- Department of Infection Control, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: To quantitatively analyze the impact of environmental hygiene on the occurrence of healthcare-associated infection (HAI).
Methods: Monitoring data of HAI and environmental hygiene from a tertiary first-class hospital from July 1, 2022, to December 31, 2024, were collected, and the impact of environmental bacterial colony forming unit (CFU) on the occurrence of HAI was analyzed by a time-series generalized additive model (GAM).
Results: The single-contamination model showed a significant positive correlation between HAI and total colony count, high-touch surface (HTS) colony count, and staff' hands colony count. The same pattern was observed in the multi-contamination model.
Conclusion: There is a significant correlation between environmental hygiene and the occurrence of HAI.
Introduction
Healthcare-associated infections (HAIs) represent a persistent global public health challenge in medical institutions over the past decade, contributing significantly to elevated morbidity, mortality rates, and healthcare system expenditure (1–3). World Health Organization (WHO) surveillance data indicate that 7%−15% of hospitalized patients acquire at least one HAI during their hospital stay, with fatal outcomes occurring in 10% of affected cases (4). Comprehensive research has identified multiple hospital environmental factors as potential reservoirs for pathogenic microorganisms, including staff hand surfaces, high-touch fomites, medical instrumentation, and water distribution systems (5–9). Notably, multidrug-resistant organisms (MDROs) such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci, and carbapenem-resistant Gram-negative bacteria demonstrate frequent environmental persistence in healthcare settings (10). Empirical evidence reveals these pathogens can maintain viability for extended periods exceeding decades in medical environments, with demonstrated capacity to form desiccated biofilm matrices. Such biofilm formation and subsequent microbial proliferation have been epidemiologically linked to HAI outbreak events (11).
Current infection control protocols, as outlined in international guidelines and domestic regulations, emphasize environmental sanitation as a critical intervention for HAI mitigation (12). This comprehensive approach encompasses standardized protocols for cleaning tool selection, validated disinfection methodologies, and optimized decontamination frequency (13). In Chinese healthcare institutions, environmental sanitation services predominantly operate under third-party contractual agreements. However, implementation efficacy remains suboptimal due to systemic challenges including variable workforce education levels among cleaning personnel and deficiencies in quality assurance mechanisms.
The existing evidence base establishing environmental contamination-HAI correlations primarily derives from microbial surveillance conducted during nosocomial infection outbreaks. A notable research gap persists regarding quantitative associations between routine environmental bioburden and baseline HAI incidence in non-outbreak scenarios (14, 15). Furthermore, while multiple determinants influence environmental hygiene outcomes—including cleaning material selection and staff training protocols—current studies predominantly analyze these variables as composite bundles rather than isolating individual factors (16). This methodological limitation constrains precise evaluation of specific intervention impacts on infection rates.
This investigation was conducted at a tertiary public teaching hospital (4,616-bed capacity) in Western China, utilizing two inpatient branches (A, B) as study sites. All branches maintained strict adherence to standardized infection control protocols issued by the National Health Commission of China, including the “Regulations for Cleaning and Disinfection Management of Environmental Surface in Healthcare” (17) and institution-specific “Standard Operating Procedure for Environmental Cleaning.” Identical staff training programs were implemented across all study units.
This investigation is structured to address three principal research objectives through systematic methodological implementation: (1) comparative evaluation of disinfection efficacy between distinct reusable cleaning equipment decontamination protocols; (2) quantitative characterization of environmental microbiome bioburden fluctuations associated with differing intervention strategies; and (3) establishment of epidemiological correlations between measurable microbial contamination parameters and healthcare-associated infection incidence patterns. The study framework enables rigorous examination of intervention effectiveness, microbial dynamics, and clinical outcomes through integrated analytical approaches.
The experimental framework provides novel insights into optimization of environmental hygiene protocols through evidence-based disinfection strategy selection, with particular relevance for healthcare systems utilizing outsourced sanitation services.
