- 1School of Social Development and Public Policy, Fudan University, Shanghai, China
- 2Institute of Population Research, Peking University, Beijing, China
Background: Cardiovascular Diseases (CVD) remain a leading threat among aging populations globally, with Cardiovascular Health (CVH) trajectories shaped by cumulative exposures across the life course. Understanding these life-course connections is urgent to inform equitable geriatric care strategies.
Objective: This study aims to examine the long-term trajectories of CVH and the association between Childhood Access to Healthcare (CAH) and CVH trajectories in Chinese older adults.
Methods: Data were obtained from Chinese Longitudinal Healthy Longevity Study (CLHLS). A composite CVH score was established based on the American Heart Association’s (AHA) guidelines. Group-Based Trajectory Modeling (GBTM) was employed to identify distinct CVH trajectories over time. Multi-logistic regression was used to analyze the association between CAH and CVH trajectories. To minimize potential confounding and selection bias, entropy balancing was applied to balance covariates between the treatment and control groups.
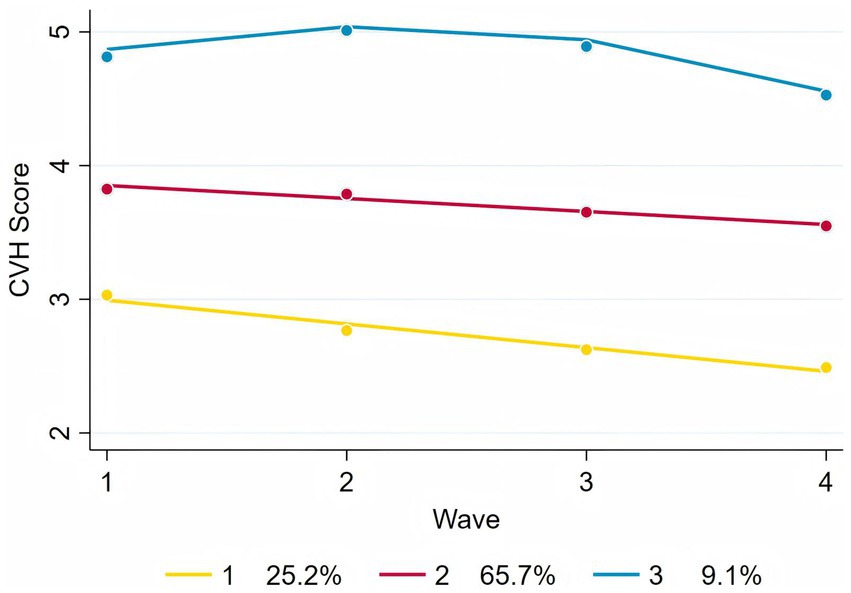
Results: Three distinct CVH trajectories were identified: Low-rapid decline (25.2%), Moderate-stable (65.7%), and High-stable (9.1%). Compared with high-stable trajectory, individuals with CAH were associated with lower likelihood in moderate-stable trajectory (Adjusted and balanced OR = 0.61, 95% CI: 0.44–0.85, p < 0.01) and low-rapid decline trajectory (Adjusted and balanced OR = 0.63, 95% CI: 0.44–0.90, p < 0.05), suggesting that CAH was associated with more favorable long-term CVH outcomes. Subgroup analysis indicated that the association was generally stable across different populations.
Conclusion: CAH significantly influences the long-term CVH trajectories of older adults in China. These findings underscore the need for public health interventions that prioritize childhood healthcare access to reduce the burden of CVD in the aging population.
1 Introduction
Population aging is a global trend, with estimates suggesting that by 2050, one in six individuals worldwide will be aged 65 years or older (1). The aging population poses a significant challenge to global public health. Cardiovascular health (CVH) is one of the key aspects. in China, It is estimated that approximately 330 million individuals are affected by Cardiovascular Disease (CVD) (2). Assessing CVH and conducting research on early pathological changes to enhance prevention, treatment, and understanding of CVD have become important areas of consensus in public health. In CVH assessment, the American Heart Association (AHA) introduced a composite marker of CVH in 2010, which consists of four behavioral and three biological metrics (3). These CVH metrics are excellent predictors of CVD, mortality, and many essential health outcomes across various populations (4, 5). The value of the total CVH metrics surpasses that of any of its individual components (6). Various types of CVD increase with age, and the outcomes tend to be more detrimental (7). CVH metrics are generally poorer in older adults, who face higher risks of CVD (8). For China, the number of older adult’s individuals is expected to reach 418 million by 2035 (9). This increase in the older adult population makes CVH a greater public health concern. Recently, some studies have examined long-term patterns in CVH metrics, showing that over time, these metrics tend to follow different trajectories (10–12). However, research on CVH metric trajectories in older adults remains limited, especially in China, where most studies have focused solely on the cross-sectional CVH status of the older adults (13, 14). Long-term patterns of CVH in older Chinese adults still require further investigation (15).
Life course theory provides an important perspective in studying aging and health. According to life course theory, experiencing adverse or positive living conditions or events during this period can influence later health outcomes including CVH (16). Healthcare is one of the major influencing factors of health throughout the life cycle (17). Adequate healthcare services during childhood are essential for ensuring health levels, which in turn affect health status in later stages of life through cumulative effects (18). During the early years of the People’s Republic of China, the country faced multiple burdens on healthcare resources, including infectious diseases and maternal, child, and infant health issues. Many children during this period were unable to access sufficient healthcare services (19). The various disadvantages experienced in early life tend to accumulate over time, and the aging process of individuals is largely shaped by the advantages and disadvantages encountered during their formative years (16). Much of the existing research has focused on the influence of childhood family environments on later-life health outcomes (20, 21), while neglecting to examine whether older individuals had their medical needs adequately met during childhood (17, 22). Furthermore, the findings of these studies are predominantly limited to midlife outcomes (22). The long-term impact of Childhood Access to Healthcare (CAH) on CVH in later life remains underexplored in the literature. Investigating the relationship between childhood healthcare and later-life CVH trajectories in Chinese older adults is crucial for developing effective public health strategies and reducing the burden of CVD (23). If childhood health exerts long-term effects on aging, then ensuring access to pediatric healthcare could yield substantial public health and economic benefits (24). This is particularly true for CVD, which impose a major global public health burden (24).
This study aimed to examine the long-term trajectories of CVH and the association between CAH and CVH trajectories in Chinese older adults. We used data from the Chinese Longitudinal Healthy Longevity Study (CLHLS) from 2008 to 2018. First, based on the AHA guidelines, we established a CVH score index. Then, we used the Group-Based Trajectory Modeling (GBTM) to analyze the long-term patterns of CVH score in Chinese older adults from 2008 to 2018. We examined the relationship between CAH and CVH score using multi-logistic regression models. To control for potential confounding factors, we also performed a sensitivity analysis with entropy balancing. Subgroup analysis has also conducted to explore the heterogeneous effect of CAH on CVH.
2 Materials and methods
2.1 Study population
The data used in this study was obtained 4 waves from 2008 to 2018 in CLHLS database. CLHLS is a longitudinal survey of the older adults organized by Peking University Center for Healthy Aging and Development, covering 23 provinces, municipalities and autonomous regions in China. The latest survey was conducted in 2018. CLHLS is the earliest and longest social science survey in China (25). In the CLHLS, a total of 2,440 participants completed all survey waves from 2008 to 2018 (26). After excluding individuals with missing data on CVH (N = 450), the final analytical sample comprised N = 1990 subjects.
