- Department of Thoracic Surgery, Icahn School of Medicine at Mount Sinai, Institute for Translational Epidemiology, New York, NY, United States
Background: Although the association between exposure to asbestos and malignant mesothelioma has been established, occupational exposure has been historically present in males, while the ascertainment of female exposures is more nuanced. We reviewed the literature to assess differences in environmental exposure in mesothelioma cases according to sex.
Methods: A new PubMed search was conducted with the key words “mesothelioma” and “environmental exposure” on October 11, 2024 with a start date of January 1, 2016, to supplement our previous qualitative review that included publications up through June 2016. Studies conducted in occupational settings were excluded.
Results: Out of the 26 eligible papers, 11 were excluded because they did not report information on exposure by sex, leaving 15 published studies that were added to the 9 from our previous qualitative synthesis (24 total studies). 19 studies were cross-sectional, 2 were cohort and 3 were case control studies. The average NIH Study Quality tool score was 7.4/14 (minimum 3, maximum 12). Occupational exposure was more frequently observed in males than in females. While a male to female ratio favored males, there was variation in the strength of the association. There was a large proportion of cases with “unknown exposure,” and these were more frequently observed among female cases. In some studies, up to 40% of female cases had unknown exposure profiles. Quality assessment showed a generalized lack of standardization in the definition of environmental exposures across studies.
Conclusion: Although recent studies have continued to improve our understanding of environmental exposure to asbestos and other elongated fibers, challenges remain, including but not limited to lack of rigorous, high-quality evidence and difficulty standardizing definitions across countries and datasets to enable appropriate comparison across studies.
Introduction
Although the association between exposure to asbestos and malignant mesothelioma has been established, the bans and restrictions on asbestos use, production and import have contributed little to curb the incidence of mesothelioma in the US and around the world in the population at large (1). Some of the reasons for this discrepancy between the implementation of stricter regulations and the observed flat curve of mesothelioma incidence may be explained by both the long latency period of the disease, which usually presents 30 to 40 years after exposure. Moreover, asbestos fibers have persisted and remained in the environment, even after the new regulations (2). Males have historically experienced occupational asbestos exposure, and while this exposure has been reduced by bans on production and use, asbestos is still present in several structures including schools, municipal buildings, and in residential areas built in close proximity to former asbestos mines, factories, and soil containing natural asbestos.
As such, it is more difficult to ascertain and quantify these forms of non-occupational exposures, which are thus understudied (3). Non-occupational asbestos exposure may affect females more often than males and may go unnoticed unless specific questions and screening are carried out in a systematic manner. Trends in U.S. mesothelioma incidence suggest that incidence among males has decreased in recent years, while female incidence rates have been stable (4). This data suggests that there may be gender disparities in terms of asbestos exposure type, length of exposure, and at-risk populations, in addition to temporal changes in other risk factors such as smoking behavior. Here, we review the literature on mesothelioma in non-occupational settings by sex to explore these disparities and offer possible future directions in mesothelioma prevention.
Methods
We previously published a comprehensive qualitative review of studies on environmental exposure and mesothelioma that included publications up to June 2016 (5). To supplement this, we updated the published manuscript by conducting a new PubMed search with the key words mesothelioma and environmental exposure on October 11, 2024 with a start date of January 1, 2016 (5). Description of asbestos exposure according to sex was also added as an additional inclusion criterium.
The flowchart of study selection and review is outlined in Supplementary Figure 1. From 1/1/2016 to 9/30/2024, there were 645 studies published in English. After reviewing the abstracts, we further excluded articles that were not pertinent to the study question (n = 516). The remaining 129 papers were reviewed, with 103 papers further excluded because they were reviews, case reports and comments, editorials, or letters. This left 26 eligible papers whose full text was assessed; 11 studies were excluded because they did not report information on asbestos exposure by sex. This left 15 studies reporting exposure data stratified by sex that were added to the 9 publications in our previous qualitative synthesis. The overall quality of the 24 papers was assessed by two investigators (K.P. and E.T.) who separately addressed 14 points from the quality assessment tool developed by National Institutes of Health for observational cohort and cross-sectional studies. The 14 points in the quality assessment tool are weighted equally, with a maximum possibility quality score of 14, and encompass criteria of methodology rigor including but not limited to clarity of research question and study population, measurements of outcomes and exposure, and blinding of outcome assessors (6).
