- 1Department of Infectious Disease, Yiwu Central Hospital, Zhejiang, China
- 2Department of Public Health and Geriatric Health Guidance, Yiwu Central Hospital, Zhejiang, China
Background: Non-alcoholic fatty liver disease (NAFLD) is a global health issue, with fast food consumption hypothesized as a risk factor. This meta-analysis aimed to explore the relationship between fast food intake and NAFLD.
Methods: This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. A comprehensive search was conducted across PubMed, Web of Science, Scopus, and Embase from inception to February 28, 2025. A total of nine eligible observational studies involving 169,771 participants were included. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using random-effects models.
Results: A higher consumption of fast food was significantly associated with a 55% increased risk of NAFLD (OR = 1.55, 95% CI: 1.51–1.59, p < 0.001, I2 = 15.6%). Moreover, fast food intake was linked to a 37% higher risk of obesity (OR = 1.37, 95% CI: 1.27–1.49, p < 0.001, I2 = 54.2%), a key metabolic factor in NAFLD pathogenesis. Sensitivity analysis confirmed the robustness of these associations, with no significant evidence of publication bias.
Conclusion: Fast food consumption is positively associated with NAFLD and obesity. Heterogeneity highlights the need for standardized methods in future large-scale studies to validate these findings and inform preventive strategies.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD), the most prevalent chronic liver condition worldwide, presents a rising burden on global health systems (1). With a global prevalence of up to 25% and a marked increase in metabolic syndrome populations, NAFLD poses a significant burden on healthcare systems (1). Characterized by excessive hepatic triglyceride accumulation, this disease can progress insidiously to non-alcoholic steatohepatitis (NASH), liver fibrosis, and hepatocellular carcinoma, underscoring its clinical relevance (2). Dietary patterns have shifted significantly in recent decades, with fast food consumption rising exponentially due to its accessibility, affordability, and convenience (2, 3). This dietary shift is particularly concerning, as fast food consumption patterns are typically rich in energy-dense, hyperpalatable components (e.g., saturated fats, refined sugars, and sodium) while lacking essential nutrients, potentially exacerbating metabolic dysfunction (3).
Although robust biological mechanisms link fast food intake to NAFLD pathogenesis—including induction of insulin resistance, promotion of visceral adiposity, and perturbation of gut microbiota—epidemiological evidence remains inconsistent (4, 5). Observational studies have reported conflicting results, with some demonstrating a positive dose-dependent association between fast food consumption and NAFLD risk (4), while others found no significant correlation (5). These discrepancies may stem from methodological heterogeneity, such as differences in dietary assessment tools (e.g., self-reported questionnaires vs. objective biomarkers) and population demographics (age, ethnicity, and baseline metabolic status).
To address the inconsistencies in the literature, we performed a systematic review and meta-analysis to examine the association between fast food consumption and NAFLD risk. By synthesizing data from high-quality observational studies, we aimed to assess the overall impact of fast food intake on NAFLD prevalence.
2 Materials and methods
2.1 Search strategy
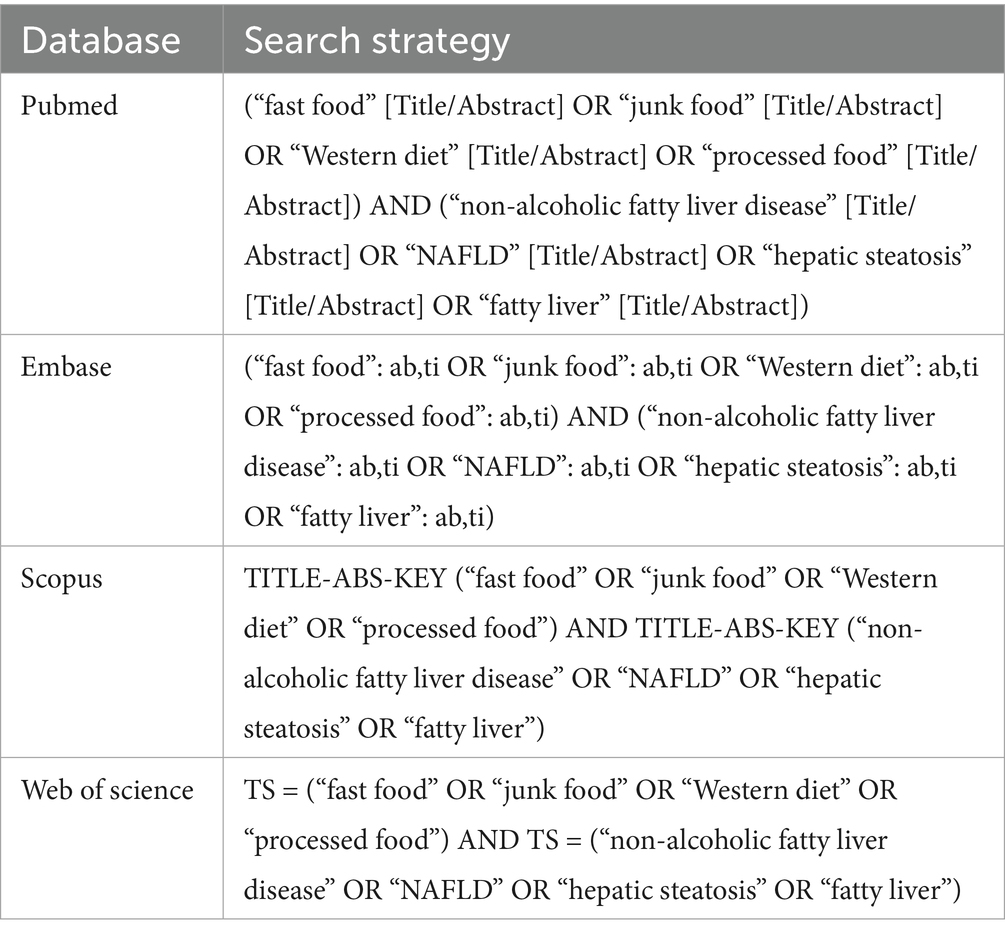
A systematic literature search was conducted across four electronic databases (PubMed, Web of Science, Scopus, and Embase) from inception to February 28, 2025, to identify all relevant studies evaluating the association between fast food consumption and NAFLD. The search strategy combined medical subject headings (MeSH terms) and free-text keywords related to both exposures and outcomes. For NAFLD, the search included terms such as “non-alcoholic fatty liver disease,” “NAFLD,” “steatohepatitis,” and “hepatic steatosis.” For fast food consumption, terms included “fast food,” “junk food,” “takeaway meals,” and “processed food intake.” Boolean operators (AND/OR) were used to combine these concepts, with truncation and wildcards applied to capture variant spellings (e.g., “consum*” for consumption/consumer). Database-specific adjustments were made to accommodate syntax differences. For example, in PubMed, MeSH terms were exploded (e.g., “fast food” [Mesh] OR “fast food” [tiab]) and combined with free-text terms. In Scopus, adjacency operators (e.g., “fast food” NEAR/3 “consum*”) were used to enhance precision. A full search syntax for each database is provided in Table 1.
