Ahlbom, A. Magnetic fields, leukemia and central nervous system tumors in Swedish adults residing near high - voltage power lines. Epidemiology. (1994) 5:501–9.
160. Greenland, S, Sheppard, AR, Kaune, WT, Poole, C, and Kelsh, MA. A pooled analysis of magnetic fields, wire codes, and childhood leukemia. Childhood leukemia-EMF study group. Epidemiology. (2000) 11:624–34. doi: 10.1097/00001648-200011000-00003
161. Kheifets, L, Ahlbom, A, Crespi, CM, Draper, G, Hagihara, J, Lowenthal, RM, et al. Pooled analysis of recent studies on magnetic fields and childhood leukaemia. Br J Cancer. (2010) 103:1128–35.
162. Miller, A, To, T, Agnew, DA, et al. Leukemia following occupational exposure to 60-Hz electric and magnetic fields among Ontario electric utility workers. Am J Epidemiol. (1996) 144:150–60. doi: 10.1093/oxfordjournals.aje.a008902
163. Savitz, DA, Wachtel, H, Barnes, F, John, EM, and Tvrdik, JG. Case-control study of childhood cancer and exposure to 60Hz magnetic fields. Am J Epidemiol. (1988) 128:21–38. doi: 10.1093/oxfordjournals.aje.a114943
164. Villeneuve, PJ, Agnew, DA, Miller, AB, and Corey, PN. Non-Hodgkin's lymphoma among electric utility workers in Ontario: the evaluation of alternate indices of exposure to 60 Hz electric and magnetic fields. Occup Environ Med. (2000) 57:249–57. doi: 10.1136/oem.57.4.249
165. Villeneuve, PJ, Agnew, DA, Miller, AB, Corey, PN, and Purdham, JT. Leukemia in electric utility workers: the evaluation of alternative indices of exposure to 60 Hz electric and magnetic fields. Am J Ind Med. (2000) 37:607–17. doi: 10.1002/(SICI)1097-0274(200006)37:6<607::AID-AJIM5>3.0.CO;2-L
166. Wertheimer, N, and Leeper, E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. (1979) 109:273–84. doi: 10.1093/oxfordjournals.aje.a112681
167. Wertheimer, N, and Leeper, E. Adult cancer related to electrical wires near the home. Int J Epidemiol. (1982) 11:345–55. doi: 10.1093/ije/11.4.345
168. Hallberg, O, and Johansson, O. Melanoma incidence and frequency modulation (FM) broadcasting. Arch Environ Health. (2002) 57:32–40. doi: 10.1080/00039890209602914
169. López, I, Félix, N, Rivera, M, Alonso, A, and Maestú, C. What is the radiation before 5G? A correlation study between measurements in situ and in real time and epidemiological indicators in Vallecas, Madrid. Environ Res. (2021) 194:110734. doi: 10.1016/j.envres.2021.110734
170. Yakymenko, I, Sidorik, E, Kyrylenko, S, and Chekhun, V. Long-term exposure to microwave radiation provokes cancer growth: evidences from radars and mobile communication systems. Exp Oncol. (2011) 33:62–70.
171. Yakymenko, I, and Tsybulin, O. Carcinogenic effects of non-thermal exposure to wireless communication electromagnetic fields In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC Press (2022)
172. Gulati, S, Yadav, A, Kumar, N, Kanupriya,, Aggarwal, NK, Kumar, R, et al. Effect of GSTM1 and GSTT1 polymorphisms on genetic damage in humans populations exposed to radiation from Mobile towers. Arch Environ Contam Toxicol. (2016) 70:615–25. doi: 10.1007/s00244-015-0195-y
173. Zothansiama,, Zosangzuali, M, Lalramdinpuii, M, and Jagetia, GC. Impact of radiofrequency radiation on DNA damage and antioxidants in peripheral blood lymphocytes of humans residing in the vicinity of mobile phone base stations. Electromagn Biol Med. (2017) 36:295–305. doi: 10.1080/15368378.2017.1350584
174. Carlberg, M, and Hardell, L. Evaluation of Mobile phone and cordless phone use and glioma risk using the Bradford Hill viewpoints from 1965 on association or causation. Biomed Res Int. (2017) 2017:9218486–17. doi: 10.1155/2017/9218486
175. Hardell, L, Carlberg, M, Söderqvist, F, Mild, KH, and Morgan, LL. Long-term use of cellular phones and brain tumours: increased risk associated with use for > or =10 years. Occup Environ Med. (2007) 64:626–32. doi: 10.1136/oem.2006.029751
176. Hardell, L, and Carlberg, M. Mobile phones, cordless phones and the risk for brain tumours. Int J Oncol. (2009) 35:5–17. doi: 10.3892/ijo_00000307
177. Hardell, L, Carlberg, M, and Hansson, MK. Epidemiological evidence for an association between use of wireless phones and tumor diseases. Pathophysiology. (2009) 16:113–22. doi: 10.1016/j.pathophys.2009.01.003
178. Hardell, L, Carlberg, M, Söderqvist, F, and Mild, KH. Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol. (2013) 43:1833–45. doi: 10.3892/ijo.2013.2111
179. Hardell, L, Carlberg, M, and Hansson Mild, K. Use of mobile phones and cordless phones is associated with increased risk for glioma and acoustic neuroma. Pathophysiology. (2013) 20:85–110. doi: 10.1016/j.pathophys.2012.11.001
180. Hardell, L. Effects of Mobile phones on Children's and Adolescents' health: a commentary. Child Dev. (2018) 89:137–40. doi: 10.1111/cdev.12831
181. Khurana, VG, Teo, C, Kundi, M, Hardell, L, and Carlberg, M. Cell phones and brain tumors: a review including the long-term epidemiologic data. Surg Neurol. (2009) 72:205–14. doi: 10.1016/j.surneu.2009.01.019
182. Miller, AB, Morgan, LL, Udasin, I, and Davis, DL. Cancer epidemiology update, following the 2011 IARC evaluation of radiofrequency electromagnetic fields (monograph 102). Environ Res. (2018) 167:673–83. doi: 10.1016/j.envres.2018.06.043
183. Miller, AB, Sears, ME, Morgan, LL, Davis, DL, Hardell, L, Oremus, M, et al. Risks to health and well-being from radio-frequency radiation emitted by cell phones and other wireless devices. Front Public Health. (2019) 7:223. doi: 10.3389/fpubh.2019.00223
184. Momoli, F, Siemiatycki, J, McBride, ML, Parent, MÉ, Richardson, L, Bedard, D, et al. Probabilistic multiple-bias modeling applied to the Canadian data from the interphone study of mobile phone use and risk of glioma, meningioma, acoustic neuroma, and parotid gland tumors. Am J Epidemiol. (2017) 186:885–93. doi: 10.1093/aje/kwx157
185. Wang, Y, and Guo, X. Meta-analysis of association between mobile phone use and glioma risk. J Cancer Res Ther. (2016) 12:298–C300. doi: 10.4103/0973-1482.200759
186. Navarro, A, et al. The microwave syndrome: a preliminary study in Spain. Electromagn Biol Med. (2003) 22:161–9. doi: 10.1081/JBC-120024625
187. Santini, R, Santini, P, Danze, JM, Le Ruz, P, and Seigne, M. Study of the health of people living in the vicinity of mobile phone base stations: I. Influences of distance and sex. Pathol Biol. (2002) 50:369–73. doi: 10.1016/S0369-8114(02)00311-5
188. Salama, OE, and Abou El Naga, RM. Cellular phones: are they detrimental? J Egypt Public Health Assoc. (2004) 79:197–223.
