- 1Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy
- 2Professional Health Care Services Department, University Hospital Policlinico Umberto I, Rome, Italy
- 3Department of Trauma, AOU G. Martino University Hospital, Messina, Italy
- 4SPIR - Professional Development and Research Implementation, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 5Strategic Steering Commitee, Centro Studi SAPIS Foundation, Italian National Federation of Orders of Radiographers and Technical, Rehabilitation, and Prevention Health Professions Research Centre, Rome, Italy
- 6Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy
- 7Department of Medical and Surgical Sciences-DIMEC, University of Bologna, Bologna, Italy
- 8Department of Life, Health and Health Professions Sciences, Link Campus University, Rome, Italy
- 9Department of Health Promotion, Mother and Childcare, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy
Introduction: Malignant fungating wounds (MFWs) are secondary chronic wounds resulting from malignant cell proliferation and migration, compromising skin integrity in patients with cancer. These wounds present a range of signs and symptoms. Although several instruments are used in their assessment, it is still unclear which tool is most appropriate for comprehensive evaluation and wound healing.
Aim: To review the existing instruments for MFW assessment, highlighting their strengths and limitations.
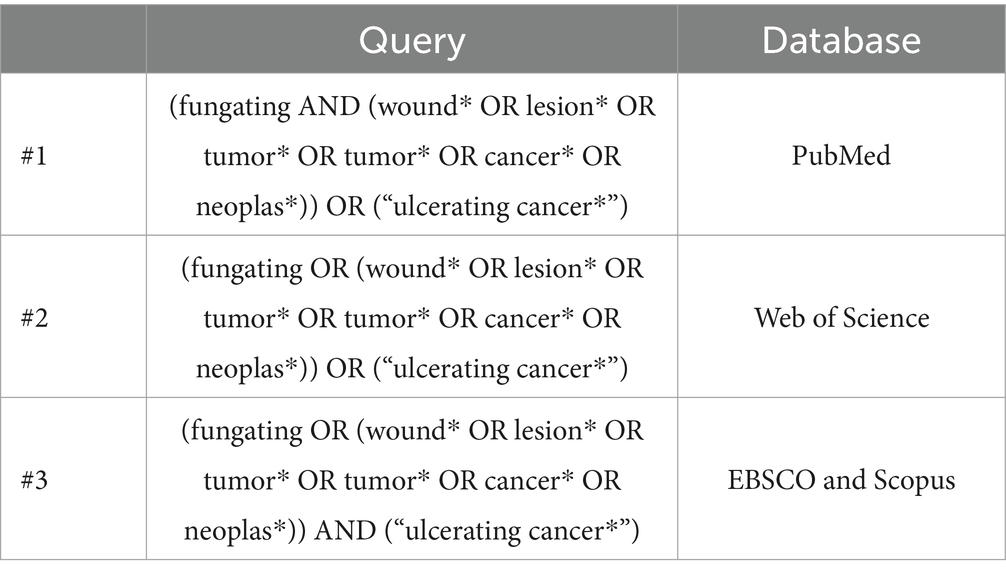
Methods: A scoping review was conducted following the Arksey and O’Malley framework (2005), the Joanna Briggs Institute guidelines (2020, 2021), and the PRISMA-ScR checklist (2018). The search was performed on four databases: Web of Science, Scopus, PubMed, and EBSCO.
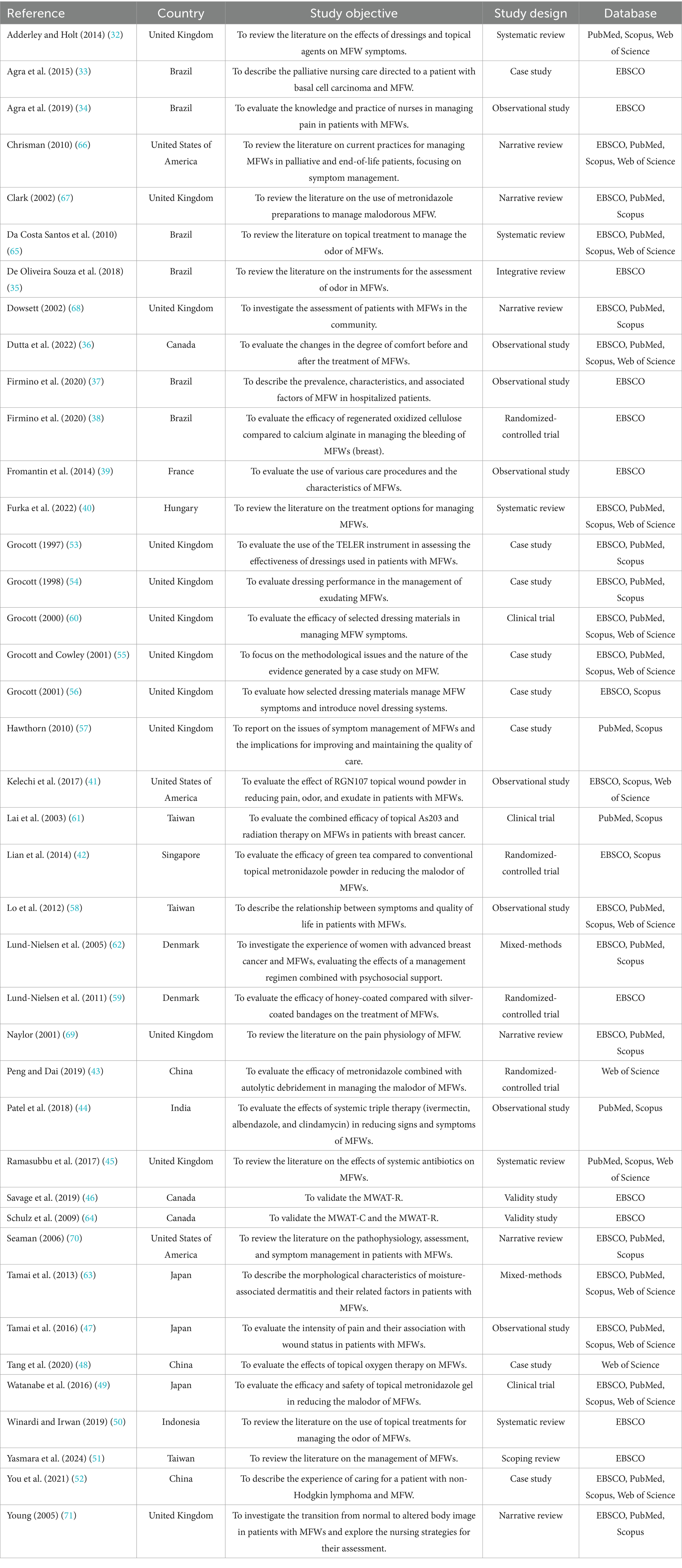
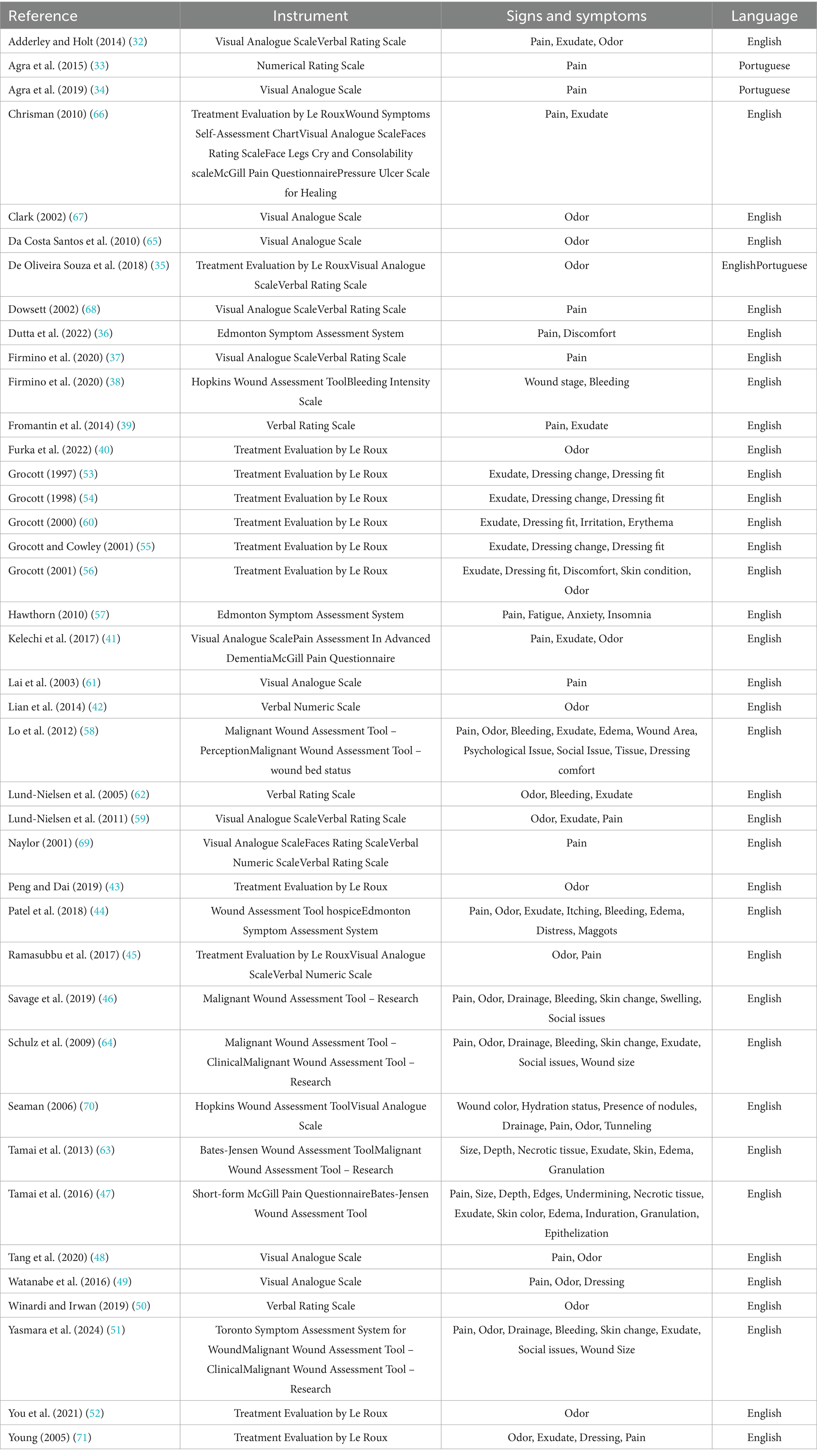
Results: Forty studies were included, describing 22 instruments. They described half targeted general symptoms, and half wound-related signs and symptoms. Four instruments were specifically designed for MFWs, all based on the Malignant Wound Assessment Tool (MWAT). These were: MWAT – Clinical; MWAT– wound bed status; MWAT– Perception; MWAT – Research. However, only the Clinical and Research versions were validated in English, but neither was subjected to psychometric validation, and lacked a comprehensive assessment, such as key symptoms.