Methods
Study design
A comparative observational study was conducted across two branches (Branch A and B) of a tertiary hospital between July 1, 2022 and December 31, 2024. Branch A comprises 3,200 inpatient beds, while Branch B operates 611 beds. The study population included all 477,831 hospitalized patients admitted to both branches during the observation period, alongside 9,167 environmental health samples collected from staff hands, air, and high-frequency contact surfaces. Additionally, reusable cleaning tools subjected to distinct disinfection protocols were analyzed as study objects: Branch A employed manual cleaning with chemical disinfection, while Branch B utilized centralized thermal disinfection systems (automated washer-disinfector technology). This controlled variation enables isolation of cleaning tool decontamination methodology as the primary experimental variable for comparative analysis of environmental microbial load and subsequent HAI incidence.
Bacterial detection in reusable cleaning tools
A total of 100 post-disinfection samples of reusable cleaning textiles (including hand towels and floor towels) were randomly selected from both branches for microbiological analysis. Specimens were processed using standardized aseptic techniques: each sample was sectioned into 1 cm × 3 cm segments using sterile instruments and immersed in 5 ml of sterile normal saline containing appropriate neutralizers. After vortex mixing, 1 ml of the eluate was transferred to a sterile Petri dish, combined with 15–18 ml of nutrient agar (45–48 °C), and homogenized. Plates were incubated at 37 °C for 48 h. Finally, calculate the colony-forming units contained in each square centimeter (CFU/cm2) of reusable cleaning textiles samples.
Data collection
Hospital-acquired infection (HAI) diagnoses adhered to the Diagnostic Criteria for Nosocomial Infection (Trial Edition). Monthly HAI incidence rates and case-specific data (gender, age, hospitalization duration, ward assignment) were retrospectively extracted from the Nosocomial Infection Surveillance Database (NISD). Concurrently, environmental hygiene metrics, encompassing staff hand hygiene, air quality, and high-touch surface (HTS) were obtained from the Environmental Hygiene Monitoring System. Monitoring covered all clinical wards and critical units surgical center, hemodialysis center, endoscopy suite, pharmacy intravenous admixture service, and central sterile supply department). Data collection was executed by certified infection prevention and control (IPC) personnel under strict confidentiality protocols, with subsequent quality verification and archival management by designated personnel.
Statistical analysis
Descriptive statistics (mean, standard deviation, median, range, and interquartile range) characterized central tendencies and dispersion of monthly microbial colony-forming units per dish (MCFU/Dish) and HAI frequencies. Normality of HAI case distribution was assessed via the Kolmogorov–Smirnov test. Spearman's rank correlation analyzed associations between MCFU/Dish and HAI incidence. A generalized additive model (GAM) evaluated the non-linear relationship between environmental microbial loads and HAI occurrence. All analyses were performed using R software (v4.3.1), with statistical significance defined as p ≤ 0.05 (two-tailed).
Results
Microbial contamination of reusable cleaning tools
Reusable cleaning textiles subjected to centralized thermal disinfection demonstrated a statistically significant reduction in bacterial colonization compared to manually processed counterparts (0.53 vs. 177.3 CFU/cm2; p < 0.001; Figure 1).
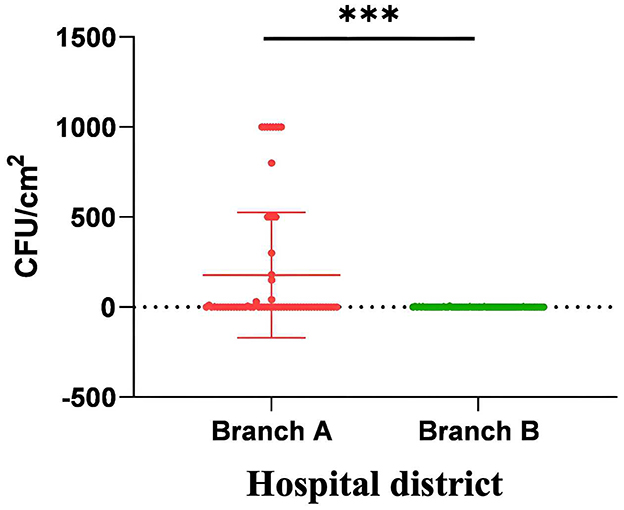
Figure 1. The microbiological monitoring comparison of reusable cleaning tools for fabrics in two branches. The bacterial colony counts on reusable sanitary ware cleaned manually were significantly higher than those on reusable sanitary ware cleaned by centralized thermal cleaning. ***p < 0.001.