189. Abdel-Rassoul, G, El-Fateh, OA, Salem, MA, Michael, A, Farahat, F, El-Batanouny, M, et al. Neurobehavioral effects among inhabitants around mobile phone base stations. Neurotoxicology. (2007) 28:434–40. doi: 10.1016/j.neuro.2006.07.012
190. Belpomme, D, and Irigaray, P. Electrohypersensitivity as a worldwide man-made electromagnetic pathology: a review of the medical evidence In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC Press (2022)
191. Blettner, M, Schlehofer, B, Breckenkamp, J, Kowall, B, Schmiedel, S, Reis, U, et al. Mobile phone base stations and adverse health effects: phase 1 of a population-based, cross-sectional study in Germany. Occup Environ Med. (2009) 66:118–23. doi: 10.1136/oem.2007.037721
192. Davis, D, Birnbaum, L, Ben-Ishai, P, Taylor, H, Sears, M, Butler, T, et al. Wireless technologies, non-ionizing electromagnetic fields and children: identifying and reducing health risks. Curr Probl Pediatr Adolesc Health Care. (2023) 53:101374. doi: 10.1016/j.cppeds.2023.101374
193. Greco, F, Garnier, O, Macioce, V, and Picot, MC. Prevalence of migraine disease in Electrohypersensitive patients. J Clin Med. (2023) 12:4092. doi: 10.3390/jcm12124092
194. Hutter, H-P, Moshammer, H, Wallner, P, and Kundi, M. Subjective symptoms, sleeping problems, and cognitive performance in subjects living near mobile phone base stations. Occup Environ Med. (2006) 63:307–13. doi: 10.1136/oem.2005.020784
195. Kundi, M, and Hutter, HP. Mobile phone base stations-effects on wellbeing and health. Pathophysiology. (2009) 16:123–35. doi: 10.1016/j.pathophys.2009.01.008
196. Viel, JF, Clerc, S, Barrera, C, Rymzhanova, R, Moissonnier, M, Hours, M, et al. Residential exposure to radiofrequency fields from mobile phone base stations, and broadcast transmitters: a population-based survey with personal meter. Occup Environ Med. (2009) 66:550–6. doi: 10.1136/oem.2008.044180
197. Irigaray, P, Caccamo, D, and Belpomme, D. Oxidative stress in electrohypersensitivity self-reporting patients: results of a prospective in vivo investigation with comprehensive molecular analysis. Int J Mol Med. (2018) 42:1885–98. doi: 10.3892/ijmm.2018.3774
198. Hardell, L, and Nilsson, M. Summary of seven Swedish case reports on the microwave syndrome associated with 5G radiofrequency radiation. Rev Environ Health. (2024) 40:147–57. doi: 10.1515/reveh-2024-0017
199. Hardell, L, and Koppel, T. Spots with extremely high radiofrequency radiation after deployment of 5G base stations in Stockholm, Sweden. Ann Clin Med Case Rep. (2024) V14:1–8.
200. Lerchl, A, Klose, M, Grote, K, Wilhelm, AFX, Spathmann, O, Fiedler, T, et al. Tumor promotion by exposure to radiofrequency electromagnetic fields below exposure limits for humans. Biochem Biophys Res Commun. (2015) 459:585–90. doi: 10.1016/j.bbrc.2015.02.151
201. Tillmann, T, Ernst, H, Streckert, J, Zhou, Y, Taugner, F, Hansen, V, et al. Indication of cocarcinogenic potential of chronic UMTS-modulated radiofrequency exposure in an ethylnitrosourea mouse model. Int J Radiat Biol. (2010) 86:529–41. doi: 10.3109/09553001003734501
202. NTP. Toxicology and carcinogenesis studies in Hsd: Sprague Dawley SD rats exposed to whole-body radio frequency radiation at a frequency (900 MHz) and modulations (GSM and CDMA) used by cell phones. USA: NTP TR 595, Department of Health and Human Services (2018).
203. Falcioni, L, Bua, L, Tibaldi, E, Lauriola, M, de Angelis, L, Gnudi, F, et al. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8GHz GSM base station environmental emission. Environ Res. (2018) 165:496–503. doi: 10.1016/j.envres.2018.01.037
204. IARC. Non-ionizing radiation, part 1: static and extremely low-frequency (ELF) electric and magnetic fields, vol. 80. Lyon, France: International Agency for Research on Cancer. (2002).
205. IARC. Non-ionizing radiation, part 2: radiofrequency electromagnetic fields, vol. 102. Lyon, France: International Agency for Research on Cancer. (2013).
