- 1Department of Clinical Laboratory Medicine, The Ding Li Clinical College of Wenzhou Medical University, Wenzhou Central Hospital, Wenzhou, China
- 2Key Laboratory of Diagnosis and Treatment of New and Recurrent Infectious Diseases of Wenzhou, Wenzhou Sixth People's Hospital, Wenzhou, China
- 3Medical Management Office, Wenzhou Municipal Public Hospital Management Center, Wenzhou, China
- 4Department of Tuberculosis Clinic, The Ding Li Clinical College of Wenzhou Medical University, Wenzhou Central Hospital, Wenzhou, China
- 5Department of Clinical Laboratory Medicine, Yueqing People's Hospital, Wenzhou, Zhejiang, China
- 6Key Laboratory of Precision Medicine of Wenzhou, Wenzhou Central Hospital, Wenzhou, China
Objective: This study aimed to analyze the epidemiological characteristics and trends of notified multidrug-resistant tuberculosis (MDR-TB) in Wenzhou City, China, from 2014 to 2023, with a focus on differences between migrant and local populations among reported TB cases.
Methods: This was a facility-based retrospective cohort study that included all bacteriologically confirmed TB cases notified between 1 January 2014 and 31 December 2023 in the Tuberculosis Information Management System (TBIMS) of the Chinese Center for Disease Control and Prevention and the hospital’s laboratory information system, provided they had available phenotypic drug-susceptibility testing (pDST) results. Pearson’s chi-square test was used to compare drug-resistance rates between groups, the trend chi-square test was applied to assess temporal changes, and a Sankey diagram was employed to illustrate the origins and intra-city distribution of MDR-TB among the migrant population.
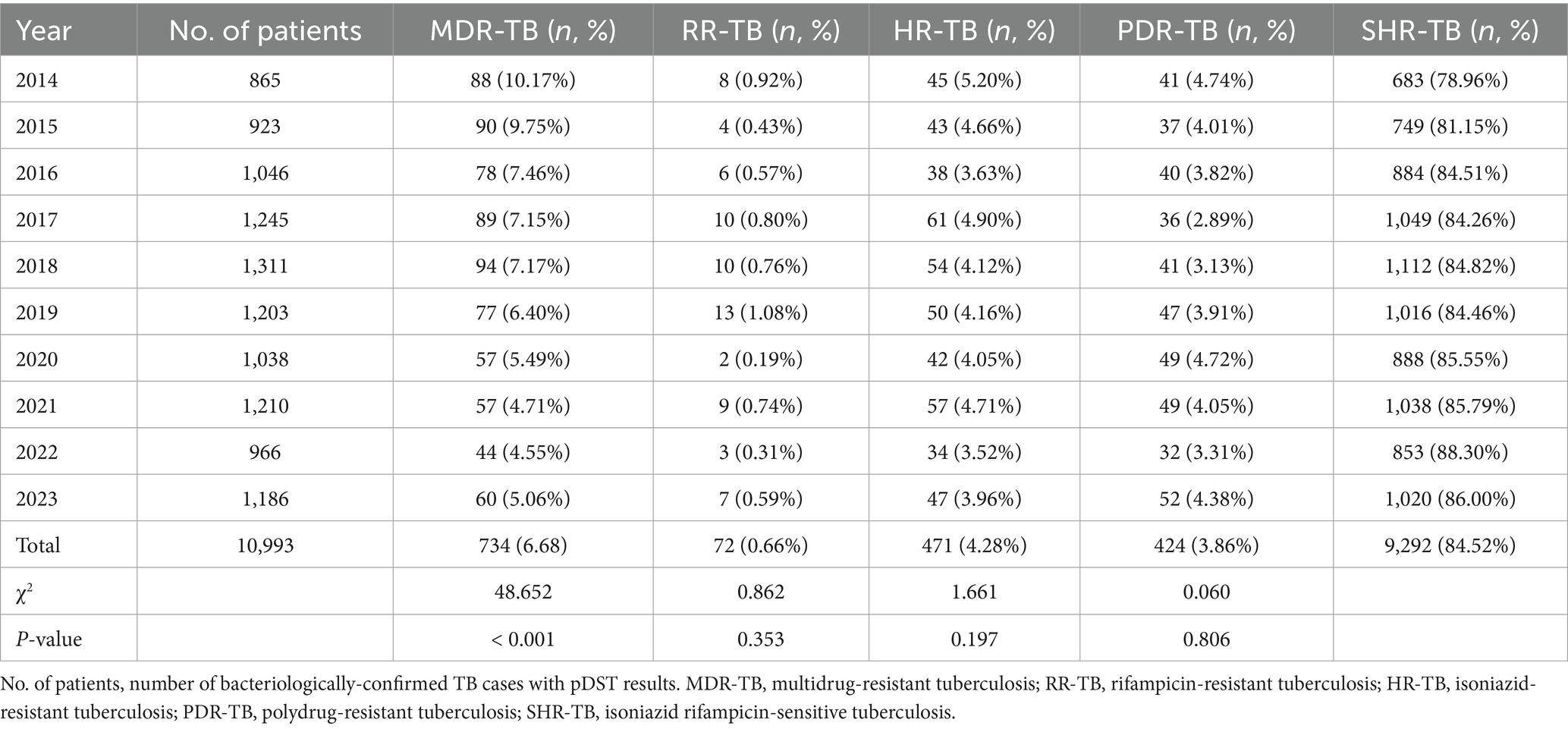
Results: Among 10,993 notified TB patients, 734 (6.68%) were classified as MDR-TB. The proportion of MDR-TB among notified cases declined over the study period (p < 0.001). Nearly half (352/734; 47.96%) of the notified MDR-TB patients were migrants; 226 (64.21%) originated from elsewhere in Zhejiang Province, and 126 (35.79%) came from outside the province. Guizhou, Jiangxi and Sichuan were the leading external contributors. Within Wenzhou, Yueqing City, Yongjia County and Ouhai District reported the highest numbers of migrant MDR-TB notifications.
Conclusion: The proportion of MDR-TB among notified TB cases in Wenzhou City has steadily decreased. Migrants account for almost half of these notified MDR-TB cases. Surveillance-driven and migrant-targeted interventions should be prioritized to further reduce MDR-TB transmission.
1 Introduction
Tuberculosis (TB), a persistent global health threat (1), caused an estimated 1.25 million deaths in 2023, surpassing COVID-19 as the leading infectious disease killer (1, 2). While global TB incidence rates declined by 2% annually from 2010 to 2020, recent years have seen a resurgence, with 10.8 million new cases projected for 2023—a 4.6% increase from 2020 levels (3). Drug-resistant TB (DR-TB), particularly multidrug-resistant TB (MDR-TB), compounds this crisis. In 2023, 400,000 new MDR/rifampicin-resistant TB (RR-TB) cases emerged globally, yet only 44% were diagnosed and treated, with treatment success rates stagnating below 70% (3). MDR-TB’s prolonged treatment duration, high costs, and socioeconomic burden underscore the urgent need for targeted interventions (4, 5).
In recent years, Wenzhou City has implemented several measures to control MDR-TB, including expanding phenotypic drug sensitivity testing (pDST) to most bacteriologically positive patients, equipping all designated TB hospitals with the GeneXpert MTB/RIF system for rapid rifampicin resistance detection, incorporating MDR pulmonary TB into medical insurance with subsidies up to 30,000 yuan per patient, and increasing financial support for designated TB hospitals. These efforts have improved local MDR-TB control, but challenges remain in providing TB services to vulnerable populations, such as migrants (6).
