- 1Department of Laboratory Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 2Department of Medical Laboratory, Qianxi People's Hospital, Qianxi, Guizhou, China
Introduction: The widely prevalent Acinetobacter baumannii is a gram-negative opportunistic pathogen, with the enormous potential to trigger multiple concurrent diseases. Due to its multidrug resistance, A. baumannii has emerged as a key monitored pathogen of WHO for surveillance in healthcare settings, particularly in intensive care units (ICUs).
Methods: To identify factors associated with A. baumannii infection in Guizhou province, China from 2015 to 2023, a retrospective cross-sectional analysis was carried out with the data from hospital records, electronic medical databases. Fisher’s exact test (gender) and Chi-square were used for statistical tests, while p-values was used to define the statistically significance of variables.
Results and discussion: Out of 460,620 patients; there were 6,944 positive infection events, with ICU-related infections accounting for 45.77%. Males had a significantly higher risk of A. baumannii infection compared to females, and host factors such as gender and increasing age were associated with greater susceptibility. In addition, infection rates were higher during warmer summer months, suggesting a possible seasonal trend in transmission. Within the population undergoing antibiotic therapy, only polymyxin B demonstrated an alarmingly high level of resistance, whereas other clinically employed antibiotics were invalid. In summary, the statistical data from these years emphasizes that gender and age are main drivers of A. baumannii outbreaks in Guizhou, and follow-up public health interventions need to target these factors to enhance infection control and reduce the spread. Also, the observation of high levels of resistance to multiple antibiotics is a matter of concern, as it presents significant challenges in terms of treatment.
Introduction
Acinetobacter baumannii is a globally prevalent opportunistic pathogen known for causing a wide range of healthcare-associated infections (1, 2). The viability of A. baumannii is remarkably robust, enabling its prolonged persistence in humid environments, as well as formidable resistance to desiccation, ultraviolet radiation, and a wide range of disinfectants, like lodophor, benzalkonium chloride and chlorhexidine (3–8). Acinetobacter baumannii primarily contributes to nosocomial infections, particularly patients in ICUs, those with compromised immune systems diseases, individuals on long-term broad-spectrum antibiotic therapy, and patients undergoing invasive medical procedures (9–12). Simultaneously, A. baumannii has developed mechanisms to evade the host immune response, facilitating rapid colonization and proliferation within the host (13, 14). The global mortality rate associated with A. baumannii infection is alarmingly high, ranging from 30% to as high as 75%, depending on the severity of the infection and the underlying health status of the patient (15, 16). The high incidence rate and mortality caused by A. baumannii pose a significant public health concern, necessitating stringent control measures to curtail its transmission.
Pneumonia is the most prevalent respiratory tract infection complications caused by A. baumannii (17–19). Prolonged immobilization, and utilization of mechanical ventilators contaminated with A. baumannii is a risk factor of pneumonia for patients admitted to the ICUs, common symptoms include fever, persistent cough, and respiratory distress. Thus, it is of paramount importance to implement regular disinfection protocols for medical devices, conduct bacterial testing, employ antibacterial coatings on medical equipment, and enhance disinfection plans to effectively control respiratory diseases caused by A. baumannii (20, 21). Acinetobacter baumannii is capable of inducing urinary tract infections such as cystitis, pyelonephritis, and prostatitis. Common symptoms include increased frequency of urination, urgency, dysuria, and lower back pain in affected individuals (22, 23). The infection of the central nervous system highlights the robust immune evasion mechanism employed by A. baumannii, despite its lack of flagellar motility assistance (24–26). When infection occurs in the central nervous, it frequently results in the development of meningitis and brain abscess in patients, accompanied by symptoms such as headache, fever, vomiting, neck stiffness, and even disturbances in consciousness or fatality. Meanwhile, A. baumannii is also a significant contributor to various environmental susceptibility diseases, such as postoperative wound infections and bacteremia in healthcare settings (27, 28).
Clinical management of A. baumannii infections is complicated by its broad antibiotic resistance (29, 30). The formulation of specific disease prevention policies depends on the epidemiology of regional diseases, and the selection of regions is typically guided by choosing representative areas as key references. For instance, regions with inadequate medical resources or those that are excessively healthy may lack sufficient representativeness. To investigate the current status of A. baumannii epidemic prevention and control in China, this study selected data recorded in the Guizhou region from 2015 to 2023 for analysis. The Guizhou region, situated in a central geographic location with relatively mid-level medical care, serves as a representative sample for this research. It has been previously reported that the incidence rate of A. baumannii infection is higher in summer and among male patients (31). In this study, we extended the analysis beyond season and gender to include age and other factors. Additionally, we evaluated the effectiveness of antibiotics in existing hospitals, aiming to establish a more comprehensive regional trend of A. baumannii epidemiology.
Materials and methods
Data sources, inclusion criteria and case definition
The study population comprised patients hospitalized or admitted to intensive care units (ICUs) across Guizhou Province with a confirmed diagnosis of A. baumannii infection from January 2015 to December 2023. Surveillance data of A. baumannii infection in both public and private hospitals in Guizhou province obtained from the National Health and Medical Big Data Western Center of Guizhou Province were retrospectively analyzed. It is a comprehensive medical information database that stores historical hospital admission cases, diagnostic and therapeutic information, as well as special disease cases from across 12 provinces in western China (including Guizhou province). Inclusion Criteria (positive case definitions): the microbiological testing clinic provides comprehensive reports on the presence of A. baumannii, wherein blood and sputum are collected to detect the content and abundance by qRT-PCR. Exclusion criteria: patients with incomplete data, repeated samples, or colonization without infection excluded. Biochemical parameters: negative oxidase, positive catalase, negative indole, non-assimilation of sugars and nitrate non-reduction of submitted samples were detected by API-20NE (32). Molecular parameters: β-lactamase (also known as cephalosporinase) coding gene AmpC, is encoded in the chromosomes or plasmids of Enterobacteriaceae or Pseudomonas aeruginosa, which was also used for accurate quantitative assessment of A. baumannii load in clinical cases by real-timepolymerase chain reaction (Forward primer: 5′-TAAACACCACTATGTTCCG-3′/Reverse primer: 5′-ACTTACTTCAACTCGCGACG-3′. Reaction procedure: 95°C 1 min; [95°C 10 s; 60°C 25 s; 72°C 30 s] × 40 circles) (33). Post biochemical and molecular analyses, bacteria were detected after cultivation of both sputum and blood samples as a confirmed case.
