- 1Department of Public Health, University “Federico II” of Naples, Naples, Italy
- 2Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy
- 3Department of Molecular Medicine and Medical Biotechnology, Federico II University of Naples, Naples, Italy
- 4Multiple Sclerosis Unit, Policlinico Federico II University Hospital, Naples, Italy
- 5Department of Advanced Medical and Surgical Sciences, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 6Department of Health Sciences, University of Genova, Genova, Italy
- 7Medical Direction, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 8Department of Primary Care and Public Health, School of Public Health, Imperial College, London, United Kingdom
- 9Interdepartmental Research Center in Healthcare Management and Innovation in Healthcare (CIRMIS), Naples, Italy
Introduction: The prevalence and costs of dementias are rising due to demographic changes. Dementia care depends largely on informal caregivers and fragmented healthcare systems that often fail to meet the needs of people with dementia.
Objectives: This systematic review aims to identify unmet needs and barriers in European dementia care, providing a framework to improve health strategies.
Methods: Following PRISMA guidelines, articles from 2013 to 2023 were screened from Embase, PsycINFO, HTA Database, and Web of Science. The Mixed Methods Appraisal Tool was used for evaluation.
Results: From 3,738 articles, 47 met the inclusion criteria. Through a narrative synthesis, the review identified unmet needs and barriers among People Living with Dementia, caregivers, and healthcare workers. Psychosocial and emotional support are essential for managing stress and ensuring quality of life. Caregivers demand education about dementia care, progression, and self-care, while healthcare workers need training, and interdisciplinary teams. Cultural sensitivity is critical for addressing stigma and facilitating inclusive care for ethnic minorities. Healthcare access remains fragmented, thereby decreasing continuity of care for families. High costs, bureaucratic complexity, and geographical inequalities, particularly in rural areas can be barrier to care for People Living with Dementia and their families. The COVID-19 pandemic disrupted social support services, increasing distress and uncertainty. About limitation, publication bias and geographical bias from focus on Europe were possible, potentially overlooking insights from other regions.
Conclusion: There is need for public policies to enhance education, community support, and dementia awareness, with a focus on culturally sensitive care.
1 Introduction
Alzheimer’s disease (AD) and other dementia constitute a complex set of progressive neurodegenerative conditions that primarily affect older adults (1). Both are recognized as leading causes of disability in the older adult (2). In 2019, approximately 14.1 million people were living with AD or other forms of dementia in Europe alone, a number projected to double by 2050 (3).
Median life expectancy is around 3 to 6 years after formal diagnosis of dementia but some individuals survive for as long as 20 years (4). Clinical deterioration is progressive and ranges from mild or early stage of dementia (e.g., forgetful, some language difficulties, and mood changes) for the first year or two, the moderate or middle stage (e.g., very forgetful, increasing difficulty with speech, and help needed with self-care activities) from the second to the fourth or fifth years, and the severe or late stage (e.g., serious memory disturbances and nearly total dependence and inactivity) from the fifth year onwards (5). Disability progression and increase in seeking-care lead to a significant drop in overall quality of life (6).
While most care needs for people living with dementia (PLWD) are satisfied by their caregivers (someone who takes care of a person, usually a family member) (6), this might have a negative impact on carer’s physical and mental wellbeing and also their social life and financial situation (7). Often, caregivers often experience elevated levels of stress and depression, and reduced employment compared to the general population (8–10). However, several factors, including resilience, post-traumatic growth, and a positive attitude, influence the disease burden (11).
Healthcare workers (HCWs) play a crucial role in delivering care to patients with advanced-stage dementia, where professional support becomes indispensable due to the progression of cognitive impairment and frailty (12). Nevertheless, this responsibility often places HCWs under considerable pressure, leading to both psychological and physical strain, including an increased risk of injury and depression (13). As a result, HCWs face a heightened higher risk of stress (14) and burnout (15), further compounding the challenges they encounter in their demanding roles. Furthermore, high staff turnover rate (16) and training programs held by more experienced professionals rather than qualified instructors (17) represent a barrier in delivering care in a highly complex setting.
Considering the complexity of dementia care and the challenges faced by all those involved, it is essential to identify and understand the specific needs encountered at different levels of the care system. The central question guiding this review is as follows: What are the needs, unmet needs, and linked barriers experienced by primary, secondary, and tertiary end-users (people living with dementia, caregivers, healthcare workers, and other stakeholders) within dementia care systems? Answering this question can provide valuable insight into the challenges encountered at different levels of engagement with dementia-related services, with the ultimate aim of informing targeted interventions and contributing to improvements in the overall care and support ecosystem.
2 Methods
This systematic review analysed studies focusing on needs, unmet needs and barriers in European dementia care-systems and was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18). Refer to the Supplementary Table 1 for additional details.
2.1 Search strategy and eligibility criteria
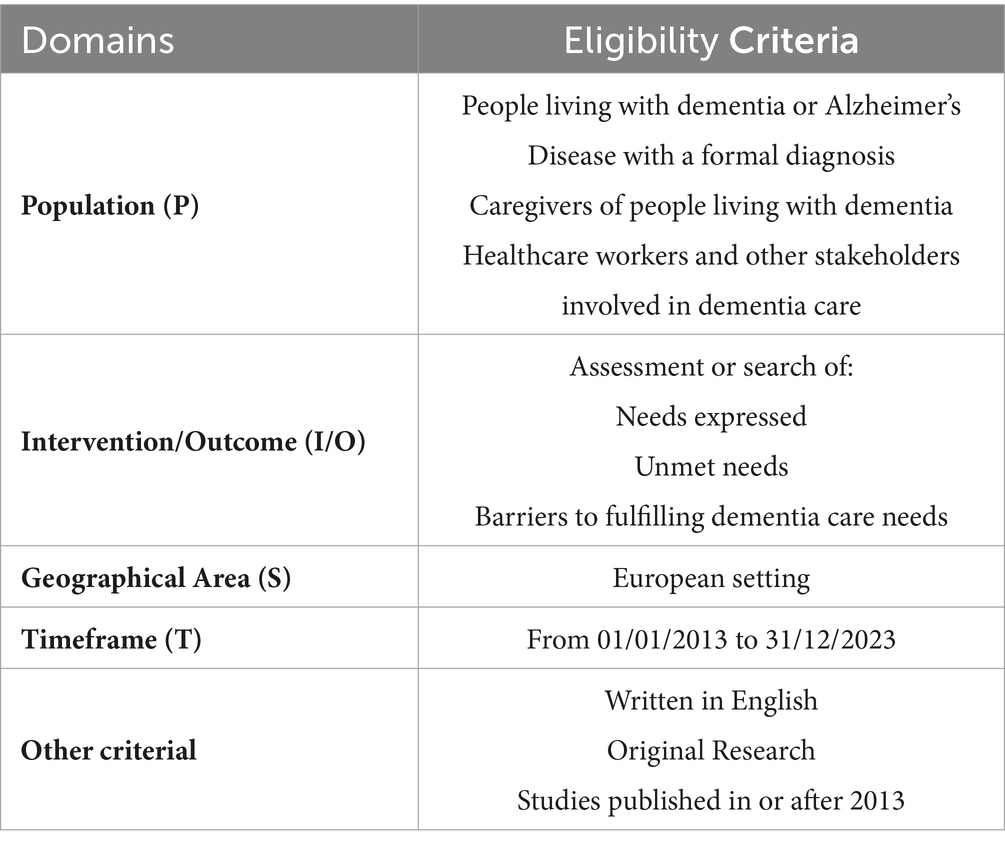
PubMed was used as a primary dataset. Additional database searches were performed in the following databases: Embase, PsycINFO (EBSCOhost), Health Technology Assessment Database, and Web of Science (Clarivate). Duplicate were eliminated using Rayyan AI (19). These searches covered 10 years (2013–2023). Eligibility criteria are summarized in Table 1.
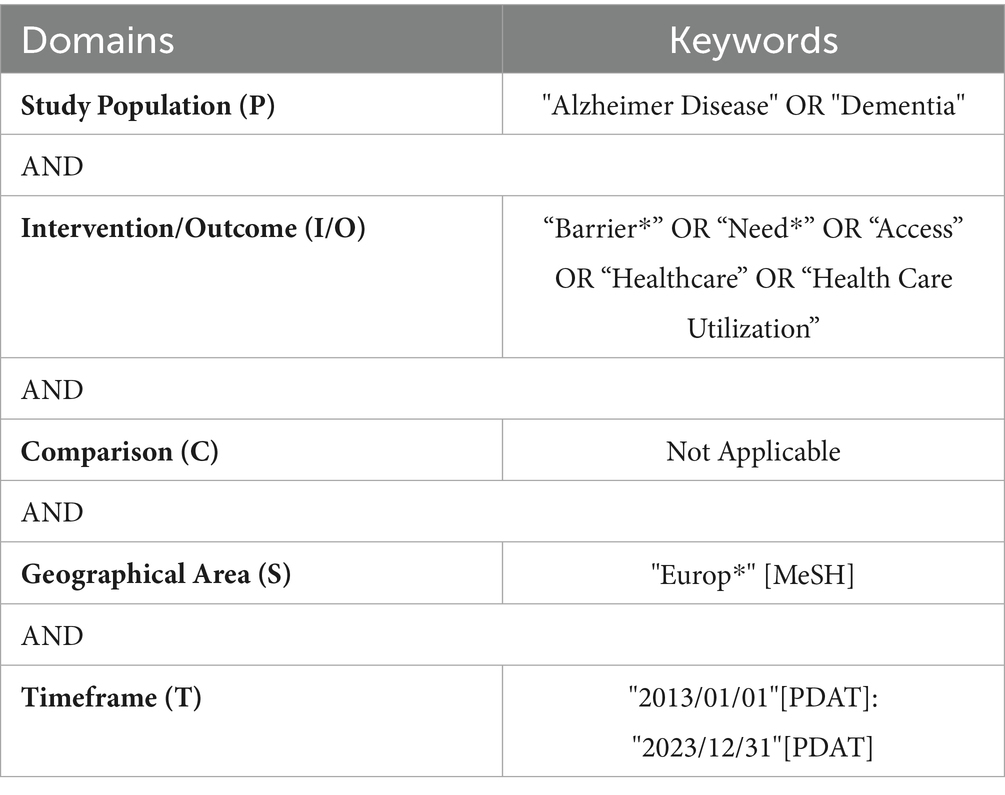
The search strategy employed a combination of keywords and Boolean operators (Table 2). The keywords used to identify the needs, the unmet needs, and the barriers that compromise the assistance of people living with dementia, their caregivers, and HCWs. The keywords, aligned the PICO framework, include the following terms: Population (P) (“Alzheimer Disease” OR “Dementia”) AND Intervention/Outcome (“Barrier*” OR “Need*” OR “Access” OR “Healthcare” OR “Health Care Utilization”) AND Geographical Area (S) (“Europ*” [MeSH]) AND Timeframe (T) (“2013/01/01” [PDAT]: “2023/12/31” [PDAT]). No comparison was made.
