- 1College of Physical Education and Health, Guangxi Normal University, Guilin, China
- 2Faculty of Education and Liberal Studies, City University, Petaling Jaya, Malaysia
- 3Faculty of Kinesiology, Sport, and Recreation, University of Alberta, Edmonton, AB, Canada
Background: Physical activity (PA) has been widely recognized as a key strategy to slow age-related cognitive decline. However, its specific effects on older adults with diabetes or prediabetes remain poorly understood. Therefore, we investigated the association between different levels of PA and cognitive function among older Americans with diabetes and prediabetes.
Methods: This cross-sectional study used data from the 2011–2014 National Health and Nutrition Examination Survey (NHANES) and included a total of 1,299 older adults aged ≥60 years. The PA levels were determined by calculating the weekly metabolic equivalent of task time (MET-min/week). The participants’ cognitive abilities were assessed using the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) Word Learning Test, Animal Fluency Test (AFT), and Digit Symbol Substitution Test (DSST). Multivariable logistic regression models were used to analyze the association between different PA levels and cognitive function in patients with diabetes and prediabetes. The study utilized the restricted cubic spline (RCS) models to explore the nonlinear correlation of PA with cognitive function.
Results: Upon controlling for confounders, DSST scores were still significantly associated with moderate-level PA (OR: 0.457, 95% CI: 0.244, 0.853, p = 0.020) and high-level PA (OR: 0.478, 95% CI: 0.240, 0.955, p = 0.039). According to the RCS models, PA showed a significant nonlinear correlation with cognitive function, and the risk of cognitive decline decreased with the increase of PA levels.
Conclusion: In older adults with diabetes and prediabetes, moderate and high levels of physical activity are associated with a lower risk of cognitive decline. Clinicians should encourage patients to participate actively in exercise to maximize the benefits of PA.
1 Introduction
Dementia, a syndrome caused by a variety of diseases, would wreak havoc on nerve cells and damage the brain over time, leading to deterioration in cognitive function. With a great impact, dementia is one of the main causes of the older adult’s incapacity and dependence on others (1). Currently, there are over 55 million dementia cases globally, with estimates suggesting a threefold rise to more than 150 million by 2050 (2). The United States is one of the countries in the world with the highest burden of diabetes and prediabetes among the older adult (3). The prevalence rate is increasing year by year, and approximately 10% of patients with prediabetes progress to diabetes each year (4). Cognitive decline is an important precursor to the onset of dementia. Numerous studies have shown that both diabetes and prediabetes contribute to a greater susceptibility to cognitive decline and dementia (5, 6). Various neuropathological mechanisms can explain this association, such as large production of β-amyloid and impaired cerebral blood flow caused by disruption of cellular metabolism due to insulin resistance or inadequate secretion (7). In addition, in studies of older adults, it was found that patients in the diabetic or pre-diabetic stage had reduced brain volume and elevated HbA1c, and this led to significant declines in cognitive domains such as memory, attention and executive function, which in turn increased risk of cognitive decline and thus dementia (8–10). Among the many dementia assessment tools, the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word Learning Test, Animal Fluency Test (AFT), and Digit Symbol Substitution Test (DSST) have been widely used, not only for diagnosing dementia but also for intuitively evaluating overall cognitive function (11).
Physical activity (PA) is defined as any motion that involves the bones and muscles, and such physical action will result in energy consumption. PA may also enhance physical fitness and promote physical and mental health (12). In the general population, PA is an effective means of preventing cognitive decline and reducing the risk of developing dementia (13, 14). Research indicates that engaging in more PA can lower the chances of developing dementia and offset the harmful consequences of a sedentary lifestyle (15). Another study has shown that, compared to inactive individuals, more physical activity among older adults reduced the risk of cognitive impairment and made them perform better on tests of overall cognitive and executive function (16). Furthermore, PA may confer multiple benefits to patients with diabetes or prediabetes, including improved insulin sensitivity, improved glycemic control, and improved cardiovascular health (17–19). At present, some studies have explored the impact of PA on cognitive function among individuals with diabetes, suggesting that a physically active lifestyle is associated with a slower rate of cognitive decline and that aerobic exercise can improve overall cognitive performance in older adults with diabetes (20, 21). However, to date, no study has utilized a nationally representative U.S. sample to examine the association between PA and multidomain cognitive function among older adults with diabetes or prediabetes. Furthermore, the dose–response relationship between PA and cognitive performance remains unclear. Therefore, investigating the effects of varying levels of PA on cognitive function in older adults with diabetes or prediabetes not only helps to elucidate the role of PA in maintaining or improving cognitive health, but also contributes to enhancing their quality of life and potentially preventing the onset of dementia.
The objective of this study is to investigate the association of PA with cognitive function among older adults with diabetes and prediabetes by employing a sample of a nationally representative U.S. population, thereby providing important guidelines for clinicians and patients.
2 Materials and methods
2.1 Study population
Data for this study were obtained from the National Health and Nutrition Examination Survey (NHANES), a biennial representative sample survey of the U.S. population conducted by the Centers for Disease Control and Prevention since 1999 using a cross-sectional, stratified, multi-stage probability sampling design to assess the health and nutritional status of the U.S. population (22). All NHANES survey protocols have been approved by the Ethics Review Board of the National Center for Health Statistics and all of the participants offered written informed consent (23).
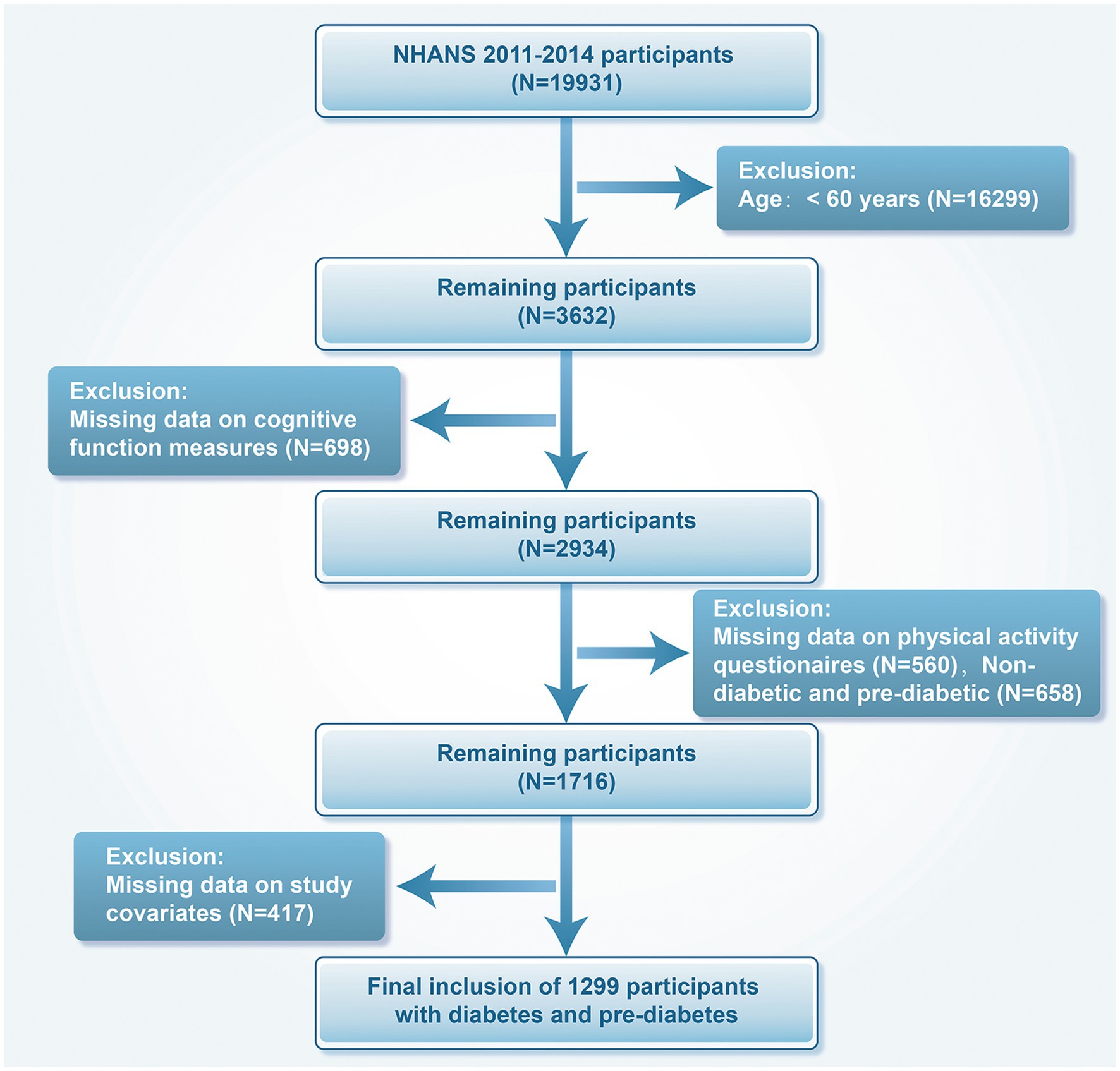
We included data from the NHANES cycles of 2011–2012 and 2013–2014, comprising 19,931 individuals aged 60 years and above with prediabetes or diabetes. We excluded 16,299 patients younger than 60 years of age, 698 with missing cognitive function measures, 560 with missing PA questionnaires, 658 patients without diabetes and prediabetes, and 417 with missing covariates. In the end, 1,299 participants were analyzed (Figure 1).