Definition of variables
Time-oriented variables, such as the year and the season (quarterly), were generated by categorizing cases according to dates of illness onset. In addition, socio-demographic factors, such as gender and age were identified. Sex was recorded as male or female, while age is divided into four groups (0–14, 15–47, 48–63, and ≥64 years). To assess the risk of infection of A. baumannii among patients with severe preexisting conditions or acute severe injuries, a comparative analysis of infection rates between ICU and non-ICU cases was conducted. As an independent factor, the efficacy of different antibiotic types has also been evaluated in relation to clinical treatment success and microbiological clearance capability.
Statistical analysis
All characteristics were presented as categorical variables and summarized using frequencies or percentages. The comparison of variables between condition and outcome was conducted using Fisher’s exact test (gender). Chi-square was used for categorical variables (age and season). p-values less than 0.05 were considered statistically significant. Time series seasonality plots (a line graph summarizing cases by year and month throughout the study period) were drawn to show trends of cases over time. To quantify the strength of the association between the explanatory and outcome variables, we computed the odds ratios apply to binary outcomes along with its corresponding 95% confidence interval and p-value. Both data analysis and visualization were conducted using GraphPad Prism 8.0 software.
Results
Trend and seasonality of Acinetobacter baumannii incidence from 2015 to 2023
A total of 6,944 positive cases of A. baumannii infection were identified among 460,620 tested hospitalized patients in Guizhou, resulting in an overall positivity rate of 1.51%. Case reporting was higher during the 2023 (14.12%) within 9 years, while positive rate was higher during the 2016 (2.34%). The detection rate of A. baumannii accounted for 8.95% of the total positive bacteria (among the pathogens identified in patients diagnosed with bacterial infections), indicating its high likelihood of being detected during infection or rapid proliferation as a relatively abundant pathogen upon detection (Figures 1A,B).
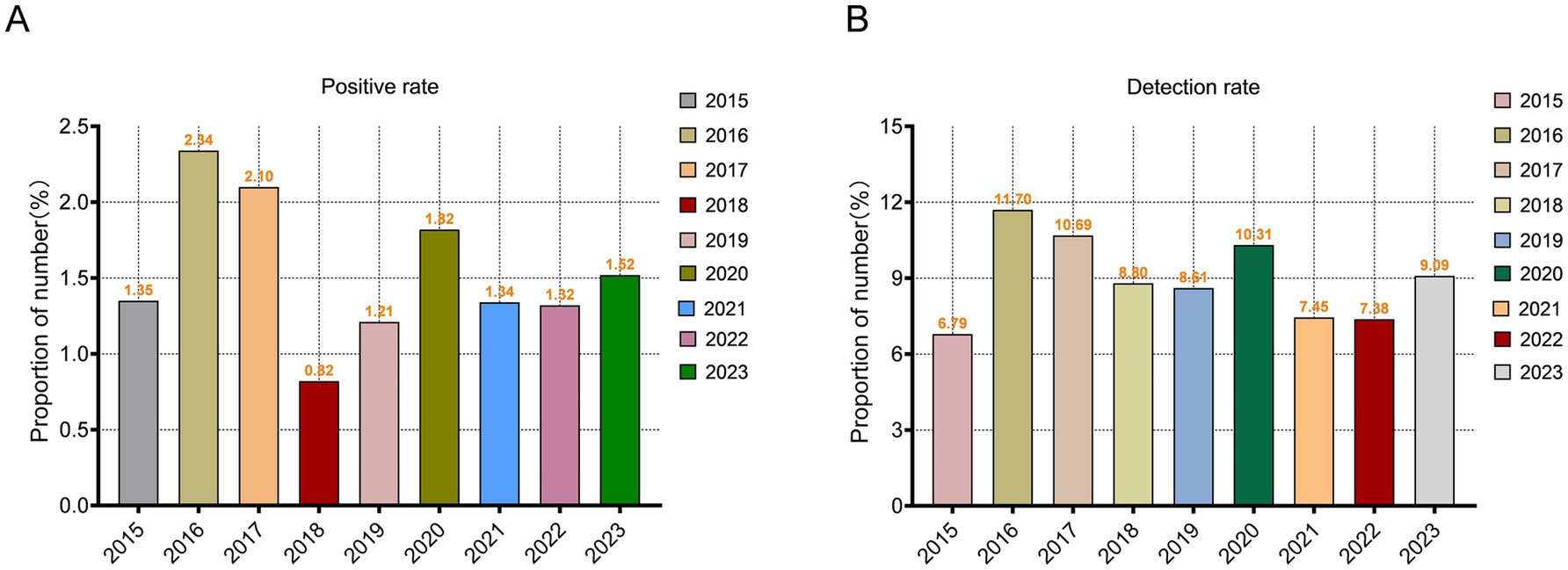
Figure 1. Temporal evolution of Acinetobacter baumannii Epidemiology in Guizhou Province from 2015 to 2023. (A) Changes in the positivity rate of A. baumannii from 2015 to 2023. (B) The detection rate of A. baumannii in hospital bacteria examination from 2015 to 2023.
There was a predominance of high positive infection cases observed during spring and summer (autumn in 2016 and 2020), while the incidence rate remained relatively low during autumn and winter. For instance, in 2015, the proportion of infections during summer constituted 34.97% of the total infection count, whereas infections during autumn accounted for merely 15.88%. Statistical analysis of variance was conducted to examine the incidence of A. baumannii across different seasons. However, revolving around the variable of season, there was no statistically significant difference in the incidence rate (p = 0.764; Figures 2A,B). The phenomenon of inconspicuous seasonal statistical analysis results may be attributable to the limited size of the dataset or regional climatic variations. With average positive cases in spring was 200 (95% Cl: 155 to 244), summer was 207 (95% Cl: 164 to 249), autumn was 181 (95% Cl: 128 to 234), and winter was 184 (95% Cl: 144 to 225).