2.2 Data extraction and quality assessment
Five reviewers (MS, MM, CF, IS, FE) examined titles and abstract to identify studies adhering to inclusion criteria. If the abstract lacked sufficient information to decide for inclusion or exclusion, full text review was performed. Conflict and uncertainties were discussed with the senior reviewer (RP). Articles selected trough title-abstract analysis were fully review. During full text review, additional data was extracted and summarized in Excel spreadsheets. The quality of the selected papers was assessed using the revised version of the Mixed Methods Appraisal Tool (MMAT) (20). This tool is designed to evaluate different dimensions of study quality according to the specific research design. Studies were not automatically excluded due to quality concerns; however, those with lower quality were closely examined to understand their potential influence on the overall findings. Each paper was independently reviewed by the evaluators to ensure an unbiased assessment of its quality.
3 Results
3.1 Study selection
The main search identified 3,854 studies. After adapting and running the research string on secondary database, duplicates were removed using Rayyan AI,1 and a database of 3,175 unique studies was compiled and 40 studies were identified through reference list. Upon review of their titles and abstracts, 3,038 studies were deemed irrelevant and excluded. Subsequently, 177 publications underwent a full-text review, resulting in the selection of 47 studies meeting the inclusion criteria. Grey literature was not considered, as well as conference papers, dissertations, letters, and editorials.
The 130 studies were excluded for the following reasons: 92 lacked assessments of needs or unmet needs, 25 were excluded due to study design/type, 8 were not set in a European nation, 3 were not focused on the population with Alzheimer’s disease and other dementias, and 2 studies were excluded due to lack of full-text availability. The list of excluded articles and the detailed reasons for their exclusion are provided in the Supplementary Table 2.
A visual representation of this selection process and the reason for exclusion is provided in the PRISMA diagram (Figure 1).
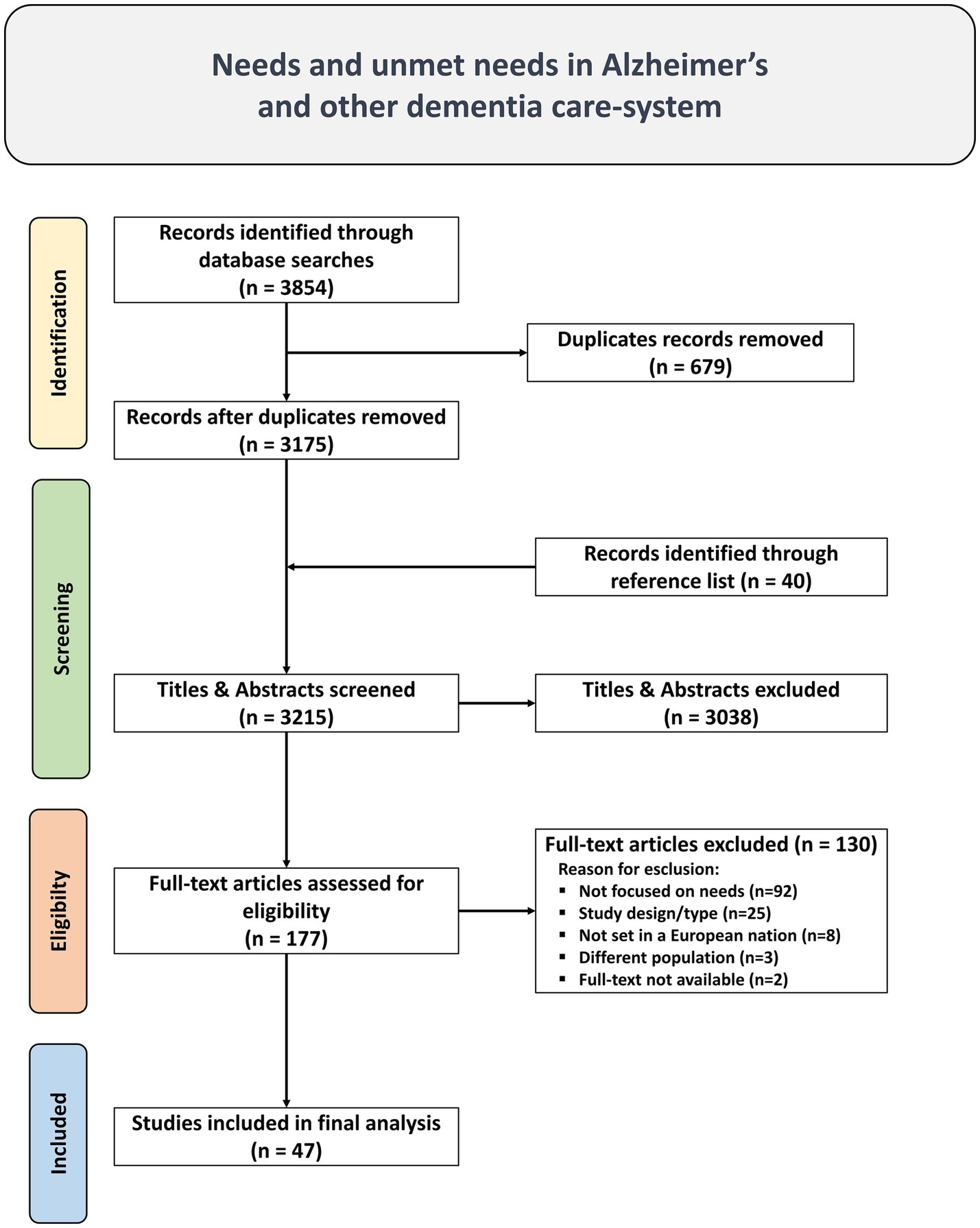
Figure 1. PRISMA Flow diagram of literature search, abstract screen, full article assessment for exclusion and inclusion criteria with most common reasons for exclusion detailed.
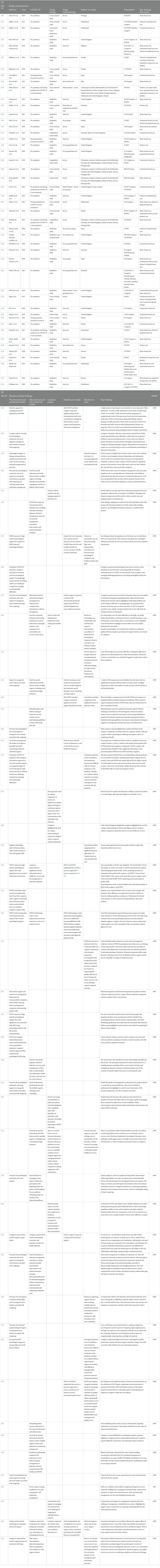
3.2 Study characteristics
The characteristics of the articles included are summarized in Table 3. 47 studies, spanning from 2013 to 2023, were selected for this review. The publication years were: five studies in 2013 (21–25), five studies in 2014 (26–30), one in 2016 (31), two in 2017 (32, 33), five in 2018 (34–38), five in 2019 (39–43), eleven in 2020 (44–54), nine in 2021 (55–63), three in 2022 (64–66), and one in 2023 (67). From the COVID-19 pandemic event, some studies were conducted during the pandemic with varying degrees of focus on COVID-19: three studies explicitly focused on COVID-19 (47, 55, 58), one studies did not mention COVID-19 despite being conducted during the pandemic (64) and three studies (62, 65, 66) combined pre-pandemic data with observations during the pandemic without focusing on COVID-19.
Most of the studies were conducted in single nations. The United Kingdom had the highest number of studies (n = 14) (21–23, 28, 29, 33, 36, 39, 43, 47–49, 56, 59), followed by the Netherlands (n = 5) (25, 26, 30, 54, 67), Spain (n = 4) (42, 44, 46, 58), Ireland (n = 3) (45, 61, 63), Germany (n = 2) (31, 51), Sweden (n = 2) (27, 34), Poland (n = 2) (41, 55), Portugal (n = 1) (40), Norway (n = 1) (52), Belgium (n = 1) (37), Estonia (n = 1) (53), Denmark (n = 1) (60), France (n = 1) (24), Italy (n = 1) (32), and Switzerland (n = 1) (62). The multi-country studies included the “Intermediate Care for Dementia in Europe” project (n = 1) (64), covering 16 countries: Bosnia, Croatia, Georgia, Greece, France, Hungary, Ireland, Israel, Italy, Latvia, Poland, Portugal, Romania, Switzerland, the United Kingdom, and Ukraine, and the “Actifcare” project (n = 4) (35, 38, 50, 66), involving eight countries: Germany, Ireland, the Netherlands, Norway, Sweden, the United Kingdom, Italy, and Portugal. One study (n = 1) (57) involved Germany, Spain, and the United Kingdom, and another study (n = 1) (65) involved the United Kingdom, France, and Cyprus, highlighting the collaborative nature of the research.
The methodologies employed in the studies varied significantly. Qualitative studies (n = 26) utilized different techniques, including interviews (n = 15) (21, 28, 29, 33, 36, 37, 47, 51–54, 56, 59, 61, 62), focus groups (n = 3) (39, 42, 45), surveys (n = 2) (32), both focus groups and interviews (n = 5) (27, 30, 48, 49, 60), and, a combination of surveys and focus groups (n = 1) (44). Cross-sectional studies (n = 15) were frequent, primarily using surveys (n = 10) (23, 25, 40, 41, 50, 55, 58, 63, 64, 67), with some employing interviews (n = 4) (22, 31, 34, 46) and one using both focus groups and interviews (n = 1) (65). Cohort studies (n = 6) demonstrated varied approaches, with some employing surveys (n = 4) (26, 35, 38, 66), one combining surveys and healthcare data (n = 1) (57), and one utilizing only healthcare data (n = 1) (43).
