- 1College of Pharmacy, Xavier University of Louisiana, New Orleans, LA, United States
- 2Elaine P Nunez College, Chalmette, LA, United States
- 3START Corporation, Covington, LA, United States
- 4C & S Family Pharmacy, Metairie, LA, United States
- 5Methodist Clinic, St. Bernard, LA, United States
- 6Independent Researcher, Bridgetown, Barbados
- 7Yale School of Medicine, Yale University, New Haven, CT, United States
- 8Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States
Introduction: Respiratory diseases, including influenza (flu) and respiratory syncytial virus, continue to be major health concerns globally. The onset of COVID-19 further compounded these issues, making it important to examine public attitudes toward vaccination and understanding of respiratory diseases. This study explores factors influencing decisions to receive the latest COVID-19 vaccine, focusing specifically on the role of prior respiratory illness diagnosis.
Methods: A follow-up survey among 299 participants from Southeastern Louisiana across 10 healthcare facilities was administered via phone and the Qualtrics platform, gathering information about the likelihood of receiving the latest COVID-19 vaccine. Quantitative data were analyzed using log-binomial and Poisson regression models to assess relationships between respiratory illness history and COVID-19 vaccine acceptance.
Results: Nearly half (47%) of the participants reported a history of respiratory illness. Individuals with prior respiratory diagnosis were more likely to accept the latest COVID-19 vaccine (62%) than those without (41%) (RR: 1.79, 95% CI: 1.26–2.56). In fully adjusted models, accounting for age, prior vaccine hesitancy, and comorbidities, influenza vaccine acceptance (RR: 1.87, 95% CI: 1.06–3.28) was associated with greater likelihood of receiving the latest COVID-19 vaccine. Key barriers to vaccination, including concerns about side effects and distrust in vaccine efficacy were identified.
Discussion: Participants with respiratory illnesses and those with positive vaccination histories, particularly regarding influenza, showed a higher likelihood of accepting the latest COVID-19 vaccines. However, significant obstacles to vaccine uptake persist. Tailored public health efforts that address these concerns are crucial to improving vaccine rates, particularly among hesitant groups.
Introduction
Respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), influenza, and respiratory syncytial virus (RSV), have long posed significant challenges for global public health, accounting for millions of deaths each year (1, 2). The COVID-19 pandemic added complexity, overwhelming healthcare systems already burdened by respiratory conditions (3). As the pandemic ended, understanding public perceptions of these respiratory diseases in conjunction with COVID-19 vaccination efforts became critical for developing future public health strategies.
Co-infections, where a patient is simultaneously infected with more than one pathogen, have been a persistent issue in the management of respiratory diseases. Historically, co-infections have been linked to worsened clinical outcomes and increased complications in treatment (4, 5). This issue gained even more importance during the COVID-19 pandemic, where patients infected with both SARS-CoV-2 and other respiratory pathogens presented significant diagnostic and treatment challenges (2). Despite these complexities, there has been minimal focus on how public awareness of these co-infections might influence behavior, particularly regarding vaccine uptake.
Several studies have highlighted the impact of co-infections on respiratory disease management during the pandemic. In Ethiopia, researchers frequently detected SARS-CoV-2 co-infections with influenza and RSV, further complicating the treatment of respiratory illnesses (6). Similarly, non-pharmacological interventions (NPIs) such as mask-wearing and social distancing were effective in reducing the spread of certain viruses, but others, like rhinovirus, continued to circulate among children (7). In Arkansas, NPIs also significantly reduced pediatric infections caused by Mycoplasma pneumoniae and other respiratory viruses during the pandemic (8). The effectiveness of NPIs in reducing respiratory viruses such as RSV and influenza, particularly in children, raised critical questions about their role, compared to vaccines, in controlling outbreaks (9, 10).
While efforts such as NPIs and vaccination campaigns have been implemented to lessen the spread of respiratory viruses (11), significant gaps remain in understanding how public perception of respiratory diseases impacts vaccine behavior. Specifically, while research has largely focused on clinical outcomes of co-infections, there is limited exploration of how public awareness of respiratory diseases might influence health behaviors, including vaccine uptake (12, 13). This gap in research limits the ability of healthcare systems to fully address the complexities of managing multiple respiratory pathogens during future outbreaks.
In the United States, including Louisiana, individuals with chronic respiratory diseases such as asthma and COPD are strongly recommended to receive routine vaccinations, including the annual influenza and pneumococcal vaccines (e.g., PCV20 or a sequential schedule of PCV15 followed by PPSV23). These vaccines are crucial in reducing the risk of severe illness and complications. However, national and regional data show variable uptake among these groups, often influenced by individual risk perception and access to care (14, 15). This study aims to explore how individuals with respiratory diseases perceive the COVID-19 vaccine and how these perceptions influence their vaccination decisions. Given the ongoing spread of respiratory pathogens, understanding how public perception affects vaccination decisions could be crucial for enhancing future public health responses. The findings will provide insights into how public awareness of respiratory conditions influences vaccine behavior, ultimately contributing to more effective public health strategies during respiratory disease outbreaks.
Materials and methods
Study design and participants
This study employed a community based participatory research approach by engaging community healthcare partners from Southeastern Louisiana. This report was the final follow-up in a longitudinal survey which assessed participants’ likelihood of keeping up to date with the COVID-19 vaccine series, i.e., receiving the latest COVID-19 vaccine, with a focus on how perceptions of respiratory illness influenced decision-making. Initially, the baseline survey identified varying levels of vaccine acceptance, influenced by demographic factors and previous health experiences, laying the groundwork for understanding shifts in attitudes over time (16). A total of 299 participants, who were part of the baseline survey, were included. A total of 79.3% of recipients were retained from baseline, with over 90% of follow-up responses occurring via in-person follow-up. Participants were recruited from 10 healthcare locations across Southeastern Louisiana, including pharmacies and clinics serving rural and marginalized communities. Data collection for this phase focused on participants’ likelihood of getting the latest COVID-19 vaccine and their perspectives on respiratory diseases.
Data collection
Trained pharmacists and healthcare workers conducted data collection in-person or through phone interviews, with surveys administered via the Qualtrics© platform (17). All baseline surveys were conducted in Summer and Fall 2022. All follow-up surveys were conducted within 12-months, from August through November 2023. The survey gathered self-reported quantitative data regarding participants’ likelihood of receiving the latest COVID-19 vaccine, along with information on respiratory illness diagnoses and other health-related concerns. All vaccine status information was validated by the healthcare worker via their health informatics system. The survey questions focused on identifying factors that influenced participants’ decision-making regarding vaccination and their general attitudes toward respiratory health.