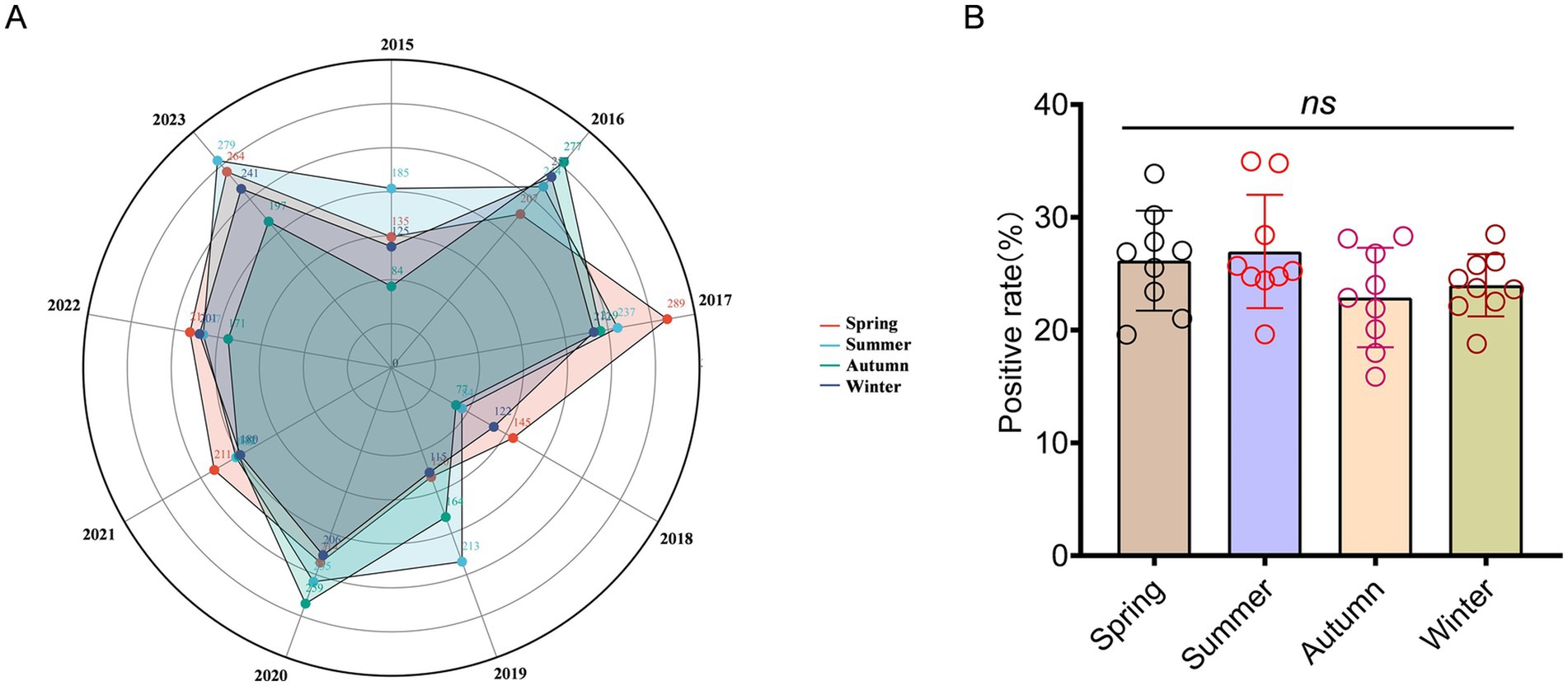
Figure 2. Seasonal distribution patterns of patients infected with A. baumannii. (A) The radius of a circle represents the number of positive patients, while solid circles of varying hues symbolize different seasons. (B) The distribution of seasonal groups among patients infected with A. baumannii. In (B), data were shown as mean ± standard deviation. Differences were assessed using two-tailed t-test.
Socio-demographic characteristics
Among the positive patients, there were 4,842 males and 2,102 females, resulting in a male to female ratio of 2.3:1. To examine the significant association between infection rate and gender, we performed a Fisher’s exact test. The overall results indicate a statistically significant difference in A. baumannii infection rates (case counts) between males and females (p < 0.001). Among them, the average annual number of infections in males was 538 (95% Cl: 431 to 645), in females was 234 (95% Cl: 172 to 295). The annual statistical data was presented in Table 1. This result indicated that there was a higher susceptibility among males.
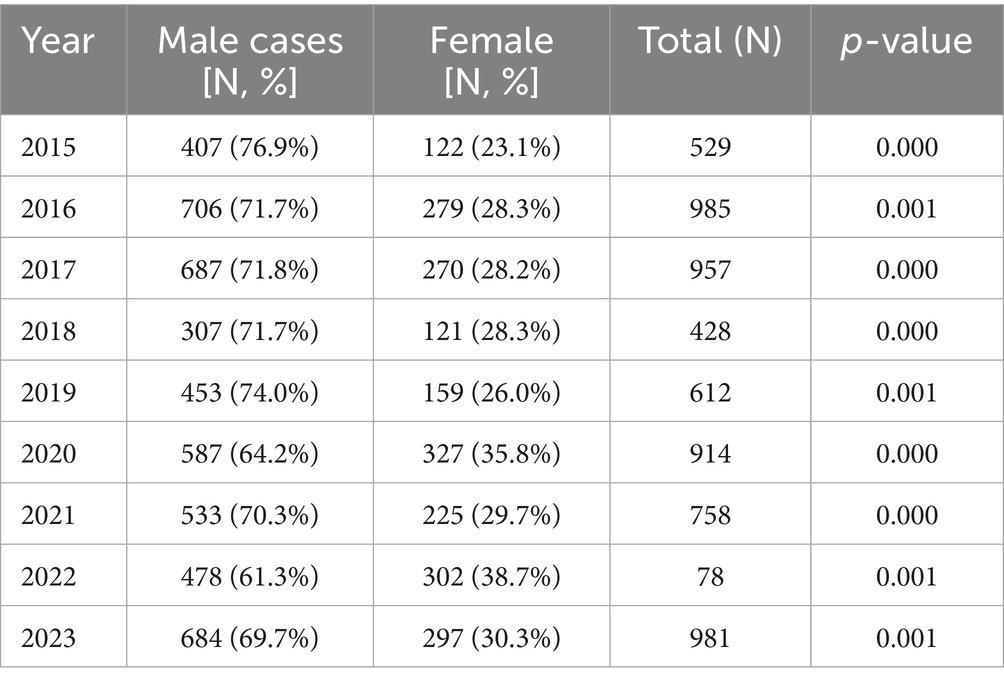
Table 1. Trends in the male-to-female ratio of Acinetobacter baumannii infection in Guizhou province from 2015 to 2023.