The reviewed studies encompassed various populations, with sample size ranging from 5 to 1,283. The focus of most studies was on caregivers (n = 13) (30, 32, 36, 42, 43, 46, 48, 51, 53, 55, 58, 65, 67), while others concentrated solely on HCWs (n = 8) (23, 24, 27, 33, 44, 45, 63, 64), and on people living with dementia (n = 7) (31, 34, 40, 49, 52, 62, 66). Both caregivers and people living with dementia participated in fourteen studies (21, 22, 25, 26, 28, 29, 35, 38, 41, 47, 50, 56, 57, 59). Some studies (n = 4) included both HCWs and caregivers (37, 39, 60, 61), and one study (n = 1) included people living with dementia, caregivers, and HCWs (54). Furthermore, five of the previous studies were focused on minority groups (30, 37, 48, 51, 60).
The studies assessed various care settings, focusing on either a single setting or multiple settings. The majority of studies (n = 15) focused on home-based care (21, 22, 28, 32, 34, 35, 40, 47, 51, 52, 54–56, 59, 66), community-based care (n = 10) (26, 36, 38, 43, 49, 50, 57, 58, 61, 65), primary care (n = 3) (24, 31, 33), clinical or hospital-based care (n = 3) (25, 29, 44), residential or long-term care facilities (n = 1) (63). Furthermore, multiple settings were explored in several studies: for instance, there were studies that combined multiple setting (n = 15) (23, 27, 30, 37, 39, 41, 42, 45, 46, 48, 53, 60, 62, 64, 67).
The majority of studies included in the analysis achieved the highest level of quality overall; however, only a small number of articles reached a quality score of 80% based on the MMAT assessment. Refer to Table 3 and Supplementary Table 3 for additional details.
3.3 Identified needs
Multiple needs have been identified. These needs were broadly categorized into psychosocial, emotional and social, educational and informational, cultural, healthcare and barriers to care. Additional details are provided in Table 3. The proposed categories did not influence the article analysis and were defined to enhance the presentation of the findings.
3.3.1 Psychosocial and emotional needs
People living with dementia expressed a high need for psychological support due to the significant stress associated with unmet social needs (50), such as activities of daily living, psychological distress, and the need for companionship (41). People living with dementia with hearing and/or visual impairment also expressed the need for psychological support (65). They rated their unmet social needs significantly lower than their caregivers (35), with a negative relationship observed between these unmet needs and both their own and their caregivers’ quality of life (35, 38), as well as the level of neuropsychiatric symptoms over time (26, 66), and demand support for their emotional and psychological well-being (66).
Caregivers expressed more unmet social needs than people living with dementia (41), highlighting the necessity for psychological, emotional, and social support to manage the stress burden associated with caregiving (22, 25, 31, 40, 42, 43, 53). As the disease progressed, the burden on caregivers gradually increased, necessitating enhanced emotional support (57). Caregivers expressed the need for support groups for emotional well-being (54), respite breaks (39), and assistance in managing the behavioral and neuropsychiatric symptoms of the people living with dementia, reducing distress and depressive symptoms, and improving quality of life (67).
During the COVID-19 pandemic, both caregivers and people living with dementia expressed an increased need for psychological support to manage high stress levels due to isolation and the new challenges arising from emerging social and health difficulties (55, 58).
3.3.2 Educational and informational needs
People living with dementia expressed several informational needs, including a general need for in-depth information about dementia (31), demanding online resources that focus on the course of the disease and related issues (62). Those with hearing and/or visual impairments specifically noted a need for more education on the use of assistive devices (65).
Caregivers demonstrated a significant need for information (50) and expressed a strong desire for training and groups to facilitate information sharing (53). Their educational needs cover managing various aspects of dementia, particularly at the beginning of the care process and throughout the care pathway, including special care situations and behavioral problems (42). They require information on dementia progression, disease management, and available care and support options (54). Additionally, caregivers expressed the need for information, awareness, and education on self-care health behaviors (39), and information on technological devices (53).
Additionally, both people living with dementia and their caregivers emphasized the necessity for specific information about the disease and the available support, including social counseling and legal assistance (22), as well as education on medication management and how to address related anxiety (28, 56).
HCWs have numerous educational needs in dementia care. These include training in dementia diagnosis and behavior management (27), as well as education on dementia management, assessment, legal and ethical aspects, interventions, and national dementia strategies (23). Additionally, HCP expressed the need for educational programs related to dementia care, and to have better communication and collaboration within the team (23, 44). General practitioners (GPs) specifically highlighted the need for continuing education and training, pointing out a lack of information and regular updates on the management of intermediate care services in their areas (64). This is particularly important for non-pharmacological management of dementia, effective communication with families and people living with dementia (24), and comprehensive information on disease management (32). Nurses require training in medication and pain management (63), while physiotherapists need further training in dementia and evidence-based physiotherapy guidelines to better understand their role (45).
3.3.3 Cultural needs
People living with dementia sought a reconsideration of public perceptions, emphasizing acknowledgment of their capabilities rather than focusing solely on their disabilities (49) and caregivers expressed the need for more culturally informed care to enhance dementia care (53). GPs expressed a need for cultural competence in society and healthcare to address stigma and improve societal perceptions of dementia (33), as well as to reduce the stigmatization families face when seeking intermediate care (64).
Across various European countries, caregivers from minority ethnic groups have expressed specific needs for cultural sensitivity and competence in dementia care. In the United Kingdom, Bangladeshi caregivers highlighted the need for a deeper understanding of cultural and religious values to improve cultural competence (48). In Belgium, Moroccan caregivers and HCWs emphasized the need for culture-sensitive tests and language support in dementia care (37). In Germany, Turkish caregivers expressed the need for better access to relevant information and the incorporation of Turkish culture into healthcare services (51). Similarly, in Denmark, HCWs and caregivers from Turkish, Pakistani, and Arabic-speaking minority groups require increased cultural sensitivity and competence in healthcare interactions, as well as improved awareness and understanding of dementia within their communities (60). In the Netherlands, caregivers from Turkish, Moroccan, and Surinamese Creole backgrounds need support in managing the emotional and social challenges of caregiving in culturally diverse contexts (30).
3.3.4 Healthcare needs
Healthcare for people living with dementia encompasses a wide range of needs, including nursing care and treatment, drug treatment and care, medical diagnosis and treatment, special therapies, social counseling, and legal support (31). People with severe dementia have a greater need for informal care for activities of daily living, and the need to utilize formal care services increases with the severity of dementia (34), as well as the need for access to healthcare resources (57). Both people living with dementia and their caregivers expressed significant physical and environmental needs, such as medication management, support for physical activity, fall prevention, sensory support, food preparation, personal hygiene, and money management (40, 59, 65).
Family caregivers expressed a strong need for both instrumental and formal support from HCWs to expedite diagnosis and ensure effective treatment (42), as well as financial support (32). The organization and coordination of services are critical to ensuring continuity of support, which plays a crucial role in promoting self-care health behaviors among people living with dementia (53).
HCWs and people living with dementia emphasized the need for a proactive approach to person-centered care (27). Integration of services is particularly necessary, with a focus on providing sensory aids to address the increasing difficulties faced by people living with dementia who also have hearing or visual impairments (65).
3.3.5 Barriers to care
Some needs expressed address barriers to dementia care. These include the need for easier access to care due to the complexity and fragmentation of the care system (54). Informal caregivers express the need to access support services (53), facing logistical barriers in organizing support and care, often involving multiple agencies, professionals, friends, and family members, and find that bureaucracy adds frustration and is time-consuming (36). Caregivers also need support to navigate advanced care planning (21).
People living with dementia face numerous barriers to physical activity due to physical health and cognitive impairment, with dementia progression increasing the need for physical activity while activity levels decrease (29). Caregivers experience difficulties in improving their quality of life, with key barriers including fear of social exclusion and isolation, concerns about the safety of the person they care for, and challenges in maintaining physical activity (29). Lack of time and their caregiving role are significant barriers to timely care (59) and to physical activity (59).
Caregivers also express the need for financial support to cope with the high costs of intermediate care for dementia (51), especially for those living in rural areas due to geographical barriers (64). Both caregivers and people living with dementia have expressed the need for support in navigating advance care planning, with difficulties finding the right time for discussions and a preference for informal plans (21).
HCWs and caregivers recognized emotional barriers to community care access due to reluctance to question authority and fear of stigma, expressing the need to improve access to community-based services for caregivers and user-centered models of care to facilitate better access and improve the quality of care (61). Barriers to intermediate care for dementia include high costs, disorientation, exacerbation of behavioral and psychological symptoms, living in rural areas, and feelings of shame, sadness, and guilt (64).
During the pandemic, access to healthcare and medications became more difficult, with new barriers arising due to the pandemic (47, 55). Families were isolated without assistance, increasing concerns about the resumption of care provision after the pandemic (47, 55).
4 Discussion
This systematic review analysed 47 studies published between 2013 and 2023, revealing a complex range of needs expressed by people living with dementia, their caregivers, and HCWs.
The findings highlight several needs, involving different domains of interest including psychosocial and emotional support, educational and information needs, cultural needs, healthcare need, and barriers to care.
The diverse geographic scope of the studies, with a primary focus on the United Kingdom, along with representation from other European countries and some global perspectives, underscores the universal relevance of the issues surrounding dementia care. Indeed, international collaboration and global coordination are crucial for addressing the unequal impact of dementia worldwide (68).
4.1 Psychosocial and emotional needs
People living with dementia expressed a high need for psychological support due to the significant stress associated with unmet social needs (41, 50). This need for psychological support (65) is negatively related to both their own and their caregivers’ quality of life (35, 38), as well as the level of neuropsychiatric symptoms over time (26, 66), which are a major predictor of caregiver burden (69, 70), leading to different distress patterns (71–73). Indeed, caregivers expressed even more unmet social needs than people living with dementia (35, 41), expressing the necessity for psychological, emotional, and social support to manage the stress burden associated with caregiving (22, 25, 31, 39, 40, 42, 43, 53, 54), a need for support that increases as the disease progresses, necessitating greater emotional support (57), particularly to manage the behavioral and neuropsychiatric symptoms of people living with dementia (67). Caregivers can develop skills and competence in coping with these symptoms, which can provide relief from negative states when facing people living with dementia demands at different stages (74). Furthermore, non-pharmacological activities, including physical activity, mental activities and music therapy, improve cognition and neuropsychiatric symptoms (75).