Variable definitions
Surveys were designed to capture demographic characteristics, respiratory diagnosis history, chronic disease history, COVID-19 experiences and perceptions, including vaccination hesitancy, and other behavioral measures, via self-report. Demographic characteristics included age (17–49 years vs. 50 years and above), race (categorized as African American, Caucasian, or Other), gender (male vs. female), and area of residence (rural/semi-rural vs. suburban/city). Respiratory illness history included conditions such as flu, cold, allergy, sinus infection, ear infection, pneumonia, throat infection, RSV, strep throat, or other respiratory diseases except COVID-19 diagnosed between Fall 2022 and Fall 2023. Respiratory illness, whether physician diagnosed or self limiting requiring only over-the counter management, were included as one predictive variable without differentiation of severity of illness. This was done to ensure capturing all clinically relevant respiratory infections. Presence of chronic health conditions, such as diabetes, hypertension, or cardiovascular diseases, were also recorded. COVID-19 diagnosis captured whether participants had tested positive for COVID-19 during the study period. Post-vaccination COVID-19 infection assessed whether participants tested positive for COVID-19 after receiving a COVID-19 vaccine. The latest COVID-19 vaccine reflected the vaccination released within the cohort year of the participation. This study spanned vaccination releases in both 2022 and 2023. Latest vaccination was referenced according to the most current release at the time of the survey administration. Vaccination status was confirmed via pharmacy and clinic records, as well as patient self-report. The vaccine hesitancy score was calculated based on participants’ responses to questions addressing concerns about vaccine safety, efficacy, and the potential for side effects. Scores were categorized into tertiles: not hesitant, moderately hesitant, and most hesitant. Behavioral measures included previous refusal of any vaccines, likelihood of receiving an influenza vaccine and willingness to get vaccinated during a new pandemic or rapidly spreading infection. Participants’ opinions about COVID-19 as a public health risk were also evaluated. All survey instruments were drawn from previously validated tools in the research groups COVID-19 studies and are described in referenced publications (16).
Data analysis
The data were analyzed using STATA version 18 (18). Descriptive statistics such as frequencies and proportions were calculated to summarize demographic characteristics, responses about vaccine acceptance, and respiratory illness history. The primary outcome variable was the participants’ likelihood of receiving the latest COVID-19 vaccine. A log-binomial regression model was used to explore associations between respiratory illness diagnoses and the likelihood of vaccine uptake. In cases where model convergence issues occurred, Poisson regression was employed. The final model was adjusted for variables that might confound the relationship