206. Leach, V, Weller, S, and Redmayne, M. A novel database of bio-effects from non-ionizing radiation. Rev Environ Health. (2018) 33:273–80. doi: 10.1515/reveh-2018-0017
207. Lerchl, A, Klose, M, and Drees, K. No increased DNA damage observed in the brain, liver, and lung of fetal mice treated with Ethylnitrosourea and exposed to UMTS radiofrequency electromagnetic fields. Bioelectromagnetics. (2020) 41:611–6. doi: 10.1002/bem.22301
208. Manna, D, and Ghosh, R. Effect of radiofrequency radiation in cultured mammalian cells: a review. Electromagn Biol Med. (2016) 35:265–301. doi: 10.3109/15368378.2015.1092158
209. Panagopoulos, DJ, Johansson, O, and Carlo, GL. Real versus simulated Mobile phone exposures in experimental studies. Biomed Res Int. (2015) 2015:607053. doi: 10.1155/2015/607053
210. Panagopoulos, DJ, Yakymenko, I, and Chrousos, GP. Electromagnetic field-induced dysfunction of voltage-gated ion channels, oxidative stress, DNA damage, and related pathologies In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC Press (2022)
211. Schuermann, D, Ziemann, C, Barekati, Z, Capstick, M, Oertel, A, Focke, F, et al. Assessment of Genotoxicity in human cells exposed to modulated electromagnetic fields of wireless communication devices. Genes. (2020) 11:347. doi: 10.3390/genes11040347
212. Verschaeve, L. Misleading scientific papers on health effects from wireless communication devices In: CD Geddes, editor. Microwave effects on DNA and proteins. Cham, Switzerland: Springer. (2017)
213. Wood, A, Mate, R, and Karipidis, K. Meta-analysis of in vitro and in vivo studies of the biological effects of low-level millimetre waves. J Expo Sci Environ Epidemiol. (2021) 31:606–13. doi: 10.1038/s41370-021-00307-7
214. McCredden, JE, Cook, N, Weller, S, and Leach, V. Wireless technology is an environmental stressor requiring new understanding and approaches in health care. Front Public Health. (2022) 10:986315. doi: 10.3389/fpubh.2022.986315
215. McCredden, JE, Weller, S, and Leach, V. The assumption of safety is being used to justify the rollout of 5G technologies. Front Public Health. (2023) 11:1058454. doi: 10.3389/fpubh.2023.1058454
216. Panagopoulos, DJ. Mobile telephony radiation effects on insect ovarian cells. The necessity for real exposures bioactivity assessment. The key role of polarization, and the ion forced-oscillation mechanism In: CD Geddes, editor. Microwave effects on DNA and proteins. Cham, Switzerland: Springer (2017) 1–48.
217. Frank, JW. Electromagnetic fields, 5G and health: what about the precautionary principle? J Epidemiol Community Health. (2021) 75:562–6. doi: 10.1136/jech-2019-213595
218. Hardell, L, and Nyberg, R. Appeals that matter or not on a moratorium on the deployment of the fifth generation, 5G, for microwave radiation. Mol Clin Oncol. (2020) 12:247–57. doi: 10.3892/mco.2020.1984
219. Hardell, L, and Carlberg, M. Health risks from radiofrequency radiation, including 5G, should be assessed by experts with no conflicts of interest. Oncol Lett. (2020) 20:15. doi: 10.3892/ol.2020.11876
220. Hardell, L, and Carlberg, M. Lost opportunities for cancer prevention: historical evidence on early warnings with emphasis on radiofrequency radiation. Rev Environ Health. (2021) 36:585–97. doi: 10.1515/reveh-2020-0168
221. Harremoes, P, Gee, D, MacGarvin, M, Stirling, A, Keys, J, Wynne, B, et al. The precautionary principle in the 20th century: late lessons from early warnings. London: Routledge (2013).
222. ICNIRP. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). Health Phys. (1998) 74:494–521.
223. ICNIRP. Guidelines for limiting exposure to time-varying electric and magnetic fields (1Hz to 100 kHz). Health Phys. (2010) 99:818–36.
224. ICNIRP. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. (2020) 118:483–524.
225. Ishai, PB, Baldwin, HZ, Birnbaum, LS, Butler, T, Chamberlin, K, Davis, DL, et al. Applying the precautionary principle to wireless technology: policy dilemmas and systemic risks. Environ Sci Policy Sustain Dev. (2024) 66:5–18. doi: 10.1080/00139157.2024.2293631
226. Read, R, and O'Riordan, T. The precautionary principle under fire. Environ Sci Policy Sustain Dev. (2017) 59:4–15. doi: 10.1080/00139157.2017.1350005
227. Baan, R, Grosse, Y, Lauby-Secretan, B, El Ghissassi, F, Bouvard, V, et al. WHO International Agency for Research on Cancer monograph working group: carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. (2011) 12:624–6. doi: 10.1016/S1470-2045(11)70147-4
228. Nyberg, NR, McCredden, JE, Weller, SG, and Hardell, L. The European Union prioritises economics over health in the rollout of radiofrequency technologies. Rev Environ Health. (2024) 39:47–64. doi: 10.1515/reveh-2022-0106
229. Francis, G. Ionization phenomena in gases. London: Butterworths Scientific Publications (1960).
231. Alberts, B, Bray, D, Lewis, J, Raff, M, Roberts, K, and Watson, JD. Molecular biology of the cell. New York, USA: Garland Publishing, Inc (1994).