Epidemiological profile of hospital-acquired infections
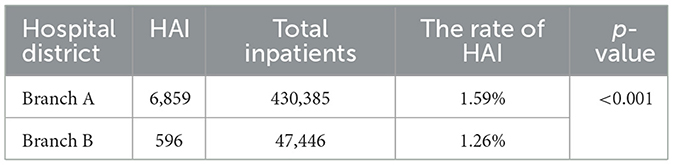
Among 477,831 hospitalized patients across both branches during the 30-month observation period, 7,455 HAIs were documented, yielding a cumulative incidence rate of 1.56% (mean: 248.5 cases/month). Branch A exhibited a significantly higher HAI incidence than Branch B (1.59 vs. 1.26%; p < 0.001; Table 1).
Environmental hygiene monitoring outcomes
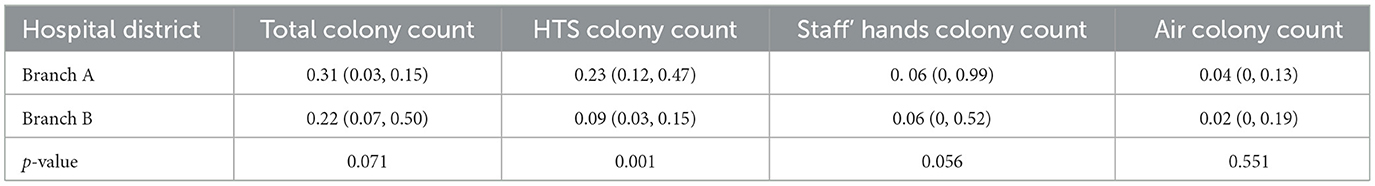
Of 9,167 environmental samples collected, 9,039 met inclusion criteria after excluding 128 contaminated or control-positive specimens. Sampling distribution included 2,308 hand hygiene evaluations (1,806 hygienic hand disinfection, 502 surgical hand disinfection), 4,138 air quality assessments, and 2,593 HTS analyses. The results revealed that except for the HTS colony count in the Branch A which was higher than that in the Branch B, there was no difference in the other two indicators (Table 2).
Association between HAI incidence and environmental contamination
After applying the Kolmogorov–Smirnov test, the monthly hospital infection occurrence numbers in both hospital branches were found to conform to the Poisson distribution (Za = 0.132, Pa = 0.191, Zb = 0.164, Pb = 0.058). Spearman correlation analysis revealed that the total environmental colony count (r = 0.676), HTS colony count (r = 0.547), and the colony count on the hands of staff (r = 496) in Branch A were positively correlated with the monthly hospital infection count (p < 0.05). While in Branch B, the total environmental colony count (r = 0.374) and HTS colony count (r = 0.451) were positively correlated with the monthly number of hospital infection cases (p < 0.05); the air colony counts in both branches were not correlated with the monthly hospital infection count (p > 0.05; Figure 2).