Conclusion: Despite the existence of specific tools for MFW assessment, a comprehensive, validated, and standardized tool is still lacking. While the Clinical and the Research versions of the MWAT offer a broad assessment of MFWs, they require refinement to address overlooked symptoms and validation in other languages. Establishing standardized, multidimensional measures could enhance clinical decision-making and improve outcomes for patients living with MFWs.
Introduction
Malignant fungating wounds (MFWs) are complex, secondary chronic wounds arising from the uncontrolled proliferation and infiltration of malignant cells, compromising skin integrity in patients with cancer (1). They can present as an ulcerated wound, raised nodules with a cauliflower appearance, or a combination of these forms, explaining the term ‘fungating’ used to describe these ulcers (2). MFWs can develop due to primary skin malignancies, like melanoma, basal cell carcinoma, or squamous cell carcinoma, direct spread from an underlying cancer, or when the tumor metastasizes and breaks the skin (3).
The epidemiology of MFWs remains uncertain due to limited data (4), but estimates suggest that 5–10% of cancer patients may develop such wounds, typically in the last 6 months of life, with minimal prospects of wound healing (1). MFWs are most frequently located on the breast (62–66%), head and neck (22–24%), or chest (1%) (5). These wounds are characterized by rapid tumoral growth, excessive exudate, necrosis, pruritus, malodor, and bleeding (6). Excessive exudate can result from increased vascular permeability, infection, or devitalized tissue (7), while necrotic tissue fosters bacterial proliferation, leading to secondary infections and malodor (8). Such odor can induce nausea, reduce appetite, drive social withdrawal, and contribute to depression among both patients and caregivers (9). Patients with MFWs experience a significant symptom burden, and studies have shown that managing these symptoms not only improves patient outcomes and physical well-being but also enhances their self-esteem (10). Bleeding commonly occurs due to friable tissue and impaired homeostasis, compounded by cancer disease and treatment that influence coagulation (7). Rapid tumor growth can also compress nearby structures, such as nerves and lymphatic vessels, leading to pain, reduced mobility, and impaired drainage. These issues can be worsened by incorrect wound dressing techniques (1, 2).
The numerous and distressing symptoms associated with MFWs can significantly affect the quality of life of patients with advanced cancer (11). Low-performance status and the challenges of ongoing wound care may further compromise essential dimensions of health, such as functional status, social relationships, and mental well-being (12, 13). Moreover, the terminal prognosis of MFWs imposes a substantial emotional burden on patients and their families, which in turn often results in patients experiencing fear and uncertainty about the future (14, 15).
Given these challenges, developing pragmatic, patient, and family-centered palliative wound care strategies is essential, beginning with a comprehensive assessment of MFWs (12). Such assessment can enable appropriate symptom evaluation, prioritizing patient safety, comfort, and quality of life (16). For instance, managing exudate and bacterial colonization through proper wound cleaning can be transformative for patients, alleviating discomfort, pain, and social isolation triggered by leakage of malodor (17). Nurses play a central role in the assessment of wounds, leveraging their expertise to identify complications early, implement personalized pain management strategies, and provide education and support to patients and their families (18). Their ability to assess wounds within the broader context of advanced illness enables them to make meaningful contributions to patient comfort and dignity (19). Therefore, an adequate assessment could support the palliative care nurses in managing these wounds, which is currently challenging given the heightened risk of complications (20). Although the literature highlights the clinical relevance of these symptoms, there are still gaps in accurately documenting their prevalence, characteristics, and impacts on patients’ functional status, underscoring the need for comprehensive assessment (21). However, to the best of our knowledge, no reviews have systematically evaluated the tools available for assessing and managing MFWs.
To address this gap, a clinical tool that facilitates the evaluation of MFW signs and symptoms is urgently required (7), both to guide interventions (22) and to overcome the barriers to evidence-based care (23). Such interventions should reflect patient and family goals of care, especially in advanced disease where the symptom burden can be invalidating, with physical disfigurement and emotional debilitation (24). Given the complexity of MFWs, it is also relevant to inform both patients and families about the scenarios, reassure them, and ensure that the healthcare providers can assess, select, and deliver tailored and appropriate treatment to best manage the condition (25). In light of these considerations, the present study seeks to answer the following question: What instruments are currently available for the comprehensive assessment of MFWs?
Aims
The primary aim of this study is to review the existing research on the comprehensive instruments used to assess and manage malignant fungating wounds (MFWs). Specifically, we aim to (1) identify how current research addresses the assessment of MFWs’ signs and symptoms, and (2) highlight the strengths and limitations of available tools to guide clinical practice and future research.
Materials and methods
This scoping review followed the five-stage framework of Arksey and O’Malley (26) and the methodology outlined by the Joanna Briggs Institute (JBI) (27, 28). We also adhered to the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist to ensure transparent reporting (29).
Eligibility criteria
In line with established scoping review methodologies (27–29), we formulated eligibility criteria and their justification. We included peer-reviewed quantitative and qualitative studies reporting on tools for MFW assessment, without limiting to specific symptoms or signs, to capture a broad range of characteristics. No temporal and language restrictions were applied, ensuring a comprehensive overview of the literature. Studies were excluded if they focused on other types of wounds (e.g., surgical), involved non-cancer populations, or lacked information on instruments relevant to MFW assessment. Gray literature and non-empirical research (e.g., editorial) were excluded to prioritize feasibility and ensure the inclusion of peer-reviewed tools, consistent with previous review (30). Eligibility criteria were refined iteratively, as recommended by Arksey and O’Malley (26), allowing for adjustment based on emerging insights from the literature.
Information sources
An extensive search was performed in four databases to identify potentially relevant studies.
Pub-Med, Web of Science, Scopus, and EBSCO were used. These databases were selected for their broad coverage of biomedical, nursing, and health sciences literature, ensuring a comprehensive and multidisciplinary retrieval of studies related to MFWs’ assessment. The initial search was conducted from 06th July 2024 to 11th February 2025.
Search strategy
Search strings were developed using a combination of controlled vocabulary (e.g., MeSH terms), free text keywords, and Boolean operators. Each search string was tailored to the specific databases consulted. Consistent with methodology, reference lists and citations of all retrieved full-text articles were screened to identify additional eligible studies. Zotero X8 software (Clarivate Analytics, Philadelphia, PA) was employed to organize records and ensure traceability. Duplicates were removed through the automated merging function of Zotero and manual checking. The final search strings used can be found in Table 1.
Selection of sources of evidence
The study selection followed a two-stage process. In the first stage, two authors (DN, FG) independently screened the titles and abstracts of all identified records against the inclusion and exclusion criteria. Records deemed potentially eligible were advanced to the second stage, and the full texts were retrieved. In the second stage, two other authors (GA, RL) reviewed the full texts to confirm their eligibility. Any disagreements in study selection were resolved through discussion with a third reviewer (AP), ensuring consensus on final inclusions.
Data charting process
A data-charting form was created in Microsoft Excel following the JBI scoping review guidelines (27) to collect relevant data from the included studies. Two authors (DN, RL) independently extracted the data, and any discrepancies were resolved by consultation with another two authors (AP, SD) until an agreement was reached.
Data items
The following information was extracted from each included study: reference (author and year of publication), country, study objective, study design, instrument used for MFWs assessment, symptoms considered, the database from which the study was identified, and the language of publication.
Critical appraisal of individual sources of evidence
Considering that scoping reviews are generally undertaken to map existing evidence rather than evaluate methodological quality or risk of bias (29), we did not formally assess the quality of the included studies. Consequently, no study was excluded based on methodological limitations, ensuring a broad representation of the available literature. Nonetheless, every study included in this review was published in a peer-reviewed scientific journal.
Synthesis of results
A narrative approach was used to synthesize and present the findings of this scoping review, in line with the framework by Arksey and O’Malley (26). Studies were organized based on whether they employed instruments explicitly designed for wound assessment or instead targeted general symptoms without a wound-specific focus. We also searched the databases consulted for this review for validation studies related to each identified instrument. The key findings are described narratively in the results section and supplemented with tables, graphs, and visual representations when appropriate.
Results
Selection of sources of evidence
The search on the four databases returned 2,699 potentially relevant records. After the duplicates were removed, 1,358 records proceeded to the first stage of screening. After screening for relevance based on title and abstract, 116 records proceeded to the full-text screening phase. All identified full-text records were retrieved. Only 40 articles met the eligibility criteria and were included in the review. A PRISMA flow diagram (31) describing the screening process is displayed in Figure 1, with the number of included and excluded records for each phase and the justification for exclusion.