2.2 Diabetes and prediabetes
Diabetic status is self-reported as diagnosed by a physician or healthcare practitioner, or as determined by higher levels of fasting blood glucose (FBG) (≥ 126 mg/dL), blood glucose 2 h post oral glucose tolerance test (≥ 200 mg/dL) and HbA1c (≥ 6.5%). Prediabetes identifies who does not have diabetes but meets one or more of the following criteria: prediabetes self-reported as diagnosed by a physician or healthcare practitioner, a FBG concentration of 100–125 mg/dL, blood glucose of 140–199 mg/dL 2 h post oral glucose tolerance test, or HbA1c at 5.7–6.4% (24).
2.3 MET and PA calculation
Metabolic equivalent of task (MET) is a measure of the oxygen needed to sustain basic metabolism and is frequently used to gage relative energy metabolism and exercise intensity in various activities (25). In this study, the Global Physical Activity Questionnaire (GPAQ) was used to evaluate PA via MET to represent exercise intensity (26). The PAQ survey involved the following PA: vigorous work-related (MET = 8.0), vigorous leisure-time (MET = 8.0), moderate work-related (MET = 4.0), walking or bicycling for transportation (MET = 4.0), and moderate leisure-time (MET = 4.0). By multiplying MET, activity type, weekly frequency, and duration of each session, MET-min/week was calculated, and the PA levels of each participant were quantified accordingly (27). PA levels (MET-min/week) were categorized into four groups according to the Physical Activity Guidelines for Americans (28): (1) no PA (No-PA): 0, (2) low-level PA (LLPA): 1–599, (3) moderate-level PA (MLPA): 600–1,200, and (4) high-level PA (HLPA): >1,200.
2.4 Cognitive function
Participants (60 years of age and above) in the NHANES survey conducted from 2011 to 2014 underwent three cognitive tests. The Immediate Word Learning (CERAD-WL) and Delayed Recall Module (CERAD-DR), the AFT, and the DSST of the CERAD were established.
The CERAD Word Learning subtest assessed the immediate and delayed ability to learn new language information (29). The test consists of three sequential learning trials and one delayed recall. Participants were tasked with reading 10 unrelated words out loud in the learning trials, one word at a time. Following the appearance of the words, the participant promptly recollected as many words as they could. The sequence of the 10 words was altered in each of the three learning trials. After finishing the AFT and DSST tasks, a delayed word recall test was conducted. The CERAD test comprised three learning trials and one delayed recall test, with each test scored on a scale of 0–10, and the total score being the sum of all four tests. The AFT assessed the ability to fluently categorize speech, which was a key aspect of executive function. Participants were asked to list as many animals as possible in 1 min, earning one point for each animal named (30). The DSST was a component of the Wechsler Adult Intelligence Scale (WAIS III) that assessed processing speed, sustained attention, and working memory (31). In each dimension of cognitive function, lower scores indicate poorer cognitive function.
Based on the previous study, the ages of 60–69, 70–79, and ≥80 were, respectively, set as three age groups to correspond to the lower cut-off values for different cognitive tests (32). The cut-off values for low cognitive performance in the three age groups were as follows: 22, 19, and 16 for the CERAD test; 14, 13, and 12 for the AFT; and 38, 34, and 29 for the DSST.
2.5 Covariates assessment
Based on prior studies, the following covariates were included to reduce the effect of potential confounders (33–36). Demographic characteristics, including age (60–69, 70–79, 80 and older), gender (male/female), race (Mexican-American, non-Hispanic white, non-Hispanic black, and other races), education (< high school diploma, high school graduate/equivalent, >high school diploma), marital status (Married, Widow/divorce/separation, Unmarried and Cohabitation) and household poverty-to-income ratio (PIR), were obtained from the NHANES survey. Smoking status was classified as never smokers, former smokers and current smokers; a participant was considered a drinker if he or she had consumed alcohol more than 12 times in 1 year. The body mass index (BMI) was determined by professional technician in physical examination by dividing the weight in kilograms by the square of the height in meters. Diet quality was assessed using the Healthy Eating Index-2015 (HEI) score, and higher scores indicated healthier diet. The nine-item Patient Health Questionnaire (PHQ-9) was used to evaluate depression (score ≥10) (37). Hypertension, stroke, and coronary heart disease (CHD) were confirmed by physician or health care professional.
2.6 Statistical analysis
Sample weights provided by NHANES were used to weight all statistical analyses in this study, and the stratification and clustering methods for complex sampling designs were employed. The analysis strictly adhered to NHANES guidelines. A weighted presentation was used to illustrate basic characteristics traits of the study population for a nationally representative estimate (38). Continuous variables that were not normally distributed were reported as median interquartile range, while categorical variables were shown as N (%). Multivariate logistic regression analysis was performed to analyze the associations between different levels of PA and three cognitive function tests in patients with diabetes or prediabetes, and the results were presented as odds ratios (OR) with 95% confidence intervals (CI). Model 1 did not adjust for confounding factors; gender, age, ethnicity, education, marital status and PIR were added to Model 2; and Model 3 further adjusted for HEI, BMI, smoke, drink, hypertension, CHD, stroke, and depression on the basis of Model 2. Secondly, we employed the restricted cubic spline (RCS) model to analyze the nonlinear relationship between the continuous variable PA and cognitive function. The number of nodes was set at 3, and the goodness of fit of the model was verified using the Akaike Information Criterion (AIC). Finally, subgroup analyses and interaction tests were conducted to investigate the association of PA with cognitive function across various populations. Based on the criteria of whether meeting the recommended amount of PA guidelines (39), the participants were assigned to two groups and classified by age, sex, race/ethnicity, marital status, education, smoking, drinking, hypertension, depression, diabetes, and prediabetes.
The R software (4.3.0 version) was utilized for the statistical analysis. All statistical tests were two-sided, and statistical significance was determined at a threshold of p < 0.05.