Besides, a significant correlation between age and infection rate was observed (p < 0.001). The annual average infection rate in the four groups is: 64 (95% Cl: 42 to 87) in the age group of 0–14, 224 (95% Cl: 178 to 270) in the age group of 15–47, 236 (95% Cl: 186 to 285) in the age group of 48–63, and 248 (95% Cl: 189 to 307) in the age group over 64. However, the incidence rate of positive cases just in the 0–14 age group exhibited a significantly lower trend compared to other age groups, and this difference was statistically significant (p = 3.41E-6; p = 1.30E-6; p = 3.77E-6; Figures 3A–E). Among the confirmed positive patients, only 8.3% were infected within the age group of 0–14, while individuals aged 15–47, 48–63, and over 64 accounted for 29.0, 30.5, and 32.2%, respectively. The prevalence among individuals aged 0–14 remained relatively low. In 2017, only 3.1% of positive cases were in the 0–14 age group, while in 2016, individuals aged over 64 represented 35.1% of positive cases.
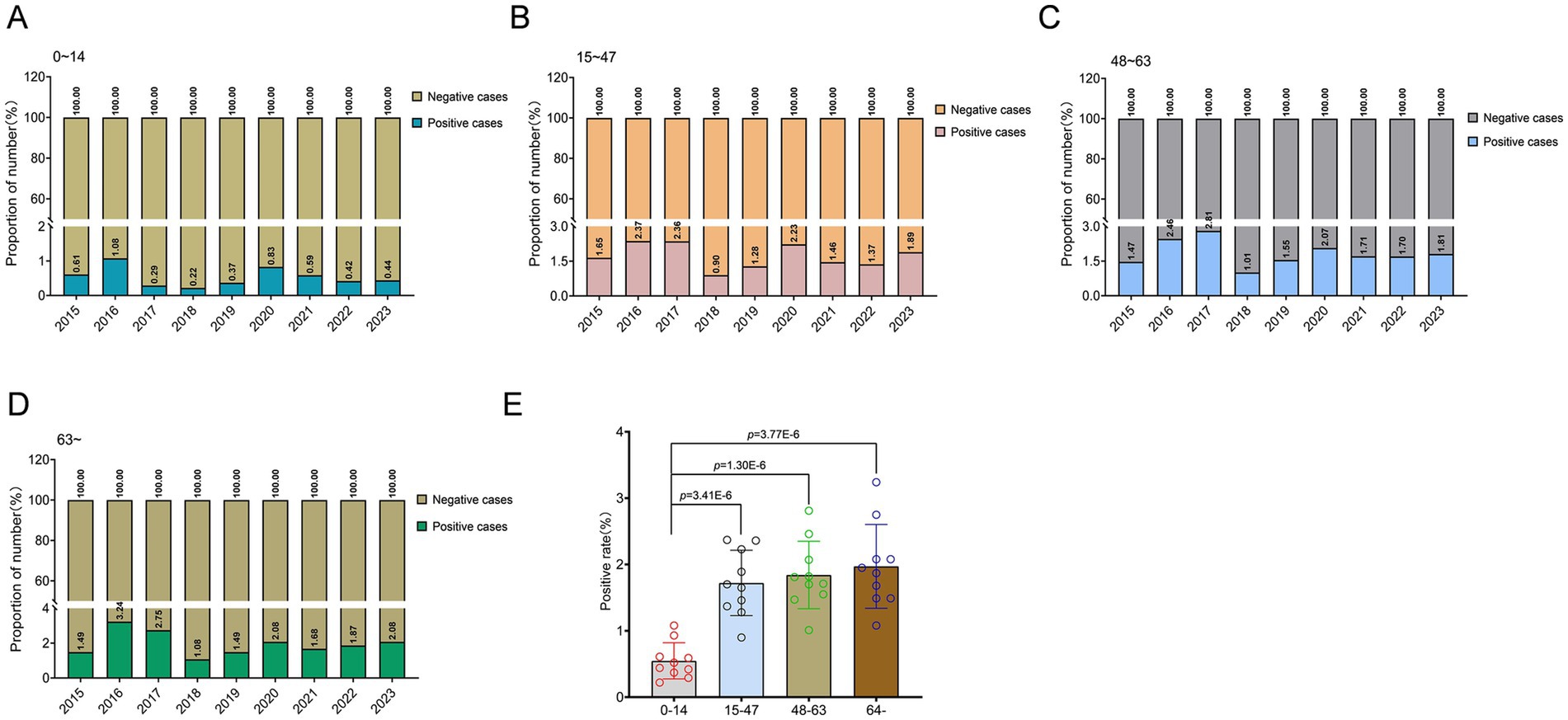
Figure 3. Age-specific variations in A. baumannii infection patterns in Guizhou Province from 2015 to 2023. (A–D) Epidemiological analysis of A. baumannii infection across different age cohorts in Guizhou province. (E) The distribution of age groups among patients infected with A. baumannii. In (E), data were shown as mean ± standard deviation. Differences were assessed using two-tailed t-test.
Acinetobacter baumannii in ICUs and antibiotic resistance
Of all confirmed A. baumannii cases, 45.77% occurred in ICU patients. The observed ICU incidences in 2015, 2022, and 2023 were remarkably high, with values of 50.66, 55.77, and 52.29% respectively, all surpassing the threshold of 50% (Figure 4A). The number of positive infections in ICUs exhibited a consistent decline from 2017 to 2019, with only 34.15% recorded in 2019, followed by a subsequent upward trend. Meanwhile, the prevalence of A. baumannii infections among ICU patients is remarkably high, reaching 27.05%. Particularly in 2016 and 2017 (Figure 4B).