2.2 Definition of CVH score
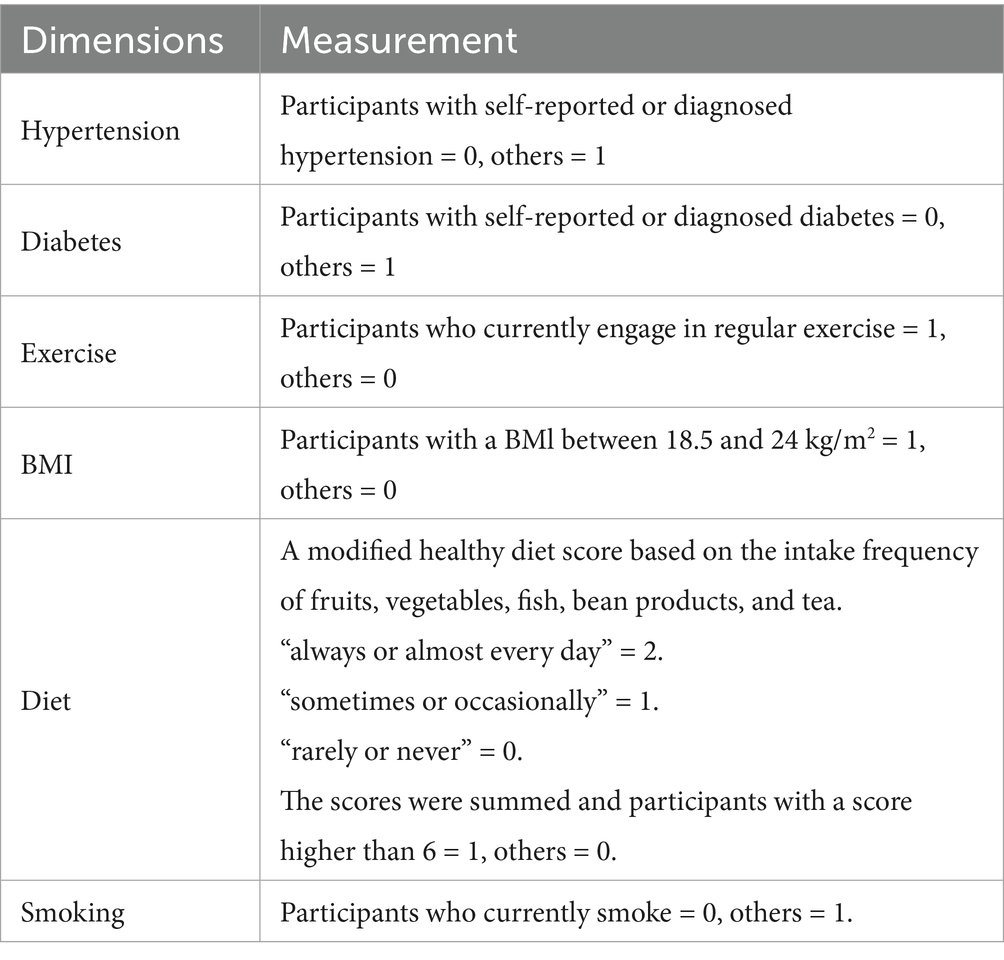
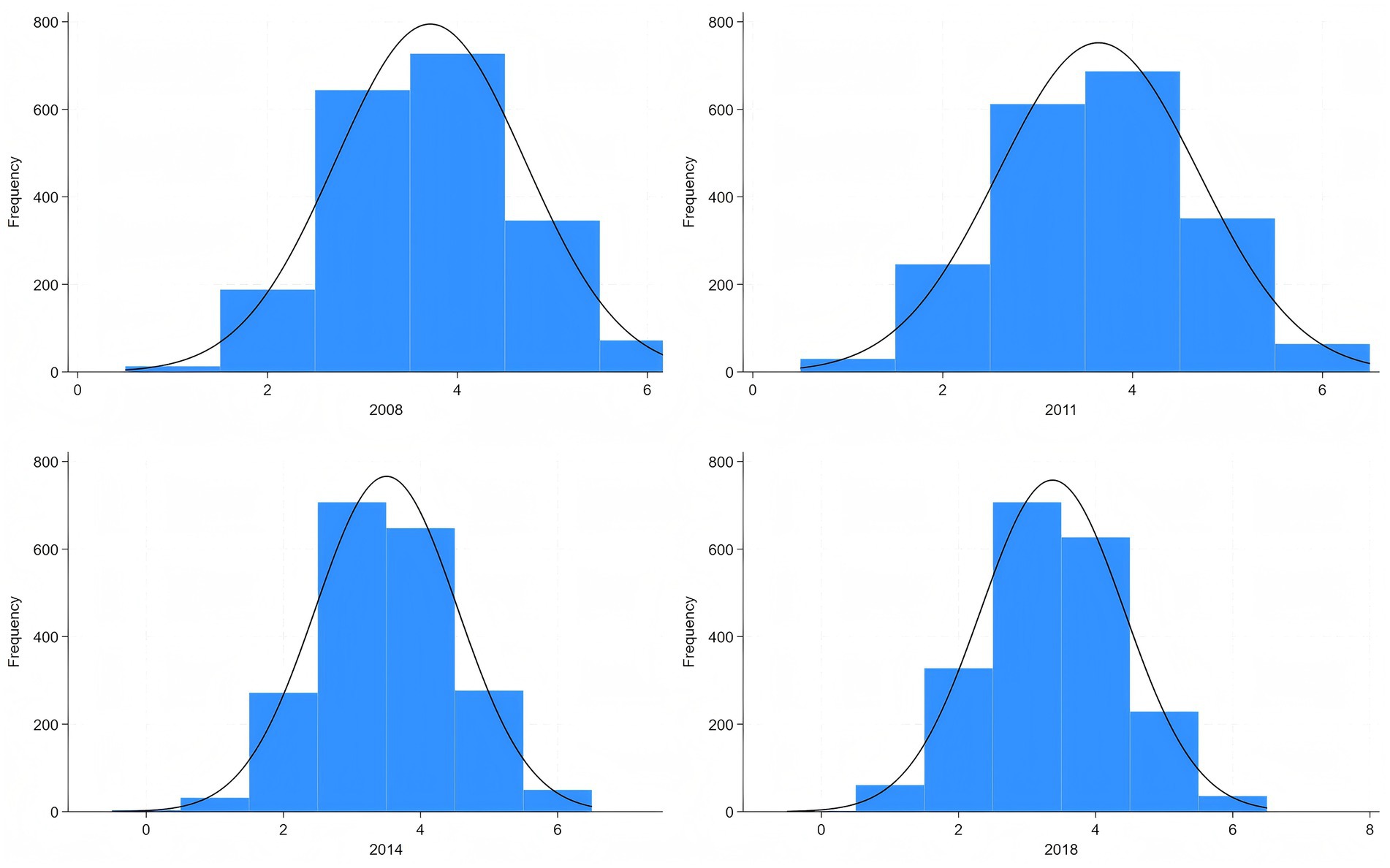
Based on the AHA guidelines and existing research on CLHLS (3, 14), CVH score is composed of six dimensions, as total Cholesterol if not applicable in CLHLS: hypertension, diabetes, exercise, BMI, diet, and smoking. We constructed a CVH score ranging from 0 to 6, with higher scores being associated with more ideal CVH conditions. The specific score construction system can be found in Table 1 and the distribution of CVH score can be found in Figure 1. Although the sleep dimension has been newly incorporated into the AHA’s updated Life’s Essential 8 metrics in 2022, the optimal sleep duration for older adult remains scientifically debated when measurement methodologies lack standardization (27). Given these methodological inconsistencies in sleep assessment approaches, we excluded the sleep component from our analytical framework.
2.3 Definition of CAH
Following the approach used in existing studies (28). We used self-perceived childhood healthcare to measure the CAH of the older adults (29). In the CLHLS, respondents were asked about their childhood healthcare condition using question F63: “Could you get adequate medical service when you were sick in childhood? “, if the answer was “Yes,” it was defined as Accessible to healthcare in childhood and assigned a value of 1; otherwise, it was assigned a value of 0. Older adults’ retrospective recall of CAH effectively captures multidimensional healthcare circumstances, providing a more comprehensive assessment than single objective indicators (29). As a critical component of childhood socioeconomic status, this recall-based measure of CAH has demonstrated criterion validity through significant associations with multiple geriatric health endpoints (30, 31).
The self-reported measure of CAH may be subject to recall bias. However, the CLHLS study design incorporated repeated measurements by readministering the same CAH question to participants across three survey waves (2008, 2011, and 2014). To further address potential recall bias, we conducted sensitivity analyses using responses from both the 2011 and 2014 waves as alternative measures.