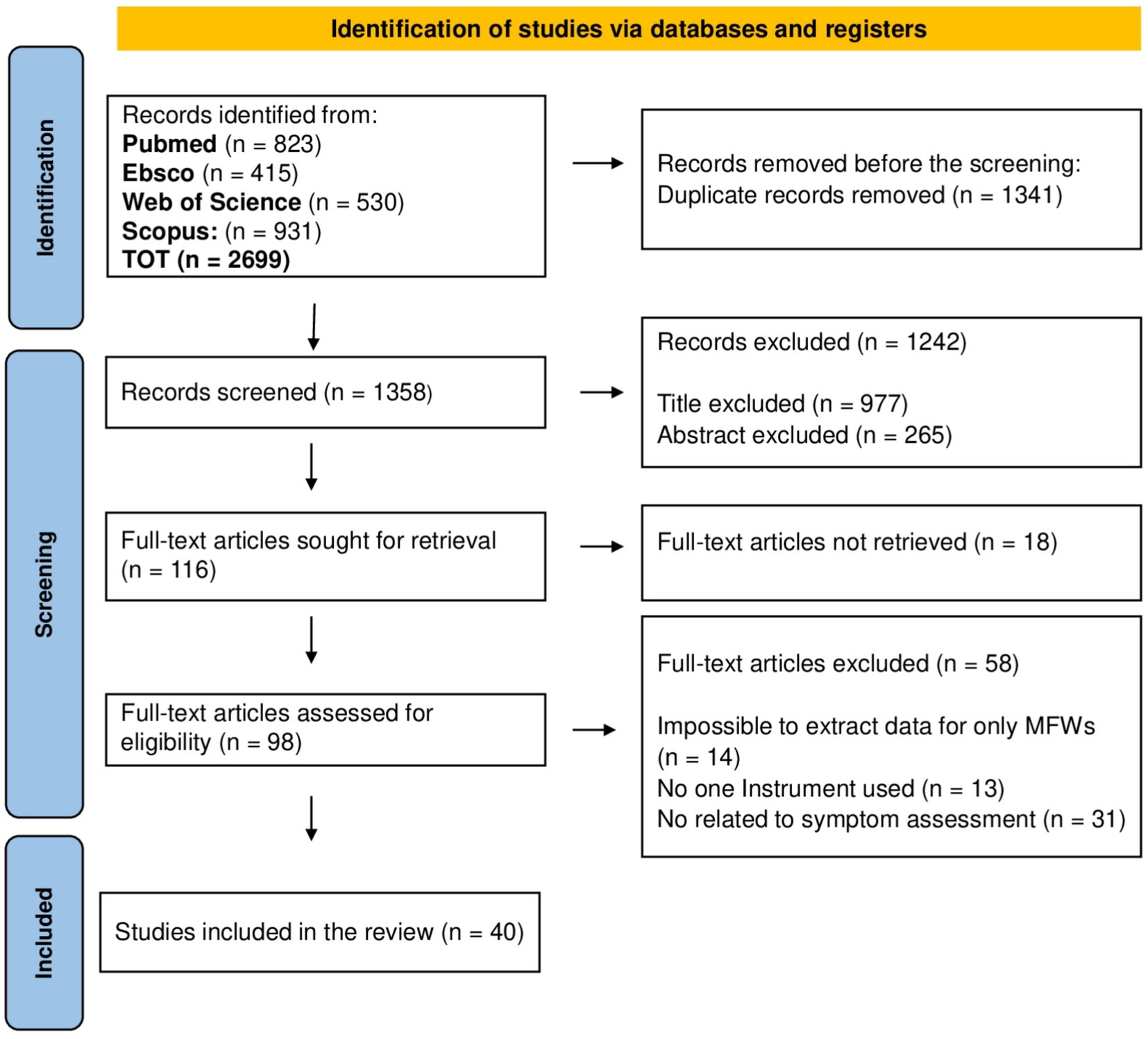
Figure 1. PRISMA flowchart illustrating the selection process and the number of sources included and excluded.
Characteristics of sources of evidence
The characteristics of the studies included in this review are presented in Tables 2, 3.
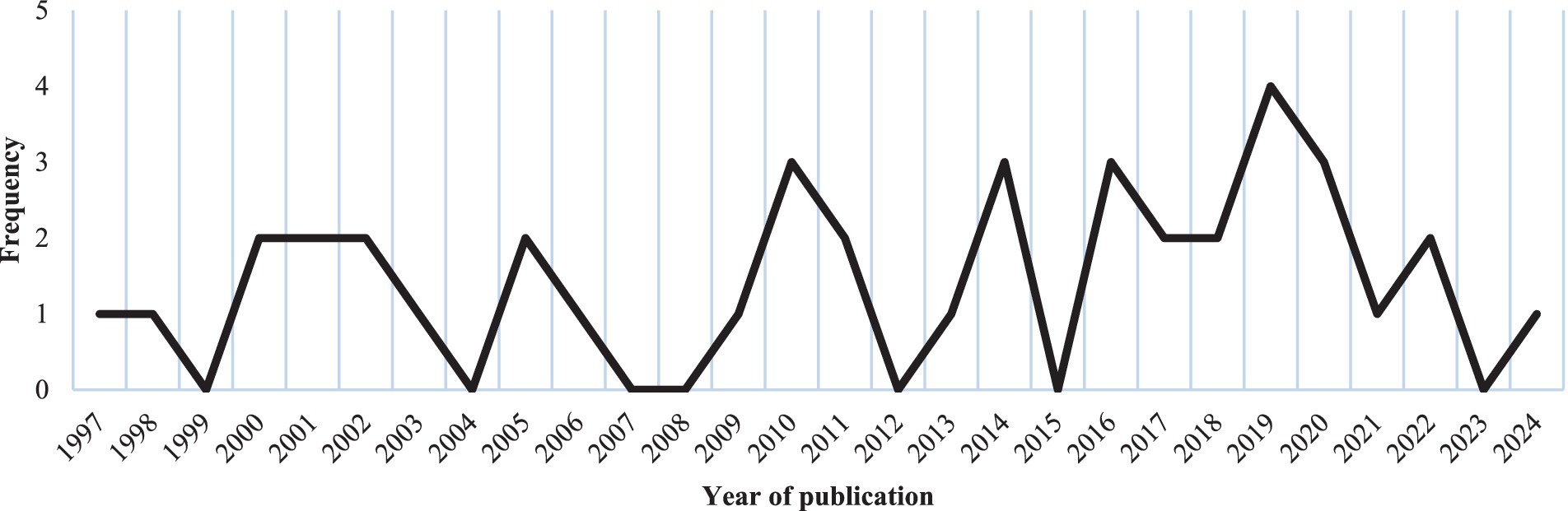
The publication period of the included studies ranges from 1997 to 2024. The distribution of studies over these years is heterogeneous, with a marked increase in research interest over the past decade (n = 21, 52.5%) (32–52). The highest number of eligible studies was published in 2019 (34, 43, 46, 50), as illustrated in Figure 2.
In terms of study design, most of the studies included were primary research (n = 27, 67.5%), comprising case studies (n = 8, 20%) (33, 48, 52–57), observational studies (n = 8, 20%) (34, 36, 37, 39, 41, 44, 47, 58), randomized controlled trials (n = 4, 10%) (38, 42, 43, 59), clinical trials (n = 3, 7.5%) (49, 60, 61), mixed-methods research (n = 2, 5%) (62, 63), and validation studies (n = 2, 5%) (46, 64). Secondary research was less common (n = 13, 32.5%), consisting primarily of systematic reviews (n = 5, 12.5%) (32, 40, 45, 50, 65) and other literature reviews (n = 8, 20%) (35, 51, 66–71).
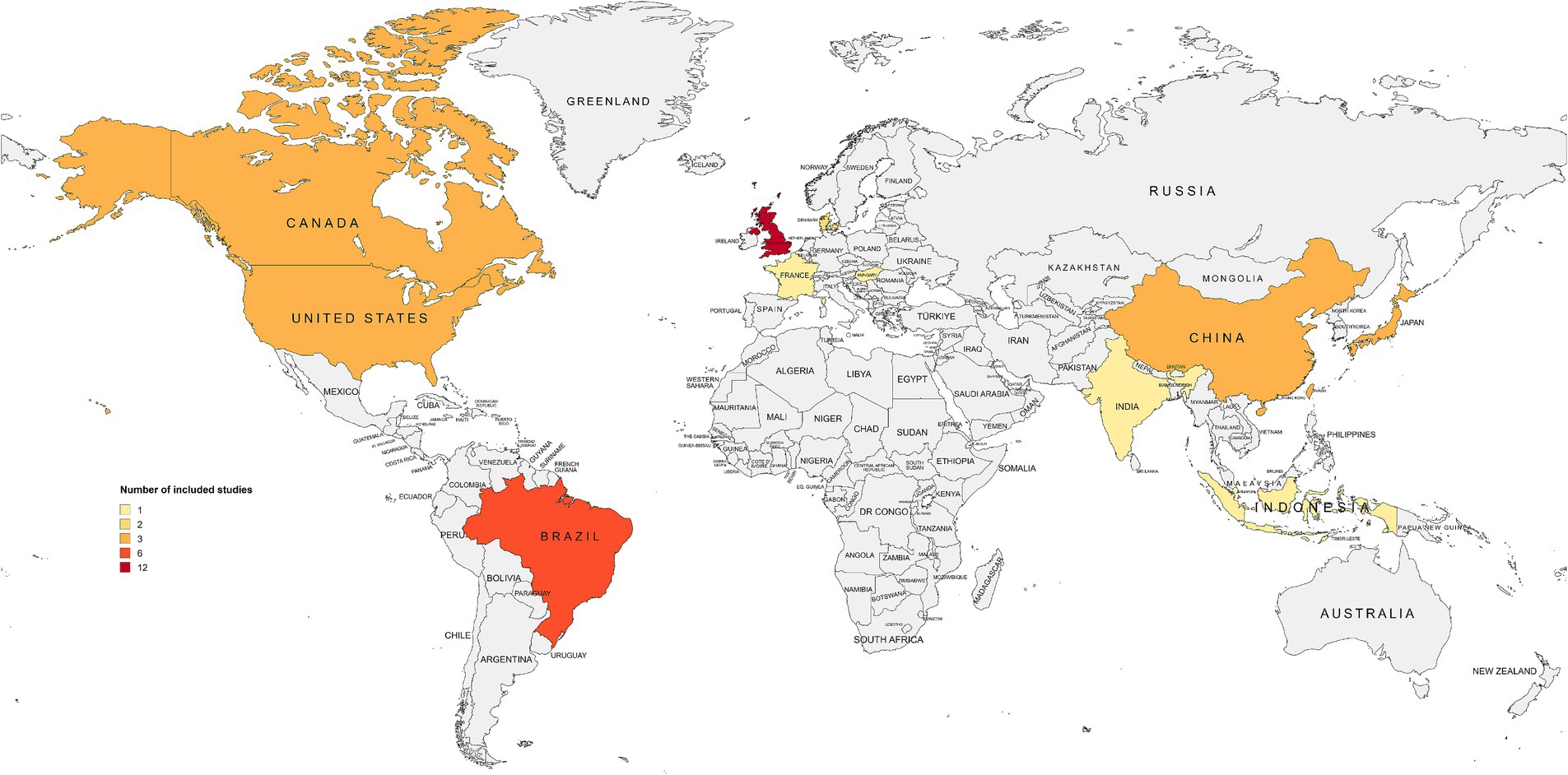
Geographically, the included studies originated from 13 countries in six macro-regions: Europe (n = 16, 40%) (32, 39, 40, 45, 53–57, 59, 60, 62, 67–69, 71), East Asia (n = 9, 22.5%) (43, 47–49, 51, 52, 58, 61, 63), North America (n = 6, 15%) (36, 41, 46, 64, 66, 70), South America (n = 6, 15%) (33–35, 37, 38, 65), the Pacific (n = 2, 5%) (42, 50), and South Asia (n = 1, 2.5%) (44). As shown in Figure 3, the United Kingdom contributed the largest number of eligible studies (n = 12, 30%) (32, 45, 53–57, 60, 67–69, 71). Most of the included articles were published in English (n = 37, 92.5%) (32, 36–46, 53–62, 64–70), followed by Portuguese (n = 2, 5%) (33, 34), while one record (n = 1, 2.5%) (35) appeared in both languages.