between respiratory illness and vaccine uptake. These adjustments included age, gender, race/ethnicity (African American, Caucasian, Others), vaccine hesitancy (not hesitant, moderately hesitant, most hesitant), prior vaccine refusal, history of COVID-19 diagnosis, perceptions of COVID-19 as a public health risk, and other health conditions. Such adjustments were critical to ensure the accuracy of our findings by mitigating potential biases and providing a clearer picture of the factors influencing vaccine uptake.
Results
A total of 299 participants were included in this final follow-up survey. Among them, 47% (141) reported a history of respiratory disease. The data in Table 1 show that there was a significant association between a previous COVID-19 diagnosis and a history of respiratory disease (RR: 1.49, 95% CI: 1.15–1.91, p = 0.002), where participants with a history of respiratory illness were more likely to have been diagnosed with COVID-19 during the study period. Participants diagnosed with respiratory illness were significantly more likely to report having chronic health conditions compared to those not diagnosed (67% vs. 46%; RR: 1.58, 95% CI: 1.21–2.06, p = 0.001). Additionally, significant differences were observed in participants’ likelihood to receive vaccinations, with a higher proportion of those diagnosed with respiratory illness indicating willingness to get the vaccine during a new pandemic or rapidly spreading infection (61% vs. 36%, RR: 1.71, 95% CI: 1.33–2.19, p < 0.001) and also indicating a higher likelihood of receiving the influenza vaccine (47% vs. 31%; RR: 1.41, 95% CI: 1.11–1.78, p = 0.004).
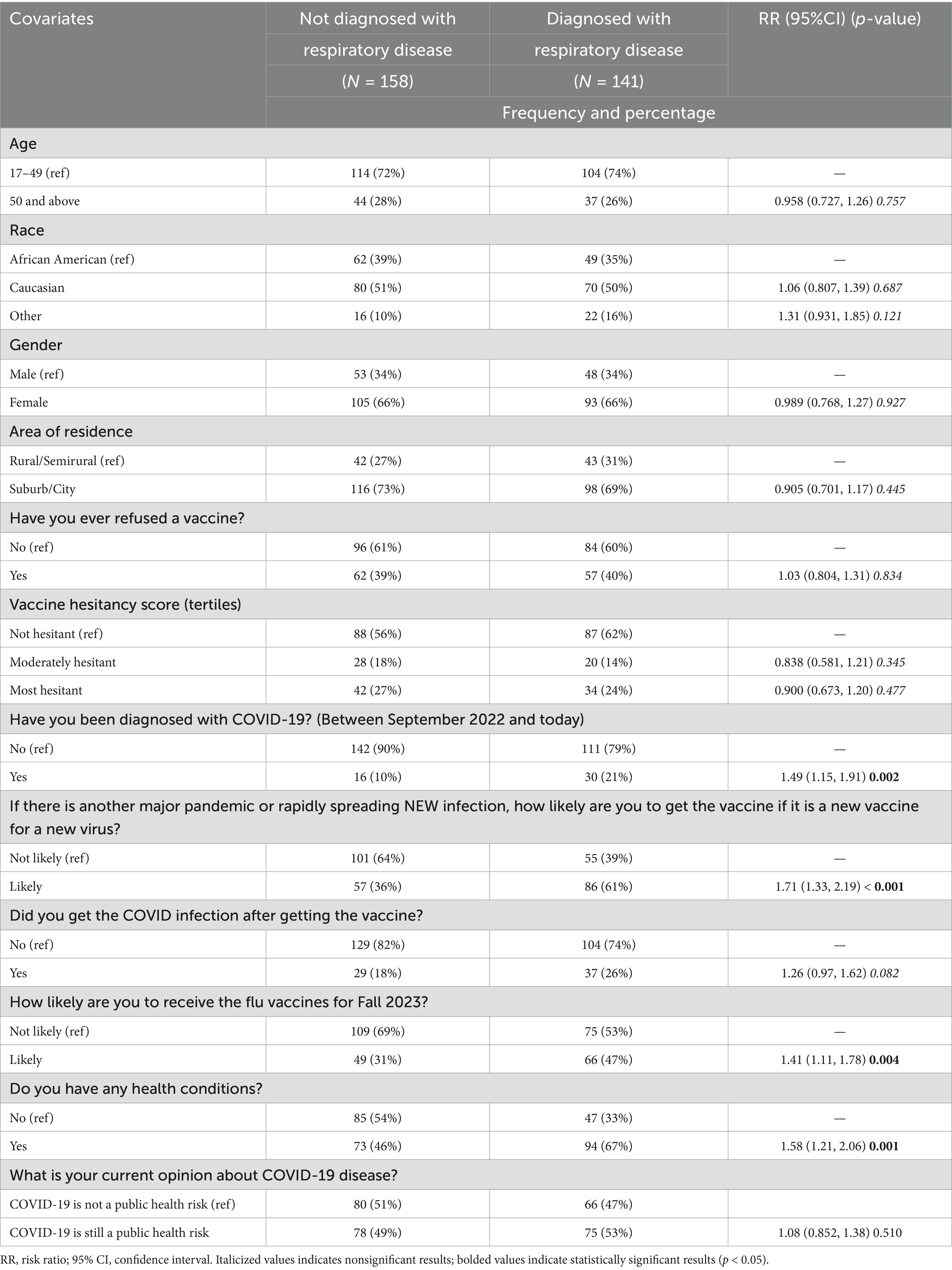
Table 1. Baseline characteristics of participants based upon exposure to respiratory disease diagnosis in Fall 2022 through Fall 2023.
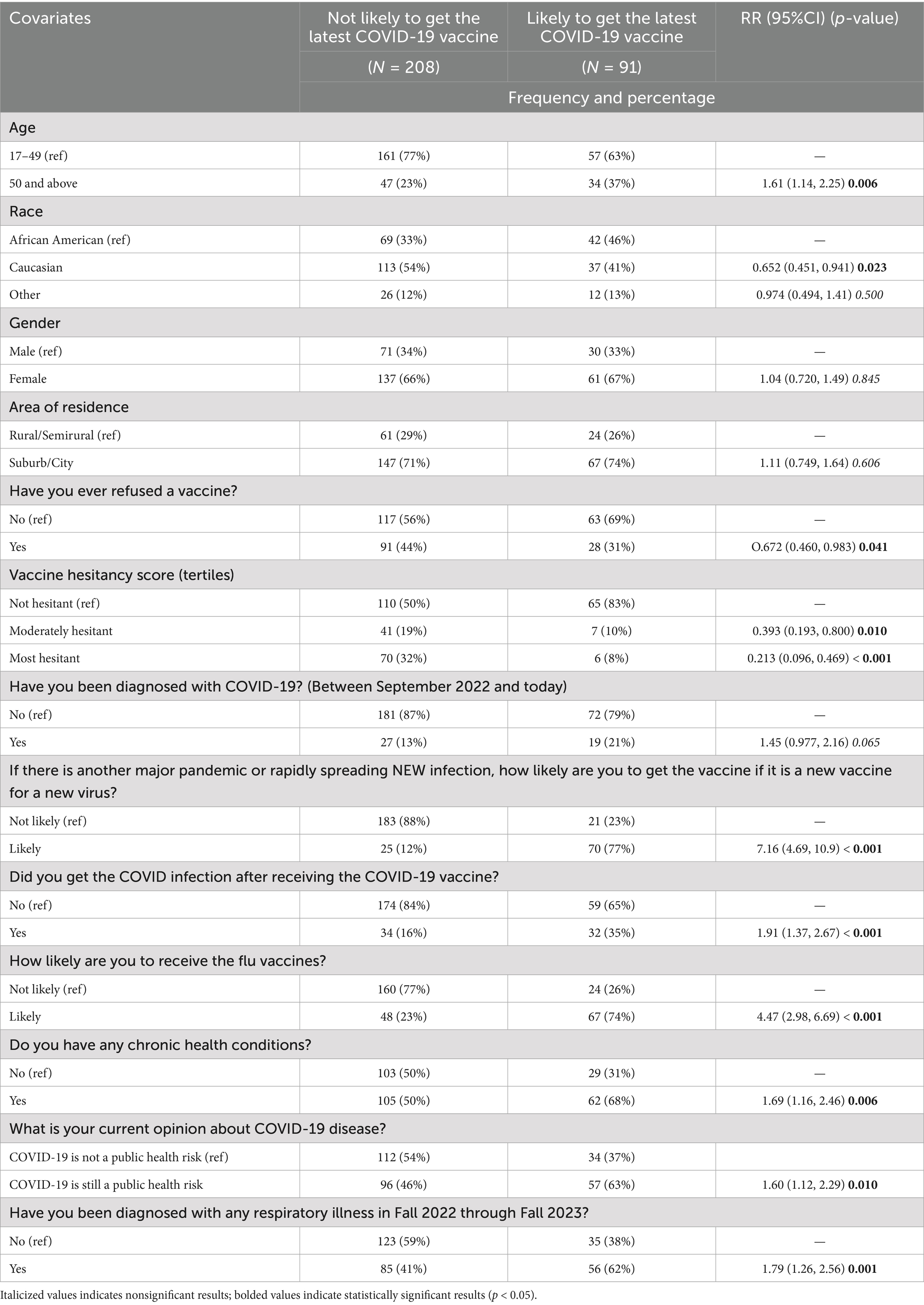
Table 2 shows that for participants aged 50 years and above, a significantly greater proportion (37% compared to 23%) were likely to get the latest COVID-19 vaccine compared to those aged under 50 years (RR: 1.61, 95% CI: 1.14–2.25, p = 0.006). Caucasians were less likely to accept the latest COVID-19 vaccine than African Americans (RR: 0.652, 95% CI: 0.451–0.941, p = 0.023). Participants with a history of refusing previous vaccines were also significantly less likely to accept the latest COVID-19 vaccine (RR: 0.672, 95% CI: 0.460–0.983, p = 0.041). Participants with moderate and high hesitancy scores showed significant associations with a lower likelihood of accepting the latest COVID-19 vaccine. Moderate hesitancy was associated with a reduced likelihood of vaccine acceptance (RR: 0.393, 95% CI: 0.193–0.800, p = 0.010), while high hesitancy further decreased the likelihood (RR: 0.213, 95% CI: 0.096–0.469, p < 0.001). Conversely, participants with a history of respiratory disease were more likely to accept the latest COVID-19 vaccine (RR: 1.79, 95% CI: 1.26–2.56, p = 0.001). Participants who were likely to get the influenza vaccine were also more likely to accept the latest COVID-19 vaccine (RR: 4.47, 95% CI: 2.98–6.69, p < 0.001).