3 Results
3.1 Baseline characteristics of participants by PA level
A total of 1,299 older adults with diabetes or prediabetes (weighted population 22,959,343) were included in the final analysis. In the study population, the proportion of patients aged 60 to 69 was 56%; that of patients aged 70 to 79 was 29%, and that of patients aged over or equal to 80 was 15%. In terms of gender, 48% were male, and 52% were female. In regards to race, the proportion of Mexican American, Non-Hispanic White, Non-Hispanic Black, and Others was 3.7, 78, 9.1, and 9.2%, respectively. About 61% of the participants were married, and 58% had a high school degree or higher. Drinkers and current smokers accounted for 73 and 10%, respectively. Table 1 illustrates the baseline traits of the weighted samples.
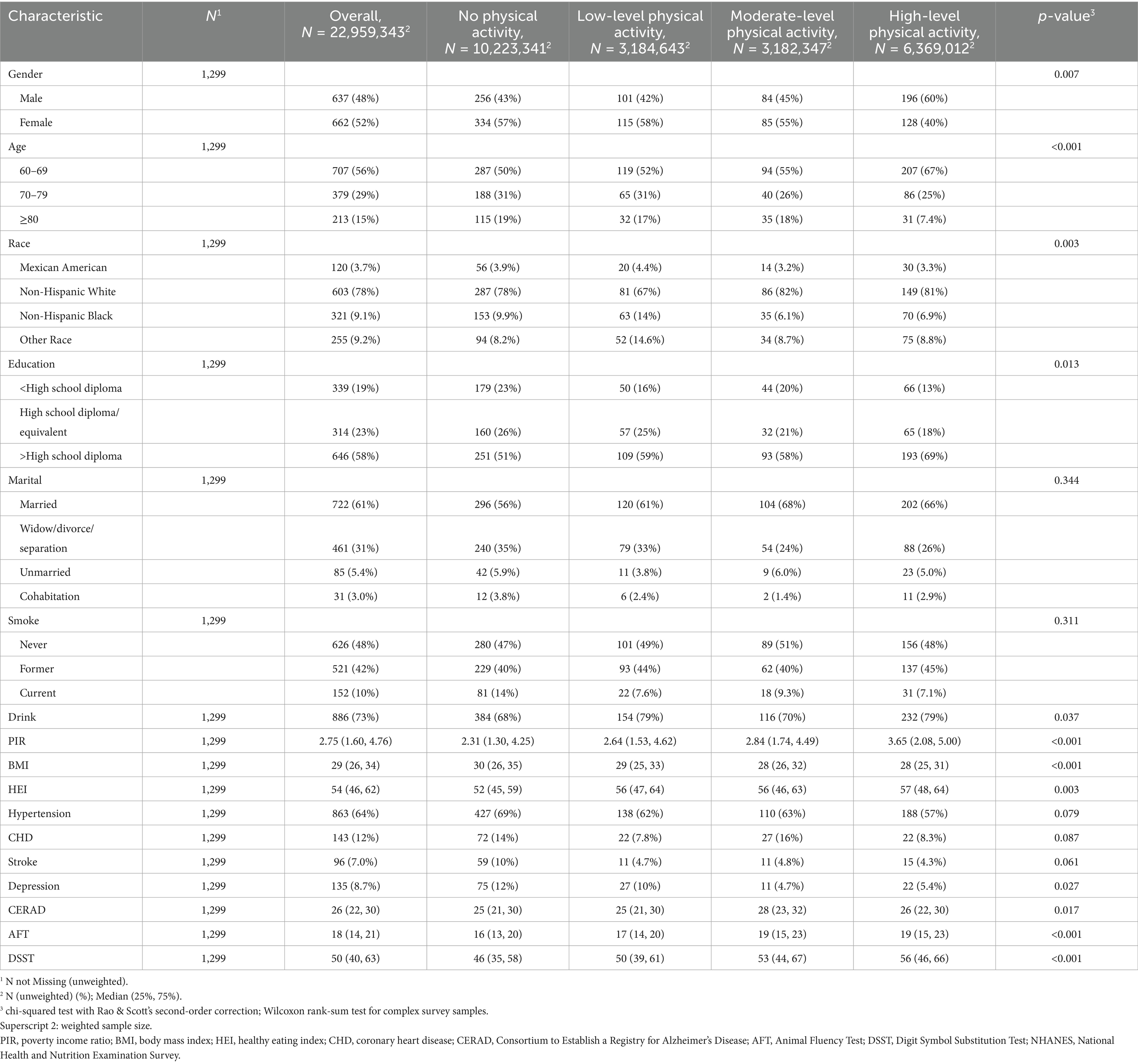
Table 1. Baseline characteristics of participants by physical activity level, from NHANES (2011–2014).
3.2 Association between physical activity level and cognitive function
Associations were found between various levels of PA and three cognitive function tests based on the weighted logistic regression model. In Model 1, there was a significant association between HLPA and CERAD scores compared with the No-PA group (OR: 0.621, 95% CI: 0.388, 0.994, p = 0.047). After adjusting for other confounding factors, the protective effect of PA on cognitive function was not observed in Model 2 and Model 3. In the AFT, participants who reported LLPA (OR: 0.571, 95% CI: 0.364, 0.894, p = 0.016), MLPA (OR: 0.365, 95% CI: 0.172, 0.775, p = 0.011), and HLPA (OR: 0.519, 95% CI: 0.284, 0.949, p = 0.034) were significantly associated with outcomes in Model 1 compared with the No-PA group; after adjusting for Model 2, LLPA (OR:0.513, 95% CI: 0.310, 0.850, p = 0.032) and MLPA (OR:0.400, 95% CI: 0.179, 0.894, p = 0.028) were still significantly correlated with cognitive ability (p < 0.05); after adjusting for Model 3, no significant association between PA and AFT scores was observed. In the DSST, participants with MLPA and HLPA were significantly associated with higher cognitive function scores in Model 1, Model 2, and Model 3 compared to the No-PA group (p < 0.05). In the fully adjusted model, the risk of cognitive decline in the moderate PA group and the high PA group was reduced by 54.3 and 52.2%, respectively (Table 2).