2.2 Eligibility criteria
2.2.1 Study design
Only observational studies were eligible for inclusion in this systematic review and meta-analysis. This specifically encompassed cohort studies, which follow a group of individuals over time to observe the development of outcomes related to exposure; case–control studies, which compare individuals with a particular outcome (cases) to those without it (controls) to investigate the association with prior exposures; and cross-sectional studies, which assess the exposure and outcome simultaneously in a defined population at a single point in time.
2.2.2 Language
All publications considered for inclusion had to be in the English language. This language restriction was applied to ensure accurate interpretation and consistent quality assessment of the included studies. While this may introduce a potential language bias, it was deemed necessary due to resource limitations and to minimize the risk of data misinterpretation.
2.2.3 Population
The studies had to involve individuals with or without a diagnosis of NAFLD. This broad population scope was chosen to comprehensively assess the relationship between fast food consumption and NAFLD. By including both groups, it was possible to examine the impact of fast food intake on the development of NAFLD in those without the disease and any potential associations with disease severity or progression in those already diagnosed. Studies that focused solely on populations with other liver diseases or conditions unrelated to NAFLD, or those that did not provide any information on the presence or absence of NAFLD, were excluded.
2.2.4 Data availability
In this meta-analysis, fast food consumption is defined based on either of the following criteria: (1) a frequency-based definition, where individuals consume fast food more than three times per week, or (2) a proportion-based definition, where fast food accounts for at least 20% of the total dietary intake. NAFLD is characterized by lipid accumulation, primarily triacylglycerol, in hepatocytes of individuals with minimal alcohol intake (≤2 drinks/day for women, ≤3 drinks/day for men), after excluding other causes of steatosis, such as chronic liver diseases (hepatitis A, B, and C, Wilson’s disease) or medication-induced effects. Studies needed to provide data on the frequency or amount of fast food intake and a corresponding diagnosis of NAFLD. For fast food consumption, this could include details such as the number of times fast food was consumed per week or month, the portion sizes, or any other measure that quantified the exposure. The diagnosis of NAFLD is based on the “Guidelines for the Prevention and Treatment of Metabolic Dysfunction-Associated (Non-Alcoholic) Fatty Liver Disease (Version 2024)” issued by the Chinese Society of Hepatology (6). Studies that lacked sufficient data on either fast food consumption or NAFLD diagnosis were not eligible for inclusion.
2.3 Study selection