In the context of rapid social and economic development in China, population movements have become increasingly frequent (7). As an economically developed coastal city, Wenzhou has attracted a significant number of migrants. The living conditions of this migrant population are often complex, characterized by overcrowding and inadequate sanitation facilities, which facilitate the spread of MDR-TB (8). Additionally, previous studies (9, 10) have indicated that the frequent relocation of migrants can lead to challenges in accessing medical care across different regions, resulting in interrupted treatment and irregular medication adherence. This situation increases the likelihood of developing MDR-TB among the migrant population. Understanding the epidemiological characteristics and trends of MDR-TB within this group is therefore essential for the timely detection and management of mobile patients and for effective epidemic control. To date, such evidence has been lacking for Wenzhou. This study aims to analyze the epidemiological features and temporal trends of MDR-TB among notified TB cases in Wenzhou from 2014 to 2023 and to compare patterns between migrant and local populations, so as to inform the development of targeted prevention and control strategies.
2 Materials and methods
2.1 Study setting, population, and data collection
The research was carried out in Wenzhou, a city located on China’s southeast coast. Wenzhou’s geographical coordinates extend from 119° 37′ to 121° 18′ east longitude and from 27° 03′ to 28° 36′ north latitude. Covering an area of 12,110 square kilometers, the city is made up of four districts, five counties, and three county-level cities. As of late 2023, the permanent population of Wenzhou is around 9.761 million, which includes a transient population of 4.42 million. This research took place at Wenzhou Central Hospital, the sole facility in Wenzhou dedicated to treating drug-resistant tuberculosis. The sociodemographic information (including gender, age, ethnicity, household registration address and category [local or migrant], current residence, and occupation), phenotypic drug susceptibility testing (pDST) outcomes, and the tuberculosis treatment history of participants were sourced from the Tuberculosis Information Management System (TBIMS) of the Chinese Center for Disease Control and Prevention and the hospital’s laboratory information system. Between January 1, 2014, and December 31, 2023, the TBIMS recorded a total of 39,356 patients diagnosed with tuberculosis in Wenzhou. Of these, 9,819 cases that depended exclusively on molecular diagnosis without results from mycobacterial cultures were removed from consideration. Furthermore, 117 cases that presented nontuberculous mycobacterial infections as indicated by positive mycobacterial culture results were also excluded, along with 18,427 cases that were culture-negative and lacked pDST results or having failed such tests. In total, 10,993 patients who met the eligibility criteria were incorporated into the study. The specifics concerning the patient inclusion and exclusion criteria are depicted in Figure 1.
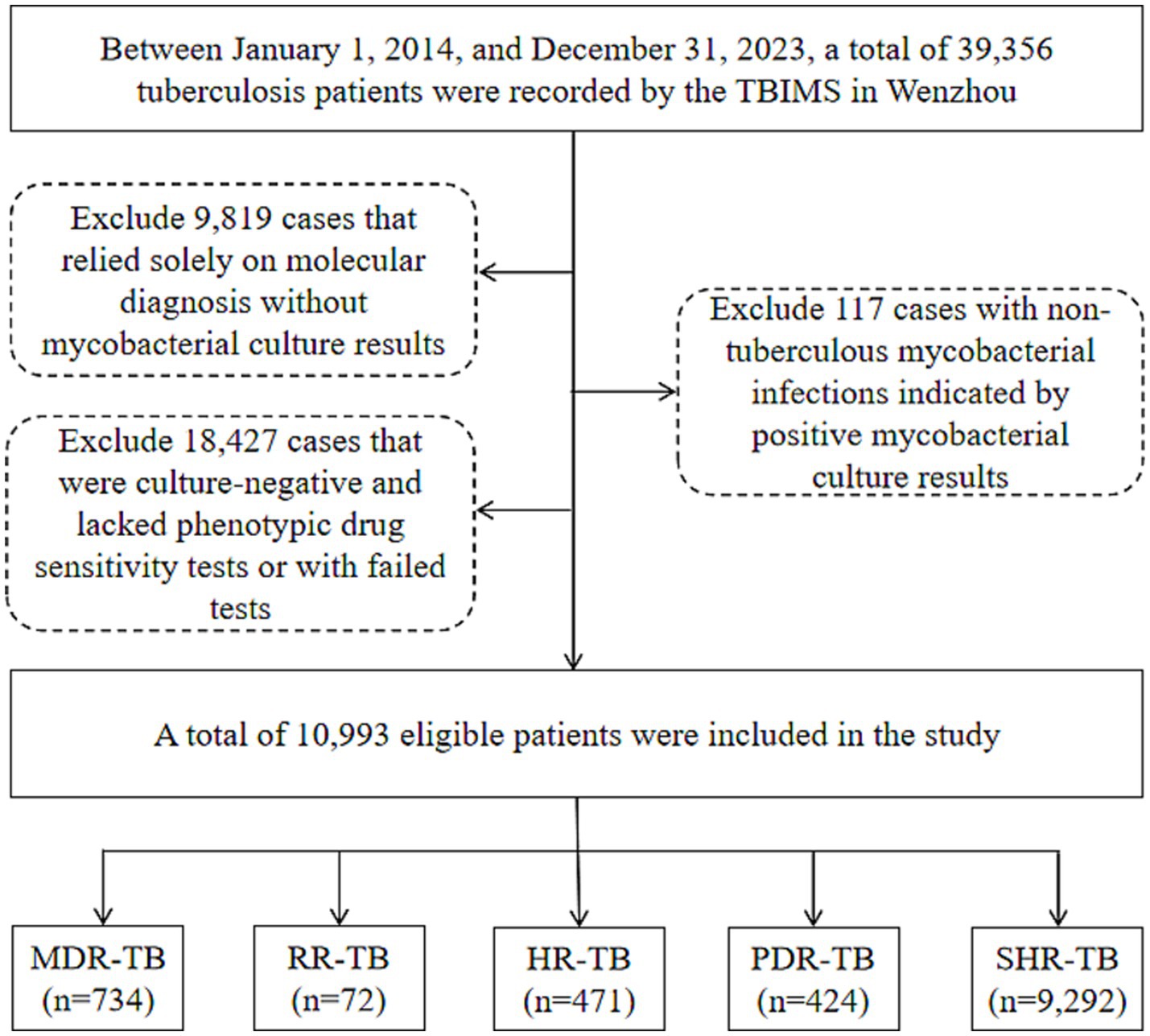
Figure 1. Flow chart of the patients enrolled in the study. TBIMS, tuberculosis information management system; MDR-TB, multidrug-resistant tuberculosis; RR-TB, rifampicin-resistant tuberculosis; HR-TB, isoniazid-resistant tuberculosis; PDR-TB, polydrug-resistant tuberculosis; SHR-TB, isoniazid rifampicin-sensitive tuberculosis.
2.2 Definitions
The migrant population refers to people whose place of residence is inconsistent with their registered household location at the township or street level, and who have been away from their registered household location for six months or more. Multidrug-resistant tuberculosis (MDR-TB) is defined as resistance to at least both isoniazid and rifampicin (11). Polydrug-resistant tuberculosis (PDR-TB) is defined as resistance to two or more first-line anti-TB drugs (except both isoniazid and rifampicin) (11). Rifampicin-resistant tuberculosis (RR-TB) is defined as resistance to rifampicin only. Isoniazid-resistant tuberculosis (HR-TB) is defined as resistance to isoniazid only. Isoniazid rifampicin-sensitive tuberculosis (SHR-TB) is defined as susceptibility to both isoniazid and rifampicin.