4.2 Impact of the COVID-19 pandemic
The COVID-19 pandemic caused substantial disruptions in healthcare systems such as a reduction in face to face consultations, an increase in remote consultations and delayed care for elective pocedures (76, 77), further exacerbating symptoms in people living with dementia, compromising their quality of life (78, 79), and increasing the care burden and psychological distress for family caregivers (80–82). Both caregivers and people living with dementia expressed an increased need for psychological support to manage high stress levels due to isolation and new challenges arising from emerging social and health difficulties (55, 58). Social and instrumental support can mediate the effects of caregivers’ stressors, leading to distinct mental reactions (83, 84).
For people living with dementia, access to comprehensive and easily accessible educational resources is essential (22, 31), particularly through online platforms (62), which, along with mass media and smartphones, are among the top sources of information, offering a variety of information independent of time and location (85). For those with sensory disabilities, information about assistive devices is also crucial (65).
4.3 Educational and informational needs
Caregivers also expressed the need for educational programs, mainly focused on dementia care (42, 54, 56), in accordance with the literature which shows that the most frequently reported information needs are information about the disease and patient care (86, 87). Furthermore, they wanted detailed guidance on medication management (28, 56), technological devices (53) self-care health practices (39) and a strong desire for training and groups to facilitate information sharing (53). This preference is supported by literature (85) reporting that HCWs are often perceived as lacking adequate training on dementia care services (88) and the information they provide is frequently considered insufficient (89–91). Education and support services can positively impact people living with dementia and their caregivers by enhancing confidence, reducing stress and depression, and improving overall well-being (92–94). However, the effectiveness of educational programs is influenced by various factors, affecting their delivery and outcomes (95).
HCWs also face significant needs, requiring ongoing training and specialized skills to deliver effective and personalized care (23, 24, 27, 32, 63): specific training is essential, such as medication management for nurses (63), evidence-based guidelines for physiotherapists (45) and regular updates on intermediate care management for general practitioners (64). Nonetheless, adequate time for training is crucial (96), yet organizations often struggle with resource constraints, including time, finances, and staff availability, which hinder the effective implementation of training initiatives (97–99).
4.4 Cultural aspects of dementia care
In many cultures, dementia is frequently perceived as a shameful condition or a normal part of aging rather than a manageable disease (100). This stigma, driven by a lack of understanding and cultural taboos, discourages families and individuals from seeking help, leading to increased isolation and exacerbating difficulties in managing the disease (101). Such social isolation contributes to higher rates of loneliness and depression, worsening mental health and creating a cycle of exclusion (102).
People living with dementia and caregivers emphasize the need for cultural competence in both societal and healthcare contexts to address this stigma and improve perceptions of dementia (49, 53). Cultural barriers and misconceptions significantly impact families’ experiences and expectations of dementia care, making culturally informed care essential (103). HCWs also recognize the importance of cultural competence, particularly in reducing stigmatization within intermediate care settings (33, 64). Fear of discrimination and social isolation can delay diagnosis and treatment, further hindering access to necessary support services (104).
Addressing these issues requires increasing dementia awareness to combat stigma and challenge the perception of dementia as a normal part of aging (105). HCWs must be trained to address stigma and fears associated with dementia to better support diagnosed individuals and their caregivers (106).
4.5 Needs of minority groups
Minority groups express a critical need for deeper cultural and religious understanding in dementia care, including effective language support (37, 48, 51, 60). The lack of cultural sensitivity and adaptation by HCWs often results in inadequate care and limited access for these groups (107, 108). Challenges such as language barriers and insufficient culturally adapted assessment tools exacerbate these issues (109–111). Consequently, individuals from these groups may avoid seeking dementia care, underscoring the need for culturally sensitive approaches and improved access to services for all ethnic groups (112–116). Research highlights the importance of understanding diverse sociocultural factors and tailoring interventions to local contexts, especially in low- and middle-income countries (117–120).
4.6 Needs to improve access to dementia care
Dementia management necessitates an integrated approach that addresses various needs, including health and treatment requirements (31, 34) and environmental considerations (40, 59, 65). However, health and care systems are often overly complex and challenging for caregivers to navigate, which can lead to delays in seeking or accessing care and increased stress (121, 122), frequently leading to frustration and overload (123). Caregivers wanted robust support from HCWs and improved coordination of services to ensure continuity of care (32, 42, 53). Fragmentation in the care pathway can result in inconsistencies in the quality and continuity of care (124), significantly increasing healthcare costs (125, 126) and the risk of comorbidity (127–129). A proactive, person-centered approach that integrates services and includes attention to disability aids is crucial for providing effective and personalized care (27, 65). Integrated care systems facilitate rapid response to the assessment and management of needs of people living with dementia, highlighting the urgent need for functional and seamless dementia care pathways (130), encompassing specialized dementia care spaces and ensuring well-coordinated care (131). A crucial aspect is focusing on the specific needs of caregivers, with flexibility and sensitivity being key components for the successful adaptation of care for individuals with AD at different stages (85, 132). This approach promotes the well-being of individuals and ensures continuity across professional boundaries, ultimately improving access to specialized care and minimizing disruptions in care plans (130, 131, 133).
4.7 Barriers in dementia care
Several barriers can compromise access to care and the fulfillment of needs, including the complexity and fragmentation of the health and care system (54), difficulties in accessing support services (53), and navigating advanced care planning (21). The involvement of multiple agencies, professionals, and administrative hurdles (36) can exacerbate logistical barriers. Another barrier is the need for improved access to community services and user-centered care to address reluctance to challenge authority, fear of stigma (61), as well as disorientation and feelings of shame and guilt (64). Inequities in healthcare access can compromise adequate care, particularly in rural and deprived areas worldwide (134, 135). Consequently, international efforts are underway to enhance access to healthcare services for dementia care. These efforts aim to address these challenges, reduce stigma, prejudice, inequalities and associated costs, and improve the quality of life for affected families (115, 136–138).
4.8 Financial barriers and inequities
Lack of financial support is another barrier, especially due to the high costs of intermediate care, particularly for those living in rural areas (64). Financial constraints can restrict access to vital health and social services and high-quality treatments (109, 139–141), which may result in suboptimal disease management and deteriorating health. Therefore, interventions aimed at reducing financial inequalities could lead to improved health outcomes for older adults (142–144).
4.9 Needs to improve quality of life
There are also barriers to improving quality of life, such as a lack of time, social challenges related to physical activities, and the progression of diseases, which exacerbate these issues (29, 59). However, physical activity has a positive effect in mitigating cognitive decline associated with dementia (145, 146). Considering the positive impact of physical activity and the challenges faced by families, it becomes even more important to incentivize programs and initiatives that support and promote physical activity (147, 148).
5 Strengths and limitations
To our knowledge, this is the first review to provide an integrated synthesis of the main unmet needs of people living with dementia, their caregivers, and healthcare workers. Whereas most previous studies have examined these groups in isolation, this review considers them collectively, highlighting the interdependent nature of their experiences within dementia care systems and supporting the development of coordinated interventions that reflect the real-world complexity of dementia care. It features several strengths that contribute to the robustness of its conclusions. The review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, ensuring a transparent and meticulous review process that minimizes bias and enhances reproducibility. A comprehensive search strategy was employed, spanning multiple databases over a decade, to maximize the retrieval of pertinent literature and provide a thorough examination of the topic. The involvement of multiple reviewers bolsters the reliability of study selection and minimizes the likelihood of errors. Additionally, the systematic assessment of the methodological quality of the included studies strengthens the review’s findings by identifying potential biases and limitations within individual studies. The studies included in the review were conducted across various European countries and covered diverse roles, including informal caregivers, HCWs, and people living with dementia, analyzing these groups both collectively and separately to produce robust findings.
However, several limitations need to be acknowledged. One study included in the sample did not exclusively consist of people with a formal diagnosis of dementia. This could limit the generalizability of the findings, as the experiences and needs of individuals without an official diagnosis might differ significantly from those of individuals with diagnosed dementia. Including participants without a diagnosis could introduce variability in the data and affect the accuracy of the conclusions, making it more challenging to identify the specific needs and challenges faced solely by people living with dementia. Most studies did not specify the race and ethnicity of the participants, which limits the understanding of the needs across different racial and ethnic groups. Nonetheless, some studies did address the specific needs of ethnic minorities, providing valuable insights. An additional limitation to consider is the high number of studies conducted exclusively in the UK. However, nearly all findings identified in these studies were also confirmed by research conducted in other European countries. Furthermore, restricting the review to studies published in English and conducted in Europe may introduce language and geographical biases, potentially overlooking valuable insights from non-English literature or studies conducted in other regions. Additionally, reliance on published literature may lead to publication bias, as studies with positive results are more likely to be published, potentially skewing the overall findings. However, the themes identified may be relevant beyond Europe, and could offer useful insights for informing dementia care strategies in other global contexts, particularly in countries facing similar demographic and health system challenges.
6 Conclusion
This systematic review underscores the broad range of needs, unmet needs and barriers within dementia care systems in Europe, affecting people living with dementia, caregivers, and healthcare professionals. The findings highlight significant challenges in social inclusion and access to support services for families and people living with dementia, and the need for continuous training for healthcare workers and professionals. The economic impact of unmet also needs to be better understood. There is an urgent need for public policies that enhance support networks, improve resource availability, and promote culturally sensitive care approaches. Future research should focus on the development integrated strategies to better address these needs and ensure a more robust and effective dementia care framework. This could include exploring the role of technology (such as telehealth, assistive devices and online support platforms) in addressing unmet needs.
Author contributions
MS: Methodology, Supervision, Conceptualization, Writing – original draft, Formal analysis. MMe: Conceptualization, Methodology, Data curation, Formal analysis, Writing – original draft. CF: Writing – original draft, Methodology, Data curation, Investigation, Formal analysis. IS: Writing – original draft, Formal analysis, Methodology, Investigation, Data curation. FE: Writing – original draft, Formal analysis, Investigation. MMo: Methodology, Writing – original draft, Investigation, Formal analysis. LL: Writing – original draft, Methodology, Formal analysis, Investigation. GA: Data curation, Investigation, Writing – original draft, Formal analysis. ES: Writing – original draft, Writing – review & editing. MPS: Writing – review & editing, Writing – original draft. AO: Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. FR: Writing – review & editing, Writing – original draft. MT: Writing – review & editing, Writing – original draft. RP: Validation, Supervision, Writing – review & editing, Project administration, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Italian Ministry of Health, through the project HubLife Science – Digital Health (LSH-DH) PNC-E3-2022-23683267 - DHEAL-COM – CUP E63C22003790001, within the “National Plan for Complementary Investments - Innovative Health Ecosystem” - Unique Investment Code: PNC-E.3.