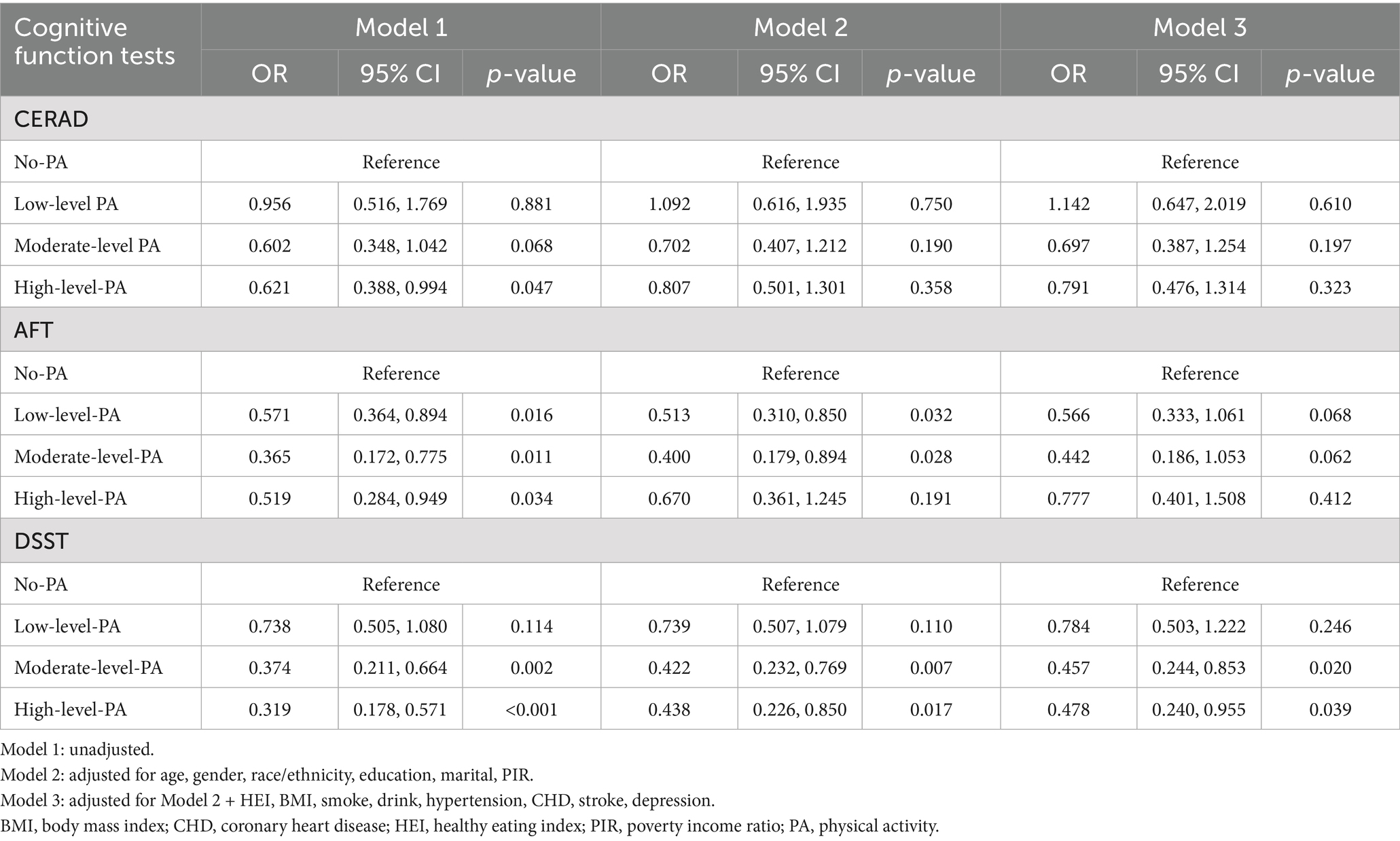
Table 2. Associations between different levels of physical activity and three measures of cognitive function.
The RCS results showed significant nonlinear dose–response correlations between PA levels and CERAD test (p for overall = 0.079, p for nonlinear = 0.024), AFT (p for overall = 0.020, p for nonlinear = 0.043), and DSST (p for overall<0.001, p for nonlinear <0.001). According to the results of CERAD, AFT and DSST, cognitive function benefited the most when the PA reached 6,200 MET-min/week, 4,600 MET-min/week and 5,400 MET-min/week (Figure 2).
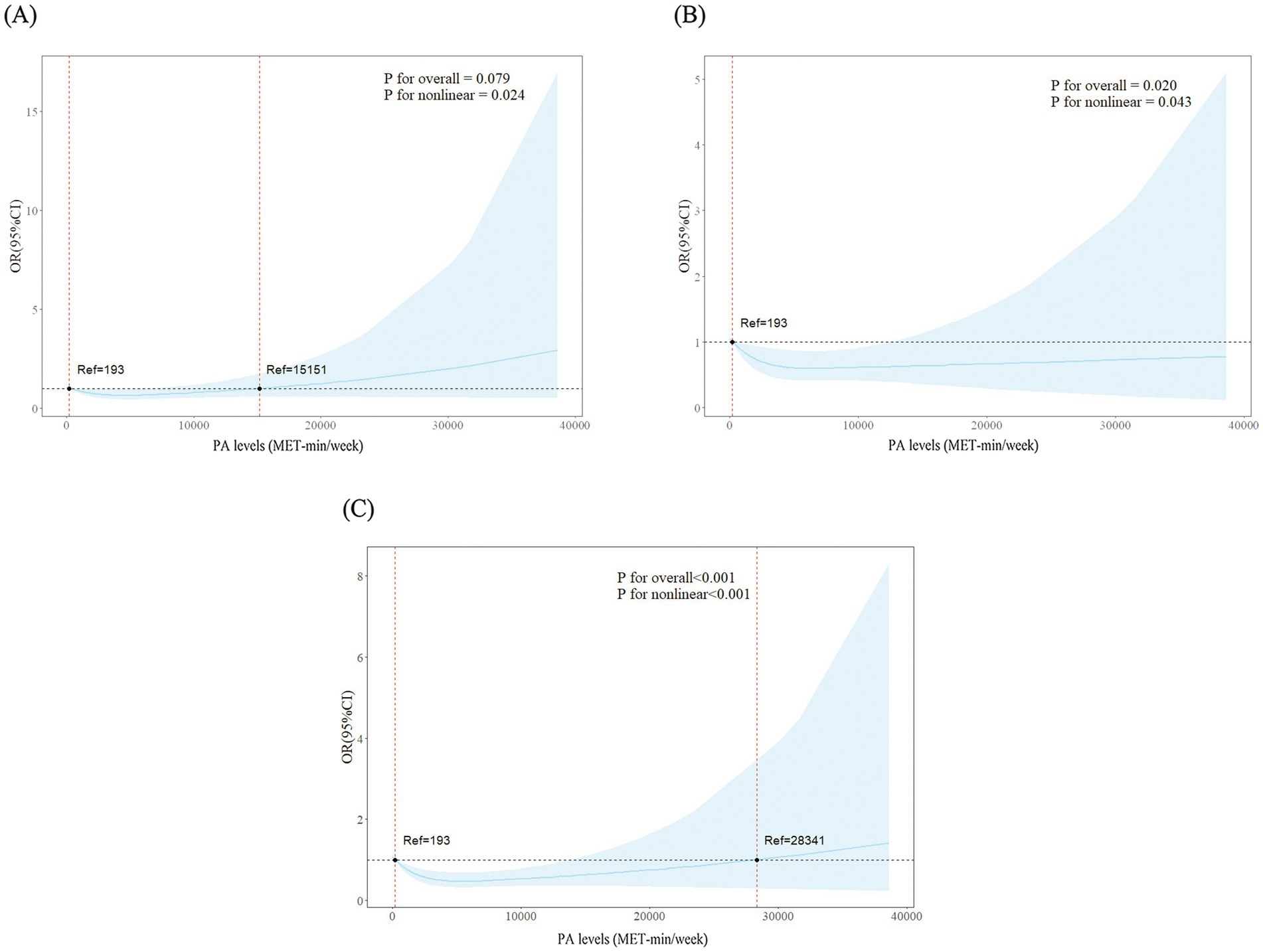
Figure 2. Dose–response associations between physical activity levels and three tests of cognitive function. (A) Consortium to establish a registry for Alzheimer’s disease test. (B) Animal fluency test. (C) Digit symbol substitution test.
3.3 Subgroup analyses
Figure 3 illustrates the associations between PA and cognitive function in different subgroups. The association of PA with cognitive function stayed consistent across various subgroups of variables, such as age, sex, race/ethnicity, education, drink, smoke, hypertension, depression, and diabetes/prediabetes, when using CERAD as the outcome; on the contrary, interaction analysis showed that marital status could affect the association of PA with cognitive ability (p interaction = 0.021). With AFT as the outcome indicator, the association between PA and cognitive level remained consistent in subgroups such as gender, ethnicity, marital marriage, educational attainment, drink, smoke, hypertension, depression, diabetes, and prediabetes; in the subgroup of age, the interaction between PA and cognitive level was significant (p interaction < 0.001). With DSST as the outcome indicator, educational level was found to influence the correlation of PA with cognitive function (p interaction = 0.005) and it remained stable in other subgroups.