Finally, 22 distinct instruments were identified across the included studies (Tables 2, 3). Out of these, 13 studies (32.5%) reported on two or more tools concurrently, resulting in a total of 68 instances of tool reporting. The Visual Analogue Scale (n = 16, 23.5%) and the Treatment Evaluation by Le Roux (n = 12, 17.6%) emerged as the most cited. Half of the instruments (n = 11) were specifically developed to assess wounds and related signs and symptoms, whereas the other half (n = 11) targeted general symptoms such as pain or insomnia, without a wound-specific focus. Furthermore, considering their validity, most of the assessment tools identified were previously validated (n = 16, 72.7%), whereas a minority lacked formal validity testing (n = 6, 27.3%). Lastly, as illustrated in Figure 4, the most common signs and symptoms evaluated by validated instruments were pain (n = 12, 78.5%), exudate (n = 8, 57.1%), and odor (n = 7, 50%). The following sections discuss each tool and its investigated dimensions, signs, and symptoms in detail, distinguishing between wound-specific and non-specific instruments.
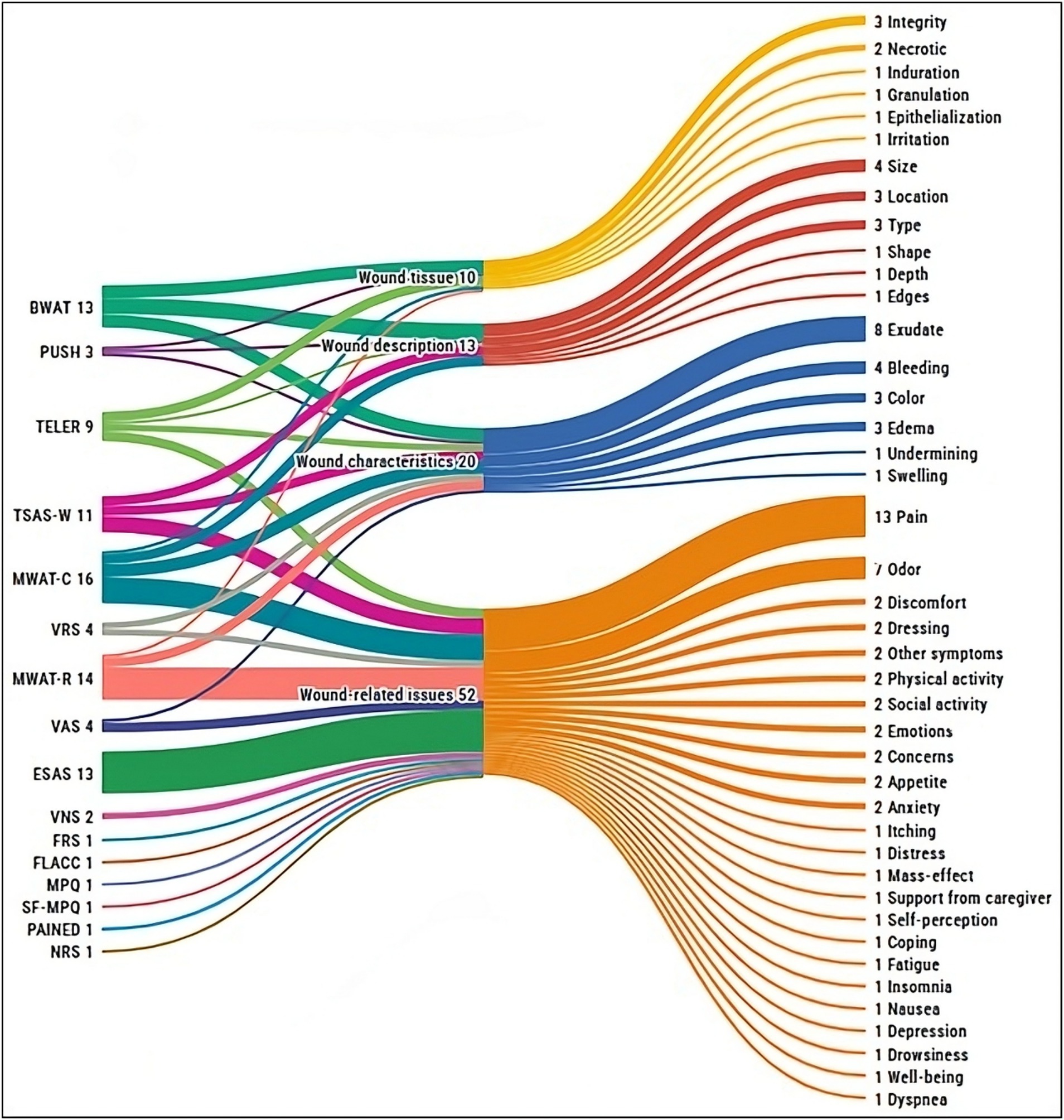
Figure 4. Overview of signs and symptoms assessed by the validated instruments, grouped by instrument and dimension evaluated. BWAT, Bates-Jensen Wound Assessment Tool; ESAS, Edmonton Symptom Assessment System; FLACC, Face Legs Cry and Consolability Scale; FRS, Faces Rating Scale; MPQ, McGill Pain Questionnaire; MWAT-C, Malignant Wound Assessment Tool – Clinical; MWAT-R, Malignant Wound Assessment Tool – Research; NRS, Numerical Rating Scale; PAINAD, Pain Assessment In Advanced Dementia; PUSH, Pressure Ulcer Scale for Healing; SF-MPQ, Short-form McGill Pain Questionnaire; TELER, Treatment Evaluation by Le Roux; TSAS-W, Toronto Symptom Assessment System for Wound; VAS, Visual Analogue Scale; VNS, Verbal Numeric Scale; VRS, Verbal Rating Scale.
Wound-specific instruments for sign and symptom assessment
Of the tools employed to assess MFWs’ signs and symptoms, most (n = 7, 66.6%) were originally developed for other wound types (e.g., pressure ulcers) and subsequently applied to MFWs. These include the Bates-Jensen Wound Assessment Tool (BWAT), the Hopkins Wound Assessment Tool (HWAT), the Pressure Ulcer Scale for Healing (PUSH), the Treatment Evaluation by Le Roux (TELER), the Toronto Symptom Assessment System for Wound (TSAS-W), the Wound Assessment Tool for hospices (WAT), and the Wound Symptoms Self-Assessment Chart (WoSSAC). A smaller subset (n = 4, 36.3%) was specifically designed for MFWs, comprising four versions of the Malignant Wound Assessment Tool (MWAT): Clinical (MWAT-C), Research (MWAT-R), Wound Bed Status (MWAT-N), and Perception (MWAT-P).
Figure 5 illustrates the dimensions covered by each validated wound-specific instrument, along with the percentage of items that address each dimension. Overall, the MWAT-R includes the broadest range of wound-related items (n = 24), capturing aspects such as pain and odor alongside their impacts on social interactions, physical activity, appetite, self-perception, and emotional well-being. MWAT-C also addresses various wound-related issues (n = 12), further detailing wound characteristics such as location, size, changes over time, exudate, bleeding, and edema.