In cases of disagreement, the two investigators convened and discussed the reasons behind their score to reach a consensus. The summary quality scores are reported in Table 1. Despite anticipation of high heterogeneity among the reviewed studies in their design and in their measures for exposure and health outcomes, a quantitative summary estimate was conducted.
Results
Of the 24 papers covering 29 datasets included in this review, 19 studies were cross-sectional, 2 were cohort, and 3 were case control studies (Table 1). The average NIH Study Quality tool score was 7.4/14 (minimum 3, maximum 12). Assessments of exposures were captured as part of this quality tool, and both male and female patients were assessed in the same way through national registries built from standardized questionnaires and survey data or direct interviews of patients. Studies were conducted in Italy, Australia, Denmark, France, Greece, various locations in the US, South Africa, Colombia, Japan, and Turkey. Manuscripts reporting on the same or overlapping sample of cases were considered together, and only the most updated version of the results was reported in Table 1 and analyzed subsequently.
Definitions of exposure
A summary of the different definitions of non-occupational exposure used by the included studies is reported in Table 2. Eight studies from the Italian registry (7–14) classified non-occupational exposure into familial, environmental and “hobby related.” Another group of investigators (15–19) used categories of “familial” or “para-occupational” relating to domestic exposure (repairs, ironing etc), as well as “environmental indoor” (material in the house) and outdoor (living near a factory). Some investigators (20–23) further defined domestic exposure or household exposure (24) as living with a person with occupational exposure, while other investigators (25) defined domestic exposure as any of having a family member occupationally exposed, having asbestos devices at home, or involvement in do-it-yourself home projects.
Characteristics of cases according to type of exposure
Of the 24 included studies, 11 reported data for pleural mesothelioma (7, 12, 13, 15, 16, 19, 22, 24–28), and the other 13 reported on mesothelioma in general without a distinction between pleural and other sites.
Male vs. female type of exposure
Six studies reported on past occupational exposure by sex in patients with pleural mesothelioma (7, 12, 15, 16, 19, 24, 25), while eight studies reported this information on mesothelioma in general without distinction by site (8, 9, 14, 18, 23, 29–32).
Occupational exposure was more frequently observed in males than in females; among studies reporting on past occupational exposure by sex, the male to female ratio favors males, although the strength of the association varies (Figures 1A,B). In studies including both sexes with more than 30 cases, the percentages of occupational exposure cases consisting of men ranged from 78.7% (18) to 96.9% (30). Moreover, not only are there a large number of cases with unknown exposure, the proportion of unknown exposure cases was seen relatively more frequently among female patients (Figures 2A,B, 3). In the majority of studies, females had greater proportions of cases with unknown exposure compared to males, with some studies reporting as high as 25% of female cases having unknown exposure (Figure 3B). Across all studies of female patient cohorts with multiple sources of exposure, the percentages of mesothelioma cases attributable to unknown exposures ranged from 9% (12) to 22% (19).

Figure 1. Percentage of cases with recorded occupational exposure to asbestos by sex*. (A) Percentage of male patients with recorded occupational exposure to asbestos. (B) Percentage of female patients with recorded occupational exposure to asbestos. *17/24 studies are shown that reported data on male and female past occupational exposure. (C) Male to female ratio, occupational exposure.