2.3 Bacteriologic examinations and drug susceptibility testing
Upon hospital admission, 3–5 mL sputum or bronchoalveolar lavage fluid samples were rigorously collected and pretreated with NALC-NaOH solution. This pretreatment step is critical for sample decontamination and significantly enhances the isolation rate of potential pathogens. The processed samples were then inoculated into BACTEC MGIT liquid culture tubes (BD, USA) or Löwenstein-Jensen solid media (Zhuhai BaiShi Biotechnology Co., Ltd.) and incubated at a constant temperature of 37°C to facilitate mycobacterial growth. For culture-positive isolates, smears were prepared and subjected to Ziehl-Neelsen acid-fast staining (KS, Seoul, Korea). This staining technique is definitive for mycobacterial identification by differentiating acid-fast bacilli from other bacteria under microscopic examination. When mycobacteria were detected, MPB64 antigen testing (Hangzhou Innovation Biotechnology Co., Ltd.) was further performed to confirm the identification of Mycobacterium tuberculosis (MTB), the causative agent of tuberculosis. In addition to strain identification, phenotypic drug susceptibility testing (pDST) for MTB was rigorously conducted using the MGIT liquid method. The procedure strictly adhered to the manufacturer’s protocols for both the instrument and reagents to ensure result reliability. The pDST evaluated the inhibitory effects of four first-line anti-tuberculosis drugs at the following concentrations: streptomycin (SM) 1.0 μg/mL, isoniazid (INH) 0.1 μg/mL, rifampicin (RFP) 1.0 μg/mL, and ethambutol (EMB) 5.0 μg/mL.
2.4 Statistical analysis
All data were imported into WPS Excel (version 12.1.0.18276, Jinshan Office Software Co., Ltd., Beijing, China) to create a database. Additionally, WPS Excel was utilized for generating trend charts related to drug resistance. Statistical analyses were carried out with SPSS software (version 26.0, IBM, New York, USA), where counting data were represented in terms of frequencies and percentages. To compare categorical variables, Pearson’s chi-square test was applied. The trend chi-square test was used to examine the temporal patterns associated with TB drug resistance. Furthermore, Origin software (version 2021, OriginLab, Massachusetts, USA) assisted in developing a Sankey diagram, depicting the sources and destinations of migrant MDR-TB. A significance level of p-value < 0.05 was deemed statistically significant.
3 Results
3.1 Demographic characteristics of the study population
A total of 10,993 subjects were included in this study, with a median age of 45 years (29, 59). Among these, 7,930 were males (72.14%) and 3,063 were females (27.86%). The majority of participants were of Han nationality, comprising 10,681 cases (97.16%), while 312 cases (2.84%) were from other ethnic minorities. Additionally, 4,786 cases (43.54%) were classified as part of the migrant population, and 6,207 cases (56.46%) were local residents. Employment status revealed that 2,965 cases (26.97%) were employed, whereas 8,028 cases (73.03%) were unemployed. Furthermore, 1,176 cases (10.70%) had previously received anti-TB treatment, while 9,817 cases (89.30%) were newly diagnosed.
3.2 Proportion and trends of drug-resistant TB among notified cases
Among 10,993 bacteriologically-confirmed TB cases notified in Wenzhou, 734 (6.68%) were classified as MDR-TB, 72 (0.66%) as RR-TB, 471 (4.28%) as HR-TB, 424 (3.86%) as PDR-TB, and 9,292 (84.52%) as SHR-TB. From 2014 to 2023, the proportion of MDR among all notified TB cases declined significantly (p < 0.001), while the proportions of RR-TB, HR-TB, and PDR-TB showed modest decreases (p > 0.05) (Table 1; Figure 2). Among newly notified patients, 434 (4.42%) were MDR; among previously treated patients, 300 (25.51%) were MDR. The difference in the proportion of MDR between the two groups was statistically significant (χ2 = 749.593, p < 0.001).
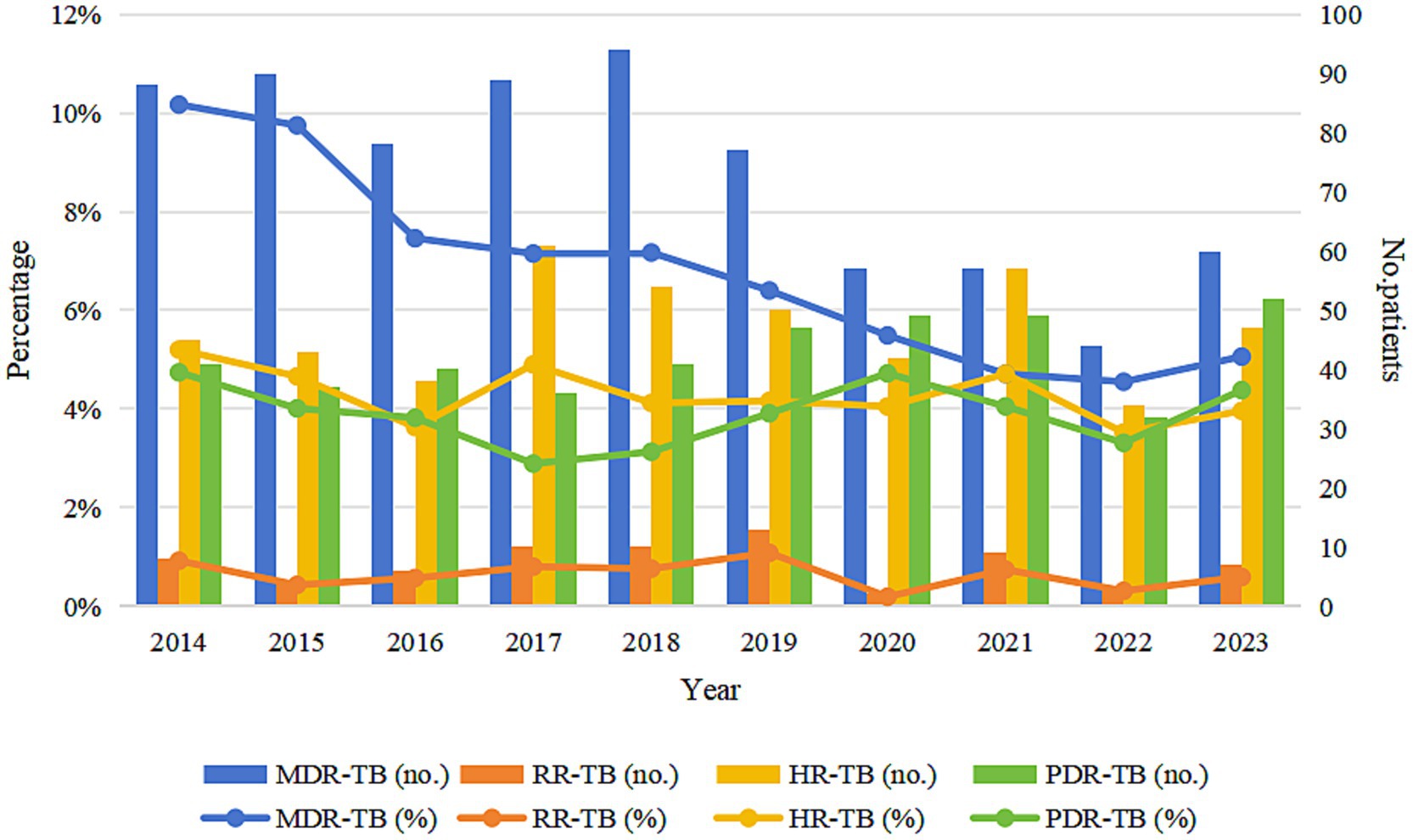
Figure 2. Trends in drug resistance patterns in Wenzhou from 2014 to 2023. MDR-TB, multidrug-resistant tuberculosis; RR-TB, rifampicin-resistant tuberculosis; HR-TB, isoniazid-resistant tuberculosis; PDR-TB, polydrug-resistant tuberculosis.