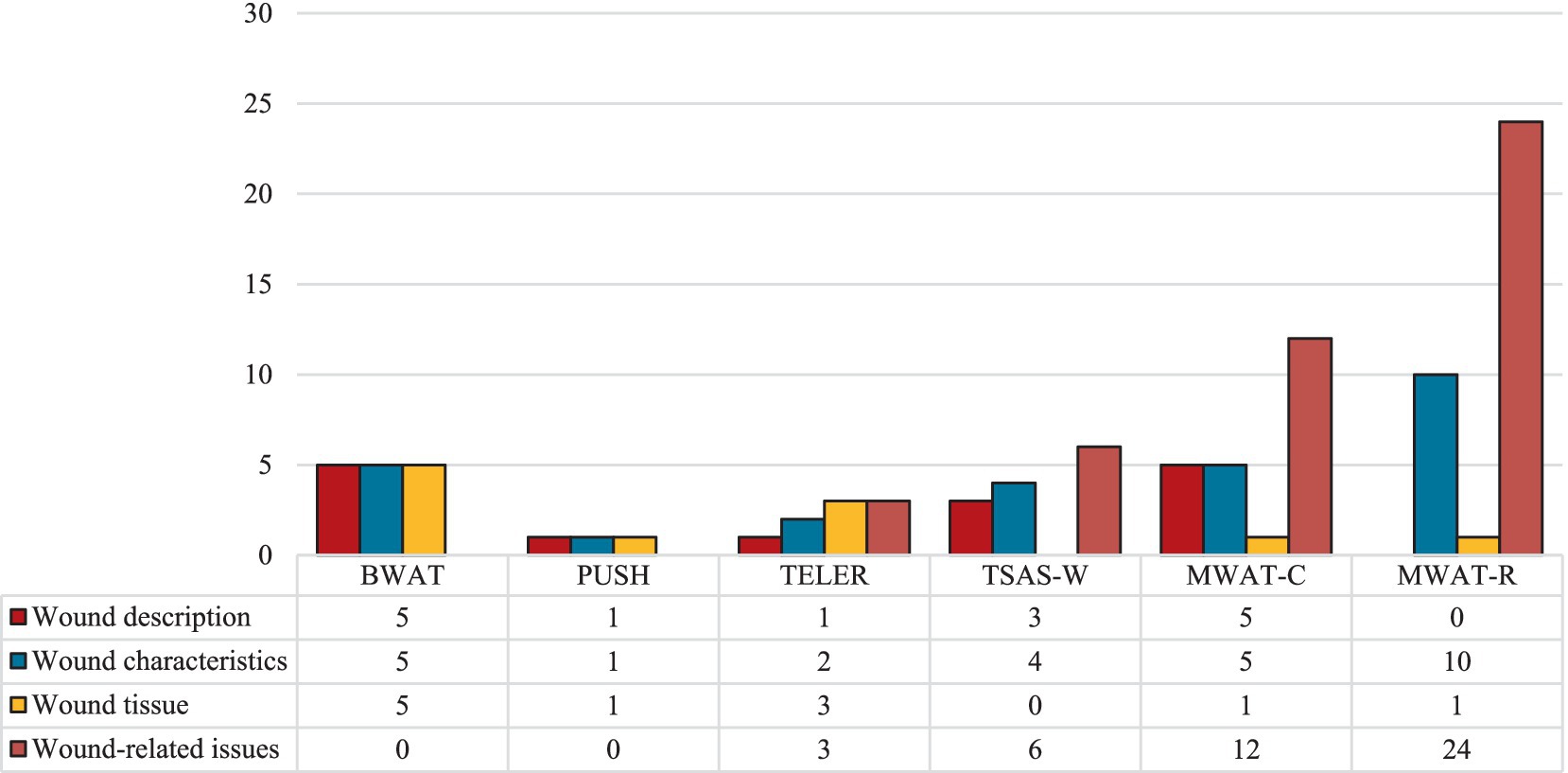
Figure 5. Signs and symptoms assessed by the validated wound-specific instruments, grouped by instrument and dimension evaluated. BWAT, Bates-Jensen Wound Assessment Tool; MWAT-C, Malignant Wound Assessment Tool – Clinical; MWAT-R, Malignant Wound Assessment Tool – Research; PUSH, Pressure Ulcer Scale for Healing; TELER, Treatment Evaluation by Le Roux; TSAS-W, Toronto Symptom Assessment System for Wound.
Non-specific instruments for sign and symptom assessment
Most non-wound-specific tools identified (n = 6, 54.5%) focus on evaluating a single symptom. These include the Bleeding Intensity Scale (BIS); Face, Legs, Activity, Cry, and Consolability (FLACC) scale; Faces Rating Scale (FRS); McGill Pain Questionnaire (MPQ); Pain Assessment in Advanced Dementia (PAINAD); and Short-Form McGill Pain Questionnaire (SF-MPQ). The remaining instruments (n = 5, 45.5%) assess multiple symptoms, such as the Edmonton Symptom Assessment System (ESAS) and widely used, simple scales like the Numerical Rating Scale (NRS), Visual Analogue Scale (VAS), Verbal Numeric Scale (VNS), and Verbal Rating Scale (VRS). These tools are valued for their feasibility and Likert-like structure, making them adaptable for various clinical contexts. Almost all (n = 10, 90.9%) have been validated in English (72–77), except the BIS. As shown in Figure 6, none of these instruments address wound characteristics or tissue integrity, but all capture at least one wound-related symptom, such as pain. Only two were also employed to describe wound characteristics: the VAS for exudate amount and the VRS for both exudate and bleeding.
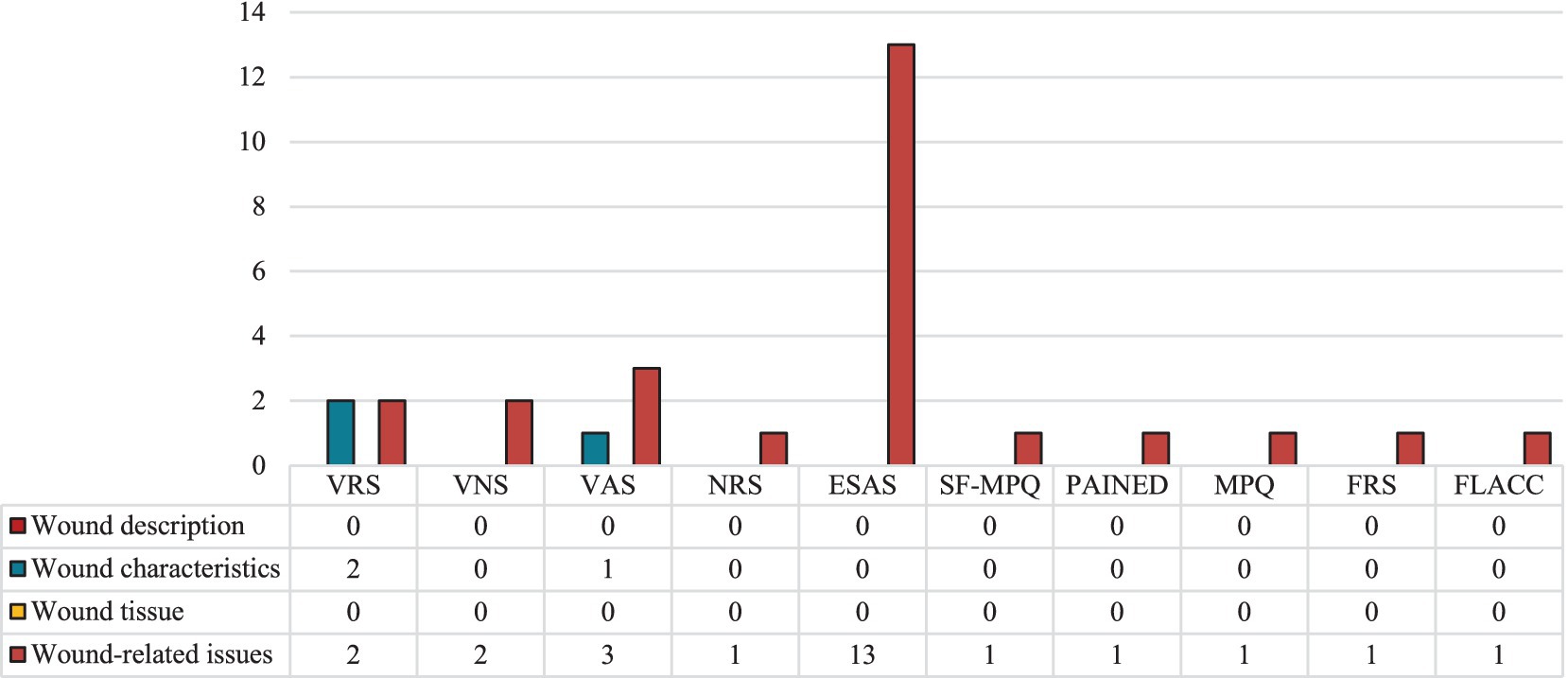
Figure 6. Signs and symptoms assessed by the validated non-specific instruments, grouped by instrument and dimension assessed. ESAS, Edmonton Symptom Assessment System; FLACC, Face Legs Cry and Consolability scale; FRS, Faces Rating Scale; MPQ, McGill Pain Questionnaire; NRS, Numerical Rating Scale; PAINAD, Pain Assessment In Advanced Dementia; SF-MPQ, Short-form McGill Pain Questionnaire; VAS, Visual Analogue Scale; VNS, Verbal Numeric Scale; VRS, Verbal Rating Scale.
Discussion
This scoping review identified the instruments used for MFWs’ assessment, which remain a significant clinical challenge in patients with advanced cancer. MFWs have a negative impact on psycho-social and emotional quality of life (78), affecting body image perception and contributing to worse distress (79), due to related symptoms, such as pain, excessive exudate, malodor, and bleeding (1). Malodor is particularly distressing, often contributing to stigma and social withdrawal, making patients reluctant to seek medical attention (10). The visible and odorous nature of MFWs can also act as a distressing reminder of disease progression and the imminence of death, exacerbating anxiety for both patients and their families (80). Stigma or fear, especially when wounds occur in sensitive areas such as the breast, face, or genitals, further discourages patients from seeking timely care (81). Given these challenges, a comprehensive and multidimensional assessment of MFWs is crucial (46), although these wounds do not have a good healing prognosis. Our results described multiple tools that have been used to assess MFWs, which most capture only a limited subset of wound-related signs and symptoms. Therefore, this review partially addresses the research question, as the available instruments, while some are specific to the assessment of MFWs, cannot achieve a comprehensive and multidimensional evaluation. Half of the instruments were specifically developed to assess wounds, and related signs and symptoms (i.e., bleeding, odor, exudate), and the other half targeted general symptoms such as pain, anxiety, and insomnia, without a wound-specific focus. Our research identified the MWAT in four different versions, but only the MWAT-C and MWAT-R (64) are specifically for assessing and managing MFWs. The MWAT-C addresses several wound-related issues, particularly wound characteristics such as location, size, changes over time, exudate, bleeding, and edema. Instead, the MWAT-R includes a broad range of wound-related items, capturing clinical characteristics of the wound and social interactions, physical activity, self-perception, and emotional well-being, and also focuses on nutritional and self-esteem. A systematic approach is optimal for the evaluation of patients presenting with MFWs, given the infrequent etiology of a singular underlying cause. A comprehensive assessment of both local and systemic contributing factors within each stage of the diagnostic work-up is critical. The principles guiding ongoing nursing and clinician assessment and monitoring of wounds generally exhibit considerable overlap (82). It is plausible that managing skin wounds with bad healing prognoses does not solicit standardized clinical and welfare responses, with careful funds to support research (83). All health professionals, and in particular nurses, are now part of the healthcare team as patient advocates. They must respond to health needs as a nursing imperative, supporting public health as a fundamental right (84).