Two independent reviewers meticulously screened the search results. First, they reviewed the titles and abstracts of all retrieved studies to identify potentially relevant ones. Studies that clearly did not meet the eligibility criteria were excluded at this stage. Subsequently, the full-text versions of the remaining potentially relevant studies were obtained and carefully evaluated. Any discrepancies in the selection process between the two reviewers were resolved through in-depth discussion. If necessary, a third reviewer was consulted to reach a consensus.
2.4 Data extraction
Once the eligible studies were selected, the two independent reviewers extracted the relevant data from each study. Any discrepancies in data extraction were resolved through discussion. If a consensus could not be reached, a third reviewer was consulted to adjudicate and finalize the extracted information. The data extracted included basic study characteristics such as the study design, year of publication, country of origin, sample size, age range of the participants, and ethnic composition. Regarding the exposure variable (fast food consumption), details about how it was measured (e.g., self-reported questionnaires with specific recall periods like weekly or monthly intake, objective measures such as food frequency records), and the actual values or categories of fast food intake were noted.
2.5 Quality assessment
The quality of each included study was evaluated using the Newcastle-Ottawa Scale (NOS) (7). The NOS assesses three main aspects of observational studies: selection of the study population, comparability of the groups, and ascertainment of the outcome. For cohort studies, points are assigned based on factors such as the representativeness of the exposed cohort, the selection of the non-exposed cohort, and the adequacy of follow-up. In case–control studies, aspects like the definition of cases and controls, the selection of controls, and the ascertainment of exposure are considered. For cross-sectional studies, the sampling method, the definition of the population, and the assessment of exposure and outcome are evaluated. Each study was given a score on a scale of 0–9, with higher scores indicating better-quality studies. To enhance transparency and allow detailed evaluation of study quality, the full NOS scoring for each included study is provided in Supplementary File 1.
2.6 Statistical analysis
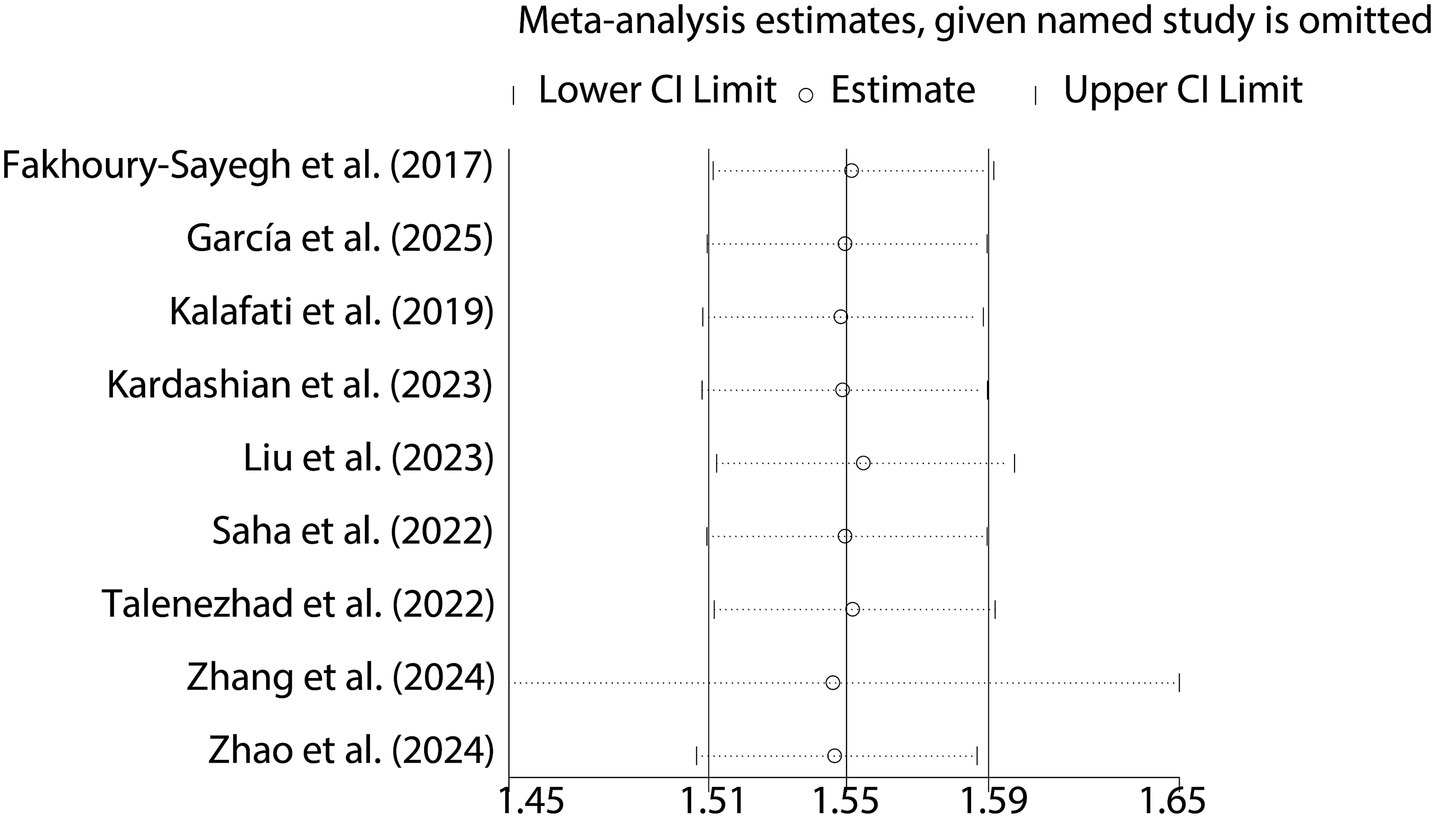
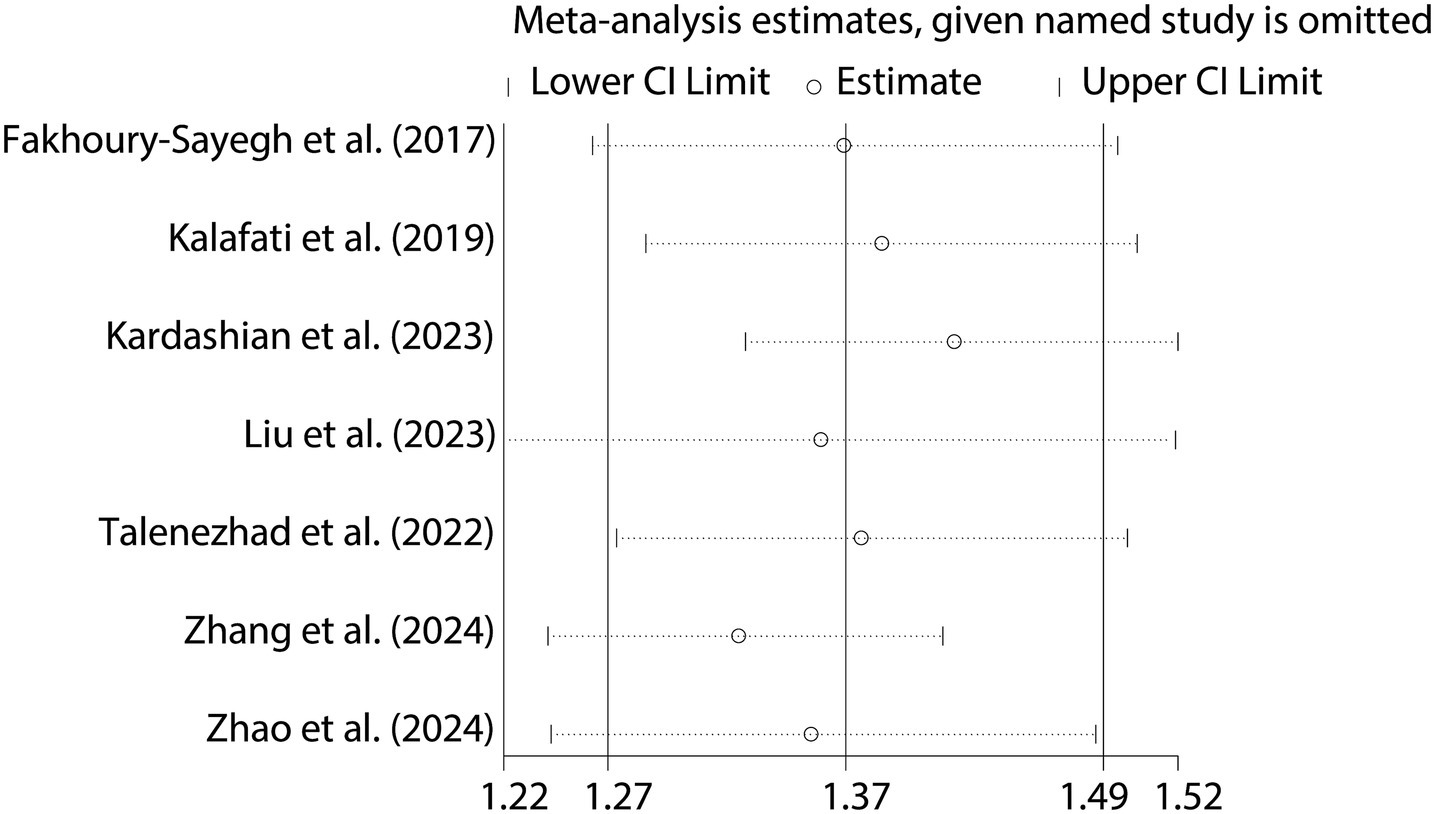
For dichotomous outcomes (presence or absence of NAFLD), odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. These ORs were used to estimate the strength of the association between fast food consumption and the risk of NAFLD. Heterogeneity was quantified using the Cochrance Q statistic and the I2 statistic. The Cochrance Q statistic tests the null hypothesis that all studies are estimating the same effect size, while the I2 statistic measures the proportion of total variation in study estimates that is due to heterogeneity rather than chance. A value of I2 > 50% and a significant Cochrance Q statistic (p < 0.05) were considered indicative of substantial heterogeneity. Sensitivity analyses were also conducted by sequentially removing each study from the meta-analysis to assess the stability of the overall results.