3.3 Trends of MDR-TB proportion among migrant and local notified cases
Among the 734 MDR-TB cases, 352 (47.96%) were notified among migrants and 382 (52.04%) among local residents. The proportion of MDR among notified migrant TB cases was 7.35%, higher than that among local notified cases (6.15%) (χ2 = 6.250, p < 0.05) (Table 2). The annual share of migrant MDR-TB cases among all MDR-TB cases rose from 2014 to 2019 and then decreased markedly after 2020 (p < 0.001) (Table 3; Figure 3).
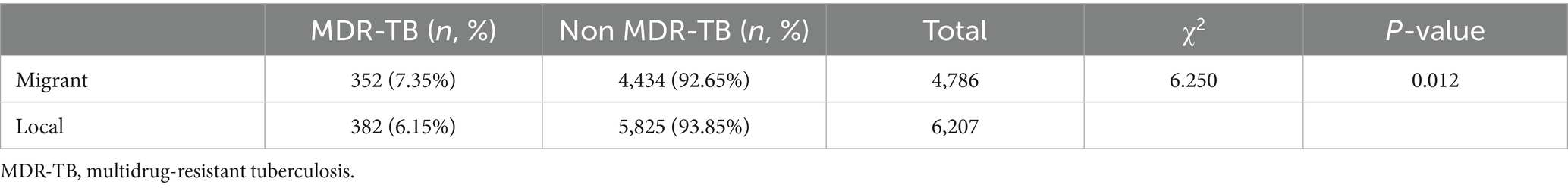
Table 2. Proportion of MDR-TB among notified TB cases in migrant and local populations, Wenzhou, 2014–2023.
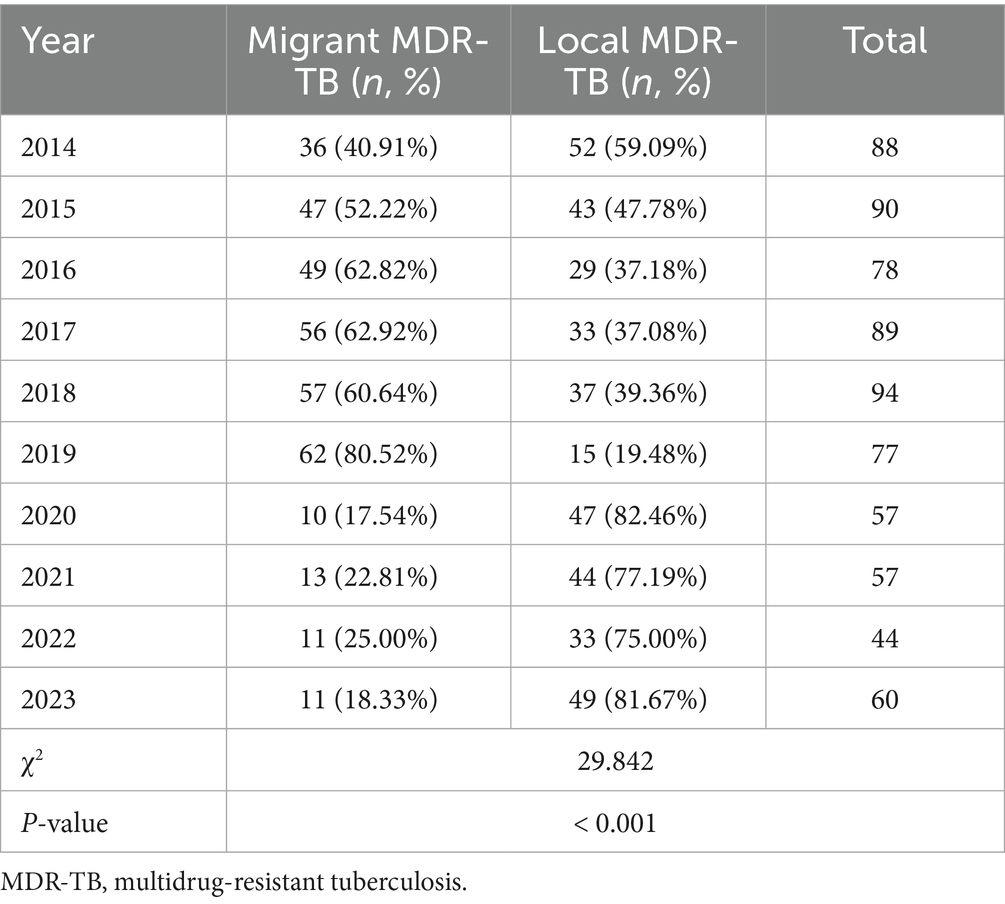
Table 3. Proportion of MDR-TB patients among migrant population and local residents in Wenzhou, 2014–2023.
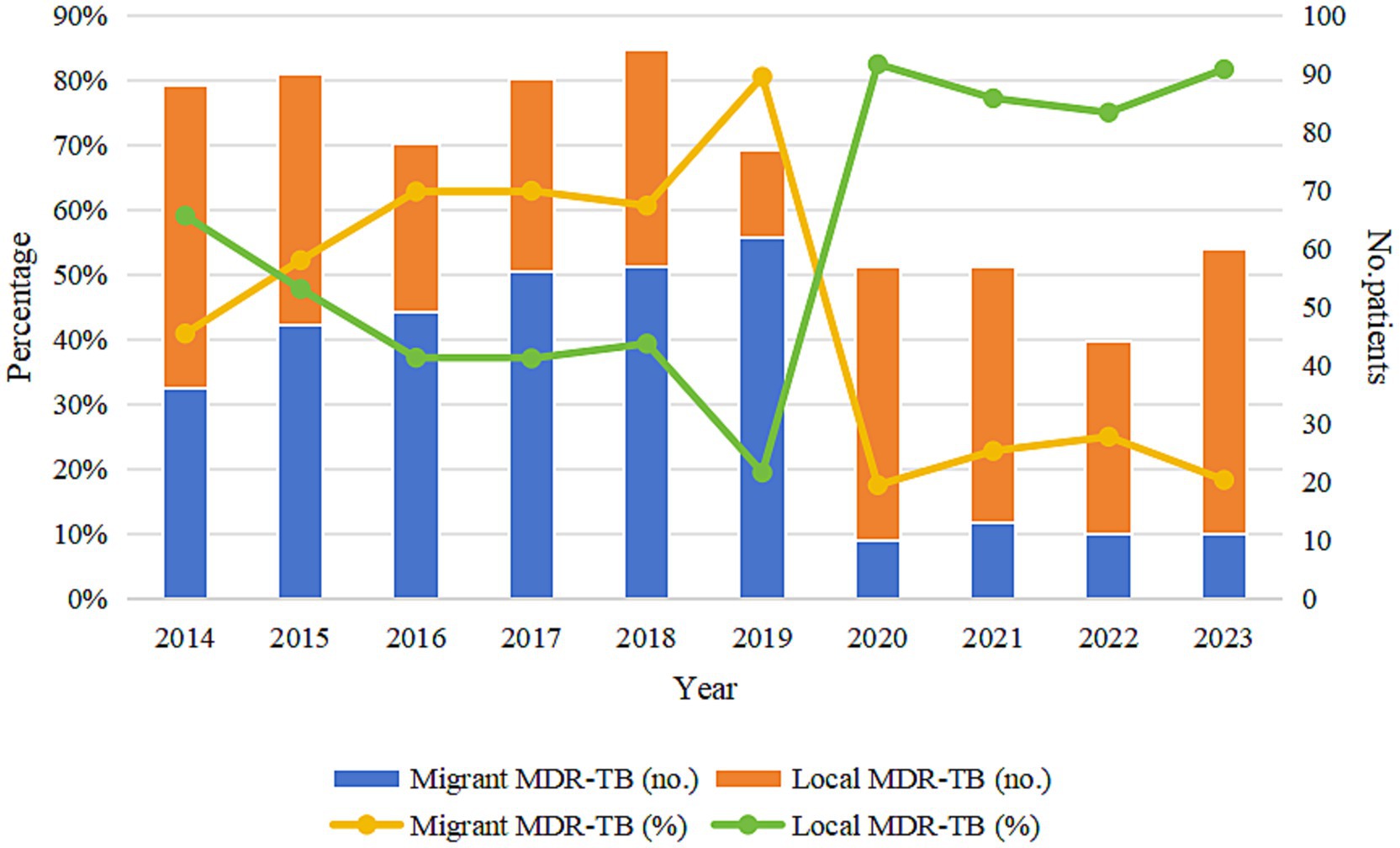
Figure 3. Trends in the proportion of migrant MDR-TB and local MDR-TB in Wenzhou from 2014 to 2023. MDR-TB, multidrug-resistant tuberculosis.