232. Finkel, T. Signal transduction by reactive oxygen species. J Cell Biol. (2011) 194:7–15. doi: 10.1083/jcb.201102095
233. Görlach, A, Bertram, K, Hudecova, S, and Krizanova, O. Calcium and ROS: a mutual interplay. Redox Biol. (2015) 6:260–71. doi: 10.1016/j.redox.2015.08.010
235. Inoue, M, Sato, EF, Nishikawa, M, Park, AM, Kira, Y, Imada, I, et al. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. (2003) 10:2495–505. doi: 10.2174/0929867033456477
236. Lushchak, VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. (2014) 224:164–75. doi: 10.1016/j.cbi.2014.10.016
237. Ischiropoulos, H, Zhu, L, and Beckman, JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. (1992) 298:446–51. doi: 10.1016/0003-9861(92)90433-W
238. Pall, ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. (2013) 17:958–65. doi: 10.1111/jcmm.12088
239. Schuermann, D, and Mevissen, M. Manmade electromagnetic fields and oxidative stress—biological effects and consequences for health. Int J Mol Sci. (2021) 22:3772. doi: 10.3390/ijms22073772
240. Checa, J, and Aran, JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res. (2020) 13:1057–73. doi: 10.2147/JIR.S275595
241. Andrés, CMC, Pérez de la Lastra, JM, Andrés Juan, C, Plou, FJ, and Pérez-Lebeña, E. Superoxide anion chemistry—its role at the core of the innate immunity. Int J Mol Sci. (2023) 24:1841. doi: 10.3390/ijms24031841
242. Koppers, AJ, De Iuliis, GN, Finnie, JM, McLaughlin, EA, and Aitken, RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metabol. (2008) 93:3199–207. doi: 10.1210/jc.2007-2616
243. Gibb, Z, Griffin, RA, Aitken, RJ, and De Iuliis, GN. Functions and effects of reactive oxygen species in male fertility. Anim Reprod Sci. (2020) 220:106456. doi: 10.1016/j.anireprosci.2020.106456
244. Brookes, PS, Yoon, Y, Robotham, JL, Anders, MW, and Sheu, SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. (2004) 287:C817–33. doi: 10.1152/ajpcell.00139.2004
245. Bertero, E, and Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res. (2018) 122:1460–78. doi: 10.1161/CIRCRESAHA.118.310082
246. Lowe, SW, and Lin, AW. Apoptosis in cancer. Carcinogenesis. (2000) 21:485–95. doi: 10.1093/carcin/21.3.485
247. Bedard, K, and Krause, KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. (2007) 87:245–313. doi: 10.1152/physrev.00044.2005
248. Gamaley, I, Augsten, K, and Berg, H. Electrostimulation of macrophage NADPH oxidase by modulated high-frequency electromagnetic fields. Bioelectrochem Bioenerg. (1995) 38:415–8. doi: 10.1016/0302-4598(95)01836-4
249. Iverson, D, De Chatelet, LR, Spitznagel, JK, and Wang, P. Comparison of NADH and NADPH oxidase activities in granules isolated from human polymorphonuclear leukocytes with a fluorometric assay. J Clin Invest. (1977) 59:282–90. doi: 10.1172/JCI108639
250. Li, JM, and Shah, AM. Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc Res. (2001) 52:477–86. doi: 10.1016/s0008-6363(01)00407-2
251. Panday, A, Sahoo, MK, Osorio, D, and Batra, S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. (2015) 12:5–23. doi: 10.1038/cmi.2014.89
252. Cross, AR, and Segal, AW. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochim Biophys Acta. (2004) 1657:1–22. doi: 10.1016/j.bbabio.2004.03.008
253. Henderson, LM. NADPH oxidase subunit gp91phox: a proton pathway. Protoplasma. (2001) 217:37–42. doi: 10.1007/BF01289411
254. Musset, B, Cherny, VV, Morgan, D, and De Coursey, TE. The intimate and mysterious relationship between proton channels and NADPH oxidase. FEBS Lett. (2009) 583:7–12. doi: 10.1016/j.febslet.2008.12.005
255. André-Lévigne, D, Modarressi, A, Pepper, MS, et al. Reactive oxygen species and NOX enzymes are emerging as key players in cutaneous wound repair. Int J Mol Sci. (2017) 18:2149. doi: 10.3390/ijms18102149
256. Fang, J, Sheng, R, and Qin, Z-H. NADPH oxidases in the central nervous system: regional and cellular localization and the possible link to brain diseases. Antioxid Redox Signal. (2021) 35:951–73. doi: 10.1089/ars.2021.0040
257. Friedman, J, Kraus, S, Hauptman, Y, Schiff, Y, and Seger, R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem J. (2007) 405:559–68. doi: 10.1042/BJ20061653
258. Pacher, P, Beckman, JS, and Liaudet, L. Nitric oxide and Peroxynitrite in health and disease. Physiol Rev. (2007) 87:315–424. doi: 10.1152/physrev.00029.2006
259. Pilla, AA. Electromagnetic fields instantaneously modulate nitric oxide signaling in challenged biological systems. Biochem Biophys Res Commun. (2012) 426:330–3. doi: 10.1016/j.bbrc.2012.08.078
260. Lai, H, and Singh, NP. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ Health Perspect. (2004) 112:687–94. doi: 10.1289/ehp.6355
261. Moon, HK, Yang, ES, and Park, JW. Protection of peroxynitrite-induced DNA damage by dietary antioxidants. Arch Pharm Res. (2006) 29:213–7. doi: 10.1007/BF02969396
262. Sakihama, Y, Maeda, M, Hashimoto, M, Tahara, S, and Hashidoko, Y. Beetroot betalain inhibits peroxynitrite- mediated tyrosine nitration and DNA strand damage. Free Radic Res. (2012) 46:93–9. doi: 10.3109/10715762.2011.641157
263. Förstermann, U, and Sessa, WC. Nitric oxide synthases: regulation and function. Eur Heart J. (2012) 33:829–37. doi: 10.1093/eurheartj/ehr304
264. Bortolotti, M, Polito, L, Battelli, MG, and Bolognesi, A. Xanthine oxidoreductase: one enzyme for multiple physiological tasks. Redox Biol. (2021) 41:101882. doi: 10.1016/j.redox.2021.101882
265. Manikandan, P, and Nagini, S. Cytochrome P450 structure, function and clinical significance: a review. Curr Drug Targets. (2018) 19:38–54. doi: 10.2174/1389450118666170125144557
266. Becchetti, A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am J Phys Cell Phys. (2011) 301:C255–65.
267. Lang, F, Föller, M, Lang, KS, Lang, PA, Ritter, M, Gulbins, E, et al. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. (2005) 205:147–57. doi: 10.1007/s00232-005-0780-5
268. Chattipakorn, N, Kumfu, S, Fucharoen, S, and Chattipakorn, S. Calcium channels and iron uptake into the heart. World J Cardiol. (2011) 3:215–8. doi: 10.4330/wjc.v3.i7.215
269. Gaasch, JA, Geldenhuys, WJ, Lockman, PR, Allen, DD, and Van der Schyf, CJ. Voltage-gated calcium channels provide an alternate route for Iron uptake in neuronal cell cultures. Neurochem Res. (2007) 32:1686–93. doi: 10.1007/s11064-007-9313-1
270. Salsbury, G, Cambridge, EL, McIntyre, Z, Arends, MJ, Karp, NA, Isherwood, C, et al. Disruption of the potassium channel regulatory subunit KCNE2 causes iron-deficient anemia. Exp Hematol. (2014) 42:1053–1058.e1. doi: 10.1016/j.exphem.2014.07.269
271. Alberts, B, Johnson, A, Lewis, J, Raff, M, Roberts, K, and Walter, P. Membrane transport of small molecules and the electrical properties of membranes-molecular biology of the cell. New York, USA: Garland Science (2002).