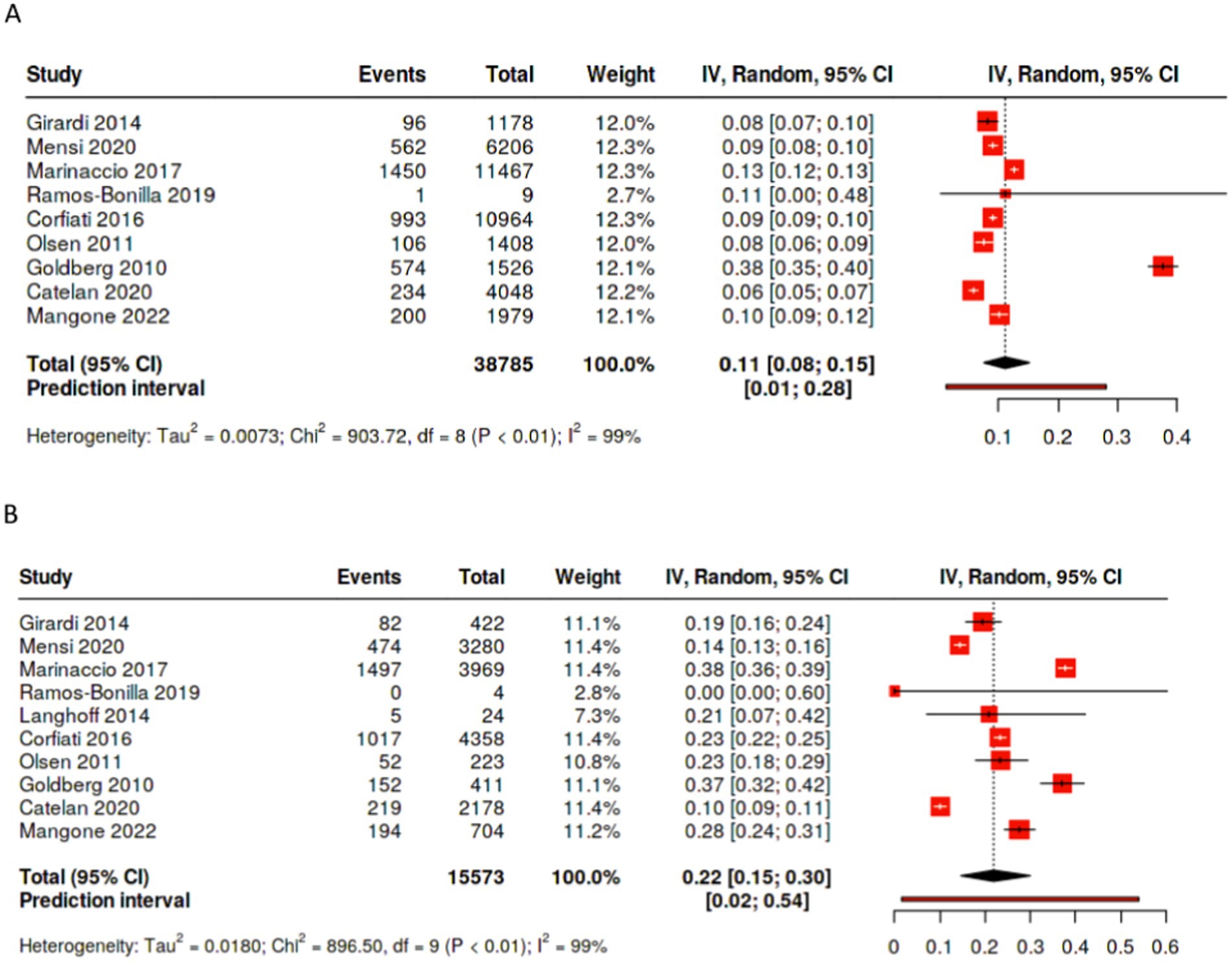
Figure 2. Percentage of cases with unknown exposure by sex. (A) Percentage of male patients with unknown exposure to asbestos. (B) Percentage of female patients with unknown exposure to asbestos.
Overall, pooling data across studies showed that the weighted pooled proportion of cases with environmental exposure was significantly higher among female (0.45 [95% CI: 0.34, 0.56], p < 0.01, n = 15,103) than male mesothelioma cases (0.24 [95% CI: 0.18, 0.30], p < 0.01, n = 35,261; Figures 4A,B). Likewise, the proportion of females mesothelioma linked with unknown exposure (0.22 [95% CI: 0.15, 0.30], p < 0.01, n = 15,573) is significantly greater the proportion of males (0.11 [95% CI: 0.08, 0.15,], p < 0.01, n = 38,785; Figures 2A,B), with large statistical heterogeneity. The pooled and weighted proportion of occupational exposure among male mesothelioma cases was 0.71 (95% CI: 0.64, 0.77, p < 0.01; n = 39, 702) significantly higher than that observed among female cases at 0.24 (95% CI: 0.20, 0.29; p < 0.01; n = 16,105; Figures 1A–C) with large statistical heterogeneity.