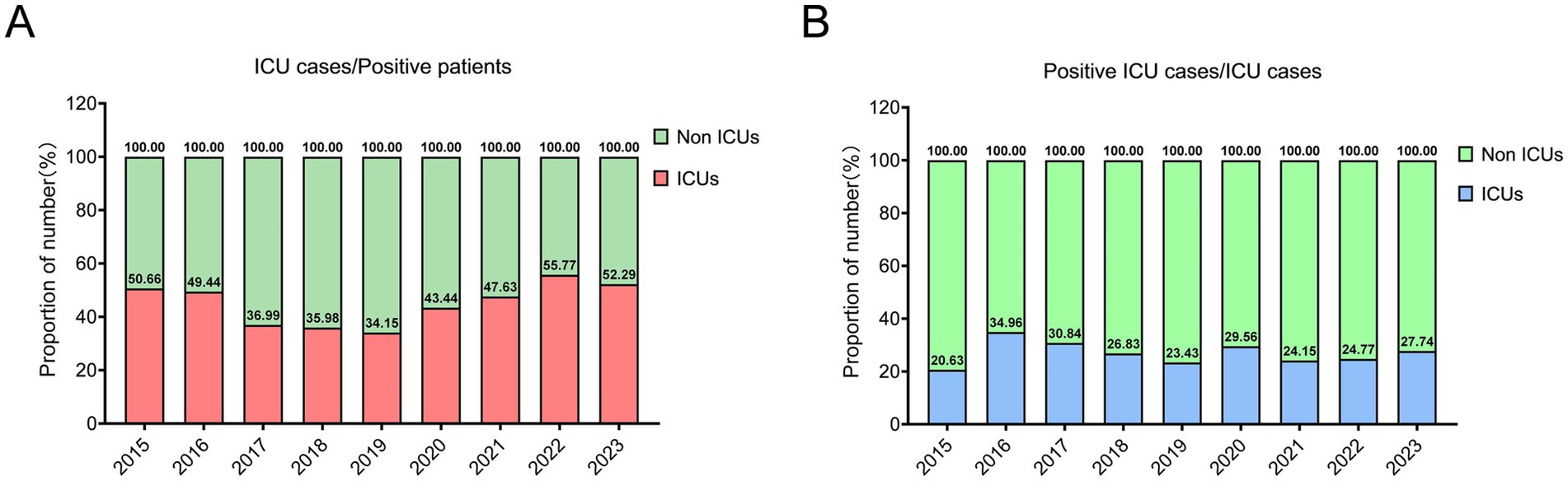
Figure 4. The epidemiological pattern of A. baumannii infection within the intensive care units (ICUs). (A) The ratio of ICU patients to the total number of positive patients. (B) The prevalence of A. baumannii infection among patients in the intensive care unit (ICU).
The majority of the tested antibiotics here exhibited limited efficacy against A. baumannii. The statistical results indicate that a significant proportion of the subjects infected with A. baumannii, ranging from 80% to even 95%, exhibit resistance toward antibiotics such as ampicillin, cefotetan, and cefazolin. However, piperacillin, gentamicin, and amikacin demonstrate ineffectiveness in over 50% of patients. The antibiotic Polymyxin B is widely recognized as a potent and broad-spectrum agent, exhibiting remained effective against A. baumannii in infected patients. Among the 3,556 subjects tested positive, infection control was achieved in 3456 individuals, underscoring the promising potential of polymyxin B for future treatment strategies targeting A. baumannii (Table 2).
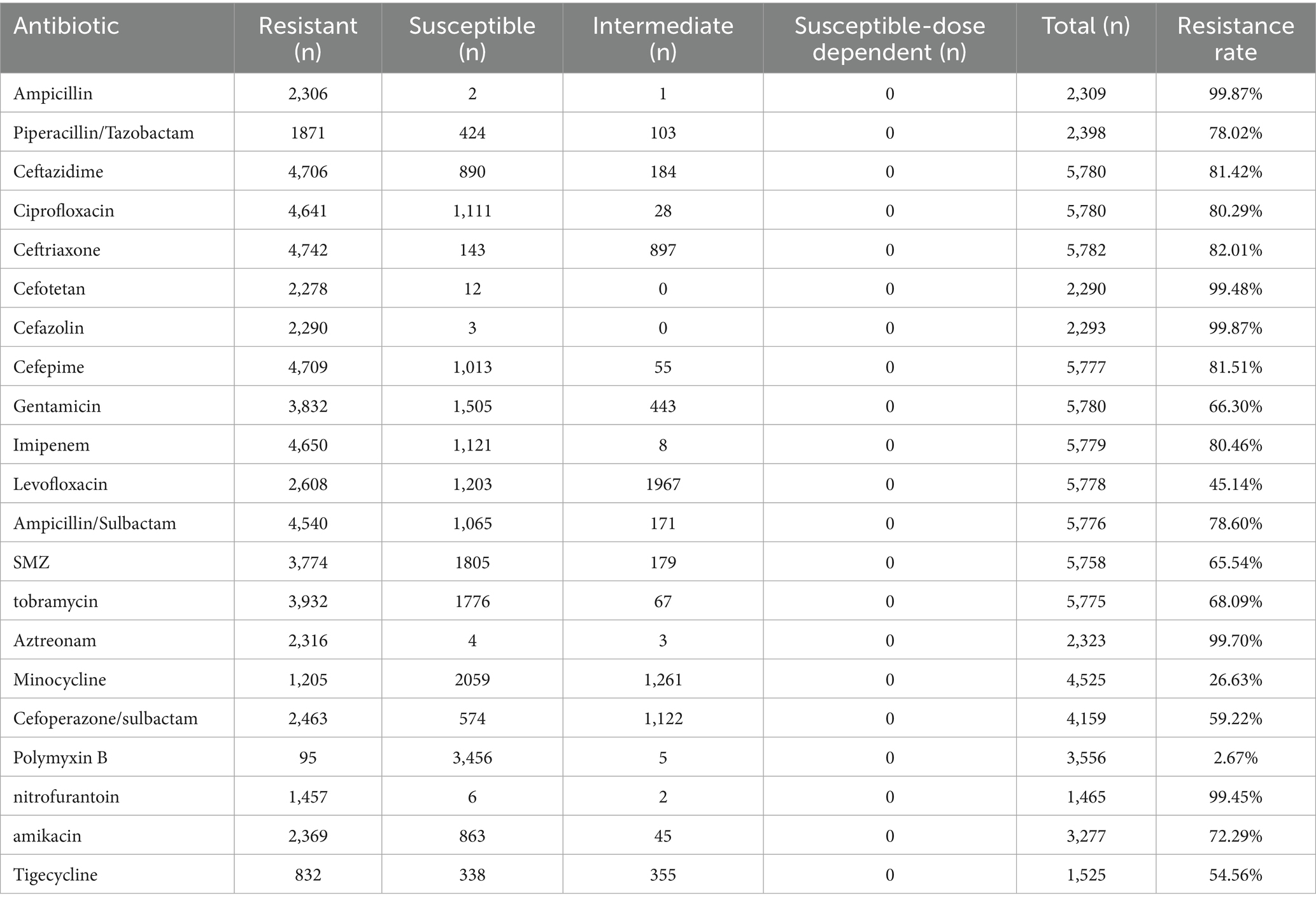
Table 2. The antibiotic resistance profile of A. baumannii against commonly prescribed medical antibiotics.