272. Blank, M, and Soo, L. Ion activation of the Na, K-ATPase in alternating currents. Bioelectrochem Bioenerg. (1990) 24:51–61. doi: 10.1016/0302-4598(80)85006-9
273. Serpersu, EH, and Tsong, TY. Activation of electrogenic Rb transport of (Na/K)-ATPase by an electric field. J Biol Chem. (1984) 259:7155–62. doi: 10.1016/S0021-9258(17)39851-4
274. Pratt, RD, Brickman, CR, Cottrill, CL, Shapiro, JI, and Liu, J. The Na/K-ATPase signaling: from specific ligands to general reactive oxygen species. Int J Mol Sci. (2018) 19:2600. doi: 10.3390/ijms19092600
275. Zhang, L, Zhang, Z, Guo, H, and Wang, Y. Na+/K+-ATPase-mediated signal transduction and Na+/K+-ATPase regulation. Fundam Clin Pharmacol. (2008) 22:615–21. doi: 10.1111/j.1472-8206.2008.00620.x
277. Barzilai, A, and Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair. (2004) 3:1109–15. doi: 10.1016/j.dnarep.2004.03.002
278. Cooke, MS, Evans, MD, Dizdaroglu, M, and Lunec, J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. (2003) 17:1195–214. doi: 10.1096/fj.02-0752rev
279. Sillar, JR, Germon, ZP, De Iuliis, GN, and Dun, MD. The role of reactive oxygen species in acute myeloid leukaemia. Int J Mol Sci. (2019) 20:6003. doi: 10.3390/ijms20236003
280. Valko, M, Leibfritz, D, Moncol, J, Cronin, MTD, Mazur, M, and Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. (2007) 39:44–84. doi: 10.1016/j.biocel.2006.07.001
281. Burney, S, Caulfield, JL, Niles, JC, Wishnok, JS, and Tannenbaum, SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. (1999) 424:37–49. doi: 10.1016/S0027-5107(99)00006-8
282. Yu, H, Venkatarangan, L, Wishnok, JS, and Tannenbaum, SR. Quantitation of four guanine oxidation products from reaction of DNA with varying doses of Peroxynitrite. Chem Res Toxicol. (2005) 18:1849–57. doi: 10.1021/tx050146h
283. Szabo, G, and Baehrle, S. Role of nitrosative stress and poly(ADP-ribose) polymerase activation in myocardial reperfusion injury. Curr Vasc Pharmacol. (2005) 3:215–20. doi: 10.2174/1570161054368599
284. Lymar, SV, Khairutdinov, RF, and Hurst, JK. Hydroxyl radical formation by O-O bond homolysis in peroxynitrous acid. Inorg Chem. (2003) 42:5259–66. doi: 10.1021/ic030104l
285. Pérez de la Lastra, JM, Juan, CA, Plou, FJ, and Pérez-Lebeña, E. The nitration of proteins, lipids and DNA by peroxynitrite derivatives-chemistry involved and biological relevance. Stress. (2022) 2:53–64. doi: 10.3390/stresses2010005
286. De Coursey, T, Morgan, D, and Cherny, V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. (2003) 422:531–4. doi: 10.1038/nature01523
287. Balasubramanian, B, Pogozelski, WK, and Tullius, TD. DNA strand breaking by hydroxyl radical is governed by the accessible surface area of the hydrogen atom of the DNA backbone. PNAS. (1998) 95:9738–43. doi: 10.1073/pnas.95.17.9738
288. Cadet, J, Delatour, T, Douki, T, Gasparutto, D, Pouget, JP, Ravanat, JL, et al. Hydroxyl radicals and DNA base damage. Mutat Res. (1999) 424:9–21. doi: 10.1016/S0027-5107(99)00004-4
289. Cadet, J, and Wagner, JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. (2013) 5:a012559. doi: 10.1101/cshperspect.a012559
290. Halliwell, B. Biochemistry of oxidative stress. Biochem Soc Trans. (2007) 35:1147–50. doi: 10.1042/BST0351147
291. Tsunoda, M, Sakaue, T, Naito, S, Sunami, T, Abe, N, Ueno, Y, et al. Insights into the structures of DNA damaged by hydroxyl radical: crystal structures of DNA duplexes containing 5-formyluracil. J Nucleic Acids. (2010) 2010:107289. doi: 10.4061/2010/107289
292. Fenton, HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc Trans. (1894) 65:899–910. doi: 10.1039/CT8946500899
293. Valko, M, Rhodes, CJ, Moncol, J, Izakovic, M, and Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. (2006) 160:1–40. doi: 10.1016/j.cbi.2005.12.009
296. Abolfath, RM, Van Duin, ACT, and Brabec, T. Reactive molecular dynamics study on the first steps of DNA damage by free hydroxyl radicals. Chem A Eur J. (2011) 115:11045–9. doi: 10.1021/jp204894m
297. Panagopoulos, DJ, Karabarbounis, A, and Chrousos, GP. Biophysical mechanism of animal magnetoreception, orientation and navigation. Sci Rep. (2024) 14:30053. doi: 10.1038/s41598-024-77883-9
298. Bertagna, F, Lewis, R, Silva, SRP, McFadden, J, and Jeevaratnam, K. Effects of electromagnetic fields on neuronal ion channels: a systematic review. Ann N Y Acad Sci. (2021) 1499:82–103. doi: 10.1111/nyas.14597
299. Liman, ER, Hess, P, Weaver, F, and Koren, G. Voltage sensing residues in the S4 region of a mammalian K+ channel. Nature. (1991) 353:752–6. doi: 10.1038/353752a0
300. Miller, C. An overview of the potassium channel family. Genome Biol. (2000) 1:reviews0004.1. doi: 10.1186/gb-2000-1-4-reviews0004
301. Noda, M, Ikeda, T, Kayano, T, Suzuki, H, Takeshima, H, Kurasaki, M, et al. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. (1986) 320:188–92. doi: 10.1038/320188a0
302. Panagopoulos, DJ. Mechanism of ion forced-oscillation and voltage-gated ion channel dysfunction by polarized and coherent electromagnetic fields In: DJ Panagopoulos, editor. Electromagnetic fields of wireless communications: biological and health effects. Boca Raton: CRC Press, Taylor and Francis (2022)
303. Sandipan, C, and Baron, C. Basic mechanisms of voltage sensing In: J Zheng and MC Trudeau, editors. Handbook of ion channels. Boca Raton: CRC Press (2015)
304. Zhang, XC, Yang, H, Liu, Z, and Sun, F. Thermodynamics of voltage-gated ion channels. Biophys Rep. (2018) 4:300–19. doi: 10.1007/s41048-018-0074-y