3.4 Provincial distribution of migrant MDR-TB patients
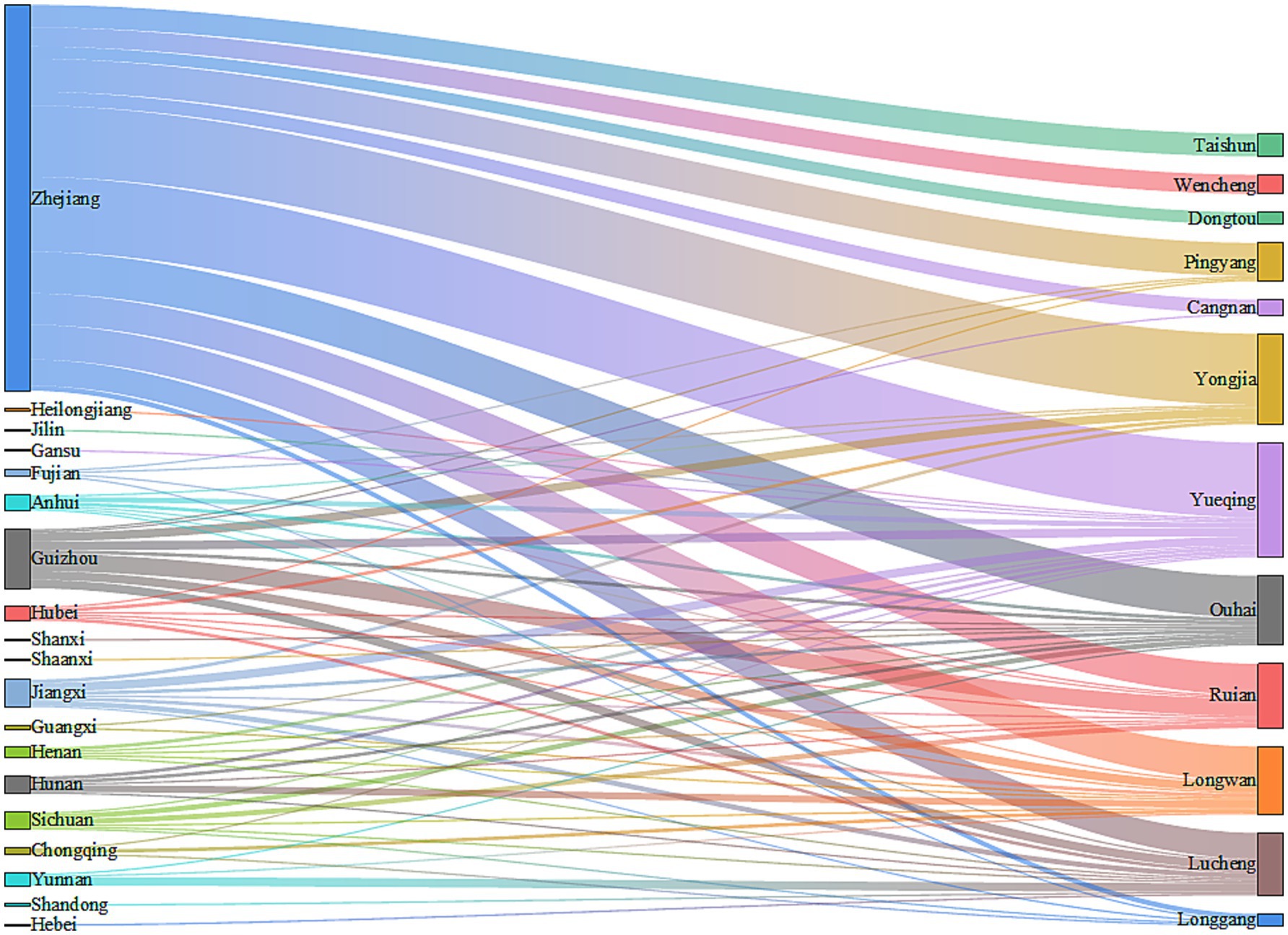
Among the 352 migrant MDR-TB patients, 226 cases (64.21%) were from the migrant population within Zhejiang Province. The migrant population from outside Zhejiang Province included 37 cases (10.51%) from Guizhou Province, 18 cases (5.11%) from Jiangxi Province, 10 cases (2.84%) from Sichuan Province, and 61 cases (17.33%) from other provinces. Of the 352 migrant MDR-TB patients, 339 cases (96.31%) currently reside in Wenzhou. Among these, 222 cases (65.49%) were registered in Zhejiang Province, 34 cases (10.03%) in Guizhou Province, 16 cases (4.72%) in Jiangxi Province, 10 cases (2.95%) in Sichuan Province, 10 cases (2.95%) in Hunan Province, and 47 cases (13.86%) in other provinces (Figure 4).
3.5 Intra-city distribution of migrant MDR-TB patients in Wenzhou
Among the 339 migrant MDR-TB patients residing in Wenzhou, 66 cases (19.47%) were reported in Yueqing City, 52 cases (15.34%) in Yongjia County, 40 cases (11.80%) in Ouhai District, 39 cases (11.50%) in Longwan District, 37 cases (10.91%) in Rui’an City, 36 cases (10.62%) in Lucheng District, and 69 cases (20.36%) in other areas of Wenzhou City (Figure 4). The distribution of patients from Zhejiang Province predominantly occurred in Yueqing City, Yongjia County, and Ouhai District. In contrast, patients from Guizhou Province primarily migrated to Rui’an City, Lucheng District, and Longwan District. Cases from Jiangxi Province were mainly found in Yueqing City and Lucheng District, while those from Sichuan Province primarily settled in Rui’an City and Ouhai District (Figure 4). Notably, migrant MDR-TB cases in Longwan District constituted 67.24% of all MDR-TB cases, whereas Dongtou District, Ouhai District, and Taishun County accounted for 63.64, 62.50, and 56.52%, respectively (Table 4).