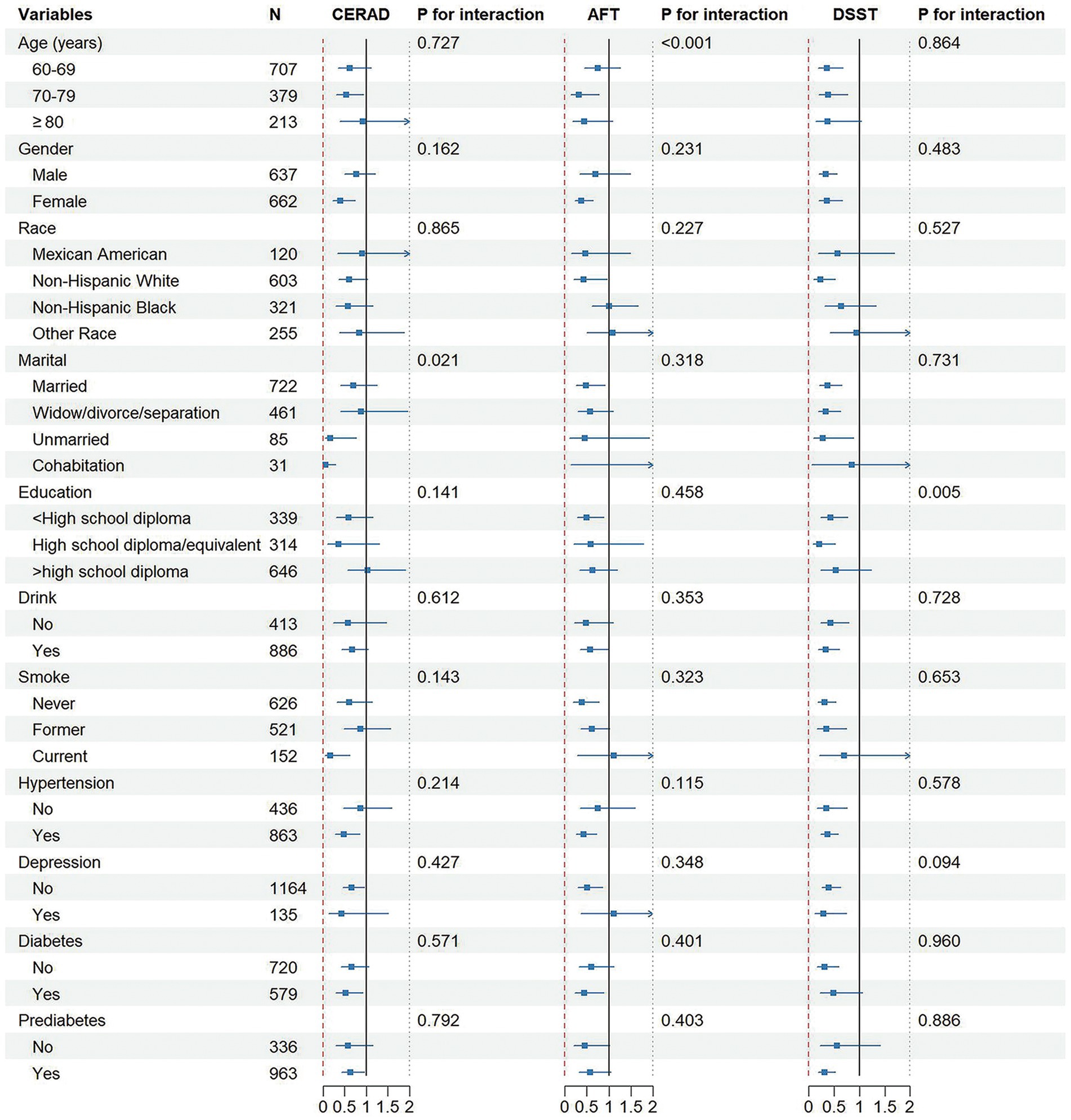
Figure 3. Associations between physical activity levels and cognitive function in different subgroups.
4 Discussion
In this study of older adults with diabetes and prediabetes in America, it was found that moderate to high levels of PA were associated with better cognitive performance and a lower prevalence of cognitive impairment. After controlling for sociodemographic factors, lifestyle behaviors, and prevalent chronic conditions, a significant correlation between MLPA and HLPA groups and DSST scores was still observed. DSST is recognized as a reliable measure of overall cognitive function. According to our study, maintaining a moderate to high level of physical activity on a weekly basis is associated with a lower risk of cognitive decline and PA could be used as a reliable measure for patients with diabetes or prediabetes to maintain cognitive function.
In America, about 53% of adults do not meet the exercise levels recommended in PA guidelines (40). Engaging in routine PA can offer many benefits to the structure and function levels of brain, including slowing down brain aging, increasing brain volume, and providing potential neuroprotection, which is extremely effective in the older adults (41, 42). PA can improve cognitive levels in individuals with disorders that impair cognitive function, such as schizophrenia, Parkinson’s disease, and stroke (43). We have also found such benefits in patients with diabetes or prediabetes. Previous studies have shown that PA can be used as a long-term protective factor against dementia and cognitive decline in the general population (14); and another randomized controlled trial also found this association, i.e., moderate PA could significantly improve verbal memory, executive function, and overall cognition in healthy older adults (44). Furthermore, the cognitive function of the senior was also shown to be associated with the type of PA, where closed exercise was associated with better selective attention and visuospatial function and open exercise was associated with better inhibitory control and cognitive flexibility (45). These provided a possible explanation for our study.
Currently, few studies have highlighted the importance of PA in preventing cognitive impairment in diabetic patients. A large cohort study in Sweden showed that an active lifestyle (including physical exercise and social activities) may reduce the harmful effects of diabetes on the brain, retain brain volume and reduce the risk of diabetes-related dementia by 30% (46). Another cross-sectional study of senior Chinese patients with type 2 diabetes showed that PA could delay the onset of cognitive impairment possibly due to improved sleep quality and reduced depressive symptoms (47). According to another multicenter cohort study conducted in Brazil, for the middle-aged and older patients with diabetes, leisure time PA was a key moderating factor in cognitive decline that weakened the harmful association between diabetes and cognitive impairment (35). This is the first time we have found this association in seniors in America. Our findings stressed the importance of public health policies that promote the participation of populations at high risk of cognitive impairment and dementia (including patients with diabetes and prediabetes) in PA.
The CERAD and AFT are highly dependent on language ability and vocabulary reserve; however, their results lost statistical significance after adjusting for confounding factors, which may be attributed to the strong influence of educational level and depressive symptoms on language-related cognitive functions (48, 49). In contrast, the DSST primarily reflects processing speed and attention, which are more directly influenced by the neurovascular and metabolic regulation of PA, such as increased cerebral blood flow and the release of brain-derived neurotrophic factor (43). These findings suggest that different cognitive domains may exhibit domain-specific responsiveness to PA. Future studies should employ path analysis to further elucidate the underlying mechanisms.