305. Panagopoulos, DJ, Messini, N, Karabarbounis, A, Filippetis, AL, and Margaritis, LH. A mechanism for action of oscillating electric fields on cells. Biochem Biophys Res Commun. (2000) 272:634–40. doi: 10.1006/bbrc.2000.2746
306. Panagopoulos, DJ, Karabarbounis, A, and Margaritis, LH. Mechanism for action of electromagnetic fields on cells. Biochem Biophys Res Commun. (2002) 298:95–102. doi: 10.1016/S0006-291X(02)02393-8
307. Balcavage, WX, Alvager, T, Swez, J, Goff, CW, Fox, MT, Abdullyava, S, et al. A mechanism for action of extremely low frequency electromagnetic fields on biological systems. Biochem Biophys Res Commun. (1996) 222:374–8. doi: 10.1006/bbrc.1996.0751
308. Honig, BH, Hubbell, WL, and Flewelling, RF. Electrostatic interactions in membranes and proteins. Ann Rev Biophys Biophys Chem. (1986) 15:163–93. doi: 10.1146/annurev.bb.15.060186.001115
309. Cleary, SF, Liu, LM, Graham, R, and Diegelmann, RF. Modulation of tendon fibroplasia by exogenous electric currents. Bioelectromagnetics. (1988) 9:183–94. doi: 10.1002/bem.2250090210
310. McLeod, KJ, Lee, RC, and Ehrlich, HP. Frequency dependence of electric field modulation of fibroblast protein synthesis. Science. (1987) 236:1465–9. doi: 10.1126/science.3589667
311. England, SJ, and Robert, D. The ecology of electricity and electroreception. Biol Rev. (2022) 97:383–413. doi: 10.1111/brv.12804
312. Johnsen, S, and Lohmann, KJ. Magnetoreception in animals. Phys Today. (2008) 61:29–35. doi: 10.1063/1.2897947
313. Cecchetto, C, Maschietto, M, Boccaccio, P, and Vassanelli, S. Electromagnetic field affects the voltage-dependent potassium channel Kv1.3. Electromagn Biol Med. (2020) 39:316–22. doi: 10.1080/15368378.2020.1799386
314. El-Swefy, S, Soliman, H, and Huessein, M. Calcium channel blockade alleviates brain injury induced by long-term exposure to an electromagnetic field. J Appl Biomed. (2008) 6:153–63. doi: 10.32725/jab.2008.019
315. Liburdy, RP. Calcium signaling in lymphocytes and ELF fields. FEBS Lett. (1992) 301:53–9. doi: 10.1016/0014-5793(92)80209-Y
316. Piacentini, R, Ripoli, C, Mezzogori, D, Azzena, GB, and Grassi, C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Cav1-channel activity. J Cell Physiol. (2008) 215:129–39. doi: 10.1002/jcp.21293
317. Zheng, Y, Xia, P, Dong, L, Tian, L, and Xiong, C. Effects of modulation on sodium and potassium channel currents by extremely low frequency electromagnetic fields stimulation on hippocampal CA1 pyramidal cells. Electromagn Biol Med. (2021) 40:274–85. doi: 10.1080/15368378.2021.1885433
318. Orfali, R, Alwatban, AZ, Orfali, RS, Lau, L, Chea, N, Alotaibi, AM, et al. Oxidative stress and ion channels in neurodegenerative diseases. Front Physiol. (2024) 15:1320086. doi: 10.3389/fphys.2024.1320086
319. Batcioglu, K, Uyumlu, AB, Satilmis, B, Yildirim, B, Yucel, N, Demirtas, H, et al. Oxidative stress in the in vivo DMBA rat model of breast cancer: suppression by a voltage-gated Sodium Channel inhibitor (RS100642). Basic Clin Pharmacol Toxicol. (2012) 111:137–41. doi: 10.1111/j.1742-7843.2012.00880.x
320. Afanas’ev, I. New nucleophilic mechanisms of ROS-dependent epigenetic modifications: comparison of aging and cancer. Aging Dis. (2014) 5:52–62. doi: 10.14336/AD.2014.050052
321. Ramírez, A, Vázquez-Sánchez, AY, Carrión-Robalino, N, and Camacho, J. Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases. Oxidative Med Cell Longev. (2016) 2016:3928714. doi: 10.1155/2016/3928714
322. O'Hare Doig, RL, Chiha, W, Giacci, MK, Yates, NJ, Bartlett, CA, Smith, NM, et al. Specific ion channels contribute to key elements of pathology during secondary degeneration following neurotrauma. BMC Neurosci. (2017) 18:62. doi: 10.1186/s12868-017-0380-1
323. Esteras, N, Kundel, F, Amodeo, GF, Pavlov, EV, Klenerman, D, and Abramov, AY. Insoluble tau aggregates induce neuronal death through modification of membrane ion conductance, activation of voltage-gated calcium channels and NADPH oxidase. FEBS J. (2021) 288:127–41. doi: 10.1111/febs.15340
324. Fouda, MA, Ghovanloo, MR, and Ruben, PC. Cannabidiol protects against high glucose-induced oxidative stress and cytotoxicity in cardiac voltage-gated sodium channels. Br J Pharmacol. (2020) 177:2932–46. doi: 10.1111/bph.15020
325. Thomas, MP, Chartrand, K, Reynolds, A, Vitvitsky, V, Banerjee, R, and Gendelman, HE. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: relevance for the pathogenesis of Parkinson’s disease. J Neurochem. (2007) 100:503–19. doi: 10.1111/j.1471-4159.2006.04315.x
326. Li, M, and Lester, HA. Ion Channel diseases of the central nervous system. CNS Drug Rev. (2001) 7:214–40. doi: 10.1111/j.1527-3458.2001.tb00196.x
327. Huang, H, and Shakkottai, VG. Targeting ion channels and Purkinje neuron intrinsic membrane excitability as a therapeutic strategy for cerebellar Ataxia. Life. (2023) 13:1350. doi: 10.3390/life13061350
328. Kourie, JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Phys. (1998) 275:C1–C24.
329. Lombardi, AA, Gibb, AA, Arif, E, Kolmetzky, DW, Tomar, D, Luongo, TS, et al. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat Commun. (2019) 10:1–17. doi: 10.1038/s41467-019-12103-x
330. Patergnani, S, Danese, A, Bouhamida, E, Aguiari, G, Previati, M, Pinton, P, et al. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int J Mol Sci. (2020) 21:8323. doi: 10.3390/ijms21218323
331. Akbarali, HI. Oxidative stress and ion channels In: I Lahers, editor. Systems biology of free radicals and antioxidants. Berlin, Heidelberg: Springer. (2014) 355–73.