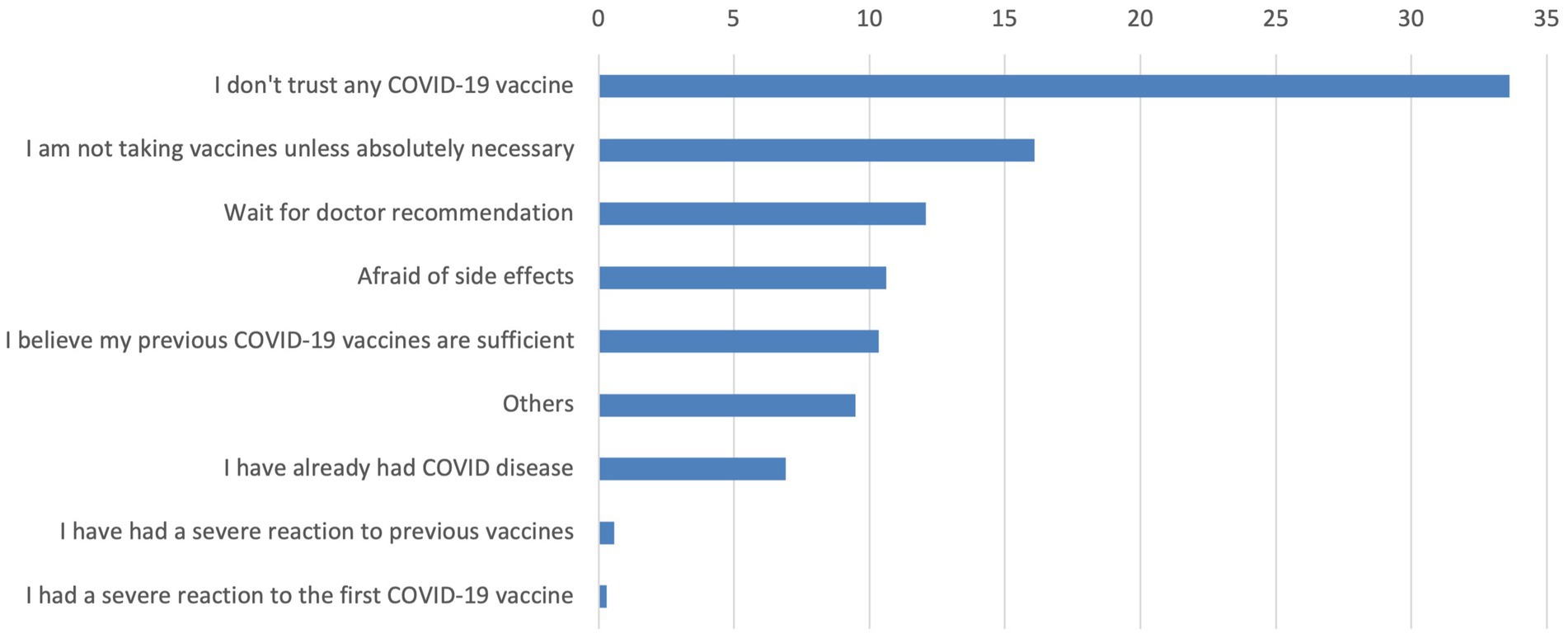
Figure 1 shows the reasons why participants indicated they were not interested in receiving the latest COVID-19 vaccine. The most frequently cited reason was not trusting any COVID-19 vaccine (33.6%), followed by views about only taking vaccines if absolutely necessary (16.1%), waiting for a doctor’s recommendation (12.1%) and concerns about side effects (10.6%).
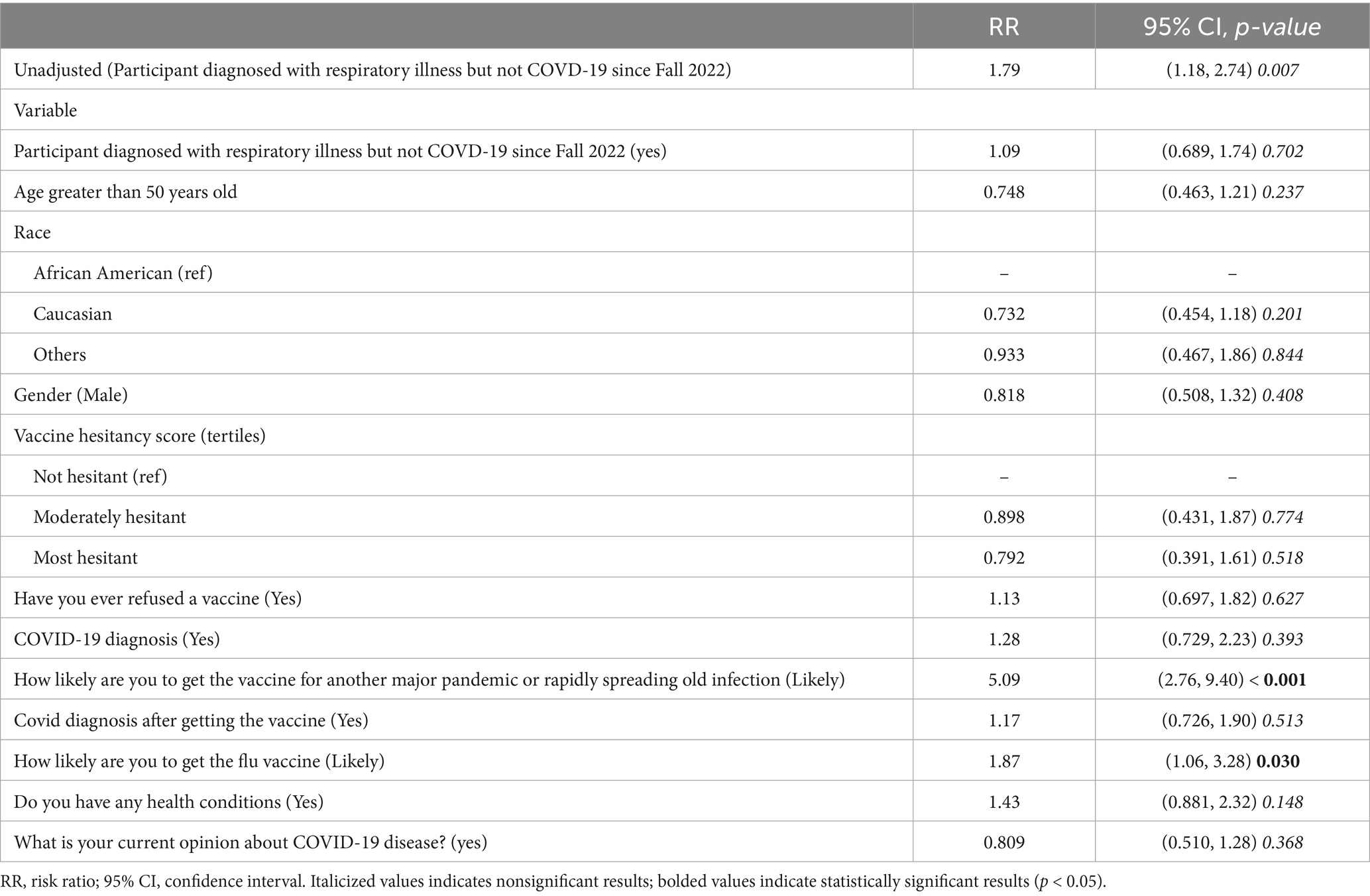
The final model variables displayed in Table 3 illustrate the likelihood of accepting the latest COVID-19 vaccine. The unadjusted model showed a significant association between respiratory illness and COVID-19 vaccine acceptance (RR: 1.79, 95% CI: 1.18–2.74, p = 0.007). While age, gender, and chronic health conditions were not statistically significant in the fully adjusted model, participants likely to get the influenza vaccine showed a significantly higher likelihood of accepting the COVID-19 vaccine (RR: 1.87, 95% CI: 1.06–3.28, p = 0.030), compared to those were unlikely. Additionally, those willing to vaccinate during a new pandemic exhibited the strongest association (RR: 5.09, 95% CI: 2.76–9.40, p < 0.001).
Discussion
Individuals with a history of respiratory illness were significantly more willing to receive the latest COVID-19 vaccine, in this sample, highlighting the role of perceived vulnerability in shaping vaccine behavior. Research consistently shows that individuals with chronic respiratory conditions prioritize preventive health measures, including vaccination, to lessen their risk of severe health outcomes (19–21). In Southeastern Louisiana, this relationship may be further amplified by the region’s high prevalence of respiratory illnesses and limited healthcare resources, creating a sense of urgency among vulnerable populations (22). This is particularly relevant in the context of COVID-19, where awareness of potential complications likely drives health-protective behaviors (23). Additionally, these findings align with health behavior theories, which emphasize that perceptions of personal risk are critical motivators for engaging in preventive actions, such as vaccination (24). Participants with respiratory disease likely have increased interaction with healthcare providers due to their underlying conditions, increasing their opportunities for vaccine education and trust-building during routine care (23, 25). This suggests that healthcare engagement plays an important role in enhancing vaccine acceptance, particularly for populations at higher risk. Although our study did not assess vaccination opportunities during hospitalization, prior research highlights the potential impact of leveraging inpatient and outpatient care settings to offer recommended vaccines. Studies have shown that routine vaccination during hospitalization for chronic patients, especially those with respiratory illnesses, can significantly increase coverage and reduce missed opportunities (15, 26). This suggests that future interventions might benefit from integrating vaccine delivery into standard chronic disease management visits. The alignment of these findings with the behavioral theories, such as the Health Belief Model, reinforces the importance of utilizing healthcare interactions to address hesitancy and promote vaccine uptake among high-risk individuals (27, 28).