Discussion
The emergence and dissemination of A. baumannii in healthcare settings have become a significant public health concern (34, 35). This opportunistic pathogen has gained notoriety due to its capacity to induce severe infections, its persistence in hospital environments, and, most alarmingly, its rapid acquisition of antimicrobial resistance. The present study is based on the documented cases of A. baumannii infection within the healthcare system of Guizhou province from 2015 to 2023. A total of 6,944 positive cases of A. baumannii infection were identified among 460,620, resulting in an overall low positivity rate of 1.51% examined patients. Among the infection risk factors, age and gender have been identified as predominant contributors. Moreover, the incidence of A. baumannii infections in the ICUs remains elevated, presenting a substantial challenge for clinical management. Apart from polymyxin B, most commonly employed antibiotics in clinical practice demonstrate limited efficacy, thereby complicating infection prevention and control strategies within the ICU environment.
Throughout the entire duration of the statistical period, the prevalence of A. baumannii infections within the entire healthcare system of Guizhou is relatively low; however, the overall detection rate remains high. Among hospitalized patients infected for various reasons, the incidence of A. baumannii infections is notably elevated, highlighting its significant infectivity and potential susceptibility in immunocompromised populations. There is a notable surge in infection rates during 2016 and 2017, followed by a subsequent decline, suggesting the probable occurrence of localized outbreaks of A. baumannii within these 2 years. This might be attributed to inadequate medical prevention and control measures, or abnormal changes in the regional environmental climate facilitating the dissemination of pathogens. The seasonal distribution of A. baumannii infection reveals a higher incidence of susceptibility during warm spring and summer months, while infection events are relatively fewer in winter. This observation also suggests the potential preference of A. baumannii for high temperature environment. Despite a discernible seasonal preference for infection, no statistically significant difference was observed across the entire study period. Previous studies have indicated that A. baumannii may exhibit seasonal preferences; however, the findings presented here differ, potentially due to regional selection pressures. The seasonal climate changes in Guizhou might not substantially facilitate its transmission. Certainly, the necessity of intensifying pathogen detection and epidemic prevention measures were also needed during vulnerable periods, while concurrently providing enhanced care for susceptible populations.
Notably, the proportion of male individuals among infected patients is significantly higher than that of females, which implies that males are more prone to becoming susceptible populations. The data encompassing the past 9 years unequivocally highlight this striking disparity. Gender has emerged as a significant risk factor for A. baumannii infection. This could potentially be attributed to disparities in the working environment, internal physiological conditions, and even genetic variations between males and females. However, the question of whether this sex predisposition stems from environment, physiological or genetic factors remains to be fully elucidated through more in-depth experimental investigation. Age emerges as a pivotal risk factor for A. baumannii infection. Among the individuals who tested positive, the prevalence of patients aged 0–14 is significantly lowers compared to other age groups. The incidence of infection is slightly higher in the population aged over 64 compared to other age groups. A positive correlation between infection events and increasing age is observed. The older adult population is particularly vulnerable to A. baumannii infection, potentially attributed to immunosenescence and heightened healthcare exposure. The elevated incidence of comorbidities and more frequent visits to medical institutions have significantly heightened the susceptibility risk.
The ICUs are equipped with isolation facilities and specialized equipment to provide optimal care, comprehensive treatment, and ancillary services for critically ill or comatose patients (36–38). The study observed a notable concentration of infected individuals in ICUs, including both community-onset and hospital-acquired cases. As commonly acknowledged, ICU equipment and the surrounding environment undergo regular sterilization and disinfection; however, this scenario still gives rise to instances of A. baumannii infection, highlighting its formidable resistance. There have been reports indicating the ability of A. baumannii to form biofilms on medical devices, including catheters, tracheas, and intubation equipment, thereby posing significant challenges in eradication and elevating the risk of nosocomial infections (39, 40). Here, out of over 40 antibiotics investigated, only polymyxin B demonstrates potent inhibitory activity against A. baumannii (97.33%), while the majority exhibit limited or no efficacy. The formidable drug resistance and intricate mechanisms of resistance pose a significant medical challenge in combating A. baumannii (41, 42). Carbapenem resistance in A. baumannii limits the efficacy of imipenem and meropenem in ICU settings, contributing to treatment challenges. Furthermore, with the evolution of drug-resistant strains, the efficacy of individual polymyxin B agents may be limited. Consequently, there is an urgent imperative to discover or develop more potent pharmaceutical formulations.
This study is subject to several limitations. Considering China’s expansive territory, the nation’s climate, environment, and other conditions display a remarkable spectrum of diversity that may not be adequately captured by this localized focus. Moreover, the geographical restriction might limit generalizability, such as changes in air quality attributable to altitude or changes in temperature attributable to seasonal variations. Besides, these variations can exhibit profound differences on a global scale. Furthermore, in the realm of data collection and analysis, age and gender were identified as salient risk factors for A. baumannii infection. However, this study did not delve into whether these factors operate independently or synergistically to influence infection rates. This oversight could potentially undermine the formulation of subsequent epidemic prevention policies and the identification of key populations that warrant heightened attention. Age and gender may act independently or synergistically, and multivariable analysis or interaction models may be needed established as a potential future approach for A. baumannii infection analysis. The dependence on historical data extracted from medical records might introduce potential biases, whereas the lack of molecular information significantly hampers our capacity to precisely elucidate the intricate transmission dynamics. Also, the lack of molecular data could restrict conclusions on transmission or resistance mechanisms, which may impose certain limitations on the development of more effective antibiotics and molecular drugs via molecular medical engineering. A more comprehensive integration of molecular biology tools will generate richer, more robust, and profoundly insightful scientific data. In order to achieve a more comprehensive understanding of the epidemiology of A. baumannii, it is advisable to complement traditional epidemiological statistical analyses with molecular biology methods, such as genomics. This integrated approach not only highlights regional infection differences but also offers deeper insights into the molecular basis of these variations.