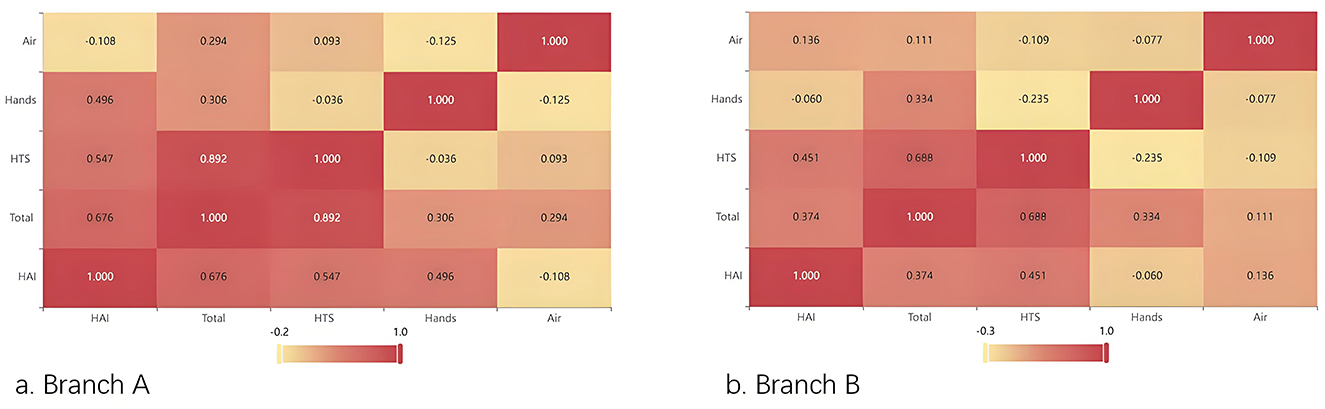
Figure 2. Heatmap of the correlation between environmental monitoring indicators and the monthly occurrence numbers of HAI cases. (a) Branch A. (b) Branch B.
GAM model
After adjusting for long-term trends and seasonal variability, GAM analysis identified significant associations between microbial loads and HAI incidence. The β1 of the Total colony counts, HTS colony counts, staff's hands colony counts and air colony count were shown in Table 3. We found that the total colony count, HTS colony count, and staff' hands colony count all had an impact on the monthly number of hospital infections in both hospital branches. The single-factor model indicated that the total colony count, HTS colony count, and staff hand colony count had a significant positive impact on the monthly number of hospital infections. For example, in Hospital Branch A, the β1 for the total colony count was 0.21 (p < 0.001), and it was estimated that the risk of hospital infection would increase by approximately 23% (95% CI) for each increase of 1 IQR.
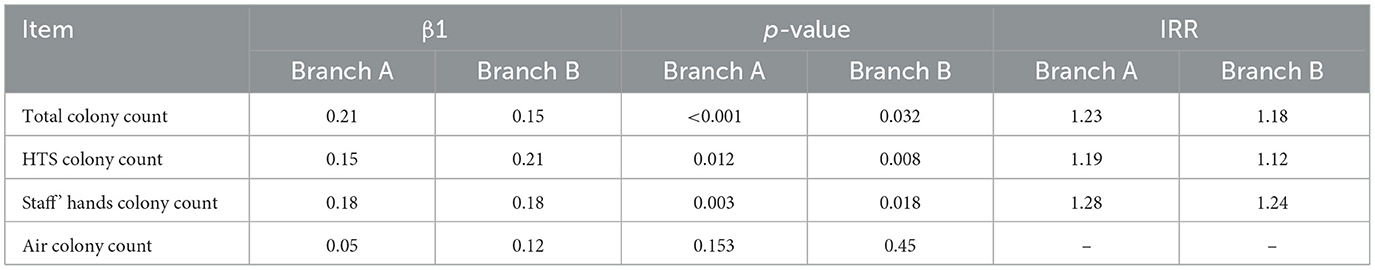
Table 3. The GAM model coefficients of environmental monitoring indicators for the two hospital branches.
The lag effect of 0–6 months shows that the lag time of total colony count is the longest (3 months in Branch A and 2 months in Branch B), the lag time of HTS colony count is 1 month in both areas, while there is no lag effect for the colony count on the hands of staff in both areas. This suggests that in the influence of the environment on hospital infections, the contamination level of staff hands has an immediate effect on the occurrence of hospital infections, and the implementation of hand hygiene for staff should be strengthened. However, the total environment, especially the HTS colony count, has a lag effect on the occurrence of hospital infections, indicating that the cleaning and disinfection of environmental surfaces should be strengthened, especially the terminal disinfection of the environment, to reduce the possibility of subsequent hospital infections in inpatients.