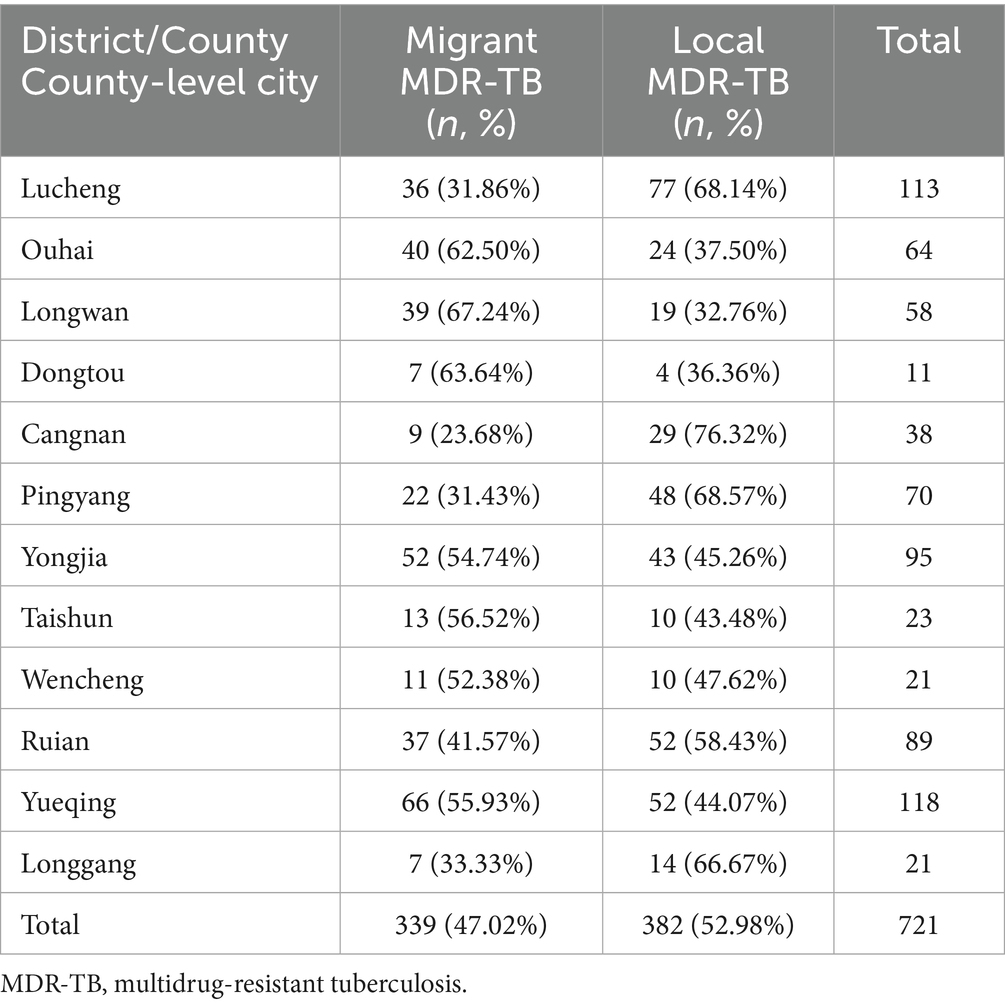
Table 4. Proportion of MDR-TB among migrant population and local residents in various districts, counties and county-level cities in Wenzhou, 2014–2023.
4 Discussion
The results of this study indicate that the overall proportion of MDR among notified TB cases in Wenzhou City from 2014 to 2023 was 6.68%, which decreased to 5.06% in 2023. A study conducted by Yao et al. (12) in Anhui Province from 2015 to 2016 reported a MDR rate of 7.63%. Another study by Wang et al. (13) in Hainan Province from 2019 to 2021 found the MDR rate to be 19.19%. Similarly, research conducted by Wang et al. (14) in Luoyang City from 2019 to 2022 revealed a MDR rate of 11.3%. Furthermore, a study by Li et al. (15) in Hangzhou City from 2012 to 2022 reported a MDR rate of 5.3%. A study by Sambas et al. (16) in Mecca, Saudi Arabia, from 2009 to 2019 showed a MDR rate of 5.0%. A systematic review and meta-analysis of the prevalence of MDR-TB in Iran from 1981 to 2023 indicated a multidrug resistance rate of 12.31% (17). Additionally, Tengan et al. (18) systematic review and meta-analysis on the prevalence of MDR-TB in Latin America and the Caribbean reported a rate of 13.0%. A prospective cohort study by Aznar et al. (19) on the prevalence and risk factors of MDR-TB in Cubal, Angola, found a MDR rate of 25%. By comparing the results of these previous studies, we conclude that the MDR rate among TB patients in Wenzhou City is relatively low both within China and in the context of the global epidemic.
This study found that the proportion of MDR among notified patients with a history of TB treatment was higher than in new patients, which aligns with the findings of Du et al. (20) in Dalian, China, and Molla et al. (21) in East Africa. Numerous studies have identified previous treatment history as a risk factor for the development of acquired MDR (22–24). However, a study by Nsofor et al. (25) in Shanghai, China, revealed that over half of patients with a history of TB treatment developed drug resistance due to primary drug resistance rather than acquired drug resistance. Consequently, drug resistance screening should be conducted during the diagnosis and treatment of cases to facilitate the timely detection of primary multidrug resistance. Additionally, comprehensive management of the treatment of new patients should be reinforced to mitigate the risk of their progression to retreatment cases. This approach aims to reduce the spread of MDR-TB through early detection and proactive management.
From 2014 to 2023, the proportion of MDR-TB among notified cases in Wenzhou City demonstrated a significant downward trend. Concurrently, the proportions of RR-TB, HR-TB, and PDR-TB among notified cases exhibited slight decreases. These trends indicate an overall reduction in the proportion of drug-resistant TB in Wenzhou City. This positive outcome can be attributed to a series of prevention and control measures implemented over the years, including the comprehensive execution of the DOTS-Plus strategy, the expansion of pDST qualifications, and the provision of government subsidies to patients with DR-TB. Furthermore, collaborative projects, such as the prospective study on the treatment of RR-TB in partnership with the infection department team at Huashan Hospital, affiliated with Fudan University, have contributed to enhancing the success rate of MDR-TB treatment, curbing the spread of MDR-TB, and consequently reducing the drug resistance rate.
The proportion of migrant MDR-TB cases among all notified MDR-TB cases in Wenzhou City constitutes 47.96% of the total MDR-TB cases. This figure is relatively high compared to findings from other regions. For instance, a study conducted in Hangzhou indicated that migrant MDR-TB accounted for 46% of the total cases (15), while a report from Shanghai revealed that this proportion was 53% (26). Additionally, research by AI Mahrouqi et al. (27) in the Sultanate of Oman reported that migrant MDR-TB cases represented 35.6% of the total, and a study by Oliveira et al. (28) in Portugal found that the figure was 32.9%. The proportion of MDR-TB among notified migrant TB cases in this study was higher than that among notified local TB cases, aligning with results from previous studies (29, 30). The significant proportion and incidence of MDR-TB in the migrant population, corroborated by various studies indicating that migration is a risk factor for the development of MDR-TB (31, 32), underscore the necessity and importance of implementing effective prevention and control strategies specifically targeted at this demographic. Notably, the proportion of migrant MDR-TB in Wenzhou City has shown a year-on-year increase from 2014 to 2019, but declined significantly after 2020. This downward trend is consistent with our recent study in an older adult cohort [citing40671759] (33), which employed time-series analysis to demonstrate that strict mobility-restriction policies during the COVID-19 pandemic (e.g., transportation limitations and community lockdowns) led to a transient decline in reported TB incidence during 2020–2021 (APC = −10.1, 95% CI: −15.3 to −4.7). Although the present study did not directly collect data on these control policies, the indirect evidence from the older adult-cohort study supports this inference. As we transition into the post-pandemic era, it remains imperative that prevention and control of MDR-TB among the migrant population continue to receive high priority.