Conclusion
Not only do ICUs account for the majority of positive cases, but also the significant incidence of A. baumannii infections among ICU-admitted patients within Guizhou province. These findings suggest that effective suppression methods for A. baumannii, such as antimicrobial stewardship, environmental cleaning and hand hygiene, compliance are still lacking in ICU system of Guizhou province, highlighting a significant burden. Moreover, A. baumannii exhibits remarkably high drug resistance to some antibiotics, such as carbapenems and aminoglycosides, and the availability of effective antibiotics in the collected data is limited (just polymyxin B). Overall, there has been limited progress in the prevention and control of A. baumannii in Guizhou province over the past 9 years, particularly concerning key populations, such as older adults, immunocompromised, and ICU patients. Efforts such as implementing molecular surveillance, optimizing infection control policies, and conducting multicentre studies remain insufficient or are progressing slowly. In summary, further enhancement of overall efforts toward drug development for prevention and treatment may be needed.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval were waived for this study, because it did not involve humans or animals. The focus of this article is solely on the analysis of statistical data, without addressing any concerns related to patient privacy.
Author contributions
ZL: Conceptualization, Writing – original draft. DT: Writing – review & editing, Formal analysis. YH: Writing – review & editing, Formal analysis. SD: Formal analysis, Writing – review & editing. AY: Writing – review & editing, Formal analysis. ZC: Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Harding, CM, Hennon, SW, and Feldman, MF. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol. (2018) 16:91–102. doi: 10.1038/nrmicro.2017.148
2. Antunes, LC, Visca, P, and Towner, KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. (2014) 71:292–301. doi: 10.1111/2049-632X.12125
3. Wang, X, Trent, MS, and Davies, BW. Desiccation tolerance assays for Acinetobacter baumannii. Methods Mol Biol. (2019) 1946:189–94. doi: 10.1007/978-1-4939-9118-1_18
4. Choudhary, M, Shrivastava, R, and Vashistt, J. Acinetobacter baumannii biofilm formation: association with antimicrobial resistance and prolonged survival under desiccation. Curr Microbiol. (2022) 79:361. doi: 10.1007/s00284-022-03071-5
5. Monem, S, Furmanek-Blaszk, B, Łupkowska, A, Kuczyńska-Wiśnik, D, Stojowska-Swędrzyńska, K, and Laskowska, E. Mechanisms protecting Acinetobacter baumannii against multiple stresses triggered by the host immune response, antibiotics and outside-host environment. Int J Mol Sci. (2020) 21:5498. doi: 10.3390/ijms21155498
6. Milani, ES, Hasani, A, Varschochi, M, Sadeghi, J, Memar, MY, and Hasani, A. Biocide resistance in Acinetobacter baumannii: appraising the mechanisms. J Hosp Infect. (2021) 117:135–46. doi: 10.1016/j.jhin.2021.09.010
7. Smitran, A, Lukovic, B, Bozic, L, Jelic, D, Jovicevic, M, Kabic, J, et al. Carbapenem-resistant Acinetobacter baumannii: biofilm-associated genes, biofilm-eradication potential of disinfectants, and biofilm-inhibitory effects of selenium nanoparticles. Microorganisms. (2023) 11:171. doi: 10.3390/microorganisms11010171
8. Ivanković, T, Goić-Barišić, I, and Hrenović, J. Reduced susceptibility to disinfectants of Acinetobacter baumannii biofilms on glass and ceramic. Arh Hig Rada Toksikol. (2017) 68:99–108. doi: 10.1515/aiht-2017-68-2946
9. Ibrahim, S, Al-Saryi, N, Al-Kadmy, IMS, and Aziz, SN. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol Biol Rep. (2021) 48:6987–98. doi: 10.1007/s11033-021-06690-6
10. Shahid, F, Ashraf, ST, and Ali, A. Reverse vaccinology approach to potential vaccine candidates against Acinetobacter baumannii. Methods Mol Biol. (2019) 1946:329–36. doi: 10.1007/978-1-4939-9118-1_29
11. Oyejobi, GK, Olaniyan, SO, Yusuf, NA, Ojewande, DA, Awopetu, MJ, Oyeniran, GO, et al. Acinetobacter baumannii: more ways to die. Microbiol Res. (2022) 261:127069. doi: 10.1016/j.micres.2022.127069
12. Ainsworth, S, Ketter, PM, Yu, JJ, Grimm, RC, May, HC, Cap, AP, et al. Vaccination with a live attenuated Acinetobacter baumannii deficient in thioredoxin provides protection against systemic Acinetobacter infection. Vaccine. (2017) 35:3387–94. doi: 10.1016/j.vaccine.2017.05.017
13. Ko, TP, Lai, SJ, Hsieh, TJ, Yang, CS, and Chen, Y. The tetrameric structure of sialic acid-synthesizing UDP-Glc NAc 2-epimerase from Acinetobacter baumannii: a comparative study with human GNE. J Biol Chem. (2018) 293:10119–27. doi: 10.1074/jbc.RA118.001971
14. Rafailidis, P, Panagopoulos, P, Koutserimpas, C, and Samonis, G. Current therapeutic approaches for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii infections. Antibiotics (Basel). (2024) 13:261. doi: 10.3390/antibiotics13030261
15. Karakonstantis, S, Gikas, A, Astrinaki, E, and Kritsotakis, EI. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J Hosp Infect. (2020) 106:447–53. doi: 10.1016/j.jhin.2020.09.009
16. Piperaki, ET, Tzouvelekis, LS, Miriagou, V, and Daikos, GL. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect. (2019) 25:951–7. doi: 10.1016/j.cmi.2019.03.014
17. Betrosian, AP, Frantzeskaki, F, Xanthaki, A, and Douzinas, EE. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Inf Secur. (2008) 56:432–6. doi: 10.1016/j.jinf.2008.04.002
18. Jean, SS, Liu, CY, Huang, TY, Lai, CC, Liu, IM, Hsieh, PC, et al. Potentially effective antimicrobial treatment for pneumonia caused by isolates of carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii complex species: what can we expect in the future? Expert Rev Anti-Infect Ther. (2024) 22:1171–87. doi: 10.1080/14787210.2024.2412637
19. Jung, SY, Lee, SH, Lee, SY, Yang, S, Noh, H, Chung, EK, et al. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: a systemic review and Bayesian network meta-analysis. Crit Care. (2017) 21:319. doi: 10.1186/s13054-017-1916-6
20. Silva Santos, K, Barbosa, AM, Pereira da Costa, L, Pinheiro, MS, Oliveira, MB, and Ferreira, PF. Silver nanocomposite biosynthesis: antibacterial activity against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii. Molecules. (2016) 21:1255. doi: 10.3390/molecules21091255
21. Ananda, T, Modi, A, Chakraborty, I, Managuli, V, Mukhopadhyay, C, and Mazumder, N. Nosocomial infections and role of nanotechnology. Bioengineering (Basel). (2022) 9:51. doi: 10.3390/bioengineering9020051
22. Iesari, S, Lai, Q, Rughetti, A, Dell'Orso, L, Clemente, K, Famulari, A, et al. Infected nonhealing wound in a kidney transplant recipient: successful treatment with topical homologous platelet-rich gel. Exp Clin Transplant. (2017) 15:222–5. doi: 10.6002/ect.2014.0236
23. Ayoub Moubareck, C, and Hammoudi Halat, D. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics. (2020) 9:119. doi: 10.3390/antibiotics9030119
24. Chen, Y, Liu, L, and Zhu, M. Intraventricular administration of antibiotics by ommaya reservoir for patients with multidrug-resistant Acinetobacter baumannii central nervous system infection. Br J Neurosurg. (2021) 35:170–3. doi: 10.1080/02688697.2020.1777255
25. Khawcharoenporn, T, Apisarnthanarak, A, and Mundy, LM. Intrathecal colistin for drug-resistant Acinetobacter baumannii central nervous system infection: a case series and systematic review. Clin Microbiol Infect. (2010) 16:888–94. doi: 10.1111/j.1469-0691.2009.03019.x
26. Liang, W, Yuan-Run, Z, and Min, Y. Clinical presentations and outcomes of post-operative central nervous system infection caused by multi-drug-resistant/extensively drug-resistant Acinetobacter baumannii: a retrospective study. Surg Infect. (2019) 20:460–4. doi: 10.1089/sur.2018.286
27. Aguirre-Avalos, G, Mijangos-Méndez, JC, and Amaya-Tapia, G. Bacteriemia por Acinetobacter baumannii [Acinetobacter baumannii bacteremia]. Rev Med Inst Mex Seguro Soc. (2010) 48:625–34.