2.4 Covariates
We selected common demographic and socioeconomic factors potentially affecting CVH in older adults as covariates (32). The covariates include sex (male / female), age (as an integer continuous variable), years of schooling (as an integer continuous variable), marital status (married / non-married), income (quantile), race (han / non-han), and living arrangement (living alone / not living alone). In addition, given that healthcare is a service utilized throughout the life course, CAH may be correlated with Access to Healthcare (AH) in different life stages. This introduces a potential for confounding, where healthcare access at subsequent life stages could influence the observed outcomes. Therefore, we incorporated variables representing healthcare access at different life stages into our model. CLHLS has investigated AH of older adults at age 60 (“Could you get adequate medical service when you were sick at around age 60? “) and at baseline (“Could you get adequate medical service when you were sick at present”), we included these two measures as covariates in our analysis.
2.5 Statistical analysis
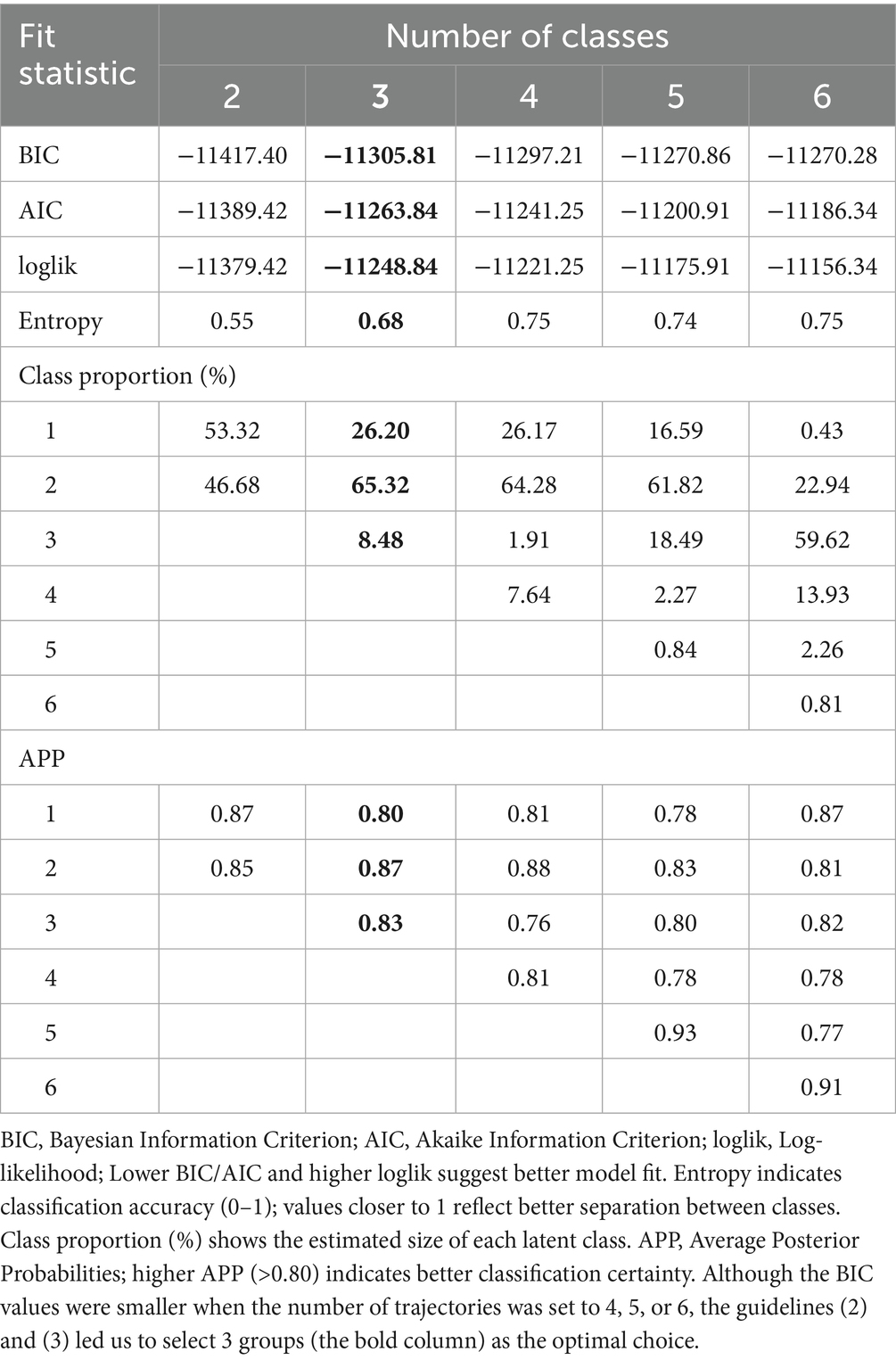
We first employed the GBTM approach to identify CVH score trajectories, which is one of the most commonly used methods for identifying subgroups within longitudinal data. Compared to alternative methods such as the Latent Growth Mixture Model, GBTM requires fewer computational resources, offers simpler fitting, and is more suitable for use with smaller sample sizes (33). In trajectory analysis, model fit is typically evaluated using statistical measures such as the Bayesian Information Criterion (BIC), Average Posterior Probability (APP), and the proportion of observations in each group (34, 35). Based on these criteria, model selection followed the guidelines: (1) choosing the optimal number of trajectories based on the minimum BIC; (2) computing posterior probabilities for each participant and assigning them to the trajectory group with the highest probability, where an APP exceeding 80% indicates an acceptable model fit; and (3) ensuring that the smallest trajectory group accounted for at least 5% of the total sample.
Kruskal-Wallis and Chi-square test were used to examine the differences in covariates among different trajectory samples. A multi-logistic regression model was used to examine the association between CAH and CVH score trajectories, with all covariates adjusted.
Some studies indicated that most research dealt inadequately with the fact that the association between adult health and early life experience was confounded by persisting social and economic disadvantage (36). To further eliminate potential confounding and sample selection bias, and to enhance the robustness of the conclusions while estimating the causal treatment effects of CAH on CVH, we applied the counterfactual causal framework by entropy balancing to weight the covariates (37). Entropy balancing, an alternative matching technique, adjusts the weights of the control group data to match the covariate distribution of the treatment group. Normally, propensity score matching (PSM) is a more popular method for estimating causal treatment effects (38). However, when the sample size in the treatment group is small, PSM can result in substantial sample loss, which may significantly impact the final conclusions (39). Entropy method directly establishes covariate balance in the weighting function, ensuring no sample loss. Compared to other techniques like propensity score matching, entropy balancing performs better in terms of bias reduction and mean squared error (40). We used the EBALANCE package in Stata to directly balance the covariates (41).
3 Results
3.1 Trajectories of CVH score
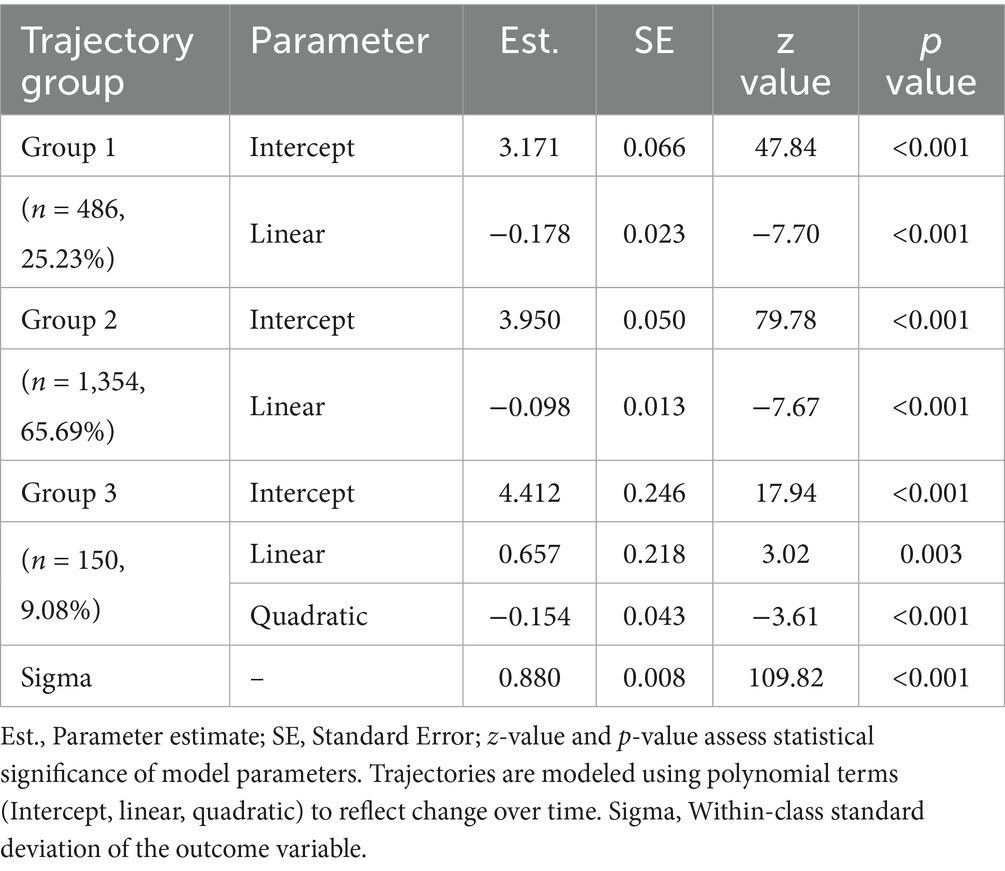
Following model selection based on goodness-of-fit criteria (Tables 2, 3), we divided the sample into three distinct trajectories with significant differences (Figure 2). The Group 1 (Low-rapid decline, approximately 25.2%) had an initial CVH score (intercept) of 3.17, and a linear slope of −0.18, indicating a continuous decline in CVH over time. This suggests that individuals in this group experienced a significant deterioration in CVH during the study period. The Group 2 (Moderate-stable, approximately 65.7%) started with a relatively higher score (intercept of 3.95), but declined at a slower rate with a slope of −0.10. This group represents the largest population in the study, where health levels were still acceptable but showed mild risk of decline. The Group 3 (High-stable, approximately 9.1%) had the highest baseline score (intercept of 4.41), with a significant positive linear term (0.66), suggesting an early upward trend, but with a negative quadratic term (−0.15), indicating a later decline. However, their overall health remained the best among the three groups.