Conflict of interest
MMo has received financial support by the MUR PNRR Extended Partnership (MNESYS no. PE00000006, and DHEAL-COM no. PNC-E3-2022-23683267); research grants from the ECTRIMS-MAGNIMS, the UK MS Society, and Merck; and honoraria from Biogen, BMS Celgene, Ipsen, Jansenn, Merck, Novartis, Roche, and Sanofi-Genzyme; and serves as editorial board member in Neurology and Multiple Sclerosis Journal. AM is supported by the NIHR Applied Research Collaboration NW London. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. In the last 3 years, RP has received support from the UK MS Society (Award 146) and has taken part in advisory boards/consultancy for MSD, Sanofi, and BMS. MaS reported personal fees from Alexion, Biogen, Immunic, Merck, Novartis, Roche, Sanofi, and Viatris outside the submitted work. MMo and AO declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1605993/full#supplementary-material
Abbreviations
AD, Alzheimer’s disease; PLWD, people living with dementia; HCWs, healthcare workers; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; MMAT, Mixed Methods Appraisal Tool; GPs, general practitioners; PICO, population, intervention, comparison, outcome; COVID-19, coronavirus disease 2019.
Footnotes
References
1. Li, K, Li, A, Mei, Y, Zhao, J, Zhou, Q, Li, Y, et al. Trace elements and Alzheimer dementia in population-based studies: A bibliometric and meta-analysis. Environ Pollut. (2023) 318:120782. doi: 10.1016/j.envpol.2022.120782
2. G. B. D. D. F. Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. (2022) 7:e105–25. doi: 10.1016/S2468-2667(21)00249-8
3. Jonsson, L. The personal economic burden of dementia in Europe. Lancet Reg Health Eur. (2022) 20:100472. doi: 10.1016/j.lanepe.2022.100472
4. Todd, S, Barr, S, Roberts, M, and Passmore, AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. (2013) 28:1109–24. doi: 10.1002/gps.3946
5. Long, S., Benoist, C., and Weidner, W. World Alzheimer Report 2023: Reducing dementia risk: never too early, never too late. London, England. (2023). Available online at: https://www.alzint.org/u/World-Alzheimer-Report-2023.pdf. (Accessed September, 2024).
6. Winblad, B, Amouyel, P, Andrieu, S, Ballard, C, Brayne, C, Brodaty, H, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. (2016) 15:455–532. doi: 10.1016/S1474-4422(16)00062-4
7. Courtin, E, Jemiai, N, and Mossialos, E. Mapping support policies for informal carers across the European Union. Health Policy. (2014) 118:84–94. doi: 10.1016/j.healthpol.2014.07.013
8. Pinquart, M, and Sorensen, S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. (2003) 18:250–67. doi: 10.1037/0882-7974.18.2.250
9. Alzheimer's, A. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. (2016) 12:459–509. doi: 10.1016/j.jalz.2016.03.001
10. Vitaliano, PP, Zhang, J, and Scanlan, JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychol Bull. (2003) 129:946–72. doi: 10.1037/0033-2909.129.6.946
11. Jordan, G, Pope, M, Lambrou, A, Malla, A, and Iyer, S. Post-traumatic growth following a first episode of psychosis: a scoping review. Early Interv Psychiatry. (2017) 11:187–99. doi: 10.1111/eip.12349
12. Vandrevala, T, Samsi, K, Rose, C, Adenrele, C, Barnes, C, and Manthorpe, J. Perceived needs for support among care home staff providing end of life care for people with dementia: a qualitative study. Int J Geriatr Psychiatry. (2017) 32:155–63. doi: 10.1002/gps.4451
13. Walsh, D. Dementia care training manual for staff working in nursing and residential settings. London and Philadelphia: Jessica Kingsley Publishers (2006).
14. Carr, S., and Rowntree, F. Joseph. Pay, conditions and care quality in residential nursing and domiciliary services. York: Joseph Rowntree Foundation (in English), (2014), p. 7.
15. Pitfield, C, Shahriyarmolki, K, and Livingston, G. A systematic review of stress in staff caring for people with dementia living in 24-hour care settings. Int Psychogeriatr. (2011) 23:4–9. doi: 10.1017/S1041610210000542
17. Hussein, S, and Manthorpe, J. The dementia social care workforce in England: secondary analysis of a national workforce dataset. Aging Ment Health. (2012) 16:110–8. doi: 10.1080/13607863.2011.596808
18. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006
19. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
20. Hong, QN, Fàbregues, S, Bartlett, G, Boardman, F, Cargo, M, Dagenais, P, et al. The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. (2018) 34:285–91. doi: 10.3233/efi-180221
21. Dickinson, C, Bamford, C, Exley, C, Emmett, C, Hughes, J, and Robinson, L. Planning for tomorrow whilst living for today: the views of people with dementia and their families on advance care planning. Int Psychogeriatr. (2013) 25:2011–21. doi: 10.1017/S1041610213001531
22. Miranda-Castillo, C, Woods, B, and Orrell, M. The needs of people with dementia living at home from user, caregiver and professional perspectives: a cross-sectional survey. BMC Health Serv Res. (2013) 13:43. doi: 10.1186/1472-6963-13-43
23. Page, S, and Hope, K. Towards new ways of working in dementia: perceptions of specialist dementia care nurses about their own level of knowledge, competence and unmet educational needs. J Psychiatr Ment Health Nurs. (2013) 20:549–56. doi: 10.1111/jpm.12029
24. Somme, D, Gautier, A, Pin, S, and Corvol, A. General practitioner's clinical practices, difficulties and educational needs to manage Alzheimer's disease in France: analysis of national telephone-inquiry data. BMC Fam Pract. (2013) 14:81. doi: 10.1186/1471-2296-14-81
25. Bakker, C, de Vugt, ME, van Vliet, D, Verhey, F, Pijnenburg, YA, Vernooij-Dassen, MJ, et al. Unmet needs and health-related quality of life in young-onset dementia. Am J Geriatr Psychiatry. (2014) 22:1121–30. doi: 10.1016/j.jagp.2013.02.006
26. Bakker, C, de Vugt, ME, van Vliet, D, Verhey, FRJ, Pijnenburg, YA, Vernooij-Dassen, MJFJ, et al. The relationship between unmet care needs in young-onset dementia and the course of neuropsychiatric symptoms: a two-year follow-up study. Int Psychogeriatr. (2014) 26:1991–2000. doi: 10.1017/S1041610213001476
27. Bokberg, C, Ahlstrom, G, Karlsson, S, Hallberg, IR, and Janlov, AC. Best practice and needs for improvement in the chain of care for persons with dementia in Sweden: a qualitative study based on focus group interviews. BMC Health Serv Res. (2014) 14:596. doi: 10.1186/s12913-014-0596-z
28. Smith, F, Grijseels, MS, Ryan, P, and Tobiansky, R. Assisting people with dementia with their medicines: experiences of family carers. Int J Pharm Pract. (2015) 23:44–51. doi: 10.1111/ijpp.12158
29. Malthouse, R, and Fox, F. Exploring experiences of physical activity among people with Alzheimer's disease and their spouse carers: a qualitative study. Physiotherapy. (2014) 100:169–75. doi: 10.1016/j.physio.2013.10.002
30. van Wezel, N, Francke, AL, Kayan-Acun, E, Ljm Deville, W, van Grondelle, NJ, and Blom, MM. Family care for immigrants with dementia: The perspectives of female family carers living in The Netherlands. Dementia (London). (2016) 15:69–84. doi: 10.1177/1471301213517703
31. Eichler, T, Thyrian, JR, Hertel, J, Richter, S, Wucherer, D, Michalowsky, B, et al. Unmet Needs of Community-Dwelling Primary Care Patients with Dementia in Germany: Prevalence and Correlates. J Alzheimer's Dis. (2016) 51:847–55. doi: 10.3233/JAD-150935
32. De Cola, MC, Lo Buono, V, Mento, A, Foti, M, Marino, S, Bramanti, P, et al. Unmet needs for family caregivers of elderly people with dementia living in Italy: what do we know so far and what should we do next? Inquiry. (2017) 54:46958017713708. doi: 10.1177/0046958017713708
33. Gove, D, Small, N, Downs, M, and Vernooij-Dassen, M. General practitioners' perceptions of the stigma of dementia and the role of reciprocity. Dementia (London). (2017) 16:948–64. doi: 10.1177/1471301215625657
34. Bokberg, C, Ahlstrom, G, and Karlsson, S. Utilisation of formal and informal care and services at home among persons with dementia: a cross-sectional study. Scand J Caring Sci. (2018) 32:843–51. doi: 10.1111/scs.12515
35. Kerpershoek, L, de Vugt, M, Wolfs, C, Woods, B, Jelley, H, Orrell, M, et al. Needs and quality of life of people with middle-stage dementia and their family carers from the European Actifcare study. When informal care alone may not suffice. Aging Ment Health. (2018) 22:897–902. doi: 10.1080/13607863.2017.1390732
36. Pini, S, Ingleson, E, Megson, M, Clare, L, Wright, P, and Oyebode, JR. A needs-led framework for understanding the impact of caring for a family member with dementia. Gerontologist. (2018) 58:e68–77. doi: 10.1093/geront/gnx148
37. Berdai Chaouni, S, and De Donder, L. Invisible realities: caring for older Moroccan migrants with dementia in Belgium. Dementia (London). (2019) 18:3113–29. doi: 10.1177/1471301218768923
38. Janssen, N, Handels, RL, Sköldunger, A, Woods, B, Jelley, H, Edwards, RT, et al. Impact of untimely access to formal care on costs and quality of life in community dwelling people with dementia. J Alzheimer's Dis. (2018) 66:1165–74. doi: 10.3233/JAD-180531
39. Oliveira, D, Zarit, SH, and Orrell, M. Health-promoting self-care in family caregivers of people with dementia: the views of multiple stakeholders. Gerontologist. (2019) 59:e501–11. doi: 10.1093/geront/gnz029