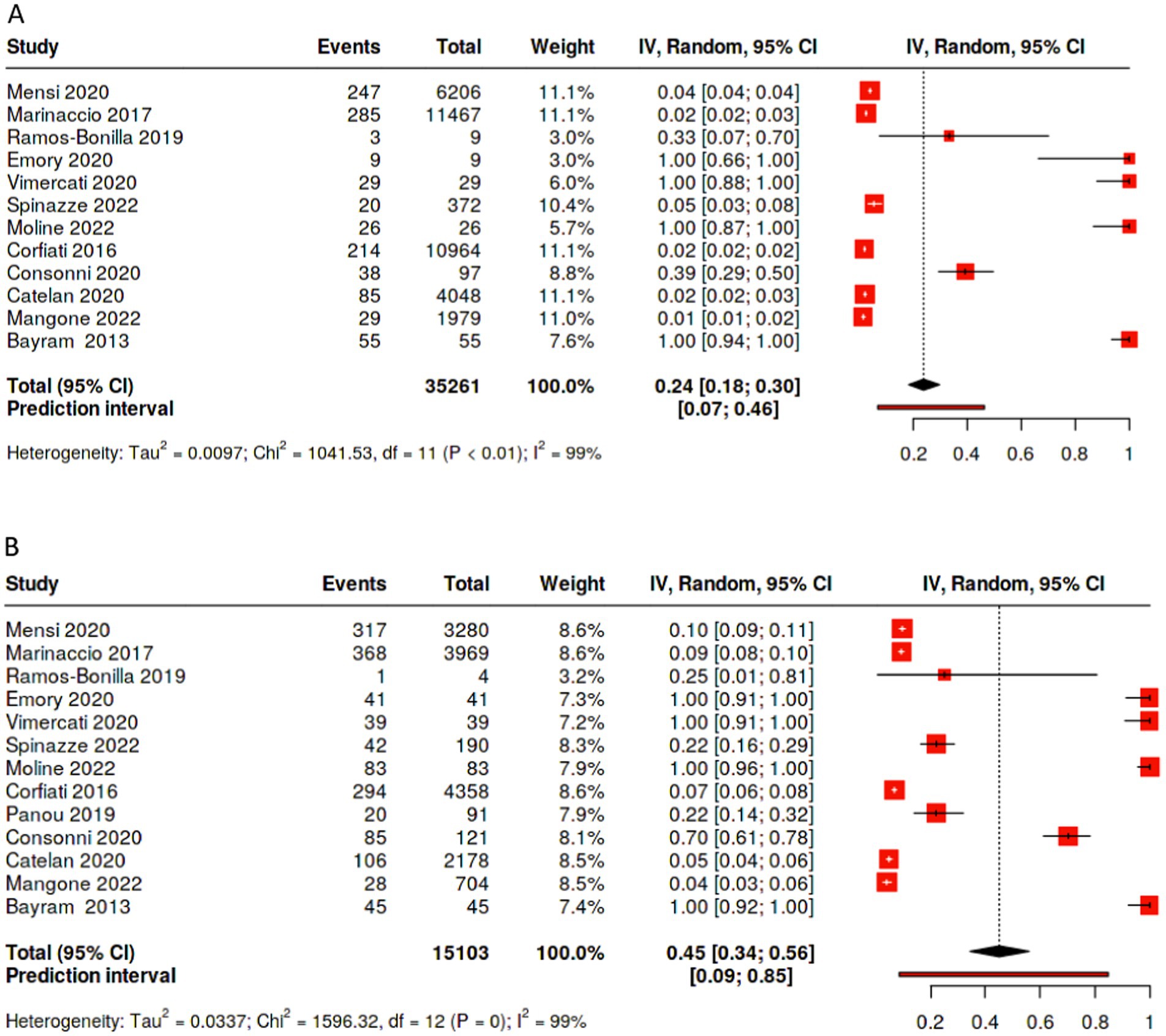
Figure 4. Percentage of cases with environmental exposure to asbestos by sex. (A) Percentage of male patients with unknown exposure to asbestos. (B) Percentage of female patients with unknown exposure to asbestos.