3.2 Baseline descriptive analysis
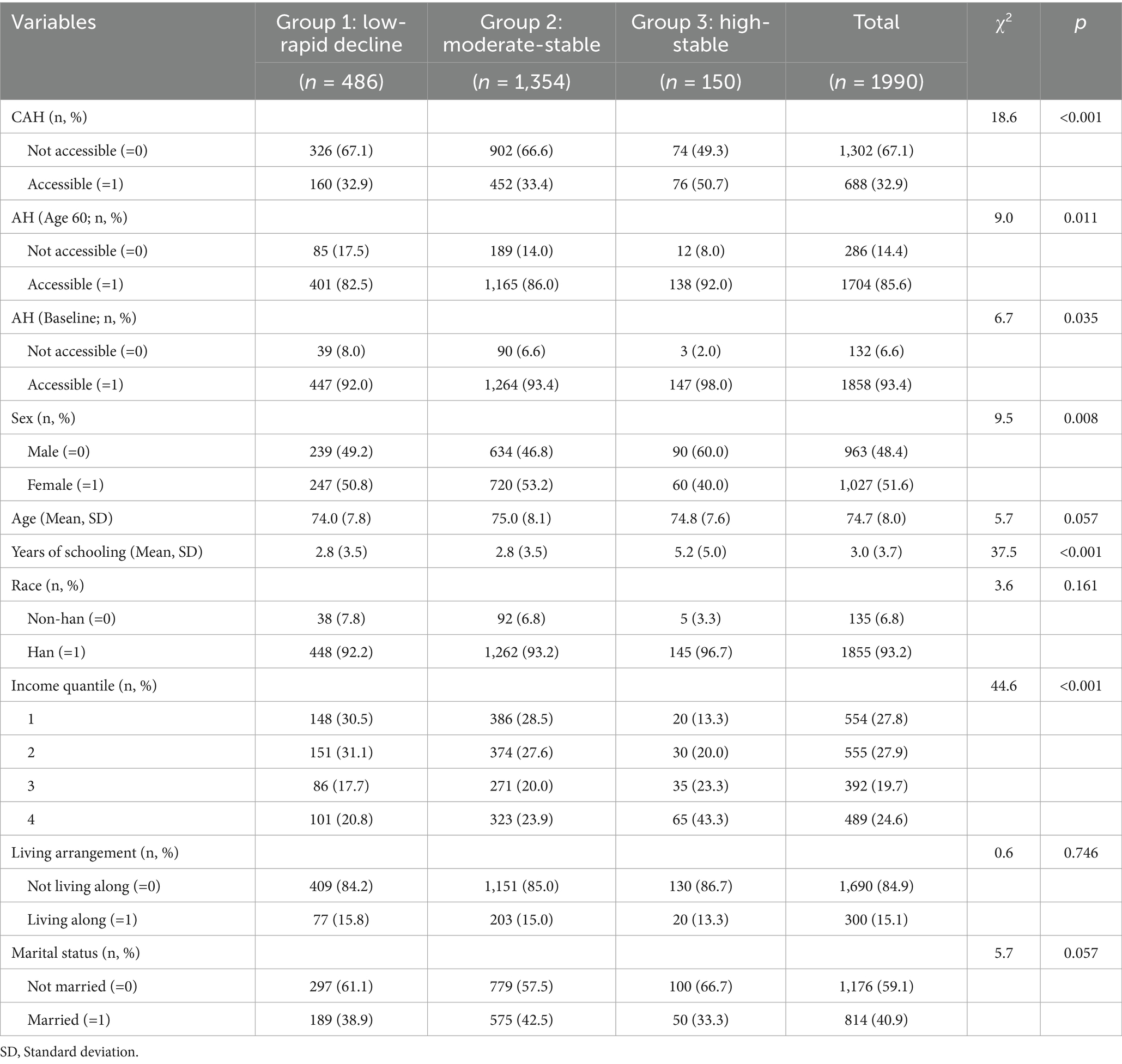
Table 4 presents the results of the descriptive statistics. Significant differences were observed across the groups in terms of CAH (p < 0.001), with Group 3 exhibited the highest prevalence of CAH among older adults (50.7%), followed by Group 2 (33.4%) and Group 1 (32.9%). Similar significant differences were also observed for AH (Age 60, p = 0.011) and AH (Baseline, p = 0.035). Significant differences were observed across the groups in terms of other covariates. In terms of sex distribution (p = 0.008), Group 1 and Group 2 exhibited a relatively balanced male-to-female ratio, whereas Group 3 had a significantly higher proportion of males (60.0%). The mean ages of the three groups were similar and the median analysis revealed a marginally significant age difference (p = 0.057). Significant differences were found in years of education across the three groups (p < 0.001), with Group 3 having significantly more years of education compared to the other two groups (5.2 years vs. 2.8 years). Han ethnicity predominated in all three groups (92.2–96.7%), and the differences between groups were not statistically significant (p = 0.161). Income levels also showed significant differences across the groups (p < 0.001), with Group 3 having a significantly higher proportion of high-income individuals compared to the other two groups. Differences in living arrangements were not statistically significant (p = 0.746), whereas marital status exhibited a marginally significant (p = 0.057). In conclusion, significant differences in several sociodemographic characteristics were observed across the trajectory groups.