40. Abreu, W, Tolson, D, Jackson, GA, Staines, H, and Costa, N. The relationship between frailty, functional dependence, and healthcare needs among community-dwelling people with moderate to severe dementia. Health Soc Care Community. (2019) 27:642–53. doi: 10.1111/hsc.12678
41. Mazurek, J, Szczesniak, D, Urbanska, K, Droes, RM, and Rymaszewska, J. Met and unmet care needs of older people with dementia living at home: personal and informal carers' perspectives. Dementia (London). (2019) 18:1963–75. doi: 10.1177/1471301217733233
42. Moreno-Camara, S, Palomino-Moral, PA, Moral-Fernandez, L, Frias-Osuna, A, Parra-Anguita, L, and Del-Pino-Casado, R. Perceived needs of the family caregivers of people with dementia in a mediterranean setting: a qualitative study. Int J Environ Res Public Health. (2019) 16:993. doi: 10.3390/ijerph16060993
43. Quinn, C, Nelis, SM, Martyr, A, Victor, C, Morris, RG, Clare, L, et al. Influence of positive and negative dimensions of dementia caregiving on caregiver well-being and satisfaction with life: findings from the IDEAL study. Am J Geriatr Psychiatry. (2019) 27:838–48. doi: 10.1016/j.jagp.2019.02.005
44. Minaya-Freire, A, Ramon-Aribau, A, Pou-Pujol, G, Fajula-Bonet, M, and Subirana-Casacuberta, M. Facilitators, barriers, and solutions in pain management for older adults with dementia. Pain Manag Nurs. (2020) 21:495–501. doi: 10.1016/j.pmn.2020.03.003
45. Foley, T, Sheehan, C, Jennings, AA, and O'Sullivan, T. A qualitative study of the dementia-care experiences and educational needs of physiotherapists in the Republic of Ireland. Physiotherapy. (2020) 107:267–74. doi: 10.1016/j.physio.2019.08.006
46. Frias, CE, Cabrera, E, and Zabalegui, A. Informal caregivers' roles in dementia: the impact on their quality of life. Life (Basel). (2020) 10:251. doi: 10.3390/life10110251
47. Giebel, C, Cannon, J, Hanna, K, Butchard, S, Eley, R, Gaughan, A, et al. Impact of COVID-19 related social support service closures on people with dementia and unpaid carers: a qualitative study. Aging Ment Health. (2021) 25:1281–8. doi: 10.1080/13607863.2020.1822292
48. Hossain, MZ, and Khan, HTA. Barriers to access and ways to improve dementia services for a minority ethnic group in England. J Eval Clin Pract. (2020) 26:1629–37. doi: 10.1111/jep.13361
49. Mitchell, G, McTurk, V, Carter, G, and Brown-Wilson, C. Emphasise capability, not disability: exploring public perceptions, facilitators and barriers to living well with dementia in Northern Ireland. BMC Geriatr. (2020) 20:525. doi: 10.1186/s12877-020-01933-w
50. Janssen, N, Handels, RL, Köhler, S, Gonçalves-Pereira, M, Marques, MJ, Irving, K, et al. Profiles of met and unmet needs in people with dementia according to caregivers' perspective: results from a European multicenter study. J Am Med Dir Assoc. (2020) 21:e1:1609–16. doi: 10.1016/j.jamda.2020.05.009
51. Monsees, J, Schmachtenberg, T, Hoffmann, W, Kind, A, Gilmore-Bykovskyi, A, Kim, AJ, et al. Dementia in people with a Turkish migration background: experiences and utilization of healthcare services. J Alzheimer's Dis. (2020) 77:865–75. doi: 10.3233/JAD-200184
52. Telenius, EW, Eriksen, S, and Rokstad, AMM. I need to be who I am: a qualitative interview study exploring the needs of people with dementia in Norway. BMJ Open. (2020) 10:e035886. doi: 10.1136/bmjopen-2019-035886
53. Varik, M, Medar, M, and Saks, K. Informal caregivers' experiences of caring for persons with dementia in Estonia: A narrative study. Health Soc Care Community. (2020) 28:448–55. doi: 10.1111/hsc.12877
54. Vullings, I, Labrie, N, Wammes, JD, de Bekker-Grob, EW, and MacNeil-Vroomen, J. Important components for Dutch in-home care based on qualitative interviews with persons with dementia and informal caregivers. Health Expect. (2020) 23:1412–9. doi: 10.1111/hex.13118
55. Rusowicz, J, Pezdek, K, and Szczepanska-Gieracha, J. Needs of Alzheimer's charges' caregivers in poland in the Covid-19 pandemic-an observational study. Int J Environ Res Public Health. (2021) 18:4493. doi: 10.3390/ijerph18094493
56. Barry, HE, McGrattan, M, Ryan, C, Passmore, AP, Robinson, AL, Molloy, GJ, et al. I just take them because I know the people that give them to me': A theory-informed interview study of community-dwelling people with dementia and carers' perspectives of medicines management. Int J Geriatr Psychiatry. (2021) 36:883–91. doi: 10.1002/gps.5488
57. Froelich, L, Lladó, A, Khandker, RK, Pedrós, M, Black, CM, Sánchez Díaz, EJ, et al. Quality of life and caregiver burden of Alzheimer's disease among community dwelling patients in europe: variation by disease severity and progression. J Alzheimers Dis Rep. (2021) 5:791–804. doi: 10.3233/ADR-210025
58. Carcavilla, N, Pozo, AS, González, B, Moral-Cuesta, D, Roldán, JJ, Erice, V, et al. Needs of dementia family caregivers in Spain during the COVID-19 pandemic. J Alzheimer's Dis. (2021) 80:533–7. doi: 10.3233/JAD-201430
59. Farina, N, Williams, A, Clarke, K, Hughes, LJ, Thomas, S, Lowry, RG, et al. Barriers, motivators and facilitators of physical activity in people with dementia and their family carers in England: dyadic interviews. Aging Ment Health. (2021) 25:1115–24. doi: 10.1080/13607863.2020.1727852
60. Nielsen, TR, Nielsen, DS, and Waldemar, G. Barriers in access to dementia care in minority ethnic groups in Denmark: a qualitative study. Aging Ment Health. (2021) 25:1424–32. doi: 10.1080/13607863.2020.1787336
61. Ryan, L. Accessing community dementia care services in Ireland: emotional barriers for caregivers. Health Soc Care Community. (2021) 29:1980–9. doi: 10.1111/hsc.13342
62. Schnelli, A, Hirt, J, and Zeller, A. Persons with dementia as internet users: what are their needs? A qualitative study. J Clin Nurs. (2021) 30:849–60. doi: 10.1111/jocn.15629
63. Timmons, S, O'Loughlin, C, Buckley, C, Cornally, N, Hartigan, I, Lehane, E, et al. Dementia palliative care: a multi-site survey of long term care STAFF'S education needs and readiness to change. Nurse Educ Pract. (2021) 52:103006. doi: 10.1016/j.nepr.2021.103006
64. Dibao-Dina, C, Oger, C, Foley, T, Torzsa, P, Lazic, V, Kreitmayer Peštiae, S, et al. Intermediate care in caring for dementia, the point of view of general practitioners: a key informant survey across Europe. Front Med (Lausanne). (2022) 9:1016462. doi: 10.3389/fmed.2022.1016462
65. Leroi, I, Wolski, L, Charalambous, AP, Constantinidou, F, Renaud, D, Dawes, P, et al. Support care needs of people with hearing and vision impairment in dementia: a European cross-national perspective. Disabil Rehabil. (2022) 44:5069–81. doi: 10.1080/09638288.2021.1923071
66. Michelet, M, Selbaek, G, Strand, BH, Lund, A, Engedal, K, Bieber, A, et al. Associations between unmet needs for daytime activities and company and scores on the Neuropsychiatric Inventory-Questionnaire in people with dementia: a longitudinal study. Aging Ment Health. (2022) 26:725–34. doi: 10.1080/13607863.2021.1910792
67. Mank, A, van Maurik, IS, Rijnhart, JJM, Rhodius-Meester, HFM, Visser, LNC, Lemstra, AW, et al. Determinants of informal care time, distress, depression, and quality of life in care partners along the trajectory of Alzheimer's disease. Alzheimers Dement (Amst). (2023) 15:e12418. doi: 10.1002/dad2.12418
68. Sexton, C, Snyder, HM, Chandrasekaran, L, Worley, S, and Carrillo, MC. Expanding representation of low and middle income countries in global dementia research: commentary from the Alzheimer's association. Front Neurol. (2021) 12:633777. doi: 10.3389/fneur.2021.633777
69. van der Lee, J, Bakker, TJ, Duivenvoorden, HJ, and Droes, RM. Multivariate models of subjective caregiver burden in dementia: a systematic review. Ageing Res Rev. (2014) 15:76–93. doi: 10.1016/j.arr.2014.03.003
70. Chiao, CY, Wu, HS, and Hsiao, CY. Caregiver burden for informal caregivers of patients with dementia: a systematic review. Int Nurs Rev. (2015) 62:340–50. doi: 10.1111/inr.12194
71. Cheng, ST. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. (2017) 19:64. doi: 10.1007/s11920-017-0818-2
72. Storti, LB, Quintino, DT, Silva, NM, Kusumota, L, and Marques, S. Neuropsychiatric symptoms of the elderly with Alzheimer's disease and the family caregivers' distress. Rev Lat Am Enfermagem. (2016) 24:e2751. doi: 10.1590/1518-8345.0580.2751
73. Pinyopornpanish, K, Soontornpun, A, Wongpakaran, T, Wongpakaran, N, Tanprawate, S, Pinyopornpanish, K, et al. Impact of behavioral and psychological symptoms of Alzheimer's disease on caregiver outcomes. Sci Rep. (2022) 12:14138. doi: 10.1038/s41598-022-18470-8
74. Jung, S, Song, JA, Kim, J, Cheon, H, and Kim, J. Family caregiver competence in managing behavioral and psychological symptoms of dementia: a concept synthesis. Jpn J Nurs Sci. (2022) 19:e12462. doi: 10.1111/jjns.12462
75. Li, B, Liu, D, Wan, Q, Sheng, C, Wang, X, Leng, F, et al. Differences in treatment for Alzheimer's disease between urban and rural areas in China. Front Neurol. (2022) 13:996093. doi: 10.3389/fneur.2022.996093
76. Lai, CC, Wang, JH, Ko, WC, Yen, MY, Lu, MC, Lee, CM, et al. COVID-19 in long-term care facilities: an upcoming threat that cannot be ignored. J Microbiol Immunol Infect. (2020) 53:444–6. doi: 10.1016/j.jmii.2020.04.008