3 Results
3.1 Basic characteristics of the included studies
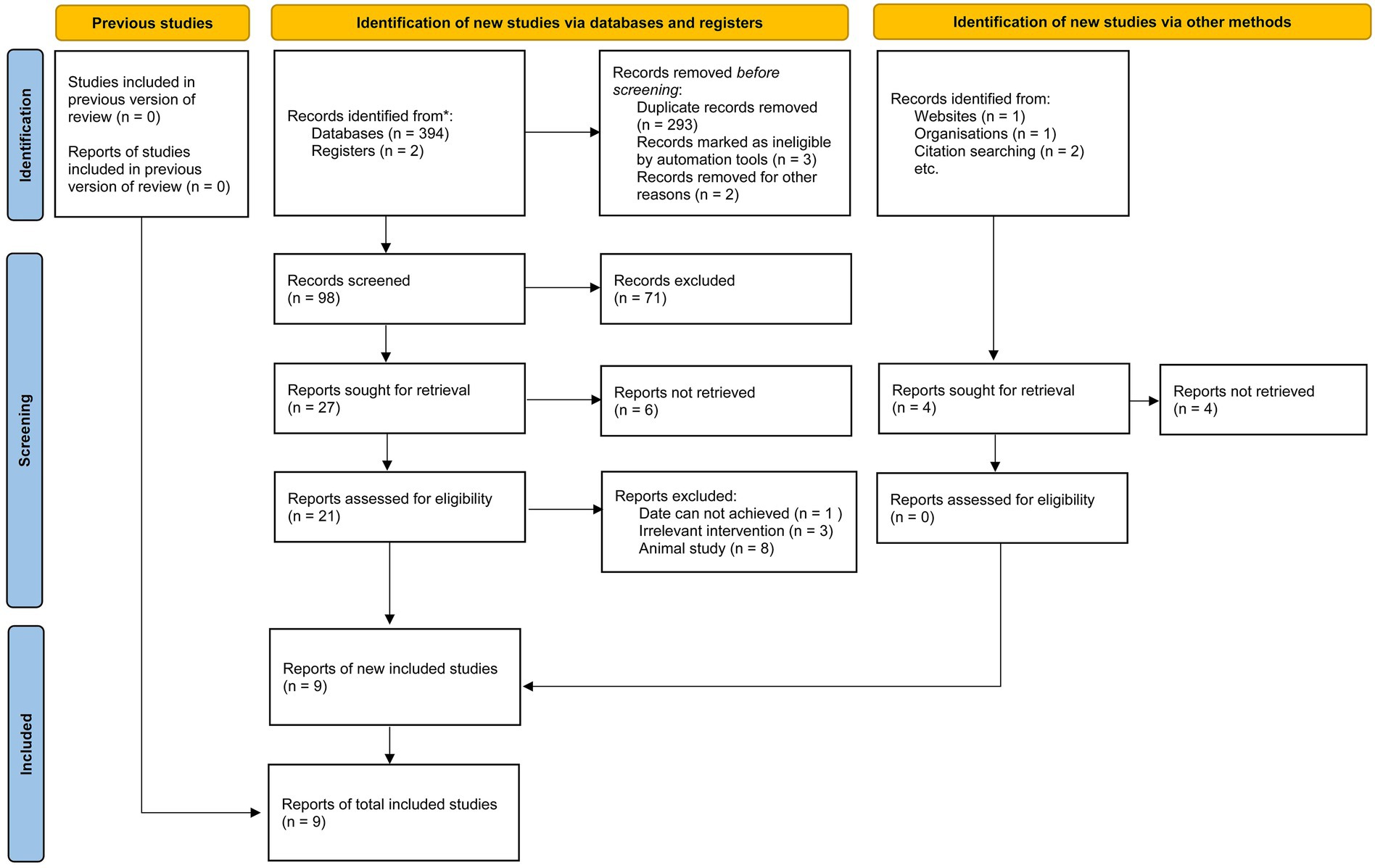
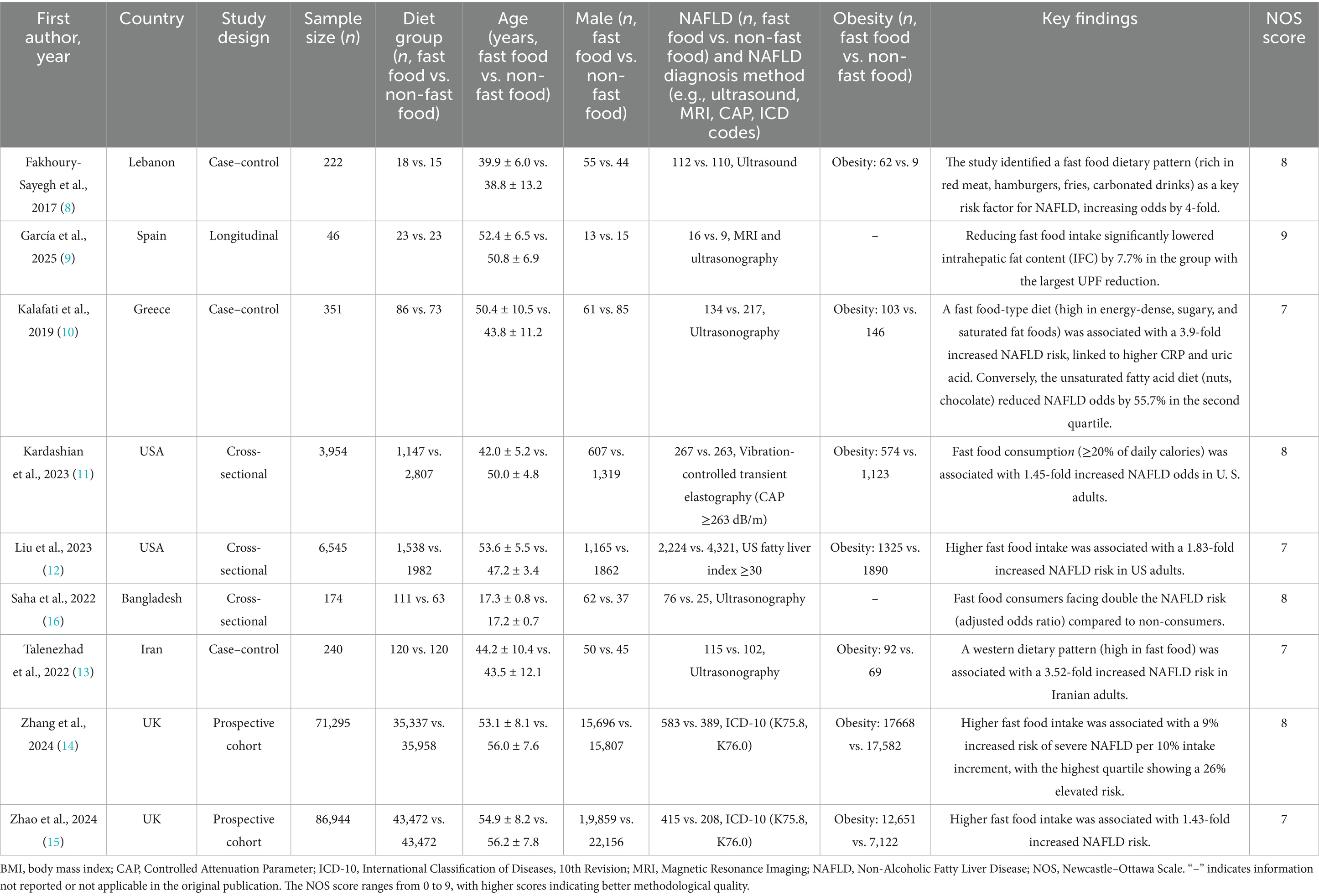
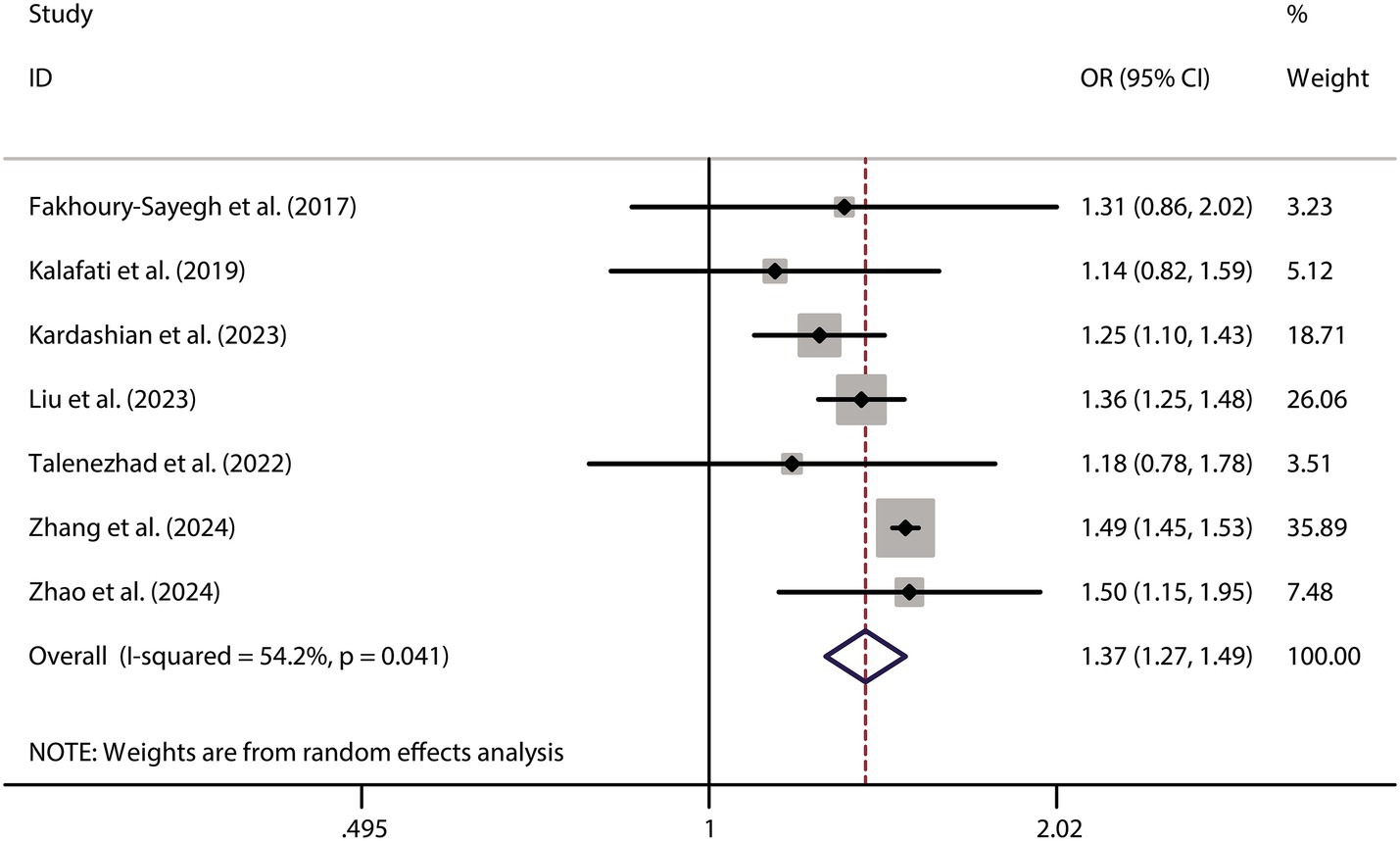
This meta-analysis included nine studies conducted across diverse geographical regions, including Lebanon, Spain, Greece, the USA, Bangladesh, Iran, and the UK (8–16). The study designs varied, comprising case–control (n = 3), cross-sectional (n = 3), longitudinal (n = 1), and prospective cohort (n = 2) methodologies. The sample sizes ranged from 46 to 86,944 participants, with a total of 169,771 individuals analyzed. Participants were categorized into fast food and non-fast food diet groups. The mean age ranged from 17.2 to 56.0 years, and the proportion of male participants varied across studies, with some reporting a near-equal gender distribution while others exhibited a male predominance. Several studies also assessed obesity and body mass index (BMI) differences between dietary groups, consistently reporting higher BMI or a greater prevalence of obesity among fast food consumers. The methodological quality of the included studies, as assessed by the NOS, ranged from 7 to 9, indicating a generally high level of study rigor. The literature screening process adhered to PRISMA_2020 guidelines (Figure 1), and the basic information of the included studies is presented in Table 2. To ensure transparency and compliance with systematic review reporting standards, the completed PRISMA 2020 checklist has been provided as Supplementary File 2.
3.2 Association between fast food consumption and the risk of NAFLD
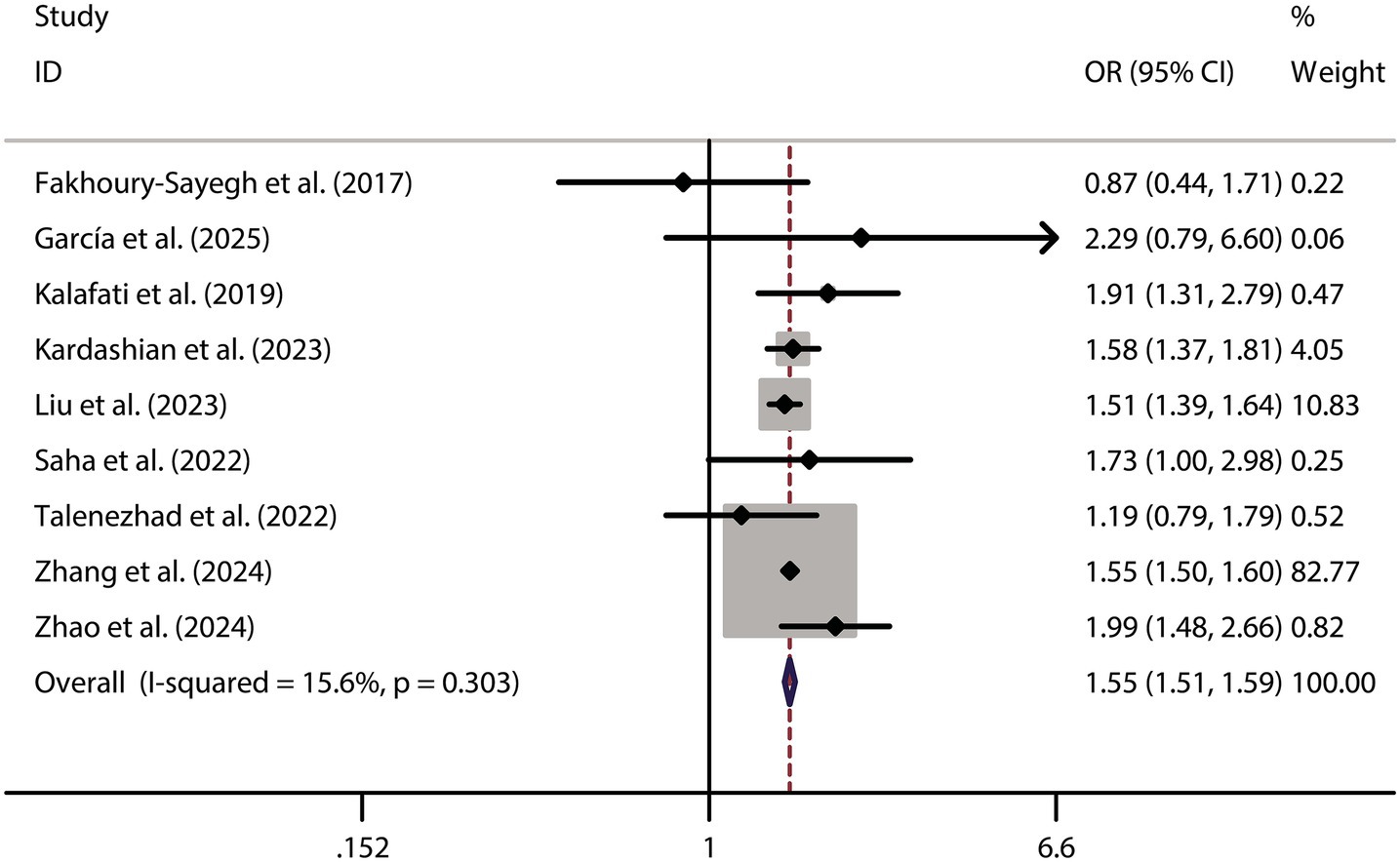