3.3 Entropy balancing process
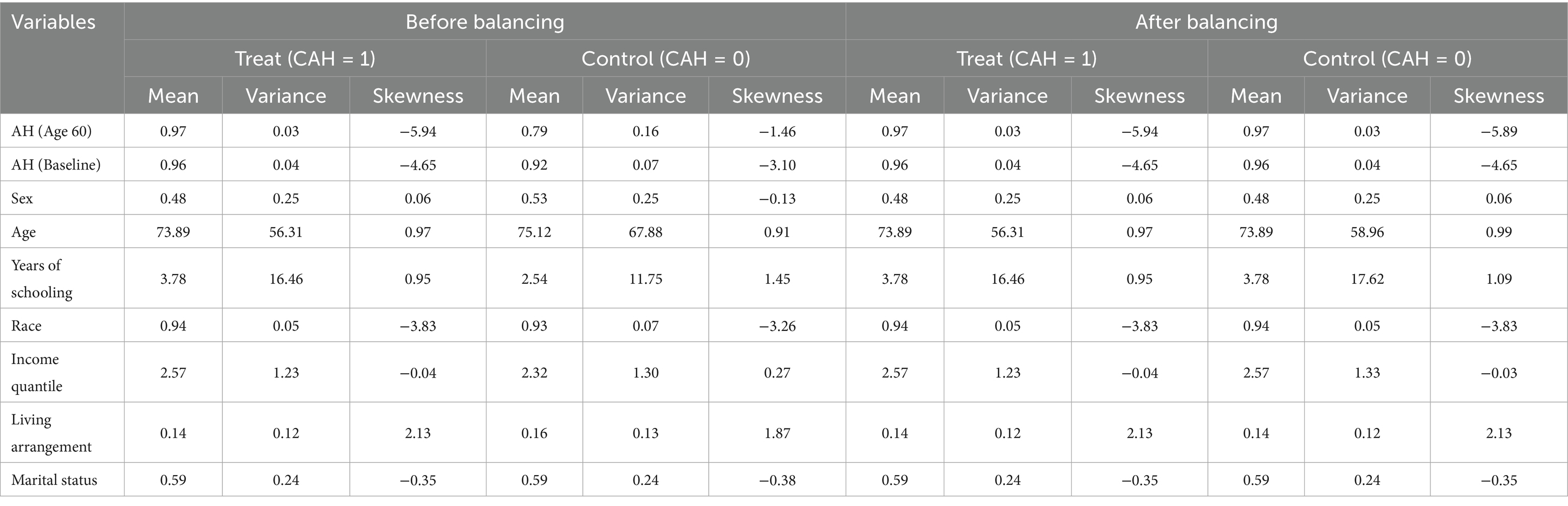
Table 5 presents the results of the entropy balancing of covariates. Before balancing, the treatment group had clear differences in covariates, which could be important sources of confounding. After entropy balancing, the differences between the treatment and control groups in covariates were significantly reduced. This indicates that entropy balancing successfully balanced these covariates, providing a more reliable foundation for subsequent causal inference.
3.4 Logistic regression analysis
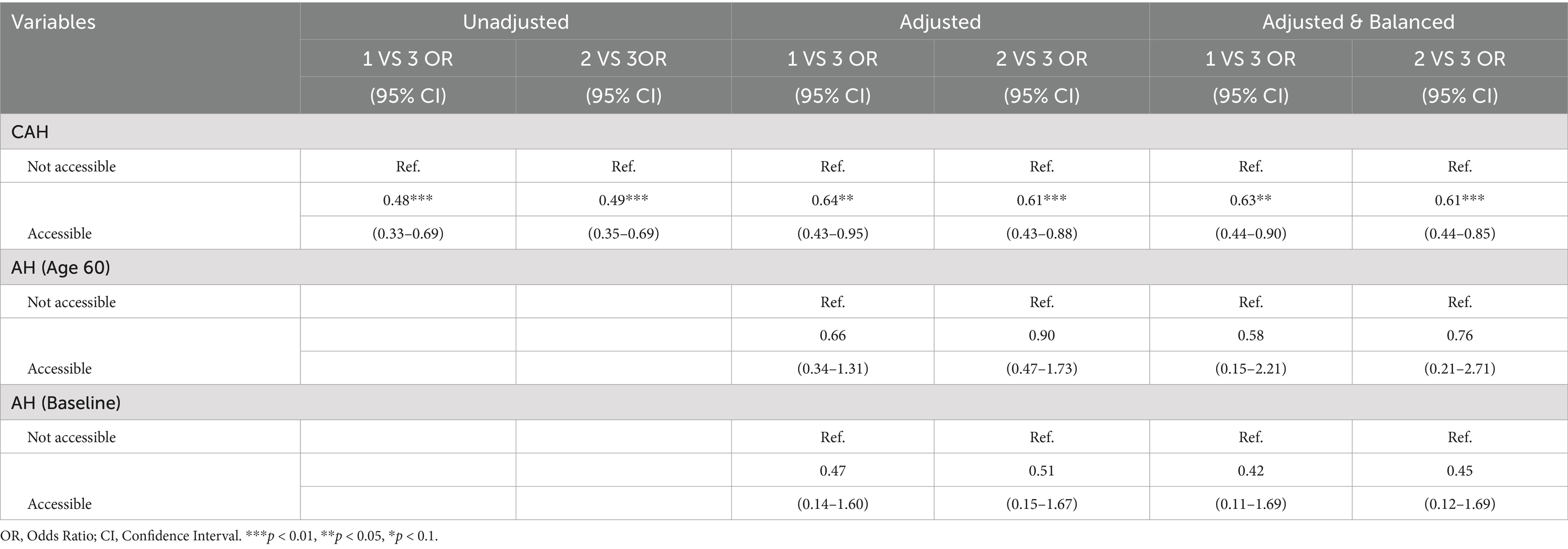
Table 6 illustrated the impact of CAH on CVH trajectories. Unadjusted, adjusted, and entropy-balanced models were used to examine how CAH influences the occurrence of three distinct CVH score trajectories. In the unadjusted model, compared to Group 3 (the best CVH trajectory group, as the reference group), individuals with CAH were significantly less likely to experience poorer health trajectories (Group 2 and Group 1). Specifically, individuals with CAH had a 51% lower probability of being in Group 2 (OR = 0.49, 95% CI: 0.35–0.69, p < 0.01) and a 52% lower probability of being in Group 1 (OR = 0.48, 95% CI: 0.33–0.69, p < 0.01). In the adjusted model, after controlling for potential confounders, the protective effect of CAH remained significant. Specifically, individuals with CAH had a 39% lower probability of being in Group 2 (OR = 0.61, 95% CI: 0.43–0.88, p < 0.01) and a 36% lower probability of being in Group 1 (OR = 0.64, 95% CI: 0.43–0.95, p < 0.05), compared to those without CAH. Entropy-balanced regression analysis further validated these findings, providing stronger causal inferences. After balancing covariates through entropy balancing, the results showed that individuals with CAH were significantly less likely to enter poorer health trajectories (Group 2 and Group 1). In the entropy-balanced model, the probability of being in Group 2 was 39% lower (OR = 0.61, 95% CI: 0.44–0.85, p < 0.01), and the probability of being in Group 1 was 37% lower (OR = 0.63, 95% CI: 0.44–0.90, p < 0.05), compared to those without CAH. In stark contrast, after controlling for all covariates, neither AH at age 60 nor at baseline showed a statistically significant association with CVH trajectories, underscoring the foundational role of CAH in shaping these long-term CVH outcomes. In Table 7, we also present sensitivity analyses utilizing alternative-year CAH recall measurements, which demonstrate complete consistency in results with our baseline regression estimates.
In conclusion, the results indicate that CAH significantly reduces the probability of entering poorer CVH trajectory groups (Group 2 and Group 1). Whether in the unadjusted, adjusted, or entropy-balanced models, individuals with CAH are less likely to be in the poorer CVH trajectory groups, highlighting the potential public health benefits of improving CAH for long-term CVH.
3.5 Subgroup analysis
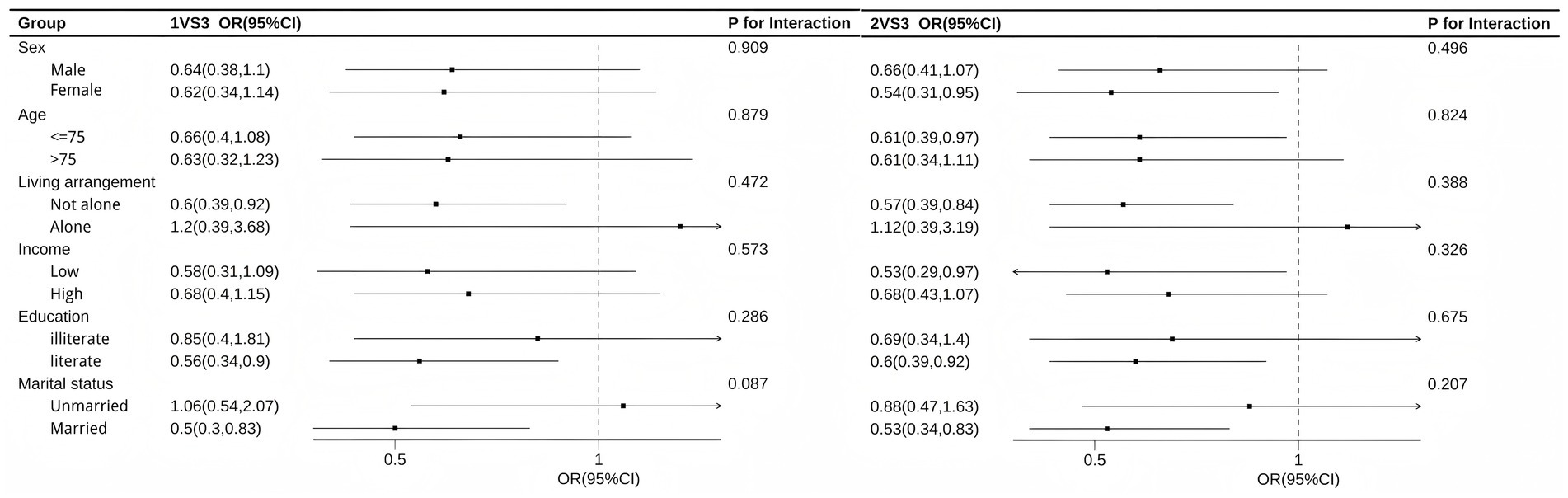
Figure 3 illustrates the results of subgroup analysis. The results indicate that the association was more pronounced among female, age under 75 years old, non-living-alone, low-income, literate, and married individuals. The P for interaction test results show that, with the exception of a marginally significant difference by marital status in the 1VS3 comparison (p = 0.087), the differences between subgroups for all other stratifying variables were not statistically significant, suggesting that the association is generally stable across different populations.