Influenza vaccine acceptance emerged as a strong predictor in latest COVID-19 vaccine uptake as well, suggesting that integrating multiple vaccine campaigns, for example, combining influenza vaccine campaigns with COVID-19 vaccine outreach, could enhance overall vaccine uptake (20, 29). Although our study did not examine booster uptake or co-administration directly, recent research supports the safety and feasibility of administering multiple vaccines simultaneously in high-risk populations, including individuals with chronic respiratory conditions. These findings highlight the value of integrated vaccine delivery models, particularly in outpatient settings, where vaccine recommendations can be reinforced during routine care (30–32). Willingness to vaccinate during a new pandemic or rapidly spreading infection was another strong predictor of COVID-19 vaccine uptake (RR: 5.09, 95% CI: 2.76–9.40, p < 0.001), emphasizing the influence of perceived vulnerability. These results highlight the complex and multifactorial nature of vaccine uptake, where factors such as prior positive vaccine behaviors, perceptions of risk, and individual trust in vaccines converge. For instance, moderate hesitancy reduced the likelihood of vaccine acceptance (RR: 0.798, 95% CI: 0.567–0.912, p = 0.021), while high hesitancy further decreased this likelihood (RR: 0.612, 95% CI: 0.433–0.873, p = 0.007).
Mistrust in vaccines emerged as a significant barrier, with 33.62% of participants citing distrust in COVID-19 vaccines as the primary reason for not accepting, followed by preferences for vaccines if it was necessary (16.09%), waiting for a doctor’s recommendation (12.07%), and concerns about side effects (10.63%). These findings underscore the deeply rooted mistrust in vaccines and highlight critical areas for targeted public health interventions to address hesitancy and misinformation. By focusing on these barriers and promoting trust in healthcare providers, public health strategies can better address the interplay of factors influencing vaccine uptake (23). Beyond addressing individual concerns, broader organizational and communication strategies are critical in reducing vaccine hesitancy. Evidence suggests that well-structured health communication campaigns, combined with the trusted role of healthcare workers (HCWs), can significantly improve vaccine confidence and uptake. HCWs serve as influential messengers, especially when interventions are personalized and culturally appropriate (33, 34). Incorporating educational outreach and clear communication strategies into routine care may help overcome barriers related to misinformation, mistrust, and limited health literacy.