The results of this study indicate that 64.21% of migrant MDR-TB patients originate from the migrant population in Zhejiang Province, while the remaining cases primarily come from areas outside Zhejiang, including Guizhou Province, Jiangxi Province, and Sichuan Province. The majority of migrant MDR-TB patients reside in Wenzhou. Our analysis identifies several contributing factors: First, Wenzhou’s economy is relatively developed, and its industrial structure is diversified (34), encompassing sectors such as manufacturing and commerce, which provide numerous employment opportunities and relatively higher income levels. This economic environment attracts labor from various levels, making it easier for the migrant population in Zhejiang Province to secure economic benefits by working and living in Wenzhou. Additionally, individuals from economically underdeveloped regions, such as Guizhou, Jiangxi, and Sichuan provinces, are drawn to Wenzhou’s economic vitality in hopes of improving their financial circumstances. Second, Zhejiang Province experiences frequent population movements, with Wenzhou, as a prominent economic hub, attracting many people from within the province. Furthermore, provinces like Guizhou, Jiangxi, and Sichuan have large population bases, contributing to significant migrant worker movements, and Wenzhou has emerged as a preferred destination for these individuals. Third, social networks formed by relatives and friends from the same village who are already residing and working in Wenzhou facilitate the migration process. Newcomers often follow these established connections, leading to a clustering effect that may result in an influx of patients into Wenzhou. These findings underscore the strong association between the migrant population and MDR-TB, highlighting the necessity for public health prevention and control strategies to account for regional economic disparities and the characteristics of the migrant population. Wenzhou has a developed economy and experiences a significant influx of population. It is advisable to enhance health screening at critical points, such as employment centers and entry points for migrant populations, to detect potential cases of MDR-TB early and prevent its spread. Additionally, it is important to increase awareness of MDR-TB in enterprises, communities, and other gathering places, thereby enhancing public understanding of prevention measures and promoting the adoption of healthy habits. Utilizing the network of local residents to train health promoters can facilitate the efficient dissemination of TB prevention and control information, establish a multi-level protection network, and ultimately reduce the risk and impact of MDR-TB transmission in Wenzhou and beyond.
Among the 339 migrant MDR-TB patients residing in Wenzhou, the primary areas of residence include Yueqing City, Yongjia County, and Ouhai District. Patients from Zhejiang Province predominantly migrate to Yueqing City, Yongjia County, and Ouhai District, while those from Guizhou Province primarily move to Rui’an City, Lucheng District, and Longwan District. Additionally, cases from Jiangxi Province mainly flow into Yueqing City and Lucheng District. Notably, migrant MDR-TB cases in Longwan District represent the highest proportion of all MDR-TB cases, accounting for 67.24%. Furthermore, Dongtou District, Ouhai District, Taishun County, Yueqing City, Yongjia County, and Lucheng District exhibit a higher prevalence of migrant MDR-TB cases, all exceeding 50%. These findings underscore the necessity of developing MDR-TB prevention and control strategies tailored for all districts and counties in Wenzhou City, providing a valuable reference for formulating effective interventions.
To translate our findings into immediate action, we propose three practical measures: Set up rapid on-site screening booths at Wenzhou South Railway Station, Shuangyu Coach Terminal, and major industrial parks; sputum smear and GeneXpert results are delivered the same day. Create a 24-h WeChat referral group linking Wenzhou with Guizhou, Jiangxi, and Sichuan to share patient information instantly and cut treatment interruption. Establish a “Migrant MDR-TB Rapid Response Team” led by Wenzhou Central Hospital together with county-level facilities to ensure referral and treatment initiation within 48 h of notification.
This study has several limitations. First, we did not assess whether these cases developed MDR-TB before or after arriving in Wenzhou, which may influence the interpretation of our results. Second, the economic structure of Wenzhou, and indeed China as a whole, is undergoing changes that could alter the composition of the migrant population in the future (35), thereby impacting the applicability of our research conclusions. Third, drug resistance was defined exclusively by phenotypic DST; the absence of genotypic data (Whole Genome Sequencing or line-probe assays) prevents us from distinguishing imported infections from local reactivation or transmission and limits insight into resistance mechanisms. Future work will integrate molecular surveillance and prospective genotypic–phenotypic linkage to refine MDR-TB control strategies.
5 Conclusion
Between 2014 and 2023, the proportion of MDR among notified TB cases in Wenzhou declined overall. Migrants contributed nearly half of all notified MDR-TB cases. Sustained, migrant-focused and district-specific interventions are required to further curb the spread of MDR-TB in Wenzhou.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Wenzhou Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because as a retrospective study, all data were anonymized prior to analysis, and the Medical Ethics Committee of Wenzhou Central Hospital approved the exemption of patient informed consent.
Author contributions
LW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Methodology. XC: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. SX: Data curation, Writing – original draft, Writing – review & editing. XL: Data curation, Writing – original draft, Writing – review & editing. SW: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. XX: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key Laboratory of Diagnosis and Treatment of New and Recurrent Infectious Diseases of Wenzhou (grant no. 2021HZSY0067) and Wenzhou Central Hospital Ding Li Talent Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Burki, T. Tuberculosis reclaims position as top infectious killer. Lancet Infect Dis. (2024) 24:e743–4. doi: 10.1016/s1473-3099(24)00761-8
2. Dheda, K, Barry, CE 3rd, and Maartens, G. Tuberculosis. Lancet. (2016) 387:1211–26. doi: 10.1016/s0140-6736(15)00151-8
4. Chin, KL, Anibarro, L, Chang, ZY, Palasuberniam, P, Mustapha, ZA, Sarmiento, ME, et al. Impacts of MDR/XDR-TB on the global tuberculosis epidemic: challenges and opportunities. Curr Res Microb Sci. (2024) 7:100295. doi: 10.1016/j.crmicr.2024.100295
5. Naidoo, K, Perumal, R, Cox, H, Mathema, B, Loveday, M, Ismail, N, et al. The epidemiology, transmission, diagnosis, and management of drug-resistant tuberculosis-lessons from the south African experience. Lancet Infect Dis. (2024) 24:e559–75. doi: 10.1016/s1473-3099(24)00144-0
6. Odone, A, Tillmann, T, Sandgren, A, Williams, G, Rechel, B, Ingleby, D, et al. Tuberculosis among migrant populations in the European Union and the European economic area. Eur J Pub Health. (2015) 25:506–12. doi: 10.1093/eurpub/cku208
7. Ma, D, Zhou, K, Xu, J, and Qian, Y. Industrial structure upgrading, population aging and migration: an empirical study based on Chinese provincial panel data. PLoS One. (2023) 18:e0291718. doi: 10.1371/journal.pone.0291718
8. Thi, SS, Parker, DM, Swe, LL, Pukrittayakamee, S, Ling, CL, Amornpaisarnloet, K, et al. Migration histories of multidrug-resistant tuberculosis patients from the Thailand-Myanmar border, 2012-2014. Int J Tuberc Lung Dis. (2017) 21:753–8. doi: 10.5588/ijtld.16.0658
9. Lin, K, and Xiang, L. Factors associated with non-adherence to treatment among migrants with MDR-TB in Wuhan, China: a cross-sectional study. Risk Manag Healthc Policy. (2024) 17:727–37. doi: 10.2147/rmhp.S448706
10. Chen, J, Qi, L, Xia, Z, Shen, M, Shen, X, Mei, J, et al. Which urban migrants default from tuberculosis treatment in Shanghai, China? PLoS One. (2013) 8:e81351. doi: 10.1371/journal.pone.0081351
11. Organization WH. Definitions and reporting framework for tuberculosis-2013 revision: Updated December 2014 and January 2020. Geneva: World Health Organization (2013).