4 Discussion
This study provides valuable insights into the long-term CVH trajectories of Chinese older adults. We identified three distinct CVH trajectories: Low-rapid decline, Moderate-stable, and High-stable. CAH was associated with better CVH outcomes.
To our knowledge, this is the first study to examine CVH trajectories in Chinese older adults. We found that CVH scores showed a gradual decline over the study period, but exhibited distinct patterns in both baseline levels and rates of decline. These findings support the notion that CVH deteriorates with advancing age (42), and demonstrate that CVH trajectories in older adults differ from those observed in other age groups (10). A meta-analysis has shown that meeting 5–7 (7 in total) CVH metrics provides the greatest protection for health while lowering the risk of CVD; however, maintaining 3–4 metrics also offers a significant protective effect (43). The three trajectories we identified in our study support this conclusion. In another study conducted in northern China, CVH was categorized into Inadequate, Average, and Optimum levels. Its conclusions similarly indicated that a higher CVH score is related to a decrease in CVD incidence, and being in a better CVH category was associated with 47% reduced odds of CVD events (44). The finding also aligns with previous studies suggesting that early-life healthcare access can influence long-term health outcomes (17, 45). Similar studies conducted in western populations have shown that CAH reduces the risk of CVD in later life (46). This study extends long-term pattern research of CVH in China, and highlighting the importance of CAH in the prevention of long-term CVH decline.
CAH may influence CVH in older adults through several interconnected mechanisms. CAH can improve long-term health outcomes by promoting the prevention and treatment of chronic conditions during childhood that increase cardiovascular risk, such as hypertension, diabetes, and obesity (47–49). Studies suggest that children who receive early interventions in managing conditions like high cholesterol, and metabolic disorders are less likely to develop cardiovascular issues in later life (50, 51). On the other hand, lack of CAH can lead to delayed diagnoses, and poorer management of childhood diseases, which could increases the risk of cardiovascular diseases in later life (52, 53). Furthermore, CAH fosters the development of health-promoting behaviors (54), which are crucial for CVH in adulthood (55). Early exposure to healthcare systems helps create better health literacy, which influences lifelong health behaviors and decision-making regarding preventive care (56). These early-life healthcare experiences, therefore, can either mitigate or exacerbate the risks of CVD in older adults, depending on the quality and consistency of CAH.
This study offers actionable implications for China’s healthcare system amid rapid population aging. In the absence of adequate childhood healthcare treatment, older adults not only exhibit lower baseline levels of CAH in later life but also experience accelerated CVH decline. Since the beginning of the 21st century, China has made substantial progress in child health security, representing a significant improvement in both health outcomes and medical coverage compared to older adults born six decades ago (57). However, persistent disparities in childhood healthcare access remain prevalent among disadvantaged populations. A cross-sectional study further highlights pronounced inequalities in child health access across different demographic groups and geographic regions in China (58). A recent study revealed that the substantial rise in incident CVD cases and mortality rates underscores the persistent burden of CVD, which disproportionately affects older adult populations (42). Advanced aging population serves as a significant exacerbating factor that amplifies the public health impact of CVD, with distinct epidemiological implications. With an estimated 418 million individuals aged over 65 by 2035, addressing CVH decline through life-course interventions is imperative. The findings advocate for prioritizing equitable access to childhood healthcare, particularly in low-income regions, to mitigate later-life health disparities. Strengthening primary healthcare infrastructure in underserved areas could ensure early disease prevention and health behavior cultivation, aligning with China’s “Healthy China 2030” goals of reducing CVD burdens (59).
This study has several significant strengths. GBTM model allows for the identification of distinct long-term patterns in CVH, which captures the dynamic nature of CVH over time and provides a deeper understanding of how CVH evolves. The application of advanced statistical methods, including entropy balancing before logistic regression, helps to control for confounding factors and estimate causal treatment effects. This study has several limitations. First, CAH was assessed based on a self-reported survey, which may be subject to recall bias and subjective evaluation. Some studies suggest that in certain scenarios, people perceive their healthcare needs better than healthcare professionals (60). However, data based on self-report may still be affected by recall bias and the judgments of older adults, especially when asking them to recall their childhood healthcare environment from over 60 years ago, which is highly subjective. This is the case even though we attempted to enhance the robustness of our conclusions using repeated measurement data. Furthermore, a comprehensive assessment of CAH involves multiple dimensions and is not merely a “yes” or “no” binary question. Elements such as the quality and efficiency of healthcare services, as well as the severity of illness and individual needs of the older adults, should also be taken into consideration (61). Future research should endeavor to investigate the childhood healthcare conditions of older adults by combining both subjective and objective measures (62). The second limitation of this study is the sample selection. Previous research has shown that the CVH of older adults deteriorates significantly during the last few years of life, with various CVD becoming particularly pronounced (63). This could potentially bias the study’s conclusions. Therefore, we only included older adults who had survived for all four waves. However, a potential issue with this approach is that the included sample may be subject to a selection bias, which could lead to a more conservative estimate of the effect. At the same time, the exclusion of these participants may also weaken the national representativeness of our sample. Future research should consider including a broader sample and further investigate whether the healthcare environment in childhood affects CVH during the end-of-life stage. Finally, while we utilized entropy balancing to control for confounding variables and estimate causal treatment effects, it is important to note that observational studies like this one cannot fully establish causality. Unmeasured or residual confounders may still influence the observed associations between CAH and CVH trajectories. Future research should explore the specific pathways through which CAH influences long-term CVH trajectories, such as its impact on lifestyle behaviors and chronic disease prevention. Additionally, studies that include more diverse populations across different regions of China and longitudinal data are needed to strengthen the generalizability and causal inference of these findings.
5 Conclusion
This study highlights the significant association between CAH and CVH trajectories in older adults in China. Our findings suggest that CAH plays a crucial role in CVH outcomes in later life, with those having CAH demonstrating more favorable CVH score trajectories. Given the rapid aging of the Chinese population, these findings highlight the need for public health policies that prioritize CAH as a long-term investment in population health. Policymakers should focus on expanding healthcare access in early childhood to mitigate future healthcare burdens and improve quality of life in older adults.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://opendata.pku.edu.cn/dataset.xhtml?persistentId=doi:10.18170/DVN/WBO7LK.
Ethics statement
The CLHLS study we used was approved by the Research Ethics Committee of Peking University (IRB00001052-13074), and all participants or their proxy respondents provided written informed consent.