Vaccine hesitancy persists as a critical barrier, specifically among underrepresented communities in Southeastern Louisiana. Concerns about vaccine side effects, mistrust in efficacy, and logistical challenges reflect longstanding issues of healthcare inequity (35, 36). These barriers align with other studies on vaccine hesitancy, highlight the impact of misinformation and logistical challenges on vaccine decisions (27, 36). Historical healthcare discriminations, particularly affecting African American communities, have exacerbated these challenges and underline the need for culturally tailored public health interventions (35, 37). In Southeastern Louisiana, addressing these issues is important given the region’s unique demographic and health disparities, that may amplify these challenges among marginalized communities (22). Public health campaigns should focus on educating individuals about vaccine safety and efficacy, addressing common fears and misconceptions that lead to hesitancy. Moreover, logistical barriers such as access to vaccination sites should be considered in future outreach efforts to ensure equitable access to vaccines.
Strengths and limitations
This study has several limitations that should be in consideration. First, the study did measure trust in healthcare providers and institutions; yet, these were not found to be significant in the bivariate or multivariate analysis and excluded from model prediction. Healthcare trust is a factor consistently highlighted in previous research as a critical determinant of vaccine acceptance, particularly in Louisiana (38, 39). Additionally, the study did not include an analysis of preventive health behaviors beyond influenza vaccine acceptance, which could have provided further insights into vaccine decision-making patterns. Addressing these gaps in future research would enhance the understanding of the multifactorial drivers of vaccine acceptance.
To address selection bias, participants were randomly sampled from various healthcare locations across Southeastern Louisiana, which aimed to minimize bias and enhance the representativeness of the sample. However, as with any study, the potential for selection bias cannot be completely ruled out. The random sampling method was intended to distribute any unmeasured confounders evenly among the study groups, thus reducing their impact on the observed associations. Future studies could improve on this by incorporating stratified or cluster sampling techniques, especially when targeting specific sub populations or regions.
Despite these limitations, the study offers significant strengths. The focus on actionable predictors, including respiratory disease history, vaccine hesitancy, and influenza vaccine behavior, allows for a detailed exploration of factors that directly influence COVID-19 vaccine uptake. Furthermore, the study’s diverse sample enhances its applicability to broader populations, providing critical insights for designing equitable public health strategies. These findings are consistent with prior research emphasizing the importance of targeted approaches to addressing disparities in vaccine acceptance (38, 39). By emphasizing predictors that are both practical and relevant, the findings contribute to a growing body of evidence supporting tailored interventions to address vaccine hesitancy and improve vaccination rates in underserved communities.
Table 3 underscores the complex relationship between predictors and vaccine acceptance. Specifically, this final model integrated key predictors such as respiratory disease history, vaccine hesitancy, and influenza vaccine behavior, providing a comprehensive perspective on the factors driving vaccine uptake. The inclusion of these variables emphasizes their theoretical and practical relevance in shaping public health strategies. This approach helps shape a clear and meaningful narrative, offering valuable insights for designing targeted interventions to address vaccine hesitancy and boost vaccine uptake in future public health efforts.
Conclusion
This study provides valuable insights into the factors influencing the likelihood of receiving the latest COVID-19 vaccine, particularly highlighting the impact of respiratory disease history and influenza vaccine behavior. Participants with a history of respiratory illness were significantly more likely to express willingness to receive the latest COVID vaccine, reflecting their increased health concerns and likelihood of engaging in preventive health behaviors (19). This increased willingness demonstrates the critical role of personal risk perceptions in shaping health-protective behaviors. In Southeastern Louisiana, a region characterized by significant health disparities, these findings emphasize the need to consider regional contexts in vaccine strategies.
Overall, future public health strategies should encourage uptake of health-promoting behaviors, improve trust through transparent communication, and address logistical barriers to ensure broad and fair vaccine coverage (23, 36, 38). By focusing on both medical and social determinants of vaccine behavior, these strategies can strengthen preparedness for future pandemics or health crises (40). Understanding how the personal perceptions of respiratory disease influence vaccine behavior will be crucial for designing effective public health interventions in Southeastern Louisiana and beyond.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Xavier University of Louisiana (IRB#850). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SB: Validation, Writing – original draft, Software, Visualization, Formal analysis, Data curation. SA-D: Funding acquisition, Software, Formal analysis, Resources, Project administration, Data curation, Writing – original draft, Conceptualization, Methodology, Supervision, Visualization. KH: Methodology, Supervision, Writing – review & editing, Conceptualization, Resources, Project administration. AT: Writing – review & editing, Resources, Methodology. AK: Methodology, Resources, Writing – review & editing, Project administration. MB: Methodology, Writing – review & editing, Validation, Investigation. B-AB: Investigation, Writing – review & editing, Resources. KC: Resources, Writing – review & editing, Investigation. HS: Investigation, Resources, Writing – review & editing. IH: Investigation, Validation, Writing – review & editing. MJ: Investigation, Data curation, Writing – review & editing. LC: Writing – review & editing, Visualization. DSar: Writing – review & editing, Funding acquisition. DSal: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This publication was made possible by the NIH-RCMI grant #U54MD007595 from the National Institute on Minority Health and Health Disparities. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. SA-D has received additional funding from Pfizer© and Roche-Genentech© not in relation to this publication. DSal has received funding from Merck© for research projects through his institution, not in relation to this project.
Acknowledgments
The researchers would like to acknowledge their community research partners: START© Corporation Louisiana, Methodist Health-Systems Foundation©, Channel Drugs, Cedar Pharmacy, C&S Family Pharmacy, Common Ground Health Clinic and Total Health Covington Clinic. This research was possible through the mentorship and guidance of the Research Center for Minority Institutions at Xavier University of Louisiana by project leads Dr. Guangdi Wang and Dr. Gene D’Amour. We would also like to acknowledge the administrative support of Dr. Kathleen Kennedy, Dean of the College of Pharmacy; Dr. Dana Jamero, Division Chair for Clinical and Administrative Sciences; Kaneisha Akinpelumi, Office of Sponsored Research; and Dr. Marguerite Giguette, Provost for Xavier University of Louisiana.