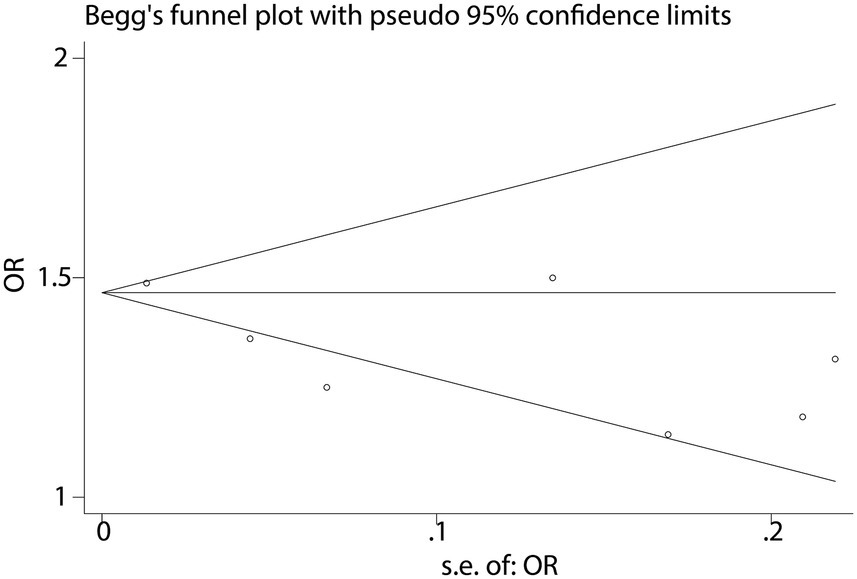
When analyzing the dichotomous outcome of NAFLD presence or absence, the pooled results from our meta-analysis demonstrated that increased fast food consumption is significantly associated with a higher risk of developing NAFLD. The combined OR was 1.55 (95% CI: 1.51–1.59, p < 0.001, I2 = 15.6%, Figure 2). Sensitivity analysis confirmed the robustness of these findings (Figure 3), and publication bias assessment suggested no significant bias affecting the results (Figure 4). These findings highlight the potential role of dietary patterns in NAFLD risk and emphasize the need for dietary interventions to mitigate this risk.
3.3 Association between fast food consumption and the risk of obesity
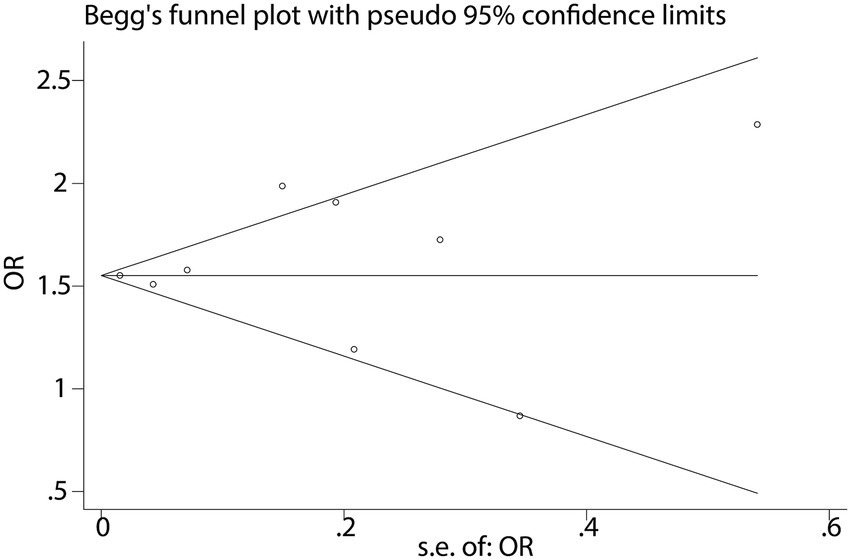
To further validate the relationship between fast food consumption and fat accumulation, we conducted a meta-analysis of seven studies that examined the association between fast food intake and obesity. The pooled results indicated that fast food consumption is a significant risk factor for obesity, with a combined OR of 1.37 (95% CI: 1.27–1.49, p < 0.001, I2 = 54.2%, Figure 5). Sensitivity analysis (Figure 6) confirmed the robustness of these findings, and no significant publication bias was detected (Figure 7).
4 Discussion
NAFLD has emerged as a global health crisis, affecting approximately 25% of the population and posing significant risks for cirrhosis and hepatocellular carcinoma (17). This epidemic has coincided with a dramatic surge in fast food consumption, which is characterized by its high content of saturated