Author contributions
BM: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. XF: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. LL: Data curation, Methodology, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the CLHLS team for collecting the data and providing an open access platform for the data and the respondents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pilleron, S, Soto-Perez-de-Celis, E, Vignat, J, Ferlay, J, Soerjomataram, I, Bray, F, et al. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int J Cancer. (2021) 148:601–8. doi: 10.1002/ijc.33232
2. WANG, Z-W. Status of cardiovascular disease in China. J Geriatr Cardiol. (2023) 20:397–8. doi: 10.26599/1671-5411.2023.06.006
3. Yang, Q, Cogswell, ME, Flanders, WD, Hong, Y, Zhang, Z, Loustalot, F, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. (2012) 307:1273–83. doi: 10.1001/jama.2012.339
4. Fang, N, Jiang, M, and Fan, Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int J Cardiol. (2016) 214:279–83. doi: 10.1016/j.ijcard.2016.03.210
5. Guo, L, and Zhang, S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta-analysis of prospective studies. Clin Cardiol. (2017) 40:1339–46. doi: 10.1002/clc.22836
6. Xanthakis, V, Enserro, DM, Murabito, JM, Polak, JF, Wollert, KC, Januzzi, JL, et al. Ideal cardiovascular health. Circulation. (2014) 130:1676–83. doi: 10.1161/CIRCULATIONAHA.114.009273
7. Ijaz, N, Buta, B, Xue, Q-L, Mohess, DT, Bushan, A, Tran, H, et al. Interventions for frailty among older adults with cardiovascular disease. J Am Coll Cardiol. (2022) 79:482–503. doi: 10.1016/j.jacc.2021.11.029
8. Han, X, Jiang, Z, Li, Y, Wang, Y, Liang, Y, Dong, Y, et al. Sex disparities in cardiovascular health metrics among rural-dwelling older adults in China: a population-based study. BMC Geriatr. (2021) 21:158. doi: 10.1186/s12877-021-02116-x
9. Tan, S, Yang, H, Xi, X, Zhou, M, Tang, Z, and Zuo, H. Associations of baseline and longitudinal changes in basic activity of daily living with risk of cardiovascular disease among older adults in China. Nutr Metab Cardiovasc Dis. (2024) 35:103804. doi: 10.1016/j.numecd.2024.103804
10. Ruiz-Ramie, JJ, Barber, JL, Lloyd-Jones, DM, Gross, MD, Rana, JS, Sidney, S, et al. Cardiovascular health trajectories and elevated C-reactive protein: the CARDIA study. J Am Heart Assoc. (2021) 10:e019725. doi: 10.1161/JAHA.120.019725
11. Sheng, Q, Ding, J, Gao, Y, Patel, RJ, Post, WS, and Martin, SS. Cardiovascular health trajectories and subsequent cardiovascular disease and mortality: the multi-ethnic study of atherosclerosis (MESA). Am J Prev Cardiol. (2023) 13:100448. doi: 10.1016/j.ajpc.2022.100448
12. Martínez-Gómez, J, de Cos-Gandoy, A, Fernández-Alvira, JM, Bodega, P, de Miguel, M, Tresserra-Rimbau, A, et al. Cardiovascular health trajectories in adolescence and their association with sociodemographic and Cardiometabolic outcomes in Spain. J Adolesc Health. (2024) 74:1039–48. doi: 10.1016/j.jadohealth.2023.12.016
13. Gao, K, Cao, L-F, Ma, W-Z, Gao, Y-J, Luo, M-S, Zhu, J, et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. eClinicalMedicine. (2022) 44:101264. doi: 10.1016/j.eclinm.2021.101264
14. Huang, S, Zhao, Z, Wang, S, Xu, Y, Wang, Z, Wang, J, et al. Hypothetical interventions on cardiovascular health metrics for abnormal cognitive aging: an application of the parametric g-formula in the CLHLS cohort study with 12 years follow-up. J Prev Alzheimers Dis. (2024) 11:1615–25. doi: 10.14283/jpad.2024.143
15. Wu, X, Liu, X, Liao, W, Kang, N, Sang, S, Abdulai, T, et al. Association of night sleep duration and ideal cardiovascular health in rural China: the Henan rural cohort study. Front Public Health. (2021) 8:606458. doi: 10.3389/fpubh.2020.606458
16. Liu, L, Mao, B, and Ji, F. From patterns to pathways: latent class trajectories of self-perceptions of aging and their causal effects on multi-state functional transitions. Arch Gerontol Geriatr. (2025) 133:105827. doi: 10.1016/j.archger.2025.105827
17. Sales, WB, Maranhão, EF, Ramalho, CST, Macêdo, SGGF, Souza, GF, and Maciel, ÁCC. Early life circumstances and their impact on health in adulthood and later life: a systematic review. BMC Geriatr. (2024) 24:978. doi: 10.1186/s12877-024-05571-4
18. Tao, T, Shao, R, and Hu, Y. The effects of childhood circumstances on health in middle and later life: evidence from China. Front Public Health. (2021) 9:642520. doi: 10.3389/fpubh.2021.642520
19. Wang, L, Wang, Z, Ma, Q, Fang, G, and Yang, J. The development and reform of public health in China from 1949 to 2019. Glob Health. (2019) 15:45. doi: 10.1186/s12992-019-0486-6
20. Su, S, Jimenez, MP, Roberts, CTF, and Loucks, EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. (2015) 17:88. doi: 10.1007/s11886-015-0645-1
21. Mannoh, I, Hussien, M, Commodore-Mensah, Y, and Michos, ED. Impact of social determinants of health on cardiovascular disease prevention. Curr Opin Cardiol. (2021) 36:572–9. doi: 10.1097/HCO.0000000000000893
22. Campbell, F, Conti, G, Heckman, JJ, Moon, SH, Pinto, R, Pungello, E, et al. Early childhood investments substantially boost adult health. Science. (2014) 343:1478–85. doi: 10.1126/science.1248429
23. Schwartz, LF, Stratton, KL, Leisenring, WM, Rodriguez, SM, Alston, S, McDonald, A, et al. Adverse childhood experiences, resilience, and cardiovascular disease in adult survivors of childhood Cancer: a report from the childhood Cancer survivor study. Cancer Epidemiol Biomarkers Prev. (2024) 33:1132–6. doi: 10.1158/1055-9965.EPI-24-0249
24. Sartori, LRM, Baker, SR, and Corrêa, MB. Beyond the horizon: exploring adverse childhood experiences and their lifelong, intergenerational influence on dental caries. Med Hypotheses. (2024) 184:111292. doi: 10.1016/j.mehy.2024.111292
25. Wang, L, Xian, X, Liu, M, Li, J, Shu, Q, Guo, S, et al. Predicting the decline of physical function among the older adults in China: a cohort study based on China longitudinal health and longevity survey (CLHLS). Geriatr Nurs. (2025) 61:378–89. doi: 10.1016/j.gerinurse.2024.11.019
26. Du, J, Zhang, M, Zeng, J, Han, J, Duan, T, Song, Q, et al. Frailty trajectories and determinants in Chinese older adults: a longitudinal study. Geriatr Nurs. (2024) 59:131–8. doi: 10.1016/j.gerinurse.2024.06.015
27. Lauderdale, DS, Chen, J-H, Kurina, LM, Waite, LJ, and Thisted, RA. Sleep duration and health among older adults: associations vary by how sleep is measured. J Epidemiol Community Health. (2016) 70:361–6. doi: 10.1136/jech-2015-206109
28. Liu, C, Hou, X, Wang, Q, Xu, X, Wu, B, and Liu, J. Impact of access to childhood health services on healthy life expectancy of the older population. Front Public Health. (2023) 11:1234880. doi: 10.3389/fpubh.2023.1234880
29. Bataineh, H, Devlin, RA, and Barham, V. Unmet health care and health care utilization. Health Econ. (2019) 28:529–42. doi: 10.1002/hec.3862
30. Yi, Z, Gu, D, and Land, KC. The association of childhood socioeconomic conditions with healthy longevity at the oldest-old ages in China. Demography. (2007) 44:497–518. doi: 10.1353/dem.2007.0033
31. Shen, K, and Zeng, Y. Direct and indirect effects of childhood conditions on survival and health among male and female elderly in China. Soc Sci Med. (2014) 119:207–14. doi: 10.1016/j.socscimed.2014.07.003
32. Burke, GL, Arnold, AM, Bild, DE, Cushman, M, Fried, LP, Newman, A, et al. Factors associated with healthy aging: the cardiovascular health study. J Am Geriatr Soc. (2001) 49:254–62. doi: 10.1046/j.1532-5415.2001.4930254.x
33. Mésidor, M, Rousseau, M-C, O’Loughlin, J, and Sylvestre, M-P. Does group-based trajectory modeling estimate spurious trajectories? BMC Med Res Methodol. (2022) 22:194–11. doi: 10.1186/s12874-022-01622-9
34. Mattsson, M, Maher, GM, Boland, F, Fitzgerald, AP, Murray, DM, and Biesma, R. Group-based trajectory modelling for BMI trajectories in childhood: a systematic review. Obes Rev. (2019) 20:998–1015. doi: 10.1111/obr.12842
35. Yang, JM, and Hwang, J. Effect of healthy lifestyle score trajectory on all-cause mortality in the late middle-aged and older population: finding from 17-year retrospective cohort study. Exp Gerontol. (2025) 200:112681. doi: 10.1016/j.exger.2025.112681
36. Elford, J, Whincup, P, and Shaper, AG. Early life experience and adult cardiovascular disease: longitudinal and case-control studies. Int J Epidemiol. (1991) 20:833–44.