Conflict of interest
B-AB was employed by START Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ndumwa, HP, Mboya, EA, Amani, DE, Mashoka, R, Nicholaus, P, Haniffa, R, et al. The burden of respiratory conditions in the emergency department of Muhimbili National Hospital in Tanzania in the first two years of the COVID-19 pandemic: a cross sectional descriptive study. PLOS Glob Public Health. (2022) 2:e0000781. doi: 10.1371/journal.pgph.0000781
2. Lai, CC, Wang, CY, and Hsueh, PR. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. (2020) 53:505–12. doi: 10.1016/j.jmii.2020.05.013
3. Keni, R, Alexander, A, Nayak, PG, Mudgal, J, and Nandakumar, K. COVID-19: emergence, spread, possible treatments, and global burden. Front Public Health. (2020) 8:216. doi: 10.3389/fpubh.2020.00216
4. Borges do Nascimento, IJ, O’Mathúna, DP, Von Groote, TC, Abdulazeem, HM, Weerasekara, I, Marusic, A, et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis. (2021) 21:525. doi: 10.1186/s12879-021-06214-4
5. Leuzinger, K, Roloff, T, Gosert, R, Sogaard, K, Naegele, K, Rentsch, K, et al. Epidemiology of severe acute respiratory syndrome coronavirus 2 emergence amidst community-acquired respiratory viruses. J Infect Dis. (2020) 222:1270–9. doi: 10.1093/infdis/jiaa464
6. Shure, W, Tayachew, A, Berkessa, T, Teka, G, Biru, M, Gebeyehu, A, et al. SARS-CoV-2 co-detection with influenza and human respiratory syncytial virus in Ethiopia: findings from the severe acute respiratory illness (SARI) and influenza-like illness (ILI) sentinel surveillance, January 01, 2021, to June 30, 2022. PLOS Glob Public Health. (2024) 4:e0003093. doi: 10.1371/journal.pgph.0003093
7. Varela, FH, Sartor, ITS, Polese-Bonatto, M, Azevedo, TR, Kern, LB, Fazolo, T, et al. Rhinovirus as the main co-circulating virus during the COVID-19 pandemic in children. J Pediatr. (2022) 98:579–86. doi: 10.1016/j.jped.2022.03.003
8. Boyanton, BL Jr, Frenner, RA, Ingold, A, Ambroggio, L, and Kennedy, JL. SARS-CoV-2 pandemic non-pharmacologic interventions temporally associated with reduced pediatric infections due to mycoplasma pneumoniae and co-infecting respiratory viruses in Arkansas. Microbiol Spectr. (2024) 12:e0290823. doi: 10.1128/spectrum.02908-23
9. Kışlal, FM, Hanilçe, Y, Altaş, B, Büyükbaşaran, ZE, and Güven, D. The disappearance of respiratory syncytial virus and influenza viruses in children during the second year of the COVID-19 pandemic - are non-pharmaceutical interventions as effective as vaccines? Eur Rev Med Pharmacol Sci. (2023) 27:3777–83. doi: 10.26355/eurrev_202304_32178
10. Shi, HJ, Kim, NY, Eom, SA, Kim-Jeon, MD, Oh, SS, Moon, BS, et al. Effects of non-pharmacological interventions on respiratory viruses other than SARS-CoV-2: analysis of laboratory surveillance and literature review from 2018 to 2021. J Korean Med Sci. (2022) 37:e172. doi: 10.3346/jkms.2022.37.e172
11. Davies, NG, Kucharski, AJ, Eggo, RM, Gimma, A, and Edmunds, WJ. The effect of non-pharmaceutical interventions on COVID-19 cases, deaths and demand for hospital services in the UK: a modelling study. medRxiv. (2020) 5:e375–e385. doi: 10.1016/S2468-2667(20)30133-X
12. Achangwa, C, Park, H, Ryu, S, and Lee, MS. Collateral impact of public health and social measures on respiratory virus activity during the COVID-19 pandemic 2020-2021. Viruses. (2022) 14:1071. doi: 10.3390/v14051071
13. Yuan, H, Yeung, A, and Yang, W. Interactions among common non-SARS-CoV-2 respiratory viruses and influence of the COVID-19 pandemic on their circulation in new York City. Influenza Other Respir Viruses. (2022) 16:653–61. doi: 10.1111/irv.12976
14. Di Lorenzo, A, Martinelli, A, Bianchi, F, Scazzi, FL, Diella, G, Tafuri, S, et al. The safety of pneumococcal vaccines at the time of sequential schedule: data from surveillance of adverse events following 13-valent conjugated pneumococcal and 23-valent polysaccharidic pneumococcal vaccines in newborns and the elderly, in Puglia (Italy), 2013-2020. Ann Ig Sanita Pubblica. (2023) 35:459–467. doi: 10.7416/ai.2022.2551
15. Gallone, MS, Infantino, V, Ferorelli, D, Stefanizzi, P, De Nitto, S, and Tafuri, S. Vaccination coverage in patients affected by chronic diseases: a 2014 cross-sectional study among subjects hospitalized at Bari Policlinico general hospital. Am J Infect Control. (2018) 46:e9–e11. doi: 10.1016/j.ajic.2017.10.004
16. Al-Dahir, S, Barri, S, Heyer, K, Taylor, AM, Khalil, A, Belkhouche, M, et al. COVID-19 testing behavior as a predictor of COVID-19 vaccination in southeastern Louisiana: a longitudinal cohort study. Vaccines (Basel). (2024) 12:1338. doi: 10.3390/vaccines12121338
17. Qualtrics Qualtrics [survey software]. (2022). Available online at: https://www.qualtrics.com
19. Lu, P-j, Euler, GL, and Callahan, DB. Influenza vaccination among adults with asthma: findings from the 2007 BRFSS survey. Am J Prev Med. (2009) 37:109–15. doi: 10.1016/j.amepre.2009.03.021
20. Smith, BA, Ricotta, EE, Kwan, JL, and Evans, NG. COVID-19 risk perception and vaccine acceptance in individuals with self-reported chronic respiratory or autoimmune conditions. Allergy, Asthma Clin Immunol. (2023) 19:37. doi: 10.1186/s13223-023-00791-6
21. Goldszmidt, R, Petherick, A, Andrade, EB, Hale, T, Furst, R, Phillips, T, et al. Protective behaviors against COVID-19 by individual vaccination status in 12 countries during the pandemic. JAMA Netw Open. (2021) 4:e2131137. doi: 10.1001/jamanetworkopen.2021.31137