fats, refined sugars, and sodium, coupled with easy accessibility and affordability (18). Mechanistically, fast food diets promote insulin resistance, visceral adiposity, and gut microbiota dysbiosis, all of which are implicated in NAFLD pathogenesis (19). However, epidemiological studies have yielded conflicting results, with some reporting a positive association between fast food intake and NAFLD (20), while others found no significant link (21). These inconsistencies likely stem from methodological variations in dietary assessment, NAFLD diagnostic criteria, and population demographics. To address this ambiguity, we conducted a systematic review and meta-analysis to quantify the association between fast food consumption and NAFLD, identify sources of heterogeneity, and inform future research.
Our meta-analysis of 9 observational studies revealed a 55% increased risk of NAFLD among individuals with higher fast food consumption (OR = 1.55, 95% CI: 1.51–1.59, p < 0.001). This finding aligns with biological plausibility, as fast food diets are known to induce hepatic steatosis through multiple pathways: (1) insulin resistance: high intake of refined sugars and trans fats impairs insulin signaling, leading to increased lipolysis and hepatic fat accumulation (22). (2) Obesity: fast food consumption was associated with a 37% higher obesity risk (OR = 1.37, 95% CI: 1.27–1.49), and obesity is a key driver of NAFLD via adipose tissue inflammation and ectopic fat deposition (3). (3) Gut microbiota dysbiosis: processed foods may alter gut microbial composition, promoting endotoxemia and hepatic inflammation (5).