37. Rubin, DB. Causal inference using potential outcomes: design, modeling, decisions. J Am Stat Assoc. (2005) 100:322–31. doi: 10.1198/016214504000001880
38. Wang, J. To use or not to use propensity score matching? Pharm Stat. (2021) 20:15–24. doi: 10.1002/pst.2051
39. Benedetto, U, Head, SJ, Angelini, GD, and Blackstone, EH. Statistical primer: propensity score matching and its alternatives†. Eur J Cardiothorac Surg. (2018) 53:1112–7. doi: 10.1093/ejcts/ezy167
40. Hainmueller, J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal. (2012) 20:25–46. doi: 10.1093/pan/mpr025
41. Hainmueller, J, and Xu, Y. Ebalance: a Stata package for entropy balancing. J. Stat. Softw (2013) 54:1–18. doi: 10.18637/jss.v054.i07
42. Qu, C, Liao, S, Zhang, J, Cao, H, Zhang, H, Zhang, N, et al. Burden of cardiovascular disease among elderly: based on the global burden of disease study 2019. Eur Heart J - Qual Care Clin Outcomes. (2024) 10:143–53. doi: 10.1093/ehjqcco/qcad033
43. Ramírez-Vélez, R, Saavedra, JM, Lobelo, F, Celis-Morales, CA, Pozo-Cruz, Bdel, and García-Hermoso, A Ideal cardiovascular health and incident cardiovascular disease among adults: a systematic review and meta-analysis Mayo Clin Proc (2018) 93 1589–1599 doi: 10.1016/j.mayocp.2018.05.035
44. Miao, C, Bao, M, Xing, A, Chen, S, Wu, Y, Cai, J, et al. Cardiovascular health score and the risk of cardiovascular diseases. PLoS One. (2015) 10:e0131537. doi: 10.1371/journal.pone.0131537
45. Bharadwaj, P, Løken, KV, and Neilson, C. Early life health interventions and academic achievement. Am Econ Rev. (2013) 103:1862–91. doi: 10.1257/aer.103.5.1862
46. Suglia, SF, Campo, RA, Brown, AGM, Stoney, C, Boyce, CA, Appleton, AA, et al. Social determinants of cardiovascular health: early life adversity as a contributor to disparities in cardiovascular diseases. J Pediatr. (2020) 219:267–73. doi: 10.1016/j.jpeds.2019.12.063
47. Hanson, MA, Gluckman, PD, Ma, RC, Matzen, P, and Biesma, RG. Early life opportunities for prevention of diabetes in low and middle income countries. BMC Public Health. (2012) 12:1025. doi: 10.1186/1471-2458-12-1025
48. Toschke, A, Kohl, L, Mansmann, U, and Von Kries, R. Meta-analysis of blood pressure tracking from childhood to adulthood and implications for the design of intervention trials. Acta Paediatr. (2010) 99:24–9. doi: 10.1111/j.1651-2227.2009.01544.x
49. Haire-Joshu, D, and Tabak, R. Preventing obesity across generations: evidence for early life intervention. Annu Rev Public Health. (2016) 37:253–71. doi: 10.1146/annurev-publhealth-032315-021859
50. Stein, AD, Wang, M, Ramirez-Zea, M, Flores, R, Grajeda, R, Melgar, P, et al. Exposure to a nutrition supplementation intervention in early childhood and risk factors for cardiovascular disease in adulthood: evidence from Guatemala. Am J Epidemiol. (2006) 164:1160–70. doi: 10.1093/aje/kwj328
51. Siegrist, M, Hanssen, H, Lammel, C, Haller, B, and Halle, M. A cluster randomised school-based lifestyle intervention programme for the prevention of childhood obesity and related early cardiovascular disease (JuvenTUM 3). BMC Public Health. (2011) 11:258. doi: 10.1186/1471-2458-11-258
52. Blackwell, DL, Hayward, MD, and Crimmins, EM. Does childhood health affect chronic morbidity in later life? Soc Sci Med. (2001) 52:1269–84. doi: 10.1016/S0277-9536(00)00230-6
53. Halfon, N, Verhoef, PA, and Kuo, AA. Childhood antecedents to adult cardiovascular disease. Pediatr Rev. (2012) 33:51–61. doi: 10.1542/pir.33-2-51
54. Jones, T, DeMore, M, Cohen, LL, O’Connell, C, and Jones, D. Childhood healthcare experience, healthcare attitudes, and optimism as predictors of adolescents’ healthcare behavior. J Clin Psychol Med Settings. (2008) 15:234–40. doi: 10.1007/s10880-008-9126-7
55. Musavian, AS, Pasha, A, Rahebi, S-M, Atrkar Roushan, Z, and Ghanbari, A. Health promoting behaviors among adolescents: a cross-sectional study. Nurs Midwifery Stud. (2014) 3:e14560. doi: 10.17795/nmsjournal14560
56. Okan, O. The importance of early childhood in addressing equity and health literacy development in the life-course. Public Health Panor. (2019) 5:170–76. Available at: https://iris.who.int/handle/10665/327054
57. Wu, M, Liu, Q, and Wang, Z. A comparative evaluation of child health care in China using multicriteria decision analysis methods. BMC Health Serv Res. (2023) 23:1217. doi: 10.1186/s12913-023-10204-4
58. Ni, X, Li, Z, Li, X, Zhang, X, Bai, G, Liu, Y, et al. Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: a cross-sectional study. Lancet. (2022) 400:1020–32. doi: 10.1016/S0140-6736(22)01541-0
59. Xiao, Y, Chen, X, Li, Q, Jia, P, Li, L, and Chen, Z. Towards healthy China 2030: modeling health care accessibility with patient referral. Soc Sci Med. (2021) 276:113834. doi: 10.1016/j.socscimed.2021.113834
60. Lee, D-W, Choi, J, Kim, H-R, Myong, J-P, and Kang, M-Y. Differential impact of working hours on unmet medical needs by income level: a longitudinal study of Korean workers. Scand J Work Environ Health. (2022) 48:109–17. doi: 10.5271/sjweh.3999
61. Zhang, K, Kumar, G, and Skedgel, C. Towards a new understanding of unmet medical need. Appl Health Econ Health Policy. (2021) 19:785–8. doi: 10.1007/s40258-021-00655-3
62. Jang, H-Y, Ko, Y, and Han, S-Y. The effects of social networks of the older adults with limited instrumental activities of daily living on unmet medical needs. Int J Environ Res Public Health. (2021) 18:27. doi: 10.3390/ijerph18010027
Keywords: childhood access to healthcare, cardiovascular health, group-based trajectory modeling, entropy balancing, older adults
Citation: Mao B, Fang X and Liu L (2025) Unclosed wound: effect of childhood access to healthcare on cardiovascular health trajectories of Chinese older adults based on entropy balancing analysis. Front. Public Health. 13:1602953. doi: 10.3389/fpubh.2025.1602953
Edited by:
Paulo Santos, University of Porto, PortugalReviewed by:
Kei Shing Ng, The University of Hong Kong, Hong Kong SAR, ChinaJovica Jovanovic, University of Niš, Serbia
Copyright © 2025 Mao, Fang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Boshu Mao, MjMyMTA3MzAwMTlAbS5mdWRhbi5lZHUuY24=
†These authors have contributed equally to this work
 Boshu Mao
Boshu Mao Xiaoyi Fang2†
Xiaoyi Fang2†