77. Flint, AJ, Bingham, KS, and Iaboni, A. Effect of COVID-19 on the mental health care of older people in Canada. Int Psychogeriatr. (2020) 32:1113–6. doi: 10.1017/S1041610220000708
78. Sundstrom, A, Adolfsson, AN, Nordin, M, and Adolfsson, R. Loneliness increases the risk of all-cause dementia and Alzheimer's disease. J Gerontol B Psychol Sci Soc Sci. (2020) 75:919–26. doi: 10.1093/geronb/gbz139
79. Wang, H, Li, T, Barbarino, P, Gauthier, S, Brodaty, H, Molinuevo, JL, et al. Dementia care during COVID-19. Lancet. (2020) 395:1190–1. doi: 10.1016/S0140-6736(20)30755-8
80. Borges-Machado, F, Barros, D, Ribeiro, O, and Carvalho, J. The effects of COVID-19 home confinement in dementia care: physical and cognitive decline, severe neuropsychiatric symptoms and increased caregiving burden. Am J Alzheimers Dis Other Dement. (2020) 35:1533317520976720. doi: 10.1177/1533317520976720
81. Altieri, M, and Santangelo, G. The psychological impact of COVID-19 pandemic and lockdown on caregivers of people with dementia. Am J Geriatr Psychiatry. (2021) 29:27–34. doi: 10.1016/j.jagp.2020.10.009
82. Carpinelli Mazzi, M, Iavarone, A, Musella, C, de Luca, M, de Vita, D, Branciforte, S, et al. Time of isolation, education and gender influence the psychological outcome during COVID-19 lockdown in caregivers of patients with dementia. Eur Geriatr Med. (2020) 11:1095–8. doi: 10.1007/s41999-020-00413-z
83. Rosa, R, Rosa, RDLD, Simões-Neto, JP, Santos, RL, Torres, B, Baptista, MAT, et al. Caregivers' resilience in mild and moderate Alzheimer's disease. Aging Ment Health. (2020) 24:250–8. doi: 10.1080/13607863.2018.1533520
84. Clay, OJ, Roth, DL, Wadley, VG, and Haley, WE. Changes in social support and their impact on psychosocial outcome over a 5-year period for African American and White dementia caregivers. Int J Geriatr Psychiatry. (2008) 23:857–62. doi: 10.1002/gps.1996
85. Soong, A, Au, ST, Kyaw, BM, Theng, YL, and Tudor Car, L. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatr. (2020) 20:61. doi: 10.1186/s12877-020-1454-y
86. McCabe, M, You, E, and Tatangelo, G. Hearing their voice: a systematic review of dementia family caregivers' needs. Gerontologist. (2016) 56:e70–88. doi: 10.1093/geront/gnw078
87. Novais, T, Dauphinot, V, Krolak-Salmon, P, and Mouchoux, C. How to explore the needs of informal caregivers of individuals with cognitive impairment in Alzheimer's disease or related diseases? A systematic review of quantitative and qualitative studies. BMC Geriatr. (2017) 17:86. doi: 10.1186/s12877-017-0481-9
88. Laparidou, D, Middlemass, J, Karran, T, and Siriwardena, AN. Caregivers' interactions with health care services - mediator of stress or added strain? Experiences and perceptions of informal caregivers of people with dementia – a qualitative study. Dementia (London). (2019) 18:2526–42. doi: 10.1177/1471301217751226
89. Huis In Het Veld, JG, Verkaik, R, van Meijel, B, Verkade, P-J, Werkman, W, Hertogh, CMPM, et al. Self-management support and eHealth when managing changes in behavior and mood of a relative with dementia: an asynchronous online focus group study of family caregivers' needs. Res Gerontol Nurs. (2018) 11:151–9. doi: 10.3928/19404921-20180216-01
90. Boughtwood, D, Shanley, C, Adams, J, Santalucia, Y, Kyriazopoulos, H, Pond, D, et al. Dementia information for culturally and linguistically diverse communities: sources, access and considerations for effective practice. Aust J Prim Health. (2012) 18:190–6. doi: 10.1071/PY11014
91. Jensen, CJ, and Inker, J. Strengthening the dementia care triad: identifying knowledge gaps and linking to resources. Am J Alzheimers Dis Other Dement. (2015) 30:268–75. doi: 10.1177/1533317514545476
92. Gaugler, JE, Reese, M, and Mittelman, MS. Effects of the NYU caregiver intervention-adult child on residential care placement. Gerontologist. (2013) 53:985–97. doi: 10.1093/geront/gns193
93. Lu, YY, Bakas, T, Yang, Z, Weaver, MT, Austrom, MG, and Haase, JE. Feasibility and effect sizes of the revised daily engagement of meaningful activities intervention for individuals with mild cognitive impairment and their caregivers. J Gerontol Nurs. (2016) 42:45–58. doi: 10.3928/00989134-20160212-08
94. Austrom, MG, Geros, KN, Hemmerlein, K, McGuire, SM, Gao, S, Brown, SA, et al. Use of a multiparty web based videoconference support group for family caregivers: innovative practice. Dementia (London). (2015) 14:682–90. doi: 10.1177/1471301214544338
95. Opfer, VD, and Pedder, D. Conceptualizing teacher professional learning. Rev Educ Res. (2011) 81:376–407. doi: 10.3102/0034654311413609
96. Brooks, HL, Pontefract, SK, Vallance, HK, Hirsch, CA, Hughes, E, Ferner, RE, et al. Perceptions and impact of mandatory elearning for foundation trainee doctors: a qualitative evaluation. PLoS One. (2016) 11:e0168558. doi: 10.1371/journal.pone.0168558
97. Gulla, C, Flo, E, Kjome, RLS, and Husebo, BS. Implementing a novel strategy for interprofessional medication review using collegial mentoring and systematic clinical evaluation in nursing homes (COSMOS). BMC Geriatr. (2019) 19:130. doi: 10.1186/s12877-019-1139-6
98. Cooper, E, Spilsbury, K, McCaughan, D, Thompson, C, Butterworth, T, and Hanratty, B. Priorities for the professional development of registered nurses in nursing homes: a Delphi study. Age Ageing. (2017) 46:39–45. doi: 10.1093/ageing/afw160
99. Griffiths, AW, Kelley, R, Garrod, L, Perfect, D, Robinson, O, Shoesmith, E, et al. Barriers and facilitators to implementing dementia care mapping in care homes: results from the DCM EPIC trial process evaluation. BMC Geriatr. (2019) 19:37. doi: 10.1186/s12877-019-1045-y
100. Cahill, S, Pierce, M, Werner, P, Darley, A, and Bobersky, A. A systematic review of the public's knowledge and understanding of Alzheimer's disease and dementia. Alzheimer Dis Assoc Disord. (2015) 29:255–75. doi: 10.1097/WAD.0000000000000102
101. Kerpershoek, L, Wolfs, C, Verhey, F, Jelley, H, Woods, B, Bieber, A, et al. Optimizing access to and use of formal dementia care: qualitative findings from the European actifcare study. Health Soc Care Community. (2019) 27:e814–23. doi: 10.1111/hsc.12804
102. Urbańska, K, Szcześniak, D, and Rymaszewska, J. The stigma of dementia. Postepy Psychiatr Neurol. (2015) 24:225–30. doi: 10.1016/j.pin.2015.10.001
103. Low, LF, and Purwaningrum, F. Negative stereotypes, fear and social distance: a systematic review of depictions of dementia in popular culture in the context of stigma. BMC Geriatr. (2020) 20:477. doi: 10.1186/s12877-020-01754-x
104. Mahoney, DF, Cloutterbuck, J, Neary, S, and Zhan, L. African American, Chinese, and Latino family caregivers' impressions of the onset and diagnosis of dementia: cross-cultural similarities and differences. Gerontologist. (2005) 45:783–92. doi: 10.1093/geront/45.6.783
105. Cations, M, Radisic, G, Crotty, M, and Laver, KE. What does the general public understand about prevention and treatment of dementia? A systematic review of population-based surveys. PLoS One. (2018) 13:e0196085. doi: 10.1371/journal.pone.0196085
106. Haralambous, B, Mackell, P, Lin, X, Fearn, M, and Dow, B. Improving health literacy about dementia among older Chinese and Vietnamese Australians. Aust Health Rev. (2018) 42:5–9. doi: 10.1071/AH17056
107. Nielsen, TR, Andersen, BB, Kastrup, M, Phung, TK, and Waldemar, G. Quality of dementia diagnostic evaluation for ethnic minority patients: a nationwide study. Dement Geriatr Cogn Disord. (2011) 31:388–96. doi: 10.1159/000327362
108. Giebel, C, Challis, D, Worden, A, Jolley, D, Bhui, KS, Lambat, A, et al. Perceptions of self-defined memory problems vary in south Asian minority older people who consult a GP and those who do not: a mixed-method pilot study. Int J Geriatr Psychiatry. (2016) 31:375–83. doi: 10.1002/gps.4337
109. Parveen, S, Peltier, C, and Oyebode, JR. Perceptions of dementia and use of services in minority ethnic communities: a scoping exercise. Health Soc Care Community. (2017) 25:734–42. doi: 10.1111/hsc.12363
110. Casas, R, Guzmán-Vélez, E, Cardona-Rodriguez, J, Rodriguez, N, Quiñones, G, Izaguirre, B, et al. Interpreter-mediated neuropsychological testing of monolingual Spanish speakers. Clin Neuropsychol. (2012) 26:88–101. doi: 10.1080/13854046.2011.640641
111. Sagbakken, M, Spilker, RS, and Nielsen, TR. Dementia and immigrant groups: a qualitative study of challenges related to identifying, assessing, and diagnosing dementia. BMC Health Serv Res. (2018) 18:910. doi: 10.1186/s12913-018-3720-7
112. Mukadam, N, Cooper, C, and Livingston, G. A systematic review of ethnicity and pathways to care in dementia. Int J Geriatr Psychiatry. (2011) 26:12–20. doi: 10.1002/gps.2484
113. Giebel, C, Cations, M, Draper, B, and Komuravelli, A. Ethnic disparities in the uptake of anti-dementia medication in young and late-onset dementia. Int Psychogeriatr. (2023) 35:381–90. doi: 10.1017/S1041610220000794
114. Cooper, C, Tandy, AR, Balamurali, TB, and Livingston, G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. (2010) 18:193–203. doi: 10.1097/JGP.0b013e3181bf9caf
115. Mukadam, N, Cooper, C, and Livingston, G. Improving access to dementia services for people from minority ethnic groups. Curr Opin Psychiatry. (2013) 26:409–14. doi: 10.1097/YCO.0b013e32835ee668