Based on the single-pollution model, we further established a multi-pollution model by adding a tensor product smoother to capture potential non-linear interaction relationships regarding environmental pollution factors and hospital infections. The interaction effect among the total environmental colony count, HTS colony count, and the colony count on staff hands showed that the interaction term te had a statistically significant impact on the monthly occurrence of hospital infections (edf = 7.366, p = 0.0313).
Discussion
In this study, the bacterial monitoring load of reusable sanitary ware using different cleaning and disinfection methods was compared, and the environmental hygiene and nosocomial infection monitoring data of the hospital for 30 month were comprehensively analyzed and evaluated.
Unsurprisingly, it was found that the bacterial load of manually cleaned reusable sanitary ware was significantly higher than that of centralized thermal cleaning, and the difference was statistically significant. The reasons for the poor effect of manual cleaning reusable sanitary ware mainly include the following two points: first, it is mainly operated by cleaning personnel, and there are disadvantages such as large individual behavior, poor implementation of norms and limited operating space; second, due to the large number of cleaning personnel involved and the wide distribution area, there are difficulties in supervision. Correspondingly, the centralized thermal cleaning and disinfection is mainly operated by the machine, the procedure is standardized, and the personnel involved and the area are centralized, which is more convenient for supervision and management. Reusable sanitary ware is the main tool for medical institutions to maintain environmental hygiene (18). Manually cleaned reusable sanitary ware may be contaminated before use, may lead to incomplete environmental cleaning, eventually excessive bacterial load on the surface of the environment. In this study, it was found that the unqualified rate of high frequency contact surface bacteria monitoring in the branch with manual cleaning was significantly higher than that in the branch with centralized heat cleaning, which also confirmed this. We also found that there were differences in the average HTS colony counts between the two hospital branches. The average HTS colony count of the district adopting centralized hot water cleaning was lower than that of the district using manual cleaning. This further indicates the direct impact of the cleaning methods for reusable sanitary fixtures on the hospital environment.
In this study, we found that the total colony count, HTS colony count and the Staff' hands colony count were positively correlated with the monthly number of hospital-acquired infections. Lee et al. (19) have shown that increased hand sanitizer use is associated with decreased rates of HAI and healthcare-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) infections. However, a recent study shows that ICU nurses must spend 17% of their working time performing proper hand hygiene, and a 100% hand hygiene compliance rate seems unattainable (20). Numerous studies have proposed that high-frequency contact surfaces of objects are directly related to the occurrence of hospital-acquired infections, especially in circumstances such as the COVID-19 pandemic and outbreaks of multi-drug resistant bacteria (21, 22). Russotto et al.'s (23) research demonstrated that if medical staff fail to correctly perform hand hygiene before and after contacting patients, it might increase the risk of environmental contamination and subsequent healthcare-associated infections (HAIs) for patients (23). Healthcare workers can cause contamination not only through direct contact with patients but also by touching inanimate surfaces and equipment in the patients' surroundings, and bacteria can survive on dry surfaces for several month. A study that screened the hands of healthcare workers and high-frequency contact areas found that the isolated yeasts underwent cross-hospital transmission, and the same clone strain was detected in the blood specimens of patients with candidemia (24). Hence, for infection control personnel, it is particularly crucial to effectively enhance the compliance and accuracy of hand hygiene among medical staff.
The average number of colonies on high-frequency contact surfaces in our hospital is lower than the requirements stipulated in the national standard GB15982 of China (25). This is attributed to the standard operating procedures and strict implementation of environmental cleaning and disinfection regulations we have adopted. However, it was still found that it has an impact on the monthly occurrence rate of nosocomial infections. This is consistent with the findings of Schmidt et al. Even when the cleaning team strictly implements cleaning measures, the microbial load on the patient's bed surface may still exceed the standard concentration, and the microbial load increases with the increase of the patient's hospitalization time (26). This may be related to the persistent existence of biofilms on dry surfaces in medical institutions (27). Maillard proposed that the use of specialized microbial cleaners can stabilize the contamination degree of object surfaces (28). Therefore, the cleaning of high-frequency contact surfaces needs to be actively and continuously implemented, and the monitoring of the colony count should be strengthened to provide comprehensive monitoring data for medical institutions and to formulate targeted intervention measures accordingly.