A key insight of this review is the lack of standardized, multidimensional instruments capable of capturing both physical and psychosocial domains of MFWs with a single tool. The MWAT-C and MWAT-R strive toward such comprehensiveness by integrating wound characteristics, social impacts, and emotional dimensions. Moreover, they fail to address certain symptoms frequently reported in the literature, such as itching and the presence of maggots (22). Although these symptoms can be recorded under an open-ended other symptoms category, this approach relies on practitioners’ awareness and assessment skills, potentially introducing variability and inconsistency in evaluations. As our review indicates, healthcare professionals often use separate instruments to evaluate different aspects, such as pain intensity, exudate volume, or odor severity, leading to fragmented assessments and repeated measurements. This fragmentation not only places an additional burden on patients but can also reduce adherence to regular assessments (20). Reflecting best practice recommendations (grade B) and recognized as a quality indicator for the physical aspects of palliative care, nurses and other healthcare professionals should utilize evidence-based assessment tools for the initial and ongoing evaluation of symptoms in end-of-life care patients (84, 85). As highlighted by the guidelines generated by the European Oncology Nursing Society (EONS) only some scales assess psychosocial aspects and their impact on daily life; therefore having an integrated and comprehensive instrument for the assessment of MFWs is vital for guiding effective palliative strategies, particularly in situations where patients are facing profound physical, emotional, and social strains. A comprehensive assessment should take into account the physical and psychosocial aspects of both the patient and caregivers, treatment such as symptoms evaluation, supportive care, and preventative measures, and a focus on local wound management such as bleeding, odor, pain/pruritus, exudate, and superficial infection (86). The substantial metabolic demands of patients with MFWs, often due to significant wound exudate or fistula fluid, necessitate a higher energy intake. Standard guidelines recommend 25–35 kcal/kg body weight per day, so frequent meals and snacks may be indicated for individuals with MFWs to satisfy these elevated energy requirements (87). The National Health Service Forth Valley guidelines for the management of MFWs suggest the M: EMPHIS Pathway approach which is specific for guiding the management of local symptoms about malignant wound (M), exudate (E), malodour (M), pain (P), hemorrhage (H), infection (I) and skin tissue related problems (S) (88). This may be a good approach to manage the core symptoms related to MFWs, but it does not address the other symptoms that occur in patients with this lesion. Nurses report difficulties in applying wound dressings and concealing their disgust at the odor, often finding the care of these patients emotionally challenging due to the sometimes incurable nature of the wounds, while also facing the complexities of patient isolation, altered body image, and holistic interdisciplinary approach guided by specialist-level palliative wound care expertise (22). Effectively managing the complex needs of patients with MFWs demands a pragmatic approach. Implementing palliative care relies on a holistic approach that spans multiple levels of assistance, involving the collaborative input of various disciplines from the health, education, business, and labor sectors, all guided by a transdisciplinary framework to address these needs. It’s vital to consider the transdisciplinary nature of palliative care, enabling diverse professions to contribute their specific knowledge toward a common objective, which includes integrating family and community. Their experiences and inherited knowledge can significantly inform the goal of addressing the needs of the individual-family dyad (89). Interdisciplinary care involves assistance where knowledge is shared at the point of care delivery and where the goal of care is agreed upon. There is communication, collaboration, and coordination among the various care providers. The importance of each discipline and the contribution each can make is recognized to deliver integrated and individualized palliative care based on the needs of the patient-family dyad (90).
Comprehensive and easy-to-use tools, and an interdisciplinary team could facilitate the use of a standardized language, help nurses and healthcare professionals to effectively assessment the complexities of MFWs, reduce wound management times, ensure the assessment of all the symptoms, and provide well-being in the patient-family dyad (91). Therefore, it is imperative to investigate the adoption and implementation of efficient tools informed by scientific evidence, which support the transition toward more efficacious care paradigms, as exemplified by transdisciplinary palliative care.
Additionally, the MWAT-C and the MWAT-R have only been validated in English and lack comprehensive psychometric testing, raising concerns about their reliability and limiting their applicability in diverse cultural contexts (92). Validation in terms of both validity and reliability is essential to ensure that an instrument accurately measures the intended constructs and can be confidently applied across different clinical and research settings. Without such validation, the use of these instruments risks introducing measurement errors, leaving practices prone to error in contexts not oriented toward evidence-based practice (93).
Strengths and limitations
The findings of this review should be interpreted in light of a few limitations. First, the search was limited to four databases, which may have resulted in the omission of relevant studies indexed elsewhere. Additionally, to ensure a timely completion while upholding methodological rigor, gray literature was not included. This may have narrowed the scope of the findings and excluded potentially valuable insights. Furthermore, the limited validation of assessment tools across different languages and cultural contexts may hinder the global applicability of current approaches to the evaluation of MFWs. Finally, the sensitive nature of the topic, where patients may hide their wounds due to stigma or embarrassment, could lead to underreporting thereby affecting the generalizability of existing evidence, and could be a potential bias (10).
Conclusion
The assessment of MFWs continue to raise significant challenges due to the lack of standardized, comprehensive, and evidence-based evaluation tools. Although various instruments have been employed, most focus only on specific dimensions of MFWs, leading to fragmented assessments and potentially suboptimal care. Among the available measures, the MWAT-C and MWAT-R demonstrate the greatest potential, due to their relatively broad scope. However, both tools still require further refinement and validation, especially to incorporate underreported symptoms and enhance applicability across diverse clinical and cultural settings. There is an urgent need for the development of a validated, multidimensional instrument specifically designed to capture the complexity of MFWs within a broader biopsychosocial framework. In the interim, in the absence of such tools, nurses are encouraged to follow evidence-based and adapt clinical guidelines in different clinical context (94), such as those from the EONS and NHS (86, 88), to guide wound assessment in clinical practice. Future research should prioritize rigorous investigations that address existing gaps in knowledge and practice, moving beyond traditional, non-standardized approaches. This process will ultimately improve the assessment of MFWs in palliative care settings and provide better support to patients, their families, and healthcare professionals.
Author contributions
DN: Conceptualization, Writing – original draft, Writing – review & editing, Project administration, Formal analysis, Methodology, Supervision, Data curation. FG: Supervision, Writing – original draft, Formal analysis, Writing – review & editing, Data curation, Project administration, Methodology, Conceptualization. GA: Writing – original draft, Writing – review & editing, Project administration, Conceptualization, Methodology, Formal analysis, Supervision, Data curation. AP: Conceptualization, Project administration, Supervision, Writing – review & editing, Data curation, Methodology, Formal analysis, Writing – original draft. SD: Data curation, Writing – review & editing, Formal analysis. MG: Writing – review & editing, Formal analysis, Data curation. VB: Data curation, Formal analysis, Writing – original draft. SQ: Formal analysis, Data curation, Writing – review & editing. LI: Project administration, Data curation, Formal analysis, Supervision, Methodology, Conceptualization, Writing – review & editing. RL: Writing – review & editing, Conceptualization, Writing – original draft, Supervision, Data curation, Formal analysis, Methodology, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work reported in this publication was funded by the Italian Ministry of Health, RC-2025-2796962 project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Starace, M, Carpanese, MA, Pampaloni, F, Dika, E, Pileri, A, Rubino, D, et al. Management of malignant cutaneous wounds in oncologic patients. Support Care Cancer. (2022) 30:7615–23. doi: 10.1007/s00520-022-07194-0
2. Mohan, S, and Khan, A. Pain and wound management in fungating Merkel cell carcinoma within a palliative setting: the first case report of this predicament. Indian J Palliat Care. (2024) 30:81–4. doi: 10.25259/IJPC_259_2023
3. Hasan, N, Nadaf, A, Imran, M, Jiba, U, Sheikh, A, Almalki, WH, et al. Skin cancer: understanding the journey of transformation from conventional to advanced treatment approaches. Mol Cancer. (2023) 22:168. doi: 10.1186/s12943-023-01854-3
4. Adderley, U, and Smith, R. Topical agents and dressings for fungating wounds In: The Cochrane Collaboration, editor. The Cochrane database of systematic reviews (protocol). Chichester, UK: John Wiley & Sons, Ltd. (2003). CD003948.
5. O’Neill, L, Nelson, Z, Ahmad, N, Fisher, AH, Denton, A, Renzi, M, et al. Malignant fungating wounds of the head and neck: management and antibiotic stewardship. OTO Open. (2022) 6:3306. doi: 10.1177/2473974X211073306
6. Tsichlakidou, A, Govina, O, Vasilopoulos, G, Kavga, A, Vastardi, M, and Kalemikerakis, I. Intervention for symptom management in patients with malignant fungating wounds – a systematic review. J BUON. (2019) 24:1301–8.