Discussion
This review of publications on mesothelioma cases from non-occupational settings provides evidence suggesting gender disparities in asbestos exposure modalities. Among cases reporting a past occupational exposure, males are by far the predominant sex, both for pleural mesothelioma and mesothelioma in general, as shown by uniform positive male to female ratios across studies. When other forms of non-occupational exposures are analyzed, females are more likely to report unknown or no exposure at all, confirming the need to more extensively query these cases about their past asbestos exposure history. It is possible that local residents may be less aware of the presence of environmental asbestos exposure, thereby reducing the likelihood of non-occupational exposures being reported when asked. The disparities by sex data suggest that there are sources of asbestos exposure that are not apparent or known to the cases. Therefore, without the necessary awareness, these individuals are more likely to have made no attempt to avoid such exposure and are therefore at increased risk of the disease. In recent years, other uncommon sources of asbestos contamination have surfaced, including cosmetic products and household items such as home decorations and textiles (27, 28). These products’ composition may be prone to contamination and content are often not appropriately labeled, and further contribute to under-reporting of exposure and lack of awareness by patients.
From a public health perspective, updating exposure questionnaires to include novel, uncommon sources of exposure, along with standardized methods to collect exposure information are needed. Mineralogical studies to identify geographic areas with natural occurring asbestos-like fibers are also needed, so that a map of exposure can be created and assessed in relation to residential history of cases, without relying on individual patient recall or knowledge of their residential exposure. Appropriate linkage between administrative health data, such as cancer registry and SPARCS, and mineralogical datasets and other useful exposure datasets provided by the U.S. Environmental Protection Agency such as the National Air Toxics Assessment (NATA) data, could help identify risk from inhalation of non-asbestos air toxins, emission sources, meteorological conditions, human activity patterns, smoking, and iatrogenic causes including radiation therapy. Finally, appropriate residential history should be coupled with emerging geospatial data-collection methods to avoid recall bias and bolster internal validity (33).
More precise measurements of asbestos exposure are urgently needed to better characterize risk, given the high proportion of cases where a clear exposure cannot be identified through questionnaires or other environmental measures. For instance, while certain biomarkers like the detection of asbestos fibers in mesothelioma tissue can be a proxy of past exposure, they are still imperfect measures of asbestos exposure and would therefore not be helpful for preventive purposes and predicting risk (34). Most of the existing biomarkers are geared toward early detection of mesothelioma rather than asbestos exposure (35). Mesothelin and osteopontin have showed early promise for early detection of asbestos-related mesothelioma; when measuring soluble mesothelin-related peptide (SMRP) levels in malignant pleural mesothelioma as well as benign pleural pathology, SMRP was able to distinguish between asbestos exposed and naïve patients (36). The prognostic capacity of osteopontin for detecting malignant mesothelioma was evaluated in a six-study meta-analysis and showed an overall specificity, sensitivity, and area under curve were 81, 65%, and 0.83, respectively. Moreover, the diagnostic odds ratio of osteopontin was 10.65 (95% CI: 7.13–15.91), and the positive and negative likelihood ratios were 3.77 (95% CI: 1.82–7.82) and 0.42 (95% CI: 0.31–0.58), respectively (37). However, none of these biomarkers can distinguish patients at risk for mesothelioma while they are still healthy.
Proteomic and epigenomic studies have also shown some promise in identifying asbestos-related profiles, but the translational potential is still in its early stages. Generic biomarkers like oxidative stress, inflammation, gene expression have not offered the specificity necessary for detection of asbestos exposure in healthy subjects (34). For instance, while one study found mir-29c mi-RNA was associated with worse prognosis independent of malignant pleural mesothelioma histology (n = 75), there is limited data linking proteomic and epigenomic studies to exposure itself (38).
Lastly, we acknowledge that the quantitative meta-analysis is limited by heterogeneity across included studies in study design, study sample sizes, and methodology for evaluating mesothelioma and health exposure. In particular, we understand that the pooled estimates and associated confidence intervals are biased by smaller studies with disproportional weighting and therefore, not representative of the true range of data. As such, we ensured that our forest plots depict the range of data across each of the included studies.