In the RCS curves, we found that PA levels showed a nonlinear correlation with cognitive function in diabetes and prediabetes patients. According to the results of three cognitive function tests, older patients benefited from low-dose PA, and the risk of their cognitive impairment decreased as PA levels increased. Previous studies have also supported our view that when the overall PA level reaches 3,000–4,000 MET/min/week, it can significantly reduce the risk of various diseases such as diabetes, heart disease, and stroke (50). In terms of preventing cognitive decline, high levels of PA are better than low to medium levels (13); a study by Endeshaw et al. came to a similar conclusion that participation in PA had a positive effect on cognitive function in older adults, and the effects of medium to high levels of PA are better than that of low levels of PA (51). However, the overall activity level of old patients should not be too high. As can be seen from the results of the CERAD test, PA no longer has a protective effect on cognitive function when the PA level is above 15,151 MET-min/week. At extremely high PA levels, the benefits will gradually stabilize and begin to reverse, which may reflect several biological phenomena. First of all, excessive PA may cause fluctuations in the patient’s blood glucose levels, leading to an increase in oxidative stress and inflammatory responses within the brain (52). These inflammatory cytokines can cross the blood–brain barrier, exacerbate neuroinflammation (53). Furthermore, overexercise without sufficient recovery can downregulate brain-derived neurotrophic factor signaling, blunting the neuroplastic adaptations that underlie exercise’s protective effects, and thereby negatively affect cognitive function (54).
In addition, variables (such as age, marital status, and education) were found to play a role in affecting the correlation of PA with cognitive function in our subgroup analysis. The brain of older adults will experience some degenerative changes with age, such as synaptic degeneration, neuronal apoptosis, reduced brain volume, and suffer from cognitive decline. Meanwhile, the nervous system of the older adult will also change; for example, nerve fibers become more fragile and prone to fracture, and the number of neurons will decrease, which will affect the normal function of cognitive function (55, 56). A significant association between cognitive impairment and marital status was also noted. A longitudinal study on the older adult in America suggested that divorce or widowhood may be a risk factor for cognitive impairment and its progression to dementia (57). Furthermore, abundant evidence showed that education also affects the level of cognitive function. In terms of cognitive performance, individuals with a high level of education have a significant advantage over those with a low level of education (48) and the risk of dementia decreases as the level of education increases (58). It was noted in our study that PA had a significant protective effect in <high school diploma group and High school diploma group, and the study by Li et al. also supported our view that PA may contribute to a significant improvement in overall cognition of people with low education level (59). This correlation is no longer significant among the highly educated group. This might be because higher education levels enhance the tolerance to the PA effect through cognitive reserves (48). Future research could combine cognitive training with PA to increase the cognitive benefits for the highly educated population. Moreover, we also found significant benefits of PA in patients with hypertension and depression, possibly because PA helped regulate blood pressure, improve mood and alleviate depressive symptoms, improve memory and concentration of patients, and thus reduce the impairment of cognitive function (49, 60).
It has been inferred that there may be multiple underlying mechanisms for the improvement of cognitive function in older adults with diabetes and prediabetes through PA. Firstly, PA helps to control blood glucose levels, improve insulin sensitivity, and increase insulin secretion, thereby maintaining the normal functioning of the brain and reducing the negative effects of diabetes and prediabetes on cognitive function (61, 62). Secondly, PA can release a variety of neurotransmitters (such as dopamine and endorphins), which help relieve negative emotions such as stress, anxiety, and depression, thereby improving mental health, a crucial factor for the brain’s learning and memory functions (63, 64). PA can also promote the release of brain-derived neurotrophic factors, maintain the structure and function of neurons, and improve the cognitive state of the brain (20). Finally, exercise has a certain anti-inflammatory effect, and the myogenic factor produced by skeletal muscle can delay brain aging and improve the redox state of the brain, thereby reducing the deposition of harmful substances such as β-amyloid and improving cognitive function (15, 65). These mechanisms interact with each other and together improve cognitive function and reduce the incidence of dementia in older adults with diabetes and prediabetes.
Building on these findings, we emphasize the importance of clinicians routinely assessing PA levels in older adults with diabetes/prediabetes and prescribing tailored, progressive exercise regimens. Secondly, we recommend that patients should actively engage in moderate to high levels of PA, increase the amount of daily activity, gradually increase the intensity of exercise, match a healthy lifestyle, have regular medical checkups to monitor blood glucose, and seek support and encouragement to work together to reduce the risk of diabetes onset and improve overall health. Our findings may offer valuable guidance for both clinicians and patients and support the integration of personalized PA plans into the routine management of diabetes to maximize the cognitive benefits of PA.
4.1 Strengths and limitations
For the first time, our study analyzed the association between PA and cognitive function in older people with diabetes and prediabetes by employing a sample that is nationally representative. In addition, we further explored the amount of exercise appropriate for patients with diabetes and prediabetes, grouped by PA level, as well as the dose–response correlation of PA with cognitive function. This provides a reliable basis that PA may lead to a reduced risk of cognitive decline in this population.
There are also some limitations of this study. First, self-reported questionnaires were used to assess PA and some covariates, which may introduce bias due to recall errors and information inaccuracies. The lack of device-measured PA data may attenuate the observed magnitude of the dose–response relationship. Future studies should incorporate device-based measures (e.g., accelerometers or wearable sensors) to improve accuracy and control for variability. In addition, due to the cross-sectional nature of the study, it is difficult to infer a causal relationship between PA and cognitive ability. Longitudinal cohort studies and randomized controlled trials are needed to determine the directionality and temporal sequence of these associations.
5 Conclusion
In conclusion, our study showed that moderate and high levels of PA can act as a key factor to maintain cognitive function in older adults with diabetes and prediabetes. Despite the need for further studies, our results strengthened support for the critical role of PA in mitigating cognitive complications associated with diabetes and prediabetes.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found at: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
Because all patient information in the NHANES database has been de-identified and is publicly accessible, the research involving the anonymous data from that database was exempt from medical ethics review and did not require informed consent.
Author contributions
YW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. DL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition. HW: Conceptualization, Methodology, Supervision, Writing – review & editing. MW: Writing – review & editing. WR: Writing – review & editing. YaH: Funding acquisition, Supervision, Writing – review & editing. YiH: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Social Science Foundation of China [grant number 21XYT018]; and Innovation Project of Guangxi Graduate Education [grant number XYCSR2024055].
Acknowledgments
We appreciate the contributions of all participants and researchers to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization . (2023). Dementia. Available online at: https://www.who.int/news-room/fact-sheets/detail/dementia (Accessed November 6, 2024).