7. Alexander, S. Malignant fungating wounds: epidemiology, aetiology, presentation and assessment. J Wound Care. (2009) 18:273–80. doi: 10.12968/jowc.2009.18.7.43110
8. Gethin, G, Vellinga, A, McIntosh, C, Sezgin, D, Probst, S, Murphy, L, et al. Systematic review of topical interventions for the management of odour in patients with chronic or malignant fungating wounds. J Tissue Viability. (2023) 32:151–7. doi: 10.1016/j.jtv.2022.10.007
9. Morris, C. Wound odour: principles of management and the use of Clini sorb. Br J Nurs. (2008) 17:S38–42. doi: 10.12968/bjon.2008.17.Sup3.28914
10. Dos Santos, WA, Fuly, PDSC, Souto, MD, Dos Santos, MLSC, and Beretta, LDL. Asociación entre olor y aislamiento social en pacientes con heridas tumorales malignas: estudio piloto. Eglobal. (2018) 18:19–65. doi: 10.6018/eglobal.18.1.322641
11. Koumaki, D, Kostakis, G, Boumpoucheropoulos, S, Ioannou, P, and Katoulis, AC. A narrative review of management of wounds in palliative care setting. Ann Palliat Med. (2023) 12:1089–105. doi: 10.21037/apm-23-138
12. Liu, X, Xie, JQ, Liao, ZY, Wei, MJ, and Lin, H. Changes in wound symptoms and quality of life of patients with newly diagnosed malignant fungating wounds. J Wound Care. (2024) 33:262–70. doi: 10.12968/jowc.2024.33.4.262
13. Tam, SH, Lai, WS, Kao, CY, and Fang, SY. Maintain professionalism: nurses’ experiences in caring for patients with malignant Fungating wounds in Taiwan. J Pain Symptom Manag. (2024) 68:69–77.e1. doi: 10.1016/j.jpainsymman.2024.04.008
14. Huang, Y, Hu, J, Xie, T, Jiang, Z, Ding, W, Mao, B, et al. Effects of home-based chronic wound care training for patients and caregivers: a systematic review. Int Wound J. (2023) 20:3802–20. doi: 10.1111/iwj.14219
15. Eyres, J. Fear of malignant fungating wounds. Br J Community Nurs. (2024) 29:S36–41. doi: 10.12968/bjcn.2024.29.Sup9.S36
16. Alexander, SJ. An intense and unforgettable experience: the lived experience of malignant wounds from the perspectives of patients, caregivers and nurses. Int Wound J. (2010) 7:456–65. doi: 10.1111/j.1742-481X.2010.00715.x
17. Cornish, L. Holistic management of malignant wounds in palliative patients. Br J Community Nurs. (2019) 24:S19–23. doi: 10.12968/bjcn.2019.24.Sup9.S19
18. Atthayasai, J, Chatchumni, M, Eriksson, H, and Mazaheri, M. Surgical nurses' perceptions of strategies to enhance pain management proficiency: a qualitative study. Nurs Rep. (2023) 13:923–33. doi: 10.3390/nursrep13020081
19. Ferrell, B, Virani, R, Malloy, P, and Kelly, K. The preparation of oncology nurses in palliative care. Semin Oncol Nurs. (2010) 26:259–65. doi: 10.1016/j.soncn.2010.08.001
20. Schmidt, FMQ, Firmino, F, Lenza, NDFB, and Santos, VLCDG. Nursing team knowledge on care for patients with fungating wounds. Rev Bras Enferm. (2020) 73:e20170738. doi: 10.1590/0034-7167-2017-0738
21. Probst, S, Arber, A, and Faithfull, S. Malignant fungating wounds: a survey of nurses’ clinical practice in Switzerland. Eur J Oncol Nurs. (2009) 13:295–8. doi: 10.1016/j.ejon.2009.03.008
22. Tilley, CP, Fu, MR, Van Cleeve, J, Crocilla, BL, and Comfort, CP. Symptoms of malignant fungating wounds and functional performance among patients with advanced cancer: an integrative review from 2000 to 2019. J Palliat Med. (2020) 23:848–62. doi: 10.1089/jpm.2019.0617
23. Chang, SL, Chung, CF, Liou, YG, Lo, SF, and Hu, SH. Improving malignant fungating wound management among oncology nurses: a best practice implementation project. JBI Evid Implement. (2025) 23:33–41. doi: 10.1097/XEB.0000000000000430
24. Tilley, C, Lipson, J, and Ramos, M. Palliative wound care for malignant fungating wounds. Nurs Clin North Am. (2016) 51:513–31. doi: 10.1016/j.cnur.2016.05.006
25. Wilkes, LM, Boxer, E, and White, K. The hidden side of nursing: why caring for patients with malignant malodorous wounds is so difficult. J Wound Care. (2003) 12:76–80. doi: 10.12968/jowc.2003.12.2.26468
26. Arksey, H, and O’Malley, L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
27. Peters, MDJ, Marnie, C, Tricco, AC, Pollock, D, Munn, Z, Alexander, L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. (2020) 18:2119–26. doi: 10.11124/JBIES-20-00167
28. Pollock, D, Davies, EL, Peters, MDJ, Tricco, AC, Alexander, L, McInerney, P, et al. Undertaking a scoping review: a practical guide for nursing and midwifery students, clinicians, researchers, and academics. J Adv Nurs. (2021) 77:2102–13. doi: 10.1111/jan.14743
29. Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
30. Anastasi, G, and Bambi, S. Utilization and effects of security technologies in mental health: a scoping review. Int J Ment Health Nurs. (2023) 32:1561–82. doi: 10.1111/inm.13193
31. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. Statement: an updated guideline for reporting systematic reviews. BMJ. (2020) 372:n71. doi: 10.1136/bmj.n71
32. Adderley, UJ, and Holt, IGS. Topical agents and dressings for fungating wounds. Cochrane Database Syst Rev. (2014) 2014:CD003948. doi: 10.1002/14651858.CD003948.pub3
33. Agra, G, de Lourdes André Gouveia, B, Tamar Oliveira Sousa, A, Lopes Costa, MM, Santos Oliveira, SH, and Guimarães Soares, MJ. Cuidados paliativos de enfermagem a paciente com carcinoma basocelular terebrante: estudo de caso. J Nurs UFPE. (2015) 9:9873–81.
34. Agra, G, de Souza Medeiros, MV, Freire de Brito, DT, Silva Pimentel, ER, Soares Formiga, N, and Lopes Costa, MM. Conhecimento e prática de enfermeiros no controle da dor de pacientes com feridas neoplásicas. Enferm Bras. (2019) 18:3–11. doi: 10.33233/eb.v18i1.1039
35. de Oliveira Souza, MA, Rodrigues de Souza, N, da Silva, T, Melo, J, Campos Absalão Xavier, MA, Lopes de Almeida, G, et al. Odor evaluation scales for odor in neoplastic wounds: an integrative review. Rev Bras Enferm. (2018) 71:2552–60. doi: 10.1590/0034-7167-2017-0428
36. Dutta, S, Ishore, K, and Ghoshal, A. Role of integrative oncology and palliative care services in improving comfort level and compliance among patients with advanced fungating breast cancer—experience from a rural hospital of north eastern India during the COVID-19 pandemic. Indian J Palliat Care. (2022) 28:256–61. doi: 10.25259/IJPC_40_2021
37. Firmino, F, Ferreira, SADC, Franck, EM, De Queiroz, WMS, Castro, DV, Nogueira, PC, et al. Malignant wounds in hospitalized oncology patients: prevalence, characteristics, and associated factors. Plast Surg Nurs. (2020) 40:138–44. doi: 10.1097/PSN.0000000000000320
38. Firmino, F, Santos, J, Meira, KC, de Araújo, JL, Júnior, VA, and de Gouveia Santos, VLC. Regenerated oxidized cellulose versus calcium alginate in controlling bleeding from malignant breast cancer wounds: randomised control trial study protocol. J Wound Care. (2020) 29:52–60. doi: 10.12968/jowc.2020.29.1.52
39. Fromantin, I, Watson, S, Baffie, A, Rivat, A, Falcou, MC, Kriegel, I, et al. A prospective, descriptive cohort study of malignant wound characteristics and wound care strategies in patients with breast cancer. Ostomy Wound Manage. (2014) 60:38–48.
40. Furka, A, Simkó, C, Kostyál, L, Szabó, I, Valikovics, A, Fekete, G, et al. Treatment algorithm for cancerous wounds: a systematic review. Cancers. (2022) 14:1177. doi: 10.3390/cancers14051177
41. Kelechi, T, Prentice, M, Madisetti, M, Brunette, G, and Mueller, M. Palliative care in the management of pain, odor, and exudate in chronic wounds at the end of life: a cohort study. J Hosp Palliat Nurs. (2017) 19:17–25. doi: 10.1097/NJH.0000000000000306
42. Lian, SB, Xu, Y, Goh, SL, and Aw, FC. Comparing the effectiveness of green tea versus topical metronidazole powder in malodorous control of fungating malignant wounds in a controlled randomised study. Proc Singapore Healthc. (2014) 23:3–12. doi: 10.1177/201010581402300102
43. Peng, L, and Dai, Y. Effect of metronidazole combined with autolytic debridement for the management of malignant wound malodor. J Int Med Res. (2019) 48. doi: 10.1177/0300060519897505
44. Patel, BC, Ostwal, S, Sanghavi, PR, Joshi, G, and Singh, R. Management of malignant wound myiasis with ivermectin, albendazole, and clindamycin (triple therapy) in advanced head-and-neck cancer patients: a prospective observational study. Indian J Palliat Care. (2018) 24:459–64. doi: 10.4103/IJPC.IJPC_92_18
45. Ramasubbu, DA, Smith, V, Hayden, F, and Cronin, P. Systemic antibiotics for treating malignant wounds. Cochrane Database Syst Rev. (2017) 8:CD011609. doi: 10.1002/14651858.CD011609.pub2
46. Savage, P, Murphy-Kane, P, Lee, CT, Suet-Lam Chung, C, and Howell, D. Validation of the malignant wound assessment tool—research (MWAT-R) using cognitive interviewing. Can Oncol Nurs J. (2019) 29:97–109. doi: 10.5737/2368807629297102
47. Tamai, N, Mugita, Y, Ikeda, M, and Sanada, H. The relationship between malignant wound status and pain in breast cancer patients. Eur J Oncol Nurs. (2016) 24:8–12. doi: 10.1016/j.ejon.2016.05.004
48. Tang, J, Qin, F, Chen, Y, Liu, Y, Nong, L, Zhong, W, et al. Topical oxygen therapy can reduce related symptoms of malignant fungating wounds in breast cancer: a retrospective observational case series study. Int J Clin Exp Med. (2020) 13:8526–34.
49. Watanabe, K, Shimo, A, Tsugawa, K, Tokuda, Y, Yamauchi, H, Miyai, E, et al. Safe and effective deodorization of malodorous fungating tumors using topical metronidazole 0.75% gel (GK567): a multicenter, open-label, phase III study (RDT.07.SRE.27013). Support Care Cancer. (2016) 24:2583–90. doi: 10.1007/s00520-015-3067-0
50. Winardi, A, and Irwan, AM. Topical treatment for controlling malignant wound odour. EWMA J. (2019) 20:7–17. doi: 10.35279/jewma201910.01
51. Yasmara, D, Tam, SH, and Fang, SY. Caring for patients with malignant fungating wounds: a scoping literature review. J Wound Ostomy Continence Nurs. (2024) 51:19–25. doi: 10.1097/WON.0000000000001046
52. You, M, Zhang, S, Ma, X, Liu, H, Lu, Y, and Li, Y. Nursing of a non-Hodgkin’s lymphoma patient with a facial malignant fungating wound. Asia Pac J Oncol Nurs. (2021) 8:581–5. doi: 10.4103/apjon.apjon-2119
53. Grocott, P. Evaluation of a tool used to assess the management of fungating wounds. J Wound Care. (1997) 6:421–4. doi: 10.12968/jowc.1997.6.9.421
54. Grocott, P. Exudate management in fungating wounds. J Wound Care. (1998) 7:445–8. doi: 10.12968/jowc.1998.7.9.445
55. Grocott, P, and Cowley, S. The palliative management of fungating malignant wounds—generalising from multiple-case study data using a system of reasoning. Int J Nurs Stud. (2001) 38:533–45. doi: 10.1016/s0020-7489(00)00098-5
56. Grocott, P. Developing a tool for researching fungating wounds. World Wide Wounds [Internet]. 2001; 2001. Available online at: https://www.scopus.com/inward/record.uri?eid=2-s2.0–4043100470&partnerID=40&md5=59d582d5c0d7eebe4b1f51a8e140d020 (Accessed February 16, 2025).