22. Al-Dahir, S, Earls, M, Gillard, C, Singleton, B, and Hall, E. Assessing the impact of COVID-19 phased vaccine eligibility on COVID-19 vaccine intent among African Americans in southeastern Louisiana: a community-based, cohort study. Int J Environ Res Public Health. (2022) 19:16737. doi: 10.3390/ijerph192416737
23. Paterson, P, Meurice, F, Stanberry, LR, Glismann, S, Rosenthal, SL, and Larson, HJ. Vaccine hesitancy and healthcare providers. Vaccine. (2016) 34:6700–6. doi: 10.1016/j.vaccine.2016.10.042
24. Brewer, NT, Chapman, GB, Rothman, AJ, Leask, J, and Kempe, A. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. (2017) 18:149–207. doi: 10.1177/1529100618760521
25. Abad, N, Messinger, SD, Huang, Q, Hendrich, MA, Johanson, N, Fisun, H, et al. A qualitative study of behavioral and social drivers of COVID-19 vaccine confidence and uptake among unvaccinated Americans in the US April-May 2021. PLoS One. (2023) 18:e0281497. doi: 10.1371/journal.pone.0281497
26. Sanftenberg, L, Brombacher, F, Schelling, J, Klug, S, and Gensichen, J. Increasing influenza vaccination rates in people with chronic illness. Dtsch Arztebl Online. (2019) 9:116. doi: 10.3238/arztebl.2019.0645
27. MacDonald, NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. (2015) 33:4161–4. doi: 10.1016/j.vaccine.2015.04.036
28. Champion, VL, and Skinner, CS. The health belief model In: Health behavior and health education: Theory, research, and practice, vol. 4 (2008) 45–65. doi: 10.1080/10410236.2013.873363
29. Tosh, PK, Boyce, TG, and Poland, GA. Flu myths: dispelling the myths associated with live attenuated influenza vaccine. Mayo Clin Proc. (2008) 83:77–84. doi: 10.4065/83.1.77
30. Lu, P-J, Srivastav, A, Vashist, K, Black, CL, Kriss, JL, Hung, M-C, et al. COVID-19 booster dose vaccination coverage and factors associated with booster vaccination among adults, United States, March 2022. Emerg Infect Dis. (2023) 29:133. doi: 10.3201/eid2901.221151
31. Fekete, M, Horvath, A, Santa, B, Tomisa, G, Szollosi, G, Ungvari, Z, et al. COVID-19 vaccination coverage in patients with chronic obstructive pulmonary disease–a cross-sectional study in Hungary. Vaccine. (2023) 41:193–200. doi: 10.1016/j.vaccine.2022.11.020
32. Moscara, L, Venerito, V, Martinelli, A, di Lorenzo, A, Toro, F, Violante, F, et al. Safety profile and SARS-CoV-2 breakthrough infections among HCWs receiving anti-SARS-CoV-2 and influenza vaccines simultaneously: an Italian observational study. Vaccine. (2023) 41:5655–61. doi: 10.1016/j.vaccine.2023.07.043
33. Hong, S. COVID-19 vaccine communication and advocacy strategy: a social marketing campaign for increasing COVID-19 vaccine uptake in South Korea. Humanit Soc Sci Commun. (2023) 10:1–9. doi: 10.1057/s41599-023-01593-2
34. Gallé, F, Quaranta, A, Napoli, C, Diella, G, de Giglio, O, Caggiano, G, et al. How do vaccinators experience the pandemic? Lifestyle behaviors in a sample of Italian public health workers during the COVID-19 era. Vaccine. (2022) 10:247. doi: 10.3390/vaccines10020247
35. Crouse Quinn, S, Jamison, AM, Freimuth, VS, An, J, and Hancock, GR. Determinants of influenza vaccination among high-risk Black and White adults. Vaccine. (2017) 35:7154–9. doi: 10.1016/j.vaccine.2017.10.083
36. Loomba, S, de Figueiredo, A, Piatek, SJ, de Graaf, K, and Larson, HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. (2021) 5:337–48. doi: 10.1038/s41562-021-01056-1
37. Röpcke, A, Brinkmann, C, Neumann-Böhme, S, Sabat, I, pita Barros, P, Schreyögg, J, et al. Understanding health risk perception: insights from an eight-country panel study during the COVID-19 pandemic. J Public Health. (2024) 46. doi: 10.7189/jogh.15.04189
38. Williams, L, Craig, LS, Peacock, E, Fields, T, al-Dahir, S, Hawkins, F, et al. Exploring the roles of trust, attitudes, and motivations in COVID-19 decision-making and vaccination likelihood: insights from the Louisiana community engagement alliance (LA-CEAL) community-academic-public health-practice (CAPP) partnership. Int J Environ Res Public Health. (2024) 22:48. doi: 10.3390/ijerph22010048
39. Peacock, E, Craig, LS, Wilson, M, Williams, LK, Dahir, SA, Tang, W, et al. COVID-19 vaccination likelihood among federally qualified health center patients: lessons learned for future health crises. Am J Med Sci. (2023) 366:321–9. doi: 10.1016/j.amjms.2023.07.013
Keywords: respiratory diseases, COVID-19, vaccination, vaccine behavior, public health interventions, attitudes
Citation: Barri S, Al-Dahir S, Heyer K, Taylor A, Khalil A, Belkhouche M, Bonvillain B-A, Caldwell K, Surcouf H, Hamed I, Jwayyed M, Craig LS, Sarpong DF and Salmon D (2025) Influence of respiratory disease experiences on COVID-19 vaccine acceptance: a study from Southeastern Louisiana. Front. Public Health. 13:1593861. doi: 10.3389/fpubh.2025.1593861
Edited by:
Belay Desye, Wollo University, EthiopiaReviewed by:
Pasquale Stefanizzi, University of Bari Aldo Moro, ItalyNour Youssef, American University of Beirut Medical Center, Lebanon
Copyright © 2025 Barri, Al-Dahir, Heyer, Taylor, Khalil, Belkhouche, Bonvillain, Caldwell, Surcouf, Hamed, Jwayyed, Craig, Sarpong and Salmon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Al-Dahir, c2FhbGRhaEB4dWxhLmVkdQ==
 Saba Barri1
Saba Barri1 Sara Al-Dahir
Sara Al-Dahir Malack Jwayyed
Malack Jwayyed Leslie S. Craig
Leslie S. Craig