This study utilized a generalized additive model (GAM) to analyze the association between environmental contamination and HAI incidence, which offers flexibility in capturing non-linear relationships and adjusting for underlying temporal trends such as seasonality and long-term changes. By incorporating a smoothing function of time, the model effectively controlled for these confounding patterns, thereby providing a more accurate estimation of the independent effects of environmental microbial loads on HAI risk. This approach is particularly suited for analyzing complex time-series data in hospital epidemiology, where infection rates may fluctuate due to both environmental factors and unobserved temporal influences. The results of both the single-factor model and the multiple factors model showed that the smoothing term s (month) of the time series was statistically significant (p < 0.001), indicating that there was a significant non-linear relationship between the occurrence of HAI and the month, which consistent with the research result of Roux. While the non-linear relationship may be more influenced by ambient temperature and humidity, air quality or other seasonal factors (29). Recently, there has been an application of the GAM model in the research related to HAI (30), which proves that GAM has a promising application in the field of HAI prevention and control.
Furthermore, the lag effect analysis revealed distinct temporal patterns between different contamination sources. The colony count on staff hands exhibited an immediate effect on HAI occurrence, indicating that hand hygiene interventions should be implemented in real time to minimize transmission risks. In contrast, the total environmental colony count and HTS colony count demonstrated lag effects of up to 3 months and 1 month, respectively, suggesting that environmental surface contamination accumulates over time and influences HAI incidence in a delayed manner. This finding underscores the importance of reinforcing terminal disinfection protocols and scheduled environmental cleaning to mitigate residual microbial burden. Consequently, infection control strategies should prioritize immediate hand hygiene compliance alongside structured environmental decontamination schedules to address both instantaneous and delayed contamination pathways effectively.
This study has some limitation. First, as the study is a single-center research, its results have certain limitations in terms of their generalizability. Second, it is a retrospective analysis involving data over a period of 30 months, there may be potential bias in the test results due to the reasons related to specimen collection. In the future, it is urgently necessary to optimize and improve the model and methods of this study through multi-center and prospective research.
Conclusions
To sum up, the innovation of this study lies in the fact that it is the first time to apply GAM to explore the association between environmental pollution factors and the risk of HAI. The GAM model has been extended to the interdisciplinary research field of environmental hygiene and hospital infection prevention and control. Moreover, the types of environmental specimens included in this study cover the types usually monitored in medical institutions. Using 30-month data for in-depth modeling analysis, it provides a new perspective for the formulation and implementation of HAI prevention and control strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was conducted in accordance with relevant guidelines and regulations. The analysis of anonymized surveillance data was approved by the Human Research Ethics Committee of The First Affiliated Hospital of Chongqing Medical University.
Author contributions
RL: Investigation, Data curation, Writing – review & editing. ZW: Writing – review & editing, Investigation, Data curation. MH: Formal analysis, Validation, Writing – review & editing. DL: Data curation, Investigation, Writing – review & editing. ZY: Writing – review & editing, Validation. KQ: Conceptualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Science and Technology Research Project of Chongqing Municipal Education Commission (KJZD-M202400405).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank HP, Ducomble T, et al. Burden of Six Healthcare-Associated Infections on European Population Health: estimating incidence-based disability-adjusted life years through a Population Prevalence-Based Modelling Study. PLoS Med. (2016) 13:e1002150. doi: 10.1371/journal.pmed.1002150
2. Tchouaket Nguemeleu E, Beogo I, Sia D, Kilpatrick K, Séguin C, Baillot A, et al. Economic analysis of healthcare-associated infection prevention and control interventions in medical and surgical units: systematic review using a discounting approach. J Hosp Infect. (2020) 106:134–54. doi: 10.1016/j.jhin.2020.07.004
3. Rosenthal VD, Yin R, Nercelles P, Rivera-Molina SE, Jyoti S, Dongol R, et al. International Nosocomial Infection Control Consortium (INICC) report of health care associated infections, data summary of 45 countries for 2015 to 2020, adult and pediatric units, device-associated module. Am J Infect Control. (2024) 52:1002–11. doi: 10.1016/j.ajic.2023.12.019
4. World Health Organization. Global Report on Infection Prevention and Control 2024: executive summary[EB/OL] (2024). Available online at: https://www.who.int/publications/i/item/B09195 (Accessed March 16, 2025).