332. Nitahara, JA, Cheng, W, Liu, Y, Li, B, Leri, A, Li, P, et al. Intracellular calcium, DNase activity and myocyte apoptosis in aging Fischer 344 rats. J Mol Cell Cardiol. (1998) 30:519–35. doi: 10.1006/jmcc.1997.0616
333. Bawin, SM, and Adey, WR. Sensitivity of calcium binding in cerebral tissue to weak environmental electric fields oscillating at low frequency. Proc Natl Acad Sci USA. (1976) 73:1999–2003.
334. Barbier, E, Vetret, B, and Dufy, B. Stimulation of Ca2+ influx in rat pituitary cells under exposure to a 50 Hz magnetic field. Bioelectromagnetics. (1996) 17:303–11. doi: 10.1002/(SICI)1521-186X(1996)17:4<303::AID-BEM6>3.0.CO;2-7
335. Dutta, SK, Subramaniam, A, Ghosh, B, and Parshad, R. Microwave radiation - induced calcium ion efflux from human neuroblastoma cells in culture. Bioelectromagnetics. (1984) 5:71–8. doi: 10.1002/bem.2250050108
336. Gobba, F, Malagoli, D, and Ottaviani, E. Effects of 50 Hz magnetic fields on fMLP-induced shape changes in invertebrate immunocytes: the role of calcium ion channels. Bioelectromagnetics. (2003) 24:277–82. doi: 10.1002/bem.10102
337. Grassi, C, D’Ascenzo, M, Torsello, A, Martinotti, G, Wolf, F, Cittadini, A, et al. Effects of 50 Hz electromagnetic fields on voltage-gated Ca2+ channels and their role in modulation of neuroendocrine cell proliferation and death. Cell Calcium. (2004) 35:307–15. doi: 10.1016/j.ceca.2003.09.001
338. Jeong, JH, Kum, C, Choi, HJ, Park, ES, and Sohn, UD. Extremely low frequency magnetic field induces hyperalgesia in mice modulated by nitric oxide synthesis. Life Sci. (2006) 78:1407–12. doi: 10.1016/j.lfs.2005.07.006
339. Lisi, A, Ledda, M, Rosola, E, Pozzi, D, Emilia, ED′, Giuliani, L, et al. Extremely low frequency electromagnetic field exposure promotes differentiation of pituitary corticotrope-derived AtT20 D16V cells. Bioelectromagnetics. (2006) 27:641–51. doi: 10.1002/bem.20255
340. Marchionni, I, Paffi, A, Pellegrino, M, Liberti, M, Apollonio, F, Abeti, R, et al. Comparison between low-level 50 Hz and 900 MHz electromagnetic stimulation on single channel ionic currents and on firing frequency in dorsal root ganglion isolated neurons. Biochim Biophys Acta. (2006) 1758:597–605. doi: 10.1016/j.bbamem.2006.03.014
341. Morgado-Valle, C, Verdugo-Dıaz, L, Garcıa, DE, et al. The role of voltage-gated Ca2+ channels in neurite growth of cultured chromaffin cells induced by extremely low frequency (ELF) magnetic field stimulation. Cell Tissue Res. (1998) 291:217–30. doi: 10.1007/s004410050992
342. De Coursey, TE. Interactions between NADPH oxidase and voltage-gated proton channels: why electron transport depends on proton transport. FEBS Lett. (2003) 555:57–61. doi: 10.1016/S0014-5793(03)01103-7
343. Seredenina, T, Demaurex, N, and Krause, KH. Voltage-gated proton channels as novel drug targets: from NADPH oxidase regulation to sperm biology. Antioxid Redox Sign. (2015) 23:490–513. doi: 10.1089/ars.2013.5806
344. Sesti, F, Liu, S, and Cai, SQ. Oxidation of potassium channels by ROS: a general mechanism of aging and neurodegeneration? Trends Cell Biol. (2010) 20:45–51. doi: 10.1016/j.tcb.2009.09.008
345. Panagopoulos, DJ, and Margaritis, LH. Theoretical considerations for the biological effects of electromagnetic fields In: P Stavroulakis, editor. Biological effects of electromagnetic fields : Springer (2003). 5–33.
346. Hall, EJ, and Giaccia, AJ. Radiobiology for the radiologist. Philadelphia: Lippincott Williams & Wilkins (2006).
348. Reisz, JA, Bansal, N, Qian, J, Zhao, W, and Furdui, CM. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. (2014) 21:260–92. doi: 10.1089/ars.2013.5489
349. Kiselyov, K, and Muallem, S. ROS and intracellular ion channels. Cell Calcium. (2016) 60:108–14. doi: 10.1016/j.ceca.2016.03.004
350. Blaustein, MP, and Lederer, WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. (1999) 79:763–854. doi: 10.1152/physrev.1999.79.3.763
351. Maliszewska-Olejniczak, K, and Bednarczyk, P. Novel insights into the role of ion channels in cellular DNA damage response. Mutation Res. (2024) 793:108488. doi: 10.1016/j.mrrev.2024.108488
352. De Luca, C, Thai, JC, Raskovic, D, et al. Metabolic and genetic screening of electromagnetic hypersensitive subjects as a feasible tool for diagnostics and intervention. Mediat Inflamm. (2014) 2014:924184
353. Baek, S, Quan, X, Kim, S, Lengner, C, Park, JK, and Kim, J. Electromagnetic fields mediate efficient cell reprogramming into a pluripotent state. ACS Nano. (2014) 8:10125–38. doi: 10.1021/nn502923s
354. Giorgi, G, and Del Re, B. Epigenetic dysregulation in various types of cells exposed to extremely low-frequency magnetic fields. Cell Tissue Res. (2021) 386:1–15. doi: 10.1007/s00441-021-03489-6
355. Liu, Y, Liu, WB, Liu, KJ, Ao, L, Zhong, JL, Cao, J, et al. Effect of 50 Hz extremely low frequency electromagnetic fields on the DNA methylation and DNA methyltransferases in mouse spermatocyte-derived cell line GC-2. Biomed Res Int. (2015) 2015:237183:1–10. doi: 10.1155/2015/237183
356. Kaur, RP, Kaur, P, and Munshi, A. Epigenetic instability caused by oxidative stress triggers tumorigenesis In: S Chakraborti, BK Ray, and S Roychoudhury, editors. Handbook of oxidative stress in cancer: Mechanistic aspects. Singapore: Springer (2022). 1639–55.