Conclusion
In summary, we reviewed literature on non-occupational exposure setting in mesothelioma, and observed a discrepancy with sex, with more female cases having unknown or no history of exposure to asbestos. Future research should address changing modalities, environmental, and occupational sources of asbestos and other elongated fibers exposure in order to deploy public health initiatives that address such exposures in an upstream and timely fashion while reducing the downstream occurrence of this malignant disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KP: Writing – original draft, Writing – review & editing, Data curation, Methodology, Validation. ST: Methodology, Writing – original draft, Writing – review & editing, Supervision, Visualization. ET: Supervision, Writing – original draft, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1588415/full#supplementary-material
References
1. Carbone, M, Yang, H, Pass, HI, and Taioli, E. Did the Ban on Asbestos Reduce the Incidence of Mesothelioma? J Thorac Oncol. (2023) 18:694–7. doi: 10.1016/j.jtho.2023.03.013
2. Alpert, N, van Gerwen, M, and Taioli, E. Epidemiology of mesothelioma in the 21(st) century in Europe and the United States, 40 years after restricted/banned asbestos use. Transl Lung Cancer Res. (2020) 9:S28–38. doi: 10.21037/tlcr.2019.11.11
3. van Gerwen, M, Alpert, N, Flores, R, and Taioli, E. An overview of existing mesothelioma registries worldwide, and the need for a US Registry. Am J Ind Med. (2020) 63:115–20. doi: 10.1002/ajim.23069
4. Taioli, E, Wolf, A, Alpert, N, Rosenthal, D, and Flores, R. Malignant pleural mesothelioma characteristics and outcomes: a SEER-Medicare analysis. J Surg Oncol. (2023) 128:134–41. doi: 10.1002/jso.27243
5. Liu, B, van Gerwen, M, Bonassi, S, and Taioli, E. Epidemiology of environmental exposure and malignant mesothelioma. J Thorac Oncol. (2017) 12:1031–45. doi: 10.1016/j.jtho.2017.04.002
6. National Institutes of Health. Quality assessment tool for observational cohort and cross-sectional studies. Available online at: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (accessed 1/2025)
7. Girardi, P, Bressan, V, and Merler, E. Past trends and future prediction of mesothelioma incidence in an industrialized area of Italy, the Veneto region. Cancer Epidemiol. (2014) 38:496–503. doi: 10.1016/j.canep.2014.08.007
8. Corfiati, M, Scarselli, A, Binazzi, A, Di Marzio, D, Verardo, M, Mirabelli, D, et al. Epidemiological patterns of asbestos exposure and spatial clusters of incident cases of malignant mesothelioma from the Italian national registry. BMC Cancer. (2015) 15:1–14. doi: 10.1186/s12885-015-1301-2
9. Marinaccio, A, Binazzi, A, Bonafede, M, Corfiati, M, Di Marzio, D, Scarselli, A, et al. Malignant mesothelioma due to non-occupational asbestos exposure from the Italian national surveillance system (ReNaM): epidemiology and public health issues. Occup Environ Med. (2015) 72:648–55. doi: 10.1136/oemed-2014-102297
10. Binazzi, A, Marinaccio, A, Corfiati, M, Bruno, C, Fazzo, L, Pasetto, R, et al. Mesothelioma incidence and asbestos exposure in Italian national priority contaminated sites. Scand J Work Environ Health. (2017) 43:550–9. doi: 10.5271/sjweh.3676
11. Pasetto, R, Zona, A, Fazzo, L, Binazzi, A, Bruno, C, Pirastu, R, et al. Proportion of mesothelioma attributable to living in industrially contaminated areas in Italy. Scand J Work Environ Health. (2019) 45:444–9. doi: 10.5271/sjweh.3809
12. Marinaccio, A, Corfiati, M, Binazzi, A, Di Marzio, D, Scarselli, A, Ferrante, P, et al. The epidemiology of malignant mesothelioma in women: gender differences and modalities of asbestos exposure. Occup Environ Med. (2018) 75:254–62. doi: 10.1136/oemed-2016-104119