5. Adams CE, Smith J, Watson V, Robertson C, Dancer SJ. Examining the association between surface bioburden and frequently touched sites in intensive care. J Hosp Infect. (2017) 95:76–80. doi: 10.1016/j.jhin.2016.11.002
6. Rawlinson S, Cloutman-Green E, Asadi F, Ciric L. Surface sampling within a pediatric ward-how multiple factors affect cleaning efficacy. Am J Infect Control. (2020) 48:740–5. doi: 10.1016/j.ajic.2019.10.023
7. Facciolà A, Pellicanò GF, Visalli G, Paolucci IA, Venanzi Rullo E, Ceccarelli M, et al. The role of the hospital environment in the healthcare-associated infections: a general review of the literature. Eur Rev Med Pharmacol Sci. (2019) 23:1266–78. doi: 10.1016/j.etap.2018.11.006
8. Najotra DK, Malhotra AS, Slathia P, Raina S, Dhar A. Microbiological surveillance of operation theatres: five year retrospective analysis from a tertiary care hospital in North India. Int J Appl Basic Med Res. (2017) 7:165–8. doi: 10.4103/ijabmr.IJABMR_281_16
9. Hayward C, Brown MH, Whiley H. Hospital water as the source of healthcare-associated infection and antimicrobial-resistant organisms. Curr Opin Infect Dis. (2022) 35:339–45. doi: 10.1097/QCO.0000000000000842
10. Perry-Dow KA, de Man TJB, Halpin AL, Shams AM, Rose LJ, Noble-Wang JA. The effect of disinfectants on the microbial community on environmental healthcare surfaces using next generation sequencing. Am J Infect Control. (2022) 50:54–60. doi: 10.1016/j.ajic.2021.08.027
11. Porter L, Sultan O, Mitchell BG, Jenney A, Kiernan M, Brewster DJ, et al. How long do nosocomial pathogens persist on inanimate surfaces? A scoping review. J Hosp Infect. (2024) 147:25–31. doi: 10.1016/j.jhin.2024.01.023
12. World Health Organization. Regional Office for the Western Pacific. Practical Guidelines for Infection Control in Health Care Facilities. Manila, Philippines: WHO Regional Office for the Western Pacific (2004).
13. Centers for Disease Control and Prevention of the United States. Guideline for Disinfection and Sterilization in Healthcare Facilities (2008).
14. Smith J, Adams CE, King MF, Noakes CJ, Robertson C, Dancer SJ. Is there an association between airborne and surface microbes in the critical care environment? J Hosp Infect. (2018) 10:e123–9. doi: 10.1016/j.jhin.2018.04.003
15. Gaudart J, Cloutman-Green E, Guillas S, D'Arcy N, Hartley JC, Gant V, et al. Healthcare environments and spatial variability of healthcare associated infection risk: cross-sectional surveys. PLoS ONE. (2013) 8:e76249. doi: 10.1371/journal.pone.0076249
16. Allen M, Hall L, Halton K, Graves N. Improving hospital environmental hygiene with the use of a targeted multi-modal bundle strategy. Infect Dis Health. (2018) 23:107–13. doi: 10.1016/j.idh.2018.01.003
17. National Health and Family Planning Commission of the People's Republic of China. Regulations for Cleaning and Disinfection Management of Environmental Surface in Healthcare:WS/T512-2025 (2025).