357. Nishida, N, and Kudo, M. Oxidative stress and epigenetic instability in human hepato-carcinogenesis. Dig Dis. (2013) 31:447–53. doi: 10.1159/000355243
358. Chalidis, B, Sachinis, N, Assiotis, A, Maccauro, G, and Graziani, F. Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: biologic responses and clinical implications. Int J Immunopathol Pharmacol. (2011) 24:17–20. doi: 10.1177/03946320110241S204
359. Daish, C, Blanchard, R, Fox, K, Pivonka, P, and Pirogova, E. The application of pulsed electromagnetic fields (PEMFs) for bone fracture repair: past and perspective findings. Ann Biomed Eng. (2018) 46:525–42. doi: 10.1007/s10439-018-1982-1
360. Pilla, AA. State of the art in electromagnetic therapeutics In: M Blank, editor. Electricity and magnetism in biology and medicine. San Francisco: San Francisco Press Inc (1993). 17–22.
361. Pilla, AA. Weak time-varying and static magnetic fields: from mechanisms to therapeutic applications In: P Stavroulakis, editor. Biological effects of electromagnetic fields. Berlin: Springer (2003). 34–75.
362. Ryabi, JT. Clinical effects of electromagnetic fields on fracture healing. Clin Orthop Relat Res. (1998) 355S:S205–15. doi: 10.1097/00003086-199810001-00021
363. Wade, B. A review of pulsed electromagnetic field (PEMF) mechanisms at a cellular level: a rationale for clinical use. Am J Health Res. (2013) 1:51–5. doi: 10.11648/j.ajhr.20130103.13
364. Funk, RH. Coupling of pulsed electromagnetic fields (PEMF) therapy to molecular grounds of the cell. Am J Transl Res. (2018) 10:1260–72.
365. Maziarz, A, Kocan, B, Bester, M, Budzik, S, Cholewa, M, Ochiya, T, et al. How electromagnetic fields can influence adult stem cells: positive and negative impacts. Stem Cell Res Ther. (2016) 7:1–12. doi: 10.1186/s13287-016-0312-5
366. Pantelis, P, Theocharous, G, Veroutis, D, Vagena, IA, Polyzou, A, Thanos, DF, et al. Pulsed electromagnetic fields (PEMFs) trigger cell death and senescence in Cancer cells. Int J Mol Sci. (2024) 25:2473. doi: 10.3390/ijms25052473
367. Meijer, DK, and Geesink, HJ. Favourable and unfavourable EMF frequency patterns in cancer: perspectives for improved therapy and prevention. J Cancer Ther. (2018) 9:188–230. doi: 10.4236/jct.2018.93019
368. Panagopoulos, DJ, and Karabarbounis, A. Comments on behavior of charged particles in a biological cell exposed to AC-DC electromagnetic fields, and on comparison between two models of interaction between electric and magnetic fields and proteins in cell membranes. Environ Eng Sci. (2011) 28:749–51. doi: 10.1089/ees.2011.2810.com
369. Panagopoulos, DJ, and Chrousos, GP. Shielding methods and products against man-made electromagnetic fields: protection versus risk. Sci Total Environ. (2019) 667:255–62. doi: 10.1016/j.scitotenv.2019.02.344
370. Persinger, MA. Brain electromagnetic activity and lightning: potentially congruent scale - invariant quantitative properties. Front Integr Neurosci. (2012) 6:1–7. doi: 10.3389/fnint.2012.00019
371. Persinger, MA. Schumann resonance frequencies found within quantitative electro-encephalographic activity: implications for earth-brain interactions. Int Lett Chem Phys Astron. (2014) 30:24–32. doi: 10.56431/p-ly2br0
372. Yan, X, Liu, X, Zhang, S, Liu, Z, and Ren, L. Study of the inhibition of Schumann resonance-inspired electromagnetic field on cancer cell proliferation. J Bionic Eng. (2025) 22:341–53.
373. Zahumenska, R, Badurova, B, Pavelek, M, Sojka, P, and Pavlisova, T. Comparison of pulsed and continuous electromagnetic field generated by WPT system on human dermal and neural cells. Scientific Reports. (2024) 14:5514.
Glossary
AOS - Antioxidant System
ATP - Adenosine Triphosphate
CNS - Central Nervous System
CYP - Cytochrome P450
DECT - Digitally Enhanced Cordless Telecommunications
DOX - Deoxyribose
EHS - Electro-hypersensitivity
ELF - Extremely Low Frequency
EMF - Electromagnetic Field
EMR - Electromagnetic Radiation
ETC - Electron Transport Chain
GEF - geoelectric field
GMF - geomagnetic field
GSM - Global System for Mobile telecommunications
IFO - Ion Forced Oscillation
LF - Low Frequency
MT - Mobile Telephony
MW - Microwave
NADH - Nicotinamide Adenine Dinucleotide
NADPH - Nicotinamide Adenine Dinucleotide Phosphate
NOS - Nitric Oxide Synthase
NOX - NADH/NADPH Oxidase
OS - Oxidative Stress
RF - Radio Frequency
ROS - Reactive Oxygen Species
SOD - Superoxide Dismutase
VGCC - Voltage-Gated Calcium Channel
VGIC - Voltage-Gated Ion Channel
VLF - Very Low Frequency
WC - Wireless Communication
Wi-Fi - Wireless Fidelity
ULF - Ultra Low Frequency
XO - Xanthine Oxidase
2G, 3G, 4G, 5G - Second, Third, Fourth, Fifth Generation of MT/WC
Keywords: electromagnetic fields, voltage-gated ion channels, oxidative stress, ion forced oscillation, IFO-VGIC mechanism, ROS, DNA damage
Citation: Panagopoulos DJ, Yakymenko I, De Iuliis GN and Chrousos GP (2025) A comprehensive mechanism of biological and health effects of anthropogenic extremely low frequency and wireless communication electromagnetic fields. Front. Public Health. 13:1585441. doi: 10.3389/fpubh.2025.1585441
Edited by:
George Louis Carlo, Longwood University, United StatesReviewed by:
Ugo Cappucci, Sapienza University of Rome, ItalyVictor Alan Leach, Oceania Radiofrequency Scientific Advisory Association, Australia
Copyright © 2025 Panagopoulos, Yakymenko, De Iuliis and Chrousos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimitris J. Panagopoulos, ZHBhbmFnb3BAYmlvbC51b2EuZ3I=
 Dimitris J. Panagopoulos
Dimitris J. Panagopoulos Igor Yakymenko
Igor Yakymenko Geoffry N. De Iuliis
Geoffry N. De Iuliis George P. Chrousos
George P. Chrousos