13. D'Agostin, F, De Michieli, P, and Negro, C. Pleural mesothelioma in household members of asbestos-exposed workers in Friuli Venezia Giulia, Italy. Int J Occup Med Environ Health. (2017) 30:419–31. doi: 10.13075/ijomeh.1896.00890
14. Mangone, L, Storchi, C, Pinto, C, Rossi, PG, Bisceglia, I, and Romanelli, A. Incidence of malignant mesothelioma and asbestos exposure in the Emilia-Romagna region, Italy. Med Lav. (2022) 113:e2022047. doi: 10.23749/mdl.v113i5.13312
15. Mensi, C, De Matteis, S, Dallari, B, Riboldi, L, Bertazzi, PA, and Consonni, D. Incidence of mesothelioma in Lombardy, Italy: exposure to asbestos, time patterns and future projections. Occup Environ Med. (2016) 73:607–13. doi: 10.1136/oemed-2016-103652
16. Mensi, C, Riboldi, L, De Matteis, S, Bertazzi, PA, and Consonni, D. Impact of an asbestos cement factory on mesothelioma incidence: global assessment of effects of occupational, familial, and environmental exposure. Environ Int. (2015) 74:191–9. doi: 10.1016/j.envint.2014.10.016
17. Consonni, D, Migliore, E, Barone-Adesi, F, Dallari, B, De Matteis, S, Oddone, E, et al. Gender differences in pleural mesothelioma occurrence in Lombardy and Piedmont. Italy Environm Res. (2019) 177:108636. doi: 10.1016/j.envres.2019.108636
18. Catelan, D, Consonni, D, Biggeri, A, Dallari, B, Pesatori, AC, Riboldi, L, et al. Estimate of environmental and occupational components in the spatial distribution of malignant mesothelioma incidence in Lombardy (Italy). Environ Res. (2020) 188:109691. doi: 10.1016/j.envres.2020.109691
19. Spinazzè, A, Consonni, D, Borghi, F, Rovelli, S, Cattaneo, A, Zellino, C, et al. Asbestos exposure in patients with malignant pleural mesothelioma included in the PRIMATE study, Lombardy, Italy. Int J Environm Res Pub Health. (2022) 19:3390. doi: 10.3390/ijerph19063390
20. Panou, V, Vyberg, M, Meristoudis, C, Hansen, J, Bøgsted, M, Omland, Ø, et al. Non-occupational exposure to asbestos is the main cause of malignant mesothelioma in women in North Jutland, Denmark. Scand J Work Environ Health. (2019) 45:82–9. doi: 10.5271/sjweh.3756
21. Dalsgaard, SB, Würtz, ET, Hansen, J, Røe, OD, and Omland, Ø. Cancer incidence and risk of multiple cancers after environmental asbestos exposure in childhood—a long-term register-based cohort study. Int J Environm Res Pub Health. (2022) 19:268. doi: 10.3390/ijerph19010268
22. Langhoff, MD, Kragh-Thomsen, MB, Stanislaus, S, and Weinreich, UM. Almost half of women with malignant mesothelioma were exposed to asbestos at home through their husbands or sons. Dan Med J. (2014) 61:A4902.
23. Kitamura, Y, Zha, L, Liu, R, Shima, M, Nakaya, T, Kurumatani, N, et al. Association of mesothelioma deaths with cumulated neighborhood exposures due to a large-scale asbestos cement plant in Amagasaki city, Japan: a nested case-control study Int. J Environm Res Pub Health. (2022) 17:2636. doi: 10.21203/rs.3.rs-574038/v2
24. Ramos-Bonilla, JP, Cely-García, MF, Giraldo, M, Comba, P, Terracini, B, Pasetto, R, et al. An asbestos contaminated town in the vicinity of an asbestos-cement facility: the case study of Sibaté. Colombia Environm Res. (2019) 176:108464. doi: 10.1016/j.envres.2019.04.031
25. Lacourt, A, Gramond, C, Rolland, P, Ducamp, S, Audignon, S, Astoul, P, et al. Occupational and non-occupational attributable risk of asbestos exposure for malignant pleural mesothelioma. Thorax. (2014) 69:532–9. doi: 10.1136/thoraxjnl-2013-203744
26. Vimercati, L, Cavone, D, Delfino, MC, Caputi, A, De Maria, L, Sponselli, S, et al. Asbestos air pollution: description of a mesothelioma cluster due to residential exposure from an asbestos cement factory. Int J Environm Res Pub Health. (2020) 17:2636. doi: 10.3390/ijerph17082636
27. Emory, TS, Maddox, JC, and Kradin, RL. Malignant mesothelioma following repeated exposures to cosmetic talc: a case series of 75 patients. Am J Ind Med. (2020) 63:484–9. doi: 10.1002/ajim.23106