116. Hurley, S, Turnbull, S, and Calia, C. Barriers and facilitators to diagnosing dementia in migrant populations: a systematic review of European health professionals' perspectives. Int J Geriatr Psychiatry. (2024) 39:e6118. doi: 10.1002/gps.6118
117. Alladi, S, and Hachinski, V. World dementia: one approach does not fit all. Neurology. (2018) 91:264–70. doi: 10.1212/WNL.0000000000005941
118. Warren, LA, Shi, Q, Young, K, Borenstein, A, and Martiniuk, A. Prevalence and incidence of dementia among indigenous populations: a systematic review. Int Psychogeriatr. (2015) 27:1959–70. doi: 10.1017/S1041610215000861
119. Zeng, F, Xie, WT, Wang, YJ, Luo, HB, Shi, XQ, Zou, HQ, et al. General public perceptions and attitudes toward Alzheimer's disease from five cities in China. J Alzheimer's Dis. (2015) 43:511–8. doi: 10.3233/JAD-141371
120. Schouten, BC, Cox, A, Duran, G, Kerremans, K, Banning, LK, Lahdidioui, A, et al. Mitigating language and cultural barriers in healthcare communication: toward a holistic approach. Patient Educ Couns. (2020) 103:2604–8. doi: 10.1016/j.pec.2020.05.001
121. Cations, M, Withall, A, Horsfall, R, Denham, N, White, F, Trollor, J, et al. Why aren't people with young onset dementia and their supporters using formal services? Results from the INSPIRED study. PLoS One. (2017) 12:e0180935. doi: 10.1371/journal.pone.0180935
122. Bauer, M, Fetherstonhaugh, D, Blackberry, I, Farmer, J, and Wilding, C. Identifying support needs to improve rural dementia services for people with dementia and their carers: a consultation study in Victoria, Australia. Aust J Rural Health. (2019) 27:22–7. doi: 10.1111/ajr.12444
123. Innes, A, Morgan, D, and Kosteniuk, J. Dementia care in rural and remote settings: a systematic review of informal/family caregiving. Maturitas. (2011) 68:34–46. doi: 10.1016/j.maturitas.2010.10.002
124. Crabtree-Ide, C, Sevdalis, N, Bellohusen, P, Constine, LS, Fleming, F, Holub, D, et al. Strategies for improving access to cancer services in rural communities: a pre-implementation study. Front Health Serv. (2022) 2:818519. doi: 10.3389/frhs.2022.818519
125. Qayed, E, and Muftah, M. Frequency of hospital readmission and care fragmentation in gastroparesis: a nationwide analysis. World J Gastrointest Endosc. (2018) 10:200–9. doi: 10.4253/wjge.v10.i9.200
126. Kaltenborn, Z, Paul, K, Kirsch, JD, Aylward, M, Rogers, EA, Rhodes, MT, et al. Super fragmented: a nationally representative cross-sectional study exploring the fragmentation of inpatient care among super-utilizers. BMC Health Serv Res. (2021) 21:338. doi: 10.1186/s12913-021-06323-5
127. Walunas, TL, Jackson, KL, Chung, AH, Mancera-Cuevas, KA, Erickson, DL, Ramsey-Goldman, R, et al. Disease outcomes and care fragmentation among patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken). (2017) 69:1369–76. doi: 10.1002/acr.23161
128. Pinheiro, LC, Reshetnyak, E, Safford, MM, Nanus, D, and Kern, LM. Healthcare fragmentation and cardiovascular risk control among older cancer survivors in the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Cancer Surviv. (2021) 15:325–32. doi: 10.1007/s11764-020-00933-4
129. Kern, LM, Rajan, M, Ringel, JB, Colantonio, LD, Muntner, PM, Casalino, LP, et al. Healthcare fragmentation and incident acute coronary heart disease events: a cohort study. J Gen Intern Med. (2021) 36:422–9. doi: 10.1007/s11606-020-06305-z
130. Samsi, K, Abley, C, Campbell, S, Keady, J, Manthorpe, J, Robinson, L, et al. Negotiating a labyrinth: experiences of assessment and diagnostic journey in cognitive impairment and dementia. Int J Geriatr Psychiatry. (2014) 29:58–67. doi: 10.1002/gps.3969
131. Tang, JY, Wong, GH, Ng, CK, Kwok, DT, Lee, MN, Dai, DL, et al. Neuropsychological profile and dementia symptom recognition in help-seekers in a community early-detection program in Hong Kong. J Am Geriatr Soc. (2016) 64:584–9. doi: 10.1111/jgs.13938
132. Lee, K, Puga, F, Pickering, CEZ, Masoud, SS, and White, CL. Transitioning into the caregiver role following a diagnosis of Alzheimer's disease or related dementia: a scoping review. Int J Nurs Stud. (2019) 96:119–31. doi: 10.1016/j.ijnurstu.2019.02.007
133. Hobfoll, SE, Halbesleben, J, Neveu, J-P, and Westman, M. Conservation of resources in the organizational context: the reality of resources and their consequences. Annu Rev Organ Psychol Organ Behav. (2018) 5:103–28. doi: 10.1146/annurev-orgpsych-032117-104640
134. Watson, J, Green, MA, Giebel, C, Darlington-Pollock, F, and Akpan, A. Social and spatial inequalities in healthcare use among people living with dementia in England (2002-2016). Aging Ment Health. (2023) 27:1476–87. doi: 10.1080/13607863.2022.2107176
135. Rhew, SH, Jacklin, K, Bright, P, McCarty, C, Henning-Smith, C, and Warry, W. Rural health disparities in health care utilization for dementia in Minnesota. J Rural Health. (2023) 39:656–65. doi: 10.1111/jrh.12700
136. Morgan, DG, Crossley, M, Kirk, A, D’Arcy, C, Stewart, N, Biem, J, et al. Improving access to dementia care: development and evaluation of a rural and remote memory clinic. Aging Ment Health. (2009) 13:17–30. doi: 10.1080/13607860802154432
137. Milders, M, Bell, S, Lorimer, A, Jackson, H, and McNamee, P. Improving access to a multi-component intervention for caregivers and people with dementia. Dementia (London). (2019) 18:347–59. doi: 10.1177/1471301216672745
138. Rosvik, J, Michelet, M, Engedal, K, Bieber, A, Broda, A, Gonçalves-Pereira, M, et al. Interventions to enhance access to and utilization of formal community care services for home dwelling persons with dementia and their informal carers. A scoping review. Aging Ment Health. (2020) 24:200–11. doi: 10.1080/13607863.2018.1523876
139. Mukadam, N, Cooper, C, Basit, B, and Livingston, G. Why do ethnic elders present later to UK dementia services? A qualitative study. Int Psychogeriatr. (2011) 23:1070–7. doi: 10.1017/S1041610211000214
140. Berwald, S, Roche, M, Adelman, S, Mukadam, N, and Livingston, G. Black African and Caribbean British Communities' Perceptions of Memory Problems: "We Don't Do Dementia.". PLoS One. (2016) 11:e0151878. doi: 10.1371/journal.pone.0151878
141. Cooper, C, Lodwick, R, Walters, K, Raine, R, Manthorpe, J, Iliffe, S, et al. Observational cohort study: deprivation and access to anti-dementia drugs in the UK. Age Ageing. (2016) 45:148–54. doi: 10.1093/ageing/afv154
142. Sommers, BD, Baicker, K, and Epstein, AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. (2012) 367:1025–34. doi: 10.1056/NEJMsa1202099
143. Damiani, G, Federico, B, Visca, M, Agostini, F, and Ricciardi, W. The impact of socioeconomic level on influenza vaccination among Italian adults and elderly: a cross-sectional study. Prev Med. (2007) 45:373–9. doi: 10.1016/j.ypmed.2007.07.007
144. Aguila, E, Kapteyn, A, and Smith, JP. Effects of income supplementation on health of the poor elderly: the case of Mexico. Proc Natl Acad Sci USA. (2015) 112:70–5. doi: 10.1073/pnas.1414453112
145. Demurtas, J, Schoene, D, Torbahn, G, Marengoni, A, Grande, G, Zou, L, et al. Physical activity and exercise in mild cognitive impairment and dementia: an umbrella review of intervention and observational studies. J Am Med Dir Assoc. (2020) 21:1415–1422.e6. doi: 10.1016/j.jamda.2020.08.031
146. Brasure, M, Desai, P, Davila, H, Nelson, VA, Calvert, C, Jutkowitz, E, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med. (2018) 168:30–8. doi: 10.7326/M17-1528
147. Middleton, LE, Pelletier, CA, Koch, M, Norman, R, Dupuis, S, Astell, A, et al. The dementia-inclusive choices in exercise project: Using participatory action research to improve physical activity supports for persons with dementia. Dementia (London). (2023) 22:1651–76. doi: 10.1177/14713012231197144
Keywords: dementia, need, systematic review, Alzheimer’s disease, caregiver, healthcare workers, unmet needs
Citation: Sorrentino M, Mercogliano M, Fiorilla C, Stilo I, Esposito F, Moccia M, Lavorgna L, Affinito G, Salvatore E, Sormani MP, Odone A, Majeed A, Rubba F, Triassi M and Palladino R (2025) The needs and unmet needs for people living with dementia, caregivers and care workers in dementia health care systems: a systematic review. Front. Public Health. 13:1605993. doi: 10.3389/fpubh.2025.1605993
Edited by:
Monica Lauren Parker, Emory University, United StatesReviewed by:
Shirley Barbara Evans, University of Worcester, United KingdomMohamed Taiebine, UEMF, Morocco
Luca Cuffaro, University of Milano-Bicocca, Italy
Copyright © 2025 Sorrentino, Mercogliano, Fiorilla, Stilo, Esposito, Moccia, Lavorgna, Affinito, Salvatore, Sormani, Odone, Majeed, Rubba, Triassi and Palladino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaele Palladino, ci5wYWxsYWRpbm9AaW1wZXJpYWwuYWMudWs=
†These authors have contributed equally to this work and share first authorship
 Michele Sorrentino
Michele Sorrentino Michelangelo Mercogliano
Michelangelo Mercogliano Claudio Fiorilla
Claudio Fiorilla Irene Stilo
Irene Stilo Federica Esposito
Federica Esposito Marcello Moccia
Marcello Moccia Luigi Lavorgna
Luigi Lavorgna Giuseppina Affinito
Giuseppina Affinito Elena Salvatore
Elena Salvatore Maria Pia Sormani
Maria Pia Sormani Anna Odone
Anna Odone Azeem Majeed8
Azeem Majeed8 Fabiana Rubba
Fabiana Rubba Maria Triassi
Maria Triassi Raffaele Palladino
Raffaele Palladino