28. Garnacho-Montero, J, Amaya-Villar, R, Ferrándiz-Millón, C, Díaz-Martín, A, López-Sánchez, JM, and Gutiérrez-Pizarraya, A. Optimum treatment strategies for carbapenem-resistant Acinetobacter baumannii bacteremia. Expert Rev Anti-Infect Ther. (2015) 13:769–77. doi: 10.1586/14787210.2015.1032254
29. Nguyen, M, and Joshi, SG. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: a scientific review. J Appl Microbiol. (2021) 131:2715–38. doi: 10.1111/jam.15130
30. Dehbanipour, R, and Ghalavand, Z. Acinetobacter baumannii: pathogenesis, virulence factors, novel therapeutic options and mechanisms of resistance to antimicrobial agents with emphasis on tigecycline. J Clin Pharm Ther. (2022) 47:1875–84. doi: 10.1111/jcpt.13787
31. Garlantézec, R, Bourigault, C, Boles, JM, Prat, G, Baron, R, Tonnelier, JM, et al. Investigation and management of an imipenem-resistant OXA-23 Acinetobacter baumannii outbreak in an intensive care unit. Med Mal Infect. (2011) 41:430–6. doi: 10.1016/j.medmal.2011.01.013
32. Dahdouh, E, Hajjar, M, Suarez, M, and Daoud, Z. Acinetobacter baumannii isolated from Lebanese patients: phenotypes and genotypes of resistance, Clonality, and determinants of pathogenicity. Front Cell Infect Microbiol. (2016) 6:163. doi: 10.3389/fcimb.2016.00163
33. Benin, BM, Hillyer, T, Aguirre, N, Sham, YY, Willard, B, and Shin, WS. Carbapenem-induced β-lactamase-isoform expression trends in Acinetobacter baumannii. Sci Rep. (2024) 14:30841. doi: 10.1038/s41598-024-81501-z
34. Doi, Y. Treatment options for Carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis. (2019) 69:S565. doi: 10.1093/cid/ciz830
35. Mea, HJ, Yong, PVC, and Wong, EH. An overview of Acinetobacter baumannii pathogenesis: motility, adherence and biofilm formation. Microbiol Res. (2021) 247:126722. doi: 10.1016/j.micres.2021.126722
36. De Jong, A, Wrigge, H, Hedenstierna, G, Gattinoni, L, Chiumello, D, Frat, JP, et al. How to ventilate obese patients in the ICU. Intensive Care Med. (2020) 46:2423–35. doi: 10.1007/s00134-020-06286-x
37. Jonkman, AH, de Vries, HJ, and Heunks, LMA. Physiology of the respiratory drive in ICU patients: implications for diagnosis and treatment. Crit Care. (2024) 28:94. doi: 10.1186/s13054-020-2776-z
38. Avdalovic, MV, and Marcin, JP. When will telemedicine appear in the ICU? J Intensive Care Med. (2019) 34:271–6. doi: 10.1177/0885066618775956
39. Eze, EC, Chenia, HY, and El Zowalaty, ME. Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect Drug Resist. (2018) 11:2277–99. doi: 10.2147/IDR.S169894
40. Cavallo, I, Oliva, A, Pages, R, Sivori, F, Truglio, M, Fabrizio, G, et al. Acinetobacter baumannii in the critically ill: complex infections get complicated. Front Microbiol. (2023) 14:1196774. doi: 10.3389/fmicb.2023.1196774
41. Kyriakidis, I, Vasileiou, E, Pana, ZD, and Tragiannidis, A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens. (2021) 10:373. doi: 10.3390/pathogens10030373
Keywords: Acinetobacter baumannii , epidemiological characteristics, Guizhou province, correlation, multi-drug resistance
Citation: Liang Z, Tian D, Huang Y, Dou S, Yang A and Chen Z (2025) Epidemiology of Acinetobacter baumannii: analysis of hazard factors associated with positivity cases in Guizhou province, China from 2015 to 2023. Front. Public Health. 13:1592783. doi: 10.3389/fpubh.2025.1592783
Edited by:
Kok Keng Tee, Kunming Medical University, ChinaReviewed by:
Sakiusa Cabe Baleivanualala, Fiji National University, FijiSusan Ibrahim, Mustansiriyah University, Iraq
Copyright © 2025 Liang, Tian, Huang, Dou, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuyi Chen, Y2hlbnp1eWlsaUAxNjMuY29t
†These authors have contributed equally to this work
 Zhongzhi Liang1†
Zhongzhi Liang1† Zuyi Chen
Zuyi Chen