12. Yao, S, Yan, J, Li, L, Ma, D, Liu, J, Wang, Q, et al. Determining Mycobacterium tuberculosis drug resistance and risk factors for multidrug-resistant tuberculosis in sputum smear-positive tuberculosis outpatients in Anhui Province, China, 2015-2016. Infect Drug Resist. (2020) 13:1023–32. doi: 10.2147/idr.S244482
13. Wang, J, Yu, C, Xu, Y, Chen, Z, Qiu, W, Chen, S, et al. Analysis of drug-resistance characteristics and genetic diversity of multidrug-resistant tuberculosis based on whole-genome sequencing on the Hainan Island, China. Infect Drug Resist. (2023) 16:5783–98. doi: 10.2147/idr.S423955
14. Wang, Z, Hou, Y, Guo, T, Jiang, T, Xu, L, Hu, H, et al. Epidemiological characteristics and risk factors of multidrug-resistant tuberculosis in Luoyang. China Front Public Health. (2023) 11:1117101. doi: 10.3389/fpubh.2023.1117101
15. Li, Q, Wu, Y, Cheng, Q, Lu, M, Huang, Y, Bai, X, et al. Prevalence and epidemic pattern of ecdemic multidrug-resistant tuberculosis during 2012-2022 in Hangzhou, China: implication for public health strategies. BMC Public Health. (2024) 24:2859. doi: 10.1186/s12889-024-20273-7
16. Sambas, M, Rabbani, U, Al-Gethamy, MMM, Surbaya, SH, Alharbi, FFI, Ahmad, RGA, et al. Prevalence and determinants of multidrug-resistant tuberculosis in Makkah, Saudi Arabia. Infect Drug Resist. (2020) 13:4031–8. doi: 10.2147/idr.S277477
17. Ayoubi, S, Farnia, P, Farnia, P, Ghanavi, J, and Velayati, AA. Prevalence and temporal trends of multidrug-resistant tuberculosis in Iran from 1981 to 2023: a systematic review and Meta-analysis. Int J Mycobacteriol. (2024) 13:320–30. doi: 10.4103/ijmy.ijmy_162_24
18. Tengan, FM, Figueiredo, GM, Leite, OH, Nunes, AK, Manchiero, C, Dantas, BP, et al. Prevalence of multidrug-resistant tuberculosis in Latin America and the Caribbean: a systematic review and meta-analysis. Trop Med Int Health. (2020) 25:1065–78. doi: 10.1111/tmi.13453
19. Aznar, ML, Rando-Segura, A, Moreno, MM, Soley, ME, Igual, ES, Bocanegra, C, et al. Prevalence and risk factors of multidrug-resistant tuberculosis in Cubal, Angola: a prospective cohort study. Int J Tuberc Lung Dis. (2019) 23:67–72. doi: 10.5588/ijtld.18.0231
20. Du, L, Zhang, Y, Lv, X, Duan, Y, Shi, X, Ji, H, et al. Prevalence of multidrug-resistant tuberculosis in Dalian, China: a retrospective study. Infect Drug Resist. (2021) 14:1037–47. doi: 10.2147/idr.S294611
21. Molla, KA, Reta, MA, and Ayene, YY. Prevalence of multidrug-resistant tuberculosis in East Africa: a systematic review and meta-analysis. PLoS One. (2022) 17:e0270272. doi: 10.1371/journal.pone.0270272
22. Dalton, T, Cegielski, P, Akksilp, S, Asencios, L, Campos Caoili, J, Cho, SN, et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. (2012) 380:1406–17. doi: 10.1016/s0140-6736(12)60734-x
23. Le, X, Qian, X, Liu, L, Sun, J, Song, W, Qi, T, et al. Trends in and risk factors for drug resistance in Mycobacterium tuberculosis in HIV-infected patients. Viruses. (2024) 16:627. doi: 10.3390/v16040627
24. Kamolwat, P, Nateniyom, S, Chaiprasert, A, Disratthakit, A, Mahasirimongkol, S, Yamada, N, et al. Prevalence and associated risk factors of drug-resistant tuberculosis in Thailand: results from the fifth national anti-tuberculosis drug resistance survey. Trop Med Int Health. (2021) 26:45–53. doi: 10.1111/tmi.13502
25. Nsofor, CA, Jiang, Q, Wu, J, Gan, M, Liu, Q, Zuo, T, et al. Transmission is a noticeable cause of resistance among treated tuberculosis patients in Shanghai, China. Sci Rep. (2017) 7:7691. doi: 10.1038/s41598-017-08061-3
26. Ge, E, Li, D, Luo, M, Tsui, KWS, Waye, MMY, Shen, X, et al. Transmission of multidrug-resistant tuberculosis in Shanghai: roles of residential status. Int J Tuberc Lung Dis. (2018) 22:1462–8. doi: 10.5588/ijtld.18.0125
27. Al Mahrouqi, S, Gadalla, A, Al Azri, S, Al-Hamidhi, S, Al-Jardani, A, Balkhair, A, et al. Drug resistant Mycobacterium tuberculosis in Oman: resistance-conferring mutations and lineage diversity. PeerJ. (2022) 10:e13645. doi: 10.7717/peerj.13645
28. Oliveira, O, Gaio, R, Carvalho, C, Correia-Neves, M, Duarte, R, and Rito, T. A nationwide study of multidrug-resistant tuberculosis in Portugal 2014-2017 using epidemiological and molecular clustering analyses. BMC Infect Dis. (2019) 19:567. doi: 10.1186/s12879-019-4189-7
29. Hargreaves, S, Lönnroth, K, Nellums, LB, Olaru, ID, Nathavitharana, RR, Norredam, M, et al. Multidrug-resistant tuberculosis and migration to Europe. Clin Microbiol Infect. (2017) 23:141–6. doi: 10.1016/j.cmi.2016.09.009
30. Riccardi, N, Antonello, RM, Ferrarese, M, Saderi, L, Besozzi, G, Sotgiu, G, et al. Tuberculosis in migrants: epidemiology, resistance and outcome in Milan, Italy. Infect Dis. (2023) 55:543–50. doi: 10.1080/23744235.2023.2217912
31. Feng, M, Xu, Y, Zhang, X, Qiu, Q, Lei, S, Li, J, et al. Risk factors of multidrug-resistant tuberculosis in China: a meta-analysis. Public Health Nurs. (2019) 36:257–69. doi: 10.1111/phn.12582
32. Huai, P, Huang, X, Cheng, J, Zhang, C, Wang, K, Wang, X, et al. Proportions and risk factors of developing multidrug resistance among patients with tuberculosis in China: a population-based case-control study. Microb Drug Resist. (2016) 22:717–26. doi: 10.1089/mdr.2015.0186
33. Wu, L, Cai, X, Xu, S, Lin, X, Peng, T, and Jiang, X. Trends in drug resistance and epidemiological patterns of tuberculosis in elderly patients in Wenzhou, China (2014-2023). Infect Drug Resist. (2025) 18:3459–70. doi: 10.2147/idr.S530067
34. Lin, S, and Li, Z. City profile: Wenzhou - a model city of transitional China. Cities. (2019) 95:102393. doi: 10.1016/j.cities.2019.102393
Keywords: multidrug-resistant tuberculosis, epidemiology, notified cases, migrant population, Wenzhou
Citation: Wu L, Cai X, Xu S, Lin X, Wu S and Xu X (2025) Prevalence and epidemiological pattern of drug-resistant tuberculosis among migrant populations in Wenzhou City, China, 2014–2023: implications for public health strategies. Front. Public Health. 13:1600214. doi: 10.3389/fpubh.2025.1600214
Edited by:
Zisis Kozlakidis, International Agency for Research on Cancer (IARC), FranceReviewed by:
Raman Swathy Vaman, National Institute of Epidemiology (ICMR), IndiaSantosh Chokkakula, Chungbuk National University, Republic of Korea
Copyright © 2025 Wu, Cai, Xu, Lin, Wu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangliao Wu, d3VzbHd6QDE2My5jb20=; Xueqin Xu, d3p4eHFAMTM5LmNvbQ==
 Lianpeng Wu
Lianpeng Wu Xiyue Cai3
Xiyue Cai3 Shuangliao Wu
Shuangliao Wu