18. Mitchell BG, Hall L, White N, Barnett AG, Halton K, Paterson DL, et al. An environmental cleaning bundle and health-care-associated infections in hospitals (REACH): a Multicentre, Randomised trial. Lancet Infect Dis. (2019) 19:410–8. doi: 10.1016/S1473-3099(18)30714-X
19. Lee YT, Chen SC, Lee MC, Hung HC, Huang HJ, Lin HC, et al. Time-series analysis of the relationship of antimicrobial use and hand hygiene promotion with the incidence of healthcare-associated infections. J Antibiot. (2012) 65:311–6. doi: 10.1038/ja.2012.20
20. Siebers C, Mittag M, Grabein B, Zoller M, Frey L, Irlbeck M. Hand hygiene compliance in the intensive care unit: hand hygiene and glove changes. Am J Infect Control. (2023) 51:1167–71. doi: 10.1016/j.ajic.2023.04.007
21. Mitchell BG, McGhie A, Whiteley G, Farrington A, Hall L, Halton K, et al. Evaluating bio-burden of frequently touched surfaces using Adenosine Triphosphate bioluminescence (ATP): results from the researching effective approaches to cleaning in hospitals (REACH) trial. Infect Dis Health. (2020) 25:168–74. doi: 10.1016/j.idh.2020.02.001
22. Wei L, Lin J, Duan X, Huang W, Lu X, Zhou J, et al. Asymptomatic COVID-19 patients can contaminate their surroundings: an environment sampling study. mSphere. (2020) 5:e00442–20. doi: 10.1128/mSphere.00442-20
23. Russotto V, Cortegiani A, Raineri SM, Giarratano A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J Intensive Care. (2015) 3:54. doi: 10.1186/s40560-015-0120-5
24. Megri Y, Arastehfar A, Boekhout T, Daneshnia F, Hörtnagl C, Sartori B, et al. Candida tropicalis is the most prevalent yeast species causing candidemia in Algeria: the urgent need for antifungal stewardship and infection control measures. Antimicrob Resist Infect Control. (2020) 9:50. doi: 10.1186/s13756-020-00710-z
25. National Health and Family Planning Commission of the People's Republic of China. Hygienic standard for disinfection in hospitals: WS/T313-2019 (2019).
26. Schmidt MG, Attaway HH, Fairey SE, Howard J, Mohr D, Craig S. Self-disinfecting copper beds sustain terminal cleaning and disinfection effects throughout patient care. Appl Environ Microbiol. (2019) 86:e01886–19. doi: 10.1128/AEM.01886-19
27. Maillard JY, Centeleghe I. How biofilm changes our understanding of cleaning and disinfection. Antimicrob Resist Infect Control. (2023) 12:95. doi: 10.1186/s13756-023-01290-4
28. Vandini A, Temmerman R, Frabetti A, Caselli E, Antonioli P, Balboni PG, et al. Hard surface biocontrol in hospitals using microbial-based cleaning products. PLoS ONE. (2014) 9:e108598. doi: 10.1371/journal.pone.0108598
29. Roux J, Nekkab N, Colomb-Cotinat M, Astagneau P, Crépey P. Time-series modelling for the quantification of seasonality and forecasting antibiotic-resistant episodes: application to carbapenemase-producing Enterobacteriaceae episodes in France over 2010–20. J Antimicrob Chemother. (2021) 76:226–32. doi: 10.1093/jac/dkaa388
Keywords: healthcare-associated infection, environmental hygiene, time-series analysis, generalized additive model, lag effect
Citation: Li R, Wang Z, Huang M, Liao D, Yuan Z and Qian K (2025) Environmental hygiene and healthcare-associated infection: a time-series study based on generalized additive model. Front. Public Health 13:1592700. doi: 10.3389/fpubh.2025.1592700
Received: 13 March 2025; Accepted: 26 September 2025;
Published: 13 October 2025.
Edited by:
Erjona Abazaj, Institute of Public Health, AlbaniaReviewed by:
Muhammad Saqib, Khyber Medical College, PakistanBhupinder Kaur, Akal Degree College Mastuana, India
Copyright © 2025 Li, Wang, Huang, Liao, Yuan and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keli Qian, cWlhbmtlbGk4NkAxNjMuY29t
 Renhua Li
Renhua Li Zhongjie Wang
Zhongjie Wang Mingqi Huang
Mingqi Huang Keli Qian
Keli Qian