57. Hawthorn, M. Caring for a patient with a fungating malignant lesion in a hospice setting: reflecting on practice. Int J Palliat Nurs. (2010) 16:70–2. doi: 10.12968/ijpn.2010.16.2.46752
58. Lo, SF, Hayter, M, Hu, WY, Tai, CY, Hsu, MY, and Li, YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs. (2012) 68:1312–21. doi: 10.1111/j.1365-2648.2011.05839.x
59. Lund-Nielsen, B, Adamsen, L, Kolmos, HJ, Rørth, M, Tolver, A, and Gottrup, F. The effect of honey-coated bandages compared with silver-coated bandages on treatment of malignant wounds—a randomized study. Wound Repair Regen. (2011) 19:664–70. doi: 10.1111/j.1524-475X.2011.00735.x
60. Grocott, P. The palliative management of fungating malignant wounds. J Wound Care. (2000) 9:4–9. doi: 10.12968/jowc.2000.9.1.25942
61. Lai, YL, Chang, HH, Huang, MJ, Chang, KH, Su, WH, Chen, HW, et al. Combined effect of topical arsenic trioxide and radiation therapy on skin-infiltrating lesions of breast cancer—a pilot study. Anti-Cancer Drugs. (2003) 14:825–8. doi: 10.1097/00001813-200311000-00008
62. Lund-Nielsen, B, Müller, K, and Adamsen, L. Qualitative and quantitative evaluation of a new regimen for malignant wounds in women with advanced breast cancer. J Wound Care. (2005) 14:69–73. doi: 10.12968/jowc.2005.14.2.26736
63. Tamai, N, Horii, M, Takehara, K, Kato, S, Yamamoto, Y, Naito, A, et al. Morphological characteristics of and factors related to moisture-associated dermatitis surrounding malignant wounds in breast cancer patients. Eur J Oncol Nurs. (2013) 17:673–80. doi: 10.1016/j.ejon.2013.05.005
64. Schulz, V, Kozell, K, Biondo, PD, Stiles, C, Tonkin, K, and Hagen, NA. The malignant wound assessment tool: a validation study using a Delphi approach. Palliat Med. (2009) 23:266–73. doi: 10.1177/0269216309102536
65. da Costa Santos, CM, de Mattos Pimenta, CA, and Nobre, MRC. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manag. (2010) 39:1065–76. doi: 10.1016/j.jpainsymman.2009.11.319
66. Chrisman, CA. Care of chronic wounds in palliative care and end-of-life patients. Int Wound J. (2010) 7:214–35. doi: 10.1111/j.1742-481X.2010.00682.x
67. Clark, J. Metronidazole gel in managing malodorous fungating wounds. Br J Nurs. (2002) 11:S54–60. doi: 10.12968/bjon.2002.11.Sup1.12249
68. Dowsett, C. Malignant fungating wounds: assessment and management. Br J Community Nurs. (2002) 7:394–400. doi: 10.12968/bjcn.2002.7.8.10641
69. Naylor, W. Assessment and management of pain in fungating wounds. Br J Nurs. (2001) 10:S33–6. doi: 10.12968/bjon.2001.10.Sup5.12325
70. Seaman, S. Management of malignant fungating wounds in advanced cancer. Semin Oncol Nurs. (2006) 22:185–93. doi: 10.1016/j.soncn.2006.04.006
71. Young, CV. The effects of malodorous fungating malignant wounds on body image and quality of life. J Wound Care. (2005) 14:359–62. doi: 10.12968/jowc.2005.14.8.26827
72. Voepel-Lewis, T, Zanotti, J, Dammeyer, JA, and Merkel, S. Reliability and validity of the face, legs, activity, cry, Consolability behavioral tool in assessing acute pain in critically ill patients. Am J Crit Care. (2010) 19:55–61. doi: 10.4037/ajcc2010624
73. Jensen, MP. The validity and reliability of pain measures in adults with cancer. J Pain. (2003) 4:2–21. doi: 10.1054/jpai.2003.1
74. Ngamkham, S, Vincent, C, Finnegan, L, Holden, JE, Wang, ZJ, and Wilkie, DJ. The mcgill pain questionnaire as a multidimensional measure in people with cancer: an integrative review. Pain Manag Nurs. (2012) 13:27–51. doi: 10.1016/j.pmn.2010.12.003
75. Gauthier, LR, Young, A, Dworkin, RH, Rodin, G, Zimmermann, C, Warr, D, et al. Validation of the short-form McGill pain questionnaire-2 in younger and older people with cancer pain. J Pain. (2014) 15:756–70. doi: 10.1016/j.jpain.2014.04.004
76. Warden, V, Hurley, AC, and Volicer, L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. J Am Med Dir Assoc. (2003) 4:9–15. doi: 10.1097/01.JAM.0000043422.31640.F7
77. Chang, VT, Hwang, SS, and Feuerman, M. Validation of the Edmonton symptom assessment scale. Cancer. (2000) 88:2164–71. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5
78. Frasier, K, Li, V, Christoforides, S, Daly, K, Loperfito, A, Stech, K, et al. The impact of psychosocial influences on chronic wound healing. Open J Med Psychol. (2024) 13:39–57. doi: 10.4236/ojmp.2024.133004
79. Wilson, CM, McGuire, DB, Rodgers, BL, Elswick, RK, and Temkin, SM. Body image, sexuality, and sexual functioning in women with gynecologic cancer: an integrative review of the literature and implications for research. Cancer Nurs. (2021) 44:E252–86. doi: 10.1097/NCC.0000000000000818
80. Vardhan, M, Flaminio, Z, Sapru, S, Tilley, CP, Fu, MR, Comfort, C, et al. The microbiome, malignant Fungating wounds, and palliative care. Front Cell Infect Microbiol. (2019) 9:373. doi: 10.3389/fcimb.2019.00373
81. Melhem, SJ, Nabhani-Gebara, S, and Kayyali, R. Latency of breast cancer stigma during survivorship and its influencing factors: a qualitative study. Front Oncol. (2023) 13:1075298. doi: 10.3389/fonc.2023.1075298
82. Nagle, SM, Stevens, KA, and Wilbraham, SC. Wound Assessment. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing (2025).
83. Lovén, M, Huilaja, L, Paananen, M, and Torkki, P. The integration of dermatology experts into primary care to assess and treat patients with skin lesions is cost-effective: a quasi-experimental study. Acad Dermatol Venereol. (2024):20451. doi: 10.1111/jdv.20451
84. The new Italian nursing code of ethics issued by the National Council of Nursing Professions (FNOPI). (2025).
85. Capelas, ML, Simões, ASAWF, Teves, CFTM, Durão, SAP, Coelho, SPF, Da Silva, SCFS, et al. Indicadores de Qualidade Prioritários para os Serviços de Cuidados Paliativos em Portugal. Cad Saude Publica. (2018) 10:11–24. doi: 10.34632/CADERNOSDESAUDE.2018.7245
86. EONS (2015). Recommendations for the care of patients with malignant fungating wounds. European Oncology Nursing Society (EONS).
87. Dryden, SV, Shoemaker, WG, and Kim, JH. Wound management and nutrition for optimal wound healing. Atlas Oral Maxillofac Surg Clin. (2013) 21:37–47. doi: 10.1016/j.cxom.2012.12.008
88. NHS Forth Valley. Guidelines for the management of malignant wounds M:EMPHIS pathway. Heather Macgowan. (2025)
89. Peñaranda, LM. The utopia of transdisciplinary palliative care. AG Salud. (2024) 2:89. doi: 10.62486/agsalud202489
90. Universidad Cooperativa de Colombia BucaramangaPeñaranda Ospina, LM, Iglesias Meza, FS, and Alvarado Garcia, AM. ¿Podemos ver el mundo igual? interdisciplinariedad en el cuidado paliativo. Rev Cuid. (2022) 13. doi: 10.15649/cuidarte.2568
91. Pramod, S, Dumville, J, Norman, G, and Stringer, J. A survey of UK nurses about their care of people with malignant fungating wounds. Eur J Oncol Nurs. (2024) 70:102609. doi: 10.1016/j.ejon.2024.102609
92. Streiner, D. Health measurement scales: a practical guide to their development and use (5th edition). Aust N Z J Public Health. (2016) 40:294–5. doi: 10.1111/1753-6405.12492
93. Park, H, Kim, KE, Moon, E, and Kang, T. Psychometric properties of assessment tools for depression, anxiety, distress, and psychological problems in breast Cancer patients: a systematic review. Psychiatry Investig. (2023) 20:395–407. doi: 10.30773/pi.2022.0316
Keywords: malignant fungating wound, symptom assessment, wound healing, tool, neoplasms, scoping review, nursing
Citation: Nigrelli D, Gambalunga F, Anastasi G, Peghetti A, Durante S, Giusti M, Biagioli V, Quirini S, Iacorossi L and Latina R (2025) Malignant fungating wounds assessment in palliative care: a scoping review. Front. Public Health. 13:1602493. doi: 10.3389/fpubh.2025.1602493
Edited by:
Ana Pires, Universidade Atlântica, PortugalReviewed by:
Claudia Oliveira, University of Algarve, PortugalRicardo Sousa Mestre, Atlântica University, Portugal
Rita Figueiredo, Escola Superior de Enfermagem de São José de Cluny, Portugal
Copyright © 2025 Nigrelli, Gambalunga, Anastasi, Peghetti, Durante, Giusti, Biagioli, Quirini, Iacorossi and Latina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Durante, c3RlZmFuby5kdXJhbnRlQGFvc3AuYm8uaXQ=
 Daniela Nigrelli
Daniela Nigrelli Francesca Gambalunga2
Francesca Gambalunga2 Giuliano Anastasi
Giuliano Anastasi Angela Peghetti
Angela Peghetti Stefano Durante
Stefano Durante Martina Giusti
Martina Giusti Silvio Quirini
Silvio Quirini Roberto Latina
Roberto Latina