28. Moline, J, Patel, K, and Frank, AL. Exposure to cosmetic talc and mesothelioma. J Occup Med Toxicol. (2023) 18:1. doi: 10.1186/s12995-023-00367-5
29. Consonni, D, De Matteis, S, Dallari, B, Pesatori, AC, Riboldi, L, and Mensi, C. Impact of an asbestos cement factory on mesothelioma incidence in a community in Italy. Environ Res. (2020) 183:108968. doi: 10.1016/j.envres.2019.108968
30. Olsen, NJ, Franklin, PJ, Reid, A, de Klerk, NH, Threlfall, TJ, Shilkin, K, et al. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med J Austr. (2011) 195:271–4. doi: 10.5694/mja11.10125
31. Goldberg, S, Rey, G, Luce, D, Ilg, AGS, Rolland, P, Brochard, P, et al. Possible effect of environmental exposure to asbestos on geographical variation in mesothelioma rates. Occup Environ Med. (2010) 67:417–21. doi: 10.1136/oem.2009.050336
32. Konen, T, Johnson, JE, Lindgren, P, and Williams, A. Cancer incidence and mortality associated with non-occupational and low dose exposure to Libby vermiculite in Minnesota. Environ Res. (2019) 175:449–56. doi: 10.1016/j.envres.2019.04.004
33. Liu, B, Niu, L, and Lee, FF. Utilizing residential histories to assess environmental exposure and socioeconomic status over the life course among mesothelioma patients. J Thor Dis. (2023) 15:6126–39. doi: 10.21037/jtd-23-533
34. Pass, HI, Alimi, M, Carbone, M, Yang, H, and Goparaju, CM. Mesothelioma biomarkers: a review highlighting contributions from the early detection research network. Cancer Epidemiol Biomarkers Prev. (2020) 29:2524–40. doi: 10.1158/1055-9965.EPI-20-0083
35. Gillezeau, CN, van Gerwen, M, Ramos, J, Liu, B, Flores, R, and Taioli, E. Biomarkers for malignant pleural mesothelioma: a meta-analysis. Carcinogenesis. (2019) 40:1320–31. doi: 10.1093/carcin/bgz103
36. Rodríguez Portal, JA, Rodríguez Becerra, E, Rodríguez, D, Alfageme Michavila, I, Quero Martínez, A, Diego Roza, C, et al. Serum levels of soluble mesothelin-related peptides in malignant and nonmalignant asbestos-related pleural disease: relation with past asbestos exposure. Cancer Epidemiol Biomarkers Prev. (2009) 18:646–50. doi: 10.1158/1055-9965.EPI-08-0422
37. Hu, ZD, Liu, XF, Liu, XC, Ding, CM, and Hu, CJ. Diagnostic accuracy of osteopontin for malignant pleural mesothelioma: a systematic review and meta-analysis. Clin Chim Acta. (2014) 433:44–8. doi: 10.1016/j.cca.2014.02.024
38. Pass, HI, Goparaju, C, Ivanov, S, Donington, J, Carbone, M, Hoshen, M, et al. Hsa-miR-29c* is linked to the prognosis of malignant pleural mesothelioma. Cancer Res. (2010) 70:1916–24. doi: 10.1158/0008-5472.CAN-09-3993
39. Gogali, A, Ntzani, E, Peristeri, S, Tzarouchi, L, Manda-Stachouli, C, Vadivoulis, T, et al. End of domestic asbestos exposure epidemic in Metsovo. NW Greece Respiration. (2017) 94:510–7. doi: 10.1159/000480151
40. Ndlovu, N, Tewater Naude, J, and Murray, J. Compensation for environmental asbestos-related diseases in South Africa: a neglected issue. Glob Health Action. (2013) 6:19410. doi: 10.3402/gha.v6i0.19410
Keywords: asbestos exposure, cancer risk, environmental risk, non-occupational exposure, epidemiology
Citation: Patel K, Tuminello S and Taioli E (2025) Sex differences in asbestos exposure. Front. Public Health. 13:1588415. doi: 10.3389/fpubh.2025.1588415
Edited by:
Kenneth A. Mundt, University of Massachusetts Amherst, United StatesReviewed by:
Mei Yong, MY EpiConsulting, GermanyWilliam Joseph Thompson, William Thompson, United States
Copyright © 2025 Patel, Tuminello and Taioli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emanuela Taioli, RW1hbnVlbGEudGFpb2xpQG1vdW50c2luYWkub3Jn
 Krishna Patel
Krishna Patel Stephanie Tuminello
Stephanie Tuminello Emanuela Taioli
Emanuela Taioli