Despite robust findings, several limitations warrant consideration: (1) Heterogeneity: the I2 statistic for NAFLD (15.6%) indicates low heterogeneity, suggesting consistent findings across studies evaluating this outcome. In contrast, the I2 for obesity (54.2%) suggests moderate heterogeneity. This may arise from multiple sources. First, differences in NAFLD diagnostic criteria (e.g., ultrasound vs. MRI vs. CAP or ICD codes) may lead to inconsistent outcome classification. Second, regional dietary habits could influence both the type and frequency of fast food consumed—fast food in Mediterranean countries (e.g., Spain, Greece) often differs in composition and preparation methods compared to Western or Asian settings, potentially moderating its metabolic impact. Third, population characteristics such as age ranges (e.g., adolescents vs. older adults), genetic predispositions, and baseline metabolic profiles may alter susceptibility to NAFLD. Fourth, dietary assessment tools varied widely, from self-reported frequency to structured food frequency questionnaires, which may further introduce measurement error and misclassification bias. These inter-study variations highlight the need for more stratified analyses and standardized methodologies in future investigations to better account for potential effect modifiers and improve comparability across studies. (2) Observational design: the reliance on observational studies precludes causal inference. Reverse causation (e.g., individuals with NAFLD altering their diet) cannot be ruled out, though sensitivity analyses and the use of prospective cohort studies partially mitigate this concern. (3) Dietary assessment: most studies used self-reported questionnaires, which are prone to recall bias and misclassification of fast food intake. Future studies should incorporate objective measures (e.g., biomarkers or food frequency records). (4) Generalizability: the included studies were conducted in diverse regions (e.g., Lebanon, Spain, USA), but the majority focused on adults. The extrapolation of findings to children or specific ethnic groups (e.g., East Asians, who may have higher NAFLD susceptibility) remains uncertain. (5) Publication bias: while funnel plots showed no significant asymmetry, the possibility of unpublished negative studies cannot be entirely dismissed.
Furthermore, recent high-quality evidence supports a broader view on the relationship between dietary patterns and metabolic health outcomes, including NAFLD. For instance, an umbrella review by Lane et al. (23) synthesized meta-analyses and found that greater exposure to ultra-processed foods is consistently associated with increased risks of metabolic disorders, including obesity and type 2 diabetes, both of which are major risk factors for NAFLD. Additionally, data from a UK population-based study by Madruga et al. (24) highlighted that over 56% of dietary energy intake derives from ultra-processed foods, underscoring their dominant role in contemporary eating patterns. However, not all studies concur. Some reports have found no significant association between fast food or ultra-processed food intake and NAFLD, likely due to variation in diagnostic tools, sample demographics, and cultural dietary differences (8, 13, 16). Moreover, the predominance of data from Western or high-income countries in our analysis (e.g., USA, UK, Spain) limits generalizability to underrepresented regions such as Africa and South America. To provide a more balanced and comprehensive perspective, future studies should include diverse geographical settings and socio-economic backgrounds. It is also essential to integrate findings from recent narrative and systematic reviews on broader dietary patterns and liver health. Examples include recent reviews examining the associations between NAFLD and various dietary patterns, such as the Mediterranean diet, plant-based dietary interventions, and dietary classifications based on the NOVA system (25–30). Additionally, as most included studies relied on self-reported dietary intake, recall bias and misclassification of fast food exposure cannot be ruled out, which may have attenuated or inflated the observed associations.
In conclusion, this meta-analysis provides robust evidence that fast food consumption is associated with increased NAFLD and obesity risk. The findings highlight the need for public health interventions targeting dietary patterns to reduce NAFLD burden. However, heterogeneity in study design and methodology underscores the importance of standardized approaches in future research to validate these associations and inform precision prevention strategies. To enhance real-world applicability, these findings warrant the implementation of specific public health strategies. For example, regulatory policies such as mandatory front-of-package labeling, taxation on ultra-processed foods, and restrictions on fast food advertising to children may help reduce consumption. Additionally, national dietary guidelines should emphasize limiting fast food intake and promote the adoption of whole-food, plant-based dietary patterns. Public health campaigns and educational programs targeting high-risk populations can further support behavior change and reduce NAFLD prevalence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JH: Supervision, Project administration, Writing – original draft, Resources, Writing – review & editing. YW: Writing – original draft, Formal analysis, Data curation, Software. FW: Methodology, Writing – original draft, Investigation, Visualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Institutional support was provided by Yiwu Central Hospital, including access to library databases and software necessary for data analysis and manuscript preparation. This work was supported by the Jinhua Science and Technology Bureau 2024 Major Science and Technology Plan Project (Grant No. 2024-3-131).
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1600826/full#supplementary-material
References
1. Vancells Lujan, P, Viñas Esmel, E, and Sacanella, ME. Overview of non-alcoholic fatty liver disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients. (2021) 13:1442. doi: 10.3390/nu13051442
2. Carpi, RZ, Barbalho, SM, Sloan, KP, Laurindo, LF, Gonzaga, HF, Grippa, PC, et al. The effects of probiotics, prebiotics and synbiotics in non-alcoholic fat liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Int J Mol Sci. (2022) 23:8805. doi: 10.3390/ijms23158805
3. Avelar-Rodríguez, D, Peña-Vélez, R, Popov, J, Hill, L, and Ryan, PM. Probiotics and non-alcoholic fatty liver disease in children and adolescents: a systematic review. Rev Esp Enferm Dig. (2023) 115:418–27. doi: 10.17235/reed.2022.8796/2022
4. Zhang, Z, Burrows, K, Fuller, H, Speliotes, EK, Abeysekera, KWM, Thorne, JL, et al. Non-alcoholic fatty liver disease and vitamin D in the UK biobank: a two-sample bidirectional Mendelian randomisation study. Nutrients. (2023) 15:1442. doi: 10.3390/nu15061442