2. GBD 2019 Dementia Forecasting Collaborators . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. (2022) 7:e105–25. doi: 10.1016/s2468-2667(21)00249-8
3. Wang, L, Li, X, Wang, Z, Bancks, MP, Carnethon, MR, Greenland, P, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. JAMA. (2021) 326:704–13. doi: 10.1001/jama.2021.9883
4. Echouffo-Tcheugui, JB, Perreault, L, Ji, L, and Dagogo-Jack, S. Diagnosis and management of prediabetes: a review. JAMA. (2023) 329:1206–16. doi: 10.1001/jama.2023.4063
5. Xue, M, Xu, W, Ou, Y-N, Cao, X-P, Tan, M-S, Tan, L, et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. (2019) 55:100944. doi: 10.1016/j.arr.2019.100944
6. Makino, K, Lee, S, Bae, S, Chiba, I, Harada, K, Katayama, O, et al. Diabetes and prediabetes inhibit reversion from mild cognitive impairment to Normal cognition. J Am Med Dir Assoc. (2021) 22:1912–8.e2. doi: 10.1016/j.jamda.2021.02.033
7. Sims-Robinson, C, Kim, B, Rosko, A, and Feldman, EL. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol. (2010) 6:551–9. doi: 10.1038/nrneurol.2010.130
8. Moheet, A, Mangia, S, and Seaquist, ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. (2015) 1353:60–71. doi: 10.1111/nyas.12807
9. Jing, J, Liu, C, Zhu, W, Pan, Y, Jiang, J, Cai, X, et al. Increased resting-state functional connectivity as a compensatory mechanism for reduced brain volume in prediabetes and type 2 diabetes. Diabetes Care. (2023) 46:819–27. doi: 10.2337/dc22-1998
10. Wang, X, Li, X, Wang, W, Shi, G, Wu, R, Guo, L, et al. Longitudinal associations of newly diagnosed prediabetes and diabetes with cognitive function among Chinese adults aged 45 years and older. J Diabetes Res. (2022) 2022:1–10. doi: 10.1155/2022/9458646
11. Fillenbaum, GG, and Mohs, R. CERAD (consortium to establish a registry for Alzheimer's disease) neuropsychology assessment battery: 35 years and counting. J Alzheimers Dis. (2023) 93:1–27. doi: 10.3233/jad-230026
12. Wang, X, Sun, M, Wang, L, Li, J, Xie, Z, Guo, R, et al. The role of dietary inflammatory index and physical activity in depressive symptoms: results from NHANES 2007–2016. J Affect Disord. (2023) 335:332–9. doi: 10.1016/j.jad.2023.05.012
13. Sofi, F, Valecchi, D, Bacci, D, Abbate, R, Gensini, GF, Casini, A, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. (2011) 269:107–17. doi: 10.1111/j.1365-2796.2010.02281.x
14. Iso-Markku, P, Kujala, UM, Knittle, K, Polet, J, Vuoksimaa, E, and Waller, K. Physical activity as a protective factor for dementia and Alzheimer’s disease: systematic review, meta-analysis and quality assessment of cohort and case–control studies. Br J Sports Med. (2022) 56:701–9. doi: 10.1136/bjsports-2021-104981
15. Zhong, Q, Zhou, R, Huang, YN, Chen, HW, Liu, HM, Huang, Z, et al. The independent and joint association of accelerometer-measured physical activity and sedentary time with dementia: a cohort study in the UK biobank. Int J Behav Nutr Phys Act. (2023) 20:59. doi: 10.1186/s12966-023-01464-8
16. Kelly, ME, Loughrey, D, Lawlor, BA, Robertson, IH, Walsh, C, and Brennan, S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. (2014) 16:12–31. doi: 10.1016/j.arr.2014.05.002
17. Balducci, S, Sacchetti, M, Haxhi, J, Orlando, G, D'Errico, V, Fallucca, S, et al. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab Res Rev. (2014) 30:13–23. doi: 10.1002/dmrr.2514
18. Jadhav, RA, Hazari, A, Monterio, A, Kumar, S, and Maiya, AG. Effect of physical activity intervention in prediabetes: a systematic review with Meta-analysis. J Phys Act Health. (2017) 14:745–55. doi: 10.1123/jpah.2016-0632
19. Rossen, J, Hagströmer, M, Larsson, K, Johansson, UB, and von Rosen, P. Physical activity patterns among individuals with prediabetes or type 2 diabetes across two years-a longitudinal latent class analysis. Int J Environ Res Public Health. (2022) 19:3667. doi: 10.3390/ijerph19063667
20. Rabinowitz, Y, Ravona-Springer, R, Heymann, A, Moshier, E, Berman, Y, Schwartz, J, et al. Physical activity is associated with slower cognitive decline in older adults with type 2 diabetes. J Prev Alzheimers Dis. (2023) 10:497–502. doi: 10.14283/jpad.2023.26
21. Chen, Y, Qin, J, Tao, L, Liu, Z, Huang, J, Liu, W, et al. Effects of tai chi Chuan on cognitive function in adults 60 years or older with type 2 diabetes and mild cognitive impairment in China: a randomized clinical trial. JAMA Netw Open. (2023) 6:e237004. doi: 10.1001/jamanetworkopen.2023.7004
22. Shi, Y, and Wen, M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011-2018 population. Cardiovasc Diabetol. (2023) 22:19. doi: 10.1186/s12933-023-01740-8
23. National Center for Health Statistics . (2022) NCHS Research Ethics Review Board (ERB) Approval. Available online at: https://www.cdc.gov/nchs/nhanes/about/erb.html?CDC_AAref_Val=https://www.cdc.gov/nchs/nhanes/irba98.htm (Accessed October 10, 2024).
24. American Diabetes Association . Standards of care in diabetes-2023 abridged for primary care providers. Clin Diabetes. (2022) 41:4–31. doi: 10.2337/cd23-as01
25. Yan, T, Ding, X, Xie, T, Lan, T, Niu, D, Li, J, et al. Physical activity (PA) influences the risk of depression associated with long working hours. J Affect Disord. (2023) 321:227–33. doi: 10.1016/j.jad.2022.10.043
26. Herrmann, SD, HK, J, DAC, A, and Ainsworth, BE. Validity and reliability of the global physical activity questionnaire (GPAQ). Meas Phys Educ Exerc Sci. (2013) 17:221–35. doi: 10.1080/1091367X.2013.805139
27. Wang, Y, Yang, X, Zhou, Y, Ruan, W, Li, H, Han, Y, et al. High-level physical activity provides protection against all-cause mortality among U.S. adults with depression. J Affect Disord. (2024) 358:458–65. doi: 10.1016/j.jad.2024.05.057
28. Piercy, KL, Troiano, RP, Ballard, RM, Carlson, SA, Fulton, JE, Galuska, DA, et al. The physical activity guidelines for Americans. JAMA. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
29. Morris, JC, Heyman, A, Mohs, RC, Hughes, JP, van Belle, G, Fillenbaum, G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. (1989) 39:1159–65. doi: 10.1212/wnl.39.9.1159
30. Clark, LJ, Gatz, M, Zheng, L, Chen, YL, McCleary, C, and Mack, WJ. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer's disease. Am J Alzheimers Dis Other Dement. (2009) 24:461–8. doi: 10.1177/1533317509345154
31. Tulsky, DS, Saklofske, DH, Wilkins, C, and Weiss, LG. Development of a general ability index for the Wechsler adult intelligence scale--third edition. Psychol Assess. (2001) 13:566–71. doi: 10.1037/1040-3590.13.4.566
32. Dong, X, Li, S, Sun, J, Li, Y, and Zhang, D. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and nutrition examination survey (NHANES) 2011-2014. Nutrients. (2020) 12:840. doi: 10.3390/nu12030840
33. Chen, Y, Liao, J, Zeng, Y, Ma, H, Jiang, C, Yu, S, et al. The combined effect of diabetes mellitus and sarcopenia on depression and cognitive function: insights from the CHARLS cohort, 2011-2020. Eur Geriatr Med. (2024) 15:1881–90. doi: 10.1007/s41999-024-01039-1
34. Alam, MT, Vásquez, E, Etnier, JL, and Echeverria, S. Dietary adherence and cognitive performance in older adults by nativity status: results from the National Health and nutrition examination survey (NHANES), 2011-2014. Geriatrics. (2024) 9:25. doi: 10.3390/geriatrics9020025
35. Feter, N, de Paula, D, Dos Reis, RCP, Raichlen, D, Patrão, AL, Barreto, SM, et al. Leisure-time physical activity may attenuate the impact of diabetes on cognitive decline in middle-aged and older adults: findings from the ELSA-Brasil study. Diabetes Care. (2024) 47:427–34. doi: 10.2337/dc23-1524
36. Casagrande, SS, Lee, C, Stoeckel, LE, Menke, A, and Cowie, CC. Cognitive function among older adults with diabetes and prediabetes, NHANES 2011-2014. Diabetes Res Clin Pract. (2021) 178:108939. doi: 10.1016/j.diabres.2021.108939
37. Sun, Y, Kong, Z, Song, Y, Liu, J, and Wang, X. The validity and reliability of the PHQ-9 on screening of depression in neurology: a cross sectional study. BMC Psychiatry. (2022) 22:98. doi: 10.1186/s12888-021-03661-w
38. Liao, J, Hu, M, Imm, K, Holmes, CJ, Zhu, J, Cao, C, et al. Association of daily sitting time and leisure-time physical activity with body fat among U.S. adults. J Sport Health Sci. (2024) 13:195–203. doi: 10.1016/j.jshs.2022.10.001
39. Bull, FC, Al-Ansari, SS, Biddle, S, Borodulin, K, Buman, MP, Cardon, G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
40. National Center for Health Statistics . (2022). Exercise or physical activity. Available online at: https://www.cdc.gov/nchs/fastats/exercise.htm (Accessed October 10, 2024).
41. Raji, CA, Meysami, S, Hashemi, S, Garg, S, Akbari, N, Ahmed, G, et al. Exercise-related physical activity relates to brain volumes in 10,125 individuals. J Alzheimers Dis. (2024) 97:829–39. doi: 10.3233/jad-230740
42. Domingos, C, Pêgo, JM, and Santos, NC. Effects of physical activity on brain function and structure in older adults: a systematic review. Behav Brain Res. (2021) 402:113061. doi: 10.1016/j.bbr.2020.113061
43. Erickson, KI, Hillman, C, Stillman, CM, Ballard, RM, Bloodgood, B, Conroy, DE, et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. (2019) 51:1242–51. doi: 10.1249/mss.0000000000001936
44. Galle, SA, Deijen, JB, Milders, MV, De Greef, MHG, Scherder, EJA, van Duijn, CM, et al. The effects of a moderate physical activity intervention on physical fitness and cognition in healthy elderly with low levels of physical activity: a randomized controlled trial. Alzheimers Res Ther. (2023) 15:12. doi: 10.1186/s13195-022-01123-3
45. Ingold, M, Tulliani, N, Chan, CCH, and Liu, KPY. Cognitive function of older adults engaging in physical activity. BMC Geriatr. (2020) 20:229. doi: 10.1186/s12877-020-01620-w
46. Marseglia, A, Darin-Mattsson, A, Kalpouzos, G, Grande, G, Fratiglioni, L, Dekhtyar, S, et al. Can active life mitigate the impact of diabetes on dementia and brain aging? Alzheimers Dement. (2020) 16:1534–43. doi: 10.1002/alz.12142
47. Zhang, H, Zhang, Y, Sheng, S, Xing, Y, Mou, Z, Zhang, Y, et al. Relationship between physical exercise and cognitive impairment among older adults with type 2 diabetes: chain mediating roles of sleep quality and depression. Psychol Res Behav Manag. (2023) 16:817–28. doi: 10.2147/prbm.S403788
48. Lövdén, M, Fratiglioni, L, Glymour, MM, Lindenberger, U, and Tucker-Drob, EM. Education and cognitive functioning across the life span. Psychol Sci Public Interest. (2020) 21:6–41. doi: 10.1177/1529100620920576
49. Hu, L, Smith, L, Imm, KR, Jackson, SE, and Yang, L. Physical activity modifies the association between depression and cognitive function in older adults. J Affect Disord. (2019) 246:800–5. doi: 10.1016/j.jad.2019.01.008
50. Kyu, HH, Bachman, VF, Alexander, LT, Mumford, JE, Afshin, A, Estep, K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the global burden of disease study 2013. BMJ. (2016) 354:i3857. doi: 10.1136/bmj.i3857
51. Endeshaw, Y, and Goldstein, F. Association between physical exercise and cognitive function among community-dwelling older adults. J Appl Gerontol. (2021) 40:300–9. doi: 10.1177/0733464820952242
52. Kirk-Sanchez, NJ, and McGough, EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. (2014) 9:51–62. doi: 10.2147/cia.S39506
53. Gleeson, M, Bishop, NC, Stensel, DJ, Lindley, MR, Mastana, SS, and Nimmo, MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. (2011) 11:607–15. doi: 10.1038/nri3041
54. Wrann, CD, White, JP, Salogiannnis, J, Laznik-Bogoslavski, D, Wu, J, Ma, D, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. (2013) 18:649–59. doi: 10.1016/j.cmet.2013.09.008
55. Greenberg, DL, Messer, DF, Payne, ME, Macfall, JR, Provenzale, JM, Steffens, DC, et al. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiol Aging. (2008) 29:290–302. doi: 10.1016/j.neurobiolaging.2006.09.016
56. Liu-Ambrose, T, Barha, CK, and Best, JR. Physical activity for brain health in older adults. Appl Physiol Nutr Metab. (2018) 43:1105–12. doi: 10.1139/apnm-2018-0260
57. Liu, H, Zhang, Y, Burgard, SA, and Needham, BL. Marital status and cognitive impairment in the United States: evidence from the National Health and aging trends study. Ann Epidemiol. (2019) 38:28–34.e2. doi: 10.1016/j.annepidem.2019.08.007
58. Xu, W, Tan, L, Wang, HF, Tan, MS, Tan, L, Li, JQ, et al. Education and risk of dementia: dose-response Meta-analysis of prospective cohort studies. Mol Neurobiol. (2016) 53:3113–23. doi: 10.1007/s12035-015-9211-5
59. Li, N, Deng, Q, Yang, Q, Wang, Y, Hu, J, Zhao, X, et al. Effect of physical activity intervention on cognitive function in China: a cluster randomized trial. Alzheimers Dement. (2023) 19:3679–87. doi: 10.1002/alz.13005
60. Frith, E, and Loprinzi, PD. Physical activity and cognitive function among older adults with hypertension. J Hypertens. (2017) 35:1271–5. doi: 10.1097/hjh.0000000000001311
61. Wang, Y, Li, H, Yang, D, Wang, M, Han, Y, and Wang, H. Effects of aerobic exercises in prediabetes patients: a systematic review and meta-analysis. Front Endocrinol. (2023) 14:1227489. doi: 10.3389/fendo.2023.1227489
62. Colberg, SR, Somma, CT, and Sechrist, SR. Physical activity participation may offset some of the negative impact of diabetes on cognitive function. J Am Med Dir Assoc. (2008) 9:434–8. doi: 10.1016/j.jamda.2008.03.014
63. Hou, M, Herold, F, Zhang, Z, Ando, S, Cheval, B, Ludyga, S, et al. Human dopaminergic system in the exercise-cognition link. Trends Mol Med. (2024) 30:708–12. doi: 10.1016/j.molmed.2024.04.011
64. Zhao, JL, Jiang, WT, Wang, X, Cai, ZD, Liu, ZH, and Liu, GR. Exercise, brain plasticity, and depression. CNS Neurosci Ther. (2020) 26:885–95. doi: 10.1111/cns.13385
Keywords: physical activity, cognitive decline, older adults, diabetes, prediabetes, NHANES
Citation: Wang Y, Liu D, Wang H, Wang M, Ruan W, Han Y and Han Y (2025) The role of physical activity in preventing cognitive decline among U.S. older adults with diabetes and prediabetes: a cross-sectional study. Front. Public Health. 13:1603627. doi: 10.3389/fpubh.2025.1603627
Edited by:
Wiktoria Staśkiewicz-Bartecka, Medical University of Silesia, PolandReviewed by:
Christian E Vazquez, University of Texas at Arlington, United StatesMengsha Sun, Tongji University, China
Yiqun Pang, Southwest Petroleum University, China
Dewi Nurhidayah, Cenderawasih University, Indonesia
Copyright © 2025 Wang, Liu, Wang, Wang, Ruan, Han and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiming Han, eWltaW5naGFuQG1haWxib3guZ3hudS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yifei Wang
Yifei Wang Dezheng Liu2†
Dezheng Liu2† Hongli Wang
Hongli Wang Mengzhao Wang
Mengzhao Wang Yanbai Han
Yanbai Han