5. Henney, AE, Gillespie, CS, Alam, U, Hydes, TJ, and Cuthbertson, DJ. Ultra-processed food intake is associated with non-alcoholic fatty liver disease in adults: a systematic review and meta-analysis. Nutrients. (2023) 15:2266. doi: 10.3390/nu15102266
6. Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of metabolic dysfunction-associated (non-alcoholic) fatty liver disease (version 2024). Chin J Hepatol. (2024) 32:418–34. doi: 10.3760/cma.j.cn501113-20240327-00163
7. Wen, X, Xiong, Y, Qu, X, Jin, L, Zhou, C, Zhang, M, et al. The risk of endometriosis after exposure to endocrine-disrupting chemicals: a meta-analysis of 30 epidemiology studies. Gynecol Endocrinol. (2019) 35:645–50. doi: 10.1080/09513590.2019.1590546
8. Fakhoury-Sayegh, N, Younes, H, Heraoui, G, and Sayegh, R. Nutritional profile and dietary patterns of Lebanese non-alcoholic fatty liver disease patients: a case-control study. Nutrients. (2017) 9:1245. doi: 10.3390/nu9111245
9. Garcia, S, Monserrat-Mesquida, M, Ugarriza, L, Casares, M, Gómez, C, Mateos, D, et al. Ultra-processed food consumption and metabolic-dysfunction-associated Steatotic liver disease (MASLD): a longitudinal and sustainable analysis. Nutrients. (2025) 17:472. doi: 10.3390/nu17030472
10. Kalafati, IP, Borsa, D, Dimitriou, M, Revenas, K, Kokkinos, A, and Dedoussis, GV. Dietary patterns and non-alcoholic fatty liver disease in a Greek case-control study. Nutrition. (2019) 61:105–10. doi: 10.1016/j.nut.2018.10.032
11. Kardashian, A, Dodge, JL, and Terrault, NA. Quantifying the negative impact of fast-food consumption on liver steatosis among United States adults with diabetes and obesity. Clin Gastroenterol Hepatol. (2023) 21:3176–3178.e3. doi: 10.1016/j.cgh.2022.11.040
12. Liu, Z, Huang, H, Zeng, Y, Chen, Y, and Xu, C. Association between ultra-processed foods consumption and risk of non-alcoholic fatty liver disease: a population-based analysis of NHANES 2011-2018. Br J Nutr. (2023) 130:996–1004. doi: 10.1017/S0007114522003956
13. Talenezhad, N, Mirzavandi, F, Rahimpour, S, Amel Shahbaz, AP, Mohammadi, M, and Hosseinzadeh, M. Empirically derived dietary pattern and odds of non-alcoholic fatty liver diseases in overweight and obese adults: a case-control study. BMC Gastroenterol. (2022) 22:158. doi: 10.1186/s12876-022-02222-z
14. Zhang, YF, Qiao, W, Zhuang, J, Feng, H, Zhang, Z, and Zhang, Y. Association of ultra-processed food intake with severe non-alcoholic fatty liver disease: a prospective study of 143073 UK biobank participants. J Nutr Health Aging. (2024) 28:100352. doi: 10.1016/j.jnha.2024.100352
15. Zhao, L, Clay-Gilmour, A, Zhang, J, Zhang, X, and Steck, SE. Higher ultra-processed food intake is associated with adverse liver outcomes: a prospective cohort study of UK biobank participants. Am J Clin Nutr. (2024) 119:49–57. doi: 10.1016/j.ajcnut.2023.10.014
16. Saha, M, Hossain, MZ, Gope, S, Ahmed, MU, Azad, KI, Chowdhury, ZR, et al. Prevalence of sonologically detected non-alcoholic fatty liver disease among school children of Sylhet City. Mymensingh Med J. (2022) 31:412–5.
17. Younossi, ZM, Zelber-Sagi, S, Henry, L, and Gerber, LH. Lifestyle interventions in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. (2023) 20:708–22. doi: 10.1038/s41575-023-00800-4
18. Kosmalski, M, Frankowski, R, Deska, K, Różycka-Kosmalska, M, and Pietras, T. Exploring the impact of nutrition on non-alcoholic fatty liver disease management: unveiling the roles of various foods, food components, and compounds. Nutrients. (2023) 15:2838. doi: 10.3390/nu15132838
19. Vogli, S, Naska, A, Marinos, G, Kasdagli, MI, and Orfanos, P. The effect of vitamin E supplementation on serum aminotransferases in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Nutrients. (2023) 15:3733. doi: 10.3390/nu15173733
20. Lv, Y, Rong, S, Deng, Y, Bao, W, Xia, Y, and Chen, L. Plant-based diets, genetic predisposition and risk of non-alcoholic fatty liver disease. BMC Med. (2023) 21:351. doi: 10.1186/s12916-023-03028-w
21. Shea, S, Lionis, C, Kite, C, Lagojda, L, Uthman, OA, Dallaway, A, et al. Non-alcoholic fatty liver disease and coexisting depression, anxiety and/or stress in adults: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2024) 15:1357664. doi: 10.3389/fendo.2024.1357664
22. Tanase, DM, Gosav, EM, Costea, CF, Ciocoiu, M, Lacatusu, CM, Maranduca, MA, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. (2020) 2020:3920196. doi: 10.1155/2020/3920196
23. Lane, MM, Gamage, E, Du, S, Ashtree, DN, McGuinness, AJ, Gauci, S, et al. Ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological meta-analyses. BMJ. (2024) 384:e077310. doi: 10.1136/bmj-2023-077310
24. Madruga, M, Martínez Steele, E, Reynolds, C, Levy, RB, and Rauber, F. Trends in food consumption according to the degree of food processing among the UK population over 11 years. Br J Nutr. (2023) 130:476–83. doi: 10.1017/S0007114522003361
25. Zeng, XF, Varady, KA, Wang, XD, Targher, G, Byrne, CD, Tayyem, R, et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: an international multidisciplinary expert consensus. Metabolism. (2024) 161:156028. doi: 10.1016/j.metabol.2024.156028
26. Li, T, Zhao, J, Cao, H, Han, X, Lu, Y, Jiang, F, et al. Dietary patterns in the progression of metabolic dysfunction-associated fatty liver disease to advanced liver disease: a prospective cohort study. Am J Clin Nutr. (2024) 120:518–27. doi: 10.1016/j.ajcnut.2024.07.015
27. Schepp, M, Freuer, D, Wawro, N, Peters, A, Heier, M, Teupser, D, et al. Association of the habitual dietary intake with the fatty liver index and effect modification by metabotypes in the population-based KORA-fit study. Lipids Health Dis. (2024) 23:99. doi: 10.1186/s12944-024-02094-0
28. Yaskolka Meir, A, Rinott, E, Tsaban, G, Zelicha, H, Kaplan, A, Rosen, P, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. (2021) 70:2085–95. doi: 10.1136/gutjnl-2020-323106
29. Moore, MP, Cunningham, RP, Dashek, RJ, Mucinski, JM, and Rector, RS. A fad too far? Dietary strategies for the prevention and treatment of NAFLD. Obesity. (2020) 28:1843–52. doi: 10.1002/oby.22964
Keywords: non-alcoholic fatty liver disease, fast food, obesity, meta-analysis, systematic review
Citation: He J, Wang Y and Weng F (2025) Fast food consumption and risk of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front. Public Health. 13:1600826. doi: 10.3389/fpubh.2025.1600826
Edited by:
Jemmyson Romario Jesus, Federal University of Viçosa, BrazilReviewed by:
Poliana Camila Marinello, University of Texas MD Anderson Cancer Center, United StatesAdane Gebeyehu, Swedish University of Agricultural Sciences, Sweden
Copyright © 2025 He, Wang and Weng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinke He, MTk4OTk3NDc1MTJAMTYzLmNvbQ==
 Jinke He
Jinke He Yingxue Wang2
Yingxue Wang2